Synthesis and biological evaluation of novel asiatic acid derivatives with anticancer activity†
Bruno M. F. Gonçalvesa,
Jorge A. R. Salvador*ab,
Silvia Maríncd and
Marta Cascante*cd
aLaboratory of Pharmaceutical Chemistry, Faculty of Pharmacy, University of Coimbra, 3000-548, Coimbra, Portugal. E-mail: salvador@ci.uc.pt; Fax: +351 239 488 503; Tel: +351 239 488 400
bCenter for Neuroscience and Cell Biology, Coimbra, Portugal
cDepartment of Biochemistry and Molecular Biology, Faculty of Biology, University of Barcelona, Diagonal 643, 08028 Barcelona, Spain. E-mail: martacascante@ub.edu; Tel: +34-934021593
dInstitute of Biomedicine of University of Barcelona (IBUB) and Associated Unit to CSIC, Barcelona, Spain
First published on 18th December 2015
Abstract
Asiatic acid (AA) is a pentacyclic triterpenoid with recognized anticancer properties. Structural modification of AA may afford derivatives with improved anticancer potency. Hence, in this paper, a series of new lactol and A-nor AA derivatives were prepared, and their antiproliferative activities against several human cancer cell lines and a non-tumoral fibroblast cell line (BJ) were tested. Among all the derivatives tested, compound 24 proved to be the most active compound, with IC50 values ranging from 0.11 μM to 0.65 μM for cancer cell lines. The molecular mechanisms underlying its antiproliferative activity were further investigated using the HeLa cell line. Our results showed that treatment of HeLa cells with compound 24 led to cell-cycle arrest at the G0/G1 phase, which was associated with an upregulation of p21cip1/waf1 and p27kip1 and a downregulation of cyclin D3. Moreover, compound 24 induced apoptotic HeLa cell death via the activation of caspase-9, caspase-8, and caspase-3. The downregulation of the Bcl-2 and Bid proteins and the upregulation of the Bax protein suggest that the mitochondrial pathway is activated during the apoptotic process.
1. Introduction
Despite recent advances in cancer therapy, this complex disease remains one of the leading causes of death worldwide.1,2 To solve this public health problem, research efforts toward the development of new therapeutic strategies and more effective anticancer drugs have been made.Pentacyclic triterpenoids (PTs) are a large class of natural products that are widely distributed in nature and have a vast range of unique biological activities.3–5 In the last decades, the anticancer and anti-inflammatory activities of PTs and their semi-synthetic derivatives have been extensively studied, and some papers support the contention that these compounds are ideal candidates for the development of new anticancer therapies.4,6–11 Asiatic acid (AA) (1) (Fig. 1) is a PT that is mainly extracted from Centella asiatica that, in addition to other important pharmacological activities, shows promising anticancer effects. The mechanisms underlying the anticancer effect of AA (1) include cell-cycle arrest,12 strong antiangiogenic activity,13 inhibition of cancer cell proliferation, and induction of apoptosis in several cancer cell lines.13–16
Apoptotic cell death induced by AA (1) has been reported to be mediated by alterations in calcium homeostasis in the HepG2, PCC-1, and U87-MG cancer cell lines;14,16,17 activation of the mitochondrial pathway in SW480 human colon cancer cells;18 and generation of reactive oxygen species (ROS), upregulation of BAX, and activation of caspase-3 in SK-MEL-2 human melanoma cells.15
Because of its promising anticancer activity, low toxicity, and commercial availability, AA (1) has been receiving increased attention from scientists who aim for the development of new anticancer drugs. Several groups performed structural modifications of the AA (1) backbone; some of the semi-synthetic derivatives obtained displayed improved antiproliferative activity in several cancer cell lines compared with AA (1).19–22 However, further investigation is needed to develop and synthesize new AA (1) derivatives as anticancer agents.
Bhagirath Sing and coworkers reported the conversion of the hexameric ring A of AA (1) into a pentameric ring containing an α,β-unsaturated carbonyl group.23 It has been well established in several studies that the presence of an α,β-unsaturated carbonyl group in the A-ring of PTs significantly enhances their biological activities.4,24–26 However, the transformations performed in the A ring of AA (1) were not thoroughly explored, which encouraged us to develop and synthesize a series of new AA (1) derivatives containing a 5-carbon ring A with an α,β-unsaturated carbonyl moiety, combined with additional modifications at C-23, C-11, and C-28, to obtain AA (1) derivatives with improved anticancer activity.
In addition, the nitrile group has been gaining great importance in the design and development of new drugs27,28, because its presence in organic molecules plays several biologically important roles.27–32 This functional group could work as a hydroxyl and carboxyl surrogate, as nitrile is a strong hydrogen acceptor.27 Taking this into account, we also designed and prepared a series of pentameric A-ring AA (1) derivatives containing a nitrile group.
The in vitro antiproliferative activities of the newly synthesized derivatives against the MCF-7, HT-29, and HeLa cell lines were tested, and a structure–activity relationship (SAR) was established. Most of the new derivatives showed improved cytotoxic activities compared with AA (1). Compound 24 exhibited the best antiproliferative profile among all new derivatives and was selected for further experiments aimed at exploring its mechanism of action in HeLa cells.
2. Results and discussion
2.1 Chemistry
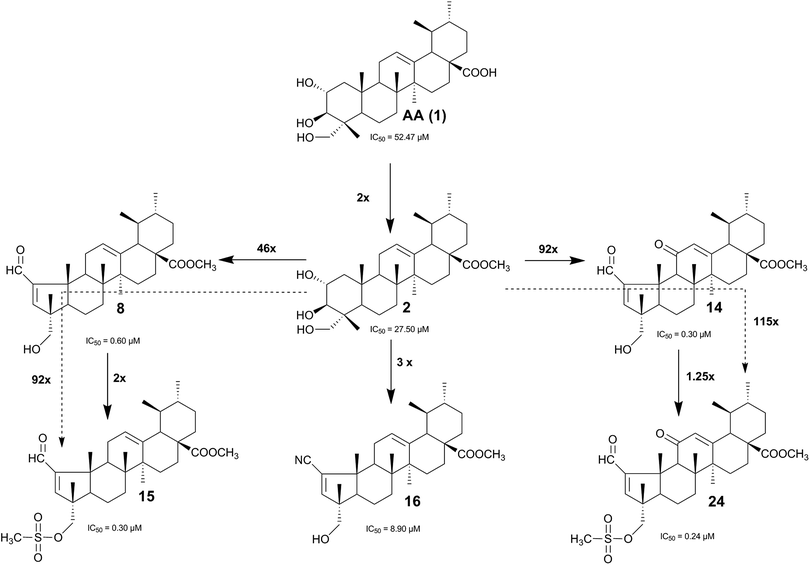
A series of new AA (1) derivatives containing a pentameric ring A were synthesized and their structures were fully elucidated. The synthesis started by the treatment of AA (1) with anhydrous potassium carbonate (K2CO3) and methyl iodide or ethyl iodide in DMF, to give the methyl derivative 2 and the ethyl derivative 3, respectively, in almost quantitative yields (Scheme 1). The lactol derivatives 4, 5, and 6 were obtained in good yield via the reaction of compounds 1, 2, and 3, respectively, with sodium periodate (NaIO4) in methanol/water.23 Further treatment of compounds 4, 5, and 6 with catalytic amounts of piperidine and acetic acid in benzene at 60 °C afforded the pentameric A-ring derivatives 7, 8, and 9, respectively.23The proton of the aldehyde group of the lactol derivatives 4–6 appeared as a singlet at δ = 9.94 ppm on 1H NMR spectra, and the 13C NMR signal for the CHO carbon was observed around δ = 206 ppm. In the 1H NMR spectra of compounds 7–9, the signals of the aldehyde and olefinic protons in the A ring were consistently observed as two singlet signals at δ = 9.72 ppm and δ = 6.66 ppm, respectively. The 13C NMR signal for the CHO carbon was observed at δ = 190.81–190.85 ppm, whereas the signals for the carbons of the A ring double bond appeared at δ = 159.24–159.29 ppm and δ = 158.90–159.95 ppm. The characteristic IR bands for the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C
O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibrations of the α,β-unsaturated aldehydes were observed around 1689 cm−1 and 1581 cm−1, respectively.
C stretching vibrations of the α,β-unsaturated aldehydes were observed around 1689 cm−1 and 1581 cm−1, respectively.
To study the impact of the introduction of a carbonyl group at C-11 on the anticancer activity, we decided to synthesize several AA (1) derivatives with an α,β-unsaturated ketone in the C ring. As shown in Scheme 2, the reaction of compound 2 with acetic anhydride in the presence of DMAP in THF at room temperature gave compound 10, which was further oxidized with a mixture of potassium permanganate and iron sulfate, to afford the 11-oxo-12-en derivative 11 in 95% yield.33,34 Compound 11 was then deacetylated with potassium hydroxide (KOH) in methanol, to afford intermediate 12. The lactol derivative 13 was prepared by reaction of compound 12 with sodium periodate (NaIO4) in methanol/water at room temperature. The α,β-unsaturated aldehyde 14 was obtained from 13 using the procedure that was described previously for the preparation of compounds 7–9.
Taking into consideration that the nitrile group can act as a carbonyl bioisostere,27 and considering its pharmaceutical importance, we prepared a panel of AA (1) nitrile derivatives. The treatment of compounds 7, 8, and 14 with iodine and 25% aqueous ammonium solution in THF at room temperature35 afforded the derivatives 17, 16, and 18, respectively, which have an α,β-unsaturated nitrile group in the pentameric A ring (Scheme 3). The direct conversion of aldehyde into nitrile was confirmed by the specific IR absorption band for CN stretching vibration observed at 2215.8–2217.7 cm−1 and by the 13C NMR signal for the CN carbon observed around δ = 117 ppm. Moreover, no signal corresponding to the proton of the CHO group was observed on the 1H NMR spectra. These structural data were consistent for all derivatives that had a pentameric A ring with an α,β-unsaturated nitrile (16–18 and 25–31).
 | ||
| Scheme 3 Synthesis of asiatic acid derivatives 15–18. Reagents and conditions: (a) methanesulfonyl chloride, triethylamine, dry CH2Cl2, r.t. (b) I2, aq. NH3, THF, r.t. | ||
In addition, we decided to investigate the influence of C-23 hydroxyl substitution on the anticancer activity of compounds 14 and 16. Thus, starting from these two compounds, we prepared a panel of C-23-substituted ester, carbamate, and mesylate derivatives (Schemes 4 and 5). The ester derivatives 19, 20, and 25–28 were prepared in moderate-to-good yields via the reaction of compound 14 or compound 16 with the corresponding anhydrides in the presence of DMAP at room temperature. The treatment of 14 or 16 with cinnamoyl chloride and DMAP in dry benzene at 60 °C afforded the 23-cinnamic ester derivatives 21 and 29, respectively. As shown in Scheme 4, compound 14 was treated with CDI or CBMI in THF at reflux, to give the carbamate derivatives 22 and 23 in 12% and 33% yield, respectively, after FCC. Derivative 30 was prepared in 59% isolated yield via the reaction of 16 with CBMI in THF at 70 °C (Scheme 5). The successful preparation of the carbamate derivatives 22, 23, and 30 was confirmed by the IR band observed at 1747–1764 cm−1, corresponding to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration, and by the 13C NMR signal for the carbamate carbonyl carbon observed at 148.56–149.47 ppm. In addition, in the 1H NMR spectra of compounds 23 and 30, the two specific hydrogen atoms of the methyl imidazole moiety appeared as two peaks at 7.27–7.28 ppm and 6.84–6.87 ppm. In the case of the derivative 22, the imidazole protons appeared as three singlets at 8.09, 7.30, and 7.06 ppm (ref. 36 and 37) on the 1H NMR spectra.
O stretching vibration, and by the 13C NMR signal for the carbamate carbonyl carbon observed at 148.56–149.47 ppm. In addition, in the 1H NMR spectra of compounds 23 and 30, the two specific hydrogen atoms of the methyl imidazole moiety appeared as two peaks at 7.27–7.28 ppm and 6.84–6.87 ppm. In the case of the derivative 22, the imidazole protons appeared as three singlets at 8.09, 7.30, and 7.06 ppm (ref. 36 and 37) on the 1H NMR spectra.
Finally, the 23-methanesulfonyloxy derivatives 15, 24, and 31 were obtained in moderate yields by treatment of compounds 8, 14, and 16, respectively, with methanesulfonyl chloride and triethylamine in dry dichloromethane at room temperature (Schemes 3–5). The successful preparation of compounds 15, 24, and 31 was confirmed by the characteristic IR absorptions for the asymmetric and symmetric S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O vibrations observed around 1355–1359 cm−1 and 1174–1176 cm−1. In the 1H NMR spectra, the protons from the mesylate methyl group were observed as a singlet at 3.01–3.03 ppm.
O vibrations observed around 1355–1359 cm−1 and 1174–1176 cm−1. In the 1H NMR spectra, the protons from the mesylate methyl group were observed as a singlet at 3.01–3.03 ppm.
2.2 Biological activities
The analysis of IC50 values (Table 1) revealed that the great majority of the new derivatives showed better antiproliferative activities than AA (1) against the tested cell lines. These new derivatives were particularly active against the HeLa cell line, with the exception of compound 29; therefore, their antiproliferative activities against HeLa cells were used to establish a SAR (Fig. 2).
| Compound | Cell linea/IC50 (μM) | ||
|---|---|---|---|
| MCF-7 | HT-29 | HeLa | |
| a The cell lines were treated with increasing concentrations of each compound for 72 h. IC50 values were determined by MTT assay and are expressed as means ± SD (standard deviation) of three independent experiments. N.D. not determined. IC50 is the concentration of compound that inhibits 50% of cell growth. | |||
| Asiatic acid (1) | 68.50 ± 2.12 | 64.33 ± 3.21 | 52.47 ± 0.06 |
| 2 | N.D. | N.D. | 27.50 ± 2.50 |
| 3 | N.D. | N.D. | 20.67 ± 1.53 |
| 4 | N.D. | N.D. | 21.67 ± 1.04 |
| 5 | N.D. | N.D. | 4.70 ± 0.40 |
| 6 | 4.00 ± 0.02 | 5.70 ± 0.46 | 4.75 ± 0.21 |
| 7 | N.D. | N.D. | 5.30 ± 0.2 |
| 8 | N.D. | N.D. | 0.60 ± 0.07 |
| 9 | 0.70 ± 0.05 | 0.64 ± 0.05 | 0.51 ± 0.03 |
| 10 | N.D. | N.D. | 0.60 ± 0.04 |
| 11 | N.D. | N.D. | 3.13 ± 0.32 |
| 12 | N.D. | N.D. | 37.17 ± 2.57 |
| 13 | 12.03 ± 0.40 | 12.20 ± 0.26 | 8.48 ± 1.31 |
| 14 | 0.74 ± 0.05 | 0.59 ± 0.04 | 0.30 ± 0.02 |
| 15 | 0.47 ± 0.02 | 0.45 ± 0.05 | 0.30 ± 0.02 |
| 16 | 14.33 ± 1.53 | 10.27 ± 1.55 | 8.90 ± 0.53 |
| 17 | >60 | >60 | 43.17 ± 3.55 |
| 18 | 16.10 ± 1.40 | 17.00 ± 1.32 | 14.60 ± 1.65 |
| 19 | 1.02 ± 0.11 | 0.97 ± 0.09 | 0.60 ± 0.05 |
| 20 | 0.88 ± 0.07 | 0.77 ± 0.03 | 0.49 ± 0.00 |
| 21 | 1.03 ± 0.04 | 0.84 ± 0.05 | 0.53 ± 0.01 |
| 22 | 1.20 ± 0.05 | 0.73 ± 0.02 | 0.53 ± 0.03 |
| 23 | 0.98 ± 0.02 | 0.66 ± 0.05 | 0.54 ± 0.02 |
| 24 | 0.65 ± 0.04 | 0.59 ± 0.02 | 0.24 ± 0.02 |
| 25 | 11.50 ± 1.32 | 12.20 ± 1.04 | 10.75 ± 0.35 |
| 26 | >30 | >60 | 12.00 ± 1.41 |
| 27 | >60 | >60 | 15.50 ± 0.71 |
| 28 | 30.00 ± 1.41 | >30 | 26.80 ± 2.34 |
| 29 | >60 | >60 | >60 |
| 30 | 7.00 ± 0.00 | 6.13 ± 0.32 | 6.33 ± 0.15 |
| 31 | 19.50 ± 2.12 | 9.77 ± 0.23 | 7.27 ± 0.91 |
| Cisplatin | 19.10 ± 4.50 | 6.11 (ref. 41) | 2.28 ± 0.26 |
According to previous studies, the small carbon chain ester derivatives at C-28 of ursane-type triterpenoids present increased cytotoxic activity.38,39 Similar results were obtained in our study, the methyl ester derivatives 2, 5, and 8 and the ethyl ester derivatives 3, 6, and 9 exhibited increased cytotoxic activity compared with the parent compounds that had a free carboxyl group (AA (1), 4, and 7, respectively). The antiproliferative activity of C-28 ester derivatives was significantly increased with the increase in the length of the carbon chain.
The direct comparison of the antiproliferative activities (against the HeLa cell line) of compounds 15 and 24, as well as compounds 8 and 14, indicated that the introduction of a keto group at position C-11 improves antiproliferative activity. However, an opposite effect was observed when we compared the activity of the following pairs of compounds: 2 and 12, 10 and 11, and 5 and 13. These results suggest that there is no direct relationship between the introduction of a keto group at C-11 and the antiproliferative activity of the compound, which is in accordance with the results of a previous study.40
The conversion of hexameric ring A into the corresponding heptameric lactol ring substantially improved the growth inhibitory activity in all tested cell lines. The lactol derivatives (4, 5, 6, and 13) showed IC50 values ranging from 4.70 μM (5) to 8.48 μM (13), which was 6–11 times lower than the IC50 of AA (1) (52.47 μM) against the HeLa cell line.
The group of derivatives that had a pentameric ring A with an α,β-unsaturated carbonyl moiety proved to be the most active among all tested derivatives in all tested cancer cell lines. These compounds presented IC50 values ranging from 0.24 μM (compound 24) to 5.30 μM (compound 7) against the HeLa cell line. Moreover, with the exception of compound 7, all the derivatives of this group were more active than cisplatin, which indicated that the α,β-unsaturated carbonyl moiety in ring A is important for the antiproliferative activity of these compounds against cancer cell lines.
Compounds 17, 16, and 18, with a pentameric ring A with an α,β-unsaturated nitrile, were 1.2-, 5.9-, and 3.6-fold more potent than was AA (1), respectively, against the HeLa cell line.
The effect of different substituents at the C-23 position of compounds 14 and 16 on their antiproliferative activities was also investigated. We found that, with the exception of compound 30, only the introduction of the methanesulfonyloxy group at C-23 (compounds 15, 24, and 31) resulted in a marked increase in antiproliferative activity. In fact, compounds 15 and 24 were the most active compounds among all the synthesized derivatives, as they were approximately 175- and 218-fold more active than AA (1), respectively, against the HeLa cell line.
The most active compounds, 9, 14, 15, and 24, were selected and their antiproliferative activity was further evaluated against a panel of other four cancer cell lines (Jurkat, PC-3, MIA PaCa-2, and A-375) and against a nontumoral fibroblast cell line (BJ), to evaluate selectivity. As depicted in Table 2, the selected compounds markedly inhibited the proliferation of all tested cancer cell lines. Compounds 14, 15, and 24 exhibited a decreased toxicity for the normal fibroblast cell line BJ compared with cancer cell lines. Compound 24 presented the best antiproliferative profile, and was especially active against the HeLa and Jurkat cell lines, with IC50 values of 0.24 μM and 0.11 μM, respectively. Thus, this compound was selected for further studies aimed at exploring the mechanism underlying its antiproliferative effect against the HeLa cell line.
| Compound | Cell linesa/IC50 (μM) | ||||
|---|---|---|---|---|---|
| Jurkat | PC-3 | MiaPaca-2 | A-375 | BJ | |
| a The cell lines were treated with increasing concentrations of each compound for 72 h. IC50 values were determined by MTT assay in PC-3, MIA PaCa-2, A375 and BJ cell lines and by XTT assay in Jurkat cell line. The results shown are expressed as means ± SD (standard deviation) of three independent experiments. N.D. not determined. IC50 is the concentration of compound that inhibits 50% of cell growth. | |||||
| Asiatic acid 1 | 37.18 ± 3.75 | 67.25 ± 0.35 | 50.67 ± 1.15 | 50.33 ± 2.57 | 88.70 ± 0.58 |
| 9 | 0.45 ± 0.04 | 0.57 ± 0.04 | 0.83 ± 0.04 | 0.63 ± 0.05 | N.D. |
| 14 | 0.18 ± 0.02 | 0.60 ± 0.05 | 0.60 ± 0.06 | 0.38 ± 0.01 | 3.33 ± 0.25 |
| 15 | 0.27 ± 0.01 | 0.41 ± 0.02 | 0.60 ± 0.06 | 0.36 ± 0.03 | 1.94 ± 0.08 |
| 24 | 0.11 ± 0.01 | 0.42 ± 0.02 | 0.46 ± 0.04 | 0.25 ± 0.01 | 2.43 ± 0.11 |
| Cisplatin | 1.94 (ref. 44) | 4.60 (ref. 45) | 5.00 ± 1.00 (ref. 46) | 3.11 ± 0.98 (ref. 47) | 10.10 ± 2.00 |
We also observed an increase of around 17% in the percentage of cells in the sub-G0/G1 phase (cells with hypodiploid DNA) after treatment of HeLa cells with 1.44 μM of compound 24, which suggests that this compound has the ability to induce cell death in a dose-dependent manner.
Upregulation of p21waf1/cip1 was also observed after treatment with 1.44 μM compound 24. These data suggest that 24 preferably targets p27kip1. Compound 24 also decreased the levels of cyclin D3 in a concentration-dependent manner, but did not affect the levels of CDK4.
Considering that activated cyclin D/CDK4 complexes promote the progression of the G1 phase of the cell cycle and that the CDKIs p21waf1/cip1 and p27kip1 inhibit the kinase activity of such complexes, our results suggest that the upregulation of p27kip1 and p21waf1/cip1 and the downregulation of cyclin D3 induced by compound 24 lead to cell-cycle arrest at the G0/G1 phase, with the consequent inhibition of cell proliferation.
We observed that treatment of HeLa cells with 1.44 μM of compound 24 led to an increase in the number of apoptotic cells, from 2.9% in control cells to 19.17% in treated cells (i.e., 7.53% of early apoptotic cells and 11.64% of late apoptotic cells) (Fig. 4A). Concomitantly, the percentage of live cells decreased from 96.53% in the control to 78.61% in treated cells. Treatment with 0.24 μM or 0.48 μM of compound 24 did not change the apoptotic rates significantly. These results suggest that compound 24 at 1.44 μM induces apoptosis in HeLa cells.
As shown in the phase-contrast microscopic pictures (Fig. 4B, upper panel), treatment of HeLa cells with compound 24 reduced the cell density and induced remarkable morphological changes. Compared with control cells, treated cells became smaller and nonadherent and acquired a rounded morphology.
To assess the nuclear morphological changes in greater detail, HeLa cells were stained with Hoechst 33258 after treatment with compound 24 and were analyzed by fluorescence microscopy. As shown in Fig. 4B, lower panel, control cells were uniformly stained and presented a normal morphology. Conversely, HeLa cells treated with 0.96 μM of compound 24 exhibited typical apoptotic morphological changes, such as chromatin condensation and cell shrinkage. Nuclear fragmentation and membrane blebbing were evident after treatment with 1.44 μM of compound 24. A decrease in the number of cells with increasing drug concentrations was also observed. The morphological changes induced by compound 24 were consistent with an apoptotic cell death process.
Subsequently, we explored the effect of compound 24 on the levels of Bcl-2 protein family members, such as Bcl-2 (antiapoptotic), Bax (proapoptotic), and Bid (proapoptotic). As depicted in Fig. 5B and C, the treatment of HeLa cells with compound 24 caused a downregulation of Bcl-2 in a concentration-dependent manner. These data support that the mitochondrial pathway is involved in compound 24-induced apoptosis. Compound 24 also downregulated the levels of Bid, suggesting the activation of Bid into t-Bid. However, bands corresponding to t-Bid were not detected on western blots, which can be justified by the short half-life and small size of t-Bid. Further studies are needed to confirm and understand better the role of the intrinsic and extrinsic pathways in this apoptotic mechanism.
3. Conclusions
In summary, we successfully synthesized a series of new pentameric A-ring AA derivatives. Our results showed that the conversion of the hexameric ring A of AA into a 5-carbon ring with α,β-unsaturated carbonyl or α,β-unsaturated nitrile moieties significantly improved the antiproliferative activity of compounds. Compound 24 displayed the best antiproliferative profile, with IC50 values ranging from 0.11 μM to 0.65 μM. This compound arrested the cell cycle at the G0/G1 phase and induced apoptosis in HeLa cells via the activation of caspases-9, -8, and -3, cleavage of PARP, and modulation of the ratio of Bax/Bcl-2. In light of our results, this compound might represent a promising lead for the development of new anticancer agents.4. Experimental
4.1 Chemistry
![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2CH3), 3.79–3.72 (m, 1H, H-2), 3.66 (d, J = 10.36 Hz, 1H, H-3), 3.44 (d, J = 9.05 Hz, 1H, H-23), 3.41 (d, J = 9.40 Hz, 1H, H-23), 1.21 (t, J = 7.00 Hz, 3H, COOCH2
2CH3), 3.79–3.72 (m, 1H, H-2), 3.66 (d, J = 10.36 Hz, 1H, H-3), 3.44 (d, J = 9.05 Hz, 1H, H-23), 3.41 (d, J = 9.40 Hz, 1H, H-23), 1.21 (t, J = 7.00 Hz, 3H, COOCH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3), 1.08 (s, 3H), 1.03 (s, 3H), 0.94 (d, J = 6.05 Hz, 3H), 0.87 (s, 3H), 0.85 (d, J = 6.20 Hz, 3H), 0.76 (s, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ = 177.53 (C28), 138.25 (C13), 125.18 (C12), 68.75, 60.02, 52.81, 47.83, 47.47, 46.24, 42.13, 39.57, 39.07, 38.85, 38.15, 38.62, 32.70, 30.58, 27.91, 24.13, 23.58, 23.34, 21.18, 18.32, 17.16, 17.11, 17.01, 14.21, 12.76 ppm; DI-ESI-MS m/z: 516.97 ([M + H]+); calcd. For C32H52O5·0.5H2O: C, 73.10; H, 10.16. Found: C, 73.31; H, 10.32%.
3), 1.08 (s, 3H), 1.03 (s, 3H), 0.94 (d, J = 6.05 Hz, 3H), 0.87 (s, 3H), 0.85 (d, J = 6.20 Hz, 3H), 0.76 (s, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ = 177.53 (C28), 138.25 (C13), 125.18 (C12), 68.75, 60.02, 52.81, 47.83, 47.47, 46.24, 42.13, 39.57, 39.07, 38.85, 38.15, 38.62, 32.70, 30.58, 27.91, 24.13, 23.58, 23.34, 21.18, 18.32, 17.16, 17.11, 17.01, 14.21, 12.76 ppm; DI-ESI-MS m/z: 516.97 ([M + H]+); calcd. For C32H52O5·0.5H2O: C, 73.10; H, 10.16. Found: C, 73.31; H, 10.32%.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)), NaIO4 (131.30 mg; 0.61 mmol, 1.5 eq.) was added. The reaction mixture was stirred at room temperature. After 2 hours the reaction mixture was evaporated under reduced pressure to remove the organic phase. The obtained crude was dispersed by water (40 mL) and extracted with ethyl acetate (3 × 40 mL). The resulting organic phase was washed with water (4 × 40 mL) and brine (40 mL), dried over Na2SO4, filtered, and concentrated under vacuum to afford 4 as a white powder (quantitative). Mp: 198.5–201.4 °C. vmax/cm−1 (KBr): 3421.1, 2948.63, 2927.41, 2871.49, 2732.64, 2630.43, 1716.34, 1695.12, 1456.99, 1378.85, 1037.52. 1H NMR (400 MHz, CDCl3): δ = 9.94 (s, 1H, CHO), 5.29 (t, J = 3.25 Hz, 1H, H-12), 5.14–5.11 (m, 1H, H-2), 3.94 (d, J = 13.41 Hz, 1H), 3.75 (d, J = 13.17 Hz, 1H), 1.08 (s, 3H), 1.06 (s, 3H), 0.99 (s, 3H), 0.95 (d, J = 5.96 Hz, 3H), 0.86 (s, 3H), 0.85 (d, J = 5.42 Hz, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ = 206.09 (CHO), 182.94 (C28), 138.05 (C13), 125.98 (C12), 93.68, 65.37, 61.16, 53.41, 62.71, 48.07, 45.15, 43.65, 42.57, 40.05, 39.98, 38.94, 38.77, 38.58, 33.57, 30.59, 27.83, 24.63, 24.06, 23.17, 21.10, 20.58, 20.37, 17.88, 16.93, 14.57 ppm; DI-ESI-MS m/z: 487.15 ([M + H]+); calcd. For C30H46O5·H2O: C, 71.39; H, 9.59. Found: C, 71.49; H, 9.85%.
1)), NaIO4 (131.30 mg; 0.61 mmol, 1.5 eq.) was added. The reaction mixture was stirred at room temperature. After 2 hours the reaction mixture was evaporated under reduced pressure to remove the organic phase. The obtained crude was dispersed by water (40 mL) and extracted with ethyl acetate (3 × 40 mL). The resulting organic phase was washed with water (4 × 40 mL) and brine (40 mL), dried over Na2SO4, filtered, and concentrated under vacuum to afford 4 as a white powder (quantitative). Mp: 198.5–201.4 °C. vmax/cm−1 (KBr): 3421.1, 2948.63, 2927.41, 2871.49, 2732.64, 2630.43, 1716.34, 1695.12, 1456.99, 1378.85, 1037.52. 1H NMR (400 MHz, CDCl3): δ = 9.94 (s, 1H, CHO), 5.29 (t, J = 3.25 Hz, 1H, H-12), 5.14–5.11 (m, 1H, H-2), 3.94 (d, J = 13.41 Hz, 1H), 3.75 (d, J = 13.17 Hz, 1H), 1.08 (s, 3H), 1.06 (s, 3H), 0.99 (s, 3H), 0.95 (d, J = 5.96 Hz, 3H), 0.86 (s, 3H), 0.85 (d, J = 5.42 Hz, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ = 206.09 (CHO), 182.94 (C28), 138.05 (C13), 125.98 (C12), 93.68, 65.37, 61.16, 53.41, 62.71, 48.07, 45.15, 43.65, 42.57, 40.05, 39.98, 38.94, 38.77, 38.58, 33.57, 30.59, 27.83, 24.63, 24.06, 23.17, 21.10, 20.58, 20.37, 17.88, 16.93, 14.57 ppm; DI-ESI-MS m/z: 487.15 ([M + H]+); calcd. For C30H46O5·H2O: C, 71.39; H, 9.59. Found: C, 71.49; H, 9.85%.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1]) and NaIO4 (645.60 mg; 3.02 mmol, 1.52 eq.) for 3 hours to afford 5 as a white powder (978.70 mg, 98%). Mp: 144.7–147.1 °C. vmax/cm−1 (KBr): 3444.24, 2948.63, 2927.41, 2871.49, 2730.71, 2626.57, 1722.12, 1454.06, 1378.85, 1037.52. 1H NMR (400 MHz, CDCl3): δ = 9.94 (s, 1H, CHO), 5.30 (t, J = 3.26 Hz, H-12), 5.13–5.09 (m, 1H, H-2), 3.93 (d, J = 13.31 Hz, 1H), 3.74 (d, J = 13.31 Hz, 1H), 3.60 (s, 3H, COO
1]) and NaIO4 (645.60 mg; 3.02 mmol, 1.52 eq.) for 3 hours to afford 5 as a white powder (978.70 mg, 98%). Mp: 144.7–147.1 °C. vmax/cm−1 (KBr): 3444.24, 2948.63, 2927.41, 2871.49, 2730.71, 2626.57, 1722.12, 1454.06, 1378.85, 1037.52. 1H NMR (400 MHz, CDCl3): δ = 9.94 (s, 1H, CHO), 5.30 (t, J = 3.26 Hz, H-12), 5.13–5.09 (m, 1H, H-2), 3.93 (d, J = 13.31 Hz, 1H), 3.74 (d, J = 13.31 Hz, 1H), 3.60 (s, 3H, COO![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3), 1.08 (s, 3H), 1.06 (s, 3H), 0.99 (s, 3H), 0.94 (d, J = 6.03 Hz), 0.85–0.83 (m, 6H) ppm. 13C NMR (100 MHz, CDCl3): δ = 205.85 (CHO), 177.99 (C28); 138.30 (C13); 125.75 (C12); 93.67; 65.41, 61.12, 53.45, 53.03, 51.49, 48.23, 45.17, 43.67, 42.59, 40.03, 39.97, 38.94, 38.81, 36.52, 33.62, 30.64, 27.86, 24.63, 24.17, 23.17, 21.10, 20.60, 20.38, 17.86, 16.97, 14.52 ppm; DI-ESI-MS m/z: 500.99 ([M + H]+); calcd. For C31H48O5·0.25H2O: C, 73.70; H, 9.68. Found: C, 73.75; H, 9.97%.
3), 1.08 (s, 3H), 1.06 (s, 3H), 0.99 (s, 3H), 0.94 (d, J = 6.03 Hz), 0.85–0.83 (m, 6H) ppm. 13C NMR (100 MHz, CDCl3): δ = 205.85 (CHO), 177.99 (C28); 138.30 (C13); 125.75 (C12); 93.67; 65.41, 61.12, 53.45, 53.03, 51.49, 48.23, 45.17, 43.67, 42.59, 40.03, 39.97, 38.94, 38.81, 36.52, 33.62, 30.64, 27.86, 24.63, 24.17, 23.17, 21.10, 20.60, 20.38, 17.86, 16.97, 14.52 ppm; DI-ESI-MS m/z: 500.99 ([M + H]+); calcd. For C31H48O5·0.25H2O: C, 73.70; H, 9.68. Found: C, 73.75; H, 9.97%.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)) and NaIO4 (299.45 mg; 1.40 mmol, 1.5 eq.) for 1 h 30 min to afford 6 as a white powder (477.90 mg, quantitative). Mp: 138.1–142.0 °C. vmax/cm−1 (KBr): 3444.24, 2950.55, 2927.41, 2971.49, 2732.64, 1720.19, 1454.06, 1378.85, 1230.36, 1141.65, 1037.52. 1H NMR (400 MHz, CDCl3): δ = 9.94 (s, 1H, CHO), 5.29 (t, J = 3.10 Hz, 1H, H-12), 5.12–5.09 (m, 1H, H-2), 4.06 (q, J = 7.15 Hz, 2H, COO
1)) and NaIO4 (299.45 mg; 1.40 mmol, 1.5 eq.) for 1 h 30 min to afford 6 as a white powder (477.90 mg, quantitative). Mp: 138.1–142.0 °C. vmax/cm−1 (KBr): 3444.24, 2950.55, 2927.41, 2971.49, 2732.64, 1720.19, 1454.06, 1378.85, 1230.36, 1141.65, 1037.52. 1H NMR (400 MHz, CDCl3): δ = 9.94 (s, 1H, CHO), 5.29 (t, J = 3.10 Hz, 1H, H-12), 5.12–5.09 (m, 1H, H-2), 4.06 (q, J = 7.15 Hz, 2H, COO![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2CH3), 3.93 (d, J = 13.29 Hz, 1H), 3.75 (d, J = 13.29 Hz, 1H), 1.21 (t, J = 7.14 Hz, 3H, COOCH2
2CH3), 3.93 (d, J = 13.29 Hz, 1H), 3.75 (d, J = 13.29 Hz, 1H), 1.21 (t, J = 7.14 Hz, 3H, COOCH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3), 1.08 (s, 3H), 1.06 (s, 3H), 0.99 (s, 3H), 0.94 (d, J = 6.10 Hz, 3H), 0.84 (m, 6H) ppm. 13C NMR (100 MHz, CDCl3): δ = 205.85 (CHO), 177.45 (C28), 138.31 (C13), 125.70 (C12), 93.65, 65.39, 61.12, 60.04, 53.43, 53.01, 47.99, 45.21, 43.68, 42.65, 40.11, 39.94, 38.97, 38.81, 36.53, 33.71, 30.68, 27.80, 24.63, 24.12, 23.07, 21.10, 20.60, 20.37, 18.05, 16.95, 14.53, 14.21 ppm. DI-ESI-MS m/z: 514.92 ([M + H]+); calcd. For C32H50O5·0.25H2O: C, 74.02; H, 9.80. Found: C, 73.84; H, 10.05%.
3), 1.08 (s, 3H), 1.06 (s, 3H), 0.99 (s, 3H), 0.94 (d, J = 6.10 Hz, 3H), 0.84 (m, 6H) ppm. 13C NMR (100 MHz, CDCl3): δ = 205.85 (CHO), 177.45 (C28), 138.31 (C13), 125.70 (C12), 93.65, 65.39, 61.12, 60.04, 53.43, 53.01, 47.99, 45.21, 43.68, 42.65, 40.11, 39.94, 38.97, 38.81, 36.53, 33.71, 30.68, 27.80, 24.63, 24.12, 23.07, 21.10, 20.60, 20.37, 18.05, 16.95, 14.53, 14.21 ppm. DI-ESI-MS m/z: 514.92 ([M + H]+); calcd. For C32H50O5·0.25H2O: C, 74.02; H, 9.80. Found: C, 73.84; H, 10.05%.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 → 1
1 → 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford 7 as a white solid (353.77 mg, 73%). Mp: 183.5–186.1 °C. vmax/cm−1 (KBr): 3428.81, 2946.7, 2925.48, 2869.56, 2726.85, 2632.36, 1689.34, 1581.34, 1454.06, 1380.78, 1041.37. 1H NMR (400 MHz, CDCl3): δ = 9.72 (s, 1H, CHO), 6.66 (s, 1H, H-3), 5.28 (t, J = 3.05 Hz, 1H, H-12), 3.62 (d, J = 10.67 Hz, 1H, H-23), 3.45 (d, J = 10.70 Hz, 1H, H-23), 1.25 (s, 3H), 1.10 (s, 3H), 1.01 (s, 3H), 0.93 (d, J = 6.24 Hz, 3H), 0.88 (s, 3H), 0.84 (d, J = 6.32 Hz, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ = 190.85 (CHO), 183.27 (C28), 159.29 (C3), 158.90 (C2), 137.49 (C13), 126.60 (C12), 69.36, 56.26, 52.57, 50.88, 49.41, 47.87, 44.10, 42.41, 41.36, 38.78 (2C), 36.63, 33.53, 30.55, 28.19, 27.09, 23.99 (2C), 21.16, 19.03, 18.74, 17.35, 16.98, 15.92 ppm; DI-ESI-MS m/z: 469.03 ([M + H]+); calcd. For C30H44O4·H2O: C, 74.04; H, 9.53. Found: C, 73.68; H, 9.75%.
1) to afford 7 as a white solid (353.77 mg, 73%). Mp: 183.5–186.1 °C. vmax/cm−1 (KBr): 3428.81, 2946.7, 2925.48, 2869.56, 2726.85, 2632.36, 1689.34, 1581.34, 1454.06, 1380.78, 1041.37. 1H NMR (400 MHz, CDCl3): δ = 9.72 (s, 1H, CHO), 6.66 (s, 1H, H-3), 5.28 (t, J = 3.05 Hz, 1H, H-12), 3.62 (d, J = 10.67 Hz, 1H, H-23), 3.45 (d, J = 10.70 Hz, 1H, H-23), 1.25 (s, 3H), 1.10 (s, 3H), 1.01 (s, 3H), 0.93 (d, J = 6.24 Hz, 3H), 0.88 (s, 3H), 0.84 (d, J = 6.32 Hz, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ = 190.85 (CHO), 183.27 (C28), 159.29 (C3), 158.90 (C2), 137.49 (C13), 126.60 (C12), 69.36, 56.26, 52.57, 50.88, 49.41, 47.87, 44.10, 42.41, 41.36, 38.78 (2C), 36.63, 33.53, 30.55, 28.19, 27.09, 23.99 (2C), 21.16, 19.03, 18.74, 17.35, 16.98, 15.92 ppm; DI-ESI-MS m/z: 469.03 ([M + H]+); calcd. For C30H44O4·H2O: C, 74.04; H, 9.53. Found: C, 73.68; H, 9.75%.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 → 2
1 → 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford 9 as a white solid (193.2 mg, 67%). Mp: 207.5–209.7 °C. vmax/cm−1 (KBr): 3550.31, 2977.55, 2960.20, 2946.70, 2925.48, 2867.63, 2809.78, 2726.85, 1708.62, 1689.34, 1581.34, 1452.11, 1238.08, 1143.58, 1045.23. 1H NMR (400 MHz, CDCl3): δ = 9.72 (s, 1H, CHO), 6.66 (s, 1H, H-3), 5.28 (t, J = 3.00 Hz, 1H, H-12), 4.09–4.02 (m, 2H, COO
1) to afford 9 as a white solid (193.2 mg, 67%). Mp: 207.5–209.7 °C. vmax/cm−1 (KBr): 3550.31, 2977.55, 2960.20, 2946.70, 2925.48, 2867.63, 2809.78, 2726.85, 1708.62, 1689.34, 1581.34, 1452.11, 1238.08, 1143.58, 1045.23. 1H NMR (400 MHz, CDCl3): δ = 9.72 (s, 1H, CHO), 6.66 (s, 1H, H-3), 5.28 (t, J = 3.00 Hz, 1H, H-12), 4.09–4.02 (m, 2H, COO![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2CH3), 3.62 (d, J = 8.50 Hz, 1H, H-23), 3.46 (d, J = 8.78 Hz, 1H, H-23), 1.25 (s, 3H), 1.22 (t, J = 7.20 Hz, 3H, COOCH2
2CH3), 3.62 (d, J = 8.50 Hz, 1H, H-23), 3.46 (d, J = 8.78 Hz, 1H, H-23), 1.25 (s, 3H), 1.22 (t, J = 7.20 Hz, 3H, COOCH2![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3), 1.09 (s, 3H), 1.02 (s, 3H), 0.93 (d, J = 6.07 Hz, 3H), 0.86 (s, 3H), 0.83 (d, J = 6.54 Hz, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ = 190.84 (CHO), 177.57 (C28), 159.29 (C3), 158.95 (C2), 137.79 (C13), 126.28 (C12), 69.39 (C23), 60.02, 56.25, 52.81, 50.91, 49.43, 47.79, 44.15, 42.49, 41.44, 38.84 (2C), 36.57, 33.62, 30.64, 28.19, 27.10, 24.11, 23.90, 21.17, 19.01, 18.81, 17.37, 17.02, 15.93, 14.18 ppm. DI-ESI-MS m/z: 497.07 ([M + H]+); calcd. For C32H48O4: C, 77.38; H, 9.74. Found: C, 76.94; H, 10.12%.
3), 1.09 (s, 3H), 1.02 (s, 3H), 0.93 (d, J = 6.07 Hz, 3H), 0.86 (s, 3H), 0.83 (d, J = 6.54 Hz, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ = 190.84 (CHO), 177.57 (C28), 159.29 (C3), 158.95 (C2), 137.79 (C13), 126.28 (C12), 69.39 (C23), 60.02, 56.25, 52.81, 50.91, 49.43, 47.79, 44.15, 42.49, 41.44, 38.84 (2C), 36.57, 33.62, 30.64, 28.19, 27.10, 24.11, 23.90, 21.17, 19.01, 18.81, 17.37, 17.02, 15.93, 14.18 ppm. DI-ESI-MS m/z: 497.07 ([M + H]+); calcd. For C32H48O4: C, 77.38; H, 9.74. Found: C, 76.94; H, 10.12%.![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3), 2.08 (s, 3H, CH3CO), 2.01 (s, 3H, CH3CO), 1.97 (s, 3H, CH3CO), 1.09 (s, 3H), 1.06 (s, 3H), 0.94 (d, J = 6.06 Hz, 3H), 0.88 (s, 3H), 0.84 (d, J = 6.23 Hz, 3H), 0.74 (s, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ = 177.95 (C28), 170.85 (OCO), 170.44 (OCO), 170.37 (OCO), 138.30 (C13), 125.01 (C12), 74.85, 69.91, 65.28, 52.81, 51.46, 48.04, 47.59, 47.49, 43.75, 41.98, 41.89, 39.50, 39.00, 38.81, 37.77, 36.54, 32.44, 30.60, 27.90, 24.12, 23.42, 23.34, 21.14, 21.06, 20.86, 20.76, 17.88, 17.00, 16.95, 16.85, 13.90 ppm; DI-ESI-MS m/z: 629.36 ([M + H]+); calcd. For C37H56O8: C, 70.67; H, 8.98. Found: C, 70.55; H, 8.98%.
3), 2.08 (s, 3H, CH3CO), 2.01 (s, 3H, CH3CO), 1.97 (s, 3H, CH3CO), 1.09 (s, 3H), 1.06 (s, 3H), 0.94 (d, J = 6.06 Hz, 3H), 0.88 (s, 3H), 0.84 (d, J = 6.23 Hz, 3H), 0.74 (s, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ = 177.95 (C28), 170.85 (OCO), 170.44 (OCO), 170.37 (OCO), 138.30 (C13), 125.01 (C12), 74.85, 69.91, 65.28, 52.81, 51.46, 48.04, 47.59, 47.49, 43.75, 41.98, 41.89, 39.50, 39.00, 38.81, 37.77, 36.54, 32.44, 30.60, 27.90, 24.12, 23.42, 23.34, 21.14, 21.06, 20.86, 20.76, 17.88, 17.00, 16.95, 16.85, 13.90 ppm; DI-ESI-MS m/z: 629.36 ([M + H]+); calcd. For C37H56O8: C, 70.67; H, 8.98. Found: C, 70.55; H, 8.98%.![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3), 2.07 (s, 3H, CH3CO), 2.00 (s, 3H, CH3CO), 1.94 (s, 3H, CH3CO), 1.28 (s, 6H), 0.96 (d, J = 6.31 Hz, 3H), 0.90 (s, 6H), 0.85 (d, J = 6.49 Hz, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ = 198.82 (C11), 177.09 (C28), 170.79 (OCO), 170.46 (OCO), 170.10 (OCO), 163.26 (C13), 130.43 (C12), 74.88, 68.96, 65.21, 61.11, 52.71, 51.87 (2C), 47.61, 47.32, 44.55, 44.17, 43.72, 41.88, 38.58, 37.65, 35.88, 32.45, 30.26, 28.29, 23.84, 20.98, 20.95 (2C), 20.88, 20.75, 18.81, 17.70, 17.05, 16.99, 13.85 ppm; DI-ESI-MS m/z: 643.12 ([M + H]+); calcd. For C37H54O9·0.25H2O: C, 68.65; H, 8.49. Found: C, 68.46; H, 8.38%.
3), 2.07 (s, 3H, CH3CO), 2.00 (s, 3H, CH3CO), 1.94 (s, 3H, CH3CO), 1.28 (s, 6H), 0.96 (d, J = 6.31 Hz, 3H), 0.90 (s, 6H), 0.85 (d, J = 6.49 Hz, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ = 198.82 (C11), 177.09 (C28), 170.79 (OCO), 170.46 (OCO), 170.10 (OCO), 163.26 (C13), 130.43 (C12), 74.88, 68.96, 65.21, 61.11, 52.71, 51.87 (2C), 47.61, 47.32, 44.55, 44.17, 43.72, 41.88, 38.58, 37.65, 35.88, 32.45, 30.26, 28.29, 23.84, 20.98, 20.95 (2C), 20.88, 20.75, 18.81, 17.70, 17.05, 16.99, 13.85 ppm; DI-ESI-MS m/z: 643.12 ([M + H]+); calcd. For C37H54O9·0.25H2O: C, 68.65; H, 8.49. Found: C, 68.46; H, 8.38%.![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3), 3.42 (d, J = 9.51 Hz, 1H, H3) 3.36 (d, J = 11.09 Hz, 2H, H-23), 1.30 (s, 3H), 1.21 (s, 3H), 0.96 (d, J = 6.10 Hz, 3H), 0.89 (s, 3H), 0.86 (d, J = 6.31 Hz, 3H), 0.82 (s, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ = 199.70 (C11), 177.19 (C28), 163.51 (C13), 130.43 (C12), 68.50, 61.04, 52.73, 51.85 (2C), 47.64, 46.89, 44.66, 43.86, 42.78, 38.63, 38.56, 38.01, 35.94, 32.54, 30.28, 28.35, 23.90, 21.19, 20.96 (2C), 18.91, 17.88, 17.21, 17.09, 12.99 ppm; DI-ESI-MS m/z: 516.93 ([M + H]+); calcd. For C31H48O6·H2O: C, 69.63; H, 9.42. Found: C, 69.19; H, 8.92%.
3), 3.42 (d, J = 9.51 Hz, 1H, H3) 3.36 (d, J = 11.09 Hz, 2H, H-23), 1.30 (s, 3H), 1.21 (s, 3H), 0.96 (d, J = 6.10 Hz, 3H), 0.89 (s, 3H), 0.86 (d, J = 6.31 Hz, 3H), 0.82 (s, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ = 199.70 (C11), 177.19 (C28), 163.51 (C13), 130.43 (C12), 68.50, 61.04, 52.73, 51.85 (2C), 47.64, 46.89, 44.66, 43.86, 42.78, 38.63, 38.56, 38.01, 35.94, 32.54, 30.28, 28.35, 23.90, 21.19, 20.96 (2C), 18.91, 17.88, 17.21, 17.09, 12.99 ppm; DI-ESI-MS m/z: 516.93 ([M + H]+); calcd. For C31H48O6·H2O: C, 69.63; H, 9.42. Found: C, 69.19; H, 8.92%.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)) and NaIO4 (1227.40 mg; 5.74 mmol, 1.52 eq.) for 1 hour at room temperature. The crude solid was purified by flash column chromatography (petroleum ether/ethyl acetate, 4
1)) and NaIO4 (1227.40 mg; 5.74 mmol, 1.52 eq.) for 1 hour at room temperature. The crude solid was purified by flash column chromatography (petroleum ether/ethyl acetate, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 → 1
1 → 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford 13 as a white solid (1280.30 mg, 66%). Mp: 154.0–157.0 °C. vmax/cm−1 (KBr): 3436.53, 2948.63, 2938.98, 2873.42, 2734.57, 1725.98, 1658.48, 1455.99, 1205.29, 1141.65, 1037.52. 1H NMR (400 MHz, CDCl3): δ = 9.97 (s, 1H, CHO), 5.64 (s, 1H, H-12), 5.36–5.33 (m, 1H, H-2), 3.98 (d, J = 13.23 Hz, 1H), 3.71 (d, J = 13.59 Hz, 1H), 3.60 (s, 3H, COO
1) to afford 13 as a white solid (1280.30 mg, 66%). Mp: 154.0–157.0 °C. vmax/cm−1 (KBr): 3436.53, 2948.63, 2938.98, 2873.42, 2734.57, 1725.98, 1658.48, 1455.99, 1205.29, 1141.65, 1037.52. 1H NMR (400 MHz, CDCl3): δ = 9.97 (s, 1H, CHO), 5.64 (s, 1H, H-12), 5.36–5.33 (m, 1H, H-2), 3.98 (d, J = 13.23 Hz, 1H), 3.71 (d, J = 13.59 Hz, 1H), 3.60 (s, 3H, COO![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3), 1.34 (s, 3H), 1.32 (s, 3H), 0.97 (m, 6H), 0.93 (s, 3H), 0.86 (d, J = 6.37 Hz, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ = 205.65 (CHO), 198.89 (C11), 177.12 (C28), 162.74 (C13), 130.85 (C12), 93.49, 65.25, 61.38, 57.40, 53.20, 52.75, 51.89, 47.71, 45.70, 44.65, 44.15, 39.21, 38.60, 38.57, 35.92, 33.16, 30.30, 28.35, 23.88, 20.93, 20.70, 20.47, 19.60, 19.28, 17.03, 14.38 ppm; DI-ESI-MS m/z: 515.42 ([M + H]+); calcd. For C31H46O6·0.75H2O: C, 70.49; H, 9.06. Found: C, 70.31; H, 9.41%.
3), 1.34 (s, 3H), 1.32 (s, 3H), 0.97 (m, 6H), 0.93 (s, 3H), 0.86 (d, J = 6.37 Hz, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ = 205.65 (CHO), 198.89 (C11), 177.12 (C28), 162.74 (C13), 130.85 (C12), 93.49, 65.25, 61.38, 57.40, 53.20, 52.75, 51.89, 47.71, 45.70, 44.65, 44.15, 39.21, 38.60, 38.57, 35.92, 33.16, 30.30, 28.35, 23.88, 20.93, 20.70, 20.47, 19.60, 19.28, 17.03, 14.38 ppm; DI-ESI-MS m/z: 515.42 ([M + H]+); calcd. For C31H46O6·0.75H2O: C, 70.49; H, 9.06. Found: C, 70.31; H, 9.41%.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 → 1
1 → 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford 14 as a white solid (142.90 mg, 49%). Mp: 144.8–146.6 °C. vmax/cm−1 (KBr): 3442.31, 2977.55, 2948.63, 2929.34, 2871.49, 1727.91, 1662.34, 1614.13, 1581.34, 1455.99, 1226.50. 1H NMR (400 MHz, CDCl3): δ = 10.15 (s, 1H, CHO), 6.35 (s, 1H, H-3), 5.67 (s, 1H, H-12), 3.62 (s, 3H, COO
1) to afford 14 as a white solid (142.90 mg, 49%). Mp: 144.8–146.6 °C. vmax/cm−1 (KBr): 3442.31, 2977.55, 2948.63, 2929.34, 2871.49, 1727.91, 1662.34, 1614.13, 1581.34, 1455.99, 1226.50. 1H NMR (400 MHz, CDCl3): δ = 10.15 (s, 1H, CHO), 6.35 (s, 1H, H-3), 5.67 (s, 1H, H-12), 3.62 (s, 3H, COO![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3, H28), 3.56 (d, J = 11.08 Hz, 1H), 3.39 (d, J = 11.08 Hz, 1H), 1.45 (s, 3H), 1.33 (s, 3H), 1.00 (s, 3H), 0.97 (m, 6H), 0.85 (d, J = 6.35 Hz, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ = 199.38 (C11), 195.49 (CHO), 177.17 (C28), 164.67 (C13), 157.47 (C2); 144.75 (C3), 129.47 (C12), 69.24, 58.26, 54.83, 52.99, 51.88, 48.70, 48.23, 47.57, 45.83, 44.44, 38.61, 38.36, 35.87, 33.44, 30.21, 28.77, 23.87, 21.39, 21.06, 20.96, 20.54, 17.11, 16.90, 16.10 ppm. DI-ESI-MS m/z: 497.47 ([M + H]+). Anal. calcd. For C31H44O5·0.5H2O: C, 73.63; H, 8.97. Found: C, 73.27; H, 8.50%.
3, H28), 3.56 (d, J = 11.08 Hz, 1H), 3.39 (d, J = 11.08 Hz, 1H), 1.45 (s, 3H), 1.33 (s, 3H), 1.00 (s, 3H), 0.97 (m, 6H), 0.85 (d, J = 6.35 Hz, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ = 199.38 (C11), 195.49 (CHO), 177.17 (C28), 164.67 (C13), 157.47 (C2); 144.75 (C3), 129.47 (C12), 69.24, 58.26, 54.83, 52.99, 51.88, 48.70, 48.23, 47.57, 45.83, 44.44, 38.61, 38.36, 35.87, 33.44, 30.21, 28.77, 23.87, 21.39, 21.06, 20.96, 20.54, 17.11, 16.90, 16.10 ppm. DI-ESI-MS m/z: 497.47 ([M + H]+). Anal. calcd. For C31H44O5·0.5H2O: C, 73.63; H, 8.97. Found: C, 73.27; H, 8.50%.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 → 2
1 → 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford 15 as a white solid (206.9 mg, 71%). Mp: 105.6–107.2 °C. vmax/cm−1 (KBr): 2948.63, 2925.48, 2871.49, 2728.78, 1722.12, 1689.34, 1585.2, 1455.99, 1357.64, 1176.36, 958.45. 1H NMR (400 MHz, CDCl3): δ = 9.73 (s, 1H, CHO), 6.60 (s, 1H, H-3), 5.26 (t, J = 3.01 Hz, 1H, H-12), 4.13 (d, J = 9.59 Hz, 1H, H-23), 4.06 (d, J = 9.59 Hz, 1H, H-23), 3.61 (s, 3H, COO
1) to afford 15 as a white solid (206.9 mg, 71%). Mp: 105.6–107.2 °C. vmax/cm−1 (KBr): 2948.63, 2925.48, 2871.49, 2728.78, 1722.12, 1689.34, 1585.2, 1455.99, 1357.64, 1176.36, 958.45. 1H NMR (400 MHz, CDCl3): δ = 9.73 (s, 1H, CHO), 6.60 (s, 1H, H-3), 5.26 (t, J = 3.01 Hz, 1H, H-12), 4.13 (d, J = 9.59 Hz, 1H, H-23), 4.06 (d, J = 9.59 Hz, 1H, H-23), 3.61 (s, 3H, COO![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3), 3.03 (s, 3H), (S–
3), 3.03 (s, 3H), (S–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3), 1.25 (s, 3H), 1.10 (s, 3H), 1.08 (s, 3H), 0.93 (d, J = 5.87 Hz, 3H), 0.84–0.83 (m, 6H) ppm. 13C NMR (100 MHz, CDCl3): δ = 190.56 (CHO), 178.03 (C28), 158.95 (C2), 155.79 (C3), 137.85 (C13), 126.10 (C12), 74.70, 56.93, 52.79, 51.45, 50.85, 48.01, 47.69, 44.29, 42.43, 41.30, 38.82, 38.78, 37.49, 36.53, 33.43, 30.56, 28.17, 27.03, 24.11, 23.86, 21.16, 18.78, 18.66, 17.30, 17.05, 15.88 ppm. DI-ESI-MS m/z: 560.91 ([M + H]+); calcd. For C32H48O6S·0.5H2O: C, 67.45; H, 8.67; S, 5.63. Found: C, 67.56; H, 8.73; S, 5.27%.
3), 1.25 (s, 3H), 1.10 (s, 3H), 1.08 (s, 3H), 0.93 (d, J = 5.87 Hz, 3H), 0.84–0.83 (m, 6H) ppm. 13C NMR (100 MHz, CDCl3): δ = 190.56 (CHO), 178.03 (C28), 158.95 (C2), 155.79 (C3), 137.85 (C13), 126.10 (C12), 74.70, 56.93, 52.79, 51.45, 50.85, 48.01, 47.69, 44.29, 42.43, 41.30, 38.82, 38.78, 37.49, 36.53, 33.43, 30.56, 28.17, 27.03, 24.11, 23.86, 21.16, 18.78, 18.66, 17.30, 17.05, 15.88 ppm. DI-ESI-MS m/z: 560.91 ([M + H]+); calcd. For C32H48O6S·0.5H2O: C, 67.45; H, 8.67; S, 5.63. Found: C, 67.56; H, 8.73; S, 5.27%.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 → 2
1 → 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford 16 as a white solid (146.20 mg, 50%). Mp: 115.1–118.1 °C. vmax/cm−1 (KBr): 3504.02 (OH), 2948.63, 2925.48, 2871.49, 2215.81, 1724.05, 1652.70, 1587.13, 1455.99, 1362.71, 1234.22, 1197.58, 1145.51, 1049.09. 1H NMR (400 MHz, CDCl3): δ = 6.48 (s, 1H, H-3), 5.26 (t, J = 3 Hz, 1H, H-12), 3.61 (s, 3H, COO
1) to afford 16 as a white solid (146.20 mg, 50%). Mp: 115.1–118.1 °C. vmax/cm−1 (KBr): 3504.02 (OH), 2948.63, 2925.48, 2871.49, 2215.81, 1724.05, 1652.70, 1587.13, 1455.99, 1362.71, 1234.22, 1197.58, 1145.51, 1049.09. 1H NMR (400 MHz, CDCl3): δ = 6.48 (s, 1H, H-3), 5.26 (t, J = 3 Hz, 1H, H-12), 3.61 (s, 3H, COO![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3), 3.55 (d, J = 10.07 Hz, 1H, H-23), 3.39 (d, J = 9.92 Hz, 1H, H-23), 1.27 (s, 3H), 1.13 (s, 3H), 1.02 (s, 3H), 0.94 (d, J = 5.36 Hz, 3H), 0.87 (d, J = 6.13 Hz, 3H), 0.83 (s, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ = 178.02 (C28), 153.59 (C3), 138.68 (C13), 127.16 (C2), 125.01 (C12), 117.64 (CN), 69.26, 55.37, 52.90 (2C), 51.48, 50.83, 48.03, 43.55, 42.39, 40.92, 38.83 (2C), 36.54, 33.41, 30.57, 28.20, 24.55, 24.09, 24.01, 21.16, 19.27, 18.44, 17.65, 17.08, 16.01 ppm. DI-ESI-MS m/z: 480.08 ([M + H]+); calcd. For C31H45NO3·0.5H2O: C, 76.19; H, 9.49; N, 2.87. Found: C, 76.41; H, 9.62; N, 2.97%.
3), 3.55 (d, J = 10.07 Hz, 1H, H-23), 3.39 (d, J = 9.92 Hz, 1H, H-23), 1.27 (s, 3H), 1.13 (s, 3H), 1.02 (s, 3H), 0.94 (d, J = 5.36 Hz, 3H), 0.87 (d, J = 6.13 Hz, 3H), 0.83 (s, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ = 178.02 (C28), 153.59 (C3), 138.68 (C13), 127.16 (C2), 125.01 (C12), 117.64 (CN), 69.26, 55.37, 52.90 (2C), 51.48, 50.83, 48.03, 43.55, 42.39, 40.92, 38.83 (2C), 36.54, 33.41, 30.57, 28.20, 24.55, 24.09, 24.01, 21.16, 19.27, 18.44, 17.65, 17.08, 16.01 ppm. DI-ESI-MS m/z: 480.08 ([M + H]+); calcd. For C31H45NO3·0.5H2O: C, 76.19; H, 9.49; N, 2.87. Found: C, 76.41; H, 9.62; N, 2.97%.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 → 2
1 → 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford 17 as a white solid (134.4 mg, 56%). Mp: 147.1–150.1 °C. vmax/cm−1 (KBr): 3423.03, 2948.63, 2925.48, 2871.49, 2217.74, 1697.05, 1455.99, 1382.71, 1043.30. 1H NMR (400 MHz, CDCl3): δ = 6.48 (s, 1H, H-3), 5.25 (t, J = 2.99 Hz, 1H, H-12), 3.54 (d, J = 10.58 Hz, 1H, H-23), 3.38 (d, J = 10.58 Hz, 1H, H-23), 1.27 (s, 3H), 1.13 (s, 3H), 1.01 (s, 3H), 0.95 (d, J = 6.04 Hz, 3H), 0.87 (m, 6H) ppm. 13C NMR (100 MHz, CDCl3): δ = 183.40 (C28), 153.72 (C3), 138.50 (C13), 127.02 (C2), 125.13 (C12), 117.62 (CN), 69.17 (C23), 55.34, 52.85, 52.65, 50.81, 47.89, 43.53, 42.37, 40.92, 38.79 (2C), 36.63, 33.38, 30.54, 28.13, 24.54, 24.00, 23.93, 21.15, 19.29, 18.46, 17.62, 17.03, 16.00 ppm. DI-ESI-MS m/z: 466.03 ([M + H]+); calcd. For C30H43NO3·0.75H2O: C, 75.20; H, 9.36; N, 2.92. Found: C, 75.10; H, 9.83; N, 2.79%.
1) to afford 17 as a white solid (134.4 mg, 56%). Mp: 147.1–150.1 °C. vmax/cm−1 (KBr): 3423.03, 2948.63, 2925.48, 2871.49, 2217.74, 1697.05, 1455.99, 1382.71, 1043.30. 1H NMR (400 MHz, CDCl3): δ = 6.48 (s, 1H, H-3), 5.25 (t, J = 2.99 Hz, 1H, H-12), 3.54 (d, J = 10.58 Hz, 1H, H-23), 3.38 (d, J = 10.58 Hz, 1H, H-23), 1.27 (s, 3H), 1.13 (s, 3H), 1.01 (s, 3H), 0.95 (d, J = 6.04 Hz, 3H), 0.87 (m, 6H) ppm. 13C NMR (100 MHz, CDCl3): δ = 183.40 (C28), 153.72 (C3), 138.50 (C13), 127.02 (C2), 125.13 (C12), 117.62 (CN), 69.17 (C23), 55.34, 52.85, 52.65, 50.81, 47.89, 43.53, 42.37, 40.92, 38.79 (2C), 36.63, 33.38, 30.54, 28.13, 24.54, 24.00, 23.93, 21.15, 19.29, 18.46, 17.62, 17.03, 16.00 ppm. DI-ESI-MS m/z: 466.03 ([M + H]+); calcd. For C30H43NO3·0.75H2O: C, 75.20; H, 9.36; N, 2.92. Found: C, 75.10; H, 9.83; N, 2.79%.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 → 1
1 → 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford 18 as a white solid (144.6 mg, 48%). Mp: 139.1–142.0 °C. vmax/cm−1 (KBr): 3444.24, 2948.63, 2929.34, 2871.49, 2217.74, 1725.98, 1670.05, 1612.2, 1581.34, 1457.92, 1226.50. 1H NMR (400 MHz, CDCl3): δ = 6.57 (s, 1H, H-3), 5.71 (s, 1H, H-12), 3.60 (s, 3H, COO
1) to afford 18 as a white solid (144.6 mg, 48%). Mp: 139.1–142.0 °C. vmax/cm−1 (KBr): 3444.24, 2948.63, 2929.34, 2871.49, 2217.74, 1725.98, 1670.05, 1612.2, 1581.34, 1457.92, 1226.50. 1H NMR (400 MHz, CDCl3): δ = 6.57 (s, 1H, H-3), 5.71 (s, 1H, H-12), 3.60 (s, 3H, COO![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3), 3.55 (d, J = 10.93 Hz, 1H, H-23), 3.38 (d, J = 10.92 Hz, 1H, H-23), 1.40 (s, 3H), 1.33 (s, 3H), 1.01 (s, 3H), 0.97 (d, J = 6.26 Hz, 3H), 0.91 (s, 3H), 0.87 (d, J = 6.43 Hz, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ = 197.16 (C11), 177.19 (C28), 163.92 (C13), 155.23 (C3), 129.34 (C12), 128.72 (C2), 117.24 (CN), 68.76, 57.20, 54.68, 52.93, 51.88, 50.86, 49.96, 47.56, 45.43, 44.25, 38.61, 38.45, 35.91, 33.14, 30.20, 28.74, 23.81, 21.51, 20.96, 20.76, 20.44, 17.23, 16.86, 15.86 ppm. DI-ESI-MS m/z: 494.37 ([M + H]+); calcd. For C31H43NO4·0.5H2O: C, 74.07; H, 8.82; N, 2.79. Found: C, 74.10; H, 8.83; N, 2.94%.
3), 3.55 (d, J = 10.93 Hz, 1H, H-23), 3.38 (d, J = 10.92 Hz, 1H, H-23), 1.40 (s, 3H), 1.33 (s, 3H), 1.01 (s, 3H), 0.97 (d, J = 6.26 Hz, 3H), 0.91 (s, 3H), 0.87 (d, J = 6.43 Hz, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ = 197.16 (C11), 177.19 (C28), 163.92 (C13), 155.23 (C3), 129.34 (C12), 128.72 (C2), 117.24 (CN), 68.76, 57.20, 54.68, 52.93, 51.88, 50.86, 49.96, 47.56, 45.43, 44.25, 38.61, 38.45, 35.91, 33.14, 30.20, 28.74, 23.81, 21.51, 20.96, 20.76, 20.44, 17.23, 16.86, 15.86 ppm. DI-ESI-MS m/z: 494.37 ([M + H]+); calcd. For C31H43NO4·0.5H2O: C, 74.07; H, 8.82; N, 2.79. Found: C, 74.10; H, 8.83; N, 2.94%.![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3), 2.05 (s, 3H, CH3CO), 1.45 (s, 3H), 1.31 (s, 3H), 1.05 (s, 3H), 0.96 (m, 6H), 0.86 (d, J = 6.51 Hz, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ = 199.12 (C11), 195.25 (CHO), 177.13 (C28), 171.05 (OCO), 164.61 (C13), 156.37 (C2), 143.96 (C3), 129.46 (C12), 70.75, 58.41, 56.75, 52.99, 51.88, 48.01, 47.56, 46.64, 45.77, 44.37, 38.61, 38.36, 35.84, 33.49, 30.19, 28.71, 23.85, 21.21, 21.02, 20.95, 20.85, 20.25, 17.12, 17.00, 16.28 ppm. DI-ESI-MS m/z: 539.88 ([M + H]+); calcd. For C33H46O6·0.75H2O: C, 71.77; H, 8.67. Found: C, 71.80; H, 8.65%.
3), 2.05 (s, 3H, CH3CO), 1.45 (s, 3H), 1.31 (s, 3H), 1.05 (s, 3H), 0.96 (m, 6H), 0.86 (d, J = 6.51 Hz, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ = 199.12 (C11), 195.25 (CHO), 177.13 (C28), 171.05 (OCO), 164.61 (C13), 156.37 (C2), 143.96 (C3), 129.46 (C12), 70.75, 58.41, 56.75, 52.99, 51.88, 48.01, 47.56, 46.64, 45.77, 44.37, 38.61, 38.36, 35.84, 33.49, 30.19, 28.71, 23.85, 21.21, 21.02, 20.95, 20.85, 20.25, 17.12, 17.00, 16.28 ppm. DI-ESI-MS m/z: 539.88 ([M + H]+); calcd. For C33H46O6·0.75H2O: C, 71.77; H, 8.67. Found: C, 71.80; H, 8.65%.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 → 5
1 → 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford 20 as a white solid (59.1 mg, 17%). Mp: 77.8–80.3 °C. vmax/cm−1 (KBr): 2950.55, 2933.20, 2873.42, 1731.76, 1662.34, 1614.13, 1585.20, 1457.92, 1224.58, 1197.58, 1174.44. 1H NMR (400 MHz, CDCl3): δ = 10.15 (s, 1H, CHO), 6.39 (s, 1H, H-3), 5.68 (s, 1H, H-12), 3.98 (d, J = 10.78 Hz, 1H, H-23), 3.90 (d, J = 10.63 Hz, 1H, H-23), 3.62 (s, 3H, COOCH3), 1.46 (s, 3H), 1.31 (s, 3H), 1.05 (s, 3H), 0.96–0.92 (m, 6H), 0.86 (d, J = 6.23 Hz, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ = 199.13 (C11), 195.22 (CHO), 177.14 (C28), 173.61 (OCO), 164.59 (C13), 156.36 (C2), 144.10 (C3), 129.49 (C12), 70.33, 58.41, 56.64, 53.00, 51.89, 47.98, 47.57, 46.73, 45.79, 44.37, 38.62, 38.38, 36.16, 35.86, 33.51, 30.20, 28.72, 23.86, 21.21, 21.01, 20.97, 20.30, 18.47, 17.14, 16.97, 16.31, 13.73 ppm. DI-ESI-MS m/z: 567.40 ([M + H]+). Anal. calcd. For C35H50O6: C, 74.17; H, 8.89. Found: C, 73.79; H, 9.21%.
1) to afford 20 as a white solid (59.1 mg, 17%). Mp: 77.8–80.3 °C. vmax/cm−1 (KBr): 2950.55, 2933.20, 2873.42, 1731.76, 1662.34, 1614.13, 1585.20, 1457.92, 1224.58, 1197.58, 1174.44. 1H NMR (400 MHz, CDCl3): δ = 10.15 (s, 1H, CHO), 6.39 (s, 1H, H-3), 5.68 (s, 1H, H-12), 3.98 (d, J = 10.78 Hz, 1H, H-23), 3.90 (d, J = 10.63 Hz, 1H, H-23), 3.62 (s, 3H, COOCH3), 1.46 (s, 3H), 1.31 (s, 3H), 1.05 (s, 3H), 0.96–0.92 (m, 6H), 0.86 (d, J = 6.23 Hz, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ = 199.13 (C11), 195.22 (CHO), 177.14 (C28), 173.61 (OCO), 164.59 (C13), 156.36 (C2), 144.10 (C3), 129.49 (C12), 70.33, 58.41, 56.64, 53.00, 51.89, 47.98, 47.57, 46.73, 45.79, 44.37, 38.62, 38.38, 36.16, 35.86, 33.51, 30.20, 28.72, 23.86, 21.21, 21.01, 20.97, 20.30, 18.47, 17.14, 16.97, 16.31, 13.73 ppm. DI-ESI-MS m/z: 567.40 ([M + H]+). Anal. calcd. For C35H50O6: C, 74.17; H, 8.89. Found: C, 73.79; H, 9.21%.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 → 4
1 → 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford 21 as a white solid (268.7 mg, 71%). Mp: 97.0–100.2 °C. vmax/cm−1 (KBr): 3083.62, 3060.48, 3025.76, 2948.63, 2931.27, 2873.42, 1718.26, 1664.27, 1637.27, 1452.14, 1382.71, 1309.43, 1272.79, 1228.43, 1201.43, 1164.79. 1H NMR (400 MHz, CDCl3): δ = 10.17 (s, 1H, CHO), 7.69 (d, J = 15.98 Hz, 1H, H-3′), 7.54–7.52 (m, 2H, H-2′′ and H-6′′), 7.40–7.38 (m, 3H, H-3′′, H-4′′, H-5′′), 6.47 (s, 1H, H-3), 6.43 (d, J = 16.02 Hz, 1H, H-2′), 5.68 (s, 1H, H-12), 4.11 (d, J = 11.01 Hz, 1H, H-23), 4.07 (d, J = 11.05 Hz, 1H, H-23), 3.62 (s, 3H, COO
1) to afford 21 as a white solid (268.7 mg, 71%). Mp: 97.0–100.2 °C. vmax/cm−1 (KBr): 3083.62, 3060.48, 3025.76, 2948.63, 2931.27, 2873.42, 1718.26, 1664.27, 1637.27, 1452.14, 1382.71, 1309.43, 1272.79, 1228.43, 1201.43, 1164.79. 1H NMR (400 MHz, CDCl3): δ = 10.17 (s, 1H, CHO), 7.69 (d, J = 15.98 Hz, 1H, H-3′), 7.54–7.52 (m, 2H, H-2′′ and H-6′′), 7.40–7.38 (m, 3H, H-3′′, H-4′′, H-5′′), 6.47 (s, 1H, H-3), 6.43 (d, J = 16.02 Hz, 1H, H-2′), 5.68 (s, 1H, H-12), 4.11 (d, J = 11.01 Hz, 1H, H-23), 4.07 (d, J = 11.05 Hz, 1H, H-23), 3.62 (s, 3H, COO![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3), 1.48 (s, 3H), 1.29 (s, 3H), 1.11 (s, 3H), 0.96–0.95 (m, 6H), 0.84 (d, J = 6.36 Hz, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ = 199.18 (C11), 195.29 (CHO), 177.14 (C28), 166.94 (OCO), 164.66 (C13), 156.39 (C2), 145.30, 144.13 (C3), 134.22, 130.43, 129.43 (C12), 128.89 (2C), 128.19 (2C), 117.61, 70.67, 58.42, 56.73, 52.98, 51.88, 48.03, 47.55, 46.92, 45.78, 44.37, 38.59, 38.32, 35.84, 33.51, 30.18, 28.69, 23.83, 21.19, 21.02, 20.94, 20.29, 17.10, 17.03, 16.37 ppm. DI-ESI-MS2 m/z: 627.36 ([M + H]+, 42%), 609.48 (62), 566.37 (72), 479.38 (100), 419.39 (29); calcd. For C40H50O6·H2O: C, 74.50; H, 8.13. Found: C, 74.10; H, 7.76%.
3), 1.48 (s, 3H), 1.29 (s, 3H), 1.11 (s, 3H), 0.96–0.95 (m, 6H), 0.84 (d, J = 6.36 Hz, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ = 199.18 (C11), 195.29 (CHO), 177.14 (C28), 166.94 (OCO), 164.66 (C13), 156.39 (C2), 145.30, 144.13 (C3), 134.22, 130.43, 129.43 (C12), 128.89 (2C), 128.19 (2C), 117.61, 70.67, 58.42, 56.73, 52.98, 51.88, 48.03, 47.55, 46.92, 45.78, 44.37, 38.59, 38.32, 35.84, 33.51, 30.18, 28.69, 23.83, 21.19, 21.02, 20.94, 20.29, 17.10, 17.03, 16.37 ppm. DI-ESI-MS2 m/z: 627.36 ([M + H]+, 42%), 609.48 (62), 566.37 (72), 479.38 (100), 419.39 (29); calcd. For C40H50O6·H2O: C, 74.50; H, 8.13. Found: C, 74.10; H, 7.76%.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford 22 as a white solid (41.4 mg, 12%). Mp: 111.6–114.1 °C. vmax/cm−1 (KBr): 3129.90, 2948.63, 2873.42, 1764.55, 1725.98, 1662.34, 1614.13, 1587.13, 1457.92, 1400.07, 1288.22, 1240.00, 1004.73. 1H NMR (400 MHz, CDCl3): δ = 10.14 (s, 1H, CHO), 8.09 (s, 1H, H-2′′), 7.30 (s, 1H, H-5′′), 7.06 (s, 1H, H-4′′), 6.37 (s, 1H, H-3), 5.67 (s, 1H, H-12), 4.29 (d, J = 10.50, 1H, H-23), 4.24 (d, J = 10.33 Hz, 1H, H-23), 3.60 (s, 3H, COO
1) to afford 22 as a white solid (41.4 mg, 12%). Mp: 111.6–114.1 °C. vmax/cm−1 (KBr): 3129.90, 2948.63, 2873.42, 1764.55, 1725.98, 1662.34, 1614.13, 1587.13, 1457.92, 1400.07, 1288.22, 1240.00, 1004.73. 1H NMR (400 MHz, CDCl3): δ = 10.14 (s, 1H, CHO), 8.09 (s, 1H, H-2′′), 7.30 (s, 1H, H-5′′), 7.06 (s, 1H, H-4′′), 6.37 (s, 1H, H-3), 5.67 (s, 1H, H-12), 4.29 (d, J = 10.50, 1H, H-23), 4.24 (d, J = 10.33 Hz, 1H, H-23), 3.60 (s, 3H, COO![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3), 1.24 (s, 3H), 1.13 (s, 3H), 0.95 (s, 6H), 0.84 (d, J = 5.41 Hz, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ = 198.78 (C11), 194.76 (CHO), 177.06 (C28), 164.76 (C13), 157.09 (C2), 148.56 (OCO), 142.24 (C3), 136.89, 130.90, 129.34 (C12), 116.90, 73.85, 58.35, 56.69, 52.95, 51.85, 48.10, 47.50, 46.95, 45.68, 44.29, 38.56, 38.29, 35.77, 33.46, 30.12, 28.66, 23.77, 21.01, 20.97, 20.90, 20.20, 17.11, 17.05, 16.20 ppm. DI-ESI-MS m/z: 591.52 ([M + H]+); calcd. For C35H46N2O6·0.25H2O: C, 70.62; H, 7.87; N, 4.71. Found: C, 70.30; H, 7.71; N, 4.35%.
3), 1.24 (s, 3H), 1.13 (s, 3H), 0.95 (s, 6H), 0.84 (d, J = 5.41 Hz, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ = 198.78 (C11), 194.76 (CHO), 177.06 (C28), 164.76 (C13), 157.09 (C2), 148.56 (OCO), 142.24 (C3), 136.89, 130.90, 129.34 (C12), 116.90, 73.85, 58.35, 56.69, 52.95, 51.85, 48.10, 47.50, 46.95, 45.68, 44.29, 38.56, 38.29, 35.77, 33.46, 30.12, 28.66, 23.77, 21.01, 20.97, 20.90, 20.20, 17.11, 17.05, 16.20 ppm. DI-ESI-MS m/z: 591.52 ([M + H]+); calcd. For C35H46N2O6·0.25H2O: C, 70.62; H, 7.87; N, 4.71. Found: C, 70.30; H, 7.71; N, 4.35%.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 → 1
1 → 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) to afford 23 as a white solid (121.4 mg, 33%). Mp: 128.7–131.0 °C. vmax/cm−1 (KBr): 3122.19, 2948.63, 2931.27, 2873.42, 1758.76, 1725.98, 1660.41, 1614.13, 1587.13, 1552.42, 1511.92, 1457.92, 1398.14, 1295.93, 1143.58. 1H NMR (400 MHz, CDCl3): δ = 10.15 (s, 1H, CHO), 7.28 (d, J = 1.47 Hz, 1H, H-5′′), 6.84 (d, J = 1.48 Hz, 1H, H-4′′), 6.39 (s, 1H, H-3), 5.67 (s, 1H, H-12), 4.27 (d, J = 10.81 Hz, 1H, H-23), 4.20 (d, J = 10.81 Hz, 1H, H-23), 3.61 (s, 3H, COO
2) to afford 23 as a white solid (121.4 mg, 33%). Mp: 128.7–131.0 °C. vmax/cm−1 (KBr): 3122.19, 2948.63, 2931.27, 2873.42, 1758.76, 1725.98, 1660.41, 1614.13, 1587.13, 1552.42, 1511.92, 1457.92, 1398.14, 1295.93, 1143.58. 1H NMR (400 MHz, CDCl3): δ = 10.15 (s, 1H, CHO), 7.28 (d, J = 1.47 Hz, 1H, H-5′′), 6.84 (d, J = 1.48 Hz, 1H, H-4′′), 6.39 (s, 1H, H-3), 5.67 (s, 1H, H-12), 4.27 (d, J = 10.81 Hz, 1H, H-23), 4.20 (d, J = 10.81 Hz, 1H, H-23), 3.61 (s, 3H, COO![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3), 2.62 (s, 3H, CH3 imidazole), 1.47 (s, 3H), 1.23 (s, 3H), 1.13 (s, 3H), 0.97–0.95 (m, 6H), 0.85 (d, J = 6.30 Hz, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ = 198.80 (C11), 194.81 (CHO), 177.08 (C28), 164.75 (C13), 157.03 (C2), 149.47 (OCO), 147.91, 142.61 (C3), 129.39 (C12), 128.20, 117.78, 73.30, 58.38, 56.42, 52.98, 51.88, 48.07, 47.52, 46.93, 45.72, 44.32, 38.59, 38.33, 35.81, 33.48, 30.15, 28.70, 23.80, 21.06, 20.98, 20.92, 20.26, 17.13, 16.94, 16.85, 16.26 ppm. DI-ESI-MS m/z: 605.40 ([M + H]+); calcd. For C36H48N2O6: C, 71.50; H, 8.00; N, 4.63. Found: C, 71.16; H, 8.46; N, 4.36%.
3), 2.62 (s, 3H, CH3 imidazole), 1.47 (s, 3H), 1.23 (s, 3H), 1.13 (s, 3H), 0.97–0.95 (m, 6H), 0.85 (d, J = 6.30 Hz, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ = 198.80 (C11), 194.81 (CHO), 177.08 (C28), 164.75 (C13), 157.03 (C2), 149.47 (OCO), 147.91, 142.61 (C3), 129.39 (C12), 128.20, 117.78, 73.30, 58.38, 56.42, 52.98, 51.88, 48.07, 47.52, 46.93, 45.72, 44.32, 38.59, 38.33, 35.81, 33.48, 30.15, 28.70, 23.80, 21.06, 20.98, 20.92, 20.26, 17.13, 16.94, 16.85, 16.26 ppm. DI-ESI-MS m/z: 605.40 ([M + H]+); calcd. For C36H48N2O6: C, 71.50; H, 8.00; N, 4.63. Found: C, 71.16; H, 8.46; N, 4.36%.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 → 1.5
1 → 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford 24 as a white solid (183.1 mg, 53%); Mp: 114.0–116.5 °C. vmax/cm−1 (KBr): 2948.63, 2933.20, 2873.42, 1725.98, 1662.34, 1614.13, 1587.13, 1457.92, 1355.71, 1174.44, 958.45 cm−1. 1H NMR (400 MHz, CDCl3): δ = 10.15 (s, 1H, CHO), 6.30 (s, 1H, H-3), 5.68 (s, 1H, H-12), 4.07 (d, J = 10.05 Hz, 1H, H-23), 4.04 (d, J = 10.05 Hz, 1H, H-23), 3.62 (s, 3H, COO
1) to afford 24 as a white solid (183.1 mg, 53%); Mp: 114.0–116.5 °C. vmax/cm−1 (KBr): 2948.63, 2933.20, 2873.42, 1725.98, 1662.34, 1614.13, 1587.13, 1457.92, 1355.71, 1174.44, 958.45 cm−1. 1H NMR (400 MHz, CDCl3): δ = 10.15 (s, 1H, CHO), 6.30 (s, 1H, H-3), 5.68 (s, 1H, H-12), 4.07 (d, J = 10.05 Hz, 1H, H-23), 4.04 (d, J = 10.05 Hz, 1H, H-23), 3.62 (s, 3H, COO![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3), 3.01 (s, 3H, S–
3), 3.01 (s, 3H, S–![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3), 1.46 (s, 3H), 1.33 (s, 3H), 1.09 (s, 3H), 0.97–0.96 (m, 6H), 0.86 (d, J = 6.21 Hz, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ = 199.04 (C11), 194.93 (CHO), 177.14 (C28), 164.93 (C13), 157.18 (C2), 141.96 (C3), 129.35 (C12), 75.09, 58.28, 56.08, 53.01, 51.89, 48.15, 47.56, 46.91, 45.75, 44.44, 38.61, 38.35, 37.55, 35.84, 33.28, 30.18, 29.69, 23.83, 21.25, 21.05, 20.95, 20.27, 17.12, 16.94, 16.06 ppm. DI-ESI-MS m/z: 575.42 ([M + H]+), 597.30 ([M + Na]+). Anal. calcd. For C32H46O7S: C, 66.87; H, 8.07; S, 5.58. Found: C, 66.48; H, 8.45; S, 5.20%.
3), 1.46 (s, 3H), 1.33 (s, 3H), 1.09 (s, 3H), 0.97–0.96 (m, 6H), 0.86 (d, J = 6.21 Hz, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ = 199.04 (C11), 194.93 (CHO), 177.14 (C28), 164.93 (C13), 157.18 (C2), 141.96 (C3), 129.35 (C12), 75.09, 58.28, 56.08, 53.01, 51.89, 48.15, 47.56, 46.91, 45.75, 44.44, 38.61, 38.35, 37.55, 35.84, 33.28, 30.18, 29.69, 23.83, 21.25, 21.05, 20.95, 20.27, 17.12, 16.94, 16.06 ppm. DI-ESI-MS m/z: 575.42 ([M + H]+), 597.30 ([M + Na]+). Anal. calcd. For C32H46O7S: C, 66.87; H, 8.07; S, 5.58. Found: C, 66.48; H, 8.45; S, 5.20%.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 → 4
1 → 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford 25 as a white solid (126.4 mg, 58%). Mp: 86.7–88.6 °C. vmax/cm−1 (KBr): 2948.63, 2925.48, 2871.49, 2215.81, 1745.26, 1724.05, 1650.77, 1589.06, 1455.99, 1382.71, 1236.15, 1039.44. 1H NMR (400 MHz, CDCl3): δ = 6.44 (s, 1H, H-3), 5.26 (t, J = 2.95 Hz, 1H, H-12), 3.97 (d, J = 11.05 Hz, 1H, H-23), 3.90 (d, J = 10.95 Hz, 1H, H-23), 3.60 (s, 3H, COO
1) to afford 25 as a white solid (126.4 mg, 58%). Mp: 86.7–88.6 °C. vmax/cm−1 (KBr): 2948.63, 2925.48, 2871.49, 2215.81, 1745.26, 1724.05, 1650.77, 1589.06, 1455.99, 1382.71, 1236.15, 1039.44. 1H NMR (400 MHz, CDCl3): δ = 6.44 (s, 1H, H-3), 5.26 (t, J = 2.95 Hz, 1H, H-12), 3.97 (d, J = 11.05 Hz, 1H, H-23), 3.90 (d, J = 10.95 Hz, 1H, H-23), 3.60 (s, 3H, COO![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3), 2.06 (s, 3H, CO
3), 2.06 (s, 3H, CO![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3), 1.26 (s, 3H), 1.11 (s, 3H), 1.05 (s, 3H), 0.94 (d, J = 5.92 Hz, 3H), 0.87 (d, J = 6.32 Hz, 3H), 0.83 (s, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ = 177.95 (C28), 170.88 (OCO), 152.65 (C3), 138.72 (C13), 127.03 (C2), 124.93 (C12), 117.46 (CN), 69.73, 56.16, 52.89, 52.61, 51.47, 48.99, 48.01, 43.79, 42.33, 40.88, 38.81, 38.81, 36.51, 33.42, 30.54, 28.12, 24.54, 24.06, 23.81, 21.15, 20.80, 19.02, 18.42, 17.49, 17.11, 16.19 ppm. DI-ESI-MS m/z: 522.31 ([M + H]+); calcd. For C33H47NO4: C, 75.97; H, 9.08; N, 2.68. Found: C, 76.22; H, 9.25; N, 2.85%.
3), 1.26 (s, 3H), 1.11 (s, 3H), 1.05 (s, 3H), 0.94 (d, J = 5.92 Hz, 3H), 0.87 (d, J = 6.32 Hz, 3H), 0.83 (s, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ = 177.95 (C28), 170.88 (OCO), 152.65 (C3), 138.72 (C13), 127.03 (C2), 124.93 (C12), 117.46 (CN), 69.73, 56.16, 52.89, 52.61, 51.47, 48.99, 48.01, 43.79, 42.33, 40.88, 38.81, 38.81, 36.51, 33.42, 30.54, 28.12, 24.54, 24.06, 23.81, 21.15, 20.80, 19.02, 18.42, 17.49, 17.11, 16.19 ppm. DI-ESI-MS m/z: 522.31 ([M + H]+); calcd. For C33H47NO4: C, 75.97; H, 9.08; N, 2.68. Found: C, 76.22; H, 9.25; N, 2.85%.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 → 6
1 → 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford 26 as a white solid (101.1 mg, 30%); Mp: 65.5–68.0 °C. vmax/cm−1 (KBr): 2948.63, 2927.41, 2873.42, 2215.81, 1735.55, 1652.70, 1589.06, 1455.99, 1172.51. 1H NMR (400 MHz, CDCl3): δ = 6.44 (s, 1H, H-3), 5.27 (t, J = 3.0 Hz, 1H, H-12), 3.98 (d, J = 10.94 Hz, 1H, H-23), 3.90 (d, J = 10.94 Hz, 2H, H-23), 3.61 (s, 3H, COO
1) to afford 26 as a white solid (101.1 mg, 30%); Mp: 65.5–68.0 °C. vmax/cm−1 (KBr): 2948.63, 2927.41, 2873.42, 2215.81, 1735.55, 1652.70, 1589.06, 1455.99, 1172.51. 1H NMR (400 MHz, CDCl3): δ = 6.44 (s, 1H, H-3), 5.27 (t, J = 3.0 Hz, 1H, H-12), 3.98 (d, J = 10.94 Hz, 1H, H-23), 3.90 (d, J = 10.94 Hz, 2H, H-23), 3.61 (s, 3H, COO![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3), 1.27 (s, 3H), 1.10 (s, 3H), 1.04 (s, 3H), 0.97–0.93 (m, 6H), 0.87 (d, J = 6.09 Hz, 3H), 0.83 (s, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ = 177.96 (C28), 173.45 (OCO), 152.81 (C3), 138.70 (C13), 126.99 (C2), 124.95 (C12), 117.47 (CN), 69.27, 55.99, 52.88, 52.60, 51.48, 49.09, 48.01, 43.76, 42.33, 40.89, 38.82 (2C), 36.52, 36.16, 33.43, 30.55, 28.13, 24.54, 24.06, 23.79, 21.15, 19.06, 18.48, 18.40, 17.44, 17.12, 16.23, 13.71 ppm. DI-ESI-MS m/z: 550.33 ([M + H]+); calcd. For C35H51NO4: C, 76.46; H, 9.35; N, 2.55. Found: C, 76.75; H, 9.62; N, 2.58%.
3), 1.27 (s, 3H), 1.10 (s, 3H), 1.04 (s, 3H), 0.97–0.93 (m, 6H), 0.87 (d, J = 6.09 Hz, 3H), 0.83 (s, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ = 177.96 (C28), 173.45 (OCO), 152.81 (C3), 138.70 (C13), 126.99 (C2), 124.95 (C12), 117.47 (CN), 69.27, 55.99, 52.88, 52.60, 51.48, 49.09, 48.01, 43.76, 42.33, 40.89, 38.82 (2C), 36.52, 36.16, 33.43, 30.55, 28.13, 24.54, 24.06, 23.79, 21.15, 19.06, 18.48, 18.40, 17.44, 17.12, 16.23, 13.71 ppm. DI-ESI-MS m/z: 550.33 ([M + H]+); calcd. For C35H51NO4: C, 76.46; H, 9.35; N, 2.55. Found: C, 76.75; H, 9.62; N, 2.58%.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 → 7
1 → 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford 27 a white solid (158.2 mg, 52%). Mp: 100.2–103.1 °C. vmax/cm−1 (KBr): 3089.40, 3060.48, 2948.63, 2925.48, 2871.49, 2215.81, 1722.12, 1602.56, 1585.20, 1452.14, 1270.86, 1112.73. 1H NMR (400 MHz, CDCl3): δ = 8.00 (d, J = 7.23 Hz, 2H, H2′′ and H6′′), 7.58 (t, J = 7.34 Hz, 1H, H4′′), 7.45 (t, J = 7.75 Hz, 2H, H3′′ and H5′′), 6.54 (s, 1H, H-3), 5.26 (t, J = 2.80 Hz, 1H, H-12), 4.24 (d, J = 11.00 Hz, 1H, H-23), 4.14 (d, J = 11.00 Hz, 1H, H-23), 3.61 (s, 3H, COO
1) to afford 27 a white solid (158.2 mg, 52%). Mp: 100.2–103.1 °C. vmax/cm−1 (KBr): 3089.40, 3060.48, 2948.63, 2925.48, 2871.49, 2215.81, 1722.12, 1602.56, 1585.20, 1452.14, 1270.86, 1112.73. 1H NMR (400 MHz, CDCl3): δ = 8.00 (d, J = 7.23 Hz, 2H, H2′′ and H6′′), 7.58 (t, J = 7.34 Hz, 1H, H4′′), 7.45 (t, J = 7.75 Hz, 2H, H3′′ and H5′′), 6.54 (s, 1H, H-3), 5.26 (t, J = 2.80 Hz, 1H, H-12), 4.24 (d, J = 11.00 Hz, 1H, H-23), 4.14 (d, J = 11.00 Hz, 1H, H-23), 3.61 (s, 3H, COO![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3), 1.30 (s, 3H), 1.15 (s, 3H), 1.03 (s, 3H), 0.94 (d, J = 5.79 Hz, 3H), 0.86 (d, J = 6.64 Hz, 3H). 0.84 (s, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ = 177.95 (C28), 166.27 (OCO), 152.76 (C3), 138.73 (C13), 133.26, 129.75, 129.54, 128.55, 127.18 (C2), 124.92 (C12), 117.45 (CN), 69.85, 56.08, 52.87, 52.67, 51.47, 49.39, 48.00, 43.88, 42.31, 40.90, 38.83, 38.78, 36.52, 33.49, 30.54, 28.09, 24.57, 24.04, 23.63, 21.11, 19.00, 18.41, 17.49, 17.12, 16.36 ppm. DI-ESI-MS m/z: 584.29 ([M + H]+); calcd. For C38H49NO4: C, 78.18; H, 8.46; N, 2.40. Found: C, 77.80; H, 8.58; 2.52%.
3), 1.30 (s, 3H), 1.15 (s, 3H), 1.03 (s, 3H), 0.94 (d, J = 5.79 Hz, 3H), 0.86 (d, J = 6.64 Hz, 3H). 0.84 (s, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ = 177.95 (C28), 166.27 (OCO), 152.76 (C3), 138.73 (C13), 133.26, 129.75, 129.54, 128.55, 127.18 (C2), 124.92 (C12), 117.45 (CN), 69.85, 56.08, 52.87, 52.67, 51.47, 49.39, 48.00, 43.88, 42.31, 40.90, 38.83, 38.78, 36.52, 33.49, 30.54, 28.09, 24.57, 24.04, 23.63, 21.11, 19.00, 18.41, 17.49, 17.12, 16.36 ppm. DI-ESI-MS m/z: 584.29 ([M + H]+); calcd. For C38H49NO4: C, 78.18; H, 8.46; N, 2.40. Found: C, 77.80; H, 8.58; 2.52%.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 → 1
1 → 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford 28 as a white solid (213.9 mg, 71%). Mp: 105.8–107.7 °C. vmax/cm−1 (KBr): 2948.63, 2927.41, 2871.49, 2657.43, 2559.08, 2215.81, 1743.33, 1727.91, 1712.48, 1589.06, 1455.99, 1201.43, 1162.87. 1H NMR (400 MHz, CDCl3): δ = 6.43 (s, 1H, H-3), 5.26 (t, J = 2.85 Hz, 1H, H-12), 4.01 (d, J = 11.01 Hz, 1H, H-23), 3.92 (d, J = 11.01 Hz, 1H, H-23), 3.60 (s, 3H, COO
1) to afford 28 as a white solid (213.9 mg, 71%). Mp: 105.8–107.7 °C. vmax/cm−1 (KBr): 2948.63, 2927.41, 2871.49, 2657.43, 2559.08, 2215.81, 1743.33, 1727.91, 1712.48, 1589.06, 1455.99, 1201.43, 1162.87. 1H NMR (400 MHz, CDCl3): δ = 6.43 (s, 1H, H-3), 5.26 (t, J = 2.85 Hz, 1H, H-12), 4.01 (d, J = 11.01 Hz, 1H, H-23), 3.92 (d, J = 11.01 Hz, 1H, H-23), 3.60 (s, 3H, COO![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3), 2.68–2.64 (m, 4H, H-2′ and H-3′), 1.26 (s, 3H), 1.11 (s, 3H), 1.05 (s, 3H), 0.94 (d, J = 5.96 Hz, 3H), 0.87 (d, J = 6.33 Hz, 3H), 0.83 (s, 3H). 13C NMR (100 MHz, CDCl3): δ = 178.00 (C28), 177.25, 171.89, 152.49 (C3), 138.71 (C13), 127.10 (C2), 124.91 (C12), 117.41 (CN), 70.06, 56.21, 52.88, 52.62, 51.49, 49.05, 48.02, 43.74, 42.33, 40.88, 38.80 (2C), 36.51, 33.40, 30.54, 28.76, 28.70, 28.10, 24.53, 24.06, 23.82, 21.15, 19.02, 18.41, 17.49, 17.10, 16.17 ppm. DI-ESI-MS m/z: 580.35 ([M + H]+); calcd. For C35H49NO6·H2O: C, 70.32; H, 8.60; N, 2.34. Found: C, 70.22; H, 8.50; N, 2.38%.
3), 2.68–2.64 (m, 4H, H-2′ and H-3′), 1.26 (s, 3H), 1.11 (s, 3H), 1.05 (s, 3H), 0.94 (d, J = 5.96 Hz, 3H), 0.87 (d, J = 6.33 Hz, 3H), 0.83 (s, 3H). 13C NMR (100 MHz, CDCl3): δ = 178.00 (C28), 177.25, 171.89, 152.49 (C3), 138.71 (C13), 127.10 (C2), 124.91 (C12), 117.41 (CN), 70.06, 56.21, 52.88, 52.62, 51.49, 49.05, 48.02, 43.74, 42.33, 40.88, 38.80 (2C), 36.51, 33.40, 30.54, 28.76, 28.70, 28.10, 24.53, 24.06, 23.82, 21.15, 19.02, 18.41, 17.49, 17.10, 16.17 ppm. DI-ESI-MS m/z: 580.35 ([M + H]+); calcd. For C35H49NO6·H2O: C, 70.32; H, 8.60; N, 2.34. Found: C, 70.22; H, 8.50; N, 2.38%.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford 29 as a white solid (189 mg, 50%) Mp: 102.3–105.0 °C. vmax/cm−1 (KBr): 3083.62, 3060.48, 2946.70, 2925.48, 2871.49, 2215.81, 1718.26, 1637.27, 1579.41, 1496.49, 1452.14, 1162.87. 1H NMR (400 MHz, CDCl3): δ = 7.69 (d, J = 16.15 Hz, 1H, H-3′), 7.54–7.52 (m, 2H, H-2′′and H-6′′), 7.41–7.39 (m, 3H, H-3′′, H-4′′ and H-5′′), 6.51 (s, 1H, H-3), 6.43 (d, J = 16.17 Hz, 1H, H-2′), 5.27 (t, J = 2.65 Hz, 1H, H-12), 4.10 (d, J = 10.93 Hz, 1H, H-23), 4.05 (d, J = 10.99 Hz, 1H, H-23), 3.61 (s, 3H, COO
1) to afford 29 as a white solid (189 mg, 50%) Mp: 102.3–105.0 °C. vmax/cm−1 (KBr): 3083.62, 3060.48, 2946.70, 2925.48, 2871.49, 2215.81, 1718.26, 1637.27, 1579.41, 1496.49, 1452.14, 1162.87. 1H NMR (400 MHz, CDCl3): δ = 7.69 (d, J = 16.15 Hz, 1H, H-3′), 7.54–7.52 (m, 2H, H-2′′and H-6′′), 7.41–7.39 (m, 3H, H-3′′, H-4′′ and H-5′′), 6.51 (s, 1H, H-3), 6.43 (d, J = 16.17 Hz, 1H, H-2′), 5.27 (t, J = 2.65 Hz, 1H, H-12), 4.10 (d, J = 10.93 Hz, 1H, H-23), 4.05 (d, J = 10.99 Hz, 1H, H-23), 3.61 (s, 3H, COO![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3), 1.29 (s, 3H), 1.11 (s, 3H), 1.08 (s, 3H), 0.93 (d, J = 6.04 Hz, 3H), 0.85–0.84 (m, 6H) ppm. 13C NMR (100 MHz, CDCl3): δ = 177.95 (C28), 166.75, 152.77 (C3), 145.50, 138.72 (C13), 134.16, 130.50, 128.91 (2C), 128.17 (2C), 127.06 (C2), 124.91 (C12), 117.48 (CN), 117.42, 69.70, 56.22, 52.88, 52.66, 51.46, 49.23, 48.01, 43.81, 42.34, 40.89, 38.81, 38.77, 36.51, 33.44, 30.53, 28.11, 24.54, 24.05, 23.85, 21.13, 19.06, 18.44, 17.54, 17.08, 16.28 ppm. DI-ESI-MS m/z: 610.33 ([M + H]+); calcd. For C40H51NO4: C, 78.78; H, 8.43; N, 2.30. Found: C, 78.82; H, 8.20; N, 2.46%.
3), 1.29 (s, 3H), 1.11 (s, 3H), 1.08 (s, 3H), 0.93 (d, J = 6.04 Hz, 3H), 0.85–0.84 (m, 6H) ppm. 13C NMR (100 MHz, CDCl3): δ = 177.95 (C28), 166.75, 152.77 (C3), 145.50, 138.72 (C13), 134.16, 130.50, 128.91 (2C), 128.17 (2C), 127.06 (C2), 124.91 (C12), 117.48 (CN), 117.42, 69.70, 56.22, 52.88, 52.66, 51.46, 49.23, 48.01, 43.81, 42.34, 40.89, 38.81, 38.77, 36.51, 33.44, 30.53, 28.11, 24.54, 24.05, 23.85, 21.13, 19.06, 18.44, 17.54, 17.08, 16.28 ppm. DI-ESI-MS m/z: 610.33 ([M + H]+); calcd. For C40H51NO4: C, 78.78; H, 8.43; N, 2.30. Found: C, 78.82; H, 8.20; N, 2.46%.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 → 1
1 → 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford 30 as a white solid (204.1 mg, 59%). Mp: 104.1–107.2 °C. vmax/cm−1 (KBr): 3166.54, 2948.63, 2925.48, 2215.81, 1747.19, 1724.05, 1455.99, 1384.64, 1249.65. 1H NMR (400 MHz, CDCl3): δ = 7.27 (s, 1H, H-5′), 6.87 (s, 1H, H-4′), 6.47 (s, 1H, H-3), 5.27 (t, J = 3.0 Hz, 1H, H-12), 4.29 (d, J = 11.04 Hz, 1H, H-23), 4.18 (d, J = 11.01 Hz, 1H, H-23), 3.60 (s, 3H, COO
1) to afford 30 as a white solid (204.1 mg, 59%). Mp: 104.1–107.2 °C. vmax/cm−1 (KBr): 3166.54, 2948.63, 2925.48, 2215.81, 1747.19, 1724.05, 1455.99, 1384.64, 1249.65. 1H NMR (400 MHz, CDCl3): δ = 7.27 (s, 1H, H-5′), 6.87 (s, 1H, H-4′), 6.47 (s, 1H, H-3), 5.27 (t, J = 3.0 Hz, 1H, H-12), 4.29 (d, J = 11.04 Hz, 1H, H-23), 4.18 (d, J = 11.01 Hz, 1H, H-23), 3.60 (s, 3H, COO![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3), 2.63 (s, 3H, CH3 Imidazole), 1.30 (s, 3H), 1.13 (s, 3H), 1.04 (s, 3H), 0.94 (d, J = 5.68 Hz, 3H), 0.86 (d, J = 6.89 Hz, 3H), 0.84 (s, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ = 177.94 (C28), 151.40 (C3), 149.37 (OCO), 147.98, 138.73 (C13), 128.33, 127.99 (C2), 124.76 (C12), 117.68, 117.01 (CN), 72.34, 55.96, 52.84, 52.72, 51.48, 49.22, 47.99, 43.91, 42.30, 40.90, 38.82, 38.76, 36.50, 33.45, 30.51, 28.10, 24.51, 24.02, 23.70, 21.13, 18.95, 18.39, 17.43, 17.13, 16.85, 16.20 ppm. DI-ESI-MS m/z: 588.63 ([M + H]+); calcd. For C36H49N3O4: C, 73.56; H, 8.40; N, 7.15. Found: C, 73.46; H, 8.79; N, 6.86%.
3), 2.63 (s, 3H, CH3 Imidazole), 1.30 (s, 3H), 1.13 (s, 3H), 1.04 (s, 3H), 0.94 (d, J = 5.68 Hz, 3H), 0.86 (d, J = 6.89 Hz, 3H), 0.84 (s, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ = 177.94 (C28), 151.40 (C3), 149.37 (OCO), 147.98, 138.73 (C13), 128.33, 127.99 (C2), 124.76 (C12), 117.68, 117.01 (CN), 72.34, 55.96, 52.84, 52.72, 51.48, 49.22, 47.99, 43.91, 42.30, 40.90, 38.82, 38.76, 36.50, 33.45, 30.51, 28.10, 24.51, 24.02, 23.70, 21.13, 18.95, 18.39, 17.43, 17.13, 16.85, 16.20 ppm. DI-ESI-MS m/z: 588.63 ([M + H]+); calcd. For C36H49N3O4: C, 73.56; H, 8.40; N, 7.15. Found: C, 73.46; H, 8.79; N, 6.86%.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 → 3
1 → 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford 31 as a white solid (179.2 mg, 62%). Mp: 109.50–112.10 °C. vmax/cm−1 (KBr): 2948.63, 2925.48, 2871.49, 2217.74, 1722.12, 1455.99, 1359.57, 1176.36, 960.38. 1H NMR (400 MHz, CDCl3): δ = 6.43 (s, 1H, H-3), 5.27 (t, J = 2.54 Hz, 1H, H-12), 4.05 (d, J = 9.84, 1H, H-23), 4.00 (d, J = 9.98 Hz, 1H, H-23), 3.60 (s, 3H, COO
1) to afford 31 as a white solid (179.2 mg, 62%). Mp: 109.50–112.10 °C. vmax/cm−1 (KBr): 2948.63, 2925.48, 2871.49, 2217.74, 1722.12, 1455.99, 1359.57, 1176.36, 960.38. 1H NMR (400 MHz, CDCl3): δ = 6.43 (s, 1H, H-3), 5.27 (t, J = 2.54 Hz, 1H, H-12), 4.05 (d, J = 9.84, 1H, H-23), 4.00 (d, J = 9.98 Hz, 1H, H-23), 3.60 (s, 3H, COO![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3), 3.02 (s, 3H, S–CH3), 1.28 (s, 3H), 1.12 (s, 3H), 1.10 (s, 3H), 0.94 (d, J = 5.88 Hz, 3H), 0.87 (d, J = 6.44 Hz, 3H), 0.83 (s, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ = 177.94 (C28), 150.86 (C3), 138.79 (C13), 128.01 (C2), 124.76 (C12), 117.08 (CN), 74.09, 55.95, 52.87, 52.79, 51.47, 49.07, 48.00, 43.72, 42.38, 40.88, 38.81, 38.79, 37.56, 36.50, 33.27, 30.53, 28.10, 24.49, 24.04, 23.87, 21.15, 19.03, 18.43, 17.52, 17.20, 15.96. DI-ESI-MS m/z: 558.28 ([M + H]+); calcd. For C32H47NO5S·0.25H2O: C, 68.35; H, 8.51; N, 2.49; S, 5.70. Found: C, 68.55; H, 8.51; N, 2.67; S, 5.30%.
3), 3.02 (s, 3H, S–CH3), 1.28 (s, 3H), 1.12 (s, 3H), 1.10 (s, 3H), 0.94 (d, J = 5.88 Hz, 3H), 0.87 (d, J = 6.44 Hz, 3H), 0.83 (s, 3H) ppm. 13C NMR (100 MHz, CDCl3): δ = 177.94 (C28), 150.86 (C3), 138.79 (C13), 128.01 (C2), 124.76 (C12), 117.08 (CN), 74.09, 55.95, 52.87, 52.79, 51.47, 49.07, 48.00, 43.72, 42.38, 40.88, 38.81, 38.79, 37.56, 36.50, 33.27, 30.53, 28.10, 24.49, 24.04, 23.87, 21.15, 19.03, 18.43, 17.52, 17.20, 15.96. DI-ESI-MS m/z: 558.28 ([M + H]+); calcd. For C32H47NO5S·0.25H2O: C, 68.35; H, 8.51; N, 2.49; S, 5.70. Found: C, 68.55; H, 8.51; N, 2.67; S, 5.30%.4.2 Biology
Asiatic acid and its derivatives were suspended in DMSO at 20 mM as stock solutions that were stored at −80 °C. To obtain final assay concentrations, the stock solutions were diluted in culture medium. The final concentration of DMSO in working solutions was always equal or lower than 0.5%. Cisplatin was obtained from Sigma Aldrich.
Primary antibodies against p21cip1/waf1 (sc-397), Bcl-2 (sc-509), Bax (sc-493), cyclin E (sc-247) and cyclin D3 (sc-182) were obtained from Santa Cruz Biotechnology, inc. Primary antibodies against for p27kip1 (#610242), Bid (#550365) and poly-(ADP-ribose)-polymerase (PARP) (#556493) were obtained from BD Biosciences. Primary antibodies against caspase 3 (#9662) and caspase 8 (#9746S) were purchased from Cell Signaling. Primary antibody against α-actin (#69100) was obtained from MP Biomedicals. Secondary antibodies anti-mouse (P0260) and anti-rabbit (NA934) were obtained from Dako and from Amersham Biosciences, respectively.
The antiproliferative activities of compounds against Jurkat cells were determined by XTT assay. Briefly, Jurkat cells were seeded at a density of 4 × 103 cells per well in 96 well plates in 100 μL of medium. After 24 h of incubation, 100 μL of medium containing the tested compounds at different concentration, in triplicate, were added. Following 72 h of incubation, 100 μL of XTT solution were added to each well and the plates were incubated for a further 4 hours at 37 °C. Relative cell viability was measured by absorbance at 450 nm on an ELISA plate reader (Tecan Sunrise MR20-301, TECAN, Salzburg, Austria).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 5 min at 4 °C. The protein content in supernatants was determined using the bicinchoninic acid (BCA) assay kit (Pierce Biotechnology, Rockford).
000 rpm for 5 min at 4 °C. The protein content in supernatants was determined using the bicinchoninic acid (BCA) assay kit (Pierce Biotechnology, Rockford).Acknowledgements
Jorge A. R. Salvador thanks Universidade de Coimbra for financial support. Bruno M. F. Goncalves thanks Fundação para a Ciência e a Tecnologia for financial support (SFRH/BD/69193/2010). MC and SM thank MINECO of Spain and FEDER Funds (grant numbers SAF2011-25726 and SAF2014-56059-R) and Agència Catalana d’Ajuts Universitaris I de Recerca (AGAUR) (2014SGR1017). MC thanks also ICREA Foundation (Icrea Academia Award programme, Generalitat de Catalunya). The authors would like to acknowledge UC-NMR facility, which is supported in part by FEDER – European Regional Development Fund through the COMPETE Programme (Operational Programme for Competitiveness) and by National Funds through FCT – Fundação para a Ciência e a Tecnologia (Portuguese Foundation for Science and Technology) through grants REEQ/481/QUI/2006, RECI/QEQ-QFI/0168/2012, CENTRO-07-CT62-FEDER-002012, and Rede Nacional de Ressonância Magnética Nuclear (RNRMN), for NRM data. The authors would like to acknowledge Centro de Apoio Científico e Tecnolóxico á Investigación (C.A.C.T.I.), Universidade de Vigo, Campos Lagoas – Marcosende, 15, 36310 Vigo, for elemental analysis and CCIT-scientific and technological centers UB for flow cytometry analysis support.Notes and references
- D. F. Ahmedin Jemal, F. Bray, M. M. Center, J. Ferlay and E. Ward, Ca-Cancer J. Clin., 2011, 61, 69–90 CrossRef PubMed.
- J. Ferlay, I. Soerjomataram, R. Dikshit, S. Eser, C. Mathers, M. Rebelo, D. M. Parkin, D. Forman and F. Bray, Int. J. Cancer, 2015, 136, E359–E386 CrossRef CAS PubMed.
- P. Dzubak, M. Hajduch, D. Vydra, A. Hustova, M. Kvasnica, D. Biedermann, L. Markova, M. Urban and J. Sarek, Nat. Prod. Rep., 2006, 23, 394–411 RSC.
- J. A. R. Salvador, V. M. Moreira, B. M. F. Gonçalves, A. S. Leal and Y. Jing, Nat. Prod. Rep., 2012, 29, 1463–1479 RSC.
- H. Sheng and H. Sun, Nat. Prod. Rep., 2011, 28, 543–593 RSC.
- M. K. Shanmugam, A. H. Nguyen, A. P. Kumar, B. K. H. Tan and G. Sethi, Cancer Lett., 2012, 320, 158–170 CrossRef CAS PubMed.
- S. M. Kamble, S. N. Goyal and C. R. Patil, RSC Adv., 2014, 4, 33370 RSC.
- A. S. Leal, R. Wang, J. A. R. Salvador and Y. Jing, Org. Biomol. Chem., 2013, 11, 1726–1738 CAS.
- J. Fu, Y. Zou, Z. Huang, C. Yan, Q. Zhou, H. Zhang, Y. Lai, S. Peng and Y. Zhang, RSC Adv., 2015, 5, 19445–19454 RSC.
- M. Kvasnica, M. Urban, N. J. Dickinson and J. Sarek, Nat. Prod. Rep., 2015, 1–28 Search PubMed.
- W. Setzer and M. Setzer, Mini-Rev. Med. Chem., 2003, 3, 540–556 CrossRef CAS PubMed.
- Y. Hsu, P. Kuo, L. Lin and C. Lin, J. Pharmacol. Exp. Ther., 2005, 313, 333–344 CrossRef CAS PubMed.
- C. V Kavitha, C. Agarwal, R. Agarwal and G. Deep, PLoS One, 2011, 6, e22745 Search PubMed.
- Y. S. Lee, D.-Q. Jin, E. J. Kwon, S. H. Park, E.-S. Lee, T. C. Jeong, D. H. Nam, K. Huh and J.-A. Kim, Cancer Lett., 2002, 186, 83–91 CrossRef CAS PubMed.
- B. C. Park, K. O. Bosire, E.-S. Lee, Y. S. Lee and J.-A. Kim, Cancer Lett., 2005, 218, 81–90 CrossRef CAS PubMed.
- C. W. Cho, D. S. Choi, M. H. Cardone, C. W. Kim, A. J. Sinskey and C. Rha, Cell Biol. Toxicol., 2006, 22, 393–408 CrossRef CAS PubMed.
- D. M. Gurfinkel, S. Chow, R. Hurren, M. Gronda, C. Henderson, C. Berube, D. W. Hedley and A. D. Schimmer, Apoptosis, 2006, 11, 1463–1471 CrossRef CAS PubMed.
- X.-L. Tang, X.-Y. Yang, H.-J. Jung, S.-Y. Kim, S.-Y. Jung, D.-Y. Choi, W.-C. Park and H. Park, Biol. Pharm. Bull., 2009, 32, 1399–1405 CAS.
- Y. Jing, G. Wang, Y. Ge, M. Xu and Z. Gong, Molecules, 2015, 20, 7309–7324 CrossRef CAS PubMed.
- J.-F. Li, R.-Z. Huang, G.-Y. Yao, M.-Y. Ye, H.-S. Wang, Y.-M. Pan and J.-T. Xiao, Eur. J. Med. Chem., 2014, 86, 175–188 CrossRef CAS PubMed.
- L. Wang, J. Xu, C. Zhao, L. Zhao and B. Feng, Chem. Pharm. Bull., 2013, 61, 1015–1023 CrossRef CAS PubMed.
- Y. Meng, Y. Li, F. Li, Y. Song, H. Wang, H. Chen and B. Cao, J. Asian Nat. Prod. Res., 2012, 14, 844–855 CrossRef CAS PubMed.
- B. Singh and R. P. Rastogi, Phytochemistry, 1969, 8, 917–921 CrossRef CAS.
- M. B. Sporn, K. T. Liby, M. M. Yore, L. Fu, J. M. Lopchuk and G. W. Gribble, J. Nat. Prod., 2011, 74, 537–545 CrossRef CAS PubMed.
- H. Chen, Y. Gao, A. Wang, X. Zhou, Y. Zheng and J. Zhou, Eur. J. Med. Chem., 2015, 92, 648–655 CrossRef CAS PubMed.
- K. T. Liby and M. B. Sporn, Pharmacol. Rev., 2012, 64, 972–1003 CrossRef CAS PubMed.
- F. F. Fleming, L. Yao, P. C. Ravikumar, L. Funk and B. C. Shook, J. Med. Chem., 2010, 53, 7902–7917 CrossRef CAS PubMed.
- A. S. Kalgutkar and D. K. Dalvie, Expert Opin. Drug Discovery, 2012, 7, 561–581 CrossRef CAS PubMed.
- S. T. Murphy, H. L. Case, E. Ellsworth, S. Hagen, M. Huband, T. Joannides, C. Limberakis, K. R. Marotti, A. M. Ottolini, M. Rauckhorst, J. Starr, M. Stier, C. Taylor, T. Zhu, A. Blaser, W. A. Denny, G.-L. Lu, J. B. Smaill and F. Rivault, Bioorg. Med. Chem. Lett., 2007, 17, 2150–2155 CrossRef CAS PubMed.
- E. Altmann, S. W. Cowan-jacob and M. Missbach, J. Med. Chem., 2004, 47, 5833–5836 CrossRef CAS PubMed.
- R. Mannhold, H. D. Höltje and V. Koke, Arch. Pharm., 1986, 319, 990–998 CrossRef CAS PubMed.
- M. Frizler, F. Lohr, N. Furtmann, J. Kläs and M. Gütschow, J. Med. Chem., 2011, 54, 396–400 CrossRef CAS PubMed.
- J. A. R. Salvador, M. L. Sá E Melo and A. S. Campos Neves, Tetrahedron Lett., 1996, 37, 687–690 CrossRef CAS.
- A. S. Leal, R. Wang, J. A. R. Salvador and Y. Jing, Bioorg. Med. Chem., 2012, 20, 5774–5786 CrossRef CAS PubMed.
- S. Talukdar, J.-L. Hsu, T.-C. Chou and J.-M. Fang, Tetrahedron Lett., 2001, 42, 1103–1105 CrossRef CAS.
- R. C. Santos, J. A. R. Salvador, S. Marín and M. Cascante, Bioorg. Med. Chem., 2009, 17, 6241–6250 CrossRef CAS PubMed.
- R. C. Santos, J. A. R. Salvador, S. Marín, M. Cascante, J. N. Moreira and T. C. P. Dinis, Bioorg. Med. Chem., 2010, 18, 4385–4396 CrossRef CAS PubMed.
- H.-Y. Tu, A.-M. Huang, B.-L. Wei, K.-H. Gan, T.-C. Hour, S.-C. Yang, Y.-S. Pu and C.-N. Lin, Bioorg. Med. Chem., 2009, 17, 7265–7274 CrossRef CAS PubMed.
- B. Siewert, E. Pianowski and R. Csuk, Eur. J. Med. Chem., 2013, 70, 259–272 CrossRef CAS PubMed.
- R. Csuk, S. Schwarz, R. Kluge and D. Ströhl, Arch. Pharm., 2012, 345, 28–32 CrossRef CAS PubMed.
- F. Chu, X. Xu, G. Li, S. Gu, K. Xu, Y. Gong, B. Xu, M. Wang, H. Zhang, Y. Zhang, P. Wang and H. Lei, Molecules, 2014, 19, 18215–18231 CrossRef PubMed.
- I. Vermes, C. Haanen, H. Steffens-Nakken and C. Reutelingsperger, J. Immunol. Methods, 1995, 184, 39–51 CrossRef CAS PubMed.
- G. Häcker, Cell Tissue Res., 2000, 301, 5–17 CrossRef.
- M. Antunovic, B. Kriznik, E. Ulukaya, V. T. Yilmaz, K. C. Mihalic, J. Madunic and I. Marijanovic, Anticancer Drugs, 2015, 26, 180–186 CrossRef CAS PubMed.
- S. Dhar, W. L. Daniel, D. A. Giljohann, C. A. Mirkin and S. J. Lippard, J. Am. Chem. Soc., 2009, 131, 14652–14653 CrossRef CAS PubMed.
- Z. Rajic, B. Zorc, S. Raic-Malic, K. Ester, M. Kralj, K. Pavelic, J. Balzarini, E. De Clercq and M. Mintas, Molecules, 2006, 11, 837–848 CrossRef CAS PubMed.
- M. Porchia, A. Dolmella, V. Gandin, C. Marzano, M. Pellei, V. Peruzzo, F. Refosco, C. Santini and F. Tisato, Eur. J. Med. Chem., 2013, 59, 218–226 CrossRef CAS PubMed.
- I. Budihardjo, H. Oliver, M. Lutter, X. Luo and X. Wang, Annu. Rev. Cell Dev. Biol., 1999, 15, 269–290 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra19120c |
| This journal is © The Royal Society of Chemistry 2016 |