Adsorption of phosphate onto amine functionalized nano-sized magnetic polymer adsorbents: mechanism and magnetic effects†
Haoyu Shen*a,
Zhejun Wanga,
Ameng Zhoua,
Junliang Chena,
Meiqin Hua,
Xinyan Donga and
Qinghua Xiab
aNingbo Institute of Technology, Zhejiang University, Ningbo, 315100, China. E-mail: hyshen@nit.zju.edu.cn; Tel: +86-574-88130130
bMinistry-of-Education Key Laboratory for the Synthesis and Applications of Organic Functional Molecules, Hubei Collaborative Innovation Center for Advanced Organochemical Materials, Hubei University, Wuhan 430062, China
First published on 19th February 2015
Abstract
A series of tetraethylenepentamine-functionalized core–shell structured nano magnetic Fe3O4 polymers (TEPA-Fe3O4-NMPs) with different amounts of the magnetic core were synthesized and characterized by XRD, EA, VSM, FTIR and XPS. Their applications as adsorbents for phosphate removal from aqueous solutions were studied. The adsorption mechanism and magnetic effects were intensively investigated. The adsorption processes of phosphate by TEPA-Fe3O4-NMPs were found to be highly pH dependent and related to the content of Fe3O4 magnetic core in the adsorbents. The optimized pH value was found to be 3.0 for TEPA-Fe3O4-NMPs, while that of TEPA-Fe3O4-NMPs-0, which was without the Fe3O4 magnetic core, was found to be 2.5. Kinetic studies showed that the adsorption of phosphate by TEPA-Fe3O4-NMPs followed a pseudo-second-order model, with the adsorption rate constant, k2, between 0.00274–0.0241 g mg−1 min−1, suggesting a chemisorption process. Activation energies (Ea) for phosphate removal varied with the content of Fe3O4 magnetic core, and were found to be 38.9–16.5 kJ mol−1, indicating that the diffusion process might be the rate-controlling step. Thermodynamic studies suggested that the adsorption processes fit the Langmuir isotherm well with the optimized maximum adsorption capacities of phosphate onto TEPA-Fe3O4-NMPs obtained when the content of Fe3O4 in TEPA-Fe3O4-NMPs was 14.55%. The Langmuir constants of apparent heat change, KL, were found to be 0.0142–0.0461 L mg−1, and varied with the content of Fe3O4 magnetic core as well. FTIR and XPS analytical results of the adsorbents before and after phosphate adsorption suggested that phosphate had been successfully adsorbed onto TEPA-Fe3O4-NMPs via electrostatic attraction. The existence of the magnetic core might be favorable for mass transfer to accelerate the adsorption process.
1. Introduction
Recently, nano magnetic polymers (NMPs) have been intensively studied, not only for scientific interest, but also for technological applications, such as magnetic fluids,1 catalysis,2 biotechnology/biomedicine,3,4 contrast agents in magnetic resonance imaging (MRI),5,6 data storage,7 and environmental remediation.8–16However, bare magnetite nanoparticles are quite susceptible to air oxidation and are easily aggregated in aqueous systems. Although there have been many significant developments in the synthesis of magnetic nanoparticles, maintaining the stability of these particles for a long time without agglomeration or precipitation is an important issue. Recent research indicated that “core–shell” structured nano-magnetic polymers (NMPs) might be potential candidates as novel adsorbents. Functional polymers coating on the magnetic core enhanced the stability of nanodispersions by preventing their aggregation, moreover, the absorption properties can be tailored by suitable functional groups.10–15
It is well known that trace amounts of phosphorus in water can lead to eutrophication problems. The phosphate concentration is regulated, and the maximum permissible is limited to 10 μg L−1 to escape eutrophication problems.16,17 With more and more strictly environmental regulations on the discharge of phosphorus and rising demands for clean water with extremely low levels of phosphorus, functionalized-magnetic nanoparticles, with enhanced adsorption rate, capacity, and selectivity, have gradually attracted researchers' great interest to be used as adsorbents for phosphorus removal.18,19 However, most researches about the magnetic core of NMPs were only focused on its intrinsic magnetic properties for facile solid–liquid separation under an applied magnetic field. Little attention was paid to the magnetic effects on the adsorption mechanism. Some results showed that magnetic composite materials presented higher adsorption capacities than a simple sum of the capacities of the non-interacting components, while some other results showed that there was no such a relationship.13,14,20–22
In the present work, TEPA-Fe3O4-NMPs with different Fe3O4 content were prepared to investigate their adsorption efficiencies towards phosphate. Batch adsorption tests were conducted to study the adsorption mechanism. The magnetic effect for phosphate removal was carried out by comparing the adsorption properties of the adsorbents with different magnetic Fe3O4 content. The results of kinetic and thermodynamic studies as well as XPS characterization suggested the Fe3O4 magnetic core in the adsorbents played a very important role during the adsorption process, not only on the arrangement of the amino groups on the surface of the adsorbents, but also on the species distribution of the phosphate in solution under different pH values.
2. Experimental
2.1 Chemicals
Glycidylmethacrylate (GMA), divinylbenzene (DVB), styrene (St) were sigma-aldrich products. Ferric chloride (FeCl3·6H2O), ferrous sulphate (FeSO4·7H2O), KH2PO4 was purchased from Sinopharm Chemical Reagent Co., Ltd (China). All the reagents were analytical grade. Distilled water was used to prepare all the solutions. 0.5 mol L−1 HNO3 and 0.5 mol L−1 NaOH solutions were used for pH adjustment.2.2 Adsorbents preparation
TEPA-Fe3O4-NMPs were synthesized according to our previous work13 after a minor modification. Briefly, Fe3O4 particles were firstly encapsulated by oleic acids to obtain OA-M (oleic acids encapsulated magnetic Fe3O4); 2.0 g of polyglycol was dissolved into 200 mL hot water, followed by adding 0.5 mL DVB (0.0035 mol), 4 mL St (0.04 mol), and 8 mL GMA (0.052 mol). Then 1.0 g of OA-M was dispersed to the above system under ultrasonication. Finally, 1.0 g of benzoyl peroxide (BPO) dissolved in 20 mL ethanol was added dropwisely under vigorously stirring. The mixture was continuously reacted at 80 °C for 3 h, yielding epoxyl-Fe3O4-co-poly(DVB-St-GMA)s, named as eO-Fe3O4-NMPs. The final adsorbents (TEPA-Fe3O4-NMPs) were obtained by ring-opening reaction with TEPA. They were named as TEPA-Fe3O4-NMPs-0, TEPA-Fe3O4-NMPs-0.5, TEPA-Fe3O4-NMPs-1, TEPA-Fe3O4-NMPs-1.5, and TEPA-Fe3O4-NMPs-2, with the usage amount of Fe3O4 at 0, 0.5, 1, 1.5 and 2 g for preparation respectively. Overall preparation procedure was given in Fig. S1 (ESI†).2.3 Characterization of TEPA-Fe3O4-NMPs
The structure and composition of the TEPA-Fe3O4-NMPs were investigated by a vibrating sample magnetometer (VSM, Lake Shore 7410), elementary analysis (EA, Thermo Fisher Flash-1112), X-ray diffractometer analysis (XRD, Bruker D8 Advance), X-ray photoelectron spectroscopy (XPS, AXIS ULTRADLD) and FTIR spectrometer (Thermo Nicolet NEXUS-470). Scanning electron microscopy (SEM) was performed using scanning electron microscopy (SEM, JSM-6700F) at an accelerating voltage of 5.0 kV. Sample dispersed at an appropriate concentration in ethanol was cast onto a silicon sheet at room temperature and sputter-coated with gold. Transmission electron microscopy (TEM) images were obtained on a Hitachi H-7650 transmission electron microscopy (TEM) (Hitachi, Japan) at an accelerating voltage of 75 kV.2.4 Batch adsorption tests and analyses
The adsorption experiments were carried out in 100 mL stoppered flasks, each of which contained 40 mL of phosphate solution prepared with KH2PO4. A 0.02 g amount of adsorbents was added to each flask and shaken at 180 rpm in a thermostatic shaker. The concentration of phosphate was analyzed spectrophotometrically by the molybdenum blue method of National Standard of China (GB/T 6913-2008) at a wavelength of 690 nm, after a minor modification.23 The amount of phosphate adsorbed per unit mass of adsorbents was evaluated as eqn (1):13
 | (1) |
To investigate the effect of pH, 40 mL of 100 mg L−1 phosphate with pH ranging from 1.5 to 7.0 were mixed with 0.02 g of magnetic adsorbents for 3 h at 308 K, respectively. In the kinetic experiments, the TEPA-Fe3O4-NMPs were also investigated with contacting time ranging from 1–180 min at pH 3.0. The pseudo-first-order model (eqn (2)) and pseudo-second-order model (eqn (3))24,25 were used to fit the experimental data.
 | (2) |
 | (3) |
The adsorption isotherm studies were investigated with phosphate initial concentrations ranging from 50 to 700 mg L−1, under pH of 3.0 and at 308 K for 3 h. Two adsorption isotherms, Langmuir model (eqn (4))26 and Freundlich model (eqn (5)),27 were applied to analyze the adsorption data.
 | (4) |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe = log qe = log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KF + (1/n)log KF + (1/n)log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce Ce
| (5) |
3. Results and discussion
3.1 Characterization of TEPA-Fe3O4-NMPs
The microspheres of TEPA-Fe3O4-NMPs with the usage amount of Fe3O4 were similar. The SEM and TEM images of TEPA-Fe3O4-NMPs-1 were shown in Fig. S2(a) and (b),† respectively. It revealed that the TEPA-Fe3O4-NMPs particles were multidispersed with an average diameter of around 300 nm.| Adsorbents | Usage amount of Fe3O4 (g) | Content of Fe3O4 (%) | Content of N (mmol g−1) | Saturation moments (emu g−1) |
|---|---|---|---|---|
| Bare Fe3O4 | — | 97.53 | — | 73.98 |
| TEPA-Fe3O4-NMPs-0 | 0 | 0 | 5.00 | — |
| TEPA-Fe3O4-NMPs-0.5 | 0.5 | 13.49 | 4.43 | 9.77 |
| TEPA-Fe3O4-NMPs-1 | 1 | 14.55 | 4.28 | 10.59 |
| TEPA-Fe3O4-NMPs-1.5 | 1.5 | 16.64 | 3.66 | 12.39 |
| TEPA-Fe3O4-NMPs-2 | 2 | 19.24 | 3.55 | 12.78 |
The elementary analysis results of the nitrogen contents in TEPA-Fe3O4-NMPs were listed in Table 1 as well. It showed that the nitrogen contents decreased from 5.00 mmol g−1 for TEPA-Fe3O4-NMPs-0 to 3.55 mmol g−1 for TEPA-Fe3O4-NMPs-2.
The vibrating sample magnetometer (VSM) was applied to test the magnetic properties of TEPA-Fe3O4-NMPs as shown in Fig. 2. The saturation moments of the four TEPA-Fe3O4-NMPs were found to be 9.77, 10.59, 12.39, 12.78 emu g−1 (listed in Table 1), respectively. Since the polymer shell was diamagnetism, it was reasonable that the ratio of paramagnetic composition reduced, which led to lower saturation moments than that of the bare Fe3O4, (73.98 emu g−1, Fig. 2(e), insert). The magnetism of the TEPA-Fe3O4-NMPs enhanced with the content of Fe3O4 increased.
 | ||
| Fig. 3 XPS spectra of: (a) survey scan, and high-resolution scan of: (b) P2p; (c) N1s; (d) O1s; (e) C1s; (f) Fe2p. | ||
| Valence state | Position BE (eV) | TEPA-Fe3O4-NMPs-0 | TEPA-Fe3O4-NMPs-0.5 | TEPA-Fe3O4-NMPs-1.0 | TEPA-Fe3O4-NMPs-1.5 | TEPA-Fe3O4-NMPs-2.0 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Atomic concentration (%) | Atomic concentration (%) | Atomic concentration (%) | Atomic concentration (%) | Atomic concentration (%) | |||||||
| Before | After | Before | After | Before | After | Before | After | Before | After | ||
| Fe2p | 709 | 0 | 0 | 0.4 | 0.42 | 0.44 | 0.48 | 0.5 | 0.53 | 0.54 | 0.58 |
| O1s | 530.5 | 17.45 | 21.02 | 14.53 | 21.46 | 15.45 | 20.59 | 18.58 | 25.32 | 18.05 | 26.03 |
| N1s | 397.85 | 4.54 | 4.42 | 4.92 | 4.73 | 5.88 | 5.76 | 7.88 | 7.16 | 8.53 | 8.23 |
| C1s | 284.1 | 78.01 | 73.98 | 80.15 | 72.63 | 78.23 | 72.05 | 73.04 | 65.46 | 72.88 | 63.4 |
| P2p | 131.25 | 0 | 0.58 | 0 | 0.76 | 0 | 1.12 | 0 | 1.53 | 0 | 1.76 |
3.2 Adsorption properties
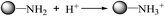 | (6) |
 | (7) |
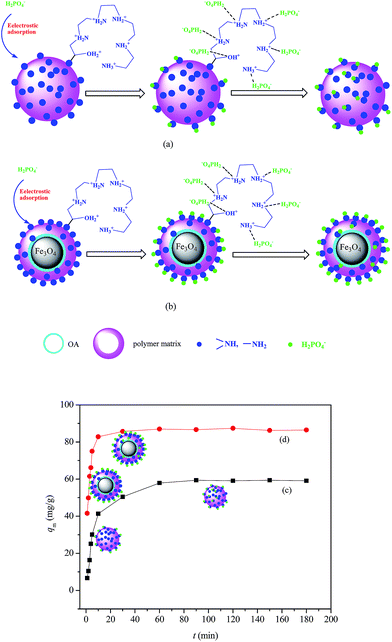
Interestingly, the optimized pH value was found at 3.0 for TEPA-Fe3O4-NMPs, while that of TEPA-Fe3O4-NMPs-0, which without Fe3O4 magnetic core, was at 2.5 (Fig. 4). Based on the experimental data of the total concentration of phosphate, we run Visual MINTEQ 3, which is widely used in recent years to simulate equilibria and speciation of inorganic solutes in aqueous solution.33,34 The speciation of phosphate under various pH was obtained, as shown in Fig. S3,† the highest fraction of H2PO4− occurred at pH = 2.5 for TEPA-Fe3O4-NMPs-0, while at pH = 3.0 for TEPA-Fe3O4-NMPs-0.5, TEPA-Fe3O4-NMPs-1.0, TEPA-Fe3O4-NMPs-1.5, and TEPA-Fe3O4-NMPs-2.0, which were consistent with the experimental results. The results inferred that the existing of magnetic core might have some effect on species distribution in solution. This could be further explained by the results of the zeta potential of the TEPA-Fe3O4-NMPs. As shown in Fig. S4,† The pH of zero point of charge (pHzpc) was observed at pH 2.48, 2.98, 3.02, 2.99, 3.02, 3.04, for TEPA-Fe3O4-NMPs-0, TEPA-Fe3O4-NMPs-0.5, TEPA-Fe3O4-NMPs-1.0, TEPA-Fe3O4-NMPs-1.5 and TEPA-Fe3O4-NMPs-2.0, respectively. Positive zeta potential was found to be at pH of 2.48 for TEPA-Fe3O4-NMPs-0, while 2.98–3.05 for other TEPA-Fe3O4-NMPs, indicating that the particles were positive charged. Thus, electrostatic interaction would be favorable for anionic ions (H2PO4−) adsorption. Negative zeta potential of TEPA-Fe3O4-NMPs was found at pH above the pHZPC. The repulsion between the negatively charged surface of the adsorbent and the anions, i.e., H2PO4−, H2PO42−, PO43−, etc., resulting in low adsorption capacities. Although the saturation moments TEPA-Fe3O4-NMPs are quite small (9.77–12.78 emu g−1), their magnetic effects on the species distribution of the phosphate in solution under different pH values are not negligible.
Pseudo-first-order and pseudo-second-order models were used to describe the adsorption kinetic data. The correlation coefficient values indicated a better fit of the pseudo-second-order model with the experimental data compared to the pseudo-first-order for all the five adsorbents (Table 3). The calculated qe values were in agreement with the theoretical ones, and the plots showed good linearity with R2 above 0.99. Therefore, the adsorption behaviors followed the pseudo-second-order model, suggesting a chemisorption process.25
| Adsorbents | qe,exp (mg g−1) | Pseudo-first-order model | Pseudo-second-order model | ||||
|---|---|---|---|---|---|---|---|
| k1 (min−1) | qe,cal (mg g−1) | R2 | k2 (g mg−1 min−1) | qe,cal (mg g−1) | R2 | ||
| TEPA-Fe3O4-NMPs-0 | 59.09 | 0.1202 | 45.51 | 0.9691 | 0.00247 | 61.34 | 0.9991 |
| TEPA-Fe3O4-NMPs-0.5 | 68.17 | 0.6771 | 62.66 | 0.8297 | 0.00795 | 68.49 | 0.9996 |
| TEPA-Fe3O4-NMPs-1 | 86.46 | 0.6241 | 58.35 | 0.9716 | 0.0132 | 86.96 | 0.9999 |
| TEPA-Fe3O4-NMPs-1.5 | 78.06 | 0.9382 | 65.71 | 0.99 | 0.0221 | 78.74 | 0.9999 |
| TEPA-Fe3O4-NMPs-2 | 81.26 | 2.0986 | 174.32 | 0.9021 | 0.0241 | 81.97 | 0.9999 |
Adsorption kinetic at different temperatures (25–45 °C) was carried out. The Arrhenius equation was applied to investigate the Ea for the adsorption process.
| k = Ae−Ea/RT | (8) |
From the linear relationships between ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k and 1/T (Fig. 6(c)), for the process of adsorbing phosphate onto the TEPA-Fe3O4-NMPs, Ea was found to be 38.9, 25.2, 22.1, 18.9, 16.5 kJ mol−1 for TEPA-Fe3O4-NMPs-0, TEPA-Fe3O4-NMPs-0.5, TEPA-Fe3O4-NMPs-1, TEPA-Fe3O4-NMPs-1.5, and TEPA-Fe3O4-NMPs-2, respectively (all were less than 42 kJ mol−1), indicating that diffusion process was the rate-controlled step.35
k and 1/T (Fig. 6(c)), for the process of adsorbing phosphate onto the TEPA-Fe3O4-NMPs, Ea was found to be 38.9, 25.2, 22.1, 18.9, 16.5 kJ mol−1 for TEPA-Fe3O4-NMPs-0, TEPA-Fe3O4-NMPs-0.5, TEPA-Fe3O4-NMPs-1, TEPA-Fe3O4-NMPs-1.5, and TEPA-Fe3O4-NMPs-2, respectively (all were less than 42 kJ mol−1), indicating that diffusion process was the rate-controlled step.35
Interestingly, except TEPA-Fe3O4-NMP-0, a fine linear relationship between k2 and the saturation moments of the TEPA-Fe3O4-NMPs, was observed, shown in Fig. 6(d). The relationship between the activation energy changes (ΔEa = Ea,TEPA-Fe3O4-NMPs − Ea,TEPA-Fe3O4-NMPs-0) and the saturation moments was further observed, shown in Fig. 6d (insert), it was found that the ΔEa linearly decreased with the increasing of the saturation moments of the TEPA-Fe3O4-NMPs, was observed, shown in Fig. 6(d). This phenomenon inferred that the higher the saturation moments of the TEPA-Fe3O4-NMPs was, the faster the adsorption process achieved. The existing of the magnetic core might be favorable the mass transfer to accelerate the adsorption process. On the contrary, k2 decreased with the increase of nitrogen contents of the TEPA-Fe3O4-NMPs linearly, including TEPA-Fe3O4-NMP-0, shown in Fig. 6(e). Although the total functional groups of amine (–NH– and –NH2) increased as the nitrogen contents increased, some of the active sites might be immersed in the polymer matrix of the TEPA-Fe3O4-NMPs, some transfer barriers occurred and the equilibrium time increased accordingly, which was consistent with the findings of the none-magnetic amino-functional polymers.19
ΔGθ = −RT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KD KD
| (9) |
 | (10) |
According to thermodynamics, the Gibb's free energy change (ΔGθ) is also related to the enthalpy change (ΔHθ) and entropy change (ΔSθ) at constant temperature by eqn (11):
| ΔGθ = ΔHθ − TΔSθ | (11) |
 | (12) |
The values of enthalpy change (ΔHθ) and entropy change (ΔSθ) were calculated from the slope and intercept of the plot of ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KD vs. (1/T). The results of the five adsorbents were shown in Fig. 7. These thermodynamic parameters were given in Table 4. As listed in Table 4, the enthalpy changes (ΔHθ) for the five adsorbents were found to be at 16.54–7.86 kJ mol−1, which indicated that the adsorption was endothermic. The entropy changes (ΔSθ) were in range of 56.78–27.60 J mol−1 K−1. The values of ΔGθ were all negative for the TEPA-Fe3O4-NMPs with different amount of magnetic nuclei, implying the spontaneous nature of the adsorption process.
KD vs. (1/T). The results of the five adsorbents were shown in Fig. 7. These thermodynamic parameters were given in Table 4. As listed in Table 4, the enthalpy changes (ΔHθ) for the five adsorbents were found to be at 16.54–7.86 kJ mol−1, which indicated that the adsorption was endothermic. The entropy changes (ΔSθ) were in range of 56.78–27.60 J mol−1 K−1. The values of ΔGθ were all negative for the TEPA-Fe3O4-NMPs with different amount of magnetic nuclei, implying the spontaneous nature of the adsorption process.
| Adsorbents | Initial concentration (mg L−1) | ΔH (kJ mol−1) | ΔS (J mol−1 K−1) | ΔG (kJ mol−1)/308 K |
|---|---|---|---|---|
| TEPA-Fe3O4-NMPs-0 | 50 | 13.55 | 47.28 | −1.02 |
| TEPA-Fe3O4-NMPs-0.5 | 50 | 11.33 | 45.54 | −2.70 |
| TEPA-Fe3O4-NMPs-1 | 50 | 7.26 | 42.19 | −5.74 |
| TEPA-Fe3O4-NMPs-1.5 | 50 | 6.38 | 40.63 | −6.13 |
| TEPA-Fe3O4-NMPs-2 | 50 | 5.15 | 33.71 | −5.23 |
| Adsorbents | Langmuir isotherm | Freundlich isotherm | ||||
|---|---|---|---|---|---|---|
| KL (L mg−1) | qm (mg g−1) | R2 | KF | 1/n | R2 | |
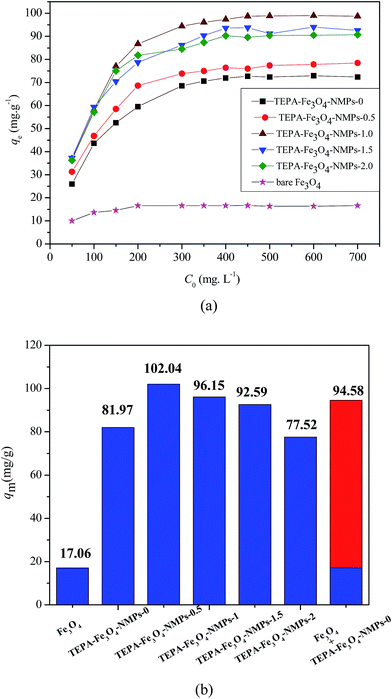
| TEPA-Fe3O4-NMPs-0 | 0.0304 | 77.52 | 0.9988 | 14.12 | 0.2775 | 0.9050 |
| TEPA-Fe3O4-NMPs-0.5 | 0.0409 | 81.97 | 0.9996 | 20.24 | 0.2284 | 0.9262 |
| TEPA-Fe3O4-NMPs-1 | 0.0523 | 102.04 | 0.9996 | 30.93 | 0.2011 | 0.9329 |
| TEPA-Fe3O4-NMPs-1.5 | 0.0654 | 96.15 | 0.9988 | 31.93 | 0.1825 | 0.9647 |
| TEPA-Fe3O4-NMPs-2 | 0.0747 | 92.59 | 0.9996 | 30.66 | 0.1866 | 0.9135 |
| Bare Fe3O4 | 0.0609 | 17.06 | 0.9987 | 6.598 | 0.8194 | 0.7710 |
As shown in Fig. 8(a) and Table 5, the usage amount of Fe3O4 could obviously affect the adsorption efficiency. The maximum adsorption capacities of phosphate onto the TEPA-Fe3O4-NMPs increased from 77.52 mg g−1 to 102.04 mg g−1 with an increase in the usage amount of Fe3O4 for TEPA-Fe3O4-NMPs preparation from 0 to 1.0 g, then decreased from 104.04 mg g−1 to 92.59 mg g−1 with increasing the usage amount of Fe3O4 from 1.0 to 2.0 g. Similarly, although TEPA-Fe3O4-NMPs-0 contained the largest amount of amine (–NH– and –NH2) groups (5.0 mmol g−1), its adsorption capacity (77.52 mg g−1) was the lowest among the present studied adsorbents. TEPA-Fe3O4-NMPs-2, with the largest amount of Fe3O4, had the second lowest adsorption capacity (92.59 mg g−1). These results indicated that the adsorption capacity would not be simply related to the amount of amine (–NH– and –NH2) groups on the adsorbents.19 The content of Fe3O4 in the adsorbents may also play an important role in the removal of phosphate. The adsorption capacity of phosphate may be an integrated result of both the amount of amine (–NH– and –NH2) groups and Fe3O4 content in the magnetic adsorbents since neither of them fully reflected the present finding. TEPA-Fe3O4-NMPs-1, with the highest adsorption capacity for phosphate removal, may have an appropriate ratio of amine (–NH– and –NH2) groups and Fe3O4 content. We further tested the adsorption capacity of bare Fe3O4 to phosphate, which was found to be 17.06 mg g−1. The results showed that the sum of adsorption capacities of bare Fe3O4 and TEPA-Fe3O4-NMPs-0 was found to be 94.58 mg g−1 (Fig. 8(b)). It clearly implied that the adsorption capacity of TEPA-Fe3O4-NMPs to phosphate was not a simple sum of the two isolated components (Fe3O4 core and polymeric layer). There might be some cooperative interactions between them. From our present investigation, the equilibrium constant KL was found to increase from 0.0304 to 0.0747 L mg−1 with the usage amount of Fe3O4 increasing from 0.5 to 2.0 g for synthesis, as shown in Table 5. The results implied that the presence of Fe3O4 in the magnetic adsorbents would be favorable for the achievement of maximum adsorption capacity. The more Fe3O4 contained in the adsorbents, the more significant the effect was.
3.3 Adsorption mechanism
To further investigate the mechanism of phosphate adsorbing onto TEPA-Fe3O4-NMPs, VSM, FTIR and XPS techniques were applied to distinguish the difference of before and after phosphate loading onto TEPA-Fe3O4-NMPs-1.The magnetic performance comparison of TEPA-Fe3O4-NMPs-1 before and after loading phosphate ions was carried out by VSM analysis (Fig. 9). As shown in Fig. 9, the saturation moment reduced from 10.59 emu g−1 to 9.58 emu g−1 after phosphate ions adsorption. Since phosphate ion was diamagnetism, it was reasonable that the ratio of paramagnetic composition reduced for the post-adsorbed magnetic adsorbent.
The IR spectra of TEPA-Fe3O4-NMPs-1 before (a) and after adsorption of phosphate (b) were showed in Fig. 10. In Fig. 10(a), the broad peak appeared at ∼3360 cm−1 and ∼1573 cm−1 can be assigned to be the stretching and bending vibrations of the –NH and –NH2 groups.36,37 While after adsorption, in Fig. 10(b), the characteristic bands at ∼1573 cm−1 disappeared along with the appearance of the bands at ∼1630 cm−1, which may be attributed to the interaction between amino groups and the phosphate groups, subsequently weakened the N–H bonding and resulted in a large shift (∼80 cm−1). The characteristic peaks of the phosphate groups at 543 cm−1 were also observed in Fig. 10(b), corresponding to the –P–O and –O–P–O groups, respectively.38 It indicated that the phosphate groups adsorbed successfully onto the TEPA-Fe3O4-NMPs-1.
XPS spectra of both survey and high-resolution scans for the key elements on TEPA-Fe3O4-NMPs-1 surfaces before and after adsorption (Fig. 3) were furtherly investigated to gain more insights on the interaction mechanism. From the survey spectra (Fig. 3(a)), and the high-resolution XPS spectra (Fig. 3(b)), the new peak, appeared at 131.8 eV, which can be assigned to P2p, clearly confirmed the successful adsorption of phosphate.39 As shown in Fig. 3(c), after adsorption, the peaks of N1s at 396.8 eV shifted to higher binding energies (∼398.8 eV) with a broader band range. The shift of the N1s peaks could be attributed to protonated amine groups (–NH3+) and the further formation of –NH3+⋯H2PO4−.40 The effects from electrostatic attraction would make the outer-shell electron density of N atoms reduced, which caused the nuclear charge on the inner electron shielding effect to be weakened. The inner electron binding energy of N1s increased, so the peaks of N1s in XPS spectra shifted to higher binding energies.13 Similar phenomena were observed in the XPS spectra of O1s (Fig. 3(d)), peaks of O1s appeared at ∼530 eV and ∼527.9 eV, assigned to C–O–C and C–OH groups, broadening with a slight shift of binding energies. In the XPS spectra of C1s (Fig. 3(e)), the carbon atoms can be found in two chemically different positions, leading to two differing C1s binding energies: C–O–C (∼282.9 eV) and C–O–C or C–OH (∼286.5 eV). After adsorption, the peaks at ∼282.9 eV shifted to higher binding energies (∼283.9 eV) with a broader band range, while the peaks at ∼286.5 eV disappeared, which may attribute to the involvement of the –OH groups in the adsorption phosphate.
Changes in atomic concentration of the key elements after the adsorption were summarized in Table 2. After adsorption of phosphate, the atomic concentration of the P element on their surfaces increased from 0.58% to 1.76%, with the usage amount of Fe3O4 increasing from 0.5 g to 2 g, which was consistent with that of nitrogen element. The presence of Fe3O4 in the magnetic adsorbents would be favorable to the achievement of maximum adsorption capacity with less equilibrium time.
Presumed adsorption mechanism was shown in Scheme 1. Under acidic conditions, the functional amino groups were first protonated. H2PO4− ions adsorbed onto the TEPA-Fe3O4-NMPs via electrostatic attraction as described above (eqn (6) and (7)). Since atomic concentration of nitrogen on the surfaces of TEPA-Fe3O4-NMPs increased from 4.53% to 8.53%, with the usage amount of Fe3O4 increasing from 0 g to 2 g, as found in XPS, the existing of the magnetic core might be favorable the arrangement of the amino groups on the surface of the TEPA-Fe3O4-NMPs, leading fewer amino groups (–NH– and –NH2) immersed in the polymer matrix, as shown in Scheme 1(a) and (b), thus comparing to TEPA-Fe3O4-NMPs-0, less time needed for other TEPA-Fe3O4-NMPs for phosphate migrating to active groups to reach equilibrium, shown in Scheme 1(c) and (d).
4. Conclusions
The removal of phosphate from aqueous solution by TEPA-Fe3O4-NMPs was studied. The effect of parameters, such as solution pH, contact time, solution temperature, Fe3O4 amount on the surface and in adsorbents, were investigated. An intensive investigation on the adsorption mechanism and the magnetic effect was studied. The results suggested that the adsorption processes were highly pH dependent. The kinetic studies revealed that the adsorption of phosphate onto TEPA-Fe3O4-NMPs followed pseudo-second-order model. Thermodynamic studies suggested that the adsorption processes of phosphate onto the TEPA-Fe3O4-NMPs were endothermic and entropy favored in nature. The content of Fe3O4 in the adsorbents could obviously affect the adsorption efficiencies. TEPA-Fe3O4-NMPs-1, with the highest adsorption capacity for phosphate removal at 102.04 mg g−1, may had an appropriate ratio of amino groups and Fe3O4 content. The adsorption capacity of phosphate by TEPA-Fe3O4-NMPs may be an integrated result of both the amount of amino groups and Fe3O4 content in the magnetic adsorbents. The existing of magnetic core in the TEPA-Fe3O4-NMPs was found to be able to eliminate the intra-particle diffusion, but accelerate and strengthen the adsorption equilibrium processes, with larger equilibrium constant KL and pseudo-second-order rate constant k2. The results from XPS analyses indicated that the existing of the magnetic core might be favorable the arrangement of the amino groups on the surface of the TEPA-Fe3O4-NMPs, which may be favorable the mass transfer to accelerate the adsorption process.Acknowledgements
We would like to thank the National Natural Science Foundation of Zhejiang Province (LY14B04003), the National Natural Science Foundation of Ningbo (2014A610092), the National College Students' innovation and entrepreneurship training program (201413022011), the Xinmiao Students' innovation training program of Zhejiang Province (2014R401190) for the financial support.References
- P. Mahato, S. Saha, P. Das, H. Agarwallad and A. Das, RSC Adv., 2014, 4, 36140 RSC
.
- N. Singh and K. Balasubramanian, RSC Adv., 2014, 4, 27691 RSC
.
- A. H. Lu, E. L. Salabas and F. Schuth, Angew. Chem., Int. Ed., 2007, 46, 1222 CrossRef CAS PubMed
.
- J. Kim, J. E. Lee, J. Lee, J. H. Yu, B. C. Kim, K. An, Y. Hwang, C. H. Shin, J. G. Park, J. Kim and T. Hyeon, J. Am. Chem. Soc., 2006, 128, 688 CrossRef CAS PubMed
.
- S. T. Xing, D. Y. Zhao, W. J. Yang, Z. C. Ma, Y. S. Wu, Y. Z. Gao, W. R. Chen and J. Han, J. Mater. Chem. A, 2013, 1, 1694 CAS
.
- M. C. K. Wiltshire, J. B. Pendry, I. R. Young, D. J. Larkman, D. J. Gilderdale and J. V. Hajnal, Science, 2001, 291, 849 CrossRef CAS PubMed
.
- Y. W. Jun, Y. M. Huh, J. S. Choi, J. H. Lee, H. T. Song, S. Kim, S. Yoon, K. S. Kim, J. S. Shin, J. S. Suh and J. Cheon, J. Am. Chem. Soc., 2005, 127, 5732 CrossRef CAS PubMed
.
- C. Paul, Inkjet printing for materials and devices, Chem. Mater., 2001, 13, 3299 CrossRef
.
- S. A. Ghosal, J. Shah, R. K. Kotnala and S. Ahmad, J. Mater. Chem. A, 2013, 1, 12868 Search PubMed
.
- R. D. Ambashta and S. Mika, J. Hazard. Mater., 2010, 180, 38 CrossRef CAS PubMed
.
- Y. G. Zhao, H. Y. Shen, S. D. Pan and M. Q. Hu, J. Hazard. Mater., 2010, 182, 295 CrossRef CAS PubMed
.
- H. Y. Shen, S. D. Pan, Y. Zhang, X. L. Huang and H. X. Gong, Chem. Eng. J., 2012, 183, 180 CrossRef CAS PubMed
.
- H. Y. Shen, J. L. Chen, H. F. Dai, L. B. Wang, M. Q. Hu and Q. H. Xia, Ind. Eng. Chem. Res., 2013, 52, 12723 CrossRef CAS
.
- S. D. Pan, Y. Zhang, H. Y. Shen and M. Q. Hu, Chem. Eng. J., 2012, 210, 564 CrossRef CAS PubMed
.
- Y. G. Zhao, X. H. Chen, S. D. Pan, H. Zhu, H. Y. Shen and M. C. Jin, J. Mater. Chem. A, 2013, 1, 11648 CAS
.
- Y. Zhang, S. D. Pan, H. Y. Shen and M. Q. Hu, Anal. Sci., 2012, 28, 887 CrossRef CAS
.
- L. A. Rodrigues and M. L. C. P. da Silva, Desalination, 2010, 263, 29 CrossRef CAS PubMed
.
- F. Özmen, P. A. Kavakli and O. Güven, J. Appl. Polym. Sci., 2011, 119, 613 CrossRef
.
- Y. Zhang, X. Q. Xi, S. N. Xu, J. C. Zhou, J. J. Zhou, Q. H. Xu and H. Y. Shen, Acta Chim. Sin., 2012, 70, 1839 CrossRef CAS
.
- M. M. Amin, B. Bina, A. M. S. Majd and H. Pourzamani, Front. Environ. Sci. Eng., 2014, 8, 345 CrossRef CAS
.
- F. Aboufazeli, H. R. L. Z. Zhad, O. Sadeghi, M. Karimi and E. Najafi, Food Chem., 2013, 141, 3459 CrossRef CAS PubMed
.
- M. E. Mahmoud, M. S. Abdelwahab and E. M. Fathallah, Chem. Eng. J., 2013, 223, 318 CrossRef CAS PubMed
.
- Ministry of Health, and Standardization Administration, the People’s Republic of China, GB/T 6913–2008 Standard examination methods for phosphate in boiler-used water and cooling water, 2008.
- J. X. Lin and L. Wang, Front. Environ. Sci. Eng. China, 2009, 3, 320 CrossRef CAS PubMed
.
- Y. S. Ho, J. Hazard. Mater., 2006, 136, 681 CrossRef CAS PubMed
.
- Y. Chen, B. Pan, H. Li, W. Zhang, L. Lv and J. Wu, Environ. Sci. Technol., 2010, 44, 3508 CrossRef CAS PubMed
.
- M. Owlad, M. K. Aroua and W. M. A. W. Daud, Bioresour. Technol., 2010, 101, 5098 CrossRef CAS PubMed
.
- W. S. Lu, Y. H. Shen, A. J. Xie, X. Z. Zhang and W. G. Chang, J. Phys. Chem. C, 2010, 114, 4846 CAS
.
- B. X. Cai and Y. W. Chen, Basical Chemistry Experiments, Science Press, Beijing, China, 2001 Search PubMed
.
- X. F. Sun, Y. Ma, X. W. Liu, S. G. Wang, B. Y. Gao and X. M. Li, Water Res., 2010, 44, 2517 CrossRef CAS PubMed
.
- S. R. Chowdhury, E. K. Yanful and A. R. Pratt, J. Hazard. Mater., 2012, 235–236, 246 CrossRef CAS PubMed
.
- D. K. Park, Y. S. Yun and J. M. Park, J. Colloid Interface Sci., 2008, 317, 54 CrossRef CAS PubMed
.
- E. Nehrenheim and J. P. Gustafsson, Bioresour. Technol., 2008, 99, 1571 CrossRef CAS PubMed
.
- E. Vasyukova, O. S. Pokrovsky, J. Viers and B. Dupre, Appl. Geochem., 2012, 27, 1226 CrossRef CAS PubMed
.
- E. I. El-Shafey, J. Hazard. Mater., 2010, 175, 319 CrossRef CAS PubMed
.
- J. W. Choi, Y. S. Choi, S. W. Hong, D. J. Kim and S. H. Lee, Water Environ. Res., 2012, 84, 596 CrossRef CAS
.
- R. Saad, K. Belkacemi and S. Hamoudi, J. Colloid Interface Sci., 2007, 311, 375 CrossRef CAS PubMed
.
- T. S. Anirudhan and P. Senan, Chem. Ecol., 2011, 27, 147 CrossRef CAS
.
- H. B. Ma, J. Li, H. Chen, G. Z. Zuo, Y. Yu, T. H. Ren and Y. D. Zhao, Tribol. Int., 2009, 42, 940 CrossRef CAS PubMed
.
- Z. Nazarpoor, S. Ma, P. T. Fanson, O. S. Alexeev and M. D. Amiridis, Polym. Degrad. Stab., 2012, 97, 439 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra14630a |
| This journal is © The Royal Society of Chemistry 2015 |