Investigation into the enantiospecific behavior of trichlorfon enantiomers during microorganism degradation†
Jing Nieb,
Li-Ye Yangb,
Xiao-kun OuYang*ab,
Wei-Jian Wub,
Yang-Guang Wang*ab and
Di Yub
aKey Laboratory of Health Risk Factors for Seafood of Zhejiang Province, Zhejiang Ocean University, Zhoushan 316022, P.R. China
bSchool of Food and Pharmacy, Zhejiang Ocean University, Zhoushan 316022, P.R. China. E-mail: xkouyang@zjou.edu.cn; ygw0510@sohu.com; Fax: +86-580-2554781; Tel: +86-580-2554781
First published on 28th December 2015
Abstract
Trichlorfon (TF) is commonly used as an antiparasitic agent in aquaculture. A chiral HPLC method for the detection of the two TF enantiomers in fish was developed using metolcarb as an internal standard. Both the enantiospecific behavior of R-(−)-TF and S-(+)-TF and the formation of the toxic metabolite dichlorvos during fish storage were investigated using the developed HPLC method. Results showed that the degradation of R-(−)-TF and S-(+)-TF during fish storage was enantiospecific at both 18 °C and 25 °C, with R-(−)-TF being preferentially degraded. However, no enantiospecific behavior of TF enantiomers was observed when fish was stored at either −18 °C or 4 °C. In addition, dichlorvos formation was detected in TF-contaminated fish at a range of storage temperatures. Furthermore, microbial activity was found to play an important role in the degradation of TF enantiomers and in the formation of dichlorvos, and contributed to the enantiospecific behavior of TF enantiomers during storage.
1. Introduction
Trichlorfon (TF, Fig. 1) is a water-soluble organophosphorus insecticide that is used as an antiparasitic agent in aquaculture.1 Although the dosage of TF is highly controlled, excess dosage is often applied in aquaculture farms, resulting in the presence of TF residues in fish. This is problematic for a number of reasons. Studies have shown that the consumption of TF-contaminated fish is hazardous to pregnant women, and may lead to the development of Down's syndrome in the fetus.2 In addition, TF tends to decompose to the toxic compound dichlorvos, which exhibits higher lipid-solubility than trichlorfon.3,4 Indeed, both TF and dichlorvos cause teratogenesis and mutagenesis in mice and rats,5 and so there is an increasing concern regarding the presence of TF residues in fish products.In terms of its chemical structure, TF contains an asymmetric carbon center and exists in two enantiomeric forms. Chiral drugs may degrade stereospecifically, which was usually leaded by the activity of microorganism,6 such as hexachlorocyclohexane, mecoprop, and phenoxyalkanoic acid herbicides.7–9 However, since fish tissues are rich in nutrients, and have high water content and water activity, microbes can easily grow and reproduce in stored fish tissues. Thus, TF residues in fish tissues may degrade stereospecifically and result in enantiomeric excess. Although there are no reports on different physiological toxicities between TF enantiomers, they exhibit stereospecificity in the inhibition of acetylcholinesterase,10 which participates in development and maturation of cells. Therefore, the expected result for enantiospecific degradation of TF in fish tissues is that more S-enantiomer is induced or more R is induced and it can be dangerous to human health. However, TF is available commercially only in the racemic form.11–13 Due to the absence of adequate information regarding the potential differences in the degradation behavior of TF enantiomers, traditional methods for the risk evaluation of TF residues in fish are inadequate.
A number of previous studies3,14–18 have reported the determination of TF in food, soil, and seawater. Moreover, a gas chromatography (GC) method for the separation of TF enantiomers has been reported.12 However, to the best of our knowledge, a validated analytical procedure for the separation and qualification of TF enantiomers for investigating the enantiospecific behavior of TF enantiomers in fish has not been reported. Thus, in order to obtain information regarding the dissipation of TF enantiomers in fish, the enantioseparation and determination of TF enantiomers in fish must be investigated.
In this paper, we have reported the influence of microorganism activity on the degradation of TF enantiomers in stored fish. In addition, we have developed and validated a chiral high-performance liquid chromatography (HPLC) technique for the determination of TF enantiomers in stored fish. The enantiospecific degradation behavior of TF enantiomers during fish storage was investigated at different temperatures, and the formation of dichlorvos, a toxic metabolite of TF, was also evaluated.
2. Materials and methods
2.1. Materials
Drug-free skinned and boned fish (Sciaenops ocellatus) was purchased from the DongShan Market (Zhoushan, China) and stored at −50 °C prior to use. (±)-TF (>98%), dichlorvos (>98%), and metolcarb (>99%) were purchased from Aladdin Reagent Co., Ltd. (Shanghai, China). Pure TF enantiomers (>98%) were obtained from Daicel (Shanghai, China). Ethanol, n-hexane, and isopropanol were of HPLC grade and purchased from Merck (Darmstadt, Germany). Deionized water was obtained using a Milli-Q® water purification system (Molsheim, France). All other chemicals and solvents were of analytical grade and were obtained from common commercial sources. The chiral analytical column (Chiralpak IC, 5 μm, 250 mm × 4.6 mm i.d.) was purchased from Daicel (Tokyo, Japan).2.2. Absolute configuration assignment of TF enantiomers
Electronic circular dichroism (ECD) spectroscopy was carried out on a Jasco-J815 spectropolarimeter at 25 °C. The ECD spectra were collected between 200 and 400 nm with a scan speed of 100 nm min−1. A quartz cell with 1 mm path length was used, and the average spectrum obtained over three scans was reported. The absolute configuration of enantiomers was important for understanding the biochemical processing of chiral compounds.19,20 Therefore, the calculated ECD spectra were used to predict the absolute configurations of the TF enantiomers. The calculated ECD spectra were obtained using time-dependent density functional theory (TDDFT) with the B3LYP functional in Gaussian 09, and were analyzed using GUIs GaussView (version 5.0).21 The calculated ECD spectra were simulated at the B3LYP/6-311G (d,p) level in methanol.2.3. Preparation of standard stock solutions
Samples of (±)-TF, R-(−)-TF, and S-(+)-TF were accurately weighed, transferred to volumetric flasks, and dissolved in n-hexane/isopropanol (91/9, v/v) to give stock solutions of 5 μg mL−1. In addition, dichlorvos and metolcarb were accurately weighed, transferred to volumetric flasks, and dissolved in the mobile phase to give stock solutions of 10 μg mL−1. The R-(−)-TF, S-(+)-TF, dichlorvos, and metolcarb solutions were thoroughly mixed and stored at 4 °C in airtight bottles until use. Working solutions for each enantiomer (0.25, 0.5, 1.0, 1.5, 2.0, and 2.5 μg mL−1) and for dichlorvos (0.25, 1.0, 2.0, 3.0, 4.0, and 5.0 μg mL−1) were prepared from the stock solutions by diluting with the mobile phase. A 1.0 μg mL−1 metolcarb internal standard (IS) was used.2.4. Sample preparation
A minced fish sample (5 g) was placed in a 50 mL polypropylene centrifuge tube and extracted using acetonitrile containing 0.1% acetic acid (15.0 mL) by vortex mixing for 2 min. Following centrifugation for 3 min at 5000 rpm, the upper organic layer was transferred to a disposable glass tube. The fish samples were then re-extracted with a further portion of 0.1% acetic acid in acetonitrile (15.0 mL) as described above. The organic layers were combined and evaporated to dryness at 27 °C. The resulting residues were dissolved in methanol/water (5.0 mL, 80/20, v/v), and the solutions loaded onto a ProElut™ PLS (500 mg, DIKMR, China) solid-phase extraction (SPE) cartridge preconditioned with methanol (5.0 mL) and water (5.0 mL). A sample flow rate of 2.0 mL min−1 was used. The samples were eluted with ethyl acetate (8.0 mL), and evaporated to dryness using a gentle stream of nitrogen. The resulting residues were dissolved in n-hexane/isopropanol (1.0 mL, 91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, v/v). Finally, the extract was filtered through a 0.22 μm nylon syringe filter (Agilent Technologies, Waldbronn, Germany), and an aliquot (25 μL) was injected into the HPLC system.
9, v/v). Finally, the extract was filtered through a 0.22 μm nylon syringe filter (Agilent Technologies, Waldbronn, Germany), and an aliquot (25 μL) was injected into the HPLC system.
2.5. Chromatographic conditions
HPLC separation experiments for method development and validation were carried out on an Agilent 1200 HPLC system (Agilent, USA), consisting of a quaternary pump (G1311A), a column thermostat (G1316A), a degasser unit (G1322A), and an autosampler (G1329A). A Chiralpak IC column (250 mm × 4.6 mm i.d., 5 μm) was used for all experiments. A mixture of n-hexane and isopropanol (91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 (v/v)) was used as the mobile phase at a flow rate of 0.8 mL min−1. The UV detector was set at 207 nm and the separation temperature was 25 °C. The HPLC system was controlled and the data analyzed by a computer equipped with the ChemStation software package (Rev. B.04.02, Agilent).
9 (v/v)) was used as the mobile phase at a flow rate of 0.8 mL min−1. The UV detector was set at 207 nm and the separation temperature was 25 °C. The HPLC system was controlled and the data analyzed by a computer equipped with the ChemStation software package (Rev. B.04.02, Agilent).
2.6. Method development and validation
Calibration curves were obtained for the TF enantiomers and for dichlorvos using a series of standard solutions (0.25–2.5 μg mL−1 and 0.25–5.0 μg mL−1, respectively). Five replicate injections of each standard solution were performed. The peak area ratio (y, TF enantiomer/IS or dichlorvos/IS) was used in conjunction with the calibration curve to derive the TF concentrations (x, μg g−1) in fish samples. To evaluate the matrix effect, matrix-matched calibration curves for the TF enantiomers (0.25–2.5 μg mL−1) and for dichlorvos (0.25–5.0 μg mL−1) were constructed for each fish sample. The limits of detection (LODs) and limits of quantitation (LOQs) for the fish samples were established at signal to noise (S/N) ratios of 3 and 10, respectively.The accuracy and precision of the method were evaluated by recovery studies using spiked drug-free samples. For the method-recovery studies, aliquots of the R-(−)-TF, S-(+)-TF, and dichlorvos working solutions (1.0 mL) were each added to 50 mL polypropylene centrifugal tubes, and were evaporated to dryness using a gentle stream of nitrogen. The residues were then homogenized with drug-free fish samples. The R-(−)-TF and S-(+)-TF nominal concentrations used in the drug-free fish samples were 0.06, 0.15, and 0.4 μg g−1. In addition, the dichlorvos nominal concentrations used in the drug-free fish samples were 0.06, 0.2, and 0.8 μg g−1, and the IS nominal concentration used was 0.2 μg g−1. The spiked fish samples were analyzed by the developed method and the recovery values were calculated. Intraday accuracy and precision for R-(−)-TF, S-(+)-TF, and dichlorvos were determined based on five replicate analyses for the three different concentrations on a single day. Furthermore, interday accuracy and precision values were determined by five replicate analyses for the three different concentrations on five different days within a 14 day period. Accuracy was calculated as the percentage difference between the concentration measured using the calibration plot and the concentration of the enantiomer or dichlorvos added to the drug-free sample. Precision was expressed as the percentage variation of the measured concentrations.
2.7. Enantioselective behavior of TF enantiomers in stored fish
To investigate the enantiospecific behavior of TF enantiomers and the formation of dichlorvos in stored fish, batch experiments were conducted with the minced fish samples by spiking with each TF enantiomer (0.5 μg g−1). The spiked fish samples were then transferred to 50 mL polypropylene centrifuge tubes, and the tubes covered with film and stored at −18 °C, 4 °C, 18 °C, or 25 °C. For the sterile experiments, the minced fish samples were irradiated by ultraviolet radiation for 0.5 h and then stored at 25 °C. Three samples were removed at different time intervals and were immediately extracted. All experiments were performed in triplicate, and the average values used for data analysis using Origin 8.0.3. Results and discussion
3.1. Absolute configuration of TF enantiomers and elution order
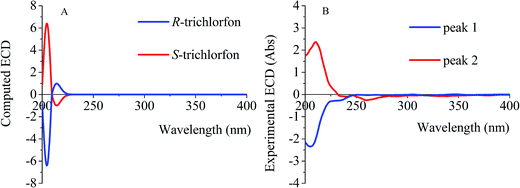
TDDFT methodology was used to calculate the ECD spectra of the TF enantiomers. Comparison of the experimental ECD spectra with the calculated spectra allowed determination of the absolute configurations of the TF enantiomers. The overall ECD curves obtained from the TDDFT calculations (Fig. 2A) and those obtained experimentally (Fig. 2B) were comparable. As the individual enantiomers of chiral compounds generally correspond to a specific circular dichroism (CD) signal at a given wavelength, the first enantiomer eluted was confirmed to be the (−)-enantiomer, whereas the second was the (+)-enantiomer (Fig. 2B). Comparison of the calculated and experimental ECD spectra allowed peaks 1 and 2 (Fig. 3) to be assigned to R-(−)-TF and S-(+)-TF, respectively.3.2. Optimization of solid-phase extraction
To determine the optimal extraction solvent, eight solvents were investigated, and the recoveries of TF and dichlorvos following extraction from the fish samples were compared. A comparison of the eight solvents (shown in Table 1) suggested that both the TF and dichlorvos recoveries were higher with acidified solvents than with unacidified solvents. Table 1 also shows that low recoveries (29.83–81.59%) were obtained for TF and dichlorvos with three of the acidified solvents (ethyl acetate, dichloromethane, and acetone). Thus, acetonitrile containing 0.1% acetic acid (91.67–99.77% recovery) was selected for the extraction of dichlorvos and the TF enantiomers from the fish samples.| Extraction solvent | Recoverya (%) | |||
|---|---|---|---|---|
| S-(+)-TF | R-(−)-TF | Dichlorvos | ||
| a Expressed as mean ± SD. | ||||
| Unacidified | Ethyl acetate | 15.02 ± 3.23 | 25.51 ± 2.56 | 38.24 ± 3.98 |
| Dichloromethane | 65.09 ± 4.84 | 68.10 ± 5.68 | 80.76 ± 2.98 | |
| Acetone | 39.13 ± 4.99 | 50.33 ± 5.36 | 60.98 ± 7.12 | |
| Acetonitrile | 51.92 ± 7.32 | 56.39 ± 6.69 | 66.65 ± 3.13 | |
| Acidified | Ethyl acetate | 29.83 ± 7.10 | 37.55 ± 3.93 | 45.98 ± 3.98 |
| Dichloromethane | 76.03 ± 4.33 | 81.59 ± 5.83 | 87.98 ± 4.87 | |
| Acetone | 50.54 ± 9.03 | 61.59 ± 7.44 | 78.09 ± 2.19 | |
| Acetonitrile | 92.69 ± 3.41 | 91.69 ± 4.70 | 99.77 ± 1.11 | |
To determine the optimal extraction volume, we examined the relationship between extraction volume and TF recovery. As shown in Table S1,† extraction volumes of 15 mL and 20 mL gave higher recoveries for all compounds. Larger extraction volumes could be used to obtain close to quantitative recovery of the target compounds, but led to an increase in solvent wastage and a longer concentration time to obtain the desired compounds. Therefore, an extraction volume of 15 mL was selected.
For the enrichment and cleanup of the target compounds obtained from the fish samples, an SPE procedure was applied. Three different classes of SPE cartridges (N-alumina, C18, and PLS) were used and compared for sample prefractionation, as shown in Table 2. Comparison of the three different SPE cartridges suggested that the ProElut™ PLS (500 mg, DIKMR) cartridge efficiently adsorbed TF and dichlorvos (see Fig. 3), and yielded higher recoveries (see Table 2) of all target compounds than the other types of SPE cartridge (ProElut™ C18 and Cleanert N-alumina). Therefore, the ProElut™ PLS cartridge (500 mg, 6 mL) was selected for the prefractionation of TF and dichlorvos from the fish samples.
Elution volume was also an important factor in the SPE process. Table 3 shows the relationship between the elution volume and the recoveries of TF and dichlorvos. As shown in Table 3, elution volumes of 8 mL and 10 mL were optimal. Larger elution volumes could be used to quantitatively elute the target compounds, but led to increased losses during drying under a flow of nitrogen. Thus, an elution volume of 8 mL was selected for use throughout.
3.3. Selectivity
Potential interference from endogenous substances was evaluated by extracting and analyzing six different sources of drug-free fish samples. Fig. 3 shows the typical chromatograms of a drug-free fish sample (Fig. 3A) and a spiked fish sample (Fig. 3B) containing 2 μg g−1 of S-(+)-TF, R-(−)-TF, dichlorvos, and IS. No endogenous substances exhibiting a significant influence on the compound retention times were detected.Matrix effects (MEs)22 were then investigated by comparison of the slopes of analytes obtained from standard solutions with those obtained from extracted samples doped with an equivalent amount of each analyte. Six different drug-free fish samples were used for these assessments. As shown in Table 4, the MEs for the two TF enantiomers and dichlorvos were 0.98, 0.97, and 1.06, respectively. Thus, the variation due to MEs was insignificant. The relative difference between the slopes of solvent and matrix-matched calibration curves was <5%, indicating that no significant matrix effect from the fish tissue was observed.23
| Analyte | Matrix | Regression equationa | Linear range (mg L−1) | Correlation coefficient (R2) | MEb | LODc (μg g−1) | LOQd (μg g−1) |
|---|---|---|---|---|---|---|---|
| a y: peak area; x: concentration of analyte (μg g−1).b Matrix effect.c Limit of detection: S/N > 3.d Limit of quantitation: S/N > 10. | |||||||
| R-(−)-TF | Solvent | y = 3.4926x + 0.0452 | 0.25–5.0 | 0.9996 | — | ||
| Fish tissue | y = 3.4227x + 0.1356 | 0.25–5.0 | 0.9989 | 0.98 | 0.016 | 0.041 | |
| S-(+)-TF | Solvent | y = 3.9534x + 0.0910 | 0.25–5.0 | 0.9992 | — | ||
| Fish tissue | y = 3.8348x + 0.0522 | 0.25–5.0 | 0.9990 | 0.97 | 0.018 | 0.047 | |
| Dichlorvos | Solvent | y = 8.9310x − 0.3842 | 0.25–10.0 | 0.9997 | — | ||
| Fish tissue | y = 9.5072x − 0.6578 | 0.25–5.0 | 0.9991 | 1.06 | 0.009 | 0.027 | |
To confirm that the chiral configurations of pure R-(−)-TF and S-(+)-TF were stable during the pretreatment process, fish samples were spiked with pure R-(−)-TF or S-(+)-TF, and then extracted and analyzed by the aforementioned methods. The obtained chromatograms (Table S2†) confirmed that in both cases, the other enantiomer was not detected. This suggests that the chiral configurations of both enantiomers were stable in the pretreatment process.
3.4. Validation of the chiral HPLC method
The chiral HPLC method was validated, and the linearity, LODs, and LOQs were determined by serial dilution of the standard solutions. The IS method was used to obtain fish sample concentrations, with each calibration sample containing 1.0 μg mL−1 of the IS. The calibration curve was generated by plotting the peak area ratio (y, TF/IS or dichlorvos/IS) against the amount of analyte injected (x, μg mL−1).As shown in Table 4, the three calibration curves were linear over the concentration range studied, with correlation coefficients (R2) greater than 0.999. The LODs were 0.016 μg g−1, 0.018 μg g−1, and 0.009 μg g−1 for S-(+)-TF, R-(−)-TF, and dichlorvos, respectively, and the LOQs were 0.041 μg g−1, 0.047 μg g−1, and 0.027 μg g−1 for S-(+)-TF, R-(−)-TF, and dichlorvos, respectively. The LODs and LOQs for the TF enantiomers were lower than the maximum residue limits (0.05 μg g−1) in animal muscle, as recommended by the Food and Agricultural Organization/World Health Organization (FAO/WHO) in 2000.13 This method could therefore be applied for the detection of TF enantiomer residues in fish tissues.
Using this method, the recoveries were determined in triplicate by external standard calibration with spiked control fish samples (0.06, 0.15, and 0.40 μg g−1 of each TF enantiomer; 0.06, 0.2, and 0.8 μg g−1 of dichlorvos). As shown in Table 5, good recoveries (89.70–99.96%) were obtained for both TF enantiomers and for dichlorvos. This not only confirmed the accuracy of this method, but also the integrity of the SPE procedure.
| Analyte | Spiked amount (μg g−1) | Recovery (n = 5) | Precision (RSD%) | |
|---|---|---|---|---|
| Mean (%) ± SDa | Intradayb | Interdayc | ||
| a Determination in one day.b n = 5.c n = 5 replicates × 5 d within a 14 d period. | ||||
| R-(−)-TF | 0.06 | 89.70 ± 7.32 | 7.58 | 9.05 |
| 0.15 | 90.05 ± 6.21 | 5.06 | 9.86 | |
| 0.40 | 91.76 ± 5.17 | 4.36 | 4.30 | |
| S-(+)-TF | 0.06 | 90.15 ± 7.74 | 7.58 | 9.91 |
| 0.15 | 99.96 ± 7.47 | 5.13 | 9.65 | |
| 0.40 | 98.60 ± 4.55 | 3.83 | 4.36 | |
| Dichlorvos | 0.06 | 95.03 ± 8.21 | 2.41 | 3.69 |
| 0.20 | 90.87 ± 3.22 | 2.34 | 2.18 | |
| 0.80 | 97.85 ± 1.22 | 1.98 | 2.10 | |
Intraday precision was evaluated by analyzing five replicate samples for three consecutive days, with three different concentrations of TF enantiomers and dichlorvos. The relative standard deviation (RSD) values for the intraday precision ranged from 1.98% to 7.58% (Table 5). The interday precision was also evaluated by analyzing five replicate samples spiked with three different concentrations of TF enantiomers and dichlorvos on five different days within a 2 week period. The RSD values for the interday precision ranged from 2.10% to 9.91%, as shown in Table 5.
3.5. Dissipation of TF enantiomers during fish storage
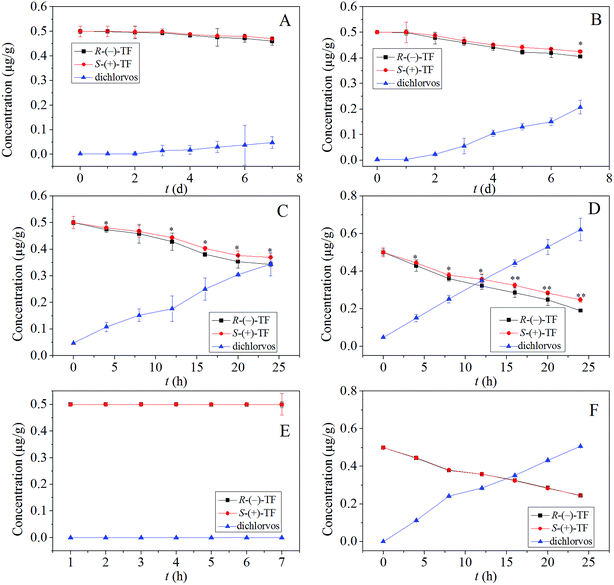
To simulate the storage of TF-contaminated fish, experiments were performed by spiking drug-free fish samples with TF then storing at −18 °C, 4 °C, 18 °C, and 25 °C for 7 d, 7 d, 24 h, and 24 h, respectively. The dissipation of S-(+)-TF and R-(−)-TF, and the formation of dichlorvos in stored fish at different temperatures are shown in Fig. 4. Increasing storage time led to a decrease in both the S-(+)-TF and R-(−)-TF concentrations in the fish samples at all four storage temperatures. Furthermore, dichlorvos concentrations in the fish samples also increased with storage time. At −18 °C and 4 °C, S-(+)-TF and R-(−)-TF concentrations decreased slowly over time (Fig. 4(A and B)), while a rapid decrease was observed at 18 °C and 25 °C (Fig. 4(C and D)), resulting in the enrichment of dichlorvos levels in the fish. As can be seen from Fig. 4, the degradation rate of S-(+)-TF and R-(−)-TF along with the formation rate of dichlorvos at different storage temperatures followed the order −18 °C < 4 °C < 18 °C < 25 °C. This suggested that storage temperature significantly affected the degradation of TF enantiomers and the resulting formation of dichlorvos in fish samples. The concentration of S-(+)-TF in the fish samples was slightly higher than that of R-(−)-TF. In addition, faster degradation of R-(−)-TF was observed at 18–25 °C, as shown in Fig. 4(C and D). Therefore, to verify whether temperature had an effect on the enantiospecific degradation of TF, we designed temperature degradation experiments of sterile fish samples at 4 °C and 25 °C, and the results are shown in Fig. 4. Fig. 4(E and F) indicate the degradation of TF enantiomers (0.5 mg kg−1) and the generation of dichlorvos in sterile fish samples at 4 °C and 25 °C. The degradation rate of S-(+)-TF and R-(−)-TF and the formation rate of dichlorvos at the different storage temperatures was lower at 4 °C than at 25 °C.Student's paired t-test between the concentrations of R-(−)-TF and S-(+)-TF at different storage times was then investigated. As shown in Fig. 4, at 4 °C, the p value was <0.05 at 7 d, and <0.05 at 18 °C after 4, 12, 16, 20, and 24 h. This indicated that TF degradation was enantiospecific during storage at 18 °C. In addition, at 25 °C, p was <0.05 after 4, 8, and 12 h, and <0.01 after 16, 20, and 24 h, indicating that the degradation of TF was highly enantiospecific during storage at 25 °C.
Several studies have reported that the enantiospecific behavior of chiral compounds is influenced by microorganisms.24 To determine whether the enantiospecific dissipation of TF enantiomers was the result of microorganism activity, we investigated the degradation behavior of TF in unsterile and sterile fish samples at 25 °C. As shown in Fig. 4(D and F), the degradation of TF enantiomers in the sterile fish samples showed no enantiospecificity, whereas the unsterile fish samples exhibited enantiospecific degradation. To assess the enantiospecific degradation of TF, we also calculated the enantiomer fraction (EF) under different conditions. The EF value (EF = concentration of R/concentration of (S + R)25) was used to characterize the enantiospecific degradation behavior of TF enantiomers in fish samples (where EF ranges from 0 to 1, while a racemic mixture is expressed as EF = 0.5). As shown in Fig. S1A,† a stable EF of 0.5 was observed at −18 °C and 4 °C, suggesting that the TF enantiomers did not degrade enantioselectively. However, at 18 °C and 25 °C (Fig. S1B†) the EF value increased with time, finally reaching 0.52 and 0.56, respectively, confirming enantiospecific degradation. In addition, in sterile fish samples, the EF remained constant at 0.5 (Fig. S1B†), indicating that microbial activity did indeed contribute to the enantiospecific degradation of TF enantiomers in fish samples.
To obtain further information regarding the influence of microorganisms on the enantiospecific degradation of TF in fish samples, the relationship between the EF and the aerobic plate count (APC) was investigated. TF enantiomer concentrations were measured following storage at 24 h, and the EF values were calculated. As shown in Fig. 5, the increasing APC values resulted in higher EF values, suggesting that a greater number of microorganisms yielded increased enantiospecific TF degradation in fish samples. In addition, the EF values were close to 0.5 at both −18 °C and 4 °C, but increased from 0.5 to 0.56 upon increasing the storage temperature from 18 °C to 25 °C. This indicated that lower storage temperatures inhibited microbial activity, with increased activity at higher temperatures causing the enantiospecific degradation of TF. Overall, the relationship between EF, APC, and storage temperature provided further evidence to support our suggestion that microorganisms in fish play an important role in the enantiospecific degradation of TF enantiomers.
4. Conclusions
A chiral HPLC method for the simultaneous determination of the concentrations of trichlorfon (TF) enantiomers and dichlorvos in fish samples was developed. The absolute configuration of the TF enantiomers was then assigned by comparing the experimental electronic circular dichroism (ECD) spectra with the calculated ECD spectra. In addition, the degradation rate of TF enantiomers in fish increased with increasing storage temperature, resulting in enrichment of the toxic metabolite dichlorvos. The enantiospecific degradation of S-(+)-TF and R-(−)-TF in fish at 18 °C and 25 °C was observed, with R-(−)-TF being preferentially degraded. However, the degradation of S-(+)-TF and R-(−)-TF showed no enantiospecificity in fish stored at −18 °C or 4 °C. These results, combined with the results from sterile and unsterile fish samples suggested that microorganisms in fish are responsible for the enantioselective degradation of S-(+)-TF and R-(−)-TF. Thus, these results provide useful information for evaluating the risk posed by the presence of TF in fish, and could be used for evaluation of the environmental risks and safety of foods containing TF. This method may therefore prove invaluable in the overall field of food safety.Acknowledgements
The authors are grateful for the support of the Zhejiang Provincial Natural Science Foundation of China (LY14C200003), the National Natural Science Foundation of China (21276240), funds of the Science and Technology department of Zhejiang province (2014C32080), and the ZheJiang education department project PD201322.References
- C. Hispao, P. Bulto and A. R. Blanch, J. Appl. Ichthyol., 2013, 29, 1139–1144 CrossRef
.
- A. E. Czeizel, C. Elek, S. Gundy, J. Metneki, E. Nemes, A. Reis, K. Sperling, L. Timar, G. Tusnady and Z. Viragh, Lancet, 1993, 341, 539–542 CrossRef CAS
.
- O. B. Samuelsen, Aquaculture, 1987, 66, 373–380 CrossRef CAS
.
- W. Hofer, Acta Pharmacol. Toxicol., 1981, 49, 7–14 CrossRef CAS
.
- K. D. Courtney, J. E. Andrews and J. Springer, J. Environ. Sci. Health, Part B, 1986, 21, 207–227 CrossRef CAS PubMed
.
- D. L. Lewis, A. W. Garrison, K. E. Wommack, A. Whittemore, P. Steudler and J. Melillo, Nature, 1999, 401, 898–901 CrossRef CAS PubMed
.
- L. M. Jantunen and T. Bidleman, J. Geophys. Res.: Atmos., 1996, 101, 28837–28846 CrossRef CAS
.
- C. Zipper, M. J. F. Suter, S. B. Haderlein, M. Gruhl and H. P. E. Kohler, Environ. Sci. Technol., 1998, 32, 2070–2076 CrossRef CAS
.
- P. Ludwig, W. Gunkel and H. Huhnerfuss, Chemosphere, 1992, 24, 1423–1429 CrossRef CAS
.
- L. Li, Doctor of Philosophy PhD dissertation, Zhejiang University of Technology, 2010 Search PubMed
.
- E. Toksen, U. Degirmenci and M. Cankurt, sB. Eur. Assoc. Fish Pat., 2010, 30, 205–210 Search PubMed
.
- N. Fidalgo-Used, E. Blanco-Gonzalez and A. Sanz-Medel, Talanta, 2006, 70, 1057–1063 CrossRef CAS PubMed
.
- G. M. Wang, H. Dai, Y. G. Li, X. L. Li, J. Z. Zhang, L. Zhang, Y. Y. Fu and Z. G. Li, Food Addit. Contam., Part A, 2010, 27, 983–988 CrossRef CAS PubMed
.
- S. Grimalt, J. V. Sancho, O. J. Pozo, J. M. Garcia-Baudin, M. L. Fernandez-Cruz and F. Hernandez, J. Agric. Food Chem., 2006, 54, 1188–1195 CrossRef CAS PubMed
.
- B. A. Khan, A. Farid, M. R. Asi, H. Shah and A. K. Badshah, Food Chem., 2009, 114, 286–288 CrossRef CAS
.
- L. Meng, X. G. Qiao, J. M. Song, Z. X. Xu, J. H. Xin and Y. Zhang, J. Agric. Food Chem., 2011, 59, 12745–12751 CrossRef CAS PubMed
.
- X. L. Wang, Q. H. Tang, Q. Q. Wang, X. G. Qiao and Z. X. Xu, J. Sci. Food Agric., 2014, 94, 1409–1415 CrossRef CAS PubMed
.
- M. A. Ngoh and R. Cullison, J. Agric. Food Chem., 1996, 44, 2686–2689 CrossRef CAS
.
- S. Ding, L. Jia, A. Durandin, C. Crean, A. Kolbanovskiy, V. Shafirovich, S. Broyde and N. E. Geacintov, Chem. Res. Toxicol., 2009, 22, 1189–1193 CrossRef CAS PubMed
.
- S. Allenmark and J. Gawronski, Chirality, 2008, 20, 606–608 CrossRef CAS PubMed
.
- P. J. Stephens, F. J. Devlin, F. Gasparrini, A. Ciogli, D. Spinelli and B. Cosimelli, J. Org. Chem., 2007, 72, 4707–4715 CrossRef CAS PubMed
.
- F. Gosetti, E. Mazzucco, D. Zampieri and M. C. Gennaro, J. Chromatogr. A, 2010, 1217, 3929–3937 CrossRef CAS PubMed
.
- Y. X. Xu, H. Zhang, S. L. Zhuang, M. Yu, H. Xiao and M. R. Qian, J. Agric. Food Chem., 2012, 60, 4173–4178 CrossRef CAS PubMed
.
- Y. P. Zhang, D. Y. Hu, H. R. Ling, L. Zhong, A. X. Huang, K. K. Zhang and B. A. Song, J. Agric. Food Chem., 2014, 62, 9066–9072 CrossRef CAS PubMed
.
- E. M. Ulrich, D. R. Helsel and W. T. Foreman, Chemosphere, 2003, 53, 531–538 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra17702b |
| This journal is © The Royal Society of Chemistry 2016 |