Open Access Article
Open Access ArticleA lysosomal chloride ion-selective fluorescent probe for biological applications†
Sang-Hyun
Park‡
,
Ji Young
Hyun‡
and
Injae
Shin
 *
*
Center for Biofunctional Molecules, Department of Chemistry, Yonsei University, Seoul 03722, Republic of Korea. E-mail: injae@yonsei.ac.kr
First published on 27th November 2018
Abstract
Lysosomal pHs are maintained at low values by the cooperative action of a proton pump and a chloride channel to maintain electroneutrality. Owing to the biological significance of lysosomal chloride ions, measurements of their levels are of great importance to understand lysosome-associated biological events. However, appropriate probes to selectively detect Cl− ions within acidic lysosomes have not been developed to date. In this study, we prepared MQAE-MP, a lysosomal Cl−-selective fluorescent probe, and applied it to gain information about biological processes associated with lysosomes. The fluorescence of MQAE-MP is pH-insensitive over physiological pH ranges and is quenched by Cl− with a Stern–Volmer constant of 204 M−1. Because MQAE-MP detects lysosomal Cl− selectively, it was employed to assess the effects of eleven substances on lysosomal Cl− concentrations. The results show that lysosomal Cl− concentrations decrease in cells treated with substances that inhibit proteins responsible for lysosomal membrane stabilization, induce lysosomal membrane permeabilization, and transport lysosomal Cl− to the cytosol. In addition, we investigated the effect of lysosomal chloride ions on the fusion of autophagosomes with lysosomes to generate autolysosomes during autophagy inhibition promoted by substances. It was found that changes in lysosomal Cl− concentrations did not affect the fusion of autophagosomes with lysosomes but an increase in the cytosolic Ca2+ concentration blocked the fusion process. We demonstrate from the current study that MQAE-MP has great potential as a lysosomal Cl−-selective fluorescent probe for studies of biological events associated with lysosomes.
Introduction
A lysosome is an intracellular organelle that plays a key role in degrading and recycling intracellular biomolecules and extracellular materials delivered via endocytosis and phagocytosis by the action of lysosomal hydrolases.1 This subcellular compartment is crucial for autophagy (or a self-eating process) which is involved in the maintenance of cellular homeostasis and cell survival under the conditions of nutrient deficiency.2 During autophagy, cytoplasmic constituents are sequestered in autophagosomes and subsequently delivered to lysosomes for digestion by lysosomal hydrolases to produce recyclable products. Lysosomal enzymes normally are stable and active in the acidic pH range.3 The low pH (4.5–5.0) inside lysosomes is achieved by the action of vacuolar H+-ATPase (V-ATPase), which pumps protons into the lysosome lumen by using energy generated by ATP hydrolysis.4 To maintain electroneutrality during proton pumping, anions must enter lysosomes or cations must exit from lysosomes. Multiple previous studies suggest that the Cl− influx into lysosomes mediated by CLC-7 (a Cl−/H+ antiporter) is the principal process occurring during lysosome acidification.5 As a consequence, the Cl− concentration (more than 80 mM) in the lysosome is higher than that in the cytosol (5–20 mM) in order to alleviate the charge imbalance resulting from the maintenance of the low lysosomal pH.6 Owing to the biological significance of lysosomal Cl−, the measurement of its level is key to understanding lysosome-associated biological events. However, fluorescent probes to selectively detect Cl− within acidic lysosomes have not been developed thus far. Consequently, it is in great demand to create fluorescent probes that are pH-insensitive over a broad pH range and selectively monitor Cl− within lysosomes.To date, engineered fluorescent protein-based probes have been constructed and utilized to detect intracellular chloride ions.7 However, these probes are not applicable to monitoring lysosomal Cl− owing to their pH-sensitivity.7,8 On the other hand, a few chemical fluorescent probes have been exploited to detect intracellular chloride ions.9–12 For example, 1-(ethoxycarbonylmethyl)-6-methoxyquinolinium bromide (MQAE) is a pH-insensitive, Cl−-selective fluorescent probe that has been employed to detect cytosolic chloride ions (Fig. 1a).9 This probe has a relatively large Stern–Volmer constant (200 M−1) for fluorescence quenching by Cl−. A bisacridinium-based fluorescent probe, with a relatively small Stern–Volmer constant (36 M−1) for Cl− quenching, was observed to be a pH-insensitive, Cl−-selective probe applicable to measuring endosomal chloride ions.10 Recently, a ratiometric fluorescent probe, 6-methoxyquinolinium-dansyl (MQ-DS), was developed to detect intracellular Cl− but is inappropriate to selectively monitor lysosomal Cl−.11 Thus, no chemical probes have been devised for selectively determining the levels of lysosomal chloride ions.
Despite the biological significance of lysosomal chloride ions, research focusing on lysosomal Cl− has not been actively performed owing to the absence of suitable fluorescent probes. In the study described below, we have remedied this deficiency by developing a fluorescent probe, MQAE-MP, which selectively detects Cl− in lysosomes. Our findings show that MQAE-MP fluorescence is pH-insensitive in the physiological pH range and that its fluorescence is efficiently quenched by Cl− with a Stern–Volmer constant of 204 M−1 without interference from cations and non-halide anions. Moreover, MQAE-MP was successfully utilized to determine the effects of various substances on lysosomal Cl− concentrations. We found that lysosomal Cl− concentrations were reduced in cells treated with substances that inhibit proteins responsible for lysosomal membrane stabilization, promote lysosomal membrane permeabilization (LMP), and transport lysosomal Cl− to the cytosol. Based on these results, we also investigated whether lysosomal chloride ions have an influence on the fusion of autophagosomes with lysosomes to generate autolysosomes during the inhibition of autophagy induced by substances. The results showed that alterations in lysosomal Cl− concentrations do not affect the fusion of autophagosomes with lysosomes but the fusion process was blocked when cytosolic Ca2+ concentrations are increased. The combined results demonstrate that MQAE-MP has great potential for use as a lysosomal Cl−-selective fluorescent probe for studies of biological events associated with lysosomes.
Results
Design, synthesis and characterization of Cl−-sensitive fluorescent probes
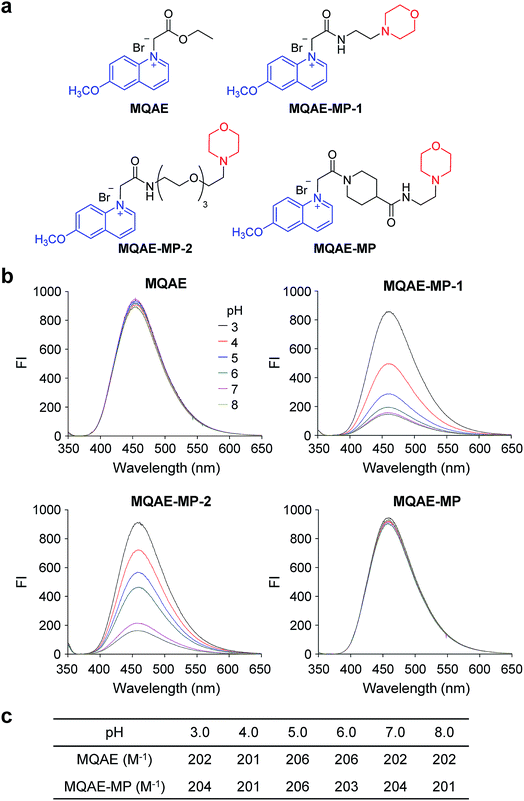
The known Cl−-sensitive fluorescent probe MQAE9 was used as the platform to design lysosome-targeting, Cl−-sensitive fluorescent probes. To enable lysosome targeting, the ethyl ester moiety in MQAE was changed to an amide group containing a morpholine moiety which is a lysosome-targeting motif.13 MQAE-MP-1 (Fig. 1a), the first probe constructed based on this design that possessed an ethylene tether between the amide NH and morpholine groups, was synthesized by using the procedure shown in Scheme 1a. The analysis of the Cl− concentration-dependent response of MQAE-MP-1 at pH 3–8 showed that its fluorescence was gradually quenched as the Cl− concentration increased (Fig. S1†) but was highly pH-sensitive unlike that of MQAE (Fig. 1b and S1, S2†).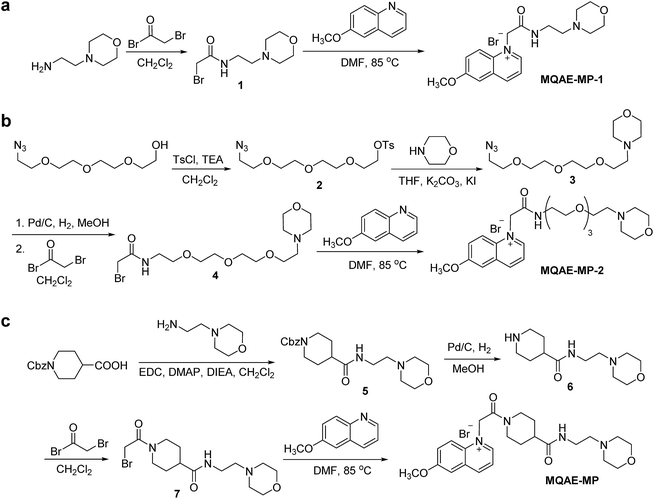 | ||
| Scheme 1 Synthesis of lysosome-targeting fluorescent Cl− probes: (a) MQAE-MP-1, (b) MQAE-MP-2 and (c) MQAE-MP. | ||
To circumvent this problem, MQAE-MP-2 (Fig. 1a) containing a relatively long oligoethylene glycol tether between the amide NH and morpholine groups was prepared by using the procedure shown in Scheme 1b. The analysis of the pH-dependent response of the fluorescence of MQAE-MP-2 to Cl− showed that its emission intensity was reduced in a concentration-dependent manner by Cl− (Fig. 1b and S3†). However, although to a lesser extent than that of MQAE-MP-1, the fluorescence of MQAE-MP-2 was still sensitive to pH. We assumed from this study that the morpholine moiety in MQAE-MP-1 and MQAE-MP-2 might affect the fluorescence of the 6-methoxyquinolinium group depending on pH. To test this proposal, the fluorescence of MQAE was measured in the presence of various concentrations of N-methylmorpholine (NMM) in a pH range of 3.0–8.0. The results showed that as the pH of solutions containing MQAE and NMM increased from 3.0 to 8.0, NMM affected the fluorescence of 6-methoxyquinolinium to a greater extent presumably through the interaction of free NMM with 6-methoxyquinolinium (Fig. S4†).
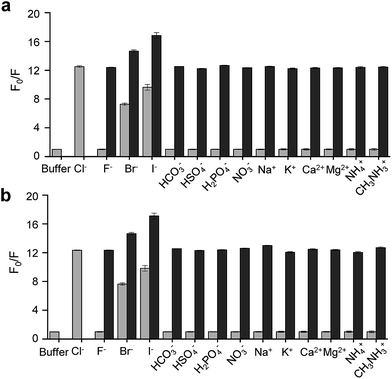
On the basis of these results, we separated morpholine and quinolinium groups by using a rigid rather than flexible tether to alleviate or eliminate the effect of a morpholine moiety on the fluorescence of a quinolinium group. MQAE-MP (Fig. 1a), which contains a rigid isonipecotic acid moiety as the tether between amide NH and morpholine groups, was prepared by using the procedure shown in Scheme 1c. To our pleasure, the analysis of the emission properties of MQAE-MP demonstrated that its fluorescence was insensitive to pH over the 3.0–8.0 range and was quenched in a Cl− concentration-dependent manner (Fig. 1b and S5a†). MQAE-MP exhibited two absorption bands with maximum wavelengths at 320 nm and 350 nm (Fig. S6†) and an emission band with a maximum wavelength at 460 nm (Fig. 1b), which were quite close to those of MQAE (Fig. 1b and S6†). In addition, emission from MQAE-MP was quenched by Cl− in the range of 0–250 mM with a Stern–Volmer quenching constant (Cl− quenching sensitivity) of 204 M−1 at pH 7.0, a value that is close to that of MQAE (202 M−1) (Fig. 1c and S2b, S5b†).9 The fluorescence response of MQAE-MP to various anions and cations was assessed. As shown in Fig. 2 and S7,† emission from MQAE-MP was not affected by cations (Na+, K+, Ca2+, Mg2+, NH4+ and CH3NH3+), F−, and non-halide anions (bicarbonate, sulfate, phosphate and nitrate) like that of MQAE. In addition, these ions did not interfere with the fluorescence response of MQAE-MP to Cl− (Fig. 2b).
Detection of lysosomal Cl− ions in cells using MQAE-MP
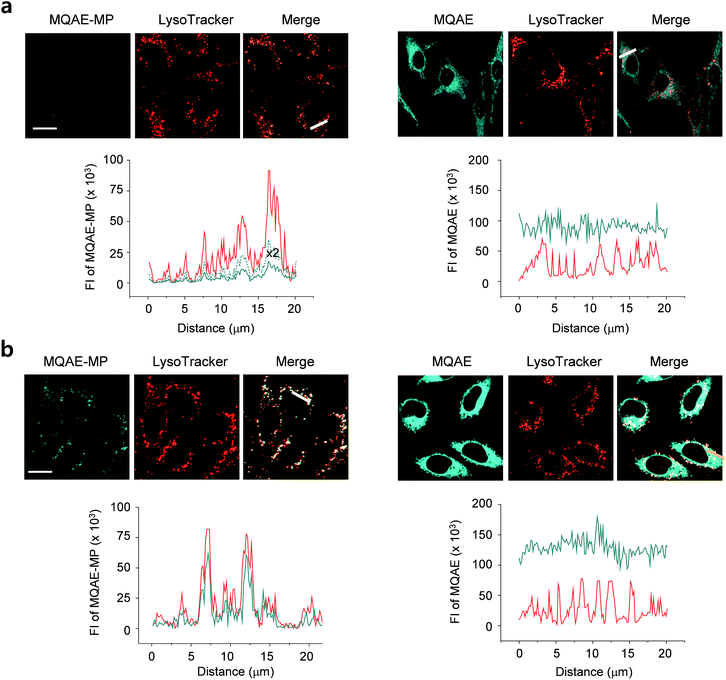
The desirable properties (pH-insensitive and Cl−-sensitive) of MQAE-MP in aqueous solutions stimulated a study probing its ability to detect Cl− in lysosomes of cells. For this study, HeLa cells cultured in normal media or Cl−-deficient buffer were treated with MQAE-MP and LysoTracker Red. Also, the cells were incubated with MQAE for comparison purposes. The analysis of confocal fluorescence microscopy images of cells showed that fluorescence arising from MQAE was distributed throughout the cytoplasm (Fig. 3). Importantly, the fluorescence of MQAE-MP overlapped well with that of LysoTracker Red (Fig. 3). Because the lysosomal Cl− concentration is normally higher (∼80 mM) than the cytosolic Cl− concentration (5–20 mM),14 the fluorescence intensity of MQAE-MP in lysosomes was observed to be weaker than that of MQAE. However, cells cultured in Cl−-deficient buffer exhibited stronger fluorescence of MQAE-MP than cells in normal media, in accordance with the fact that the lysosomal Cl− concentration is reduced when cells are cultured in Cl−-deficient buffer.15 It should be noted that because lysosomal Cl− concentrations are normally reduced in cells treated with substances that disrupt the function of lysosomes, changes in the levels of lysosomal Cl− could be readily determined by using MQAE-MP (vide infra). Overall, the findings indicate that MQAE-MP accumulates mainly in lysosomes owing to the lysosome-targeting morpholine group and that it selectively and efficiently detects lysosomal Cl−.Also, a study was conducted to elucidate the time-dependence of detection of lysosomal Cl− using MQAE-MP. For this purpose, HeLa cells cultured in Cl−-deficient buffer were treated with MQAE-MP while confocal fluorescence microscopy images of cells were collected during a 2 h time period. The results showed that the fluorescence intensity of MQAE-MP in cells rapidly increased and nearly reached saturation after ca. 30 min incubation (Fig. S8†). The combined findings indicate that MQAE-MP is applicable for monitoring Cl− inside acidic lysosomes.
Analysis of the effects of substances on lysosomal Cl− concentrations using MQAE-MP
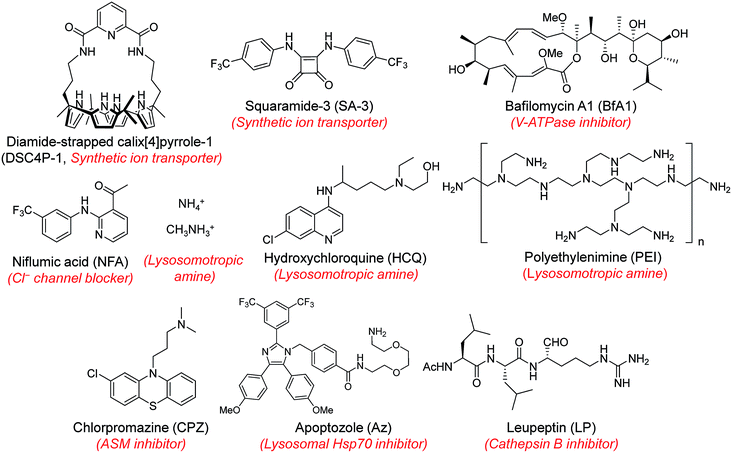
Next, MQAE-MP was employed to determine changes in lysosomal Cl− concentrations promoted by eleven substances that affect the function of lysosomes (Fig. 4). The substances used for this study include synthetic ion transporters (diamide-strapped calix[4]pyrrole-1 (DSC4P-1) and squaramide-3 (SA-3)), inhibitors of a proton pump V-ATPase (bafilomycin A1 (BfA1)) and a chloride channel blocker (niflumic acid (NFA)), lysosomotropic amines (ammonium ions, methylammonium ions, hydroxychloroquine (HCQ) and polyethylenimine (PEI)), and inhibitors of lysosomal proteins (chlorpromazine (CPZ), apoptozole (Az) and leupeptin (LP)). HeLa cells were incubated for 6 h with several concentrations of each substance in normal culture media and then treated for 30 min with non-cytotoxic MQAE-MP (Fig. S9†) and acridine orange, which is used to detect acidic vesicles such as lysosomes.16 Cell images were obtained by using confocal fluorescence microscopy.The effects of two synthetic ion transporters, DSC4P-1 and SA-3, on lysosomal Cl− concentrations and pH were assessed first. Our previous studies showed that DSC4P-1 and SA-3 promoted increases in the cytosolic Cl− concentration by their ability to transport extracellular Cl− with higher concentrations (120 mM) to the cytosol with lower Cl− concentrations (5–20 mM).17,18 However, the determination of changes in lysosomal Cl− concentrations caused by these transporters was not conducted at that time owing to the lack of a suitable lysosomal Cl− fluorescent probe. The results of the current effort using MQAE-MP clearly indicate that DSC4P-1 does not affect the lysosomal Cl− concentration and pH (Fig. 5a and S10†). The findings suggest that this transporter does not have the ability to transport lysosomal Cl− to the cytosol.
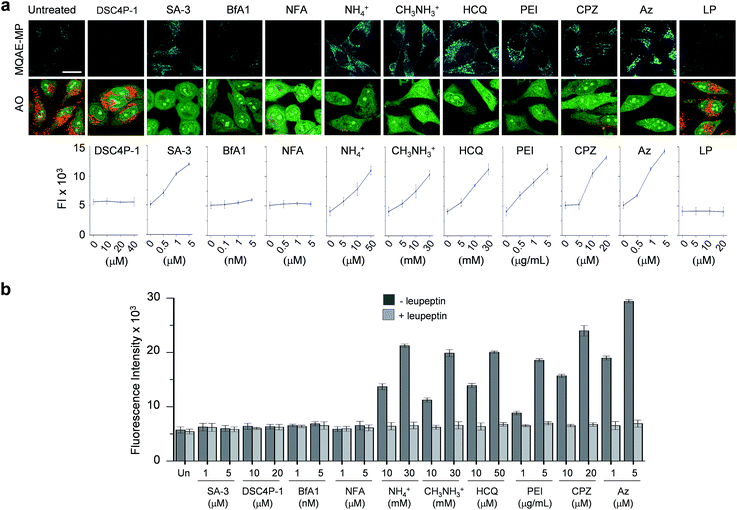 | ||
| Fig. 5 Detection of lysosomal chloride ions in cells treated with various substances using MQAE-MP. (a) HeLa cells were incubated for 6 h with each substance (concentration: 40 μM DSC4P-1, 5 μM SA-3, 5 nM BfA1, 5 μM NFA, 30 mM NH4+, 30 mM CH3NH3+, 50 μM HCQ, 5 μg mL−1 PEI, 20 μM CPZ, 5 μM Az and 50 μM LP) and then treated for 30 min with 5 mM MQAE-MP or 100 nM acridine orange (AO). Cell images were obtained by using confocal fluorescence microscopy (scale bar: 10 μm). Graph shows the fluorescence intensities (FI) of cells treated with indicated concentrations of each substance for 6 h (see Fig. S10† for complete images). Fluorescence intensities of MQAE-MP in the cells were analyzed by using ZEN software. (b) HeLa cells were treated with each substance for 6 h. Cathepsin B activity of the isolated cytosolic fraction was measured by using a fluorogenic substrate MR-(RR)2 in the absence and presence of leupeptin (mean ± s.d., n = 3). | ||
In marked contrast to DSC4P-1, SA-3 promoted a dose-dependent decrease in the level of lysosomal Cl−, as judged from an increase in the intensity of fluorescence from MQAE-MP, and increased the lysosomal pH, based on the observation of the disappearance of red fluorescence from acridine orange (Fig. 5a and S10†).15 The reduced level of lysosomal Cl− caused by SA-3 could be a result of the previous suggestion17 that SA-3 has the ability to transport Cl− from lysosomes with a higher concentration (more than 80 mM) to the cytosol with a lower concentration (5–20 mM).6,14 However, it could be possible that the decrease in the lysosomal Cl− concentration is caused by its ready exit across lysosomal membranes disrupted by SA-3. To determine whether SA-3 induces LMP, the release of lysosomal cathepsin B into the cytosol of treated cells, which is evidence for LMP,19 was examined. HeLa cells were incubated for 6 h with SA-3, along with DSC4P-1, and the cathepsin B activity of the isolated cytosolic fraction of the treated HeLa cells was then measured by using a fluorogenic substrate, MR-(RR)2.17 The results showed that cathepsin B activity was not detected in the cytosol of the cells treated with SA-3, a phenomenon also seen in cells treated with DSC4P-1 (Fig. 5b). The findings indicate that SA-3 does not induce LMP and, consequently, promotes a decrease in the lysosomal Cl− concentration through its lysosomal Cl− transport activity. Concomitantly, SA-3 increased the lysosomal pH as a result of proton leakage which takes place in lysosomes.20,21 Together with the previous results, the findings indicate that while DSC4P-1 transports extracellular Cl− to the cytosol without transporting lysosomal Cl− to the cytosol, SA-3 has the ability to transport extracellular Cl− as well as lysosomal Cl− to the cytosol.
Next, confocal fluorescence microscopy images of cells treated with BfA1 and niflumic acid were analyzed to determine their effects on lysosomal Cl− concentrations and pH. BfA1 is an inhibitor of the proton pump V-ATPase and thus causes an increase in lysosomal pH.22,23 Niflumic acid is a cellular chloride channel inhibitor that regulates the Cl− concentration in cells.24 However, the effects of these substances on lysosomal Cl− concentrations have not been determined. The results of cell image analyses showed that BfA1 and niflumic acid did not alter the levels of lysosomal Cl− but they did induce an increase in the lysosomal pH in a dose-dependent manner (Fig. 5a and S10†). To determine whether BfA1 and niflumic acid promote LMP, cathepsin B activities in the cytosol of HeLa cells treated with these substances were determined. In each case, cathepsin B activity was not detected in the cytosol of treated cells (Fig. 5b), indicating that both BfA1 and niflumic acid do not induce LMP. Collectively, the findings suggest that either BfA1 or niflumic acid blocks Cl− entry into lysosomes because V-ATPase and CLC-7 cooperatively function, thus leading to no changes in lysosomal Cl− concentrations (Fig. S11b and c†).4 Moreover, the lysosomal pH increased in cells treated with either BfA1 or niflumic acid is likely to be a consequence of proton leakage under conditions where pumping of protons into lysosomes is suppressed by either BfA1 or niflumic acid (Fig. S11b and c†).5
The effects of lysosomotropic amines (ammonium ions, methylammonium ions, hydroxychloroquine and polyethylenimine (PEI)) on lysosomal Cl− levels and pH were assessed.25 It is known that uncharged forms of lysosomotropic amines freely diffuse across membranes and their protonated (non-diffusible) forms accumulate in acidic intracellular compartments such as lysosomes.26 When trapped in lysosomes, lysosomotropic amines induce lysosomal osmotic stress, leading to the disruption of lysosomal membranes and induction of LMP.25,27–29 Cell image analyses revealed that each of the tested lysosomotropic amines caused a dose-dependent decrease in lysosomal Cl− concentrations and an increase in lysosomal pH (Fig. 5a and S10†). As expected, lysosomotropic amines caused LMP, as judged by observations that cathepsin B activities were detected in the isolated cytosol of the treated cells (Fig. 5b). However, when leupeptin, an inhibitor of cathepsin B,30 was present in the isolated cytosol of the treated cells, cathepsin B activities were not detected. On the basis of these observations, we conclude that lysosomal H+ and Cl− exit from lysosomes to the cytosol across disrupted lysosomal membranes of the cells treated with lysosomotropic amines.
The effects of the three inhibitors of lysosomal proteins (chlorpromazine for acid sphingomyelinase (ASM), apoptozole for lysosomal Hsp70, and leupeptin for cathepsin B) on lysosomal Cl− concentrations and pH were determined. Chlorpromazine is an inhibitor of ASM responsible for the cleavage of sphingomyelin into ceramide and phosphorylcholine that are involved in the stabilization of lysosomal membranes.31,32 Apoptozole is an inhibitor of lysosomal Hsp70,33,34 which acts as a lysosomal membrane stabilizer through its interaction with lysosomal membranes.35 Leupeptin is a naturally occurring inhibitor of cathepsin B, which degrades proteins inside lysosomes.30 As shown in Fig. 5a and S10,† the treatment of cells with chlorpromazine led to a reduction in the level of lysosomal Cl− and an increase in lysosomal pH. In addition, chlorpromazine induced LMP,36 as judged by the observation that cathepsin B was released from lysosomes to the cytosol of the treated cells (Fig. 5b). Consequently, lysosomal H+ and Cl− ions exited across disrupted membranes of lysosomes of the cells treated with chlorpromazine. Similarly, apoptozole caused a decrease in the level of lysosomal Cl− ions and an increase in lysosomal pH (Fig. 5a and S10†) as a result of the ability of apoptozole to destabilize lysosomal membranes and the consequent induction of LMP (Fig. 5b).34 In contrast to chlorpromazine and apoptozole, leupeptin did not cause any change in the lysosomal Cl− concentration and pH (Fig. 5a and S10†).
Time-dependent changes in the levels of lysosomal Cl− promoted by these substances were also investigated using MQAE-MP as the probe. HeLa cells were incubated for various times with SA-3, four lysosomotropic amines, chlorpromazine and apoptozole, all of which affect the levels of lysosomal chloride ions. The results showed that whereas SA-3 promoted a relatively slow decrease in the lysosomal Cl− concentration, lysosomotropic amines, chlorpromazine and apoptozole caused relatively rapid decreases in lysosomal Cl− concentrations (Fig. 6 and S12†). The findings suggest that substances with the ability to induce LMP accelerate Cl− efflux from lysosomes into the cytosol more rapidly than the synthetic ion transporter SA-3 which does not induce LMP. It is worth mentioning that although LMP-inducing agents promote the release of cathepsin B into the cytosol, the fluorescence arising from Lysotracker and MQAE-MP is detectable in lysosomes.37,38
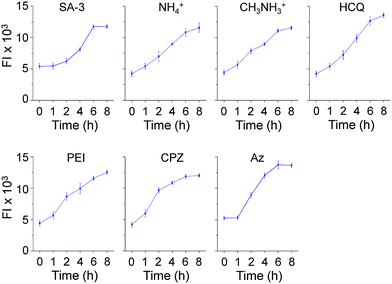 | ||
| Fig. 6 Time-dependent detection of lysosomal chloride ions in cells treated with various substances using MQAE-MP. HeLa cells were incubated with each compound (concentration: 5 μM SA-3, 30 mM NH4+, 30 mM CH3NH3+, 50 μM HCQ, 5 μg mL−1 PEI, 20 μM CPZ and 5 μM Az) for indicated times and then treated with 5 mM MQAE-MP for 30 min (mean ± s.d., n = 3). Graphs show the fluorescence intensities (FI) of cells treated with each substance for indicated times (see Fig. S12† for cell images). | ||
Collectively, the results of the study aimed at determining the effects of substances on lysosomal Cl− concentrations using MQAE-MP show that (1) the synthetic ion transporter, SA-3, promotes decreases in lysosomal Cl− and H+ concentrations but DSC4P-1 does not affect lysosomal Cl− and H+ concentrations, (2) inhibitors of a proton pump V-ATPase (BfA1) and a chloride channel (NFA) cause decreases in H+ concentrations without affecting Cl− concentrations, and (3) substances that induce LMP cause decreases in lysosomal Cl− and H+ concentrations.
Effect of lysosomal Cl− on the fusion of autophagosomes with lysosomes
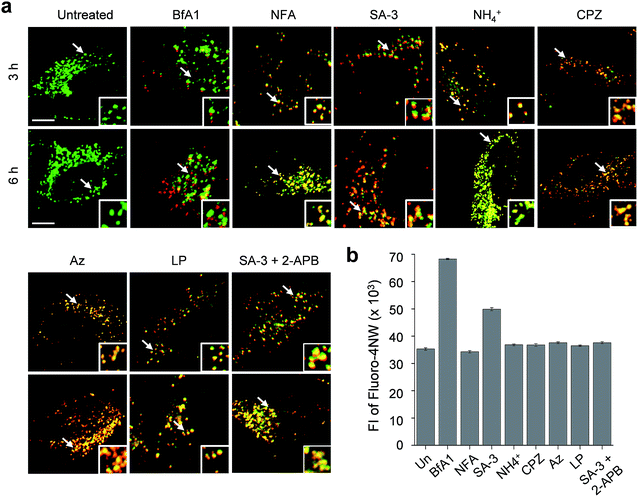
The lysosome is a crucial intracellular compartment for autophagy during which autophagosomes containing cytoplasmic constituents are fused with lysosomes to generate autolysosomes.39 In autolysosomes, engulfed cytoplasmic components are degraded by lysosomal enzymes, which normally are stable and active at an acidic pH.40 Recently, it was shown that the fusion of autophagosomes with lysosomes is suppressed at high cytosolic Ca2+ concentrations.23 In light of this, we wondered whether lysosomal chloride ions also have an influence on the fusion process to generate autolysosomes.Thus, we assessed the effect of lysosomal Cl− on the fusion of autophagosomes with lysosomes in cells undergoing autophagy inhibition promoted by SA-3, BfA1, niflumic acid, NH4+, chlorpromazine, apoptozole and leupeptin (see Fig. S13† for the inhibition of autophagy by these substances). To accomplish this, HeLa cells were individually treated for 3 or 6 h with each substance. The treated cells were immunostained with LAMP2A and p62 antibodies serving as respective lysosome and autophagosome markers.41,42 If lysosomes and autophagosomes are fused in the treated cells, yellow fluorescence arising from colocalization of lysosome (LAMP2A, green) and autophagosome (p62, red) markers will be observed. However, discrete fluorescence arising from LAMP2A and p62 without detecting yellow fluorescence will be seen in the cells treated with substances that suppress the fusion of autophagosomes with lysosomes.
The analysis of confocal fluorescence microscopy images of the cells showed that discrete LAMP2A and p62 puncta existed in the cells treated with BfA1 which does not affect the lysosomal Cl− concentration (Fig. 7a). The finding indicates that BfA1 blocks the fusion of autophagosomes with lysosomes, which is in agreement with the recent finding.23 As expected, the cytosolic Ca2+ concentration greatly increased (∼2 times) in the cells treated with BfA1,23 as judged from the observation of an increase in the fluorescent intensity of Ca2+-sensitive probe Fluoro-4NW (Fig. 7b).17 In contrast to BfA1, the cells treated with niflumic acid which does not affect the lysosomal Cl− concentration exhibited significant colocalization of LAMP2A and p62 (Fig. 7a), indicating that niflumic acid allows the fusion of lysosomes with autophagosomes. In addition, the cytosolic Ca2+ concentration was found to be unaltered in the cells treated with niflumic acid (Fig. 7b). Consequently, the results show that the two substances which do not change lysosomal Cl− concentrations differently affect the fusion of autophagosomes with lysosomes, suggesting that lysosomal Cl− may have no influence on the fusion process.
Cell image analysis also revealed that the fusion of autophagosomes with lysosomes took place in cells treated with LMP-inducing substances, such as ammonium ions, chlorpromazine and apoptozole (Fig. 7a). In the cells treated with each of LMP-inducing substances, cytosolic Ca2+ concentrations were not changed (Fig. 7b). These phenomena were also observed in cells incubated with leupeptin (Fig. 7b). As a consequence, the LMP-inducing substances, which caused decreases in lysosomal Cl− concentrations, as well as leupeptin, which did not affect the lysosomal Cl− concentration, allow the fusion of autophagosomes with lysosomes. The findings also support the conclusion that the fusion process may not be affected by lysosomal Cl−.
Interestingly, cells treated with SA-3 with lysosomal chloride transport activity exhibited partially fused vesicles (or puncta) even after 6 h incubation (Fig. 7a). The cytosolic Ca2+ concentration in the cells treated with SA-3 was measured to increase by half of that caused by BfA1 (Fig. 7b). Recently, we have found that SA-3 activates the inositol trisphosphate receptor (IP3R)43 responsible for Ca2+ efflux from the ER to the cytosol and, consequently, causes an increase in the cytosolic Ca2+ concentration. On this basis, we examined whether an increase in the cytosolic Ca2+ concentration in the cells treated with SA-3 affected the fusion of autophagosomes with lysosomes. For this purpose, cells were co-treated with SA-3 and an IP3R inhibitor, 2-aminoethoxydiphenyl borate (2-APB).44 When 2-APB was present in the SA-3 treated cells, the cytosolic Ca2+ concentration was reduced to a normal level and the fusion of autophagosomes with lysosomes was comparable to that in the cells treated with niflumic acid, LMP-inducing substances or leupeptin (Fig. 7). Because the cytosolic Ca2+ concentration in the cells treated with SA-3 increased to a lesser extent than that in the cells treated with BfA1, the fusion of autophagosomes with lysosomes was not completely suppressed in the cells treated with SA-3 but partially fused vesicles were observed. Taken together, the findings suggest that changes in lysosomal Cl− concentrations have no influence on the fusion process but high cytosolic Ca2+ concentrations are critical to block the fusion process.
Conclusions
We developed the first lysosomal Cl−-selective fluorescent probe by linking a quinolinium fluorophore to a lysosome-targeting morpholine group. It was found that the nature of the tether between the quinolinium fluorophore and morpholine moiety was key to generating the desired fluorescence properties. Specifically, the fluorescence of probes, MQAE-MP-1 and MQAE-MP-2, containing flexible tethers is sensitive to pH, whereas the probe MQAE-MP containing a rigid tether is pH-insensitive. Moreover, the fluorescence of MQAE-MP is quenched by Cl− with a Stern–Volmer constant of 204 M−1 but is not responsive to cations, F− and non-halide anions. Because MQAE-MP accumulates mainly in lysosomes and successfully responds to lysosomal Cl− by undergoing a decrease in fluorescence intensity, it was applied to assess the effects of eleven substances on lysosomal Cl− concentrations. Among the substances, a DSC4P-1 based synthetic ion transporter did not change both lysosomal Cl− concentration and pH, but a SA-3 based synthetic ion transporter altered both. This observation might result from the difference in the ability of these substances to transport lysosomal Cl− to the cytosol. In addition, chlorpromazine and apoptozole, which inhibit respective ASM and lysosomal Hsp70 responsible for lysosomal membrane stabilization, cause changes in both lysosomal Cl− concentration and pH by inducing LMP. Similar results also arise from studies of cells treated with lysosomotropic amines, which promote the induction of LMP. However, inhibitors of V-ATPase (BfA1) and a chloride channel (niflumic acid) increase lysosomal pH without affecting lysosomal Cl− concentrations. In contrast, an inhibitor of cathepsin B (leupeptin) does not change either lysosomal Cl− concentration or pH. Collectively, the findings indicate that lysosomal Cl− concentrations decrease in cells treated with substances that inhibit proteins responsible for lysosomal membrane stabilization, induce LMP, and transport lysosomal Cl− to the cytosol.We also examined whether lysosomal Cl− concentrations affected the fusion of autophagosomes with lysosomes to form autolysosomes. Our results showed that BfA1, which does not affect the lysosomal Cl− concentration but increases the cytosolic Ca2+ concentration, suppressed the fusion of autophagosomes with lysosomes. However, ammonium ions, chlorpromazine and apoptozole, which cause decreases in lysosomal Cl− concentrations but do not change cytosolic Ca2+ concentrations, allowed the fusion of autophagosomes with lysosomes. In addition, niflumic acid and leupeptin, which affect neither the lysosomal Cl− concentrations nor cytosolic Ca2+ concentrations, also allowed the fusion process. Interestingly, SA-3, which induces a decrease in the lysosomal Cl− concentration, caused the partial fusion of autophagosomes with lysosomes because the cytosolic Ca2+ concentration in the SA-3 treated cells increases by half of that caused by BfA1. Collectively, the findings provide evidence to support the notion that alterations in lysosomal Cl− concentrations do not affect the fusion of lysosomes with autophagosomes but increases in cytosolic Ca2+ concentrations are critical to block the fusion process. It should be noted that the disruption of lysosomal membranes and an increase in lysosomal pH are also not critical for the suppression of fusion of autophagosomes with lysosomes. It is clear that the investigation described above has demonstrated the usefulness of MQAE-MP in studies of how substances affect the levels of lysosomal Cl− ions. It is anticipated that MQAE-MP will serve as a useful fluorescent probe to explore more lysosome-associated biological systems.
Experimental section
Spectral characterization of fluorescent Cl− probes
Fluorescence quenching measurements with fluorescent Cl− probes were performed by excitation at 350 nm. Microliter aliquots of NaCl (5 M stock) were added to 3 mL of each fluorescent probe (100 μM in 50 mM sodium phosphate buffer) at various pHs (pH 3–8). Stern–Volmer constants were calculated from the slope of F0/F versus [Cl−] plots, where F0 is the fluorescence of each Cl− probe in the absence of Cl− and F in the presence of Cl−.MQAE-MP staining
HeLa cells in culture media were incubated with each substance at the indicated concentrations and for the indicated times. The cells were washed three times with DPBS and then incubated with 5 mM of MQAE-MP and 100 nM of LysoTracker Red (ThermoFisher Scientific) for 30 min at 37 °C. The cells were washed twice with DPBS and imaged by using confocal fluorescence microscopy (Zeiss LSM 800). The fluorescence intensities of fluorescent dyes in the cells were quantified using the mean region of interest (ROI) tool with ZEN software. Specifically, a constant circular ROI was chosen to encompass the cell of interest and the same ROI area size was used for background subtraction.Acridine orange staining
HeLa cells were incubated for 6 h with each substance at the indicated concentrations. The treated cells were washed three times with DPBS and then incubated with 100 nM of acridine orange (AO) for 30 min at 37 °C. The cells were washed twice with DPBS and imaged by using confocal fluorescence microscopy (Zeiss LSM 800).Measurements of cathepsin B activity in the cytosolic fraction
HeLa cells were incubated with each compound for 6 h at 37 °C. After removal of culture media, an extraction buffer (250 mM sucrose, 20 mM HEPES, 10 mM KCl, 1.5 mM MgCl2, 1 mM EDTA and 1 mM EGTA) containing 25 μg mL−1 digitonin (Sigma-Aldrich) was added to the cells and the resulting cells were shaken on a rocker on ice for 12 min. The extraction buffer was collected and used as the cytosolic fraction. The cytosolic fraction was treated with 1 μL MR-(RR)2 (ImmunoCytochemistry Technologies) in the absence and presence of 20 μM leupeptin for an additional 4 h at 37 °C. The enzyme-catalyzed release of the fluorescent probe was monitored by using an Infinite® 200 PRO multimode microplate reader (excitation wavelength: 540 nm; emission wavelength: 600 nm).Immunocytochemistry
HeLa cells were incubated for 3 or 6 h with each compound at the indicated concentrations. The treated cells were fixed with 4% formaldehyde in PBS buffer for 15 min. The cells were incubated with mouse LAMP2A monoclonal (1:200, Santa Cruz) and guinea pig p62 polyclonal (1:200, PROGEN) for 1 h at room temperature followed by incubation with Alexa-Fluoro 488 conjugated goat anti-mouse IgG (1:200, Invitrogen, Molecular Probes) and Alexa-Fluoro 555 conjugated goat anti-guinea pig IgG (1:200, Invitrogen, Molecular Probes) for 1 h at room temperature. The cells were imaged by using confocal fluorescence microscopy (Zeiss LSM 800).Detection of calcium ions
HeLa cells were incubated with 10 μM Fluo-4NW in culture media for 1 h. After washing with PBS to remove remaining Fluo-4NW, the cells were incubated with an indicated compound for 12 h. Fluo-4 fluorescence was measured using a microplate reader (excitation wavelength: 485 nm; emission wavelength: 538 nm).Conflicts of interest
The authors declare no competing financial interests.Acknowledgements
This study was financially supported by a grant from the National Creative Research Initiative (2010-0018272) Program in Korea.References
- J. P. Luzio, P. R. Pryor and N. A. Bright, Nat. Rev. Mol. Cell Biol., 2007, 8, 622 CrossRef CAS PubMed.
- N. Mizushima and M. Komatsu, Cell, 2011, 147, 728 CrossRef CAS PubMed.
- A. R. Graves, P. K. Curran, C. L. Smith and J. A. Mindell, Nature, 2008, 453, 788 CrossRef CAS PubMed.
- S. Ohkuma, Y. Moriyama and T. Takano, Proc. Natl. Acad. Sci. U. S. A., 1982, 79, 2758 CrossRef CAS.
- J. A. Mindell, Annu. Rev. Physiol., 2012, 74, 69 CrossRef CAS PubMed.
- H. Xu and D. Ren, Annu. Rev. Physiol., 2015, 77, 57 CrossRef CAS PubMed.
- D. Arosio and G. M. Ratto, Front. Cell. Neurosci., 2014, 8, 258 Search PubMed.
- P. Bregestovski, T. Waseem and M. Mukhtarov, Front. Mol. Neurosci., 2009, 2, 15 Search PubMed.
- A. S. Verkman, M. C. Sellers, A. C. Chao, T. Leung and R. Ketcham, Anal. Biochem., 1989, 178, 355 CrossRef CAS PubMed.
- N. D. Sonawane, J. R. Thiagarajah and A. S. Verkman, J. Biol. Chem., 2002, 277, 5506 CrossRef CAS PubMed.
- P. Li, S. Zhang, N. Fan, H. Xiao, W. Zhang, W. Zhang, H. Wang and B. Tang, Chem.–Eur. J., 2014, 20, 11760 CrossRef CAS PubMed.
- P. Li, T. Xie, N. Fan, K. Li and B. Tang, Chem. Commun., 2012, 48, 2077 RSC.
- J. Y. Hyun, S. Kim, H. S. Lee and I. Shin, Cell Chem. Biol., 2018, 25, 1255 CrossRef CAS PubMed.
- T. Stauber and T. J. Jentsch, Annu. Rev. Physiol., 2013, 75, 453 CrossRef CAS PubMed.
- S. Hosogi, K. Kusuzaki, T. Inui, X. Wang and Y. Marunaka, J. Cell. Mol. Med., 2014, 18, 1124 CrossRef CAS PubMed.
- M. G. Palmgren, Anal. Biochem., 1991, 192, 316 CrossRef CAS PubMed.
- N. Busschaert, S. H. Park, K. H. Baek, Y. P. Choi, J. Park, E. N. W. Howe, J. R. Hiscock, L. E. Karagiannidis, I. Marques, V. Felix, W. Namkung, J. L. Sessler, P. A. Gale and I. Shin, Nat. Chem., 2017, 9, 667 CrossRef CAS PubMed.
- S. K. Ko, S. K. Kim, A. Share, V. M. Lynch, J. Park, W. Namkung, W. Van Rossom, N. Busschaert, P. A. Gale, J. L. Sessler and I. Shin, Nat. Chem., 2014, 6, 885 CrossRef CAS PubMed.
- C. Oberle, J. Huai, T. Reinheckel, M. Tacke, M. Rassner, P. G. Ekert, J. Buellesbach and C. Borner, Cell Death Differ., 2010, 17, 1167 CrossRef CAS PubMed.
- D. E. Johnson, P. Ostrowski, V. Jaumouille and S. Grinstein, J. Cell Biol., 2016, 212, 677 CrossRef CAS PubMed.
- D. Deng, N. Jiang, S. J. Hao, H. Sun and G. J. Zhang, Biochim. Biophys. Acta, 2009, 1788, 470 CrossRef CAS PubMed.
- L. Jahreiss, F. M. Menzies and D. C. Rubinsztein, Traffic, 2008, 9, 574 CrossRef CAS PubMed.
- C. Mauvezin, P. Nagy, G. Juhasz and T. P. Neufeld, Nat. Commun., 2015, 6, 7007 CrossRef PubMed.
- Y. Ishida, S. Nayak, J. A. Mindell and M. Grabe, J. Gen. Physiol., 2013, 141, 705 CrossRef CAS PubMed.
- P. D. Hart and M. R. Young, J. Exp. Med., 1991, 174, 881 CrossRef CAS PubMed.
- P. Boya and G. Kroemer, Oncogene, 2008, 27, 6434 CrossRef CAS PubMed.
- P. Boya, R. A. Gonzalez-Polo, D. Poncet, K. Andreau, H. L. Vieira, T. Roumier, J. L. Perfettini and G. Kroemer, Oncogene, 2003, 22, 3927 CrossRef CAS PubMed.
- A. E. Solheim and P. O. Seglen, Biochem. J., 1983, 210, 929 CrossRef CAS PubMed.
- D. W. Pack, A. S. Hoffman, S. Pun and P. S. Stayton, Nat. Rev. Drug Discovery, 2005, 4, 581 CrossRef CAS PubMed.
- A. Baici and M. Gyger-Marazzi, Eur. J. Biochem., 1982, 129, 33 CrossRef CAS PubMed.
- D. Canals, D. M. Perry, R. W. Jenkins and Y. A. Hannun, Br. J. Pharmacol., 2011, 163, 694 CrossRef CAS PubMed.
- N. H. Petersen, O. D. Olsen, L. Groth-Pedersen, A. M. Ellegaard, M. Bilgin, S. Redmer, M. S. Ostenfeld, D. Ulanet, T. H. Dovmark, A. Lonborg, S. D. Vindelov, D. Hanahan, C. Arenz, C. S. Ejsing, T. Kirkegaard, M. Rohde, J. Nylandsted and M. Jaattela, Cancer Cell, 2013, 24, 379 CrossRef CAS PubMed.
- S. K. Ko, J. Kim, D. C. Na, S. Park, S. H. Park, J. Y. Hyun, K. H. Baek, N. D. Kim, N. K. Kim, Y. N. Park, K. Song and I. Shin, Chem. Biol., 2015, 22, 391 CrossRef CAS PubMed.
- S.-H. Park, K.-H. Baek, I. Shin and I. Shin, Cell Chem. Biol., 2018, 25, 1242 CrossRef CAS PubMed.
- T. Kirkegaard, A. G. Roth, N. H. Petersen, A. K. Mahalka, O. D. Olsen, I. Moilanen, A. Zylicz, J. Knudsen, K. Sandhoff, C. Arenz, P. K. Kinnunen, J. Nylandsted and M. Jaattela, Nature, 2010, 463, 549 CrossRef CAS PubMed.
- A. C. Johansson, H. Appelqvist, C. Nilsson, K. Kagedal, K. Roberg and K. Ollinger, Apoptosis, 2010, 15, 527 CrossRef CAS PubMed.
- K. S. Cho, Y. H. Yoon, J. A. Choi, S. J. Lee and J. Y. Koh, Invest. Ophthalmol. Visual Sci., 2012, 53, 5344 CrossRef CAS PubMed.
- P. Boya, K. Andreau, D. Poncet, N. Zamzami, J. L. Perfettini, D. Metivier, D. M. Ojcius, M. Jaattela and G. Kroemer, J. Exp. Med., 2003, 197, 1323 CrossRef CAS PubMed.
- N. Mizushima, Genes Dev., 2007, 21, 2861 CrossRef CAS PubMed.
- J. A. Cardelli, J. Richardson and D. Miears, J. Biol. Chem., 1989, 264, 3454 CAS.
- C. T. Hua, J. J. Hopwood, S. R. Carlsson, R. J. Harris and P. J. Meikle, Clin. Chem., 1998, 44, 2094 CAS.
- N. Mizushima, T. Yoshimori and B. Levine, Cell, 2010, 140, 313 CrossRef CAS PubMed.
- J. Schlossmann, A. Ammendola, K. Ashman, X. Zong, A. Huber, G. Neubauer, G. X. Wang, H. D. Allescher, M. Korth, M. Wilm, F. Hofmann and P. Ruth, Nature, 2000, 404, 197 CrossRef CAS PubMed.
- L. Missiaen, G. Callewaert, H. De Smedt and J. B. Parys, Cell Calcium, 2001, 29, 111 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8sc04084b |
| ‡ S.-H. P. and J. Y. H. contributed equally. |
| This journal is © The Royal Society of Chemistry 2019 |