DOI:
10.1039/D4RA03507K
(Review Article)
RSC Adv., 2024,
14, 30411-30439
Insights into micro-and nano-zero valent iron materials: synthesis methods and multifaceted applications
Received
16th May 2024
, Accepted 28th August 2024
First published on 24th September 2024
Abstract
The growing threat of environmental pollution to global environmental health necessitates a focus on the search for sustainable wastewater remediation materials coupled with innovative remediation strategies. Nano and micro zero-valent iron materials have attracted substantial researchers' attention due to their distinct physiochemical properties. This review article delves into novel micro- and nano-zero valent iron (ZVI) materials, analysing their synthesis methods, and exploring their multifaceted potential as a powerful tool for environmental remediation. This analysis contributes to the ongoing search of effective solutions for environmental remediation. Synthesis techniques are analysed based on their efficacy, scalability, and environmental impact, providing insights into existing methodologies, current challenges, and future directions for optimisation. Factors influencing ZVI materials' physicochemical properties and multifunctional engineering applications, including their role in wastewater and soil remediation, are highlighted. Environmental concerns, pros and cons, and the potential industrial applications of these materials are also discussed, accenting the importance of understanding the synthesis methods, materials' applications and their impacts on humans and the environment. The review is designed to provide insights into nano-and micro-ZVI materials, and their potential engineering applications, as well as guide researchers in the choice of ZVI materials' synthesis methods from a variety of nanoparticle synthesis strategies fostering nexus between these methods and industrial applications.
1 Introduction
In the past two decades, significant progress has been made in developing efficient materials and synthesis strategies for environmental remediations to address soil and wastewater pollution.1–3 Nano and micro zero-valent iron (ZVI) materials have emerged as effective materials due to their exceptional capacity to degrade a wide range of environmental contaminants.4–8 These novel materials are derived from their precursor iron materials through a plethora of synthesis strategies.9–12 Iron is a naturally occurring material that ranks as the fourth most abundant element after oxygen, silicon, aluminium, and constitutes about 5% of Earth's crust by mass.13–15 Iron is naturally found in the form of ore, a naturally occurring mineral aggregate combined with gangues. Iron ores enveloped vital minerals, such as magnetite Fe3O4.,13,16–20 Haematite Fe2O4,21–23 Goethite Fe2O3·H2O,19,24 Pyrrhotite Fe(1−x)S17,19,25 Limonite 2Fe2O3·3H2O,26,27 Siderite FeCO3,28,29 Pyrite FeS2,30,31 and Ilmenite FeTiO3 (ref. 32 and 33) with their iron contents decreasing order from magnetite to Ilmenite.34 Iron minerals have a wide range of engineering applications and utilized in several industries.35,36 Iron occurs in either 0, +2, or +3 oxidation states which are more occurrent than +4, +5 and +6 oxidations. However, significant tendencies of reduced oxidation (see eqn (1)) exist even at standard conditions which is most common in the case of aqueous Fe3+ to Fe2+.
Iron oxides particularly magnetite and haematites usually release metallic irons when heated in the presence of a reducing agent.13,18,37 However, the reconditeness of this approach lies on the purity of the metal compounds produced as well as their physical and chemical properties since various applications require distinct chemical and morphological characteristics of iron compounds. In addition to chemical reaction, magnetism significantly influences the role of iron compounds in many engineering applications like catalysis, biomedicine, and magnetic fluids. As a result, several synthesis strategies of producing iron materials are explored. Apart from conventional utilization of iron materials in agriculture, metal, mechanic, and steel industries, recent technological advancement has seen the use of iron precursors in the extraction of crucial metal compounds which are used as catalysts for environmental remediation14,15,38 among a plethora of other applications.23 These advancements include the fabrication of micro and nano zerovalent iron materials (nano and micro-ZVI).39 Micro-ZVI (micro-zero valent iron) materials were discovered to be significantly effective in groundwater remediations since 1997.40 And have ever since attracted researchers' attention owing to their cost effectiveness, non-toxicity,41,42 and potentials for industrial effluents and wastewater remediation.43,44 Fig. 1 provides research advances on nano and micro-ZVI and their composites.
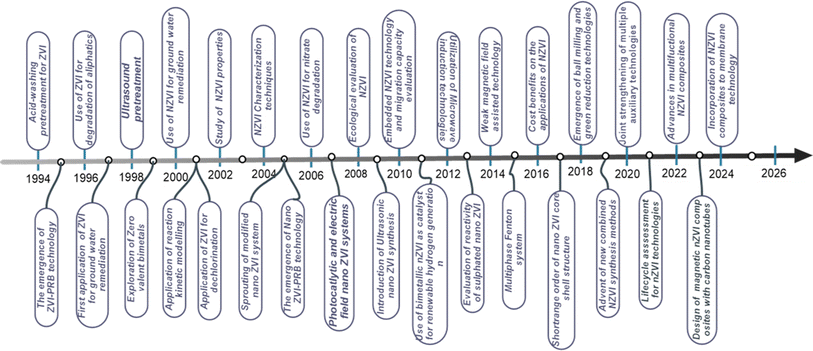 |
| | Fig. 1 History and research advances on nano and micro ZVI and their composites. | |
Despite the potentials of micro-ZVI materials, rapid passivation and demand for special material properties necessitated their encapsulation with supportive materials,45–48 forming stabilized nanocomposites with enhanced reactivity and reduced passivation.49–53 However, chemical reactivity and photocatalytic activity were yet hindered by limited active sites in addition to particle agglomeration.54,55 Unlocking the full potential of these materials, producing nanoparticles with multiple active sites coupled with enhanced physicochemical properties viz. strong reducing power, and diverse functionalities to suite demanding engineering applications, hinges on a comprehensive understanding of their physicochemical properties, evolving synthesis strategies, and application fields. Although previous reviews have addressed individual aspects,56,57 a critical and consolidated analysis of both synthesis strategies and application landscapes remains elusive. This review bridges this gap in the literature by providing a concise insight into nano and micro-ZVI materials, synthesis methods, and their extensive and developing range of applications, including their functions in groundwater, industrial wastewater, soil, sludge, and waste treatment. The review explores the basic concepts of these synthesis methods, their environmental impacts, influence synthesis methods on properties and surface characteristics of the synthesized materials, and the existing and prospective engineering applications of nano and micro-ZVI materials. The article is organised into four sections. The first section provides basic background on ZVI materials and their iron precursors, followed by brief overview of nano and micro-ZVI materials, factors influencing nano and micro-ZVI materials properties and performance, a general overview of nanoparticle synthesis methods. Section two provides comprehensive overview of nano and micro-ZVI materials synthesis strategies and comparison of physico-chemical properties and performance of materials synthesized. Section three discusses existing and prospectives applications with environmental considerations. Section four finalized with conclusions and future perspectives.
2 Nano and micro zerovalent irons (nano and micro-ZVI)
2.1 Micro zero valent iron (micro-ZVI)
Micro zero-valent iron (mZVI) is a fine, black powder derived from iron that exhibits a zero oxidation state, it is characterized by high surface area,58 chemical reactivity,59,60 and employed in various environmental and engineering applications, particularly in the field of contaminant remediation.61 ZVI materials are favourable option for environmental remediation owing to their unique combination of desirable properties, viz. They are considerably non-toxic, readily available, inexpensive, and easily produced while requiring minimal maintenance for their chemical reductive processes.62 Their reactivity stem from their standard redox potential (E0 = −0.44 V), rendering them effective reductants for oxidizing contaminants like Cr(VI).63,64 The primary reaction mechanism of mZVI materials involve direct electron transfer from mZVI to the contaminant.65 Extensive researches established the efficacy of advanced oxidation processes (AOPs) of ZVI in wastewater treatment.66 In these processes, ZVI materials degrade organic contaminants into smaller, less harmful molecules.43 Additionally, zero valent iron Fe0-based Fenton-like reactions generate reactive oxygen species (ROSs) capable of decomposing organic pollutants present in wastewater.67 Moreso, under acidic conditions, in the presence of dissolved oxygen (O2), zero-valent iron (Fe0) produces hydrogen peroxide (H2O2), a key precursor for ROS generation in Fenton-like systems.68 These reaction mechanisms, from eqn (1), are outlined below.| | |
Fe0 + O2 + 2H+ → Fe2+ + H2O2
| (2) |
| | |
Fe2+ + H2O2 → Fe3+ + ˙OH + OH−
| (3) |
As shown in eqn (1) to (3), the hydrogen peroxide (H2O2) which subsequently reacted with iron(II), was formed through electron transfer from ZVI in the presence of oxygen. ZVI exhibits the ability to degrade and oxidize various organic compounds in the presence of dissolved oxygen (DO) as shown in the reactions above. This process involves ZVI transferring two electrons to O2, resulting in the formation of hydrogen peroxide (H2O2). Subsequently, H2O2 undergoes chemical reduction to produce water via another two-electron transfer from ZVI. Interestingly, the Fenton reaction, involving the combination of H2O2 and Fe2+ generates hydroxyl radicals (˙OH) with potent oxidizing capabilities against diverse organic compounds, which are otherwise generated through either photo-Fenton of iron(ii or iii) or UV irradiation.69 In addition to Fenton reaction mechanism, ZVI core–shell model70 emerged for contaminant removal. Vast evidences support the presence of a core–shell structure in ZVI and several chemical reactions occurring on its surface.70–72 While the metallic iron core serves as the electron donor, facilitating pollutant reduction,70,73,74 the surrounding iron oxide shell acts as an adsorption platform for contaminant accumulation.73 This conceptual model has proven adept at explaining phenomena such as adsorption, reduction, oxidation, and precipitation that occur near the ZVI surface. However, the core–shell framework currently falls short of providing the quantitative insights necessary for designing and optimizing ZVI-based wastewater treatment processes.75 To achieve this, dynamic reaction kinetic models capturing the complex phenomena around ZVI particles are a requisite, particularly for quantifying the impacts of operating parameters on heavy metal removal efficiency. Coincidently, the suitability of modified Fenton like reaction was assessed.76 Zhou et al. (2018) incorporated magnetic field and ZVI/ethylenediaminetetraacetic acid (EDTA) Fenton like system to quantify ZVI degradation of nonsteroidal anti-inflammatory diseases (NSAIDs). The magnetic field primarily influences surface-bound reactions on heterogeneous ZVI material surface thereby accelerating its corrosion. Interestingly, this influence remains restricted to surface phenomena, with no impact observed on the homogeneous iron cycle or Fenton-like reactions within the bulk ZVI material.76 However, the oxidation potential depends on the type and structural properties of ZVI materials. The modified reactions are as follows.
| | |
Fe2+ + EDTA + H2O → [Fe2+(EDTA)(H2O)]˙2−
| (4) |
| | |
[Fe2+(EDTA)(O2)]˙2− → [Fe3+(EDTA)(O2−)]˙2−
| (5) |
| | |
[(EDTA)Fe3+(O22−)Fe3+(EDTA)]˙4− → 2[Fe3+(EDTA)(H2O)]˙− + H2O2
| (6) |
| | |
[Fe2+(EDTA)(H2O)]˙2−[Fe3+(EDTA)(O22−)]˙2− → [(EDTA)Fe3+(O22−)Fe3+(EDTA)Fe3+]˙4− + H2
| (7) |
| |
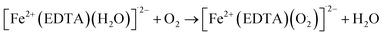 | (8) |
Zero-valent iron (ZVI) materials possesses remarkable flexibility in their ability to transform a diverse range of environmental contaminants through direct contact.34,77 Among these contaminants include halogenated hydrocarbons such as chlorinated methane. The direct contact reaction mechanism of ZVI in the degradation of chlorinated methane was reported in ref. 78, the mechanism is briefly described here as follows.
| | |
RCl + H+ + 2e− → RH + Cl−
| (10) |
| | |
RCl + Fe0 + H+ → RH + Fe2+ + Cl
| (11) |
| | |
C2Cl4 + 5Fe0 + 6H+ → C2H6 + 5Fe2+ + 4Cl−
| (12) |
From the above reactions, R represent an alkyl group such as methane, ethane etc. ZVI (zero valent iron) initiates the reaction by donating electrons to the chlorinated hydrocarbon (RCl), which subsequently underwent de-chlorination leading to the formation of RH + Cl−. Introducing ZVI to this step produces an oxidized iron with a chlorine byproduct indicating ZVI requirements and its favourable conditions in completely degrading chlorinated hydrocarbons like tetrachloromethane (C2Cl4).79 Several factors including operating conditions and intrinsic metal characteristics influence the performance of ZVI materials in the removal of environmental contaminants.65 Operating conditions such as temperature, iron concentrations and pH are more pronounced (see Fig. 2 and 3). Shimizu et al.,81 revealed the mechanism of phenol removal by zero-valent iron (ZVI) in the presence of dissolved oxygen by varying the pH from 2 to 8.1, pH and dissolved oxygen was found to significantly influence iron dissolution while OH radical production was an important parameter. At pH 3, 91% phenol removal was achieved with a 24% reduction in total organic carbon (TOC), of which 77% was attributed to the Fenton reaction, while at pH 4 and 5, adsorption/precipitation dominated DOC removal, and minimal TOC reduction was observed at pH 2 and 8.1.81 Other research findings show that 3.0 is the optimal pH for NB degradation within the tested pH range of 3.0 to 12.0. While the rate of formation of aniline, a major reductive product of NB, follows zero-order kinetics at various pH levels.82,83
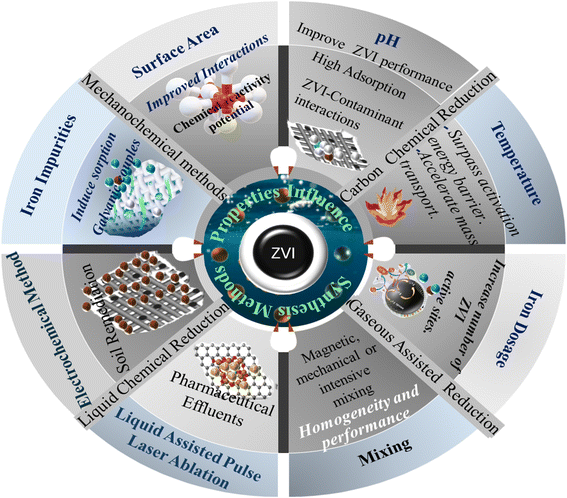 |
| | Fig. 2 Strategies and influence of process conditions and iron characteristics on the performance of micro-ZVI materials. | |
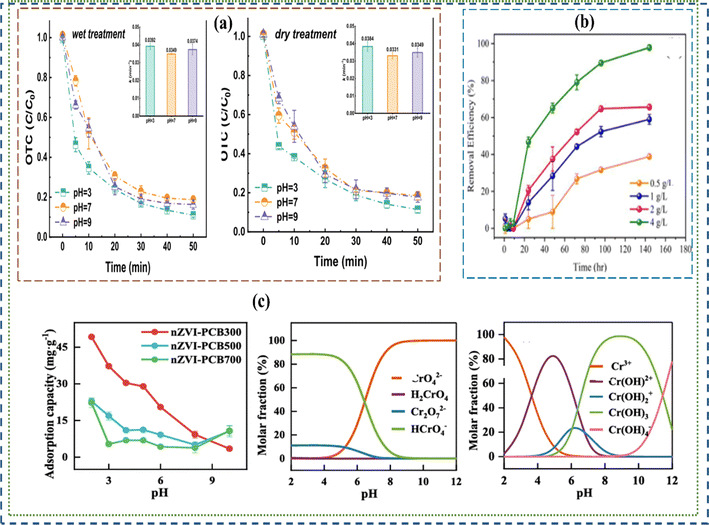 |
| | Fig. 3 Influence of concentrations and pH on the performance of nano and micro-ZVI materials in the removal and adsorption of contaminants. (a–c) show the impacts of pH variation on removal chromium, and the influence of concentration of nano and micro-ZVI composites on oxytetracycline and chromium degradation80 Copyright: 2024, Elsevier. | |
Similarly, Wang et al., observed the efficiency in the treatment of lead contaminated soils by zero valent iron materials to decrease with increasing pH from 3 to 9.84 Fig. 3 shows how varying concentration of nano and micro-ZVI influenced their performance. Micro ZVI materials can be used to transform organic dyes and pesticides: including dichlorodiphenyltrichloroethane, (DDT),85 lindane, and other dye molecules,86–89 inorganic anions like dichromate, perchlorate, nitrate, and arsenic. ZVI material performance in the degradation of persistent organic pollutants such as polychlorinated biphenyls, dioxins, pentachlorophenol, N-nitrosodimethylamine and TNT were also reported.34,78 However, ZVI materials are limited by low sorption affinity in degrading organic contaminants, which is a typical characteristic of pristine iron oxides.85 Hence, supportive adsorbent such as graphene or activated carbon90 are often incorporated to ZVI materials when applied in an emerging organic pollutants medium.
ZVI (Zero Valent Iron) materials' applications span across many fields, profoundly in groundwater and soil remediations like the removal of organophosphates,91 heavy metals,92 dyes, anti-biotics,93 and other organic contaminants.85,94–97 Emerging contaminants such as polyhalogenated carbazoles (PHCZs) are persistent, bio-accumulative, and toxic environmental contaminants lacking efficient and sustainable degradation method, coupling micro-ZVI with supportive adsorbents has indicated a promising activity in degrading these types of contaminants.75,82,98–100 Such as the use of sulfidated zero-valent iron combined with peroxymonosulfate (S-ZVI/PMS).101 Similar research by Wang et al., (2023)102 and103,104 with 96.6% Cr(VI) removal, thiobencarb removal,105 degradation of oxytetracycline106,107 and degradation of clopyralid and MTBE, tetrachloroethene.95 By and large, it is imperative to note that both micro and nano ZVI material's characteristics depend on the intrinsic properties and mineralogical compositions of the precursors. Consequently, the core–shell model, Fenton reaction mechanism and modified Fenton like mechanisms have found common use in revealing the efficacy and mechanism of ZVI interactions with contaminants owing to their understood technology plus the availability of research findings on these mechanisms since the introduction of PRB technologies in the early 1990s (refer to Fig. 1). Distinct material properties requirements such as increased chemical reactivity, higher surface area to volume ratio for an increased number of active sites coupled with low agglomeration demands led to the emergence of nano zero-valent iron materials or simply nZVI as a research area.
2.2 Nano zero valent iron (nano-ZVI)
Nano ZVI (Zero Valent Iron) is a fine, black powder derived from micro-ZVI through chemical and physical synthesis methods and characterized by smaller particle sizes within the nano scale (between 1 nm to 100 nm) range.72,108 nZVI is distinguished by its excellent surface area with higher chemical reactivity, and numerous active sites (see Fig. 4). It provides sufficient surface area and excellent interaction with emerging contaminants.109,110 The high surface area and reactivity of nZVI materials translate to improved effects and superior performance compared to their microscale counterpart.34,111
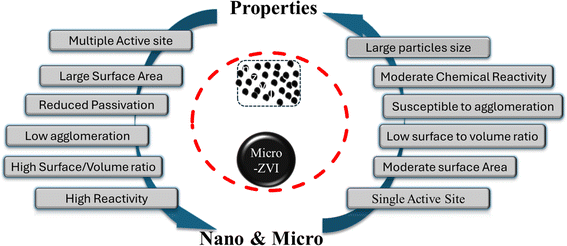 |
| | Fig. 4 Comparison of reaction active sites and properties of micro and nano ZVI materials. | |
The unique attributes of nano-ZVI materials that distinguish them from micro-ZVI stems predominantly from their increased surface-to-volume ratio and/or the enhanced reactivity of their surface sites.6 As ZVI (Zero Valent Iron) particle size diminishes, the proportionate contribution of surface atoms increases, significantly amplifying their propensity to adsorb, interact, and react with other atoms, molecules, and complexes (refer to Fig. 4). This amplified reactivity can be ascribed to the greater access and availability of active sites on the nZVI particle surface, aiding charge stabilization through electronic interactions with surrounding atoms.112 Recent researches highlight the remarkable potentials of nano ZVI for degrading diverse inorganic contaminants,110 including metal ions,113 like Cd,114 Cr,115–118 complex anions like perchlorate and nitrate.82,119–122 Compared to conventional sorbents and their interactions with other iron particles, nZVI materials offer significant advantages and exhibit substantially higher capacity for contaminant removal as also exemplified by their ability to remove mixed dyes86,123,124 and heavy metals like Cr(VI)125 and Ni(II) efficiently from wastewater through reduction and co-precipitation usually at both lower and higher pH126 Moreover, nZVI possesses faster reaction rates, with studies showing at least 25–30 times higher efficiency in Cr(VI) removal compared to microscale ZVI.127 In addition, n-ZVI when stabilized with other materials such as carboxymethyl cellulose, CMC, and nZVI materials significantly enhances dye degradation from textile wastewater.128
Nanosized zero-valent iron (nZVI) materials offer enhanced environmental remediation capabilities due to their increased specific surface area, leading to a greater abundance of active reaction sites (refer to Fig. 4) compared to their corresponding micro-scaled counterpart. However, a key challenge arises owing to the inherent magnetic properties of nZVI, which often cause particle aggregation.75 These formed aggregates possess paradoxical properties and enhance magnetic mobility for targeted delivery with a reduced surface area, causing nZVI material's reactivity. To address this and optimize both reactivity and mobility, extensive research efforts have focused on tailoring the synthesis methods of nZVI particles.
2.3 Overview of nanoparticle synthesis methods
Nanoparticle synthesis methods are classified into either top-down and bottom-up approaches or simply physical and chemical synthesis methods.108 Fig. 5 gives an overview these classifications.
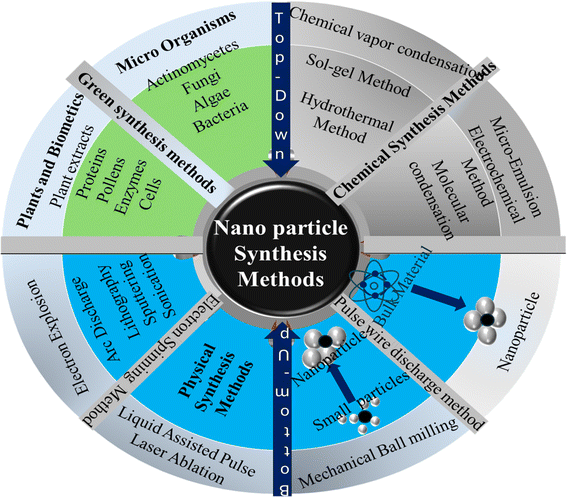 |
| | Fig. 5 Overview of nanoparticles synthesis methods: top-down and bottom-up approaches. | |
Physical/top-down methods are effective in fabricating nanomaterials with distinct properties and applications.108 However, in addition to being energy intensive, the properties of the developed nanoparticles are often altered, limiting their applications in material synthesis that require stringent control over morphological structures.108 Bottom-up/chemical synthesis methods involve the use of chemical reactions to manipulate atoms or molecules to form nanoparticles. In contrast, chemical methods allow a degree of control over the morphological structure of the synthesized nanomaterials.108 Green synthesis methods are recently discovered viable,108,129–131 paving the way for utilizing biobased materials as a sustainable source of nanoparticles for various applications.108
2.4 Synthesis methods of zerovalent iron micro and nano materials
There are several synthesis methods of nano and micro zerovalent iron materials ranging from bottom-up approaches to green synthesis and combined technologies each with its distinguishing applications, materials properties, and limitations.132–138
2.4.1 Mechanical ball milling. Ball milling refers to the breaking down of iron precursor material into micro or nano-scale particles through a high-speed rotating chamber.108,139,140 The ball-milling approach is regarded as one of the most sustainable for synthesizing nanocomposites and wear spray coatings.141–143 Imperative factors in this approach are the container size, and the energy input.108,144,190 Ball milled nanoparticles are highly efficient in the degradation of wastewater contaminants,77,145 and their composites recorded high efficacy in soil remediations.125,146
2.4.2 Mechanochemical method. Mechanochemical synthesis is a combined synthesis method that involves the use of mechanical energy such as friction or shear force for the initiation of chemical reactions.133 It's usually combined with ball milling to provide sufficient mechanical energy for the bulk or micro-scale iron particles to disintegrate into nano-scaled particles133,147 and provides sufficient access to active sites of nanocomposites materials.132 Mechanochemical method produces ZVI materials with an increased particle reactivity potential compared to conventional mechanical ball milling procedure.134
2.4.3 Chemical vapor deposition method. Chemical vapor deposition can be physical or chemical process depending on the nature and compositions of the reacting materials.148 The procedure involves vaporizing target materials and condensing them with chemical methods altering the target material's composition to form nanocomposite materials.149,150 The produced nanoparticles condense into liquid nitrogen before subsequent morphological changes.148 In addition to control over material properties, chemical vapor deposition produces nano materials with significantly lower agglomeration potential.
2.4.4 Liquid -assisted pulsed laser ablation. Liquid-assisted pulsed laser ablation (LA-PLA) synthesis method refers to the formation of nanoparticles with varying sizes, crystallinity, and shell composition using femtosecond pulse laser in water, or a nanosecond pulse laser on bulk iron targets immersed in different organic solvents. The properties of the synthesized materials are proportional to the nature of the solvent used and the pulse overlap.151 Pulse laser is cost effective, and environmentally benign.151,152
2.4.5 Liquid assisted chemical reduction. The liquid chemical reduction approach is the most utilized method of synthesizing micro-nano ZVIs (Zero Valent Iron),153 by chemically reacting suitable iron precursor with sodium borohydride (NaBH4) or any other suitable reducing agent,154 the reaction is conducted under nitrogen condition.155 Black colour particles emerge immediately after the addition of sufficient solution of the reducing agent.,.156 Fig. 6 shows the synthesis route in the liquid-assisted chemical reduction of selected nano and micro-ZVI materials.
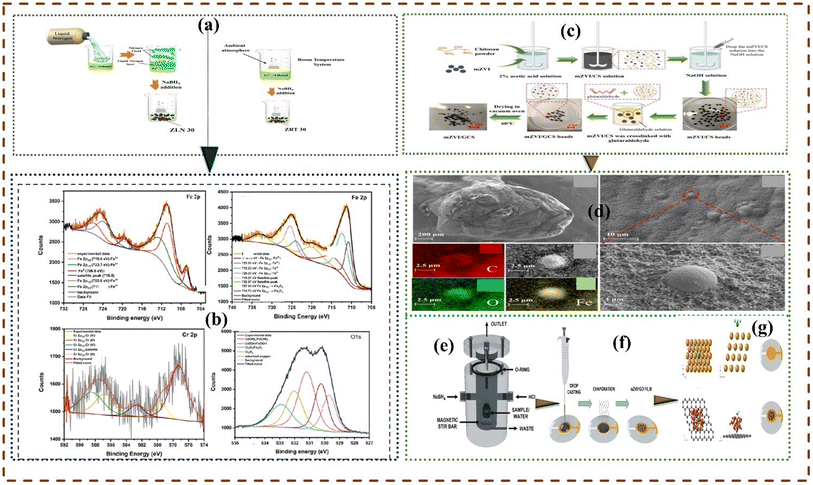 |
| | Fig. 6 Sodium borohydride (NaBH4) assisted chemical reduction methods. Methodology for synthesizing liquid nitrogen-assisted zerovalent iron (ZLN) (a and b), energy peaks and chemical composition of ZLN observed from iron 2p before and after reactions (b)157 Copyright: 2023, Elsevier. Synthesis pathway of micro-ZVI coupled glutaraldehyde crosslinked chitosan (c) micro-ZVI morphological structures (d).158 Synthesis route of nano ZVI coated reduced graphene oxide (nZVI/rGO) (e and f), lateral, top, and unmodified adsorption of arsine AsH3 by nZVI/rGO (g)159 Copyright: 2024, Elsevier. | |
2.4.6 Gaseous chemical reduction. In the gaseous chemical reduction method, hydrogen is the primary reducing agent.160 Iron precursors such as goethite,161 magnetite162 or limonite27 are first produced through precipitation of ferrous salts and then dehydrated or heated to prepare them for chemical reduction. The method starts by reducing the iron precursors (obtained through precipitation), at elevated temperatures with hydrogen gas,37 followed by chemical reduction in a hydrogen or nitrogen gas-controlled environment.160 The gaseous chemical reduction synthesis method yields nano and micro-ZVI materials with controlled surface properties.150
2.4.7 Carbo-thermal reduction. The carbo-thermal synthesis method is a high-temperature reduction of iron precursors using thermal energy in the presence of gaseous reducing agents.163–165 The resulting coupled ZVI-carbon products are obtained through chemical reactions with carbon materials.166,167 The synthesized nZVI-supported carbon particles exhibit enhanced physicochemical properties,168 low agglomeration characteristics,169–171 and high degradation activity in relation to non-carbon encapsulated ZVI materials.172 Carbothermal synthesis method is highly suitable for producing carbon encapsulated iron materials with chemical byproducts compared to conventional chemical reduction methods.173
2.4.8 One spot chemical method. One spot synthesis method is a combined technology usually accompanied by liquid chemical reduction where iron precursors such as FeCl3·6H2O are dissolved and mixed with reacting/supporting material under nitrogen gas, N2 atmosphere.154,174 It is cost-effective and produces iron materials with significant stability and low aggregation. Fig. 7 shows one spot chemical synthesis routes for producing nano zero-valent iron composite materials.
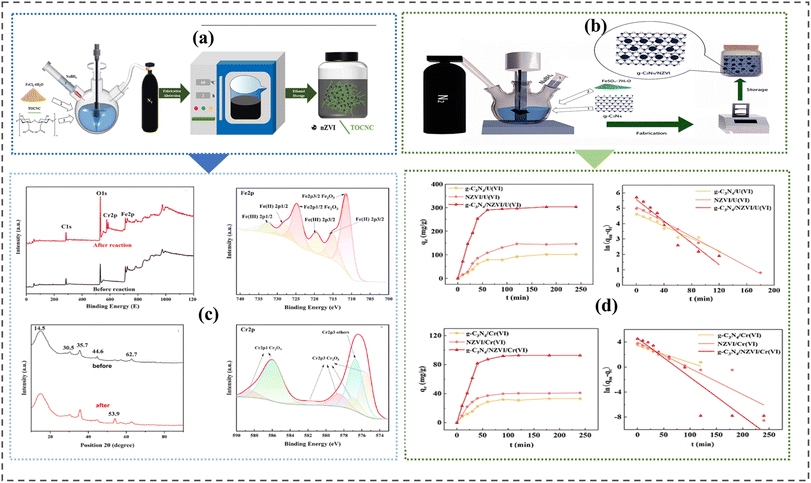 |
| | Fig. 7 One spot synthesis of nZVI nanocomposites. (a) synthesis procedure of TEMPO-oxidized cellulose nanocrystal. (c) One spot scaffolding of zero-valent iron onto graphene carbon nitride, g-C3N4. In both methods nanocomposites' fabrications were conducted under nitrogen gas N2 conditions to rid dissolved oxygen and maintain anaerobic conditions, TOCNC adequately absorbed Cr(VI) due to their large surface areas and abundant active sites. Charged nZVI facilitated the conversion of adsorbed Cr(VI) to Cr(III) through the oxidation of Fe0 to Fe2+ and Fe3+ with adsorption rate depicted in (d). The binding energy and adsorption reaction kinetics of the zero-valent iron coupled graphene carbon nitrite are shown in (b)174 Copyright: 2024, Elsevier., and154 Copyright: 2023, Elsevier. | |
2.4.9 Electrochemical reduction. The electrochemical method uses an electrolysis process to produce nano and micro-ZVI materials.175–177 This method involves introducing iron precursors like iron pentacarbonyl, argon gas, ethylene, acetylene, and ethyl materials into a reaction chamber while gas current is rapidly expanded in two phases to control nanoparticle growth and agglomeration.178 The ZVI (Zero Valent Iron) materials nanoparticles condense into a liquid nitrogen substrate and transferred to a delivery system for collection after undergoing structural changes through purification, and crystallization.150,175 Electrochemical method is cost effective, however it generates copious amount of chlorine gas byproducts.
2.4.10 Ultrasonic wave method. The ultrasonic wave method involves the reduction of micro-ZVI particle size and increasing surface area and uniformity. It is applied in laboratories alongside other methods like chemical reduction with sodium borohydride. The process involves preparing solutions of precursor and reducing agent with ammonium hydroxide solutions and deionized water, applying ultrasonic waves through a titanium probe, and maintaining the solution temperature. The resulting nZVI is filtered, washed, and dried to avoid oxidation.108,179,180 Ultrasonic method is cost effective and provides significant access to the morphological structure of nano and micro materials.
2.4.11 Microemulsion method. The micro-emulsion method involves the formation of uniformly dispersed inorganic phase, particle size is controlled by adjusting reaction parameters.181 The reduction reaction forms an emulsion which is separated through centrifugation or magnetic methods. The average nanoparticle size depends on droplet size, iron precursor concentration, and surfactant flexibility.40
2.4.12 Green synthesis method. Recently, there has been increasing research interest in the green synthesis of zero-valent iron materials.182–185 These methods involve utilizing plant extracts that are rich in reducing polyphenols and flavonoids components such as green tea (camellia Senensis),186 Verbascum Thapsus,185 Syzygium aromaticum extracts,183 to fabricate nano and micro-ZVI. These biobased extracts efficiently reduce Fe2+ and Fe3+ and other contaminants while preventing agglomeration of zero valent iron nanoparticles. In addition to being environmentally benign, green synthesis methods are efficient and cost effective (Table 1).
Table 1 Synthesis methods, methodologies, and properties of selected nano and micro ZVIs and their composites
| Synthesis method/material synthesized and precursor |
Methodology (with reaction conditions) |
Properties of the synthesized materials |
Ref |
| Green synthesis method nZVI particles |
The procedure involved the co-addition of an optimized amounts of sodium borohydride (NaBH4) and H. caffrum extract to ferric chloride (FeCl3) under an inert nitrogen atmosphere and titration at 20 °C. Following a 30 minutes stirring period, the product was washed with deionized water and dilute ethanol (50%) and freeze-dried |
Higher reactivity, stability, and well dispersed nano ZVI particles with strong Fenton catalytic properties |
187 |
| Chemical reduction method nZVI-SBA15 mesoporous silica composite |
The SBA-15 silica was first prepared using sol–gel method with P123 as a structure-directing agent. Subsequently, iron was deposited onto the silica surface via controlled hydrolysis of iron III nitrate nonahydrate (Fe(NO3)3·9H2O). Finally, the deposited iron was reduced to zero-valent state using sodium borohydride under acidic conditions |
Averaged size of nZVI-SBA15 mesoporous silica composite in the nanometre range. The material exhibited a mixed composition of iron and silica oxides, with iron content slightly exceeding 10%. The isoelectric point, influenced by the dominant silica component, was found to be around 2.0 |
188 |
| Mechanochemical method |
10 g of reduced iron powder and NGB at a mass ratio of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was mixed in a stainless-steel jar with agate balls. The jar was sealed, evacuated, and purged with pure nitrogen gas three times before subjected to ball milling at 200 rpm for 12 hours using a planetary ball mill. The resulting composite was collected in a glovebox and stored in an air-proof desiccator filled with argon gas to prevent oxidation 1 was mixed in a stainless-steel jar with agate balls. The jar was sealed, evacuated, and purged with pure nitrogen gas three times before subjected to ball milling at 200 rpm for 12 hours using a planetary ball mill. The resulting composite was collected in a glovebox and stored in an air-proof desiccator filled with argon gas to prevent oxidation |
The mZVI/NGB composite demonstrated exceptional efficiency in the removal of tetracycline TC, reaching near-complete degradation under circumneutral pH conditions (5.0–6.8), the composited displayed significant tolerance to co-existing anions such as Cl−, SO42− and humic acid |
|
| Micro zero valent iron grafted nitrogen doped biochar-like graphene (mZVI/GBN composite |
| Combined method involving initial liquid-phase reduction |
Firstly, Banana peels carbonated at 250 °C for 2 h was utilized to prepare phosphoric acid-activated biochar (BC) followed by nano zerovalent iron synthesis via liquid phase reduction method. The nZVI-BC composite was subsequently fabricated via in situ formation of nZVI on the BC surface using deionized water, ferric nitrate, and sodium borohydride under nitrogen condition |
Compared to microbubbles alone, tetracycline degradation performance using nano zero valent particles incorporated microbubbles or conventional microbubbles (MBs-nZVI or BC-nZVI) demonstrated significant efficiency. Removing 80% tetracycline contaminants from wastewater within 2 h |
189 |
| Biochar incorporated nano zero valent iron (BC-nZVI composite |
2.5 Physico-chemical properties and performance of ZVI materials synthesized through different synthesis methods
The ability of Zero-Valent Iron (ZVI) materials are emerging materials that effectively remediate a plethora of environmental contaminants.4,53 The removal performance of ZVI materials is inextricably intertwined with their physicochemical properties, which in turn is significantly influenced by the synthesis method employed.104 This section provides a concise review of the enhanced removal performance of ZVI materials prepared using different synthesis techniques. Physical or top-down approaches such as the ball milling process are reported to generate ZVI particles with irregular shapes and a broad particle size distribution with an improved specific surface area due to the particle size reduction.77 A study by Zhang et al. (2023) reported that ball-milled ZVI coupled with biochar (ZVI/BC) exhibited an improved adsorption capacity for Cr(VI) of 117.7 mg g−1 at 298 K, which was 2.08 times higher than the pristine ZVI/BC. Similarly, Fang et al. (2022)142 found that ball-milled ZVI composite showed enhanced removal of hydrophobic organic compounds (HOCs), with a maximum removal efficiency of 99%. In a combined mechanical chemical approach, the method generates ZVI particles with smaller, higher surface area, and more uniform size distribution compared to the ball milling method.133 Calderón Bedoya et al. (2023) demonstrated that ZVI materials produced by the mechanochemical method exhibit improved reactivity and contaminant removal efficiency, along with excellent magnetic properties (55–57 emu g−1) and very low coercivity (12–19 Oe). Ref. 147 reported the mechanochemically modified micro-ZVI material with 88.8% efficiency in the removal of phenols. In similar studies, ZVI materials were fabricated through top-down or chemical synthesis methods such as liquid-assisted pulsed laser ablation where high purity and controlled size ZVI materials are produced, resulting in a narrow particle size distribution and high specific surface area. Coincidently, Lahoz et al. (2020)151 reported the ZVI nanoparticles produced by this approach with polydispersity indices lower than 10 nm and 0.10, respectively, and exhibited over 99.9% performance when utilized in medicine and other environmental remediations. Another promising chemical synthesis method is the liquid-assisted chemical reduction method. This method produces ZVI nanoparticles with high chemical reactivity and dispersibility. However, there is a strong potential for agglomeration and rapid passivation when utilizing this method over time.57 Other synthesis methods, such as the one-pot chemical method, electrochemical reduction, ultrasonic wave method, and microemulsion method enhance the removal performance of ZVI materials.137,174,177,181,185 These methods offer various advantages, including high reactivity, controlled particle size, improved dispersion, and the use of environmentally friendly reducing and stabilizing agents. Table 2 summarizes the physicochemical properties and performance of ZVI materials synthesized via different synthesis methods.
Table 2 Comparison of physico-chemical properties and performance of ZVI Materials synthesized through different synthesis methods
| Synthesis method |
Material physico-chemical properties |
Removal performance |
Reference |
| Mechanical ball milling |
ZVI particles with irregular shapes and broad size distribution |
Ball milled ZVI/BC recorded an improved adsorption capacity for Cr(VI) to 117.7 mg g−1 (298 K), 2.08 times higher than the pristine ZVI/BC |
190 |
| High specific surface area due to particle size reduction potential for agglomeration and loss of reactivity |
| Mechanochemical method |
ZVI particles with smaller and more uniform size distribution |
The method produces ZVI materials with improved reactivity and contaminant removal efficiency |
133 |
| Higher specific surface area compared to ball milling |
The method produces ZVI materials with excellent magnetic properties (55–57 emu g−1) and very low coercivity (12–19 Oe) |
| Liquid-assisted pulsed laser ablation |
ZVI nanoparticles with high purity and controlled size |
Produces ZVI materials with polydispersity indices lower than 10 nm and 0.10, respectively >99.9% performance for medicine and environmental remediation |
151 |
| Narrow particle size distribution and high specific surface area |
| Liquid-assisted chemical reduction |
ZVI nanoparticles with high reactivity and dispersion |
ZVI coupled zinc incorporated silica bn titania dioxides synthesized via chemical reduction method showed >99.8% arsenic removal at 5.0 mg L−1 |
191 |
| Potential for agglomeration and loss of reactivity over time |
| Gaseous chemical reduction |
Controlled particle size and morphology through gas-phase reactions |
The size of zero valent iron materials synthesized via this method are approximately 60 nm with significantly controlled morphologies |
150 |
| Generates ZVI particles with high purity but high environmental impacts |
| Carbo-thermal reduction |
ZVI particles with high purity, crystallinity with exceptionally high chemical reactivity and regenerative capacity |
The nZVI@MOF-CN demonstrated significant reactivity achieving bromate reduction efficiency of 80% after five successive regeneration cycles |
249 |
| Potential for carbon contamination and agglomeration |
| One-pot chemical method |
ZVI nanoparticles with high reactivity and dispersion |
The sequestration of U(VI) and Cr(VI) by NZVI nanocomposite was greater than that of pure NZVI or g-C, demonstrating a significant enhancement in the performance of NZVI composites |
39 |
| Relatively simple and scalable synthesis process |
| Potential for agglomeration and uncontrolled particle size distribution |
| Electrochemical reduction |
ZVI nanoparticles with high purity and controlled size |
Moratalla et al., reported zero-valent iron (ZVI) facilitated conversion of 95% iopamidol into C17H25N3O8 with nearly total elimination after electrolysis of the initial pollutant |
177 |
| Enables in situ generation and application of ZVI |
| Ultrasonic wave method |
ZVI nanoparticles with high specific surface area |
99.76% of Rh B degradation within 12 min at Ph 4 and 1.0 g per L ZVI concentration |
137 |
| Improved dispersion and reactivity compared to conventional methods |
| Microemulsion method |
ZVI nanoparticles with controlled size and narrow distribution |
Produce nanoparticles with exceptional superparamagnetic and ferromagnetic properties |
181 |
| Enhances stability and dispersibility of ZVI in aqueous media |
| Green synthesis method |
Utilizes environmentally friendly reducing and stabilizing agents |
Enhanced performance and complete reduction of Cr(VI) after 30 min under 1 g per L green synthesized nZVI |
185 |
| Generates ZVI nanoparticles with high purity and biocompatibility |
3 Applications of nano and micro materials and environmental considerations
Nano and micro-ZVI materials have recently recorded an increasing utilization in a plethora of industries. Moreso in groundwater treatment, wastewater and environmental remediations,56,192–197 degrading obnoxious and contemporary contaminants such as sulfamethoxazole198 chromium,104,116,146,154,199 wastewater antibiotics,200 trichloroethane,109 petroleum hydrocarbon, soil contamination,201 lindane202 nickel203 arsenic204 zinc, lead and cadmium contaminated soil.205–207 Table 3 shows selected applications of nano and micro-ZVI and their composites in the removal various contaminants present in ground water, wastewater, soil, and other domestic and industrial effluents.
Table 3 Selected applications of nano and micro-ZVI materials
| Type of contaminant |
Type of zero valent iron material |
Efficiency in contaminants removal and conditions |
Remediated material |
Ref. |
| Uranium, U(VI) |
Fe-PANI-GA (zero-valentiron-polyaniline graphene aerogel) |
Excellent reduction of U(IV) in in acid solutions through sorption and partial precipitation |
Aqueous solution containing radionuclide pollutant, U(IV) |
208 |
| Uranium, U(VI) |
Fe–Ni/graphene; nZVI loaded chitosan (NZVI/CS); Fe–Cu/MBC; Fe–Cu |
Maximum sorption and reduction of U(VI) with maximum removal capacity |
Uranium contaminated wastewater |
209–211 |
| Chromium |
Sulfidized nZVI supported Oyster shell powder |
Excellent performance, Cr(VI) removal capacity of 164.7 mg g−1 in acidic solution |
Wastewater |
212 |
| Oxytetracycline (OTC) |
Dry and wet Pre-ZVI-activated peroxymonosulfate (PMS) |
Varying the pH values (3,7 and 9). Both dry and wet Pre-ZVI/PMS systems achieved high initial OTC removal efficiency (>43%) at pH 3 |
Organic matter contaminated water |
107 |
| Long-term OTC degradation was significantly lower for Pre-ZVI/PMS (8–9%) compared to PMS alone (44%) |
| Further research is needed to improve the long-term effectiveness of Pre-ZVI/PMS for organic matter removal |
| Trivalent and pentavalent antimony (Sb) |
Sulfidated nano zero valent iron coupled with graphene oxide (S-nZVI@GO) |
Varying pH from (3–9), excellent contaminant removal of 96.7% under aerobic condition with absorption capacity of Qmax = 311.75 mg g−1 |
Sb contaminated wastewater |
110 |
| Orange II sodium salt (OR2) |
Green synthesized zero valent iron (gNZVI) |
Inert (nitrogen) controlled environment, 65% 20 ppm OR2 dye degradation in 1 hour, with excellent Fenton catalytic properties |
For dye removal such as in textile wastewater (effluent) treatment |
187 |
| Xenobiotics |
nZVI-SBA-SiO2 |
Acid controlled polymer reaction, promising properties with high xenobiotics degradation potential |
Xenobiotics contaminated groundwater and wastewater |
188 |
| Copper ions |
Core–shell zero valent iron, CS-nZVI |
For batch process, adsorption of copper ions was significantly controlled by the operational parameters however, CS-nZVI recorded high efficacy in treating copper ions contaminated water |
Wastewater |
70 |
| Lead (Pb) |
Nano zerovalent iron, nZVI coupled low molecular weight organic acid |
Lead removal rate of 64% and 83% with rapid adsorption rate in 4 hours. Efficiency decreases with increasing pH |
|
84 |
| Metal and/or metalloid contaminants; Cu(II), Zn(II), Cr(VI), and As(V) |
Nano zerovalent iron |
Removal efficiency varied depending on the interaction mechanisms and solution speciation while increasing the ionic strength decreased the rate of removal |
Hydraulic fracturing wastewater, saline wastewater |
213 |
| Arsenic (As) |
Goethite nanospheres(nGoethite) coupled nano zero valent iron (Nzvi) |
nGoethite and nZVI effectively immobilized As in the tested brownfield soil with an 89.5% decrease in As concentration however, high concentration of Goethite nanospheres increase the phytotoxicity of the polluted soil |
Arsenic polluted soils |
161 |
| DDT (1,1,1-trichloro-2,2-bis(p-chlorophenyl) ethane) |
Nano zero valent iron |
nZVI treatment degraded 50% of 20 mg DDT contaminants in the spiked sandy soils and 24% degradation in 24 mg aged DDT contaminated soil |
DDT-polluted soil and spiked sandy soil |
214 |
| Trinitroglycerin (TNG) |
Nano zero valent iron and nZVI supported nanostructured silica SBA-15 |
ZVI-nanoparticles and ZVI-nanoparticles/SBA-15 efficiently degrade TNG in water, resulting in the production of glycerol and ammonium with respective normalised rate constant of 0.36 and 0.33 L h−1 m−2 |
Trinitroglycerin (TNG) contaminated soils and water |
215 |
| Direct black G (DBG), a model azo dye |
Kaolin supported nanoscale zero-valent iron, K-nZVI |
Excellent degradation efficiency with mechanisms involving prompt adsorption of DBG onto the K-nZVI surface and the subsequent oxidation of DBG by hydroxyl radicals at the K-nZVI surface |
Wastewater containing azo dyes |
216 |
| 1,1,1-Trichloro-2,2-bis(p-chlorophenyl) ethane or (DDT) |
nZVI, produced through chemical reduction method using precipitation with borohydride, and nZVI, produced through gas phase reduction of iron oxides under H2 |
Both types of nZVI effectively degraded DDT in water but were slow in the remediation of DDT contaminated soils |
DDT contaminated water and soils |
217 |
| Hexavalent chromium (Cr(VI)) |
Nano-zero-valent iron (ZVI) encapsulated in a carbon shell (ZVI-C) |
pH and concentration of the contaminant (Cr(IV)) influenced the electron utilization efficiency of ZVI and the utilization efficiency was observed to increase up to 80% at 2000 mg per L Cr(VI) concentration and pH 3.0. Core–shell structure of ZVI-C shielded ZVI from the acidic conditions and the formation of undesired precipitates |
Cr(VI)-contaminated wastewater |
118 and 218 |
| Escherichia coli (E. coli) |
Sulphur modified nano zero valent iron (S-nZVI) |
Reduced toxicity of zero valent iron materials used ground water treatments, although other ions presence in ground water could interfere with the reactions, however coupling sulphur to nano ZVI was observed to lose inherent toxicity over time |
Groundwater |
219 |
| Dewaterability of aerobic digested sludge |
Persulfate combined ZVI (PS-ZVI) |
Persulfate combined zero valent iron technique recorded 80% reduction in capillary suction time (CST), with an efficient removal of hydrophilic organics compounds (HOC) presence in extracellular polymeric substances (EPS) |
Sludge |
220 |
| Tetracycline contaminants |
Biochar supported nano ZVI |
Micro-nanobubbles supported nano zero valent iron (MBs-nZVI composite) recorded 80% tetracycline degradation within 2 h from wastewater effluent which was significantly greater compared to the tetracycline degradation efficiency via microbubbles alone |
Wastewater |
189 |
| Chromium(VI) |
Zero valent iron biochar (ZVI-BC) composite |
High efficiency in converting chromium contaminants to stable compounds thereby reducing their detrimental impacts and rejuvenating soil richness and its microbial diversity |
Chromium contaminated soil |
125 |
| DDT and DDT byproducts |
Zero valent iron coupled EDTA, (ethylenediaminetetraacetic acid) |
Fenton-like system efficiently remediated DDT contaminated soils while an increase in reactants concentrations (EDTA and ZVI) significantly increased the efficacy of DDT degradation as well as the removal of DDT byproducts contaminants with a risk of the production of secondary pollutants at excess DDT concentrations |
Soil contaminated with dichlorodiphenyltrichloroethane and its reaction byproducts |
221 |
| Trichloroacetic acid from family of Halo acetic acids or simply HAAs |
ZVI encapsulated biological active carbon (Fe0-BAC) |
Fe0-BAC completely removed TCAA and its byproducts, dichloroacetic acid (DCAA) and monochloroacetic acid (MCAA) contaminants through combined chemical dehalogenation and biodegradation of ZVI and BAC |
Wastewater, halogenated/chlorinated drinking water, and swimming pool water |
222 |
| Nitrate contaminants |
Nano magnetite supported zero valent iron |
Nano-sized magnetite substantially improved nitrate reduction in groundwater. The rate of nitrate reduction increases proportionally with the loading of magnetite nanoparticles. The presence of magnetite nanoparticles aids electron transport from Fe0 to adsorbed nitrate, thus supporting the reduction process |
Ground water |
223 |
| Nitrate contaminants |
Nano zero valent iron |
Removal efficiency of nitrate contaminants of over 90% using laboratory scale continuous flow systems |
River and ground water |
224 |
| Thiobencarb contaminants |
Zero valent iron powder |
Through reduction and adsorption ZVI effectively treated 10 ml sample solution containing 10 μg ml−1 of thiobencarb at room temperature for 12 hours |
Thiobencarb contaminated water/wastewater |
105 |
| Uranium, copper, and cadmium |
Nano zero valent iron |
Zero valent nanoparticle efficiently degraded the studied contaminants. The efficiency increases with increase and reduction of pH and oxidation–reduction potential |
Acid mine water |
225 |
| Arsenic, copper, and other heavy metals |
Nanoparticles zero valent iron |
High removal capacity to over 99.5% of arsenic and copper using gravitational separation and zero valent nanoparticles recirculation process |
Heavy metals contaminated wastewater |
113 |
| 2,20,5,50-Tetrachlorinated biphenyl (PCB-52) |
Zero valent iron encapsulated anionic/cationic surfactants |
The higher the concentration of surfactants the greater efficiency and the more the reduction of PCB-52 contaminants |
Soil and/or sediment solutions |
226 |
| Nitrate and total nitrogen |
Zero valent iron nanoparticles coupled activated carbon (nZVI-AC) |
Total nitrogen removal efficiency proportionately increased with an increase in nZVI/AC concentration but declined above 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 nZVI/AC ratio. When utilized alone, nZVI recorded 100% nitrate removal while its efficacy in terms of total nitrogen (TN) was virtually the same at about 35% at acidic pH like nZVI/AC composite 1 nZVI/AC ratio. When utilized alone, nZVI recorded 100% nitrate removal while its efficacy in terms of total nitrogen (TN) was virtually the same at about 35% at acidic pH like nZVI/AC composite |
Nitrate contaminated ground water |
227 |
| Chlorinated dense non-aqueous phase liquids, DNAPLs |
Zero valent iron nanoparticles coupled polyethyleneimine PEIe/nZVI |
Significant transformation of DNAPLs contaminants into non-toxic elements. The reduction reactions with contaminated ground water occurred prior to the oxidations of nano zero valent iron composite |
Contaminated groundwater |
228 |
| Nitrobenzene, NB |
Zero valent iron nanoparticles supported ordered mesoporous carbon (nZVI/OMC) |
NZVI/OMC composite exhibited enhanced removal efficiency, which was attributed to its combined adsorption and synergistic reduction properties towards nitrobenzene |
Wastewater and groundwater |
121 and 229 |
| Organic pollutants (azo-dye orange II) |
Nanoscale zero valent iron |
Significant formation of iron oxide/hydroxide layers on the nZVI surface, and the adsorption of the pollutant and its intermediates. Rapid decolorization of orange II with nZVI, but the removal of total organic carbon (TOC) observed was slower, with maximum removal achieved at pH 9.0 |
Wastewater |
230 |
| Copper |
Zero valent iron, ZVI |
Cu removal was quick and efficiently completed at pH 2–5. Complete Cu removal was achieved within 35 minutes at pH 4 for Cu loadings ranging from 0.393 to 4.72 mM. The concentrations of Cu2+ and dissolved oxygen were found to be strongly connected |
Wastewater |
231 |
| Organophosphate pesticides |
Zero valent iron |
Complete removal of toxicity with rapid degradation of malathion, ethyl parathion and methyl parathion |
Groundwater |
91 |
| Phycocyanin |
Zero valent iron |
High phycocyanin removal at pH value below 6 which decreases as pH increased. The removal efficiency and mechanism were governed by the coagulations of the dissolved ZVI ions as compared to the conventional adsorption mechanism where contaminants are adsorbed on material surface |
Drinking water |
100 |
3.1 Soil and groundwater remediation
Of the most dominant applications of nano and micro zero valent iron materials are in soil and groundwater remediation (see Fig. 8).3,91,155,176,192,223,232,233 Their chemical reactive ability to degrade chlorinated solvents through reductive de-chlorination,65,92,134,175,228 degrade nitrates,223 pesticides,91 micropollutants,176 reduction of sulphides and leads,109,115,195 adsorb heavy metals,22,234 and organic contaminants7,9,68,95,127,186,230,235 suggests a promising alternative to conventional methods.92 However, factors like contaminant type, soil properties, and aging effects significantly influence their performance.84,102,103,164,214 ZVI materials are particularly highly efficient in degrading metal contaminants present soil contaminated soil.236 Coincidently, Alhadidi et al., evaluated the efficiency of ZVI materials in the remediation of metal based contaminated soil, the results revealed 80% removal efficiency for various metals categorized by the Nieboer–Richardson method.237 Similar and better performance ZVI materials in soil remediations is also reported.238
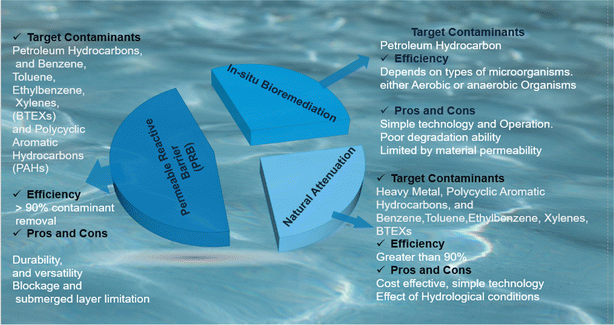 |
| | Fig. 8 Nano and micro-ZVI Materials' applications in groundwater remediation in situ technologies, pros and cons, and their target contaminants. | |
However, despite this promising performance and applications of ZVI materials, it is imperative to assess their impact on soil microorganisms when applied for soil remediations. Interestingly, Saccà et al., (2014) coupled molecular and classical methods to investigate the impact of nano zero valent materials on soil microorganism, from their findings, classical toxicity tests using nematodes (Caenorhabditis elegans) revealed no negative effects of nano ZVI on microorganisms. However, molecular analysis of soil microbial communities showed significant changes in gene expression associated with nano ZVI materials exposure.239 Interestingly, following their conclusion, these gene expressions changes of soil microorganisms varied depending on soil characteristics, hence highlighting the need for case-by-case evaluation. Generally, potential environmental risks associated with iron oxide formation and unintended contaminant mobilization necessitate a thorough evaluation before large-scale application.240,241
3.2 Water treatment
Contemporary challenges posed by emerging contaminants in water sources presents a new frontier for nano and micro zero valent iron materials. ZVI materials have showed potentials for removing pharmaceutical waste,71,176,242 and pesticides91,103 washed and transported to water bodies by rain or through municipal effluents.242,243 Surface modification strategies can further enhance their selectivity and efficiency for targeted contaminant removal.71,244,245 Liu et al., investigated the effectiveness of zero-valent iron (ZVI) for removing phycocyanin from water. The results revealed more than 80% removal efficiency in acidic environments, further analysis proposed two reaction mechanisms viz. adsorption onto the ZVI surface and coagulation by iron ions released from ZVI materials.100 Like ZVI materials' applications in soil remediations, significant challenges remain, particularly in ZVI materials' separation and re-generation after water treatment operations, hence, limiting their widespread applications.
3.3 Industrial catalysts
Nano and micro-ZVI materials as catalysts for hydrogen production from water splitting or hydrolysis reactions is a sustainable route towards clean energy generation.246 ZVI materials offer benefits in terms of their cost and material abundance compared to conventional catalytic materials.247 Chen et al., explored the potential of nano and micro-ZVI materials for hydrogen production, their results revealed nano-ZVI to exhibits significantly higher iron-normalized hydrogen production rates of 15.2–58.3 mgH2 kgFe−1 h−1 compared to their Micro-ZVI counterpart. Interestingly, doping nano-ZVI with 1% noble metals viz. Pd, Ni, Cu, or Ag was observed to further accelerates hydrogen production from 2–39 times, with Pd–Fe0 achieving optimal rate of 1490 mgH2 kgFe−1 h−1. Nano ZVI materials system is cost effective and operates under ambient conditions with superior volumetric hydrogen storage density (279 kgH2 m−3) compared to conventional catalytic materials.247 However, their efficiency and long-term stability require significant improvement,247,248 for crucial economic viability, developing efficient regeneration and separation strategies of nano- and micro- ZVI materials after use in industrial processes is highly essential.
3.4 Industrial wastewater effluents
Nano and micro materials are emerging materials in degrading organic and inorganic obnoxious pollutants present in pharmaceuticals,71 and other industrial wastewater streams for cleaner engineering processes.249–252 Zhang et al., evaluated the suitability of ZVI materials in the remediation of Swine wastewater (SWW), high removal efficiency was observed at acidic pH (3) and in the presence of dissolved oxygen.253 However, similar studies on the engineering applications of these novel materials recommended optimizing materials' particle size and surface properties for efficient pollutants degradation and improved catalytic selectivity.55,254,255
3.5 Organic pollutant removal
Nano and micro-ZVI materials degrade various organic pollutants present in industrial wastewater streams, such as dyes, pharmaceuticals, and pesticides (see Table 2). These pollutants can be harmful to ecosystems if released untreated.108,256 The degradation reaction mechanism involves nano and micro-ZVI materials acting as a reducing agent, and hence breaking down the complex organic molecules into simpler and less harmful compounds.241
De-sulfurization: Although there is barely any study with direct application of nano and micro-ZVI materials for removing sulphur impurities from fuels, potential utilization of these materials when coupled with other materials could contribute to cleaner fuel combustion.
3.6 Potential engineering applications
3.6.1 Battery technology. Future research should explore the potential of utilizing nano and micro-ZVI materials as anodic material in lithium-ion batteries considering their high physicochemical and theoretical capacity for lithium storage. Challenges to overcome could include overcoming volume changes during charge/discharge cycles and improving electrode stability.
3.6.2 Sensors. The distinguished reactivity and surface properties of nano and micro-ZVI materials make them potential materials for developing sensitive and selective sensors for wide process engineering applications.
3.6.3 Biomedical applications. Emerging research should investigate the potential of nano and micro-ZVI materials in biomedical engineering applications like biocatalysis and targeted drug delivery considering their biocompatible modifications and efficacy in pharmaceuticals. However, comprehensive toxicity assessment is highly essential for safe biomedical utilization.
3.6.4 Construction materials. Nano and micro-ZVI materials can be incorporated into building materials to enhance their fire resistivity or conductivity. However, the long-term stability, and durability, of such materials need further investigation.
3.7 Environmental considerations
Despite the promising and versed applications of these emerging materials significant efforts need to be placed in evaluating their environmental implications and detrimental effect on both human, animal, and aquatic lives. Since chemical methods for synthesising nano and micro-ZVI materials involve the use of various reagents, such as iron chloride,70,187 sodium borohydride, and iron sulphate,257 as well as gases like; argon,258 nitrogen,259 and hydrogen.260 These reagents have reported environmental impacts due to their production and can result in waste, wastewater, and emissions containing these compounds. Some of these compounds, such as sodium borohydride, isooctane, polyvinylpyrrolidone, cetylpyridinium chloride, and sulphur pose risks to human health and the environment.261 Water consumption in most methods is low, but the ultrasonic wave method has a high-water consumption rate. Wastewater generation is directly related to water consumption, with methods like chemical reduction with sodium borohydride, micro-emulsions, and ultrasonic waves generating more wastewater byproducts. The wastewater contains chemical components that can be hazardous, requiring proper treatment before reuse or disposal.262 Energy consumption is another important factor in process engineering applications, with gas reduction using hydrogen gas and the ultrasonic wave method having high energy consumption (refer to Section 2). High energy consumption contributes to environmental impacts, including resource use, gas emissions, and climate change. The composition of the energy matrix also affects the environmental impacts, with countries relying more on renewable energy sources experiencing lower impacts. Solid waste generation is primarily associated with filtration processes, where the filter used may contain chemical compounds from the process reagents. Proper treatment, such as incineration, is necessary before final disposal.
4 Conclusions and future perspectives
In conclusion, nano and micro-ZVI materials, and synthesis methods coupled with their multifaceted applications are concisely reviewed herein. The crucial points are summarized as follows.
4.1 Synthesis strategies
A diverse range of nano- and micro-ZVI materials synthesis methods were reviewed, each with distinct advantages and limitations. Selecting the optimal method depends on factors like the targeted production scale, economic feasibility, technological requirements, and desired nano- and micro-ZVI material characteristics. Future research should prioritize optimizing existing methods, exploring eco-friendly approaches, and evaluating their industrial applicability. Table 2 provides an insight into recent methodologies of selected synthesis methods with their recorded efficiencies and the properties of the synthesized materials to serve as a reference in the selection process.
4.2 Tuning synthesis methods for specific engineering applications
Selection of the right synthesis method for a given nano and micro-ZVI materials' properties requirement and particular engineering application is highly crucial for ensuring the efficacy of the fabricated nano and micro-ZVI materials in process operations. This review found that by tuning nano and micro-ZVI materials properties such as size and morphological structures researchers can enhance material performance, selectivity, and catalytic activity. The smaller the particle size, the higher the surface area, and the greater the contaminant's adsorption. Other findings revealed that modification of material surface properties enhances the stability of nano and micro ZVI materials, hence improving their prospects in a plethora of engineering applications. pH, concentration, and temperature, among other factors, are found to proportionately influence overall ZVI materials performance (X. Sun et al., 2015).
4.3 Environmental remediation
Nano and micro-ZVI materials are unveiled to act as highly effective materials for soil, groundwater, and wastewater remediations, effectively degrading organic pollutants, heavy metals, and other emerging contaminants (see Table 3) through reductive degradation and adsorption processes.
4.4 Challenges
Despite the invaluable potential of nano and micro-ZVI materials, contemporary challenges persist in their utilizations in many areas. These challenges revolve around three key factors viz. aggregation, selectivity, and potential environmental impacts.
Improved Stability and applicability: investigating sustainable strategies will help in mitigating material aggregation and improve their stability for diverse environmental utilization, particularly via modification of morphological structure or encapsulation with other supportive materials. Additionally, synthesizing stable nano and micro-ZVI materials with improved multifunctional surface properties can widen the prospective applications of these novel materials beyond environmental remediations such as energy storage, drug delivery, and sensing materials in electronics devices as well as magnetics materials thereby extending and utilizing the full potential of these emerging materials.
Combined technologies: integrating nano and micro-ZVI technologies with other engineering techniques, such as electrocatalysis or bioremediation can help foster the establishment of synergy in developing a robust environmental remediation system.
Life cycle assessment: performing comprehensive life cycle assessments to gauge the environmental impact and sustainability of nano and micro-ZVI materials production, utilization, and environmental limitations is highly essential for sustainable industrial applications.
Regulatory frameworks: like all other engineering materials, determining clear standards and protocols for the safe and responsible utilization of these materials to warrant environmental protection against waste generation, waste disposal, and community health is crucial.
Therefore, by concentrating on these outlined fundamental features, nano and micro-ZVI materials and their synthesis technologies would uncover a sustainable and transformative means of environmental remediation systems coupled with resource recovery, and the development of advanced materials.
Conflicts of interest
There are no conflicts to declare.
Acknowledgements
This work was funded by Universiti Malaysia Sarawak under the Vice Chancellor Higher Impact Research Scheme, Grant Number (UNI/FO2/VC-HIRG/85508/P10-03).
References
- Y. Wang, H. Wang and H. Chen, Understanding role and mechanisms of zero-valent iron (ZVI) in activated sludge responses under glyphosate exposure, J. Water Proc. Eng., 2023, 55(August), 104210, DOI:10.1016/j.jwpe.2023.104210
 .
. - Y. Arbid, M. Usman, N. T. Luong, B. Mathon, B. Cedat and J. F. Boily, et al., Use of iron-bearing waste materials in laundry wastewater treatment, J. Water Proc. Eng., 2024, 57(December 2023), 104717, DOI:10.1016/j.jwpe.2023.104717
 .
. - Y. Hu, K. Ke, H. Sun, Z. Wang, X. Zhang and W. Shen, et al., Coffee grounds modified zero-valent iron for efficient heavy metal removal, J. Water Proc. Eng., 2023, 56(October), 104397, DOI:10.1016/j.jwpe.2023.104397
 .
. - W. Xu, D. Huang, L. Du, G. Wang, Y. Chen and R. Xiao, Recent advances on the incorporation of N into zero-valent and atomic iron for contaminants transformation, Coord. Chem. Rev., 2024, 505, 215671, DOI:10.1016/j.ccr.2024.215671
 .
. - F. Zhang, C. Chen, J. Zhou, C. Zheng, Q. Zhu and F. Peng, et al., Enhancing O2 resistance during storage and 2, 4-dichlorophenol degradation reaction of nano zero-valent iron by in-situ formation on the partially delignified stalk, Sep. Purif. Technol., 2024, 332(October 2023), 125818, DOI:10.1016/j.seppur.2023.125818
 .
. - D. Ding, Y. Zhao, Y. Chen, C. Xu, X. Fan and Y. Tu, et al., Recent advances in bimetallic nanoscale zero-valent iron composite for water decontamination: Synthesis, modification and mechanisms, J. Environ. Manag., 2024, 353(December 2023), 120187, DOI:10.1016/j.jenvman.2024.120187
 .
. - X. Yang, F. Ming, J. Wang and L. Xu, Amino acids modified nanoscale zero-valent iron: Density functional theory calculations, experimental synthesis and application in the Fenton-like degradation of organic solvents, J. Environ. Sci., 2024, 135, 296–309, DOI:10.1016/j.jes.2022.11.013
 .
. - Z. Zhao, Y. Hou, C. Wang, Z. Man, G. Sun and J. Shang, et al., Sulfite activated by in situ preparation of zero-valent iron agar composite membrane for the removal of metronidazole: Synergistic effect of radical and nonradical pathways, Sep. Purif. Technol., 2024, 334(December 2023), 125964, DOI:10.1016/j.seppur.2023.125964
 .
. - Y. Wu, C. Y. Guan, N. Griswold, L. yuan Hou, X. Fang and A. Hu, et al., Zero-valent iron-based technologies for removal of heavy metal(loid)s and organic pollutants from the aquatic environment: Recent advances and perspectives, J. Clean. Prod., 2020, 277, 123478, DOI:10.1016/j.jclepro.2020.123478
 .
. - G. Kozma, A. Rónavári, Z. Kónya and Á. Kukovecz, Environmentally Benign Synthesis Methods of Zero-Valent Iron Nanoparticles, ACS Sustain. Chem. Eng., 2016, 4(1), 291–297 CrossRef
 .
. - P. A. R. Puthukkara, T. S. Jose and S. D. lal, Plant mediated synthesis of zero valent iron nanoparticles and its application in water treatment, J. Chem. Environ. Eng., 2021, 9(1), 104569, DOI:10.1016/j.jece.2020.104569
 .
. - G. Zhang, F. Yang, W. Yang and Y. Li, N-doped carbon nanotube encapsulated nZVI as a high-performance bifunctional catalyst for oxidative desulfurization, Particuology, 2023, 81, 109–118, DOI:10.1016/j.partic.2023.01.002
 .
. - Q. L. Liang, X. Y. Song, V. Smolkin, N. A. Krivolutskaya, S. Y. Yu and L. M. Chen, Mineralogy and geochemistry of magnetite in the Zhdanov Ni-Cu-(PGE) deposit in the Pechenga ore field, Russia: Implications for the formation of magmatic sulfide ores, J. Asian Earth Sci., 2024, 259(August 2023), 105903, DOI:10.1016/j.jseaes.2023.105903
 .
. - X. Gao, L. Zhang, M. Sun, Y. Xiao and J. Su, Fast zero-order hydro-cracking reaction of X-3B over crystal Al-Fe alloys: Effect of electrochemical corrosion behaviors, Mater. Des., 2016, 109, 570–579, DOI:10.1016/j.matdes.2016.07.087
 .
. - M. Boisvert, D. Christopherson, P. Beaulieu and G. L'Espérance, Treatment of ferrous melts for the improvement of the sphericity of water atomized powders, Mater. Des., 2017, 116, 644–655, DOI:10.1016/j.matdes.2016.12.059
 .
. - A. S. Astuti, M. Muldarisnur and U. S. R. A. Zulhadjri, Enhancement in photoluminescence performance of carbon-based Fe3O4@ZnO–C nanocomposites, Vacuum, 2023, 211(December 2022), 111935, DOI:10.1016/j.vacuum.2023.111935
 .
. - C. Xu, X. Zhao, J. Marten Huizenga, J. Wei, Y. Hu and Z. Zhao, Garnet U-Pb dating and magnetite geochemistry: Constraints on the origin of Fe mineralization in the Huogeqi polymetallic deposit, Northern China, Ore Geol. Rev., 2023, 163(May), 105747, DOI:10.1016/j.oregeorev.2023.105747
 .
. - S. Vyhnáleková, M. B. Miglierini, J. Dekan, M. Bujdoš, E. Dobročka and B. Farkas, et al., Encapsulating magnetite nanopowder with fungal biomass: Investigating effects on chemical and mineralogical stability, Sep. Purif. Technol., 2024, 333, 125899 CrossRef
 .
. - R. A. Harris, Phase transformation of magnetite and goethite nanoparticles controlled by pH : Experimental and simulation study of cuboid magnetic nanoparticles prepared with NaOH, Solid State Sci., 2024, 148(August 2023), 107416, DOI:10.1016/j.solidstatesciences.2023.107416
 .
. - T. Madrakian, A. Afkhami, M. A. Zolfigol, M. Ahmadi and N. Koukabi, Application of modified silica coated magnetite nanoparticles for removal of iodine from water samples, Nano-Micro Lett., 2012, 4(1), 57–63 CrossRef
 .
. - N. A. Rosin, J. A. M. Demattê, R. R. Poppiel, N. E. Q. Silvero, H. S. Rodriguez-Albarracin and J. T. F. Rosas, et al., Mapping Brazilian soil mineralogy using proximal and remote sensing data, Geoderma, 2023, 432(January), 116413, DOI:10.1016/j.geoderma.2023.116413
 .
. - I. A. Okewale and H. Grobler, Assessment of heavy metals in tailings and their implications on human health, Geosyst. Geoenviron., 2023, 2(4), 100203, DOI:10.1016/j.geogeo.2023.100203
 .
. - E. Tomasini, I. Costantini, V. Careaga, C. R. Landa, K. Castro and J. M. Madariaga, et al., Identification of pigments and binders of a 17th century mural painting (Bolivia). New report on pigments associated with Andean minerals, J. Cult. Herit., 2023, 62, 206–216, DOI:10.1016/j.culher.2023.05.030
 .
. - M. Pilavtepe, L. Dela Vernhe, A. Steudel, R. Schuhmann, N. Willenbacher and K. Emmerich, Formation of arrested states in natural di-and trioctahedral smectite dispersions compared to those in synthetic hectorite – A macro-and microrheological study, Clays Clay Miner., 2018, 66(4), 339–352 CrossRef
 .
. - M. Mn, F. Ti, S. Kumar, R. Prakash, H. K. Choi and B. H. Koo, et al., Influence of Ti 4 + doping on hyperfine field parameters of, J. Cent. South Univ., 2010, 4, 1139–1143, DOI:10.1007/s11771-010-0609-y
 .
. - R. Castroviejo, A Practical Guide to Ore Microscopy—Volume 1, Springer, 2023, vol. 1, pp. 419–424, DOI:10.1007/978-3-031-12654-3
 .
. - P. Li, X. Zhu, Y. Han and W. Li, Dehydroxylation of Limonite Ore for Magnetization Roasting: Phase Transformation and Kinetics Analysis, Min. Metall. Explor., 2023, 40(6), 2477–2486, DOI:10.1007/s42461-023-00870-7
 .
. - A. S. Vusikhis, L. I. Leont’ev and D. Z. Kudinov, Use of Bakal Deposit Siderite Ore in Iron and Steel Production, Metallurgist, 2017, 61(1–2), 116–120 CrossRef
 .
. - A. S. Vusikhis, L. I. Leont’ev, D. Z. Kudinov and V. S. Gulyakov, Metallization of siderite ore in reducing roasting, Russ. Metall., 2016, 2016(5), 404–408 CrossRef
 .
. - Q. Zhao, H. Yang, L. Tong, Z. Jin and R. Jin, New Insights into Improving the Physicochemical Properties and Flotation Behavior of Pyrite Interfaces from Cyanide Tailings, J. Sustain. Metall., 2023, 9(3), 1155–1167, DOI:10.1007/s40831-023-00713-1
 .
. - Q. Zhao, H. Yang, L. Tong and R. Jin, Pollution Characteristics of Pyrite Surface
in Cyanide Tailings by PCA-Assisted ToF-SIMS and Their Correlation with the Contact Angle, JOM, 2023, 76(1), 547–557, DOI:10.1007/s11837-023-06143-4
 .
. - M. H. Sadeghi and E. M. Nasr, Variable Surface Reactivity Model for Leaching Kinetics of Activated Ilmenite Particles, JOM, 2023, 76(1), 532–539, DOI:10.1007/s11837-023-06219-1
 .
. - F. Tornos, J. M. Hanchar, M. Steele-MacInnis, E. Crespo, V. S. Kamenetsky and C. Casquet, Formation of magnetite-(apatite) systems by crystallizing ultrabasic iron-rich melts and slag separation, Miner. Deposita, 2023, 59, 189–225, DOI:10.1007/s00126-023-01203-w
 .
. - J. Lee, B. C. Kim, H. J. Cho and C. Lee, Environmental Applications of Nanomaterials, Nanobiomaterials, 2013, 385–415 Search PubMed
 .
. - H. Alijani and Z. Shariatinia, Effective aqueous arsenic removal using zero valent iron doped MWCNT synthesized by in situ CVD method using natural α-Fe2O3as a precursor, Chemosphere, 2017, 171, 502–511, DOI:10.1016/j.chemosphere.2016.12.106
 .
. - L. X. Ou, M. Y. Liu, L. Y. Zhu, D. W. Zhang and H. L. Lu, Recent Progress on Flexible Room-Temperature Gas Sensors Based on Metal Oxide Semiconductor, Nano-Micro Lett., 2022, 14, 1–42, DOI:10.1007/s40820-022-00956-9
 .
. - S. K. Kuila, R. Chatterjee and D. Ghosh, Kinetics of hydrogen reduction of magnetite ore fines, Int. J. Hydrogen Energy, 2016, 41(22), 9256–9266, DOI:10.1016/j.ijhydene.2016.04.075
 .
. - Y. Wu, L. Xu, J. Shi, J. Cui, S. Han and C. Xia, et al., Cobalt ferrite/cellulose membrane inserted catalytic syringe filter for facile in-situ filtration/degradation of emerging organic pollutants in water via activating peroxymonosulfate, Mater. Des., 2022, 220, 110817, DOI:10.1016/j.matdes.2022.110817
 .
. - X. Zhang, Z. Yao, Y. Gao, S. Yan, X. Peng and W. Shen, Efficient Cu(II) removal with boronated zero-valent iron via inhibition of oxygen adsorption and enhanced electron transfer, Appl. Surf. Sci., 2024, 654(January), 159466, DOI:10.1016/j.apsusc.2024.159466
 .
. - P. Wang, F. Fu and T. Liu, A review of the new multifunctional nano zero-valent iron composites for wastewater treatment: Emergence, preparation, optimization and mechanism, Chemosphere, 2021, 285(July), 131435, DOI:10.1016/j.chemosphere.2021.131435
 .
. - L. Wang, Y. Li, R. Zou, R. Sun, H. Tian and G. Luo, et al., Insight into the influence of environmental factors on selenite removal from desulfurization wastewater by NZVI@AC: An experimental and theoretical study, J. Water Proc. Eng., 2024, 57(October 2023), 104674, DOI:10.1016/j.jwpe.2023.104674
 .
. - J. Zeng, J. Liu, W. Su, J. Tang, Z. Luo and F. Tang, et al., Persulfate activation by sulfide-modified nanoscale zero-valent iron for metronidazole degradation: Mechanism, major radicals and toxicity assessment, J. Water Proc. Eng., 2023, 53(February), 103733, DOI:10.1016/j.jwpe.2023.103733
 .
. - Y. Lu, M. Feng and Y. Wang, Enhancing the heterogeneous electro-Fenton degradation of methylene blue using sludge-derived biochar-loaded nano zero-valent iron, J. Water Proc. Eng., 2024, 59(January), 104980, DOI:10.1016/j.jwpe.2024.104980
 .
. - J. Phanthasri, D. Y. S. Yan, K. Wantala, R. Khunphonoi, P. Khamdahsag and V. Tanboonchuy, Selenate removal via continuous fixed-bed column with nanoscale zero-valent iron supported on bentonite-zeolite pellets, J. Water Proc. Eng., 2023, 53(May), 103843, DOI:10.1016/j.jwpe.2023.103843
 .
. - X. Wang, P. Liu, M. Fu, J. Ma and P. Ning, Novel sequential process for enhanced dye synergistic degradation based on nano zero-valent iron and potassium permanganate, Chemosphere, 2016, 155, 39–47, DOI:10.1016/j.chemosphere.2016.04.022
 .
. - G. Sheng, J. Hu, H. Li, J. Li and Y. Huang, Enhanced sequestration of Cr(VI) by nanoscale zero-valent iron supported on layered double hydroxide by batch and XAFS study, Chemosphere, 2016, 148, 227–232, DOI:10.1016/j.chemosphere.2016.01.035
 .
. - L. Xu and Y. Huang, A simple and novel method to enhance As (V) removal by zero valent iron and activated iron media through air injection at intervals, Chemosphere, 2019, 222, 415–421, DOI:10.1016/j.chemosphere.2019.01.183
 .
. - M. Zhang, Y. Dong, S. Gao, P. Cai and J. Dong, Effective stabilization and distribution of emulsified nanoscale zero-valent iron by xanthan for enhanced nitrobenzene removal, Chemosphere, 2019, 223, 375–382, DOI:10.1016/j.chemosphere.2019.02.099
 .
. - S. Feng, B. Zhang, J. Wang and J. Jeanne, Journal of Water Process Engineering Zero-valent iron in phosphate removal : Unraveling the role of particle size and dissolved oxygen, J. Water Proc. Eng., 2024, 60(March), 105180, DOI:10.1016/j.jwpe.2024.105180
 .
. - J. Zhang, X. Zhao, W. Wang, Z. Song, Y. Mao and J. Sun, et al., Removal of p-nitrophenol by double-modified nanoscale zero-valent iron with biochar and sulfide: Key factors and mechanisms, J. Water Proc. Eng., 2023, 51(December 2022), 103398, DOI:10.1016/j.jwpe.2022.103398
 .
. - Q. Jing, W. You, S. Qiao, Y. Ma and Z. Ren, Comprehensive understanding of adsorption and reduction on 2,4-DCP and Cr(VI) removal process by NZVI-rGO: Performance and mechanism, J. Water Proc. Eng., 2023, 51(December 2022), 103413, DOI:10.1016/j.jwpe.2022.103413
 .
. - W. Ma, Z. Cao, X. Shi, S. H. Deng, H. Dai and B. Xie, Synergistic effects of zero-valent iron-carbon galvanic cells on the microalgal-bacterial symbiosis system for efficient anaerobic digestion effluent treatment, J. Water Proc. Eng., 2023, 56(May), 104296, DOI:10.1016/j.jwpe.2023.104296
 .
. - X. Wang, P. Huang, P. Zhang, C. Wang, F. He and H. Sun, Synthesis of stabilized zero-valent iron particles and role investigation of humic acid-Fex+ shell in Fenton-like reactions and surface stability control, J. Hazard Mater., 2024, 465(October 2023), 133296, DOI:10.1016/j.jhazmat.2023.133296
 .
. - Z. Peng, C. Xiong, W. Wang, F. Tan, X. Wang and X. Qiao, et al., Hydrophobic modification of nanoscale zero-valent iron with excellent stability and floatability for efficient removal of floating oil on water, Chemosphere, 2018, 201, 110–118 CrossRef PubMed
 .
. - B. Chen, N. Lv, W. Xu, L. Gong, T. Sun and L. Liang, et al., Transport of nanoscale zero-valent iron in saturated porous media: Effects of grain size, surface metal oxides, and sulfidation, Chemosphere, 2023, 313(October 2022), 137512, DOI:10.1016/j.chemosphere.2022.137512
 .
. - F. Fu, D. D. Dionysiou and H. Liu, The use of zero-valent iron for groundwater remediation and wastewater treatment: A review, J. Hazard Mater., 2014, 267, 194–205, DOI:10.1016/j.jhazmat.2013.12.062
 .
. - W. Xue, D. Huang, G. Zeng, J. Wan, M. Cheng and C. Zhang, et al., Performance and toxicity assessment of nanoscale zero valent iron particles in the remediation of contaminated soil: A review, Chemosphere, 2018, 210, 1145–1156, DOI:10.1016/j.chemosphere.2018.07.118
 .
. - S. Patra, A. Pranudta, N. Chanlek, T. T. Nguyen, N. H. Nhat and M. M. El-Moselhy, et al., Denitrification of nitrate in regeneration waste brine using hybrid cation exchanger supported nanoscale zero-valent iron with/without palladium nanoparticles, Chemosphere, 2023, 310(October 2022), 136851, DOI:10.1016/j.chemosphere.2022.136851
 .
. - C. Kim, Y. P. Chin, J. Y. Ahn, M. Wei-Haas, B. McAdams and I. Hwang, Reciprocal influences of dissolved organic matter and nanosized zero-valent iron in aqueous media, Chemosphere, 2018, 193, 936–942, DOI:10.1016/j.chemosphere.2017.11.097
 .
. - J. Ji, S. Xu, Z. Ma and Y. Mou, Trivalent antimony removal using carbonaceous nanomaterial loaded with zero-valent bimetal (iron/copper) and their effect on seed growth, Chemosphere, 2022, 296(January), 134047, DOI:10.1016/j.chemosphere.2022.134047
 .
. - K. P. Kowalski and E. G. Søgaard, Implementation of zero-valent iron (ZVI) into drinking water supply - Role of the ZVI
and biological processes, Chemosphere, 2014, 117(1), 108–114, DOI:10.1016/j.chemosphere.2014.05.088
 .
. - W. Zhou, D. Huang, S. Chen, L. Du, G. Wang and R. Li, et al., Modified nano zero-valent iron reduce toxicity of polystyrene microplastics to ryegrass (Lolium Perenne L.), Chemosphere, 2023, 337(June), 139152, DOI:10.1016/j.chemosphere.2023.139152
 .
. - E. Petala, M. Baikousi, M. A. Karakassides, G. Zoppellaro, J. Filip and J. Tuček, et al., Synthesis, physical properties and application of the zero-valent iron/titanium dioxide heterocomposite having high activity for the sustainable photocatalytic removal of hexavalent chromium in water, Phys. Chem. Chem. Phys., 2016, 18(15), 10637–10646 RSC
 .
. - Q. Zhou, K. Zhao, Y. Wu, S. Li, J. Guo and B. Zhou, et al., Rapid magnetic enrichment and sensitive detection of Sudan pollutants with nanoscale zero valent iron-based nanomaterials in combination with liquid chromatography-ultraviolet detector, Chemosphere, 2021, 281(February), 130900, DOI:10.1016/j.chemosphere.2021.130900
 .
. - C. Kim, T. T. Thao, J. H. Kim and I. Hwang, Effects of the formation of reactive chlorine species on oxidation process using persulfate and nano zero-valent iron, Chemosphere, 2020, 250, 126266, DOI:10.1016/j.chemosphere.2020.126266
 .
. - Y. fan Su, C. Y. Hsu and Y. hsin Shih, Effects of various ions on the dechlorination kinetics of hexachlorobenzene by nanoscale zero-valent iron, Chemosphere, 2012, 88(11), 1346–1352, DOI:10.1016/j.chemosphere.2012.05.036
 .
. - M. Raji, S. A. Mirbagheri, F. Ye and J. Dutta, Nano zero-valent iron on activated carbon cloth support as Fenton-like catalyst for efficient color and COD removal from melanoidin wastewater, Chemosphere, 2021, 263, 127945, DOI:10.1016/j.chemosphere.2020.127945
 .
. - X. Yang, G. Yu, L. Xu and J. Wang, Degradation of the mixed organic solvents of tributyl phosphate and n-dodecane by heterogeneous Fenton-like oxidation using nanoscale zero-valent iron as the catalyst, Chemosphere, 2022, 292(October 2021), 133449, DOI:10.1016/j.chemosphere.2021.133449
 .
. - J. Azizi and R. Davarnejad, Electro-Fenton technique associated with core-shell Fe@Fe2O3 nanoparticles-bentonite catalyst for pre-treating a super-pollutant industrial wastewater, J. Water Proc. Eng., 2023, 51(November 2022), 103385, DOI:10.1016/j.jwpe.2022.103385
 .
. - A. O. Dada, F. A. Adekola, E. O. Odebunmi, F. E. Dada, O. S. Bello and A. S. Ogunlaja, Bottom-up approach synthesis of core-shell nanoscale zerovalent iron (CS-nZVI): Physicochemical and spectroscopic characterization with Cu(II) ions adsorption application, MethodsX, 2020, 7, 100976, DOI:10.1016/j.mex.2020.100976
 .
. - S. Krithika Shree, S. K. R. Namasivayam and A. Pandian, Sustainable developmental measures for the treatment of pharmaceutical industry effluent using nano zero valent iron technology (nZVI) – A review, J. Water Proc. Eng., 2023, 56(August), 104390, DOI:10.1016/j.jwpe.2023.104390
 .
. - L. Di, X. Chen, J. Lu, Y. Zhou and Y. Zhou, Removal of heavy metals in water using nano zero-valent iron composites: A review, J. Water Proc. Eng., 2023, 53(May), 103913, DOI:10.1016/j.jwpe.2023.103913
 .
. - S. H. Ammar, A. Ibrahim Elaibi and S. Mohammed I, Core/shell Fe3O4@Al2O3-PMo magnetic nanocatalyst for photocatalytic degradation of organic pollutants in an internal loop airlift reactor, J. Water Proc. Eng., 2020, 37(June), 101240, DOI:10.1016/j.jwpe.2020.101240
 .
. - N. S. Al-Radadi, Microwave assisted green synthesis of Fe@Au core–shell NPs magnetic to enhance olive oil efficiency on eradication of helicobacter pylori (life preserver), Arab. J. Chem., 2022, 15(5), 103685, DOI:10.1016/j.arabjc.2022.103685
 .
. - X. Guan, Y. Sun, H. Qin, J. Li, I. M. C. Lo and D. He, et al., The limitations of applying zero-valent iron technology in contaminants sequestration and the corresponding countermeasures: The development in zero-valent iron technology in the last two decades (1994-2014), Water Res., 2015, 75, 224–248 CrossRef PubMed
 .
. - T. Zhou, K. Feng, W. Xiang, Y. Lv, X. Wu and J. Mao, et al., Rapid decomposition of diclofenac in a magnetic field enhanced zero-valent
iron/EDTA Fenton-like system, Chemosphere, 2018, 193, 968–977, DOI:10.1016/j.chemosphere.2017.11.090
 .
. - Y. Zhang, D. Li, L. She, F. Guo, F. Jia and L. Zhang, et al., Ball-milled zero-valent iron with formic acid for effectively removing Cu(II)-EDTA accomplished by EDTA ligands oxidative degradation and Cu(II) removal, J. Hazard Mater., 2024, 465(November 2023), 133009, DOI:10.1016/j.jhazmat.2023.133009
 .
. - X. Q. Li, D. W. Elliott and W. X. Zhang, Zero-valent iron nanoparticles for abatement of environmental pollutants: Materials and engineering aspects, Crit. Rev. Solid State Mater. Sci., 2006, 31(4), 111–122 CrossRef
 .
. - J. Gao, W. Wang, A. J. Rondinone, F. He and L. Liang, Degradation of Trichloroethene with a Novel Ball Milled Fe-C Nanocomposite, J. Hazard Mater., 2015, 300, 443–450, DOI:10.1016/j.jhazmat.2015.07.038
 .
. - D. Xu, S. Zheng, T. Sun, G. Gao, Y. Sun and H. Jia, et al., Simultaneous adsorption and reduction of Cr(VI) on Potamogeton·crispus biochar supported nanoscale zero-valent iron: Electro- and sepctro-chemical mechanism, J. Taiwan Inst. Chem. Eng., 2024, 155(November 2023), 105259, DOI:10.1016/j.jtice.2023.105259
 .
. - A. Shimizu, M. Tokumura, K. Nakajima and Y. Kawase, Phenol removal using zero-valent iron powder in the presence of dissolved oxygen: Roles of decomposition by the Fenton reaction and adsorption/precipitation, J. Hazard Mater., 2012, 201–202, 60–67, DOI:10.1016/j.jhazmat.2011.11.009
 .
. - Y. Mu, H. Q. Yu, J. C. Zheng, S. J. Zhang and G. P. Sheng, Reductive degradation of nitrobenzene in aqueous solution by zero-valent iron, Chemosphere, 2004, 54(7), 789–794 CrossRef PubMed
 .
. - Z. Yang, X. Ma, C. Shan, Z. Fang and B. Pan, Enhanced Nitrobenzene reduction by zero valent iron pretreated with H2O2/HCl, Chemosphere, 2018, 197, 494–501, DOI:10.1016/j.chemosphere.2018.01.068
 .
. - G. Wang, S. Zhang, X. Xu, T. Li, Y. Li and O. Deng, et al., Efficiency of nanoscale zero-valent iron on the enhanced low molecular weight organic acid removal Pb from contaminated soil, Chemosphere, 2014, 117(1), 617–624, DOI:10.1016/j.chemosphere.2014.09.081
 .
. - F. D. Kopinke, S. Sühnholz, A. Georgi and K. Mackenzie, Interaction of zero-valent iron and carbonaceous materials for reduction of DDT, Chemosphere, 2020, 253, 126712, DOI:10.1016/j.chemosphere.2020.126712
 .
. - X. Man, X. an Ning, H. Zou, J. Liang, J. Sun and X. Lu, et al., Removal of polycyclic aromatic hydrocarbons (PAHs) from textile dyeing sludge by ultrasound combined zero-valent iron/EDTA/Air system, Chemosphere, 2018, 191, 839–847, DOI:10.1016/j.chemosphere.2017.10.043
 .
. - J. Fan, Y. Guo, J. Wang and M. Fan, Rapid decolorization of azo dye methyl orange in aqueous solution by nanoscale zerovalent iron particles, J. Hazard. Mater., 2009, 166(2–3), 904–910 CrossRef PubMed
 .
. - M. Yaghoobi, F. Asjadi and M. Sanikhani, A facile one-step green hydrothermal synthesis of paramagnetic Fe3O4 nanoparticles with highly efficient dye removal, J. Taiwan Inst. Chem. Eng., 2023, 144, 104774, DOI:10.1016/j.jtice.2023.104774
 .
. - M. Suzuki, Y. Suzuki, K. Uzuka and Y. Kawase, Biological treatment of non-biodegradable azo-dye enhanced by zero-valent iron (ZVI) pre-treatment, Chemosphere, 2020, 259, 127470, DOI:10.1016/j.chemosphere.2020.127470
 .
. - M. Vogel, F. D. Kopinke and K. Mackenzie, Acceleration of microiron-based dechlorination in water by contact with fibrous activated carbon, Sci. Total Environ., 2019, 660, 1274–1282, DOI:10.1016/j.scitotenv.2019.01.070
 .
. - A. S. Fjordbøge, A. Baun, T. Vastrup and P. Kjeldsen, Zero valent iron reduces toxicity and concentrations of organophosphate pesticides in contaminated groundwater, Chemosphere, 2013, 90(2), 627–633 CrossRef PubMed
 .
. - Z. Wu, Y. Tang, X. Yuan and Z. Qiang, Reduction of bromate by zero valent iron (ZVI) enhances formation of brominated disinfection by-products during chlorination, Chemosphere, 2021, 268, 129340, DOI:10.1016/j.chemosphere.2020.129340
 .
. - Y. X. Song, S. Chen, N. You, H. T. Fan and L. N. Sun, Nanocomposites of zero-valent Iron@Activated carbon derived from corn stalk for adsorptive removal of tetracycline antibiotics, Chemosphere, 2020, 255, 126917, DOI:10.1016/j.chemosphere.2020.126917
 .
. - Y. Rashtbari, F. Sher, S. Afshin, A. Hamzezadeh bahrami, S. Ahmadi and O. Azhar, et al., Green synthesis of zero-valent iron nanoparticles and loading effect on activated carbon for furfural adsorption, Chemosphere, 2022, 287(P1), 132114, DOI:10.1016/j.chemosphere.2021.132114
 .
. - K. P. Sedlazeck, D. Vollprecht, P. Müller, R. Mischitz, J. Gill and W. Trois, et al., Decomposition of dissolved organic contaminants by combining a boron-doped diamond electrode, zero-valent iron and ultraviolet radiation, Chemosphere, 2019, 217, 897–904, DOI:10.1016/j.chemosphere.2018.11.043
 .
. - Y. Song, Y. Zeng, J. Liao, J. Chen and Q. Du, Efficient removal of sulfamethoxazole by resin-supported zero-valent iron composites with tunable structure: Performance, mechanisms, and degradation pathways, Chemosphere, 2021, 269, 128684, DOI:10.1016/j.chemosphere.2020.128684
 .
. - Y. J. Shih, K. F. Hsia, C. W. Chen, C. F. Chen and C. Di Dong, Characteristics of trichloroethene (TCE) dechlorination in seawater over a granulated zero-valent iron, Chemosphere, 2019, 216, 40–47, DOI:10.1016/j.chemosphere.2018.10.059
 .
. - T. Zhang, Y. Zhao, S. Kang, H. Bai, W. Gu and D. Fang, et al., Mechanical activation of zero-valent iron (ZVI) in the presence of CaCO3: Improved reactivity of ZVI for enhancing As(III) removal from water, J. Clean. Prod., 2021, 286, 124926, DOI:10.1016/j.jclepro.2020.124926
 .
. - R. Cheng, C. Cheng, G. hua Liu, X. Zheng, G. Li and J. Li, Removing pentachlorophenol from water using a nanoscale zero-valent iron/H2O2 system, Chemosphere, 2015, 141, 138–143, DOI:10.1016/j.chemosphere.2015.06.087
 .
. - C. Liu, D. Chen, R. Y. yuan and W. Chen, Removal efficiency and mechanism of phycocyanin in water by zero-valent iron, Chemosphere, 2019, 218, 402–411, DOI:10.1016/j.chemosphere.2018.11.101
 .
. - Z. Sun, D. Geng, X. Wu, L. Zhu, J. Wen and L. Wang, et al., Degradation of 3-chlorocarbazole in water by sulfidated zero-valent iron/peroxymonosulfate system: Kinetics, influential factors, degradation products and pathways, Chemosphere, 2022, 296(August 2021), 134016, DOI:10.1016/j.chemosphere.2022.134016
 .
. - X. Wang, Y. Zhang, Y. Zhang and C. Xu, Remediation of Cr(VI)-contaminated soil by sulfidated zero-valent iron: The effect of citric acid as eluant and modifying agent, Chemosphere, 2023, 313(November 2022), 137436, DOI:10.1016/j.chemosphere.2022.137436
 .
. - P. Zhang, D. Song, H. Y. XuejingXu, X. Shang and C. Wang, et al., Sulfidated zero valent iron as a persulfate activator for oxidizing organophosphorus pesticides (OPPs) in aqueous solution and aged contaminated soil columns, Chemosphere, 2021, 281(May), 130760, DOI:10.1016/j.chemosphere.2021.130760
 .
. - N. Liu, Y. Zhang, C. Zheng, C. Tang, J. Guan and Y. Guo, Sulfidated nanoscale zero valent iron for in situ immobilization of hexavalent chromium in soil and response of indigenous microbes, Chemosphere, 2023, 344(October), 140343, DOI:10.1016/j.chemosphere.2023.140343
 .
. - A. M. Nurul, S. Kaneco, T. Kato, H. Katsumata, T. Suzuki and K. Ohta, Removal of thiobencarb in aqueous solution by zero valent iron, Chemosphere, 2008, 70(3), 511–515 CrossRef
 .
. - Y. Wu, X. Fang, X. T. Yang, C. Y. Guan, X. R. Sun and H. Y. Wu, et al., Comparative study on the removal
of organic pollutants by magnetic composite and pre-magnetized zero-valent iron activated persulfate, Chemosphere, 2022, 286(P2), 131722, DOI:10.1016/j.chemosphere.2021.131722
 .
. - Y. Wu, J. Bai, J. Zhu, Z. Li, S. Y. Fan and X. Qing, Unveiling the traits of dry and wet pre-magnetized zero-valent iron-activated peroxymonosulfate: Degradation of oxytetracycline, Chemosphere, 2023, 344(October), 140348, DOI:10.1016/j.chemosphere.2023.140348
 .
. - M. Namakka, M. R. Rahman, K. A. M. B. Said, M. Abdul Mannan and A. M. Patwary, A review of nanoparticle synthesis methods, classifications, applications, and characterization, Environ. Nanotechnol. Monit. Manag., 2023, 20(November), 100900 CAS
 . 10.1016/j.enmm.2023.100900, https://linkinghub.elsevier.com/retrieve/pii/S2215153223001241.
. 10.1016/j.enmm.2023.100900, https://linkinghub.elsevier.com/retrieve/pii/S2215153223001241. - J. Tang, W. Su, J. Liu, F. Tang and X. Yang, Reductive dechlorination of trichloroethene by sulfided microscale zero-valent iron in fresh and saline groundwater: Reactivity, pathways, and selectivity, Chemosphere, 2023, 340(August), 139900, DOI:10.1016/j.chemosphere.2023.139900
 .
. - Z. Chi, S. Ju, X. Liu, F. Sun and Y. Zhu, Graphene oxide supported sulfidated nano zero-valent iron (S-nZVI@GO) for antimony removal: The role of active oxygen species and reaction mechanism, Chemosphere, 2022, 308(August), 136253, DOI:10.1016/j.chemosphere.2022.136253
 .
. - P. Mandade, Introduction, basic principles, mechanism, and challenges of photocatalysis. Handbook of Nanomaterials for Wastewater Treatment: Fundamentals and Scale up Issues, Elsevier Inc., 2021, pp. 137–154, DOI:10.1016/B978-0-12-821496-1.00016-7
 .
. - D. Ribas, M. Cernik, V. Martí and J. A. Benito, Improvements in nanoscale zero-valent iron production by milling through the addition of alumina, J. Nanopart. Res., 2016, 18(7), 181, DOI:10.1007/s11051-016-3490-2
 .
. - S. Li, W. Wang, F. Liang and W. X. Zhang, Heavy metal removal using nanoscale zero-valent iron (nZVI): Theory and application, J. Hazard Mater., 2017, 322, 163–171, DOI:10.1016/j.jhazmat.2016.01.032
 .
. - W. Li, Y. Yang, X. Lin, W. Yin, Z. Fang and P. Li, et al., Effective immobilization of Cd(II) in soil by biotic zero-valent iron and coexisting sulfate, Chemosphere, 2023, 310(September 2022), 136915, DOI:10.1016/j.chemosphere.2022.136915
 .
. - R. Fu, Y. Yang, Z. Xu, X. Zhang, X. Guo and D. Bi, The removal of chromium (VI) and lead (II) from groundwater using sepiolite-supported nanoscale zero-valent iron (S-NZVI), Chemosphere, 2015, 138, 726–734, DOI:10.1016/j.chemosphere.2015.07.051
 .
. - D. Jiang, X. Hu, X. Jin, A. Ma and D. Yin, Oxidized nanoscale zero-valent iron changed the bioaccumulation and distribution of chromium in zebrafish, Chemosphere, 2021, 263, 128001, DOI:10.1016/j.chemosphere.2020.128001
 .
. - R. Zhang, R. Napolano, B. Xi, A. M. Salazar, Q. Shi and Y. Zhao, et al., Mechanistic insights into Cr(VI) removal by a combination of zero-valent iron and pyrite, Chemosphere, 2023, 330(February), 138693, DOI:10.1016/j.chemosphere.2023.138693
 .
. - N. Zhou, K. Gong, Q. Hu, X. Cheng, J. Zhou and M. Dong, et al., Optimizing nanocarbon shell in zero-valent iron nanoparticles for improved electron utilization in Cr(VI) reduction, Chemosphere, 2020, 242, 125235, DOI:10.1016/j.chemosphere.2019.125235
 .
. - A. F. Florea, C. Lu and H. C. B. Hansen, A zero-valent iron and zeolite filter for nitrate recycling from agricultural drainage water, Chemosphere, 2022, 287(P1), 131993, DOI:10.1016/j.chemosphere.2021.131993
 .
. - O. Eljamal, R. Eljamal, I. Maamoun, A. M. E. Khalil, T. Shubair and O. Falyouna, et al., Efficient treatment of ammonia-nitrogen contaminated waters by nano zero-valent iron/zeolite composite, Chemosphere, 2022, 287(P1), 131990, DOI:10.1016/j.chemosphere.2021.131990
 .
. - X. Ling, J. Li, W. Zhu, Y. Zhu, X. Sun and J. Shen, et al., Synthesis of nanoscale zero-valent iron/ordered mesoporous carbon for adsorption and synergistic reduction of nitrobenzene, Chemosphere, 2012, 87(6), 655–660, DOI:10.1016/j.chemosphere.2012.02.002
 .
. - L. Wang and A. Li, Impact of zero-valent iron on nitrifying granular sludge for 17-ethinylestradiol removal and its mechanism, Chemosphere, 2023, 333(January), 138904, DOI:10.1016/j.chemosphere.2023.138904
 .
. - J. Liu, A. Liu, W. Wang, R. Li and W. Zhang, Feasibility of nanoscale zero-valent iron (nZVI) for enhanced biological treatment of organic dyes, Chemosphere, 2019, 237, 124470, DOI:10.1016/j.chemosphere.2019.124470
 .
. - H. Kamani, M. Hosseinzehi, M. Ghayebzadeh, A. Azari, S. D. Ashrafi and H. Abdipour, Degradation of reactive red 198 dye from aqueous solutions by combined technology advanced sonofenton with zero valent iron: Characteristics/effect of parameters/kinetic studies, Heliyon, 2024, 10(1), e23667, DOI:10.1016/j.heliyon.2023.e23667
 .
. - P. Guo, J. Zhang, Y. Zhou, C. Tang, X. Wang and X. Gao, et al., Journal of Environmental Chemical Engineering Remediation of Cr ( VI ) -contaminated soil by ball milling modified zero-valent iron biochar composites : Insights into long-term stability and microbial community, J. Chem. Environ. Eng., 2023, 11(6), 111279, DOI:10.1016/j.jece.2023.111279
 .
. - N. Bounab, L. Duclaux, L. Reinert, A. Oumedjbeur, C. Boukhalfa and P. Penhoud, et al., Improvement of zero valent iron nanoparticles by ultrasound-assisted synthesis, study of Cr(VI) removal and application for the treatment of metal surface processing wastewater, J. Chem. Environ. Eng., 2021, 9(1), 104773, DOI:10.1016/j.jece.2020.104773
 .
. - W. Chen, X. Xu, J. Cui, Z. Zhou and Y. Yao, Porous boron nitride intercalated zero-valent iron particles for highly efficient elimination of organic contaminants and Cr (VI), Chemosphere, 2022, 306(June), 135501, DOI:10.1016/j.chemosphere.2022.135501
 .
. - Z. Cai, J. Fu, P. Du, X. Zhao, X. Hao and W. Liu, et al., Reduction of nitrobenzene in aqueous and soil phases using carboxymethyl cellulose stabilized zero-valent iron nanoparticles, Chem. Eng. J., 2018, 332(August 2017), 227–236, DOI:10.1016/j.cej.2017.09.066
 .
. - S. Kadaikunnan, N. S. Alharbi, J. M. Khaled and A. S. Alobaidi, Biocontrol property of Streptomyces parvulus VRR3 in green gram plant (Vigna radiata L.) against Fusarium solani in greenhouse, Physiol. Mol. Plant Pathol., 2023, 128(July), 102128, DOI:10.1016/j.pmpp.2023.102128
 .
. - D. Gupta, A. Boora, A. Thakur and T. K. Gupta, Green and sustainable synthesis of nanomaterials: Recent advancements and limitations, Environ. Res., 2023, 231(June), 116316, DOI:10.1016/j.envres.2023.116316
 .
. - K. K. Bharadwaj, B. Rabha, S. Pati, T. Sarkar, B. K. Choudhury and A. Barman, et al., Green synthesis of gold nanoparticles using plant extracts as beneficial prospect for cancer theranostics, Molecules, 2021, 26, 6389, DOI:10.3390/molecules26216389
 .
. - Y. Liang, X. Min, L. Chai, M. Wang, W. Liyang and Q. Pan, et al., Stabilization of arsenic sludge with mechanochemically modified zero valent iron, Chemosphere, 2017, 168, 1142–1151, DOI:10.1016/j.chemosphere.2016.10.087
 .
. - P. A. Calderón Bedoya, P. M. Botta, P. G. Bercoff and M. A. Fanovich, Influence of the milling materials on the mechanochemical synthesis of magnetic iron oxide nanoparticles, J. Alloys Compd., 2023, 939, 168720, DOI:10.1016/j.jallcom.2023.168720
 .
. - S. Wu, S. Cai, F. Qin, F. He, T. Liu and X. Yan, et al., Reductive dechlorination of chlorinated ethenes by ball milled and mechanochemically sulfidated microscale zero valent iron: A comparative study, J. Hazard Mater., 2023, 446(January), 130730, DOI:10.1016/j.jhazmat.2023.130730
 .
. - K. Wang, G. Wang, L. Zhou, Y. Zeng, Y. Zhang and Z. Fang, Rapid Removal of Decabromodiphenyl ether by Mechanochemically Prepared Submicron Zero-valent Iron with FeC2O4·2H2O layers: Kinetics, Mechanisms and Pathways, J. Hazard Mater., 2023, 465(December 2023), 133309, DOI:10.1016/j.jhazmat.2023.133309
 .
. - Y. Dai, L. Duan, W. Du, X. Yang, S. Sun and Q. Xiu, et al., Morphology and structure of in situ FeS affect Cr(VI) removal by sulfidated microscale zero-valent iron with short-term ultrasonication, Chemosphere, 2022, 290(November 2021), 133372, DOI:10.1016/j.chemosphere.2021.133372
 .
. - Y. Pang, Y. Ruan, Y. Feng, Z. Diao, K. Shih and L. Hou, et al., Ultrasound assisted zero valent iron corrosion for peroxymonosulfate activation for Rhodamine-B degradation, Chemosphere, 2019, 228, 412–417, DOI:10.1016/j.chemosphere.2019.04.164
 .
. - D. Dong, O. Kyung Choi and L. J. Woo, Influence of the continuous addition of zero valent iron (ZVI) and nano-scaled zero valent iron (nZVI) on the anaerobic biomethanation of carbon dioxide, Chem. Eng. J., 2022, 430(P3), 132233, DOI:10.1016/j.cej.2021.132233
 .
. - M. Du, Y. Zhang, X. Zeng, H. Kuang and S. Huang, Enhancement of ball-miling on pyrite/zero-valent iron for arsenic removal in water: A mechanistic study, Chemosphere, 2020, 249, 126130, DOI:10.1016/j.chemosphere.2020.126130
 .
. - S. Ratso, A. Zitolo, M. Käärik, M. Merisalu, A. Kikas and V. Kisand, et al., Non-precious metal cathodes for anion exchange membrane fuel cells from ball-milled iron and nitrogen doped carbide-derived carbons, Renew. Energy, 2021, 167, 800–810 CrossRef CAS
 .
. - Y. Yao, Y. Yu, L. Wan, C. Du, Y. Zhang and J. Chen, et al., Structurally-stable Mg-Co-Ni LDH grown on reduced graphene by ball-milling and ion-exchange for highly-stable asymmetric supercapacitor, J. Colloid Interface Sci., 2023, 649, 519–527 CrossRef CAS PubMed
 .
. - C. Xue, L. Zhou and Z. Fang, Remediation of polybrominated diphenyl ethers contaminated soil in the e-waste disposal site by ball milling modified zero valent iron activated persulfate, Chemosphere, 2023, 324(January), 138376, DOI:10.1016/j.chemosphere.2023.138376
 .
. - M. Liu, J. Chen, B. Li, B. Wang, Q. Han and S. Wei, et al., Preparation of microcrystalline graphite/zinc ferrite composites with enhanced and tunable electromagnetic wave absorption using a high-temperature ball milling method, Mater. Res. Bull., 2023, 161, 112170, DOI:10.1016/j.materresbull.2023.112170
 .
. - P. Wang, J. Hu, Y. Wang and T. Liu, Enhanced elimination of V5+ in wastewater using zero-valent iron activated by ball milling: The overlooked crucial roles of energy input and sodium chloride, J. Hazard Mater., 2022, 435(March), 129050, DOI:10.1016/j.jhazmat.2022.129050
 .
. - J. Gao, W. Wang, A. J. Rondinone, F. He and L. Liang, Degradation of Trichloroethene with a Novel Ball Milled Fe-C Nanocomposite, J. Hazard Mater., 2015, 300, 443–450, DOI:10.1016/j.jhazmat.2015.07.038
 .
. - M. Hou, Y. Zhang, X. Jiao, N. Ding, Y. Jiao and Y. Pan, et al., Polyphenol-modified zero-valent iron prepared using ball milling technology for hexavalent chromium removal: Kinetics and mechanisms, Sep. Purif. Technol., 2023, 326(August), 124874, DOI:10.1016/j.seppur.2023.124874
 .
. - S. Wu, S. Yang, Q. Li, M. Wang, Y. Xue and D. Zhao, Iron(II) sulfate crystals assisted mechanochemical modification of microscale zero-valent aluminum (mZVAl) for oxidative degradation of phenol in water, Chemosphere, 2021, 274, 129767, DOI:10.1016/j.chemosphere.2021.129767
 .
. - J. Zhang, J. Wang, G. Zhang, Z. Huo, Z. Huang and L. Wu, A review of diamond synthesis, modification technology, and cutting tool application in ultra-precision machining, Mater. Des., 2024, 237(September 2023), 112577, DOI:10.1016/j.matdes.2023.112577
 .
. - M. Sohail, N. Baig, M. Sher, R. Jamil, M. Altaf and S. Akhtar, et al., A Novel Tin-Doped Titanium Oxide Nanocomposite for Efficient Photo-Anodic Water Splitting, ACS Omega, 2020, 5(12), 6405–6413 CrossRef CAS PubMed
 .
. - C. Visentin, A. W. da S. Trentin, A. B. Braun and A. Thomé, Nano scale zero valent iron production methods applied to contaminated sites remediation: An overview of production and environmental aspects, J. Hazard. Mater., 2021, 410(September 2020), 24614, DOI:10.1016/j.jhazmat.2020.124614
 .
. - R. Lahoz, A. Naghilou, W. Kautek and O. Bomati-Miguel, Study of the physicochemical surface alterations and incubation phenomena induced on iron targets by nanosecond pulsed laser ablation in liquids: Effect on productivity and characteristics of the synthesized nanoscale zero-valent iron (nZVI) particles, Appl. Surf. Sci., 2020, 511(August 2019), 145438, DOI:10.1016/j.apsusc.2020.145438
 .
. - X. Gao, C. Xu, H. Yin, P. Chen, X. Wang and Q. Song, et al., Synthesis of nano titanium oxide with controlled oxygen content using pulsed discharge in water, Adv. Powder Technol., 2020, 31(3), 986–992 CrossRef CAS
 .
. - L. Liu, J. Zhao, X. Liu, S. Bai, H. Lin and D. Wang, Reduction and removal of As(V) in aqueous solution by biochar derived from nano zero-valent-iron (nZVI) and sewage sludge, Chemosphere, 2021, 277, 130273, DOI:10.1016/j.chemosphere.2021.130273
 .
. - X. Hu, M. Song, S. Li, Y. Chu, W. Zhang and Z. Deng, TEMPO oxidized cellulose nanocrystal (TOCNC) scaffolded nanoscale zero-valent iron (nZVI) for enhanced chromium removal, Chemosphere, 2023, 343(April), 140212, DOI:10.1016/j.chemosphere.2023.140212
 .
. - M. H. Park, J. Lee and J. Y. Kim, Oxidation resistance of nanoscale zero-valent iron supported on exhausted coffee grounds, Chemosphere, 2019, 234, 179–186, DOI:10.1016/j.chemosphere.2019.06.035
 .
. - Y. P. Sun, X. qin Li, J. Cao, W. xian Zhang and H. P. Wang, Characterization of zero-valent iron nanoparticles, Adv. Colloid Interface Sci., 2006, 120(1–3), 47–56 CrossRef PubMed
 .
. - N. K. Mathew, V. K. Rohith, G. Theertharaman, M. Navaneethan and S. Balakumar, Probing the influence of liquid nitrogen assisted chemical reduction on the nature of passivation layer, magnetic properties, and Cr (VI) remediation performance of nanoscale zero valent iron, J. Chem. Environ. Eng., 2023, 11(1), 109096, DOI:10.1016/j.jece.2022.109096
 .
. - Y. Duan, F. Liu, X. Liu and M. Li, Removal of Cr(VI) by glutaraldehyde-crosslinked chitosan encapsulating microscale zero-valent iron: Synthesis, mechanism, and longevity, J. Environ. Sci., 2023, 142, 115–128 CrossRef PubMed
 .
. - J. Gutiérrez, Y. N. Robein, J. Juan, M. S. Di Nezio, C. Pistonesi and E. A. González, et al., A combined experimental and DFT study on the zero valent iron/reduced graphene oxide doped QCM sensor for determination of trace concentrations of As using a Flow-batch system, Sens. Actuators, B, 2023, 404(December 2023), 135233, DOI:10.1016/j.snb.2023.135233
 .
. - H. S. Kim, T. Kim, J. Y. Ahn, K. Y. Hwang, J. Y. Park and T. T. Lim, et al., Aging characteristics and reactivity of two types of nanoscale zero-valent iron particles (FeBH and FeH2) in nitrate reduction, Chem. Eng. J., 2012, 197, 16–23, DOI:10.1016/j.cej.2012.05.018
 .
. - D. Baragaño, J. Alonso, J. R. Gallego, M. C. Lobo and M. Gil-Díaz, Zero valent iron and goethite nanoparticles as new promising remediation techniques for As-polluted soils, Chemosphere, 2020, 238, 124624 CrossRef
 .
. - A. S. Lozhkomoev, O. V. Bakina, S. O. Kazantsev, E. A. Glazkova, N. G. Rodkevich and M. I. Lerner, Antibacterial electro-explosive Co/CoO composite nanoparticles: Synthesis, structure, magnetic and antibacterial properties, J. Magn. Magn. Mater., 2023, 15, 580 Search PubMed
 .
. - L. Qian, X. Shang, B. Zhang, W. Zhang, A. Su and Y. Chen, et al., Chemosphere Enhanced removal of Cr ( VI ) by silicon rich biochar-supported nanoscale zero-valent iron, Chemosphere, 2019, 215, 739–745, DOI:10.1016/j.chemosphere.2018.10.030
 .
. - F. H. dos Santos, M. B. Soares and L. R. F. Alleoni, Pristine and biochar-supported nano zero-valent iron to immobilize As, Zn and Pb in soil contaminated by smelting activities, J. Environ. Manage., 2022, 321(May), 116017 CrossRef CAS PubMed
 .
. - X. Shang, L. Yang, D. Ouyang, B. Zhang, W. Zhang and M. Gu, et al., Enhanced removal of 1,2,4-trichlorobenzene by modified biochar supported nanoscale zero-valent iron and palladium, Chemosphere, 2020, 249, 126518, DOI:10.1016/j.chemosphere.2020.126518
 .
. - T. Zeng, H. Nong, H. Sha, S. Chen, X. Zhang and J. Liu, Performance and mechanism of Cr(VI) removal by sludge-derived biochar loaded with nanoscale zero-valent iron, Acta Mater. Compositae Sin., 2023, 40(2), 1037–1049, DOI:10.1016/j.dwt.2024.100035
 .
. - E. Longo and F. de Almeida La Porta, Recent advances in complex functional materials: From design to application, Recent Advances in Complex Functional Materials: from Design to Application, 2017, pp. 1–454 Search PubMed
 .
. - Y. Liu, R. Ma, J. Wang, G. Wang, G. Li and D. Wuyun, et al., Effect of nano zero-valent iron, potassium persulphate, and biochar on maturity and gaseous emissions during multi-material co-composting, Environ. Technol. Innovat., 2023, 32, 103309, DOI:10.1016/j.eti.2023.103309
 .
. - M. Wu, X. Teng, X. Liang, Y. Zhang, Z. Huang and Y. Yin, Supporting nanoscale zero-valent iron onto shrimp shell-derived N-doped biochar to boost its reactivity and electron utilization for selenite sequestration, Chemosphere, 2023, 319(January), 137979, DOI:10.1016/j.chemosphere.2023.137979
 .
. - L. Ma, Y. Du, S. Chen, D. Du, H. Ye and T. C. Zhang, Highly efficient removal of Cr(VI) from aqueous solution by pinecone biochar supported nanoscale zero-valent iron coupling with Shewanella oneidensis MR-1, Chemosphere, 2022, 287(P2), 132184, DOI:10.1016/j.chemosphere.2021.132184
 .
. - L. Zhu, L. Tong, N. Zhao, J. Li and Y. Lv, Coupling interaction between porous biochar and nano zero valent iron/nano -hydroxyl iron oxide improves the remediation efficiency of cadmium in aqueous solution, Chemosphere, 2019, 219, 493–503, DOI:10.1016/j.chemosphere.2018.12.013
 .
. - Y. Wang, W. Jiang, Y. Tang, Z. Liu, Q. Qin and Y. Xu, Biochar–supported sulfurized nanoscale zero–valent iron facilitates extensive dechlorination and rapid removal of 2,4,6–trichlorophenol in aqueous solution, Chemosphere, 2023, 332(May), 138835, DOI:10.1016/j.chemosphere.2023.138835
 .
. - L. Ren, Y. Li, K. Wang, K. Ding, M. Sha and Y. Cao, et al., Recovery of phosphorus from eutrophic water using nano zero-valent iron-modified biochar and its utilization, Chemosphere, 2021, 284(308), 131391, DOI:10.1016/j.chemosphere.2021.131391
 .
. - X. Zhang, K. Du, L. Deng, Z. Zhang, Z. Chen and H. Dong, One-pot fabrication of nanoscale zero-valent iron scaffolded onto graphitic carbon nitride as bifunctional nanohybrid in sequestering U(VI) and Cr(VI) with boost performance and intrinsic mechanism, Appl. Surf. Sci., 2024, 654(November 2023), 159437, DOI:10.1016/j.apsusc.2024.159437
 .
. - A. Pavelková, V. Cencerová, J. Zeman, V. Antos and J. Nosek, Reduction of chlorinated hydrocarbons using nano zero-valent iron supported with an electric field. Characterization of electrochemical processes and thermodynamic stability, Chemosphere, 2021, 265, 128764 CrossRef
 .
. - F. L. Souza, J. B. Attig, L. Latrous, C. Sáez, P. Cañizares and M. A. Rodrigo, et al., Electrochemical removal of pharmaceutical micropollutants from groundwater, J. Electroanal. Chem., 2022, 910(February), 116173 CrossRef CAS
 .
. - Á. Moratalla, S. E. Correia, S. Cotillas, E. Lacasa, P. Cañizares and M. A. Rodrigo, et al., The integration of ZVI-dehalogenation and electrochemical oxidation for the treatment of complex effluents polluted with iodinated compounds, J. Environ. Chem. Eng., 2022, 10(3), 107587 CrossRef
 .
. - Q. Yu, H. Mao, Z. Zhao and Y. Zhang, Electro-polarization of the sludge with dynamic magnetic field enhanced the interspecies electron transfer in ZVI-added anaerobic digesters, Renew. Energy, 2023, 215(July), 119012, DOI:10.1016/j.renene.2023.119012
 .
. - M. R. Jamei, M. R. Khosravi and B. Anvaripour, A novel ultrasound assisted method in synthesis of NZVI particles, Ultrason. Sonochem., 2014, 21(1), 226–233, DOI:10.1016/j.ultsonch.2013.04.015
 .
. - Y. Quintero, E. Mosquera, J. Diosa and A. García, Ultrasonic-assisted sol–gel synthesis of TiO2 nanostructures: Influence of synthesis parameters on morphology, crystallinity, and photocatalytic performance, J. Solgel Sci. Technol., 2020, 94(2), 477–485 CrossRef
 .
. - H. Beygi and A. Babakhani, Microemulsion synthesis and magnetic properties of FexNi(1−x) alloy nanoparticles, J. Magn. Magn. Mater., 2017, 421, 177–183, DOI:10.1016/j.jmmm.2016.07.071
 .
. - X. Weng, X. Jin, J. Lin, R. Naidu and Z. Chen, Removal of mixed contaminants Cr(VI) and Cu(II) by green synthesized iron based nanoparticles, Ecol. Eng., 2016, 97, 32–39, DOI:10.1016/j.ecoleng.2016.08.003
 .
. - K. A. P. Gaminda, I. B. K. Thomas, P. Lakmauri, T. Abeysinghe, C. Jayasinghe and R. Senthilnithy, Green synthesis of iron nanoparticles using Syzygium aromaticum extracts and their applications: Nitrate removal, malachite green degradation and antibacterial activity, Environ. Nanotechnol. Monit. Manag., 2024, 21(October 2023), 100925, DOI:10.1016/j.enmm.2024.100925
 .
. - A. Wang, J. Hou, Q. Xu, J. Wu and B. Xing, Green synthesis of zero valent iron using tannins to activate persulfate for sulfamethoxazole degradation, Environ. Pollut., 2023, 336(June), 122418, DOI:10.1016/j.envpol.2023.122418
 .
. - M. Saleh, Z. Isik, Y. Aktas, H. Arslan, M. Yalvac and N. Dizge, Green synthesis of zero valent iron nanoparticles using Verbascum thapsus and its Cr (VI) reduction activity, Bioresour. Technol. Rep., 2021, 13(December 2020), 100637 CrossRef CAS
 .
. - W. Moyo and T. T. I. Nkambule, Green synthesis of kaolin-supported nanoscale zero-valent iron using camellia sinensis extract for effective adsorption of dissolved organic matter: Preparation, adsorption, and Fenton regenerative valorization of “spent” adsorbent, Environ. Nanotechnol. Monit. Manag., 2022, 18(September 2021), 100697, DOI:10.1016/j.enmm.2022.100697
 .
. - K. O. Badmus, E. Coetsee-Hugo, H. Swart and L. Petrik, Synthesis and characterisation of stable and efficient nano zero valent iron, Environ. Sci. Pollut. Res., 2018, 25(24), 23667–23684 CrossRef CAS
 .
. - F. S. D. Santos, F. R. Lago, L. Yokoyama and F. V. Fonseca, Synthesis and characterization of zero-valent iron nanoparticles supported on SBA-15, J. Mater. Res. Technol., 2017, 6(2), 178–183, DOI:10.1016/j.jmrt.2016.11.004
 .
. - C. Chi, B. Huo, Z. Liang, C. Hu, Q. Sun and S. Zhou, Study on tetracycline degradation in wastewater based on zero-valent nano iron assisted micro-nano bubbles, Alex. Eng. J., 2024, 86(December 2023), 577–583, DOI:10.1016/j.aej.2023.12.004
 .
. - J. Zhang, L. Xie, Q. Ma, Y. Liu, J. Li and Z. Li, et al., Ball milling enhanced Cr(VI) removal of zero-valent iron biochar composites: Functional groups response and dominant reduction species, Chemosphere, 2023, 311(P2), 137174, DOI:10.1016/j.chemosphere.2022.137174
 .
. - Y. Huang, W. Zhang, M. Zhang, X. Zhang and Y. Zhao, Hydroxyl-functionalized TiO2@SiO2@Ni/nZVI nanocomposites fabrication, characterization and enhanced simultaneous visible light photocatalytic oxidation and adsorption of arsenite, Chem. Eng. J., 2018, 338(August 2017), 369–382 CrossRef CAS
 .
. - Z. Yu, L. Hu and I. M. C. Lo, Transport of the arsenic (As)-loaded nano zero-valent iron in groundwater-saturated sand columns: Roles of surface modification and As loading, Chemosphere, 2019, 216, 428–436, DOI:10.1016/j.chemosphere.2018.10.125
 .
. - K. Pandey and S. Saha, Encapsulation of zero valent iron nanoparticles in biodegradable amphiphilic janus particles for groundwater remediation, J. Hazard Mater., 2023, 445(November 2022), 130501, DOI:10.1016/j.jhazmat.2022.130501
 .
. - P. Rao, M. S. H. Mak, T. Liu, K. C. K. Lai and I. M. C. Lo, Effects of humic acid on arsenic(V) removal by zero-valent iron from groundwater with special references to corrosion products analyses, Chemosphere, 2009, 75(2), 156–162, DOI:10.1016/j.chemosphere.2008.12.019
 .
. - K. He, R. Sun, D. Yang, S. Wang, J. Shu and W. Wan, et al., Effect of sulfidation on nitrobenzene removal from groundwater by microscale zero-valent iron: Insights into reactivity, reaction sites and removal pathways, Chemosphere, 2023, 310(September 2022), 136819, DOI:10.1016/j.chemosphere.2022.136819
 .
. - Q. Xie, L. Li, H. Dong, R. Li, R. Tian and J. Chen, Influence of several crucial groundwater components on the toxicity of nanoscale zero-valent iron towards Escherichia coli under aerobic and anaerobic conditions, Chemosphere, 2021, 285(May), 131453, DOI:10.1016/j.chemosphere.2021.131453
 .
. - J. Hou, Y. Li, H. Ci, L. Miao, G. You and J. Wu, et al., Influence of aggregation and sedimentation behavior of bare and modified zero-valent-iron nanoparticles on the Cr(VI) removal under various groundwater chemistry conditions, Chemosphere, 2022, 296, 133905 CrossRef CAS
 .
. - Z. Zhou, J. Ma, X. Liu, C. Lin, K. Sun and H. Zhang, et al., Activation of peroxydisulfate by nanoscale zero-valent iron for sulfamethoxazole removal in agricultural soil: Effect, mechanism and ecotoxicity, Chemosphere, 2019, 223, 196–203, DOI:10.1016/j.chemosphere.2019.02.074
 .
. - J. Wu, M. Yan, S. Lv, W. Yin, H. Bu and L. Liu, et al., Preparation of highly dispersive and antioxidative nano zero-valent iron for the removal of hexavalent chromium, Chemosphere, 2021, 262, 127733, DOI:10.1016/j.chemosphere.2020.127733
 .
. - F. Furia, M. Minella, F. Gosetti, F. Turci, R. Sabatino and A. Di Cesare, et al., Elimination from wastewater of antibiotics reserved for hospital settings, with a Fenton process based on zero-valent iron, Chemosphere, 2021, 283(November 2020), 131170, DOI:10.1016/j.chemosphere.2021.131170
 .
. - R. Bajagain and S. W. Jeong, Degradation of petroleum hydrocarbons in soil via advanced oxidation process using peroxymonosulfate activated by nanoscale zero-valent iron, Chemosphere, 2021, 270, 128627, DOI:10.1016/j.chemosphere.2020.128627
 .
. - P. Shao, Y. Chen, D. Gu, J. Zeng, S. Zhang and Y. Wu, et al., Resistance and resilience of soil bacterial community to zero-valent iron disposal of lindane contamination, Chemosphere, 2022, 306(January), 135612, DOI:10.1016/j.chemosphere.2022.135612
 .
. - X. Zhao, L. Sang, H. Song, W. Liang, K. Gong and C. Peng, et al., Stabilization of Ni by rhamnolipid modified nano zero-valent iron in soil: Effect of simulated acid rain and microbial response, Chemosphere, 2023, 341(May), 140008, DOI:10.1016/j.chemosphere.2023.140008
 .
. - J. Qiao, T. Liu, X. Wang, F. Li, Y. Lv and J. Cui, et al., Simultaneous alleviation of cadmium and arsenic accumulation in rice by applying zero-valent iron and biochar to contaminated paddy soils, Chemosphere, 2018, 195(808), 260–271, DOI:10.1016/j.chemosphere.2017.12.081
 .
. - A. Mitzia, M. Vítková and M. Komárek, Assessment of biochar and/or nano zero-valent iron for the stabilisation of Zn, Pb and Cd: A temporal study of solid phase geochemistry under changing soil conditions, Chemosphere, 2020, 242, 125248 CrossRef PubMed
 .
. - T. Guha, S. Barman, A. Mukherjee and R. Kundu, Nano-scale zero valent iron modulates Fe/Cd transporters and immobilizes soil Cd for production of Cd free rice, Chemosphere, 2020, 260, 127533, DOI:10.1016/j.chemosphere.2020.127533
 .
. - V. Khum-in, J. Suk-in, P. In-ai, K. Piaowan, Y. Praimeesub and K. Rintachai, et al., Combining magnet-assisted soil washing and soil amendment with zero-valent iron to restore safe rice cultivation in real cadmium-contaminated paddy fields, Chemosphere, 2023, 340(May), 139816, DOI:10.1016/j.chemosphere.2023.139816
 .
. - L. Chen, S. Feng, D. Zhao, S. Chen, F. Li and C. Chen, Efficient sorption and reduction of U(VI) on zero-valent iron-polyaniline-graphene aerogel ternary composite, J. Colloid Interface Sci., 2017, 490, 197–206, DOI:10.1016/j.jcis.2016.11.050
 .
. - Q. Zhang, D. Zhao, S. Feng, Y. Wang, J. Jin and A. Alsaedi, et al., Synthesis of nanoscale zero-valent iron loaded chitosan for synergistically enhanced removal of U(VI) based on adsorption and reduction, J. Colloid Interface Sci., 2019, 552, 735–743, DOI:10.1016/j.jcis.2019.05.109
 .
. - L. Wang, D. Zhao, D. Ding, C. Wu and Y. Chen, Highly improved removal of U(VI) from water by Fe-Cu nanoparticles anchored on corn straw biochar: Influencing factors and mechanism studies, Inorg. Chem. Commun., 2023, 153(November 2022), 110771, DOI:10.1016/j.inoche.2023.110771
 .
. - Q. Zhang, D. Zhao, Y. Ding, Y. Chen, F. Li and A. Alsaedi, et al., Synthesis of Fe–Ni/graphene oxide composite and its highly efficient removal of uranium(VI) from aqueous solution, J. Clean. Prod., 2019, 230, 1305–1315, DOI:10.1016/j.jclepro.2019.05.193
 .
. - H. Hu, D. Zhao, C. Wu and R. Xie, Sulfidized Nanoscale Zerovalent Iron Supported by Oyster Powder for Efficient Removal of Cr (VI): Characterization, Performance, and Mechanisms, Materials, 2022, 15(11), 3898 CrossRef PubMed
 .
. - Y. Sun, C. Lei, E. Khan, S. S. Chen, D. C. W. Tsang and Y. S. Ok, et al., Nanoscale zero-valent iron for metal/metalloid removal from model hydraulic fracturing wastewater, Chemosphere, 2017, 176, 315–323, DOI:10.1016/j.chemosphere.2017.02.119
 .
. - Y. S. El-Temsah and E. J. Joner, Effects of nano-sized zero-valent iron (nZVI) on DDT degradation in soil and its toxicity to collembola and ostracods, Chemosphere, 2013, 92(1), 131–137, DOI:10.1016/j.chemosphere.2013.02.039
 .
. - R. Saad, S. Thiboutot, G. Ampleman, W. Dashan and J. Hawari, Degradation of trinitroglycerin (TNG) using zero-valent iron nanoparticles/nanosilica SBA-15 composite (ZVINs/SBA-15), Chemosphere, 2010, 81(7), 853–858, DOI:10.1016/j.chemosphere.2010.08.012
 .
. - J. Lin, M. Sun, X. Liu and Z. Chen, Functional kaolin supported nanoscale zero-valent iron as a Fenton-like catalyst for the degradation of Direct Black G, Chemosphere, 2017, 184, 664–672, DOI:10.1016/j.chemosphere.2017.06.038
 .
. - Y. S. El-Temsah, A. Sevcu, K. Bobcikova, M. Cernik and E. J. Joner, DDT degradation efficiency and ecotoxicological effects of two types of nano-sized zero-valent iron (nZVI) in water and soil, Chemosphere, 2016, 144, 2221–2228, DOI:10.1016/j.chemosphere.2015.10.122
 .
. - Z. Ren, H. Tang, H. Li and Q. Jing, Journal of Water Process Engineering Column experimental study on the removal of hexavalent chromium from water by modified cellulose filter paper loaded with nano zero-valent iron, J. Water Proc. Eng., 2024, 59(January), 104920, DOI:10.1016/j.jwpe.2024.104920
 .
. - Y. Cheng, H. Dong, Y. Lu, K. Hou, Y. Wang and Q. Ning, et al., Toxicity of sulfide-modified nanoscale zero-valent iron to Escherichia coli in aqueous solutions, Chemosphere, 2019, 220, 523–530, DOI:10.1016/j.chemosphere.2018.12.159
 .
. - B. J. Ni, X. Yan, J. Sun, X. Chen, L. Peng and W. Wei, et al., Persulfate and zero valent iron combined conditioning as a sustainable technique for enhancing dewaterability of aerobically digested sludge, Chemosphere, 2019, 232, 45–53, DOI:10.1016/j.chemosphere.2019.05.148
 .
. - M. Cao, L. Wang, L. Wang, J. Chen and X. Lu, Remediation of DDTs contaminated soil in a novel Fenton-like system with zero-valent iron, Chemosphere, 2013, 90(8), 2303–2308, DOI:10.1016/j.chemosphere.2012.09.098
 .
. - S. Tang, X. mao Wang, H. W. Yang and Y. F. Xie, Haloacetic acid removal by sequential zero-valent iron reduction and biologically active carbon degradation, Chemosphere, 2013, 90(4), 1563–1567, DOI:10.1016/j.chemosphere.2012.09.046
 .
. - D. W. Cho, H. Song, F. W. Schwartz, B. Kim and B. H. Jeon, The role of magnetite nanoparticles in the reduction of nitrate in groundwater by zero-valent iron, Chemosphere, 2015, 125, 41–49, DOI:10.1016/j.chemosphere.2015.01.019
 .
. - A. M. E. Khalil, O. Eljamal, B. B. Saha and N. Matsunaga, Performance of nanoscale zero-valent iron in nitrate reduction from water using a laboratory-scale continuous-flow system, Chemosphere, 2018, 197, 502–512, DOI:10.1016/j.chemosphere.2018.01.084
 .
. - S. Klimkova, M. Cernik, L. Lacinova, J. Filip, D. Jancik and R. Zboril, Zero-valent iron nanoparticles in treatment of acid mine water from in situ uranium leaching, Chemosphere, 2011, 82(8), 1178–1184, DOI:10.1016/j.chemosphere.2010.11.075
 .
. - Y. Wu, Y. Wang, X. Huang, M. O. Simonnot, W. Wu and X. Cai, et al., Surfactant-facilitated dechlorination of 2,2′′,5,5′′-tetrachlorinated biphenyl using zero-valent iron in soil/sediment solution: Integrated effects of plausible factors, Chemosphere, 2018, 212, 845–852, DOI:10.1016/j.chemosphere.2018.08.113
 .
. - N. Song, J. Xu, Y. Cao, F. Xia, J. Zhai and H. Ai, et al., Chemical removal and selectivity reduction of nitrate from water by (nano) zero-valent iron/activated carbon micro-electrolysis, Chemosphere, 2020, 248, 125986 CrossRef
 .
. - N. V. Mdlovu, K. S. Lin, C. Y. Chen, F. A. Mavuso, S. C. Kunene and E. M. J. Carrera, In-situ reductive degradation of chlorinated DNAPLs in contaminated groundwater using polyethyleneimine-modified zero-valent iron nanoparticles, Chemosphere, 2019, 224, 816–826, DOI:10.1016/j.chemosphere.2019.02.160
 .
. - J. Dong, Y. Dong, C. Wen, S. Gao, L. Ren and Q. Bao, A 2D tank test on remediation of nitrobenzene-contaminated aquifer using in-situ reactive zone with emulsified nanoscale zero-valent iron, Chemosphere, 2018, 206, 766–776, DOI:10.1016/j.chemosphere.2018.05.067
 .
. - N. Fujioka, M. Suzuki, S. Kurosu and Y. Kawase, Linkage of iron elution and dissolved oxygen consumption with removal of organic pollutants by nanoscale zero-valent iron: Effects of pH on iron dissolution and formation of iron oxide/hydroxide layer, Chemosphere, 2016, 144, 1738–1746, DOI:10.1016/j.chemosphere.2015.10.064
 .
. - H. Yoshino, S. Kurosu, R. Yamaguchi and Y. Kawase, A phenomenological reaction kinetic model for Cu removal from aqueous solutions by zero-valent iron (ZVI), Chemosphere, 2018, 200, 542–553, DOI:10.1016/j.chemosphere.2018.02.127
 .
. - H. L. Lien and R. T. Wilkin, High-level arsenite removal from groundwater by zero-valent iron, Chemosphere, 2005, 59(3), 377–386 CrossRef PubMed
 .
. - H. Song, B. H. Jeon, C. M. Chon, Y. Kim, I. H. Nam and F. W. Schwartz, et al., The effect of granular ferric hydroxide amendment on the reduction of nitrate in groundwater by zero-valent iron, Chemosphere, 2013, 93(11), 2767–2773, DOI:10.1016/j.chemosphere.2013.09.033
 .
. - K. Li, C. Wang, H. Hu and Q. Zhang, Selective removal of copper from heavy-metals-containing acidic solution by a mechanochemical reduction with zero-valent silicon, Chem. Eng. J., 2023, 466(October 2022), 143246, DOI:10.1016/j.cej.2023.143246
 .
. - P. Xu, L. Wang, X. Liu, S. Xie and B. Hou, Vitamin C promoted refractory organic contaminant elimination in the zero-valent iron/peracetic acid system: Efficiency, mechanism and effects of various parameters, Chemosphere, 2023, 326(November 2022), 138481, DOI:10.1016/j.chemosphere.2023.138481
 .
. - C. Zhang, D. Shi, C. Wang, G. Sun, H. Li and Y. Hu, et al., Pristine/magnesium-loaded biochar and ZVI affect rice grain arsenic speciation and cadmium accumulation through different pathways in an alkaline paddy soil, J. Environ. Sci., 2024, 147, 630–641 CrossRef
 .
. - Q. A. Alhadidi, Z. Zhou, K. Y. Quiñones Deliz, H. Y. Greenslet and J. C. J. Bonzongo, Removal of type-A, type-B, and borderline metals from contaminated soils using zero valent iron and magnetic separation technology: A predictive approach for metal resources recovery, Chemosphere, 2021, 274, 129980 CrossRef PubMed
 .
. - K. Kumar Kesari, R. Soni, Q. M. S. Jamal, P. Tripathi, J. A. Lal, N. Kumar Jha, M. H. Siddiqui, P. Kumar, V. Tripathi and J. Ruokolainen, Sci-Hub | Wastewater Treatment and Reuse: a Review of its Applications and Health Implications, Water, Air, Soil Pollut., 2021, 232(5), 208, DOI:10.1007/s11270-021-05154-8
 .
. - M. L. Saccà, C. Fajardo, G. Costa, C. Lobo, M. Nande and M. Martin, Integrating classical and molecular approaches to evaluate the impact of nanosized zero-valent iron (nZVI) on soil organisms, Chemosphere, 2014, 104, 184–189 CrossRef
 .
. - C. Fajardo, M. L. Saccà, M. Martinez-Gomariz, G. Costa, M. Nande and M. Martin, Transcriptional and proteomic stress responses of a soil bacterium bacillus cereus to nanosized zero-valent iron (nZVI) particles, Chemosphere, 2013, 93(6), 1077–1083, DOI:10.1016/j.chemosphere.2013.05.082
 .
. - L. Yuan, K. Wang, Q. Zhao, L. Yang, G. Wang and M. Jiang, et al., An overview of in situ remediation for groundwater co-contaminated with heavy metals and petroleum hydrocarbons, J. Environ. Manag., 2024, 349(June 2023), 119342, DOI:10.1016/j.jenvman.2023.119342
 .
. - I. R. Bautitz, A. C. Velosa and R. F. P. Nogueira, Zero valent iron mediated degradation of the pharmaceutical diazepam, Chemosphere, 2012, 88(6), 688–692, DOI:10.1016/j.chemosphere.2012.03.077
 .
. - J. Wu, J. Zheng, K. Ma, C. Jiang, L. Zhu and X. Xu, Tertiary treatment of municipal wastewater by a novel flow constructed wetland integrated with biochar and zero-valent iron, J. Water Proc. Eng., 2022, 47(April), 102777 CrossRef
 .
. - M. Du, Y. Zhang, I. Hussain, X. Du, S. Huang and W. Wen, Effect of pyrite on enhancement of zero-valent iron corrosion for arsenic removal in water: A mechanistic study, Chemosphere, 2019, 233, 744–753, DOI:10.1016/j.chemosphere.2019.05.197
 .
. - P. A. R. Puthukkara, T. S. Jose and S. D. lal, Plant mediated synthesis of zero valent iron nanoparticles and its application in water treatment, J. Chem. Environ. Eng., 2021, 9(1), 104569, DOI:10.1016/j.jece.2020.104569
 .
. - E. J. Reardon, Capture and storage of hydrogen gas by zero-valent iron, J. Contam. Hydrol., 2014, 157, 117–124, DOI:10.1016/j.jconhyd.2013.11.007
 .
. - K. F. Chen, S. Li and W. X. Zhang, Renewable hydrogen generation by bimetallic zero valent iron nanoparticles, Chem. Eng. J., 2011, 170(2–3), 562–567, DOI:10.1016/j.cej.2010.12.019
 .
. - H. Qin, X. Guan, J. Z. Bandstra, R. L. Johnson and P. G. Tratnyek, Modeling the Kinetics of Hydrogen Formation by Zerovalent Iron: Effects of Sulfidation on Micro- and Nano-Scale Particles, Environ. Sci. Technol., 2018, 52(23), 13887–13896 CrossRef CAS
 .
. - L. Li, Y. He, H. Fu, X. Qu and Z. Xu, Efficient and reductive removal of bromate using a novel and stable nanoscale zero-valent iron embedded in N-doped carbon derived from metal-organic frameworks, Chemosphere, 2022, 306(June), 135503, DOI:10.1016/j.chemosphere.2022.135503
 .
. - S. H. Kang and W. Choi, Oxidative degradation of organic compounds using zero-valent iron in the presence of natural organic matter serving as an electron shuttle, Environ. Sci. Technol., 2009, 43(3), 878–883 CrossRef CAS
 .
. - K. O. Badmus, N. Irakoze, O. R. Adeniyi and L. Petrik, Synergistic advance Fenton oxidation and hydrodynamic cavitation treatment of persistent organic dyes in textile wastewater, J. Chem. Environ. Eng., 2020, 8(2), 103521, DOI:10.1016/j.jece.2019.103521
 .
. - Y. Li, L. Liu, Q. Wang, J. Wu, T. Liu and H. Liu, et al., Enhanced anaerobic co-metabolism of coal gasification wastewater via the assistance of zero-valent iron, J. Water Proc. Eng., 2021, 40(December 2020), 101817, DOI:10.1016/j.jwpe.2020.101817
 .
. - Q. Zhang, X. Ye, D. Chen, W. Xiao, S. Zhao and J. Li, et al., Chromium(VI) removal from synthetic solution using novel zero-valent iron biochar composites derived from iron-rich sludge via one-pot synthesis, J. Water Proc. Eng., 2022, 47(January), 102720, DOI:10.1016/j.jwpe.2022.102720
 .
. - Y. Zhang, X. Lu, R. Yu, J. Li and F. Wang, Immobilization of Sb in a smelting residue by micro-sized zero-valent iron: Long-term performance under accelerated exposure to strong acid rain, Chemosphere, 2022, 291(P1), 132699, DOI:10.1016/j.chemosphere.2021.132699
 .
. - Q. Xiu, S. Zhao, X. Yang, S. Sun, Y. Dai and L. Duan, et al., Warrior's armor: Study on the aging of sulfidated micro-sized zero valent iron in air and its subsequent reactivity for chloramphenicol degradation in different acid systems, Chemosphere, 2021, 285(June), 131422, DOI:10.1016/j.chemosphere.2021.131422
 .
. - T. A. Formentini, G. Cornelis, J. P. Gustafsson, K. Leicht, C. Tiberg and B. Planer-Friedrich, et al., Immobilizing arsenic in contaminated anoxic aquifer sediment using sulfidated and uncoated zero-valent iron (ZVI), J. Hazard Mater., 2024, 462(June 2023), 132743, DOI:10.1016/j.jhazmat.2023.132743
 .
. - J. Shen, H. Chen, N. Xu, Y. Liu, W. Sun and X. Ma, et al., Molybdate modified nano zero-valent iron via green synthesis enhances Cr(VI) reduction during their cotransport in water-saturated porous media, Chem. Eng. J., 2024, 479(September 2023), 147599, DOI:10.1016/j.cej.2023.147599
 .
. - R. Wirecka, D. Lachowicz, K. Berent, M. M. Marzec and A. Bernasik, Ion distribution in iron oxide, zinc and manganese ferrite nanoparticles studied by XPS combined with argon gas cluster ion beam sputtering, Surface. Interfac., 2022, 30, 101865 CrossRef CAS
 .
. - H. Yang, L. Deng, H. Yang, Y. Xiao and D. Zheng, Promotion of nitrogen removal in a zero-valent iron-mediated nitrogen removal system operated in co-substrate mode, Chemosphere, 2022, 307(P2), 135779, DOI:10.1016/j.chemosphere.2022.135779
 .
. - Y. An, Q. Dong and K. Zhang, Bioinhibitory effect of hydrogenotrophic bacteria on nitrate reduction by nanoscale zero-valent iron, Chemosphere, 2014, 103, 86–91 CrossRef CAS PubMed
 .
. - X. Sheng and S. Lyu, Insights into enhanced removal of fluoranthene by sulfidated nanoscale zero-valent iron: In aqueous solution and soil slurry, Chemosphere, 2023, 312(P1), 137172, DOI:10.1016/j.chemosphere.2022.137172
 .
. - O. Mohammed, K. G. Mumford and B. E. Sleep, Effects of hydrogen gas production, trapping and bubble-facilitated transport during nanoscale zero-valent iron (nZVI) injection in porous media, J. Contam. Hydrol., 2020, 234(June), 103677, DOI:10.1016/j.jconhyd.2020.103677
 .
.
|
| This journal is © The Royal Society of Chemistry 2024 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article *a,
Khairul Anwar Bin Mohamad Saida and
Adamu Muhammadc
*a,
Khairul Anwar Bin Mohamad Saida and
Adamu Muhammadc
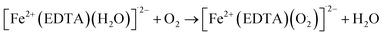




![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was mixed in a stainless-steel jar with agate balls. The jar was sealed, evacuated, and purged with pure nitrogen gas three times before subjected to ball milling at 200 rpm for 12 hours using a planetary ball mill. The resulting composite was collected in a glovebox and stored in an air-proof desiccator filled with argon gas to prevent oxidation
1 was mixed in a stainless-steel jar with agate balls. The jar was sealed, evacuated, and purged with pure nitrogen gas three times before subjected to ball milling at 200 rpm for 12 hours using a planetary ball mill. The resulting composite was collected in a glovebox and stored in an air-proof desiccator filled with argon gas to prevent oxidation![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 nZVI/AC ratio. When utilized alone, nZVI recorded 100% nitrate removal while its efficacy in terms of total nitrogen (TN) was virtually the same at about 35% at acidic pH like nZVI/AC composite
1 nZVI/AC ratio. When utilized alone, nZVI recorded 100% nitrate removal while its efficacy in terms of total nitrogen (TN) was virtually the same at about 35% at acidic pH like nZVI/AC composite
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
. 10.1016/j.enmm.2023.100900, https://linkinghub.elsevier.com/retrieve/pii/S2215153223001241.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.



