Progress in polymeric separators for lithium ion batteries
Hong Zhang
,
Ming-Yong Zhou
,
Chun-Er Lin
and
Bao-Ku Zhu
*
Key Laboratory of Macromolecule Synthesis and Functionalization (MOE), ERC of Membrane and Water Treatment (MOE), Department of Polymer Science and Engineering, Zhejiang University, Hangzhou 310027, China. E-mail: zhubk@zju.edu.cn; Fax: +86 571 87953723; Tel: +86 571 87953723
First published on 30th September 2015
Abstract
This study reviews the recent developments and the characteristics of polymeric separators used for lithium ion batteries. According to the structure and composition of the separators, they are broadly divided into four types: (1) polyolefin microporous separators, (2) heterochain polymer microporous separators, (3) polymer electrolytes and (4) non-woven separators. In particular, polymer electrolytes were defined as one category of separators for convenient description in this review; these feature intermediates between the two electrodes and possess transport properties comparable with the separator in liquid LIBs. For each category, the structure, characteristics, modification, and performance of separators are described. Finally, guidelines for further improvements in this research are outlined.
1. Introduction
The constant increase in global energy demand, together with an awareness of the finite supply of fossil fuels, has sparked the need to explore alternative green energy sources (such as solar, wind and geothermal energy). Besides these factors, increasing pollution due to the growing CO2 content in the atmosphere has boosted technological efforts to replace the combustion-engine car with electric or hybrid vehicles. However, the success of these technologies relies on the availability of suitable storage systems such as electrochemical batteries.Among the different varieties of batteries, the lithium ion battery (LIB) is one of the more vigorous competitors due to its high energy and power density, low self-discharge, and long cycle life. LIBs have been already widely used in various portable electronic devices such as cellular phones, laptops, and PDAs. Recently, their applications have expanded to electric and hybrid vehicles. Basically, a LIB consists of four functional components, the anode, the cathode, the separator and the electrolyte, as illustrated in Fig. 1.1
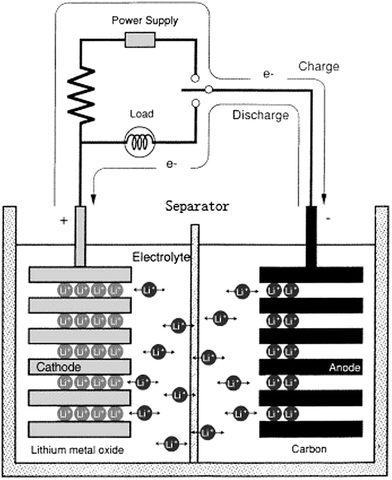 | ||
| Fig. 1 Schematic of a typical LIB. This figure was edited from Fig. 2 of ref. 1, with permission from Elsevier. | ||
A separator is placed between the anode and cathode. Its primary function is to prevent physical contact between the electrodes so that free ions rather than electrons can be transported between them.2 Although the separator itself is not involved in any cell reactions, its structure and properties considerably influence the interfacial structure and internal resistance of the LIB, thus affecting capacity, polarization, cycle life, and safety. Therefore, the fabrication of a high-performance separator is necessary to develop the overall performance of LIBs.
In principle, an ideal LIB separator must satisfy some basic requirements such as electronic insulation, mechanical and dimensional stability, chemical and electrochemical reliability, excellent wettability in liquid electrolytes, and uniform thickness. Detailed descriptions have been extensively discussed elsewhere and are not included in this review.3–5 However, it is not easy to achieve all the requirements simultaneously. For example, with the reduction of the separator thickness, the battery's energy and power densities increase, whereas its mechanical strength and safety may deteriorate. Herein, the order of importance of the various requirements varies depending on the application.
2. Types and properties
Based on their structure and composition, we broadly divide polymeric separators into four types: (1) polyolefin microporous separators, (2) heterochain polymer separators, (3) polymer electrolytes, and (4) non-woven separators. In this article, we focus on the influence of the material, processing method, and structure on the properties of the abovementioned separators. In addition, some remarks on the main strengths and weaknesses of each separator as well as future directions are presented. Prior to introducing each system, however, one important concept still has to be clarified. As mentioned earlier, strictly speaking, there are some obvious differences between polymer electrolytes and traditional separators. Polymer electrolytes were defined as one category of separators in this review that features intermediates between the two electrodes and possesses transport properties comparable with the separators in liquid LIBs.6,72.1 Polyolefin microporous separators
Currently, commercial separators are mainly made of polyolefins, including polyethylene (PE) or polypropylene (PP). The dry process and the wet process are the most widely-used methods for preparing the polyolefin separators. In general, separators made using the dry process exhibit distinct slit-pore and straight microstructures, whereas those made by the wet process show interconnected spherical or elliptical pores. Typical surface morphologies of polyolefin separators made by both processes are displayed in Fig. 2.4 From the viewpoint of the porous structure, separators made by the dry process are more suitable for high-power-density batteries due to their open and straight-through porous structure, whereas the tortuous and interconnected porous structures of the wet-process separators are more advantageous with respect to suppressing the growth of lithium dendritic structures during long cycle processes. Detailed descriptions of the various fabrication processes as well as the relationships between the structure and properties of polyolefin separators have been reported in numerous monographs and reviews.8–10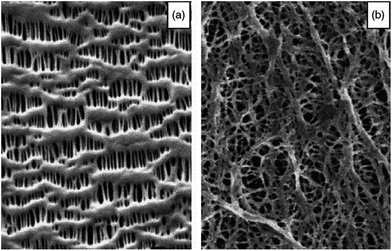 | ||
| Fig. 2 Surface morphologies of polyolefin separators made by (a) dry process and (b) wet process. This figure was edited from Fig. 3 of ref. 5, with permission from Elsevier. | ||
Although polyolefin separators are basically reliable for portable applications, two major drawbacks have been identified. First, due to their inherent hydrophobicity and low surface energy, the polyolefin separators show poor wettability and retention of liquid electrolytes containing polar solvents such as ethylene carbonate (EC), dimethyl carbonate (DMC), γ-butyrolactone (γ-GBL) and propylene carbonate (PC). Second, the polyolefin separators suffer from severe thermal shrinkage at high temperatures because of their low melting points. Although they have the features of thermal shut-down, which can turn the polyolefin separator into a nonporous insulating film around its melting point, the temperature of the cell often continues to increase even after shutdown before it actually begins to cool.11 Thus, the cell may experience an internal short-circuit or even an explosion, especially when it is exposed to abusive conditions such as overcharging, crushing or overheating. Therefore, a separator with excellent wettability in liquid electrolyte and high thermal stability is highly desired for high performance LIBs.
A multilayer separator made from PE and PP has been patented as a highly heat-resistant separator; this is a perfect combination, providing both safety assurance and sufficient mechanical strength.12–14 In a multilayered structure (as shown in Fig. 3), the low melting PE layer (135 °C) can act as a thermal fuse and block the pathway of ions when the temperature is close to its melting temperature. At the same time, the higher-melting PP layer (165 °C) retains its dimensional structure and mechanical strength, and thus prevents short circuiting between two electrodes.
 | ||
| Fig. 3 Scanning electron micrographs of the Celgard 2325 (PP/PE/PP) separators: (a) surface and (b) cross-section. This figure was edited from Fig. 5 of ref. 14, with permission from the American Chemical Society. | ||
In addition, many methods have been attempted in the lab such as surface coating, surface grafting and blending.
| Polyolefin substrate | Coating materials | Liquid electrolyte | Electrolyte uptake (%) | Ionic conductivity (mS cm−1) | Ref. |
|---|---|---|---|---|---|
| PE | AN-MMA | LiClO4–EC/DMC | 91 | 1.00 | 15 |
| PE | PVDF-HFP/PMMA | LiClO4–EC/DEC | 403 | 1.69 | 16 |
| PE | PVDF–CAB | LiPF6–EC/EMC | 152 | 2.48 | 17 |
| PE | PDA | LiPF6–DEC/EC/PC | 126 | 0.41 | 18 |
| PP | Catechol/polyamine | LiPF6–EC/DMC | 270 | — | 19 |
| PE | PVDF | LiPF6–EC/EMC/DMC | — | 0.89 | 20 |
| PE | Ethylcellulose (EC) | LiPF6–EC/DMC | — | 0.68 | 21 |
| PE | PI (BTDA–TDI/MDI) | LiPF6–EC/DEC | 106 | 0.24 | 22 |
| PE | SiO2/PMMA | LiPF6–EC/DEC | 180 | 0.74 | 23 |
| PE | Al2O3 (cPET binder) | LiPF6–EC/DEC | — | — | 24 |
| PE | Al2O3 (SBR–CMC binder) | — | 82 | 1.00 | 25 |
| Polyolefin | PMOTPA | LiPF6–EC/EMC/DMC | — | — | 26 |
| PE | SiO2/poly(lithium 4-styrenesulfonate) | LiPF6–EC/DEC | 242 | 0.75 | 27 |
| PP | SiO2/Al2O3 | EC/DEC | 120 | 0.78 | 28 |
| PE | SiO2/PEI | LiPF6–EC/EMC/DEC | 398 | 0.49 | 29 |
| PE | Al2O3/PVDF-HFP | LiPF6–EC/DEC | — | 0.53 | 30 |
| PE | DMAET | 108 | 0.29 | 31 | |
| PP | Polyamine/catechol | LiPF6–EC/DMC | 270 | — | 32 |
| PP | Tannic acid | LiPF6–EC/DMC | 125 | 0.46 | 33 |
Jeong et al.15 reported a method of reducing interfacial resistance by coating a gellable acrylonitrile (AN)–methyl methacrylate (MMA) copolymer onto the PE separator by dip-coating followed by phase inversion, which can trap a large amount of liquid electrolyte, further facilitating adherence of the electrodes to the separator. Moreover, the cell assembled with this modified separator displayed a stable discharge capacity and an excellent rate performance.
In general, the coating process produces a new interface between the support separator and the coating material, which inevitably compromises ionic conductivity and the electrochemical kinetics of the modified separator. To modify the interfacial properties between the support PE separator and the coated material, Kim et al.34 used gamma-ray irradiation to treat the PE separator before coating the gel polymer electrolyte (PVDF–12% HFP). Compared to the non-irradiated separator, the electrolyte uptake, the ionic conductivity and the cycle performance of the irradiated separator were improved.
Despite success in achieving superior wettability in liquid electrolytes, the all-covered gel coating layer usually causes serious pore blockage and strong resistance to ion transportation, which in turn generates serious negative effects on the ionic conductivity and cycle performances. Park et al.35 introduced closely packed poly(methyl methacrylate) (PMMA) nanoparticle arrays on the surface of the PE separator. In contrast to the conventional dense coating layer, the highly ordered nanoporous structure, i.e. well-connected interstitial voids formed between the closely packed PMMA nanoparticles, not only favored liquid electrolyte wettability but also facilitated ionic conduction.
Inspired by the outstanding adhering ability of mussel, Ryou et al.18 prepared a polydopamine (PDA)-coated PE separator using a facile and “green” dipping process. The PDA coating decreased the contact angle significantly from 108° ± 1.4° to 39° ± 1.7°, suggesting that the modified PE became more hydrophilic. Afterwards, Wang19 et al. reduced the manufacturing cost by replacing dopamine with catechol and a polyamine binary system.
Among all the standards that need to be satisfied, the safety issue is directly associated with human life and can thus eventually depress the entire market, which is more critical than others. In fact, the temperature-related safety issue is usually related to the dimensional shrinking or melting of the separator.36 This would result in direct contact and chemical reactions between the strongly oxidative cathode and the reductive anode.
To overcome this drawback, remarkable efforts have been carried out to improve the thermal stability of the polyolefin separators. Chung et al.11 coated a cross-linked polymer synthesized from diethylene glycol dimethacrylate (DEGDMA) on the PE separator. For the uncoated separator, the reduction in the sample size (shrinking), which started at around 120 °C, continued as the temperature increased, and then the sample was completely melted at around 150 °C. On the other hand, the sample coated with polymer synthesized from DEGDMA maintained its original shape until around 150 °C.
In addition, a variety of high heat resistant polymers have also been investigated as coating materials.21,22 Song et al.22 coated the PE separator with a co-polymerized polyimide (PI), which is composed of 3,3′,4,4′-benzophenone tetracarboxylic dianhydride having 80% toluene diisocyanate and 20% methylene diphenyl diisocyanate. The shrinkage ratio of the PI coated separator was only 10.0% after heating at 140 °C, whereas the bare PE separator showed a significant thermal shrinkage ratio of 83.3% under the same conditions.
Many types of inorganic nanoparticles, such as Al2O3,37,38 SiO2,31,39,40 TiO2 (ref. 41) and BaTiO3,42 were directly coated onto the polyolefin separators to enhance their mechanical strength, wettability and thermal stability. Several properties of the inorganic composite separators, especially ionic conductivity, are influenced by the type and size of the ceramic particle incorporated. Choi et al.43 investigated the effects of SiO2 particle size on the electrochemical performance of inorganic composite separators. In comparison to 530 nm SiO2 composite separators, the 40 nm SiO2 system showed a higher porosity and a shorter tortuous path, which could lead to higher liquid electrolyte uptake and ionic conductivity.
A salient feature of these inorganic nanoparticle-coated composite separators is their unusual porous structure, i.e. the interstitial voids formed between the closely packed nanoparticles are interconnected by a binder. Lee et al.44 controlled the morphology of the coating layer by varying the SiO2/PVDF-HFP ratio. At low SiO2/PVDF-HFP ratios, SiO2 domains in PVDF-HFP matrices with nonporous morphologies were developed, whereas PVDF-HFP serves as a binder to interconnect SiO2 nanoparticles and highly percolated interstitial voids are formed between closely packed SiO2 nanoparticles at high ratios. In comparison, the composite separator with a high SiO2/PVDF-HFP ratio showed a higher ionic conductivity and a superior cell performance.
However, the thick inorganic nanoparticle-coated layer inevitably increases the thickness and weight and decreases the ionic conductivity of the separator, although it is helpful in improving the thermal stability.45 To reduce the thickness of the inorganic layer, Jung et al.46 employed an atomic layer deposition method to introduce inorganic materials into the pores of the PP separator. By this method, the thermal stability of the PP separator is enhanced without any significant change in the thickness.
As the abovementioned inorganic nanoparticles are introduced, the improvement in the separator's properties is mainly related to physical actions without including lithium ion transport processes. Kim et al.27,47 used core–shell structured SiO2 particles containing lithium ions in their shells as the functional fillers. The presence of hydrophilic poly(lithium 4-styrenesulfonate) (PLSS) in the shells of the SiO2 (Li+) particles holds the solvent more effectively, which leads to enhancement of interfacial stability. In this coated separator, the liquid electrolyte is not only encapsulated in the pores, but is also retained in the coating layer composed of SiO2 (Li+) particles and P(VDF-co-HFP). As a result, liquid electrolyte uptake is greater than that of the PE separator. As lithium ions dissociated from the SiO2 (Li+) particles can also contribute to the ionic conductivity, the maximum value of the ionic conductivity of the coated separator can be up to 6.0 × 10−3 S cm−1 at room temperature.
Potential issues related to inorganic nanoparticle-coated separators are the increased separator weight due to the introduction of heavy inorganic nanoparticles, particle detachment due to insufficient binding, and the unknown electrochemical stability of the inorganic nanoparticles and the binder in the highly oxidizing and reducing environments encountered in LIBs.10 In addition, during the drying process, care is taken because any drying temperature above 100–120 °C can cause a permanent morphological change in the polyolefin separator skeleton.48
Some electroactive redox coating materials, such as poly(4-methoxytriphenylamine) (PMOTPA),26 polytriphenylamine (PTPAn),49 and polydiphenylamine (PDPAn),50 which can make the microporous separators to effectively shut down during overcharging, have also been coated onto the polyolefin separators. Once the cell is overcharged (i.e. the cathode potential exceeds the oxidation potential of the electroactive polymer), the incorporated polymer will be electrochemically p-doped so as to convert it into a conducting state. This in turn produces a current bypass for the charging reaction through the conductive polymer bridge formed between the two electrodes.
| Polyolefin materials | Grafting materials | Grafting method | Liquid electrolyte | Electrolyte uptake (%) | Ionic conductivity (mS cm−1) | Ref. |
|---|---|---|---|---|---|---|
| PE | MMA | Electron beam radiation | LiPF6–EC/DMC | 210 | 1.01 | 55 |
| PP | PEG | Mussel-inspired dopamine coating | LiPF6–EC/DMC/EMC | 145 | 0.99 | 56 |
| PE | MMA | γ-Rays | LiClO4–EC/DEC | 320 | 2.0 | 54 |
| PE | AN | Plasma-induced pre-irradiation | LiPF6–EC/EMC/DEC | — | 1.4 | 53 |
| PP/PE/PP | MMA | Electron beam syn-irradiation technique | LiPF6–DMC/EC | 240 | 1.21 | 57 |
| PE | MMA | Surface-initiated ATRP | LiPF6–EC/DMC/DEC | 195 | 1.25 | 58 |
| PE | GMA | Electron beam technology | LiPF6–EC/DMC | — | — | 59 |
| PE | Siloxane | Electron beam technology | LiPF6–EC/DMC | — | 0.7 | 60 |
| PP | AA + DEGDM | Electron beam technology | LiAsF6–PC/EC/DME | — | — | 61 |
| PE | PVDF-HFP/PEGDMA | Electron beam technology | LiClO4–EC/DEC | 125 | 0.38 | 62 |
| PE | PMMA | γ-Rays | LiClO4–EC/PC | 380 | 1.3 | 63 |
Gao et al.55 prepared PE-g-MMA separators with different degrees of grafting (DG) using the electron beam radiation technique. The DG was controlled by varying the total electron dose. However, the electrolyte uptake did not increase linearly on increasing DG. The reason can be explained as follows. At a low DG, the separators still retained their pore structures. With an increase in DG, the micropores are gradually filled by PMMA and the porosity decreases.
Li et al.57 introduced a pre-irradiation method to avoid the generation of a thick and compact PMMA-grafted layer. In this method, a high-energy radiation source was used to chemically activate the ungrafted polyolefin separators before the graft copolymerization process. Compared with the electron beam-induced syn-irradiation technique, this method produced a much looser and spongier grafted PMMA layer, which could apparently reduce the activation time. Moreover, the grafted separator showed a smooth surface morphology and excellent adhesion properties toward electrodes, which led to a low interfacial resistance.
In a UV-irradiation-assisted grafting process, the introduction of a photo-initiator would inevitably inhibit the electrochemical reactions during battery operation. In a high energy radiation-induced graft polymerization, usual uncontrolled and high energy projectiles, such as electrons, ions, and radicals, often result in the degradation of the polymer backbone (–C–C– bond) as well as the formation of undesirable chemical functionalities and damage to the target molecules. Even worse, these surface grafting approaches usually reduce the separator's mechanical strength. To bypass these disadvantages, Man et al.64 developed a hyperthermal hydrogen induced cross-linking (HHIC) technology to graft PEO onto the PP separator. In the HHIC process, energy-controlled hydrogen projectiles collided with both PEO and PP chains to selectively cleave only the –C–H– bonds. The adjacent carbon radicals generated from –C–H– bond cleavages would bond with each other, leading to effective and efficient cross-linking. The PEO layer on the PP separator obtained by HHIC treatment was extremely thin such that the pore size and structure remained intact.
Shi65 successfully prepared hydrophilic PE/poly(ethylene-block-ethylene glycol) (PE-b-PEG) blend separators via a thermally-induced phase separation (TIPS) process. It was found that the incorporated polyether chains were greatly enriched in the surface layer and improved the compatibility between separator/liquid electrolyte and the electrodes. Furthermore, such a modified separator matrix could absorb liquid electrolyte and swell to some extent, showing the characteristics of an “active separator” (as shown in Fig. 4).
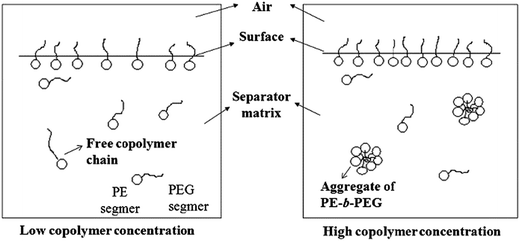 | ||
| Fig. 4 Model of the configurations of PE-b-PEG chains in blend separators with different additive contents. This figure was edited from Fig. 3 of ref. 65, with permission from Elsevier. | ||
2.2 Heterochain polymer microporous separators
To develop a high-performance separator with excellent wettability in liquid electrolyte and high heat resistance, various heterochain polymer microporous separators have also been studied such as brominated poly(phenylene oxide) (BPPO),66 isotactic poly(4-methyl-1-pentene),67 polyoxymethylene,68 polystyrene (PS)–PP69 blend, and poly(ethylene terephthalate) (PET)–PP69 blend polymers.A series of sponge-like porous polyimide separators have been prepared by Wang et al. using a wet phase inversion process.70 The obtained separators exhibited excellent thermal stability and their thermal shrinkage was less than 1% after storage at 200 °C. Moreover, due to their high surface polarity and high porosity, the liquid electrolyte uptake can be up to 190–378%.
A polyetherimide (PEI)-based three dimensional heat resistant skeleton has been constructed via a vapor-induced phase inversion process.71 This PEI separator can provide good short circuit protection with negligible deformation of the dimensions even at 180 °C due to its relatively high heat deflection temperature (210 °C).
Xiao et al.72 prepared pure PVA microporous separators by a non-solvent-induced phase separation (NIPS) process, with notable features such as lower thermal shrinkage, superior electrolyte adsorption/retention capacity and higher ionic conductivity compared to a commercial PP separator.
2.3 Polymer electrolytes
Because of the high oxidation potential of metal oxide cathodes in LIBs, the cell with the abovementioned micropore separator may suffer from decomposition reactions of the liquid electrolyte solutions. Therefore, replacement of the liquid electrolyte with a polymer electrolyte would be highly desirable. The motivations and advantages of using a polymer electrolyte can be listed as follows: (1) suppression of growth of lithium dendritic structures; (2) enhancement of endurance of the varying electrode volume during the cycle process; (3) avoidance of reactivity of liquid electrolytes; (4) improvement in security; and (5) better shape flexibility and manufacturing integrity.73–75The development of polymer electrolytes has gone through three stages: (1) solid polymer electrolytes, in which the polymer host itself is used as a solid solvent along with a lithium salt and does not contain any organic liquids; (2) gel polymer electrolytes, in which an organic electrolyte solution is introduced into a polymeric matrix such as PEO,76 PVDF,77–79 and PMMA;80,81 and (3) composite polymer electrolytes, formed by incorporating inorganic particles into the polymeric matrix.
| Polymer electrolytes | Lithium salt | Ionic conductivity (S cm−1) | Ref. |
|---|---|---|---|
| POSS–PEG/methyl cellulose | LiClO4 | 1.6 × 10−3 | 89 |
| HPG–PPEGMA | LiTFSI | 1 × 10−4 | 90 |
| Oligo(ethyleneoxy)cyclotriphosphazenes | LiN–(SO2CF3)2 | 1.98 × 10−4 | 91 |
| Oligo(ethyleneoxy)cyclotriphosphazenes | LiSO3CF3 | 1.16 × 10−4 | 91 |
| Poly(oxetane)–polyDDOE | LiBF4 | 3.7 × 10−5 | 92 |
| PVA | LiCF3SO3 | — | 93 |
| P(EO/MEEGE) | LiTFSI | 1 × 10−4 | 88 |
| PMPEA | LiTFSI | 10−3 | 94 |
| P(EO/MEEGE) | LiClO4 | 3.3 × 10−4 | 95 |
| P(EO/PO) | LiTFSI | 2.2 × 10−5 | 96 |
| TEC | LiClO4 | 1.5 × 10−4 | 97 |
| PEP | LiClO4 | 10−4 | 98 |
| P(EO/MEEGE/AGE) | LiTFSI | — | 99 |
| PME–GE | LiClO4 | 10−4 | 100 |
| PEO–PEGEEM–MMA | LiClO4 | — | 101 |
| Siloxy-aluminate polymer | LiAlH4 | 2.3 × 10−5 | 102 |
| PLMA-b-POEM | LiCF3SO3 | 10−5 | 103 |
| Cyanuric chloride/PEO | LiTFSI | 3.9 × 10−5 | 104 |
| PEG–PDMS | LiTFSI | 6.7 × 10−4 | 105 |
| PEO | POSS–phenyl-(BF3Li)3 | 4 × 10−4 | 106 |
| TPEO–MA9LC19 | LiClO4 | 2.2 × 10−5 | 107 |
To improve the mechanical strength of the solid polymer electrolytes, block copolymer electrolytes, containing both well-defined conducting pathways and a sturdy supporting matrix, have been proposed.108 This class of polymer electrolytes includes poly(styrene-b-(styrene-g-ethylene oxide)-b-styrene),109 poly(styrene-b-oligooxyethylene methacrylate-b-styrene),110 poly(styrene trifluoromethanesulfonylimide of lithium-b-ethylene oxide-b-styrene trifluoromethanesulfonylimide of lithium) (P(STFSILi)–PEO–P(STFSILi))111 and poly(ethylene glycol)methyl ether methacrylate–polystyrene–poly(ethylene glycol)methyl ether methacrylate (POEM–PS–POEM).112
Xue86 and coworkers reported a branched PI–PEO copolymer and confirmed the possibility of using it as a solid polymer electrolyte for LIBs. Herein, the PEO coil phase allowed high ionic conductivity, whereas the PI rod phase provided good mechanical strength.
Higa et al.87 prepared a graft copolymer electrolyte consisting of a PI main chain and POEM side chains using an atom-transfer radical polymerization method. Compared with the solid polymer electrolyte with the PMMA main chain, the tensile strength of this solid polymer electrolyte is about 15 times higher, with an ionic conductivity of approximately 6.5 × 10−6 S cm−1 at 25 °C.
All the abovementioned solid polymer electrolytes are dual-ion conductors, in which both cation and anion are mobile.113 The motion of lithium ions is only a small fraction of the overall ionic current, which may lead to a strong concentration gradient and generate internal polarization. To improve the lithium ion transference number of the polymer electrolytes, Bouchet et al.111 designed a BAB triblock copolymer P(STFSILi)–PEO–P(STFSILi), in which the anions were immobilized by covalently linking to the polymeric backbone, such that the lithium ion transference number could approach 0.85.
Zhu et al.114 synthesized a novel single-ion conducting polymer electrolyte, lithium polyvinyl alcohol oxalate borate (LiPVAOB), whose monomer is structurally similar to lithium bis(oxalate)borate LiBOB. The obtained polymer electrolyte showed a good electrochemical performance and a stable electrochemical window up to 7 V (vs. Li+/Li).
In addition, plastic crystal electrolytes (PCEs), which are prepared by doping lithium salts into a plastic crystalline matrix, have been studied in the laboratory. Due to rotational disorder and the existence of vacancies in the lattice, they usually exhibit fast lithium ion motion.115 For example, a succinonitrile (SN, NC–CH2–CH2–CN) plastic crystal electrolyte exhibited a high ionic conductivity of more than 10−3 S cm−1 at room temperature.116
Currently, these available homopolymers cannot simultaneously attain the optimal requirements for mechanical strength, ionic conductivity, and electrochemical stability. To overcome these drawbacks to some extent, copolymers formed by cross-linking or copolymerization with different functional monomers have been used as the matrixes of GPE. These include poly(methyl methacrylate acrylonitrile) (P(MMA-AN)),121,122 poly(butyl methacrylate styrene) (P(BMA-St)),123 poly(methyl methacrylate acrylonitrile vinyl acetate) (P(MMA-AN-VAc))124,125 and poly(methyl methacrylate vinyl acetate)-co-poly(ethylene glycol) diacrylate (P(MMA-VAc)–PEGDA).126
Costa et al.3 summarized the developments and main characteristics of PVDF and its copolymers in LIB separators. Being a semi-crystalline polymer, PVDF does not easily incorporate liquid electrolyte into the polymer matrix. Moreover, the affinity between PVDF and the liquid electrolyte is not strong enough to hold the entrapped liquid electrolyte. Thus, various PVDF/copolymer blending systems have been prepared to decrease the crystallinity of PVDF as well as to increase its affinity with the liquid electrolyte, and the “active separator” concept was proposed. Herein, the copolymers are mainly amphiphilic copolymers such as P(hexafluorobutyl methacrylate-co-poly(ethylene glycol)methacrylate) (P(HFBMA-co-PEGMA))77 and P(methyl methacrylate–poly(ethylene glycol) methacrylate) P(MMA-co-PEGMA).127 Table 4 shows PVDF-based gel polymer electrolytes and their properties.
| Gel polymer electrolytes | Liquid electrolyte | Electrolyte uptake (%) | Ionic conductivity (mS cm−1) | Ref. |
|---|---|---|---|---|
| PVDF | LiPF6–EC/EMC/DMC | 230 | 1.3 | 128 |
| PVDF-HFP | LiPF6–EC/DEC | — | 1.2 | 129 |
| PVDF/PEO-b-PMMA | LiClO4–EC/PC | 211 | 2.79 | 130 |
| PVDF/PEO–PPO–PEO | LiPF6–DMC/EMC/EC | 188 | 2.94 | 131 |
| PVDF-HFP/PSx–PEO3 | LiTFSI–EC/DMC | 520 | 0.42 | 132 |
| PVDF–TrFE/PEO | LiPF6–EC/DMC | 45 | 2.3 | 133 |
| PVDF/P(MMA-co-PEGMA) | LiPF6–EC/EMC/DMC | 372 | 3.01 | 134 |
| PVDF/PDMS-g-(PPO–PEO) | LiPF6–EC/EMC/DMC | 520 | 4.5 | 135 |
| PVDF/PMMA | LiPF6–EC/EMC/DMC | 129.3 | 3.38 | 136 |
| PVDF/PSF | LiPF6–EC/EMC/DMC | 129.7 | 2.03 | 137 |
| PVDF/P(HFBMA-co-PEGMA) | LiPF6–EC/EMC/DMC | 387 | 3.19 | 138 |
| PVDF/PVDF–PEO | LiClO4–EC/PC | 387 | 3.03 | 139 |
To explain the stability mechanism of a liquid electrolyte in the PVDF-based blend, GPE, Li et al.127 synthesized a brush-like amphiphilic copolymer P(MMA-co-PEGMA) and blended it into the PVDF matrix. They classified the status of the electrolyte in GPEs into three phases: filled-in pores, swollen in the amorphous phase (swollen phase), and absorbed by the PEG chains that enrich at the separator surface and pore walls (fixed phase), as shown in Fig. 5.
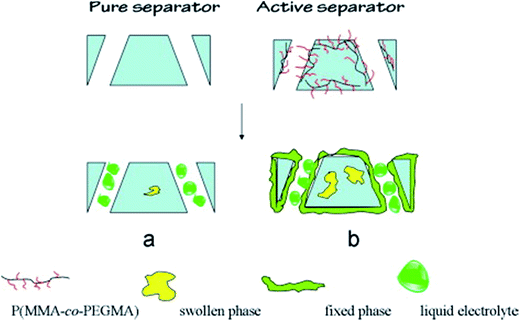 | ||
| Fig. 5 Status of electrolyte in pure and active separators. This figure was edited from Fig. 11 of ref. 127, with permission from Elsevier. | ||
A dense instead of a porous gel polymer electrolyte for LIBs has been reported by Li et al.140 Hydroxyethyl cellulose (HEC) has been chosen as the host for this gel polymer electrolyte. The results demonstrated that this dense gel polymer electrolyte can effectively avoid short circuiting in LIBs.
Cai et al.141 used a lithiated perfluorinated sulfonic (PFSA-Li) ion-exchange membrane swollen with non-aqueous solvent as the polymer electrolyte for LIBs. The results demonstrated that a LIB can be operated using only ion-exchange membranes as electrolytes with no doped salts.
It has been well established that several parameters influence the electrochemical properties of composite polymer electrolytes: the nature and content of the lithium salt (LiCF3SO3, LiClO4 and LiPF6), the nature of the nanoparticles (TiO2, SiO2 and Al2O3), and the size of the nanoparticles. In general, ceramic fillers for the composite polymer electrolytes are broadly classified into two categories: active and passive. Active component materials, such as Li2N and LiAl2O3, are involved in the conduction process, whereas materials such as Al2O3, SiO2 and MgO are passive. Raghavan et al.143 prepared a series of nanocomposite polymer electrolytes comprising nanoparticles of BaTiO3, Al2O3 or SiO2 by an electrospinning technique. Among these particle fillers, BaTiO3, which has a significant Lewis-acid character, gave the best electrochemical performance. The effect of these ferroelectric ceramic fillers appeared to be highly dependent on the nature of the salt used in the PEO-based systems, with LiClO4 showing the most dramatic effects.144 However, in both the cases, the particle size and the characteristics of the ceramic fillers play a vital role in the electrochemical properties of composite polymer electrolytes. The smaller the size of the ceramic fillers, the more they influence the crystallization kinetics of the polymer chains. It has also has been proved that nanosized ceramic fillers showed better compatibility with lithium metal than micron-sized fillers.145 Table 5 shows the properties of some of the composite polymer electrolytes.146
| LiX | Ceramic fillers | Conductivity (S cm−1) | Ref. |
|---|---|---|---|
| LiCF3SO3 | γ-LiAlO2 | 9.0 × 10−4 | 146 |
| LiBF4 | γ-LiAlO2 | 6.0 × 10−4 | 146 |
| LiClO4 | TiO2 | 1.8 × 10−3 | 146 |
| LiClO4 | Al2O3 | 1.1 × 10−3 | 146 |
| LiClO4 | BaTiO3 | 1 × 10−5 | 42 |
| LiCF3SO3 | ZrO2–TiO2 | 1.2 × 10−5 | 147 |
| LiN(C2F5SO2)2 | Nanochitin | — | 148 |
| Li(C2F5SO2)2N | BaTiO3 | 1.5 × 10−5 | 149 |
| Li-montmorillonite | Li-montmorillonite | 1.6 × 10−6 | 150 |
Kumar et al.145 reviewed state-of-the-art composite polymer electrolytes based on ionic conductivity, lithium ion transference number and electrode–electrolyte interfacial reactions. Stephan et al.151 also reviewed various CPE hosts in view of their electrochemical and physical properties with respect to application in LIBs, mainly focusing on PEO, PAN, PMMA, and PVDF.
2.4 Non-woven separators
Non-woven separators are traditionally prepared by wet processes, such as the paper-making method,152,153 the solution extrusion method,154 and the wet-laid method,155 or by dry processes such as the melt-blown method.156 In general, these non-woven separators have certain advantages such as relatively low processing costs, high porosity, and low mass. They are not, however, able to compete with polyolefin separators in LIBs due to their inferior tensile strength and excessively large pore size.155 The large pores would cause internal short circuits, overgrowth of lithium dendrites and self-discharge, and would further reduce the battery cycle performance and safety. Several attempts have made to tackle the abovementioned shortcomings, including the incorporation of inorganic powders,157,158 fibrillated fibers (poly(paraphenylene terephthalamide) (PPTA)),155 and gel polymer electrolytes159,160 into the non-woven separators. Table 6 shows the properties of some non-woven microporous separators.| Non-woven matrix | Liquid electrolyte | Electrolyte uptake (%) | Ionic conductivity (mS cm−1) | Ref. |
|---|---|---|---|---|
| Glass-microfiber/PVA–β-CN | LiTFSI–EC/DMC | — | 0.89 | 161 |
| PP/PVDF | LiPF6–EC/DMC | 116 | 0.65 | 79 |
| PP/PVDF-HFP/PMMA | LiPF6–EC/DMC | 212 | 1.85 | 162 |
| PI/PEG | LiPF6 | 140 | 1.45 | 163 |
| PVDF/PEGDA | LiPF6–EC/DMC | — | 3.3 | 164 |
| PET/PVDF-HFP | LiPF6–EC/DEC | — | 0.86 | 2 |
| PET/PVA-co-PE | LiPF6–EC/DMC | 160 | 0.55 | 165 |
| PE/P(AN-Vac) | LiPF6–DMC/DEC/EC | 380 | 3.8 | 166 |
| TPU/PVDF | LiClO4–EC/PC | 342 | 3.2 | 167 |
| PVDF–PVC | LiClO4–PC/EC | 290 | 2.25 | 168 |
| PVDF–10CTFE | LiPF6–EC/DMC | 800 | 2.57 | 169 |
| PAN | LiPF6–EC/DEC | — | 2.6 | 170 |
| PET | LiPF6–EC/DMC | 484 | 2.27 | 171 |
| SiO2/PVDF-HFP coated PET | LiPF6–EC/DEC | 1.08 g cm−3 | 0.91 | 43 |
| PMMA coated PET | LiPF6–EC/DEC | 160 | 0.96 | 172 |
| SiO2 coated PET | LiPF6–EC/DEC | — | 0.91 | 173 |
| PVDF coated PET | LiPF6–EC/DEC | 185 | 0.81 | 174 |
| PI/PET | LiPF6–EC/DMC/EMC | 200 | 0.66 | 175 |
| PBT | LiPF6–EC/EMC | — | 0.27 | 176 |
| Cellulose | LiPF6–EC/DEC | 0.77 | 177 | |
| Cellulose/PSA | LiPF6–EC/DEC | 260 | 1.2 | 178 |
| PI–PEO | LiPF6–EC/PC/DMC/EA | 178 | 0.65 | 159 |
| Carboxymethyl cellulose | LiPF6–EC/DMC | 340 | 1.75 | 179 |
| PPESK | LiPF6–EC/DMC | 1210 | 3.79 | 180 |
Degussa has developed a series of Separion (a trade name) separators by coating a porous ceramic layer on each side of a flexible perforated non-woven separator, as illustrated in Fig. 6. In general, the ceramic materials can be alumna, silica, zirconia, or their mixtures and their particle size is required to be a few nanometers. It is proven that the Separion separators have excellent wettability with liquid electrolyte and show negligible shrinkage at high temperatures.
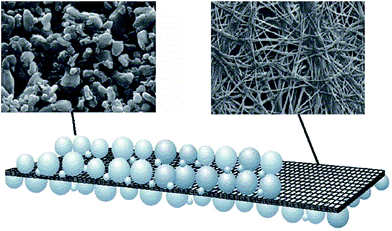 | ||
| Fig. 6 Schematic of the Separion separators. This figure was edited from Fig. 6 of ref. 5, with permission from Elsevier. | ||
Lee et al.44,157,181,182 developed various types of colloidal particles or inorganic nanoparticle-coated non-woven separators. The introduction of poly(methyl methacrylate) (PMMA) colloidal particle arrays on non-woven poly(ethylene terephthalate) (PET) effectively helped to decrease the pore size of the non-woven substrate.181 Moreover, due to the well-connected interstitial voids between the PMMA particles and the strong affinity between PMMA and the liquid electrolyte, the composite non-woven separator allowed facile ion transport and high electrolyte retention. However, it has also been pointed out that the long-term durability of colloidal particle-embedded non-woven separators still needs to be improved.163
Zhu et al.118 coated PVDF onto PP/PE non-woven separators. The surface pore size was effectively reduced from 50 mm to 100 nm by the formation of the PVDF gel layer. However, the PVDF gel also permeated into the inner space of the non-woven separator that suppresses the rapid diffusion of lithium ions. To retain large pores in the interior of the non-woven separator, Shi et al.163 designed a sandwich-like composite separator, i.e. a crosslinked polyethylene glycol (PEG) skin layer was formed on both sides of the non-woven separator by in situ polymerization. It was demonstrated that the large pores remaining in the interior of non-woven separators could provide quick access for the fast transportation of lithium ions, and thus contribute to a superior rate capability.
Among all the preparation methods of non-woven separators, electrospinning has drawn considerable attention due to its capacity to create a fully interconnected pore structure with a large surface area and high porosity. By controlling the processing parameters, the obtained electrospun separators will be endowed with porosities in the range of 30–90% and pore sizes in the range of sub-micrometers to a few micrometers.183
However, the single layer non-woven separator shows unsatisfactory mechanical properties (not free-standing) with respect to its use in LIBs. To address this problem, Angulakshmi et al.184 prepared a PVDF-HFP/PVC/PVDF-HFP trilayer non-woven separator by electrospinning technology. By adding a second PVC and a third PVDF-HFP layer to the PVDF non-woven separator, the tensile strength of this trilayer non-woven separator became superior to the commercial Celgard separator. Furthermore, due to the increase in fiber diameter, the formation of inter fiber bonding and enhanced crystallinity, heat treatment can be used to improve the mechanical properties of this electrospun non-woven separator.185
A polymethylpentene (PMP) non-woven separator has been formed directly on the anode by an electrospinning process by Huang.48 This PMP separator exhibited much improved thermal stability and interfacial adhesion between the separator and the deposited electrode compared to the commercial polyolefin separator.
Non-woven separators with heat-resistant engineering polymers, such as polyimide (PI),186–189 polyester (PET),43,171,190 poly(phthalazinone ether sulfone ketone) (PPESK),180,191 cellulose,152,177,192,193 poly(m-phenylene isophthalamide) (PMIA),194,195 and aramid,153 as the matrix have also attracted lot of attention in recent years. These types of non-woven separators could still retain dimensional stability and effectively isolate the electrodes, further preventing battery short-circuit and explosion at high temperature. They are proven to be promising separators for highly heat-resistant lithium ion batteries.
3. Conclusions and further studies
The separator is a critical component in LIBs and is placed between the cathode and anode to prevent physical contact between the electrodes, so as to isolate the electronic flow while enabling free ionic transport. Although the separator itself is not involved in any cell reactions, its structure and properties considerably affect the internal resistance and cycle performance of LIBs.Polyolefin separators have been the most dominant ones in commercial lithium ion batteries over the last few decades due to superior properties such as low cost, electrochemical stability, good mechanical strength and pore structure. However, to date, these properties have not all been completely optimized. One of the most serious issues is their hydrophobic nature and lower surface energy, which cause poor compatibility with the liquid electrolyte. Moreover, polyolefin separators suffer from severe thermal shrinkage at high temperatures due to their low melting points. Accordingly, it is important to develop some novel separators for a high-performance and safe LIB.
Polyolefin separators have been modified using various techniques such as surface coating, surface grafting and blending. The modified separators are endowed with certain improved properties such as thermal stability, wettability, and ionic conductivity. Compared to the polyolefin separators, there are two advantages of incorporating inorganic particles. One is suppression of thermal shrinkage and other is enhanced liquid electrolyte wettability. However, the increased thickness and weight inevitably reduces the power and energy densities of the corresponding cell.
Solid polymer electrolytes have several advantages over their liquid counterparts such as no leakage of electrolytes, better shape flexibility, and non-combustible reaction products at the electrode surface that exists in the liquid electrolytes. Furthermore, the incorporation of substantial amounts of organic solvents to form a gel polymer electrolyte has effectively improved the ionic conductivity of polymer electrolytes. However, the requirements of high ion conductivity and good mechanical strength could still not be met simultaneously.
Non-woven separators are now deemed to be important candidates for separators to be used in high power lithium ion batteries due to their low processing cost, high porosity, and low mass. However, to date, non-woven separators are mainly used in rechargeable alkaline batteries. Their application in lithium ion batteries is limited by their low tensile strength and excessively large pore size.
In future, the development of lithium ion battery separators needs to balance performance against the safety and cost. Separators that combine the features of high ionic conductivity and excellent thermal stability are highly desirable for high-performance lithium ion batteries. Moreover, the high cost of separators is mainly due to their production process. Therefore, developing a more cost-effective process is very important with respect to reducing battery separator costs.
References
- M. Wakihara, Mater. Sci. Eng., R, 2001, 33, 109–134 CrossRef.
- J. HunáKim, J. Mater. Chem., 2010, 20, 9180–9186 RSC.
- C. M. Costa, M. M. Silva and S. Lanceros-Mendez, RSC Adv., 2013, 3, 11404–11417 RSC.
- H. Lee, et al., Energy Environ. Sci., 2014, 7, 3857–3886 CAS.
- S. S. Zhang, J. Power Sources, 2007, 164, 351–364 CrossRef CAS PubMed.
- A. M. Stephan, Eur. Polym. J., 2006, 42, 21–42 CrossRef PubMed.
- W. H. Meyer, Adv. Mater., 1998, 10, 439–448 CrossRef CAS.
- M. Weighall, J. Power Sources, 1991, 34, 257–268 CrossRef CAS.
- G. Venugopal, et al., J. Power Sources, 1999, 77, 34–41 CrossRef CAS.
- X. Huang, J. Solid State Electrochem., 2010, 15, 649–662 CrossRef.
- Y. Chung, S. Yoo and C. Kim, Ind. Eng. Chem. Res., 2009, 48, 4346–4351 CrossRef CAS.
- W.-C. Yu, Shutdown, US Pat., 5691077, 1997.
- S. E. Hux and W.-C. Yu, US Pat., 5952120, 1999.
- P. Arora and Z. Zhang, Chem. Rev., 2004, 104, 4419–4462 CrossRef CAS.
- Y.-B. Jeong and D.-W. Kim, J. Power Sources, 2004, 128, 256–262 CrossRef CAS PubMed.
- J.-Y. Sohn, et al., J. Solid State Electrochem., 2012, 16, 551–556 CrossRef CAS.
- J. Liu, et al., J. Power Sources, 2013, 226, 101–106 CrossRef CAS PubMed.
- M. H. Ryou, et al., Adv. Mater., 2011, 23, 3066–3070 CrossRef CAS PubMed.
- H. Wang, et al., ACS Appl. Mater. Interfaces, 2014, 6, 5602–5608 CAS.
- L.-F. Fang, et al., J. Appl. Polym. Sci., 2014, 21, 41036 Search PubMed.
- M. Xiong, et al., Carbohydr. Polym., 2014, 101, 1140–1146 CrossRef CAS PubMed.
- J. Song, et al., Electrochim. Acta, 2012, 85, 524–530 CrossRef CAS PubMed.
- J.-H. Park, et al., J. Power Sources, 2010, 195, 8306–8310 CrossRef CAS PubMed.
- Y. Ko, H. Yoo and J. Kim, RSC Adv., 2014, 4, 19229–19233 RSC.
- C. Shi, et al., J. Power Sources, 2014, 270, 547–553 CrossRef CAS PubMed.
- H. Zhang, et al., Electrochim. Acta, 2013, 108, 191–195 CrossRef CAS PubMed.
- W.-K. Shin and D.-W. Kim, J. Power Sources, 2013, 226, 54–60 CrossRef CAS PubMed.
- H. Liu, et al., Ceram. Int., 2014, 40, 14105–14110 CrossRef CAS PubMed.
- Z. Wang, et al., ACS Appl. Mater. Interfaces, 2015, 5, 3314–3322 Search PubMed.
- H.-S. Jeong, et al., J. Power Sources, 2010, 195, 6116–6121 CrossRef CAS PubMed.
- S. M. Kang, et al., Chem. Mater., 2012, 24, 3481–3485 CrossRef CAS.
- H. Wang, et al., ACS Appl. Mater. Interfaces, 2014, 6, 5602–5608 CAS.
- L. Pan, et al., ACS Appl. Mater. Interfaces, 2015, 29, 16003–16010 Search PubMed.
- K. J. Kim, et al., J. Power Sources, 2012, 198, 298–302 CrossRef CAS PubMed.
- J.-H. Park, et al., J. Power Sources, 2011, 196, 7035–7038 CrossRef CAS PubMed.
- M. Kim and J. H. Park, J. Power Sources, 2012, 212, 22–27 CrossRef CAS PubMed.
- Y. Liao, et al., Electrochim. Acta, 2013, 89, 461–468 CrossRef CAS PubMed.
- T. Lee, et al., Macromol. Res., 2014, 11, 1190–1195 CrossRef.
- H.-S. Jeong and S.-Y. Lee, J. Power Sources, 2011, 196, 6716–6722 CrossRef CAS PubMed.
- J.-H. Park, et al., J. Power Sources, 2010, 195, 8306–8310 CrossRef CAS PubMed.
- K. M. Kim, et al., J. Electroceram., 2013, 2–3, 146–153 Search PubMed.
- H. Y. Sun, et al., J. Electrochem. Soc., 1999, 146, 1672–1676 CrossRef CAS PubMed.
- E.-S. Choi and S.-Y. Lee, J. Mater. Chem., 2011, 21, 14747–14754 RSC.
- H.-S. Jeong, E.-S. Choi and S.-Y. Lee, Electrochim. Acta, 2012, 86, 317–322 CrossRef CAS PubMed.
- L.-F. Fang, et al., RSC Adv., 2014, 4, 22501–22508 RSC.
- Y. S. Jung, et al., Adv. Energy Mater., 2012, 2, 1022–1027 CrossRef CAS PubMed.
- S. H. Ju, et al., J. Mater. Chem. A, 2013, 1, 395–401 CAS.
- X. Huang, J. Membr. Sci., 2014, 466, 331–337 CrossRef CAS PubMed.
- S. Li, et al., J. Power Sources, 2009, 189, 771–774 CrossRef CAS PubMed.
- S. Li, et al., J. Power Sources, 2008, 184, 553–556 CrossRef CAS PubMed.
- M. Yang, et al., Solid State Ionics, 2005, 176, 2829–2834 CrossRef CAS PubMed.
- H.-S. Kim, et al., J. Power Sources, 2003, 124, 221–224 CrossRef CAS.
- J. Y. Kim, Y. Lee and D. Y. Lim, Electrochim. Acta, 2009, 54, 3714–3719 CrossRef CAS PubMed.
- S.-J. Gwon, et al., Nucl. Instrum. Methods Phys. Res., Sect. B, 2008, 266, 3387–3391 CrossRef CAS PubMed.
- K. Gao, et al., Electrochim. Acta, 2006, 52, 443–449 CrossRef CAS PubMed.
- L.-F. Fang, et al., J. Membr. Sci., 2013, 448, 143–150 CrossRef CAS PubMed.
- S. Li and K. Gao, Surf. Coat. Technol., 2010, 204, 2822–2828 CrossRef CAS PubMed.
- J.-L. Shi, et al., J. Membr. Sci., 2013, 437, 160–168 CrossRef CAS PubMed.
- J. Ko, et al., Electrochim. Acta, 2004, 50, 367–370 CrossRef CAS PubMed.
- J. Y. Lee, et al., Electrochim. Acta, 2009, 54, 4312–4315 CrossRef CAS PubMed.
- J. L. Gineste, J. Membr. Sci., 1995, 107, 155–164 CrossRef CAS.
- J.-Y. Sohn, et al., Radiat. Phys. Chem., 2009, 78, 505–508 CrossRef CAS PubMed.
- S.-J. Gwon, et al., Nucl. Instrum. Methods Phys. Res., Sect. B, 2009, 267, 3309–3313 CrossRef CAS PubMed.
- C. Man, et al., J. Mater. Chem. A, 2014, 2, 11980–11986 CAS.
- J.-L. Shi, et al., J. Membr. Sci., 2013, 429, 355–363 CrossRef CAS PubMed.
- J.-J. Woo, et al., Electrochem. Commun., 2013, 35, 68–71 CrossRef CAS PubMed.
- M. B. Johnson and G. L. Wilkes, J. Appl. Polym. Sci., 2002, 83, 2095–2113 CrossRef CAS PubMed.
- S. Wu, et al., Polym. Bull., 2014, 9, 2205–2217 CrossRef.
- C. Chandavasu, et al., U.S. Pat., 6824680, 2004.
- H. Wang, et al., Polymer, 2013, 54, 6339–6348 CrossRef CAS PubMed.
- J. Shi, et al., Sci. Rep., 2015, 5, 8255 CrossRef CAS PubMed.
- W. Xiao, et al., J. Membr. Sci., 2015, 487, 221–228 CrossRef CAS PubMed.
- A. Manuel Stephan, Eur. Polym. J., 2006, 42, 21–42 CrossRef CAS PubMed.
- J. Song, Y. Wang and C. Wan, J. Power Sources, 1999, 77, 183–197 CrossRef CAS.
- F. B. Dias, L. Plomp and J. B. Veldhuis, J. Power Sources, 2000, 88, 169–191 CrossRef CAS.
- H. Li, et al., Electrochim. Acta, 2011, 56, 2641–2647 CrossRef CAS PubMed.
- H. Zhang, et al., RSC Adv., 2014, 4, 33713–33719 RSC.
- A. Hofmann, M. Schulz and T. Hanemann, Electrochim. Acta, 2013, 89, 823–831 CrossRef CAS PubMed.
- J. Hao, et al., Electrochim. Acta, 2014, 125, 450–456 CrossRef CAS PubMed.
- O. Bohnke, et al., Solid State Ionics, 1993, 66(1), 97–104 CrossRef CAS.
- H. Zhang, et al., Electrochem. Commun., 2007, 9, 1700–1703 CrossRef CAS PubMed.
- D. Fenton, J. Parker and P. Wright, Polymer, 1973, 14, 589 CrossRef CAS.
- M. B. Armand, et al., Fast ion transport in solids, ed. P. Vashishta, J. N. Mundy and G. K. Shenoy, North Holland, Amsterdan, 1979 Search PubMed.
- H.-J. Ha, et al., Energy Environ. Sci., 2012, 5, 6491–6499 CAS.
- J. Li and I. M. Khan, Macromolecules, 1993, 26, 4544–4550 CrossRef CAS.
- C. Xue, et al., Polymer, 2006, 47, 6149–6155 CrossRef CAS PubMed.
- M. Higa, K. Yaguchi and R. Kitani, Electrochim. Acta, 2010, 55, 1380–1384 CrossRef CAS PubMed.
- A. Nishimoto, et al., Macromolecules, 1999, 32, 1541–1548 CrossRef CAS.
- P. R. Chinnam, H. Zhang and S. L. Wunder, Electrochim. Acta, 2015, 170, 191–201 CrossRef CAS PubMed.
- T. Zheng, et al., Ionics, 2015, 21, 917–925 CrossRef CAS.
- D. He, et al., Macromolecules, 2012, 45, 7931–7938 CrossRef CAS.
- Y. Miwa, H. Tsutsumi and T. Oishi, Polym. J., 2001, 33, 927–933 CrossRef CAS.
- H. Every, et al., Electrochim. Acta, 1998, 43, 1465–1469 CrossRef CAS.
- M. Kono, E. Hayashi and M. Watanabe, J. Electrochem. Soc., 1998, 145, 1521–1527 CrossRef CAS PubMed.
- M. Watanabe, et al., J. Power sources, 1999, 81, 786–789 CrossRef.
- M. Watanabe and A. Nishimoto, Solid State Ionics, 1995, 79, 306–312 CrossRef CAS.
- Y. Ikeda, et al., Electrochim. Acta, 2000, 45, 1167–1174 CrossRef CAS.
- L. Marchese, et al., Electrochim. Acta, 1992, 37, 1559–1564 CrossRef CAS.
- S. Matsui, et al., J. Power Sources, 2001, 97, 772–774 CrossRef.
- M. Kono, et al., Polym. Adv. Technol., 1993, 4, 85–91 CrossRef CAS PubMed.
- J. Gnanaraj, et al., Polymer, 1997, 38, 3709–3712 CrossRef CAS.
- T. Fujinami, et al., Chem. Mater., 1997, 9, 2236–2239 CrossRef CAS.
- P. P. Soo, et al., J. Electrochem. Soc., 1999, 146, 32–37 CrossRef CAS PubMed.
- D. M. Tigelaar, et al., Macromolecules, 2006, 39, 120–127 CrossRef CAS.
- C. N. Walker, et al., ACS Macro Lett., 2012, 1, 737–741 CrossRef CAS.
- P. R. Chinnam and S. L. Wunder, J. Mater. Chem. A, 2013, 1, 1731–1739 CAS.
- Y. Tong, et al., Appl. Surf. Sci., 2012, 258, 10095–10103 CrossRef CAS PubMed.
- W. S. Young, W. F. Kuan and T. H. Epps, J. Polym. Sci., Part B: Polym. Phys., 2014, 52(1), 1–16 CrossRef CAS PubMed.
- C. Wang, et al., J. Electrochem. Soc., 2003, 150, A1166–A1170 CrossRef CAS PubMed.
- T. Niitani, et al., Electrochem. Solid-State Lett., 2005, 8, A385–A388 CrossRef CAS PubMed.
- R. Bouchet, et al., Nat. Mater., 2013, 12, 452–457 CrossRef CAS PubMed.
- I. M. Khan, et al., Macromol. Chem., 1989, 190, 1069–1078 CrossRef CAS PubMed.
- S. Feng, et al., Electrochim. Acta, 2013, 93, 254–263 CrossRef CAS PubMed.
- Y. Zhu, et al., Electrochim. Acta, 2013, 87, 113–118 CrossRef CAS PubMed.
- D. R. MacFarlane, J. Huang and M. Forsyth, Nature, 1999, 402, 792–794 CrossRef CAS.
- P.-J. Alarco, et al., Nat. Mater., 2004, 3, 476–481 CrossRef CAS PubMed.
- N. Shubha, et al., J. Power Sources, 2014, 267, 48–57 CrossRef CAS PubMed.
- Y. Zhu, et al., Energy Environ. Sci., 2013, 6, 618–624 CAS.
- F. Lian, et al., J. Membr. Sci., 2014, 456, 42–48 CrossRef CAS PubMed.
- H.-y. Guan, et al., J. Power Sources, 2014, 245, 95–100 CrossRef CAS PubMed.
- M. Rao, et al., J. Membr. Sci., 2008, 322, 314–319 CrossRef CAS PubMed.
- P. Zhang, et al., Electrochem. Commun., 2008, 10, 1052–1055 CrossRef CAS PubMed.
- Y. Liao, et al., J. Membr. Sci., 2010, 352, 95–99 CrossRef CAS PubMed.
- Y. Liao, et al., Electrochim. Acta, 2009, 54, 6396–6402 CrossRef CAS PubMed.
- Y. Liao, et al., J. Power Sources, 2009, 189, 139–144 CrossRef CAS PubMed.
- Y. Liao, et al., in Meeting Abstracts, The Electrochemical Society, 2010, vol. 1, p. 170 Search PubMed.
- H. Li, et al., Electrochim. Acta, 2014, 115, 317–325 CrossRef CAS PubMed.
- G.-L. Ji, et al., Polymer, 2007, 48, 6415–6425 CrossRef CAS PubMed.
- W. Pu, et al., J. Membr. Sci., 2006, 272, 11–14 CrossRef CAS PubMed.
- Q. Xiao, et al., J. Membr. Sci., 2009, 334, 117–122 CrossRef CAS PubMed.
- Z.-Y. Cui, et al., J. Membr. Sci., 2008, 325, 957–963 CrossRef CAS PubMed.
- S. Seidel, et al., Chem. Commun., 2015, 51, 12048–12051 RSC.
- C. Costa, et al., J. Power Sources, 2014, 245, 779–786 CrossRef CAS PubMed.
- H. Li, et al., Electrochim. Acta, 2014, 115, 317–325 CrossRef CAS PubMed.
- H. Li, et al., Electrochim. Acta, 2014, 116, 413–420 CrossRef CAS PubMed.
- T. Ma, et al., J. Membr. Sci., 2013, 444, 213–222 CrossRef CAS PubMed.
- Q. Cheng, et al., J. Power Sources, 2014, 266, 401–413 CrossRef CAS PubMed.
- H. Zhang, et al., RSC Adv., 2014, 4, 33713–33719 RSC.
- F. Deng, et al., J. Membr. Sci., 2015, 491, 82–89 CrossRef CAS PubMed.
- M. Li, et al., J. Membr. Sci., 2015, 476, 112–118 CrossRef CAS PubMed.
- Z. Cai, et al., Energy Environ. Sci., 2012, 5, 5690–5693 CAS.
- A. Best, et al., Solid State Ionics, 1999, 126, 269–276 CrossRef CAS.
- P. Raghavan, et al., Electrochim. Acta, 2008, 54, 228–234 CrossRef CAS PubMed.
- M. Forsyth, et al., Solid State Ionics, 2002, 147, 203–211 CrossRef CAS.
- B. Kumar and L. G. Scanlon, J. Power Sources, 1994, 52, 261–268 CrossRef CAS.
- F. Croce, et al., Electrochim. Acta, 2001, 46, 2457–2461 CrossRef CAS.
- L. TianKhoon, et al., Solid State Ionics, 2015, 276, 72–79 CrossRef CAS PubMed.
- N. Angulakshmi, et al., Electrochim. Acta, 2010, 55, 1401–1406 CrossRef CAS PubMed.
- Q. Li, et al., Ionics, 2002, 8, 79–84 CrossRef CAS.
- R. A. Vaia, et al., Adv. Mater., 1995, 7, 154–156 CrossRef CAS PubMed.
- A. Manuel Stephan and K. S. Nahm, Polymer, 2006, 47, 5952–5964 CrossRef PubMed.
- Q. Xu, et al., RSC Adv., 2014, 4, 7845–7850 RSC.
- J. Zhang, et al., Solid State Ionics, 2013, 245–246, 49–55 CrossRef CAS PubMed.
- S. J. Law, H. Street and G. J. Askew, US Pat., 6358461, 2002.
- W. Yi, et al., J. Power Sources, 2009, 189, 616–619 CrossRef PubMed.
- K. Singha, et al., Int. J. Textil. Sci., 2012, 1(1), 7–14 Search PubMed.
- J.-R. Lee, et al., J. Power Sources, 2012, 216, 42–47 CrossRef CAS PubMed.
- X. Huang, D. Bahroloomi and X. Xiao, J. Solid State Electrochem., 2014, 18, 133–139 CrossRef CAS.
- J. Shi, et al., J. Mater. Chem. A, 2014, 2, 9134–9141 CAS.
- Y. Zhu, et al., Energy Environ. Sci., 2013, 6, 618–624 CAS.
- Q. Wang, et al., Electrochim. Acta, 2015, 157, 191–198 CrossRef CAS PubMed.
- D. Wu, et al., J. Power Sources, 2015, 290, 53–60 CrossRef CAS PubMed.
- J. Shi, et al., J. Power Sources, 2014, 271, 134–142 CrossRef CAS PubMed.
- K. M. Kim, et al., Electrochim. Acta, 2014, 120, 159–166 CrossRef CAS PubMed.
- M. Xia, et al., J. Power Sources, 2014, 266, 29–35 CrossRef CAS PubMed.
- X. Li, et al., J. Appl. Electrochem., 2010, 40, 2185–2191 CrossRef CAS.
- N. Wu, et al., J. Power Sources, 2011, 196, 8638–8643 CrossRef CAS PubMed.
- Z. Zhong, et al., Mater. Sci. Eng., B, 2012, 177(1), 86–91 CrossRef CAS PubMed.
- F. Croce, et al., Energy Environ. Sci., 2011, 4, 921–927 CAS.
- T.-H. Cho, et al., J. Power Sources, 2008, 181, 155–160 CrossRef CAS PubMed.
- J. Hao, et al., J. Membr. Sci., 2013, 428, 11–16 CrossRef CAS PubMed.
- J. HunáKim, J. Mater. Chem., 2011, 21, 8192–8198 RSC.
- H.-S. Jeong, et al., J. Membr. Sci., 2012, 415, 513–519 CrossRef PubMed.
- H.-S. Jeong, et al., Electrochim. Acta, 2011, 56, 5201–5204 CrossRef CAS PubMed.
- J. Ding, Y. Kong and J. Yang, J. Electrochem. Soc., 2012, 159, A1198–A1202 CrossRef CAS PubMed.
- C. J. Orendorff, et al., Adv. Energy Mater., 2013, 3, 314–320 CrossRef CAS PubMed.
- S.-J. Chun, et al., J. Mater. Chem., 2012, 22, 16618–16626 RSC.
- Q. Xu, et al., ACS Sustainable Chem. Eng., 2013, 2, 194–199 CrossRef.
- J. Zhang, et al., ACS Appl. Mater. Interfaces, 2012, 5, 128–134 Search PubMed.
- W. Qi, et al., Mater. Lett., 2012, 66, 239–241 CrossRef CAS PubMed.
- J.-H. Cho, et al., J. Mater. Chem., 2011, 21, 8192–8198 RSC.
- H.-S. Jeong, et al., J. Membr. Sci., 2012, 415–416, 513–519 CrossRef CAS PubMed.
- J. R. Kim, et al., Electrochim. Acta, 2004, 50, 69–75 CrossRef CAS PubMed.
- N. Angulakshmi and A. M. Stephan, Electrochim. Acta, 2014, 127, 167–172 CrossRef CAS PubMed.
- Y. Liang, et al., J. Power Sources, 2013, 240, 204–211 CrossRef CAS PubMed.
- J. Lee, et al., J. Power Sources, 2014, 248, 1211–1217 CrossRef CAS PubMed.
- Y.-E. Miao, et al., J. Power Sources, 2013, 226, 82–86 CrossRef CAS PubMed.
- Q. Wang, et al., Electrochim. Acta, 2014, 132, 538–544 CrossRef CAS PubMed.
- J. Lee, et al., J. Power Sources, 2014, 248, 1211–1217 CrossRef CAS PubMed.
- M. Xia, et al., J. Power Sources, 2014, 266, 29–35 CrossRef CAS PubMed.
- C. Lu, et al., J. Appl. Electrochem., 2013, 43, 711–720 CrossRef CAS.
- J. Zhang, et al., Sci. Rep., 2014, 4, 3939 Search PubMed.
- G. Ding, et al., J. Electrochem. Soc., 2015, 162, A834–A838 CrossRef CAS PubMed.
- Y. Zhai, et al., J. Mater. Chem. A, 2014, 2, 14511–14518 CAS.
- K. S. Jeon, et al., Mater. Lett., 2014, 132, 384–388 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |




