Thermo-responsive poly(ethylene-co-vinyl alcohol) based asymmetric membranes
Riyasudheen Nechikkattu and
Sujith Athiyanathil*
Materials Research Laboratory, Department of Chemistry, National Institute of Technology Calicut, Calicut-673601, Kerala, India. E-mail: athiyanathil.sujith@gmail.com; Fax: +91 495 2287250; Tel: +91 984 6475675
First published on 30th November 2016
Abstract
This paper reports surface modification of hydrophilic poly(ethylene-co-vinyl alcohol) (EVAL) microporous membranes, by surface-initiated atom transfer radical polymerization (SI-ATRP) of N-isopropylacrylamide (NIPAAm) and its subsequent applications in thermo responsive permeation. The surface thermo responsive behaviour of the membrane was characterized by water contact angle and bovine serum albumin (BSA) adsorption measurements at different temperatures. It was found that the hydrophilicity of the membrane is improved and the BSA adsorption was significantly suppressed by the poly N-isopropylacrylamide (pNIPAAm) NIPAAm grafting. Below the lower critical solution temperature (LCST) of pNIPAAm, a smaller amount of protein was adsorbed on the membranes, and a diametrically opposite effect with larger amounts of protein adsorption was evident above the LCST. The water flux and protein rejection at different temperatures were also determined for modified and unmodified membranes. The permeation results showed an obvious increase in the water flux in the temperature range 31–34 °C for the modified membranes due to the LCST of pNIPAAm. Protein rejection is found to be switched when the temperature is changed through the LCST of grafted pNIPAAm. Thermo responsive release of vitamin B12 has been also confirmed. These results indicate that the pore size of the membrane can be regulated in response to the temperature.
1. Introduction
Stimulus responsive membranes have gained more attention due to their change in physical, chemical and biological properties in response to external stimuli.1 Surface modification of membranes can be achieved by grafting the stimulus responsive polymers onto them using techniques such as plasma and UV induced polymerization.2,3 These graft polymers can undergo fast and reversible structural or morphological transformations in response to the surrounding environmental stimulus. The environmental stimuli may be temperature, pH, light intensity, current density, glucose level or magnetic field.4–7 Temperature is an easily manipulated stimulus for a wide variety of applications. Poly(N-isopropylacrylamide) (pNIPAAm) is one of the most studied stimulus sensitive polymers which can respond to external temperature changes.8 The lower critical solution temperature (LCST) of pNIPAAm is around 32 °C, below which it forms hydrogen bonds with water and becomes soluble by attaining a coil like chain conformation. Above the LCST, thermodynamic equilibrium favors the formation of intra-molecular hydrogen bonding and results in a globular conformation.9,10 Below lower critical solution temperature (LCST), pNIPAAm swells in water but above LCST it expels water to the surroundings by shrinking. The closeness of LCST of pNIPAAm to the human body temperature makes it a suitable candidate for biomedical applications. Reversible hydrophilic/hydrophobic surface of such membranes enable it to be used in drug delivery systems, artificial organs, chemical sensors and in the separation of biomolecules.11–14Controlled living radical polymerization (CRP) is a widely accepted technique for surface initiated graft polymerization due to its better control over molecular weight and molecular weight distribution.15,16 Atom transfer radical polymerization (ATRP) is the most versatile technique among CRP due to its feasibility for synthesizing a wide variety of polymers with different architecture on different substrates. ATRP is based on the equilibrium that exists between the dormant species (alkyl halides) and a living species (alkyl free radical) in presence of a transition metal complex catalyst.17 Reversible homolytic cleavage of alkyl halides generates alkyl free radicals and halide which are transferred to the complex species. The generated alkyl free radical reacts with monomer and propagates polymerization. The equilibrium is more towards dormant species and hence radical concentration is very low which results in suppressed termination.18 Many research groups have recently reported the applications of controlled living polymerization techniques for surface grafting on silicon wafers,19 porous glasses, ceramics and polymer substrates such as polystyrene (PS),20 polycarbonate (PC),21 polypropylene (PP),22 polyethylene (PE),23 nylon-6,24 poly(ethylene terephthalate) (PET),25 polyvinylidene fluoride (PVDF)26 and poly(tetrafluoro ethylene).27
In this study, the micro porous EVAL membrane is chosen as the substrate for grafting of pNIPAAm by surface initiated ATRP. Poly(ethylene-co-vinyl alcohol) (EVAL) is a semi crystalline polymer that possesses excellent biocompatibility and mechanical strength even in wet state and hence it has been used as a membrane material for biomedical applications.28 Its hydrophilicity can be controlled by changing the monomer ratio of hydrophilic vinyl alcohol and hydrophobic ethylene segments. EVAL microfiltration membranes have been utilized in blood purification techniques like plasmaphoresis, haemodyalysis and plasma protein separation.29,30 Nakamae et al.31 found that the EVAL polymer segments are asymmetrically distributed across the cross section of the membrane formed by immersion precipitation. Hydroxyl group enriched phase is found to be directed towards top surface which is in contact with the coagulation medium. These reactive hydroxyl groups are capable for further surface modifications. Zhou et al.32 introduced phosphorylcholine (PC) moieties on the surface of EVAL micro porous membrane by the reaction of hydroxyl groups with 2-chloro-2-oxo-1,3,2-dioxaphospholane (COP) followed by a ring-opening reaction with trimethylamine in tetrahydrofuran. Chemical surface modification is found to be more effective and feasible for pore surfaces as compared to irradiation induced modifications. Our research group also has reported the formation behaviour and performance studies of EVAL/PVP blend membranes prepared by non-solvent induced phase inversion method.33,34
Thermo responsive membranes have been prepared by graft polymerization of N-isopropyl acrylamide on the membrane surface by different techniques such as UV irradiation, plasma treatment, γ-ray, ozone treatment and chemical reaction.2,35,36 Wang et al.23 grafted pNIPAAm tubular-type porous polyethylene membrane by low temperature plasma method. The studies showed that the grafted pNIPAAm could respond instantly to environmental temperature changes. By varying UV irradiation time and monomer concentration, the graft yield of pNIPAAm graft polymerization onto porous PE membranes can be controlled.37 Wan et al.38 prepared thermo-responsive microporous polypropylene membranes by grafting pNIPAAm chains by a surface-initiated ATRP process after generating hydroxyl groups by the UV-induced graft polymerization of hydroxyethyl methacrylate. Liang et al.39 prepared temperature-sensitive membranes by photo-induced graft polymerization of NIPAAm monomer on the surface of PP microfiltration membrane. Curti et al.40 graft polymerized NIPAAm monomers on previously oxidized PET and PS surfaces. It is found that the grafted surface hydrophobic/hydrophilic character depends on the environmental temperature.
The objective of the work is to prepare and characterize a novel thermo-responsive membrane that has the advantages of both hydrophilic and temperature sensitive polymeric membranes. This can overcome the disadvantages of radiation induced graft polymerization like, occurrence of secondary reactions under the influence of ionizing radiation, polymer substrate degradation, radiolysis of monomer and need of stringent equipments.
The specific aim of the work is to prepare a novel membrane by the graft polymerization of NIPAAm on the EVAL asymmetric membrane previously immobilized by ATRP initiator. The chemical composition of the modified membrane surface, surface morphology and thermo responsive behaviour of the membranes were studied in detail. Protein adsorption characteristics, temperature responsive water permeation and protein rejection of modified EVAL membranes were also evaluated. Modified EVAL membrane with temperature responsive behaviour invokes its successful usage in various biomedical devices.
2. Experimental
2.1. Materials
EVAL-copolymer (from Sigma Aldrich) with an average of 44 mol% ethylene groups was used for the membrane preparation. Monomer, NIPAAm for graft polymerization, pentamethyldiethylenetriamine (PMDETA), 2-bromoisobutyryl bromide (2-BIBB), triethylamine (TEA), CuBr and CuBr2, acetonitrile and tetrahydrofuran (THF) were purchased from Sigma Aldrich. Dimethyl sulphoxide (DMSO) was obtained from Merck India Pvt. Ltd.2.2. Preparation of membrane
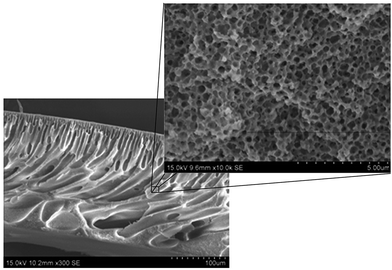
EVAL asymmetric membrane was prepared by non-induced phase separation (NIPS) method by using DMSO (solvent) and water (coagulation medium). The detailed description is given elsewhere,33 in brief as follows. Membrane casting solutions were prepared by dissolving 15 wt% EVAL in DMSO at 60 °C. Bubbles removed by sonication for 1½ hours. The solutions were casted on glass plate with a doctor blade with a clearance of 150 μm. The nascent membrane was subsequently immersed in distilled water coagulation bath and kept for 2 hours. The remaining solvent was removed by keeping in distilled water for 24 hours and further kept in 0.01 M formalin solution until it is used, to prevent microbial contamination. All the membranes used for graft polymerization were thoroughly washed with distilled water and cut into circular discs with a diameter of 4.40 cm. The membrane samples were washed again with acetone for 30 minutes to remove any impurity adsorbed on the surface, dried in a vacuum oven at 40 °C to until they attain constant weight, and then stored in a desiccator. Fig. 1 shows the scanning electron micrograph of the prepared EVAL asymmetric membrane and magnified image of its inner pores.2.3. Polymerization of NIPAAm on the surface of EVAL by ATRP
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 acetonitrile
1 acetonitrile![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water mixtures. Monomer solution was bubbled with nitrogen for 12 hours and transferred to the Schlenk flask using a cannula. The mixture was kept for reaction at room temperature for a predetermined time. The reaction was terminated by exposure to air. After the reaction, membranes were taken out from the flask, washed with acetonitrile–water mixture three times followed by water and vacuum dried at 60 °C and weighed (Wa). The reactant ratio, reaction time and sample abbreviations were given in the Table 1.
water mixtures. Monomer solution was bubbled with nitrogen for 12 hours and transferred to the Schlenk flask using a cannula. The mixture was kept for reaction at room temperature for a predetermined time. The reaction was terminated by exposure to air. After the reaction, membranes were taken out from the flask, washed with acetonitrile–water mixture three times followed by water and vacuum dried at 60 °C and weighed (Wa). The reactant ratio, reaction time and sample abbreviations were given in the Table 1.
| Sample | Reactant ratio [NIPAAm]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [CuBr] [CuBr]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [CuBr2] [CuBr2]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [PMDETA] [PMDETA] |
Reaction time (hour) |
|---|---|---|
| EN100t24 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
24 |
| EN100t12 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
12 |
| EN50t24 | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
24 |
| EN50t12 | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
12 |
2.4. Characterizations
 | (1) |
 | (2) |
The atomic force microscopic images of the membrane surface were obtained by scanning under ambient conditions using an AFM, Park Model XE 100.
 | (3) |
The water flux through each membrane was determined at different temperatures ranging from 25 °C to 40 °C. Permeation experiments of the membranes were repeated three times and its average was taken as water flux.
Protein rejection of the modified membranes were measured at 25 °C and 40 °C by using immunoglobulin (IgG) from rabbit serum (Mw-166 kDa), albumin from bovine serum (66 kDa) and cytochrome C from bovine heart (12.3 kDa). Proteins were dissolved (0.1 wt%) in phosphate buffer (0.1 M, pH 7.0) solution and filtered through each membrane. The solution rejections of proteins were calculated by determining the concentration of proteins in the feed and in the permeate by UV-spectrophotometer (Perkin-Elmer, Lambda 35) at a wavelength of 278 nm. The solution rejection ratio (R%) was calculated by the following equation:42
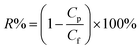 | (4) |
 | (5) |
It is obvious that the item −ln[1 − (C2)t(1 + V2/V1)/(C1)0] in eqn (5) is a function of permeation time t. All the curves of −ln[1 − (C2)t(1 + V2/V1)/(C1)0] against t are linear in our permeation experiments. The diffusional coefficient D calculated by the slopes of lines and eqn (6) can be changed to the following form:
 | (6) |
 | (7) |
3. Results and discussions
3.1. Surface modification of NIPAAm on EVAL membranes
One-step procedure was applied to anchor the initiator molecules on the EVAL membrane surface. The reaction of the hydroxyl groups of the EVAL membrane with BIBB produced alkyl bromide-grafted EVAL membranes. Surface initiated polymerization of NIPAAm on the EVAL-Br membrane is done by ATRP method. The whole chemical process is schematically showed in Fig. 4. The initiator immobilization and grafting of NIPAAm on the membrane is further confirmed by FTIR spectral analysis. Fig. 5 shows FTIR spectra of virgin, initiator immobilized and pNIPAAm terminated EVAL-Br membrane. | ||
| Fig. 5 FTIR-ATR spectra of (a) pristine EVAL, (b) 2BBIB immobilized EVAL and (c) NIPAAm-grafted EVAL (EN100t12) membranes. | ||
Fig. 5b has a new sharp peak at 1737 cm−1 which could be due to the stretching vibrations of ester carbonyl bond formed by the immobilization of initiator 2-BIBB on the membrane. In Fig. 5c, the peak at 1652 cm−1 is attributed to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations of amide I. The N–H and C–N combined vibration of amide II is at 1549 cm−1.37 As compared with the spectrum of EVAL membrane, a double peak is found at 1385 cm−1 and 1368 cm−1 which arose by the symmetrical bending vibrations and the coupling split due to bimethyl group of the isopropyl group of pNIPAAm in the spectra of the pNIPAAm grafted EVAL membrane.38 The broad absorption peak at ∼3295 cm−1 is attributed to the stretch of the hydrogen-bonded NH group.
O stretching vibrations of amide I. The N–H and C–N combined vibration of amide II is at 1549 cm−1.37 As compared with the spectrum of EVAL membrane, a double peak is found at 1385 cm−1 and 1368 cm−1 which arose by the symmetrical bending vibrations and the coupling split due to bimethyl group of the isopropyl group of pNIPAAm in the spectra of the pNIPAAm grafted EVAL membrane.38 The broad absorption peak at ∼3295 cm−1 is attributed to the stretch of the hydrogen-bonded NH group.
The anti-symmetric stretching vibration of the CH3 group occurs at 2934 cm−1 and the antisymmetric bending deformation of CH3 occurs at 1460 cm−1.45
The graft yield (GY), of the membranes is given in Table 2. Each of the disc membranes has surface area of 30.67 ± 0.03 cm2 and specific surface area of 1769.82 ± 105.23 cm2. The graft yield is increased with monomer concentration. The reaction time dependency on graft yield can be deduced from the results shown in the Table 2.
| Sample | Graft yield | |
|---|---|---|
| GYSA (mg cm−2) | GYSSA (μg cm−2) | |
| EN100t24 | 1.4188 | 8.2910 |
| EN100t12 | 0.7989 | 4.5973 |
| EN50t24 | 0.9632 | 6.0803 |
| EN50t12 | 0.5818 | 3.7882 |
The corresponding GYSA and GYSSA values for a particular monomer concentration at two reaction time 12 h and 24 h indicates the livingness of graft polymerization.
3.2. Surface morphology
Fig. 6 shows two dimensional and three dimensional AFM images of the membrane surface. Surface roughness of the membranes was determined from the figures for a scan area of 5 μm × 5 μm is found to be increased with increase in grafting yield. Figure shows that the pNIPAAm brush is appeared as closely packed domains. The surface root mean square (RMS) roughness of pNIPAAm brushes were 16.101 nm and 19.188 nm for EN50t24 and EN100t24 membranes respectively in the scanned area. Polymer brush formed on the membrane leads to a relatively rough surface and membranes with higher graft yield have higher rough surface.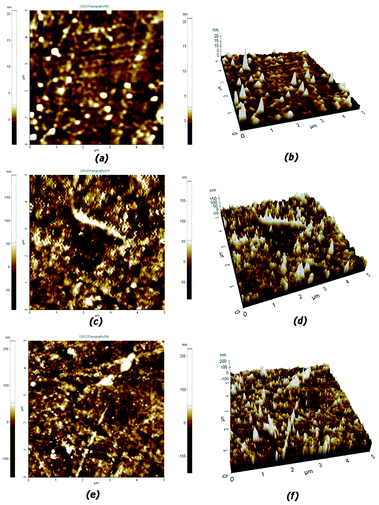 | ||
| Fig. 6 AFM images of surface for pristine EVAL (a & b) EN50t24 (c & d) and EN100t24 (e & f) membranes. | ||
Fig. 7 shows the SEM images of EVAL, EVAL-Br, EN50t24 and EN100t24. After the immobilization of 2-BIBB, the membrane surface become inhomogeneous and pore size gets reduced. This is due to the formation of a dense layer of ATRP initiators, which is appropriate for the surface modification of membranes. A thin layer of grafted polymer has been formed as seen in Fig. 7c and d. Most of the pores are also covered with the pNIPAAm brushes. This is further increased with increase in graft yield due to its denser/longer grafted NIPAAm polymer brushes.
 | ||
| Fig. 7 FESEM micrographs showing surface for (a) pristine EVAL, (b) initiator immobilized EVAL-Br, (c) EN50t24 and (d) EN100t24 EVAL membranes. | ||
3.3. Stimulus-responsive behaviour
From Fig. 10, it is clear that the nonspecific adsorption of BSA on EVAL membrane surface is lowered by 82.16% and 75.67% for EN50t24 and EN100t24 membranes respectively at 25 °C. The larger amount of the protein adsorption on the unmodified EVAL membrane can be attributed to the stronger hydrophobic interaction due to the presence of ethylene part in the copolymer.32 The marked reduction in the BSA adsorption observed for the EN50t24 and EN100t24 membranes indicate good resistance of the grafted membranes to protein adsorption in the swollen state below LCST of grafted NIPAAM chains. EN100t24 membrane is more resistant to protein adsorption than EN50t24 membrane, which indicates that, the higher graft yield can reduce the protein adsorption further.
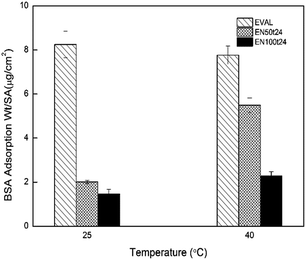 | ||
| Fig. 10 Amount of protein adsorbed on the pristine and poly(NIPAAm) grafted EVAL membranes at 25 °C and 40 °C. | ||
The question of interest was how the protein adsorption to the pNIPAAm grafted membranes is affected by the temperature for transitions of grafted chains from hydrated to hydrophobic state or vice versa. Fig. 10 shows that, at 25 °C EN50t24 membrane adsorbs 2.01 ± 0.08 μg cm−2 and at 40 °C it takes 5.49 ± 0.34 μg cm−2 of BSA. EN100t24 membranes are also showing this switching behaviour, the values being 1.47 ± 0.20 μg cm−2 and 2.28 ± 0.21 μg cm−2 at 25 °C and 40 °C respectively. The amount of protein adsorbed on these membranes is found to be related with their hydrophobicity. As the protein adsorption is due to the hydrophobic interaction between substrate and the protein, membranes at less hydrophilic state can adsorb larger amount of protein. Lowering of the temperature below the LCST allow the bound proteins to be released which enables to separate or purify the targeted proteins.
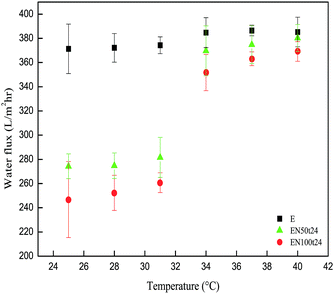 | ||
| Fig. 11 Thermo-responsive water flux of poly(NIPAAm) grafted EVAL membranes at different temperatures (between 25 °C and 40 °C). | ||
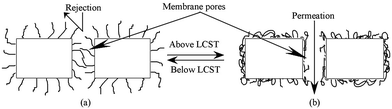 | ||
| Fig. 12 Schematic representation of thermo-responsive permeation through pNIPAAm surface grafted EVAL porous membrane. | ||
Fig. 13 shows percentage rejection of proteins cytochrome C, BSA and immunoglobulin with molecular weights 12.3 kDa, 66 kDa and 116 kDa respectively. The thermo response behaviour of the modified membrane is quite obvious in protein rejection studies. Since, the pore size of unmodified membranes does not change with temperature they have no pronounced difference in the rejection values at 25 °C and 40 °C. Modified membranes have higher rejection values at 25 °C than 40 °C. This can be due to the swelling and deswelling mechanism of the grafted pNIPAAm chains with water molecules. Below LCST, grafted pNIPAAm chains form hydrogen bonds with water molecules and its chains extend. Polymer chains grafted at the inner pores reduces its pore size and hence more protein is rejected. At temperature above LCST, hydrogen bond ruptures, shrinks the grafted polymer chains and hence pore size increases which lead to lesser rejection. Rejection values of cytochrome C for modified membranes are increased by about 15% when temperature switches from 40 °C to 25 °C. BSA rejection values for unmodified membrane at both 25 °C and 40 °C are approximately 77%. But for modified membranes rejection value is above 90% at 25 °C, while it is below 90% at 40 °C. This indicates that, the molecular weight cutoff of modified membranes at 25 °C is below 66 kDa. Both modified and unmodified membranes rejected more than 90% of immunoglobulin at the two experimental temperatures. It indicates, the molecular weight cutoff of this membrane is below 116 kDa. Even below LCST of pNIPAAm, a small fraction of immunoglobulin (IgG) is permeated through the modified membranes indicating the presence of some larger pores or polydispersity of solute.
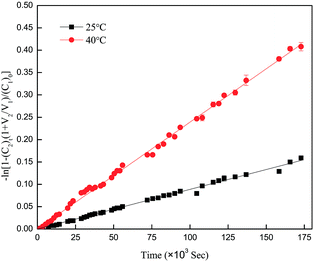 | ||
| Fig. 14 Vitamin B12 drug release plots of −ln[1 − (C2)t(1 + V2/V1)/(C1)0] vs. time at 25 °C and 40 °C through the pNIPAAm grafted EVAL membrane (EN50t24). | ||
The diffusion coefficients for modified membranes are shown in the Fig. 15. It is clear from this figure that the modified membranes have shown low permeation rate of the model drug vitamin B12 through the membrane. This can be due to narrowing of pores and partial blocking of pores by surface grafting.
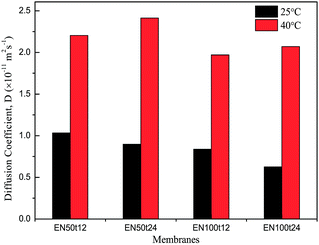 | ||
| Fig. 15 Thermo-responsive diffusional permeation of vitamin B12 trough pNIPAAm grafted EVAL membranes. | ||
Diffusion coefficients of vitamin B12 through the modified membranes at 40 °C are remarkably high as compared with that at 25 °C. The obvious change in the diffusional coefficient is due to the swelling and de-swelling mechanism of the grafted polymer chains. Poly N-isopropylacrylamide swells in water by forming hydrogen bonds below their LCST. Above their LCST pNIPAAm chains undergo entropically driven collapse by hydrophobic interaction. Grafted pNIPAAm on the surface of membrane including pores undergo these mechanisms upon change in the environmental temperature above and below the LCST say 40 °C and 25 °C.
The extent of thermo-responsive permeability of drugs can be defined by a parameter called thermo-responsive factor. Thermo-responsive factors of modified membranes are plotted in Fig. 16. Thermo-responsive factor of the membrane EN50t24 is higher than that of the membrane EN50t12. This indicates that the responsive behaviour of the membrane increased with the grafting time. As grafting time increased, more amount of NIPAAm can be graft polymerized. This high degree of grafting can have high thermo-responsive factor. EN100t24 and EN100t12 have higher thermo-responsive factor than that of EN50t24 and EN50t12 respectively. This indicates for this particular condition the concentration of the monomer improved their graft degree. Likewise, the release rate of drug can be tuned by controlling their grafting time and monomer concentration.
4. Conclusions
N-Isopropylacrylamide was graft polymerized on asymmetric EVAL membrane by surface initiated ATRP technique. Direct immobilization of ATRP initiator was achieved by utilizing surface enriched hydroxyl groups. Characteristic peaks of pNIPAAm in ATR-FTIR spectra revealed the successful grafting on the membrane surface. Graft yield is high for higher concentration of monomer in the reaction mixture at a particular polymerization time. With time graft yield gets improved, due to the livingness of polymerization. Surface roughness is increased due to the formation of brush like structure. Contact angle studies revealed that the wettability of the modified membranes is switchable with temperature. Controlled thermo-responsive water permeation and solute rejections through EVAL membrane are achieved by surface grafting of pNIPAAm. The pore size and thermo-responsive behavior of the EVAL membranes can be easily regulated by controlling grafting degree. The drug release rate is depending on the degree of grafted chains in the membrane. This membrane can be used to release drugs in response to its environmental temperature changes. These hydrophilic-thermo-responsive membranes can be potential candidates in biomedical devices.Acknowledgements
The authors would like to acknowledge Dr Anas Abdulaziz, Scientist, CSIR-National Institute of Oceanography, India for his helps to perform FTIR characterization.References
- M. Liu, L. Zhao, S. Li, H. Ye, H. Anb and Y. Zhang, RSC Adv., 2016, 6, 10704 RSC.
- S. Y. Kim, T. Kanamori and T. Shinbo, J. Appl. Polym. Sci., 2002, 84, 1168 CrossRef CAS.
- L. Liang, P. C. Rieke, G. E. Fryxell, J. Liu, M. H. Engelhard and K. L. Alford, J. Phys. Chem. B, 2000, 104, 11667 CrossRef CAS.
- H. Iwata, M. Oodate, Y. Uyama, H. Amemiya and Y. Ikada, J. Membr. Sci., 1991, 55, 119 CrossRef CAS.
- Q. Shi, Y. Su, X. Ning, W. Chen, J. Peng and Z. Jiang, J. Membr. Sci., 2010, 347, 62 CrossRef CAS.
- L. Y. Chu, Y. J. Liang, W. M. Chen, X. Ju and H. D. Wang, Colloids Surf., B, 2004, 37, 9 CrossRef CAS PubMed.
- Q. Yang, H. H. Himstedt, M. Ulbricht, X. Qian and S. R. Wickramasinghe, J. Membr. Sci., 2013, 430, 70 CrossRef CAS.
- H. Guo and M. Ulbricht, J. Membr. Sci., 2011, 372, 331 CrossRef CAS.
- M. Heskins, J. E. Julliet and E. James, J. Macromol. Sci., Chem., 1968, 2, 1441 CrossRef CAS.
- T. Yakushiji, K. Sakai, A. Kikuchi, T. Aoyagi, Y. Sakurai and T. Okano, Langmuir, 1998, 14, 4657 CrossRef CAS.
- J. C. Cuggino, M. C. Strumia and C. I. A. Igarzabal, React. Funct. Polym., 2011, 71, 440 CrossRef CAS.
- F. J. Xu, Y. Q. Zheng, W. J. Zhen and W. T. Yang, Colloids Surf., B, 2011, 85, 40 CrossRef CAS PubMed.
- M. T. Nistor, A. P. Chiriac, C. Vasile, L. Verestiuc and L. E. Nita, Colloids Surf., B, 2011, 87, 382 CrossRef CAS PubMed.
- C. Yi, N. Liu, J. Zheng, J. Jiang and X. Liu, J. Colloid Interface Sci., 2012, 380, 90 CrossRef CAS PubMed.
- W. A. Braunecker and K. Matyjaszewski, Prog. Polym. Sci., 2007, 32, 93 CrossRef CAS.
- N. Hadjichristidis, H. Iatrou, M. Pitsikalis and J. Mays, Prog. Polym. Sci., 2006, 31, 1068 CrossRef CAS.
- K. Matyjaszewski and J. Xia, Chem. Rev., 2001, 101, 2921 CrossRef CAS.
- K. Matyjaszewski, Macromolecules, 2012, 45, 4015 CrossRef CAS.
- N. Ishida, Adv. Powder Technol., 2007, 18, 631 CrossRef CAS.
- A. Mizutani, A. Kikuchi, M. Yamato, H. Kanazawa and T. Okano, Biomaterials, 2073, 29, 2073 CrossRef PubMed.
- S. J. Lue, C. H. Chen, C. M. Shih, M. C. Tsai, C. Y. Kuo and J. Y. Lai, J. Membr. Sci., 2011, 379, 330 CrossRef CAS.
- S. M. Desai, S. S. Solanky, A. B. Mandale, K. Rathore and R. P. Singh, Polymer, 2003, 44, 7645 CrossRef CAS.
- J. Huang, X. L. Wang, W. S. Qi and X. H. Yu, Desalination, 2002, 146, 345 CrossRef CAS.
- F. J. Xu, J. P. Zhao, E. T. Kang, K. G. Neoh and J. Li, Langmuir, 2007, 23, 8585 CrossRef CAS PubMed.
- H. Alem, A. S. Duwez, P. Lussis, P. Lipnik, A. M. Jonas and S. D. Champagne, J. Membr. Sci., 2008, 308, 75 CrossRef CAS.
- N. Singh, S. M. Husson, B. Zdyrko and I. Luzinov, J. Membr. Sci., 2005, 262, 81 CrossRef CAS.
- C. C. Han, T. C. Wei, C. S. Wu and Y. L. Liu, J. Membr. Sci., 2010, 358, 60 CrossRef CAS.
- Y. Sakurada, A. Sueoka and M. Kawahashi, Polym. J., 1987, 19, 501 CrossRef CAS.
- R. A. Ward, R. M. Schaefer, D. Falkenhagen, M. S. Joshua, A. Heidland and H. Klinkmann, Nephrol., Dial., Transplant., 1993, 8, 47 CrossRef CAS.
- M. E. Avramescu, W. F. C. Sager and M. Wessling, J. Membr. Sci., 2003, 216, 177 CrossRef CAS.
- K. Nakamae, T. Miyata and T. Matsumoto, J. Membr. Sci., 1992, 75, 163 CrossRef CAS.
- J. Zhou, S. Meng, Z. Guo, Q. Du and W. Zhong, J. Membr. Sci., 2007, 305, 279 CrossRef CAS.
- N. Riyasudheen and A. Sujith, Desalination, 2012, 29, 17 CrossRef.
- Z. B. Zhang, X. L. Zhu, F. J. Xu, K. G. Neoha and E. T. Kang, J. Membr. Sci., 2009, 342, 300 CrossRef CAS.
- T. Peng and Y. L. Chen, J. Appl. Polym. Sci., 1998, 70, 2133 CrossRef CAS.
- J. Z. Yu, L. P. Zhu, B. K. Zhu and Y. Y. Xu, J. Membr. Sci., 2011, 366, 276 CrossRef.
- M. C. Koetting, J. T. Peters, S. D. Steichen and N. A. Peppas, Mater. Sci. Eng., R, 2015, 93, 1 CrossRef PubMed.
- L. S. Wan, Y. F. Yang, J. Tian, M. Xin and H. Z. Xu, J. Membr. Sci., 2009, 327, 174 CrossRef CAS.
- L. Liang, X. D. Feng, L. M. Peurrung and V. Viswanathan, J. Membr. Sci., 1999, 162, 235 CrossRef CAS.
- P. S. Curti, M. R. de Moura, W. Veiga, E. Radovanovic, A. F. Rubira and E. C. Muniz, Appl. Surf. Sci., 2005, 245, 223 CrossRef CAS.
- S. Sun, Y. Yue, X. Huang and D. Meng, J. Membr. Sci., 2003, 222, 3 CrossRef CAS.
- H. Y. Yu, J. Zhou, J. S. Gu and S. Yang, J. Membr. Sci., 2010, 384, 203 CrossRef.
- E. Turan, S. Demirci and T. Caykara, Thin Solid Films, 2010, 518, 5950 CrossRef CAS.
- Y. C. Chen, R. Xie, M. Yang, P. F. Li, X. L. Zhu and L. Y. Ch, Chem. Eng. Technol., 2009, 32, 622 CrossRef CAS.
- A. Khan, J. Colloid Interface Sci., 2007, 313, 697 CrossRef CAS PubMed.
- F. Abbasi, H. Mirzade and A. A. Katbab, Polym. Int., 2001, 50, 1279 CrossRef CAS.
- M. Hesampour, T. Huuhilo, K. Makinen, M. Manttari and M. Nystrom, J. Membr. Sci., 2008, 310, 85 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |