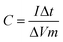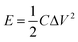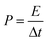Rapid microwave-assisted fabrication of 3D cauliflower-like NiCo2S4 architectures for asymmetric supercapacitors†
Yanling Xiaoa,
Ying Leib,
Baozhan Zhenga,
Li Gua,
Yanyan Wangb and
Dan Xiao*ab
aCollege of Chemistry, Sichuan University, 29 Wangjiang Road, Chengdu 610064, China. E-mail: xiaodan@scu.edu.cn; Fax: +86-28-85415029; Tel: +86-28-85416029
bCollege of Chemical Engineering, Sichuan University, 29 Wangjiang Road, Chengdu 610064, China
First published on 19th February 2015
Abstract
In this study, 3D cauliflower-like NiCo2S4 architectures have been synthesized through a facile, one-step and template-free microwave method. The cauliflower-like NiCo2S4 materials are made up of 3D microstructures with an average diameter of around 600 nm and each nanostructure is found to be constructed of many intertwined nanoparticles. The NiCo2S4-modified electrode was successfully applied to a pseudocapacitor. The electrochemical performance of the NiCo2S4 material was studied by cyclic voltammetry, galvanostatic charge–discharge and electrical impedance spectroscopy. The 3D cauliflower-like NiCo2S4 materials exhibit a maximum capacitance of 1471 F g−1 at 1 A g−1 and also show remarkable rate capability and prominent cycling stability. To improve the energy density of the supercapacitor, a NiCo2S4-modified electrode and activated carbon-modified electrode were used to assemble an asymmetric capacitor. The asymmetric capacitor demonstrates remarkable properties with a maximum energy density of 44.8 W h kg−1 and a maximum power density of 16.0 kW kg−1. Furthermore, two capacitors assembled together can successfully light up a red light-emitting diode (LED) and last for more than 10 min. The excellent capacitance performance demonstrates that the cauliflower-like NiCo2S4 has potential applications in supercapacitors.
Introduction
In order to address the rapidly increasing energy crisis, much effort has been devoted to exploring and designing new sustainable and environmentally friendly products by scientists for decades.1,2 Electrochemical capacitors (ECs), as promising candidates for power source storage devices, fill the gap between batteries and traditional electrostatic capacitors, and have attracted much attention due to their higher power and energy densities, fast charging, and long cycle life.3–5 The electrode materials of ECs usually consist of conducting polymers, transition metal oxides/hydroxides and carbon materials. However, carbon materials and conducting polymers usually have low specific capacitance and poor cycling stability, respectively. The poor electrochemical property of these materials impedes their practical applications.6,7 Interestingly, transition metal oxides/hydroxides usually have a variety of oxidation states which facilitate faradaic reactions, achieving much more prominent specific capacitance.8,9Among transition metal oxide materials, binary metal oxides, such as NiCo2O4, are a potential material for supercapacitors. It has been reported that NiCo2O4 is endowed with higher conductive ability, at least ∼100 times higher than Co3O4 and NiO, which enables it to be an outstanding electrode material.10,11 Similar to NiCo2O4, nickel cobalt sulphide (NiCo2S4) materials are also applied to supercapacitors and other applications.12–15 NiCo2S4 possesses richer redox ability than the single phase of metal sulfides due to its synergistic effect from both nickel and cobalt ions.16 Furthermore, NiCo2S4 shows much higher conductivity compared to NiCo2O4.17 Up to now, many methods for synthesizing NiCo2S4 electrode materials have been reported, such as hydrothermal method and electrodeposition.10,17–20 For example, Xia et al. have synthesized 3D urchin-like NiCo2S4 using a multistep hydrothermal method without any templates.17 Furthermore, Ruan and co-workers have fabricated porous NiCo2S4 nanotubes via hydrothermal method and sacrificial templates.19 In addition, Alshareef et al. have proposed NiCo2S4 nanosheet arrays by electrodeposition.20 Although the electrode materials fabricated by the two strategies usually have relatively high specific capacitances, their some disadvantages still greatly hinder their practical applications. The hydrothermal method generally involves multistep procedure and even need some templates, which make the preparation of the electroactive materials complicated and time-consuming. What's more, hydrothermal reaction has relatively rigorous requirements for safety and quality of the equipments due to the generated relatively high temperature and high pressure during hydrothermal reaction, which will increase the cost undoubtedly. For electrodeposition, it needs a long time to deposit one electrode and uses the expensive equipment (electrochemical workstation). Therefore, the electrodeposition is also costly and time-consuming. By contrast, microwave-assisted heating (MWH) method involves simple and easy operation, greatly shortening the reaction time and easily forming a uniform reaction system. Moreover, most materials can be prepared by a simple one-step microwave-assisted method just by employing a household microwave oven without any templates. In a word, the MWH is a facile, low-cost, template-free, one-step method, and can be used for large-scale application. Therefore, MWH can be applied to the preparation of different inorganic materials.21,22
In this paper, we present a facile MWH method to prepare 3D cauliflower-like NiCo2S4. The cauliflower-like NiCo2S4 acting as supercapacitor electrode material displays high specific capacitance, remarkable rate performance and prominent cycle stability. To further study the practical application of NiCo2S4, an asymmetric capacitor was assembled. Fortunately, the as-fabricated asymmetric supercapacitor exhibits high energy and power densities, which is able to drive a low voltage device such as LED.
Experimental section
Synthesis of 3D cauliflower-like NiCo2S4 architectures
All the analytical grade chemicals were used directly. In this typical experiment, 5 mmol of Ni(NO3)2·6H2O, 10 mmol of Co(NO3)2·6H2O, 10 mmol of citric acid (H3Cit) and 30 mmol of thioacetamide (CH3CSNH2) were dissolved in 100 mL of ethylene glycol (EG) solution with magnetic stirring. Then, the mixture was treated for 5 min at 700 W in the household microwave oven. After that, the products were obtained by centrifugal filtration and rinsed with deionized water and absolute ethanol, respectively. The final products were dried at 50 °C overnight under vacuum conditions.Characterization
The composition and crystal structure of NiCo2S4 materials were investigated by X-ray powder diffractometer (XRD, Tongda TD-3500, Liaoning, China, Cu-Kα radiation, λ = 0.15148 nm) operated at 30.0 kV and 20.0 mA. The morphology and structure of NiCo2S4 materials were conducted using a scanning electron microscope (SEM, Hitachi S4800, Tokyo, Japan) attached with energy dispersive spectra (EDS) and a transmission electron microscopy (TEM), high resolution transmission electron microscopy (HRTEM, Hitachi H-800, operated at 200.0 kV). The X-ray photoelectron spectra (XPS, Kratos XSAM 800, Manchester, U.K., Mg-Ka, 1253.6 eV) were acquired to investigate the composition on the near-surface of NiCo2S4 materials. The Brunauer–Emmett–Teller (BET) surface area and pore size distribution of the NiCo2S4 materials were conducted from N2 adsorption–desorption at 77.3 K through a Automated Surface Area and Pore Size Analyzer (Quadrasorb SI).Electrochemical characterization
The electrochemical performance of resultant NiCo2S4 material was measured in a traditional three-electrode system, where NiCo2S4-modified electrode, a graphite sheet and a Hg/HgO electrode act as the working, counter and reference electrodes, respectively. And the working electrodes were fabricated as follows. 80 wt% of NiCo2S4 material (electroactive material), 15 wt% of acetylene black (conducting material) and 5 wt% of poly (vinylidene fluoride, PVDF, binder) were mixed and dissolved in a certain amount of N-Methyl-2-pyrrolidne (NMP, solvent). Then, the mixture was loaded onto the nickel foam substrate (surface, 1.0 cm × 1.0 cm). The as-prepared NiCo2S4-modified electrodes were dried at 60 °C for 12 h under vacuum. After that, the NiCo2S4-modified electrodes were pressed under a pressure of 10 MPa. The mass loading of NiCo2S4 electroactive materials was about 3.5–5.0 mg.In half-cell tests, all electrochemical experiments of the NiCo2S4-modified electrode were conducted by an Autolab PGSTAT 30/302 electrochemical workstation (Eco Chemie B.V., Amsterdam, the Netherlands). Cyclic voltammetry (CV), galvanostatic charge–discharge and electrical impedance spectroscopy (EIS) were carried out to investigate the electrochemical performance of the obtained NiCo2S4-modified electrodes. EIS tests of the as-synthesized NiCo2S4-modified electrodes were performed with a frequency loop 10 kHz to 10 mHz under open circuit voltage with an ac amplitude of 10 mV. The electrodes are first activated for a while until the specific capacitance basically doesn't increase.
For full cell tests, the asymmetric capacitors were fabricated to evaluate the NiCo2S4 electrode for practical application. The electrochemical performance was conducted in two-electrode system. The NiCo2S4 electrodes were acted as the positive electrode and the activated carbon (the synthetic details and the BET surface area details in Fig. S7, ESI†) electrodes served as the negative electrode. The two asymmetric supercapacitors could power a LED indicator.
Results and discussion
Structure and morphology of 3D cauliflower-like NiCo2S4 architectures
To investigate the crystallinity and phase purities of the product, we resorted to XRD measurement and the corresponding result is demonstrated in Fig. 1. As depicted in Fig. 1, the distinctive peaks at 26.8°, 31.5°, 38.1°, 50.4° and 55.2°can be observed and they accord with (220), (311), (400), (511) and (440) planes, respectively. All the reflection peaks of the as-prepared products can be well indexed to the NiCo2S4 (JCPDS card no. 20-0782) according to the previous reports.13,14,19 The diffraction peaks can be assigned to cubic type NiCo2S4. Near absence of impurity peaks appeared in the XRD pattern indicates that high purity NiCo2S4 is obtained through our proposed synthesis method.To further understand the composition of the NiCo2S4 samples, the EDS and XPS tests were carried out and the corresponding results are demonstrated in Fig. 2. Only the peaks of Ni, Co, S elements appear in the EDS spectrum (Fig. 2a), which suggests that the NiCo2S4 material is mainly composed of Ni, Co, S elements. Fig. 2b–d present the XPS spectra of Ni, Co and S. As shown in Fig. 2b, two strong peaks in the Co 2p spectrum at 778.7 eV and 793.9 eV are observed and can be assigned to Co 2p3/2 and Co 2p1/2.12,23 The Co 2p3/2 and Co 2p1/2 with a spin-energy separation of 15.2 eV demonstrates the coexistence of Co2+ and Co3+.23 From the Ni 2p spectrum (Fig. 2c), we can clearly observe two kinds of Ni species (Ni2+ and Ni3+). The fitting peaks at 853.2 and 871.0 eV are assigned to Ni2+, and another two peaks at 854.9 and 872.6 eV are ascribed to Ni3+.24,25 In addition, the relative content of Co2+ and Co3+ as well as Ni2+ and Ni3+ in the NiCo2S4 materials is presented in the Table S1 in the ESI.† The amount of Co2+, Co3+, Ni2+ and Ni3+ are 32.00, 68.00, 64.90, and 35.1%, respectively. The peak at 163.4 eV is a typical of metal–sulphur bond.17,18 Obviously, the XPS data demonstrate that the near-surface of the NiCo2S4 materials have a composition containing S2−, Ni3+, Ni2+, Co3+, Co2+, which is consistent with the conclusions in the reported literatures for NiCo2S4.17
 | ||
| Fig. 2 (a) EDS image, (b) Ni 2p, (c) Co 2p, and (d) S 2p XPS spectra of the 3D cauliflower-like NiCo2S4 architectures. | ||
The morphology and the crystalline structure of the obtained NiCo2S4 materials were demonstrated by the SEM, TEM and HRTEM. Fig. 3a presents a representative SEM image of the NiCo2S4 materials. Apparently, the SEM image presents that the NiCo2S4 is made up of 3D microstructures with an average diameter of approximately 600 nm. Furthermore, it is found that each nanostructure is made up of numerous NiCo2S4 nanoparticles. Fig. 3b is the picture of cauliflower. Comparing the Fig. 3a and b, it can be observed that the shape of the NiCo2S4 materials is extremely similar to that of cauliflower. As is depicted in Fig. 3c, we can clearly see that the 3D cauliflower-like NiCo2S4 samples attach to each other and form some nanoclusters. The TEM images in Fig. 3d and e display that there are some obvious small protuberances on the surface of cauliflower-like NiCo2S4 samples. Apparently, it is also found that the cauliflower-like architectures are inclined to aggregate in groups according to the TEM images, which is in agreement with the images of SEM. The HRTEM image (Fig. 3f) presents that the lattice phase grows along with random orientation. The interlayer spacings between adjacent lattice stripes are calculated to be around 0.285 and 0.235 nm, which can respectively be ascribed to the (311) and (400) planes of the cubic NiCo2S4, indicating the polycrystalline characteristic of the 3D cauliflower-like NiCo2S4 architectures. The result is consistent with other literatures.13,17 The as-synthesized cauliflower-like NiCo2S4 with polycrystalline structures are expected to deliver outstanding electrochemical characteristics.
 | ||
| Fig. 3 (a and c) SEM images, (b) the picture of cauliflower, (d and e) TEM images, (f) HRTEM image of the 3D cauliflower-like NiCo2S4 architectures. | ||
Growth mechanism of 3D cauliflower-like NiCo2S4 architectures
In order to present the growth mechanism of the 3D cauliflower-like NiCo2S4 architectures clearly, a series of experiments were performed in different stages of reaction. The corresponding SEM images of the materials obtained at various reaction stages are shown in Fig. 4. As shown in Fig. 4a, the species consisting of numerous nanoparticles was obtained after a very short time (1.5 min). As the reaction time continues to 3 min, some solid microspheres (Fig. 4b) are formed from the cluster of the nanoparticles. After 5 min, the typical cauliflower-like NiCo2S4 architectures (Fig. 4c) are formed gradually. These SEM images suggest that the shape and size of the materials change gradually along with the reaction time. The XRD patterns of the samples collected at different reaction time were also conducted and the corresponding results are shown in Fig. S1.† As shown in the black curve in Fig. S1,† there is no obvious diffraction peaks presented in the XRD pattern of the samples collected at the early stage of the reaction (1.5 min). As the reaction continues (3 min), the diffraction peaks of NiCo2S4 with some impurity peaks in the XRD pattern are presented (Fig. S1,† red curve). At a reaction time of 5 min, all of the diffraction peaks are well indexed to the NiCo2S4. These results suggest that the formation of the NiCo2S4 crystal could be manipulated by orientation growth kinetics.26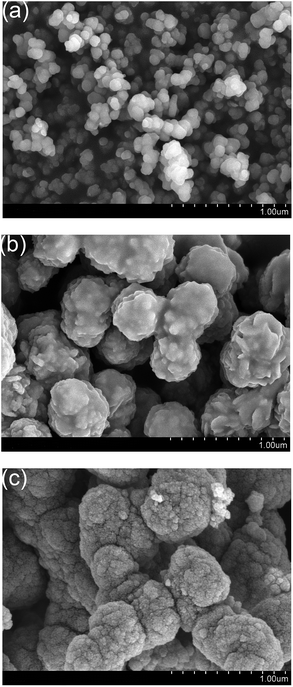 | ||
| Fig. 4 SEM images of the materials obtained at different reaction stages: (a) 1.5 min, (b) 3 min, (c) 5 min. | ||
Based on the results obtained above, the formation mechanism of the cauliflower-like NiCo2S4 architectures is tentatively presented. Also, the plausible growth process is depicted in Fig. 5. As is well-known, the functional group –COOH of the H3Cit in a homogenous system, could be inclined to react with Co2+ and Ni2+ to form stable complexes.27 At the early stage of the reaction, the sulfur atoms of the thioacetamide are replaced by the oxygen atoms from H2O and produce H2S. The H2S could react with the complexes to produce the initial NiCo2S4 seeds. As the reaction proceeds, the secondary nucleations will be likely to grow along with the surface of the NiCo2S4 initial nuclei rather than scattering in the solution.13 At the same time, along with an Ostwald ripening process, the small NiCo2S4 nanoparticles gradually dissolve and gather again around some agglomerates, and thus larger structures are formed, which is driven by the minimization of surface energy.27,28 As the reaction continues, the bigger nanoparticles further aggregate to form sphere-like superstructures and these superstructures prefer to cluster in groups. After further prolonged reaction time, 3D well-rounded cauliflower-like hierarchical microstructures are formed.
 | ||
| Fig. 5 Schematic illustration of possible growth process of the 3D cauliflower-like NiCo2S4 architectures. | ||
Electrochemical property of the NiCo2S4 materials
To explore the performance of the NiCo2S4 in supercapacitors, the electrochemical property is tested by the CV method. Through electrochemical measurement, we optimized the experimental conditions, such as reaction time, reaction power, and with citric acid or not (Fig. S2–S4, ESI†). The NiCo2S4 materials prepared at the optimal experimental conditions displays prominent electrochemical performance. Fig. 6a presents typical CV curves of the NiCo2S4 electrode with various scan rates (2, 5, 10, 20 and 30 mV s−1) in the voltage range from −0.1 to 0.6 V. Apparently, the distinct redox peaks in each CV curve reveal that the pseudo-capacitive characteristics of the NiCo2S4 samples. The redox peaks may mostly result from the faradaic redox reactions related to Co2+/Co3+/Co4+ and Ni2+/Ni3+ redox couples. The faradaic reactions are proposed as follows:18,19| CoS + OH− ↔ CoSOH + e− |
| CoSOH + OH− ↔ CoSO + H2O + e− |
| NiS + OH− ↔ NiSOH + e− |
Because the redox potentials of Co2+/Co3+ and Ni2+/Ni3+ are near, the two redox peaks overlap together.26 The first pair of redox peaks might be assigned to the redox reaction among from NiCo2S4 to NiSOH and CoSOH. Another pair probably come from the conversion between CoSOH and CoSO.29 Moreover, the acetylene black added in the electrode can broad some redox peaks. Then the three pairs of redox peaks are not obvious.26 The symmetrical CV curves indicate the good kinetic reversibility of NiCo2S4. As the scan rates increased, the current densities of the CV curves gradually increase and the oxidation and reduction peaks shift to more positive and negative potential, respectively. This may be attributed to the diffusion of OH− ions. The OH− ions diffuse more slowly at low scan rates, then the active materials can react fully with OH− ions and have a higher utilization ratio.18 As shown in the inset of Fig. 6a, good linear dependence of the current densities of anodic and cathodic peaks on the square root of the scan rates is shown, suggesting that the diffusion of OH− is indeed the rate controlling process.30
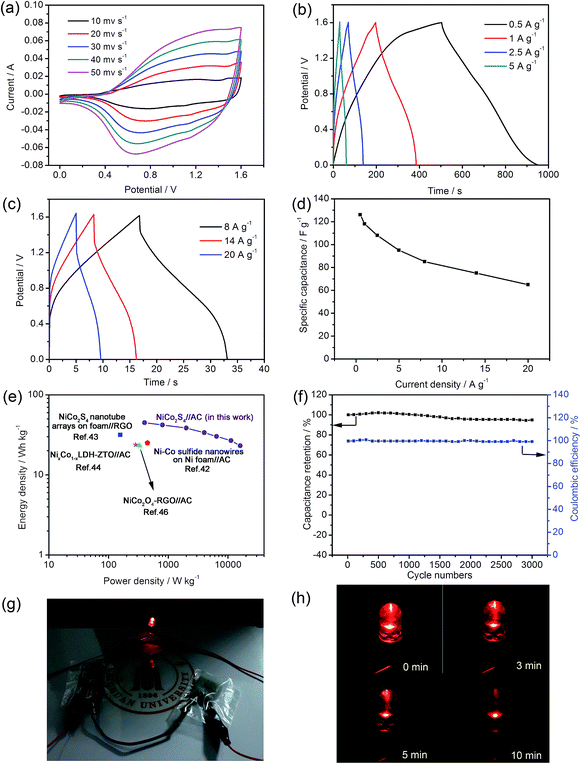
Further evaluate the capacitive properties of NiCo2S4 sample, the charge–discharge measurements were performed at a voltage range from 0 to 0.5 V with various current densities (vs. Hg/HgO). As depicted in Fig. 6b and c, the shapes of galvanostatic charge–discharge curves show a representative pseudo-capacitive behaviour coming from the faradaic redox reactions, which is consistent with the CV results.25 From the Fig. 6b and c, no obvious iR drops are observed at low current densities, indicating the good conductivity of the NiCo2S4.17 The specific capacitances are calculated from the galvanostatic charge–discharge curves employing the following equation:31
The stability of the NiCo2S4 sample is also important for supercapacitors. Consecutive galvanostatic charge–discharge was investigated at 10 A g−1 between 0 and 0.5 V for 1000 repetitive cycles, the corresponding result is shown in Fig. 7a. For the NiCo2S4 electrode, the specific capacitance hardly changes in the first 500 cycles. As the cycle numbers continue to increasing, the specific capacitance gradually decreases. After 1000 cycles, the specific capacitance can still retain 94.9% of the initial value, indicating its remarkable cycling stability. What's more, during the entire cycling test, the coulombic efficiency (η) almost remains unchanged at ∼100%, suggesting the redox process within the NiCo2S4 materials is highly reversible. These results reveal that the as-obtained 3D cauliflower-like NiCo2S4 have excellent performance in pseudocapacitors.
Further understand electrochemical properties of the NiCo2S4-modified electrode, the EIS measurements were carried out before and after the cycle tests and the corresponding Nyquist plots are displayed in Fig. 7b. The EIS spectra are composed of a sloped line at the low-frequency and one small arc at the high-frequency. The enlarge view of the high-frequency and the equivalent circuit according to the EIS are shown in the insets of Fig. 7b, where Rs composed of the inherent resistance of electrode materials, electrolyte resistance, and contact resistance at the interface between materials and current collector,40 is a bulk solution resistance. Rct is the charge-transfer resistance, CPE and CF respectively represent a double-layer capacitor, a faradaic pseudocapacitance. The Warburg impedance is attributed to the diffusive resistance of the electrolyte ion within the NiCo2S4 modified electrodes.40 Rs and Rct are characterized by the high-frequency intercept on the real axis at Rs and (Rs + Rct), respectively. Before and after the cycling tests, the resistances of the bulk solution (Rs) are 0.491 and 0.518 Ω, respectively. And the values of Rct are 0.290 and 0.301 Ω, respectively. The results are in good accordance with the slight decrease of the specific capacitance after the cycle test and reveal that the 3D cauliflower-like NiCo2S4 architectures have excellent electrochemical performance.
Electrochemical performance of the NiCo2S4//AC asymmetric capacitors
To further investigate the practical application of the NiCo2S4 material, an asymmetric capacitor was fabricated employing the NiCo2S4-modified electrode as the positive electrode and the activated carbon-modified electrode (the electrochemical performance details in Fig. S8, ESI†) as the negative electrode. The typical weight ratio between the positive and negative is calculated based on the following equation:41where ΔE+ and ΔE− are the potential range for positive and negative electrodes, respectively. C+ and C− are the specific capacitance measured in the same condition for positive and negative electrodes, respectively. m+ and m− represent the weight of electroactive materials for positive and negative electrodes, respectively. Based on the equation above, the weight ratio between AC and NiCo2S4 is calculated to be 3.03. Fig. 8a presents the CV curves of the NiCo2S4//AC asymmetric capacitor with various scan rates. The voltage window of the asymmetric supercapacitor can be extend up to 1.6 V, according to the electrochemical performance of NiCo2S4 and AC electrodes measured in a three-electrode system (Fig. S9, ESI†). These CV curves show electric double layer capacitive and pseudo-capacitive properties between AC and NiCo2S4, respectively. Fig. 8b and c show the galvanostatic charge–discharge curves of the asymmetric capacitor with different current densities and the results are shown in Fig. 8d. Obviously, the asymmetric supercapacitor presents the maximum capacitance value of 126 F g−1 at 0.5 A g−1. Moreover, the specific capacitance at 20 A g−1 is still around 52% of that at 0.5 A g−1, indicating remarkable rate capability.
Based on the galvanostatic charge–discharge measurements, the energy density and power density of the asymmetric supercapacitor were estimated based on the following equations:40
where E, P, C, Δt, and ΔV are the energy density, power density, specific capacitance, discharge time, and potential change during discharge process, respectively. Fig. 8e presents the Ragone plot of asymmetric supercapacitor. Remarkably, the asymmetric capacitor presents the maximum energy density of 44.8 W h kg−1 at 0.5 A g−1, and the corresponding power density is 401 W kg−1. After 40-time increase in the current density, the power density can reach 16.0 kW kg−1, while the energy density can still retain 23.1 W h kg−1, which displays their superiority as a potential candidate for supercapacitor. The large capacitance value and large potential window make great contributions to the high energy density. Obviously, our NiCo2S4//AC supercapacitor presents remarkable performance compared to other Ni–Co compounds assembled asymmetric capacitors, such as Ni–Co sulfide nanowires on Ni foam//AC (25.0 W h kg−1),42 NiCo2S4 nanotube arrays on Ni foam//RGO (31.5 W h kg−1),43 NixCo1−xLDH–ZTO heterostructure//AC (23.7 W h kg−1),44 porous CQDs/NiCo2O4 composite//AC (27.8 W h kg−1),45 and NiCo2O4–RGO composite//AC (23.3 W h kg−1).46
The cycle performance and coulombic efficiency of the NiCo2S4//AC-based asymmetric capacitor were carried out at 6 A g−1 for 3000 cycles. As depicted in Fig. 8f, the asymmetric supercapacitor shows remarkable cycle stability with only 5% decrease of the initial value after 3000 cycles. The prominent cycling characteristics of the asymmetric capacitor might be ascribed to the incorporation of activated carbon (AC). In the three-electrode system, both the NiCo2S4-modified electrode and AC-modified electrode exhibit prominent cycling characteristics (Fig. 7a and Fig. S8d†). So in the two-electrode system, the synergy of the two electrodes makes the NiCo2S4//AC-based asymmetric capacitor show better cycle stability, and the similar phenomenon was also performed in previous reports.47,48 Furthermore, the asymmetric supercapacitor exhibits a high coulombic efficiency of ∼99%. These results demonstrate the outstanding electrochemical performance of the NiCo2S4//AC asymmetric supercapacitor. What's more, two assembled asymmetric capacitors can light up a 5 mm-diameter red (2.0 V, 20 mA) round light-emitting diode (LED) and last for more than 10 min (Fig. 8g and h). It clearly indicates that the assembled NiCo2S4//AC asymmetric capacitor has remarkable electrochemical characteristics and the NiCo2S4 materials have a great potential in practical application of supercapacitors.
Conclusions
In conclusion, 3D cauliflower-like NiCo2S4 architectures were successfully fabricated by a facile, rapid MWH method. The NiCo2S4 samples exhibit high specific capacitance (1471 F g−1 at 1 A g−1), as well as remarkable rate capability and cycling characteristics (94.9% retention after 1000 cycles). The as-fabricated asymmetric supercapacitor exhibits the highest energy density of 44.8 W h kg−1 and the highest power density of 16 kW kg−1. In addition, the asymmetric supercapacitor also presents prominent cycle performance with ∼95% retained after 3000 charging–discharging cycles. The prominent capacitance performance of the 3D cauliflower-like NiCo2S4 indicate that it is a potential material for supercapacitor.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (nos 21177090, 21275104) and the China Postdoctoral Science Foundation (2013M531962).Notes and references
- N. S. Lewis and D. G. Nocera, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 15729–15735 CrossRef CAS PubMed.
- C. Liu, F. Li, L. P. Ma and H. M. Cheng, Adv. Mater., 2010, 22, E28–E62 CrossRef CAS PubMed.
- H. Jiang, T. Zhao, J. Ma, C. Y. Yan and C. Z. Li, Chem. Commun., 2011, 47, 1264–1266 RSC.
- H. Jiang, J. Ma and C. Z. Li, Adv. Mater., 2012, 24, 4197–4202 CrossRef CAS.
- L. Li, Y. Q. Zhang, F. Shi, Y. J. Zhang, J. H. Zhang, C. D. Gu, X. L. Wang and J. P. Tu, ACS Appl. Mater. Interfaces, 2014, 6, 18040–18047 CAS.
- W. Wang, S. R. Guo, M. Penchev, I. Ruiz, K. N. Bozhilov, D. Yan, M. Ozkan and C. S. Ozkan, Nano Energy, 2013, 2, 294–303 CrossRef CAS PubMed.
- G. A. Snook, P. Kao and A. S. Best, J. Power Sources, 2011, 196, 1–12 CrossRef CAS PubMed.
- S. K. Meher, P. Justin and G. R. Rao, Nanoscale, 2011, 3, 683–692 RSC.
- X. Wang, C. Y. Yan, A. Sumboja and P. S. Lee, Nano Energy, 2014, 3, 119–126 CrossRef CAS PubMed.
- Y. F. Zhang, M. Z. Ma, J. Yang, H. Q. Su, W. Huang and X. C. Dong, Nanoscale, 2014, 6, 4303–4308 RSC.
- H. L. Wang, Q. M. Gao and L. Jiang, Small, 2011, 7, 2454–2459 CAS.
- J. W. Xiao, L. Wan, S. H. Yang, F. Xiao and S. Wang, Nano Lett., 2014, 14, 831–838 CrossRef CAS PubMed.
- S. J. Peng, L. L. Li, C. C. Li, H. T. Tan, R. Cai, H. Yu, S. Mhaisalkar, M. Srinivasan, S. Ramakrishna and Q. Y. Yan, Chem. Commun., 2013, 49, 10178–10180 RSC.
- Z. Y. Zhang, X. G. Wang, G. L. Cui, A. H. Zhang, X. H. Zhou, H. X. Xu and L. Gu, Nanoscale, 2014, 6, 3540–3544 RSC.
- Y. Liu, J. N. Zhang, S. P. Wang, K. X. Wang, Z. M. Chen and Q. Xu, New J. Chem., 2014, 38, 4045–4048 RSC.
- J. F. Li, S. L. Xiong, Y. R. Liu, Z. C. Ju and Y. T. Qian, ACS Appl. Mater. Interfaces, 2013, 5, 981–988 CAS.
- H. C. Chen, J. J. Jiang, L. Zhang, H. Z. Wan, T. Qi and D. D. Xia, Nanoscale, 2013, 5, 8879–8883 RSC.
- J. Pu, T. T. Wang, H. Y. Wang, Y. Tong, C. C. Lu, W. Kong and Z. H. Wang, ChemPlusChem, 2014, 79, 577–583 CrossRef CAS.
- H. Z. Wan, J. J. Jiang, J. W. Yu, K. Xu, L. Miao, L. Zhang, H. C. Chen and Y. J. Ruan, CrystEngComm, 2013, 15, 7649–7651 RSC.
- W. Chen, C. Xia and H. N. Alshareef, ACS Nano, 2014, 8, 9531–9541 CrossRef CAS PubMed.
- Z. H. Ai, K. J. Deng, Q. F. Wan, L. Z. Zhang and S. C. Lee, J. Phys. Chem. C, 2010, 114, 6237–6242 CAS.
- S. K. Meher, P. Justin and G. Ranga Rao, ACS Appl. Mater. Interfaces, 2011, 3, 2063–2073 CAS.
- J. W. Xiao, X. W. Zeng, W. Chen, F. Xiao and S. Wang, Chem. Commun., 2013, 49, 11734–11736 RSC.
- C. Z. Yuan, J. Y. Li, L. Hou, L. R. Yang, L. F. Shen and X. G. Zhang, J. Mater. Chem., 2012, 22, 16084–16090 RSC.
- J. F. Marco, J. R. Gancedo, M. Gracia, J. L. Gautier, E. I. Ríos, H. M. Palmer, C. Greaves and F. J. Berry, J. Mater. Chem., 2001, 11, 3087–3093 RSC.
- Y. Lei, J. Li, Y. Y. Wang, L. Gu, Y. F. Chang, H. Y. Yuan and D. Xiao, ACS Appl. Mater. Interfaces, 2014, 6, 1773–1780 CAS.
- F. L. Luo, J. Li, H. Y. Yuan and D. Xiao, Electrochim. Acta, 2014, 123, 183–189 CrossRef CAS PubMed.
- L. S. Zhang, W. Z. Wang, Z. G. Chen, L. Zhou, H. L. Xu and W. Zhu, J. Mater. Chem., 2007, 17, 2526–2532 RSC.
- L. F. Shen, J. Wang, G. Y. Xu, H. S. Li, H. Dou and X. G. Zhang, Adv. Energy Mater., 2014, 5, 1400977 Search PubMed.
- J. Li, F. L. Luo, Q. Zhao, Z. P. Li, H. Y. Yuan and D. Xiao, J. Mater. Chem. A, 2014, 2, 4690–4697 CAS.
- D. P. Dubal, V. J. Fulari and C. D. Lokhande, Microporous Mesoporous Mater., 2012, 151, 511–516 CrossRef CAS PubMed.
- W. M. Du, Z. Q. Zhu, Y. B. Wang, J. N. Liu, W. J. Yang, X. F. Qian and H. Pang, RSC Adv., 2014, 4, 6998–7002 RSC.
- L. Yu, H. B. Wu, T. Wu and C. Z. Yuan, RSC Adv., 2013, 3, 23709–23714 RSC.
- T. Zhu, Z. Y. Wang, S. J. Ding, J. S. Chen and X. W. D. Lou, RSC Adv., 2011, 1, 397–400 RSC.
- L. Zhang, H. B. Wu and X. W. Lou, Chem. Commun., 2012, 48, 6912–6914 RSC.
- H. Jiang, J. Ma and C. Z. Li, Chem. Commun., 2012, 48, 4465–4467 RSC.
- G. X. Hu, C. H. Tang, C. X. Li, H. M. Li, Y. Wang and H. Gong, J. Electrochem. Soc., 2011, 158, A695–A699 CrossRef CAS PubMed.
- H. C. Chen, J. J. Jiang, Y. D. Zhao, L. Zhang, D. Q. Guo and D. D. Xia, J. Mater. Chem. A, 2015, 3, 428–437 CAS.
- D. W. Wang, F. Li, M. Liu, G. Q. Lu and H. M. Cheng, Angew. Chem., 2008, 120, 379–382 CrossRef.
- Y. Y. Wang, Y. Lei, J. Li, L. Gu, H. Y. Yuan and D. Xiao, ACS Appl. Mater. Interfaces, 2014, 6, 6739–6747 CAS.
- V. Khomenko, E. Raymundo-Pinero and F. Béguin, J. Power Sources, 2006, 153, 183–190 CrossRef CAS PubMed.
- Y. H. Li, L. J. Cao, L. Qiao, M. Zhou, Y. Yang, P. Xiao and Y. H. Zhang, J. Mater. Chem. A, 2014, 2, 6540–6548 CAS.
- H. C. Chen, J. J. Jiang, L. Zhang, D. D. Xia, Y. D. Zhao, D. Q. Guo, T. Qi and H. Z. Wan, J. Power Sources, 2014, 254, 249–257 CrossRef CAS PubMed.
- X. Wang, A. Sumboja, M. F. Lin, J. Yan and P. S. Lee, Nanoscale, 2012, 4, 7266–7272 RSC.
- Y. R. Zhu, Z. B. Wu, M. J. Jing, H. S. Hou, Y. C. Yang, Y. Zhang, X. M. Yang, W. X. Song, X. N. Jia and X. B. Ji, J. Mater. Chem. A, 2015, 3, 866–877 CAS.
- X. Wang, W. S. Liu, X. H. Lu and P. S. Lee, J. Mater. Chem., 2012, 22, 23114–23119 RSC.
- M. J. Jing, Y. C. Yang, Y. R. Zhu, H. S. Hou, Z. B. Wu and X. B. Ji, Electrochim. Acta, 2014, 141, 234–240 CrossRef CAS PubMed.
- J. Yan, Z. J. Fan, W. Sun, G. Q. Ning, T. Wei, Q. Zhang, R. F. Zhang, L. J. Zhi and F. Wei, Adv. Funct. Mater., 2012, 22, 2632–2641 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Preparation of Activated Carbon (AC), the relative content of Co2+ and Co3+ as well as Ni2+ and Ni3+ in NiCo2S4, XRD patterns of the samples collected at different time, the electrochemical performance of the samples collected at different time, different reaction power and with citric acid or not, cycling ability of NiCo2S4, BET of NiCo2S4, BET of AC, the electrochemical performance of AC, the CV of NiCo2S4 and AC, a table including specific capacitance and capacitance retention of electroactive materials. See DOI: 10.1039/c5ra00665a |
| This journal is © The Royal Society of Chemistry 2015 |