Facile synthesis of nitrogen-doped hierarchical porous lamellar carbon for high-performance supercapacitors†
Wang Yang‡
ab,
Zhiling Du‡ab,
Zhipeng Maa,
Guiling Wanga,
Heping Baic and
Guangjie Shao*ab
aHebei Key Laboratory of Applied Chemistry, College of Environmental and Chemical Engineering, Yanshan University, Qinhuangdao 066004, China. E-mail: shaoguangjie@ysu.edu.cn; Fax: +86-335-8061569; Tel: +86-335-8061569
bState Key Laboratory of Metastable Materials Science and Technology, Yanshan University, Qinhuangdao 066004, China
cCollege of Real Estate, Beijing Normal University, Zhuhai 519000, China
First published on 14th December 2015
Abstract
Three-dimensional (3D) interconnected N-enriched hierarchical porous lamellar carbon (NPLC) with a multilevel pore structure has been fabricated by a wet impregnation method using waste nitrogen-containing mantis shrimp shell as a carbon precursor and KOH as an impregnation solution. The synthesized NPLC-2 shows a large surface area of 1222.961 m2 g−1 calculated by the BET method, a hierarchical porous structure analyzed by the density functional theory (DFT) model, and a high nitrogen content of 1.78% quantified by X-ray photoelectron spectroscopy (XPS). Moreover, the NPLC-2 sample exhibits an ultra-high specific capacitance of 312.62 F g−1 at 0.3 A g−1, excellent rate capability with a specific capacitance of 272.56 F g−1 at 20.0 A g−1 and outstanding cycling stability with around 96.26% capacitance retention after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at a high current density of 20.0 A g−1. In addition, NPLC-2 presents a high energy density of 15.05 W h kg−1 at 270 W kg−1, and up to 10.12 W h kg−1 even at a large power density of 14
000 cycles at a high current density of 20.0 A g−1. In addition, NPLC-2 presents a high energy density of 15.05 W h kg−1 at 270 W kg−1, and up to 10.12 W h kg−1 even at a large power density of 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 580 W kg−1. Therefore, the prepared material can be applied in high energy density and high power density demanding fields.
580 W kg−1. Therefore, the prepared material can be applied in high energy density and high power density demanding fields.
1. Introduction
Porous carbon is considered to be an advanced electrode material for supercapacitors owing to its high specific surface area, unique structure, strong thermal and chemical stability and simple charge storage mechanism.1–5 The precursor sources of porous carbon are various, including organics, inorganics and complex compounds. Some reports6–9 have successfully prepared the porous carbon using carbide and polymers as raw materials, but the cost and jeopardy were tremendous and the preparation procedure was tedious.Recently, with the rapid development of society and the significant improvement of living standards, biological wastes are increasing. In terms of resource recycling and environment conservation, biological wastes have great potential for applications in various aspects covering energy materials and functional materials.10–13 Compared with other resources of carbon materials, biomass materials as a green resource possess the remarkable properties of a short renewal cycle and natural texture structure, which endow them with the opportunities of reutilization in the future with resource scarcity. Due to the sustainability and economical efficiency of porous carbon production, biological materials have gradually developed into promising raw material candidates. Huang et al.14 reported that hierarchical porous carbon obtained from animal bone had a high surface area (SBET = 2157 m2 g−1) and a large total volume (Vt = 2.26 cm3 g−1). Sun et al.15 revealed that porous carbon derived from coconut shells possessed a much higher specific capacitance (268 F g−1 at 1 A g−1) than ordinary activated carbon (210 F g−1) and excellent cycle durability. Additionally, different categories of biomass have their own fine texture characteristics and natural compositions. It is supposed that porous carbon with superior properties can be fabricated by electing appropriate biomass. Research16–20 showed that nitrogen-modification could optimize the structure and improve the performance of porous carbon. Therefore, nitrogen-doped porous carbon has attracted increasing attention as an ideal electrode material for supercapacitors. White et al.21 successfully obtained nitrogen-doped porous carbon with a high surface area (SBET > 300 m2 g−1) and large pore volume (Vpore > 0.3 cm3 g−1) using prawn shells as a carbon resource. At the same time, the product preserved the original texture of the carbon resource. In terms of the porous carbon electrode materials used for supercapacitors, the most fundamental question is its pore structure because the structure determines performance. Of most concern is finding better supercapacitor parameters for charge storage by exploring pore size distribution, the pore-network connectivity and the doping of carbon materials. Therefore, we plan to select a kind of special biomass waste to synthesize an electrode material for supercapacitors with better pore structure as well as a superior electrochemical performance.
Mantis shrimp shell is enriched with abundant chitin (poly-β(1,4)-N-acetyl-2-amino-2-deoxidation-D-glucose) and natural inorganic calcium salt which can act as natural templates in preparing porous materials. Herein, we prepared a nitrogen-doped hierarchical porous lamellar carbon via a facile wet impregnation method using mantis shrimp shell as a nitrogen-rich carbon precursor and KOH as an auxiliary pore-forming agent. The obtained material exhibits an ultra-high specific capacitance of 312.62 F g−1 at 0.3 A g−1, and an excellent rate capability with a specific capacitance of 272.56 F g−1 at 20.0 A g−1, which are superior to other reported literature.14,15 This is attributed to the reasonable microstructure, including the appropriate aperture size, hierarchical pore size distribution, three-dimensional pore structure and the nitrogen doping. Such results give us certain inspiration and references to design and optimize the structure of porous carbon electrode materials for supercapacitors.
2. Experimental
2.1. Chemicals
In this experiment, the chemicals KOH and HCl used were of analytical grade and they were used directly as received without any further processing. Deionized water was used as a cleaning agent in the whole experiment.2.2. Preparation of NPLCs
NPLC materials were synthesized via an alkali solution impregnation method using N-enriched mantis shrimp shell as a sustainable and economic carbon precursor and KOH as an impregnation solution. In the pretreatment procedure, 15.0 g of the washed mantis shrimp shell was mixed with 50 ml of 10 wt% KOH solution (ca. 5.0 g KOH), and the mixture was oscillated for 3 h using an ultrasonic cleaner to assist KOH in soaking into the internal structure of the mantis shrimp shell. Then the mixture was evaporated at 80 °C overnight in an oven to obtain an impregnated carbon precursor. Subsequently, the impregnated product was transferred into three ceramic crucibles and was carbonized in a furnace at the desired temperatures of 700 °C(NPLC-700), 750 °C(NPLC-750) and 800 °C(NPLC-800) for 1 h under an inert N2 atmosphere. Then the calcined products were etched with 15 wt% HCl, filtrated using a vacuum pump and rinsed with deionized water until they became neutral. Finally the products were dried at 80 °C for 12 h to obtain the final samples of NPLC-700, NPLC-750 and NPLC-800, which are labeled NPLC-1, NPLC-2 and NPLC-3, respectively.2.3. Structure characterization
The structures of the as-prepared NPLCs were characterized by X-ray diffraction (XRD) on a Rigakud/MAX-2500/pc X-ray diffractometer with Cu Kα radiation. The Raman spectra were recorded on a Horiva (LabRam HR-800) spectrometer (532 nm, 50 mW excitation laser). Field-emission scanning electron microscopy (FE-SEM, Hitachi Modle S-4800, KV) and transmission electron microscopy (TEM, JEM2010) were employed to observe the morphology and microstructure of the materials. Fourier transform infrared spectroscopy (FTIR) was used to analyze the surface functional groups using a Nicolet IS Fourier transformation infrared spectrometer with the wave number range of 400–4000 cm−1. Elemental quantitative analysis was achieved using X-ray photoelectron spectroscopy (XPS) with a Kratos XSAM-800 spectrometer with an Al Kα radiation source. High-resolution spectra of the individual elements were acquired with the analyzer pass energy set at 20 eV. The pressure of the vacuum system was set at 1 × 10−9 during all XPS operation. N2 sorption isotherms were carried out to analyze the porous structures of the samples using a NOVA instrument (Quantachrome Instruments version 10.01). The specific surface area was calculated using the BET (Brunauer–Emmett–Teller) method in the relative pressure range of 0.04–0.20. The pore volume and pore size distributions (PSDs) were obtained using the density functional theory (DFT) model from the adsorption branches of the isotherms.2.4. Electrochemical characterization
The electrochemical characterizations were carried out using a three-electrode system in 6 M KOH electrolyte. A Hg/HgO electrode and an active carbon electrode were used as the reference electrode and as the counter electrode, respectively. To prepare the working electrode NPLCs, acetylene black and polytetrafluorothylene (PTFE) binder were mixed at a weight ratio of 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 into a uniform slurry which was coated onto current collectors of 1 cm2 in area nickel foam, and dried at 80 °C for 12 h. The total mass loading of each NPLC-based electrode was 3 mg in which the active material was 2.4 mg. The fabricated electrodes were pressed at 4 MPa pressure for 30 s, and marinated in 6 M KOH electrolyte for 24 h.
5 into a uniform slurry which was coated onto current collectors of 1 cm2 in area nickel foam, and dried at 80 °C for 12 h. The total mass loading of each NPLC-based electrode was 3 mg in which the active material was 2.4 mg. The fabricated electrodes were pressed at 4 MPa pressure for 30 s, and marinated in 6 M KOH electrolyte for 24 h.
A CHI660E electrochemical workstation (Chenhua, Shanghai China) was employed to carry out the cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) tests. The CV was tested at various scanning rates of 5, 20, 50 and 100 mV s−1. The frequency range for the EIS measurement was from 1 mHz to 1 MHz. The galvanostatic charge–discharge measurements were recorded on a NEWARE auto-cycler and the potential window was −1.0 to 0.11 V. The specific gravimetric capacitance of the working electrodes was obtained from the galvanostatic discharge process via Cgd = IΔt/(mΔV), where I (A) is the discharge current, Δt (s) is the discharge time, ΔV (V) is the discharge voltage change excluding the IR drop during the discharge process, and m (g) is the mass of the active material for each electrode. The specific capacitance could also be calculated from the CV curve via  where Ccv (F g−1) is the specific capacitance, I (A) is the discharge current, m (g) is the mass of active material of the working electrode, v (V s−1) is the scan rate, and Va and Vc represent the low and high potential limits from the CV test, respectively. In particular, the symmetrical total cell was used to analyze the power and energy density in a symmetrical two-electrode system. The energy density was calculated using the equation E = (CcellV2)/2, where Ccell (F g−1) is the total cell specific capacitance and V (V) is the cell-operation potential. The average power density was calculated via P = E/Δt, where E is the energy density and Δt is the discharge time.
where Ccv (F g−1) is the specific capacitance, I (A) is the discharge current, m (g) is the mass of active material of the working electrode, v (V s−1) is the scan rate, and Va and Vc represent the low and high potential limits from the CV test, respectively. In particular, the symmetrical total cell was used to analyze the power and energy density in a symmetrical two-electrode system. The energy density was calculated using the equation E = (CcellV2)/2, where Ccell (F g−1) is the total cell specific capacitance and V (V) is the cell-operation potential. The average power density was calculated via P = E/Δt, where E is the energy density and Δt is the discharge time.
3. Results and discussion
3.1. Structure and morphology analysis
XRD and Raman spectra were carried out to analyze the structure characteristics of the prepared materials. Fig. 1a presents the XRD patterns of the NPLCs synthesized at 700 °C, 750 °C and 800 °C. An inconspicuous peak located at near 43° corresponds to a superposition of the graphitic (101) and (100) planes and a broad hump positioned at 2θ = ∼23° is attributed to the graphitic (002) reflection.22 The peak at around 23° shifts to a lower diffraction angle in comparison to the ideal graphitic (002) peak at 26°, indicating that the interlayer spacing (002) of the NPLCs is larger than the ideal value of 0.34 nm for the ordered graphite (002), and the materials present an amorphous structure.23 Moreover, it can be detected that the humps shift to the lower diffraction angle with an increasing calcination temperature. It is speculated that the increasing interlayer spacing is caused by the higher gas pressure during the reaction with increasing temperature. The Raman spectra present the characteristic peaks of the D (defect), G (graphite) and 2D bands, as shown in Fig. 1b. The D band centered at 1350 cm−1 is related to defects in the carbon materials (sp3-coordinated), and the G band at 1590 cm−1 corresponds to the ordered graphitic structure of carbon (sp2-coordinated).24 The relative intensities (ID/IG) can be used to evaluate the orderly degree of atomic arrangement of carbon. The ID/IG value of the NPLCs changes from 0.916 to 0.968 and the 2D peak also gets weak with the temperature increasing from 700 °C to 800 °C, suggesting the increase of amorphous degree, which is consistent with the XRD analysis.Fig. 2a–d shows the SEM and TEM images of NPLC-2 at different magnifications. As seen from the SEM images of Fig. 2a and b, the sample has a typical 3D web-like porous structure, and the pores with different sizes play different roles in optimizing the electrochemical properties of the product. The numerous pores originated from the etched natural mineral salt of CaCO3, the corrosive action of KOH and the decomposition of chitin during the pyrolysis process. The TEM images in Fig. 2c and d show the layered structure of NPLC-2 with abundant hierarchical porosity. The porous lamellar structure can provide more accessible paths which are favorable for the fast charge transfer and a higher surface area contributing to more charge absorption sites. It is deduced that the porous lamellar structure is closely related to the natural skeleton and chemical component of the mantis shrimp shell. More importantly, KOH impregnated into its internal structure plays a significant role in the formation of the porous graphite-like lamellar structure. A possible mechanism of action is proposed in Scheme 1 with the included illustrations.
Firstly, KOH is decomposed into K2O and H2O at a temperature of 400 °C (eqn (1)).25 The generated H2O reacts with C resulting in the consumption of carbon according to eqn (2). Simultaneously, the reaction of eqn (3) and (4) are happening.
| 2KOH → K2O + H2O | (1) |
| C + H2O → CO + H2 | (2) |
| CO + H2O → CO2 + H2 | (3) |
| K2O + CO2 → K2CO3 | (4) |
When the calcination temperature exceeds 700 °C, K2CO3 can be highly decomposed (eqn (5)).2 Consecutively, the reaction products of CO2 and K2O are reduced by carbon according to eqn (6) and (7).
| K2CO3 → K2O + CO2 | (5) |
| CO2 + C → CO | (6) |
| K2O + C → 2K + CO | (7) |
It is notable that considerable amounts of gas and pores are introduced in the activation-KOH process, some of which are formed in the internal texture. The pressure in the interior of the precursor grows as the reaction progresses. As a result, the natural elaborate texture of the raw material expands to form a porous lamellar structure during the carbonization process. Certainly, the natural template of the inorganic salt and the release of small molecules of CO2, H2O and H2 have a vital impact on the formation of the pore structure.
A large surface area and favorable pore size distribution are known to be significant factors in advanced electrode materials for supercapacitors. In this test, the nitrogen adsorption–desorption test is carried out to investigate the textural properties, and the density functional theory (DFT) model is used to analyze the pore volume and pore size distribution. The main parameters of the pore structure of NPLC are listed in Table S1.† The as-obtained NPLC materials show a cross between type-I and type-IV N2-adsorption isotherms as depicted in Fig. 3a, which implies that the NPLC samples are enriched with abundant micropores and mesopores. Obviously, the isotherms of NPLCs present H4 hysteresis loops at a relatively high pressure section of above 0.9P/P0. The result indicates that pores with narrow and slit-like shapes exist in NPLC materials,26 which is consistent with the result of the TEM. Fig. 3b demonstrates that hierarchical pores with diameters of 3 nm, 12 nm, 14 nm, 17 nm and less than 2 nm exist in the fabricated materials, indicating the coexistence of micropores and mesopores in the carbon. Additionally, Fig. 3b reveals that, with the increased temperature from 700 °C to 800 °C, the amount of micropores decreases and that of different levels of mesopores increases. The surface areas of the samples are calculated using the general BET method and the values of NPLC-1, NPLC-2 and NPLC-3 are 853.195 m2 g−1, 1222.961 m2 g−1 and 1329.112 m2 g−1, respectively. The increase in the surface area with a higher calcination temperature is because the increase in the amount of the mesopores is higher than the decrease in the amount of the micropores. It has been proved that the micropores (<2 nm) contribute to the large surface area which leads to the high specific capacitance.27 Moreover, the small mesopores of about 3 nm can accelerate the electrolyte ion transfer and the large mesopores (>10 nm) could store electrolyte on standby for fast electrochemical reactions.28
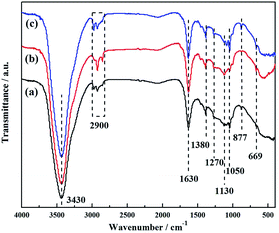
FTIR spectra were taken for the qualitative analysis of the chemical constitution of NPLCs, as shown in Fig. 4. The typical peak positioned at 3430 cm−1 is assigned to the O–H stretching vibration stemming from H2O and the three peaks at 2970 cm−1, 2920 cm−1 and 2850 cm−1 relate to the stretching vibration of the C–H bond. The sharp absorption peak observed at 1630 cm−1 is attributed to the stretching vibration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) X (X = C, N, or O),20 while the distinct peaks at 1380 cm−1/1270 cm−1 are identified as the C–N vibration modes.8 In addition, the other peaks are centered at 1050 cm−1 (the C–N bending vibration),29 1130 cm−1 (the C–O stretching vibration) and 877 cm−1/669 cm−1 (N–H out-of-plane deformation vibration).30 The above results confirm that the biomass-derived nitrogen-doped carbon has been synthesized.
X (X = C, N, or O),20 while the distinct peaks at 1380 cm−1/1270 cm−1 are identified as the C–N vibration modes.8 In addition, the other peaks are centered at 1050 cm−1 (the C–N bending vibration),29 1130 cm−1 (the C–O stretching vibration) and 877 cm−1/669 cm−1 (N–H out-of-plane deformation vibration).30 The above results confirm that the biomass-derived nitrogen-doped carbon has been synthesized.
To quantify the heteroatoms enriched in the as-synthesized carbon material, the XPS technique was employed, as shown in Fig. 5. It can be observed that NPLCs are enriched with nitrogen and oxygen elements, which can boost electrochemical performances including introducing additional pseudocapacitances and enhancing interface wettability. The element compositions of the NPLCs are determined by XPS measurement and the content of different elements is listed in Table S1.† The N content of the prepared carbons decreases from 1.99% to 0.81% with the increase of temperature. Apparently, the O content first increases from 6.77% to 19.21% and then decreases to 10.56%, which is due to the harsh redox reactions between the potassium compounds and carbon at 750 °C, and when the temperature increase continues, the oxygen containing functional groups will be reduced. Fig. 5a shows that the XPS curves of NPLCs consist of three obvious peaks of C 1s (284.0 eV), N 1s (400.0 eV), and O 1s (532.0 eV), which agrees well with the FTIR result. The nitrogen atoms in different chemical environments respectively play their own roles. The high-resolution spectra of N 1s is used to analyze the types of nitrogen species and the curve-fitting method is carried out by XPS peak-fit software. It is seen that the N 1s spectra of the as-prepared carbon materials is divided into three typical component peaks including N-6 (pyridinic, 398.4 eV), N-5 (pyrrolic/pyridine, 400.1 eV) and N-Q (quaternary, 401.0 eV), as described in Fig. 5b–d. The relative content of each nitrogen type is listed in Table S1.† The percentages of N-5, N-6 and N-Q range from 79.01% to 44.69%, 16.68 to 17.49% and 4.31 to 37.82% of the total nitrogen content with increasing calcination temperatures. It is noticeable that the N-5 content decreases, while the N-6 and N-Q content increases. Studies found that N-5 and N-6 could enhance the capacitance performance by producing pseudocapacitance and they are situated at the edges of the graphite layer, while N-Q located at both the centre and the edges of the graphite layer could facilitate the fast transfer of electrons and ions by increasing the electronic conductivity of the as-obtained materials and improve the wettability of the interface between the electrode materials and the electrolyte.31 Fig. S1† clearly presents the structure mode of different types of nitrogen atoms in a carbon matrix. In brief, the nitrogen-doped porous carbon material originating from mantis shrimp shells has been successfully fabricated and it has prospects for a broad application in various fields such as adsorption, hydrogen storage, batteries, and supercapacitors thanks to its porous structure, high specific surface area, and definite nitrogen content as well as good electrochemical characteristics which will be described below.
 | ||
| Fig. 5 XPS spectra of NPLCs (a) and N 1s XPS spectra: (b) NPLC-1; (c) NPLC-2; (d) NPLC-3 (pyridinic (N-6), pyrrolic/pyridone (N-5) and quaternary (N-Q)). | ||
3.2. Electrochemical behavior
To investigate the causality relationship of the sample microstructure and its electrochemical performance, cyclic voltammetry (CV) measurements were carried out in a three-electrode configuration in 6 M KOH. Fig. 6a presents the CV graphs of the NPLCs synthesized at the different temperatures of 700 °C, 750 °C and 800 °C at a scan rate of 50 mV s−1. The CV curves appear rectangular with broad humps at a relatively low potential, and the hump becomes inconspicuous with an increase in calcination temperature, as shown in Fig. 6a. It means that the specific capacitance of the NPLCs includes EDLC and pseudocapacitance, but the contribution of pseudocapacitance decreases with the enhanced calcination temperature due to the loss of nitrogen atoms at a relatively high temperature, which is in accordance with the XPS analysis. Moreover, it can be observed that the specific capacitance of NPLC-2 is superior to the other samples and its specific capacitance reaches up to 280.93 F g−1 at 50 mV s−1. Fig. 6c reveals the CV curves of NPLC-2 with a typical rectangular shape at different scan rates. Even at a high scan rate of 200 mV s−1, the CV curve still retains an ideal rectangular shape, indicating the excellent capacitive behavior of NPLC-2 with a fast charge and discharge process due to the favorable pore size distribution providing short and unresisting ion diffusion paths. The CV curves display fast capacitance responses at switching potentials, which means that the electrical double layer can be re-organized rapidly when the potential changes.32 Furthermore, an obvious hump gradually appears at the potentials below −0.35 V and a deviation from the perfect rectangular shape is more apparent with an increasing scan rate. It certifies that the capacitance of the as-obtained material contains not only EDLC but also pseudocapacitance which results from the produced nitrogen. It is calculated that NPLC-2 has a considerably high specific capacitance of 332.71 F g−1 at 5 mV s−1 and the capacitance still remains up to 225.21 F g−1 even at a relatively large scan rate of 200 mV s−1, as shown in Fig. 6d, manifesting the superior rate capability. As known from pore analysis, numerous micropores dwell in NPLC-2 and they can furnish abundant charge absorption sites which improve capacitance properties. Additionally, the small mesopores (ca. 2–4 nm) and the relatively big mesopores (centered at 12 nm, 14 nm, 17 nm) provide open routes for rapid ion diffusion as well as a storage bank of electrolyte ions for reasonable supplement of charges. Therefore, NPLC-2 exhibits an ultra-high specific capacitance and prominent rate capability.To further investigate the electrochemical properties of the samples, galvanostatic charge–discharge was conducted, as shown in Fig. 6b. The charge–discharge curves of NPLCs are almost linear and with good symmetry, revealing the nice capacitive ability and high discharge efficiency, and that minor distortion is generated by the doped nitrogen atom.
The discharge time of NPLC-2 is the longest among all the samples which displays the fact that the specific capacitance of NPLC-2 exceeds that of the other samples. Fig. 6e shows the V–t graphs of NPLC-2 with an increasing current density from 1.0 to 20.0 A g−1. From the intersection of the discharge curve branch and the horizontal axis, it can be seen that the discharge time decreases with the increase of current density due to the presence of internal impedance from NPLC-2. Fig. 6f demonstrates that the as-obtained material has a remarkable specific capacitance and superior rate capability. Its specific capacitance is up to 312.62 F g−1 at 0.3 A g−1 and still remains at 272.56 F g−1 at a high current density of 20.0 A g−1 (ca.87.16% of capacitance retention). The galvanostatic charge–discharge analysis is consistent with the result of CV.
The cycle durability occupies a vital position in practical applications and it is explored in detail. Fig. S2† shows the cycle life trace of the obtained samples at a current density of 20 A g−1. It can be seen that all the samples display outstanding cycle durability. The specific capacitance of NPLC-2 remains at 262.37 F g−1 even after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles (ca. 96.26% of capacitance retention), as shown in Fig. 6g. In addition, the coulombic efficiency of NPLC-2 is near 100% which is due to the rapid absorption and desorption of ions in the interior walls of the pores as well as the reversible chemical modification of the N-functional group.
000 cycles (ca. 96.26% of capacitance retention), as shown in Fig. 6g. In addition, the coulombic efficiency of NPLC-2 is near 100% which is due to the rapid absorption and desorption of ions in the interior walls of the pores as well as the reversible chemical modification of the N-functional group.
Energy density and power density tests were performed by a two-electrode system in 6 M KOH. Fig. 6h provides a comparison of the power and energy characteristics of the NPLC-2-based supercapacitors with the porous carbon materials that can be found in the literature.15,33–35 NPLC-2 exhibits a high energy density of 15.05 W h kg−1 at 270 W kg−1, and still retains a value of 10.12 W h kg−1 at a large power density of 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 580 W kg−1. This indicates that the prepared NPLC-2 has a high energy density and power density. Generally, the superior electrochemical capability is closely related to the fine microstructure with connectivity of pores in the 3D level, large surface area, definite nitrogen content and hierarchical porous size distribution.
580 W kg−1. This indicates that the prepared NPLC-2 has a high energy density and power density. Generally, the superior electrochemical capability is closely related to the fine microstructure with connectivity of pores in the 3D level, large surface area, definite nitrogen content and hierarchical porous size distribution.
The following EIS test was to investigate the resistance and the capacitive property of the prepared samples. Fig. 7a shows that the plots of NPLC-2 and NPLC-3 in the low-frequency region are more perpendicular to the real axis than that of NPLC-1, which illustrates that NPLC-2 possesses the optimal capacitive behavior. The inset of Fig. 7a demonstrates that each Nyquist plot contains three segments, including a clear semicircle, a gently sloped line and a steeply sloped line. In comparison, the semicircle at the high-frequency region becomes inconspicuous with increasing pyrolysis temperature and the semicircle almost disappears at a high calcination temperature of 800 °C, which indicates that the material prepared at a high temperature has a small charge transfer resistance. Moreover, the existence of the semicircle reflects that the as-obtained materials contains both pseudocapacitance and EDLC, which agrees well with the CV analysis and galvanostatic charge–discharge result. We have shown the equivalent circuit in the inset of Fig. 7a and have modelled the corresponding circuit for the three types of carbons. The model fitting parameters are provided in Table S2.† The equivalent series resistance (ESR) includes the resistance of electron/ion transfer. It is found that the ESR of NPLC-2 is the smallest among all the samples, and its value is about 0.771 ohm. The slope of all the lines in the medium-frequency region is nearly 45° and the length of NPLC-1 and NPLC-2 is shorter than that of NPLC-3, which indicates that the efficiency of the electrolyte in NPLC-1 and NPLC-2 is higher when diffusing into pores. To describe the capacitive property more directly, a Bode plot is shown in Fig. 7b. It can be observed that the phase angles of NPLC-2 and NPLC-3 are about −87°and they are close to the ideal value of full capacity of −90°. The relaxation time constant, τ0 (the minimum time needed for all the energy to be discharged from supercapacitor cells with an efficiency >50%) = 1/2πf, and we can see from Fig. 7b that f1 < f2 < f3, so the relaxation time constant of NPLC-1 > NPLC-2 > NPLC-3, which means that the NPLC-2 and NPLC-3 have the more appropriate pore size distribution for the transport of ions. From the whole analysis of the EIS test, the performance of NPLC-2 is superior to that of NPLC-1 and NPLC-3. In conclusion, its excellent electrochemical performance has benefited from the collaborative effect of a 3D interconnected porous structure with different levels of pores, introduced nitrogen atoms and a large surface area.
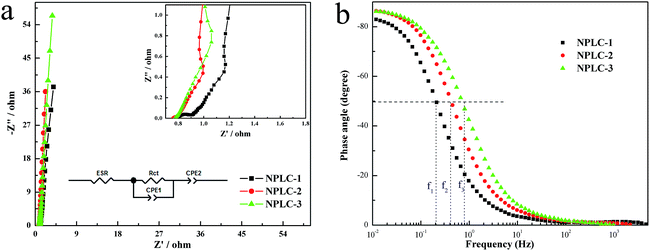 | ||
| Fig. 7 (a) Nyquist plots (the inset shows the amplified part of the Nyquist plots), and (b) Bode plots of NPLCs. | ||
4. Conclusions
In this work, we successfully obtained 3D N-doped hierarchical porous lamellar carbon from mantis shrimp shell using a facile KOH activation method. The samples exhibit a high surface area, definite nitrogen atom percentage and an ideal hierarchical pore size distribution, including numerous micropores, small mesopores and relatively big mesopores. The rational structure of the material leads to outstanding electrochemical properties when used as a supercapacitor: a high specific capacitance of 312.62 F g−1 at 0.3 A g−1; as indicated by a residual capacitance of 272.56 F g−1 at 20.0 A g−1 and a considerably superior cycling durability with a high capacitance retention of 96.26% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 charge–discharge cycles. Moreover, the obtained sample presents a high energy density and power density. The energy density of NPLC-2 can reach 10.12 W h kg−1 even at a large power density of 14
000 charge–discharge cycles. Moreover, the obtained sample presents a high energy density and power density. The energy density of NPLC-2 can reach 10.12 W h kg−1 even at a large power density of 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 580 W kg−1 in a symmetrical two-electrode system in 6 M KOH solution. The excellent electrochemical performance is due to the synergistic effect of a 3D web-like porous lamellar structure with a required moderate pore size distribution, high nitrogen doped content and satisfactory surface area. The hierarchical porous structure can provide open routes for rapid ion diffusion as well as a storage bank of electrolyte ions for the reasonable supplement of charges. Such a result can give us certain inspiration and references to design and optimize the structure of porous carbon electrode materials for supercapacitors.
580 W kg−1 in a symmetrical two-electrode system in 6 M KOH solution. The excellent electrochemical performance is due to the synergistic effect of a 3D web-like porous lamellar structure with a required moderate pore size distribution, high nitrogen doped content and satisfactory surface area. The hierarchical porous structure can provide open routes for rapid ion diffusion as well as a storage bank of electrolyte ions for the reasonable supplement of charges. Such a result can give us certain inspiration and references to design and optimize the structure of porous carbon electrode materials for supercapacitors.
Acknowledgements
We are grateful for the financial support from the Natural Science Foundation of Hebei Province (B2012203069) and support from the education department of Hebei province on natural science research key projects for institution of higher learning (ZH2011228).Notes and references
- Y. Zhai, Y. Dou, D. Zhao and S. Dai, Adv. Mater., 2011, 23, 4828 CrossRef CAS PubMed.
- J. Wang and S. Kaskel, J. Mater. Chem., 2012, 22, 23710 RSC.
- B. Xu, S. S. Hou, G. P. Cao and F. Wu, J. Mater. Chem., 2012, 22, 19088 RSC.
- L. L. Zhang, Y. Gu and X. S. Zhao, J. Mater. Chem. A, 2013, 1, 9395 CAS.
- M. Sevilla, W. Gu and G. Yushin, J. Power Sources, 2014, 267, 26 CrossRef CAS.
- J. Xu, R. Zhang, P. Chen and S. Ge, J. Power Sources, 2014, 246, 132 CrossRef CAS.
- Y. Tan, Q. Xie and N. Zheng, ACS Appl. Mater. Interfaces, 2013, 5, 2241 CAS.
- M. Li and J. Xue, J. Phys. Chem. C, 2014, 118, 2507 CAS.
- Y. Su, H. Jiang and J. Chen, J. Power Sources, 2014, 265, 246 CrossRef CAS.
- R. L. Liu, W. J. Ji and Z. Q. Zhang, Carbon, 2014, 76, 84 CrossRef CAS.
- Z. Li, Z. Xu, H. Wang and D. Mitlin, Energy Environ. Sci., 2013, 6, 871 CAS.
- J. McKittrick, P. Y. Chen, L. Tombolato, E. E. Novitskaya and G. A. Hirata, Mater. Sci. Eng., C, 2010, 30, 331 CrossRef CAS.
- G. S. Sailaja, K. Sreenivasan and H. K. Varma, Acta Biomater., 2009, 5, 1647 CrossRef CAS PubMed.
- W. Huang, H. Zhang, Y. Huang and W. Wang, Carbon, 2011, 49, 838 CrossRef CAS.
- L. Sun, C. G. Tian, M. T. Li and H. G. Fu, J. Mater. Chem. A, 2013, 1, 6462 CAS.
- Y. S. Yun, J. Shim and H. J. Jin, RSC Adv., 2012, 2, 4353 RSC.
- C. Wu, X. Wang and B. Ju, J. Power Sources, 2013, 227, 1 CrossRef CAS.
- J. Lu, L. Yang, B. Xu and X. Wang, ACS Catal., 2014, 4, 613 CrossRef CAS.
- B. Xu, D. Zheng and M. Jia, Electrochim. Acta, 2013, 98, 176 CrossRef CAS.
- C. Wang, G. Shao, Z. Ma and S. Liu, Electrochim. Acta, 2014, 130, 679 CrossRef CAS.
- R. J. White, M. Antonietti and M. M. Titirici, J. Mater. Chem., 2009, 19, 8645 RSC.
- H. Guo and Q. Gao, J. Power Sources, 2009, 186, 551 CrossRef CAS.
- J. Xu, C. Wu and S. Ge, Microporous Mesoporous Mater., 2014, 198, 74 CrossRef CAS.
- M. H. Ryu, K. N. Jung and S. Yoon, J. Phys. Chem. C, 2013, 117, 8092 CAS.
- T. Otowa, R. Tanibata and M. Itoh, Gas Sep. Purif., 1993, 7, 241 CrossRef CAS.
- M. Li, J. Ding and J. M. Xue, J. Mater. Chem. A, 2013, 1, 7469 CAS.
- K. S. Xia, Q. M. Gao, J. H. Jiang and J. Hu, Carbon, 2008, 46, 1718 CrossRef CAS.
- P. Hao, Z. H. Zhao, Y. H. Leng, C. P. Wong, H. Liu and B. Yang, Nano Energy, 2015, 15, 9 CrossRef CAS.
- S. T. Navale, G. D. Khuspe, M. A. Chougule and V. B. Patil, J. Phys. Chem. Solids, 2014, 75, 236 CrossRef CAS.
- A. Kumar, R. K. Singh, H. K. Singh and P. Srivastava, J. Power Sources, 2014, 246, 800 CrossRef CAS.
- M. Zhou, F. Pu and S. Y. Guan, Carbon, 2014, 68, 185 CrossRef CAS.
- L. P. Wang, Y. Zhou and J. S. Qiu, Microporous Mesoporous Mater., 2013, 174, 67 CrossRef CAS.
- W. J. Qian, F. X. Sun, Y. H. Xu and F. Yan, Energy Environ. Sci., 2014, 7, 379 CAS.
- Y. R. Liang, F. X. Liang, H. Zhong and D. C. Wu, J. Mater. Chem. A, 2013, 1, 7000 CAS.
- W. Xing, S. Z. Qiao, R. G. Ding, G. Q. Lu and Z. F. Yan, Carbon, 2006, 44, 216 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra21431a |
| ‡ Wang Yang and Zhiling Du contributed equally. |
| This journal is © The Royal Society of Chemistry 2016 |