Fluorine-free electrolytes in batteries: principles, strategies, and advances
Boligarla
Vinay
 a,
Yosef
Nikodimos
a,
Yosef
Nikodimos
 *ac,
Tripti
Agnihotri
a,
Shadab Ali
Ahmed
b,
Teklay Mezgebe
Hagos
*ac,
Tripti
Agnihotri
a,
Shadab Ali
Ahmed
b,
Teklay Mezgebe
Hagos
 a,
Rehbar
Hasan
a,
Elango Balaji
Tamilarasan
a,
Wei-Nien
Su
a,
Rehbar
Hasan
a,
Elango Balaji
Tamilarasan
a,
Wei-Nien
Su
 *bc and
Bing Joe
Hwang
*bc and
Bing Joe
Hwang
 *acd
*acd
aNano-electrochemistry Laboratory, Department of Chemical Engineering, National Taiwan University of Science and Technology, Taipei City 106, Taiwan. E-mail: yosefni2001@gmail.com
bNano-electrochemistry Laboratory, Graduate Institute of Applied Science and Technology, National Taiwan University of Science and Technology, Taipei City 106, Taiwan. E-mail: wsu@mail.ntust.edu.tw
cSustainable Electrochemical Energy Development (SEED) Center, National Taiwan University of Science and Technology, Taipei City 106, Taiwan. E-mail: bjh@mail.ntust.edu.tw
dNational Synchrotron Radiation Research Center (NSRRC), Hsinchu 30076, Taiwan
First published on 10th April 2025
Abstract
Electrolytes play a pivotal role in battery technologies, influencing performance and safety. However, electrolytes containing fluorine present adverse environmental risks due to their high greenhouse gas emissions, which contribute to global warming. Hence, developing fluorine-free alternatives is imperative to design net-zero fluorine electrolytes. This review addresses the need for sustainable, low-toxicity electrolytes by exploring strategies for eliminating fluorine in the electrolyte system. Studies on the choice of electrolyte ingredients, such as fluorine-free salts, green solvents, safe additives, and fluorine-free binders, have demonstrated that specific electrolyte ingredients can effectively enhance battery performance and safety. Recent progress highlights significant improvements in the environmental impact and functionality of fluorine-free electrolytes (FFEs), demonstrating their potential for practical applications. Despite these advancements, challenges remain in matching the performance and stability of traditional fluorinated electrolytes. Future research encourages us to focus on developing fluorine-free materials, understanding functional degradation processes, and ensuring commercial scalability. This review provides an in-depth look at recent innovations and promotes design principles for complete fluorine elimination strategies. It guides future pathways for creating high-performance, non-flammable, low-cost, environmentally sustainable FFEs for advanced rechargeable batteries. The summary and perspectives emphasize the importance and future directions of a sustainable circular economy in advancing sustainable electrolyte engineering.
Broader contextElectrolytes are a key component of batteries, playing a crucial role in determining the performance and stability of batteries. Fluorinated electrolytes are commonly used in traditional batteries due to their high electrochemical stability and conductivity. However, these fluorinated electrolytes present significant challenges, as they are toxic and harmful to the environment. This has spurred ongoing research into finding alternatives, specifically fluorine-free electrolytes (FFEs), to develop fluorine-free batteries (FFBs) that are both environmentally friendly and cost-effective. Despite the need for FFEs, most current research still focuses on partially fluorinated components, whether in the form of salts, solvents, diluents, or additives. Only a limited number of studies have fully explored the use of FFEs. This review seeks to address this gap by highlighting the critical issues in designing FFBs and examining the latest advancements in fundamental research. It proposes several innovative design strategies that could help overcome current challenges, offering a bridge to future sustainable and efficient battery technologies. By focusing on FFEs, this review aims to contribute to developing safer batteries for both people and the planet. |
1. Introduction
In recent years, the global demand for advanced energy storage technologies has experienced a remarkable surge, driven by an increasing emphasis on sustainable energy solutions and the need for efficient power systems across various sectors.1 This growing demand is a direct consequence of the ever-expanding range of applications that rely on rechargeable batteries, which have become an integral part of modern society.2 From portable electronics that power our daily lives to electric vehicles that promise a greener future and even large-scale grid storage systems that stabilize our energy infrastructure, rechargeable batteries are at the forefront of technological innovation.3 However, despite their widespread adoption, several critical challenges have impeded these technologies’ broader implementation and optimization. Among the most pressing concerns are the environmental impact and safety risks associated with the materials used in these energy storage devices.4At the heart of any battery lies the electrolyte, a vital component that significantly influences the device's overall performance, safety, and longevity.5 The electrolyte's primary function is to facilitate the movement of ions between the battery's electrodes during charge and discharge cycles, thereby enabling the electrochemical reactions necessary for energy storage and release.6 Traditionally, the electrolytes used in commercial batteries have been predominantly based on fluorinated compounds.7–9 This preference for fluorinated electrolytes stems from their exceptional chemical and thermal stability, which ensures consistent performance even under demanding conditions. Additionally, these compounds offer high ionic conductivity, which is crucial for efficient charge transfer, and the ability to form stable interphases with the battery's electrodes, thereby enhancing the device's lifespan.10–12
Common fluorinated components found in traditional electrolytes include lithium hexafluorophosphate (LiPF6) salts and a variety of fluorinated solvents.13 LiPF6, in particular, has been a staple in the battery industry due to its favorable properties, such as good solubility in organic solvents and the formation of a protective solid electrolyte interphase (SEI) on the anode surface.14,15 Fluorinated solvents, on the other hand, contribute to the overall stability and conductivity of the electrolyte solution.16 Despite these advantageous characteristics, the use of fluorinated electrolytes is not without significant drawbacks.17,18 The production and disposal of fluorinated compounds present substantial environmental challenges, as they can lead to the release of toxic and persistent pollutants.19 These pollutants pose severe risks to both human health and the environment, as they can accumulate over time and cause long-term ecological damage.20 Recent advances have focused on improving performance by increasing the fluorine content of electrolytes and electrode components, which has been accomplished through the use of fluorinated salts, solvents, additives, and binders.8,21 These fluorinated chemicals, namely perfluoroalkyl and polyfluoroalkyl substances (PFAS), have played an important role in enhancing battery's thermal stability, chemical resistance, and overall performance, particularly under high-voltage conditions.8 However, the European Chemicals Agency (ECHA) has announced intentions to adopt a dramatic regulation change, limiting the use of almost 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 fluorinated compounds, including PFAS, in the battery industry.22,23 Notably, substances like lithium bis(trifluoromethanesulfonyl)imide (LiTFSI), poly(vinylidene fluoride) (PVDF), and 1,1,2,2-tetrafluoroethyl 2,2,3,3-tetrafluoropropyl ether (TTE), which are frequently used as salts, binders, and solvents in lithium-ion and other advanced battery systems, are especially problematic because of their toxic and bioaccumulative nature.24,25 The internal operation of cells releases hazardous HF and POF3 which then degrade the other battery components.26 In addition, toxic HF gas emission is a concern during the recycling of traditional batteries.27 These ongoing challenges influence to shut down the fluorinated electrolytes as shown in Fig. 1.27,28 Moreover, fluorinated electrolytes are associated with safety concerns, particularly regarding their thermal stability.29,30 In the event of thermal abuse, such as overheating or short-circuiting, fluorinated electrolytes can undergo decomposition reactions that generate hazardous gases and trigger exothermic reactions.31–33 This can result in thermal runaway, a dangerous phenomenon where the battery's temperature rapidly escalates, potentially leading to fire or explosion.34 These safety issues underscore the urgent need for safer, more environmentally friendly alternatives in battery technology.35–37
000 fluorinated compounds, including PFAS, in the battery industry.22,23 Notably, substances like lithium bis(trifluoromethanesulfonyl)imide (LiTFSI), poly(vinylidene fluoride) (PVDF), and 1,1,2,2-tetrafluoroethyl 2,2,3,3-tetrafluoropropyl ether (TTE), which are frequently used as salts, binders, and solvents in lithium-ion and other advanced battery systems, are especially problematic because of their toxic and bioaccumulative nature.24,25 The internal operation of cells releases hazardous HF and POF3 which then degrade the other battery components.26 In addition, toxic HF gas emission is a concern during the recycling of traditional batteries.27 These ongoing challenges influence to shut down the fluorinated electrolytes as shown in Fig. 1.27,28 Moreover, fluorinated electrolytes are associated with safety concerns, particularly regarding their thermal stability.29,30 In the event of thermal abuse, such as overheating or short-circuiting, fluorinated electrolytes can undergo decomposition reactions that generate hazardous gases and trigger exothermic reactions.31–33 This can result in thermal runaway, a dangerous phenomenon where the battery's temperature rapidly escalates, potentially leading to fire or explosion.34 These safety issues underscore the urgent need for safer, more environmentally friendly alternatives in battery technology.35–37
 | ||
| Fig. 1 Crisis to shut down the fluorinated electrolytes: regulatory restrictions, HF shutdown, and recycling risks. | ||
The research community has increasingly focused on developing FFEs in response to these pressing challenges.38–40 This emerging area of study aims to address the environmental and safety issues associated with traditional fluorinated compounds while striving to maintain or even enhance the electrochemical performance of batteries.21 FFEs encompass various materials, including alternative salts, solvents, additives, and diluents.41,42 Each component is carefully designed to be environmentally benign, cost-effective, and compatible with various battery chemistries. This offers a promising pathway toward more sustainable and safer energy storage solutions.43
Exploring FFEs is not merely a response to current challenges but also represents a proactive approach to future-proofing battery technology.44,45 As the world transitions toward cleaner energy sources and electric mobility, the demand for high-performance batteries will only increase.46,47 Therefore, the development of next-generation electrolytes that do not rely on fluorine is crucial for meeting future energy needs while minimizing environmental impact and enhancing safety.48,49 While various review studies have explored greener, safer batteries, there has been limited focus on FFEs. This comprehensive review delves into the principles underlying FFEs, the strategies employed in their design and synthesis, and the latest advances in this exciting field. By exploring the potential of fluorine-free alternatives, we aim to highlight the opportunities and challenges in creating a new paradigm for safe and sustainable energy storage.
Herein, we compare the characteristics and performance of fluorinated and FFEs, highlighting the motivations for transitioning to fluorine-free alternatives. Next, we explore various strategies for eliminating fluorine from electrolytes, including fluorine-free salts, solvents, additives, and diluents. We explore recent innovations in fluorine-free batteries (FFBs), emphasizing cutting-edge developments in material design and electrolyte formulation strategies. Furthermore, we examine the performance of FFEs in different battery chemistries, including lithium (Li), sodium (Na), magnesium (Mg), potassium (K), calcium (Ca), and zinc (Zn) batteries. The review also addresses the challenges and limitations of implementing FFEs, such as electrochemical stability and ionic conductivity. Finally, we present conclusions and outlooks on the future directions of FFE research, emphasizing the potential for these materials to enable safer, more sustainable, and high-performance battery systems. This comprehensive review aims to provide a valuable resource for researchers and industry professionals working towards developing sustainable next-generation battery technologies.
2. Fluorinated vs. fluorine-free electrolytes (FFEs)
The selection of an appropriate electrolyte is crucial for the design and performance optimization of battery systems.50 It plays an essential role in defining the device's efficiency, safety, and longevity.50,51 Among the various components of a battery, the electrolyte serves as the medium that facilitates the movement of ions between the anode and the cathode, thus enabling the electrochemical reactions necessary for energy storage and release.52 In commercial battery systems, particularly lithium-ion batteries (LIBs), fluorinated compounds have historically been the preferred electrolyte choice.7 These compounds are celebrated for their remarkable chemical stability, high ionic conductivity, and the formation of stable interphases with electrodes.53 These properties are critical for ensuring the reliable operation of batteries across various applications, ranging from consumer electronics to electric vehicles.54–56 However, the widespread use of fluorinated electrolytes has increasingly come under scrutiny due to their associated environmental and safety concerns. As a result, there has been a growing interest in exploring fluorine-free alternatives.44,57,58 This section delves into the fundamental differences between fluorinated and FFEs, examining their respective properties, performance, and the challenges they present.57Fluorinated electrolytes typically include fluorine-rich salts, such as LiPF6, commonly employed in LIBs. These salts are prized for their ability to efficiently facilitate ionic transport and their excellent solubility in non-aqueous solvents.59,60 LiPF6 is mainly known for forming a stable passivation layer, known as the SEI, on the surface of the anode.61 The SEI layer acts as a protective barrier, preventing unwanted reactions between the electrolyte and the electrode, safeguarding the electrode material and extending the battery's lifespan.62,63 The formation of the SEI layer predominantly relies on the presence of fluorinated species derived from various sources, including the salts, solvents, and additives used in the electrolyte composition. These fluorinated components collectively contribute to the robust performance characteristics of the battery.
One of the key features of fluorinated electrolytes is their ability to enhance battery performance by providing stability and safety. For example, the incorporation of 1-fluoroethylene carbonate (FEC) as a co-solvent with dimethyl carbonate (DMC) has been demonstrated to significantly improve the stability of high-rate stripping/plating processes and prolong the operational life of batteries, especially in anode-free lithium metal battery configurations.64–68 FEC is particularly advantageous for anodes made from silicon and lithium metal, which are susceptible to substantial volume changes and challenging chemical environments during cycling.69,70 The SEI layer formed in the presence of FEC typically consists of compounds such as lithium fluoride (LiF), lithium oxide (Li2O), and other fluorinated organic species.71 These components enhance the kinetics of lithiation and improve the battery's coulombic efficiency (CE), making fluorinated electrolytes a popular choice for high-performance applications.72,73 Fluorinated electrolytes, such as those containing LiPF6 in organic solvents, have been the standard in LIBs for several reasons.74 Firstly, fluorinated electrolytes exhibit high ionic conductivity, facilitating efficient ion transport between the anode and the cathode. This is crucial for achieving high power density and fast charging capabilities.44,75 Secondly, the strong C–F bond in fluorinated compounds provides excellent chemical stability.7,76 This stability is essential for maintaining the integrity of the electrolyte under various operating conditions, including high temperatures and voltages. Thirdly, fluorinated electrolytes can form a stable SEI layer on the anode surface, which protects the electrode from further reactions with the electrolyte, thereby enhancing the battery's cycle life and safety.11
Despite the numerous benefits of fluorinated electrolytes, they also present drawbacks such as generation of harmful byproducts66 like hydrogen fluoride (HF) and phosphorus pentafluoride (PF5).16 Over the past decade, non-aqueous electrolytes containing fluorinated ingredients have faced challenges due to their sensitivity to moisture during electrochemical reactions because of hydrolysis of fluorinated salts, solvent decomposition, or contamination. For instance, the hydrolysis of LiPF6 with trace water exacerbates this problem by producing corrosive HF, damaging key battery components and reducing efficiency.16,28,68,77 Additionally, releasing these gases heightens safety risks, potentially triggering thermal runaway, which can result in fire or explosion. Furthermore, the reaction generates toxic by-products, posing serious threats to both human health and the environment.28,77,78 Although many studies emphasize the degradation mechanism of fluorinated ingredients, especially LiPF6, the solutions to address the issue remain unclear.79–81 For example, Di Muzio et al.'s computational studies reveal that LiBOB (lithium bis(oxalato)borate) is more resistant to hydrolysis than LiBF4, which degrades via LiF/HF formation.82 The stability of the fluorine-free BOB− anion enhances moisture resistance, ensuring safer, long-lasting performance.
Beyond operational hazards, the disposal and recycling of PFAS-containing battery components pose significant environmental challenges, as demonstrated in Fig. 1. The release of toxic substances such as HF and PF5 during the disposal or recycling of spent batteries can have long-term adverse effects on the environment and public health.83 The management of these batteries requires stringent safety protocols to prevent contamination and ensure safe handling. In confined spaces, such as during electric vehicle accidents, releasing toxic gases can pose immediate dangers to first responders and the public, underscoring the need for safer electrolyte alternatives.84 Eventually, with growing concerns over the toxicity and environmental impact of PFAS, regulatory bodies like ECHA are intensifying efforts to impose stricter controls, promoting the development of fluorine-free alternatives.25 Economic concerns and potential slowdowns in battery innovation further complicate regulatory efforts.
In light of these challenges, there has been a burgeoning interest in developing FFEs that can deliver comparable performance while mitigating the environmental and safety issues associated with fluorinated compounds.54 The development of FFEs is centered around eliminating fluorine-containing compounds, which reduces the environmental footprint of batteries and enhances their safety profile.45
FFEs are characterized by their environmentally friendly nature, producing toxic-free and persistent decomposition products as illustrated in Fig. 2. Moreover, these electrolytes are less likely to generate hazardous gases upon decomposition, thereby reducing the risk of thermal runaway and improving the overall safety of the battery system.85 Eventually, the development of sustainable and effective energy storage systems has attracted attention, which has prompted research into cutting-edge materials that improve battery performance. For example, with recent advances in FFEs, LiBOB is a potential electrolyte salt for lithium-ion batteries due to its outstanding thermal stability and high-voltage endurance, and is a cost-effective PFAS-free alternative.25 It enables a stable CEI that prevents oxidative degradation and shields cathode materials at voltages as high as 5.3 V.86 LiBOB has been utilized as an HF scavenger, improving battery safety and cycle performance by lowering the production of dangerous free radicals.87 Additionally, it enhances compatibility with manganese and iron cathodes, inhibits electrolyte deterioration, and promotes improved cycle stability in high-energy systems.87 Particularly in traditional batteries, minute amounts of water in the electrolyte can lead to hydrolysis and affect the development of the solid electrolyte interface (SEI) on electrode surfaces.80 However, the hydrolysis of LiBOB offers significant benefits.88 LiBOB breaks down in water and forms non-toxic byproducts, creating a stable SEI that safeguards the battery's performance over time. LiBOB hydrolysis is slower and more regulated compared to other lithium salts, improving electrolyte stability.89 This characteristic not only improves cycle stability but also reduces negative environmental consequences by limiting the production of toxic PFAS.25 While LiBOB has great stability and is non-toxic, it confronts problems such as poor solubility in low-dielectric solvents and low ionic conductivity at low temperatures, resulting in increased resistance and performance decay.88 At voltages greater than 4.7 V, the failure of the CEI causes side reactions, significantly lowering performance.90
Consequently, the development of effective FFEs is not without its own set of challenges. One of the primary hurdles is achieving electrochemical stability and ionic conductivity. FFEs may be more susceptible to oxidation and reduction, potentially affecting the battery's performance and cycle life.45 Additionally, fluorine-free salts and solvents often exhibit lower ionic conductivity, which can limit the power density and efficiency of the battery.44,45
To address these challenges, researchers have been exploring a variety of non-fluorinated salts and solvents.44 Several anions, including those based on elements such as boron (B), carbon (C), oxygen (O), sulfur (S), chlorine (Cl), phosphorus (P), and nitrogen (N), have been investigated as potential alternatives to fluorinated compounds. While many of these anions contain fluorine, a few notable fluorine-free options have emerged, such as nitrate (NO3), dicyanotriazolate, bis(oxalato)borate (BOB), perchlorate (ClO4), and tris(oxalato) phosphate (TOP).91 These anions vary in their ionic conductivity and stability, with some showing promising results in laboratory settings.92
A critical aspect of designing effective FFEs is selecting suitable salts and solvents to form stable interphases at the electrode–electrolyte interface.58 The interaction between anions and solvent molecules is pivotal in determining the dissolution behavior of electrolyte salts in non-aqueous solvents.93 Weakly coordinating anions with delocalized charges are particularly desirable, as they can lower the dissociation energy of salts, thereby enhancing ionic conductivity.94,95 Furthermore, these anions can promote the formation of stable interphases at the electrode–electrolyte interface, which is crucial for maintaining the physical and electrochemical stability of the SEI layer.
Recent research has emphasized the significance of solvent structure and its influence on the reactivity of the electrolyte in lithium-metal batteries.96 The interactions between anions and solvent molecules can significantly impact the composition and stability of the SEI layer. For instance, a shift from solvent-dominant to anion-dominant interfacial reactions can alter the composition of the SEI, transforming it from organic-rich to inorganic-rich.54,97 This shift can enhance the physical and electrochemical stability of the SEI, thereby optimizing the performance and safety of lithium-metal battery systems.98 Despite the progress in developing FFEs, challenges remain, such as effectively passivating aluminum cathode current collectors at high potentials. In LIBs, LiPF6 is often favored over fluorinated salts like lithium bis(trifluoromethanesulfonyl)imide (LiTFSI) and lithium bis(fluorosulfonyl)imide (LiFSI) due to its superior ability to passivate aluminum surfaces.75,99–107 However, recent studies have suggested that some non-fluorinated salts can effectively passivate aluminum, providing a viable alternative to fluorinated salts.108–110
Table 1 provides a foundation for understanding the comparative landscape of fluorinated and FFEs. FFEs aim to address the limitations of fluorinated electrolytes by eliminating the use of fluorine-containing compounds.57 The development of FFEs focuses on maintaining desirable electrochemical properties while improving environmental sustainability and safety. Key characteristics of FFEs include their environmental friendliness, as they reduce the conservation footprint of batteries by eliminating fluorinated compounds.8,44,111 They are designed to be more benign, with less toxic and persistent decomposition products. Moreover, FFEs are less likely to produce hazardous gases upon decomposition, reducing the risk of thermal runaway and improving overall battery safety.111 However, developing effective FFEs presents several challenges. Achieving comparable electrochemical stability without fluorine can be difficult, as FFEs may have lower resistance to oxidation and reduction, affecting the battery's performance and cycle life. Additionally, fluorine-free salts and solvents may have lower ionic conductivity than their fluorinated counterparts, potentially limiting the power density and efficiency of the battery.7 Therefore, future advancements in materials science, electrolyte formulations, and battery engineering will be critical to overcoming these challenges and realizing the full potential of FFEs.
| Fluorinated electrolytes | Characteristics | FFEs |
|---|---|---|
| High | Ionic conductivity | Variable (generally lower than that of fluorinated counterparts) |
| Excellent (due to strong C–F bonds) | Chemical stability | Variable (depends on the composition) |
| Decompose to harmful gases | Thermal stability | Decompose to less hazardous products |
| Negative (toxic and persistent pollutants) | Environmental impact | More environmentally friendly, fewer toxic by-products |
| Can decompose into hazardous gases (e.g., HF, PF5) | Safety concerns | Lower risk of producing harmful gases and reduced thermal runaway risk |
| Wide voltage window | Electrochemical stability | Variable, generally narrower than that of fluorinated counterparts |
| Form a stable SEI on the anode | SEI formation | SEI formation varies; alternative protective interphases may be needed |
| High (due to specialized manufacturing and raw materials) | Cost | Potentially lower (depending on the materials used) |
| Long (due to the stable SEI) | Cycle life | Variable, depending on SEI formation and electrolyte stability |
| Widely used in commercial LIBs | Applications | Emerging and research-focused batteries |
3. Roadmap of progress and challenges in fluorine-free electrolytes
3.1. Roadmap of progress in fluorine-free electrolytes
Several recent studies have focused on pollution-free electrolyte design as a key aspect of next-generation research in the context of climate change. FFBs are increasingly popular for their sustainable and eco-friendly properties, and they often utilize metals such as Li, Na, Mg, Al, Si, K, Ca, and Zn. These batteries aim to provide high performance, cost-efficiency, and long cycle life while eliminating harmful fluorine compounds. Innovations include lithium bis(oxalato)borate (LiBOB) for LIBs, sodium bis(oxalato)borate (NaBOB) for Na-ion batteries, and magnesium borohydride (Mg(BH4)2) for Mg batteries. Additionally, researchers are exploring aluminum chloride (AlCl3) in ionic liquids, potassium bis(oxalato)borate (KBOB), calcium tetrafluoroborate (Ca (BF4)2), and zinc sulfate (ZnSO4) for their respective metal batteries. Challenges such as lower ionic conductivity, thermal stability, and effective interphase formation compared to fluorinated electrolytes persist. Several studies show optimized electrolyte formulations and synergistic additives to boost stability and conductivity. Innovative strategies, including ionic liquids and customized solvent systems, are being developed to ensure that these fluorine-free alternatives meet or surpass the performance of traditional batteries while maintaining environmental safety. Earlier studies on FFBs have motivated the pursuit of high performance, and fortunately, a few studies achieved highly reliable practical prospects. Among all FFBs, FFE studies are frequently performed in lithium-based batteries. As shown in Fig. 3, the developed FFBs showed enhanced oxidative stability owing to optimized electrolyte composition, engineered robust interphases, and widened potential window, all of which help prevent breakdown under extreme conditions. | ||
| Fig. 3 Roadmap showing the progress of FFE-based batteries.45,58,112–127 LMBs Reproduced with permission,45 Copyright (2024) American Chemical Society. LIBs Reproduced with permission,58 Copyright (2020) American Chemical Society. LIBs Reproduced with permission,112 Copyright (2024) Elsevier. LMBs Reproduced with permission,113 Copyright (2014) Elsevier. LIBs, Reproduced with permission,114 Copyright (2018) Springer Nature. Li–S battery, Reproduced with permission,115 Copyright (2019) Wiley-VCH. LIBs, Reproduced with permission,123 Copyright (2024) Wiley-VCH. LMBs, Reproduced with permission,126 Copyright (2025) Wiley-VCH. LIBs, Reproduced with permission,122 Copyright (2024) Wiley-VCH. Na battery Reproduced with permission,116 Copyright (2020) American Chemical Society. Na battery Reproduced with permission,117 Copyright (2021) American Chemical Society. Ca battery Reproduced with permission,118 Copyright (2021) Springer Nature. Micro-Si anode battery Reproduced with permission,120 Copyright (2023) Wiley-VCH. KMB Reproduced with permission,121 Copyright (2023) Springer Nature. Zn battery Reproduced with permission,119 Copyright (2023) Wiley-VCH. Zn battery Reproduced with permission,124 Copyright (2024) American Chemical Society. For monovalent and multivalent cations, Reproduced with permission,125 Copyright (2025) Wiley-VCH. | ||
To overcome PFAS toxicity, an FFE is a promising approach for future generations where all components are fluorine-free species. Recently, an FFE study was undertaken by Jiang et al. by using the borate anion in an ether solvent (1,2-dimethoxyethane (DME)), and a good cycling performance at high temperatures was demonstrated.45 In addition, incorporating a fluorine-free binder with good self-healing capabilities at a high loading LFP cathode boosted the capacity retention (CR) of Li-LFP cells to 82%, at 60 °C, as shown in Fig. 4a.45 The FFE study was conducted for the first time using the novel strategy as bilayer phenomena for robust SEI formation. This bilayer approach consists of organic (ROCO2, C–C, Li2CO3) and inorganic components (LiBxOy, Li2O, Li3N, LiNxOy). While thin electrolyte–electrode interfaces (EEL) with organic components (inner layer) offer fast ion transport and stable chemical and mechanical support, thick EEL with organic components can lead to an unstable SEI and impede ion transport. The inorganic components (outer layer) function as a protective shield for the battery components; they are crucial for forming a protective SEI, which provides excellent electronic insulation, promotes ionic conductivity, and reduces electrolyte consumption. Although their FFE emphasizes a novel strategy focused on bi-layered SEI formation which offers several synergistic benefits, their sole-solvent DME has a flash point ≤ 0, meaning the resulting electrolyte is highly flammable.128 Shuai et al. developed FFEs by using 3-methyl-2-oxazolidinone (MO) as the sole solvent, which has a high donor number (DN: 27), meaning that it has high LiNO3 solubility to facilitate high ionic conductivity (3.2 × 10−3 S cm−1) and promote an inorganic-rich SEI with Li3N and LiNxOy species. As shown in Fig. 4b, their SEI stability enabled 91.4% capacity retention after 1000 cycles in Li‖NMC622. However, their sole solvent is limited to >1 M LiNO3 due to increasing viscosity and deterioration in cycling performance.
 | ||
Fig. 4 Electrochemical performance of fluorine-free batteries; (a) lithium metal battery Li‖LFP@1 M LiBOB + 0.5 M LiNO3 in a DME electrolyte, Reproduced with permission,45 Copyright (2024) American Chemical Society. (b) High-voltage lithium metal battery pouch cell Li‖NMC622@1 M LiNO3 MO electrolyte, Reproduced with permission,127 Copyright (2025) Elsevier. (c) High-voltage lithium metal battery Li‖NMC811@1 M LiClO4 + 0.2 M LiNO3 TEP electrolyte, Reproduced with permission,126 Copyright (2025) Wiley-VCH. (d) Sodium-ion battery Prussian white|hard carbon‖Na@0.5 M NaBOB/TMP electrolyte, Reproduced with permission,116 Copyright (2020) American Chemical Society. (e) KMB K‖PTCDA@0.1 M KBPh4 EC/DEC, Reproduced with permission,121 Copyright (2023) Springer Nature. (f) Calcium battery Ca‖S/C@0.5 M CMC, DME/THF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) 0.5 to 3.2 V; dQ/dV in the inset, Reproduced with permission,118 Copyright (2021) Springer Nature. (g) Zinc battery Zn‖Zn@1.0 M Zn(dca)2/DMSO electrolyte, Reproduced with permission,119 Copyright (2023) Wiley-VCH. 1, v/v) 0.5 to 3.2 V; dQ/dV in the inset, Reproduced with permission,118 Copyright (2021) Springer Nature. (g) Zinc battery Zn‖Zn@1.0 M Zn(dca)2/DMSO electrolyte, Reproduced with permission,119 Copyright (2023) Wiley-VCH. | ||
Gabert et al. studied the interfacial decomposition of the triethyl phosphate (TEP) based FFE system in LIBs, revealing that >30% of TEP caused strong Li+ solvation leading to graphite co-intercalation, solvent decomposition, more gas evolution and unstable SEI formation.122 In addition, Xu et al. demonstrated the in situ formation of heterostructure Li3N/Li2O interfaces by utilizing dual salts (1 M LiClO4 + 0.2 M LiNO3) in sole solvent TEP for high-voltage LMBs.126 Their studies evidently showed that bilayer SEI engineering significantly contributed to several advantages; the SEI comprised of inorganic species of Li3N/Li2O facilitated high ionic conductivity and fast Li+ transport, whereas that consisting of organic species formed a protective elasticity layer to reduce the electron tunnelling. In addition, LiCl/Li3N inorganic CEI species reinforce the interfacial layer by improving ionic conductivity and also the high-voltage Li‖NMC811 cell showed excellent electrochemical performance, achieving 82.11% capacity retention after 500 cycles at 60 °C as shown in Fig. 4c.126 These studies all utilized TEP solvent, showing both the promise and challenges of TEP-based electrolytes, underscoring the need for strategies to mitigate decomposition while promoting non-flammable and solvation benefits.122,126 Khan et al. have developed a biomass-based electrolyte, paving the way for the next generation to incorporate bio-based materials and achieve a sustainable scale.38 Furthermore, such promising approaches need more investigations towards electrochemical stability. The development of FFE has been reported by utilizing the commonly used fluorine-free ingredients to establish a benchmark of FFEs in lithium-based battery. Although FFEs with LiBxOy, Li2O, Li3N, and LiCl species have shown promise in stabilizing lithium metal interfaces, their anionic frameworks remain constrained by poor solubility, gas evolution, and electrode corrosion, limiting their applicability to lithium-based systems. This challenge becomes more pronounced with the demand for multivalent batteries, where electrolyte compatibility plays a pivotal role. Therefore, the rational design of innovative fluorine-free anions is imperative to unlock broader energy storage systems, paving the way for next-generation multivalent battery technologies.
In this new era, sodium-based batteries are critical in propelling a cleaner, more sustainable society. The switch from lithium to sodium represents a significant technological advancement, promoting a more sustainable and resource-efficient future. Greener battery development has persisted due to using FFEs in sodium batteries. Few studies of FFEs in sodium batteries have been explored in the past years. Building on advances in lithium-based FFE systems, similar approaches have also been investigated in sodium batteries. Mogensen et al. developed a fluorine-free sustainable electrolyte based on 0.5 M NaBOB in trimethyl phosphate (TMP).116 The electrolyte displayed a high ionic conductivity (5 mS cm−1) and superior electrochemical performance with a CE of 80% in the first cycle. It increased to 97% in the following cycles, as shown in Fig. 4b, and this combination has produced a secure and economical solution.116 This work was extended by incorporating N-methyl-2-pyrrolidone (NMP) as a co-solvent, which attained a high conductivity of 8.83 mS cm−1.117 This combination boosts battery safety and efficiency, with vinylene carbonate (VC) additive further enhancing performance. Furthermore, Buckel et al. investigated the same combination (NaBOB-TMP) for long cycling by high mass loading Prussian white|hard carbon cathode.129 Although sodium batteries have achieved total fluorine removal through sustainable strategies (non-flammable, non-toxic, eco-friendly), their FFE combinations and electrochemical performance are still constrained. The potassium metal battery (KMB) study has recently been explored in the FFE design. Previous studies of KMB development have identified challenges like dendrite formation and fast capacity fading.121,130 Liu et al. designed an ultra-low concentrated FFE using EC and DEC carbonate solvent with 0.1 M KBPh4. The fluorine-free anion generated a stable SEI layer and allowed reversible cycling for the PTCDA/K full cell, as shown in Fig. 4e. This promising approach could stimulate future research to deepen the understanding of anion-dominant chemistry and interfacial stability of K-batteries. Due to concerns about resource availability and safety issues with LIBs, Ca and Mg batteries are being explored as substitutes. Ca is superior to Mg in ion transport and has a higher energy density. Nevertheless, there aren’t many appropriate room temperature electrolytes for Ca. Ponrouch et al. designed an electrolyte, Ca(BF4)2, in EC/PC at ∼100 °C which enabled reversible plating and stripping.131 However, CaF2 formation alongside Ca metal deposition impedes migration, restricting plating/stripping efficiency.131,132 Conventional electrolytes create films of blockage that impede their effectiveness. Recent developments include Ca(BH4)2 in THF, which is fluorine-free but less stable, and Ca[B(hfip)4]2 (hfip![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexafluoroisopropyloxy) in DME, which has high stability but CaF2 problems.133–135 Creating a fluorine-free, stable, high-conductivity Ca electrolyte is essential. For example, Kisu et al. developed a Ca electrolyte devoid of fluorine that contained calcium monocarborane (CMC@ Ca[CB11H12]2). It provided a broad potential range (up to 4 V vs. Ca2+/Ca) and high conductivity (4 mS cm−1) with reversible Ca plating/stripping at room temperature. Fig. 4f shows the outstanding electrochemical performance of a Ca–S battery using a fluorine-free (0.5 M CMC, DME/THF) electrolyte system, which produced an initial discharge capacity of 805 mA h g−1.
hexafluoroisopropyloxy) in DME, which has high stability but CaF2 problems.133–135 Creating a fluorine-free, stable, high-conductivity Ca electrolyte is essential. For example, Kisu et al. developed a Ca electrolyte devoid of fluorine that contained calcium monocarborane (CMC@ Ca[CB11H12]2). It provided a broad potential range (up to 4 V vs. Ca2+/Ca) and high conductivity (4 mS cm−1) with reversible Ca plating/stripping at room temperature. Fig. 4f shows the outstanding electrochemical performance of a Ca–S battery using a fluorine-free (0.5 M CMC, DME/THF) electrolyte system, which produced an initial discharge capacity of 805 mA h g−1.
As a safer, more affordable, and plentiful substitute, zinc batteries show great promise. Though they have problems with zinc dendrites and electrolyte stability, they provide larger capacities. A novel electrolyte that exhibited superior performance, high conductivity, stability, and smooth zinc deposits is zinc dicyanamide (Zn(dca)2) in dimethyl sulfoxide (DMSO) by Kar et al., as shown in Fig. 4g.119 As the concentration of Zn(dca)2 increased, a zinc nitride (Zn2N3) rich SEI was formed on the anode. While sodium intercalation improved zinc's sluggish reaction kinetics in hybrid batteries with polyanion cathodes, mixed salts, such as Zn and Na, enhanced ion transport and electrochemical reversibility, and widened the potential window. They extended studies on a dual-cation electrolyte, [1.0 M Na(dca) + 1.0 M Zn(dca)2]/DMSO (NaZn), which facilitated stable cycling over 100 cycles at a 0.1C rate in Zn|NFP (t-NaFePO4).124 The addition of Na(dca) improved Zn2+ transport, resulting in high ionic conductivity (7.9 mS cm−1) and a high zinc transference number (t(zn2+) = 0.83) at 50 °C, underscoring the promise of FFEs in zinc hybrid batteries. Li et al. have pioneered a novel bifunctional FFE tailored for micro-Si anodes, transforming battery efficiency by pre-lithiating the native SiOx layer and reducing LiF-free interphase buildup.120 Their research demonstrated remarkable outcomes, with the optimized micro-Si anode achieving over 5 mA h cm−2, boasting a high specific capacity of 2900 mA h g−1, an impressive initial coulombic efficiency of 94.7%, and a stable CR of 94.3% after 100 cycles at 0.2C. The key innovation lies in the 2 M LiBH4/THF–MeTHF electrolyte, which chemically pre-lithiated the oxide layer, stimulating the SEI and enhancing lithium-ion transfer. This breakthrough has set the stage for developing eco-friendly, cost-effective electrolytes for next-generation batteries.120
Remarkably, the partial fluorine electrolyte strategy has also been explored in many studies and achieved a high-performance battery.136–139 This state-of-the-art system incorporates comparatively fewer fluorination species into electrolytes for high cycling performance, non-flammable, and high voltage applications.137,140–142 However, this system also shows toxicity from fluorination species, adversely affecting climate change. The existing literature studies on FFEs for corresponding alkaline battery systems strongly oppose practical outlooks of the development of novel ingredients to enhance efficiency with low cost. Therefore, future directions are highly encouraging to explore various strategies to design and develop innovative/discover studies of FFEs in all types of alkaline metal batteries.
3.2. Fluorine-free electrolytes in mono and multivalent batteries
The above roadmap discussion visibly demonstrated that FFEs transitioning from lithium-based batteries are promising for future generations. The development of FFEs in monovalent and multivalent battery systems gives prominence to exploring novel battery chemistry and, eventually, gaining insight into the pivotal challenge of pursuing sustainable energy. While lithium-based batteries have established the foundational principles for electrolyte design, extending these concepts to Na, K, and multivalent systems presents distinct difficulties due to variations in ion chemistry and solvent interactions. In monovalent systems, sodium and potassium-based electrolytes are hindered by differences in ion–solvent interactions compared to lithium. Despite similarities in the physicochemical characteristics of Li+, Na+, and K+, their reactivity diverges owing to differences in charge density, solvation structures, and Lewis acidity. For example, Okoshi et al. compared de-solvation energies of mono-valent Li+ and Na+ ions and di-valent Mg-ions in 26 fluorine-free solvents (carbonates, lactones, and amides), finding that Na-ions had lower energies than Li due to weaker Lewis acidity, while divalent Mg-ions had higher energies and additional interactions due to their double charge.143 Furthermore, their extended studies on monovalent K+ ions revealed even lower interaction energies, reflecting K's larger ionic radius and weaker Lewis acidity, contributing to K-ion batteries’ high rate capability and low solubility compared to other monovalent (Li+, Na+) and divalent (Mg2+) systems.144 Beyond solvent interactions, anion chemistry plays a crucial role in electrolyte performance, particularly in enabling efficient cation dissociation and transport. For instance, substituting –CF3 in TFSI− with –n-C4F9 in NFSI− reduces cation affinity, lowering Li+ dissociation energy (591 → 576 kJ mol−1), enhancing ion mobility and electrochemical efficiency.145 FFEs eventually lead to a unique anion chemistry design by replicating existing anions for advancing monovalent cations into multivalent rechargeable batteries. The BOB− anion has been extensively studied for its structural and electrochemical performance. For instance, boron-containing monovalent salts like LiBOB and NaBOB are primarily explored in FFEs,44 while Ca(BH4)2 and Mg(BH4)2 show potential in multivalent systems, though the exact role of boron remains unclear.146 Xu et al. innovatively developed the orthoboron-based anion bis(glycolato)borate (BGB), emphasizing its application for both monovalent (Li+, Na+, K+) and divalent (Mg2+, Ca2+) cations as shown in Fig. 5.125 Their research highlights the superior moisture stability of LiBGB and NaBGB compared to LiBOB. BGB salts' thermal stability follows the trend Li > Na > K > Mg > Ca, with LiBGB having the highest decomposition temperature (370 °C) and CaBGB the lowest. However, >1 M LiBGB electrolytes reduce ionic conductivity due to ion-pairing and an increase in viscosity.125 The 1 M LiBGB–TEP electrolyte shows excellent electrochemical stability. At the same time, 1 M LiBGB–TMP demonstrates the best long-term performance for Li plating-stripping. In addition to appreciation, further exploration of these anions should focus on their structural, physicochemical, and electrochemical performance, advancing electrolyte design for the transition from monovalent to multivalent rechargeable batteries.125 | ||
| Fig. 5 A novel fluorine-free bis(glycolato)borate anion-based salt for monovalent and multivalent cations, Reproduced with permission,125 Copyright (2025) Wiley-VCH. | ||
3.3. Challenges and limitations
Designing FFBs presents several significant challenges. Without fluorine, achieving similar stability and performance becomes difficult. FFEs often struggle with issues like lower ionic conductivity, reduced electrochemical stability, and increased susceptibility to degradation. Additionally, forming a stable SEI without fluorine is challenging, leading to problems such as lithium dendrite growth and poor cycle life. Studies are exploring various strategies, such as using alternative solvents and additives, to overcome these hurdles. However, significant advancements are still needed to match the performance of fluorinated systems.1384. Strategies for eliminating fluorine from electrolytes
Developing sustainable FFBs presents significant challenges, including the limited availability of FFE ingredients, electrochemical stability at high voltages, and inadequate interfacial engineering.150 Since FFE systems are still in the initial stages, studies emphasize incorporating partially fluorinated compounds or hybrid blend approaches. In addition, studies highlight the integration of fluorine-free components such as co-salts, co-solvents, additives, or diluents within fluorinated systems to enhance performance. Examining the fluorinated electrolytes provides critical insights into existing technologies and the ongoing transition toward fluorine-free solutions. Analyzing these hybrid systems is essential for understanding their role and accelerating the systematic shift toward fully PFAS-free electrolytes, marking an essential step in fluorine elimination. This section highlights the future generation research to design and develop innovative FFE ingredients to create enormous eco-friendly batteries with efficient benefits that drain out the pollutants, making it easy to follow a sustainable circular economy.151–1534.1. Fluorine-free salts
The choice of lithium salt significantly impacts the stability and performance of rechargeable LIBs, notably electrolyte oxidation. Lithium salts have varied degrees of stability during oxidation processes, affecting the battery system's overall safety and efficiency. The fluorine-free lithium salt anion stabilizes and forms the SEI, influencing cycle life, voltage stability, and operational dependability.154 Chemical stability varies across lithium salts, influencing their susceptibility to breakdown and side reactions, compromising electrolyte performance over time, and limiting battery life. Although the options for salts in the FFE system are limited, the most employed anions include (1) perchlorate-based salts, (2) phosphate-based salts, (3) nitrile-based salts, (4) borate-based salts, and (5) nitrate-based salts.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EMC
EMC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMC (1
DMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 wt), additives (1% VC + 1% PS + 0.1% LiBOB) and a newly synthesized aromatic polyamide (APA) binder.112 Notably, the CEI layer formed in the APA–FFE system incorporated B and Cl species, which enhanced ionic conductivity and provided a stable, uniform protective layer, as shown in Fig. 6b.112 Their APA–FFE system retained 75.2% capacity after 200 cycles at 1C, addressing high voltage performance concerns with high nickel NCM cathodes and underlining the requirement for fluorine-free materials to fulfill environmental laws. In the pursuit of improved performance and environmental friendliness, this strategy creates new avenues for future electrolyte designs that go beyond fluorine-based frameworks as the industry transitions to sustainable battery technologies.161
1 wt), additives (1% VC + 1% PS + 0.1% LiBOB) and a newly synthesized aromatic polyamide (APA) binder.112 Notably, the CEI layer formed in the APA–FFE system incorporated B and Cl species, which enhanced ionic conductivity and provided a stable, uniform protective layer, as shown in Fig. 6b.112 Their APA–FFE system retained 75.2% capacity after 200 cycles at 1C, addressing high voltage performance concerns with high nickel NCM cathodes and underlining the requirement for fluorine-free materials to fulfill environmental laws. In the pursuit of improved performance and environmental friendliness, this strategy creates new avenues for future electrolyte designs that go beyond fluorine-based frameworks as the industry transitions to sustainable battery technologies.161
 | ||
Fig. 6 Design of the fluorine-free halogen anion; (a) summary of CEs for LHCEs with various salts and the normalized amounts of Li2O and LiF by capacity loss in select electrolytes, Reproduced with permission,161 Copyright (2024) Springer Nature. (b) Enhanced ionic conductivity in Li-ion batteries with fluorine-free ClO4− anion and aromatic binders, compared to PVDF, Reproduced with permission,112 Copyright (2024) Elsevier. (c) Fluorine-free phosphate anion; (i) i-E profiles of PC solutions at 10 mV s−1, (ii) discharge curves in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 EC–THF with 0.5 mol dm−3 LBNB, LBBB, and LTBP for Li/V2O5 cells at 25 °C, 1.0 mA cm−2, Reproduced with permission,162 Copyright (1999) The Electrochemical Society. 1 EC–THF with 0.5 mol dm−3 LBNB, LBBB, and LTBP for Li/V2O5 cells at 25 °C, 1.0 mA cm−2, Reproduced with permission,162 Copyright (1999) The Electrochemical Society. | ||
The chelate complex of phosphorus, particularly lithium tris[1,2-benzenediolato(2)-O,O′]phosphate (LTBP), offers unique advantages in lithium battery electrolytes, significantly enhancing battery performance.162 LTBP's chelating effect with bidentate ligands boosts thermal stability, making it more resistant to decomposition than traditional lithium salts like LiPF6.162 This enhanced stability contributes to improved safety, ensuring more reliable and stable battery operation. Additionally, LTBP demonstrates superior electrochemical performance, exhibiting better discharge characteristics in Li/V2O5 cells. This leads to higher energy density and capacity, as shown in Fig. 6c-(i) and (ii).162 A comparison of the salt LTBP with its fluorinated counterpart, lithium tris[3-fluoro-1,2-benzenediolato(2-)-O,O′] phosphate (3-FLTBP), reveals that 3-FLTBP exhibits higher conductivity due to fluorine's electron-withdrawing effect. LTBP offers lower viscosity and potentially improved electrolyte stability, highlighting a balance between conductivity enhancement and practical electrolyte characteristics in lithium battery applications.165
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N groups, making them viable for high-voltage battery electrolytes.167 While theoretical studies indicate low solubility and conductivity, evidence from experiments is imminent. Additional potential borate salt, lithium tetracyanoborate (LiB(CN)4), has high oxidative stability and poor Li coordination. Experimental studies with PEGDME demonstrated outstanding stability in Li‖LiFePO4 cells at 4 V vs. Li+/Li, with 99% CR after 22 cycles.113 However, its performance in glyme-based gel polymer electrolytes discloses its LiPF6-based equivalents, with instability and low CE above 4 V vs. Li+/Li.168 For example, Scheers et al. (2014) developed electrolytes from lithium salts with nitrile-based anions, such as LiB(CN)4:PAN:PEGDME or LiDCTA:PAN:PEGDME, and tested them through electrospun PAN membranes, attaining up to 98% and 96% average CE after 11 to 15 cycles in Li/LiFePO4 cells at ambient temperature.113 The addition of Al2O3 particles had minimal effects on the PAN membranes, suggesting potential long-term performance repercussions. The impact of solvent and Li+ concentration on anion stability and the challenge of substituting traditional Li-salts with fluoro-based anions without sacrificing stability are obstacles to boosting anode stability. Identifying fluorine-free salts with increased stability at the anodic interface through quantum chemical calculations, combined with experimental measurements and exploring various combinations, are all potential solutions.113 LiB(CN)4 and LiDCTA are crucial in forming the electrolytes, contributing to ionic conductivity and stability.
N groups, making them viable for high-voltage battery electrolytes.167 While theoretical studies indicate low solubility and conductivity, evidence from experiments is imminent. Additional potential borate salt, lithium tetracyanoborate (LiB(CN)4), has high oxidative stability and poor Li coordination. Experimental studies with PEGDME demonstrated outstanding stability in Li‖LiFePO4 cells at 4 V vs. Li+/Li, with 99% CR after 22 cycles.113 However, its performance in glyme-based gel polymer electrolytes discloses its LiPF6-based equivalents, with instability and low CE above 4 V vs. Li+/Li.168 For example, Scheers et al. (2014) developed electrolytes from lithium salts with nitrile-based anions, such as LiB(CN)4:PAN:PEGDME or LiDCTA:PAN:PEGDME, and tested them through electrospun PAN membranes, attaining up to 98% and 96% average CE after 11 to 15 cycles in Li/LiFePO4 cells at ambient temperature.113 The addition of Al2O3 particles had minimal effects on the PAN membranes, suggesting potential long-term performance repercussions. The impact of solvent and Li+ concentration on anion stability and the challenge of substituting traditional Li-salts with fluoro-based anions without sacrificing stability are obstacles to boosting anode stability. Identifying fluorine-free salts with increased stability at the anodic interface through quantum chemical calculations, combined with experimental measurements and exploring various combinations, are all potential solutions.113 LiB(CN)4 and LiDCTA are crucial in forming the electrolytes, contributing to ionic conductivity and stability.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7) for LIBs.58 The cycle life and discharge capacity are significantly impacted by using an electrolyte devoid of fluorine in LIBs. The study found that an FFE based on LiBOB and VC performed better than a highly fluorinated electrolyte, including LiPF6. Specifically, the fluorine-free variant demonstrated a greater discharge capacity of 147 mA h g−1 and an 84.4% CR with 99.51% CE after 200 cycles at C/10, as shown in Fig. 7b-(i) and (ii).58 Furthermore, more oxygen-rich compounds created an SEI layer in the FFE, raising interface resistance while stabilizing the silicon-based anode for improved long-term cycling. The electrolyte without fluorine showed increased resistance and overpotential at higher currents, but overall, it improved discharge capacity and cycle life.58
7) for LIBs.58 The cycle life and discharge capacity are significantly impacted by using an electrolyte devoid of fluorine in LIBs. The study found that an FFE based on LiBOB and VC performed better than a highly fluorinated electrolyte, including LiPF6. Specifically, the fluorine-free variant demonstrated a greater discharge capacity of 147 mA h g−1 and an 84.4% CR with 99.51% CE after 200 cycles at C/10, as shown in Fig. 7b-(i) and (ii).58 Furthermore, more oxygen-rich compounds created an SEI layer in the FFE, raising interface resistance while stabilizing the silicon-based anode for improved long-term cycling. The electrolyte without fluorine showed increased resistance and overpotential at higher currents, but overall, it improved discharge capacity and cycle life.58
 | ||
| Fig. 7 Fluorine-free borate anions; (a) BOB− anion solubility in ether-based solvents (DME/DOL), Reproduced with permission,179 Copyright (2023) American Chemical Society. (b) FFE@NMC111/Si–graphite at C/10;(3.0–4.2) V with electrolytes LP57–1 M LiPF6–EC/EMC(3/7) (black), LP57 + FEC + VC–1 M LiPF6–EC/EMC(3/7) + 10 vol% FEC + 2 vol% VC (red), LiPF6 + LiBOB + FEC + VC – 0.38 M LiPF6–EC/EMC(3/7) + 0.5 M LiBOB + 10 vol% FEC + 2 vol% VC (yellow), and LiBOB + VC–0.7 M LiBOB–EC/EMC(3/7) + 2 vol% VC (blue); (i) CE, (ii) median discharge resistance, Reproduced with permission,58 Copyright (2020) American Chemical Society. (c) BOB− anion decomposition in a multistep breakdown on graphite anodes forms semi-carbonate-B (V) and (VI), Reproduced with permission,173 Copyright (2003) The Electrochemical Society. (d)-(i) Lactone effect influence on 1.70 V irreversible capacity in Li-ion cells, (ii) LiBOB stability performance at 60 °C on NCA, LCO, NMC, spinel (LixMn2O4) and LiFePO4, Reproduced with permission,181 Copyright (2008) The Electrochemical Society. (e) Absorbance and IR spectra of a porous GC electrode in LiClO4/DME with LiBOB at OCP, (f) model study of LiBOB's reduction mechanism, SEI formation, and reactions with EC, Reproduced with permission,178 Copyright (2024) American Chemical Society. | ||
The SEI composition using LiBOB-based electrolytes is generally investigated by XPS and FTIR; however, sample preparation is an issue due to salt precipitation in DMC, necessitating the usage of a γ-BL/DMC combination, which might cause SEI dissolution.173 Remarkably, the LiBOB-based SEI has an oxygen-rich layer that protects solvent intercalation and graphite degradation. This layer is ascribed to semi-carbonate-like compounds and orthoborates from anion breakdown.173 While LiBOB forms a stable SEI on graphite that requires EC for initial layer development and BOB− anion reduction, its increased cell resistance remains a constraint. However, LiBOB outperforms LiPF6-based electrolytes at higher temperatures, extending cycle life.
Additionally, studies on the interactions of LiBOB with different cathode materials have shown promise for better thermal stability and CR.100,173 This is particularly applicable for iron-based and silicon electrodes, which hold out the prospect of improved performance in electrolyte systems without fluorine. They have proposed a multi-step breakdown process for the BOB anion, generated by a single electron and occurring on graphite anode surfaces.173 This technique might provide a variety of semi-carbonate-like compounds containing B in oxidation states V–VI (Fig. 7c).173
LiBOB-based electrolytes are evaluated in Li-metal batteries with LiFePO4 cathodes, and they outperformed fluorinated electrolytes in cycle life without dendrite development.58 Accelerated rate calorimetry tests found higher initial temperatures of self-heating reactions for LiBOB, indicating improved thermal stability.182 Even with greater resistances and a lower rate capability, LiBOB provides greater thermal stability, safety, and sustainability. Thereafter, solvent and additive optimization is required.182 Extensive works by Kang Xu focus on modifying the electrolyte composition for LiBOB in LIBs.169,181 Xu's studies aim to produce electrolyte formulations with enhanced solubility, ionic conductivity, and interfacial characteristics by investigating the potential of LiBOB as an alternative salt and exploring lactone-based cosolvents to dissolve it efficiently.181 Lactone-based co-solvents, such as γ-butyrolactone (GBL), play an important role in increasing the solubility of LiBOB in electrolytes, allowing for the development of LiBOB-rich electrolytes. However, using lactone-based cosolvents may result in an increase in irreversible capacity due to the decrease of the BOB anion on carbonaceous anode surfaces, particularly with GBL.183,184 Furthermore, elevated temperatures may impact battery performance when utilizing electrolytes with a higher lactone concentration.181 This study reveals the importance of the varied properties of different lactones when designing the electrolyte for LiBOB-based systems because of the influence on the irreversible capacity and overall performance of the battery.181,185 The voltage profiles of Li-ion cells with different lactones during the early part of the first charge cycle highlight irreversible capacities from 1.70 V reductions, as shown in Fig. 7d-(i).181 The cycling stability of Li-ion cells with various cathode surfaces, using 1 M LiBOB in a EC/DMC 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 electrolyte at 60 °C, was also assessed. After 40 cycles, LCO and NMC cells retained capacities better than others, indicating faster LiBOB decomposition (Fig. 7d-(ii)).181 This incompatibility with Co-rich cathode surfaces has been frequently reported.186 A preliminary study suggests that Co may catalyze the decomposition of the BOB anion, leading to poor performance in LCO and NMC cathodes.90 The study observed several lactones, including valerolactones, that exhibited intermediate effects, butyrolactone (BL), which significantly increased irreversible capacity, and caprolactones, which revealed irreversible decreases.181
50 electrolyte at 60 °C, was also assessed. After 40 cycles, LCO and NMC cells retained capacities better than others, indicating faster LiBOB decomposition (Fig. 7d-(ii)).181 This incompatibility with Co-rich cathode surfaces has been frequently reported.186 A preliminary study suggests that Co may catalyze the decomposition of the BOB anion, leading to poor performance in LCO and NMC cathodes.90 The study observed several lactones, including valerolactones, that exhibited intermediate effects, butyrolactone (BL), which significantly increased irreversible capacity, and caprolactones, which revealed irreversible decreases.181
Compared to LiPF6, LiBOB has superior thermal stability up to 302 °C and produces less hazardous breakdown products.100,181 Since high-humidity storage of LiBOB and other contaminants, such as lithium oxalate from salt manufacture, can undermine stability and increase cell impedance, it is imperative to ensure purity.187 Despite these obstacles, LiBOB-based electrolytes show improved SEI development on graphite anodes, improving cell performance when compared to LiPF6.100 The electrochemical behavior shows an irreversible plateau due to oxalate ester impurities, which is modified by solvent choice, emphasizing the need for thorough salt purification and solvent selection in LiBOB electrolytes.100,181,182 Even so, the researchers are interested in exploring the lactones in the context of ideal electrolyte design. For example, Teoh et al. recently developed a novel FFE for a lithium-ion capacitor system with a unique combination of lithium bis(oxalato)borate (LiBOB) and γ-valerolactone (GVL). The electrolyte showed a conductivity of 4.9 mS cm−1 at 20 °C, indicating effective ion transport due to the high conductivity of the electrolyte.188 This electrolyte exhibits a flash point of 90 °C, indicating higher thermal stability and lower flammability than the 25 °C flash point of 1 M LiPF6 in EC/DMC, highlighting its safer, more sustainable design. A protective passivation layer was also formed on the aluminum current collector at high potentials owing to LiBOB breakdown, which was successfully prevented by the electrolyte from anodic dissolution. Further, the full cell assembly developed by activated carbon graphite achieved 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles with 80% retention of initial capacity. In addition, LiBOB attained a high solubility of up to 2 M in GVL, which makes it easier to use as the main salt in the electrolyte.188 Due to irreversible processes, a CE of 53.2% was demonstrated in the first cycle.188 However, consecutive cycles consistently displayed an average CE of 96% over 5 cycles, showing the BOB anion's role in forming the SEI, as verified by XPS studies on graphite electrodes. By realizing how these lactones affect battery performance, electrolyte compositions for LiBOB-based systems may be optimized, possibly improving LIB solubility, stability, and performance.188,189 LiBOB has shown promise for both cathodes and anodes, but its reaction mechanisms remain unclear. Melin et al. conducted an operando study using ATR-FTIR, as shown in Fig. 7e, to investigate LiBOB's reduction process, which began around 1.8 V and produced lithium oxalate and oxalatoborates, along with CO2, that influenced the interphase on the negative electrode.178 This research shed light on the reduction pathways of LiBOB and their role in interphase formation. The electrochemical reduction mechanism for LiBOB is shown in Fig. 7f, emphasizing the side reactions and SEI generation.178 According to the study, there was a reduction peak at less than 1.8 V.178,189 Advanced operando methods are used to monitor the process as LiBOB forms an SEI mostly composed of Li-oxalate and minor byproducts from reactions inside the electrolyte.178 Future research directions include optimizing electrolyte composition to reduce irreversible capacity losses and improve thermal stability and investigating alternative solvent systems to scale up LiBOB-based electrolytes for commercial use.
000 cycles with 80% retention of initial capacity. In addition, LiBOB attained a high solubility of up to 2 M in GVL, which makes it easier to use as the main salt in the electrolyte.188 Due to irreversible processes, a CE of 53.2% was demonstrated in the first cycle.188 However, consecutive cycles consistently displayed an average CE of 96% over 5 cycles, showing the BOB anion's role in forming the SEI, as verified by XPS studies on graphite electrodes. By realizing how these lactones affect battery performance, electrolyte compositions for LiBOB-based systems may be optimized, possibly improving LIB solubility, stability, and performance.188,189 LiBOB has shown promise for both cathodes and anodes, but its reaction mechanisms remain unclear. Melin et al. conducted an operando study using ATR-FTIR, as shown in Fig. 7e, to investigate LiBOB's reduction process, which began around 1.8 V and produced lithium oxalate and oxalatoborates, along with CO2, that influenced the interphase on the negative electrode.178 This research shed light on the reduction pathways of LiBOB and their role in interphase formation. The electrochemical reduction mechanism for LiBOB is shown in Fig. 7f, emphasizing the side reactions and SEI generation.178 According to the study, there was a reduction peak at less than 1.8 V.178,189 Advanced operando methods are used to monitor the process as LiBOB forms an SEI mostly composed of Li-oxalate and minor byproducts from reactions inside the electrolyte.178 Future research directions include optimizing electrolyte composition to reduce irreversible capacity losses and improve thermal stability and investigating alternative solvent systems to scale up LiBOB-based electrolytes for commercial use.
NO3−, with its high donor number and dielectric constant, improves interaction with Li+, accelerating de-solvation and forming a Li3N-rich SEI at the anode due to its low LUMO energy.208 Increasing the concentration of lithium salts in electrolytes boosts anion levels relative to solvents, promoting the creation of a strong, anion-derived SEI with better conductivity and mechanical strength, crucial for preventing dendrite formation.209 Two primary strategies have been developed to modulate the solvation structure and interfacial chemistry in the electrolyte: increasing the anion concentration or altering the interaction between anions and solvents without changing the overall salt concentration.202,210 These methods facilitate forming a stable, conductive SEI rich in Li3N and Li2O, optimizing solvation and ensuring robust protection for the lithium metal anode.211 Incorporating LiNO3 into carbonate electrolytes increases cycling performance by optimizing Li+ solvation and SEI formation.212 LiNO3, along with other nitrates such as KNO3 and NaNO3, augments Li+ de-solvation and mitigates polysulfide shuttle effects, thereby improving battery efficiency.199,213,214 The Liu research group demonstrated that an FFE integrating 3.5 M LiNO3 into a carbonate electrolyte with DMI, without additional stirring, substantially elevates lithium-metal battery performance by generating a stable SEI enriched with lithium salts, leading to an average CE of 99.1% for 80 hours.209 The high donor number of DMI also aids in stabilizing the electrolyte by neutralizing PF5 and curbing HF formation, as shown in Fig. 8a.209 Under severe circumstances (45 μm ultrathin Li foil and high-loading cathode of ∼1.5 mA h cm−2), the anode retains 96.5% of its capacity after 2200 cycles at a 5C rate and 97.9% after 240 cycles at 1C rate.209
 | ||
| Fig. 8 Fluorine-free nitrate anion; (a) schematic showing how LiNO3/DMI inhibits PF6 decomposition, Reproduced with permission,209 Copyright (2022) American Chemical Society. (b) Summary of solvent DN and CE with LiNO3, Reproduced with permission,215 Copyright (2023) American Chemical Society. (c) LiNO3 solubility in carbonate solvents, Reproduced with permission,216 Copyright (2021) Wiley-VCH. (d) Schematic of LiNO3 solubility: (a) enhanced dissociation, (b) equilibrium state, and (c) prevention of recombination, Reproduced with permission,192 Copyright (2021) American Chemical Society. (e) Li–S@cycling performance of fluorine-free solvent in NO3− anion: (i) 1 M LiTFSI in DMI–DMI–LNO-0 M, (ii) 1 M LiTFSI + 0.2 M LiNO3 in DMI–DMI–LNO-0.2 M, and (iii) 1 M LiTFSI+0.5 M LiNO3 in DMI–DMI–LNO-0.5 M, Reproduced with permission,217 Copyright (2020) Wiley-VCH. (f) Electrolyte solubility of Li chippings in 3 M LiNO3/DMI: (a) day 0 and (b) day 20. (g) Columnar Li growth in 3 M LiNO3/DMI: (a) initial cation/anion setup, (b) N-rich SEI on Cu, (c) small Li nuclei formation, and (d) strong SEI supporting columnar Li. (h) SEM images of Li on Cu in 3 M LiNO3/DMI: (a), (b), (d) and (e) top view and (c) and (f) cross-sectional view at 1 and 2 mA cm−2 for 2 h, Reproduced with permission,149 Copyright (2021) American Chemical Society. | ||
The FFE with LiNO3 as the main salt offers the following characteristics: (i) it has a lower molar mass than LiPF6, LiFSI, and LiTFSI, which reduces electrolyte weight at comparable concentrations.218 (ii) NO3− exhibits high Li+ affinity, displacing solvent molecules and reducing their decomposition. (iii) Decreasing the nucleation overpotential and promoting granular development facilitates the homogeneous deposition of Li metal.219 However, its use as a main salt has been restricted due to its poor solubility and dissociation in popular solvents. Zhou et al. emphasized the importance of donor number (DN) in maximizing Li plating/stripping using LiNO3.215 LiNO3 is difficult for low-DN solvents to dissolve, which reduces conductivity and cycling.215,220,221 In contrast, high-DN solvents over-coordinate with Li+ ions, resulting in excessive solvent breakdown. Trimethyl phosphate (TMP) (DN = 23.0 kcal mol−1) and TEP (DN = 26.0 kcal mol−1) were chosen due their moderate DN, as shown in Fig. 8b, demonstrating good cycling performance and offering more solvent and salt alternatives for use in FFE systems.209,215 Interestingly, Zhang et al. developed a simple and scalable way for stabilizing water-containing FFEs by demonstrating that LiNO3 can effectively restore the electrolyte.222 The NO3− anion has two functions: it inhibits hexafluorophosphate anion hydrolysis and forms a SEI, and ultimately improves the electrochemical reversibility of Li plating and stripping trial.117 Their findings address water-related hazards in the battery industry and provide insight that excess water can be turned into functional component for sustainable, high-performance batteries, aiding the shift away from fluorinated materials.222
Zhao et al. demonstrated how EC molecules are liberated to dissolve LiNO3 by strong interactions with complex anions, such as those involving the Lewis acids, as shown in Fig. 8c.216 The substitution of ethylene glycol diacetate (EGD) with DMC in mixed solvents shows that modifying the solvation structure of Li+ to prevent recombination with NO3− can greatly increase solubility.192,223 Despite its low permittivity, EGD has high LiNO3 solubility owing to its distinctive solvation structure, which repels NO3− and inhibits recombination for dynamic equilibrium, as shown in Fig. 8d.192 Baek et al. introduced an FFE in a Li–S battery with different concentrations of LiNO3, tested using galvanostatic methods. This study showed that greater LiNO3 contents improved cycling performance over 50 cycles and enhanced specific capacity with better CR, as shown in Fig. 8e.217 The constant breaking and fixing of the SEI depleted the electrolyte, causing severe lithium corrosion and reduced CE.149,224 Unrestrained dendrite development during sustained cycling can result in short circuits and possibly detrimental battery failures. Many fluorinated electrolyte systems/LiF have been developed to combat this situation and create an artificial SEI.225–233 However, these approaches greatly impact environmental strategies, especially for large-scale development. DMI solvent has a high DN property, which can enhance the solubility of LiNO3. Zhou et al. developed a complete FFE system for studying dendrite growth in LMBs for the first time using a fluorine-free approach.149 Owing to the strong Li+ and NO3− interactions that obstruct dissociation in most solvents, LiNO3 is rarely employed as the dominant salt. DMI enables extraordinary solubility of LiNO3, reaching up to 7 M. Compared to commercial LiPF6-based electrolytes, FFE (DMI/LiNO3) retained a high viscosity (51.7 cP) and moderate ionic conductivity (2.23 mS cm−1) at ambient temperature at 3 M concentration.149 As shown in Fig. 8f, both 3 M LiNO3/DMI electrolyte and pure DMI solvent remained colorless even after 20 days, while submerged lithium metals maintained a shiny appearance. Even with greater current densities and areal capacities (2 mA cm−2, 4 mA h cm−2), SEM analysis shows consistent and uniform lithium deposition with columnar formations perpendicular to the copper substrate surface, as shown in Fig. 8g.149 Similarly, Fig. 8h demonstrates the functional degradation, suggesting strong reversibility and modest column size decrease due to faster nucleation and growth at higher current density.149
Pham et al. designed an FFE with LiNO3 as the main salt in GBL and VC pentafluoro(phenoxy)cyclotriphosphazene (FPPN) as additives for flame retardance and SEI/CEI development.202 In GBL, strong Li+ and NO3− interactions dominate contact ion pairs and aggregates.202,234–237 Forming SEI/CEI layers rich in conductive inorganic species improves interfacial stability.139,238,239 At 3.5 wt% FPPN and 5 wt% VC, the electrolyte exhibited an oxidation stability up to 5.0 V and did not catch fire. The NF-LiNO3/GBL (0.8 M LiNO3-GBL/FPPN (96.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.5 wt%) + 5 wt% VC) electrolyte exhibited a great CE of 98.3% in Li‖Cu configuration and an excellent CR of ∼90% after 200 cycles in Li‖NMC622, as shown in Fig. 9a. Furthermore, this study achieved partial fluorine removal, which indicates that the primary goal of the study is a non-flammable FFE design achieved by incorporating the fluorinated additive.237 In recent times, Chen et al. introduced an electron-donation modulation strategy that uses low donor-number solvents (PC) to sustain solvation and high donor-number solvents (DMSO) for LiNO3 dissolution (Fig. 9b).146 PC exhibits a minor role in Li+ solvation compared to NO3− and DMSO, as shown by spectroscopy and theoretical models.237,240 The LiNO3–DMSO@PC electrolyte showed stable cycling across 200 cycles with graphite, LiFePO4, and LiCoO2 electrodes.240 The recent studies on fluorine-free salts illuminate promising pathways for advancing novel FFE systems for practical applications. Despite the progress, there remains a notable lack of research on these fluorine-free salt strategies. This underscores the urgent need for future investigations to synthesize and develop fluorine-free salts that can effectively address the limitations of fluorinated systems and foster the evolution of sustainable battery technologies.
3.5 wt%) + 5 wt% VC) electrolyte exhibited a great CE of 98.3% in Li‖Cu configuration and an excellent CR of ∼90% after 200 cycles in Li‖NMC622, as shown in Fig. 9a. Furthermore, this study achieved partial fluorine removal, which indicates that the primary goal of the study is a non-flammable FFE design achieved by incorporating the fluorinated additive.237 In recent times, Chen et al. introduced an electron-donation modulation strategy that uses low donor-number solvents (PC) to sustain solvation and high donor-number solvents (DMSO) for LiNO3 dissolution (Fig. 9b).146 PC exhibits a minor role in Li+ solvation compared to NO3− and DMSO, as shown by spectroscopy and theoretical models.237,240 The LiNO3–DMSO@PC electrolyte showed stable cycling across 200 cycles with graphite, LiFePO4, and LiCoO2 electrodes.240 The recent studies on fluorine-free salts illuminate promising pathways for advancing novel FFE systems for practical applications. Despite the progress, there remains a notable lack of research on these fluorine-free salt strategies. This underscores the urgent need for future investigations to synthesize and develop fluorine-free salts that can effectively address the limitations of fluorinated systems and foster the evolution of sustainable battery technologies.
 | ||
| Fig. 9 Fluorine-free solvent-salt; (a) electrochemical performance in Li‖NMC622: discharge capacities for LiFSI/GBL, LiNO3/GBL, and NF-LiNO3/GBL electrolytes, Reproduced with permission,139 Copyright (2023) Elsevier. (b) LiNO3 dissolution in solvents: (a) DN values, (b) electron pair donation, (c) low donor-number solvents, (d) low donor-number solvents, (e) solubility picture as salt-to-solvent ratios and molarity, Reproduced with permission,240 Copyright (2024) Wiley-VCH. | ||
4.2. Solvents for fluorine-free electrolytes
Solvent selectivity is crucial in electrolyte design, as 70–80% of the electrolyte's mass consists of solvents, which are vital for lithium-based battery performance and safety.11,241 Solvents must dissolve lithium salts, have high dielectric constants, low viscosity for efficient ion transport, and ensure stability across the battery's voltage range.242,243 Research focuses on minimizing interactions between Li+ ions and solvents, increasing ionic conductivity, and widening the liquid range.244 Using cosolvents with a low freezing point and viscosity can enhance conductivity at low temperatures.245–249 Maintaining the SEI is essential for stability.238 Hereafter, current research focuses on solvents' physicochemical and thermomechanical properties.243,250,251 However, given the sustainability concerns, there is a push for fluorine-free alternatives to improve safety and environmental impact, with FFEs being a promising but still developing area.11,138 Developing innovative, high-performance FFEs is crucial for sustainable battery technologies. Fig. 10 shows possible fluorine-free diluents or solvents, emphasizing the importance of selecting optimized solvents or diluents with appropriate dielectric and viscosity properties for developing efficient FFEs.FFEs, compared to fluorinated ones, contain a greater variety of solvents. These solvents offer superior thermal characteristics and create an optimal solvation structure.252,253 Alkali metal batteries frequently utilize organic liquid electrolytes due to their electrochemical stability, robust ionic conductivity, prevention of irreversible capacity loss, and adaptability to many electrode materials.254–257 The main types of solvents used in these electrolytes include (1) cyclic carbonate (CC) – linear carbonate (LC) solvents, (2) ether-based solvents (EBs), (3) ester@lactone based solvents, (4) phosphate-based solvents (PBs), (5) nitrile-based solvents (NBs), (6) sulfone-based solvents, and (7) ionic liquid-based solvents (ILBs).
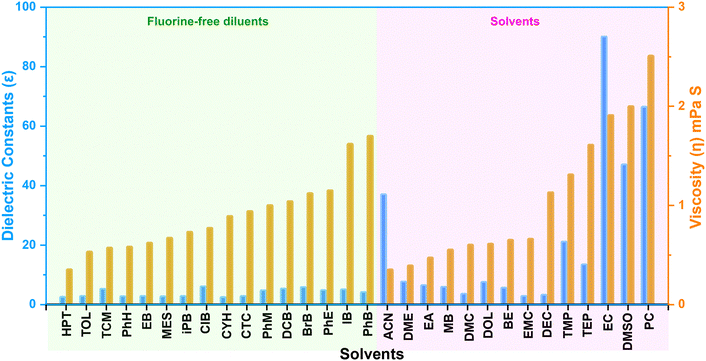 | ||
| Fig. 10 Comparison of dielectric constant and viscosity of different diluents or solvents for fluorine-free LHCE design. | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, v/v) electrolyte improved the SEI by balancing Li2CO3 and ROCO2Li, enhancing mechanical properties. Adding 0.05 M LiPF6 generated sufficient LiF without disrupting this balanced ratio. However, excessive LiPF6 (1 M) increased Li2CO3 and reduced ROCO2Li, as shown in Fig. 11a. Consequently, this electrolyte produced an optimal SEI with high stability and low impedance, outperforming other combinations in cycling stability. Lithium salts are efficiently solvated by LCs, facilitating ion transport in electrolytes. DEC improves battery performance by stabilizing the SEI on lithium electrodes of moderate viscosity. In contrast, EMC provides viscosity–stability balancing across all battery chemistry. LC challenges include low oxidative stability at high voltages and breakdown, which results in gas production and reduces electrolyte longevity and safety. Eventually, optimizing the usage of LCs in lithium batteries requires addressing these problems. For example, Lee et al. introduced molecular engineering via LCs to mitigate the thermal runways of LIBs with safe electrolyte engineering.262 Interestingly, this work used alkoxy substitution and alkyl-chain extension, as shown in Fig. 11b, to apply the equilibrium strategy and confirm that methoxy substituents play a vital role in Li-ion transport for developing an ideal electrolyte system for real-time perspective.262 This methoxy electrolyte provided stable cycling without excess gas/heat generation upon exposure to mechanical, electrical, and thermal abuse, emphasizing its exceptional electrochemical performance. Bicarbonate solvents were identified as by-products of the EC–LC (EC/DMC, EC/EMC, EC/DEC) electrolyte system formed by transesterifying LC and EC ring-opening products.263 Its structure integrates the SEI film-forming ability of EC, the efficient de-solvation of DMC, and the high anodic stability of EMC.264,265 Zhang et al. introduced dimethyl 2,5-dioxahexanedioate (DMDOHD) solvent as an alternative to traditional carbonate-based electrolytes.265 This novel electrolyte demonstrated dendrite-free lithium deposition, anodic stability up to 5.2 V, and more than 97% capacity retention after 250 cycles in Li‖LiNi0.5Mn1.5O4 cells. Many studies state that carbonate-based electrolytes are promising approaches to transport lithium ions. Furthermore, these interesting work strategies and potential electrolyte optimization gives more support for developing future standards for carbonate-based solvents.
3, v/v) electrolyte improved the SEI by balancing Li2CO3 and ROCO2Li, enhancing mechanical properties. Adding 0.05 M LiPF6 generated sufficient LiF without disrupting this balanced ratio. However, excessive LiPF6 (1 M) increased Li2CO3 and reduced ROCO2Li, as shown in Fig. 11a. Consequently, this electrolyte produced an optimal SEI with high stability and low impedance, outperforming other combinations in cycling stability. Lithium salts are efficiently solvated by LCs, facilitating ion transport in electrolytes. DEC improves battery performance by stabilizing the SEI on lithium electrodes of moderate viscosity. In contrast, EMC provides viscosity–stability balancing across all battery chemistry. LC challenges include low oxidative stability at high voltages and breakdown, which results in gas production and reduces electrolyte longevity and safety. Eventually, optimizing the usage of LCs in lithium batteries requires addressing these problems. For example, Lee et al. introduced molecular engineering via LCs to mitigate the thermal runways of LIBs with safe electrolyte engineering.262 Interestingly, this work used alkoxy substitution and alkyl-chain extension, as shown in Fig. 11b, to apply the equilibrium strategy and confirm that methoxy substituents play a vital role in Li-ion transport for developing an ideal electrolyte system for real-time perspective.262 This methoxy electrolyte provided stable cycling without excess gas/heat generation upon exposure to mechanical, electrical, and thermal abuse, emphasizing its exceptional electrochemical performance. Bicarbonate solvents were identified as by-products of the EC–LC (EC/DMC, EC/EMC, EC/DEC) electrolyte system formed by transesterifying LC and EC ring-opening products.263 Its structure integrates the SEI film-forming ability of EC, the efficient de-solvation of DMC, and the high anodic stability of EMC.264,265 Zhang et al. introduced dimethyl 2,5-dioxahexanedioate (DMDOHD) solvent as an alternative to traditional carbonate-based electrolytes.265 This novel electrolyte demonstrated dendrite-free lithium deposition, anodic stability up to 5.2 V, and more than 97% capacity retention after 250 cycles in Li‖LiNi0.5Mn1.5O4 cells. Many studies state that carbonate-based electrolytes are promising approaches to transport lithium ions. Furthermore, these interesting work strategies and potential electrolyte optimization gives more support for developing future standards for carbonate-based solvents.
 | ||
| Fig. 11 Fluorine-free solvents; (a) FTIR analysis for different electrolytes – after 100 cycles of Li/NMC cells, Reproduced with permission,136 Copyright (2019) American Chemical Society. (b) Strategy to reduce flammability and maintain ionic conductivity in LCs, Reproduced with permission,262 Copyright (2023) The Royal Society of Chemistry. (c) Molecular structures, ESP maps, and properties of (a) DME, (b) DMC, (c) their blend, and (d) BMC solvent with ether and ester groups for high-voltage, safe LMBs, Reproduced with permission,266 Copyright (2024) Springer Nature. (d) Fluorine-free ether electrolyte solvation power of ether solvents in 1.8 M LiFSI salt, Reproduced with permission,267 Copyright (2023) Springer Nature. (e) Schematic of the molecular anchoring diluent electrolyte (MADE) strategy, Reproduced with permission,268 Copyright (2024) Springer Nature. (f) Experimental design to assess the impact of electrolyte leakage, Reproduced with permission,269 Copyright (2023) Wiley-VCH. (g) Methylation strategy design for fluorine-free ether electrolytes, Reproduced with permission,138 Copyright (2024) Springer Nature. | ||
EBs can achieve partial elimination of fluorine in electrolyte systems with outstanding performance. EBs, including DME and 1,3-dioxolane (DOL), are preferred in the development of FFEs for lithium batteries due to their low viscosity, strong ionic conductivity, and excellent solvation of lithium ions and are worthy of comparison with CC–LC based electrolytes.137 DME is notable for its substantial ion transport and SEI production on lithium anodes. It aids lithium–sulfur and lithium–air batteries by dissolving active species. In contrast, DOL supports DME by stabilizing lithium metal, minimizing dendrite development, and improving battery safety due to its unique ring structure. EB and CC–LC mixed systems are promising approaches for ideal FFE design.270–272 For example, in 1984 and 1987, Tobishima studied FFEs using fluorine-free salt LiClO4 in an EB–CC mixed solvent system for a lithium battery.270,271 These studies explored different electrolyte compositions, including 1 M LiClO4 with different optimized ratios of EC/THF, PC/THF, EC/DME, PC/DME, EC/PC, and PC alone. Comparing the EC/ether combination to the EC/PC/ether and PC/ether systems, it was found that the conductivity and lithium cycling efficiency of the mixture rose dramatically. Because of the low viscosity of ether and the high dielectric constant of EC, the improvement in conductivity became particularly noticeable at a realistic solute concentration, such as 1 M. The adhesion of ether and EC on the deposited lithium, which has lower reactivity than PC, is responsible for increased lithium cycle efficiency for EC/ether combinations.270 Still, a similar approach is promising for studying different combinations of EBs and CCs incorporating electrolyte designs. Perhaps researchers grasped this in the fluorination era. However, this approach causes huge toxicity emissions.272 In recent times, Chen's research group investigated the performance and safety of high-voltage lithium metal batteries (LMBs) by developing a new FFE, bis(2-methoxyethyl)carbonate (BMC).266 The electrostatic potential (ESP) map in Fig. 11c shows a less negative charge on ether oxygens in BMC as compared to those in DME, and less positive and negative charge was exhibited by the carbonyl carbon and carbonyl oxygen in BMC relative to DMC. This molecular hybridization improved electron distribution, resulting in increased oxidative and reductive stability, thermal stability, and flash points. BMC exhibited comparatively low solvating capacity and a limited ability to dissolve LiNO3. In LMBs, the BMC-based electrolyte enabled stable operation at high voltages up to 4.4 V and obtained a dendrite-free battery with a high average CE of 99.4% over 10 cycles.266 This work stimulates future researchers to design an FFE contribution by hybridizing EB–CC–LC solvent in molecular design engineering. In contrast to ester-based substitutes, EB electrolytes have a distinct reduction stability, which makes them a good choice for organic electrolytes. Generally, EBs are classified as cyclic (DOL, THF) or linear (DME, triglyme (T3GM), and tetraglyme (T4GM)), as shown in Fig. 11d, but their strong chemical reactivity makes them prone to open-ring polymerization in the presence of Lewis acids, which has a deleterious influence on ionic conductivity.111,269 Not many studies have been done on T3GM and T4GM, which often exhibit unsatisfactory battery performance.273,274 Despite having robust ionic conductivity and excellent ionic solvation, DME electrolytes have poor oxidative stability and aluminum corrosion, which causes significant oxidative breakdown at 4.0 V.45,137,138 To improve DME electrolytes’ electrochemical performance, researchers have changed their spatial arrangement. Li's research team compared non-polar and polar solvents towards a series of EBs for high-voltage practical applications and concluded a solvating power order as DPE < DEE < DME < DIG.267 It is noteworthy that a pre-passivated cathode (99.53%) exhibited steady cycling and greater CEs than a pristine one (98.54%) over 120 cycles when a known incompatible electrolyte (1.8 M DME) was used. The DME-based highly concentrated electrolyte (HCE) system demonstrated that cathode passivation cannot completely avoid dilute ether oxidation.267 Most DME molecules are prone to oxidation due to their low concentration and cation repulsion. Although the DPE-originated cathode electrolyte interphase (CEI) boosted CE by 0.99%, the solvent-rich EDL structure of DME failed to prevent ether oxidation, leading to lower CEs after exchange despite a well-formed CEI layer from the DPE electrolyte. To improve the stability of nickel-rich cathodes at 4.7 V and increase the thermal runaway temperature of LMBs, it is crucial to limit the reactivity of free solvent molecules, especially in ether solvents with low oxidation stability (<4.0 V vs. Li+/Li). As shown in Fig. 11e, a novel molecular anchoring method employing hydrogen-bonding interactions was proposed to suppress ether side reactions.268
Comprehending the influence of electrolyte constituents on solvation structure and interfacial chemistry is crucial for creating efficient electrolytes for high-energy-density LMBs. Localized high-concentration electrolytes (LHCEs) form nanometric aggregates that increase LiF content in the SEI, improving uniform Li deposition and doubling the cycle life compared to advanced ether-based LHCEs.275 This was achieved using a fluorine-free EB, 1,3-dimethoxypropane (DMP), which has a weaker solvating power but high Li salt solubility compared to DME.276 Although a fluorine-free solvent (DME) is frequently utilized in LMBs, it has drawbacks such as poor stability at high voltage and low CE. This work presents a novel design approach using 1,2-diethoxyethane (DEE) by replacing the methoxy groups in DME with further ethoxy groups, altering lithium ions' solvation structure using steric hindrance.277 DEE exhibited improved cycling performance and more stable interfacial characteristics due to its reduced solvation capacity. Under severe circumstances, 4 M LiFSI/DEE outperformed 4 M LiFSI/DME by achieving 182 cycles at 80% CR. At the same time, the pristine counterpart could only complete 94 cycles.277 For high-voltage LMBs, DEE is a viable substitute for DME, demonstrating the potential of steric effects in the electrolyte design. Jinfan et al.'s studies on a potassium ion battery introduced a fluorine-free solvent system by investigating the interaction between weak and strong ions of EBs with K+ intercalation at low concentrations.278,279 This work tunes the solvent–ion interactions for a high performance electrolyte strategy. Similarly, Ma and colleagues developed green EBs with carbon chain modulation that regulates steric hindrance for an effective solvation structure.269 When compared to diethylene glycol dimethyl ether (DGM) and diethylene glycol diethyl ether (DGT) solvents, the electron density around the middle oxygen atom in the ESP of dipropylene glycol dimethyl ether (DMM) was lower.111,269 This structure considerably decreased the solvent's bio-toxicity, and the tailored solvation structure produced long-lasting high-voltage electrolytes for alkali metal-ion batteries. Potassium-ion batteries benefit from a stable interphase formation on both the anode and cathode, due to preferred anion-dominated solvation structure. This determination interestingly provides molecular design ideas of a sustainable world created via novel electrolyte development, as shown in Fig. 11f.
In recent years, many research studies have focused on a series of fluorine-free ether-based electrolytes for high-voltage FFBs. Li et al. explored an FFEE system via methylation of primary ether-solvent DME in series. This eventually promoted high oxidation stability by anion reduction, as shown in Fig. 11g.138 Methylation contributes to solving problems like cathode cracking and lithium dendrite formation in high-voltage LMBs by facilitating the development of ether-based electrolyte systems. Although fluorinated solvents extend the life of batteries by raising LiF in the SEI, there are numerous concerns about their high cost and potential adverse environmental effects. To improve oxidation stability and inhibit dendrite development, methylating DME reduces currents by increasing contact ion pair (CIP) and aggregate (AGG) formation, enhancing LiF-rich SEI, and lowering the anodic current in DEP. This methylation method strikes a compromise between antioxidation and the increase of ionic conductivity in the order of DEP < DPE < DEE < DME, making these solvents suitable alternatives to fluorinated ones.138 In addition, molecular dynamics simulations demonstrated that Li+ is predominantly coordinated by ether oxygen (EO) atoms and the oxygen of the FSI− anion. The coordination number for Li+–EO decreases monotonically, while that for Li+–O (FSI) increases in the solvent order of DME < DEE < DPE < DEP.138 While the fraction of free Li+ cations is similar in DME and DEE, it is substantially lower in DPE and DEP. DME has the lowest fraction of Li+ bonded to two FSI− anions, whereas DEE, DPE, and DEP have higher levels.138 As the solvent changes, there is a visible intensification in aggregation and reduction in free FSI− ions, as confirmed through Raman analysis. Methylation of DME's α-H atoms improved CE (>99.9%, 150 cycles for pouch cell). It ensured excellent cycle performance in 4.3 V Li‖NCA full cells (600 cycles, 99.8% CE for coin cell).138 Furthermore, EBs have superior stability at Li metal anodes than CB-based electrolytes. However, their low anodic stability prevents usage in high-voltage applications.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), where X stands for one of the esters. Compared to their lower molecular weight equivalents (MA and EA), which tended to form insulating films and exhibited persistent reactivity with the anode, higher molecular weight esters (EP and BE) generated more stable and less resistive SEI layers. The cells with greater molecular weight esters showed lower interfacial impedance and improved low-temperature performance. Ionic liquids containing methyl acetate groups have high conductivity and electrochemical stability.286,287 These liquids interact well with lithium ions, which may enhance the performance of the battery electrolyte.286,288–290 For example, Xia et al. designed an electrolyte in partially fluorinated linear carboxylate esters (FLCEs) as co-solvents for non-flammable, high-voltage electrolytes.291 While methyl 3,3,3-trifluoropropanoate (F1) had acceptable solubility for LiPF6, other FLCEs could not dissolve the salt well and failed to create stable SEI films on lithium metal, necessitating their usage in combination with another solvent. High oxidation and reduction potentials shown by the FLCEs qualified them for use in high-voltage applications. In addition, F1-based electrolytes showed remarkable wettability, cyclability, and safety, demonstrating the promise of partial FLCEs as useful co-solvents to improve battery performance.
1), where X stands for one of the esters. Compared to their lower molecular weight equivalents (MA and EA), which tended to form insulating films and exhibited persistent reactivity with the anode, higher molecular weight esters (EP and BE) generated more stable and less resistive SEI layers. The cells with greater molecular weight esters showed lower interfacial impedance and improved low-temperature performance. Ionic liquids containing methyl acetate groups have high conductivity and electrochemical stability.286,287 These liquids interact well with lithium ions, which may enhance the performance of the battery electrolyte.286,288–290 For example, Xia et al. designed an electrolyte in partially fluorinated linear carboxylate esters (FLCEs) as co-solvents for non-flammable, high-voltage electrolytes.291 While methyl 3,3,3-trifluoropropanoate (F1) had acceptable solubility for LiPF6, other FLCEs could not dissolve the salt well and failed to create stable SEI films on lithium metal, necessitating their usage in combination with another solvent. High oxidation and reduction potentials shown by the FLCEs qualified them for use in high-voltage applications. In addition, F1-based electrolytes showed remarkable wettability, cyclability, and safety, demonstrating the promise of partial FLCEs as useful co-solvents to improve battery performance.
 | ||
Fig. 12 (a) Summary of fluorine-free ester solvents relative to fluorinated solvents, Reproduced with permission,285 Copyright (2001) Elsevier. (b) TEP-modified electrolyte and Gr electrode performance, Reproduced with permission,292 Copyright (2023) American Chemical Society. (c)-(i) Conventional electrolyte vs. P-MSE: stability and long-life cycling for PIBs; (ii) solvent structures with different steric hindrance, Reproduced with permission,293 Copyright (2024) Wiley-VCH. (d)-(i) Solvation structure in electrolytes; (ii) Raman spectra of P–O and S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, Reproduced with permission,294 Copyright (2024) American Chemical Society. (e) Interfacial chemistry: carbonate vs. tri-anion phosphate, Reproduced with permission,295 Copyright (2024) Elsevier. (f) TEP effects on Li+ solvation, SEI, and gas generation in electrolytes, Reproduced with permission,122 Copyright (2024) Wiley-VCH. O, Reproduced with permission,294 Copyright (2024) American Chemical Society. (e) Interfacial chemistry: carbonate vs. tri-anion phosphate, Reproduced with permission,295 Copyright (2024) Elsevier. (f) TEP effects on Li+ solvation, SEI, and gas generation in electrolytes, Reproduced with permission,122 Copyright (2024) Wiley-VCH. | ||
Sulfone–ester (SL–EA) electrolytes possess higher anodic stability than EC-EMC electrolytes. However, their lithium cell charge–discharge performance is lower. A study focused on the sulfone–ester (SL–EA) mixed solvent with LiBF4 solute because of LiBF4's greater oxidation tolerance than LiPF6.237 Organic additives such as cycloalkanes, triethylene glycol derivatives, and VC can significantly increase cycling performances for high-voltage LiCoO2 and LiNi0.5Mn1.5O4 cells up to 4.5 V and beyond.237 To improve sulfone-ester mixed solvents for 5.6 V-class lithium batteries, further study is needed on altering solvent chemical structures or electrolyte additions. Phosphate-based solvents (PBs) have the potential for developing a non-flammable electrolyte design. In addition, the PBs such as TMP, TEP, and DMMP are widely used as flame retardants.279,296–298 In the study of DMMP as a flame retardant in LIBs, Feng et al. found that the graphite anode had a high CE of 84% in the 1st cycle and better cycle stability.299 This stability was ascribed to the electrolyte additive chloro-ethylene carbonate, which promoted the development of the SEI layer on the graphite and prevented the electrochemical reduction of DMMP.299 Phosphate-esters are appealing due to their high lithium salt solubility, broad voltage window, low viscosity, and nonflammability, making them prospective low-cost, high-safety solvents.300 However, reductive breakdown on lithium metal anodes is difficult, resulting in dendrite formation and low CE. Previous studies found that tributyl phosphate (TBP) mixed with LiFSI performed poorly because of its high viscosity and low conductivity.300 Incorporating DME as a cosolvent increased lithium-ion conductivity and kinetic diffusion. This novel method cleared the way for creating safer, higher-temperature electrolytes for LMBs by demonstrating exceptional electrochemical performance and safety in Li‖NMC811 cells. TEP is a non-flammable, cost-effective co-solvent and is a potential solution to the flammability of carbonate-based electrolytes in LIBs.301–307 The study found that although TEP strongly coordinates with lithium ions, impeding graphite electrode intercalation, these problems may be minimized by enhancing TEP's competitive coordination with ethylene carbonate (EC), as shown in Fig. 12b.308,309 After 150 cycles with a graphite anode, the resultant TEP-modified carbonate electrolyte exhibited strong ionic conductivity and nonflammability, maintaining approximately 100% of its capacity.292 This formulation greatly minimized fire and explosion hazards in high-capacity pouch cells, providing a safer approach for energy storage in LIBs.310
Phosphate-based solvents have emerged as potential energy storage options, notably in PIBs and LIBs. These electrolytes have various benefits, including nonflammability, strong ionic conductivity, and excellent electrochemical stability.238,279,311–313 Tris(1-propyl) phosphate (TPP) is a noteworthy phosphate solvent that balances solvation capability with salt dissociation, improving battery performance. Due to its mild solvation qualities, TPP has been chosen above alternative candidates, such as TEP and tributyl phosphate (TBP), for phosphate-based electrolytes. This feature enables better lithium or potassium ion coordination while limiting excessive solvation, which might impair conductivity. TPP's structural features also aid in creating stable electrode–electrolyte interphases, which reduce side reactions and improve battery lifetime. For example, Zhang et al. provided a new non-flammable electrolyte for PIBs that used TPP as the ideal fluorine-free solvent.293 TPP was chosen beyond TEP and TMP due to its moderate solvation capacity, as shown in Fig. 12c, which achieved a compromise between efficient ion coordination and high salt dissociation, critical for improving electrochemical performance and safety.293,314 With a salt content of 0.6 M, the designed electrolyte had low viscosity, good ionic conductivity, and excellent oxidative stability, facilitating protective interphases that reduced side reactions. PIBs containing this electrolyte displayed extraordinary longevity by maintaining 80% capacity after 2000 cycles at 4.2 V and demonstrating TPP's promise in creating safe, high-performance energy storage technologies. Similarly, Cheng et al. developed a TMP-based electrolyte. They highlighted that the Li+ solvation structure analysis revealed a decrease in coordinated TMP (Li+-TMP) from 56% in Li+[TMP]4.9[PF6−] to 36% in the Li+[TMP]2.5[EMC]2.2[EC]0.73[DTD]0.4[PF6−] electrolyte, which was due to the sequential inclusion of ethylene sulfate (DTD), EMC, and EC in the electrolyte design.294 Raman spectroscopy verified the attenuated Li+–TMP interactions with P–O vibrational frequencies, as shown in Fig. 12d.294 Recently, Ni et al. conducted anion comparison of carbonate and phosphate-based electrolytes by using fluorine-free single-anion, dual-anion, and tri-anion strategies, as shown in Fig. 12e, which eventually motivated the development of the multi-anion combination strategy.295 Moreover, optimized tri-anion by incorporating TEP achieved an excellent electrochemical performance of full cell Li|NMC811 with 98.6% CR after 300 cycles. Gebert et al. developed fluorine-free TEP-based electrolytes for interfacial investigation, and a threshold effect was observed at 30 vol% TEP when inadequate carbonate-ester solvated Li+ initiated poor SEI production and TEP breakdown.122 At increased TEP concentration (>30%), this resulted in immense ethane production and irreversible capacity loss, as shown in Fig. 12f. Perhaps additive incorporation could stabilize the TEP for safer and high-performance batteries. Therefore, fluorine-free phosphate-based solvents are a major component in developing safe flame retardant, multifunctional additives for sustainable energy systems by achieving high CR and extended cycle life.279
Electrolytes with great thermal stability and high flashpoints were produced by using sulfolane (SL) in adiponitrile (ADN).317,318 Excellent oxidation stability was demonstrated by electrolytes containing LiDCTA and LiTDI up to 4.5 V.317 Fluorine-free ASE (ADN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SL
SL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EC at 2
EC at 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) electrolytes, notably LiDCTA, achieved ∼145 mA h g−1 discharge capacities, stabilizing after initial losses with a CE of 97.5% (LiTDI:ASE) and 95.8% (LiDCTA:ASE) after 5–20 cycles due to side reactions in Li/LiFePO4, as shown in Fig. 13a-(i) and (ii). The extended cut-off voltage of 4.5 V reduced reaction reversibility. However, AS (ADN
1) electrolytes, notably LiDCTA, achieved ∼145 mA h g−1 discharge capacities, stabilizing after initial losses with a CE of 97.5% (LiTDI:ASE) and 95.8% (LiDCTA:ASE) after 5–20 cycles due to side reactions in Li/LiFePO4, as shown in Fig. 13a-(i) and (ii). The extended cut-off voltage of 4.5 V reduced reaction reversibility. However, AS (ADN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SL at 2
SL at 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) electrolytes had a higher average CE of 99.8% after 5–20 cycles despite slightly reduced capacities. However, adding lithium salt made EC break down into CO2, emphasizing the need for purification.319 Xu and Angell created various cyclic and acyclic sulfones in an effort to better understand their physical and electrochemical characteristics and evaluated them as electrolyte solvents for LIBs.320 However, their compatibility with graphitic anodes, which occasionally match the performance of carbonate-based electrolytes, is identified by their alkyl substituent structures.321 Jiang et al. developed a novel aqueous deep eutectic solvent (DES) based on methylsulfonylmethane (MSM), LiClO4, and water that exhibits increased conductivity and stability in LIBs, as shown in Fig. 13b.322,323 The electrolyte's improved performance resulted in approximately 100% CE and 72.2% CR after 1000 cycles at 4.5C. For example, in recent times, Kreth and coworkers introduced a new FFE, 1 M LiBOB in EiPS, for use in high-temperature capacitive and faradaic energy storage devices.123 The given electrolyte has superior safety characteristics (boiling point 265 °C, flash point 137 °C) and strong SEI layer formation on graphite electrodes, leading to outstanding performance at 60 °C. The capacity of the LFP half-cell in LIBs with LFP-positive electrodes was 150 mA h g−1 at 0.1C and reduced to 123 mA h g−1 at 5C, as shown in Fig. 13c-(i) and (ii). Less than 2% of capacity was lost across 200 cycles of long-term cycling at 1C and 60 °C, signifying the high-temperature adaptability of the electrolyte. Fig. 13c-(iii) shows the promising performance of a partially optimized LIB cell with a massive LFP cathode and graphite anode, which maintained roughly 80% of the initial capacity for the first 90 cycles.123 Due to their increased viscosity compared to conventional CB systems, fluorine-free sulfone-based electrolytes are widely opted for high-voltage. Moreover, wettability issues with polypropylene or polyethylene separators are mitigated by mixing CBs or EBs or surface modification of the separator, enhancing the stability of the high voltage application. Furthermore, future directions are highly motivated to develop such solvents in bi-functional additive case studies rather than as main solvent or co-solvent to empower potential strategies.324
1) electrolytes had a higher average CE of 99.8% after 5–20 cycles despite slightly reduced capacities. However, adding lithium salt made EC break down into CO2, emphasizing the need for purification.319 Xu and Angell created various cyclic and acyclic sulfones in an effort to better understand their physical and electrochemical characteristics and evaluated them as electrolyte solvents for LIBs.320 However, their compatibility with graphitic anodes, which occasionally match the performance of carbonate-based electrolytes, is identified by their alkyl substituent structures.321 Jiang et al. developed a novel aqueous deep eutectic solvent (DES) based on methylsulfonylmethane (MSM), LiClO4, and water that exhibits increased conductivity and stability in LIBs, as shown in Fig. 13b.322,323 The electrolyte's improved performance resulted in approximately 100% CE and 72.2% CR after 1000 cycles at 4.5C. For example, in recent times, Kreth and coworkers introduced a new FFE, 1 M LiBOB in EiPS, for use in high-temperature capacitive and faradaic energy storage devices.123 The given electrolyte has superior safety characteristics (boiling point 265 °C, flash point 137 °C) and strong SEI layer formation on graphite electrodes, leading to outstanding performance at 60 °C. The capacity of the LFP half-cell in LIBs with LFP-positive electrodes was 150 mA h g−1 at 0.1C and reduced to 123 mA h g−1 at 5C, as shown in Fig. 13c-(i) and (ii). Less than 2% of capacity was lost across 200 cycles of long-term cycling at 1C and 60 °C, signifying the high-temperature adaptability of the electrolyte. Fig. 13c-(iii) shows the promising performance of a partially optimized LIB cell with a massive LFP cathode and graphite anode, which maintained roughly 80% of the initial capacity for the first 90 cycles.123 Due to their increased viscosity compared to conventional CB systems, fluorine-free sulfone-based electrolytes are widely opted for high-voltage. Moreover, wettability issues with polypropylene or polyethylene separators are mitigated by mixing CBs or EBs or surface modification of the separator, enhancing the stability of the high voltage application. Furthermore, future directions are highly motivated to develop such solvents in bi-functional additive case studies rather than as main solvent or co-solvent to empower potential strategies.324
 | ||
Fig. 13 (a)-(i) CE (2.5–4.4) V and (ii) Specific capacity at different C-rates (2.5–4.0) V for Li/LFP cells with AS, ASE, and LiDCTA ASE electrolytes, Reproduced with permission,317 Copyright (2016) Elsevier. (b) DFT structure of MSM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LiClO4 DES (2 LiClO4 DES (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and MSM 1) and MSM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LiClO4 LiClO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O DES (2 H2O DES (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), Reproduced with permission,322 Copyright (2019) American Chemical Society. (c) Electrochemical performance at 60 °C with 1 M LiBOB in EiPS electrolyte: (i) rate capability; (ii) 200-cycle CR at 1C in LFP; (iii) 100-cycle CR at 1C in graphite‖oversized LFP cathode (1 1), Reproduced with permission,322 Copyright (2019) American Chemical Society. (c) Electrochemical performance at 60 °C with 1 M LiBOB in EiPS electrolyte: (i) rate capability; (ii) 200-cycle CR at 1C in LFP; (iii) 100-cycle CR at 1C in graphite‖oversized LFP cathode (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 ratio), Reproduced with permission,123 Copyright (2024) Wiley-VCH. 7 ratio), Reproduced with permission,123 Copyright (2024) Wiley-VCH. | ||
 | ||
| Fig. 14 Fluorine-free ionic liquids: (a)-(i) conductivity; (ii) viscosity vs. temperature for PT@P111i4TCM and LiDCA–P111i4TCM@LDPT electrolyte; (iii) linear sweep voltammograms of Li/PT/Pt and Li/LDPT/Pt cells, Reproduced with permission,334 Copyright (2022) American Chemical Society. (b) Oxidation limit of aromatic IL anions, Reproduced with permission,342 Copyright (2020) American Chemical Society. (c) Schematic of the steps for production of IL from biomass, Reproduced with permission,38 Copyright (2021) American Chemical Society. (d) Structure of fluorine-free ionic liquid anion, Reproduced with permission,38 Copyright (2021) American Chemical Society; Reproduced with permission,334 Copyright (2022) American Chemical Society; Reproduced with permission,341 Copyright (2020) American Chemical Society; Reproduced with permission,342 Copyright (2020) American Chemical Society. | ||
FFEs are eco-friendly alternatives to conventional toxic fluorinated electrolytes.195 Perhaps the in-depth insights from inspiring works on biomass-derived IL electrolytes for future greener battery applications could pave the way for net zero emissions by mitigating hazardous substances in the battery environment. For example, Khan and colleagues explored a new method for eliminating fluorine in LIBs, called “bio-sustainability”, which entails using enormous eco-friendly, low-cost, and readily available natural materials, such as agricultural waste and biomass.38 In comparison to non-aromatic anions, tetra(n-butyl)phosphonium and tetra(n-butyl)ammonium, together with aromatic furan-2-carboxylate and thiophene-2-carboxylate anions, show better thermal and electrochemical stability.342,343 Based on the (FuA)− anion, a tetra(n-butyl)phosphonium furoate [(P4444)(FuA)]-based fluorine-free IL was prepared and combined in different ratios with lithium 2-furoate, as shown in Fig. 14c.38 Furthermore, this fluorine-free IL with specific cations–anions, listed in Fig. 14d, was thoroughly investigated for potential battery applications due to its better synergistic ion interaction, ion diffusivity, thermal stability, and electrochemical characteristics.38,342,343 Despite the sustainability advantage of these electrolytes, their performance is limited compared to conventional electrolytes.
Liu et al. developed a PFAS-free locally concentrated ionic liquid electrolyte to study the challenges, dendrite formation, and low reversibility in both monovalent LMBs and multivalent batteries (aluminium-metal batteries (AMBs)).344 By incorporating fluorine-free non-solvating cosolvents such as aromatic organic cations, it is promoted to reduce viscosity, improving ion transport and anode compatibility.345,346 Their studies enhanced the lithium stripping/plating CE to 99.7%, providing good electrochemical stability and safety compared to traditional systems.25 In addition, FFE systems are insightfully demonstrated in terms of compatibility with high-energy cathodes, including Ni-rich layered oxides and sulfurized polyacrylonitrile (SPAN), enabling high-performance LMBs by improving ion transport and EEI formation in AMBs.346–348 Their studies facilitated the transition from monovalent to multivalent batteries, significantly promoting cost-effective batteries through greener approaches. However, the interactions at the electrode interface and their impact on reversibility and performance, particularly in the aluminum-metal anode, remain unclear.348 Investigating ionic liquid cations, Lewis basic ligands, and non-solvating cosolvents could provide valuable insights into enhancing multivalent battery performance.344 Nonetheless, these studies truly herald a new era in developing IL-based formulations for greener batteries, addressing various challenges for future generations.
4.3. Additives for fluorine-free electrolytes
By improving the inherent characteristics of electrolytes, electrolyte additives, even in small amounts, can significantly increase battery performance. These additives are classified according to their specialized functions, which include producing protective coatings, stabilizing electrolytes, preventing fire and overcharging, and promoting conductivity. Fluorinated additives are highly promising in most aspects for overcoming such obstacles.151 However, a minimal amount of fluorine greatly impacts the environment. Under such circumstances, promoting fluorine-free additives (FFAs) in FFEs could scale-up the sustainable strategy. In addition, developing new eco-friendly functional additives influences eliminating high toxicity in the energy source field. Moreover, there are a few FFAs, such as carbonate additives (VC),58 nitrate-based additives (LiNO3),139,200,349 potassium nitrate (KNO3),213 rubidium nitrate (RbNO3),350,351 dinitriles (adiponitrile, glutaronitrile, and succinonitrile),352–355 phosphorus-based additives (TMP, TEP),356–360 sulfone-based additives (SBAs),361,362 and aromatic based additives (benzene, chlorobenzene),363 which are widely used for appropriate issues.58,139,142,213,364–370 These additives have received recent attention by achieving a sustainable pathway. This is essential for lithium metal anodes to enrich the SEI layer, inhibit dendrite growth, and enable smooth lithium deposition. Despite these advancements, high-performance FFAs are still limited, highlighting the need for bifunctional and multifunctional additives in future battery technologies. Bifunctional additives are essential for stabilizing the SEI layer and enhancing lithium-ion transport, addressing dendrite formation and capacity retention challenges. Multifunctional additives further optimize electrolyte properties, thereby improving battery safety, cyclability, and energy density.For example, Zhou et al. introduced N,O-bis(trimethylsilyl)acetamide (BSA) as a bifunctional fluorine-free additive for high-voltage LMBs to overcome challenges such as dendrite growth and lithium anode expansion.141 Their DFT analysis revealed BSA's high reduction stability (LUMO: 0.0311 eV) and strong Li-ion affinity (−1.024 eV), promoting uniform deposition and stable SEI formation. In addition, BSA scavenged harmful HF byproducts, enhanced ionic conductivity, and reinforced the SEI with SiOx and Li3N components, as shown in Fig. 15a, improving mechanical strength and ion transport. Eventually, their approach proved more sustainable and cost-effective than fluorinated additives, signifying the potential of Si−O bond-based compounds.141 BSA enhanced SEI composition by 0.5 wt%, allowing successful cycling at a high loading (6 mg cm−2), 4.3 V at ∼2C, and 72.64% CR after 200 cycles.141 Interfacial characteristics are influenced mainly by the solvation shell of Li+. Hence, the cation's solvation structure must be carefully controlled to achieve reversible cycling in various batteries. This may be done by adjusting the type and concentration of solvents, additives, and anions. Moreover, adding a few additives like SBAs increases the viscosity and thermal stability issues despite improving the electrode stability and performance. Chagnes’ research group reported the synthesis of 22 bi-functional additives for LIBs.324 Their work achieved good electrode stability but limited ionic conductivity due to the high viscosity of the electrolyte. More importantly, the fast capacity fading issue associated with an anode-free battery system can eventually be solved by an FFA system.213,371–373 Our research group introduced a bi-functional FFA, KNO3, in the commercial electrolyte (1 M LiPF6 EC DEC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v)).213 As shown in Fig. 15b, our study demonstrated the synergistic shielding effect of K+ and NO3− in countering the dendrite formation and inhibiting capacity fading, which contributed to effective cycling performance.213 Similarly, Rahman et al. investigated the use of CsNO3 as a bi-functional additive to improve the performance of LMBs by forming stable interphases.374 Unlike traditional fluorinated electrolytes, which form LiF-rich SEIs that hinder fast charging due to low ionic conductivity, the electrolyte with 3 wt% CsNO3 enhances ion transport by utilizing 1,2-dimethoxyethane (DME) as the sole solvent. As shown in Fig. 15c, under the electric field, Cs+ and NO3− ions are transferred to the electron-rich Li metal surface and the electron-deficient NMC811 surface, facilitating protective interphase formation on both electrodes. The larger ionic radius of Cs+ (1.67 Å) compared to that of Li+ (0.59 Å) weakens the interaction with DME, forming a larger solvation shell that allows more anions to contribute to forming a robust interphase.374 In addition, while compared with other alkali metals (3 wt% Li, 2 wt% Na, 3 wt% K, 3 wt% Rb) nitrate additives show some improvement, CsNO3 outperforms them, challenging the conventional role of LiF in SEI formation and demonstrating the promising potential of FFE systems for advancing sustainable batteries.374 This work indicates future directions to develop a fluorine-free multi-functional additive (FF-MFA) to enhance safety, eco-friendliness, and reliable battery performance. In recent years, FF-MFA has been a promising approach to enhance pristine electrolyte properties towards sustainability. Liu et al. developed a sustainable and multifunctional FF-MFA for LIBs.142 The designed solvents for FF-MFA and bis(2-methoxyethyl)methylphosphonate (BMOP) functioned as flame retardants and were compatible with graphite, while also demonstrating solvation structure dominance.142 Analysing theoretical and practical data aided in understanding how BMOP affects graphite's electrochemical performance. To investigate the decomposition reactions, the blank electrolyte (1 M LiPF6 in DMC/EMC/EC, v
1 v/v)).213 As shown in Fig. 15b, our study demonstrated the synergistic shielding effect of K+ and NO3− in countering the dendrite formation and inhibiting capacity fading, which contributed to effective cycling performance.213 Similarly, Rahman et al. investigated the use of CsNO3 as a bi-functional additive to improve the performance of LMBs by forming stable interphases.374 Unlike traditional fluorinated electrolytes, which form LiF-rich SEIs that hinder fast charging due to low ionic conductivity, the electrolyte with 3 wt% CsNO3 enhances ion transport by utilizing 1,2-dimethoxyethane (DME) as the sole solvent. As shown in Fig. 15c, under the electric field, Cs+ and NO3− ions are transferred to the electron-rich Li metal surface and the electron-deficient NMC811 surface, facilitating protective interphase formation on both electrodes. The larger ionic radius of Cs+ (1.67 Å) compared to that of Li+ (0.59 Å) weakens the interaction with DME, forming a larger solvation shell that allows more anions to contribute to forming a robust interphase.374 In addition, while compared with other alkali metals (3 wt% Li, 2 wt% Na, 3 wt% K, 3 wt% Rb) nitrate additives show some improvement, CsNO3 outperforms them, challenging the conventional role of LiF in SEI formation and demonstrating the promising potential of FFE systems for advancing sustainable batteries.374 This work indicates future directions to develop a fluorine-free multi-functional additive (FF-MFA) to enhance safety, eco-friendliness, and reliable battery performance. In recent years, FF-MFA has been a promising approach to enhance pristine electrolyte properties towards sustainability. Liu et al. developed a sustainable and multifunctional FF-MFA for LIBs.142 The designed solvents for FF-MFA and bis(2-methoxyethyl)methylphosphonate (BMOP) functioned as flame retardants and were compatible with graphite, while also demonstrating solvation structure dominance.142 Analysing theoretical and practical data aided in understanding how BMOP affects graphite's electrochemical performance. To investigate the decomposition reactions, the blank electrolyte (1 M LiPF6 in DMC/EMC/EC, v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v
v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v = 1
v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and the 15% BMOP electrolyte were characterized after their Li/Cu cells were charged to 2 mA h cm−2 at 0.5 mA cm2. The blank electrolyte had produced dimethyl 2,5-dioxahexane dicarboxylate (DMDOHC), which could have contributed to oligomer formation for SEI construction. In contrast, 15% BMOP electrolyte had generated various organic by-products, including dimethyl methylphosphonate (DMMP), lithium 2-methoxyethyl methylphosphonite (LMMP), and lithium 2-methoxyethan-1-olate (LMO), as shown in Fig. 15c. These by-products and their derivatives from BMOP decomposition are expected to have improved SEI stability compared to BE. This configuration improves the graphite anode's long-term cycle stability by enhancing Li+ migration, lowering charge transfer impedance, and suppressing lithium dendrites and fractures.142 Kim et al. have recently introduced nano-Si3N4 as an innovative FF-MFA that significantly enhances the stability and performance of LMBs.375 Their approach precisely modulates Li+ solvation dynamics to promote the formation of a robust Si, N-based SEI through alloying and conversion reactions.375 This SEI not only improves ionic transport but also reinforces interfacial stability, both of which are critical for long-term cycling of LMBs. In addition, the nano-Si3N4 additive mitigates dendrite formation by facilitating uniform lithium deposition and suppresses electrolyte degradation through effective HF scavenging, thereby addressing a major safety concern associated with moisture exposure or the decomposition of fluorinated salts.375 Furthermore, such multi-functional approaches can significantly enhance overall performance.
1) and the 15% BMOP electrolyte were characterized after their Li/Cu cells were charged to 2 mA h cm−2 at 0.5 mA cm2. The blank electrolyte had produced dimethyl 2,5-dioxahexane dicarboxylate (DMDOHC), which could have contributed to oligomer formation for SEI construction. In contrast, 15% BMOP electrolyte had generated various organic by-products, including dimethyl methylphosphonate (DMMP), lithium 2-methoxyethyl methylphosphonite (LMMP), and lithium 2-methoxyethan-1-olate (LMO), as shown in Fig. 15c. These by-products and their derivatives from BMOP decomposition are expected to have improved SEI stability compared to BE. This configuration improves the graphite anode's long-term cycle stability by enhancing Li+ migration, lowering charge transfer impedance, and suppressing lithium dendrites and fractures.142 Kim et al. have recently introduced nano-Si3N4 as an innovative FF-MFA that significantly enhances the stability and performance of LMBs.375 Their approach precisely modulates Li+ solvation dynamics to promote the formation of a robust Si, N-based SEI through alloying and conversion reactions.375 This SEI not only improves ionic transport but also reinforces interfacial stability, both of which are critical for long-term cycling of LMBs. In addition, the nano-Si3N4 additive mitigates dendrite formation by facilitating uniform lithium deposition and suppresses electrolyte degradation through effective HF scavenging, thereby addressing a major safety concern associated with moisture exposure or the decomposition of fluorinated salts.375 Furthermore, such multi-functional approaches can significantly enhance overall performance.
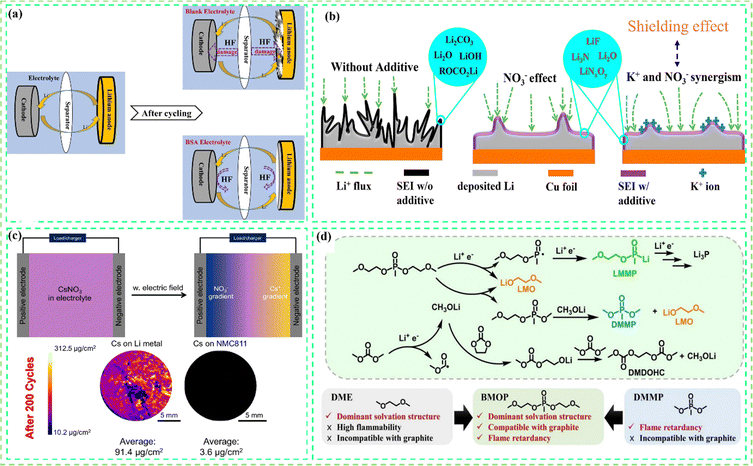 | ||
| Fig. 15 Fluorine-free additives: bifunctional additives’ mechanism (a) BSA in LMBs, Reproduced with permission,141 Copyright (2023) Wiley-VCH. (b) K+ prevents dendrites via electrostatic shielding, Reproduced with permission,213 Copyright (2019) Elsevier. (c) CsNO3 in LMBs, Reproduced with permission,374 Copyright (2023) Springer Nature. (d) Multifunctional additive BMOP degradation mechanism, Reproduced with permission,142 Copyright (2023) Elsevier. | ||
Simultaneously, several studies have been investigating the incorporation of fluorinated or fluorine-free scavengers to neutralize species such as HF, PF5, and H2O in traditional batteries.376 Although traditional scavengers can effectively neutralize HF, they often degrade over time, limiting their long-term efficiency.377 Moreover, decomposed fluorinated species complicate internal operation by deteriorating the battery components, including the cathode (particularly Co-free Ni-rich), separator (high-temperature shutdown), and high-concentration, high-voltage electrolytes. These components' interaction can obscure the underlying chemical mechanisms.28,87 Furthermore, the potential cost increase associated with adding scavenging materials, their byproducts, and interactions with other electrolyte additives in current LIB systems necessitates thorough evaluation before commercialization.376 Similarly, many studies have been conducted to increase the fluorine components in order to improve cycling performance. This consideration, particularly in the practical application, could intensely impair the eco-system. Here, our concern is not limited to scavengers only.
Despite the promising attributes of FFAs in terms of environmental and safety benefits, their integration into fluorinated electrolytes to meet viable electrochemical performance remains an unclear interfacial contribution.141,142 Furthermore, incorporating FFAs in such an HF environment may not provide insight into developing an eco-friendly battery.141 This situation underscores the urgent need for a more sustainable approach to electrolyte design. One should focus on developing advanced FFAs that replace fluorinated species and maintain compatibility with other battery components. Such advancements are essential for realizing high-performance, cost-effective, environmentally benign batteries that meet the global demand for clean and efficient solutions.
4.4. Diluents for fluorine-free electrolytes
To overcome the high viscosity and expense of HCEs, LHCEs are incorporated into lithium batteries. LHCEs increase wettability and ionic conductivity by lowering viscosity, encouraging the battery's efficiency. They also reduce the total cost by decreasing the amount of lithium salt required. Diluent electrolytes assist in stabilizing the electrolyte, resulting in improved battery system performance and safety. Fluorinated diluents (FDs) are widely used for designing various electrolyte systems, including low-concentration electrolytes, HCEs, and LHCEs, to overcome the issue to attain high performance.67,378–380 However, these FDs are highly toxic and adversely affect global integrity.111,359,381–384 Generally, the massive amount of diluent used in electrolyte design, such as FDs, presents obstacles like high toxicity, environmental pollution, and cost. Introducing non-fluorinated diluents may be a more reliable strategy. In recent years, fluorine-free diluents (FFDs) have shown promise in developing LHCEs. For example, Hai and colleagues designed an interesting LHCE using benzene as an FFD.140 This system addresses the drawbacks of FDs for high-loading (9 mg cm−2) LMBs.Owing to the absence of electron-withdrawing groups, benzene's (PhH) distinct conjugated structure offers exceptional oxidative (>4.7 V) and reduction stability. This diluent method eliminates fluorinated solvents' high cost, density, and environmental contamination. PhH-LHCE has strong ion conductivity, low density, cheap price, great lithium metal stability, and minimal dendrite formation compared to fluorinated electrolytes. This allows for stable cycling for 450 cycles in SC811‖Li cells, as shown in Fig. 16a.140 However, benzene has a very low flash point and is highly flammable, so caution is needed.
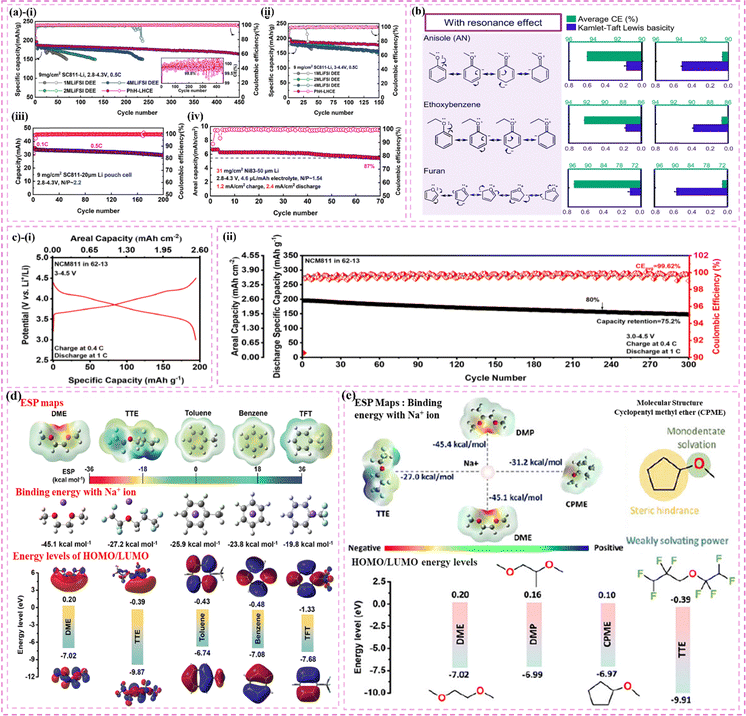 | ||
| Fig. 16 (a) Electrochemical performance of FFDs for long cycling Li-SC811 (2.8–4.3 V), Reproduced with permission,140 Copyright (2024) Wiley-VCH. (b) Resonance structures: purple (with effect), orange (without effect), average CE and Kamlet–Taft (β) values, Reproduced with permission,385 Copyright (2022) Springer Nature. (c)-(i) Voltage profiles, (ii) cycling performance, Li/NCM811 in 0.6 M LiFSI + 0.2 M LiDFOB-1,3-DIOX electrolyte, Reproduced with permission,137 Copyright (2024) Wiley-VCH. (d) Structural analysis of FFDs for sodium batteries, Reproduced with permission,386 Copyright (2024) Wiley-VCH. (e) Structural analysis of cosolvent as FFDs for sodium batteries,387 Reproduced with permission, Copyright (2025) Wiley-VCH. | ||
Researchers are focusing on lithium metal electrodes for their high energy density and low electrochemical potential despite the challenge of dendritic growth. A strong SEI can prevent this issue by limiting unwanted reactions. LHCEs with non-solvating cosolvents initiate compact solvation pairs, forming a robust SEI with lower viscosity and higher lithium-ion conductivity than the HCE. Although 1,2-difluorobenzene has a higher dielectric constant and dipole moment than THF, it is less effective at solvating lithium ions.388 To enhance cosolvent design, new solvent descriptors that account for interactions within the electrolyte are suggested.389 Moon et al.'s study on FFD electrolytes motivates future researchers to explore the FFD system.385 This study comprised 14 diluents in a LiTFSI–EC/DEC system. It revealed that stabilization energy, reflecting Lewis basicity (β), strongly correlates with solvation structures and battery performance.385 Moreover, this method showed that β is a better predictor of battery performance than traditional metrics.
As shown in Fig. 16b, the Kamlet–Taft parameter is proposed for evaluating solvent miscibility in polar electrolytes, aiding in selecting optimal diluents. It was found that both 3 M LiFSI DME![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Furan-(1
Furan-(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) and 3 M LiFSI DME
2) and 3 M LiFSI DME![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PhM (anisole)-(1
PhM (anisole)-(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) performed the best, with a high CE of 99.0% for 1400 cycles and 98.5% for 500 cycles, respectively.385 Furan-based electrolytes showed impressive performance even at higher current densities and capacities, maintaining 99.4% efficiency for 500 cycles. Similarly, Yang and his team developed high-voltage fluorine-free ether-based electrolytes in low-concentration fluorinated salts using 1,3-dioxacyclohexane (1,3-DIOX) as a diluent. An electrolyte (0.8 M) with 0.2 M LiDFOB + 0.6 M LiFSI in 1,3-DIOX achieved a high CE of 99.47%. It supported high-loading Li‖NCM811 cells up to 4.5 V, retaining 75.2% capacity after 300 cycles, as shown in Fig. 16c-(i) and (ii). DFOB− anions formed stable interphases that mitigated transition metal dissolution and gas release. This approach offered a sustainable alternative to traditional methods using hazardous fluorinated solvents. Furthermore, many studies focus on leveraging fluorination to achieve excellent performance; however, some challenges still exist. On the other hand, little investigation has been carried out into effectively removing fluorine from FFD electrolytes.
2) performed the best, with a high CE of 99.0% for 1400 cycles and 98.5% for 500 cycles, respectively.385 Furan-based electrolytes showed impressive performance even at higher current densities and capacities, maintaining 99.4% efficiency for 500 cycles. Similarly, Yang and his team developed high-voltage fluorine-free ether-based electrolytes in low-concentration fluorinated salts using 1,3-dioxacyclohexane (1,3-DIOX) as a diluent. An electrolyte (0.8 M) with 0.2 M LiDFOB + 0.6 M LiFSI in 1,3-DIOX achieved a high CE of 99.47%. It supported high-loading Li‖NCM811 cells up to 4.5 V, retaining 75.2% capacity after 300 cycles, as shown in Fig. 16c-(i) and (ii). DFOB− anions formed stable interphases that mitigated transition metal dissolution and gas release. This approach offered a sustainable alternative to traditional methods using hazardous fluorinated solvents. Furthermore, many studies focus on leveraging fluorination to achieve excellent performance; however, some challenges still exist. On the other hand, little investigation has been carried out into effectively removing fluorine from FFD electrolytes.
In the large-scale manufacture of alkaline metal batteries, this strategy promises to lower price and reduce environmental impact. For instance, Pai et al. studied the aromatic hydrocarbon toluene as a diluent for Na–S batteries.386 Their computational analysis revealed a similar solvation structure and exceptional binding energy of both FDs (TTE) and FFDs (toluene) towards the Na+ ion, as shown in Fig. 16d. In addition, toluene promotes an inorganic-rich SEI, which is significantly dominated by anions, further contributing to practically viable performance for Na-SPAN cells. Their fluorine-free LHCE in the sodium–sulfur battery emphasizes cost-effective and practical viable high-energy-density batteries. However, aromatic hydrocarbon-based diluents are limited in practical implementation by their low flash point and high flammability, posing significant safety concerns. Despite its promising electrochemical properties, the risks associated with benzene necessitate careful evaluation of its thermal stability and flammability before widespread adoption. Consequently, further research is needed to optimize electrolyte formulations that retain benzene's advantages while ensuring safe and scalable application. Furthermore, fluorine-free ethers often exhibit higher reductive stability and offer more possibilities for solvation structure modulation. There is interest in finding fluorine-free solvents with extremely low solvating power that exhibit remarkable compatibility with sodium metal while reasonably priced. For example, Pai et al. recently examined the LHCE by utilizing ether cosolvent DMP and cyclopentyl methyl ether (CPME)) as a diluent for sodium–sulfur (Na–SPAN) batteries.387 CPME has high reduction stability, weak solubility with polysulfides, and minimal solvating interactions with salt anions due to lower electron density of the five-carbon ring naphthene, which provides substantial steric hindrance and steadily contributes to anion-rich solvation shell, as shown in Fig. 16e.387 Furthermore, stable, realistically feasible Na–SPAN batteries have been developed, revealing a method that uses mixed fluorine-free ether electrolytes with LHCE behavior. However, this ether is highly flammable due to low flash points, rising safety issues. Although fluorine-free monovalent batteries (Li+, Na+) employing LHCEs have been extensively reported to have satisfactory performance and are cost-effective by utilizing aromatic hydrocarbons and mixed ether cosolvents, they face safety issues. These challenges signify that future advances should focus on sustainable LHCE development to promote safety and superior performance. In addition, designed LHCEs can be made viable for multivalent batteries to make cost-efficient and durable performance batteries.
Numerous studies have been conducted, focusing on fluorine-free solvents, salts, diluents, and additives across a range of formulations, as outlined in Table 2. These investigations have yielded promising results, showcasing the potential of fluorine-free alternatives in battery technology. However, despite these encouraging advancements, significant challenges remain. The path toward the commercial realization of FFBs is still long, as further research and optimization are necessary to ensure their efficiency, stability, and cost-effectiveness in large-scale applications.
| Battery | Electrolyte | Temperature | Cut-off voltage [V] | Electrochemical measurement | Ref. | ||
|---|---|---|---|---|---|---|---|
| Cell configuration | Capacity [mA h g−1]/C-rate | Avg. CE [%]/CR [%]/cycle number | |||||
| Fluorine-free lithium-based battery | 0.7 M LiBOB EC/EMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) 1, v/v) |
65 | 3.3–4.35 | Li‖LiMn2O4 | 60/0.5 | — | 390 |
1.0 M in LiBOB EC/PC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) 1, v/v) |
60 | 3.5–4.2 | Li‖LiMn2O4 | —/0.5 | —/—/100 | 391 | |
0.7 M LiBOB SL/DMS (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) 1, v/v) |
60 | 2.7–4.2 | Li‖LiFePO4 | —/0.5 | —/94.0/100 | 392 | |
0.7 M LiBOB SL/DES (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) 1, v/v) |
—/66.5/100 | ||||||
0.7 M LiBOB SL/DEC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) 1, v/v) |
RT | 2.7–4.2 | Li‖LiFePO4 | —/0.5 | —/89.7/342 | 393 | |
| LiB(CN)4:PAN:6% Al2O3:PEGDME | 30 | 2.5–4.4 | Li‖LiFePO4 | —/10 | ∼97/—/15 | 113 | |
0.7 M LiBOB EC/EMC (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, v/v) + 2 vol% VC 7, v/v) + 2 vol% VC |
RT | 3–4.2 | Silicon–graphite‖LiNi1/3Mn1/3Co1/3O2 (NMC111) | 147/1 | 99.51/84.4/200 | 58 | |
| 0.305 M [Li(FuA)]0.100 mol%; [(P4444)(FuA)]0.900 mol% | 20–80 | — | Glassy carbon/Pt/Ag–AgCl | — | — | 38 | |
LiClO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MSM MSM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1 H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.8 1.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol%) 1 mol%) |
— | 2.4–3.3 | Li4Ti5O12‖LiMn2O4 | —/4.5 | 99.5/85.2/1000 | 322 | |
| 1 M LiBOB GVL | — | 2.0–4.2 | Graphite‖AC | 2 | —/80/25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
188 | |
| 1 M LiBOB EiPS | 60 | 2.0–4.2 | LiFePO4|graphite|Li | —/0.1 | —/80/100 | 123 | |
| 1 M LiBOB + 0.5 M LiNO3 DME | 60 | 2.0–4.2 | Li‖LiFePO4 | —/50 | —/75/500 | 45 | |
| 1 M LiNO3/MO | — | ∼3–4.25 | Li‖|NCM622 | —/0.5 | 99/91.4/1000 | 127 | |
| 1 M LiClO4 + 0.2 M LiNO3 TEP | 60 | 4.3 | Li‖NCM811 | —/1 | 99.72/82.11/500 | 126 | |
| 25 | 4.3 | Li‖NCM811 | —/1 | 99.84/80.63/800 | |||
LiPAA 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) wt% wt% |
— | — | LiTi2(PO4)3‖LiMn2O4 | —/0.5 | —/—/100 | 114 | |
| 1 M LiClO4–2H2O–3.9PED | 25 | 1.5–2.8 | LMO‖LTO | —/0.5 | 93.5/92.4/300 | 394 | |
1 M LiClO4 EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EMC EMC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMC (1 DMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, w/w/w) + 1% VC + 1% PS + 0.1% LiBOB 1, w/w/w) + 1% VC + 1% PS + 0.1% LiBOB |
25 | 2.8–4.3 | Gr‖NCM811 | 145.9/1 | —/75.2/200 | 112 | |
| LiTCM PEO | 70 | 1.6–2.6 | Li–S‖Li | 800/0.2 | 100/—/1 | 115 | |
0.4 mol LiNO3/0.5 mol Li2S8 1 L DOL/DME (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) 1, v/v) |
— | 1.8–2.8 | CMK3|S|Li–S | 800/0.1 | —/—/>500 cycles | 395 | |
| 3 M LiNO3 DMI | 25 | 2.5–4.0 | Li‖LiFePO4 | 120/— | —/—/700 | 149 | |
| 1 M Li2SO4 0.1 M LiOH/H2O | — | — | LiTi2(PO4)3‖LiFePO4 | —/6 | —/90/1000 | 396 | |
0.7 M LiBOB EC:EMC (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, v/v) + 30 vol% TEP 7, v/v) + 30 vol% TEP |
RT | 3–4.3 | NMC622‖Gr | 180/0.2 | —/—/100 | 122 | |
| 2.27 M LiClO4 0.74 Anisole (AN)/0.26DME | RT | — | Li‖Cu | — | 98.9/—/— | 397 and 398 | |
| 1 M LiClO4 + 3 wt% LiNO3 DOL/DME | 99.1/—/— | ||||||
| Partial fluorination lithium-based battery | 0.6 M LiFSI + 0.2 M LiDFOB 1,3-DIOX | RT | 3–4.5 | Li‖NCM811 | —/0.4 | 99.47/75.2/300 | 137 |
| DME + 0.96 M LiFSI + 12 mM TFP ClO4 | RT | 3–4.2 | Li‖NCM811 | —/0.1 | 99.6/94/275 | 399 | |
1 M LiPF6 + 0.5 wt% BSA EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DME DME![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EMC (1 EMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v/v) 1, v/v/v) |
RT | 2.8–4.3 | Li‖NMC622 | 100.99/0.2–1,2,3 cycle/2 | 98/72.64/200 | 141 | |
0.8 M LiNO3 + 5 wt% VC GBL/FPPN (96.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.5 wt%) 3.5 wt%) |
25 | 2.7–4.3 | Li‖NMC622 | 143/0.619 | —/90/200 | 139 | |
| 2.0 M LiFSI DEP | 25 to 27 °C | 2.8–4.3 | Li‖NCA | —/0.3 | 99.68/80/600 | 138 | |
1 M LiPF6 + 15% BMOP DMC/EMC/EC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v/v) 1, v/v/v) |
— | 3.0–4.3 | Li‖NCM811 | —/4 | 90.9/—/500 | 142 | |
2 M LiFSI DEE/PhH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) 1, v/v) |
— | 2.8–4.3 | Li‖LiNi0.8Co0.1Mn0.1O2 (SC811) | —/0.5 | 99.8/87.3/450 | 140 | |
0.6 M LiTFSI/0.4 M LiBOB/0.05 M LiPF6 EC/PC (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, v/v) 3, v/v) |
RT | 2.8–4.3 | Li‖NMC111 | 140.1/1 | 99.7/92.8/200 | 136 | |
2 M LiFSI + 0.15 M LiDFP DX EGDBE (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) 1, v/v) |
— | 2.6–4.6 | Li‖NCM811 | —/0.5 | 99.85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) /80/250 /80/250 |
400 | |
| 1 M LiFSI + 0.2 M LiNO3 MODOL | 25 | 2.7–3.85 | Li‖LFP | —/1 | —/83/150 | 97 | |
| −20 | 2.7–3.85 | Li‖LFP | —/0.2 | —/90/110 | |||
| LiFSI-2CPME | 25 | 2.8–3.8 | Li‖LFP | —/0.2/0.5 | —/—/400 | 95 | |
1 M LiPF6 EC/DEC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) + FEC 5 wt% + 4.0 wt% 3.5 M LiNO3/DMI 1 v/v) + FEC 5 wt% + 4.0 wt% 3.5 M LiNO3/DMI |
60 | 2.8–3.65 | Li‖LiFePO4 | —/5 | —/95.6/2200 | 209 | |
3 Msolv LiFSI DME:AN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v) 2, v/v) |
25 | 2.7–4.2 | Li‖LiFePO4 | —/0.1 | —/93.7/300 | 385 | |
2 Msolv LiFSI DME:Furan (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v) 2, v/v) |
—/86.2/300 | ||||||
3 Msolv LiFSI DME![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethoxybenzene(1 ethoxybenzene(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v) 2, v/v) |
—/71.4/300 | ||||||
| Fluorine-free sodium-based battery | 0.7 M NaBOB NMP/TMP (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) 1, v/v) |
55 | 1.3–3.6 | Prussian white|hard carbon‖Na | —/0.2 | —/50/100 | 117 |
| 0.5 M NaBOB TMP | — | 1.0–3.8 | Prussian white|hard carbon|Na | —/0.2 | 97/—/2 | 401 | |
| Partial fluorine-free sodium-based battery | 2 M NaFSI DMP/CPME (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) 1, v/v) |
RT | 0.8–2.8 | Na‖SPAN | 720/0.5 | —/—/300 | 387 |
2 M NaFSI DME/toluene (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, v/v) 3, v/v) |
RT | 0.8–2.8 | Na‖SPAN | 740/0.5 | —/85/200 | 386 | |
| Fluorine-free potassium-based battery | 0.1 M KBPh4 EC/DEC (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, v/v) 7, v/v) |
— | 1.5–3.2 | K‖PTCDA | — | 92.6/200 | 121 |
| Fluorine-free calcium-based battery | 0.5 M CMC DME/THF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) 1, v/v) |
RT | 0.5–3.2 | Ca‖S/C | 805/0.1C per S | — | 118 |
| Fluorine-free zinc-based battery | 1.0 M Zn(dca)2 DMSO | — | 0.05–0.17 | Zn‖Zn | — | —/—/90 | 119 |
| 1.0 M Na(dca) + 1.0 M Zn(dca)2 DMSO | 50 | 0.2–2.0 | Zn‖NaFePO4 | —/0.1 | >99/—/100 | 124 | |
5. Paths toward greener battery materials
The transition to greener energy storage solutions is a critical step in addressing the environmental challenges posed by traditional battery technologies. A key focus in this journey is the development of fluorine-free materials and electrolytes, which offer significant advantages in terms of sustainability, safety, and environmental impact. This chapter briefly explores the path to FFB technologies, emphasizing the innovative approaches in material design and electrolyte formulation that pave the way for more eco-friendly and efficient batteries. The following sections delve into these developments, highlighting how cutting-edge research transforms the energy storage landscape by integrating sustainable practices and advanced technological solutions.5.1. Material design
The quest for safe and ecologically friendly battery solutions has resulted in extensive research towards fluorine-free materials. Fluorine-containing compounds are commonly used in traditional batteries owing to their excellent electrochemical stability and ability to generate a stable SEI.21 However, the environmental effect, toxicity, and high cost of fluorine have prompted an attempt for other materials that might provide comparable performance while avoiding these downsides. Significant environmental benefits are revealed by the life cycle assessment (LCA) of batteries designed using fluorine-free components.402 It is possible to reduce greenhouse gas emissions and harmful byproducts in the production process by removing fluorine and making it less energy-intensive.403 Besides reducing hazardous waste and disposal costs, the lack of fluorine makes end-of-life recycling easier. LCA demonstrates that finding and producing fluorine-free products frequently involves less ecologically harmful operations, such as rare element mining and processing, as shown in Fig. 17.404,405 In LCA, cradle-to-gate covers extraction to production, cradle-to-grave includes the entire life cycle to disposal, and grave-to-cradle focuses on recycling for new products.405 Thus, switching to FFB technology promotes the use of safer and more abundant materials while also improving sustainability and being consistent with the ideas of the circular economy. For example, Jiang et al. pioneered a significant advancement in battery technology by developing a net-zero fluorine battery that incorporates a fluorine-free binder in its cathode material, notably enhancing cycling performance.45 Cui et al. introduced a groundbreaking zero-strain electrode material that achieves 100% active material utilization in all-solid-state lithium batteries.406 This innovation not only boosted energy density but also extended cycle life by eliminating polymeric binders. The use of such 100% active materials is a highly promising approach for advancing FFB technology. The ongoing pursuit of FFBs necessitates a comprehensive approach that integrates cutting-edge material design, sophisticated characterization techniques, and a steadfast commitment to sustainability. Researchers are exploring alternatives such as LiF-free electrolytes and polymeric binders to develop high-performance batteries that meet rigorous environmental and safety standards. These advancements are set to transform the energy storage landscape, reducing the LCA footprint and paving the way for greener, more efficient battery technologies.5.2. Electrolyte formulation
The drive towards eco-friendly battery technologies has led to exciting advancements in FFE formulations as summarized in Table 2. The FFE system has explored different strategies for various metal batteries. Xu et al. have crafted innovative FFE “solvent-in-salt” (SIS) electrolytes for sodium batteries using NaDEEP and TEOP.407 This combination enhanced ionic conductivity and thermal stability up to 270 °C, pushing the boundaries of sodium battery performance.407 In the realm of lithium batteries, Li et al. have pioneered fluorine-free solvents by methylating DME, promoting the formation of stable inorganic LiF-rich interphases.138 This advancement addressed high-voltage stability concerns while reducing fluorine content.138 Meanwhile, Kim et al. developed a cost-effective sodium metal anode FFE, 1 M NaBH4 in an ether-based solvent, as shown in Fig. 18a, enabling a robust SEI layer crucial for stability and longevity.408 Moreover, Hong et al. proposed a strategy to significantly reduce the amount of fluorine used in lithium batteries by using diluted fluorinated cations (0.1 wt%) to produce robust battery interfaces with no effect on the environment, as shown in Fig. 18b.399 | ||
| Fig. 18 FFE advancements (a) seawater battery mechanisms; M1 and M2 reduce the oxide layer to NaH and H2, while M3 shows H2 reacting with sodium to form NaH, Reproduced with permission,408 Copyright (2022) The Royal Society of Chemistry. (b) FFE SEI interphase with Li-fluorinated cation, Reproduced with permission,399 Copyright (2024) The Royal Society of Chemistry. (c) LiBOB-based electrolyte (FFE) vs. LiPF6-based electrolyte (fluorinated electrolyte) preferential degradation on a SiGr-composite electrode, Reproduced with permission,147 Copyright (2024) The Electrochemical Society. | ||
Kisu et al. developed an FFE based on a hydrogen monocarborane cluster as a possible substitute for lithium-ion technology in calcium batteries.118 The FFE in alternative alkaline batteries works with outstanding performance. Liu et al. have developed an ultra-low concentration FFE electrolyte with exceptional stability and performance in a KMB.121 Furthermore, Weng et al. extended their studies to implement FFEs as safer substitutes for LiPF6, looking at how they affected graphite and silicon electrodes.147Fig. 18c shows that LiBOB-based electrolytes, while forming a thicker SEI layer, degraded more on graphite than silicon, leading to poorer performance compared to LiPF6.58,147 EDX mapping showed higher boron concentration on graphite, indicating preferential degradation.147 This interfacial engineering study reveals the need to match electrolyte chemistry with electrode materials to improve SEI formation and battery performance.147 Consequently, innovations in electrolyte formulations advance FFBs, incentivizing the circular economy, as shown in Fig. 19. Implementing a circular economy has significantly reduced waste by allowing the recovery and reuse of FFB materials throughout a product's life cycle. This strategy has been fostering resource conservation, priority, and efficiency.152
6. Summary and future perspectives
6.1. Summary
The development of FFEs for batteries has gained significant attention in recent years due to the environmental and safety concerns associated with traditional fluorinated components. This review emphasizes the need for sustainable alternatives that maintain high electrochemical performance. The transition to greener battery materials, especially in the design and formulation of electrolytes, is crucial for reducing environmental impact while maintaining or enhancing battery performance. To this end, several strategies have been explored to eliminate fluorine, including developing fluorine-free salts based on various anions such as halogen, phosphate, nitrile, borate, and nitrate. In addition, fluorine-free solvent systems, such as carbonate, ether, ester-phosphate, nitriles, sulfones, and ionic liquids, have been investigated for their potential to replace fluorinated counterparts. Each class of salts and solvents has been evaluated for its electrochemical properties, stability, and compatibility with high-performance batteries. Moreover, fluorine-free additives and diluents have been explored as potential enhancers to improve the overall performance of FFEs further. While fluorinated electrolytes offer notable benefits in terms of stability and conductivity, they pose significant environmental risks, including greenhouse gas emissions and challenges in managing the life cycle of fluorine-containing compounds.As concerns about climate change escalate, the development of fully “fluorine-free” electrolytes offers a compelling solution. However, “fluorine-free” is often inaccurately applied when fluorinated components remain in use, highlighting the importance of precision in terminology. This review advocates for adopting “partially fluorine-free electrolytes” where applicable, urging researchers to focus on eliminating fluorine over the long term. Achieving this goal requires optimizing ion transport, interfacial chemistry, and battery safety, aligning battery innovation with sustainability frameworks such as the 3Rs (reduce, reuse, recycle) and 3Es (energy, environment, economy). The ongoing development of FFEs has resulted in significant improvements in reducing environmental impact, aligning with the global shift towards greener technologies and cradle-to-cradle design. While there has been visible progress in developing FFEs, challenges remain, particularly in achieving adequate ionic conductivity and electrochemical stability without using fluorine-based salts and solvents. Overcoming these obstacles will require a deeper understanding of SEI formation mechanisms, degradation mechanisms, optimization of electrolyte formulations, and scaling up production processes for commercial viability. Despite these hurdles, the progress made thus far provides a solid foundation for future-generation battery technologies.
6.2. Future perspectives
As we have discussed above, FFE studies have recently been given attention, towards either partial or complete elimination of fluorine. The progress achieved on FFEs has been promising, showing significant potential for providing safer and more environmentally friendly battery systems. However, their role in enabling truly sustainable energy for the future needs attention. The future of FFEs presents a significant research opportunity as the field aims for environmentally friendly alternatives. The following are key challenges and potential research topics that should be explored in this area.An innovation of FFBs relies on the novel anion design and selection of non-fluorinated salts, solvents, additives, diluents, and binders to optimize FFEs. Materials that withstand elevated temperatures and voltage ranges must be chosen, ensuring long-term stability. The compatibility with electrodes and separators is key to minimizing degradation and providing reliable performance throughout the battery's life cycle. In addition, limited research studies on transitioning FFEs beyond lithium-based batteries present significant challenges. However, they also offer exciting opportunities for innovation.25,26,344 These breakthroughs would demonstrate the feasibility of creating FFBs that perform reliably under practical conditions, achieving safe, high-energy, cost-effective and environmentally friendly battery technologies.
6.2.1.1. Strategic innovations for fluorine-free electrolytes (FFEs). One of the main challenges in FFEs is the lack of fluorine-free ingredients, which contribute to efficient battery building with outstanding SEI/CEI construction. For instance, fluorine-free salts, including LiBOB, LiNO3, and LiClO4, commonly face issues with limited dissolution, gas evolution, and electrode corrosion. Eventually, a novel anion design should be developed strategically to overcome fundamental anion design challenges. Rational anion framework engineering can improve ionic conductivity and interfacial stability by incorporating electron-withdrawing groups or delocalized charge distribution. Moreover, a hybrid salt strategy that integrates multiple functional groups offers a pathway to optimize SEI formation while mitigating parasitic reactions. In addition, anion optimization should focus on enhancing solubility, oxidative stability, and electrochemical compatibility. Apart from lithium-based battery systems, the designed anion must evolve to support multivalent chemistries like the BGB anion, requiring deeper insights into solvation structures and transport properties. Beyond salt development, overcoming solvent design challenges is crucial, as non-fluorinated solvents often struggle with poor anodic stability, high viscosity, or low ionic dissociation. Solvent innovation should optimize electron-donating/withdrawing effects, steric hindrance, and solvation structure to improve ion transport and interphase formation. Addressing these challenges through molecular-level tuning and asymmetric solvent design approaches drives the next-generation batteries. Furthermore, designed novel FFEs underscore that they are free from expensive scavengers without compromising performance. Incorporating synergistic solvent–additive combinations or LHCE approaches may further mitigate stability issues, ensuring that FFEs can rival fluorinated counterparts in high-energy-density applications.
6.2.1.2. Scaling the synthesis of fluorine-free batteries (FFBs). The full potential of FFEs could be realized when integrated with advanced electrode materials, such as high-energy-density cathodes and dendrite resistant anodes. Although current fluorine-free ingredients are promising in performance, they suffer from limitations such as compatibility. Innovations in synthesis methodology necessitate developing moisture-free ingredients, which are crucial for performance and life cycle. In addition, novel material preparation methods and optimizing techniques should be needed to reduce resource use and manufacturing costs for broader adoption. For instance, fluorine-free disordered rock salt (DRX) cathodes, like Li1.1Mn0.8Ti0.1O2, demonstrate high specific capacities and strong cycling stability but require carefully optimized electrolyte composition to ensure efficient Li+ transport and interphase formation.409 The absence of fluorine in these systems eliminates Li–F clustering effects, thus simplifying interfacial chemistry and enhancing the long-term structural integrity of the electrode. By synergistically integrating F-free electrolyte formulations with manganese-rich DRX cathodes, the approach circumvents the limitations of conventional fluorinated systems. Furthermore, new FFB materials should be advanced in two ways: benchmarking FFEs and advanced tools, as stated below.
6.2.1.3. Comprehensive benchmarking FFEs and advanced tools for fluorine-free batteries (FFBs). Benchmarking FFBs against conventional systems is critical for validating their viability with regard to energy density, cycle life, safety, and cost-effective and scalable formulations. Advanced tools like artificial intelligence (AI) or machine learning (ML) accelerate discovery by predicting key properties such as dielectric constant, HOMO/LUMO levels, viscosity, flashpoints, solubility, and decomposition temperatures from large-scale databases. Moreover, higher energy density batteries’ operating voltages ≥4.5 V necessitate viable oxidative stability to construct a robust SEI/CEI via effective FFE design and protective cathode coatings, especially for nickel-rich cathodes prone to degradation. Simultaneously, emerging in situ and operando characterization techniques will be indispensable for elucidating real-time electrode–electrolyte interfacial evolution, thereby guiding the optimization of FFE formulations in a short-term period for sustainable technologies.
FFEs exhibit distinct LiF-free interfacial chemistry that promotes durable battery performance. Advanced techniques, such as spectroscopy, imaging, and molecular dynamics simulations, are necessary to explore the surface chemistry of these systems, revealing key insights into reaction products and interfacial phenomena. For example, Lu et al. studied the formation and evolution of the SEI by utilizing fluorine-free LiBOB-based electrolytes, with and without VC additives.410 Using operando ex situ X-ray photoelectron spectroscopy (XPS) and X-ray reflectivity (XRR), three stages of SEI evolution were observed: formation, densification, and thinning during delithiation, stated as “breathing” behavior.410 VC was found to inhibit LiBOB decomposition and promote a smoother SEI, reduce electron tunneling, and prevent lithium trapping. Their findings suggest that LiBOB-based electrolytes form distinct SEIs compared to traditional LiPF6 systems, with VC enhancing stability and electrochemical performance. Furthermore, this understanding emphasizes advanced tools’ utility in identifying optimal materials and configurations, allowing for the development of FFEs that enhance battery efficiency and longevity. By integrating advanced characterization tools, researchers can easily understand the complex mechanisms governing fluorine-free ingredients.
6.2.2.1. Expansion of FFEs beyond lithium-based batteries. One of the most promising limits for FFEs is their application in multivalent batteries. These batteries hold significant potential due to their constituent materials' abundance and low cost, making them ideal for large-scale energy storage applications. For example, Liu et al. worked on developing fluorine-free locally concentrated ionic liquid electrolytes, which hold great promise for multivalent AMBs, including aluminum–sulfur (Al–S) batteries.340,345,347,348 By incorporating fluorine-free cosolvents and aromatic organic cations, these electrolytes reduce viscosity and enhance ion transport.340 However, further research is needed to optimize electrode/electrolyte interphase formation, molecular-level interactions, and electrolyte stability.125,344 Improving organic cations, cosolvent design, and SEI evolution is critical to advancing the performance and stability, especially in Al–S batteries.344 Ongoing advancements in ionic liquids, deep eutectic solvents, and borate-based anions are key to improving ion transport and dendrite suppression. This requires the development of electrolytes specifically tailored for multivalent systems. Research must focus on understanding the de-solvation kinetics and interfacial reactions of multivalent ions, which are crucial for optimizing ion transport and ensuring stability in the electrolyte. For instance, integrating FFEs into sodium-ion and magnesium-ion batteries could provide a sustainable alternative to lithium-ion batteries. Sodium-ion batteries offer a high degree of cost-effectiveness due to the abundance of sodium. In contrast, magnesium-ion batteries have the potential for high energy density. Successful adaptations of FFEs for multivalent batteries would open new possibilities for sustainable and efficient energy storage systems that scale across various sectors.
6.2.2.2. Synergizing electrolyte and electrode innovations. The advancement of multivalent FFBs requires an integrated approach to electrolyte and electrode engineering to achieve superior electrochemical performance and stability. FFEs, utilizing alternative multivalent salts like borates (e.g., bis(glycolato)borate (BGB), bis(malonato)borate (BMB)) or sulfonimides, promote an HF-free environment while improving interfacial stability and ionic conductivity.123,411,412 Concurrently, multivalent battery electrodes such as calcium manganese dioxide (CaMn2O4) for Ca-ion and SPAN for Na–S and Al–S are gaining attention for future viability. Additionally, PFAS-free binders like hydroxyl-rich siloxane nanohybrid (SNH) and recyclable alternatives such as poly(ethyl cyanoacrylate) (PECA) promote sustainable battery designs.413,414 As these innovations continue to address existing limitations, FFBs could achieve real-world applicability, offering a viable pathway toward environmentally responsible energy storage solutions.25,26
Overall, the shift toward FFEs is driven by environmental concerns and the need for better battery performance. Developing these systems requires a comprehensive approach, from material selection to recycling strategies, leveraging innovative technologies to meet the demands of modern energy storage applications. Focusing on these areas will drive the next generation of sustainable and high-performing FFB technologies.
Abbreviations
| 1,3-DIOX | 1,3-Dioxacyclohexane |
| 3-FLTBP | Lithium tris[3-fluoro-1,2-benzenediolato(2-)-O,O′]phosphate |
| AC | Activated carbon |
| ACN | Acetonitrile |
| ADN | Adiponitrile |
| AGG | Aggregate |
| AMBs | Aluminium-metal batteries |
| BB | Butyl butyrate |
| BE | Ethyl butyrate |
| BGB | Bis(glycolato)borate |
| BL | Butyrolactone |
| BMC | Bis(2-methoxyethyl)carbonate |
| BMEC | Bis(2-methoxyethyl)carbonate |
| BMOP | Bis(2-methoxyethyl)methylphosphonate |
| BN | Butyronitrile |
| BOB | Bis(oxalato)borate |
| BrB | Bromobenzene |
| BSA | N,O-Bis(trimethylsilyl)acetamide |
| CCs | Cyclic carbonate solvents |
| CE | Coulombic efficiency |
| CEI | Cathode electrolyte interphase |
| CIB | Chlorobenzene |
| CIP | Contact ion pair |
| CMC | Calcium monocarborane |
| CMK3 | Mesoporous carbon |
| CPME | Cyclopentylmethyl ether |
| CR | Capacity retention |
| CTC | Tetrachloromethane |
| CYH | Cyclohexane |
| DBB | 1,3-Dibromobenzene |
| DBC | Dibutyl carbonate |
| DCA | Dicyanamide |
| DCB | 1,3-Dichlorobenzene |
| DEC | Diethyl carbonate |
| DEE | 1,2-Diethoxyethane |
| DEE | Ethylene glycol diethyl ether |
| DEP | 1,2-Diethoxypropane |
| DES | Diethyl sulfite |
| DGM | Diethylene glycol dimethyl ether |
| DGT | Diethylene glycol diethyl ether |
| DMC | Dimethyl carbonate |
| DMDOHD | Dimethyl 2,5-dioxahexanedioate |
| DME | 1,2-Dimethoxyethane |
| DMM | Dipropylene glycol dimethyl ether |
| DMMP | Dimethyl methylphosphonate |
| DMP | 1,3-Dimethoxypropane |
| DMS | Dimethyl sulfite |
| DMSO | Dimethyl sulfoxide |
| DN | Donor number |
| DOL | 1,3-Dioxolane |
| DPE | Dipropyl ether |
| DX | 1,3-dioxane |
| EA | Ethyl acetate |
| EB | Ethylbenzene |
| EBs | Ether-based solvents |
| EC | Ethylene carbonate |
| ECHA | European Chemicals Agency |
| EEL | Electrolyte–electrode interfaces |
| EGD | Ethylene glycol diacetate |
| EGDBE | Ethylene glycol dibutyl ether |
| EiBS | Ethyl-iso-butyl sulfone |
| EiPS | Ethyl isopropyl sulfone |
| EMC | Ethyl methyl carbonate |
| EMEES | Ethyl methoxyethoxyethyl sulfone |
| EMES | Ethyl methoxyethyl sulfone |
| EsBS | Ethyl-sec-butyl sulfone |
| ESCP | Ethyl sulfonyl cyclopentane |
| ESP | Electrostatic potential |
| FDs | Fluorinated diluents |
| FEC | 1-Fluoroethylene carbonate |
| FFA | Fluorine-free additive |
| FFBs | Fluorine-free batteries |
| FFD | Fluorine-free diluent |
| FFE | Fluorine-free electrolyte |
| FPPN | Pentafluoro(phenoxy)cyclotriphosphazene |
| GBL | γ-Butyrolactone |
| Gr | Graphite |
| GVL | γ-Valerolactone |
| HCE | Highly concentrated electrolytes |
| HF | Hydrogen fluoride |
| HPT | Heptane |
| IB | Iodobenzene |
| ILs | Ionic liquids |
| iPB | Cumene |
| KBPh4 | Potassium tetraphenylborate |
| KMB | Potassium metal battery |
| LCA | Life cycle assessment |
| LCs | Linear carbonate solvents |
| LHCEs | Localized high-concentration electrolytes |
| Li(FuA) | Lithium furan-2-carboxylate |
| LIBs | Lithium-ion batteries |
| LiDCTA | Lithium 4,5-dicyano-1,2,3-triazolate |
| LiFSI | Lithium bis(fluorosulfonyl)imide |
| LiPAA | Lithiated substituted polyacrylic acid |
| LiPF6 | Lithium hexafluorophosphate |
| LiTCM | Lithium tricyanomethanide |
| LiTFSI | Lithium bis(trifluoromethanesulfonyl)imide |
| LiTOP | Lithium tris(catecholato)phosphates |
| LMBs | Lithium metal batteries |
| LMMP | Lithium 2-methoxyethyl methylphosphonite |
| LMO | Lithium 2-methoxyethan-1-olate |
| LTBP | Lithium tris[1,2-benzenediolato(2)-O,O′]phosphate |
| MA | Methyl acetate |
| MADE | Molecular anchoring diluent electrolyte |
| MAN | Malononitrile |
| MB | Methyl butyrate |
| MEMS | Methoxyethyl methyl sulfone |
| MES | Mesitylene |
| MME | 3-Methoxyperfluoro(2-methylpentane) |
| MO | 3-Methyl-2-oxazolidinone |
| MODOL | 2-Methoxy-1,3-dioxolane |
| MOFs | Metal–organic frameworks |
| MP | Methyl propionate |
| MSM | Methylsulfonylmethane |
| MTS | 1-Methyltrimethylene sulfone |
| NaDEEP | Sodium bis(2-(2-ethoxyethoxy)ethyl)phosphate |
| NMP | N-Methyl-2-pyrrolidone |
| OOE | 1,1,2,2,5,5,6,6-Octafluoro-3-oxahexane |
| P4444(FuA) | Tetra(n-butyl)phosphonium furan-2-carboxylate |
| PAN | Polyacrylonitrile |
| PC | Propylene carbonate |
| PEGDME | Poly(ethylene glycol)dimethyl ether |
| PEO | Polyethylene oxide |
| PFAS | Perfluoroalkyl and polyfluoroalkyl substances |
| PhE | Phenetole |
| PhH | Benzene |
| PhM | Anisole |
| PTCDA | Perylene-3,4,9,10-tetracarboxylic dianhydride |
| PVDF | Poly(vinylidene fluoride) |
| RT | Room temperature |
| S/C | Sulfur/carbon composites |
| SCN | Succinonitrile |
| SEI | Solid electrolyte interphase |
| SIS | Solvent-in-salt |
| SL | Sulfolane |
| SL–EA | Sulfone–ester |
| SPAN | Sulfurized polyacrylonitrile |
| T3GM | Triglyme |
| T4GM | Tetraglyme |
| TBP | Tributyl phosphate |
| TCM | Chloroform |
| TEOP | Tris(2-(2-ethoxyethoxy)ethyl)phosphate |
| TEP | Triethyl phosphate |
| TFP ClO4 | N-Methyl-2,4,6-trifluoropyridinium perchlorate |
| THF | Tetrahydrofuran |
| TMP | Trimethyl phosphate |
| TOL | Toluene |
| TOP | Tris(oxalato)phosphate |
| TPP | Tris(1-propyl)phosphate |
| TriMS | Trimethylene sulfone |
| TTE | 1,1,2,2-Tetrafluoroethyl 2,2,3,3-tetrafluoropropyl ether |
| VC | Vinylene carbonate |
Data availability
Due to the nature of a review article, the primary data supporting this article can be obtained upon contact with the corresponding authors. All the data cited from published papers can be referred to through the given copyright permission and citation.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge the valuable financial support from the National Taiwan University of Science and Technology, Taiwan. Financial support from the National Science and Technology Council of Taiwan (NSTC 113-2639-E-011-001-ASP, 113-2923-E-011-002, 113-2222-E-011-004; 112-2923-E-011-001, 112-2923-E-011-004-MY3; 112-2218-E-011-011), the Ministry of Education of Taiwan (the Sustainable Electrochemical Energy Development Center (SEED Center) from the Featured Areas Research Center Program), as well as the supporting facilities from National Taiwan University of Science and Technology (NTUST), National Center for High-performance Computing (NCHC), and National Synchrotron Radiation Research Centre (NSRRC), are all gratefully acknowledged. V. Boligarla thanks “The Ministry of Education (MOE) Taiwan Scholarship Program.”References
- R. M. Dell and D. A. J. Rand, J. Power Sources, 2001, 100, 2–17 CrossRef CAS
.
-
D. A. Hanaor, Computational Design of Battery Materials, Springer, 2024, pp. 1–12 Search PubMed
.
- J. Liu, J. Xiao, J. Yang, W. Wang, Y. Shao, P. Liu and M. S. Whittingham, Next Energy, 2023, 1, 100015 CrossRef
.
- D. Larcher and J.-M. Tarascon, Nat. Chem., 2015, 7, 19–29 CrossRef CAS PubMed
.
- Z. Song and H. Zhou, Energy Environ. Sci., 2013, 6, 2280–2301 RSC
.
- F. Cheng, J. Liang, Z. Tao and J. Chen, Adv. Mater., 2011, 23, 1695–1715 CrossRef CAS
.
- N. Von Aspern, G. V. Röschenthaler, M. Winter and I. Cekic-Laskovic, Angew. Chem., Int. Ed., 2019, 58, 15978–16000 CrossRef CAS
.
- Y. Wang, Z. Wu, F. M. Azad, Y. Zhu, L. Wang, C. J. Hawker, A. K. Whittaker, M. Forsyth and C. Zhang, Nat. Rev. Mater., 2024, 9, 119–133 CrossRef CAS
.
- K. Xu, S. Zhang, J. L. Allen and T. R. Jow, J. Electrochem. Soc., 2002, 149, A1079 CrossRef CAS
.
- M.-Y. Qi, Y.-S. Xu, S.-J. Guo, S.-D. Zhang, J.-Y. Li, Y.-G. Sun, K.-C. Jiang, A.-M. Cao and L.-J. Wan, Small Sci., 2021, 1, 2100066 CrossRef CAS
.
- Y. Wang, Z. Li, Y. Hou, Z. Hao, Q. Zhang, Y. Ni, Y. Lu, Z. Yan, K. Zhang and Q. Zhao, Chem. Soc. Rev., 2023, 52, 2713–2763 RSC
.
- J. Chen, A. Naveed, Y. Nuli, J. Yang and J. Wang, Energy Storage Mater., 2020, 31, 382–400 CrossRef
.
- H. Wu, Z. Xie, G. Li, L. Zheng, Z. Zhao, J. He, Y. Shen, J. Hu, Z. Peng and G. Zhong, J. Energy Chem., 2024, 93, 193–201 CrossRef CAS
.
- G. J. Chung, J. Han and S.-W. Song, ACS Appl. Mater. Interfaces, 2020, 12, 42868–42879 CrossRef CAS PubMed
.
- Y. Sun, J. Li, S. Xu, H. Zhou and S. Guo, Adv. Mater., 2024, 36, 2311687 CAS
.
- O. Lavi, S. Luski, N. Shpigel, C. Menachem, Z. Pomerantz, Y. Elias and D. Aurbach, ACS Appl. Energy Mater., 2020, 3, 7485–7499 CrossRef CAS
.
- Y. Lin, Z. Yu, W. Yu, S.-L. Liao, E. Zhang, X. Guo, Z. Huang, Y. Chen, J. Qin and Y. Cui, J. Mater. Chem. A, 2024, 12, 2986–2993 CAS
.
- Z. Yu, P. E. Rudnicki, Z. Zhang, Z. Huang, H. Celik, S. T. Oyakhire, Y. Chen, X. Kong, S. C. Kim and X. Xiao, Nat. Energy, 2022, 7, 94–106 CrossRef CAS
.
- T. Stoiber, S. Evans and O. V. Naidenko, Chemosphere, 2020, 260, 127659 CrossRef CAS PubMed
.
-
M. Priyadarshanee, U. Mahto and S. Das, Microbial biodegradation and bioremediation, Elsevier, 2022, pp. 33–53 Search PubMed
.
- Y. Wang, X. Yang, Y. Meng, Z. Wen, R. Han, X. Hu, B. Sun, F. Kang, B. Li and D. Zhou, Chem. Rev., 2024, 124, 3494–3589 CrossRef CAS PubMed
.
- M. Ateia and M. Scheringer, Science, 2024, 385, 256–258 CrossRef CAS
.
- X. Lim, Nature, 2023, 620, 24–27 CrossRef CAS
.
- F. Xiao, B. Deng, D. Dionysiou, T. Karanfil, K. O’Shea, P. Roccaro, Z. J. Xiong and D. Zhao, Nat. Water, 2023, 1, 1004–1015 CrossRef
.
- E. K. Savvidou, A. Rensmo, J. P. Benskin, S. Schellenberger, X. Hu, M. Weil and I. T. Cousins, Environ. Sci. Technol., 2024, 58, 21908–21917 CrossRef CAS
.
- S. L. Ramesh and J. Lopez, ACS Energy Lett., 2025, 10, 1671–1679 CrossRef CAS
.
- X. Ma, Z. Meng, M. V. Bellonia, J. Spangenberger, G. Harper, E. Gratz, E. Olivetti, R. Arsenault and Y. Wang, Nat. Rev. Clean Technol., 2025, 1, 75–94 CrossRef
.
- F. Larsson, P. Andersson, P. Blomqvist and B.-E. Mellander, Sci. Rep., 2017, 7, 10018 CrossRef
.
- Z. B. Muche, Y. Nikodimos, T. M. Tekaligne, S. K. Merso, T. Agnihotri, G. G. Serbessa, S.-H. Wu, W.-N. Su and B. J. Hwang, Chem. Eng. J., 2023, 476, 146400 CrossRef CAS
.
- K. N. Shitaw, C.-J. Huang, S.-C. Yang, Y. Nikodimos, N. T. Temesgen, S. K. Merso, S.-K. Jiang, C.-H. Wang, S.-H. Wu and W.-N. Su, ACS Appl. Energy Mater., 2022, 5, 7770–7783 CrossRef CAS
.
- D. Ouyang, J. Guan, X. Wan, B. Liu, C. Miao and Z. Wang, ACS Appl. Mater. Interfaces, 2024, 16, 42894–44290 CAS
.
- H. Du, Y. Wang, Y. Kang, Y. Zhao, Y. Tian, X. Wang, Y. Tan, Z. Liang, J. Wozny and T. Li, Adv. Mater., 2024, 2401482 CAS
.
- L. Kong, Y. Li and W. Feng, Electrochem. Energy Rev., 2021, 4, 633–679 CAS
.
- F. Zhai, Q. Zhou, Z. Lv, Y. Wang, X. Zhou and G. Cui, EnergyChem, 2022, 4, 100082 CrossRef CAS
.
- E. Jiaqiang, H. Xiao, S. Tian and Y. Huang, Renewable Energy, 2024, 120762 Search PubMed
.
- M. C. Appleberry, J. A. Kowalski, S. A. Africk, J. Mitchell, T. C. Ferree, V. Chang, V. Parekh, Z. Xu, Z. Ye and J. F. Whitacre, J. Power Sources, 2022, 535, 231423 CrossRef CAS
.
- J. N. Lim, G. J. Lim, Y. Cai, R. Chua, Y. Guo, Y. Yan and M. Srinivasan, J. Mater. Chem. A, 2023, 11, 22688–22717 RSC
.
- I. A. Khan, O. I. Gnezdilov, A. Filippov and F. U. Shah, ACS Sustainable Chem. Eng., 2021, 9, 7769–7780 CrossRef CAS
.
- I. A. Khan and F. U. Shah, ACS Sustainable Chem. Eng., 2020, 8, 10212–10221 CrossRef CAS
.
- P. Jaumaux, J. Wu, D. Shanmukaraj, Y. Wang, D. Zhou, B. Sun, F. Kang, B. Li, M. Armand and G. Wang, Adv. Funct. Mater., 2021, 31, 2008644 CrossRef CAS
.
- Y. Zhang, J. Feng, J. Qin, Y. L. Zhong, S. Zhang, H. Wang, J. Bell, Z. Guo and P. Song, Adv. Sci., 2023, 10, 2301056 CrossRef CAS
.
- J. T. Frith, M. J. Lacey and U. Ulissi, Nat. Commun., 2023, 14, 420 CrossRef CAS PubMed
.
- J. Lin, X. Zhang, E. Fan, R. Chen, F. Wu and L. Li, Energy Environ. Sci., 2023, 16, 745–791 RSC
.
- G. Hernández, R. Mogensen, R. Younesi and J. Mindemark, Batteries Supercaps, 2022, 5, e202100373 CrossRef
.
- Z. Jiang, C. Li, T. Yang, Y. Deng, J. Zou, Q. Zhang and Y. Li, ACS Energy Lett., 2024, 9, 1389–1396 CrossRef CAS
.
- Y. Gao, Z. Pan, J. Sun, Z. Liu and J. Wang, Nano-Micro Lett., 2022, 14, 94 CrossRef CAS
.
- P. Greim, A. Solomon and C. Breyer, Nat. Commun., 2020, 11, 4570 CrossRef CAS
.
- G. S. Seck, E. Hache and C. Barnet, Resour. Policy, 2022, 75, 102516 CrossRef
.
- M. Muratori, M. Alexander, D. Arent, M. Bazilian, P. Cazzola, E. M. Dede, J. Farrell, C. Gearhart, D. Greene and A. Jenn, Prog. Energy, 2021, 3, 022002 CrossRef
.
- E. M. Erickson, E. Markevich, G. Salitra, D. Sharon, D. Hirshberg, E. de la Llave, I. Shterenberg, A. Rosenman, A. Frimer and D. Aurbach, J. Electrochem. Soc., 2015, 162, A2424 CAS
.
- B. Markovsky, F. Amalraj, H. E. Gottlieb, Y. Gofer, S. K. Martha and D. Aurbach, J. Electrochem. Soc., 2010, 157, A423 CrossRef CAS
.
- C. Wang, Y. S. Meng and K. Xu, J. Electrochem. Soc., 2019, 166, A5184–A5186 CAS
.
- G. G. Eshetu, G. A. Elia, M. Armand, M. Forsyth, S. Komaba, T. Rojo and S. Passerini, Adv. Energy Mater., 2020, 10, 2000093 CAS
.
- D. Ruan, L. Tan, S. Chen, J. Fan, Q. Nian, L. Chen, Z. Wang and X. Ren, JACS Au, 2023, 3, 953–963 CrossRef CAS
.
- W. Liu, T. Placke and K. Chau, Energy Rep., 2022, 8, 4058–4084 CrossRef
.
- Z. P. Cano, D. Banham, S. Ye, A. Hintennach, J. Lu, M. Fowler and Z. Chen, Nat. Energy, 2018, 3, 279–289 CrossRef
.
- M. Ateia, J. V. Buren, W. Barrett, T. Martin and G. G. Back, ACS Sustainable Chem. Eng., 2023, 11, 7986–7996 CrossRef CAS
.
- G. Hernández, A. J. Naylor, Y.-C. Chien, D. Brandell, J. Mindemark and K. Edström, ACS Sustainable Chem. Eng., 2020, 8, 10041–10052 CrossRef
.
- K. Kim, I. Park, S.-Y. Ha, Y. Kim, M.-H. Woo, M.-H. Jeong, W. C. Shin, M. Ue, S. Y. Hong and N.-S. Choi, Electrochim. Acta, 2017, 225, 358–368 CrossRef CAS
.
- C. Xu, G. Hernández, S. Abbrent, L. Kobera, R. Konefal, J. Brus, K. Edstrom, D. Brandell and J. Mindemark, ACS Appl. Energy Mater., 2019, 2, 4925–4935 CrossRef CAS
.
- N. N. Sinha, J. Burns and J. Dahn, J. Electrochem. Soc., 2013, 160, A709 CrossRef CAS
.
- C. L. Campion, W. Li and B. L. Lucht, J. Electrochem. Soc., 2005, 152, A2327 CrossRef CAS
.
- S. Wilken, M. Treskow, J. Scheers, P. Johansson and P. Jacobsson, RSC Adv., 2013, 3, 16359–16364 RSC
.
- B. T. Hotasi, T. M. Hagos, C. J. Huang, S.-K. Jiang, B. A. Jote, K. N. Shitaw, H. K. Bezabh, C.-H. Wang, W.-N. Su and S.-H. Wu, J. Power Sources, 2022, 548, 232047 CrossRef CAS
.
- C.-J. Huang, B. Thirumalraj, H.-C. Tao, K. N. Shitaw, H. Sutiono, T. T. Hagos, T. T. Beyene, L.-M. Kuo, C.-C. Wang and S.-H. Wu, Nat. Commun., 2021, 12, 1452 CrossRef CAS PubMed
.
- T. M. Hagos, T. T. Hagos, H. K. Bezabh, G. B. Berhe, L. H. Abrha, S.-F. Chiu, C.-J. Huang, W.-N. Su, H. Dai and B. J. Hwang, ACS Appl. Energy Mater., 2020, 3, 10722–10733 CrossRef CAS
.
- T. T. Hagos, B. Thirumalraj, C.-J. Huang, L. H. Abrha, T. M. Hagos, G. B. Berhe, H. K. Bezabh, J. Cherng, S.-F. Chiu and W.-N. Su, ACS Appl. Mater. Interfaces, 2019, 11, 9955–9963 CrossRef CAS PubMed
.
- T. Agnihotri, T.-H. Chu, S.-K. Jiang, S. A. Ahmed, A. Ranjan, E. B. Tamilarasan, S.-C. Yang, T. M. Hagos, Z. B. Muche and J.-C. Jiang, Energy Storage Mater., 2024, 103787 CrossRef
.
- R. Gond, W. van Ekeren, R. Mogensen, A. J. Naylor and R. Younesi, Mater. Horiz., 2021, 8, 2913–2928 RSC
.
- B. Aktekin, R. Younesi, W. Zipprich, C. Tengstedt, D. Brandell and K. Edström, J. Electrochem. Soc., 2017, 164, A942 CrossRef CAS
.
- T. T. Hagos, W.-N. Su, C.-J. Huang, B. Thirumalraj, S.-F. Chiu, L. H. Abrha, T. M. Hagos, H. K. Bezabh, G. B. Berhe and W. A. Tegegne, J. Power Sources, 2020, 461, 228053 CrossRef CAS
.
- R. Zhou, J. Huang, S. Lai, J. Li, F. Wang, Z. Chen, W. Lin, C. Li, J. Wang and J. Zhao, Sustainable Energy Fuels, 2018, 2, 1481–1490 RSC
.
- C. Zhan, T. Wu, J. Lu and K. Amine, Energy Environ. Sci., 2018, 11, 243–257 RSC
.
- Y. Wang, Z. Wu, F. M. Azad, Y. Zhu, L. Wang, C. J. Hawker, A. K. Whittaker, M. Forsyth and C. Zhang, Nat. Rev. Mater., 2024, 9, 119–133 CrossRef CAS
.
- H.-B. Han, S.-S. Zhou, D.-J. Zhang, S.-W. Feng, L.-F. Li, K. Liu, W.-F. Feng, J. Nie, H. Li and X.-J. Huang, J. Power Sources, 2011, 196, 3623–3632 CrossRef CAS
.
- S. Ha, C. Lim and Y.-S. Lee, J. Ind. Eng. Chem., 2022, 111, 1–17 CrossRef CAS
.
- J. Xu, Q. Duan, L. Zhang, Y. Liu, J. Sun and Q. Wang, J. Loss Prev. Process Ind., 2022, 77, 104784 CrossRef CAS
.
- F. Diaz, Y. Wang, R. Weyhe and B. Friedrich, Waste Manage., 2019, 84, 102–111 CrossRef CAS PubMed
.
- Y. Ha, C. Stetson, S. P. Harvey, G. Teeter, B. J. Tremolet de Villers, C.-S. Jiang, M. Schnabel, P. Stradins, A. Burrell and S.-D. Han, ACS Appl. Mater. Interfaces, 2020, 12, 49563–49573 CrossRef CAS PubMed
.
- L. Sheng, D. Zhu, K. Yang, Y. Wu, L. Wang, J. Wang, H. Xu and X. He, Nano Lett., 2023, 24, 533–540 CrossRef PubMed
.
- L. J. Krause, W. Lamanna, J. Summerfield, M. Engle, G. Korba, R. Loch and R. Atanasoski, J. Power Sources, 1997, 68, 320–325 CrossRef CAS
.
- S. Di Muzio, O. Palumbo, S. Brutti and A. Paolone, J. Electrochem. Soc., 2022, 169, 070523 CrossRef
.
- C. M. Costa, J. C. Barbosa, R. Gonçalves, H. Castro, F. Del Campo and S. Lanceros-Méndez, Energy Storage Mater., 2021, 37, 433–465 CrossRef
.
- J. Zhao, X. Feng, M.-K. Tran, M. Fowler, M. Ouyang and A. F. Burke, J. Power Sources, 2024, 598, 234111 CrossRef CAS
.
- X. Tian, Y. Yi, B. Fang, P. Yang, T. Wang, P. Liu, L. Qu, M. Li and S. Zhang, Chem. Mater., 2020, 32, 9821–9848 CrossRef CAS
.
- X. Cui, H. Zhang, S. Li, X. Li and H. Feng, Ionics, 2014, 20, 789–794 CrossRef CAS
.
- B. Zhang, L. Wang, X. Wang, S. Zhou, A. Fu, Y. Yan, Q. Wang, Q. Xie, D. Peng and Y. Qiao, Energy Storage Mater., 2022, 53, 492–504 CrossRef
.
- J. Li, J. Yang, Z. Ji, M. Su, H. Li, Y. Wu, X. Su and Z. Zhang, Adv. Energy Mater., 2023, 13, 2301422 CrossRef CAS
.
- Z. Li, F. Xu, C. Li, P. Wang, W. Yi, S. Wang, L. Yang, C. Yan and S. Li, ACS Appl. Energy Mater., 2021, 4, 1199–1207 CrossRef CAS
.
- Y. Wang, L. Xing, X. Tang, X. Li, W. Li, B. Li, W. Huang, H. Zhou and X. Li, RSC Adv., 2014, 4, 33301–33306 RSC
.
- K. Xu, S. Zhang, T. R. Jow, W. Xu and C. A. Angell, Electrochem. Solid-State Lett., 2001, 5, A26 CrossRef
.
- J. Scheers, P. Johansson and P. Jacobsson, J. Electrochem. Soc., 2008, 155, A628 CrossRef CAS
.
- K. L. Gering, Electrochim. Acta, 2006, 51, 3125–3138 CrossRef CAS
.
- Z. Song, X. Wang, W. Feng, M. Armand, Z. Zhou and H. Zhang, Adv. Mater., 2024, 2310245 CrossRef CAS PubMed
.
- H. Zhang, Z. Zeng, F. Ma, Q. Wu, X. Wang, S. Cheng and J. Xie, Angew. Chem., 2023, 135, e202300771 CrossRef
.
- H. Wan, J. Xu and C. Wang, Nat. Rev. Chem., 2024, 8, 30–44 CrossRef CAS PubMed
.
- X. Zhu, J. Chen, G. Liu, Y. Mo, Y. Xie, K. Zhou, Y. Wang and X. Dong, Angew. Chem., Int. Ed., 2025, 64, e202412859 CrossRef CAS PubMed
.
- W. Xiao, P. Shi, Z. Li, C. Xie, J. Qin, H. Yang, J. Wang, W. Li, J. Zhang and X. Li, J. Energy Chem., 2023, 78, 589–605 CrossRef CAS
.
- Y. Li, X.-W. Zhang, S. A. Khan and P. S. Fedkiw, Electrochem. Solid-State Lett., 2004, 7, A228 CrossRef CAS
.
- Z. Chen, W. Lu, J. Liu and K. Amine, Electrochim. Acta, 2006, 51, 3322–3326 CAS
.
- H. Xiang, P. Shi, P. Bhattacharya, X. Chen, D. Mei, M. E. Bowden, J. Zheng, J.-G. Zhang and W. Xu, J. Power Sources, 2016, 318, 170–177 CrossRef CAS
.
- R. Miao, J. Yang, X. Feng, H. Jia, J. Wang and Y. Nuli, J. Power Sources, 2014, 271, 291–297 CAS
.
- I. Phiri, J. Kim, D.-H. Oh, M. Ravi, H.-S. Bae, J. Hong, S. Kim, Y.-C. Jeong, Y. M. Lee and Y.-G. Lee, ACS Appl. Mater. Interfaces, 2021, 13, 31605–31613 CrossRef CAS PubMed
.
- J. Zheng, M. H. Engelhard, D. Mei, S. Jiao, B. J. Polzin, J.-G. Zhang and W. Xu, Nat. Energy, 2017, 2, 1–8 Search PubMed
.
- X. Li, J. Zheng, M. H. Engelhard, D. Mei, Q. Li, S. Jiao, N. Liu, W. Zhao, J.-G. Zhang and W. Xu, ACS Appl. Mater. Interfaces, 2018, 10, 2469–2479 CAS
.
- W. Li, H. Yao, K. Yan, G. Zheng, Z. Liang, Y.-M. Chiang and Y. Cui, Nat. Commun., 2015, 6, 7436 CAS
.
- S. Xiong, K. Xie, Y. Diao and X. Hong, Electrochim. Acta, 2012, 83, 78–86 CrossRef CAS
.
- X. Zhang and T. M. Devine, J. Electrochem. Soc., 2006, 153, B365 CrossRef CAS
.
- H. Xie, Z. Tang, Z. Li, Y. He, H. Wang and Y. Liu, Electrochem. Solid-State Lett., 2007, 11, C19 CrossRef
.
- W. Lu, Z. Chen, H. Joachin, J. Prakash, J. Liu and K. Amine, J. Power Sources, 2007, 163, 1074–1079 CrossRef CAS
.
- X. Ma, D. Zhang, J. Wen, L. Fan, A. M. Rao and B. Lu, Chem. – Eur. J., 2024, e202400332 CrossRef CAS PubMed
.
- S. Nam, H. Seong, Y. Kim, K. Kim, C. Kim, S. Kwon and S. Park, Chem. Eng. J., 2024, 497, 154790 CrossRef CAS
.
- J. Scheers, D.-H. Lim, J.-K. Kim, E. Paillard, W. A. Henderson, P. Johansson, J.-H. Ahn and P. Jacobsson, J. Power Sources, 2014, 251, 451–458 CrossRef CAS
.
- X. He, B. Yan, X. Zhang, Z. Liu, D. Bresser, J. Wang, R. Wang, X. Cao, Y. Su and H. Jia, Nat. Commun., 2018, 9, 5320 CrossRef CAS PubMed
.
- H. Zhang, X. Judez, A. Santiago, M. Martinez-Ibañez, M. Á. Muñoz-Márquez, J. Carrasco, C. Li, G. G. Eshetu and M. Armand, Adv. Energy Mater., 2019, 9, 1900763 CrossRef
.
- R. Mogensen, S. Colbin, A. S. Menon, E. Björklund and R. Younesi, ACS Appl. Energy Mater., 2020, 3, 4974–4982 CrossRef CAS
.
- R. Mogensen, A. Buckel, S. Colbin and R. Younesi, Chem. Mater., 2021, 33, 1130–1139 CrossRef CAS
.
- K. Kisu, S. Kim, T. Shinohara, K. Zhao, A. Züttel and S.-I. Orimo, Sci. Rep., 2021, 11, 7563 CrossRef CAS PubMed
.
- M. Kar, T. A. Ha, D. Duncan, F. Chen, P. Cherepanov, J. Sun and C. Pozo-Gonzalo, Batteries Supercaps, 2023, 6, e202200412 CrossRef CAS
.
- Q. Li, J. Ruan, S. Weng, X. Zhang, J. Hu, H. Li, D. Sun, X. Wang, F. Fang and F. Wang, Angew. Chem., Int. Ed., 2023, 62, e202310297 CrossRef CAS PubMed
.
- Q. Liu, M. Geng, T. Yu, L. Zhang, C. Wu, J. Liu, S. Zhao, Q. Yang, R. Song and J. Ye, Nano Res., 2023, 16, 8290–8296 CrossRef CAS
.
- F. Gebert, R. Lundström, W. van Ekeren and A. J. Naylor, Batteries Supercaps, 2024, e202400342 CrossRef CAS
.
- F. A. Kreth, L. Köps, C. Leibing, S. Darlami Magar, M. Hermesdorf, K. Schutjajew, C. Neumann, D. Leistenschneider, A. Turchanin and M. Oschatz, Adv. Energy Mater., 2024, 14, 2303909 CrossRef CAS
.
- M. Kar, T. A. Ha, C. Nguyen, D. Duncan, L. A. O’Dell, S. B. Ravindranath, M. Galceran, A. Kumar, M. Amores and F. Chen, ACS Appl. Mater. Interfaces, 2024, 16, 46289–46301 CrossRef CAS PubMed
.
- Y. Xu, A. Filippov, M. R. Shimpi, F. Ullah Shah and P. Johansson, Batteries Supercaps, 2024, e202400672 CrossRef
.
- S. Xu, S. Xu, X. Guo, J. Xiong, Z. Wei, S. Zhu, J. Xu, S. Gong, P. Shi and S. Guo, Adv. Funct. Mater., 2025, 2500335, DOI:10.1002/adfm.202500335
.
- Y. Shuai, Y. Hu, X. Gong, Z. Xu, L. Li, L. Zhang, M. Li, J. Zhou and M. Li, Chem. Eng. J., 2025, 505, 159101 CrossRef CAS
.
- H. Zhao, J. Li, Q. Zhao, X. Huang, S. Jia, J. Ma and Y. Ren, Electrochem. Energy Rev., 2024, 7, 11 CrossRef CAS
.
- A. Buckel, C. A. Hall, L. A. Ma, L. S. Colbin, H. Eriksson, R. Mogensen and R. Younesi, Batteries Supercaps, 2024, 7, e202300533 CrossRef CAS
.
- Q. Wang, M. Zhu, G. Chen, N. Dudko, Y. Li, H. Liu, L. Shi, G. Wu and D. Zhang, Adv. Mater., 2022, 34, 2109658 CrossRef CAS PubMed
.
- A. Ponrouch, C. Frontera, F. Bardé and M. R. Palacín, Nat. Mater., 2016, 15, 169–172 CrossRef CAS PubMed
.
- J. Forero-Saboya, C. Davoisne, R. Dedryvère, I. Yousef, P. Canepa and A. Ponrouch, Energy Environ. Sci., 2020, 13, 3423–3431 RSC
.
- Z. Li, O. Fuhr, M. Fichtner and Z. Zhao-Karger, Energy Environ. Sci., 2019, 12, 3496–3501 RSC
.
- D. Wang, X. Gao, Y. Chen, L. Jin, C. Kuss and P. G. Bruce, Nat. Mater., 2018, 17, 16–20 CrossRef CAS PubMed
.
- F. Yang, X. Feng, Z. Zhuo, L. Vallez, Y.-S. Liu, S. A. McClary, N. T. Hahn, P.-A. Glans, K. R. Zavadil and J. Guo, Arabian J. Sci. Eng., 2023, 48, 7243–7262 CrossRef CAS
.
- T. Jin, Y. Wang, Z. Hui, B. Qie, A. Li, D. Paley, B. Xu, X. Wang, A. Chitu and H. Zhai, ACS Appl. Mater. Interfaces, 2019, 11, 17333–17340 CrossRef CAS PubMed
.
- Y. Yang, X. Wang, J. Zhu, L. Tan, N. Li, Y. Chen, L. Wang, Z. Liu, X. Yao and X. Wang, Angew. Chem., Int. Ed., 2024, e202409193 CAS
.
- A.-M. Li, O. Borodin, T. P. Pollard, W. Zhang, N. Zhang, S. Tan, F. Chen, C. Jayawardana, B. L. Lucht and E. Hu, Nat. Chem., 2024, 16, 922–929 CrossRef CAS PubMed
.
- T. D. Pham, A. B. Faheem, J. Kim, K. Kwak and K.-K. Lee, Electrochim. Acta, 2023, 458, 142496 CrossRef CAS
.
- F. Hai, Y. Yi, Z. Xiao, J. Guo, X. Gao, W. Chen, W. Xue, W. Hua, W. Tang and M. Li, Adv. Energy Mater., 2024, 14, 2304253 CrossRef CAS
.
- H. Zhou, T. Li, W. Liu, Z. Guo, Z. Su, J. Gao, M. Qu and G. Peng, ChemSusChem, 2023, 16, e202300186 CrossRef CAS PubMed
.
- Q.-S. Liu, Y.-Z. Quan, M.-C. Liu, G.-R. Zhu, X.-L. Wang, G. Wu and Y.-Z. Wang, J. Energy Chem., 2023, 83, 239–246 CrossRef CAS
.
- M. Okoshi, Y. Yamada, A. Yamada and H. Nakai, J. Electrochem. Soc., 2013, 160, A2160 CrossRef CAS
.
- M. Okoshi, Y. Yamada, S. Komaba, A. Yamada and H. Nakai, J. Electrochem. Soc., 2016, 164, A54 CrossRef
.
- H. Zhang, O. Arcelus and J. Carrasco, Electrochim. Acta, 2018, 280, 290–299 CrossRef CAS
.
- N. T. Hahn, J. Self, K. S. Han, V. Murugesan, K. T. Mueller, K. A. Persson and K. R. Zavadil, J. Phys. Chem. B, 2021, 125, 3644–3652 CrossRef CAS PubMed
.
- Y.-C. Weng, R. Andersson, M.-T. Lee, J. Mindemark, A. Lindblad, M. Hahlin and G. Hernández, J. Electrochem. Soc., 2024, 171, 060527 CrossRef CAS
.
- Y. Lu, C. Z. Zhao, H. Yuan, X. B. Cheng, J. Q. Huang and Q. Zhang, Adv. Funct. Mater., 2021, 31, 2009925 CrossRef CAS
.
- J. Zhou, J. Chen, J. Yang, Y. Nuli and J. Wang, ACS Appl. Energy Mater., 2021, 4, 11336–11342 CrossRef CAS
.
- M. Valvo, A. Liivat, H. Eriksson, C. W. Tai and K. Edström, ChemSusChem, 2017, 10, 2431–2448 CrossRef CAS PubMed
.
- A. M. Haregewoin, A. S. Wotango and B.-J. Hwang, Energy Environ. Sci., 2016, 9, 1955–1988 RSC
.
- B. Ramasubramanian, J. Ling, R. Jose and S. Ramakrishna, Cell Rep. Phys. Sci., 2024, 102032 CrossRef CAS
.
- P. Jenis, T. Zhang, B. Ramasubramanian, S. Lin, P. R. Rayavarapu, J. Yu and S. Ramakrishna, Circ. Econ., 2024, 3, 100087 Search PubMed
.
- P. Johansson, J. Phys. Chem. A, 2006, 110, 12077–12080 CrossRef CAS PubMed
.
- G. Newman, R. Francis, L. Gaines and B. Rao, J. Electrochem. Soc., 1980, 127, 2025 CrossRef CAS
.
- P. Lanz and P. Novák, J. Electrochem. Soc., 2014, 161, A1555 CrossRef CAS
.
- S. Bhattacharya, A. R. Riahi and A. T. Alpas, Carbon, 2014, 77, 99–112 CAS
.
- M. Matsui, K. Dokko and K. Kanamura, J. Power Sources, 2008, 177, 184–193 CAS
.
- I. Epelboin, M. Froment, M. Garreau, J. Thevenin and D. Warin, J. Electrochem. Soc., 1980, 127, 2100 CAS
.
- R. Marom, O. Haik, D. Aurbach and I. C. Halalay, J. Electrochem. Soc., 2010, 157, A972 CAS
.
- G. M. Hobold, C. Wang, K. Steinberg, Y. Li and B. M. Gallant, Nat. Energy, 2024, 1–12 Search PubMed
.
- M. Handa, M. Suzuki, J. Suzuki, H. Kanematsu and Y. Sasaki, Electrochem. Solid-State Lett., 1998, 2, 60 Search PubMed
.
- U. Wietelmann, W. Bonrath, T. Netscher, H. Nöth, J. C. Panitz and M. Wohlfahrt-Mehrens, Chem. – Eur. J., 2004, 10, 2451–2458 CAS
.
- T. Agnihotri, S. A. Ahmed, E. B. Tamilarasan, R. Hasan, W. N. Su and B. J. Hwang, Adv. Funct. Mater., 2024, 34, 2311215 CrossRef CAS
.
- N. Nanbu, K. Tsuchiya and Y. Sasaki, J. Power Sources, 2005, 142, 333–338 CrossRef CAS
.
- M. Armand, S. Grugeon, S. Laruelle, M. Bukowska, P. Szczecinski, W. Wieczorek, L. Niedzicky, B. Scrosati, S. Panero and P. Realle, US Pat., US8927160B2, 2015.
- M. Armand and P. Johansson, J. Power Sources, 2008, 178, 821–825 CrossRef CAS
.
- Y. Nikodimos, W.-N. Su, K. N. Shitaw, S.-K. Jiang, L. H. Abrha, M. A. Weret, S. K. Merso, T. M. Hagos, C.-J. Huang and K. Lakshmanan, Energy Storage Mater., 2023, 61, 102861 CrossRef
.
- K. Xu, S. S. Zhang, U. Lee, J. L. Allen and T. R. Jow, J. Power Sources, 2005, 146, 79–85 CrossRef CAS
.
- K. Xu, U. Lee, S. Zhang, J. L. Allen and T. R. Jow, Electrochem. Solid-State Lett., 2004, 7, A273 CrossRef CAS
.
- K. Xu, B. Deveney, K. Nechev, Y. Lam and T. R. Jow, J. Electrochem. Soc., 2008, 155, A959 CrossRef CAS
.
- W. Xu and C. Angell, Electrochem. Solid-State Lett., 2001, 4, E1–E4 CrossRef CAS
.
- K. Xu, U. Lee, S. Zhang, M. Wood and T. R. Jow, Electrochem. Solid-State Lett., 2003, 6, A144 CrossRef CAS
.
- K. Xu, S. Zhang and R. Jow, J. Power Sources, 2005, 143, 197–202 CrossRef CAS
.
- K. Xu, S. Zhang and T. R. Jow, Electrochem. Solid-State Lett., 2003, 6, A117 CrossRef CAS
.
- K. Xu, U. Lee, S. S. Zhang and T. R. Jow, J. Electrochem. Soc., 2004, 151, A2106 CrossRef CAS
.
- K. Xu, S. Zhang and T. R. Jow, Electrochem. Solid-State Lett., 2005, 8, A365 CrossRef CAS
.
- T. Melin, R. Lundström and E. J. Berg, J. Phys. Chem. Lett., 2024, 15, 2537–2541 CrossRef CAS PubMed
.
- Z. Jiang, Y. Deng, J. Mo, Q. Zhang, Z. Zeng, Y. Li and J. Xie, Nano Lett., 2023, 23, 8481–8489 CrossRef CAS PubMed
.
- G. Zhuang, K. Xu, T. Jow and P. Ross, Electrochem. Solid-State Lett., 2004, 7, A224 CrossRef CAS
.
- K. Xu, J. Electrochem. Soc., 2008, 155, A733 CrossRef CAS
.
- J. Jiang and J. Dahn, Electrochem. Commun., 2004, 6, 724–728 CrossRef CAS
.
- Y. Gu, S. Fang, L. Yang and S.-I. Hirano, Electrochim. Acta, 2021, 394, 139120 CrossRef CAS
.
- P. Ping, Q. Wang, J. Sun, X. Feng and C. Chen, J. Power Sources, 2011, 196, 776–783 CrossRef CAS
.
- M. L. Lazar and B. L. Lucht, J. Electrochem. Soc., 2015, 162, A928 CrossRef CAS
.
- Y. Subaşı and S. Afyon, ACS Appl. Energy Mater., 2022, 5, 10098–10107 CrossRef
.
- L. Yang, M. Furczon, A. Xiao, B. Lucht, Z. Zhang and D. Abraham, J. Power Sources, 2010, 195, 1698–1705 CrossRef CAS
.
- K. S. Teoh, M. Melchiorre, S. Darlami Magar, M. Hermesdorf, D. Leistenschneider, M. Oschatz, F. Ruffo, J. L. Gómez Urbano and A. Balducci, Adv. Mater., 2024, 36, 2310056 CrossRef CAS PubMed
.
- X. Kou, J. Zhang, C. Li, R. Li, T. Ruan and W. Wang, Sci. China Mater., 2024, 67, 804–815 CrossRef CAS
.
- K. Wang, W. Ni, L. Wang, L. Gan, J. Zhao, Z. Wan, W. Jiang, W. Ahmad, M. Tian and M. Ling, J. Energy Chem., 2023, 77, 581–600 CrossRef CAS
.
- X. Zhang, M. Jia, Q. Zhang, N. Zhang, X. Wu, S. Qi and L. Zhang, Chem. Eng. J., 2022, 448, 137743 CrossRef CAS
.
- H. Yang, X. Chen, N. Yao, N. Piao, Z. Wang, K. He, H.-M. Cheng and F. Li, ACS Energy Lett., 2021, 6, 1413–1421 CrossRef CAS
.
- S. Liu, X. Ji, N. Piao, J. Chen, N. Eidson, J. Xu, P. Wang, L. Chen, J. Zhang and T. Deng, Angew. Chem., Int. Ed., 2021, 60, 3661–3671 CrossRef CAS PubMed
.
- T. D. Pham, A. Bin Faheem, J. Kim, H. M. Oh and K. K. Lee, Small, 2022, 18, 2107492 CrossRef CAS PubMed
.
- X. Q. Zhang, T. Li, B. Q. Li, R. Zhang, P. Shi, C. Yan, J. Q. Huang and Q. Zhang, Angew. Chem., Int. Ed., 2020, 59, 3252–3257 CrossRef CAS PubMed
.
- Z. Guo, X. Song, Q. Zhang, N. Zhan, Z. Hou, Q. Gao, Z. Liu, Z. Shen and Y. Zhao, ACS Energy Lett., 2022, 7, 569–576 CrossRef CAS
.
- G. Huang, Y. Liao, X. Zhao, X. Jin, Z. Zhu, M. Guan and Y. Li, Adv. Funct. Mater., 2023, 33, 2211364 CAS
.
- C. Liao, L. Han, W. Wang, W. Li, X. Mu, Y. Kan, J. Zhu, Z. Gui, X. He and L. Song, Adv. Funct. Mater., 2023, 33, 2212605 CrossRef CAS
.
- W. Wahyudi, V. Ladelta, L. Tsetseris, M. M. Alsabban, X. Guo, E. Yengel, H. Faber, B. Adilbekova, A. Seitkhan and A. H. Emwas, Adv. Funct. Mater., 2021, 31, 2101593 CAS
.
- C. Yan, Y. X. Yao, X. Chen, X. B. Cheng, X. Q. Zhang, J. Q. Huang and Q. Zhang, Angew. Chem., Int. Ed., 2018, 57, 14055–14059 CrossRef CAS PubMed
.
- L. P. Hou, N. Yao, J. Xie, P. Shi, S. Y. Sun, C. B. Jin, C. M. Chen, Q. B. Liu, B. Q. Li and X. Q. Zhang, Angew. Chem., Int. Ed., 2022, 61, e202201406 CrossRef CAS PubMed
.
- Y. Liu, D. Lin, Y. Li, G. Chen, A. Pei, O. Nix, Y. Li and Y. Cui, Nat. Commun., 2018, 9, 3656 CrossRef PubMed
.
- V. Giordani, D. Tozier, J. Uddin, H. Tan, B. M. Gallant, B. D. McCloskey, J. R. Greer, G. V. Chase and D. Addison, Nat. Chem., 2019, 11, 1133–1138 CrossRef CAS PubMed
.
- Q. Liu, Y. Xu, J. Wang, B. Zhao, Z. Li and H. B. Wu, Nano-Micro Lett., 2020, 12, 1–12 Search PubMed
.
- X. Wang, H. Wang, M. Liu and W. Li, Small, 2020, 16, 2000769 CrossRef CAS PubMed
.
- Y. Wen, J. Ding, Y. Yang, X. Lan, J. Liu, R. Hu and M. Zhu, Adv. Funct. Mater., 2022, 32, 2109377 CrossRef CAS
.
- J.-I. Lee, S. Cho, T. T. Vu, S. Kim, S. Ryu, J. Moon and S. Park, Energy Storage Mater., 2021, 38, 509–519 CrossRef
.
- Y. Quan, X. Cui, M. Wang, L. Hu, D. Zhao, N. Zhang, F. Zhang and S. Li, Chem. Eng. J., 2024, 489, 151539 CrossRef CAS
.
- P. Zhou, Y. Xia, Y. Wu, W.-H. Hou, Y. Lu, S. S. Yan, H.-Y. Zhou, W. Zhang and K. Liu, ACS Appl. Mater. Interfaces, 2022, 14, 38921–38930 CrossRef CAS PubMed
.
- J. Wei, Z. Guo, F. Wang, X. Zhao, S. Chen, X. Zhang, X. Wang, Y. Liang and X. Wang, J. Power Sources, 2024, 603, 234452 CrossRef CAS
.
- S. Stuckenberg, M. M. Bela, C. T. Lechtenfeld, M. Mense, V. Küpers, T. T. K. Ingber, M. Winter and M. C. Stan, Small, 2024, 20, 2305203 CrossRef CAS PubMed
.
- C. Wang, Q. Sun, Y. Liu, Y. Zhao, X. Li, X. Lin, M. N. Banis, M. Li, W. Li and K. R. Adair, Nano Energy, 2018, 48, 35–43 CrossRef CAS
.
- N. A. Sahalie, A. A. Assegie, W.-N. Su, Z. T. Wondimkun, B. A. Jote, B. Thirumalraj, C.-J. Huang, Y.-W. Yang and B.-J. Hwang, J. Power Sources, 2019, 437, 226912 CrossRef CAS
.
- J. Chen, Y. Fu and J. Guo, Adv. Mater., 2024, 2401263 CrossRef CAS PubMed
.
- P. Zhou, W. Hou, Y. Xia, Y. Ou, H.-Y. Zhou, W. Zhang, Y. Lu, X. Song, F. Liu and Q. Cao, ACS Nano, 2023, 17, 17169–17179 CrossRef CAS PubMed
.
- Q. Zhao, N. W. Utomo, A. L. Kocen, S. Jin, Y. Deng, V. X. Zhu, S. Moganty, G. W. Coates and L. A. Archer, Angew. Chem., Int. Ed., 2022, 61, e202116214 CrossRef CAS PubMed
.
- M. Baek, H. Shin, K. Char and J. W. Choi, Adv. Mater., 2020, 32, 2005022 CrossRef PubMed
.
- T. Agnihotri, S. A. Ahmed, E. B. Tamilarasan, R. Hasan, B. T. Hotasi, H. K. Bezabh, S. Suwito, Y. Nikodimos, S.-K. Jiang and K. N. Shitaw, Chem. Eng. J., 2024, 484, 149608 CrossRef CAS
.
- B. N. Olana, S. D. Lin and B.-J. Hwang, Electrochim. Acta, 2022, 416, 140266 CrossRef CAS
.
- K. N. Shitaw, S. C. Yang, S. K. Jiang, C. J. Huang, N. A. Sahalie, Y. Nikodimos, H. H. Weldeyohannes, C. H. Wang, S. H. Wu and W. N. Su, Adv. Funct. Mater., 2021, 31, 2006951 CrossRef CAS
.
- B. A. Jote, K. N. Shitaw, M. A. Weret, S.-C. Yang, C.-J. Huang, C.-H. Wang, Y.-T. Weng, S.-H. Wu, W.-N. Su and B. J. Hwang, J. Power Sources, 2022, 532, 231303 CrossRef CAS
.
- X. Zhang, Y. Wang, Z. Ouyang, S. Wang, X. Zhao, Q. Xu, B. Yuan, X. Yue, Z. Liang and S. Geng, Adv. Energy Mater., 2024, 14, 2303048 CrossRef CAS
.
- Y. Jie, X. Liu, Z. Lei, S. Wang, Y. Chen, F. Huang, R. Cao, G. Zhang and S. Jiao, Angew. Chem., 2020, 132, 3533–3538 CrossRef
.
- B.-J. Hwang, T. M. Tekaligne, S.-C. Yang and W.-N. Su, US Pat., US20240243295A1, 2024 Search PubMed.
- R. Xu, X. Q. Zhang, X. B. Cheng, H. J. Peng, C. Z. Zhao, C. Yan and J. Q. Huang, Adv. Funct. Mater., 2018, 28, 1705838 CrossRef
.
- N. W. Li, Y. X. Yin, C. P. Yang and Y. G. Guo, Adv. Mater., 2016, 28, 1853–1858 CrossRef CAS PubMed
.
- H. Yang, C. Guo, A. Naveed, J. Lei, J. Yang, Y. Nuli and J. Wang, Energy Storage Mater., 2018, 14, 199–221 CrossRef
.
- X. Q. Zhang, X. Chen, R. Xu, X. B. Cheng, H. J. Peng, R. Zhang, J. Q. Huang and Q. Zhang, Angew. Chem., 2017, 129, 14395–14399 CrossRef
.
- X. Ren, Y. Zhang, M. H. Engelhard, Q. Li, J.-G. Zhang and W. Xu, ACS Energy Lett., 2017, 3, 14–19 CrossRef
.
- J. Qian, W. Xu, P. Bhattacharya, M. Engelhard, W. A. Henderson, Y. Zhang and J.-G. Zhang, Nano Energy, 2015, 15, 135–144 CrossRef CAS
.
- Y. Zhang, J. Qian, W. Xu, S. M. Russell, X. Chen, E. Nasybulin, P. Bhattacharya, M. H. Engelhard, D. Mei and R. Cao, Nano Lett., 2014, 14, 6889–6896 CrossRef CAS PubMed
.
- H. Yang, A. Naveed, Q. Li, C. Guo, J. Chen, J. Lei, J. Yang, Y. Nuli and J. Wang, Energy Storage Mater., 2018, 15, 299–307 CrossRef
.
- X. B. Cheng, R. Zhang, C. Z. Zhao, F. Wei, J. G. Zhang and Q. Zhang, Adv. Sci., 2016, 3, 1500213 CrossRef PubMed
.
- Y. X. Yao, X. Chen, C. Yan, X. Q. Zhang, W. L. Cai, J. Q. Huang and Q. Zhang, Angew. Chem., Int. Ed., 2021, 60, 4090–4097 CrossRef CAS PubMed
.
- J. Zheng, X. Fan, G. Ji, H. Wang, S. Hou, K. C. DeMella, S. R. Raghavan, J. Wang, K. Xu and C. Wang, Nano Energy, 2018, 50, 431–440 CrossRef CAS
.
- N. Sun, R. Li, Y. Zhao, H. Zhang, J. Chen, J. Xu, Z. Li, X. Fan, X. Yao and Z. Peng, Adv. Energy Mater., 2022, 12, 2200621 CrossRef CAS
.
- Y. Watanabe, S.-i Kinoshita, S. Wada, K. Hoshino, H. Morimoto and S.-i Tobishima, J. Power Sources, 2008, 179, 770–779 CrossRef CAS
.
- K. Xu, Chem. Rev., 2014, 114, 11503–11618 CrossRef CAS PubMed
.
- Z. L. Brown, S. Heiskanen and B. L. Lucht, J. Electrochem. Soc., 2019, 166, A2523–A2527 CrossRef CAS
.
- H. Chen, K. Chen, L. Luo, X. Liu, Z. Wang, A. Zhao, H. Li, X. Ai, Y. Fang and Y. Cao, Angew. Chem., Int. Ed., 2024, 63, e202316966 CrossRef CAS PubMed
.
- K. Xu, Chem. Rev., 2004, 104, 4303–4418 CrossRef CAS PubMed
.
- L. Dong, S. Zhong, B. Yuan, Y. Ji, J. Liu, Y. Liu, C. Yang, J. Han and W. He, Research, 2022, 1–52 Search PubMed
.
- J. Kalhoff, G. G. Eshetu, D. Bresser and S. Passerini, ChemSusChem, 2015, 8, 2154–2175 CrossRef CAS PubMed
.
- Z. Wang, Z. Sun, J. Li, Y. Shi, C. Sun, B. An, H.-M. Cheng and F. Li, Chem. Soc. Rev., 2021, 50, 3178–3210 RSC
.
- W. Lin, M. Zhu, Y. Fan, H. Wang, G. Tao, M. Ding, N. Liu, H. Yang, J. Wu and J. Fang, J. Alloys Compd., 2022, 905, 164163 CrossRef CAS
.
- M. Smart, B. Ratnakumar and S. Surampudi, J. Electrochem. Soc., 2002, 149, A361 CrossRef CAS
.
- S. Chen, K. Wen, J. Fan, Y. Bando and D. Golberg, J. Mater. Chem. A, 2018, 6, 11631–11663 RSC
.
- M. Smart, B. Ratnakumar, K. Chin and L. Whitcanack, J. Electrochem. Soc., 2010, 157, A1361 CrossRef CAS
.
- X. Dong, Y. Lin, P. Li, Y. Ma, J. Huang, D. Bin, Y. Wang, Y. Qi and Y. Xia, Angew. Chem., Int. Ed., 2019, 58, 5623–5627 CrossRef CAS PubMed
.
- B. Flamme, G. R. Garcia, M. Weil, M. Haddad, P. Phansavath, V. Ratovelomanana-Vidal and A. Chagnes, Green Chem., 2017, 19, 1828–1849 RSC
.
- J. Forero-Saboya, E. Marchante, R. Araujo, D. Monti, P. Johansson and A. Ponrouch, J. Phys. Chem. C, 2019, 123, 29524–29532 CrossRef CAS PubMed
.
- Z. Tian, Y. Zou, G. Liu, Y. Wang, J. Yin, J. Ming and H. N. Alshareef, Adv. Sci., 2022, 9, 2201207 CrossRef CAS PubMed
.
- M. Zhang, X. Zhang, Y. Liu, K. Wu, Y. Zhu, H. Lu and B. Liang, Environ. Sci. Pollut. Res., 2021, 28, 35537–35563 CrossRef CAS PubMed
.
- E. Peled, J. Electrochem. Soc., 1979, 126, 2047 CrossRef CAS
.
- E. Peled, D. Golodnitsky and G. Ardel, J. Electrochem. Soc., 1997, 144, L208 CrossRef CAS
.
-
P. B. Balbuena and Y. X. Wang, Lithium-ion batteries: solid-electrolyte interphase, World Scientific, 2004 Search PubMed
.
- M. Winter, Z. Phys. Chem., 2009, 223, 1395–1406 CrossRef CAS
.
- Y. Yamada, K. Usui, C. H. Chiang, K. Kikuchi, K. Furukawa and A. Yamada, ACS Appl. Mater. Interfaces, 2014, 6, 10892–10899 CrossRef CAS PubMed
.
- B.-J. Hwang, F.-M. Wang and C.-H. Lee, US Pat., US20130122379A1, 2013.
- S. Hess, M. Wohlfahrt-Mehrens and M. Wachtler, J. Electrochem. Soc., 2015, 162, A3084 CrossRef CAS
.
- J. Tarascon and D. Guyomard, Solid State Ionics, 1994, 69, 293–305 CrossRef CAS
.
- J. Lee, A.-R. Jeon, H. J. Lee, U. Shin, Y. Yoo, H.-D. Lim, C. Han, H. Lee, Y. J. Kim and J. Baek, Energy Environ. Sci., 2023, 16, 2924–2933 RSC
.
- H. Yoshida, T. Fukunaga, T. Hazama, M. Terasaki, M. Mizutani and M. Yamachi, J. Power Sources, 1997, 68, 311–315 CrossRef CAS
.
- A. Hofmann, F. Müller, S. Schöner and F. Jeschull, Batteries Supercaps, 2023, 6, e202300325 CrossRef CAS
.
- X. Zhang, P. Xu, J. Duan, X. Lin, J. Sun, W. Shi, H. Xu, W. Dou, Q. Zheng and R. Yuan, Nat. Commun., 2024, 15, 536 CrossRef CAS PubMed
.
- J. Chen, D. Zhang, L. Zhu, M. Liu, T. Zheng, J. Xu, J. Li, F. Wang, Y. Wang and X. Dong, Nat. Commun., 2024, 15, 3217 CrossRef CAS PubMed
.
- Z. Li, H. Rao, R. Atwi, B. M. Sivakumar, B. Gwalani, S. Gray, K. S. Han, T. A. Everett, T. A. Ajantiwalay and V. Murugesan, Nat. Commun., 2023, 14, 868 CrossRef CAS PubMed
.
- Z. Cui, Z. Jia, D. Ruan, Q. Nian, J. Fan, S. Chen, Z. He, D. Wang, J. Jiang and J. Ma, Nat. Commun., 2024, 15, 2033 CrossRef CAS PubMed
.
- X. Ma, H. Fu, J. Shen, D. Zhang, J. Zhou, C. Tong, A. M. Rao, J. Zhou, L. Fan and B. Lu, Angew. Chem., Int. Ed., 2023, 62, e202312973 CrossRef CAS PubMed
.
- S.-I. Tobishima, J.-I. Yamaki and T. Okada, Electrochim. Acta, 1984, 29, 1471–1476 CrossRef CAS
.
- S. Tobishima, M. Arakawa, T. Hirai and J. Yamaki, J. Power Sources, 1987, 20, 293–297 CrossRef CAS
.
- Y. Chen, Z. Ma, Y. Wang, P. Kumar, F. Zhao, T. Cai, Z. Cao, L. Cavallo, H. Cheng and Q. Li, Energy Environ. Sci., 2024, 5613–5626 RSC
.
- K. Yoshida, M. Nakamura, Y. Kazue, N. Tachikawa, S. Tsuzuki, S. Seki, K. Dokko and M. Watanabe, J. Am. Chem. Soc., 2011, 133, 13121–13129 CrossRef CAS PubMed
.
- W. A. Henderson, N. R. Brooks, W. W. Brennessel and V. G. Young, Chem. Mater., 2003, 15, 4679–4684 CrossRef CAS
.
- Y. Zhao, T. Zhou, M. Mensi, J. W. Choi and A. Coskun, Nat. Commun., 2023, 14, 299 CrossRef CAS PubMed
.
- J.-L. Liang, S.-Y. Sun, N. Yao, Z. Zheng, Q.-K. Zhang, B.-Q. Li, X.-Q. Zhang and J.-Q. Huang, Sci. China:Chem., 2023, 66, 3620–3627 CrossRef CAS
.
- Y. Chen, Z. Yu, P. Rudnicki, H. Gong, Z. Huang, S. C. Kim, J.-C. Lai, X. Kong, J. Qin and Y. Cui, J. Am. Chem. Soc., 2021, 143, 18703–18713 CrossRef CAS PubMed
.
- J. Li, Y. Hu, H. Xie, J. Peng, L. Fan, J. Zhou and B. Lu, Angew. Chem., Int. Ed., 2022, 61, e202208291 CrossRef CAS PubMed
.
- L. Fan, H. Xie, Y. Hu, Z. Caixiang, A. M. Rao, J. Zhou and B. Lu, Energy Environ. Sci., 2023, 16, 305–315 RSC
.
- X. Ma, R. S. Arumugam, L. Ma, E. Logan, E. Tonita, J. Xia, R. Petibon, S. Kohn and J. Dahn, J. Electrochem. Soc., 2017, 164, A3556 CrossRef CAS
.
- J. Holoubek, M. Yu, S. Yu, M. Li, Z. Wu, D. Xia, P. Bhaladhare, M. S. Gonzalez, T. A. Pascal and P. Liu, ACS Energy Lett., 2020, 5, 1438–1447 CrossRef CAS
.
- D. Xiao, Q. Li, D. Luo, G. Li, H. Liu, L. Shui, S. Gourley, G. Zhou, X. Wang and Z. Chen, Small, 2020, 16, 2004688 CrossRef CAS PubMed
.
- S. Chen, Y. Xiang, G. Zheng, Y. Liao, F. Ren, Y. Zheng, H. He, B. Zheng, X. Liu and N. Xu, ACS Appl. Mater. Interfaces, 2020, 12, 27794–27802 CrossRef CAS PubMed
.
- M. S. Ding and T. R. Jow, J. Electrochem. Soc., 2005, 152, A1199 CrossRef CAS
.
- J.-I. Yamaki, I. Yamazaki, M. Egashira and S. Okada, J. Power Sources, 2001, 102, 288–293 CrossRef CAS
.
- X. Pan, J. Hou, L. Liu, P. Yang, J. Zhang, M. An and N. Li, Ionics, 2017, 23, 3151–3161 CrossRef CAS
.
- J. Lee, N. Quan, J. Hwang, J. Bae, H. Kim, B. Cho, H. Kim and H. Lee, Electrochem. Commun., 2006, 8, 460–464 CrossRef CAS
.
- X. Cao, S. Röser, B. Rezaeirad, X. He, B. Streipert, M. Winter and I. Cekic-Laskovic, Z. Anorg. Allg. Chem., 2015, 641, 2536–2542 CrossRef CAS
.
- T. Nirmale, N. Khupse, R. Gore, J. Ambekar, M. Kulkarni, A. Varma and B. Kale, ChemistrySelect, 2018, 3, 6255–6261 CrossRef CAS
.
- M. T. Garcia, I. Ribosa, L. Perez, A. Manresa and F. Comelles, Langmuir, 2013, 29, 2536–2545 CrossRef CAS PubMed
.
- L. Xia, M. Chen, F. Wang, H. Miao and J. Yuan, J. Power Sources, 2022, 526, 231152 CrossRef CAS
.
- M. Liu, Z. Zeng, C. Gu, F. Ma, Y. Wu, Q. Wu, X. Yang, X. Chen, S. Cheng and J. Xie, ACS Energy Lett., 2023, 9, 136–144 CrossRef
.
- D. Zhang, H. Fu, X. Ma, X. Yu, F. Li, J. Zhou and B. Lu, Angew. Chem., 2024, e202405153 CAS
.
- H. Cheng, Z. Ma, P. Kumar, H. Liang, Z. Cao, H. Xie, L. Cavallo, Q. Li and J. Ming, ACS Energy Lett., 2024, 9, 1604–1616 CrossRef CAS
.
- Z. Ni, C. Wei, Z. Wang, Y. Li, X. Zhang, S. Xiong and J. Feng, Energy Storage Mater., 2024, 71, 103603 CrossRef
.
- J. Zheng, Y. Yang, W. Li, X. Feng, W. Chen and Y. Zhao, J. Mater. Chem. A, 2020, 8, 22962–22968 RSC
.
- P. Yan, Y. Zhu, X. Pan and H. Ji, Int. J. Energy Res., 2021, 45, 2776–2784 CrossRef CAS
.
- N. von Aspern, M. Leissing, C. Wölke, D. Diddens, T. Kobayashi, M. Börner, O. Stubbmann-Kazakova, V. Kozel, G. V. Röschenthaler and J. Smiatek, ChemElectroChem, 2020, 7, 1499–1508 CrossRef CAS
.
- J. Feng, X. Sun, X. Ai, Y. Cao and H. Yang, J. Power Sources, 2008, 184, 570–573 CrossRef CAS
.
- L. Liao, Z. Han, X. Feng, P. Luo, J. Song, Y. Shen, X. Luo, X. Li, X. Wen and B. Yu, J. Energy Chem., 2024, 97, 156–165 CrossRef CAS
.
- Z. Zeng, V. Murugesan, K. S. Han, X. Jiang, Y. Cao, L. Xiao, X. Ai, H. Yang, J.-G. Zhang and M. L. Sushko, Nat. Energy, 2018, 3, 674–681 CrossRef CAS
.
- A. Yang, C. Yang, K. Xie, S. Xin, Z. Xiong, K. Li, Y.-G. Guo and Y. You, ACS Energy Lett., 2023, 8, 836–843 CrossRef CAS
.
- Y. Dong, N. Zhang, C. Li, Y. Zhang, M. Jia, Y. Wang, Y. Zhao, L. Jiao, F. Cheng and J. Xu, ACS Appl. Energy Mater., 2019, 2, 2708–2716 CrossRef CAS
.
- K. Takada, Y. Yamada and A. Yamada, ACS Appl. Mater. Interfaces, 2019, 11, 35770–35776 CrossRef CAS PubMed
.
- A. Pan, Z. Wang, F. Zhang, L. Wang, J. Xu, J. Zheng, J. Hu, C. Zhao and X. Wu, Nano Res., 2023, 16, 8260–8268 CrossRef CAS
.
- K. Xu, M. S. Ding, S. Zhang, J. L. Allen and T. R. Jow, J. Electrochem. Soc., 2002, 149, A622 CrossRef CAS
.
- J. Feng, P. Ma, H. Yang and L. Lu, Electrochim. Acta, 2013, 114, 688–692 CrossRef CAS
.
- S.-J. Tan, Y.-F. Tian, Y. Zhao, X.-X. Feng, J. Zhang, C.-H. Zhang, M. Fan, J.-C. Guo, Y.-X. Yin and F. Wang, J. Am. Chem. Soc., 2022, 144, 18240–18245 CrossRef CAS PubMed
.
- X. Bogle, R. Vazquez, S. Greenbaum, A. V. W. Cresce and K. Xu, J. Phys. Chem. Lett., 2013, 4, 1664–1668 CrossRef CAS PubMed
.
- M. Liu, F. Ma, Z. Ge, Z. Zeng, Q. Wu, H. Yan, Y. Wu, S. Lei, Y. Zhu and S. Cheng, Sci. China:Chem., 2024, 67, 724–731 CrossRef CAS
.
- N. Gogoi, E. Bowall, R. Lundstrom, N. Mozhzhukhina, G. Hernández, P. Broqvist and E. J. Berg, Chem. Mater., 2022, 34, 3831–3838 CrossRef CAS
.
- C. Peebles, R. Sahore, J. A. Gilbert, J. C. Garcia, A. Tornheim, J. Bareño, H. Iddir, C. Liao and D. P. Abraham, J. Electrochem. Soc., 2017, 164, A1579 CrossRef CAS
.
- Q. Zheng, Y. Yamada, R. Shang, S. Ko, Y.-Y. Lee, K. Kim, E. Nakamura and A. Yamada, Nat. Energy, 2020, 5, 291–298 CrossRef CAS
.
- Y. Geng, H. Fu, Y. Hu, A. M. Rao, L. Fan, J. Zhou and B. Lu, Appl. Phys. Lett., 2024, 124, 063901 CrossRef CAS
.
- C. Krause, P. Röring, S. Röser, D. Diddens, J. Thienenkamp, I. Cekic-Laskovic, G. Brunklaus and M. Winter, J. Chem. Phys., 2020, 152, 174701 CrossRef CAS PubMed
.
- T. Morin Caamano, M. S. Houache, M. G. Sandoval, M. Couillard, A. Weck, E. A. Baranova and Y. Abu-Lebdeh, J. Phys. Chem. C, 2023, 127, 7230–7238 CrossRef CAS
.
- M. Kerner, D.-H. Lim, S. Jeschke, T. Rydholm, J.-H. Ahn and J. Scheers, J. Power Sources, 2016, 332, 204–212 CrossRef CAS
.
- H. Zhi, L. Xing, X. Zheng, K. Xu and W. Li, J. Phys. Chem. Lett., 2017, 8, 6048–6052 CrossRef CAS PubMed
.
- A. S. Kumar, M. Pupo, K. V. Petrov, M. Ramdin, J. R. van Ommen, W. de Jong and R. Kortlever, J. Phys. Chem. C, 2023, 127, 12857–12866 CrossRef CAS PubMed
.
- K. Xu and C. A. Angell, J. Electrochem. Soc., 2002, 149, A920 CrossRef CAS
.
- S. Tan, Y. J. Ji, Z. R. Zhang and Y. Yang, ChemPhysChem, 2014, 15, 1956–1969 CrossRef CAS PubMed
.
- P. Jiang, L. Chen, H. Shao, S. Huang, Q. Wang, Y. Su, X. Yan, X. Liang, J. Zhang and J. Feng, ACS Energy Lett., 2019, 4, 1419–1426 CrossRef CAS
.
- P. Jaumaux, X. Yang, B. Zhang, J. Safaei, X. Tang, D. Zhou, C. Wang and G. Wang, Angew. Chem., Int. Ed., 2021, 60, 19965–19973 CrossRef CAS PubMed
.
- B. Flamme, M. Haddad, P. Phansavath, V. Ratovelomanana-Vidal and A. Chagnes, ChemElectroChem, 2018, 5, 2279–2287 CrossRef CAS
.
- M. A. Navarra, MRS Bull., 2013, 38, 548–553 CrossRef CAS
.
- D. R. MacFarlane, M. Forsyth, E. I. Izgorodina, A. P. Abbott, G. Annat and K. Fraser, Phys. Chem. Chem. Phys., 2009, 11, 4962–4967 RSC
.
- D. R. MacFarlane, N. Tachikawa, M. Forsyth, J. M. Pringle, P. C. Howlett, G. D. Elliott, J. H. Davis, M. Watanabe, P. Simon and C. A. Angell, Energy Environ. Sci., 2014, 7, 232–250 RSC
.
- X. Wang, M. Salari, D.-E. Jiang, J. Chapman Varela, B. Anasori, D. J. Wesolowski, S. Dai, M. W. Grinstaff and Y. Gogotsi, Nat. Rev. Mater., 2020, 5, 787–808 CrossRef CAS
.
- M. Armand, P. Axmann, D. Bresser, M. Copley, K. Edström, C. Ekberg, D. Guyomard, B. Lestriez, P. Novák and M. Petranikova, J. Power Sources, 2020, 479, 228708 CrossRef CAS
.
- N. Karimi, M. Zarrabeitia, A. Mariani, D. Gatti, A. Varzi and S. Passerini, Adv. Energy Mater., 2021, 11, 2003521 CrossRef CAS
.
- G. M. Girard, M. Hilder, H. Zhu, D. Nucciarone, K. Whitbread, S. Zavorine, M. Moser, M. Forsyth, D. R. Macfarlane and P. C. Howlett, Phys. Chem. Chem. Phys., 2015, 17, 8706–8713 RSC
.
- G. M. Girard, M. Hilder, D. Nucciarone, K. Whitbread, S. Zavorine, M. Moser, M. Forsyth, D. R. MacFarlane and P. C. Howlett, J. Phys. Chem. C, 2017, 121, 21087–21095 CrossRef CAS
.
- M. Hilder, G. Girard, K. Whitbread, S. Zavorine, M. Moser, D. Nucciarone, M. Forsyth, D. MacFarlane and P. Howlett, Electrochim. Acta, 2016, 202, 100–109 CrossRef CAS
.
- N. Karimi, M. Zarrabeitia, M. Hosseini, T. Ates, B. Iliev, T. J. Schubert, A. Varzi and S. Passerini, ACS Appl. Mater. Interfaces, 2022, 14, 20888–20895 CrossRef CAS PubMed
.
- H. Yoon, G. H. Lane, Y. Shekibi, P. Howlett, M. Forsyth, A. S. Best and D. R. Macfarlane, Energy Environ. Sci., 2013, 6, 979–986 RSC
.
- V. V. Chaban, Phys. Chem. Chem. Phys., 2015, 17, 31839–31849 RSC
.
- L. Zhao, Y. S. Hu, H. Li, Z. Wang and L. Chen, Adv. Mater., 2011, 23, 1385–1388 CrossRef CAS PubMed
.
- D. R. Macfarlane, S. A. Forsyth, J. Golding and G. Deacon, Green Chem., 2002, 4, 444–448 RSC
.
- S. Shen, S. Fang, L. Qu, D. Luo, L. Yang and S.-I. Hirano, RSC Adv., 2015, 5, 93888–93899 RSC
.
- X. Liu, A. Mariani, T. Diemant, M. E. Di Pietro, X. Dong, P.-H. Su, A. Mele and S. Passerini, ACS Energy Lett., 2024, 9, 3049–3057 CrossRef CAS
.
- F. U. Shah, O. I. Gnezdilov, I. A. Khan, A. Filippov, N. A. Slad and P. Johansson, J. Phys. Chem. B, 2020, 124, 9690–9700 CrossRef CAS PubMed
.
- I. A. Khan, O. I. Gnezdilov, Y.-L. Wang, A. Filippov and F. U. Shah, J. Phys. Chem. B, 2020, 124, 11962–11973 CrossRef CAS PubMed
.
- O. I. Gnezdilov, A. Filippov, I. A. Khan and F. U. Shah, J. Mol. Liq., 2022, 345, 117001 CrossRef CAS
.
- X. Liu, C. Xu, H. Adenusi, Y. Wu and S. Passerini, Acc. Chem. Res., 2025, 3049–3057 Search PubMed
.
- X. Liu, A. Mariani, T. Diemant, X. Dong, P. H. Su and S. Passerini, Angew. Chem., 2023, 135, e202305840 CrossRef
.
- X. Liu, A. Mariani, T. Diemant, M. E. Di Pietro, X. Dong, P.-H. Su, A. Mele and S. Passerini, ACS Energy Lett., 2024, 9, 3049–3057 CrossRef CAS
.
- X. Liu, T. Diemant, A. Mariani, X. Dong, M. E. Di Pietro, A. Mele and S. Passerini, Adv. Mater., 2022, 34, 2207155 CrossRef CAS PubMed
.
- C. Xu, T. Diemant, A. Mariani, M. E. Di Pietro, A. Mele, X. Liu and S. Passerini, Angew. Chem., 2024, 136, e202318204 CrossRef
.
- D. W. Kang, J. Moon, H.-Y. Choi, H.-C. Shin and B. G. Kim, J. Power Sources, 2021, 490, 229504 CrossRef CAS
.
- S. Gu, S. W. Zhang, J. Han, Y. Deng, C. Luo, G. Zhou, Y. He, G. Wei, F. Kang and W. Lv, Adv. Funct. Mater., 2021, 31, 2102128 CrossRef CAS
.
- S. Yu, Z. Wu, J. Holoubek, H. Liu, E. Hopkins, Y. Xiao, X. Xing, M. H. Lee and P. Liu, Adv. Sci., 2022, 9, 2104829 CrossRef CAS PubMed
.
- Y. Abu-Lebdeh and I. Davidson, J. Electrochem. Soc., 2008, 156, A60 CrossRef
.
- C. Feng and T. Kyu, Electrochim. Acta, 2020, 330, 135320 CrossRef CAS
.
- G.-Y. Kim and J. Dahn, J. Electrochem. Soc., 2015, 162, A437 CrossRef CAS
.
- H. Duncan, N. Salem and Y. Abu-Lebdeh, J. Electrochem. Soc., 2013, 160, A838 CAS
.
- X. Chang, Z. Yang, Y. Liu, J. Chen, M. Wu, L. Li, S. Chou and Y. Qiao, Energy Storage Mater., 2024, 103407 Search PubMed
.
- Z. Q. Zeng, X. P. Ai, H. X. Yang and Y. L. Cao, J. Electrochem., 2020, 26, 9 Search PubMed
.
- B. Wu, W. A. Martin and R. Feng, Safe Electrolytes for Batteries, Pacific Northwest National Laboratory (PNNL), Richland, WA (United States), 2023.
- J. Xie and Y. C. Lu, Adv. Mater., 2024, 2312451 Search PubMed
.
- Z. Gao, S. Rao, T. Zhang, W. Li, X. Yang, Y. Chen, Y. Zheng, Y. Ding, T. Dong and S. Li, J. Electrochem. Energy Convers. Storage, 2022, 19, 030910 CrossRef CAS
.
- S. Liang, K. Hofman, M. Friedrich, J. Keller and G. Manolikakes, ChemSusChem, 2021, 14, 4878–4902 CrossRef CAS PubMed
.
- X. Ou, D. Gong, C. Han, Z. Liu and Y. Tang, Adv. Energy Mater., 2021, 11, 2102498 CrossRef CAS
.
- H. Wang, J. Liu, G. Jiang, J. Huang, D. Wu, G. Yang and J. Ma, Adv. Energy Mater., 2024, 2400067 CrossRef CAS
.
- C.-C. Chang, S.-H. Hsu, Y.-F. Jung and C.-H. Yang, J. Power Sources, 2011, 196, 9605–9611 CrossRef CAS
.
- D. Aurbach, K. Gamolsky, B. Markovsky, Y. Gofer, M. Schmidt and U. Heider, Electrochim. Acta, 2002, 47, 1423–1439 CrossRef CAS
.
- J.-Y. Eom, I.-H. Jung and J.-H. Lee, J. Power Sources, 2011, 196, 9810–9814 CrossRef CAS
.
- N. Karimi, M. Zarrabeitia, H. Geaney, K. M. Ryan, B. Iliev, T. J. Schubert, A. Varzi and S. Passerini, J. Power Sources, 2023, 558, 232621 CrossRef CAS
.
- W. Jia, C. Fan, L. Wang, Q. Wang, M. Zhao, A. Zhou and J. Li, ACS Appl. Mater. Interfaces, 2016, 8, 15399–15405 CrossRef CAS PubMed
.
- J. Park, H. Kang, M. Agostini, S. Xiong, S. Kansara, X. Xu, Y. Liu and J. Y. Hwang, Energy Storage Mater., 2024, 69, 103443 CrossRef
.
- C. Liao, L. Han, X. Mu, Y. Zhu, N. Wu, J. Lu, Y. Zhao, X. Li, Y. Hu and Y. Kan, ACS Appl. Mater. Interfaces, 2021, 13, 46783–46793 CrossRef CAS PubMed
.
- T. Lai, A. Bhargav and A. Manthiram, Adv. Funct. Mater., 2023, 33, 2304568 CrossRef CAS
.
- Y. Lin, J. Chen, H. Zhang and J. Wang, J. Energy Chem., 2023, 80, 207–214 CrossRef CAS
.
- F. Ming, Y. Zhu, G. Huang, A.-H. Emwas, H. Liang, Y. Cui and H. N. Alshareef, J. Am. Chem. Soc., 2022, 144, 7160–7170 CrossRef CAS PubMed
.
- M. M. Rahman, S. Tan, Y. Yang, H. Zhong, S. Ghose, I. Waluyo, A. Hunt, L. Ma, X.-Q. Yang and E. Hu, Nat. Commun., 2023, 14, 8414 CrossRef CAS PubMed
.
- J. Kim, D. G. Lee, J. H. Lee, S. Kim, C.-Y. Park, J. Lee, H. Kwon, H. Cho, J. Lee and D. Son, Energy Environ. Sci., 2025, 18, 3148–3159 RSC
.
- J. G. Han, K. Kim, Y. Lee and N. S. Choi, Adv. Mater., 2019, 31, 1804822 CrossRef PubMed
.
- X. Qi, L. Tao, H. Hahn, C. Schultz, D. R. Gallus, X. Cao, S. Nowak, S. Röser, J. Li and I. Cekic-Laskovic, RSC Adv., 2016, 6, 38342–38349 RSC
.
- T. T. Beyene, H. K. Bezabh, M. A. Weret, T. M. Hagos, C.-J. Huang, C.-H. Wang, W.-N. Su, H. Dai and B.-J. Hwang, J. Electrochem. Soc., 2019, 166, A1501–A1509 CrossRef CAS
.
- H. K. Bezabh, M.-C. Tsai, T. T. Hagos, T. T. Beyene, G. B. Berhe, T. M. Hagos, L. H. Abrha, S.-F. Chiu, W.-N. Su and B. J. Hwang, Electrochem. Commun., 2020, 113, 106685 CrossRef CAS
.
- A. Clarisza, H. K. Bezabh, S.-K. Jiang, C.-J. Huang, B. W. Olbasa, S.-H. Wu, W.-N. Su and B. J. Hwang, ACS Appl. Mater. Interfaces, 2022, 14, 36644–36655 CrossRef CAS PubMed
.
- P. J. Bugryniec, E. G. Resendiz, S. M. Nwophoke, S. Khanna, C. James and S. F. Brown, J. Energy Storage, 2024, 87, 111288 CrossRef
.
- Y. Li, M. Sun, Y. Cao, K. Yu, Z. Fan and Y. Cao, ChemSusChem, 2024, e202301953 CrossRef CAS PubMed
.
- L. Haozheng, Y. Shanfa, Z. Hongna and Z. Yuxin, J. Environ. Occup. Med., 2024, 41, 579–585 Search PubMed
.
- M. Wu, S. Han, S. Liu, J. Zhao and W. Xie, Energy Storage Mater., 2024, 103174 CrossRef
.
- J. Moon, D. O. Kim, L. Bekaert, M. Song, J. Chung, D. Lee, A. Hubin and J. Lim, Nat. Commun., 2022, 13, 4538 CrossRef CAS PubMed
.
- M. H. Pai, T. Lai and A. Manthiram, Adv. Funct. Mater., 2024, 34, 2407450 CrossRef CAS
.
- M. H. Pai and A. Manthiram, Adv. Energy Mater., 2025, 2500026, DOI:10.1002/aenm.202500026
.
- D. J. Yoo, S. Yang, K. J. Kim and J. W. Choi, Angew. Chem., Int. Ed., 2020, 59, 14869–14876 CrossRef CAS PubMed
.
- B. Zhuang, G. Ramanauskaite, Z. Y. Koa and Z.-G. Wang, Sci. Adv., 2021, 7, eabe7275 CrossRef CAS PubMed
.
- S. Wang, W. Qiu, T. Li, B. Yu and H. Zhao, Int. J. Electrochem. Sci., 2006, 1, 250–257 CrossRef CAS
.
- S. Zhang, K. Xu and T. Jow, J. Power Sources, 2006, 154, 276–280 CrossRef CAS
.
- L. Mao, B. Li, X. Cui, Y. Zhao, X. Xu, X. Shi, S. Li and F. Li, Electrochim. Acta, 2012, 79, 197–201 CrossRef CAS
.
- S. Li, Y. Zhao, X. Shi, B. Li, X. Xu, W. Zhao and X. Cui, Electrochim. Acta, 2012, 65, 221–227 CrossRef CAS
.
- R. Lin, J. Chen, C. Ke, S. Liu and J. Wang, J. Energy Chem., 2023, 77, 180–190 CrossRef CAS
.
- M. Agostini, D. H. Lim, M. Sadd, J. Y. Hwang, S. Brutti, J. Heo, J. H. Ahn, Y. K. Sun and A. Matic, ChemSusChem, 2018, 11, 2981–2986 CrossRef CAS PubMed
.
- J.-Y. Luo, W.-J. Cui, P. He and Y.-Y. Xia, Nat. Chem., 2010, 2, 760–765 CrossRef CAS PubMed
.
- B. D. Adams, J. Zheng, X. Ren, W. Xu and J. G. Zhang, Adv. Energy Mater., 2018, 8, 1702097 CrossRef
.
- G. M. Hobold, C. Wang, K. Steinberg, Y. Li and B. M. Gallant, Nat. Energy, 2024, 9, 580–591 CrossRef CAS
.
- C. N. Hong, M. Yan, O. Borodin, T. P. Pollard, L. Wu, M. Reiter, D. G. Vazquez, K. Trapp, J. M. Yoo and N. Shpigel, Energy Environ. Sci., 2024, 17, 4137–4146 RSC
.
- Z. Wang, X. Che, D. Wang, Y. Wang, X. He, Y. Zhu and B. Zhang, Angew. Chem., 2024, 136, e202404109 CrossRef
.
- H. S. Hirsh, B. Sayahpour, A. Shen, W. Li, B. Lu, E. Zhao, M. Zhang and Y. S. Meng, Energy Storage Mater., 2021, 42, 78–87 CrossRef
.
- J. F. Peters, M. Baumann, J. R. Binder and M. Weil, Sustainable Energy Fuels, 2021, 5, 6414–6429 RSC
.
- S. S. Sharma and A. Manthiram, Energy Environ. Sci., 2020, 13, 4087–4097 RSC
.
- S. Duehnen, J. Betz, M. Kolek, R. Schmuch, M. Winter and T. Placke, Small Methods, 2020, 4, 2000039 CrossRef CAS
.
- X. Zhang, L. Li, E. Fan, Q. Xue, Y. Bian, F. Wu and R. Chen, Chem. Soc. Rev., 2018, 47, 7239–7302 RSC
.
- L. Cui, S. Zhang, J. Ju, T. Liu, Y. Zheng, J. Xu, Y. Wang, J. Li, J. Zhao and J. Ma, Nat. Energy, 2024, 1–11 Search PubMed
.
- Y. Xu, A. Filippov, S. Bhowmick, P. Johansson and F. U. Shah, Energy Adv., 2024, 3, 564–573 RSC
.
- J. Kim, J. Kim, J. Jeong, J. Park, C.-Y. Park, S. Park, S. G. Lim, K. T. Lee, N.-S. Choi and H. R. Byon, Energy Environ. Sci., 2022, 15, 4109–4118 RSC
.
- E. C. Barbosa, J. Du, A. S. Pal, A. Kondrakov and T. Brezesinski, Adv. Sustainable Syst., 2025, 2400931 CrossRef CAS
.
- Z. Lu, T. Patranika, A. J. Naylor, J. Mindemark, S. Tardif, G. Hernández and S. Lyonnard, Small, 2025, 2410654 CrossRef CAS PubMed
.
- Z. Song, X. Wang, W. Feng, M. Armand, Z. Zhou and H. Zhang, Adv. Mater., 2024, 36, 2310245 CrossRef CAS PubMed
.
- S. Zhang, X. Zhuang, X. Du, X. Zhang, J. Li, G. Xu, Z. Ren, Z. Cui, L. Huang and S. Wang, Adv. Mater., 2023, 35, 2301312 CrossRef CAS PubMed
.
- F. Leibetseder, J. Xie, E. Leeb, G. Hesser, K. H. Pettinger and K. Bretterbauer, Adv. Energy Mater., 2024, 14, 2401074 CrossRef CAS
.
- J. Jang, J. Ahn, J. Ahn, U. Jeong, J. Yoon, J. K. Park, W. Shin, M. J. Kang, M. K. Cho and D. J. Kang, Adv. Funct. Mater., 2024, 34, 2410866 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2025 |