Polyurethane types, synthesis and applications – a review
John O. Akindoyo*a,
M. D. H. Beg*a,
Suriati Ghazali*a,
M. R. Islam*b,
Nitthiyah Jeyaratnama and
A. R. Yuvarajc
aFaculty of Chemical and Natural Resources Engineering, Universiti Malaysia Pahang Lebuhraya Tun Razak, Gambang 26300, Kuantan, Malaysia. E-mail: dhbeg@yahoo.com; blessedbode@ymail.com
bMalaysian Institute of Chemical and Bioengineering Technology, University of Kuala Lumpur, Bandar vendor, Taboh Naning, Alor Gajah 78000, Melaka, Malaysia. E-mail: muhammad.remanul@unikl.edu.my
cFaculty of Industrial Sciences and Technology, Universiti Malaysia Pahang Lebuhraya Tun Razak, Gambang 26300, Kuantan, Malaysia
First published on 24th November 2016
Abstract
Polyurethanes (PUs) are a class of versatile materials with great potential for use in different applications, especially based on their structure–property relationships. Their specific mechanical, physical, biological, and chemical properties are attracting significant research attention to tailoring PUs for use in different applications. Enhancement of the properties and performance of PU-based materials may be achieved through changes to the production process or the raw materials used in their fabrication or via the use of advanced characterization techniques. Clearly, modification of the raw materials and production process through proper methods can produce PUs that are suitable for varied specific applications. The present study aims to shed light on the chemistry, types, and synthesis of different kinds of PUs. Some of the important research studies relating to PUs, including their synthesis method, characterization techniques, and research findings, are comprehensively discussed. Herein, recent advances in new types of PUs and their synthesis for various applications are also presented. Furthermore, information is provided on the environmental friendliness of the PUs, with a specific emphasis on their recyclability and recoverability.
1. Introduction
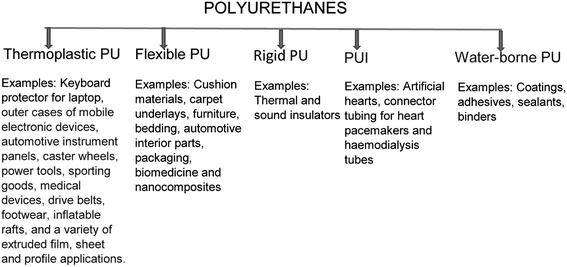
Polyurethanes (PUs) are a special group of polymeric materials that are in many ways different from most of the other plastic types. They can be incorporated into many different items, such as paints, liquid coatings, elastomers, insulators, elastic fibres, foams, integral skins, etc. Several forms in which PUs appear today are mere improvements in the invention of the German professor (Professor Dr Otto Bayer) and his co-workers.1 Fig. 1 illustrates the most important types of PUs and some common examples of their uses. The invention of the diisocyanate polyaddition technique by these researchers led to the creation of the PU industry in 1937, with PU produced through the reaction between diisocyanate and polyester diol.2,3PU was first developed as an alternative for rubber during World War II. The versatility of this material as well as its suitability to replace other scarce materials led to its incorporation in several applications. For instance, PU coatings were specifically used for impregnating paper and producing garments that were resistant to mustard gas and corrosion. They were also used as chemically-resistant coatings for wood, masonry, and metal, and as high gloss finishes for airplanes.4 The early industrial production of PU coatings began with different formulations for specific purposes. By the mid-1950s, PU coatings were commonly found in elastomers, coatings, rigid foams and adhesives.5 Towards the later part of the 1950s, comfortable and convenient cushions made from flexible foams were commercially available.4 Additionally, the development of flexible foams from cheap polyether polyols led to several automotive and upholstery applications that are still relevant today. Continuous improvements in processing techniques, additives types and in the formulations have contributed to these materials being used in a wide range of applications.6,7 Currently, PUs are one of the most common, versatile and researched materials in the world.8 These materials combine the durability and toughness of metals with the elasticity of rubber, making them suitable to replace metals, plastics and rubber in several engineered products.7,9 They have been widely applied in biomedical applications, building and construction applications, automotive, textiles and in several other industries due to their superior properties in terms of hardness, elongation, strength and modulus.6,8,10–14
The urethane group is the major repeating unit in PUs, and is produced from the reaction between alcohol (–OH) and isocyanate (NCO); albeit PUs also contain other groups, such as ethers, esters, urea and some aromatic compounds.8,15 Due to the wide variety of sources from which PUs can be synthesized and given their wide range of specific applications, they can be grouped into several different classes based on the desired properties: rigid, flexible, thermoplastic, waterborne, binders, coating, adhesives, sealants and elastomers.16 Among the major applications, PU foam is one of the most prominent PU-based products, and is used globally in significant amounts. About 50% of all polyurethane foam production is consumed by the market demand for rigid PU foam.17 Worldwide there are different types of PU production, with an estimated forecast up to 2020 given herein in Fig. 2.18 PU foams can be easily tailored to obtain specific products by merely changing the types and quantities of the surfactants, catalysts, blowing agents, isocyanate and polyol used in their fabrication, as well as the extent of intercalation and exfoliation between the fillers and matrices to meet the desired purpose.19,20 Specifically, PUs find wide application in coatings due to their specific properties, such as their excellent mechanical strength, toughness, good abrasion, corrosion and chemical resistance and low temperature flexibility.21 One of the most important categories of PUs is that of “PU elastomers”, which have been widely incorporated into different engineered products and have been proven to offer highly impeccable properties.22 They are malleable polymers and can be easily processed, both by extrusion and injection moulding, and also offer a high possibility for recycling.23
 | ||
| Fig. 2 Worldwide PU production and an estimated forecast up to 2020.18 | ||
PUs have been prepared from different diisocyanates, polyols and chain extenders and their properties investigated.8 Initially, most of the polyols used to prepare PUs were obtained from petroleum sources, but the high energy demands of the production process as well as environmental concerns have increased the necessity for more suitable and environmentally friendly substitutes. This has recently drawn enormous commercial and academic attention to renewable resources, such as vegetable oils.24–28 Vegetable oils mainly consist of triglyceride molecules with different reactive sites, such as carbon–carbon double bonds, ester and hydroxyl groups. The conversion of these oils to polyols may be achieved through different techniques, such as epoxidation and ring-opening polymerization,29 ozonolysis,30 transesterification31 and hydroformylation.32 Polyols obtained from vegetable oils have been found to be capable of partially replacing petroleum-derived polyols, especially when they are cross-linked with different isocyanates for PU production. As an advancement in product development, attention has been drawn to the manufacture of nanomaterials-based PUs following the novel production of a nanocomposite from nylon and clay for use in Toyota cars.33,34 Thus, the incorporation of nanomaterials has been suggested to offer several advantages related to desirable performance in a wide range of areas.35,36
Although a huge amount of research has been carried out on PUs, to the best of our knowledge, there are no reports in the literature compiling information on the improvement in the types and synthetic routes of PUs. Therefore, this review provides a summary of the advances made in the types and synthesis of PUs, as well as their recent incorporation into several engineered products. The importance of the individual components of PUs is fully highlighted, including how each component may determine the application for which the synthesized PU may be used.
1.1 Chemistry of polyurethanes
The chemistry of PUs leads to them being grouped with other compounds that are often collectively referred to as reaction polymers. These compounds include phenolics, unsaturated polyesters and epoxies.37,38 Generally, PUs are often synthesized from the reaction between an isocyanate and polyol molecule in the presence of either a catalyst or ultraviolet light activation.39 These isocyanate and polyol molecules should necessarily contain two or more isocyanate groups (R–(N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)n≥2) and hydroxyl groups (R′–(OH)n≥2), respectively.37 The exhibited properties of the PUs usually depend on the types of polyols and isocyanates from which they were made.40 Generally, soft elastic polymers can be produced from flexible long segments of polyols, whereas rigid and tough polymers are obtained via a higher amount of cross-linking. Stretchy polymers can be obtained through long chains with low cross-linking, whereas hard polymers can be obtained from shorter chains with high cross-linking. On the other hand, a combination of long chains with average cross-linking would produce polymers that are suitable for foam making.10 Due to the cross-linking in PUs, they often possess an infinite molecular weight with a three-dimensional (3D) network build-up. This is the reason why a small fraction of PUs may be referred to as a giant molecule and this explains why typical PUs often will not go soft or melt when they are heated. The incorporation of different additives alongside the isocyanates and polyols, as well as modifications to the processing conditions, makes it possible to obtain a wide range of characteristic features, which makes them suitable for various applications.41
O)n≥2) and hydroxyl groups (R′–(OH)n≥2), respectively.37 The exhibited properties of the PUs usually depend on the types of polyols and isocyanates from which they were made.40 Generally, soft elastic polymers can be produced from flexible long segments of polyols, whereas rigid and tough polymers are obtained via a higher amount of cross-linking. Stretchy polymers can be obtained through long chains with low cross-linking, whereas hard polymers can be obtained from shorter chains with high cross-linking. On the other hand, a combination of long chains with average cross-linking would produce polymers that are suitable for foam making.10 Due to the cross-linking in PUs, they often possess an infinite molecular weight with a three-dimensional (3D) network build-up. This is the reason why a small fraction of PUs may be referred to as a giant molecule and this explains why typical PUs often will not go soft or melt when they are heated. The incorporation of different additives alongside the isocyanates and polyols, as well as modifications to the processing conditions, makes it possible to obtain a wide range of characteristic features, which makes them suitable for various applications.41
Polyols used for PU synthesis often consist of two or more –OH groups. There are different kinds of polyols available that can be prepared in laboratories by various ways. For example, polyether polyols are obtained through the copolymerization of propylene oxide and ethylene oxide with a compatible polyol precursor,42 whereas polyester polyols are synthesized in a similar manner to the way polyesters polymers are prepared. A distinct type of polyether polyol, poly(tetramethylene ether)glycols, can be prepared by polymerizing tetrahydrofuran for usage in highly efficient elastomeric applications.43 An example of preparing isocyanate-terminate prepolymers using polytetrahydrofuran and their characterization studies were reported by Rajendran and co-workers.44 Polyols are often used as mixtures of molecules that are similar in nature but with different molecular weights. Their molecules possess different number of –OH groups. Therefore, it is often necessary to state the average functionality of the polyols.42 Despite the mixture complexity of polyols, industrial grade polyols have compositions that have been carefully controlled to obtain consistent properties, which are necessary for producing PUs with specific properties. For example, rigid PUs are made from low molecular weight polyols (a few hundred units), whereas flexible PUs are obtained from high molecular weight polyols (around ten thousand and above units).42 Different structures of various polyols are presented in Fig. 3.45 A comparison of the advantages and disadvantages of different polyols from various sources are listed in Table 1.45
 | ||
| Fig. 3 Comparison of basic polyol structures.45 | ||
| Polyol type | Advantages | Disadvantages |
|---|---|---|
| Polyether polyols based on propylene oxide and ethylene oxide | Hydrolytic stability, cost, viscosity, flexibility | Oxidative stability, modulus/strength, thermal instability, flammability |
| Aliphatic polyester polyol | Oxidative stability, modulus/strength | Viscosity, hydrolytic stability |
| Aromatic polyester polyol | Flame retardance, modulus/stiffness | Viscosity, low flexibility |
| Polyether polyols based on tetrahydrofuran | Hydrolytic stability, modulus/strength | Oxidative stability, viscosity, cost |
| Polycarbonate polyols | Hydrolytic stability, oxidative stability, modulus/strength | Viscosity, cost |
| Acrylic polyols | Hydrolytic/oxidative stability, hardness | Viscosity, cost, low flexibility |
| Polybutadiene polyol | Low temperature flexibility, solvent resistance | Viscosity, thermal oxidizable (unless hydrogenated), cost |
On the other hand, isocyanates are incorporated into PU synthesis via a hydroxyl-group-containing compound due to their high reactivity, although the reaction is slow at room temperature.45 This slow speed may be due to the phase incompatibility of the polar and less dense polyol phase and the relatively non-polar and denser isocyanate phase. Therefore, a suitable surfactant and suitable catalysts are required to get a faster reaction rate between them. The actions of catalysts based on polarization of either the isocyanate or hydroxyl compound through polar interactions are shown in Fig. 4.45 As per the model, enhancement of the electrophilic nature of isocyanate can be performed by the removal of electron density from the nitrogen or oxygen of the NCO group. Aromatic isocyanates, such as toluene diisocyanate (TDI) and diphenylmethane diisocyanate (MDI), are usually more reactive compared to their aliphatic counterparts, such as isophorone diisocyanate (IPDI) and hexamethylene diisocyanate (HDI). Most isocyanates are difunctional in nature (each molecule possesses two isocyanate groups), except for a few members, such as diphenylmethane diisocyanate, which comprises molecule mixtures containing either two or more isocyanate groups (in this case, the compound usually possess an average functionality higher than two, usually 2.7 due to the higher number of isocyanate groups). Modifications and changes to the types of raw materials and to the synthesis routes of PU determine the properties shown by the final product.
 | ||
| Fig. 4 Examples of bond polarization mechanisms of catalyst action.45 | ||
2. Types of polyurethanes
2.1 Rigid polyurethane foams
Rigid PU foams represent one of the most commonly known versatile and energy saving insulation materials. These foams can significantly reduce energy costs on the one hand and can make commercial and residential appliances more comfortable and efficient on the other. Reports from the U.S. Energy Department show that heating and cooling is one of the major consumers of energy in the majority of homes46 and are responsible for around 48% of the total energy consumption in a typical U.S. home.47 To ensure a stable temperature as well as a reduced noise level for both home and commercial appliances, builders often resort to using polyisocyanurate and PU foams. These foams have been proven to be effective as insulation materials, and hence have been applied in window insulations and wall and roof insulations as well as in barrier sealants for air and doors.The preparation of rigid PU foams can be performed using petroleum-based polyols as well as with bio-based polyols from vegetable oils or plant-based lignin. The properties of the formulated PUs depend on the category of the hydroxyl group present in the polyols. For example, glycerine, which is a petroleum-based polyol, contains a primary hydroxyl group. On the other hand, vegetable oil (for example, castor oil)-based polyols contain secondary hydroxyl groups. Thus, PUs synthesized from these two categories of polyols exhibit different physical and mechanical properties.48 In addition, the reaction between a secondary hydroxyl-group-containing polyol and isocyanate is slower compared to the reaction between a primary hydroxyl-group-containing polyol and isocyanate. Therefore, a mixture of primary and secondary hydroxyl-group-containing polyols is often used to reduce the consumption of petroleum-based polyol.48 For example, rigid PU foams with high physical and mechanical properties have been reportedly produced through a mixture of glycerine and castor oils.10 Moreover, the presence of different polyols may affect the physical properties of the PUs. For example, the presence of transesterified palm olein-based polyol, which contains a secondary hydroxyl group, can decrease the reactivity of the foaming profile of rigid PU.49 The reported consequences are an increased gel time, rise time, cream time and tack-free time of the formulated PU foam compared to petroleum-based polyol-prepared PU foams.
To obtain properties comparable to petroleum-based polyols from vegetable-based ones, a significant amount of structural modifications or changes is often required. For instance, in a research study, the hydroxylation of soybean oil was performed using formic acid and peroxide.50 Furthermore, transesterification was carried out through the help of the addition of some polyfunctional alcohol to increase the –OH functionality.50 In another study, high flame-retardant rigid PU foams were synthesized from phosphorylated polyol obtained from epoxydized soybean oil. The properties of the product were comparable with commercial polyol-based foams and were found to have a high flame-retardant capacity.48 Similarly, in another research study, the flame-retardant properties of rigid PU foams were improved through the incorporation of certain nitrogen–phosphorus-based flame retardants, such as dihydro oxa phosphaphenanthrene oxide-benzylideneaniline (DOPO-BA). Beyond the flame-retardant properties, the thermal and physical properties of the resulting foam were reported to be reasonably improved. The limiting oxygen index (LOI) value of the synthesized rigid PU was specifically found to increase from 20.01% to 28.1% when 20 wt% DOPO-BA was incorporated into the rigid PU foam formulation.51 The synthesis route for the high flame-retardant DOPO-BA material is illustrated in Fig. 5. Likewise, rigid PU foams have been produced from cardanol- and melamine-derived polyols. The material was found to possess high flame-retardant properties, with highly improved compressive strength and thermal stability compared to other conventional PU foams.52 The full synthetic route of the melamine/cardanol Mannich-based polyol is illustrated in Fig. 6. Also in another research study, flame retardants obtained from castor oil were used to synthesize rigid PU foams. The produced PU foams were found to suit a wide range of applications based on the many observed property improvements.53
 | ||
| Fig. 5 Synthetic route for dihydro oxa phosphaphenanthrene oxide-benzylideneaniline (DOPO-BA).50 | ||
In another vein, a literature survey revealed that nanoclays, such as montmorillonite, can also impart properties, such as high thermal stability, light weight, improved compressive strength and good flame-retardant properties, to several polymeric systems.54–56 It was observed, however, that the hydrophilic nature of nanoclay often leads to poor interfacial adhesion between the polymer matrix and the nanoclay filler.57,58 This makes it necessary to modify nanoclays before they are incorporated into polymeric systems. Modification could help to improve the efficiency of load transfer through better compatibility and improved dispersion of the filler within the matrix.59 In a bid to investigate this, a study was carried out on the production of rigid bio-based PU foams using nanoclay as the reinforcing agent. Different weight contents of nanoclay were incorporated into oil-palm-based PU to investigate its influence on the thermal and mechanical properties of the PU foam. The addition of up to 4% modified nanoclay (diaminopropane montmorillonite) (DAP-MMT) was found to improve the thermal, morphological and compressive properties of the rigid PU foam.41 Overall, modification of the nanoclay was found to enhance compatibility and dispersion of the filler within the matrix.41
2.2 Flexible polyurethane foams
Flexible PU (FPU) foams comprise some block copolymers whose flexibility is based on the phase separations between the soft and hard segments.60 Thus, PU foams may be modified through deliberate control of the individual compositional ratios of these segments. Depending on some physical characteristics they may be classified as flexible PUs; for example, in terms of density, durability, firmness, tearing resistivity, combustibility, surface elasticity, etc., where a combination of these properties can ensure a good flexibility in the PU compound. Flexible PU foams find application as cushion materials for a wide range of consumer and commercial products, including carpet underlays, furniture, bedding, automotive interior parts, packaging, biomedicine and nanocomposites.61–64The synthesis of flexible PU foams often involves two major steps: blowing and gelling. From the blowing reaction, carbon dioxide and urea are produced, which expand and are entrapped by the reaction mixture, while the urethane linkages are formed by reactions of the isocyanate and hydroxyl group of the polyol. There are a few parameters that dictate the morphology and microstructure of the PU foam, including the degree of cross-linking after the reaction between the polyol and diisocyanate, the segmental movement of the urea group, the nature of the interaction between the polyol and urea, etc. In a research study, special attention was given to the preparation of flexible PU foam from lignin or oxypropylated lignin.65 Some technical aspects were reported that could improve the flexibility of the materials; for example, the cross-linking density may be kept low by reducing the NCO/OH ratio, also, introducing a flexible chain to the main backbone of PU through a chain extender could help reduce the glass transition temperature to obtain a highly flexible PU. These types of flexible PUs are chemically resistant due to the high degree of cross-linking and adequate crystallinity, but they are weak in terms of their tensile and tear properties. To overcome these shortcomings, hybrid laminated high flexible PU foam was prepared and analysed.66 It was then suggested that the flexible PU foam needs to be reinforced with textile-based fibres, such as aramid, carbon, basalt and glass. Furthermore, due to the high combustible properties of FPUs, large volumes and toxic gases, such as CO, NOx and HCN, may be released to the environment during their combustion. Therefore, anti-flammable properties need to be incorporated into their formulation during production.66
2.3 Thermoplastic polyurethanes
Thermoplastic polyurethanes (TPUs) reveal vast combinations of both physical properties and processing applications. Usually, they are flexible and elastic with good resistance to impact, abrasion and weather. With TPUs, there is the possibility for colouring as well fabrication using a wide range of techniques. The incorporation of TPUs could therefore improve the overall durability of many products.67,68 TPUs are melt-processable, like other thermoplastic elastomers. They may be fabricated using extrusion, blow, compression and injection-moulding equipment.69 They may also be solution-coated or vacuum-formed, which makes them suitable to be made by a large range of fabrication techniques. The several property combinations of TPUs makes them suitable for many applications, such as in automotive, footwear and construction.67,70The synthesis of TPUs includes from novel fatty-acids-based diisocyanates, where the synthesized material was reported to display considerable thermal stability without any significant loss of weight at temperatures below 235 °C.67 The successful synthesis of rigid spiroacetal-moieties-based renewable thermoplastic has also been reported, with the corresponding materials produced in a very high yield.71 Analysis of the product with DMTA and DSC revealed a glass transition temperature of around 85 °C. Also, during hydrolytic stability analysis, there was no noticeable acid-mediated degradation of the synthesized PU. In another research study, fully bio-based thermoplastic PU was synthesized from dimer-fatty-acid-based diisocyanate and some other renewable diols. A one-step bulk synthesis method was used to produce the PU material, which was found to be suitable for coatings, automotive, building, adhesives and textile applications.40 Furthermore, due to the water-insoluble, non-ionic and inert properties of TPUs, they have been successfully utilized in applications such as polymer controllers for drug release in vaginal rings72 and in medical tubing, because their high mechanical properties could permit the use of tubes with thin walls without necessarily incorporating a plasticizer.69
2.4 Polyurethane ionomers
The presence of ionic groups in the polyurethane backbone chain has many advantages, such as better dispersion in polar solvents due to their enhanced hydrophobicity and improved thermal and mechanical properties.73 In particular, the shape memory and biocompatible features provide the materials the facilities to be used in biomedical devices.73–89 Shape memory PUs (SMPUs)79–84 possess a thermo-responsive shape memory effect (SME), and consequently exhibit different mechanical properties than the other PUs. The presence of hard (responsible for the frozen phase) and soft segments (responsible for the reversible phase) enable the PUs to ‘memorize’ the permanent shape.73,85 The permanent shape can be recovered from the temporary shape after heating the materials above a switch temperature.73 The soft segment and its glass transition temperature are related to the switch temperature and temporary deformation, whereas the hard segment is responsible for permanent shape memory. The content of hard and soft segments in the PU molecules and their molecular structure has an effect on the PU's SME. The variation of the glass transition temperature of the soft segment and crystallization of the hard segment have important effects on the SME. Those properties can be changed due to the presence of ionomers in PUs. The incorporation of ionic groups can be performed by using either ionic diols or ionic groups containing diisocyanate during the PU preparation.73,74 Anionomers can be prepared by post-functionalization of the PU.75 An example of a sulfonic ionomer prepared by Fragiadakis et al. and its synthesis route are presented in Fig. 7 (ref. 74) and 8,90 respectively. PU-Based cationomers can be prepared by ternization of a sulfur atom or quaternization of a nitrogen atom. In this case, the diol used for PU preparation should contain nitrogen or sulfur.76–78 A shape memory PU was synthesized from MDI and α,ω-poly(butylene adipate)diol. Chain extension of the diol was carried out by using MDEA and butane diol, whereas quaternization was carried out using acetic acid at 40 °C.77,78 | ||
| Fig. 7 Sulfonic ionomer.74 | ||
 | ||
| Fig. 8 Synthesis of sulfonic ionomer.90 | ||
Another important feature of polyurethane ionomers (PUIs) is their biocompatibility. Sulfonate and phosphatidylcholine groups have been studied to develop PUIs for blood compatibility.86–88 An improved haemocompatibility of segmented PU and poly(urethane-ureas) was observed by Li et al., where a comparison with medical-grade PU showed a better performance of the former.88 The successful application of these materials is found in different medical applications for artificial hearts, connector tubing for heart pacemakers and haemodialysis tubes.73
2.5 Coatings, adhesives, sealants and elastomers
There is a growing range of applications and advantageous markets that may be derived from the use of PUs as coatings, adhesives, sealants or elastomers (CASE). This is because PUs often reveal excellent and versatile mechanical, chemical and physical properties.91,92 PU adhesives can offer good bonding properties, whereas very tight seals may be obtained from PU sealants. For a PU to be suitable for coating applications, it needs to possess good adhesive properties, high chemical resistivity, excellent drying, low temperature flexibility and adequate scratch resistivity.93,94 Sometimes, to impart anti-corrosive properties into the material, different types of nanoparticles, such as titanium oxide, silicon dioxide, may be used for high-performance coating applications. The appearance of this product may be improved and also the lifespan may be extended. It is noteworthy that despite the suitability of PU coatings to offer certain desirable properties, their impact resistance insufficiency and susceptibility to UV degradation when used for outdoor purposes can reduce their use.95–97 Consequently, improvements in these shortcomings using several synthetic methods for producing PU coatings with enhanced properties have been reported in the literature.98 Recently, environmental adhering PU coatings were synthesized from vegetable oils (cotton seed and karanja oil) using a green solvent approach. The product was thoroughly characterized for its thermal and physico-chemical properties. Results obtained for the material in terms of its adhesion, impact resistance, flexibility and gloss properties showed that it is suitable for coating applications, even at the industrial scale.99 Also in a recent research study, certain isocyanate-free consumer-applied novel bonding and adhesive materials were obtained from a hybrid of PU and polyhydroxyurethane. This material was also found to be a suitable replacement for isocyanate-based PUs.100Most of the adhesive materials commonly found in wood composites and other sandwich materials are based on phenol formaldehyde and urea formaldehyde.101 They are often used as binders for interior and exterior solid wood and plywood components, but are found to cause severe damage to the integrity of the environment due to the release of organic solvents.102 They are found to also contribute to the large dependence of industries on petroleum consumption.101 Recently, research has shifted towards the production of adhesives and other binders from renewable materials, especially plant oils. This is based on their environmental, societal and economic advantages.101,103 Jatropha oil, for example, has been reported to offer various advantages as adhesive materials. This is due to its oil-containing gums, which may be converted into renewable-material-based adhesives.101 In fact, other literature reports have also revealed that PU wood adhesives have been successfully synthesized from jatropha oil, and the adhesive was found to have highly desirable properties.102,104 Also, the jatropha-oil-based adhesive is renewable, which makes it conveniently possible to incorporate in adhesive applications. This also enhances its potential replacement of conventional petroleum-based materials.101 Furthermore, the cheap cost of jatropha oil in comparison with other oils, such as rapeseed, and soybean,105 has attracted the attention of researchers, particularly for its potential in wood adhesives applications. Apart from jatropha oil, other oils have also been widely investigated, such as palm oil.106,107
PU elastomers are another type of important materials, which may be used for a variety of useful application, such as shoe soles, household items, surfboards, goggles and ski boots. They may be fashioned into a wide variety of shapes, colours and design. They are lighter compared to metals, and can provide highly desirable stress recovery properties as well as can withstand several environmental factors.15,23 Although they have an elastic property, they also possess some degree of plastic nature. Thus, in practice, the highly desired elastic property cannot be maximally obtained. In a bid to overcome this challenge, the incorporation of graphene oxide into the PU formulation was investigated.108 Also, hybrid fillers, such as titanium oxide or carbon nanotubes, are added to serve specific purposes. In most cases, PU-elastomer-based nanocomposites are prepared via the common solution cast method. From the literature, it was found that the elastomeric properties of PUs were exploited to produce graphene-oxide-based dielectric materials.109 The suitability of the material for this application is based one the ease of their actuation in an electric field. Thus this kind of materials are very useful for their tolerance to high physical strains, such as during shrinkage and expansion during the application of electric voltage.
2.6 Binders
PU binders are often used to bond different types of fibres and other materials to each other. Binders made from PU help to provide a permanent gluing effect between organic materials and long-strand lumbers, oriented strand boards, laminated veneer lumber, medium density fibre boards, particle boards and straw boards. As a binding material, the ratio of the hard-/soft segments of PUs should be high and good thermal stability is required. Sometime a specific or moderate acid number (not too high or not too low), weak crystallinity, limited molecular weight and narrow particle distribution (if PU dispersion) are preferred for a good quality binder. To impart excellent chemical resistivity in PU binders, hybridization with acrylic polymer is also preferable. The main areas of application are in elastomeric or rubbery flooring surfaces, wood panel manufacturing, ink-jet printing, foundry industries and sand casting.110,111 Among these, the major application to which PU binders are put is in the production of oriented strand board (OSB). The use of these panels cut across flooring and structural sheathing, shop panels, joist and beams and other manufactured housing applications. Also, the fabrication of rebonded foams, which are used as carpet underlay, mainly take advantage of PU binders and other chemicals to adhere flexible scrap PU foams to the underlay carpeting. Due to its excellent binding properties, PU has been proposed as a suitable alternative to binders based on organic solvents.112,113 Apart from the use of PUs as adhesives, sealants, foams and coatings, they may be used also as rocket propellants and polymer-bonded explosives (PBX).114–116 The polyfunctional groups present in the material makes it easily to cure by a diisocyanate.117 Among the various used ones, prepolymers containing hydroxyl-terminated polybutadiene (HTPB) groups have been widely used as binders for solid composite propellants as well as PBX.114,118 This binder was reported to offer structural integrity as well as dimensional stability to the explosive material. This could be accrued to the mechanical properties contributed by the urethane reaction between the HTPB end chain hydroxyl groups and isocyanates, which produced the PU elastomer. However, due to the pot life limitations of propellants and PBX, whenever TDI is applied as a curative agent, IPDI (which is less reactive) could be used as a suitable alternative if a longer pot life is desired for the PBX paste during the manufacture of large-sized products.119 Whichever the case, the mechanical properties of the material are majorly dependent on the extent of the reactions between the PU components.114,1202.7 Waterborne polyurethane dispersions
Coatings and adhesives that make use of water primarily as the solvent are often referred to as waterborne polyurethanes (WPUs).121,122 There are several pieces of legislation that place restrictions on the amount of allowed volatile organic solvents and other hazardous air pollutants that may be released into the environment. Most commercial and industrial applications are therefore dependent on polyurethane dispersions (PUDs), or waterborne polyurethane dispersions (WPUDs).123–125 PUDs have the unique advantage that the viscosity of the dispersion is not dependent on the molecular weight of the polymer. Therefore, high solid-content WPUs (HSCWPUs) can be prepared by the drying process only. The dispersion is a two-phase colloidal system, which includes the polyurethane particles and the water medium. Several pendent acid or tertiary nitrogen groups in the PU chain are neutralized to form salts, which basically create centres for water dispersibility. The types and amount of polyol, isocyanate, ionomers and chain extender used are responsible for different properties of this dispersion.Recently, a new method (a two-step emulsification process) was developed for the synthesis of HSCWPUs,126 where distribution of the bimodal particle size was strictly controlled. This was due to the high importance of particle size distribution as a parameter in the determination of the viscosity and the solid content interrelationship.127 This type of high solid-content materials has also been reported to raise the space and time yield of reactors, as well as reduce the time needed for film forming.128 One recent research study involved the synthesis of new WPU novel medium-length fluorinated diols. For this study, the fluorinated diol 3-(bis-(N,N-dihydroxyethyl))dodecafluoroheptyl acrylate (DEFA) was first produced from dodecafluoroheptyl acrylate and diethanolamine using the Michael addition method, as shown in Fig. 9. Several fluorinated WPU emulsions were then synthesized, as illustrated in Fig. 10. The organic solvent/water resistance as well as the mechanical and thermal properties of the produced material were found to be greatly improved. For instance, the tensile strength was observed to increase from 9 MPa to 15 MPa, whereas the extensibility was found to decrease from 520% to 280%.129 Other notable WPUs that have been investigated include a polycarbonatediol-based WPU, which was reinforced with silica. The produced material was reported to have suitable coating application for flexible materials, such as fabrics, paper and leather, especially in cases where high abrasion resistance is required.130 A novel synthesis route had also been provided for fully bio-based WPUs. The method was cleared to adhere strictly with requirements for environment safety, and the synthesized product was shown to possess great hydrophobic and thermal properties. It was also proposed as a suitable alternative for conventional petroleum-based materials.131
 | ||
| Fig. 9 Synthesis of dihydroxyethyl dodecafluoroheptyl acrylate (DEFA).129 | ||
 | ||
| Fig. 10 Synthetic route for isophorone diisocyanate dihydroxyethyl dodecafluoroheptyl acrylate polyurethane (IPDI-DEFA-PU) aqueous emulsion.129 | ||
3. Polyurethane synthesis
PUs may be produced through different routes.38 The most important and more useful method is through the reaction between a polyol (an alcohol that has two or more hydroxyl groups within a molecule) and a diisocyanate.1,10,132 Fig. 11 illustrates the synthesis of a typical PU. Other suitable additives and catalysts may also be incorporated for the PU synthesis.Additives that may be incorporated into the PU synthesis include flame retardants, pigments, cross-linkers, fillers, blowing agents and surfactants. PUs may be fabricated into any fashion with a variety of properties, such as hardness and density, by merely varying the quantity and types of the polyol, isocyanate or additives. The most common components that may be found in typical PUs and the reasons for their inclusion are presented in Table 2.
| Additives | Reasons for use | Ref. |
|---|---|---|
| Isocyanate | Responsible for the PU reactivity and curing properties | 124 |
| Polyols | Contributes flexible long segments, which produces soft elastic polymers | 135 |
| Catalysts | To speed up the reaction between the isocyanate and polyols and to allow reaction at a lower reaction temperature | 6 |
| Plasticisers | To reduce material hardness | 69 |
| Pigments | To produce coloured PU materials, especially for aesthetic purposes | 116 |
| Cross-linkers/chain extenders | For structural modification of the PU molecule and to offer mechanical support that will enhance the material properties | 136 and 137 |
| Blowing agents/surfactants | To aid the production of PU foams, to help control the formation of bubbles during synthesis and to control the foam cell structure | 138 |
| Fillers | To minimize cost and to improve the material properties, such as stiffness and tensile strength | 19 |
| Flame retardants | To reduce material flammability | 139 |
| Smoke retardants | To reduce the rate of possible smoke generation when the material is burnt | 51 |
3.1 Polyols
Polyols may be largely grouped into either polyether polyols or polyester polyols. Polyether polyols are obtained from the reaction between an epoxide and an active hydrogen-containing compound. They can also be prepared from the ring-opening polymerization of epoxy monomers.133,134 Another category is polyester polyols, which can be obtained from the polycondensation of hydroxyl compounds and multifunctional carboxylic acids. Also, polyols may be classified based on their end use. The high molecular weight polyols (MW ranging from 2000 to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) are mainly used for the synthesis of flexible PUs, whereas low molecular weight PUs are used for producing rigid PUs.10,140,141 Polyols that are applied for flexible PUs often make use of initiators with low functionality, such as glycerine (f = 3), dipropylene glycol (f = 2) or a solution of water and sorbitol (f = 2.75). On the other hand, polyols for rigid PUs require initiators with higher functionality, such as sorbitol (f = 6), Mannich bases (f = 4), sucrose (f = 8) and toluenediamine (f = 4). Usually, ethylene oxide and/or propylene oxide is incorporated into the initiators until the expected molecular weight is reached. It is noteworthy, however, that the amount and order in which the oxide is added can define many of the polyol properties.142 This includes its water solubility, reactivity and compatibility. Polyols produced from propylene oxide only, are often terminated with secondary –OH groups12 and they are usually less reactive compared to polyols containing ethylene oxide (containing primary –OH groups). Graft polyols, otherwise called polymer or filled polyols, comprise finely distributed acrylonitrile, styrene-acrylonitrile or polyurea polymer particles, which are chemically grafted onto a polyether ketone with a higher molecular weight. Most often, they are incorporated into high-resiliency low density foams, to improve their load-bearing capacity. They may also be applied for adding toughness to cast elastomers and other microcellular foams. Rigid foam polyols with low molecular weight may also be produced by using triethanolamine or ethylenediamine as initiators. These kinds of polyols possess inherent catalytic properties due to the nitrogen atoms present in the backbone. Another class of very important polyether polyol is called poly(tetramethylene ether) glycol (PTMG), which is obtained from the polymerization of tetrahydrofuran, and is mainly applied for high-performance wetting, elastomers and coating applications.
000) are mainly used for the synthesis of flexible PUs, whereas low molecular weight PUs are used for producing rigid PUs.10,140,141 Polyols that are applied for flexible PUs often make use of initiators with low functionality, such as glycerine (f = 3), dipropylene glycol (f = 2) or a solution of water and sorbitol (f = 2.75). On the other hand, polyols for rigid PUs require initiators with higher functionality, such as sorbitol (f = 6), Mannich bases (f = 4), sucrose (f = 8) and toluenediamine (f = 4). Usually, ethylene oxide and/or propylene oxide is incorporated into the initiators until the expected molecular weight is reached. It is noteworthy, however, that the amount and order in which the oxide is added can define many of the polyol properties.142 This includes its water solubility, reactivity and compatibility. Polyols produced from propylene oxide only, are often terminated with secondary –OH groups12 and they are usually less reactive compared to polyols containing ethylene oxide (containing primary –OH groups). Graft polyols, otherwise called polymer or filled polyols, comprise finely distributed acrylonitrile, styrene-acrylonitrile or polyurea polymer particles, which are chemically grafted onto a polyether ketone with a higher molecular weight. Most often, they are incorporated into high-resiliency low density foams, to improve their load-bearing capacity. They may also be applied for adding toughness to cast elastomers and other microcellular foams. Rigid foam polyols with low molecular weight may also be produced by using triethanolamine or ethylenediamine as initiators. These kinds of polyols possess inherent catalytic properties due to the nitrogen atoms present in the backbone. Another class of very important polyether polyol is called poly(tetramethylene ether) glycol (PTMG), which is obtained from the polymerization of tetrahydrofuran, and is mainly applied for high-performance wetting, elastomers and coating applications.
Polyester polyols are derived from virgin raw materials and are often produced through the direct polyesterification of very pure diacids and glycols. One example is 1,4-butanediol and adipic acid. Usually, polyester polyols are more viscous,142 as well as more expensive, compared to polyether polyols. However, they are still very important because they produce PUs with better abrasion, solvent and cut resistance. Another group of polyester polyols are derived from raw materials that have been reclaimed. They are produced through transesterification, otherwise known as the glycolysis of recycled poly(ethyleneterephthalate) (PET) or distillation bottoms of dimethyl terephthalate (DMT) with glycols (for example, diethylene glycol). These aromatic polyols, which also have a low molecular weight, are often used for the production of rigid foams because they offer reduced cost and good flammability properties to polyisocyanurate (PIR) boardstock foam as well as to PU insulation foams.
A group of polyols called the speciality polyols are required in the manufacture of sealants, elastomers and adhesives that need superior qualities to withstand chemical and environmental factors. Some of these polyols are polysulfide polyols, polycaprolactone polyols, polycarbonate polyols and polybutadiene polyols.
Different polyols may be obtained from natural and renewable sources, such as vegetable oils. These renewable materials can either be fatty acids or dimer fatty acids.140,143 The vegetables oils from which polyols may be obtained include castor, soybean, Pongamia glabra, neem and cotton seed.144 Oils derived from these vegetable oils are mainly used to produce flexible moulded foams, flexible bunstocks and elastomers. The presence of triacylglycerides in vegetables oils makes them suitable for manufacturing several polymeric materials.145–147 Polyols from renewable sources can be reacted with isocyanates to produce PUs with special properties that are suitable for a wide range of applications.148 In one study, modifications were made to both soya bean oil and castor oil to make them suitable for manufacturing rigid PU foams. The mechanical properties of the synthesized foams were found to be reduced compared to commercial polyol-based foams. However, it was found to present significant application opportunities for rigid PU foam production since it was obtained from sources that are renewable.149
In another vein, the copolymerization of tetrafluoroethylene or chlorotrifluoroethylene with vinyl ethers containing hydroxyalkyl vinyl ethers could lead to the production of fluoroethylene vinyl ethers (FEVE) polyols. Fluorinated PUs synthesized from two components and involving the reaction of polyisocyanate with FEVE fluorinated polyols have been explored for the synthesis of ambient cure coating/paints. Due to the high amount of fluorine–carbon bonds (the strongest chemical bond) in fluorinated PUs, they have been observed to possess good resistance to UV, alkalis, chemicals, acids, solvents, corrosion, weathering, fungi and other microbial attacks. These qualities make them highly desirable for high quality paints/coatings.
3.2 Isocyanates and non-isocyanates
Isocyanates are very necessary components for PU preparation. They can be categorized as difunctional or heterofunctional and aromatic or aliphatic in nature. Among the several available options, the most commonly used ones are methylene diphenyl diisocyanate (MDI), toluene diisocyanate (TDI) and aliphatic diisocyanates. The structures of some common isocyanates are illustrated in Table 3. Generally, MDI and TDI are cheaper and more reactive compared to other isocyanates. Industrial grade MDI and TDI, which are isomeric mixtures, most often comprise polymeric materials. They are usually used for producing flexible foams, such as moulded foams used for car seats or as slabstock foam for mattress production.2 They can also be used for producing rigid foams, such as refrigerator insulating materials, and for producing elastomers (such as for shoe soles), etc. Modification to isocyanates may be achieved through the partial reaction with polyols or by incorporating certain materials to reduce the volatility and invariably the toxicity of the isocyanates. This could also reduce the freezing point, such that they become easier to handle, as well as enhance the properties of the resulting polymers.Other groups of less often used isocyanates are the aliphatic and cycloaliphatic isocyanates. These find applications in coatings and other areas where transparency and colour are highly desired. This is because aromatic isocyanate-based PUs usually darken when exposed to light.116 Common among the aliphatic and cycloaliphatic isocyanates are 1-isocyanato-3-isocyanatomethyl-3,5,5-trimethyl-cyclohexane (isophorone diisocyanate, IPDI) 4,4′-diisocyanato dicyclohexylmethane, (hydrogenated MDI or H12MDI) and 1,6-hexamethylene diisocyanate (HDI).
Despite the importance of diisocyanates, environmental concerns have led researchers to investigate better means of reducing or possibly avoiding their use, specifically to reduce the environmental problems and other diisocyanate-related toxicity issues. For example, a study was carried out to produce sustainable PU from carbonated soybean oil, 3-aminopropyltriethoxysilane and lignin.150 The non-isocyanate route followed the reaction between cyclic carbonate with amines,151 and the polyol could be prepared from lignin via an oxypropylation method.152 In another study, oligomeric polybutadiene diisocyanate was used for the preparation of lignin-based PU.153 In a similar study, lignin-aminated polyol and diphenyl diisocyanates were reacted to prepare PUs. Most of the reports revealed that the properties of the formulated non-isocyanate-based PUs depend mostly on the lignin content. The lignin content determines the cross-linking and modulus of the materials. The typical synthetic routes are shown in the scheme in Fig. 12.
 | ||
| Fig. 12 Synthetic route for PU preparation using castor oil and lignin in an isocyanate-free mechanism. | ||
3.3 Catalysts
The catalysts that are often incorporated into PUs may be grouped into two main categories: metal complexes and amine compounds. Amine catalysts traditionally consist of tertiary amines, such as dimethylcyclohexylamine (DMCHA), dimethylethanolamine (DMEA), 1,4-diazabicyclo[2.2.2]octane (DABCO) and triethylenediamine (TEDA). The selection of tertiary amine catalysts is based on their ability to drive either the urea, urethane or isocyanate trimerization reactions. Metal complexes from compounds of bismuth, lead, zinc, tin and mercury may also be used as catalysts for urethanes. For the production of PU sealants, coatings and elastomers and mercury carboxylates have been found to be specifically effective. This is due to their preferential selection towards the polyol and isocyanate-related reactions. However, they are reportedly toxic, consequently leading to the recent use of carboxylates from zinc and bismuth as replacements. Several types of application also make use of alkyl tin carboxylates, mercaptides and oxides. Specifically, tin mercaptides are usually incorporated into water-containing formulations because carboxylates of tin may be undesirably influenced by hydrolysis.Most often, catalysts are used in the formulation of different kinds of PUs for selective purposes. For example, novel CuCo2O4/graphitic carbon nitride nanohybrids have been used for the reduction of CO generation and fire hazards.153 The reactivity of these catalysts is different in terms of their nature. A comparison was drawn for the case of the catalytic activity shown by two catalysts, namely zirconium and tin, for the preparation of isophorone diisocyanate (IPDI)-based waterborne polyurethanes.154 It was found that in the case of the tin catalyst, the reactivity of the isocyanates was different, while in the case of the zirconium catalyst, it was the same.
3.4 Chain extenders and cross-linkers
Another group of compounds that often play important roles in the polymeric morphology of PU are the chain extenders (f = 2) and cross-linkers (f = 3 or more). These compounds are usually amine and hydroxyl terminated, with low molecular weights. They are highly useful for improving the morphology of PU adhesives, elastomers, fibres and some other important microcellular and skin foams.155,156 The elastomeric features of these compounds are obtained from the copolymer interface of the soft and hard segments of the polymer. As such, the domains of the hard segment urethane serve as cross-linkers for the domains of the soft segment amorphous polyester (or polyether). This interface separation arises due to the incompatibility and immiscibility (while both phases are amorphous) of the soft segments (low melting and non-polar) with the hard segments (high melting). Therefore, crystallization does not have any effect on phase separation.Generally, the hard segments, which are produced from isocyanate and chain extenders, are immobile and stiff, while on the other hand, the soft segments, which are produced from the polyols (high molecular weight), can move freely and often appear in foil forms. Covalent coupling between the hard and soft segments leads to plastic flow inhibition within the polymer chains, thereby producing elastomeric resiliency. Mechanical deformation of these compounds lead to the uncoiling of certain portions of the stressed soft segment, making the hard segments align along the direction of the stress. The realignment of hard segments coupled with a subsequent strong hydrogen bond produces high tensile strength, tear resistance and good elongation properties.157,158 Proper selection of the chain extender could also influence the chemical resistance, heat and flexural properties of the PU. Some of the most commonly used chain extenders include 1,4-butanediol (BDO), cyclohexane dimethanol, ethylene glycol, hydroquinone bis(2-hydroxyethyl)ether (HQEE) and 1,6-hexanediol. Some examples of biodegradable PUs and their synthetic routes are presented in Fig. 13 and 14 using water and ethylene glycol as the chain extenders. These glycols can be used to manufacture thermoplastic PUs. They also form well-organised hard segment domains, which separate well and can be processed in the molten state. The only exception is ethylene glycol, whose derived bis-phenyl compound is susceptible to undesirable degradation if the level of hard segments becomes too high.37
3.5 Surfactants
Surfactants are often used to improve the properties of foam as well as non-foam PU polymers. They resemble block polymers of polydimethylsiloxane–polyoxyalkylene, nonylphenol ethoxylates, silicone oils and some other organic compounds. In applications that involve foams, they are applied for the emulsification of liquid components, the regulation of cell sizes and for stabilization of cell structures to guide against collapse as well as against voids at the sub surface. For non-foam applications, they are applied as anti-foaming and air release agents, and as wetting agents. They may also be used to remove surface imperfections, such as sink marks, orange peels and pin holes. There are different kinds of surfactants available for the preparation of PU materials, including non-ionic159 and cationic160 surfactants. The findings of using non-ionic surfactants revealed an excellent surface activity without having a fixed critical micelle concentration. On the other hand, cationic surfactants were found to be better to use for corrosion resistivity. There are a few drawbacks involved with the usage of surfactants for the synthesis of PU. For example, low molecular weight conventional surfactants are responsible for delamination and corrosion.161 Also, they can sometimes migrate easily to the surface of PU materials. Therefore, a surfactant-free PU was also proposed by a different researcher.1624. Advances in polyurethane synthesis
4.1 Click chemistry
Nowadays, PUs can be prepared following a new reaction approach called ‘click chemistry’. Click chemistry is well known for producing a single product with a very high yield and high tolerance of functional groups. There are many beneficial aspects of the click reaction compared to other traditional processes. For example, it has been observed to be fast, highly selective, with a high possibility of working with either homogeneous or heterogeneous systems, with an insensitivity to the solvent as well as it can proceed with a moderate reaction temperature.Following this technique approach, flame-retardant PUs were prepared by using a copper(I)-catalyzed azid–alkyne cycloaddition (CuAAC) technique with alkyne-polyol and azidoalkylmonophosphonate.163,164 The experimental process involved four steps: preparation of azidoalkyl monophosphonate compounds through the nucleophilic substitution of bromoalkylphosphonates with NaN3; formation of terminal alkyne groups attached to polyols from the reaction of glycidyl propargyl ether and propylene oxide via a mechanism of anionic ring-opening copolymerization; ‘clicking’ the azidoalkylphosphonate to the polyol and finally synthesis of the PU foam with 2.4 wt% of ‘click polyol’. The schemes for all the four steps are illustrated in Fig. 15. Recently, castor-oil-based polyfunctional polyurethane acrylate was prepared by following photo-click chemistry.165 The functionalization was performed via thiol–ene photo-click chemistry with β-mercaptoethanol, which was very efficient compared to the traditional method. The result showed a 100% conversion of the double bonds of castor oil, which was confirmed by real-time Fourier transform infrared spectroscopy (FTIR). A similar study was performed using click chemistry to obtain highly branched PUs by different researchers.166,167 The improvement in thermal, mechanical, anti-microbial and anti-corrosion properties are associated with the use of 1,2,3-triazole-rich polyether polyols and the incorporation of carbon nanotubes. In a different study, click chemistry and atom transfer radical polymerization were used for the deposition of a dopamine-assisted lubricating and antifouling coating on PU surfaces.168 A grafting process was involved by using poly(N,N′-dimethyl-(methylmethacryloyl ethyl)ammonium propanesulfonate) (PDMAPS) and poly(2-methacryloyloxyethyl phosphorylcholine) (PMPC) in order to improve the surface hydrophilicity and lubricating properties. Click chemistry was also used for the functionalization of waterborne PU by using Cu(I)-mediated azide–alkyne through a cycloaddition reaction.169 Some other examples of research work using click chemistry include the preparation of methoxy polyethylene glycol (MPEG)-functionalized polyurethanes (PUs) (PU-g-MPEG),170 the preparation of waterborne siloxane–polyurethane nanocomposites reinforced with nanosilica171 and the synthesis of a rigid PU foam from novel renewable polyols based on limonene.172
4.2 Nitrogen- and phosphorus-containing polyurethanes
Due to the susceptibility of fire attack, PU and PU-based materials need to be fire and flame retardant for safe use. To serve this purpose, usually halogen-based compounds are added. These compounds are, however, considered to be toxic and not environmentally friendly. Furthermore, besides the toxicological effects of using halogen-related compounds or additive-type fillers for their anti-flammable properties, they can also reduce the mechanical properties of the PU. Therefore, reactive-type fillers for anti-flammable properties are preferable instead of the additive types. To justify this assertion, environmentally friendly materials made from nitrogen and phosphorus are being exploited. A novel phosphorus–nitrogen flame-retardant-based PU was prepared from benzaldehyde, aniline and 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (DOPO) by using the condensation reaction.173 A schematic illustration of the preparation of the flame-retardant-based PU is shown in Fig. 5. The flame-retardant rosin-based rigid polyurethane foams were prepared from DOPO-BA. Similar research work was performed to prepare ricinoleic-acid-based phosphorous- and nitrogen-containing polyol, which was later used to synthesize a PU for sealing applications. Reports showed that the PU synthesized from phosphorus- and nitrogen-based materials could offer the dual benefit of environmental protection as well as improved mechanical properties.173 In a different study, a heat release study was conducted for PU containing a phosphorous flame-retardant.174 The flame retardants were mixed with PU via solvent mixing and the copolymerization method. Per the results, it was found that the mixing condition had an effect on the heat release rate. The type of reaction and type of bonding (covalently or non-covalently bonded) between the flame retardant and PU are other important factors in reducing the release of heat. An interesting work was performed using both phosphorous (BHPP)- and nitrogen (MADP)-containing polyol to improve the flame retardancy of rigid PU foam.175 The results showed that the optimal weight percentage of BHPP and MADP was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to achieve an improved thermal stability while limiting the oxygen index. In addition, a small amount (15 wt%) of expandable graphite added extra value by improving the limiting oxygen index to 33.5% and reduced the rate of heat release by 52.4%. Expandable graphite and phosphorous were also used to improve the mechanical and thermal properties of the PU.176 Besides flame retardancy, an improvement in the adhesion property of PU dispersion was also documented by using phosphorous in a previous study.177 (Bis)phosphonic acid moieties were used as adhesion promoting reactive sites to build covalent bonds through an end-capping reaction to isocyanate-reactive polyurethane particles under aqueous conditions. A detailed analysis of the fire toxicity of PU foam can be obtained from the previous literature.178
1 to achieve an improved thermal stability while limiting the oxygen index. In addition, a small amount (15 wt%) of expandable graphite added extra value by improving the limiting oxygen index to 33.5% and reduced the rate of heat release by 52.4%. Expandable graphite and phosphorous were also used to improve the mechanical and thermal properties of the PU.176 Besides flame retardancy, an improvement in the adhesion property of PU dispersion was also documented by using phosphorous in a previous study.177 (Bis)phosphonic acid moieties were used as adhesion promoting reactive sites to build covalent bonds through an end-capping reaction to isocyanate-reactive polyurethane particles under aqueous conditions. A detailed analysis of the fire toxicity of PU foam can be obtained from the previous literature.178
4.3 Carbon- and nanomaterial-based polyurethanes
To improve many materials' characteristics, the incorporation of nanoparticles or nanomaterials is now the new trend among researchers. There are a variety of nanoparticles that can serve specific purposes. However, a reasonable amount of intensive studies over the years have concentrated on nanostructures of nanocrystalline cellulose (NCC).179–181 Some of the properties that favour this material include the fact that it is a biopolymer with great natural abundance. Its industrial production has been estimated to be around 1012 tonnes annually combined with its renewability and biodegradation features.181 Furthermore, the structural geometry of NCC offers highly desirable mechanical properties, with elastic properties similar or even higher than Kevlar. Its tensile strength has been reportedly estimated to be about 103 MPa or above.182However, it has been suggested that if the full potential of NCC is to be exploited, there is a need to incorporate it as a reinforcing material into nanocomposites.182 Extensive studies have been carried out on this subject.183–187 As part of this orchestrated research, studies have also been conducted on bio-based PUs reinforced with NCC, with a number of publications produced. One group of researchers worked on the production of NCC-based PU material using different ranges of concentrations (0.2–5 wt%) of the reinforcement.188–191 Based on the results obtained from using FTIR, the authors reported that there was hydrogen bonding between the NCC and the hard segments with an increased phased separation of the soft and hard segments as the NCC was incorporated. The diameters of the cells were reported to decrease as the NCC content was increased; however, there was a non-monotonous effect on the density with respect to the NCC content. It was also stated that despite the NCC being believed to act as a nucleating agent, there was no significant change to the existing chemical structure of the PU with the NCC addition. Overall, the incorporation of NCC was reportedly found to suitably improve the mechanical properties of the PU material. A group of other researchers also studied the incorporation of micro/nanocrystals of cellulose into PU based on rapeseed oil.192 It was reported that incorporating micro-crystalline cellulose (MCC) did not offer much observable modification to either the thermal conductivity, the closed cell or the apparent density of the resulting PU. However, there was reportedly an increase in water absorption in line with the concurrent increase in the MCC content.192 Furthermore, it was observed that there was an increased glass transition temperature as well as rigidity towards compression. Other research studies have also been carried out on the incorporation of cellulose fibres into PU. However, the materials produced were classified as composites instead of nanocomposites.193,194
Recently, research on the incorporation of nanomaterials has grown wider than just the scope of cellulosic materials alone. Other materials, such as carbon nanofibres (CNFs), carbon nanotubes (CNTs) and clays, are attracting significant interest as important additions into polyurethane foams.195,196 This is based on the suitability of these nanofillers to achieve nano-scale dispersion and thereby improving the properties of PU materials.195 These properties include mechanical properties, such as stiffness, toughness, mould shrinkage and hardness,195–197 thermal properties,198,199 water solubility,200 barrier properties201,202 and other functional properties.196,201 It should be noted, however, that the influence of nanofillers on the properties of PU and other composites depends on several factors, such as aggregate size, particle size, shape, morphological characteristics and the degree of dispersion.196,202,203 Among the most commonly investigated, nanoclays, such as mica, hectorite, montmorillonite and saponite, have been the choice of many researchers.204 This is because they have been perceived to offer specific properties, such as flame retardancy, improved thermal stability, improved compressive strength and light weight, to the composite material.205
However, there are constraints to these types of materials due to the hydrophilic nature of the nanoclay, which could lead to weak interfacial bonding between the filler and the hydrophobic polymer matrix.59 It is therefore necessary to modify the nanoclay to enhance its compatibility and dispersion within the matrix, such that the overall load transfer capacity of the composite material can be improved. Among the very many possible methods, the use of organic modifiers for the ion exchange process59 and ultrasonic treatment206,207 have been reported to be suitable for effective modifications of nanoclays. Some of the organic modifiers that have been used include diamine-based modifiers, trihexyl tetradecylphosphonium (THTDP) and alkylammonium.208
Moreover, in a bid to reduce the cost of production, to minimize the synthetic fibre usage and to produce more property-tailored products, there is presently research ongoing into hybrid nanocomposites. This is because hybridization can overcome the shortcomings of one-material reinforcement through the incorporation of other reinforcing materials.209,210
5. Recent advances in polyurethane applications
The general properties (physical and chemical) of any PU are dependent on the nature of the individual reactants (especially the R1 and R2 groups) from which it is produced. Generally, the properties of the polyols, such as the number of functional groups that are reactive within each molecule, their molecular structure and their molecular mass, all define the characteristic features of the final PU material as well as how it will be used. Several research studies have been carried out on the production of PU and its potential applications. Some of the recently reported types of PU and their methods of synthesis are presented in Table 4.| Different synthesis methods | Different types of PU and references |
|---|---|
| Two-step emulsification process | High solid content WPUs126 |
| Thiol–ene coupling | PUs based on aromatic cardanol-based polyols,213 vegetable-oil-based WPUs131 |
| Step growth polymerization | Non-isocyanate PUs from secondary amines214 |
| Prepolymer | Vegetable-oil- and phosphorylated-polyols-based PUs,48 tannic-acid-based PUs,215 phosphinated PUs,216 biodegradable and electroactive PUs,217 WPU based on UV absorption groups,218 isocyanate-trimers- and polyester-polyols-based PUs,219 folate-conjugated PUs,220 iodo PUs,221 high solid content WPUs,222 hyperbranched WPUs,223 PU/polyhydroxyurethane hybrids,100 transparent PUs films from fatty acid,92 polycarbonatediols-based WPUs,224 biodegradable polycaprolactone/PUs,225 polyrotaxanes cross-linked PUs,226 biodegradable low cost aliphatic PUs,227 carbohydrate cross-linked PUs,228 renewable thermoplastic based on rigid spirocetal moieties,71 environment-friendly WPUs,229 hyperbranched PUs230 |
| Prepolymer (one step) | Fluorine-based PUs,231 |
| Prepolymer (two step) | Sulfadiazine-based PUs,232 PU based on cellulose nanofibres,233 PUs based on cardanol- and melamine-derived polyol52 |
| Solvent/emulsifier free | Fluorinated WPU acrylate234 |
| Inverse emulsification | PU based on side-chain triethoxysilane and colloidal silica235,236 |
| Hydroxylation and a subsequent alcoholysis/epoxidation | Jatropha-oil-based PUs101 |
| Microwave-assisted | Cyclodextrin PUs237 |
| Michael addition reaction followed by self-emulsification | PUs based on medium length fluorinated diols129 |
| Polycondensation | Chitosan-based PUs,238 polyester-polyols-based PUs,239 polyfunctional PU foams138 |
| Polyaddition | Natural-rubber-based PUs240 |
| Hydrolysis and condensation | WPUs based on PU/silica hybrids130 |
| Green solvent | Cotton-seed- and karanja-oil-based PUs99 |
| Non-isocyanate reaction | Soya-bean- and lignin-based PU241 |
| One shot | Liquefied-lignin-based PUs60 |
| Sol–gel synthesis method followed by supercritical CO2 drying | PU aerogels242 |
| Free rise | Modified-tung-oil-based PUs foams243 |
| Cross-linking | Terpene-based PUs244 |
5.1 Building and construction applications
Present day buildings need to meet certain requirements in terms of the use of construction materials, including high-performance strong materials, light weight, easy to install, durable and versatile. These may be achieved through the incorporation of PUs into building and construction materials. In fact, the use of PUs could offer great conservation of natural resources and help the environment through reduced energy consumption. The use of PUs for construction and building applications is on the increase due to their specific properties, such as excellent heat insulation capacity, highly desirable strength-to-weight ratio, versatility and durability. An experimental work was performed to determine the reduction of heat loss through a building envelope for the case of thermoregulating microcapsules contained in PU foam.211 Analysis showed that with an incorporation of 40% microcapsules it was possible to produce a thermoregulating foam with two possible advantages: energy accumulation and insulation during the transient state. Furthermore, the cheap cost of these high-performance materials coupled with their comfort ability have made PUs an integral part of many homes. PUs can be used in almost any part of the home, such as for floors, e.g. in the form of pads of a flexible cushion for carpets, or for roofing, e.g. in form of heat and light reflecting materials. In the roofing application, the plastic coverings on the PU surface can help to keep the house cool on the one hand, and help to reduce energy usage on the other hand. Generally, PU materials help to add flexibility to new homes, such as the entry door and garage doors, which contains panels with foam cores. The foam-core panels also provide a lot of colour variation and profiling for roofs and walls.5.2 Automotive applications
The areas of PU application in the automotive industry are vast. Aside from its common use as a foam to make vehicle seats more comfortable, it may also be used in car bodies, bumpers, doors, windows and ceiling sections. PUs also help to provide better automobile mileage through reduced weight, increased fuel economy, good insulation with proper sound absorption,212 great comfort for passengers245 and high corrosion resistance properties. Deng R. and his colleague opined that since a clear majority of vehicular seats are mainly foams, the dynamic comfort of users may be controlled by modifying the foam properties to obtain the desired quasi-static features.245 PU foams have been reported to occupy the largest fraction of the global polymeric foam market.212 Due to the low density of PU foams, they are suitable for the manufacture of stiff and light components, which may then be used as interior panels in aircrafts, structural shapes, such as bulkhead cores, stringers and transform cores in reinforced plastic boats, etc.212 Several other sandwich materials found in high-end sporting cars, ships, aircrafts and racing cars are also based on PU. This is because the PU material can help to provide heat shielding and structural stiffness as well as crash energy management.212 PU adhesives are also used as PU–aluminium laminates for automotive applications.246 These adhesives are prepared from polycaprolactone polyols and a mixture of aromatic and cycloaliphatic diisocyanates. The adhesion property has been found to be influenced by the structure of the PU used. Coatings are another prime need for automobiles and can also be prepared by using PU. The development of modern technology related to nanofillers or nanoparticles can add some important features in PU-based advanced coating materials for automobiles. The relationship between the hard segments of a PU chain and fillers was determined by Verma et al.,247 who showed that intercalated and exfoliated clay platelets have a preferential relationship with the hard segments of the PU chains.247 Furthermore, the dispersion and morphology of clay can determine the effective sites for interfacing with PU chains.5.3 Marine applications
PU materials have contributed a large innovation to the recent development in boat technology. PU-Based epoxy resins help to protect boat hulls from weather, corrosion and water as well as other substances that may increase drag. In addition, PU-based rigid foam helps to insulate boats from extreme temperatures and noise. It helps to provide increased tear and abrasion resistance, and offers good load-bearing properties even at minimum weight. Based on these, the maritime industries often incorporate several thermoplastic PUs into various products for the specific advantages they provide, including elasticity, durability and ease of processing ability with good suitability for cable and wire coatings, drive belts, hydraulic seals and hoses and engine tubing as well as ship construction. Some PUs can also be used to recognize certain active materials,248 and for removing certain organic substances from water bodies. Cyclodextrin PU, for example, has been reported to be effective towards the removal of certain organic materials, such as paraben, from water.249 Recently, a microwave-assisted technique was used to produce cyclodextrin PU. The material was fully characterized using 1H and 13C NMR spectroscopic methods and was found to be soluble in organic matters, but insoluble in water. Therefore, the synthesized PU was reported to be efficient for removing phenol as well as Nile red dye from water. It was also projected for further possible application in the removal of toxic substances from the environment.237 In a similar research, iodo PUs were synthesized and were reported to be efficient for removing dyes, such as crystal violet and aniline blue, from laundry waste water.221For marine applications, accelerated weathering or ageing analyses of the materials are very important. The possible outcomes of these analyses may include swelling, debonding of the fillers, hydrolysis, plasticization and loss of mechanical strength. There are different approaches for measuring the performance activities during the period of usage in the contact water or seawater. According to the ISO standard test method 11346, accelerated ageing can be performed at elevated temperature along with applying the Arrhenius expression to determine the relationship with the material's behaviour at low temperature or high duration. Sometime a linear extrapolation of a fixed time frame can be used for lifetime prediction. A study was conducted over a long period (2 and 5 years) observing the polyether-based PU materials under seawater and adverse conditions.250 The findings indicated that under seawater conditions, the tensile properties could be retained 100% during the stated period of immersion in seawater. This suggested that the PU material could help retain the mechanical integrity of products even under adverse environmental conditions.
5.4 Coating applications
Over the years, there has been continuous research on suitable materials for coating applications. PUs have been reported to possess great potential as paint and surface-coating materials.251,252 Research in this area saw the development of certain non-linear hyperbranched polymers, which have metamorphosed into other hyperbranched PUs with gloss, high solubility and flexible coating properties.253 However, reports in the literature revealed that most of the synthesized hyperbranched polymers cannot withstand fire outbreak as they are non-flame retardants. To modify these hyperbranched materials for certain flame-retardant coating applications, compounds containing nitrogen, halogen or phosphorus may be incorporated into them.252,254 Recently, triol, tris(bisphenol-A)mono phosphate, which contained phosphorus, was reacted with polyethylene glycol and castor oil using different diisocyanates, such as toluene diisocyanate (TDI), hexamethylene diisocyanate (HMDI) and isophorone diisocyanate (IPDI). A highly flame-retardant hyperbranched PU was produced, which was suitable for application in nanocomposites and nanocoatings.230 In another research, a two-step, one-pot prepolymerization approach was used to manufacture hyperbranched castor-oil-based PUs, which were observed to have highly desirable potential to be used as advanced surface-coating materials.255 Another type of coating material suitable as a marine antifouling material was produced from polyester-based polyol. Pentaerythritol and trimethylolpropane were used as initiators and polycondensation was done with ε-caprolactone, using the cross-linker hexamethylene diisocyanate trimer. The synthesized antifouling coating material was also found to be highly degradable.239 Other sources from which PU have been recently synthesized for coating applications include fatty acids,92,143 soybean and lignin241 isocyanate trimmers and polyester polyols.2195.5 Medical applications
PUs are used in several medicine-related applications, including, but not limited to, general purpose tubing, surgical drapes, catheters, hospital bedding, wound dressing and several other injection-moulded equipment. They are used for these applications due to their availability, good mechanical and physical properties and biocompatibility.256–258 However, the most frequent use is in short-period implants. The incorporation of PUs in medicine-related application helps to offer cost effectiveness and provides adequate room for toughness and longevity of materials.259 This feature has allowed polymeric materials to replace the conventional materials, such as metals, ceramics and metal alloys. The global bio-based PU market was 1534 tonnes in 2012.18 Polyurethane hence obtained has a bio content ranging from 30% to 70%, depending largely on the type of bio-based feedstock employed for manufacturing the polyols. The global polyols market in 2015 reached almost USD 19.5 billion, with an annual growth of 8.5%. The bio-polyol market is currently worth USD 5.03 billion.260 The global bio-based polyurethane market is expected to reach USD 37.5 million by 2020, or less than 0.07% of the total PU market according to a new study by Grand View Research, Inc.261In one study, crystalline prepolymers were used to produce biodegradable PU, using water as a chain extender. Properties of the synthesized PU were compared with those obtained via a polyaddition reaction using ethylene glycol as a chain extender. It could be seen that there was an improvement in mechanical and degradation properties of the new material, which was also found to possess suitable application as an element for joint endoprostheses.142 Full synthetic routes for the production of biodegradable PU using water and ethylene glycol as chain extenders are shown in Fig. 8 and 9, respectively. Also, due to the pH changes that often occur during sexual intercourse, special drug delivery systems, such as vaginal pessaries and microbicides, which could help to prevent the spread of sexually transmitted diseases, including HIV-AIDS, have been suggested.262,263 For this purpose, highly sensitive and smart PUs for vaginal drug delivery were synthesized.264 Moreover, PUs have been conveniently used for other purposes, such as drug delivery systems specifically made for the colon265,266 and as intra-vaginal rings.267
Recently, the suitability of carbohydrates as biomedical devices was investigated. Castor-oil-based biodegradable and biocompatible PUs were synthesized using polypropylene glycols (PGs) as the polyol and with different carbohydrates incorporated as cross-linkers.228 The properties of the produced PUs were investigated and it was found that the incorporation of carbohydrates influenced the thermal, mechanical and degradation properties of the material due to the variety in carbohydrate structures.264 The characterizations performed revealed that the carbohydrates served as suitable components of biodegradable and biocompatible PUs. This strategy could therefore be used for developing certain biomedical devices.228 This report conforms to the observations reported by other researchers who reported that incorporating modified starch and cellulose crystals into PUs could enhance their biocompatibility and biodegradability as well as their mechanical properties.268,269 Other research studies on the applicability of PU for medical devices can also be found in the literature, including work reporting on the production of a low-cost biodegradable aliphatic PU, which had a high saline stability up to about 37 °C without a significant decrease in mechanical strength.227 Furthermore, other medical-application-based studies were performed, including studies on a chitosan-based PU for antibacterial properties238 and biodegradable electroactive PUs for cardiac tissue engineering.217 From these research studies on the medicinal applications of PUs, it was observed that some of the produced materials often perform only at a moderate level, especially in terms of their resistance towards bacterial adhesion. This is because most of them are susceptible to bacterial attack, thereby leading to the risk of infection.238 New strategies for producing antibacterial PUs have therefore become necessary. These could be achieved via the incorporation of certain surfaces that have the capability to resist or repel the attachment of bacterial to the material surface.238 These bacterial-resisting surfaces could be produced either through the incorporation of some antibacterial coatings or via some other surface modifications that could enhance the antibacterial or anti-biofouling properties of the materials.
5.6 Appliances, flooring and packaging applications
Most of the appliances that consumers use these days are based on PUs. Rigid PU foams lead the way in the number of applications as they can be used as thermal insulators for refrigerators and freezers. These materials have become so essential due to their cost effectiveness, which make them suitable for use to meet the required energy ratings in most freezers and refrigerators. The advantages that rigid PU foams provide to these appliances are due to the combination of cell gases and fine foams with a closed-cell structure, which helps to prevent heat transfer.For flooring purposes, PUs have several specific applications, such as top coatings or as carpet underlay foams. They can help to make floors more durable, aesthetically pleasing and easy to maintain. The lifespan of carpets and their appearance can be increased though the use of PU foam underlays, which can also help to provide better comfort with reduced ambient noise. PU-Based protective finishes can also be used as floor coatings, where they can provide solvent and abrasion resistance on the one hand and ease of cleaning and maintenance on the other hand. Except for those properties, the lifetime or service period is also equally important to consider. In one study, the time–temperature–stress dependent shear creep behaviour of PU foam was analysed and Findley's power law, extended to include Arrhenius equations, was used to propose a model for the temperature dependency on the viscoelastic parameters.270 Combining all the properties, PU finishes can offer a better look to new wood, cement or parquet floors, and can also offer a regenerated appearance to older floors.
For packaging applications, PU can also be used as a printing ink or as packaging foams. A PU plasticizer was prepared from palm olein and castor oil for packaging applications.271 This PU plasticizing resin showed high flexibility with good mechanical and freeze resistivity. On the other hand, PU packaging foams (PPFs) offer a wide range of packaging options, which should help to overcome most onsite packaging challenges. The versatility of these foams has also been explored for the cost-effective packaging of items that demand special protection during transit, including medical equipment, electronics, large machine parts and delicate glassware. Custom-fit packaging materials have also been made available to almost all shipments using PUs.
5.7 Apparels applications
Initially when PU was discovered to be a good fit for apparels, where PUs are converted to thin threads and incorporated into nylon to produce garments that are stretchable and lightweight. Recently, PUs have been technologically developed into more improved spandex fibres and thermoplastic elastomers. With the advancement in techniques for producing PUs, it has opened up the possibilities for producers to manufacture a wide variety of PU-based leathers, bra cups and man-made skins, which may be also used for several sport attires and a wide range of accessories. Among the PU types, aqueous dispersions of WPUs have been widely incorporated into textile-related applications.218 Properties that favour the use of WPUs as finishing agents include permeability, the special structure of their molecules, abrasive resistance and softness. Also, crock fastness, fastness of washing and the soap fastness of reactive dyes, acid dyes and direct dyes on dyed fabrics may be greatly improved by using WPUs as dye finishing agents.272,273 Recently, UV absorption groups were incorporated into a WPU to enhance its washing fastness, UV protection and rubbing fastness of the material. It was also aimed at ensuring the retention of the wrinkle recovery angle of the WPU. For this purpose, N,N-dimethyl allyl p-benzoyl benzyl ammonium bromide was used as a UV absorber.218 The product was found to offer great UV dyeing protection to cotton fabrics, and it also showed great suitability for several other textile applications.218 In a different study, a low molecular weight of chitosan was used to extend the PU prepolymer chain for the preparation of a chitosan–PU dispersion.274 This dispersion was applied on different quality plain weave poly-cotton dyed and printed fabric pieces to obtain improved stiffness, pilling resistance and better mechanical properties. It was suggested that the quality of pure cotton and woollen fabrics can also be improved by applying this technique.5.8 Wood composite applications
PUs are very important inclusions in many present day materials, including wood composites. Recently PU-based flat composites were prepared by using activated carbon for electromagnetic interference (EMI) shielding.275 Different amounts of activated carbon were loaded into PUs for microwave absorption and complex permittivity. The results showed the suitability of the composites in place of materials based on polyethylene and polyester filled with metal additives. In a different study, PU/wood composites were prepared from wood wastes and polyols.276 The polyols were obtained from the chemical modification of poly(ethylene terephthalate) (PET) and commercial polyols. The chemical modification of PET was achieved through glycolysis. Although effective load transfer was found from the matrix to the dispersed phase, there was, however, no improvement in the thermal stability. However, the modification of PET-produced materials was also performed with different molecular weights and various physical characteristics. These changes were associated with several factors, such as the glycerol content, condition of the reaction and the stoichiometric ratio of the reactants.277 The significance behind the use of natural fibres or wood for PU-based composites is that they are hydroxyl-enriched substances, which can undergo chemical bonding easily with diisocyanate.278 In accordance with the findings, cellulose nanocrystals (CNC) were used in a very low amount (0.5 wt%) with high solid-content PUs to obtain higher values for the glass transition temperature (76 °C), Young's modulus (1.52 GPa), abrasion resistance, etc.279 A covalent bond was confirmed between the PU chains and CNC during the polymerization process.6. Recycling of polyurethanes
The demand for PU products is increasing day by day. Consequently, recovery and recycling processes have become important to attend to the pressing demands for more environmentally friendly materials.280–282 Indeed, the recycling process for PU is beneficial both in terms of the environmental as well as from an economical point of view.13 Usually, the recycling process is done under four classes:283 advanced chemical and thermochemical recycling, mechanical recycling, energy recovery and product recycling (Fig. 16). | ||
| Fig. 16 PU recycling using a closed loop.284 | ||
All these four methods contribute unique advantages to PU fabrication and utilization.280,281 The material recycling process needs physical treatment, whereas chemical and thermochemical recycling need chemical treatment to generate feedstock chemicals for industry.280–282 Also, energy recovery involves the partial or complete oxidation of waste materials,285 which result in the production of electricity and gaseous fuels.286 The by-products from the recycling process are non-hazardous and thus are disposable to the environment.286 However, chemical, mechanical and thermochemical recycling and energy recovery are the main pathways to recycle PUs. Usually, mechanical recycling can be achieved by regrinding the PU foams into powder wherein the PU can be used again.284 The processes used are compression moulding, adhesive pressing and flexible foam bonding.284 On the other hand, PU granules can be coated with a glue (binder) and further cured under pressure and heat. This process is carried out to fabricate floor mats and tyres.285,286 Pump and mother housing are, however, made by the compression moulding of PU granules under high pressure and heat. In the energy recovery process, the PU is burned fully to generate the maximum amount of electricity.285 In particular, the thermochemical and chemical recycling processes are based on several chemical reactions, such as, hydrogenation, pyrolysis,265,287 hydrolysis288 and glycolysis.289,290 The recycling process of PU is economical285 and practical283 because of the PU's rigid and semi-rigid nature. Hence, recycled PU may be successfully used in the manufacture of quarter panels, wheel covers, steering wheels, bumper covers and cores in automotive vehicles as well as for the manufacture of other domestic and industrial parts.283 PU is environmentally non-hazardous and more economical285 compared to other conventional polymers due to its recycling and recovery.
7. Conclusion
Polyurethanes (PUs) are some of the most common, versatile and researched materials in the world. They combine the durability and toughness of metals with the elasticity of rubber, making them suitable replacements for metals, plastics and rubber in several engineered products. They have been incorporated into many types of industrial equipment and for making numerous items, such as paints, liquid coatings, elastomers, rigid insulations, elastic fibres, soft flexible foams and even as integral skins. PUs may be produced from a wide range of diisocyanates, a variety of polyols and other chain extenders and cross-linking agents. This makes it possible to obtain a large range of tailored materials that can serve many specific applications. Initially, most of polyols used to prepare PUs were obtained from petroleum sources, but the high cost and energy demands as well as environmental concerns have increased the necessity for a more suitable and environmentally friendly substitute. This has recently drawn enormous commercial and academic attention to renewable sources, such as vegetable oils. The last decade has witnessed a clear majority of studies appearing in the literature on the use of vegetable oils as alternatives to petroleum-based materials for PU production. However, there are certain shortcomings associated with these kinds of materials, especially in terms of performance. The use of nanomaterials has been suggested to offer additional advantages for desirable performance. Hence, the incorporation of nanoparticles that can suitably replace the hard segments from isocyanate precursors has therefore been widely investigated. Thus, materials such as carbon nanofibres (CNFs), carbon nanotubes (CNTs) and clays are attracting significant interest as important additions into PU products. With all this enormously diverse research on PU, recyclability of the product is very important. Fortunately, the recycling processes of PU have been reported to be economical and practical. Thus, PU could be considered to be environmentally non-hazardous and more economical compared to other conventional polymers, due to its good recycling and recoverable properties.Abbreviations
| 13C NMR | Carbon nuclear magnetic resonance |
| 1H NMR | Hydrogen nuclear magnetic resonance |
| 31P NMR | Phosphorus nuclear magnetic resonance |
| AFM | Atomic force microscopy |
| ATR-FTIR | Attenuated total reflection Fourier transform infrared |
| AV | Acid value |
| BDO | Butanediol |
| CASE | Coatings, adhesives, sealants and elastomers |
| CNF | Cellulose nanofibre |
| CNF | Carbon nanofibre |
| CNT | Carbon nanotube |
| CO2 | Carbon dioxide |
| CPU | Cardanol-based polyurethane |
| DABCO | Diazabicyclo octane |
| DAP-MMT | Diaminopropane montmorillonite |
| DEA | Diethanolamine |
| DEFA | Dihydroxyethyl dodecafluoroheptyl acrylate |
| DFHA | Dodecafluoroheptyl acrylate |
| DLS | Dynamic light scattering |
| DMA | Dynamic mechanical analysis |
| DMCHA | Dimethylcyclohexylamine |
| DMEA | Dimethylethanolamine |
| DMPA | Dimethylolpropanoic acid |
| DMT | Dimethyl terephthalate |
| DMTA | Dynamic mechanical thermal analysis |
| DOPO-BA | Dihydro oxa phosphaphenanthrene oxide-benzylideneaniline |
| DSC | Differential scanning calorimetry |
| EDX | Energy dispersive X-ray analysis |
| ESCA | Electron spectroscopy for chemical analysis |
| ESR | Electron spin resonance spectrometry |
| f | Functionality |
| FEVE | Fluoroethylene vinyl ether |
| FPU | Flexible polyurethane |
| FTIR | Fourier transform infrared spectroscopy |
| GPC | Gel permeation chromatography |
| HDI | Hexamethylene diisocyanate |
| HQEE | Hydroquinone hydroxyethyl ether |
| HR-MS | High resolution mass spectrometry |
| HTPB | Hydroxyl-terminated polybutadiene |
| HSCWPU | High solid-content waterborne polyurethane |
| ICP-OES | Inductively coupled plasma optical emission spectrometry |
| IPDI | Isophorone diisocyanate |
| IPDA-DEFA-PU | Isophorone diisocyanate dihydroxyethyl dodecafluoroheptyl acrylate polyurethane |
| IR | Infrared |
| LOI | Limiting oxygen index |
| MALDI-TOF MS | Matrix-assisted laser desorption ionization time-of-flight mass spectrometry |
| MCC | Micro-crystalline cellulose |
| MCMP | Melamine-modified cardanol-based Mannich polyether polyol |
| MDI | Methylene diphenyl diisocyanate |
| MDSC | Differential scanning calorimetry in modulated mode |
| Mw | Molecular weight |
| NCC | Nanocrystalline cellulose |
| NCO | Isocyanate |
| NMR | Nuclear magnetic resonance |
| OSB | Oriented strand board |
| PBX | Polymer-bonded explosive |
| PCDL | Polycarbonatediol |
| PCL | Polycaprolactone |
| PDI | Particle polydispersity index |
| PDM | Pyridinedimethanol |
| PEG | Polyethylene glycol |
| PET | Poly(ethyleneterephthalate) |
| PG | Polypropylene glycol |
| PIR | Polyisocyanurate |
| PLLA | Polylactic acid |
| PPF | Polyurethane packaging foam |
| PSD | Particle size distribution |
| PTMC | Poly(trimethylene carbonate) |
| PTMG | Poly(tetramethylene oxide glycol) |
| PU | Polyurethane |
| PUD | Polyurethane dispersion |
| PUF | Polyurethane foam |
| PUI | Polyurethane ionomer |
| RIM | Reaction injection moulding |
| SAXS | Small-angle X-ray scattering |
| SEC | Size exclusion chromatography |
| SEM | Scanning electron microscopy spectroscopy |
| SME | Shape memory effect |
| SMPU | Shape memory polyurethane |
| SPU | Segmented polyurethane |
| TDI | Toluene diisocyanate |
| TEA | Triethylamine |
| TEDA | Triethylenediamine |
| TEM | Transmission electron microscopy |
| TGA | Themogravimetric analysis |
| THTDP | Trihexyl tetradecylphosphonium |
| TPU | Thermoplastic polyurethane |
| UV | Ultraviolet |
| UV-VIS | Ultraviolet visible |
| WPUD | Waterborne polyurethane dispersion |
| WPU | Waterborne polyurethane |
References
- O. Bayer, Das di-isocyanat-polyadditionsverfahren(polyurethane), Angew. Chem., 1947, 59(9), 257–272, DOI:10.1002/ange.19470590901.
- M. R. Islam, M. D. H. Beg and S. S. Jamari, Development of vegetable-oil-based polymers, J. Appl. Polym. Sci., 2014, 131(18), 40787–40790, DOI:10.1002/app.40787.
- E. Delebecq, J.-P. Pascault, B. Boutevin and F. O. Ganachaud, On the versatility of urethane/urea bonds: reversibility, blocked isocyanate, and non-isocyanate polyurethane, Chem. Rev., 2012, 113, 80–118, DOI:10.1021/cr300195n.
- R. B. Seymour and G. B. Kauffman, Polyuretanes: A class of modern versitile materials, J. Chem. Educ., 1992, 69, 909 CrossRef CAS.
- Z. S. Petrović and J. Ferguson, Polyurethane elastomers, Prog. Polym. Sci., 1991, 16, 695–836 CrossRef.
- D. Chattopadhyay and K. Raju, Structural engineering of polyurethane coatings for high performance applications, Prog. Polym. Sci., 2007, 32, 352–418 CrossRef CAS.
- Z. Rafiee and V. Keshavarz, Synthesis and characterization of polyurethane/microcrystalline cellulose bionanocomposites, Prog. Org. Coat., 2015, 86, 190–193 CrossRef CAS.
- K. M. Zia, S. Anjum, M. Zuber, M. Mujahid and T. Jamil, Synthesis and molecular characterization of chitosan based polyurethane elastomers using aromatic diisocyanate, Int. J. Biol. Macromol., 2014, 66, 26–32 CrossRef CAS PubMed.
- C. Prisacariu, Polyurethane elastomers: from morphology to mechanical aspects, Springer Science & Business Media, 2011 Search PubMed.
- M. Ionescu, Chemistry and technology of polyols for polyurethanes, Rapra Technology, Shrewsbury, UK, Polymer International, 2007, vol. 56 Search PubMed.
- S. A. Madbouly and J. U. Otaigbe, Recent advances in synthesis, characterization and rheological properties of polyurethanes and POSS/polyurethane nanocomposites dispersions and films, Prog. Polym. Sci., 2009, 34, 1283–1332 CrossRef CAS.
- P. Vermette, H. J. Griesser, G. Laroche and R. Guidoin, Biomedical applications of polyurethanes, Landes Bioscience Georgetown, TX, 2001, vol. 6 Search PubMed.
- G. T. Howard, Biodegradation of polyurethane: a review, Int. Biodeterior. Biodegrad., 2002, 49, 245–252 CrossRef CAS.
- T. Romaškevič, S. Budrienė, K. Pielichowski and J. Pielichowski, Application of polyurethane-based materials for immobilization of enzymes and cells: a review, Chemija, 2006, 17, 74–89 Search PubMed.
- D. Chattopadhyay and D. C. Webster, Thermal stability and flame retardancy of polyurethanes, Prog. Polym. Sci., 2009, 34, 1068–1133 CrossRef CAS.
- M. Szycher, Handbook of polyurethanes, CRC Press, Boca Raton, FL, 1999 Search PubMed.
- U. Lochner, H. Chin and Y. Yamaguchi, Polyurethane foams, Chemical Economics Handbook, Report No. 580.1600 A, IHS Group, Englewood, CO, 2012 Search PubMed.
- http://www.grandviewresearch.com/industry-analysis/bio-based-polyurethane-industry.
- N. Taheri and S. Sayyahi, Effect of clay loading on the structural and mechanical properties of organoclay/HDI-based thermoplastic polyurethane nanocomposites, e-Polym., 2016, 16(1), 65–73 CAS.
- D. Sridaeng, B. Sukkaneewat, N. Chueasakol and N. Chantarasiri, Copper-amine complex solution as a low-emission catalyst for flexible polyurethane foam preparation, e-Polym., 2015, 15(2), 119–126 CAS.
- E. A. Ismail, A. Motawie and E. Sadek, Synthesis and characterization of polyurethane coatings based on soybean oil–polyester polyols, Egypt. J. Pet., 2011, 20, 1–8 CrossRef CAS.
- K. M. Zia, M. Zuber, M. J. Saif, M. Jawaid, K. Mahmood and M. Shahid, Chitin based polyurethanes using hydroxyl terminated polybutadiene, part III: Surface characteristics, Int. J. Biol. Macromol., 2013, 62, 670–676 CrossRef CAS PubMed.
- K. M. Zia, H. N. Bhatti and I. A. Bhatti, Methods for polyurethane and polyurethane composites, recycling and recovery: A review, React. Funct. Polym., 2007, 67, 675–692 CrossRef CAS.
- T. Gurunathan, S. Mohanty and S. K. Nayak, Effect of reactive organoclay on physicochemical properties of vegetable oil-based waterborne polyurethane nanocomposites, RSC Adv., 2015, 5, 11524–11533 RSC.
- P. Alagi, Y. J. Choi and S. C. Hong, Preparation of vegetable oil-based polyols with controlled hydroxyl functionalities for thermoplastic polyurethane, Eur. Polym. J., 2016, 78, 46–60 CrossRef CAS.
- P. Alagi and S. C. Hong, Vegetable oil-based polyols for sustainable polyurethanes, Macromol. Res., 2015, 23(12), 1079–1086 CrossRef CAS.
- A. Fridrihsone-Girone, U. Stirna, M. Misāne, B. Lazdiņa and L. Deme, Spray-applied 100% volatile organic compounds free two component polyurethane coatings based on rapeseed oil polyols, Prog. Org. Coat., 2016, 94, 90–97 CrossRef CAS.
- M. Ionescu, D. Radojčić, X. Wan, M. L. Shrestha, Z. S. Petrović and T. A. Upshaw, Highly functional polyols from castor oil for rigid polyurethanes, Eur. Polym. J., 2016, 88, 736–749 CrossRef.
- A. Guo, Y. Cho and Z. S. Petrović, Structure and properties of halogenated and nonhalogenated soy-based polyols, J. Polym. Sci., Part A: Polym. Chem., 2000, 38, 3900–3910 CrossRef CAS.
- Z. S. Petrovic, W. Zhang and I. Javni, Structure and properties of polyurethanes prepared from triglyceride polyols by ozonolysis, Biomacromolecules, 2005, 6, 713–719 CrossRef CAS PubMed.
- U. Schuchardt, R. Sercheli and R. M. Vargas, Transesterification of vegetable oils: a review, J. Braz. Chem. Soc., 2008, 9, 199–210 Search PubMed.
- A. Guo, D. Demydov, W. Zhang and Z. S. Petrovic, Polyols and polyurethanes from hydroformylation of soybean oil, J. Polym. Environ., 2002, 10, 49–52 CrossRef CAS.
- Y. Kojima, A. Usuki, M. Kawasumi, V. Okada, Y. Fukushima and T. Kurauchi, Mechanical properties of nylon 6-clay hybrid, J. Mater. Res., 1993, 8, 1185–1189 CrossRef CAS.
- A. Usuki, Y. Kojima, M. Kawasumi, A. Okada, Y. Fukushima and T. Kurauchi, Synthesis of nylon 6-clay hybrid, J. Mater. Res., 1993, 8, 1179–1184 CrossRef CAS.
- F. Bergaya, C. Detellier, J.-F. Lambert and G. Lagaly, Introduction to clay–polymer nanocomposites (CPN), Handbook of Clay Sci., 2013, vol. 5, pp. 655–677 Search PubMed.
- B. Chen, J. R. Evans, H. C. Greenwell, P. Boulet, P. V. Coveney and A. A. Bowden, A critical appraisal of polymer–clay nanocomposites, Chem. Soc. Rev., 2008, 37, 568–594 RSC.
- E. D. Weil, Reaction polymers, ed. W. F. Gum, H. Ulrich and W. Riese, Hanser Publishers, Oxford University Press, Munich, Germany, New York, 1992, p. 838, Wiley Online Library, 1993 Search PubMed.
- H. Ulrich, Chemistry and technology of isocyanates, Wiley, 1996 Search PubMed.
- M. Soto, R. M. Sebastián and J. Marquet, Photochemical Activation of Extremely Weak Nucleophiles: Highly Fluorinated Urethanes and Polyurethanes from Polyfluoro Alcohols, J. Org. Chem., 2014, 79, 5019–5027 CrossRef CAS PubMed.
- M. Charlon, B. Heinrich, Y. Matter, E. Couzigné, B. Donnio and L. Avérous, Synthesis, structure and properties of fully biobased thermoplastic polyurethanes, obtained from a diisocyanate based on modified dimer fatty acids, and different renewable diols, Eur. Polym. J., 2014, 61, 197–205 CrossRef CAS.
- N. N. P. N. Pauzi, R. A. Majid, M. H. Dzulkifli and M. Y. Yahya, Development of rigid bio-based polyurethane foam reinforced with nanoclay, Composites, Part B, 2014, 67, 521–526 CrossRef.
- Z. S. Petrović, Polyurethanes from vegetable oils, Polym. Rev., 2008, 48, 109–155 CrossRef.
- B. F. Richard and B. A. R. D. Edmund, Mechanically Frothed Gel Elastomers and Methods of Making and Using Them, US 20160017084 A1, US 14/730,867, Jan 21, 2016.
- G. P. Rajendran, V. Mahadevan and M. Srinivasan, Synthesis of some low glass transition temperature polytetrahydrofuran polymers, Eur. Polym. J., 1989, 25(5), 461–463 CrossRef CAS.
- M. F. Sonnenschein, Polyurethanes. Science, Technology, Markets, and Trends, The Dow Chemical Company, Midland, MI, USA, 2014 Search PubMed.
- http://www.eia.gov/todayinenergy/detail.php?id=10271#.
- M. R. Anisur, M. A. Kibria, M. H. Mahfuz, R. Saidur and I. H. S. C. Metselaar, Latent Heat Thermal Storage (LHTS) for Energy Sustainability, Energy Sustainability Through Green Energy, Part of the series Green Energy and Technology, 2015, pp. 245–263 Search PubMed.
- M. Heinen, A. E. Gerbase and C. L. Petzhold, Vegetable oil-based rigid polyurethanes and phosphorylated flame-retardants derived from epoxydized soybean oil, Polym. Degrad. Stab., 2014, 108, 76–86 CrossRef CAS.
- M. Z. Arniza, S. S. Hoong, Z. Idris, S. K. Yeong, H. A. Hassan, A. K. Din and Y. M. Choo, Synthesis of Transesterified Palm Olein-Based Polyol and Rigid Polyurethanes from this Polyol, J. Am. Oil Chem. Soc., 2015, 92, 243–255 CrossRef CAS PubMed.
- V. B. Veronese, R. K. Menger, M. M. C. Forte and C. L. Petzhold, Rigid polyurethane foam based on modified vegetable oil, J. Appl. Polym. Sci., 2011, 120, 530–537 CrossRef CAS.
- M. Zhang, Z. Luo, J. Zhang, S. Chen and Y. Zhou, Effects of a novel phosphorus–nitrogen flame retardant on rosin-based rigid polyurethane foams, Polym. Degrad. Stab., 2015, 120, 427–434 CrossRef CAS.
- M. Zhang, J. Zhang, S. Chen and Y. Zhou, Synthesis and fire properties of rigid polyurethane foams made from a polyol derived from melamine and cardanol, Polym. Degrad. Stab., 2014, 110, 27–34 CrossRef CAS.
- L. Zhang, M. Zhang, L. Hu and Y. Zhou, Synthesis of rigid polyurethane foams with castor oil-based flame retardant polyols, Ind. Crops Prod., 2014, 52, 380–388 CrossRef CAS.
- S. Semenzato, A. Lorenzetti, M. Modesti, E. Ugel, D. Hrelja and S. Besco, A novel phosphorus polyurethane FOAM/montmorillonite nanocomposite: Preparation, characterization and thermal behaviour, Appl. Clay Sci., 2009, 44, 35–42 CrossRef CAS.
- S. K. Wai, A. H. Sahrim and S. A. Zubir, Synergism between Flame Retardant and Phosphonium Salt Modified Layered Silicate on Properties of Rigid Polyurethane Foam Nanocomposite, Adv. Mater. Res., 2012, 8–12 CrossRef CAS.
- M. Modesti, A. Lorenzetti, S. Besco, D. Hrelja, S. Semenzato and R. Bertani, Synergism between flame retardant and modified layered silicate on thermal stability and fire behaviour of polyurethane nanocomposite foams, Polym. Degrad. Stab., 2008, 93, 2166–2171 CrossRef CAS.
- J. Ma, Z. Qi and Y. Hu, Synthesis and characterization of polypropylene/clay nanocomposites, J. Appl. Polym. Sci., 2001, 82, 3611–3617 CrossRef CAS.
- A. El-Sabbagh, Effect of coupling agent on natural fibre in natural fibre/polypropylene composites on mechanical and thermal behaviour, Composites, Part B, 2014, 57, 126–135 CrossRef CAS.
- Z. Zhang, L. Liao and Z. Xia, Ultrasound-assisted preparation and characterization of anionic surfactant modified montmorillonites, Appl. Clay Sci., 2010, 50, 576–581 CrossRef CAS.
- P. Cinelli, I. Anguillesi and A. Lazzeri, Green synthesis of flexible polyurethane foams from liquefied lignin, Eur. Polym. J., 2013, 49, 1174–1184 CrossRef CAS.
- P. Singhal, W. Small, E. Cosgriff-Hernandez, D. J. Maitland and T. S. Wilson, Low density biodegradable shape memory polyurethane foams for embolic biomedical applications, Acta Biomater., 2014, 10, 67–76 CrossRef CAS PubMed.
- R. Hodlur and M. Rabinal, Self assembled graphene layers on polyurethane foam as a highly pressure sensitive conducting composite, Compos. Sci. Technol., 2014, 90, 160–165 CrossRef CAS.
- S. Kang, S. Kwon, J. Park and B. Kim, Carbon nanotube reinforced shape memory polyurethane foam, Polym. Bull., 2013, 70, 885–893 CrossRef CAS.
- H. D. Liu, Z. Y. Liu, M. B. Yang and Q. He, Surperhydrophobic polyurethane foam modified by graphene oxide, J. Appl. Polym. Sci., 2013, 130, 3530–3536 CrossRef CAS.
- T. Chen, J. Qiu, K. Zhu and J. Li, Electro-mechanical performance of polyurethane dielectric elastomer flexible micro-actuator composite modified with titanium dioxide–graphene hybrid fillers, Mater. Des., 2016, 90, 069–1076 Search PubMed.
- R. Yan, R. Wang, C. W. Lou, S. Y. Huang and J. H. Lin, Quasi-static and dynamic mechanical responses of hybrid laminated composites based on high-density flexible polyurethane foam, Composites, Part B, 2015, 83, 253–263 CrossRef CAS.
- A. S. More, T. Lebarbé, L. Maisonneuve, B. Gadenne, C. Alfos and H. Cramail, Novel fatty acid based di-isocyanates towards the synthesis of thermoplastic polyurethanes, Eur. Polym. J., 2013, 49, 823–833 CrossRef CAS.
- D. V. Palaskar, A. Boyer, E. Cloutet, C. Alfos and H. Cramail, Synthesis of biobased polyurethane from oleic and ricinoleic acids as the renewable resources via the AB-type self-condensation approach, Biomacromolecules, 2010, 11, 1202–1211 CrossRef CAS PubMed.
- B. Claeys, A. Vervaeck, X. K. Hillewaere, S. Possemiers, L. Hansen and T. D. Beer, Thermoplastic polyurethanes for the manufacturing of highly dosed oral sustained release matrices via hot melt extrusion and injection molding, Eur. J. Pharm. Biopharm., 2015, 90, 44–52 CrossRef CAS PubMed.
- M. Unverferth, O. Kreye, V. Prohammer and M. A. Meier, Renewable Non-Isocyanate Based Thermoplastic Polyurethanes via Polycondensation of Dimethyl Carbamate Monomers with Diols, Macromol. Rapid Commun., 2013, 34, 1569–1574 CrossRef CAS PubMed.
- S. Lingier, P. Espeel, S. S. Suarez, O. Türünç, S. D. Wildeman and F. E. D. Prez, Renewable thermoplastic polyurethanes containing rigid spiroacetal moieties, Eur. Polym. J., 2015, 70, 232–239 CrossRef CAS.
- T. J. Johnson, K. M. Gupta, J. Fabian, T. H. Albright and P. F. Kiser, Segmented polyurethane intravaginal rings for the sustained combined delivery of antiretroviral agents dapivirine and tenofovir, Eur. J. Pharm. Sci., 2010, 39, 203–212 CrossRef CAS PubMed.
- O. Jaudouin, J. J. Robin, J. M. Lopez-Cuesta, D. Perrin and C. Imbert, Ionomer-based polyurethanes: a comparative study of properties and applications, Polym. Int., 2012, 61, 495–510 CrossRef CAS.
- D. Fragiadakis, S. Dou, R. H. Colby and J. Runt, Molecular mobility, ion mobility, and mobile ion concentration in poly(ethylene oxide)-based polyurethane ionomers, Macromolecules, 2008, 41(15), 5723–5728, DOI:10.1021/ma800263b.
- T. Buruiana, A. Airinei, E. C. Buruiana and G. Robila, Polyurethane cationomers containing anthryl and nitroaromatic chromophores, Eur. Polym. J., 1997, 33(6), 877–880 CrossRef CAS.
- A. G. Charnetskaya, G. Polizos, V. I. Shtompel, E. G. Privalko, Y. Y. Kercha and P. Pissis, Phase morphology and molecular dynamics of a polyurethane ionomer reinforced with a liquid crystalline filler, Eur. Polym. J., 2003, 39(11), 2167–2174 CrossRef CAS.
- Y. Zhu, J. L. Hu, K. F. Choi, Q. H. Meng, S. J. Chen and K. W. Yeung, Shape memory effect and reversible phase crystallization process in SMPU ionomer, Polym. Adv. Technol., 2008, 19(4), 328–333 CrossRef CAS.
- Y. Zhu, J. L. Hu, J. Lu, L. Y. Yeung and K. W. Yeung, Shape memory fiber spun with segmented polyurethane ionomer, Polym. Adv. Technol., 2008, 19(12), 1745–1753 CrossRef CAS.
- J. Raasch, M. Ivey, D. Aldrich, D. S. Nobes and C. Ayranci, Characterization of polyurethane shape memory polymer processedby material extrusion additive manufacturing, Additive Manufacturing, 2015, 8, 132–141 CrossRef.
- C. C. Wang, Y. Zhao, H. Purnawali, W. M. Huang and L. Sun, Chemically induced morphing in polyurethane shape memory polymer micro fibers/springs, React. Funct. Polym., 2012, 72, 757–764 CrossRef CAS.
- U. M. Casado, R. M. Quintanilla, M. I. Aranguren and N. E. Marcovich, Composite films based on shape memory polyurethanes and nanostructured, polyaniline or cellulose–polyaniline particles, Synth. Met., 2012, 162, 1654–1664 CrossRef CAS.
- J. Chen, Z. X. Zhang, W. B. Huang, J. L. Li, J. H. Yang, Y. Wang, Z. W. Zhou and J. H. Zhang, Carbon nanotube network structure induced strain sensitivity and shape memory behavior changes of thermoplastic polyurethane, Mater. Des., 2015, 69, 105–113 CrossRef CAS.
- S. Gu, B. Yan, L. Liu and J. Ren, Carbon nanotube–polyurethane shape memory nanocomposites with low trigger temperature, Eur. Polym. J., 2013, 49, 3867–3877 CrossRef CAS.
- L. Peponi, I. Navarro-Baena, A. Sonseca, E. Gimenez, A. Marcos-Fernandez and J. M. Kenny, Synthesis and characterization of PCL–PLLA polyurethane with shape memory behavior, Eur. Polym. J., 2013, 49, 893–903 CrossRef CAS.
- T. Takahashi, N. Hayashi and S. Hayashi, Structure and properties of shape-memory polyurethane block copolymers, J. Appl. Polym. Sci., 1996, 60, 1061–1069 CrossRef CAS.
- A. Takahara, R. W. Hergenrother, A. J. Coury and S. L. Cooper, J. Biomed. Mater. Res., 1992, 26, 801–818 CrossRef CAS PubMed.
- M. Yamada, Y. J. Li and T. Nakaya, Macromol. Rapid Commun., 1995, 16, 25–30 CrossRef CAS.
- Y. J. Li, N. Nakamura, Y. F. Wang, M. Kodama and T. Nakaya, Chem. Mater., 1997, 9, 1570–1577 CrossRef CAS.
- L. Zhang, N. R. Brostowitz, K. A. Cavicchi and R. A. Weiss, Perspective: Ionomer Research and Applications, Macromol. React. Eng., 2014, 8, 81–99 CrossRef CAS.
- W. H. Zhu, X. L. Wang, B. Yang, L. Wang, X. Z. Tang and C. Z. Yang, J. Mater. Sci., 2001, 36, 5137–5141 CrossRef CAS.
- Z. S. Petrović, X. Wan, O. Bilić, A. Zlatanić, J. Hong and I. Javni, Polyols and polyurethanes from crude algal oil, J. Am. Oil Chem. Soc., 2013, 90, 1073–1078 CrossRef.
- S. D. Rajput, D. G. Hundiwale, P. P. Mahulikar and V. V. Gite, Fatty acids based transparent polyurethane films and coatings, Prog. Org. Coat., 2014, 77, 1360–1368 CrossRef CAS.
- M. Szycher, Basic concepts in polyurethane chemistry and technology. Szycher's handbook of polyurethanes, CRC Press, Taylor & Francis, Boca Raton, FL, 1999 Search PubMed.
- Y. Xu, Z. Petrovic, S. Das and G. L. Wilkes, Morphology and properties of thermoplastic polyurethanes with dangling chains in ricinoleate-based soft segments, Polymer, 2008, 49, 4248–4258 CrossRef CAS.
- D. Rosu, L. Rosu and C. N. Cascaval, IR-change and yellowing of polyurethane as a result of UV irradiation, Polym. Degrad. Stab., 2009, 94, 591–596 CrossRef CAS.
- P. Davies and G. Evrard, Accelerated ageing of polyurethanes for marine applications, Polym. Degrad. Stab., 2007, 92, 1455–1464 CrossRef CAS.
- X. F. Yang, J. Li, S. Croll, D. Tallman and G. Bierwagen, Degradation of low gloss polyurethane aircraft coatings under UV and prohesion alternating exposures, Polym. Degrad. Stab., 2003, 80, 51–58 CrossRef CAS.
- V. Gite, P. Mahulikar and D. Hundiwale, Preparation and properties of polyurethane coatings based on acrylic polyols and trimer of isophorone diisocyanate, Prog. Org. Coat., 2010, 68, 307–312 CrossRef CAS.
- M. S. Gaikwad, V. V. Gite, P. P. Mahulikar, D. G. Hundiwale and O. S. Yemul, Eco-friendly polyurethane coatings from cottonseed and karanja oil, Prog. Org. Coat., 2015, 86, 64–172 CrossRef.
- E. K. Leitsch, W. H. Heath and J. M. Torkelson, Polyurethane/Polyhydroxyurethane Hybrid Polymers and T. Applications as Adhesive Bonding Agents, Int. J. Adhes. Adhes., 2016, 64, 1–8 CrossRef CAS.
- M. M. Aung, Z. Yaakob, S. Kamarudin and L. C. Abdullah, Synthesis and characterization of Jatropha (Jatropha curcas L.) oil-based polyurethane wood adhesive, Ind. Crops Prod., 2014, 60, 177–185 CrossRef CAS.
- S. Saalah, L. C. Abdullah, M. M. Aung, M. Z. Salleh, D. R. A. Biak and M. Basri, Waterborne polyurethane dispersions synthesized from jatropha oil, Ind. Crops Prod., 2015, 64, 194–200 CrossRef CAS.
- M. S. Kumar, Z. Yaakob, S. Maimunah and S. Abdullah, Synthesis of alkyd resin from non-edible Jatropha seed oil, J. Polym. Environ., 2010, 18, 539–544 CrossRef CAS.
- H. A. Khalil, N. S. Aprilia, A. H. Bhat, M. Jawaid, M. Paridah and D. A. Rudi, Jatropha biomass as renewable materials for biocomposites and its applications, Renewable Sustainable Energy Rev., 2013, 22, 667–685 CrossRef.
- S. Z. Erhan, Industrial uses of vegetable oils, AOCS Press, Champaign, 2005 Search PubMed.
- K. Badri, S. Ahmad and S. Zakaria, Production of a high-functionality RBD palm kernel oil-based polyester polyol, J. Appl. Polym. Sci., 2001, 81, 384–389 CrossRef CAS.
- M. H. Mahmood, Z. Abdullah, Y. Sakurai, K. Zaman and H. M. Dahlan, Effects of monomers on the properties of palm-oil-based radiation curable pressure sensitive adhesives (PSA)—A prepolymer method, Radiat. Phys. Chem., 2001, 60, 129–137 CrossRef.
- Q. Jing, Q. Liu, L. Li, Z. Dong and V. V. Silberschmidt, Effect of graphene-oxide enhancement on large-deflection bending performance of thermoplastic polyurethane elastomer, Composites, Part B, 2016, 89, 1–8 CrossRef CAS.
- S. Liu, H. Sun, N. Ning, L. Zhang, M. Tian, W. Zhu and T. W. Chan, Aligned carbon nanotubes stabilized liquid phase exfoliated graphene hybrid and their polyurethane dielectric elastomers, Compos. Sci. Technol., 2016, 125, 30–37 CrossRef CAS.
- S. A. Madbouly, Y. Xia and M. R. Kessler, Rheological behavior of environmentally friendly castor oil-based waterborne polyurethane dispersions, Macromolecules, 2013, 46, 4606–4616 CrossRef CAS.
- S. A. Madbouly and J. U. Otaigbe, Kinetic analysis of fractal gel formation in waterborne polyurethane dispersions undergoing high deformation flows, Macromolecules, 2006, 39, 4144–4151 CrossRef CAS.
- L. Lei, L. Zhong, X. Lin, Y. Li and Z. Xia, Synthesis and characterization of waterborne polyurethane dispersions with different chain extenders for potential application in waterborne ink, Chem. Eng. J., 2014, 253, 518–525 CrossRef CAS.
- L. Lei, Z. Xia, C. Ou, L. Zhang and L. Zhong, Effects of crosslinking on adhesion behavior of waterborne polyurethane ink binder, Prog. Org. Coat., 2015, 88, 155–163 CrossRef CAS.
- S. Lee, J. H. Choi, I.-K. Hong and J. W. Lee, Curing behavior of polyurethane as a binder for polymer-bonded explosives, J. Ind. Eng. Chem., 2015, 21, 980–985 CrossRef CAS.
- M. Patri, S. Rath and U. Suryavansi, A novel polyurethane sealant based on hydroxy-terminated polybutadiene, J. Appl. Polym. Sci., 2006, 99, 884–890 CrossRef CAS.
- D. Randall and S. Lee, The polyurethanes book, Huntsman Polyurethanes, Belgium, 2002 Search PubMed.
- K. Ninan, V. Balagangadharan and K. B. Catherine, Studies on the functionality distribution of hydroxyl-terminated polybutadiene and correlation with mechanical properties, Polymer, 1991, 32, 628–635 CrossRef CAS.
- H. Bankaitis and P. Smith, HTPB propellants for large booster applications, 1971 Search PubMed.
- A. Ajaz, Hydroxyl-Terminated Polybutadiene Telechelic Polymer (HTPB): Binder for Solid Rocket Propellants, Rubber Chem. Technol., 1995, 68, 481–506 CrossRef CAS.
- S. S. Panicker and K. Ninan, Effect of reactivity of different types of hydroxyl groups of HTPB on mechanical properties of the cured product, J. Appl. Polym. Sci., 1997, 63, 1313–1320 CrossRef CAS.
- G. N. Chen and K. N. Chen, Self-curing behaviors of single pack aqueous-based polyurethane system, J. Appl. Polym. Sci., 1997, 63, 1609–1623 CrossRef CAS.
- J. Zhang, X. Y. Zhang, J. B. Dai and W. H. Li, Synthesis and characterization of yellow water-borne polyurethane using a diol colorant as extender, Chin. Chem. Lett., 2010, 21, 143–145 CrossRef CAS.
- J. Huber and S. Mecking, Aqueous poly(arylacetylene) dispersions, Macromolecules, 2010, 43, 8718–8723 CrossRef CAS.
- C. Fang, X. Zhou, Q. Yu, S. Liu, D. Guo and R. Yu, Synthesis and characterization of low crystalline waterborne polyurethane for potential application in water-based ink binder, Prog. Org. Coat., 2014, 77, 61–71 CrossRef CAS.
- Y. Fangcq and S. Zhous, The effect of additives to the polyurethane water-based ink, Res. J. Chem. Environ., 2011, 15, 377–379 Search PubMed.
- S.-J. Peng, Y. Jin, X.-F. Cheng, T.-B. Sun, R. Qi and B.-Z. Fan, A new method to synthesize high solid content waterborne polyurethanes by strict control of bimodal particle size distribution, Prog. Org. Coat., 2015, 86, 1–10 CrossRef CAS.
- F. Chu and A. Guyot, High solids content latexes with low viscosity, Colloid Polym. Sci., 2001, 279, 361–367 CAS.
- Z. Ai, R. Deng, Q. Zhou, S. Liao and H. Zhang, High solid content latex: Preparation methods and application, Adv. Colloid Interface Sci., 2010, 159, 45–59 CrossRef CAS PubMed.
- J. Li, X. Zhang, Z. Liu, W. Li and J. Dai, Studies on waterborne polyurethanes based on new medium length fluorinated diols, J. Fluorine Chem., 2015, 175, 12–17 CrossRef CAS.
- W. Fan, W. Du, Z. Li, N. Dan and J. Huang, Abrasion resistance of waterborne polyurethane films incorporated with PU/silica hybrids, Prog. Org. Coat., 2015, 86, 125–133 CrossRef CAS.
- C. Fu, Z. Zheng, Z. Yang, Y. Chen and L. Shen, A fully bio-based waterborne polyurethane dispersion from vegetable oils: From synthesis of precursors by thiol-ene reaction to study of final material, Prog. Org. Coat., 2014, 77, 53–60 CrossRef CAS.
- O. Bayer, Polyurethanes, Mod. Plast., 1947, 24, 149–152 CAS.
- A. L. Brocas, C. Mantzaridis, D. Tunc and S. Carlotti, Polyether synthesis: From activated or metal-free anionic ring-opening polymerization of epoxides to functionalization, Prog. Polym. Sci., 2013, 38, 845–873 CrossRef CAS.
- L. C. Bailosky, L. M. Bender, D. Bode, R. A. Choudhery, G. P. Craun, K. J. Gardner, C. R. Michalski, J. T. Rademacher, G. L. Stella and D. J. Telford, Synthesis of polyether polyols with epoxidized soy bean oil, Prog. Org. Coat., 2013, 76, 1712–1719 CrossRef CAS.
- G. T. Cardoso, S. C. Neto and F. Vecchia, Rigid foam polyurethane (PU) derived from castor oil (Ricinus communis) for thermal insulation in roof systems, Frontiers of Architectural Research, 2012, 1, 348–356 CrossRef.
- J. Blackwell, M. Nagarajan and T. Hoitink, The Structure of the Hard Segments in MDI/diol/PTMA Polyurethane Elastomers, 1981 Search PubMed.
- H. Sheikhy, M. Shahidzadeh, B. Ramezanzadeh and F. Noroozi, Studying the effects of chain extenders chemical structures on the adhesion and mechanical properties of a polyurethane adhesive, J. Ind. Eng. Chem., 2013, 19, 1949–1955 CrossRef CAS.
- Y. Savelyev, V. Veselov, L. Markovskaya, O. Savelyeva, E. Akhranovich and N. Galatenko, Preparation and characterization of new biologically active polyurethane foams, Mater. Sci. Eng., C, 2014, 45, 127–135 CrossRef CAS PubMed.
- R. Patel, M. Shah and H. Patel, Synthesis and characterization of structurally modified polyurethanes based on castor oil and phosphorus-containing polyol for flame-retardant coatings, Int. J. Polym. Anal. Charact., 2011, 16, 107–117 CrossRef CAS.
- D. Klempner and K. C. Frisch, Handbook of polymeric foams and foam technology, Hanser, Munich, 1991, vol. 404 Search PubMed.
- O. Gunter, Polyurethane handbook, Hanser, Munich, 1985 Search PubMed.
- A. Domanska and A. Boczkowska, Biodegradable polyurethanes from crystalline prepolymers, Polym. Degrad. Stab., 2014, 108, 175–181 CrossRef CAS.
- S. D. Rajput, P. P. Mahulikar and V. V. Gite, Biobased dimer fatty acid containing two pack polyurethane for wood finished coatings, Prog. Org. Coat., 2014, 77, 38–46 CrossRef CAS.
- A. Chaudhari, A. Kuwar, P. Mahulikar, D. Hundiwale, R. Kulkarni and V. Gite, Development of anticorrosive two pack polyurethane coatings based on modified fatty amide of Azadirachta indica Juss oil cured at room temperature – a sustainable resource, RSC Adv., 2014, 4, 17866–17872 RSC.
- Z. Gao, J. Peng, T. Zhong, J. Sun, X. Wang and C. Yue, Biocompatible elastomer of waterborne polyurethane based on castor oil and polyethylene glycol with cellulose nanocrystals, Carbohydr. Polym., 2012, 87, 2068–2075 CrossRef CAS.
- S. Oprea, Dependence of fungal biodegradation of PEG/castor oil-based polyurethane elastomers on the hard-segment structure, Polym. Degrad. Stab., 2010, 95, 2396–2404 CrossRef CAS.
- Y. Mülazim, E. Çakmakçı and M. V. Kahraman, Preparation of photo curable highly hydrophobic coatings using a modified castor oil derivative as a sol–gel component, Prog. Org. Coat., 2011, 72, 394–401 CrossRef.
- S. V. Levchik and E. D. Weil, Thermal decomposition, combustion and fire-retardancy of polyurethanes—a review of the recent literature, Polym. Int., 2004, 53, 1585–1610 CrossRef CAS.
- V. B. Veronese, R. K. Menger, M. M. D. C. Forte and C. L. Petzhold, Rigid polyurethane foam based on modified vegetable oil, J. Appl. Polym. Sci., 2011, 120, 530–537 CrossRef CAS.
- A. Lee and Y. Deng, Green polyurethane from lignin and soybean oil through non-isocyanate reactions, Eur. Polym. J., 2015, 63, 67–73 CrossRef CAS.
- G. Rokicki and A. Piotrowska, A new route to polyurethanes from ethylene carbonate, diamines, and diols, Polymer, 2002, 43, 2927–2935 CrossRef CAS.
- C. Cateto, M. Barreiro, A. Rodrigues and M. Belgacem, Optimization study of lignin oxypropylation in view of the preparation of polyurethane rigid foams, Ind. Eng. Chem. Res., 2009, 48, 2583–2589 CrossRef CAS.
- Y. Shi, B. Yu, K. Zhou, R. K. K. Yuen, Z. Gui, Y. Hu and S. Jiang, Novel CuCo2O4/graphitic carbon nitride nanohybrids: Highly effective catalysts for reducing CO generation and fire hazards of thermoplastic polyurethane nanocomposites, J. Hazard. Mater., 2015, 293, 87–96 CrossRef CAS PubMed.
- H. Sardon, L. Irusta and M. J. Fernández-Berridi, Synthesis of isophorone diisocyanate (IPDI) based waterborne polyurethanes: Comparison between zirconium and tin catalysts in the polymerization process, Prog. Org. Coat., 2009, 66, 291–295 CrossRef CAS.
- S. Kirchmeyer, H.-P. Müller, M. Ullrich and U. Liesenfelder, Homogeneously mixing polyisocyanate, zerewitinoff active hydrogen compound, and chain extender for condensation polymerization, continuous degassing and extrusion, Grant, US Pat., US6,417,312 B1, 2002.
- S. Yamasaki, D. Nishiguchi, K. Kojio and M. Furukawa, Effects of polymerization method on structure and properties of thermoplastic polyurethanes, J. Polym. Sci., Part B: Polym. Phys., 2007, 45, 800–814 CrossRef CAS.
- J. Blackwell and K. H. Gardner, Structure of the hard segments in polyurethane elastomers, Polymer, 1979, 20, 13–17 CrossRef CAS.
- S. Musselman, T. Santosusso and L. Sperling, Structure versus performance properties of cast elastomers, in Polyurethanes' 98 Conference Proceedings, 1998 Search PubMed.
- J. Zheng, J. Luo, D. Zhou, T. Shen, H. Li, L. Liang and M. Lu, Preparation and properties of non-ionic polyurethane surfactants, Colloids Surf., A, 2010, 363, 16–21 CrossRef CAS.
- A. R. Motamedi and M. M. Tehrani-Bagha, The effect of cationic surfactants in acid cleaning solutions onprotective performance and adhesion strength of the subsequent polyurethane coating, Prog. Org. Coat., 2014, 77, 712–718 CrossRef.
- L. Jin, Z. Liu, Q. Xu and Y. J. Li, J. Appl. Polym. Sci., 2006, 99, 1111–1116 CrossRef CAS.
- Y. Lu, Y. Xia and R. C. Larock, Surfactant-free core–shell hybrid latexes from soybean oil-based waterborne polyurethanes and poly(styrene-butyl acrylate), Prog. Org. Coat., 2011, 71, 336–342 CrossRef CAS.
- A. M. Borreguero, P. Sharma, C. Spiteri, M. M. Velencoso, M. S. Carmona, J. E. Moses and J. F. Rodriguez, A novel click-chemistry approach to flame retardant polyurethanes, React. Funct. Polym., 2013, 73, 1207–1212 CrossRef CAS.
- D. Fournier, B. G. D. Geest and F. E. D. Prez, On-demand click functionalization of polyurethane films and foams, Polymer, 2009, 50, 5362–5367 CrossRef CAS.
- G. Chen, X. Guan, R. Xu, J. Tian, M. He, W. Shen and J. Yang, Synthesis and characterization of UV-curable castor oil-based polyfunctional polyurethane acrylate via photo-click chemistry andisocyanate polyurethane reaction, Prog. Org. Coat., 2016, 93, 11–16 CrossRef CAS.
- S. Kantheti, P. S. Sarath, R. Narayan and K. V. S. N. Raju, Synthesis and characterization of triazole rich polyether polyols using click chemistry for highly branched polyurethanes, React. Funct. Polym., 2013, 73, 1597–1605 CrossRef CAS.
- S. K. Yadav, S. S. Mahapatra and J. W. Cho, Synthesis of mechanically robust antimicrobial nanocomposites by click coupling of hyperbranched polyurethane and carbon nanotubes, Polymer, 2012, 53, 2023–2031 CrossRef CAS.
- J. Wang and J. Li, Dopamine-assisted deposition of lubricating and antifouling coatings on polyurethane surfaces by one-pot ATRP and click chemistry, Mater. Lett., 2017, 186, 178–181 CrossRef CAS.
- X. J. Li, D. X. Sun and Y. H. Zhang, The Cu(I)-mediated azide–alkyne cycloadditions as a facile and efficient route for functionalization of waterborne polyurethane, J. Polym. Res., 2014, 21, 320 CrossRef.
- Y. Xue, D. Ma, T. Zhang, S. Lin, S. Shao and N. Gu, Synthesis and Characterization of Comblike Methoxy Polyethylene Glycol-grafted Polyurethanes via ‘Click’ Chemistry, J. Macromol. Sci., Part A: Pure Appl.Chem., 2014, 51(5), 456–464 CrossRef CAS.
- X. Li, J. Hu, D. Sun and Y. Zhang, Nanosilica reinforced waterborne siloxane-polyurethane nanocomposites prepared via “click” coupling, J. Coat. Technol. Res., 2014, 11(4), 517–531 CrossRef CAS.
- R. K. Gupta, M. Ionescu, D. Radojcic, X. Wan and Z. S. Petrovic, Novel Renewable Polyols Based on Limonene for Rigid Polyurethane Foams, J. Polym. Environ., 2014, 22, 304–309 CrossRef CAS.
- M. Zhang, Z. Luo, J. Zhang, S. Chen and Y. Zhou, Effects of a novel phosphorusenitrogen flame retardant on rosin-based rigid polyurethane foams, Polym. Degrad. Stab., 2015, 120, 427–434 CrossRef CAS.
- V. Benin, B. Gardelle and A. B. Morgan, Heat release of polyurethanes containing potential flame retardants based on boron and phosphorus chemistries, Polym. Degrad. Stab., 2014, 106, 108–121 CrossRef CAS.
- Y. Yuan, H. Yang, B. Yu, Y. Shi, W. Wang, L. Song, Y. Hu and Y. Zhang, Phosphorus and Nitrogen-Containing Polyols: Synergistic Effect on the Thermal Property and Flame Retardancy of Rigid Polyurethane Foam Composites, Ind. Eng. Chem. Res., 2016, 55(41), 10813–10822 CrossRef CAS.
- A. Wolska, M. Goz'dzikiewicz and J. Ryszkowska, Thermal and mechanical behaviour of flexible polyurethane foams modified with graphite and phosphorous fillers, J. Mater. Sci., 2012, 47, 5627–5634 CrossRef CAS.
- L. Breucker, K. Landfester and A. Taden, Phosphonic Acid-Functionalized Polyurethane Dispersions with Improved Adhesion Properties, ACS Appl. Mater. Interfaces, 2015, 7, 24641–24648 CAS.
- S. T. McKenna and T. R. Hull, The fire toxicity of polyurethane foams, Fire Sci. Rev., 2016, 5, 3 CrossRef.
- L. Brinchi, F. Cotana, E. Fortunati and J. Kenny, Production of nanocrystalline cellulose from lignocellulosic biomass: technology and applications, Carbohydr. Polym., 2013, 94, 154–169 CrossRef CAS PubMed.
- V. Alvarez and A. Vázquez, Thermal degradation of cellulose derivatives/starch blends and sisal fibre biocomposites, Polym. Degrad. Stab., 2004, 84, 13–21 CrossRef CAS.
- D. Klemm, B. Heublein, H. P. Fink and A. Bohn, Cellulose: fascinating biopolymer and sustainable raw material, Angew. Chem., Int. Ed., 2005, 44, 3358–3393 CrossRef CAS PubMed.
- A. I. Cordero, J. I. Amalvy, E. Fortunati, J. M. Kenny and L. M. Chiacchiarelli, The role of nanocrystalline cellulose on the microstructure of foamed castor-oil polyurethane nanocomposites, Carbohydr. Polym., 2015, 134, 110–118 CrossRef CAS PubMed.
- M. I. Aranguren, N. E. Marcovich, W. Salgueiro and A. Somoza, Effect of the nano-cellulose content on the properties of reinforced polyurethanes. A study using mechanical tests and positron anihilation spectroscopy, Polym. Test., 2013, 32, 115–122 CrossRef CAS.
- V. Wik, M. Aranguren and M. Mosiewicki, Castor oil-based polyurethanes containing cellulose nanocrystals, Polym. Eng. Sci., 2011, 51, 1389–1396 CAS.
- J. Juntaro, S. Ummartyotin, M. Sain and H. Manuspiya, Bacterial cellulose reinforced polyurethane-based resin nanocomposite: a study of how ethanol and processing pressure affect physical, mechanical and dielectric properties, Carbohydr. Polym., 2012, 87, 2464–2469 CrossRef CAS.
- L. Rueda, A. Saralegui, B. F. D'Arlas, Q. Zhou, L. A. Berglund and M. Corcuera, Cellulose nanocrystals/polyurethane nanocomposites. Study from the viewpoint of microphase separated structure, Carbohydr. Polym., 2013, 92, 751–757 CrossRef CAS PubMed.
- U. Casado, N. Marcovich, M. Aranguren and M. Mosiewicki, High-strength composites based on tung oil polyurethane and wood flour: Effect of the filler concentration on the mechanical properties, Polym. Eng. Sci., 2009, 49, 713–721 CAS.
- Y. Li and A. J. Ragauskas, Cellulose nano whiskers as a reinforcing filler in polyurethanes, Algae, 2011, 75, 10–15 Search PubMed.
- Y. Li and A. J. Ragauskas, Ethanol organosolv lignin-based rigid polyurethane foam reinforced with cellulose nanowhiskers, RSC Adv., 2012, 2, 3347–3351 RSC.
- Y. Li, H. Ren and A. J. Ragauskas, Rigid polyurethane foam reinforced with cellulose whiskers: Synthesis and characterization, Nano-Micro Lett., 2010, 2, 89–94 CrossRef CAS.
- Y. Li, H. Ren and A. J. Ragauskas, Rigid polyurethane foam/cellulose whisker nanocomposites: preparation, characterization, and properties, J. Nanosci. Nanotechnol., 2011, 11, 6904–6911 CrossRef CAS PubMed.
- M. A. Mosiewicki, P. Rojek, S. Michałowski, M. I. Aranguren and A. Prociak, Rapeseed oil-based polyurethane foams modified with glycerol and cellulose micro/nanocrystals, J. Appl. Polym. Sci., 2015, 132 Search PubMed.
- M. Mosiewicki, U. Casado, N. Marcovich and M. Aranguren, Polyurethanes from tung oil: polymer characterization and composites, Polym. Eng. Sci., 2009, 49, 685 CAS.
- M. Silva, J. Takahashi, D. Chaussy, M. Belgacem and G. Silva, Composites of rigid polyurethane foam and cellulose fiber residue, J. Appl. Polym. Sci., 2010, 117, 3665–3672 CAS.
- E. S. Ali and S. Ahmad, Bionanocomposite hybrid polyurethane foam reinforced with empty fruit bunch and nanoclay, Composites, Part B, 2012, 43, 2813–2816 CrossRef CAS.
- M. Abdollahi, M. Rezaei and G. Farzi, A novel active bionanocomposite film incorporating rosemary essential oil and nanoclay into chitosan, J. Food Eng., 2012, 111, 343–350 CrossRef CAS.
- M. Barmar, M. Barikani and M. Fereidounnia, Study of polyurethane/clay nanocomposites produced via melt intercalation method, Iran. Polym. J., 2006, 15, 709–714 CAS.
- M. Darder, M. Colilla and E. Ruiz-Hitzky, Biopolymer-clay nanocomposites based on chitosan intercalated in montmorillonite, Chem. Mater., 2003, 15, 3774–3780 CrossRef CAS.
- S. Wang, L. Shen, Y. Tong, L. Chen, I. Phang and P. Lim, Biopolymer chitosan/montmorillonite nanocomposites: preparation and characterization, Polym. Degrad. Stab., 2005, 90, 123–131 CrossRef CAS.
- A. Casariego, B. Souza, M. Cerqueira, J. Teixeira, L. Cruz and R. Díaz, Chitosan/clay films' properties as affected by biopolymer and clay micro/nanoparticles' concentrations, Food Hydrocolloids, 2009, 23, 1895–1902 CrossRef CAS.
- J. W. Rhim, S. I. Hong, H. M. Park and P. K. Ng, Preparation and characterization of chitosan-based nanocomposite films with antimicrobial activity, J. Agric. Food Chem., 2006, 54, 5814–5822 CrossRef CAS PubMed.
- J. O. Akindoyo, M. D. H. Beg, S. Ghazali and M. R. Islam, Effects of poly(dimethyl siloxane) on the water absorption and natural degradation of poly(lactic acid)/oil-palm empty-fruit-bunch fiber biocomposites, J. Appl. Polym. Sci., 2015, 132(45) DOI:10.1002/app.42784/full.
- J. O. Akindoyo, M. D. H. Beg, S. Ghazali, M. R. Islam and A. Mamun, Preparation and Characterization of Poly(Lactic Acid) Based Composites Reinforced with Poly Dimethyl Siloxane/Ultrasound Treated Oil Palm Empty Fruit Bunch, Polym.-Plast. Technol. Eng., 2015, 54(13), 1321–1333 CrossRef CAS.
- K. Liang and S. Q. Shi, Nanoclay filled soy-based polyurethane foam, J. Appl. Polym. Sci., 2011, 119(3), 1857–1863 CrossRef CAS.
- M. Berta, C. Lindsay, G. Pans and G. Camino, Effect of chemical structure on combustion and thermal behaviour of polyurethane elastomer layered silicate nanocomposites, Polym. Degrad. Stab., 2006, 91, 1179–1191 CrossRef CAS.
- W. Seo, Y. Sung, S. Kim, Y. Lee, K. Choe and S. Choe, Effects of ultrasound on the synthesis and properties of polyurethane foam/clay nanocomposites, J. Appl. Polym. Sci., 2006, 102, 3764–3773 CrossRef CAS.
- S. Kim, M. Lee, H. Kim, H. Park, H. Jeong and K. Yoon, Nanoclay reinforced rigid polyurethane foams, J. Appl. Polym. Sci., 2010, 117, 1992–1997 CrossRef CAS.
- P. Singla, R. Mehta and S. N. Upadhyay, Clay modification by the use of organic cations, Green Sustainable Chem., 2012, 2, 21 CrossRef CAS.
- M. Jacob, S. Thomas and K. T. Varughese, Mechanical properties of sisal/oil palm hybrid fiber reinforced natural rubber composites, Compos. Sci. Technol., 2004, 64, 955–965 CrossRef CAS.
- M. Huda, L. Drzal, A. Mohanty and M. Misra, The effect of silane treated-and untreated-talc on the mechanical and physico-mechanical properties of poly(lactic acid)/newspaper fibers/talc hybrid composites, Composites, Part B, 2007, 38, 367–379 CrossRef.
- A. Serrano, A. M. Borreguero, I. Garrido, J. F. Rodríguez and M. Carmona, Reducing heat loss through the building envelope by using polyurethane foams containing thermoregulating microcapsules, Appl. Therm. Eng., 2016, 103, 226–232 CrossRef CAS.
- J. Njuguna, S. Michałowski, K. Pielichowski, K. Kayvantash and A. C. Walton, Fabrication, characterization and low-velocity impact testing of hybrid sandwich composites with polyurethane/layered silicate foam cores, Polym. Compos., 2011, 32, 6–13 CrossRef CAS.
- C. Fu, J. Liu, H. Xia and L. Shen, Effect of structure on the properties of polyurethanes based on aromatic cardanol-based polyols prepared by thiol-ene coupling, Prog. Org. Coat., 2015, 83, 19–25 CrossRef CAS.
- F. Camara, S. Benyahya, V. Besse, G. Boutevin, R. Auvergne and B. Boutevin, Reactivity of secondary amines for the synthesis of non-isocyanate polyurethanes, Eur. Polym. J., 2014, 55, 17–26 CrossRef CAS.
- E. Moawed, A. Abulkibash and M. El-Shahat, Synthesis of tannic acid azo polyurethane sorbent and its application for extraction and determination of atrazine and prometryn pesticides in foods and water samples, Environmental Nanotechnology, Monitoring & Management, 2015, 3, 61–66 Search PubMed.
- A. Lorenzetti, M. Modesti, E. Gallo, B. Schartel, S. Besco and M. Roso, Synthesis of phosphinated polyurethane foams with improved fire behaviour, Polym. Degrad. Stab., 2012, 97, 2364–2369 CrossRef CAS.
- N. Baheiraei, H. Yeganeh, J. Ai, R. Gharibi, M. Azami and F. Faghihi, Synthesis, characterization and antioxidant activity of a novel electroactive and biodegradable polyurethane for cardiac tissue engineering application, Mater. Sci. Eng., C, 2014, 44, 24–37 CrossRef CAS PubMed.
- S. Xinrong, W. Nanfang, S. Kunyang, D. Sha and C. Zhen, Synthesis and characterization of waterborne polyurethane containing UV absorption group for finishing of cotton fabrics, J. Ind. Eng. Chem., 2014, 20, 3228–3233 CrossRef.
- J. Zhang, W. Tu and Z. Dai, Synthesis and characterization of transparent and high impact resistance polyurethane coatings based on polyester polyols and isocyanate trimers, Prog. Org. Coat., 2012, 75, 579–583 CrossRef CAS.
- L. Yu, L. Zhou, M. Ding, J. Li, H. Tan and Q. Fu, Synthesis and characterization of novel biodegradable folate conjugated polyurethanes, J. Colloid Interface Sci., 2011, 358, 376–383 CrossRef CAS PubMed.
- E. Moawed, A. Abulkibash and M. El-Shahat, Synthesis and characterization of iodo polyurethane foam and its application in removing of aniline blue and crystal violet from laundry wastewater, Journal of Taibah University for Science, 2015, 9, 80–88 CrossRef.
- H. Lijie, D. Yongtao, Z. Zhiliang, S. Zhongsheng and S. Zhihua, Synergistic effect of anionic and nonionic monomers on the synthesis of high solid content waterborne polyurethane, Colloids Surf., A, 2015, 467, 46–56 CrossRef.
- Y. Lin, Y. Zhou, C. Xu, A. Xie, M. Yang and S. Yang, Study on synthesis and thickening property of hyperbranched waterborne polyurethane, Prog. Org. Coat., 2013, 76, 1302–1307 CrossRef CAS.
- N. Liu, Y. Zhao, M. Kang, J. Wang, X. Wang and Y. Feng, The effects of the molecular weight and structure of polycarbonatediols on the properties of waterborne polyurethanes, Prog. Org. Coat., 2015, 82, 46–56 CrossRef CAS.
- C. H. Tsou, H. T. Lee, H. A. Tsai, H. J. Cheng and M. C. Suen, Synthesis and properties of biodegradable polycaprolactone/polyurethanes by using 2,6-pyridinedimethanol as a chain extender, Polym. Degrad. Stab., 2013, 98, 643–650 CrossRef CAS.
- H. Murakami, R. Baba, M. Fukushima and N. Nonaka, Synthesis and characterization of polyurethanes crosslinked by polyrotaxanes consisting of half-methylated cyclodextrins and PEGs with different chain lengths, Polymer, 2015, 56, 368–374 CrossRef CAS.
- W. Q. Qu, Y. R. Xia, L. J. Jiang, L. W. Zhang and Z. S. Hou, Synthesis and characterization of a new biodegradable polyurethanes with good mechanical properties, Chin. Chem. Lett., 2015, 27(1), 135–138 CrossRef.
- A. Solanki, J. Mehta and S. Thakore, Structure–property relationships and biocompatibility of carbohydrate crosslinked polyurethanes, Carbohydr. Polym., 2014, 110, 338–344 CrossRef CAS PubMed.
- A. Santamaria-Echart, A. Arbelaiz, A. Saralegi, B. Fernández-d'Arlas, A. Eceiza and M. Corcuera, Relationship between reagents molar ratio and dispersion stability and film properties of waterborne polyurethanes, Colloids Surf., A, 2015, 482, 554–561 CrossRef CAS.
- R. H. Patel and K. S. Patel, Synthesis and characterization of flame retardant hyperbranched polyurethanes for nano-composite and nano-coating applications, Prog. Org. Coat., 2015, 88, 283–292 CrossRef CAS.
- X. Wang, J. Hu, Y. Li, J. Zhang and Y. Ding, The Surface Properties and Corrosion Resistance of Fluorinated Polyurethane Coatings, J. Fluorine Chem., 2015, 176, 14–19 CrossRef CAS.
- S. Oprea, P. Gradinariu, A. Joga and V. Oprea, Synthesis, structure and fungal resistance of sulfadiazine-based polyurethane ureas, Polym. Degrad. Stab., 2013, 98, 1481–1488 CrossRef CAS.
- K. Benhamou, H. Kaddami, A. Magnin, A. Dufresne and A. Ahmad, Bio-based polyurethane reinforced with cellulose nanofibers: A comprehensive investigation on the effect of interface, Carbohydr. Polym., 2015, 122, 202–211 CrossRef CAS PubMed.
- M. Shin, Y. Lee, M. Rahman and H. Kim, Synthesis and properties of waterborne fluorinated polyurethane-acrylate using a solvent-/emulsifier-free method, Polymer, 2013, 54, 4873–4882 CrossRef CAS.
- S. Zhang, Z. Chen, M. Guo, H. Bai and X. Liu, Synthesis and characterization of waterborne UV-curable polyurethane modified with side-chain triethoxysilane and colloidal silica, Colloids Surf., A, 2015, 468, 1–9 CrossRef CAS.
- S. Zhang, A. Yu, X. Song and X. Liu, Synthesis and characterization of waterborne UV-curable polyurethane nanocomposites based on the macromonomer surface modification of colloidal silica, Prog. Org. Coat., 2013, 76, 1032–1039 CrossRef CAS.
- A. Biswas, M. Appell, Z. Liu and H. Cheng, Microwave-assisted synthesis of cyclodextrin polyurethanes, Carbohydr. Polym., 2015, 133, 74–79 CrossRef CAS PubMed.
- F. Kara, E. A. Aksoy, Z. Yuksekdag, N. Hasirci and S. Aksoy, Synthesis and surface modification of polyurethanes with chitosan for antibacterial properties, Carbohydr. Polym., 2014, 112, 39–47 CrossRef CAS PubMed.
- J. Yi, C. Huang, H. Zhuang, H. Gong, C. Zhang and R. Ren, Degradable polyurethane based on star-shaped polyester polyols (trimethylolpropane and ε-caprolactone) for marine antifouling, Prog. Org. Coat., 2015, 87, 161–170 CrossRef CAS.
- K. V. Baratha, A. Nourry and J. F. Pilard, Synthesis of NR based Polyurethanes containing phosphorylated polymers as chain extenders, Eur. Polym. J., 2015, 70, 317–330 CrossRef CAS.
- A. Lee and Y. Deng, Green polyurethane from lignin and soybean oil through non-isocyanate reactions, Eur. Polym. J., 2015, 63, 67–73 CrossRef CAS.
- N. Diascorn, S. Calas, H. Sallee, P. Achard and A. Rigacci, Polyurethane aerogels synthesis for thermal insulation – textural, thermal and mechanical properties, J. Supercrit. Fluids, 2015, 106, 76–84 CrossRef CAS.
- V. R. Da Silva, M. A. Mosiewicki, M. I. Yoshida, M. C. Da Silva, P. M. Stefani and N. E. Marcovich, Polyurethane foams based on modified tung oil and reinforced with rice husk ash I: synthesis and physical chemical characterization, Polym. Test., 2013, 32, 438–445 CrossRef.
- G. Liu, G. Wu, C. Jin and Z. Kong, Preparation and antimicrobial activity of terpene-based polyurethane coatings with carbamate group-containing quaternary ammonium salts, Prog. Org. Coat., 2015, 80, 150–155 CrossRef CAS.
- R. Deng, P. Davies and A. Bajaj, Flexible polyurethane foam modelling and identification of viscoelastic parameters for automotive seating applications, J. Sound Vib., 2003, 262, 391–417 CrossRef.
- N. M. Zain, E. N. Roslin and S. Ahmad, Preliminary study on bio-based polyurethane adhesive/aluminum laminated composites for automotive applications, Int. J. Adhes. Adhes., 2016, 71, 1–9 CrossRef CAS.
- G. Verma, A. Kaushik and A. K. Ghosh, Nano-interfaces between clay platelets and polyurethane hard segments in spray coated automotive nanocomposites, Prog. Org. Coat., 2016, 99, 282–294 CrossRef CAS.
- P. Xiao, Y. Dudal, P. F. X. Corvini, U. Pieles and P. Shahgaldian, Cyclodextrin-based polyurethanes act as selective molecular recognition materials of active pharmaceutical ingredients (APIs), Polym. Chem., 2011, 2, 1264–1266 RSC.
- Y. P. Chin, S. Mohamad and M. R. B. Abas, Removal of parabens from aqueous solution using β-cyclodextrin cross-linked polymer, Int. J. Mol. Sci., 2010, 11, 3459–3471 CrossRef CAS PubMed.
- P. Davies and G. Evrard, Accelerated ageing of polyurethanes for marine applications, Polym. Degrad. Stab., 2007, 92, 1455–1464 CrossRef CAS.
- S. Dutta and N. Karak, Synthesis, characterization of poly(urethane amide) resins from Nahar seed oil for surface coating applications, Prog. Org. Coat., 2005, 53, 147–152 CrossRef CAS.
- S. Dutta, N. Karak and T. Jana, Evaluation of Mesua ferrea L. seed oil modified polyurethane paints, Prog. Org. Coat., 2009, 65, 131–135 CrossRef CAS.
- R. A. Van Benthem, Novel hyperbranched resins for coating applications, Prog. Org. Coat., 2000, 40, 203–214 CrossRef CAS.
- L. Chen and Y. Z. Wang, A review on flame retardant technology in China. Part I: development of flame retardants, Polym. Adv. Technol., 2010, 21, 1–26 Search PubMed.
- S. Thakur and N. Karak, Castor oil-based hyperbranched polyurethanes as advanced surface coating materials, Prog. Org. Coat., 2013, 76, 157–164 CrossRef CAS.
- B. Zhou, Y. Hu, J. Li and B. Li, Chitosan/phosvitin antibacterial films fabricated via layer-by-layer deposition, Int. J. Biol. Macromol., 2014, 64, 402–408 CrossRef CAS PubMed.
- X. Zhou, T. Zhang, D. Guo and N. Gu, A facile preparation of poly(ethylene oxide)-modified medical polyurethane to improve hemocompatibility, Colloids Surf., A, 2014, 441, 34–42 CrossRef CAS.
- Y. Wang, Q. Hong, Y. Chen, X. Lian and Y. Xiong, Surface properties of polyurethanes modified by bioactive polysaccharide-based polyelectrolyte multilayers, Colloids Surf., B, 2012, 100, 77–83 CrossRef CAS PubMed.
- J. C. Middleton and A. J. Tipton, Synthetic biodegradable polymers as orthopedic devices, Biomaterials, 2000, 21, 2335–2346 CrossRef CAS PubMed.
- http://www.cleantechday.fi/wp-content/uploads/2016/05/Polylabs-2.pdf.
- http://bioplasticsnews.com/2015/02/13/bio-based-polyurethane-pu-market-analysis-and-forecasts-to-2020/.
- D. H. Owen and D. F. Katz, A vaginal fluid simulant, Contraception, 1999, 59, 91–95 CrossRef CAS PubMed.
- C. Tevi-Bénissan, L. Belec, M. Levy, V. Schneider-Fauveau, A. S. Mohamed and M. C. Hallouin, In vivo semen-associated pH neutralization of cervicovaginal secretions, Clin. Diagn. Lab. Immunol., 1997, 4, 367–374 Search PubMed.
- A. Solanki and S. Thakore, Cellulose crosslinked pH-responsive polyurethanes for drug delivery: α-hydroxy acids as drug release modifiers, Int. J. Biol. Macromol., 2015, 80, 683–691 CrossRef CAS PubMed.
- M. Naeem, W. Kim, J. Cao, Y. Jung and J. W. Yoo, Enzyme/pH dual sensitive polymeric nanoparticles for targeted drug delivery to the inflamed colon, Colloids Surf., B, 2014, 123, 271–278 CrossRef CAS PubMed.
- T. Yamaoka, Y. Makita, H. Sasatani, S. I. Kim and Y. Kimura, Linear type azo-containing polyurethane as drug-coating material for colon-specific delivery: its properties, degradation behavior, and utilization for drug formulation, J. Controlled Release, 2000, 66, 187–197 CrossRef CAS PubMed.
- M. R. Clark, T. J. Johnson, R. T. Mccabe, J. T. Clark, A. Tuitupou and H. Elgendy, A hot-melt extruded intravaginal ring for the sustained delivery of the antiretroviral microbicide UC78, J. Pharm. Sci., 2012, 101, 576–587 CrossRef CAS PubMed.
- R. Lalwani and S. Desai, Sorption behavior of biodegradable polyurethanes with carbohydrate crosslinkers, J. Appl. Polym. Sci., 2010, 115, 1296–1305 CrossRef CAS.
- S. J. Lee and B. K. Kim, Covalent incorporation of starch derivative into waterborne polyurethane for biodegradability, Carbohydr. Polym., 2012, 87, 1803–1809 CrossRef CAS.
- M. Garrido, J. R. Correia and T. Keller, Effect of service temperature on the shear creep response of rigid polyurethane foam used in composite sandwich floor panels, Construct. Build. Mater., 2016, 118, 235–244 CrossRef.
- M. A. Mekewi, A. M. Ramadan, F. M. ElDarse, M. H. A. Rehim, N. A. Mosa and M. A. Ibrahim, Preparation and characterization of polyurethane plasticizer for flexible packaging applications: Natural oils affirmed access, Egypt. J. Pet., 2016 DOI:10.1016/j.ejpe.2016.02.002.
- Y. Yu and Y. Zhang, Review of study on resin dye-fixatives on cotton fabrics, Modern App. Sci., 2009, 3, 9 CAS.
- H. H. Wang and C. T. Gen, Synthesis of anionic water-borne polyurethane with the covalent bond of a reactive dye, J. Appl. Polym. Sci., 2002, 84, 797–805 CrossRef CAS.
- S. Muzaffar, I. A. Bhatti, M. Zuber, H. N. Bhatti and M. Shahid, Synthesis, characterization and efficiency evaluation ofchitosan-polyurethane based textile finishes, Int. J. Biol. Macromol., 2016, 93, 145–155 CrossRef CAS PubMed.
- A. Shaaban, S. M. Se, I. M. Ibrahim and Q. Ahsan, Preparation of rubber wood sawdust-based activated carbon and its use as a filler of polyurethane matrix composites for microwave absorption, New Carbon Mater., 2015, 30(2), 167–175 CrossRef.
- M. Fornasieri, J. W. Alves, E. C. Muniz, A. Ruvolo-Filho, H. Otaguro, A. F. Rubira and G. M. De Carvalho, Synthesis and characterization of polyurethane composites of wood waste and polyols from chemically recycled pet, Composites, Part A, 2011, 42, 189–195 CrossRef.
- M. R. Patel, J. V. Patel and V. K. Sinha, Polymeric precursors from PET waste and their application in polyurethane coatings, Polym. Degrad. Stab., 2005, 90, 111–115 CrossRef CAS.
- B. Mohebby, M. Gorbani-Kokandeh and M. Soltani, Springback in acetylated wood based composites, Construct. Build. Mater., 2009, 23(9), 3103–3106 CrossRef.
- X. Kong, L. Zhao and J. M. Curtis, Polyurethane nanocomposites incorporating biobased polyols andreinforced with a low fraction of cellulose nanocrystals, Carbohydr. Polym., 2016, 152, 487–495 CrossRef CAS PubMed.
- J. Scheirs, Polymer recycling: science, technology and applications, John Wiley & Sons Ltd, Journals, Baffins Lane, Chichester, Sussex PO 19 1 UD, UK, 1998, vol. 591 Search PubMed.
- H. Stone, K. Frisch, D. Klempner and G. Prentice, Advances in Plastic Recycling, Recycling of Polyurethanes, ed. K. C. Frisch, D. Klempner and G. Prentice, Technomic Publishing Company, Lancaster, PA, 1998, vol. 1 Search PubMed.
- K. C. Frisch, D. Klemper and G. Prentice, Advances in Plastic Recycling: Recycling of Polyurethanes, Technomic Publishing Company, Lancaster, PA, United States, 1999, vol. 1 Search PubMed.
- E. Weigand, Properties and applications of recycled polyurethanes, Recycling and recovery of plastics, J. Branderup, M. Bittner, G. Menges and W. Micheali, Hanser, Münich, Germany, 1996 Search PubMed.
- ISOPA, http://www.isopa.org/isopa/uploads/Documents/documents/TECHNICAL%.
- J. Troitzsch, International plastics flammability handbook, Hanser, Munich, 1990, pp. 43–62 Search PubMed.
- Alliance for the Polyurethanes Industry, http://www.polyurethane.org/recycling.
- M. Szycher, Szycher's handbook of polyurethanes, CRC press, 1999 Search PubMed.
- L. R. Mahoney, S. A. Weiner and F. C. Ferris, Hydrolysis of polyurethane foam waste, Environ. Sci. Technol., 1974, 8, 135–139 CrossRef CAS.
- C. H. Wu, C. Y. Chang and J. K. Li, Glycolysis of rigid polyurethane from waste refrigerators, Polym. Degrad. Stab., 2002, 75, 413–421 CrossRef CAS.
- C. H. Wu, C. Y. Chang, C. M. Cheng and H. C. Huang, Glycolysis of waste flexible polyurethane foam, Polym. Degrad. Stab., 2003, 80, 103–111 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |