Designing and preparation of ferulic acid surface-imprinted material and its molecular recognition characteristics
Baojiao Gao*,
Suqing Meng and
Liqin Zhang
Department of Chemical Engineering, North University of China, Taiyuan 030051, People's Republic of China. E-mail: gaobaojiao@126.com; Fax: +86-0351-3922118; Tel: +86-0351-3921414
First published on 8th December 2015
Abstract
Ferulic acid (FA) is a phenolic acid with a styrene-type structure, which has many important bioactive and pharmacological functions. In this study, due to the presence of a polymerizable double bond in FA, FA surface-imprinting was realized successfully by adopting a new surface imprinting technology of “pre-graft polymerization and post-cross-linking/imprinting”. Dimethylaminoethylmethacrylate (DMAEMA) was first graft-polymerized onto the surface of micron-sized silica gel particles via surface initiated graft-polymerization to obtain the grafted PDMAEMA/SiO2 particles, and then FA surface-imprinting was successfully carried out using dibromohexane as a crosslinker to obtain the FA surface-imprinted MIP-PDMAEMA/SiO2 material. This imprinting process completely avoided the problem that the polymerizable double bond in FA takes part in the polymerization reaction. The binding and recognition characteristics of the MIP-PDMAEMA/SiO2 particles towards FA were investigated using a batch method, column method and competitive adsorption method. The experimental results show that in an acidic solution, there are strong interactions between the PDMAEMA/SiO2 particles and FA, and they involve electrostatic interaction as the main driving force and cation–π interactions as well as hydrogen bonding, constituting a supermolecular system. On this basis, FA surface-imprinting was smoothly performed. The surface-imprinted MIP-PDMAEMA/SiO2 particles have special recognition selectivity and excellent binding affinity for FA. The selectivity coefficients of the MIP-PDMAEMA/SiO2 particles for FA relative to chlorogenic acid and caffeic acid, which were used as two contrast phenolic acid compounds, were 6.47 and 2.75, respectively. The binding capacity of the MIP-PDMAEMA/SiO2 particles for FA reaches up to 142 mg g−1. The MIP-PDMAEMA/SiO2 particles still have excellent elution property, which is convenient for their reuse.
1. Introduction
Molecular imprinting is a technology to elaborately set up recognition sites in a polymer matrix. A great deal of caves that are highly matched with the template molecule in size, shape and chemical functionality are distributed within molecularly imprinted polymers (MIPs). MIPs have specific molecular recognition ability and high binding affinity for template molecules. Therefore, MIPs are described as artificial antibodies or receptors.1–4 MIPs are regarded as a group of biomimetic substances. MIPs can specifically recognize and bind the template molecule on a molecular level from mixed systems. Such characteristics of MIPs let them have very important applications in many areas of science and technology. Especially, in the various areas involving the recognition, separation, purification, enrichment and removal of substances from mixtures, the study of MIPs has received an astonishing amount of attention5–9 and molecular imprinting solid-phase extraction (MISPE) technology has developed greatly.Phenolic acids are a large class of natural compounds with many bioactivities and pharmacological activities, whereas ferulic acid (4-hydroxy-3-methoxycinnamic acid) is considered as one of the most important phenolic acids. It belongs to the cinnamic acid-type or styrene-type of phenolic acids because there is a polymerizable double bond in its structure like that found in styrene. Ferulic acid (FA) exhibits beneficial physiological effects such as antioxidant, anti-microbial, anti-thrombosis and anti-cancer activities.10–13 Thus, it is widely used in food, pharmaceutical and cosmetic industries. Ferulic acid is present in many crop residues such as wheat bran (0.5%), sugar-beet pulp (0.9%) and corn kernel (5%).14,15 FA is cross-linked with lignin and polysaccharides via ester and ether bonds, forming lignin/phenolics–carbohydrate complexes in crop residues and can be released by alkaline hydrolysis with dilute NaOH solution so as to form the crude extracts. However, the separation and purification of FA from the alkaline extracts is challenging. The composition of the crude extracts is very complicated and therefore the separation of ferulic acid is very difficult. For the separation and purification of FA from the crude extracts, some solid adsorbents, such as active charcoal, macroporous resin and anion exchange resin, have been used.16–18 However, all these solid adsorbents have a common weakness, which is that their adsorptions are non-selective leading to low separation efficiency. To date, MIPs are the solid adsorption materials with the best adsorption selectivity and if MIPs are introduced into the extraction and separation of phenolic acids, the separation efficiency of these natural compounds will be improved greatly. Unfortunately, to date, FA-imprinted materials have not been reported.
The traditional method to prepare MIPs is via an entrapment strategy. The monomer, cross-linking agent and template molecule are dissolved in a solvent and via polymerization, monolithic cross-linked polymers are obtained. Then, by crushing, grounding and sieving, fine particles with an appropriate size are acquired so as to suit their specific use. During the crushing and grounding process, a great deal of the imprinted caves is destroyed; moreover, the matrix is thick and the pore canal is deep within the imprinted particles. Consequently, some serious drawbacks occur, such as small binding capacity, poor site accessibility, greater diffusion resistance and slow mass transfer, and these drawbacks severely limit the applications of MIPs.19–21 To overcome the above mentioned drawbacks, in recent years, molecule surface-imprinting technology has been developed greatly and researchers have tried to constitute imprinted caves on the surfaces of solid particles or membranes.22–24 Our group also contributed to the development of the surface-imprinting technique and a new surface imprinting technique of “pre-graft polymerization and post-cross-linking/imprinting” was proposed. Based on this method, various MIPs with high performance were successively prepared.25–28
As described above, there is a polymerizable double bond in the structure of FA, as shown in Scheme 1, and so for the molecule imprinting of FA, general polymerization methods cannot be adopted. This is an important reason why FA-imprinted polymers as well as the imprinted polymers of other compounds containing a polymerizable double bond are seldom reported to date. However, the “pre-graft polymerization and post-cross-linking/imprinting” method developed by our group is ideal for the molecular imprinting of FA and the difficulties of FA imprinting described above can be effectively overcome. In this study, we have used the new surface imprinting technique of “pre-graft polymerization and post-cross-linking/imprinting” to realize the imprinting of FA. The ternary amine monomer dimethylaminoethyl methacrylate (DMAEMA) was first graft-polymerized on the surface of micron-sized silica gel particles and then the grafted PDMAEMA macromolecule produced a strong adsorption action for FA via strong electrostatic interaction and cation–π interaction as well as hydrogen bonding, constituting a supramolecular system. Finally, using dibromohexane as cross-linker, FA imprinting was realized. That way, the problem that the polymerizable double bond in FA would take part in the polymerization during the imprinting process was completely avoided. Therefore, the FA imprinted material with high performance was prepared successfully, fully displaying the superiority of the new surface imprinting technique of “pre-graft polymerization and post-cross-linking/imprinting”. The imprinted material has excellent molecular recognition characteristics for FA and is a good material used for the solid phase extraction of FA from the crude extracts. To the best of our knowledge, this is the first report on the molecular imprinting of compounds containing a polymerizable double bond, and similar studies have not been reported to date.
The research results in this study have important scientific significance and application value for the preparation of the surface-imprinted materials of phenolic acid compounds with a styrene-type structure as well as other compounds, in whose molecules contain polymerizable double bonds, and it can effectively accelerate the extraction and separation of natural pharmacologic active substances from natural plant extracts.
2. Experimental
2.1. Materials and instruments
Silica (120–160 mesh, about 125 μm in diameter, and 300–450 m2 g−1 of surface area) was obtained from Qingdao Ocean Chemical Limited Company (China). γ-Aminopropyltrimethoxysilane (AMPS) was supplied by Nanking Chuangshi Chemical Aux Co., Ltd. (China). Dimethylaminoethylmethacrylate (DMAEMA) was purchased from Jiangsu Feixiang chemical Co., Ltd. (China), and purified by vacuum distillation before use. Ferulic acid, chlorogenic acid and caffeic acid were supplied by Shaanxi Luqing bio-engineering Limited company (China). Dibromohexane was obtained from Shanghai Zhixin Chemical Co., Ltd. (China). Glacial acetic acid (with a purity of 98%) and methanol were purchased from by Tientsin University Chemical Reagent Plant (China) and they were used to prepare a mixed solution in a volume ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 to be used as an eluent for the FA template molecule. Other reagents were all commercial chemicals of analytical purity and purchased from Chinese companies.
9 to be used as an eluent for the FA template molecule. Other reagents were all commercial chemicals of analytical purity and purchased from Chinese companies.
The instruments used in this study were as follows: Unic-2602 UV-vis spectrophotometer (Unic Company, Shanghai), Perkin-Elmer 1700 infrared spectrometer (Perkin-Elmer Company, USA), LEO-438VP scanning electronic microscope (SEM, LEO Company, UK), Zetasizer Nano-Zeta potential analyzer (Malvern Instrument Company, UK), PHS-2 acidimeter (The Second Analytical Instrument Factory, Shanghai, China), THZ-92C constant temperature shaker equipped with gas bath (Boxun Medical Treatment Equipment Factory, Shanghai, China) and STA449 thermogravimetry analyzer (TGA, Netzsch Company, German) using an air atmosphere at a heating rate of 10 °C min−1.
2.2. Preparation and characterization of PDMAEMA/SiO2 grafted particles
The PDMAEMA/SiO2 grafted particles were prepared using the surface-initiation method29 and the main procedure is described as follows: silica gel particles were surface-modified with the coupling agent AMPS to obtain the modified AMPS-SiO2 particles whose surface contained primary amino –NH2 groups. The modified AMPS-SiO2 particles were added into an aqueous solution containing the DMAEMA monomer, cross-linker and initiator (NH4)2S2O8 to give a surface initiating system of –NH2/S2O82−. Free radicals were produced on the surface of the modified AMPS-SiO2 particles and the graft-polymerization of DMAEMA carried out under an N2 atmosphere and at a constant temperature of 35 °C for 6 h, resulting in the grafted PDMAEMA/SiO2 particles. Their FTIR spectrum was determined using the KBr pellet method. The grafting degree of PDMAEMA was determined by TGA like that described in ref. 29 and it was 24 g/100 g. The zeta potentials of the grafted PDMAEMA/SiO2 particles at different pH values were recorded using a potential analyzer and the zeta potential curve was plotted.2.3. Preparation and characterization of FA surface-imprinted MIP-PDMAEMA/SiO2 material
About 0.2 g of the grafted PDMAEMA/SiO2 particles was weighted accurately and placed into an aqueous solution of FA at a concentration of 5 mmol L−1 and pH = 4. The adsorption of the grafted particles for FA was conducted in a constant temperature oscillator for 4 h and the adsorption was made to reach saturation. The resultant particles were collected by filtration and dried under vacuum. The resulting particles were placed into an aqueous solution of FA at a concentration of 5 mmol L−1 and pH = 4 to avoid desorption and 2.2 mL of bromohexane was added. The cross-linking reaction between the grafted PDMAEMA macromolecules was performed at 40 °C with stirring for 8 h. After finishing the reaction, the resultant particles were washed repeatedly with a mixed solution of acetic acid and methanol (v/v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) to remove the FA template molecules, then washed with distilled water and dried under vacuum. The obtained particles were namely FA surface-imprinted MIP-PDMAEMA/SiO2 particles. The FTIR of the imprinted particles was determined using the KBr pellet method to confirm the structural changes and their morphology was observed using SEM.
9) to remove the FA template molecules, then washed with distilled water and dried under vacuum. The obtained particles were namely FA surface-imprinted MIP-PDMAEMA/SiO2 particles. The FTIR of the imprinted particles was determined using the KBr pellet method to confirm the structural changes and their morphology was observed using SEM.
For comparison, non-imprinted NMIP-PDMAEMA/SiO2 particles were also prepared under the same conditions only in the absence of FA, namely, the grafted PDMAEMA macromolecules of the PDMAEMA/SiO2 particles that did not adsorb FA were cross-linked to obtain NMIP-PDMAEMA/SiO2 particles.
2.4. Investigating recognition and binding characteristics of the MIP-PDMAEMA/SiO2 particles for FA
 | (1) |
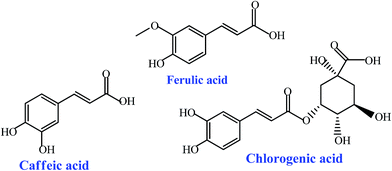
Chlorogenic acid (CHA) is a depside and can be formed using an esterification reaction between caffeic acid and quinate. CHA is also a natural compound with many biological activities and there exist similarities to a certain degree between the chemical structures of CHA and FA. Caffeic acid (CA) is also a phenolic acid with pharmacological activity and its chemical structure is very similar to that of FA. Therefore, in this study, CHA and CA were selected as two contrast substances to investigate the molecular recognition characteristics of the MIP-PDMAEMA/SiO2 particles for FA. The chemical structures of the three phenolic acid compounds are presented in Scheme 1. Using the same method described above, the isothermal binding experiments of the MIP-PDMAEMA/SiO2 particles for CHA and CA were performed and their isothermal binding curves were also plotted. The concentrations of CHA and CA in the supernatants were also determined by spectrophotometry. The characteristic absorption wavelengths were at 322 nm for CHA and at 286 nm for CA.
 | (2) |
The selectivity coefficient k of the MIP-PDMAEMA/SiO2 particles for FA relative to the two competition species, CHA and CA (assigned as B), can be obtained from the distribution coefficient data according to eqn (3).30,31 The value of k marks the recognition selectivity of MIP-PDMAEMA/SiO2 for the FA template and it is one of the most important parameters of imprinted materials.
 | (3) |
2.5. Desorption experiments
A given amount of the MIP-PDMAEMA/SiO2 particles on which the adsorption of FA had reached saturation were packed into a piece of glass pipe with an internal diameter of 10 mm. The bed volume (BV) of the packed column was 2 mL. A mixed solution of acetic acid and ethanol (v/v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) was used as the eluent and was allowed to flow upstream through the column at a rate of four bed volumes per hour (4BV per h), and the desorption of FA was allowed to occur. The effluents at one-bed volume intervals were collected and the concentrations of FA in these effluents were determined by spectrophotometry. The dynamic desorption curve was plotted and the elution properties of FA on the MIP-PDMAEMA/SiO2 particles were estimated.
9) was used as the eluent and was allowed to flow upstream through the column at a rate of four bed volumes per hour (4BV per h), and the desorption of FA was allowed to occur. The effluents at one-bed volume intervals were collected and the concentrations of FA in these effluents were determined by spectrophotometry. The dynamic desorption curve was plotted and the elution properties of FA on the MIP-PDMAEMA/SiO2 particles were estimated.
3. Results and discussion
3.1. The chemical structure of grafted PDMAEMA/SiO2 particles and their interactions with FA
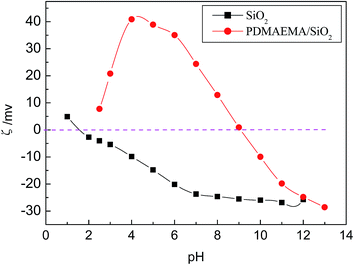
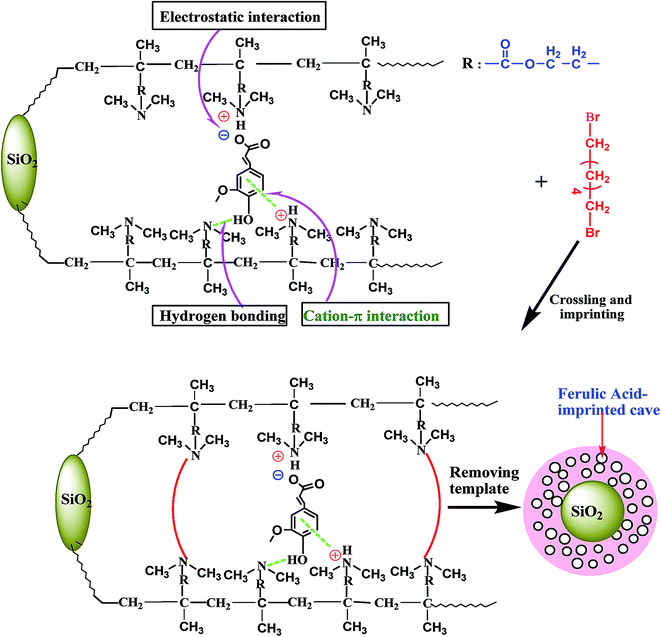
It can be observed from Fig. 2 that the adsorption ability of silica gel particles for FA is very poor or the silica gel particles nearly do not adsorb FA molecules. However, the grafted PDMAEMA/SiO2 particles have very strong adsorption ability for FA in an acidic solution and the adsorption capacity reaches up to 0.74 mmol g−1 (144 mg g−1), suggesting that there exist strong interactions between the grafted PDMAEMA/SiO2 particles and FA. In an acidic solution, the grafted PDMAEMA/SiO2 particles are negatively charged because of the protonation of the N atoms in the grafted PDMAEMA macromolecule as described above and indicated in Fig. 1. The carboxyl groups in FA can ionize basically in an acidic solution at pH = 4 (the ionization constant of cinnamic acid as the precursor of FA is in a range of pH 4.37–4.44 and it can be taken as a reference). Therefore, strong electrostatic interaction between the grafted PDMAEMA/SiO2 particles and FA is inevitably produced. Moreover, cation–π interaction between the benzene ring in FA and the protonated N atoms of the grafted PDMAEMA macromolecule will be also produced. Perhaps there is still hydrogen bond interaction between the phenolic hydroxyl group in FA and the ternary amino group in PDMAEMA. Clearly, there exists the synergy of the three interactions between the host–guest molecules. It is this synergy that leads to the very strong adsorption ability of the grafted particles for FA and this is the fundamental basis of imprinting FA on the surface of the grafted PDMAEMA/SiO2 particles. Among the three interactions, the electrostatic interaction is the main driving force. The interaction model of PDMAEMA/SiO2 with FA is schematically shown in Scheme 3. It is the strong secondary bond forces between the PDMAEMA/SiO2 particles and FA that lay a sufficient foundation for realizing the surface-imprinting of FA by adopting the novel surface imprinting method of “pre-graft polymerization and post-cross-linking/imprinting”.
To determine the optimum pH value for surface-imprinting, the isothermal adsorption experiments of the grafted PDMAEMA/SiO2 particles for FA at different pHs were conducted. Fig. 3 shows the saturated adsorption amount as function of pH.
It can be observed that the adsorption capacity of the grafted PDMAEMA/SiO2 particles for FA first increases and then decreases with the increase of pH value, and there is a maximum adsorption capacity at pH 4. This change rule reflects the variation of the interaction between the host–guest molecules with the pH of the medium. At a lower pH, the protonation degree of the N atoms in the grafted PDMAEMA macromolecule is high, whereas the ionization degree of the carboxyl groups in FA is low. With gradually increasing pH value of the medium, the ionization degree of the carboxyl groups of FA will increase, and it inevitably leads to the strengthening of the electrostatic interactions between the host–guest molecules, resulting in the increase of the adsorption capacity. However, at pH > 4, the protonation degree of the N atoms in the grafted PDMAEMA macromolecule becomes weaker and the electropositivity of the PDMAEMA/SiO2 particles begins to decrease, as shown in Fig. 1. Consequently, at pH > 4, the electrostatic interaction as well as cation–π interaction between the host–guest molecules begins to weaken with the increase of pH value, resulting in a decrease in the adsorption capacity. It is possible that there exist the strongest interactions between the host–guest molecules at pH = 4 and therefore pH = 4 was selected as the suitable pH value for the following surface-imprinting process.
3.2. Chemical process used to prepare imprinted MIP-PDMAEMA/SiO2 particles and their characterization
(1) Infrared spectra. Fig. 4 shows the infrared spectra of the three particles, SiO2, the grafted PDMAEMA/SiO2 particles and the imprinted MIP-PDMAEMA/SiO2 particles.
When compared with the spectrum of the SiO2 particles, in the spectrum of the grafted PDMAEMA/SiO2 particles, three new absorption bands appear at 1732, 1395 and 2960 cm−1. As described above, the first two bands belong to the characteristic vibration absorptions of the carbonyl group of the carboxyl group and the C–N bond of the ternary amino group in the grafted PDMAEMA macromolecule, and the last is attributed to the stretching vibration absorption of the methyl –CH3 group and methylene –CH2– group in the grafted PDMAEMA macromolecule, indicating the formation of the grafted PDMAEMA/SiO2 particles. By comparing the spectrum of the MIP-PDMAEMA/SiO2 particles with that of PDMAEMA/SiO2, the following facts can be found. The characteristic absorption of the C–N–C bond in the quaternary ammonium structure appears at 2720 cm−1 and the characteristic vibration absorption of the C–N bond in the ternary amino group has disappeared. Moreover, the absorption of the methylene groups at 2927 and 2853 cm−1 were strengthened greatly because of the formation of the crosslinking linkage of dibromohexane. This spectral data demonstrates that the quaterisation reaction between the grafted PDMAEMA macromolecule and dibromohexane has occurred and the imprinting of FA has been realized.
It needs to be pointed out that all the various absorption bands of PDMAEMA/SiO2 and MIP-PDMAEMA/SiO2 look very weak. The reason for this is that the absorption background of SiO2 is very strong and therefore it negatively affects the absorption bands of PDMAEMA/SiO2 and MIP-PDMAEMA/SiO2.
(2) SEM imaging. Fig. 5(A) and (B) present the SEM images of the raw silica gel particles and the imprinted MIP-PDMAEMA/SiO2 particles. It can be clearly observed that the surface of the raw silica gel particles was rough and scraggy, whereas the surface of the MIP-PDMAEMA/SiO2 particles was smoother and flatter. This was caused by the filling up action of the grafted and cross-linked macromolecules.
3.3. Recognition and binding characteristics of imprinted MIP-PDMAEMA/SiO2 particles for FA
 | ||
| Fig. 6 Adsorption isotherms of NMIP-PDMAEMA/SiO2 particles for FA, CHA and CA. Temperature: 25 °C; pH = 4. | ||
It can be observed that for all the three phenolic acid compounds, the non-imprinted particles NMIP-PDMAEMA/SiO2 have a high adsorption capacity, which is in the range of 0.70–0.74 mmol g−1, and therefore the NMIP-PDMAEMA/SiO2 particles have no adsorption selectivity. The reason for this lies in that there also exist the synergy of the three interactions, electrostatic interactions, cation–π interactions and hydrogen bonding, between the non-imprinted NMIP-PDMAEMA/SiO2 particles and the three phenolic acid compounds, leading to a high adsorption capacity.
However, Fig. 7 displays that the binding isotherm of FA on the imprinted MIP-PDMAEMA/SiO2 particles is greatly different from that of CHA and CA. The binding capacity of the MIP-PDMAEMA/SiO2 particles for FA still remains high (0.73 mmol g−1, 142 mg g−1). However, the binding capacities of CHA and CA decreases dramatically, decreasing to 0.19 mmol g−1 for CHA and 0.31 mmol g−1 for CA. Therefore, the experimental results fully displays that the imprinted MIP-PDMAEMA/SiO2 particles possess specific recognition selectivity and an excellent binding affinity for FA, whereas their recognition and binding ability for CHA and CA are very poor (especially for CHA) or the imprinted particles nearly do not recognize and bind CHA.
The reason for the above mentioned fact can be explained as follows: for the MIP-PDMAEMA/SiO2 particles, a great quantity of the imprinted caves of the FA molecule is distributed within the thin polymer layer on the surface of the silica gel particles. These caves are highly matched with FA in the size, shape and spatial arrangement of the action sites, and it leads to the specific recognition ability and strong binding action of the MIP-PDMAEMA/SiO2 particles towards FA. However, it can be observed from Scheme 1 that although the carboxyl group and phenol structure are also contained in the CHA molecule, the size of the CHA molecule is larger than that of FA, and there is a certain difference in the spatial structure between the CHA and CA molecules. Therefore, the FA-imprinted caves are unmatched with the CHA molecules in the size, shape and spatial arrangement of the action sites, the CHA molecules are difficult to enter into these caves or these imprinted caves, and for these caves it is difficult to accept and bind the CHA molecules, leading to the very low binding capacity of the MIP-PDMAEMA/SiO2 particles for CHA. For CA, although its molecular structure is close to that of FA and its molecule size is smaller than that of FA, there is still difference in the substituent groups on the phenol ring. This difference affects the matching in steric configuration and limits the binding ability of the imprinted caves for CA. The binding ability of the MIP-PDMAEMA/SiO2 particles for CA is higher than that for CHA because of the greater similarity between the molecular structures of CA and FA, but it is still much lower than that found for the FA template molecule.
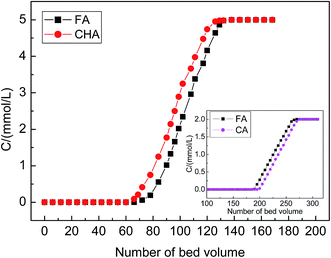
Fig. 8 displays that as the solutions of FA and CHA with the same concentration (5 mmol L−1) flows upstream through the column packed with the non-imprinted NMIP-PDMAEMA/SiO2 particles, both their leaking volumes are greater and are close to each other. They were determined to be 51BV for CHA and 54BV for CA. The results of the dynamic adsorption experiments once again confirms that the non-imprinted NMIP-PDMAEMA/SiO2 particles have no adsorption selectivity.
However, it can be observed clearly that for the column packed with the imprinted MIP-PDMAEMA/SiO2 particles, the leaking curve of CHA is clearly different from that of FA. The leaking volume of CHA is only 12BV, and this implies that once the solution of CHA goes on the column, it leaks rapidly. It shows that the column packed with the imprinted MIP-PDMAEMA/SiO2 material basically does not recognize and does not bind the contrast substance CAH. However, the leaking volume of the FA template still remains high (54BV) and is much higher than that of CAH. Clearly, the column packed with the imprinted MIP-PDMAEMA/SiO2 particles exhibits special recognition selectivity and an excellent binding affinity. The reason for this still lies in that a great deal of the FA-imprinted caves is distributed within the thin polymer layer on the surface of the MIP-PDMAEMA/SiO2 particles and that these imprinted caves are highly matched with FA and unmatched with CHA in the size, shape (steric configuration) and action sites. The calculated leaking and saturated adsorption amounts of CHA are 0.12 mmol g−1 and 0.18 mmol g−1, respectively, whereas the leaking and saturated adsorption amounts of FA reach up to 0.54 mmol g−1 and 0.74 mmol g−1, respectively.
The dynamic adsorption curves of NMIP-PDMAEMA/SiO2 and the dynamic binding curves (or leaking curves) of MIP-PDMAEMA/SiO2 for FA and CA are also presented in Fig. 8 and 9 as inserted diagrams, respectively. Fig. 8 displays that for the NMIP-PDMAEMA/SiO2 particles-packed column, the leaking volumes of FA and CA are also close to each other (190BV for FA and 195BV for CA), indicating that the non-imprinted NMIP-PDMAEMA/SiO2 particles have no adsorption selectivity. However, Fig. 9 shows that for the MIP-PDMAEMA/SiO2 particles-packed column, the leaking curve of CA is different from that of FA. The leaking volume of CA is only 75BV and that of the FA template still remains high (195BV), exhibiting the special recognition selectivity of the MIP-PDMAEMA/SiO2 particles-packed column for FA.
| Adsorbing material | PDMAEMA/SiO2 | MIP-PDMAEMA/SiO2 | ||
| Adsorbate | FA | CHA | FA | CHA |
| Kd/(L g−1) | 0.397 | 0.337 | 0.398 | 0.0615 |
| k | 1.178 | 6.47 | ||
| Adsorbing material | PDMAEMA/SiO2 | MIP-PDMAEMA/SiO2 | ||
| Adsorbate | FA | CA | FA | CA |
| Kd/(L g−1) | 0.735 | 0.778 | 0.736 | 0.267 |
| k | 0.946 | 2.752 | ||
The following facts can be found from the data shown in Tables 1 and 2. (1) As the competitive adsorption experiments were carried out in the binary mixed solutions using the non-imprinted NMIP-PDMAEMA/SiO2 particles, each component has strong competitive adsorption ability so that both the selectivity coefficients of the NMIP-PDMAEMA/SiO2 particles for FA relative to CHA (1.18) and relative to CA (0.95) are close to 1. Clearly, there is no recognition selectivity. (2) As the competitive adsorption experiments were carried out using the imprinted MIP-PDMAEMA/SiO2 particles, the selectivity coefficients of the MIP-PDMAEMA/SiO2 particles for FA relative to CHA and relative to CA are 6.47 and 2.75, respectively, displaying that the MIP-PDMAEMA/SiO2 particles possess recognition selectivity, especially relative to CHA. The high selectivity coefficient (6.47) shows the molecule recognition specificity of the MIP-PDMAEMA/SiO2 particles.
The molecule recognition specificity of the imprinted materials is very important in the separation of the target substance from the crude plant extracts. In general, the crude extracts of phenolic acids contain multiple components. If the FA-imprinted material is used as solid absorbent and the solid phase extraction is performed, the MIP-PDMAEMA/SiO2 particles will specifically recognize and bind FA in the crude extract. It can be imagined that if multiple columns packed with MIP-PDMAEMA/SiO2 particles are combined, the effective separation and purification of FA can be finally realized. In a word, by adopting the method of “pre-graft polymerization and post-cross-linking/imprinting”, the surface imprinted materials of styrene-type phenolic acid compounds with high performance can be prepared. By taking advantage of the special recognition selectivity and excellent binding properties of these imprinted materials for the target substance, it can be expected that the target pharmacologic activity constituents will be effectively separated from crude extracts via molecularly imprinted solid-phase extraction.
3.4. Effect of the amount of crosslinker used on recognition selectivity of the MIP-PDMAEMA/SiO2 particles
In the traditional imprinting method, the suitable amount of cross-linker used needs to be determined by experiments. However, in the new surface imprinting method of “pre-graft polymerization and post-cross-linking/imprinting”, the suitable amount of cross-linker used can be estimated basically from the binding model of the grafted macromolecule with the template molecule and from the number of average combination action sites of the grafted macromolecule towards the template molecule.32 It can be observed from Scheme 3 that three ternary ammonium groups in the grafted PDMAEMA macromolecule interact with one FA molecule via electrostatic interaction and cation–π interaction as well as hydrogen bonding, namely, three chain units interact with one FA molecule. Moreover, for one imprinted cave, the cross-linking action needs to spend four chain units of the grafted PDMAEMA macromolecule. Therefore, for imprinting one FA molecule, seven chain units of the grafted PDMAEMA macromolecule and two dibromohexane molecules as the cross-linker are needed. As a result, a suitable ratio of the chain unit of the grafted PDMAEMA macromolecule to the cross-linker can be estimated and it should be equal to 3.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
The FA molecule surface-imprinting was conducted with different amounts of the cross-linking agent used (dibromohexane) by fixing the other reaction conditions. Fig. 10 gives the selectivity coefficient of the MIP-PDMAEMA/SiO2 particles for FA relative to CHA as a function of the amounts of dibromohexane used (it is expressed as the molar ratio of the chain unit of the grafted PDMAEMA macromolecule on the PDMAEMA/SiO2 particles to dibromohexane).
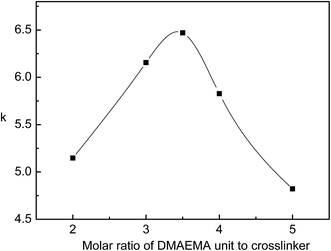 | ||
| Fig. 10 Selectivity coefficient as a function of molar ratio of chain unit of PDMAEMA to cross-linker. Temperature: 25 °C; pH = 4. | ||
It can be observed from Fig. 10 that the selectivity coefficient of the MIP-PDMAEMA/SiO2 particles first increases and then decreases with the increase of the molar ratio, and there is a maximum selectivity coefficient of 6.47 when the ratio is 3.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, implying that with this ratio, the imprinting effect is the best. Clearly, the pre-estimated result from the binding model between the host–guest molecules is in good agreement with the experimental value. The above mentioned facts show that the new surface imprinting method of “pre-graft polymerization and post-cross-linking/imprinting” is greatly different from the conventional imprinting method and in this new surface imprinting system there is a basic characteristic of “feed quantifying”, and the most suitable amount of the cross-linker used can be pre-estimated.
1, implying that with this ratio, the imprinting effect is the best. Clearly, the pre-estimated result from the binding model between the host–guest molecules is in good agreement with the experimental value. The above mentioned facts show that the new surface imprinting method of “pre-graft polymerization and post-cross-linking/imprinting” is greatly different from the conventional imprinting method and in this new surface imprinting system there is a basic characteristic of “feed quantifying”, and the most suitable amount of the cross-linker used can be pre-estimated.
3.5. Elution properties of MIP-PDMAEMA/SiO2 particles
The elution experiment of the MIP-PDMAEMA/SiO2 particles was conducted to estimate their elution properties. A mixed solution of acetic acid/methanol (v/v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) was used as the eluent and it was allowed to pass upstream through a column packed with the MIP-PDMAEMA/SiO2 particles, which had adsorbed FA in a saturated state. Fig. 11 shows the dynamic elution curve.
9) was used as the eluent and it was allowed to pass upstream through a column packed with the MIP-PDMAEMA/SiO2 particles, which had adsorbed FA in a saturated state. Fig. 11 shows the dynamic elution curve.
Fig. 11 indicates that the elution curve is cuspidal and without tailing phenomenon. The desorption ratios in 16BV and 18BV reach 98.9% and 99.6%, respectively, indicating that the FA molecules combined on the MIP-PDMAEMA/SiO2 particles are easily washed off and the surface-imprinted material has excellent elution properties. The reason is that the FA molecule-imprinted caves are distributed within the thin polymer layer on the surface of the MIP-PDMAEMA/SiO2 particles. And there is a small diffusion resistance for the template molecules so that the bound FA molecules are easily eluted.
4. Conclusions
PDMAEMA macromolecules were first grafted on micro-sized silica gel particles by adopting a surface initiated graft-polymerization method, obtaining the grafted PDMAEMA/SiO2 particles. In an acidic solution, there are strong interactions between the grafted PDMAEMA/SiO2 particles and FA involving electrostatic interaction and cation–π interaction as well as hydrogen bonding. Therefore, the grafted PDMAEMA/SiO2 particles can produce a strong adsorption for FA. On this basis, FA surface imprinting was performed smoothly using the new surface imprinting method of “pre-graft polymerization and post-crosslinking/imprinting”, obtaining the FA imprinted MIP-PDMAEMA/SiO2 particles. In the meantime, the issue of the polymerizable double bond taking part in the polymerization is completely avoided. The imprinted MIP-PDMAEMA/SiO2 particles have a specific recognition selectivity and excellent binding affinity for FA. It can be expected that MIP-PDMAEMA/SiO2 can be used as solid adsorbent with high performance and FA can be separated from the crude plant extract using the molecularly imprinted solid-phase extraction method.References
- D. R. Kryscio and N. A. Peppas, Acta Biomaterialia, 2012, 8(2), 461–473 CrossRef CAS PubMed.
- B. B. Prasad, P. S. Sharma and D. Lakshmi, J. Chromatogr. A, 2007, 1173, 18–26 CrossRef CAS PubMed.
- S. M. Ng and R. Narayanaswamy, Sens. Actuators, B, 2009, 139(1), 156–165 CrossRef CAS.
- N. Karimian, A. P. F. Turner and A. Tiwari, Biosens. Bioelectron., 2014, 59, 160–165 CrossRef CAS PubMed.
- I. Bakas, A. Hayat, S. Piletsky, E. Piletska, M. M. Chehimi, T. Noguer and R. Rouillon, Talanta, 2014, 13, 294–298 CrossRef PubMed.
- H. A. Akdamar, N. Y. Sarıözlü, A. A. Özcan, A. Ersöz, A. Denizli and R. Say, Mater. Sci. Eng., C, 2009, 29, 1404–1408 CrossRef CAS.
- L. Uzun, R. Say, S. ünal and A. Denizli, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2009, 877, 181–188 CrossRef CAS PubMed.
- Y. Yuan, Y.-Z. Wang, M.-d Huang, R. Xu, H. Zeng, C. Nie and J.-H. Kong, Anal. Chim. Acta, 2011, 695, 63–72 CrossRef CAS PubMed.
- Y. Lin, Y. Shi, M. Jiang, Y. Jin, Y. Peng, B. Lu and K. Dai, Environ. Pollut., 2008, 153, 483–491 CrossRef CAS PubMed.
- N. Du, S.-W. Cao and Y.-Y. Yu, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2011, 879, 1697–1703 CrossRef CAS PubMed.
- S. Srivastava, A. P. Singh and A. K. S. Rawat, Asian Pac. J. Trop. Biomed., 2012, S12–S14 CrossRef.
- S.-Y. Ou, Y.-L. Luo, F. Xue, C.-H. Huang, N. Zhang and Z.-L. Liu, J. Food Eng., 2007, 78, 1298–1304 CrossRef CAS.
- B. Max, A. M. Torrado, A. B. Moldes, A. Converti and J. M. Domínguez, Biochem. Eng. J., 2009, 43, 129–134 CrossRef CAS.
- A. U. Buranov and G. Mazza, Food Chem., 2009, 115, 1542–1548 CrossRef CAS.
- J. M. Salgado, B. Max, R. Rodríguez-Solana and J. M. Domínguez, Ind. Crops Prod., 2012, 39, 52–61 CrossRef CAS.
- M. L. Soto, A. Moure, H. Domínguez and J. C. Parajó, J. Food Eng., 2011, 105, 1–27 CrossRef CAS.
- N. E. Dávila-Guzman, F. J. Cerino-Córdova, P. E. Diaz-Flores, J. R. Rangel-Mendez, M. N. Sánchez-González and E. Soto-Regalado, Chem. Eng. J., 2012, 183, 112–116 CrossRef.
- S.-Y. Ou, Y.-L. Luo, F. Xue, C.-H. Huang, N. Zhang and Z.-L. Liu, J. Food Eng., 2007, 78, 1298–1304 CrossRef CAS.
- Y.-Z. Yang, X.-G. Liu, M.-C. Guo, S. Li, W.-F. Liu and B.-S. Xu, Colloids Surf., A, 2011, 377, 379–385 CrossRef CAS.
- T. E. Milj, K. P. Prathish and T. P. Rao, J. Hazard. Mater., 2011, 188, 384–390 CrossRef PubMed.
- X.-Y. Gong and X.-J. Cao, J. Biotechnol., 2011, 153, 8–14 CrossRef CAS PubMed.
- G.-F. Zhu, J. Fan, Y.-B. Gao, X. Gao and J.-N. Wang, Talanta, 2011, 84, 1124–1132 CrossRef CAS PubMed.
- H.-M. Liu, C.-H. Liu, X.-J. Yang, S.-J. Zeng, Y.-Q. Xiong and W.-J. Xu, Anal. Chim. Acta, 2008, 628, 87–94 CrossRef CAS.
- V. Kochkodan, N. Hilal, V. Melnikc, O. Kochkodan and A. Remizovska, J. Membr. Sci., 2011, 377, 151–158 CrossRef CAS.
- B.-J. Gao, J.-H. Lu, Z.-P. Chen and J.-F. Guo, Polymer, 2009, 50, 3275–3284 CrossRef CAS.
- B.-J. Gao, Y.-X. Chen and J.-Y. Men, J. Chromatogr. A, 2011, 1218, 5441–5448 CrossRef CAS PubMed.
- B.-J. Gao, X.-H. Wang and Y.-Y. Zhang, Mater. Chem. Phys., 2013, 140, 478–486 CrossRef CAS.
- B.-J. Gao, C.-C. Bi and L. Fan, Appl. Surf. Sci., 2015, 332, 430–439 CrossRef CAS.
- B.-J. Gao, N. Shi and X.-J. Shi, J. Polym. Res., 2013, 20, 260–267 CrossRef.
- A. Ersöz, R. Say and A. Denizli, Anal. Chim. Acta, 2004, 502, 91–97 CrossRef.
- R. Say, A. Ersöz, H. Türk and A. Denizli, Sep. Purif. Technol., 2004, 40, 9–14 CrossRef CAS.
- B.-J. Gao, J. Wang and Y. Yang, Chromatographia, 2009, 69, 1353–1361 CAS.
| This journal is © The Royal Society of Chemistry 2016 |