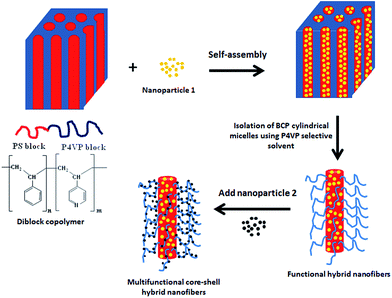
Multifunctional core–shell polymer–inorganic hybrid nanofibers prepared via block copolymer self-assembly†
Sunita Sanwariaa,
Sajan Singha,
Andriy Horechyyb,
Petr Formanekb,
Manfred Stammbc,
Rajiv Srivastavaa and
Bhanu Nandan*a
aDepartment of Textile Technology, Indian Institute of Technology Delhi, Hauz Khas, New Delhi 110016, India. E-mail: nandan@textile.iitd.ac.in
bLeibniz-Institut für Polymerforschung Dresden e.V., Hohe Strasse 6, 01069 Dresden, Germany
cTechnische Universität Dresden, Physical Chemistry of Polymer Materials, 01062 Dresden, Germany
First published on 15th October 2015
Abstract
We demonstrate a simple and robust approach for preparing multifunctional core–shell hybrid nanofibers via block copolymer self-assembly. The approach utilizes the different chemistry and solubilities of the two blocks of a diblock copolymer and different affinity of functional inorganic nanoparticles towards block copolymer constituents. In the first step, the silver nanoparticles (Ag) modified with short-chain polystyrene (PS) ligand are incorporated in the cylindrical domains of a polystyrene-block-poly(4-vinylpyridine) (PS-b-P4VP) block copolymer, constituted of PS blocks. The Ag-loaded cylindrical domains are then isolated as nanofibers by swelling the matrix forming P4VP phase using a selective solvent. The isolated nanofibers exhibit core–shell morphology with the core constituted of Ag-loaded PS phase and shell consisting of P4VP chains. The reactive P4VP shell of the nanofibers is subsequently used as a host for depositing a second type of nanoparticles. The second type of nanoparticles could be either directly synthesized on the P4VP shell or deposited from an aqueous dispersion of pre-synthesized nanoparticles. In this work, gold (Au) and cadmium sulfide (CdS) nanoparticles were deposited on the nanofiber shell. The approach is versatile and, in principle, could be extended to the fabrication of various combinations of targeted functionalities in a single nanofiber with core–shell morphology.
1. Introduction
The requirement of different properties from a single material has led to a lot of interest in the preparation of multifunctional nanomaterials. These nanomaterials, also referred to as nanohybrids, are composed of two or more different components and, hence, the properties of such nanomaterials are dictated by the resulting interaction (combination) of the properties (optical, magnetic, electrical) of the constituent materials.1–4 Such hybrid nanostructures can possess superior characteristic or, possibly, even new properties as compared to single component systems. These multifunctional nanostructures are most commonly formed through a seeded growth of one material on seed particles of another one, which may be tuned to yield composite nanoparticles of different shapes.5–9 The other approaches include simultaneous or stepwise embedding of different nanomaterials (e.g. plasmonics or quantum dots) into inorganic or polymer particles or capsules.10–12 Recently, the use of block copolymer micelles as host for growing such inorganic hybrid structures have received significant attention.13–23 This approach has the advantage that it allows for a control over the morphologies and release of the encapsulated materials. Such structures offer particular promise for applications in nanomedicine since they can accomplish multiple objectives such as imaging and therapy or can perform a single advanced function in one delivery system.24–29 The bulk self-assembly of block copolymer also has been used to fabricate multifunctional nanomaterials by mixing two different nanoparticles such that they preferentially segregate in the different phases of the microphase separated structure. Such systems have been studied both theoretically as well as experimentally.30–34The hybrid structures, using block copolymer micelle based approach, are generated by co-encapsulation of different types of nanoparticles in the hydrophobic core of the block copolymer micelles. In the past, such an approach has been used to produce multifunctional hybrid particles from quantum dots, magnetic or gold nanoparticles being co-encapsulated in the core of the same micelle.35–37 However, the approach usually allows fabrication of only spherical particles. Recently Bae et al. have used evaporation-induced instabilities in droplets at the solvent/water interface, to efficiently encapsulate pre-synthesized nanoparticles of iron oxide, CdSe, and Au, including co-encapsulation of different particle types in worm-like cylindrical micelles of polystyrene-block-poly(ethylene oxide) (PS-b-PEO).38 Such cylindrical micelles allow for a higher loading capacity of multiple functional materials per micelle and also have been proposed to have long in vivo circulation time. However, the above approaches mostly lead to hybrid materials where the encapsulated functionalities are randomly distributed in the micelle core. In a somewhat different approach, Mei et al. recently demonstrated the fabrication of core–shell multifunctional particles with Au nanoparticles encapsulated in the core and Pd nanoparticle decorating the shell.39 The approach involved the infiltration of Au loaded PS-b-P2VP block copolymers in anodized alumina oxide (AAO) membranes. The multifunctional nanoparticles so fabricated were demonstrated to work as an efficient catalyst.
One-dimensional core–shell hybrid structures, where one of the functionalities resides in the core and the second one – at the shell of cylindrical micelles, provide additional application opportunities as well as synthetic challenges. In such systems, the nanoparticles present at the shell might be targeted to be accessible for desired interactions or chemical reactions, whereas the nanoparticles to be protected or for controlled release could be encapsulated in the core. Core–shell cylindrical micelles of block copolymers with chemically different core and shell constituents could act as templates for the formation of such core–shell hybrid structures. The cylindrical micelles formed by block copolymer self-assembly in solution have been used in the past to host functionalities, like inorganic nanoparticles, either in the core or at the shell of the micelles. The nanoparticles have been synthesized either in the core or in the shell or have been physically adsorbed on the shell of the cylindrical micelles.40–45 Recently, Zhu and co-workers used supramolecular assembly approach to isolate nanoparticle loaded cylindrical BCP domains using selective solvent.46,47 However, to the best of our knowledge, cylindrical block copolymer micelles with core and shell functionalized with different nanoparticles have not been reported. But given different chemistries of core and shell of a block copolymer micelle, preparation of such a multifunctional, in principle, looks feasible.
Recently, we have shown that the cylindrical domains of a polystyrene-block-poly(4-vinylpyridine) (PS-b-P4VP) could be isolated as cylindrical micelles or nanofibers simply by swelling the block copolymer in a selective solvent.48 For block copolymer with PS as the minority block, the nanofibers had the core composed of PS block and the shell of P4VP block. It was further shown that the reactive P4VP shell of the nanofibers could be used as reactor and, hence, a targeted functionality could be loaded in the shell.49 Furthermore, the core of the nanofibers could also be used as host for the nanoparticles with suitably modified surface.50 In the present work, we demonstrate that the isolation of cylindrical domains from the self-assembled block copolymer structures by selective swelling could be used as a simple approach for fabricating multifunctional core–shell polymer nanofibers. In the two step approach, cylindrical domains of PS-b-P4VP block copolymer, pre-loaded with PS-stabilized silver (Ag) nanoparticles, are first isolated in form of nanofibers in P4VP-selective solvent. In the next step, the second type of nanoparticles (Au or CdS) are either directly synthesized or physically adsorbed at the shell of the nanofibers. The approach is versatile and, in principle, could be extended to prepare multifunctional core–shell nanofibers with a range of different combinations of functionalities.
2. Experimental
Materials
Polystyrene-block-poly(4-vinylpyridine) was purchased from Polymer source, Canada. The PS-b-P4VP purchased had Mn = 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 and Mn = 40
500 and Mn = 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 for PS and P4VP, respectively, and a polydispersity index of 1.10. The calculated volume fractions of the PS and P4VP blocks were 0.3 and 0.7, respectively. Tetrachloroauric acid (HAuCl4), cadmium acetate, silver acetate and oleylamine were procured from Sigma-Aldrich. Thiol-terminated polystyrene (PSSH, Mw = 1100 g mol−1, PDI = 1.12) was procured from Polymer Source, Canada. All anhydrous solvents were purchased from Merck chemicals and used as received.
500 for PS and P4VP, respectively, and a polydispersity index of 1.10. The calculated volume fractions of the PS and P4VP blocks were 0.3 and 0.7, respectively. Tetrachloroauric acid (HAuCl4), cadmium acetate, silver acetate and oleylamine were procured from Sigma-Aldrich. Thiol-terminated polystyrene (PSSH, Mw = 1100 g mol−1, PDI = 1.12) was procured from Polymer Source, Canada. All anhydrous solvents were purchased from Merck chemicals and used as received.
Synthesis of PS stabilized Ag nanoparticles
Polystyrene-coated silver nanoparticles were prepared adopting synthetic procedure reported by Hiramatsu and Osterloh.51 Briefly, 50 mg (0.3 mmol) of silver acetate (AgAc) was dissolved in 2.5 mL of oleylamine (OlAm) and purged with argon for 30 minutes. Separately, 50 mL of toluene was purged with argon in a round-bottom flask equipped with reflux condenser for 30 min and then heated to reflux under inert argon atmosphere. Next, AgAc/OlAm mixture was injected rapidly into a boiling toluene and refluxed for next 24 hours. After cooling the reaction mixture to room temperature, toluene was removed on rotary evaporator and 50 mL of chloroform containing 10 mg of thiol-terminated polystyrene was added drop-wise and mixture was left for stirring for the next 24 hours. Afterwards, the reaction mixture was concentrated on rotary evaporator to a volume of ca. 5 mL and AgNP were flocculated by adding of 50 mL of ethanol and separated by centrifugation. To remove oleylamine and excess of PSSH, the nanoparticles were washed with acetone by repeated re-dispersion/centrifugation (5 times with 50 mL). Finally, the nanoparticles were re-dispersed in 50 mL of chloroform, filtered through 0.2 μm PTFE membrane filter, concentrated on a rotary evaporator to a volume of ca. 5 mL and precipitated in ethanol again. After centrifugation, the PS-stabilized AgNPs were dried in vacuum at 50 °C overnight, resulting in a fine powder readily dispersible in organic solvents.Preparation of polymer nanofibers loaded with Ag nanoparticles in the core
PS-b-P4VP block copolymer was dissolved in chloroform (a common solvent for both PS and P4VP) at room temperature and then chloroform solution of PS-stabilized Ag nanoparticles was added drop wise to a block copolymer solution under continuous stirring. The whole solution was further kept for the stirring for 24 hours. Subsequently, the solution was kept in a saturated vapour atmosphere of chloroform in a closed chamber with a very small opening for slow solvent evaporation. After complete evaporation of chloroform, the sample was kept for drying in a vacuum oven for 48 h. For isolating the Ag-loaded individual nanofibers with PS core and P4VP shell, the bulk sample was dispersed in ethanol, a selective solvent for P4VP block. After short sonication in ultrasonic bath, the nanofiber dispersion in ethanol was spin coated or drop casted on a silicon substrate or TEM carbon-coated copper grid for further analysis.Preparation of multifunctional nanofibers
Multifunctional nanofibers were prepared by deposition of Au or CdS nanoparticles on the P4VP shell of the nanofibers with encapsulated Ag nanoparticles. The Au nanoparticles were deposited by loading the HAuCl4 precursor into the P4VP shell of the nanofibers and its further reduction either in the dispersed state in ethanol, or after immobilization of the nanofibers on the substrates (silicon wafers or TEM carbon-coated copper grid). The nanofibers obtained from PS-b-P4VP or Ag@PS-b-P4VP were first dispersed in ethanol (1 mg mL−1). Subsequently, HAuCl4 was added in the dispersion such that the HAuCl4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4VP molar ratio of 1
4VP molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was maintained. The solution was magnetically stirred at room temperature for 24 h. In the next step, reduction process was carried out by adding freshly prepared solution of NaBH4 (10 mg mL−1) under stirring. After further stirring for another 24 h, the nanofibers were separated by centrifugation and redispersed in ethanol to the concentration nearly same as before centrifugation. In an another approach the HAuCl4 loaded Ag@PS-b-P4VP nanofiber dispersion, prepared in ethanol, were drop casted on a silicon substrate or TEM grid and then dipped in NaBH4 solution for further reduction process. The substrate was further washed and dried. In a different approach, polymer nanofibers, immobilized on a substrate, were directly dipped into aqueous dispersion of the pre-synthesized citrate stabilized gold nanoparticles. The Au nanoparticles were found to adsorb on the P4VP shell in this case.
1 was maintained. The solution was magnetically stirred at room temperature for 24 h. In the next step, reduction process was carried out by adding freshly prepared solution of NaBH4 (10 mg mL−1) under stirring. After further stirring for another 24 h, the nanofibers were separated by centrifugation and redispersed in ethanol to the concentration nearly same as before centrifugation. In an another approach the HAuCl4 loaded Ag@PS-b-P4VP nanofiber dispersion, prepared in ethanol, were drop casted on a silicon substrate or TEM grid and then dipped in NaBH4 solution for further reduction process. The substrate was further washed and dried. In a different approach, polymer nanofibers, immobilized on a substrate, were directly dipped into aqueous dispersion of the pre-synthesized citrate stabilized gold nanoparticles. The Au nanoparticles were found to adsorb on the P4VP shell in this case.
The basic approach for depositing the CdS nanoparticles over P4VP shell of the nanofibers was same as for depositing of gold. Here, the CdS precursor, cadmium acetate, was added to the already prepared dispersion of nanofibers from PS-b-P4VP or Ag@PS-b-P4VP in ethanol (1 mg mL−1). During the addition, Cd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4VP molar ratio of 1
4VP molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was maintained and the solution was kept for stirring overnight. Subsequently, two different approaches were used to synthesize CdS quantum dots within the P4VP shell of the nanofibers. In the first approach, Cd salt loaded nanofibers were deposited onto the substrates and then H2S gas was passed over the substrate. In the second approach, H2S was passed directly through the dispersion of the nanofibers in ethanol. After centrifugation and redispersion of the solid residue in ethanol, a drop of dispersion was deposited onto the substrates for further investigations.
1 was maintained and the solution was kept for stirring overnight. Subsequently, two different approaches were used to synthesize CdS quantum dots within the P4VP shell of the nanofibers. In the first approach, Cd salt loaded nanofibers were deposited onto the substrates and then H2S gas was passed over the substrate. In the second approach, H2S was passed directly through the dispersion of the nanofibers in ethanol. After centrifugation and redispersion of the solid residue in ethanol, a drop of dispersion was deposited onto the substrates for further investigations.
Characterization
Scanning electron microscopy (SEM) images were obtained with FEI Quanta 200F with Oxford-EDS system IE 250 X Max 80 equipped with field-emission gun (FEG). The samples prepared on silicon substrates were viewed under the SEM without any additional coating. Energy-dispersive X-ray spectroscopy (EDX) mapping was performed in Zeiss Ultra55 SEM equipped with Bruker XFlash 5060 Spectrometer. The SEM was operated with a transmitted electrons detector for EDX mapping, thus will be referred as STEM further on. Conventional transmission electron microscopy (TEM) was performed using JEOL JEM-2100F and Zeiss Libra 200 transmission electron microscopes at an accelerating of 200 kV. The samples for TEM imaging were prepared on the carbon-coated copper grid by drop casting method. UV-vis absorption spectra were recorded on Perkin-Elmer Lambda 1050 spectrophotometer.3. Results and discussion
The schematic illustration of the two-step approach used in this work is shown in Fig. 1. The Ag nanoparticles stabilized with short chain PS ligand were mixed with a cylinder forming PS-b-P4VP block copolymer with PS as the minority block. The favourable interaction drives the Ag nanoparticles to locate selectively in the cylindrical PS domains, of self-assembled block copolymer matrix. The Ag@PS-b-P4VP composites then were treated with ethanol, a P4VP selective solvent. This led to swelling of the P4VP matrix and, hence, to disintegration and release of isolated cylindrical domains in the form of nanofibers. The core–shell nanofibers have their core composed of PS block and shell composed of P4VP block. In the next step, the second type of nanoparticles were deposited on the P4VP shell, by implementing either in situ or ex situ nanoparticle synthesis. The deposition of the second type nanoparticles could be carried out either in dispersion or in dried state, i.e. after immobilization of the nanofibers on substrates.Fig. 2 shows TEM images depicting the morphology of the Ag@PS-b-P4VP bulk sample as well as that of the Ag-loaded isolated nanofiber drop cast from its ethanol dispersion. TEM image of bulk sample (Fig. 2a) clearly shows that the Ag nanoparticles locate selectively in one phase of self-assembled BCP matrix. The location of nanoparticles in PS core of the nanofiber is also evident from the TEM image of the isolated nanofiber (Fig. 2b). One of the interesting observations was regarding the closely packed arrangement of nanoparticles, induced by the confinement effects provided by the BCP cylindrical domains. Furthermore, we have shown that the close packing of the nanoparticles corresponds to a helical morphology, where the central nanoparticle chain is surrounded by five other nanoparticle chains twisted with respect to each other along the cylinder axis.50
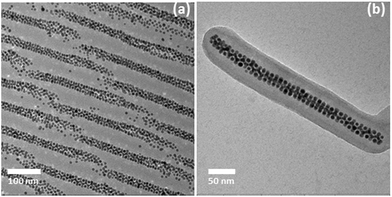 | ||
| Fig. 2 TEM micrographs of Ag@PS-b-P4VP composites (a) microtomed bulk sample; (b) cylindrical domains isolated as nanofibers from the bulk samples. | ||
Thus, isolated Ag@PS-b-P4VP nanofibers had a dense PS core loaded with Ag nanoparticles and the shell composed of P4VP chains. Now, poly(vinylpyridine) polymers (P2VP and P4VP) are well-known to coordinate with various types of nanoparticles, including metals, semiconductors or dielectrics.52 This is attributed to the possibility of the pyridine group to interact with different species. Hence, the hairy P4VP shell of the polymer nanofibres could be used to attach different functionalities on the surface of the nanofibres. Immobilization of the nanoparticles on the P4VP shell could either be done by coordinating the precursor with the pyridine units of P4VP (in situ synthesis) or by directly adsorbing pre-synthesized nanoparticles on the P4VP shell (ex situ method). Fig. 3 shows the TEM micrograph of the multifunctional nanofibers where the Au nanoparticles were in situ synthesized on the P4VP shell using HAuCl4 as gold precursor.
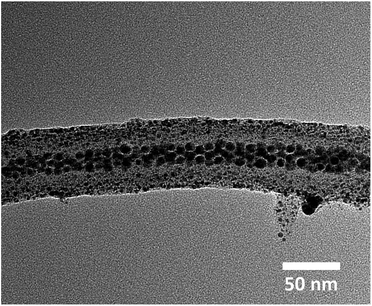 | ||
| Fig. 3 HR TEM image of multifunctional Ag@PS-b-P4VP@Au nanofiber loaded with pre-synthesized Ag nanoparticles and in situ synthesized Au nanoparticles. | ||
The TEM micrograph clearly shows the presence of Au nanoparticles on the shell. Moreover, the close-packed arrangement of Ag nanoparticles inside the PS core remained intact. The presence of Ag and Au nanoparticles in the core and in the shell, respectively, was further confirmed by EDX analysis using STEM. Fig. 4 shows the STEM image and EDX elemental maps of both Ag and Au in Ag@PS-b-P4VP@Au multifunctional nanofiber.
 | ||
| Fig. 4 STEM micrographs of Ag@PS-b-P4VP@Au multifunctional nanofibers. The Ag and Au elements present in the nanofibers were mapped using EDX analysis. | ||
The EDX analysis unambiguously proves the presence of Au and Ag nanoparticles in the shell and core of the polymer nanofiber, respectively. Although, the distribution of the Au nanoparticles within the shell was relatively uniform, the in situ method does not allow a fine control over the nanoparticle size and shape. Alternatively, the nanoparticles with desired size, shape and surface chemistry could be pre-synthesized and then allowed to physically adsorb on the P4VP shell. As an example of such approach, we demonstrated this by using, pre-synthesized citrate stabilized Au nanoparticles. These Au nanoparticles could be effectively adsorbed on the P4VP shell by dipping the substrate with immobilized nanofibers in the aqueous dispersion of the nanoparticles (see ESI†). Moreover, this approach might be also extended to other type of metal nanoparticles, like Pd or Pt, which possess affinity towards P4VP.53
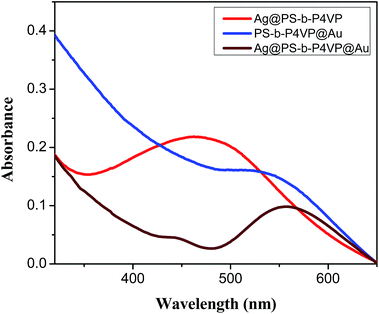
The UV-vis absorption spectrum of the Ag@PS-b-P4VP@Au multifunctional nanofibers, containing silver in the core and gold in the shell, is shown in Fig. 5. The UV-vis spectra of Ag@PS-b-P4VP and PS-b-P4VP@Au nanofibers are also given for comparison. The UV-vis spectra of nanofibers with either only Au nanoparticles in the shell or Ag nanoparticles in the core have a single absorption peak with maxima at 535 and 470 nm, respectively, originating from the surface plasmon resonance (SPR) absorptions of the respective metal nanoparticles. The position SPR absorption peak of Au in PS-b-P4VP@Au nanofibers was close to that reported for Au nanoparticles. However, the absorption band of nanofibers containing Ag nanoparticles in the core was substantially broader and red shifted as compared to the same type of Ag nanoparticles in dispersion with maximum at 420 nm (see ESI†). The red shift in SPR peak observed in case of Ag nanoparticles encapsulated in the polymer nanofibers plausibly results from the close packing of the nanoparticles. It must be noted that though the peak position of absorption peaks originating from in situ synthesized nanoparticles (AuNP) could be compared, their absolute intensities may be difficult to compare. This is because the concentration of solution especially with respect to the in situ synthesized AuNPs may not be same because of the nature of the process and limited aggregation observed during the reduction stage.
In the case of Ag@PS-b-P4VP@Au multifunctional nanofibers, two distinct absorption peaks were observed corresponding each to the SPR of Au and Ag nanoparticles. It has been shown in the past that for Au/Ag hybrid nanoparticles, the UV spectra shows a single absorption peak in between the absorption bands of the respective pure nanoparticles if an alloy is formed. However, in case of core–shell structures, two absorption peaks corresponding to those of neat Au and Ag nanoparticles are observed.54–57 Two absorption peaks observed in Ag@PS-b-P4VP@Au case corroborates the core–shell structure of the nanofibers. The core–shell structure is further exemplified from the fact that the SPR absorption peak of core encapsulated Ag nanoparticles is significantly suppressed due to masking effect from the optically active Au nanoparticles present in the shell. The absorption band of Ag nanoparticles was found to be blue shifted to 445 nm in multifunctional nanofibers compared to that in nanofibers with only Ag nanoparticles. The blue shift plausibly could be a result of absorption behaviour of Au nanoparticles present in the shell. It must be noted that compared to absorption of neat Ag nanoparticles, the absorption peak in multifunctional nanofiber still exhibited a red shift.
To demonstrate that the above approach is versatile and could be applied for preparing multifunctional nanofibers with a range of targeted nanoparticle combinations, we also deposited CdS nanoparticles on the nanofiber shell. Fig. 6a shows the TEM micrograph of Ag@PS-b-P4VP@CdS multifunctional nanofibers with Ag in the core and CdS particles generated in situ within the P4VP shell, whereas Fig. 6b depicts a high resolution TEM image taken at the edge of the nanofiber to visualise individual crystalline CdS nanoparticles. Due to the higher density of CdS as compared to the block copolymer, the edge of the nanofiber appears darker after loading with CdS (see for comparison Fig. 2b). Furthermore, similar to Ag@PS-b-P4VP@Au case, the initial closely packed arrangement of Ag nanoparticles in the nanofiber core remains intact after CdS synthesis.
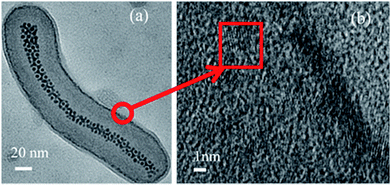 | ||
| Fig. 6 (a) TEM micrograph of Ag@PS-b-P4VP@CdS multifunctional nanofiber and (b) high resolution TEM image of the shell region of the nanofiber showing crystalline CdS nanoparticles. | ||
The respective presence and distribution of CdS and Ag nanoparticles in shell and core of the nanofibers were further validated using EDX analysis during STEM measurements. Fig. 7 shows the EDX elemental mapping of Ag@PS-b-P4VP@CdS multifunctional nanofibers. The EDX results clearly show the presence of Ag in the core and Cd and S in the shell of the nanofiber.
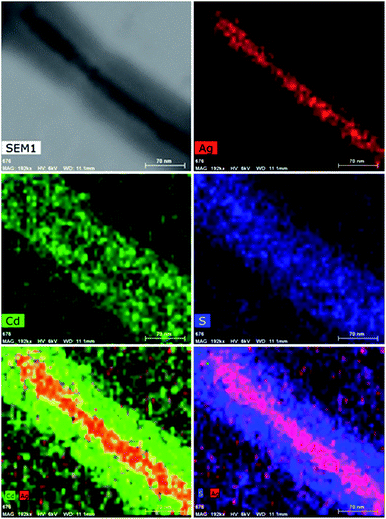 | ||
| Fig. 7 STEM micrographs of Ag@PS-b-P4VP@CdS multifunctional nanofibers. The Cd, S and Au elements present in the nanofibers were mapped using EDX analysis. Scale bars are 70 nm. | ||
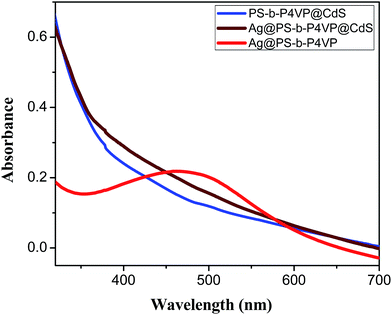
The Ag@PS-b-P4VP@CdS multifunctional nanofibers were further characterized by UV-vis spectroscopy. The corresponding UV-vis spectrum is plotted together with that of Ag@PS-b-P4VP and PS-b-P4VP@CdS nanofibers in Fig. 8. As revealed from the UV-vis studies, Ag-loaded nanofiber displays the characteristic SPR peak of Ag nanoparticle at 470 nm, while a broad absorption peak between 380 and 460 nm was observed for nanofibers functionalized with CdS nanoparticles, which is in accordance with absorption of CdS nanoparticles reported in literature.58 The weak and broad absorption of CdS observed in the UV-vis spectra plausibly is because of broad size distribution of the CdS NPs. However, UV-vis absorption spectrum of Ag@PS-b-P4VP@CdS multifunctional nanofibers only exhibited a single broad absorption band similar to that of PS-b-P4VP@CdS sample. Intuitively, it was expected that these nanofibers with core–shell structure will exhibit two absorption peaks corresponding to CdS and Ag nanoparticles respectively. The disappearance of SPR absorption peak of silver in this case might be the results of an increase of the refractive index of the nanofiber shell after incorporation of CdS quantum dots, whose refractive index is much higher as compared to that of PS-b-P4VP. The higher refractive index of the shell material is known to result in a red-shift of the plasmon resonance peak and is well explained by the Mie theory.58 Moreover, in case of a thicker and irregular shell, the surface plasmon of the core material may resonate over a wide range of frequencies and, hence, may also lead to either a broadening or complete suppression of the plasmon resonance peak.59–61 In this particular case, the distribution of in situ synthesized CdS nanoparticles present in the shell is not uniform, inducing thus certain thickness variation over the length of nanofibers, as also is clear from the STEM images in Fig. 7. However, a more clear understanding of the optical properties of such systems will require a more detailed study involving the variation of thickness and uniformity of CdS shell, which was beyond the scope of the present work.
A slight increase in the absorption intensity in multifunctional nanofibers corresponding to CdS nanoparticles as compared to that in PS-b-P4VP@CdS nanofibers could plausibly be due to a slightly different size distribution of the nanoparticles in two cases. However, as already explained in the case of Ag@PS-b-P4VP@Au, a limited aggregation during the in situ synthesis of CdS nanoparticles and variable dispersion state of polymer nanofibers may also affect the intensities in the UV-vis spectra. The size and band gap of the CdS nanoparticles, as determined from the sizing curves using the first absorption peak positions obtained from UV experiments, were found to be 3.2 nm and 2.68 eV, respectively.62
4. Conclusions
We have demonstrated the fabrication of multifunctional core–shell nanofibers via self-assembly of a cylinder forming block copolymer. The first step of the process involves the incorporation of the nanoparticles in the cylindrical domains and subsequent isolation of the cylindrical domains in form of nanofibers using selective solvent approach. The shell of the isolated nanofibers, in the next step, is used as host for the deposition of the second type of nanoparticles, which could be loaded either by in situ or ex situ approaches. The present approach for preparing multifunctional core–shell nanofibers is versatile and could easily be extended to a range of different targeted combination of nanoparticles. Moreover, with the available tools for tuning the diameter and length/orientation of the cylindrical domains, the aspect ratio of the multifunctional nanofibers could also be tuned.Acknowledgements
This research was supported by a grant from Department of Science and Technology, India (Project No. SB/S1/PC-016/2013) and Deutsche Forschungsgemeinschaft, Germany (Project No. STA 324/51-1).Notes and references
- N. C. Bigall, W. J. Parak and D. Dorfs, Nano Today, 2012, 7, 282 CrossRef CAS.
- P. D. Cozzoli, T. Pellegrino and L. Manna, Chem. Soc. Rev., 2006, 35, 1195 RSC.
- J. Kim, Y. Piao and T. Hyeon, Chem. Soc. Rev., 2009, 38, 372 RSC.
- R. Jiang, B. Li, C. Fang and J. Wang, Adv. Mater., 2014, 26, 5274 CrossRef CAS PubMed.
- W. Shi, H. Zeng, Y. Sahoo, T. Y. Ohulchanskyy, Y. Ding, Z. L. Wang, M. Swihart and P. N. Prasad, Nano Lett., 2006, 6, 875 CrossRef CAS PubMed.
- H. Yu, M. Chen, P. M. Rice, S. X. Wang, R. L. White and S. H. Sun, Nano Lett., 2005, 5, 379 CrossRef CAS PubMed.
- E. Khon, A. Mereshchenko, A. N. Tarnovsky, K. Acharya, A. Klinkova, N. N. Hewa-Kasakarage, I. Nemitz and M. Zamkov, Nano Lett., 2011, 11, 1792 CrossRef CAS PubMed.
- E. V. Shevchenko, M. I. Bodnarchuk, M. V. Kovalenko, D. V. Talapin, R. K. Smith, S. Aloni, W. Heiss and A. P. Alivisatos, Adv. Mater., 2008, 20, 4323 CrossRef CAS.
- P. D. Cozzoli, T. Pellegrino and L. Manna, Chem. Soc. Rev., 2006, 35, 1195 RSC.
- N. Insin, J. B. Tracy, H. Lee, J. P. Zimmer, R. M. Westervelt and M. G. Bawendi, ACS Nano, 2008, 2, 197 CrossRef CAS PubMed.
- D. K. Yi, S. T. Selvan, S. S. Lee, G. C. Papaefthymiou, D. Kundaliya and J. Y. Ying, J. Am. Chem. Soc., 2005, 127, 4990 CrossRef CAS PubMed.
- A. Quarta, R. Di Corato, L. Manna, A. Ragusa and T. Pellegrino, IEEE Transactions on NanoBioscience, 2007, 6, 298 CrossRef PubMed.
- S. Jain and F. S. Bates, Science, 2003, 300, 460 CrossRef CAS PubMed.
- R. C. Hayward and D. J. Pochan, Macromolecules, 2010, 43, 3577 CrossRef CAS.
- A. Blanazs, S. P. Armes and A. J. Ryan, Macromol. Rapid Commun., 2009, 30, 267 CrossRef CAS PubMed.
- J. Rodríguez-Hernández, F. Chécot, Y. Gnanou and S. Lecommandoux, Prog. Polym. Sci., 2005, 30, 691 CrossRef.
- L. Zhang, W. G. Liu, L. Lin, D. Y. Chen and M. H. Stenze, Biomacromolecules, 2008, 9, 3321 CrossRef CAS PubMed.
- G. Fuks, R. M. Talom and F. Gauffre, Chem. Soc. Rev., 2011, 40, 2475 RSC.
- R. K. O'Reilly, C. J. Hawker and K. L. Wooley, Chem. Soc. Rev., 2006, 35, 1068 RSC.
- Y. Bae and K. Kataoka, Adv. Drug Delivery Rev., 2009, 61, 768 CrossRef CAS PubMed.
- M. H. Stenzel, Chem. Commun., 2008, 3486 RSC.
- P. K. Sudeep and T. Emrick, ACS Nano, 2009, 3, 2870 CrossRef CAS PubMed.
- H.-D. Koh, S. Park and T. P. Russell, ACS Nano, 2010, 4, 1124 CrossRef CAS PubMed.
- J. Shi, A. R. Votruba, O. C. Farokhzad and R. Langer, Nano Lett., 2010, 10, 3223 CrossRef CAS PubMed.
- P. T. Hammond, ACS Nano, 2011, 5, 681 CrossRef CAS PubMed.
- G. Ruan, D. Thakur, S. Deng, S. Hawkins and J. O. Winter, J. Nanoeng. Nanosyst., 2010, 223, 81 Search PubMed.
- J. H. Park, G. von Maltzahn, E. Ruoslahti, S. N. Bhatia and M. J. Sailor, Angew. Chem., Int. Ed., 2008, 47, 7284 CrossRef CAS PubMed.
- H. Koo, M. S. Huh, J. H. Ryu, D. E. Lee, I. C. Sun, K. Choi, K. Kim and I. C. Kwon, Nano Today, 2011, 6, 204 CrossRef CAS.
- Z. Gu, A. Biswas, M. X. Zhao and Y. Tang, Chem. Soc. Rev., 2011, 40, 3638 RSC.
- H. Chen and E. Ruckenstein, Polymer, 2010, 51, 5869 CrossRef CAS.
- M. R. Bockstaller, H. J. Ryu, S. Ojha and J. Choi, J. Mater. Chem., 2010, 20, 9339 RSC.
- H. Acharya, J. Sung, B.-H. Sohn, D. H. Kim, K. Tamada and C. Park, Chem. Mater., 2009, 21, 4248 CrossRef CAS.
- J. G. Son, W. K. Bae, H. Kang, P. F. Nealey and K. Char, ACS Nano, 2009, 3, 3927 CrossRef CAS PubMed.
- T. Pietsch, P. Müller-Buschbaum, B. Mahltig and A. Fahmi, ACS Appl. Mater. Interfaces, 2015, 7, 12440 Search PubMed.
- A. C. Kamps, B. L. Sanchez-Gaytan, R. J. Hickey, N. Clarke, M. Fryd and S. J. Park, Langmuir, 2010, 26, 14345 CrossRef CAS PubMed.
- B.-S. Kim and T. A. Taton, Langmuir, 2007, 23, 2198 CrossRef CAS PubMed.
- R. Di Corato, N. C. Bigall, A. Ragusa, D. Dorfs, A. Genovese, R. Marotta, L. Manna and T. Pellegrino, ACS Nano, 2011, 5, 1109 CrossRef CAS PubMed.
- J. Bae, J. Lawrence, C. Miesch, A. Ribbe, W. Li, T. Emrick, J. Zhu and R. C. Hayward, Adv. Mater., 2012, 24, 2735 CrossRef CAS PubMed.
- S. Mei, J. Cao and Y. Lu, J. Mater. Chem. A, 2015, 3, 3382 RSC.
- H. Wang, X. S. Wang, M. A. Winnik and I. Manners, J. Am. Chem. Soc., 2008, 130, 12921 CrossRef CAS PubMed.
- R. Djalali, S. Y. Li and M. Schmidt, Macromolecules, 2002, 35, 4282 CrossRef CAS.
- A. Walther, J. Yuan, V. Abetz and A. H. E. Muller, Nano Lett., 2009, 9, 2026 CrossRef CAS PubMed.
- M. Zhang, L. R. Boisneuf, Y. W. Hu, K. Moozeh, Y. Hassan, G. Scholes and M. A. Winnik, J. Mater. Chem., 2011, 21, 9692 RSC.
- M. Zhang, M. F. Wang, S. He, J. S. Qian, A. Saffari, A. Lee, S. Kumar, Y. Hassan, A. Guenther, G. Scholes and M. A. Winnik, Macromolecules, 2010, 43, 5066 CrossRef CAS.
- M. F. Wang, M. Zhang, J. Li, S. Kumar, G. C. Walker, G. D. Scholes and M. A. Winnik, ACS Appl. Mater. Interfaces, 2010, 2, 3160 Search PubMed.
- W. Li, S. Liu, R. Deng and J. Zhu, Angew. Chem., Int. Ed., 2011, 50, 5865 CrossRef CAS PubMed.
- W. Li, P. Zhang, D. Ming, J. He, T. Babu, Y. L. Xu, R. Deng, R. Liang, M. H. Lu, Z. Nie and J. Zhu, Macromolecules, 2013, 46, 2241 CrossRef CAS.
- J. Pal, S. Sanwaria, R. Srivastava, B. Nandan, A. Horechyy, M. Stamm and H. L. Chen, J. Mater. Chem., 2012, 22, 25102 RSC.
- S. Sanwaria, J. Pal, R. Srivastava, P. Formanek, M. Stamm, A. Horechyy and B. Nandan, RSC Adv., 2013, 3, 24009 RSC.
- S. Sanwaria, A. Horechyy, D. Wolf, C. Y. Chu, H. L. Chen, P. Formanek, M. Stamm, R. Srivastava and B. Nandan, Angew. Chem., Int. Ed., 2014, 53, 9090 CrossRef CAS PubMed.
- H. Hiramatsu and F. E. Osterloh, Chem. Mater., 2004, 16, 2509 CrossRef CAS.
- S. Malynych, I. Luzinov and G. Chumanov, J. Phys. Chem. B, 2002, 106, 1280 CrossRef CAS.
- A. Horechyy, B. Nandan, N. E. Zafeiropoulos, P. Formanek, U. Oertel, N. C. Bigall, A. Eychmüller and M. Stamm, Adv. Funct. Mater., 2013, 23, 483 CrossRef CAS.
- A. Uppal, B. Ewing and P. Parkin, Eur. J. Inorg. Chem., 2011, 4534 CrossRef.
- J. Wilcoxon, J. Phys. Chem. B, 2009, 113, 2647 CrossRef CAS PubMed.
- S. Shore, J. Wang, C. Johnston-Peck, L. Oldenburg and B. Tracy, Small, 2011, 7, 230 CrossRef PubMed.
- Y. Yang, J. Shi, G. Kawamura and M. Nogami, Scr. Mater., 2008, 58, 862 CrossRef CAS.
- U. Kreibig and M. Vollmer, Optical Properties of Metal Clusters, Springer, Berlin, Germany, 1995 Search PubMed.
- H. M. Gong, X. H. Wang, Y. M. Du and Q. Q. Wang, J. Chem. Phys., 2006, 125, 024707 CrossRef PubMed.
- H. Duan and Y. Xuan, Sol. Energy Mater. Sol. Cells, 2014, 121, 8 CrossRef CAS.
- P. K. Jain, W. Huang and M. A. El-Sayed, Nano Lett., 2007, 7, 2080 CrossRef CAS.
- W. Yu, L. Qu, W. Guo and X. Peng, Chem. Mater., 2003, 15, 2854 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra17161j |
| This journal is © The Royal Society of Chemistry 2015 |