Determining the impact of hydrofluoric acid on surface states of as-prepared and chemically modified Si nanocrystals†
B. V. Oliinyk*a,
V. Lysenkob and
S. Alekseeva
aFaculty of Chemistry, National Taras Shevchenko University of Kyiv, 64 Volodymyrska Str., 01601 Kyiv, Ukraine. E-mail: oliinykb@gmail.com
bUniversité de Lyon, Institut des Nanotechnologies de Lyon (INL), UMR-5270, CNRS, INSA de Lyon, Bat. Blaise Pascal, 7 avenue Jean Capelle, Villeurbanne F-69621, France
First published on 21st December 2015
Abstract
Introduction of hydrofluoric acid before and during chemical functionalization of silicon nanocrystals with hydrocarbon chains is found to be very efficient for removing original surface defects of the nanocrystals as well as for preserving them from oxidation induced defects appearing on their surface during photoinitiated hydrosilylation procedure. In consequence, stable sols of highly luminescent silicon quantum dots in organic solvents with photoluminescence quantum yield up to 20% are shown to be easily obtained. Moreover, our approach allows considerable improvement of electric transport through meso-porous silicon nanostructures which is still a serious challenge for their applications in photovoltaics and thermoelectrics. Finally, the use of hydrofluoric acid appears as a cheap and efficient alternative to development of painstaking procedures and set-ups preventing oxidation induced deterioration of physico-chemical properties of the individual and interconnected silicon nanocrystals during their chemical functionalization.
Introduction
Being one of the most widespread non-toxic elements on earth, silicon (Si) is already widely used in the microelectronics industry.1 Although bulk silicon, as an indirect bandgap semiconductor, has poor light emitting properties at room temperature, the photoluminescence (PL) of Si nanocrystals (NCs), also called Si quantum dots,2 is much more efficient due to quantum confinement effects. In particular, being largely governed by quantum dot dimensions which are comparable or smaller than the exciton Bohr radius (about 4.2–4.6 nm for Si3), the confinement degree of photogenerated charge carriers strongly influences the emission wavelength and lifetime as well as the radiative recombination rate and efficiency.4 Thus, for example, luminescent Si NCs have already attracted the special attention of researchers for bio-imaging applications.5,6However, even if more than two decades have passed since the first reports on room temperature photo-stimulated emission of the Si NCs,7,8 the main challenge related to finding a way for simple fabrication of the NCs with tuneable emission and high quantum yield (QY) values above 10–15% still persists.5 One of the major actual problems concern perfect control of surface electronic states of the NCs. Indeed, because of a high surface to volume ratio, impact of the surface states on the overall properties of the Si NCs can be really huge.
In general, existence of any structural imperfections and defects at the NC surface can strongly cause significant QY reduction by providing various alternative ways for non-radiative electronic transitions.9 For example, spontaneous instability of H-terminated Si NCs against oxidation in air or water containing environments systematically leads to considerable decrease of their PL QY due to appearance of harmful defect states in the NC bandgap.10 Recent studies have been pointed to the significant influence of surface oxidation of the Si NCs on their optical properties.11–15 Over the past few years there has been mounting evidence that a further improvement of the optical properties of the Si NCs requires a careful chemical passivation of their surface.16–20
Globally, the need of defect-free surface engineering for Si NCs has been clearly perceived by research communities from various fields, such as: optoelectronics, photovoltaics, bio-imaging, etc. For example, original studies demonstrating feasibility of hybrid solar cells and thermoelectric devices based on Si NCs have shown that their achievable efficiencies are strongly limited by transport barriers due to a native oxide shell and/or relatively high concentration of surface defects of 7 × 1011 cm−2 in the Si NC networks,21–24 acting as recombination and trapping centers.25,26 Therefore, reduction of the surface defect density and optimization of the Si NC surfaces for an efficient inter-NC charge transfer are also the ongoing challenges.27–30
Light-promoted hydrosilylation is known to be one of the promising, simple, clean and direct chemical approaches allowing stabilization of the Si NC surface via formation of covalent Si–C bonds efficiently protecting the NCs from any further spontaneous chemical evolution as well as generally ensuring a reduced number of surface defects.31 However, due to its enhanced chemical reactivity, initially hydrogenated surface of Si NCs is substantially contaminated with oxygen-containing surface species (Si–OH, Si–O–Si, etc.) provoking significant reduction of their PL QY values and rendering instable overall electronic properties of the NCs. Anyway, some traces of surface silicon oxide are almost unavoidable even if special harsh means are undertaken to exclude oxygen and water during a grafting process.32
In this letter, we point out that a continuous permanent treatment of Si NCs with hydrofluoric acid (HF) before and during their chemical functionalization with hydrocarbon chains allows not only to efficiently remove original surface defects produced during the fabrication of the NCs by electrochemical etching of bulk Si substrates but also to avoid appearance of the oxidation induced defects on their surface during photoinitiated hydrosilylation procedure. In consequence, (i) considerable improvement of the electric transport through the meso-porous silicon nanostructures has been achieved and (ii) stable sols of highly luminescent Si NCs in organic solvents with PL QY up to 20% are shown to be easily obtained.
Experimental
Si NCs used in our work were synthesized by electrochemical etching (anodization) of p-type (100)-oriented silicon wafers (see Experimental details in the ESI†). Surface chemical modifications of the Si NCs with 1-octadecene and 10-undecylenic acid were carried out in frames of photoinitiated hydrosilylation procedure in two different ways (see Fig. 1). The first way, called “HF-free standard procedure (SP)”, corresponds to well-known hydrosilylation reaction performed on the as-prepared Si NCs (all process details can be found in the ESI†). The second way, called “HF-based modified procedure (MP)”, is one-pot synthesis comprised of two consecutive stages: (i) HF-based treatment of the as-prepared Si NCs under UV light and (ii) photoinitiated hydrosilylation reaction in presence of HF acid. | ||
| Fig. 1 Surface chemical modifications of Si NCs with 1-octadecene and 10-undecylenic acid carried out in frames of this work (used co-solvents: THF, DME). | ||
Results and discussion
The basic idea behind these two experimental ways was to check if the preliminary HF-based treatment and HF acid presence during the subsequent chemical modification of the Si NCs with alkyl groups will really improve electronic quality of the NC surface. In particular, HF acid was expected not only to remove defect states created by oxygen atoms on the NC surface but also to etch out completely the whole damaged near-surface region of the hydrogenated NCs with numerous structural defects appeared during the NC formation by anodization of bulk Si. Only a few attempts aiming introduction of HF acid in the hydrosilylation of Si NCs have been already declared in literature.33,34 For example, Sato and Swihart33 have found the mixing of HF with acrylic acid to be essential to ensure reproducibility of the hydrosilylation process. Miyano et al.34 have reported an increase of the hydrosilylation reaction efficiency due to addition of the HF acid into styrene solution containing Si nanoparticles. In order to check an impact of the preliminary HF-based treatment of Si NCs under UV light, concentration of free charge carriers in meso-PS free layers constituted by interconnected Si NCs has been monitored by infra-red (IR) absorption spectroscopy. Indeed, concentration of the free carriers (holes in the case of p+-type Si wafers used in our work for the meso-PS fabrication) is very sensitive to the nature and concentration level of the NC surface defects. According to Drude's model, characteristic IR absorption by free carriers is manifested in meso-PS samples by spectral dependence of absorption coefficient according to the power law with an exponent about 2.35 Typical IR transmittance spectra of a meso-PS free layer before and after the HF treatment under UV illumination are shown in Fig. 2. The observed characteristic background decrease of the transmitted IR intensities at lower wavenumbers reflects the IR absorption by the free holes. As one can see, this decrease is much more pronounced for the meso-PS sample treated with HF under UV light (the dashed blue line represents the background spectral evolution according to the Drude's model). It means that the holes concentration is significantly increased in the meso-PS layer after the treatment. This fact is also supported by a huge reduction of general electrical resistivity (more than two orders of magnitude) of the treated porous layer. This increase of the free carrier concentration in the treated samples can be explained by a considerable decrease of the donor-like and amphoteric surface defects responsible for the hole trapping in the p-type Si NCs. Indeed, these kinds of defects are known to be especially dominant in the as-prepared hydrogenated and partially oxidized meso-PS.36–38 As a result, the holes concentrations are very low in the as-prepared meso-PS samples because the holes are efficiently trapped by the surface states. The HF treatment efficiently removes the surface defects playing the role of electronic traps for the free charge carriers. For example, the HF-induced disappearance of the donor-like states ensured by water and oxygen39 is clearly manifested on the spectra in Fig. 2 by absence of the ν(OH) and ν(SiO) vibrational bands in the treated porous layers. The observed effect will allow remarkable improvement of the electric transport through the porous Si network which is the issue for various photovoltaic and thermoelectric applications.So we may assume that under such treatment conditions a well-known photo-induced chemical etching of silicon surface occurs. It could take place even without UV light but in the case of illumination the etching process is much more enhanced and can be efficiently controlled by the illumination conditions. As for a microscopic mechanism, the UV light very efficiently absorbed by Si generates electron–hole pairs accelerating Si dissolution in HF-based media. In this work we use this effect to promote silicon dissolution causing removing of surface defects and damaged areas.
In order to fix the improved electronic quality of the NC surface achieved after the preliminary first stage treatment in HF acid, the second stage of the MP functionalization approach aiming chemical modification of the Si NCs with alkyl groups has been also carried out in presence of HF acid. Fig. 3 shows IR transmission spectra of the Si NCs functionalized with 1-octadecene and 10-undecylenic acid in frames of the SP and MP procedures sketched in Fig. 1. First of all, the successful derivatization is confirmed by the following characteristic spectral bands:40 νs(CH2) at 2853 cm−1, νas(CH2) at 2925 cm−1, and δ(CH2) at 1467 cm−1 for both samples; νas(CH3) at 2962 cm−1 for Si–C18H37 NCs and ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) at 1715 cm−1 for Si–C10H20COOH NCs. Absence of a ν(C
O) at 1715 cm−1 for Si–C10H20COOH NCs. Absence of a ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) band at 1645 cm−1 in the shown spectra indicates on total absence of any physically adsorbed molecules of the organic reagents with the C
C) band at 1645 cm−1 in the shown spectra indicates on total absence of any physically adsorbed molecules of the organic reagents with the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds. Presence of SiHx vibration bands (ν(SiH3) at 2137 cm−1; ν(SiH2) at 2114 cm−1; ν(SiH) at 2088 cm−1; δ(SiH2) at 906 cm−1; ω(SiH2) at 660 cm−1 and ω(SiH) at 625 cm−1) in the IR spectra of the hydrosilylated samples reflects only partial consumption of the SiHx groups of the initial Si NCs. However, much higher ratios between the intensities of the ν(SiHx) and ν(CHx) bands obtained for the NCs functionalized in frames of the MP procedure testify to considerably increased number of hydrogen atoms substituted by the organic groups. Furthermore, the MP functionalization strategy allows almost complete disappearance of the spectral bands related to OxSiHy groups (ν(SiH) at 2264 cm−1; ν(Si–O) at 1000–1200 cm−1 and ω(SiH) at 875 cm−1), while substantial oxidation of the NC surface occurs during the HF-free SP approach despite a lot of taken precautions.
C bonds. Presence of SiHx vibration bands (ν(SiH3) at 2137 cm−1; ν(SiH2) at 2114 cm−1; ν(SiH) at 2088 cm−1; δ(SiH2) at 906 cm−1; ω(SiH2) at 660 cm−1 and ω(SiH) at 625 cm−1) in the IR spectra of the hydrosilylated samples reflects only partial consumption of the SiHx groups of the initial Si NCs. However, much higher ratios between the intensities of the ν(SiHx) and ν(CHx) bands obtained for the NCs functionalized in frames of the MP procedure testify to considerably increased number of hydrogen atoms substituted by the organic groups. Furthermore, the MP functionalization strategy allows almost complete disappearance of the spectral bands related to OxSiHy groups (ν(SiH) at 2264 cm−1; ν(Si–O) at 1000–1200 cm−1 and ω(SiH) at 875 cm−1), while substantial oxidation of the NC surface occurs during the HF-free SP approach despite a lot of taken precautions.
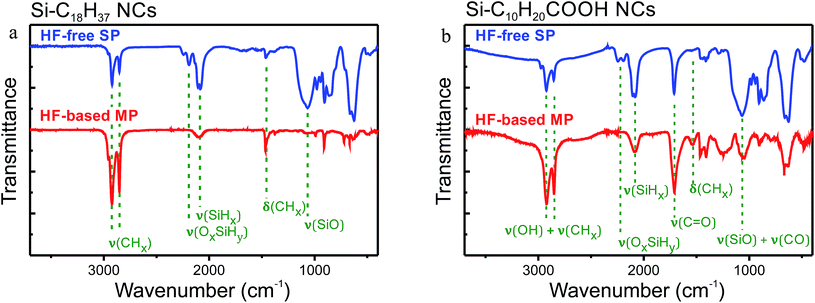 | ||
| Fig. 3 IR transmittance spectra of Si NCs functionalized with (a) 1-octadecene and (b) 10-undecylenic acid in frames of the SP and MP procedures. | ||
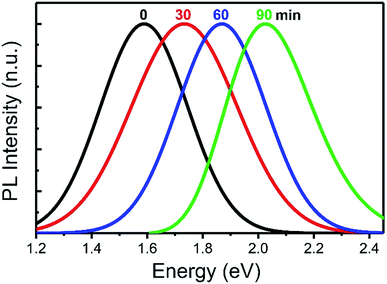
Fig. 4 shows steady-state PL and PL lifetime spectra of the as-prepared and chemically modified Si NCs. In particular, only a tiny PL spectral difference (40 meV shift of the PL maxima) between the as-prepared Si NCs and those chemically modified according to the HF-free SP protocol can be stated. As for the PL spectrum of the Si NCs functionalized according to the HF-based MP protocol, it is much more blue-shifted (about 150 meV) compared to the as-prepared sample.
Such a shift can be precisely tuned by control of time of the first treatment stage related to etching of the NCs in HF-based solutions leading to progressive decrease of the NC sizes. Indeed, as illustrated in Fig. 5, spectral position of the PL maxima unambiguously correlates to the etching time. Inset in Fig. 4a shows excitation and absorption spectra of Si NCs modified with 1-octadecene by the HF-based procedure and dispersed in hexane. All the samples with Si NCs have the similar typical spectra with only some slight spectral differences. Fig. 4b illustrates PL lifetimes (τmeas) of the Si NCs for different photon energies. Determination of the lifetime values was carried out by fitting of experimental time-resolved PL curves by a stretched exponential decay according to the general equation indicated in Fig. 4b. The order of magnitude and the observed monotonous decrease of the PL lifetimes with the increasing energy well correspond to the case of Si NCs and emphasize domination of zero phonon recombination processes and reduction of the PL lifetimes for smaller NCs emitting at higher energies.41–43 It is worth to remark that the surface chemical modifications of the Si NCs as well as their dispersion in various solvents do not provoke any changes in the spectral dependence of the measured PL lifetimes. Thus, one can conclude that the global PL mechanism taking place in the as-prepared colloidal Si NCs remains unchanged after the chemical modifications of their surface and only absolute values of non-radiative (τnr) and radiative (τr) lifetimes are changed, defining τmeas as:
| τmeas = τnr × τr/(τnr + τr) |
In addition, as summarized in Table 1, QY values of the functionalized NCs are found to be systematically higher in comparison with QY values of the as-prepared Si NCs. Moreover, the QYs of the samples modified in frames of the HF-based MP approach are 2 times higher (20%) than the QYs (10%) characterizing photo-stimulated emission of the Si NCs modified according to the HF-free SP protocol. Taking into account the QY definition:
| QY = τnr/(τnr + τr), |
| Sample | Solvent | λmax [eV] | QY [%] | τmeas [μs] | τnr/τr |
|---|---|---|---|---|---|
| As prepared | |||||
| Si NCs | Ethanol | 1.66 | 5 | 58 | 0.05 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| HF-free standard procedure | |||||
| Si–C18H37 NCs | Hexane | 1.59 | 10 | 85 | 0.11 |
| Si–C10H20COOH NCs | Ethanol | 1.59 | 9 | 88 | 0.10 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| HF-based modified procedure | |||||
| Si–C18H37 NCs (30 min) | Hexane | 1.72 | 20 | 44 | 0.25 |
| Si–C18H37 NCs (90 min) | Hexane | 2.03 | 20 | 9 | 0.25 |
| Si–C10H20COOH NCs (30 min) | Ethanol | 1.85 | 20 | 24 | 0.25 |
As one can see from Table 1, τnr/τr ratio is significantly increased for the NCs chemically modified in presence of HF acid. Since the τmeas values for these Si NCs luminescing at higher energies are shorter, the stated improvement of their PL efficiency can be explained by increase of τnr and/or decrease of τr due to the HF-induced removing of non-radiative surface states as well as by their efficient chemical passivation with the alkyl groups.
Conclusions
In conclusion, a continuous permanent treatment of Si NCs by HF acid before and during their chemical functionalization with hydrocarbon chains allows not only to efficiently remove original surface defects produced during the fabrication of the NCs by electrochemical etching of bulk Si substrates but also to avoid appearance of the oxidation induced defects on their surface during photoinitiated hydrosilylation procedure. In consequence, stable sols of highly luminescent Si NCs in organic solvents with PL QY up to 20% are shown to be easily obtained. In addition, time control of the HF action allows precise tuning of the PL wavelengths. Moreover, application of the same HF-based treatment to meso-porous Si (meso-PS) free layers leads to significant concentration increase of free charge carriers due to their liberation from the numerous electronic traps located at the near-surface defect regions. Such the observed effect will unavoidably ensure considerable improvement of the electric transport through the meso-PS network which is still an issue for various photovoltaic and thermoelectric applications. Finally, our approach allows to completely avoid development of painstaking procedures and set-ups preventing oxidation induced deterioration of physical properties of the individual and interconnected Si NCs during chemical stabilization/passivation of their surface.Acknowledgements
B. Oliinyk, V. Lysenko and S. Alekseev contributed equally to this work.Notes and references
- M. Schulz, Nature, 1999, 399, 729–730 CrossRef CAS.
- A. P. Alivisatos, Science, 1996, 271, 933–937 CAS.
- A. D. Yoffe, Adv. Phys., 1993, 42, 173–266 CrossRef CAS.
- M. Nirmal and L. Brus, Acc. Chem. Res., 1999, 32, 407–414 CrossRef CAS.
- X. Cheng, S. B. Lowe, P. J. Reece and J. J. Gooding, Chem. Soc. Rev., 2014, 43, 2680–2700 RSC.
- F. Peng, Y. Su, Y. Zhong, C. Fan, S. T. Lee and Y. He, Acc. Chem. Res., 2014, 47, 612–623 CrossRef CAS PubMed.
- W. L. Wilson, P. F. Szajowski and L. E. Brus, Science, 1993, 262, 1242–1244 CAS.
- B. Delley and E. Steigmeier, Phys. Rev. B: Condens. Matter Mater. Phys., 1993, 47, 1397–1400 CrossRef CAS.
- M. V. Wolkin, J. Jorne, P. M. Fauchet, G. Allan and C. Delerue, Phys. Rev. Lett., 1999, 82, 197–200 CrossRef CAS.
- A. Gupta and H. Wiggers, Nanotechnology, 2011, 22, 055707 CrossRef PubMed.
- S. Yang, W. Li, B. Cao, H. Zeng and W. Cai, J. Phys. Chem. C, 2011, 115, 21056–21062 CAS.
- B. Ghosh and N. Shirahata, Sci. Technol. Adv. Mater., 2014, 15, 014207 CrossRef.
- K. Dohnalová, A. N. Poddubny, A. A. Prokofiev, W. D. de Boer, C. P. Umesh, J. M. Paulusse, H. Zuilhof and T. Gregorkiewicz, Light: Sci. Appl., 2013, 2, e47 CrossRef.
- D. Mariotti, S. Mitra and V. Švrček, Nanoscale, 2013, 5, 1385 RSC.
- V. Svrcek, K. Dohnalova, D. Mariotti, M. T. Trinh, R. Limpens, S. Mitra, T. Gregorkiewicz, K. Matsubara and M. Kondo, Adv. Funct. Mater., 2013, 23, 6051–6058 CrossRef CAS.
- J. D. Holmes, K. J. Ziegler, R. C. Doty, L. E. Pell, K. P. Johnston and B. A. Korgel, J. Am. Chem. Soc., 2001, 123, 3743–3748 CrossRef CAS PubMed.
- D. Jurbergs, E. Rogojina, L. Mangolini and U. Kortshagen, Appl. Phys. Lett., 2006, 88, 233116 CrossRef.
- E. J. Henderson, A. J. Shuhendler, P. Prasad, V. Baumann, F. Maier-Flaig, D. O. Faulkner, U. Lemmer, X. Y. Wu and G. A. Ozin, Small, 2011, 7, 2507–2516 CAS.
- C. M. Hessel, E. J. Henderson and J. G. C. Veinot, Chem. Mater., 2006, 18, 6139–6146 CrossRef CAS.
- E. J. Henderson, J. A. Kelly and J. G. C. Veinot, Chem. Mater., 2009, 21, 5426–5434 CrossRef CAS.
- R. Lechner, H. Wiggers, A. Ebbers, J. Steiger, M. S. Brandt and M. Stutzmann, Phys. Status Solidi RRL, 2007, 1, 262–264 CrossRef CAS.
- Z. C. Holman, C. Y. Liu and U. R. Kortshagen, Nano Lett., 2010, 10, 2661–2666 CrossRef CAS PubMed.
- S. Niesar, R. Dietmueller, H. Nesswetter, H. Wiggers and M. Stutzmann, Phys. Status Solidi A, 2009, 206, 2775–2781 CAS.
- A. Baumer, M. S. Brandt, M. Stutzmann and H. Wiggers, US 2010/0221544 A1, 2010.
- M. A. Rafiq, Y. Tsuchiya, H. Mizuta, S. Oda, S. Uno, Z. A. K. Durrani and W. I. Milne, Appl. Phys. Lett., 2005, 87, 182101 CrossRef.
- A. R. Stegner, R. N. Pereira, K. Klein, R. Lechner, R. Dietmueller, M. S. Brandt, M. Stutzmann and H. Wiggers, Phys. Rev. Lett., 2008, 100, 18–21 CrossRef PubMed.
- X. Zhou, K. Usami, M. A. Rafiq, Y. Tsuchiya, H. Mizuta and S. Oda, J. Appl. Phys., 2008, 104, 024518 CrossRef.
- X. Zhou, K. Uchida, H. Mizuta and S. Oda, J. Appl. Phys., 2009, 105, 124518 CrossRef.
- D. Herrmann, S. Niesar, C. Scharsich, A. Köhler, M. Stutzmann and E. Riedle, J. Am. Chem. Soc., 2011, 133, 18220–18233 CrossRef CAS PubMed.
- S. Niesar, W. Fabian, N. Petermann, D. Herrmann, E. Riedle, H. Wiggers, M. S. Brandt and M. Stutzmann, Green, 2011, 1, 339–350 CrossRef CAS.
- J. M. Buriak, Chem. Mater., 2014, 26, 763–772 CrossRef CAS.
- F. Hua, M. T. Swihart and E. Ruckenstein, Langmuir, 2005, 21, 6054–6062 CrossRef CAS PubMed.
- S. Sato and M. T. Swihart, Chem. Mater., 2006, 18, 4083–4088 CrossRef CAS.
- M. Miyano, S. Endo, H. Takenouchi, S. Nakamura, Y. Iwabuti, O. Shiino, T. Nakanishi and Y. Hasegawa, J. Phys. Chem. C, 2014, 118, 19778–19784 CAS.
- V. Timoshenko, T. Dittrich, V. Lysenko, M. Lisachenko and F. Koch, Phys. Rev. B: Condens. Matter Mater. Phys., 2001, 64, 085314 CrossRef.
- T. Dittrich, M. Schwartzkopff, E. Hartmann and J. Rappich, Surf. Sci., 1999, 437, 154–162 CrossRef CAS.
- P. M. Lenahan, Microelectron. Eng., 1993, 22, 129–138 CrossRef CAS.
- J. Kočka, J. Non-Cryst. Solids, 1987, 90, 91–98 CrossRef.
- V. F. Kiselev and O. V. Krylov, Electronic Phenomena in Adsorption and Catalysis on Semiconductors and Dielectrics, Springer-Verlag Berlin Heidelberg, Berlin, 1st edn, 1987 Search PubMed.
- G. Socrates, Infrared and Raman Characteristic Group Frequencies: Tables and Charts, John Wiley and Sons, Chichester, UK, 3rd edn, 2004 Search PubMed.
- D. C. Hannah, J. Yang, P. Podsiadlo, M. K. Y. Chan, A. Demortière, D. J. Gosztola, V. B. Prakapenka, G. C. Schatz, U. Kortshagen and R. D. Schaller, Nano Lett., 2012, 12, 4200–4205 CrossRef CAS PubMed.
- P. Hapala, K. Kůsová, I. Pelant and P. Jelínek, Phys. Rev. B: Condens. Matter Mater. Phys., 2013, 87, 195420 CrossRef.
- M. L. Mastronardi, F. Maier-Flaig, D. Faulkner, E. J. Henderson, C. Kübel, U. Lemmer and G. A. Ozin, Nano Lett., 2012, 12, 337–342 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed experimental protocols and measurement methods. See DOI: 10.1039/c5ra24556g |
| This journal is © The Royal Society of Chemistry 2016 |