Preparation and characterization of surface molecularly imprinted film coated on a magnetic nanocore for the fast and selective recognition of the new neonicotinoid insecticide paichongding (IPP)
M. Zhang†
ab,
H. T. Zhao†a,
X. Yang*a,
W. T. Zhanga,
J. Wang*ab,
G. Y. Liua,
H. Zhanga and
A. J. Donga
aDepartment of Food Sciences and Engineering, School of Chemical Engineering and Technology, Harbin Institute of Technology, 150090 Harbin, PR China. E-mail: yangxin@hit.edu.cn
bKey Laboratory of Agro-Product Quality and Safety, Institute of Quality Standard and Testing Technology for Agro-Product, Chinese Academy of Agricultural Sciences, 100081 Beijing, PR China. E-mail: w_jing2001@126.com
First published on 7th December 2015
Abstract
In this work, we present a general method to prepare surface molecularly imprinted film on a magnetic nanocore for new neonicotinoid insecticide paichongding (IPP) recognition. First, magnetic Fe3O4 nanoparticles were synthesized by a hydrothermal method and coated with SiO2 on the surface, then were vinyl-modified to be Fe3O4@SiO2@C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C which was the magnetic core. After that, magnetic molecularly imprinted nanoparticles (MMIPs) were synthesized by surface-imprinted polymerization in airtight tubes at 60 °C for 24 h, using IPP as the template, methacrylic acid as the functional monomer and ethylene glycol dimethacrylate as cross-linkers. The resulting IPP-MMIPs possess specific recognition ability, fast adsorption kinetics, high adsorption capacity, and can be easily collected under an external magnetic field. The IPP-MMIPs were characterized by scanning electron microscopy (SEM), Fourier transform infrared spectroscopy (FT-IR), thermogravimetric analysis (TGA), X-ray diffraction (XRD) and vibrating sample magnetometry (VSM). The binding experiments showed a relatively high adsorption capacity (17.30 mg g−1) and specific recognition ability over structurally related compounds. Therefore, IPP-MMIPs have the potential to become a sensitive and selective approach for IPP recognition and separation.
C which was the magnetic core. After that, magnetic molecularly imprinted nanoparticles (MMIPs) were synthesized by surface-imprinted polymerization in airtight tubes at 60 °C for 24 h, using IPP as the template, methacrylic acid as the functional monomer and ethylene glycol dimethacrylate as cross-linkers. The resulting IPP-MMIPs possess specific recognition ability, fast adsorption kinetics, high adsorption capacity, and can be easily collected under an external magnetic field. The IPP-MMIPs were characterized by scanning electron microscopy (SEM), Fourier transform infrared spectroscopy (FT-IR), thermogravimetric analysis (TGA), X-ray diffraction (XRD) and vibrating sample magnetometry (VSM). The binding experiments showed a relatively high adsorption capacity (17.30 mg g−1) and specific recognition ability over structurally related compounds. Therefore, IPP-MMIPs have the potential to become a sensitive and selective approach for IPP recognition and separation.
Introduction
The selective recognition and fast isolation of pesticide residue targets from complex samples during the timely determination and removal of them is an extremely important issue in food safety as well as in environment disciplines.1–5 As a new third-generation neonicotinoid pesticide, the residue of 1-[6-chloro-3-methyl-pyridyl-8-nitro-7-methyl-1,2,3,5,6,7-hexahydro imidazo-(1,2a)]-pyridine (paichongding, IPP) results in hazardous concentrations of pesticide in surface water and soils, along with a terrible effect on human health.6–9 Our group have already evaluated the effect of IPP on a wheat plant’s antioxidant defense system10 and established a multi-residue method for the determination of seven kinds of neonicotinoid insecticides.11 It is obviously difficult to separate IPP residue from complex agricultural, food product and environmental substrates. The pre-treatment techniques for neonicotinoid pesticides are always solid-phase extraction, dispersive liquid–liquid micro-extraction and immunoassay methods, which are tedious and time consuming.12,13Thanks to the obvious advantages of predictable specific recognition, chemical stability, high binding capacity and quick solid–liquid phase separation rate, the magnetic surface molecular imprinting approach provides separation and enrichment methods for the pesticide residue analysis and removal field.14–16 Molecular imprinting has been known as a versatile technique tailor-made toward a given target or group of target molecules, and the resulting molecularly imprinted polymers (MIPs) act as molecular recognition elements.17–19 As a solution to the drawbacks of incomplete template removal, low binding capacity, and the slow mass transfer of traditional molecular imprinting technology,20–22 adopting a surface imprinting approach produces a MIP nanoshell on a solid support such as magnetic iron oxide nanomaterials.23–25 By encapsulating the magnetic components into MIPs, specific molecular recognition can be combined with magnetic properties, which allows the nanoparticles to be easily isolated from a matrix by using an external magnetic field instead of using centrifugation and filtration to obtain a novel solid phase extraction material with highly selective recognition, fast adsorption, and fast kinetic separation.26
Based on this approach, we present a general method to prepare a thin MIP layer on superparamagnetic nanoparticles with a uniform core–shell structure by using surface imprinting. These MMIPs have lots of attractive features for the purpose of the specific and efficient enrichment of IPP residue. The MMIPs can easily separate target molecules using a magnet on the bottom of the reaction bottle which makes the enrichment strategy very convenient and easy to handle. IPP was chosen as the model for this purpose. Methacrylic acid (MAA), which was chosen as the functional group for capturing the target molecules via a hydrogen bonding effect with the template molecules. Furthermore, the template molecules were removed by repetitive washing with copious amounts of a mixture of methanol and acetic acid. The morphology, adsorption, and recognition properties of these molecularly imprinted nanoparticles have been investigated.
Experimental
Materials and apparatus
Iron(III) chloride hexahydrate (FeCl3·6H2O) was purchased from Tianli Chemical Reagent Co. (Tianjing, China). Tetraethyl orthosilicate (TEOS), methacrylic acid (MAA, 98%) and 2,2-azo-bisisobutyronitrile (AIBN, 98%) were purchased from Kemiou Chemical Reagent Co. (Tianjing, China). 3-(Trimethoxysilyl)propyl methacrylate (99%) and ethylene glycol dimethacrylate (EGDMA, 98%) were obtained from Sigma (China). IPP was purchased from the Kwin Company Ltd. Nantong, Jiangsu Province PR China. The other solvents were of analytical grade. Deionized ultra-water was used from a laboratory purification system.A TU-1900 double-beam UV-vis spectrophotometer (Persee, China) was used to determine the absorbance of IPP and other neonicotinoid insecticides. Scanning electron microscopy (SEM) images were obtained on a Quanta 200FEG (FEI, U.S.) to determine the size and morphology of the nanoparticles. Fourier transform infrared spectra (FT-IR) were recorded on an Avatar 360 (Nicolet, U.S.) instrument to confirm whether the MIP film was coated onto the surface of the modified magnetic nanoparticles. Thermogravimetric analysis (TGA) was performed for powder samples (∼10 mg) with a heating rate of 10 °C min−1, up to 800 °C, using a Netzsch STA 449C (Germany) thermogravimetric analyzer under a nitrogen flow. X-ray powder diffraction (XRD) analysis was conducted on a Bruker AXS D8-advance X-ray diffractometer using Cu Kα radiation. The magnetic properties were analyzed with a LDJ 9600-1 (USA) vibrating sample magnetometer (VSM).
Synthesis of monodisperse Fe3O4 nanoparticles
The magnetic Fe3O4 nanoparticles were synthesized according to a previous report with a little modification.27 In a typical process, 13.5 g of FeCl3·6H2O was dissolved in 500 mL of ethylene glycol, followed by 36 g of sodium acetate. After vigorously stirring for 30 min, the resulting homogeneous mixture was transferred into a Teflon-lined stainless-steel autoclave, sealed, heated at 200 °C for 12 h, and Fe3O4 was then collected with a magnet. 0.4 g of the as-prepared Fe3O4 was added to 15 mL of trisodium citrate (0.5 M) and 15 mL of hydrochloric acid (2 M) under ultrasonication for 20 min. The modified Fe3O4 nanoparticles were collected with a magnet and dried at 45 °C under vacuum for further use.Synthesis of vinyl-modified magnetic nanoparticles (Fe3O4@SiO2@C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/h3_char_e001.gif) C)
C)
The Fe3O4@SiO2@C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C nanoparticles were synthesized according to the reported Stöber method28 and were subsequently vinyl-modified according the literature.14 Briefly, 400 mg of Fe3O4 nanoparticles were dissolved in 100 mL of 2-propanol and 8 mL of highly purified water, followed by 5 mL of ammonium hydroxide and 1 mL of TEOS. The mixture was put under continuous stirring for 12 h at room temperature. Then the Fe3O4@SiO2 product was obtained by magnetic separation, thoroughly rinsed six times with deionized water and dried to a powder under vacuum. After that, Fe3O4@SiO2 was dissolved in 100 mL of anhydrous toluene, mixed with 2 mL of triethylamine and 4 mL of 3-(trimethoxysilyl)propyl methacrylate, and reacted for 24 h at 130 °C under the protection of N2. At last, the resultant Fe3O4@SiO2@C
C nanoparticles were synthesized according to the reported Stöber method28 and were subsequently vinyl-modified according the literature.14 Briefly, 400 mg of Fe3O4 nanoparticles were dissolved in 100 mL of 2-propanol and 8 mL of highly purified water, followed by 5 mL of ammonium hydroxide and 1 mL of TEOS. The mixture was put under continuous stirring for 12 h at room temperature. Then the Fe3O4@SiO2 product was obtained by magnetic separation, thoroughly rinsed six times with deionized water and dried to a powder under vacuum. After that, Fe3O4@SiO2 was dissolved in 100 mL of anhydrous toluene, mixed with 2 mL of triethylamine and 4 mL of 3-(trimethoxysilyl)propyl methacrylate, and reacted for 24 h at 130 °C under the protection of N2. At last, the resultant Fe3O4@SiO2@C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C product was collected by an external magnetic field, washed 3 times with both ethanol and toluene, and dried under vacuum at 45 °C.
C product was collected by an external magnetic field, washed 3 times with both ethanol and toluene, and dried under vacuum at 45 °C.
Synthesis of IPP-imprinted magnetic nanoparticles (IPP-MMIPs)
0.1 mmol IPP and 0.4 mL MAA (1.0 M in acetonitrile) were dissolved in 16.6 mL of acetonitrile, and the mixture was incubated at room temperature for 12 h, and then 40 mg of Fe3O4@SiO2@C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, 1 mL of EGDMA (1.0 M in acetonitrile), and 2 mL of AIBN (10 mg mL−1 in acetonitrile) were added. The polymerization mixture was purged with N2 gas for 10 min to remove the molecular oxygen and the polymerization process was executed at 50 °C for 24 h. The IPP imprinted polymer was collected by an external magnetic field and washed with a methanol–acetic acid mixture (90
C, 1 mL of EGDMA (1.0 M in acetonitrile), and 2 mL of AIBN (10 mg mL−1 in acetonitrile) were added. The polymerization mixture was purged with N2 gas for 10 min to remove the molecular oxygen and the polymerization process was executed at 50 °C for 24 h. The IPP imprinted polymer was collected by an external magnetic field and washed with a methanol–acetic acid mixture (90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 = v/v) to remove the template molecules until no IPP was detected. Then the particles were dried under vacuum at 45 °C for 12 h, and stored for further use. As a control, non-molecularly imprinted nanoparticles (IPP-MNIPs) were synthesized under the same conditions except that no template was added.
10 = v/v) to remove the template molecules until no IPP was detected. Then the particles were dried under vacuum at 45 °C for 12 h, and stored for further use. As a control, non-molecularly imprinted nanoparticles (IPP-MNIPs) were synthesized under the same conditions except that no template was added.
Adsorption kinetics
To investigate the adsorption dynamics of the IPP-MMIPs, 40 mg of IPP-MMIPs and 40 mg of IPP-MNIPs were each dispersed in 20 mL of a 0.5 mM IPP standard acetonitrile solution. The mixtures were placed at room temperature for 10, 20, 30, 40, 60, 90, 120, 150, 180, 210 and 240 min. Immediately, each mixture was magnetically separated and the concentration of the supernatant in the tube was determined by a UV instrument. Then the adsorption efficiency was calculated based on the difference in the IPP concentration before and after adsorption.Three kinetic equations including the pseudo-first-order rate equation of the Lagergren model (eqn (1)), the pseudo-second-order equation (eqn (2)) and the Weber and Morris intraparticle diffusion equation (eqn (3)) were used to model the kinetics of the IPP adsorption on IPP-MMIPs, providing more detailed insight into the adsorption process. It is assumed that the measured concentrations are equal to the adsorbent surface concentrations.
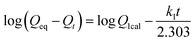 | (1) |
 | (2) |
| Qt = kpt1/2 + C | (3) |
Adsorption isotherm
To investigate the adsorption equilibrium, 40 mg of IPP-MMIPs was equilibrated with various initial concentrations of IPP in each tube at room temperature. After 150 min, the saturated polymer was separated by a magnet and the IPP concentration was measured by UV detection at 350 nm. The Langmuir and Freundlich models were employed to evaluate the adsorption isotherm of IPP by means of eqn (4) and (5), respectively.
 | (4) |
| Qeq = kfC1/neq | (5) |
Selective specificity
The competitive recognition studies were performed with IPP and other three kinds of neonicotinoid insecticides which are similar in structure. The IPP-MMIPs (40 mg) films were placed in 20 mL solutions of known concentrations of different competitive molecules (IPP, imidacloprid, thiamethoxam, and thiacloprid). The mixtures were placed for 12 h at room temperature and the polymers were isolated by a magnet. The concentrations of the neonicotinoid insecticides were detected using a UV-vis spectrophotometer at 350, 270, 255 and 235 nm respectively.11 The IPP-MNIPs were also examined using the same procedure.Results and discussion
Preparation and characterization of IPP-MMIPs
The size and shape of Fe3O4, Fe3O4@SiO2 and IPP-MMIPs were examined by scanning electron microscopy (SEM) as shown in Fig. 1. Fig. 1a and b show that the mean diameter of Fe3O4 is about 220 nm and Fe3O4@SiO2 is 280 nm, which reveals that the silica is fully coated on the surface of Fe3O4. Fig. 1c and e show the low-powered and high-powered SEM images of the IPP-MMIPs. The mean diameter is 320 nm. The thickness of the imprinted polymer layer is 40 nm, which was sufficient for mass transport between the solution and the surface of the IPP-MMIPs. Fig. 1d and f show the low-powered and high-powered SEM images of the IPP-MNIPs. This shows the difference between the IPP-MNIPs and the IPP-MNIPs when it comes to the template molecule. It shows that the synthesis of the surface molecularly imprinted polymer is successful. | ||
Fig. 1 SEM images of Fe3O4 ((a) ×100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), Fe3O4@SiO2 ((b) ×100 000), Fe3O4@SiO2 ((b) ×100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), IPP-MMIPs ((c) ×100 000), IPP-MMIPs ((c) ×100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), IPP-MNIPs ((d) ×100 000), IPP-MNIPs ((d) ×100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), IPP-MMIPs ((e) ×200 000), IPP-MMIPs ((e) ×200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) and IPP-MNIPs ((f) ×200 000) and IPP-MNIPs ((f) ×200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000). 000). | ||
Fourier transform infrared (FT-IR) spectra of Fe3O4, Fe3O4@SiO2, Fe3O4@SiO2@C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, and IPP-MMIPs were recorded using the KBr method (Fig. 2). The characteristic peaks of Si–O–Si at 1091 cm−1, the Si–O–H group at 958 cm−1, and the Si–O group at 800 cm−1 indicate the formation of the silica coating on the surface of the Fe3O4 nanocore (Fig. 2b). The peak at 1731 cm−1 was attributed to a C
C, and IPP-MMIPs were recorded using the KBr method (Fig. 2). The characteristic peaks of Si–O–Si at 1091 cm−1, the Si–O–H group at 958 cm−1, and the Si–O group at 800 cm−1 indicate the formation of the silica coating on the surface of the Fe3O4 nanocore (Fig. 2b). The peak at 1731 cm−1 was attributed to a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group, verifying the successful modification with 3-(trimethoxysilyl) propyl methacrylate (Fig. 2c). In comparison with Fig. 2c, the peak at 1731 cm−1 in Fig. 2d is relatively strong, and the peaks at 2952 cm−1 are attributed to the C–H groups of the crosslinking agent EDGMA. All peaks showed that every step of the synthesis was ideal and that the imprinted polymers were obtained with binding sites on the template.
O group, verifying the successful modification with 3-(trimethoxysilyl) propyl methacrylate (Fig. 2c). In comparison with Fig. 2c, the peak at 1731 cm−1 in Fig. 2d is relatively strong, and the peaks at 2952 cm−1 are attributed to the C–H groups of the crosslinking agent EDGMA. All peaks showed that every step of the synthesis was ideal and that the imprinted polymers were obtained with binding sites on the template.
The thermal stability measurements were performed by a STA 449C instrument from room temperature to 800 °C with a heating rate of 10 °C min−1 under a nitrogen flow. The thermogravimetric analysis (TGA) curves of Fe3O4@SiO2 and IPP-MMIPs are given in Fig. 3. Curve (a) illustrates that the weight loss of Fe3O4@SiO2 was only 9.88%, while the decrease of solvent or water in weight was 2.89% at 100 °C, the bonded water loss was 1.58% at 100–200 °C, and the decrease from the loss of others was 5.34% at 200–800 °C. Because the TEOS was hydrolyzed to Si(OH)4 under alkaline conditions, the Si(OH)4 formed a cross-linked structure on the surface of the Fe3O4@SiO2 nanoparticles. The weight loss of the saccharin IPP-MMIPs was 15.67% at 800 °C, while the decrease of solvent or water in weight was about 4.7% at 200 °C and the polymer layer released corresponded to approximately 10.87% (curve (b)), which originated from the imprinted polymer on the surface of Fe3O4. Hence, the results fully demonstrate the existence of the imprinted polymer.
The crystalline structures of the IPP-MMIPs were next analyzed by X-ray diffraction (XRD). As shown in Fig. 4, the XRD patterns of Fe3O4, Fe3O4@SiO2, Fe3O4@SiO2@MPS and IPP-MMIPs displayed several relatively strong reflection peaks in the 2θ region of 10–90°, with six characteristic peaks for Fe3O4 (2θ = 30.3°, 35.7°, 43.5°, 53.4°, 57.3° and 62.9°) observed for the four samples. These peaks positions were indexed to (220), (311), (400), (422), (511), and (440) (JCPDS card: 19-629). The XRD patterns revealed that the crystalline structures of the Fe3O4, Fe3O4@SiO2, Fe3O4@SiO2@MPS and IPP-MMIPs materials were essentially maintained.
A vibrating sample magnetometer (VSM) was used to investigate the magnetic properties of the Fe3O4, Fe3O4@SiO2 and IPP-MMIPs materials. Fig. 5A shows the absence of hysteresis and indicates that the samples have superparamagnetism to facilitate magnetic separation and reusability. Magnetic measurements show that Fe3O4, Fe3O4@SiO2 and IPP-MMIPs have saturation magnetization values of 96.66, 45.06 and 36.35 emu g−1 at room temperature, respectively. Although the magnetization value decreased after the modification process, the IPP-MMIPs with less magnetite encapsulation also reacted to the external magnetic field. As can be seen from the photo in Fig. 5B, the IPP-MMIPs could be completely separated from the solution to obtain a clear solid–liquid interphase.
 | ||
| Fig. 5 (A) VSM of Fe3O4 (a), Fe3O4@SiO2 (b), and IPP-MMIPs (c); (B) photos before (a) and after (b) magnetic separation. | ||
Adsorption kinetics
The nature of an adsorption process depends on the physical or chemical characteristics of the adsorbent system, as well as the system conditions. To describe the mechanism of adsorption in the IPP solutions, attention needs to be focused on the adsorption kinetics behavior. Fig. 6a shows the adsorption kinetics curves for 40 mg L−1 IPP solutions with IPP-MMIPs and IPP-MNIPs. It can be seen that the adsorption capacity increased rapidly in the first 60 min and approximately reached an adsorption equilibrium at 150 min for the template. Usually, it would take 12–24 h for traditionally imprinted materials to reach adsorption equilibrium, while 15–200 min for the surface imprinting technique which improves the binding kinetics.32 The equilibrium time for the IPP-MMIPs is 150 min, demonstrating that a uniform thin imprinted layer on the surface of a magnetic nanocore presents the advantages of faster mass transfer and binding kinetics, and overcomes some disadvantages of the traditionally imprinted process. From Fig. 6a, it can be seem that the maximum adsorption capacity of the IPP-MMIPs is 17.30 ± 0.40 mg g−1, while for the IPP-MNIPs it is 8.43 ± 0.55 mg g−1. The pseudo-first-order and pseudo-second-order models were adopted to describe the sorption kinetics data. According to the pseudo-first-order model (Fig. 6b), log(Qeq − Qt) was plotted versus rebinding time t (min) resulted in a straight line with coefficient of determination, R2, (0.9794). k1 was calculated from the slope of the linear plot as −0.0216 min−1. According to the pseudo-second-order model (Fig. 6c), t/Qt was plotted versus the coefficient of determination, R2, (0.9872). k2 was calculated from the intercept of the linear plot as −0.0007 min−1. The results show that the coefficients of the pseudo-first-order and second-order mechanisms are both close to 1. However, the actual maximum absorption capacity (Qeq, 17.30 mg g−1) is closer to Qeq calculated from the pseudo-first-order mechanism (19.81 mg g−1) than from the second-order (22.33 mg g−1). | ||
| Fig. 6 Curve of adsorption kinetics (■-MIPs, ●-NIPs) (a), the pseudo-first-order model (b), the pseudo-second-order model (c), and the Weber and Morris model (d). | ||
As the above kinetics models were not able to identify the diffusion mechanism, the intraparticle diffusion model based on the theory proposed by Weber and Morris was applied. The regression equation was Qt = 0.9093 + 1.2024t1/2 with a correlation coefficient of 0.9424. If the intercept was zero, it would illustrate that the adsorption process was mainly controlled by the diffusion mechanism. But now the intercept is 0.9093, which means that the diffusion mechanism is not the important influencing factor.
Adsorption isotherm
The thermodynamic adsorption properties of the IPP-MMIPs were investigated in the concentration range of 0.02–2.00 mmol L−1. Fig. 7 shows the adsorption isotherms of the IPP solutions with IPP-MMIPs and IPP-MNIPs. It can be seen that the amounts of the template IPP bound to the IPP-MMIPs increased rapidly with increasing initial concentration C0 and reached saturation when C0 was above 0.5 mmol L−1. At the same time, the amount of IPP bound to the IPP-MNIPs was lower than the IPP-MMIPs under the same conditions. The saturation capacity of the IPP-MMIPs and IPP-MNIPs for IPP were found to be 17.305 ± 0.403 mg g−1 and 8.43 ± 0.551 mg g−1, respectively. The adsorption capacity of the IPP-MMIPs is about two times higher than that of the IPP-MNIPs. It implies that the MIPs have highly specific sites for the template molecule.In order to further understand the mechanism of adsorption, the adsorption isotherm models which describe the manner of molecular interactions with the surface of the adsorbent were carried out in the study. The Langmuir isotherm indicates that the adsorbent’s surface is covered by a monolayer of molecules in a horizontal position. It assumes that the adsorbent is structurally homogeneous and that all the active sites are identical and energetically equivalent. The binding sites will not adsorb the template molecules further once the template molecules occupy the sites. The Freundlich model is basically based on absorption onto a heterogeneous surface. The parameters of the Langmuir and Freundlich models are shown in Table 1. The correlation coefficients of the two linear equations indicate that the Langmuir model provides a better fit for IPP. It can be concluded that the IPP-MMIPs are monolayer and the structure is homogeneous.
| Isotherm | Correlation | Parameters | ||
|---|---|---|---|---|
| Langmuir | 1/Qeq = 0.06365 + 0.002921/Ceq | R2 | b | Qm |
| 0.9399 | 21.80 | 15.71 | ||
| Freundlich | log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Qeq = 1.4937 + 0.5054 Qeq = 1.4937 + 0.5054![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ceq Ceq |
R2 | n | kf |
| 0.8777 | 1.979 | 31.17 | ||
Selectivity of IPP-MMIPs
The selectivity of the prepared MIP was evaluated via structural analogue of IPP. The analogue chosen should have similar functional groups, etc. As such, we selected imidacloprid, thiamethoxam and thiacloprid as the controls. The template fits into the imprinted cavity in this study, driven by electrostatic interaction followed by alignment of the functional groups on the MIP around the template molecule’s conformational orientation. In NIP, although the functional groups are also present in the polymer, they are randomly arranged in such a manner that it is ineffective for correct binding with the template. Similarly, an interferon is different in size and conformation to IPP and might lack the key structural features responsible for fitting into the recognition cavities of MIP. This leads to much greater affinity for the template molecule by molecular imprinting, in comparison with the non-imprinted one. The result of the special selective recognition is shown in Fig. 8, the absorption capacity of the IPP-MMIPs toward IPP was 17.775 mg g−1, about 4.51, 4.24 and 3.77 times than that of imidacloprid, thiamethoxam and thiacloprid. The imprinting factor of IPP (αIPP = 2.00) exhibited much higher adsorption than the imprinting factor of the other three neonicotinoid insecticides (α = 1.02–1.28). These results further verify the satisfactory imprinting efficiency of the present method for the preparation of MIP-coated magnetic nanoparticles. The four analogues belong to the neonicotinoid family which are similarly structured compounds. They have the same 6-chloropyridin group and the difference is that only IPP has a special ether group. Besides, the molar weight of IPP (366) is much larger than the other three compounds (255, 270 and 252). Accordingly, the satisfying selective adsorption ability of the IPP-MMIPs is due to the interaction between the polymer matrix and the target which is not only from hydrogen bonding but also the complementary size and shape of the IPP template and MIP.33 | ||
| Fig. 8 Selective recognition of the IPP-MMIPs and IPP-MNIPs for four kinds of neonicotinoid insecticides. | ||
Reusability of IPP-MMIPs
To examine the important reusability features of the IPP-MMIPs, the adsorption and desorption cycles were repeated five times using the same IPP-MMIPs, as shown in Fig. 9. In this study, methanol–acetic acid (90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 = v/v) was used to remove the template molecules. The results show that the IPP-MMIPs lost only 2.25–6.55% of their adsorption capacity on average over four cycles and 14.70% on the fifth cycle. These results demonstrate the reusability of the IPP-MMIPs over four adsorption/desorption cycles as they still have a high stability and retain almost the same adsorption capacity for the template. After four times, the effect of washing the MIPs was negligible because of the reduced affinity for the template. It is possible that some recognition sites are dependent on the three-dimensional distribution of the functional groups and the overall complementary interactions of the MMIPs could be blocked after regeneration or destroyed after rewashing.
10 = v/v) was used to remove the template molecules. The results show that the IPP-MMIPs lost only 2.25–6.55% of their adsorption capacity on average over four cycles and 14.70% on the fifth cycle. These results demonstrate the reusability of the IPP-MMIPs over four adsorption/desorption cycles as they still have a high stability and retain almost the same adsorption capacity for the template. After four times, the effect of washing the MIPs was negligible because of the reduced affinity for the template. It is possible that some recognition sites are dependent on the three-dimensional distribution of the functional groups and the overall complementary interactions of the MMIPs could be blocked after regeneration or destroyed after rewashing.
Conclusions
In this work, a surface MMIP was successfully developed for the selective recognition of the target molecule, i.e. the new neonicotinoid insecticide IPP. Polymerization on the surface was propagated by means of layer-by-layer self-assembly onto the surface of a magnetic nanocore. FT-IR spectroscopy, SEM, XRD, VSM and TGA were used to characterize the IPP-MMIPs prepared. The resulting IPP-MMIPs possessed a high adsorption capacity and specific recognition. The experimental results obtained in this work demonstrate that MMIPs can be used for the selective detection of IPP. The specific selectivity of the synthesized MMIPs imply potential applications in analytical separation techniques.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (no. 31000831), the National Science Foundation for Post-doctoral Scientists of China (20090450478), the Natural Science Research and Innovation Funds of Harbin Institute of Technology (HIT.NSRIF.2008.30) and the Postdoctoral Science-Research Developmental Foundation of Heilongjiang Province (LBH-Q12085).References
- J. Tang, M. Zhang, G. Cheng and Y. Lu, Development and application of molecularly imprinted polymer as solid phase extraction of imidacloprid in environmental samples, J. Liq. Chromatogr. Relat. Technol., 2009, 32, 59–71 CrossRef CAS.
- T. Alizadeh, M. R. Ganjali, P. Nourozi and M. Zare, Multivariate optimization of molecularly imprinted polymer solid-phase extraction applied to parathion determination in different water samples, Anal. Chim. Acta, 2009, 638, 154–161 CrossRef CAS PubMed.
- C. García-Ruiz, G. Alvarez-Llamas and Á. Puerta, Enantiomeric separation of organophosphorus pesticides by capillary electrophoresis: application to the determination of malathion in water samples after preconcentration by off-line solid-phase extraction, Anal. Chim. Acta, 2005, 543, 77–83 CrossRef.
- R. Rial-Otero, E. M. Gaspar and I. Moura, Chromatographic-based methods for pesticide determination in honey: an overview, Talanta, 2007, 71, 503–514 CrossRef CAS.
- D. N. Bertoncini and M. C. Hennion, Immunoaffinity solid-phase extraction for pharmaceutical and biomedical trace-analysis-coupling with HPLC and CE-perspectives, J. Pharm. Biomed. Anal., 2004, 34, 717–736 CrossRef.
- X. Y. Xu, X. S. Shao, Z. Y. Wu and W. Wei, Novel insecticide-paichongding, World Pestic., 2009, 31, 52 Search PubMed.
- G. R. B. Miranda, C. G. Raetano and E. Silva, Environmental fate of neonicotinoids and classification of their potential risks to hypogean, epygean, and surface water ecosystems in Brazil, Hum. Ecol. Risk Assess., 2011, 17, 981–995 CrossRef CAS.
- H. M. Selim, C. Y. Jeong and T. A. Elbana, Transport of imidacloprid in soils: miscible displacement experiments, Soil Sci., 2010, 175, 375–381 CrossRef CAS.
- S. T. Kurwadkar, D. Dewinne and R. Wheat, Time dependent sorption behavior of dinotefuran, imidacloprid and thiamethoxam, J. Environ. Sci. Health, Part B, 2013, 48, 37–242 CrossRef PubMed.
- P. Wang, X. Yang, W. W. Huang, M. Zhang, W. H. Lu, H. T. Zhao, J. Wang, H. L. Liu, A. J. Dong, H. Zhang, R. B. Xu, P. Zou, C. L. Cheng, Y. C. Zhang and J. Jing, Effect of pesticide 1-[6-chloro-3-methyl-pyridyl-8-nittro-7-methyl-1,2,3,5,6,7-hexahydro imidazo (1,2-a)]-pyridine when responding to a wheat plant’s antioxidant defense system, Food Chem., 2014, 146, 569–576 CrossRef CAS PubMed.
- P. Wang, X. Yang, J. Wang, J. Cui, A. J. Dong, H. T. Zhao, L. W. Zhang, Z. Y. Wang, R. B. Xu, W. J. Li, Y. C. Zhang, H. Zhang and J. Jing, Multi-residue method for determination of seven neonicotinoid insecticides in grains using dispersive solid-phase extraction and dispersive liquid–liquid micro-extraction by high performance liquid chromatography, Food Chem., 2012, 13, 1691–1698 CrossRef PubMed.
- Z. Xiao, X. Li and X. Wang, Determination of neonicotinoid insecticides residues in bovine tissues by pressurized solvent extraction and liquid chromatography-tandem mass spectrometry, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2011, 879, 117–122 CrossRef CAS PubMed.
- E. Watanabe, K. Baba and S. Miyake, Analytical evaluation of enzyme-linked immunosorbent assay for neonicotinoid dinotefuran for potential application to quick and simple screening method in rice samples, Talanta, 2011, 84, 1107–1111 CrossRef CAS PubMed.
- S. Xu, C. Guo, Y. Li, Z. Yu, C. Wei and Y. Tang, Methyl parathion imprinted polymer nanoshell coated on the magnetic nanocore for selective recognition and fast adsorption and separation in soils, J. Hazard. Mater., 2014, 264, 34–41 CrossRef CAS PubMed.
- A. H. Lu, E. L. Salabas and F. Schüth, Magnetic nanoparticles: synthesis, protection, functionalization, and application, Angew. Chem., Int. Ed., 2007, 46, 1222–1244 CrossRef CAS PubMed.
- S. Xu, L. Chen and J. Li, Preparation of hollow porous molecularly imprinted polymers and their applications to solid-phase extraction of triazines in soil samples, J. Mater. Chem., 2011, 21, 12047–12053 RSC.
- F. A. Ishkuh, M. Javanbakht, M. Esfandyari-Manesh, R. Dinarvand and F. Atyabi, Synthesis and characterization of paclitaxel-imprinted nanoparticles for recognition and controlled release of an anticancer drug, J. Mater. Sci., 2014, 49, 6343–6352 CrossRef CAS.
- Y. H. Yun, H. K. Shon and S. D. Yoon, Preparation and characterization of molecularly imprinted polymers for the selective separation of 2,4-dichlorophenoxyacetic acid, J. Mater. Sci., 2009, 44, 6206–6211 CrossRef CAS.
- N. Basar, L. Uzun, A. Guner and A. Denizli, Lysozyme purification with dye-affinity beads under magnetic field, Int. J. Biol. Macromol., 2007, 41, 234–242 CrossRef CAS PubMed.
- J. Lee, S. Bernard and X. C. Liu, Nanostructured biomimetic catalysts for asymmetric hydrogenation of enamides using molecular imprinting technology, React. Funct. Polym., 2009, 69, 650–654 CrossRef CAS.
- S. A. Piletsky, S. Alcock and A. P. F. Turner, Molecular imprinting: at the edge of the third millennium, Trends Biotechnol., 2001, 46, 1222–1244 Search PubMed.
- W. Li and S. Li, Molecular imprinting a versatile tool for separation, sensors and catalysis, Adv. Polym. Sci., 2007, 206, 191–210 CrossRef CAS.
- L. Chang, S. Wu, S. Chen and X. Li, Preparation of graphene oxide-molecularly imprinted polymer composites via atom transfer radical polymerization, J. Mater. Sci., 2011, 46, 2024–2029 CrossRef CAS.
- A. Mehdinia, T. B. Kayyal and A. Jabbari, Magnetic molecularly imprinted nanoparticles based on grafting polymerization for selective detection of 4-nitrophenol in aqueous samples, J. Chromatogr. A, 2013, 1283, 82–88 CrossRef CAS PubMed.
- R. Gao, X. Kong and F. Su, Synthesis and evaluation of molecularly imprinted core–shell carbon nanotubes for the determination of triclosan in environmental water samples, J. Chromatogr. A, 2010, 1217, 8095–8102 CrossRef CAS PubMed.
- W. Huang, X. Yang, S. Zhao, M. Zhang, X. Hu, J. Wang and H. Zhao, Fast and selective recognizes polysaccharide by surface molecularly imprinted film coated onto aldehyde modified magnetic nanoparticles, Analyst, 2013, 138, 6653–6661 RSC.
- H. Deng, X. Li and Q. Peng, Monodisperse magnetic single-crystal ferrite microspheres, Angew. Chem., 2005, 117, 2842–2845 CrossRef.
- W. Stöber, A. Fink and E. Bohn, Controlled growth of monodisperse silica spheres in the micron size range, J. Colloid Interface Sci., 1968, 26, 62–69 CrossRef.
- M. Otero, F. Rozada and L. F. Calvo, Kinetic and equilibrium modelling of the methylene blue removal from solution by adsorbent materials produced from sewage sludges, Biochem. Eng. J., 2003, 15, 59–68 CrossRef CAS.
- Y. Bulut and H. Aydin, A kinetics and thermodynamics study of methylene blue adsorption on wheat shells, Desalination, 2006, 194, 259–267 CrossRef CAS.
- H. Freundlich, Over the adsorption in solution, J. Phys. Chem. C, 1906, 57, 385–470 CAS.
- L. Li, X. W. He, L. X. Chen and Y. K. Zhang, Preparation of core–shell magnetic molecularly imprinted polymer nanoparticles for recognition of bovine haemoglobin, Chem.–Asian J., 2009, 4, 286–293 CrossRef CAS.
- Y. Zhao, C. Bi, X. He, L. Chen and Y. Zhang, Preparation of molecularly imprinted polymers based on magnetic carbon nanotubes for determination of sulfamethoxazole in food samples, RSC Adv., 2015, 5, 70309–70318 RSC.
Footnote |
| † These authors contributed equally to this work and should be considered as co-first authors. |
| This journal is © The Royal Society of Chemistry 2016 |





