Facile synthesis of the Basolite F300-like nanoscale Fe-BTC framework and its lithium storage properties†
Xiaoshi Hua,
Xiaobing Lou a,
Chao Lia,
Yanqun Ninga,
Yuxing Liaoa,
Qun Chena,
Eugène S. Manangabc,
Ming Shen
a,
Chao Lia,
Yanqun Ninga,
Yuxing Liaoa,
Qun Chena,
Eugène S. Manangabc,
Ming Shen *a and
Bingwen Hua
*a and
Bingwen Hua
aState Key Laboratory of Precision Spectroscopy, Shanghai Key Laboratory of Magnetic Resonance, Engineering Research Center for Nanophotonics & Advanced Instrument, School of Physics and Materials Science, Ministry of Education, East China Normal University, PR China. E-mail: mshen@phy.ecnu.edu.cn
bThe Graduate Center, Physics, The City University of New York, 365 Fifth Avenue, New York, New York 10016, USA
cThe Graduate Center, The City University of New York, 365 Fifth Avenue, New York, New York 10016, USA
First published on 2nd December 2016
Abstract
The Fe-BTC material commercialized as Basolite F300 is one of the most studied MOFs due to its unique features and wide range of industrial applications. In this article, Basolite F300-like Fe-BTC MOF materials were prepared directly with the protonated carboxylated ligand, circumventing the use of an alkaline solution as in previous work, by selecting an appropriate iron source. Results from the detailed characterization indicate that the obtained Fe-BTC was very similar to the commercial counterpart and the one prepared under the alkaline conditions in terms of many physicochemical properties. Besides, the Fe-BTC reported herein was scaled down to the nano-regime to afford nanoscale metal–organic frameworks (nMOFs), which is advantageous for its potential applications. More importantly, the current interest in MOFs in the area of rechargeable batteries has driven us to investigate its electrochemical performance with respect to lithium storage. It was shown that the nanoscale Fe-BTC MOF exhibits an outstanding electrochemical performance with a high reversible capacity up to 1021 mA h g−1 after 100 cycles at a current density of 100 mA g−1 and capacities up to 436 and 408 mA h g−1 after 400 cycles at a higher current density of 500 and 1000 mA g−1, respectively. Our results on the Fe-BTC MOF highlight the potential for high power Li-ion batteries (LIBs) applications.
Introduction
Metal–organic frameworks (MOFs) are currently being widely exploited in numerous catalytic, sensing, separation and sorptive applications.1,2 Fe-based structures are amongst the most promising type of MOF materials, mostly ascribed to their relatively high thermal stability, low cost, and low toxicity. Fe-BTC (BTC: 1,3,5-benzenetricarboxylate), which has already been scaled up through an electrochemical route and commercialised by BASF SE under the name of Basolite F300,3,4 is a disordered network of locally ordered units composed by acetate-model moieties with Fe3+ irons.5 Notably, it is one of the most widely and successfully applied metal–organic framework (MOF)-based catalysts for reactions requiring either acidic or redox centers.6,7 Moreover, this material has attracted great interest in the area of biomedical drug delivery.8 Recently, Fe-BTC was successfully employed in separation of small organic compounds in liquid phase.9,10 These applications are based on its exchangeable coordination positions, biocompatibility, water stability, high specific surface area, relatively strong acid sites, redox centers, and notable framework stability.3,6,11To the best of our knowledge, only two publications concerning the preparation of Fe-BTC materials are reported in the literature of material science.3,7 Majano et al. indirectly prepared Fe-BTC materials through a mixed Fe2+/Fe3+ layered double hydroxide (LDH) precursor.3 The most recently reported synthesis procedure is based on a linker salt approach12 adapted to the ferric chloride-trimesate, through pH modification by adding some bases (e.g., NaOH).7 When aiming at the future expanding applications, the simplification of experimental procedures and the present commercial synthesis strategies that involve the use of electrochemical cells is highly desirable.
Nowadays, there is much interest in exploiting the applications of MOFs in energy storage.13–15 More specifically, pure MOFs are promising for LIBs due to exceptional high surface area and porosity, high thermal stability, tunable redox properties, and controllable structures for lithium-ion intercalation and deintercalation.13–15 Moreover, both the inorganic building units16–22 (metal ions or clusters) and the electron-donating organic ligands22–27 in MOFs can serve as active sites for electrochemical processes. However, their applications in practical LIBs is hindered by their poor electrochemical activity and poor cycling performance arised from structural instability in battery discharge and charge processes.23 Recent work on the catalytic properties of Fe-BTC has encouraged us to address its potential for electrochemical performance as electrode materials because of its notable framework stability and redox reactivity.6
In this article, Basolite F300-like Fe-BTC material was readily prepared by mixing a selected iron source (ferric nitrate) with the 1,3,5-benzenetricarboxylic acid (H3BTC) ligand in water at ambient temperature, without the pH modification or the use of a precursor. In general, the potential applications of MOFs are strongly related to their textural properties such as topologies, compositions, and microstructures.7 Therefore, the Fe-BTC material prepared in this work was subjected to a detailed and combined characterization by means of power X-ray powder diffraction (PXRD), Fourier transform infrared spectroscopy (FTIR), thermogravimetric analysis (TGA), nitrogen adsorption/desorption isotherms, field emission scanning electron microscopy (FESEM), transmission electron microscopy (TEM), and energy dispersive X-ray (EDX) spectroscopy. For comparison purposes, the features of the commercial Basolite F300 were also investigated. We then carried out for the first time the investigation of the Fe-BTC MOF as an anode material for Li-ion batteries.
Experimental
All chemicals were purchased from commercial suppliers and used as received.Synthesis of the Fe-BTC materials
The synthesis of Fe-BTC material was carried out via the reaction of H3BTC with two different ferric salts (ferric nitrate nonahydrate (Fe(NO3)3·9H2O) and ferric chloride hexahydrate (FeCl3·6H2O)) in water at room temperature. We have found that the use of Fe(NO3)3·9H2O as Fe source increased the rate of reaction when compared with that of FeCl3·6H2O, as explained in the next section. Typically, 3.26 g (9.2 mmol) Fe(NO3)3·9H2O and 1.13 g (5.4 mmol) H3BTC were mixed into 40 mL deionized water. The resultant suspension was maintained under stirring at room temperature, leading to immediate formation of light orange solids. In this experiment, the reaction time was varied from 10 min to 24 h in order to track the crystallization reaction. Afterwards, the product obtained with different reaction time were filtered, washed with deionized water and ethanol, and finally dried under air.Electrochemical measurements
Prior to the electrode fabrication, the as-synthesized Fe-BTC MOF was purified three times by solvent extraction treatment with deionized water (350 mL) and ethanol (350 mL) at 70 °C for 24 h. Then the product was dried in a vacuum desiccator at 150 °C for 12 h to remove any water molecules adsorbed at the surface or coordinated to the metal centers. For electrochemical testing, the activated Fe-BTC sample, Super-P carbon black and polyacrylic acid (PAA) binder, with a weight ratio of 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, were homogenously mixed in N-methyl-2-pyrrolidone (NMP) to produce a uniform slurry. Next, the resultant slurry was coated on a 15 μm thick copper foil using a doctor blading method. Finally, these electrodes were dried at 110 °C in vacuum for 12 h, and punched into 14.0 mm-diameter disks. The active material contained in the electrode weighted about 2.0 mg. Coin-type cells (CR2032) were assembled in the glove box with oxygen and water levels of less than 0.1 ppm, using lithium metal foil as the counter and reference electrode in addition to the Celgard 2325 membrane (diameter of 19.0 mm) as separator. The electrolyte was 1 M LiPF6 in ethylene carbonate (EC)/diethylene carbonate (DEC)/ethylmethyl carbonate (EMC) (1
10, were homogenously mixed in N-methyl-2-pyrrolidone (NMP) to produce a uniform slurry. Next, the resultant slurry was coated on a 15 μm thick copper foil using a doctor blading method. Finally, these electrodes were dried at 110 °C in vacuum for 12 h, and punched into 14.0 mm-diameter disks. The active material contained in the electrode weighted about 2.0 mg. Coin-type cells (CR2032) were assembled in the glove box with oxygen and water levels of less than 0.1 ppm, using lithium metal foil as the counter and reference electrode in addition to the Celgard 2325 membrane (diameter of 19.0 mm) as separator. The electrolyte was 1 M LiPF6 in ethylene carbonate (EC)/diethylene carbonate (DEC)/ethylmethyl carbonate (EMC) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 vol%). The assembled cells were allowed to rest for 24 h prior to electrochemical measurements.
1 vol%). The assembled cells were allowed to rest for 24 h prior to electrochemical measurements.
Galvanostatic charge–discharge cycles were recorded on a multichannel LAND cycler (Wuhan Kingnuo Electronic Co., China) in the voltage range of 0.01–3.0 V. Cyclic voltammetry (CV) was performed on a CHI 660e electrochemical workstation (ChenHua Instruments Co., China) between the voltage range of 0.001–3.0 V at a scan rate of 0.2 mV s−1. EIS spectra were recorded with the frequency range of 1 × 105 Hz to 1 × 10−2 Hz.
Characterizations
PXRD patterns were recorded with a Rigaku Ultima IV X-ray Diffractometer using Cu-Kα radiation (V = 35 kV, I = 25 mA, λ = 1.5418 Å). FTIR spectra were recorded on a Nexus670 infrared spectrometer (Nicolet) within the region of 400–4000 cm−1 with samples prepared in the form of potassium bromide pellets. TGA was measured under air flow from room temperature to 800 °C at a heating rate of 10 °C min−1 using a STA 449 F3 Jupiter® simultaneous thermo-analyzer (NETZSCH Gerätebau GmbH). N2 adsorption/desorption isotherms were measured at 77 K in a 02108-KR-1 system (Quantachrome) after degassing the samples at 150 °C under high vacuum for 12 h. Surface areas were estimated by using the Brunauer–Emmett–Teller (BET) method. External surface areas were estimated by applying the t-plot method and pore size distributions were calculated by applying the Barrett–Joyner–Halenda (BJH) method to the adsorption branches of the N2 isotherms. The microstructure and morphology of the samples were observed through FESEM and TEM measurements, which were carried out on a Hitachi S-4800 and a JEOL JEM-2100F instrument, respectively. The composition of the samples was analysed by using an EDX spectrometer attached to SEM. Soft X-ray absorption spectroscopy (sXAS) was performed in beamline BL08U1A at Shanghai Synchrotron Radiation Facility (SSRF, BL08U1A). For ex situ PXRD and sXAS tests, the cycled electrodes were disassembled in an Ar-filled glovebox, rinsed with anhydrous dimethyl carbonate, dried naturally in the argon-filled glovebox for a few hours, and then transferred directly to the PXRD or sXAS sample chamber.Results and discussion
The PXRD patterns of the Fe-BTC samples prepared by using ferric nitrate at room temperature for different crystallization times are shown in Fig. 1, in comparison with that of the commercial F300 sample (supplied by Sigma-Aldrich). It is noteworthy that characteristic Fe-BTC reflections identical to commercial Basolite F300 in terms of the position and intensities occurred in XRD patterns of samples obtained with crystallization times as short as 10 min. This suggests that a material that resembles the Basolite F300 can be formed instantaneously by using our new synthetic approach (see Fig. S1† for the Fe-BTC building block). The presence of relatively broad bands is due to the small size of the crystals and the disordered structure of these materials. In fact, the average crystal size of the as-synthesized Fe-BTC was estimated to be 7.5 nm by applying the Scherrer equation to the XRD data which is very close to that of commercial Basolite F300 (7.4 nm). Furthermore, no obvious change of the XRD patterns was found for samples obtained with reaction time up to 7 d, which proves the stability of the crystals formed during the synthesis. It should be pointed out that, Basolite F300-like material can also be directly obtained using ferric chloride hexahydrate as Fe source, though the final product yielded was low (46%) as can be seen in Fig. S2.† This may be ascribed to the stronger coordination ability of Cl− than that of NO3−, which led to a slow precipitation rate of Fe3+ with the carboxylate. It is widely accepted that the PXRD technique is mostly employed as a fingerprint to identify/refute some already-known and solved structure(s).7,28 Unfortunately, because of the low-resolution of patterns in these disordered materials, XRD characterization is quite limited, which could only serve as a rough fingerprinted evidence of the crystalline nature.7 Taking this into account, extra and unequivocal indications (about structure features) from other techniques are required to assess the similarities and differences between the commercial and the synthetic Fe-BTC materials. | ||
| Fig. 1 PXRD patterns of the commercial Basolite F300 and the Fe-BTC materials prepared with different indicated precipitation times. | ||
IR spectroscopy, commonly employed as fingerprint of material nature, is regarded as a complementary technique to PXRD due to its advantage of observing short-range information of organic entities.28,29 Fig. 2 shows the FTIR spectra of the nanocrystalline samples Basolite F300 and the as-synthesized Fe-BTC. The spectra of free protonated linker H3BTC is also included as a reference. As expected, IR spectra of the linker differ greatly from that of the two iron trimesates, due to the deprotonation of the carboxylate groups and consequent bond to the metal iron. It is shown that there is a very good agreement on band position and relative intensity between IR spectrum of the two iron trimesates. In addition, the broad trimesate bands at 1112 and 941 cm−1 of the two Fe-BTC are consistent with the low crystallinity of these samples.11 Therefore, the here-described Fe-BTC material not only has a similar crystalline ordering (or topology) to the commercial Basolite F300 material (Fig. 1), but also exhibit similar linker environment (Fig. 2). This suggests that the nature of our Fe-BTC material is probably equivalent to Basolite F300.
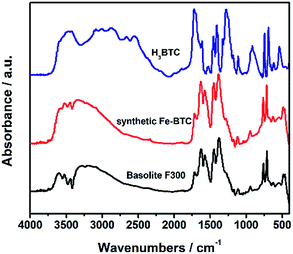 | ||
| Fig. 2 FTIR spectra of commercial Basolite F300, the as-synthesized Fe-BTC, and the free linker H3BTC. | ||
Thermal stability is of vital importance for practical applications of MOF materials. Fig. 3 shows the TGA profiles of the two Fe-BTC samples, whereas Table 1 shows the corresponding quantification data extracted from the TGA plots. These results also indicate the strong similarity between the two Fe-BTC materials. It is shown that the two Fe-BTC materials decompose exactly at the same temperatures (307 °C) below which the removal of solvents took place. The difference in the solvent content of the MOF materials is expected since the samples were not exposed to a controlled atmosphere. Indeed, the profiles of the lab-made Fe-BTC sample and the organic/residual loss ratio (Table 1) estimated from TG analysis were very similar with those of the commercial one.
| Sample | Weight loss 1 (solvent) | Weight loss 2 (linker) | Residual wt% | BTC/Fea | ||
|---|---|---|---|---|---|---|
| T/°C | wt% | T/°C | wt% | |||
| a Fe/BTC molar ratio estimated from TGA curves assuming that the weight loss is exclusively due to the decomposition BTC3− and that the residue is Fe2O3. | ||||||
| Basolite F300 | Ambient temperature | 14.2 | 307 | 50.4 | 35.4 | 0.55 |
| Synthetic Fe-BTC | Ambient temperature | 29.6 | 307 | 40.1 | 30.3 | 0.51 |
We then applied nitrogen adsorption–desorption isotherms to assess the porosity parameter which can be important for lithium storage applications. Fig. 4 shows the results on the two Fe-BTC samples measured at 77 K, whereas Table 2 complies the corresponding porosity parameters estimated from the N2 adsorption/desorption isotherms. It can be seen that the synthetic Fe-BTC material achieves a higher pore volume of 0.61 cm3 g−1 as well as a high total surface area (SBET) of 1125 m2 g−1, which is slightly larger than that of Basolite F300 (1055 m2 g−1, in accordance with previous reports).3,7,11,30,31 The external surface areas of the two materials were similar. The larger value of total SBET and Vpore for the synthesized Fe-BTC may be due to the reduced unreacted carboxylic acid linker within the MOF pores, as suggested by the slightly lower BTC/Fe ratio (Table 1).3,7,11,12 In addition, the corresponding pore size distributions (PSD) curve (inset in Fig. 4) of the synthetic Fe-BTC indicates the existence of some cavities with narrow pore distribution and a diameter of 1.2 nm,7,30,31 which is also consistent with that of Basolite F300.
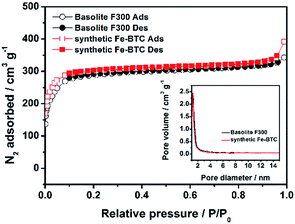 | ||
| Fig. 4 Nitrogen adsorption–desorption isotherms and the corresponding pore size distribution plots (inset) of the Basolite F300 and the synthetic Fe-BTC. | ||
| Sample | SBETa/m2 g−1 | Sextb/m2 g−1 | Vporec/m3 g−1 | PSD peaksd/nm |
|---|---|---|---|---|
| a Total surface area estimated by BET method.b BET external surface area form t-plot. External SBET is the difference between the total SBET and the microporous SBET.c Total pore volume, measured at p/p0 = 0.99.d Peaks found in pore size distribution by the applying BJH method to the adsorption branches. It should be note that the BJH method used in the generation of PSD plots from the isotherm may systematically underestimates the pore diameter.7 | ||||
| Basolite F300 | 1055 | 113 | 0.53 | 1.2 |
| Synthetic Fe-BTC | 1125 | 92 | 0.61 | 1.2 |
The samples were also studied by EDS elemental microanalysis. As shown in the spectrum (Fig. S4†), the synthetic samples contain only Fe, C and O elements. The elemental mapping image show well matched spatial distributions of these elements. In contrast, Basolite F300 showed some traces of S as an unexpected impurity element11 (Fig. S3†).
It is noted here that nanoscale metal–organic frameworks (nMOFs) with a uniform size and good dispersion are highly desirable and extremely important for areas of application such as liquid-phase catalysis,32 energy storage33,34 and biomedicine.35–40 Despite the similar textural properties of the two Fe-BTC materials according to characterization discussed above, the lab-made Fe-BTC have the additional advantage of being nanometer-scaled for potential applications. Fig. 5 shows some representative SEM and TEM images of Basolite F300 and the synthetic Fe-BTC. It was found that Basolite F300 is formed by two types of particles with the size around 10 μm (ref. 7) (Fig. 5a and b). The predominant particles, shown at the top of Fig. 5a and enlarged in Fig. S5a,† correspond to nanocrystals covering the surface. The less predominant particles, shown at the left bottom of Fig. 5a and enlarged in Fig. S5b,† scarcely contain some nanocrystals on the surface. In contrast with this bulk property and morphology heterogeneity, the lab-made Fe-BTC is formed by homogeneous and well-dispersed nanoparticles, having a size of about 200 nm (Fig. 5c and d). Each nanoparticle takes the form on a granular shape. These results indicate that the as-prepared Fe-BTC nanoparticles are well-separated in bulk state, which is not observed elsewhere.3,7 According to the estimated crystal size for this sample (ca. 7 nm), the Fe-BTC nanoparticles must be formed by a few Fe-BTC nano-crystals/domains12 (Fig. S6†).
 | ||
| Fig. 5 SEM (a and c) and TEM (b and d) of Fe-BTC samples: (a and b) Basolite F300; (c and d) as-synthesized Fe-BTC. | ||
It is worth mentioning that, the only two reported semiamorphous Fe-BTC materials3,7 in the literature have similar crystallinity, thermal stability and BET areas to the commercial one. However, the scale of the Fe-BTC prepared in an aqueous sodium hydroxide solution (in the range of 10–50 μm) is obviously larger than what we have found in this work.7 Likewise, the Fe-BTC crystals obtained from layered iron hydroxides (also under alkaline conditions) agglomerated in a similar manner as the commercial Basolite F300.3 It is assumed that the pH modification or the use of base or acid in the reaction system has a significant influence on the particle sizes of resulting MOFs, which has also been observed in previous work.40 Therefore, the present study may provide the most straightforward and efficient approach to prepare Basolite F300-like Fe-BTC material with high quality.
Recently, MOFs have attracted considerable attention in the areas of lithium storage.13–15 Inspired by previous work, we have also tested the electrochemical properties of as-obtained Fe-BTC MOF. We firstly examined the oxidation state of Fe elements during cycling by ex situ sXAS measurements, as shown in Fig. 6e. It was found that the Fe3+ has been reduced to Fe2+ after the first discharge, as suggested by the increase of the intensity ratio of Fe-L3 feature at 706 and 708 eV.41,42 However, Fe2+ was not reversibly oxidized back to Fe3+ after the charge process, which might be explained by the fact that the oxidation of Fe2+ in MOF materials usually occurs at a high voltage (ca. 3 V), as found in previous work.21,43 Fig. 6a shows the CV curves of initial five cycles of the synthesized samples. It is evident that there is distinct difference between the first cycle and the following cycles, especially for the discharge branches. The first cathodic scan results in a small peak at 1.04 V, a broad one at 0.37 V and a sharp high-intensity peak at 0.14 V. These peaks might be attributed, respectively, to lithium intercalation in the nanoparticle sites,46 formation of solid electrolyte interface (SEI) layer, and reduction of Fe3+ to Fe2+ as well as insertion of Li+ ion to the organic moiety.44–47 The first anodic scan shows only one main peak at 1.00 V which might be ascribed to the Li deinsertion from the organic ligand without the occurrence of Fe2+ oxidation based on the sXAS results.44–47 The redox participation of the conjugated organic carboxylates has already been proved in previous work by us and other researchers.27,44,45 For the second scan, the cathodic peak at 1.04 V and 0.37 V disappeared and the intensity of cathodic one at 0.14 V decreased, leaving an initial irreversible capacity. Furthermore, the proposed redox pair peaks ascribed to the reversible Li-ion insertion/deinsertion to organic moiety shifted slightly to higher potentials. The small shift in the peak potentials is an indication of the ‘formation’ or ‘conditioning’ of the electrode in the first few cycles, during which the active material undergoes minor structural rearrangement and makes good electrical contact with the conducting carbon particles in the composite electrode, the current-collector, and the liquid electrolyte.48 After the second scan, the CV curves mostly overlap, indicating good reversibility and capacity stability.
Discharge–charge cycling is firstly carried out in the voltage window of 0.01–3.0 V (vs. Li/Li+) at a current of 100 mA g−1 up to 100 cycles, and the corresponding profiles are shown in Fig. 6b. During the first discharge, the voltage steeply dropped to 0.89 V. Then, a clear plateau at around 0.79 V, followed by a sloping voltage regions located at ca. 0.46–0.01 V, were observed, which might be assigned to the formation of SEI film, reduction of Fe3+ to Fe2+ and insertion of Li+ into the benzenedicarboxylate groups, respectively. When the discharged electrode was charged to 3.0 V, a sloping voltage plateau at ca. 1.05 V was observed, suggesting the extraction of Li+ from the benzenedicarboxylate groups. The initial discharge and charge capacities were found to be 1765.5 and 683.2 mA h g−1, respectively. The low coulombic efficiency of 38.70% may be due to the irreversible capacity loss, including decomposition of electrolyte to form SEI layers, interfacial lithium storage, and irreversible reduction of Fe3+.25,27 The second discharge curve was different in shape from the first discharge curve and the sloping region was slightly higher, whereas the two charge curves were analogous. These results are consistent with the cyclic voltammetry (CV) curves shown in Fig. 6a. Further work will be carried out to investigate in detail on the Li+ insertion and extraction mechanism. The capacity vs. cycle number plot, as shown in Fig. 6d, indicates that a reversible capacity of 1021.5 mA h g−1 is stable up to 100 cycles. Such a high reversible capacity has scarcely been reported previously on the MOFs-based anode materials (Table S1†). Furthermore, its coulombic efficiency was maintained at ∼99.20% (Fig. 6d) after the 1st cycle, and the nearly 100% coulombic efficiency implies the stability of the SEI layer and minimal side reactions.49–51 The rate performance of the Fe-BTC electrode was measured by a multiple-step galvanostatic strategy at various current densities, as illustrated in Fig. 6c. With the gradually increase of current densities from 100 to 200, 400, 800, 1200, and to 2000 mA g−1, the Fe-BTC electrode could deliver an average charge/discharge capacity of 873.8/915.7, 990.1/996.3, 877.2/880.5, 694.0/694.8, 523.2/525.0, and 302.8/304.8 mA h g−1, respectively. More important is that, a high value of 1000.5/1001.2 mA h g−1 was recovered when the current density was reversed back to 100 mA g−1, which demonstrated the robustness of the MOF structure in response to abrupt current changes.
High-rate anode materials hold a great promise for actual applications in LIBs. In our case, even if the electrode was discharged using a specific current of 0.5 A g−1 and 1 A g−1, it could still deliver a discharge capacity of 436.0 mA h g−1 and 408.8 mA h g−1 after 400 cycles, while maintaining a nearly 100% coulombic efficiency (Fig. S7†). Such a high capacity retention at high charge/discharge rates is also significantly better than previously reported MOFs-based anode materials (Table S1†). To confirm the cycle stability, ex situ PXRD patterns of the Fe-BTC electrode before and after the electrochemical test were measured (Fig. 6f). It was shown that the structure of Fe-BTC was robust, despite Fe3+-to-Fe2+ reduction, which facilitates the Li-ion insertion/extraction and the electrochemical stability of electrode.
For comparison, the lithium storage performance of the bulk counterpart Basolite F300 was also examined at a current of 500 mA g−1. As expected, a much lower reversible capacity of 300.0 mA h g−1 was obtained, which suggests the importance of high-power electrode materials by reducing the dimension down to the nano-metre scale.33,34
The EIS spectra of the Fe-BTC electrode at different cycles at delithiated state with an enlarged view of the high frequency region, as well as the equivalent circuit used for fitting are shown in Fig. 7. The semicircle in the medium frequency region in the Nyquist plots are assigned to the charge-transfer resistance (Rct), caused by the faradic reaction, which was correlated with the intercalation and deintercalation of cations, while that at the high frequency is attributed to the solid electrolyte interface (SEI) film resistance and contact resistance (Rs).52,53 The linear region in low frequency region corresponds to the Warburg impedance (Zw), which is ascribed to solid state diffusion of Li+ into the electrode. The Rs and Rct were relatively small and changed slightly with cycling, which implies that the diffusion of the Li-ions and electrons of the Fe-BTC electrode is fast during charge/discharge process, resulting in outstanding electrochemical rate capability.54,55
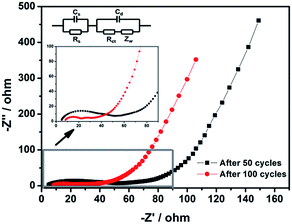 | ||
| Fig. 7 Impedance spectroscopic study of Fe-BTC electrode after different numbers of cycles in a fully charged state. | ||
It should be pointed out that the abnormal increase of the specific capacity during unremitting cycles (Fig. 6c and d and S7†) might be attributed to the formation of a polymer/gel-like organic layer and the activation of the active material, which was also observed elsewhere.56–61 A detailed investigation of the capacity rise phenomenon is in progress.
The high capacity and high-rate performance of the Fe-BTC MOF material might be attributed to the following factors. Firstly, the robust structure of the Fe-BTC MOF, which has been revealed in heterogeneous catalysis oxidation and the PXRD results, may enable Li-ion insertion/deinsertion with long cycle life. Secondly, the high porosity of Fe-BTC MOF offers open structures for migration of lithium ions within the framework and active reaction sites accessible for lithium ion insertion/desertion during charge and discharge. Lastly, the nanoscale sizes (200 nm) of the Fe-BTC material can provide short diffusion length for both Li ions and electrons, and, the large external surface areas (92.21 m2 g−1) of the Fe-BTC material may facilitate fast Li ion transport at the interface between the electrode and the electrolyte, both of which benefit the charge-transfer rate and thus enhance the rate capability.
Conclusions
We found that high-quality Basolite F300-like Fe-BTC material can be readily prepared by using a new scheme in which ferric nitrate was employed as iron source instead of ferric chloride. This approach circumvents the use of additional base to deprotonate the groups of the linker for coordination. It was shown that the new Fe-BTC sample was very much similar to the commercial product prepared under alkaline condition (also including the one conversed from a LDH) in many physicochemical properties. More specifically, the long- and short-range structure features, thermal stability, Fe and BTC environment, the BTC conformation, surface area and pore volume are very similar. However, the herein reported Fe-BTC are nano-sized (200 nm), separated in bulk sate, which should be advantageous for many potential applications. We believe that, this simplified and straightforward preparation of high-quality Basolite F300-like Fe-BTC will be extraordinarily attractive for its industrial applications. Notably, we carried out, for the first time, a preliminary investigation into its electrochemical performance toward lithium storage. It was found that this material shows attractive electrochemical activity with respect to lithium, offering great promise as a high capacity and high rate anodic material.Acknowledgements
This work was supported by National Natural Science Foundation of China (21373086), National Key Basic Research Program of China (2013CB921800), National High Technology Research and Development Program of China (2014AA123401), Basic Research Project of Shanghai Science and Technology Committee (14JC1491000), National Natural Science Foundation of China for Excellent Young Scholars (21522303), and Large Instruments Open Foundation of East China Normal University. We thank Jean-Paul Amoureux to polish the English.Notes and references
- U. Mueller, M. Schubert, F. Teich, H. Puetter, K. Schierle-Arndt and J. Pastré, J. Mater. Chem., 2006, 16, 626–636 RSC.
- P. Kumar, A. Deep and K. Kim, TrAC, Trends Anal. Chem., 2015, 73, 39–53 CrossRef CAS.
- G. Majano, O. Ingold, M. Yulikov, G. Jeschke and J. Perez-Ramirez, CrystEngComm, 2013, 15, 9885–9892 RSC.
- N. Stock and S. Biswas, Chem. Rev., 2012, 112, 933–969 CrossRef CAS PubMed.
- L. Sciortino, A. Alessi, F. Messina, G. Buscarino and F. M. Gelardi, J. Phys. Chem. C, 2015, 119, 7826–7830 CAS.
- A. Dhakshinamoorthy, M. Alvaro and H. Garcia, Chem. Commun., 2012, 48, 11275–11288 RSC.
- M. Sanchez-Sanchez, I. de Asua, D. Ruano and K. Diaz, Cryst. Growth Des., 2015, 15, 4498–4506 CAS.
- J. G. Yanuk, M. L. Alomar, M. M. Gonzalez, F. Simon, R. Erra-Balsells, M. Rafti and F. M. Cabrerizo, Phys. Chem. Chem. Phys., 2015, 17, 12462–12465 RSC.
- C. De Smedt, P. Spanoghe, S. Biswas, K. Leus and P. Van Der Voort, Adsorption, 2015, 21, 243–254 CrossRef CAS.
- M. Du, L. Li, M. Li and R. Si, RSC Adv., 2016, 6, 62705–62716 RSC.
- A. Dhakshinamoorthy, M. Alvaro, P. Horcajada, E. Gibson, M. Vishnuvarthan, A. Vimont, J. Grenèche, C. Serre, M. Daturi and H. Garcia, ACS Catal., 2012, 2, 2060–2065 CrossRef CAS.
- M. Sánchez-Sánchez, N. Getachew, K. Díaz, M. Díaz-García, Y. Chebude and I. Díaz, Green Chem., 2015, 17, 1500–1509 RSC.
- W. Liu and X. Yin, TrAC, Trends Anal. Chem., 2016, 75, 86–96 CrossRef CAS.
- S. Li and Q. Xu, Energy Environ. Sci., 2013, 6, 1656–1683 CAS.
- L. Wang, Y. Han, X. Feng, J. Zhou, P. Qi and B. Wang, Coord. Chem. Rev., 2016, 307, 361–381 CrossRef CAS.
- Y. Jin, C. Zhao, Z. Sun, Y. Lin, L. Chen, D. Wang and C. Shen, RSC Adv., 2016, 6, 30763–30768 RSC.
- X. Li, F. Cheng, S. Zhang and J. Chen, J. Power Sources, 2006, 160, 542–547 CrossRef CAS.
- K. Saravanan, M. Nagarathinam, P. Balaya and J. J. Vittal, J. Mater. Chem., 2010, 20, 8329 RSC.
- J. Shin, M. Kim, J. Cirera, S. Chen, G. J. Halder, T. A. Yersak, F. Paesani, S. M. Cohen and Y. S. Meng, J. Mater. Chem. A, 2015, 3, 4738–4744 CAS.
- W. Kaveevivitchai and A. J. Jacobson, J. Power Sources, 2015, 278, 265–273 CrossRef CAS.
- G. Férey, F. Millange, M. Morcrette, C. Serre, M. Doublet, J. Grenèche and J. Tarascon, Angew. Chem., 2007, 119, 3323–3327 CrossRef.
- Z. Zhang, H. Yoshikawa and K. Awaga, J. Am. Chem. Soc., 2014, 136, 16112–16115 CrossRef CAS PubMed.
- L. Bai, B. Tu, Y. Qi, Q. Gao, D. Liu, Z. Liu, L. Zhao, Q. Li and Y. Zhao, Chem. Commun., 2016, 52, 3003–3006 RSC.
- Y. Lin, Q. Zhang, C. Zhao, H. Li, C. Kong, C. Shen and L. Chen, Chem. Commun., 2015, 51, 697–699 RSC.
- C. Zhao, C. Shen and W. Han, RSC Adv., 2015, 5, 20386–20389 RSC.
- S. Maiti, A. Pramanik, U. Manju and S. Mahanty, Microporous Mesoporous Mater., 2016, 226, 353–359 CrossRef CAS.
- S. Maiti, A. Pramanik, U. Manju and S. Mahanty, ACS Appl. Mater. Interfaces, 2015, 7, 16357–16363 CAS.
- M. Díaz-García, Á. Mayoral, I. Díaz and M. Sánchez-Sánchez, Cryst. Growth Des., 2014, 14, 2479–2487 Search PubMed.
- M. Díaz-García and M. Sánchez-Sánchez, Microporous Mesoporous Mater., 2014, 190, 248–254 CrossRef.
- M. V. Shamzhy, M. V. Opanasenko, H. Garcia and J. Čejka, Microporous Mesoporous Mater., 2015, 202, 297–302 CrossRef CAS.
- M. Opanasenko, A. Dhakshinamoorthy, M. Shamzhy, P. Nachtigall, M. Horáček, H. Garcia and J. Čejka, Catal. Sci. Technol., 2013, 3, 500–507 CAS.
- Y. Qi, Y. Luan, J. Yu, X. Peng and G. Wang, Chem.–Eur. J., 2015, 21, 1589–1597 CrossRef CAS PubMed.
- C. He, S. Wu, N. Zhao, C. Shi, E. Liu and J. Li, ACS Nano, 2013, 7, 4459–4469 CrossRef CAS PubMed.
- P. G. Bruce, B. Scrosati and J. Tarascon, Angew. Chem., Int. Ed., 2008, 47, 2930–2946 CrossRef CAS PubMed.
- K. M. L. Taylor-Pashow, J. D. Rocca, Z. Xie, S. Tran and W. Lin, J. Am. Chem. Soc., 2009, 131, 14261–14263 CrossRef CAS PubMed.
- P. Horcajada, T. Chalati, C. Serre, B. Gillet, C. Sebrie, T. Baati, J. F. Eubank, D. Heurtaux, P. Clayette, C. Kreuz, J. Chang, Y. K. Hwang, V. Marsaud, P. Bories, L. Cynober, S. Gil, G. Férey, P. Couvreur and R. Gref, Nat. Mater., 2009, 9, 172–178 CrossRef PubMed.
- P. Horcajada, R. Gref, T. Baati, P. K. Allan, G. Maurin, P. Couvreur, G. Férey, R. E. Morris and C. Serre, Chem. Rev., 2012, 112, 1232–1268 CrossRef CAS PubMed.
- K. E. DeKrafft, Z. Xie, G. Cao, S. Tran, L. Ma, O. Z. Zhou and W. Lin, Angew. Chem., Int. Ed., 2009, 48, 9901–9904 CrossRef CAS PubMed.
- D. Liu, R. C. Huxford and W. Lin, Angew. Chem., Int. Ed., 2011, 50, 3696–3700 CrossRef CAS PubMed.
- Y. Lu, B. Yan and J. Liu, Chem. Commun., 2014, 50, 9969–9972 RSC.
- X. S. Liu, D. D. Wang, G. Liu, V. Srinivasan, Z. Liu, Z. Hussain and W. L. Yang, Nat. Commun., 2013, 4, 141–155 Search PubMed.
- S. L. Yang, D. N. Wang, G. X. Liang, Y. M. Yiu, J. J. Wang, L. J. Liu, X. L. Sun and T.-K. Sham, Energy Environ. Sci., 2012, 5, 7007 CAS.
- A. Fateeva, P. Horcajada, T. Devic, C. Serre, J. Marrot, J.-M. Grenèche, M. Morcrette, J.-M. Tarascon, G. Maurin and G. Férey, Eur. J. Inorg. Chem., 2010, 3789–3794 CrossRef CAS.
- C. Li, X. B. Lou, M. Shen, X. S. Hu, Z. Guo, Y. Wang, B. W. Hu and Q. Chen, ACS Appl. Mater. Interfaces, 2016, 8, 15352–15360 CAS.
- C. Li, X. S. Hu, X. B. Lou, L. J. Zhang, Y. Wang, J.-P. Amoureux, M. Shen, Q. Chen and B. W. Hu, J. Mater. Chem. A, 2016, 4, 16245–16251 CAS.
- J. Chen, L. Xu, W. Li and X. Gou, Adv. Mater., 2005, 17, 582–586 CrossRef CAS.
- H. Fei, X. Liu, Z. Li and W. Feng, Electrochim. Acta, 2015, 174, 1088–1095 CrossRef CAS.
- M. V. Reddy, G. V. Subba Rao and B. V. R. Chowdari, J. Power Sources, 2010, 195, 5768–5774 CrossRef CAS.
- F. Zheng, Y. Yang and Q. Chen, Nat. Commun., 2014, 5, 5261 CrossRef CAS PubMed.
- Z. Cai, L. Xu, M. Yan, C. Han, L. He, K. M. Hercule, C. Niu, Z. Yuan, W. Xu, L. Qu, K. Zhao and L. Mai, Nano Lett., 2015, 15, 738–744 CrossRef CAS PubMed.
- C. Wang, H. Wu, Z. Chen, M. T. McDowell, Y. Cui and Z. Bao, Nat. Chem., 2013, 5, 1042–1048 CrossRef CAS PubMed.
- L. Mai, F. Yang, Y. Zhao, X. Xu, L. Xu and Y. Luo, Nat. Commun., 2011, 2, 381 CrossRef PubMed.
- C. Li, T. Chen, W. Xu, X. Lou, L. Pan, Q. Chen and B. Hu, J. Mater. Chem. A, 2015, 3, 5585–5591 CAS.
- C. Chen, B. Liu, Q. Ru, S. Ma, S. Hu and X. Hou, RSC Adv., 2016, 6, 4914–4924 RSC.
- L. Hu, Y. Huang, F. Zhang and Q. Chen, Nanoscale, 2013, 5, 4186 RSC.
- B. Guo, Q. Kong, Y. Zhu, Y. Mao, Z. Wang, M. Wan and L. Chen, Chem.–Eur. J., 2011, 17, 14878–14884 CrossRef CAS PubMed.
- Q. Zhang, Z. Shi, Y. Deng, J. Zheng, G. Liu and G. Chen, J. Power Sources, 2012, 197, 305–309 CrossRef CAS.
- X. W. Li, L. Qiao, X. H. Wang, W. H. Xie and D. Y. He, J. Mater. Chem. A, 2013, 1, 6400–6406 CAS.
- X. Hu, H. Hu, C. Li, T. Li, X. Lou, Q. Chen and B. Hu, J. Solid State Chem., 2016, 242, 71–76 CrossRef CAS.
- X. Hu, C. Li, X. Lou, X. Yan, Y. Ning, Q. Chen and B. Hu, RSC Adv., 2016, 6, 54270–54276 RSC.
- G. Zhang, L. Yu, H. B. Wu, H. E. Hoster and X. W. D. Lou, Adv. Mater., 2012, 24, 4609–4613 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Structure of the Basolite F300 (Fig. S1), PXRD patterns of Fe-BTC samples synthesized using FeCl3·6H2O as the iron source (Fig. S2), EDX results for the commercial Basolite F300 and the synthetic Fe-BTC (Fig. S3 and S4), high-resolution SEM imagines of the commercial Basolite F300 and the synthetic Fe-BTC (Fig. S5 and S6), cycle performance of the synthetic Fe-BTC MOF electrode at high current density (Fig. S7), cycling performance of the bulk material Basolite F300 at the current density of 500 mA g−1 (Fig. S8), and metal–organic frameworks (MOFs) as anode materials in LIBs (Table S1). See DOI: 10.1039/c6ra22738d |
| This journal is © The Royal Society of Chemistry 2016 |


