Magnesium substituted hydroxyapatite whiskers: synthesis, characterization and bioactivity evaluation
Gong Liangzhi†
,
Zhang Weibin† and
Shen Yuhui *
*
Department of Orthopaedics, Shanghai Institute of Orthopaedics & Traumatology, Shanghai Ruijin Hospital, Shanghai Jiaotong University School of Medicine, 200025, Shanghai, China. E-mail: yuhuiss@163.com; Fax: +86-21-54660217; Tel: +86-21-64370045-666085
First published on 5th December 2016
Abstract
Magnesium (Mg) substituted hydroxyapatite (Mg-HAp) whiskers were hydrothermally synthesized using acetamide as a homogeneous precipitation reagent. The synthesized materials were characterized by FTIR, XRD, ICP-AES and SEM. The influence of the Mg substitution level on the lattice constants, and the stimulation of the substituted Mg ions on the proliferation of human osteoblast cells (MG-63) were investigated. The results showed that Mg-HAp whiskers with diameters of 0.2–5.0 μm and lengths up to 50 μm were obtained, and the Mg substitution amount could be well tailored by simply adjusting the initial molar ratio of Mg/(Mg + Ca) in aqueous solution. The Mg2+ incorporated into the Ca2+ crystal site, and the lattice constants decreased with the increase of the Mg-incorporation level. The biocompatibility of the synthetic products was confirmed by a culture of the osteoblasts in the extraction solutions and the suspensions of the whiskers. In addition, the Mg substitution could stimulate the proliferation of MG-63 at certain extracted concentrations. Moreover, the interactions of the whisker suspensions with MG-63 were characterized by SEM and fluorescence microscopy. The present study showed that the synthetic Mg-HAp whiskers might be applied as bioactive materials and mechanical reinforcements for bone tissue regenerations.
Introduction
Hydroxyapatite [Ca10(PO4)6(OH)2, HAp] materials and their composites are widely used as bone grafts and bone tissue engineering applications due to their excellent biocompatibility, osteo-conductive properties and similarity to the inorganic component of human bones and teeth.1–4 However, the low mechanical properties of HAp, especially the toughness severely hinder their wider clinical applications under load bearing situations.5,6 So far, many methods have been applied to solve this issue, such as using Al2O3, ZrO2, metals, carbon nanotubes and graphenes, etc. as mechanical reinforcements.5,7,8 However, most of these reinforcements are bioinert and/or non-biocompatible, which might lead to toxicity for the fabricated composites. It is considered that the whisker-shape HAp materials are the ideal choice to be used as mechanical reinforcements due to their excellent biocompatibility.4,9–11On the other hand, the HAp materials are still considered to be lack of the osteo-inductive ability and the stimulation of new bone formation, which is adverse to bone regeneration especially for large bone defects, nonunion and follow-up function restoration.4,7,12 It is well known that, as a trace element in human bone and the second most abundant intracellular divalent cation, the magnesium (Mg) plays critical roles in calcification process and mineral metabolism.5,13,14 In addition, around 67% Mg ions in vivo is located in bone tissue. While the remaining 30% Mg2+ ions present on the crystal surface of the bone, which is exchangeable for bone metabolism. The Mg involves in diverse mechanisms (e.g. acting over 300 enzymes as a cofactor or in metabolic pathways) and plays a structural role in the cell membrane and chromosomes.15–17 The studies confirm that the Mg ions can increase the adhesion, growth and alkaline phosphatase (ALP) activity of osteoblasts and bone mesenchymal stem cells (BMSCs), which facilitate osteogenic differentiation and subsequently increase bone formation and integration with host bone.18–20 So far, the studies and applications of the biomaterials with Mg component is still a hot research theme.19,21 Moreover, in the previous studies, the properties of the Mg-substituted hydroxyapatite were studied in the field of ferroelectric/pyro/piezoelectric, and the results suggested that the Mg-substituted hydroxyapatite might be the ideal potential candidate.22,23 Therefore, the Mg-substituted hydroxyapatite (Mg-HAp) whiskers can be expected as a new type biomaterial with well mechanical strengths and biological properties.
Recently, the studies suggest that the hydrolysis rate of acetamide is apparently lower than that of the traditional urea additive in the hydrothermal homogeneous precipitation method, which is beneficial to the rapid growth of whiskers at a low supersaturation.7,24,25 Hydrothermal homogeneous precipitation combines the best characteristics of the hydrothermal and homogeneous precipitation methods, which can produce the HA whiskers with a high aspect ratio. The previous studies suggested that the HA crystals preferred growth along the c-axis under this condition, using low concentration of calcium and phosphate ions as raw materials and acetamide as homogeneous precipitation reagent.23 In this study, the Mg-HAp whiskers were hydrothermally prepared using acetamide as homogeneous precipitation reagent. Then the effect of Mg substitution on lattice constants and osteoblast proliferation of the whiskers was further investigated.
Materials and methods
Synthesis and characterization of Mg-HAp whiskers
The Mg-HAp whiskers designed Mg2+/(Ca2+ + Mg2+) molar ratios of 0, 0.025, 0.05 and 0.1 were hydrothermally synthesized using acetamide as homogeneous precipitation reagent. Aqueous solutions containing 50 mmol (Ca2+ + Mg2+) ions and 29.94 mmol HPO42− were prepared by dissolving analytical grade reagents of Ca(NO3)2·4H2O, Mg(NO3)2 and NH4H2PO4 in acetamide aqueous solution with 1 mol L−1 concentration. The 0.1 mol L−1 HNO3 solution was used to adjust the pH to 2.65–2.80 to obtain clear solutions. Then around 85 mL of the obtained solution was transferred into 100 mL Teflon autoclaves and heated at 180 °C for 10 h, followed by cooling to room temperature naturally. After hydrothermal reaction, the obtained white suspensions were filtered and washed with distilled water and anhydrous ethanol for three times, respectively, and then dried at 200 °C for 48 h.The obtained products were characterized by X-ray diffraction (XRD: D/max 2550V, Rigaku, Japan) using mono-chromated Cu-Kα radiation, then the lattice constants were calculated from the determined XRD diffractions with MDI Jade 6.1 software (Materials Data Inc., USA).26 The whiskers were also characterized using Fourier transform infrared spectroscopy (FTIR: Nicolet Co., USA). The morphology and size of the whiskers were characterized with scanning electron microscopy (SEM: JSM-6700F, JEOL, Japan). The chemical compositions and molar ratio of Mg2+/(Mg2+ + Ca2+) for the whiskers were analyzed by inductively coupled plasma atomic emission spectroscopy (ICP-AES; VISTA AX, Varian Co., USA) after dissolving in 0.1 mol L−1 hydrochloric acid aqueous solution.
The effect of the Mg2+ ions released from Mg-HAp whiskers on MG-63 proliferation
To primarily evaluate the effect of the substitution of Mg2+ ions on biological responses in vitro, the effect of the ionic extracted from the whiskers on the proliferation of the human osteoblast cells (MG-63, Cell bank, Shanghai, China) were investigated. The stock extract solutions with 100 mg mL−1 concentration were first prepared by adding the whiskers into DMEM (GIBCO Invitrogen, Grand Island, NY) culture medium. After incubation for 24 h at 37 °C, the mixtures were centrifuged and the supernatant were collected. Then the diluted extracts with concentrations of 50, 25 and 12.5 mg mL−1 were prepared by diluting the stock solutions with serum-free DMEM. Subsequently, these extracts were sterilized by filtration through 0.2 μm filter membranes for cell culture experiments.The MG-63 were cultured in 96-well plates medium containing a-MEM (89%, GIBCO, Invitrogen, Grand Island, NY, USA), fetal bovine serum (10%, FBS; Gibco, USA), and penicillin streptomycin (1%, PS; Gibco, USA) at a density of 5 × 103 cell per well and cultured by incubation at 37 °C for 5 days with 5% CO2, 95% air at 100% RH. After culture for 24 h, the medium in the well was replaced by the prepared extracts.7 Subsequently, the culture medium was changed every day. After 3 days of culture, 10 μL (5 mg mL−1) of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT, Dojindo, Kumamoto, Japan) plus 100 μL of DMEM were added into each well for 4 h of additional incubation. Then the MTT solution was removed and replaced with 100 μL of dimethylsulfoxide (DMSO). After 10 min of slow shaking (Vibramax 100, Metrohm, USA), the absorbance was read at 570 nm against the reference value at 630 nm, and the results were expressed as optical density (OD).7 The average data were obtained from triplicate samples in all experiment.
The interactions of whisker suspensions with osteoblasts
The interactions of whisker suspensions with osteoblasts were further examined using FESEM and fluorescence microscopy observations. Mg-HAp whisker powders (0.3 g per sample) were dissolved in 2 mL DMEM (GIBCO Invitrogen, Grand Island, NY) culture medium, and then were seeded on 24-well plates, laid with glass coverslip at the bottom of it, for FESEM or confocal dishes for fluorescence microscopy observation. The same concentration of HAp whisker was used as the control sample in the tests. MG-63 cells were cultured in DMEM containing 10 vol% fetal bovine serum (FBS) at 37 °C with 5% CO2, 95% air at 100% RH. The cells were then seeded at a concentration of 5 × 104 cells per well on samples. After culture for 1 day, cell constructs for SEM observation were fixed with 1% glutaraldehyde, and dehydrated by sequential washing with 50%, 70%, 90%, 95% and 100% ethanol. Specimens were critical point dried using liquid CO2 and then sputter-coated with gold. As for the fluorescence microscopy observations, the cells after 24 h of culture were washed thrice with the phosphate buffer saline (PBS), then stained with 2 mmol L−1 calcein-AM (Thermo Fisher Scientific, USA) and 2 mmol L−1 PI (Thermo Fisher Scientific, USA) for 5 min. The live cells and dead cells were examined on a fluorescence microscopy (Olympus, Japan).8,27Statistical analysis
Data were analyzed for statistical significance using an analysis of variance. Differences at p < 0.05 were considered significant.Results and discussion
Characterization of Mg-HAp whiskers
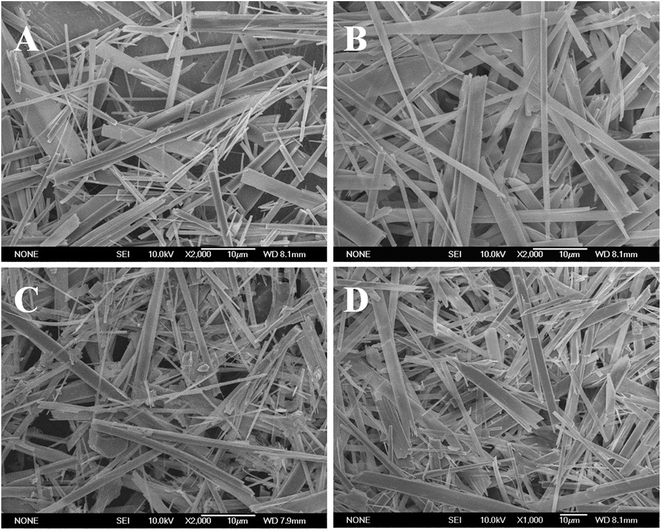
Fig. 1 presents the images of the synthetic products. The results showed that morphology in all products are whisker-like shape with diameter of 0.2–5.0 μm and length up to 50 μm. Meanwhile without particles were presented within the images, suggesting the morphology of the products was maintained after Mg-substitution. So far, the morphologies of the Mg substituted HAp materials were usually in particle- or short rod-like shapes. In present study, the Mg-HAp whiskers with length up to 50 μm were successfully synthesized by hydrothermally homogeneous precipitation method using acetamide as homogeneously precipitated reagent. The growth of the products with whisker-like shapes was due to the lower hydrolysis rate of the acetamide under the hydrothermal situation, which allows better and easier control and gives rise to rapid growth of whiskers at a low supersaturation.7,24,25XRD patterns of the synthetic HAp and Mg-HAp whiskers are shown in Fig. 2. The results revealed that all of the synthetic materials could be well identified as pure HAp phase (JCPDS card: no. 09-0432). Comparing with the pure HAp whiskers (Fig. 2a), the XRD patterns with small angle scanning (Fig. 2b) apparently showed that the corresponding peaks of the obtained Mg-HAp whiskers shifted to higher degree. In addition, the shifting degree increased with the increase of the Mg-substitution level, which was due to the decrease of the lattice constants (Table 1).2 The lattice constants calculated from the XRD characterization results further confirmed that the lattice constant c-axis and unit cell volumes (V) of the synthetic Mg-HAp whiskers was smaller than those of the pure HAp whiskers. Moreover, the V values decreased with the increase of Mg-substitution amount (Table 1). The decrease of the unit cell volumes (V) was caused by the replacement of Ca2+ ions by smaller diameter of Mg2+ ions.2 The shifting of the patterns and the deviation of lattice constants confirmed that the Mg2+ ions replaced and occupied the Ca2+ crystal positions of the HAp crystals.2,3
| Samples | Lattice constants | 2θ (°) for (300) reflection | Chemical composition | Size of the whiskers | ||||
|---|---|---|---|---|---|---|---|---|
| a (Å) | c (Å) | V (Å3) | Ca replacement by Mg (mol%) | (Ca + Mg)/P molar ratio | Length (μm) | Diameter (μm) | ||
| HAp | 9.426 | 6.895 | 530.56 | 32.763 | 0 | 1.62 | 16.81 ± 8.64 | 1.40 ± 0.80 |
| Mg2.5HAp | 9.429 | 6.886 | 530.30 | 32.783 | 1.61 | 1.65 | 30.85 ± 12.02 | 1.69 ± 0.97 |
| Mg5HAp | 9.428 | 6.887 | 530.09 | 32.822 | 2.98 | 1.63 | 37.29 ± 12.62 | 2.19 ± 1.04 |
| Mg10HAp | 9.427 | 6.883 | 529.76 | 32.863 | 6.14 | 1.62 | 34.45 ± 18.33 | 2.88 ± 1.40 |
The FTIR spectra of the obtained HAp and Mg-HAp whiskers are demonstrated in Fig. 3. The spectra were in accordance with the reported FTIR data for HAp. The adsorption peaks at 475, 564, 604, 963, 1031 and 1095 cm−1 were the characteristic bands for PO43−.1–3 The peaks at 3445 and 1638 cm−1 could be assigned to bending mode of the absorbed water. While the peak at 867 cm−1 was the characteristic band for carbonate ion in the B-site, which might come from the dissolved CO2 in aqueous solution.28 The peak at 633 cm−1 and the wide adsorption between 3200 and 3550 cm−1 were the characteristic OH bands of HAp.3 The FTIR results further confirmed that the positions of the peaks were not affected by Mg-substitution and the products were HAp crystals.
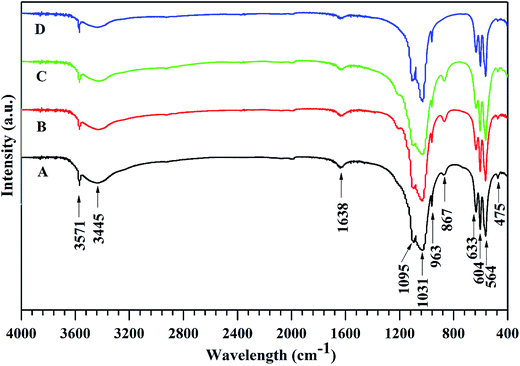 | ||
| Fig. 3 The FTIR spectra of the synthetic HAp (A), Mg2.5-HAp (B), Mg5-HAp (C) and Mg10-HAp (D) whiskers. | ||
The chemical compositions of the synthetic HAp and Mg-HAp whiskers determined by ICP-AES are showed in Table 1. The results confirmed that the prepared Mg-HAp whiskers contained Mg component, and the Mg amount increased with the increase of the initial molar ratio of Mg/(Mg + Ca) in raw materials. In addition, the (Mg + Ca)/P molar ratio of the obtained Mg-HAp whiskers was between 1.62 and 1.65, which slightly deviated from the stoichiometric HAp (Ca/P = 1.67).
The effect of the Mg2+ ions released from Mg-HAp whiskers on MG-63 proliferation
Fig. 4 shows that the proliferation rate of MG-63 can be promoted by the Mg2+ ions released from Mg-HAp whiskers at appropriate concentrations comparing with the pure HAp whiskers. The best promotion effects were found in Mg5-HAp whiskers comparing with other materials between the extract concentration of 25 and 100 mg mL−1. The results suggested that the synthetic Mg5-HAp whiskers might be the ideal potential candidate for bone regeneration. The previous studies have confirmed that Mg-doping could promote the proliferation and osteogenic differentiation of the osteoblasts and BMSCs in vitro as compared with the pure HAp materials.21,29 Mg2+ ion plays significant role in controlling the growth and metabolism of the animal cells.30 The study of Park et al.31 further confirmed that the biocompatibility and resorption of HAp grafts were enhanced by Mg-doping in vivo tests of New Zealand White rabbits. In present study, the HAp whiskers with appropriate concentrations of Mg-substitution showed well response with MG-63 cells, which might benefit for bone regeneration.The interactions of whisker suspensions with osteoblasts
Fig. 5 shows SEM images of the interactions between the whiskers and the osteoblasts. The results showed that the osteoblasts well spread on and attached to the whisker specimen surface or imbedded into the specimen. The lengths of cytoplasmic extensions were observed to range from about 20 to 60 mm. The cytoplasmic extensions are regions of the cell plasma membrane that contain a meshwork or bundles of actin-containing microfilaments, which permit the movement of the migrating cells along a substratum. These features were observed to be similar on all the three materials tested. In addition, the cell adhesion and viability on the Mg-HAp whiskers were similar to those on the HAp whiskers as control sample.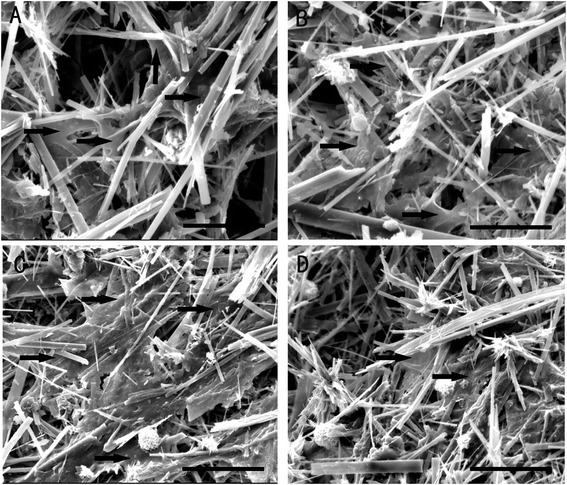 | ||
| Fig. 5 MG-63 osteoblasts growing on Mg-HAp (A), Mg2.5-HAp (B), Mg5-HAp (C), and Mg10-HAp (D) whiskers after 1 day of culture. *bar = 50 μm. | ||
Cells cultured for 1 day on the synthetic HAp and Mg-HAp whiskers were further observed with fluorescence microscopy (Fig. 6). The live cells (stained green) appeared to have adhered when seeded on all four materials. Visual examination indicated that the density of live cells adherent to each material appeared to be similar. All four materials had very few dead cells (stained red).32 The SEM and fluorescence microscopy observation results might further preliminary suggest the biocompatibility of the synthetic Mg-HAp whiskers.
 | ||
| Fig. 6 Fluorescent images of MG-63 cultured on Mg-HAp (A), Mg2.5-HAp (B), Mg5-HAp (C), and Mg10-HAp (D) whisker surfaces for 24 h with live and dead cell staining. *bar = 100 μm. | ||
Conclusions
In this study, the Mg substituted HAp (Mg-HAp) whiskers with 0–6.14 mol% of Ca substituted by Mg, and length up to 50 μm were successfully hydrothermally synthesized using acetamide as homogeneous precipitation reagent. The Mg substitution level could be facilely tailored by changing the initial molar ratio of Mg/(Mg + Ca) in the raw materials. In addition, the Mg2+ ions replaced part of Ca2+ ions, and the lattice constants decreased with the increase of the Mg-substitution amount. The cell culture results showed that the ionic products of Mg-HAp whiskers stimulated the proliferation of MG-63 at certain concentrations of Mg2+ ions. Especially the Mg5-HAp component was optimal for cell activity. Our study suggests that the Mg-HAp whiskers might be a potential candidate as a new bioactive material in bone regeneration application field.Acknowledgements
The authors gratefully acknowledge the support of the Natural Science Foundation of China (Grant no. 81371937), Science and Technology Commission of Shanghai Municipality (Grant no. 13ZR1437700 & 14JC1492400), and Shanghai Municipal Commission of Health and Family Planning (20134224).References
- J. C. K. Lin, Y. Zhu, W. Wu, G. Cheng, Y. Zeng and M. Ruan, Cryst. Growth Des., 2009, 9(1), 177–181 Search PubMed.
- K. Lin, P. Liu, L. Wei, Z. Zou, W. Zhang, Y. Qian, Y. Shen and J. Chang, Chem. Eng. J., 2013, 222, 49–59 CrossRef CAS.
- K. Lin, Y. Zhou, Y. Zhou, H. Qu, F. Chen, Y. Zhu and J. Chang, J. Mater. Chem., 2011, 21, 16558–16565 RSC.
- K. Lin, C. Wu and J. Chang, Acta Biomater., 2014, 10, 4071–4102 CrossRef CAS PubMed.
- M. Bricha, Y. Belmamouni, K. E. Mabrouk and J. M. F. Ferreira, Int. J. Appl. Ceram. Technol., 2013, 12, 264–272 CrossRef.
- K. Lin, L. Chen and J. Chang, Int. J. Appl. Ceram. Technol., 2012, 9, 479–485 CrossRef CAS.
- J. Xu, Y. Yang, R. Wan, Y. Shen and W. Zhang, Sci. World J., 2014, 2014, 863137 Search PubMed.
- G. Jin, H. Qin, H. Cao, S. Qian, Y. Zhao, X. Peng, X. Zhang, X. Liu and P. K. Chu, Biomaterials, 2014, 35, 7699–7713 CrossRef CAS PubMed.
- A. Azhari, E. Toyserkani and C. Villain, Int. J. Appl. Ceram. Technol., 2014, 12, 8–17 CrossRef.
- K. Lin, J. Chang, R. Cheng and M. Ruan, Mater. Lett., 2007, 61, 1683–1687 CrossRef CAS.
- S. Bose, A. Banerjee, S. Dasgupta and A. Bandyopadhyay, J. Am. Ceram. Soc., 2009, 92, 323–330 CrossRef CAS.
- W. Zhang, Y. Shen, H. Pan, K. Lin, X. Liu, B. W. Darvell, W. W. Lu, J. Chang, L. Deng, D. Wang and W. Huang, Acta Biomater., 2011, 7, 800–808 CrossRef CAS PubMed.
- H. Yuan, H. Fernandes, P. Habibovic, J. de Boer, A. M. Barradas, A. de Ruiter, W. R. Walsh, C. A. van Blitterswijk and J. D. de Bruijn, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 13614–13619 CrossRef CAS PubMed.
- L. A. Martini, Nutr. Rev., 1999, 57, 227–229 CAS.
- S. Ziani, S. Meski and H. Khireddine, Int. J. Appl. Ceram. Technol., 2014, 11, 83–91 CrossRef CAS.
- L. Wu, B. J. Luthringer, F. Feyerabend, A. F. Schilling and R. Willumeit, Acta Biomater., 2014, 10, 2843–2854 CrossRef CAS PubMed.
- B. M. Altura, Magnesium Trace Elem., 1991, 10, 167–171 CAS.
- M. E. Shils, M. Shike, A. C. Ross, B. Caballero and R. J. Cousins, Modern Nutrition in Health and Disease, Lippincott Williams and Wilkins, UK, 10th edn, 2006 Search PubMed.
- H. M. Wong, S. Wu, P. K. Chu, S. H. Cheng, K. D. Luk, K. M. Cheung and K. W. Yeung, Biomaterials, 2013, 34, 7016–7032 CrossRef CAS PubMed.
- Z. Wu, T. Tang, H. Guo, S. Tang, Y. Niu, J. Zhang, W. Zhang, R. Ma, J. Su, C. Liu and J. Wei, Colloids Surf., B, 2014, 120, 38–46 CrossRef CAS PubMed.
- M. Shimaya, T. Muneta, S. Ichinose, K. Tsuji and I. Sekiya, Osteoarthr. Cartil., 2010, 18, 1300–1309 CrossRef CAS PubMed.
- J. Yoo, S. Dang le, B. Chon, T. Joo and G. C. Yi, Nano Lett., 2012, 12, 556–561 CrossRef CAS PubMed.
- S. Zheng, Y. Kong, H. Liu, S. Chen, L. Zhang, S. Liu and J. Xu, Opt. Express, 2012, 20, 29131–29136 CAS.
- Y. Yan, Q. Ding, Y. Huang, S. Han and X. Pang, Appl. Surf. Sci., 2014, 305, 77–85 CrossRef CAS.
- H. Zhang and B. W. Darvell, Acta Biomater., 2011, 7, 2960–2968 CrossRef CAS PubMed.
- H. Zhang, Y. Wang, Y. Yan and S. Li, Ceram. Int., 2003, 29, 413–418 CrossRef CAS.
- W. K. Yeung, I. V. Sukhorukova, D. V. Shtansky, E. A. Levashov, I. Y. Zhitnyak, N. A. Gloushankova, P. V. Kiryukhantsev-Korneev, M. I. Petrzhik, A. Matthews and A. Yerokhin, RSC Adv., 2016, 6, 12688–12698 RSC.
- S. Koutsopoulos, J. Biomed. Mater. Res., 2002, 62, 600–612 CrossRef CAS PubMed.
- K. Lin, J. Chang, X. Liu, L. Chen and Y. Zhou, CrystEngComm, 2011, 13, 4850–4855 RSC.
- Y. L. Cai, J. J. Zhang, S. Zhang, S. S. Venkatraman, X. T. Zeng, H. J. Du and D. Mondal, Biomed. Mater., 2010, 5, 054114 CrossRef CAS PubMed.
- J. W. Park, Y. J. Kim, J. H. Jang and H. Song, Clin. Oral Impt. Res., 2010, 21, 1278–1287 CrossRef PubMed.
- R. Plowright, N. Dinjaski, S. Zhou, D. J. Belton, D. L. Kaplan and C. C. Perry, RSC Adv., 2016, 6, 21776–21788 RSC.
Footnote |
| † These authors contributed equally to this study, and should be considered as co-first authors. |
| This journal is © The Royal Society of Chemistry 2016 |