DOI:
10.1039/C4RA12599A
(Paper)
RSC Adv., 2015,
5, 21700-21709
Microwave and hydrothermal syntheses of WSe2 micro/nanorods and their application in supercapacitors
Received
17th October 2014
, Accepted 13th February 2015
First published on 13th February 2015
Abstract
WSe2 micro/nanorods were synthesized using one step microwave and hydrothermal methods. The as synthesized micro/nanorod samples of WSe2 were characterized using various characterization techniques such as SEM, TEM, Raman spectroscopy, X-ray diffraction, UV-visible and PL spectroscopy. The as synthesized samples were also tested for their applicability to use as cathode materials for supercapacitor applications. The WSe2 samples prepared by the microwave and hydrothermal methods (with use of tungstic acid as precursor) show noteworthy performance towards supercapacitor application. Our work opens a new avenue to use these simple methods to prepare various morphologies of inorganic nanomaterials and utilize them for various energy and nanoelectronics applications.
Introduction
Supercapacitors, which are also known as electrochemical capacitors, are considered as essential power sources due to promising energy-storage device applications. The important parameters of these devices are high power densities, rate of charge–discharge, life cycle stability, durability and low cost. Owing to these remarkable properties, researchers have been attracted to the area of supercapacitors, to fabricate electrodes using various nanomaterials for advanced energy storage devices. Over the past few years, there has been remarkable growth in various perspectives of engineering applications of one-dimensional (1D) and two-dimensional (2D) inorganic oxide layered materials especially transition metal dichalcogenides1–7 such as MoS2,8–24 WS2,25–28 MoSe2,29–32 WSe2,33–39 GaS,15,40 GaSe15,40 and other 2D insulating41–44 and oxide materials45,46 which have attracted much attention from the scientific community due to their extraordinary electrical, optical and magnetic properties. Among all, the WSe2 layered materials in 1D nanometric form have not yet been widely investigated for energy storage and other applications due to difficulties in the synthesis and other related issues. The 2D form of WSe2 has been recently reported as field effect transistors,8–13 photodetectors,16 for photocatalytic hydrogen production47 in solar cells48–50 and heterostructures.11 The bulk WSe2 is a layered semiconductor with indirect bandgaps ∼1.21 eV,51 in monolayer form it exhibits direct bandgaps of 1.67 eV.52 The WSe2 belongs to the transition metal dichalcogenides (TMDCs) crystal structure made up of hexagonal layers of metal atoms (M = Mo, W, V, Ga, Ta, Sn) sandwiched in between two layers of chalcogen atoms (X = S, Se, Te).8–24 The WSe2 is a naturally occurring metal selenide with intriguing electronic, electrochemical and electrocatalytic properties, formed by 2D covalently bonded Se–W–Se layers separated by a van der Waals gap. The WSe2 possesses a hexagonal crystal structure with space group P63/mmc and each WSe2 monolayer contains an individual layer of W atoms with 6-fold coordination symmetry, which is hexagonally packed between two trigonal atomic layers of Se atoms (Fig. 1).
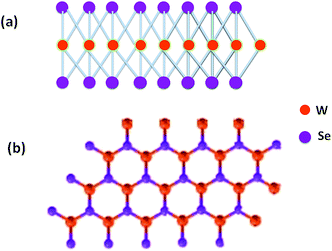 |
| | Fig. 1 Schematic of WSe2 structure: (a) side view and (b) top view. | |
The atomically thin layered WSe2 has attracted much attention for diverse applications in nanoelectronic devices because of its suitable wide and direct band gap.53 It is possible that the layered WSe2 can offer easy electron transport through MX2 nanostructures, which gives high specific capacitance values by faster ion diffusion between the layers as reported for other TMDCs.54,55 Graphene and its composites with other materials have been utilized for its suitability for supercapacitor applications.54,55 Moreover it is important that for high performance supercapacitors, materials should have properties such as high power density, fast power delivery or uptake, and excellent cycle-to-cycle stability etc. These properties are essential for material to be used in high performance energy storage devices.56–61 The TMDCs materials like MoS2 and WS2 have been recently reported for their supercapacitor behaviour,54,55 but WSe2, which also belongs to the same layered chalcogenide family, has not yet been explored till date for its application as a supercapacitor.
In the present investigations, we report the synthesis of WSe2 nanorods and nanoparticles using a simple microwave assisted route and a hydrothermal method and their supercapacitor behaviours. The WSe2 exhibited enhanced and stable supercapacitive behaviour in three electrode capacitance measurement geometry.
Results and discussion
The WSe2 samples were prepared using three different routes. Here after the sample prepared by the microwave-assisted method is named as WSe2-A, next the sample prepared using the hydrothermal method using tungstic acid and elemental selenium salts is called as WSe2-B and finally the sample prepared using the hydrothermal method with ammonium tungstate and elemental selenium salts is called as WSe2-C. Here after samples are described by above nomenclature. Fig. 2(a–d) shows the typical SEM images of the WSe2-A sample prepared using the microwave method depicting the rod-like morphology with typical dimensions 10–80 μm in length and 1–2 μm in diameter. Fig. 3(a–d) shows the typical TEM images of the WSe2-A sample, depicting the rod-like morphology. Fig. 3(e and f) shows HR-TEM images of the WSe2-A sample showing the good quality of the samples prepared using the microwave route. The inset of Fig. 3(f) shows the typical diffraction pattern of the WSe2-A sample, showing hexagonal structure and highly oriented along the <001> axis. Fig. 4(a) shows the typical XRD pattern of the WSe2-A sample, which matches well with the JCPDS data card no 06-0080. The sample also shows some other phases in addition to WSe2 which indicates that our sample is slightly impure. This needs additional detailed X-ray photoelectron spectroscopy and other investigations. The WSe2-A sample prepared using the microwave method has been further characterized using Raman spectroscopy as shown in Fig. 4(b). The typical Raman spectrum of the WSe2-A sample excited using a 514.5 nm laser source shows Raman bands at A1g-LA (142 cm−1), E2g1 (240 cm−1). It has been reported that the A1g and E2g1 bands of single-layer WSe2 are separated by ∼11 cm−1.54 The light absorption properties of the as-synthesized WSe2-A sample were characterized by the UV-Vis absorption spectrum. Fig. 4(c) shows the UV-Vis absorption spectrum of WSe2-A. The absorption of WSe2-A was extended to the UV region. From the optical absorption spectrum, it is evident that the WSe2-A showed an absorption onset of 220 nm. The room temperature PL spectrum of the WSe2-A sample is shown in Fig. 4(d). It exhibits a strong PL peak at ∼728 nm.
 |
| | Fig. 2 (a–d) SEM images of WSe2-A synthesized by the microwave method using ammonium molybdate salt. | |
 |
| | Fig. 3 (a–d) TEM images of WSe2 synthesized by the microwave method. (e–f) HRTEM images and inset of (f) shows the typical SAED pattern showing the high crystalline quality of the as synthesized sample. | |
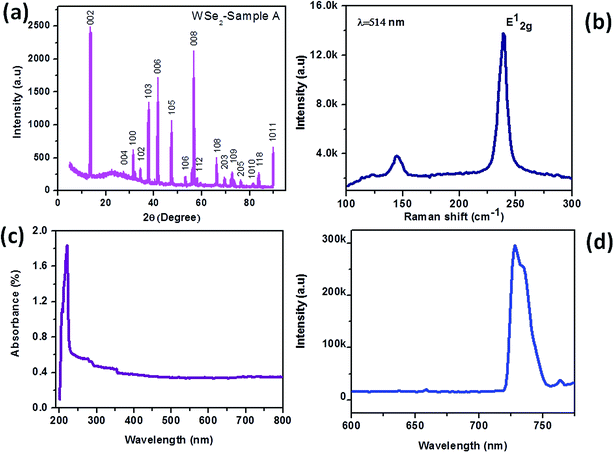 |
| | Fig. 4 WSe2 microrod sample synthesized by the microwave method. (a) XRD, (b) Raman spectrum, (c) UV-visible spectrum and (d) PL spectrum. | |
The supercapacitive performances of the WSe2-A samples were measured in a three-electrode system in 0.5 M KOH aqueous electrolyte. The specific capacitances of the WSe2 electrode have been calculated from the following equation:
where
m is the mass of single electrode material,
v is the scan rate (mV s
−1),
ν1 and
ν2 are the integration limits (potential window), and
I(
ν) denotes the response current (A). The capacitive performance of WSe
2-A was measured in a three-electrode system in 0.5 M KOH aqueous electrolyte.
Fig. 5(a) shows the comparative cyclic voltammograms (CVs) of WSe
2-A at different scan rates. The CV curve consists of an area under the curve which shows the charge storage capacity of the electrode therefore the value of specific capacitance calculated at a scan rate 100 mV s
−1 is found to be 1.939 F g
−1.
Fig. 5(b) shows the typical comparative charge–discharge profiles of WSe
2-A at current densities of 1 and 5 μA.
Fig. 5(c) depicts the single charging–discharging cycle for the 400 cycles. The electron/ion transport at the electrode/electrolyte interface and charge transfer resistance (
Rct) is further investigated by electrochemical impedance spectroscopy (EIS) techniques; the Warburg impedance can be determined using the straight line inclined to the real axis.
62 The slope of the Warburg impedance depicts the diffusion of electrolyte into the electrodes. The
Rct values of WSe
2-A samples were measured in 0.5 KOH at 10.0 Ω which is shown in
Fig. 5(d).
 |
| | Fig. 5 Supercapacitor based on WSe2 microrods synthesized by the microwave method. (a) Cyclic voltammograms at different scan rates, (b) charging–discharging profiles at different current densities, (c) charging–discharging profile for 400 cycles and (d) impedance spectroscopy. | |
Fig. 6(a)–(e) shows the typical SEM images of the WSe2-B sample depicting microrod-like morphology which is similar to WSe2-A, with dimensions 10–90 μm in length and 1–2 μm diameters. Fig. 7(a)–(c) shows typical TEM images of the WSe2-B sample and Fig. 7(d) shows the typical SAED pattern of the sample representing the single crystalline nature of the WSe2-B sample. Fig. 8(a) shows the typical XRD pattern of WSe2-B which matches with the JCPDS data card no. 06-0080. Fig. 8(b) depicts the typical Raman spectrum of the WSe2-B sample which matches with reported spectra in the literature.56 The light absorption study was carried out using the UV-Vis absorption spectrum and is shown in Fig. 8(c). The absorption of WSe2-B was observed in the UV region and the absorption onset of 220 nm was noted. The PL spectrum of the WSe2-B sample was taken at room temperature as shown in Fig. 8(d). It exhibits a strong PL peak centred at 728 nm. The capacitive performance of WSe2-B was also investigated in a three-electrode system in 0.5 M KOH aqueous electrolyte. Fig. 9(a) shows the comparative cyclic voltammograms (CVs) of the WSe2-B sample at different scan rates. The area under the CV curve represents the charge storage capacity of the electrode therefore the value of specific capacitance calculated at a scan rate of 100 mV s−1 is found to be 2.44 F g−1. The comparative charge–discharge profiles of WSe2-B at different current densities are showed in Fig. 9(b). Fig. 9(c) depicts the single charging–discharging cycle from the 400 cycles which is shown in Fig. 9(d). The Rct values of WSe2-B nanorods were measured in 0.5 KOH at 11.0 Ω.
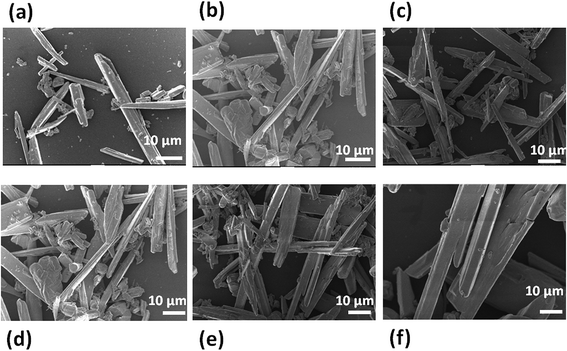 |
| | Fig. 6 (a–f) SEM images of WSe2 microrods synthesized by the hydrothermal method using tungstic acid as source material. | |
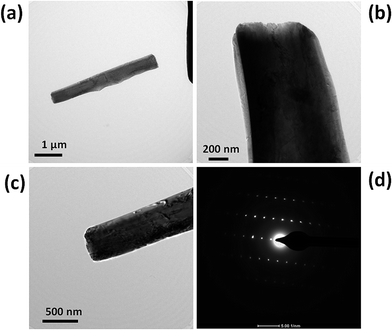 |
| | Fig. 7 (a–c) TEM images of a WSe2 microrod synthesized by the hydrothermal method and (d) typical SAED pattern of the WSe2 micro/nanorod sample. | |
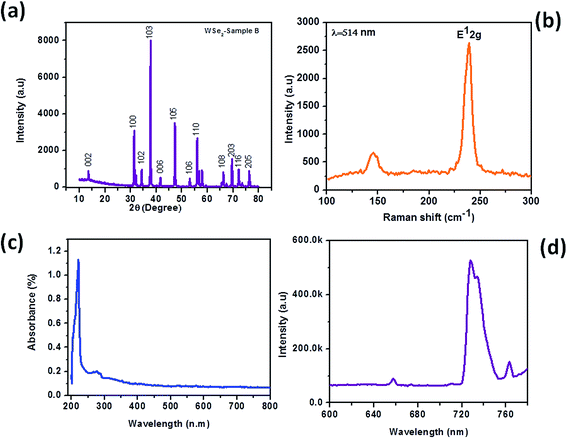 |
| | Fig. 8 WSe2 micro/nanorods synthesized by the hydrothermal method using tungstic acid as the source. (a) XRD pattern, (b) Raman spectrum, (c) UV-visible spectrum and (d) PL spectrum. | |
 |
| | Fig. 9 Supercapacitor based on WSe2 micro/nanorod sample synthesized by the hydrothermal method using tungstic acid as source: (a) cyclic voltammograms at different scan rates, (b) charging–discharging profiles at different current densities, (c) charging–discharging profile for 400 cycles and (d) impedance spectroscopy. | |
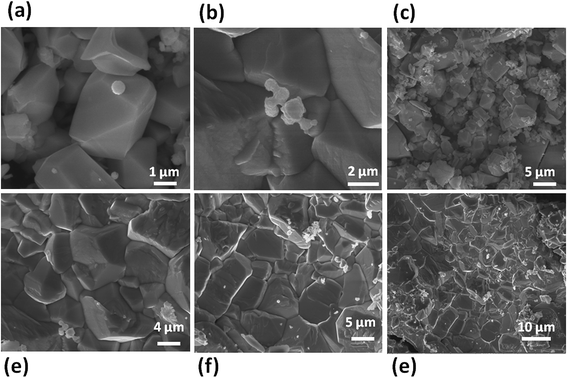
Fig. 10(a)–(f) shows the typical SEM images of the WSe2-C sample, showing micron size particle-like morphology with dimensions 2–5 μm which were further observed to be decorated with small nanoparticles on the micron size particle. Fig. 11(a) and (b) depicts typical TEM images of the WSe2-C sample, which shows good uniform growth of particle-like morphology. Fig. 11(c) and (d) shows HR-TEM images of the WSe2-C sample in high resolution. The crystal qualities of the samples were analysed using the diffraction pattern which is shown in the inset of Fig. 11(d). Fig. 12(a) shows the typical XRD pattern of the WSe2-C sample confirming the high quality of the as synthesized sample. The WSe2-C sample was further examined by Raman spectroscopy. Fig. 12(b) shows the Raman spectrum of WSe2-C matches very well with the earlier results in this manuscript. The light absorption characteristics were examined using the UV-Vis absorption spectrum which is shown in Fig. 12(c). The absorption of the WSe2-C sample was characterized using UV absorption with an observed onset of 220 nm. The WSe2-C sample was further characterized by the PL spectrum at room temperature, it exhibits a strong PL peak centred at 727.9 nm, depicted in Fig. 12(d). The capacitive performance of WSe2-C was also measured in a three-electrode system in 0.5 M KOH aqueous electrolyte. Fig. 13(a) shows the comparative cyclic voltammograms (CVs) of WSe2-C at different scan rates. The area under the CV curve represents the charge storage capacity of the electrode therefore the value of specific capacitance calculated at a scan rate of 100 mV s−1 is found to be 2.59 F g−1. Fig. 13(b) shows the typical comparative charge–discharge profiles of the sample at different current densities. Fig. 13(c) depicts the single charging–discharging cycle from the 400 cycles. Fig. 13(d) shows the impedance spectrum of WSe2-C. The results typically consist of a curve at high frequencies and a straight line at lower frequencies. The Rct values of WSe2-C were measured in 0.5 KOH at 37.0 Ω. Fig. 13(e) shows the comparative CVs of all three sample WSe2-A, WSe2-B and WSe2-C at 100 mV s−1 and Fig. 13(f) depicts the comparative charge–discharge profiles at the same current density of 5 μA. Table 1 summarizes the results of supercapacitor performances at different voltage sweep rates (50, 100, 200, 500, and 1000 mV s−1) showing the specific capacitances which were examined for all three samples of WSe2.
 |
| | Fig. 10 (a–f) SEM images of WSe2 micro/nanoparticles synthesized by the hydrothermal method using ammonium molybdate as source material. | |
 |
| | Fig. 11 WSe2 micro/nanoparticles synthesized by the hydrothermal method using ammonium molybdate as source: (a–b) TEM images, (c–d) HR-TEM images. Inset of (d) shows the typical SAED pattern of WSe2 micro/nanoparticles. | |
 |
| | Fig. 12 WSe2 micro/nanoparticles synthesized by the hydrothermal method using ammonium molybdate as source material: (a) XRD pattern, (b) Raman spectrum, (c) UV-visible spectrum and (d) PL spectrum. | |
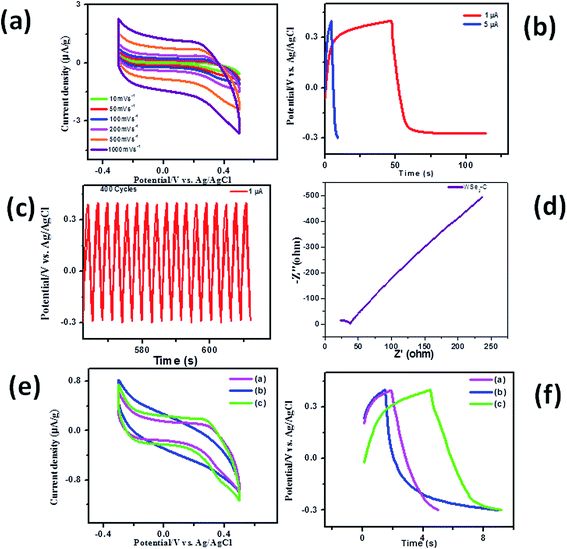 |
| | Fig. 13 Supercapacitor based on WSe2 micro/nanoparticles synthesized by the hydrothermal method using ammonium molybdate. (a) Cyclic voltammograms at different scan rates, (b) charging–discharging profiles at different current densities, (c) charging–discharging profile for 400 cycles and (d) impedance spectroscopy; (e and f) show the comparative study of WSe2-A, WSe2-B and WSe2-C samples for cyclic voltammograms at 100 mV s−1 and charge–discharge profiles at a current density of 5 μA, respectively. | |
Table 1 Specific capacitance for all samples of WSe2 at different scan rates
| Sample |
Voltage scan rate 10 mV s−1 |
Voltage scan rate 50 mV s−1 |
Voltage scan rate 100 mV s−1 |
Voltage scan rate 200 mV s−1 |
Voltage scan rate 500 mV s−1 |
Voltage scan rate 1000 mV s−1 |
| WSe2-A |
10.41 |
2.48 |
1.939 |
1.58 |
1.26 |
1.06 |
| WSe2-B |
10.41 |
3.76 |
2.44 |
1.50 |
0.88 |
0.64 |
| WSe2-C |
6.125 |
3.19 |
2.59 |
2.12 |
1.61 |
1.28 |
Conclusions
In conclusion, we have synthesized WSe2 micro/nanorods using two different methods namely one step microwave and hydrothermal methods. We have also successfully synthesized WSe2 micro/nanoparticles covered with small WSe2 nanoparticles (size ∼ 100 nm) using the hydrothermal method. In addition to pure phase some impurities in the sample were also noted, which need further detailed investigations. The samples were tested for their suitability to use in supercapacitor applications. The WSe2 microrods prepared using the microwave and hydrothermal methods show better performance towards cyclic stability and good performance towards charging and discharging cycles. Impedance spectroscopy results of all samples reveal that a lower Rct value and thicker electrical double layer formations are responsible for its superior capacitive behaviour. Our results open a new avenue to prepare WSe2 and various other inorganic layered materials using simple hydrothermal and microwave methods and the use of novel morphologies of these materials for energy applications including supercapacitors to replace conventional batteries.
Experimental section
(a) Microwave synthesis of WSe2 nanorods
In the typical microwave reaction the stoichiometry quantities of the precursor powders elemental selenium (2 mM) and ammonium tungstate (1 mM) and sodium borohydride (3 mM) were dissolved in 10 mL ethylene glycol followed by purging inert N2 gas for a few minutes prior to switching on the microwave. The microwave-assisted reaction was carried out in a Spectra 750 W microwave oven, with a 2.45 GHz working frequency. The oven was modified to include a refluxing system. During the experiments, the microwave oven was operated for switching on for 30 s and off for the next 15 s, with use of total power ∼750 W up to 3 minutes. For the post reaction, the product was centrifuge using ethanol solvent for several times at 4500 rpm followed by washing using DI water and drying in vacuum for 3 hours.
(b) Hydrothermal synthesis of WSe2 microrods
The second method for WSe2 microrod synthesis was the hydrothermal reaction. In a typical reaction, we used 1 g of ammonium tungstate [(NH4)10H2(W2O7)6], 0.89 g of elemental selenium and 8 mL of hydrazine monohydrate which were added in a 25 mL capacity Teflon-lined stainless steel autoclave and distilled water was added to fill 80% of the total volume of the autoclave. In the hydrothermal reaction, typical temperature was maintained at 150–170 °C for 48 h.
(c) Hydrothermal synthesis of WSe2 nanoparticles
The third method to synthesize WSe2 nanostructures was the same as the hydrothermal method with a different source of tungsten as tungstic acid was used. In the hydrothermal method, the typical temperature of Teflon-lined steel autoclave was maintained at 150–170 °C for 48 h. For the post reaction the autoclaves were cooled naturally followed by washing with distilled water and ethanol successively and then the final product was dried under vacuum at 50 °C for 2 h.
(d) Characterization
All WSe2 samples were further characterized using several analytical tools such as XRD, Raman, SEM, TEM, UV-Vis and PL etc. The crystallographic structures and phase confirmation of WSe2 samples were analysed by X-ray diffraction (XRD, Philips powder diffractometer PW3040/60 with Cu Kα radiation). Scanning electron microscopy (SEM) images were acquired using an FEI ESEM QUANTA 200 3D instrument. High resolution-transmission electron microscopy (HR-TEM) images were obtained using an FEI TECNAI TF-30 (FEG) instrument. The Raman spectra were recorded at room temperature, with a (LabRAM HR) using an Ar laser (514.5 nm) in the back scattering geometry. The adsorption capacities of the samples were analysed using a HORIBA Fluorolog 3 spectrofluorometer instrument. The photoluminescence was measured by exciting the samples with a 400 nm solid-state laser of few milliwatts power.
Acknowledgements
Dr D. J. Late would like to thank Department of Science and Technology (Government of India) for Ramanujan Fellowship (Grant no. SR/S2/RJN-130/2012). The research work was primarily supported by NCL-MLP project grant 028626, DST-SERB Fast-track Young scientist project Grant no. SB/FT/CS-116/2013 and the partial support by INUP IITB project sponsored by DeitY, MCIT.
Notes and references
- S. V. Morozov and A. K. Geim, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 10451 CrossRef PubMed
 .
. - K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Science, 2004, 306, 666 CrossRef CAS PubMed
 .
. - K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, M. I. Katsnelson, I. V. Grigorieva, S. V. Dubonos and A. A. Firsov, Nature, 2005, 438, 197 CrossRef CAS PubMed
 .
. - F. Schwierz, Nat. Nanotechnol., 2010, 5, 487 CrossRef CAS PubMed
 .
. - A. Ghosh, D. J. Late, L. S. Panchakarla, A. Govindaraj and C. N. R. Rao, J. Exp. Nanosci., 2009, 4, 313 CrossRef CAS
 .
. - D. J. Late, U. Maitra, L. S. Panchakarla, U. V. Waghmare and C. N. R. Rao, J. Phys.: Condens. Matter, 2011, 23, 055303 CrossRef PubMed
 .
. - F. Schedin, A. K. Geim, S. V. Morozov, E. W. Hill, P. Blake, M. I. Katsnelson and K. S. Novoselov, Nat. Mater., 2007, 6, 652 CrossRef CAS PubMed
 .
. - B. Radisavljevic, A. Radenovic, J. Brivio, V. Giacometti and A. Kis, Nat. Nanotechnol., 2011, 6, 147 CrossRef CAS PubMed
 .
. - D. J. Late, P. A. Shaikh, R. Khare, R. V. Kashid, M. Chaudhary, M. A. More and S. B. Ogale, ACS Appl. Mater. Interfaces, 2014, 6, 15881 CAS
 .
. - D. J. Late, B. Liu, H. S. S. R. Matte, V. P. Dravid and C. N. R. Rao, ACS Nano, 2012, 6, 5635 CrossRef CAS PubMed
 .
. - K. Roy, M. Padmanabhan, S. Goswami, T. P. Sai, G. Ramalingam, S. Raghavan and A. Ghosh, Nat. Nanotechnol., 2013, 8, 826 CrossRef CAS PubMed
 .
. - H. Wang, L. Yu, Y. Lee, Y. Shi, A. Hsu, M. L. Chin, L. Li, M. Dubey, J. Kong and T. Palacios, Nano Lett., 2012, 12, 4674 CrossRef CAS PubMed
 .
. - D. Jariwala, V. K. Sangwan, D. J. Late, J. E. Johns, V. P. Dravid, T. J. Marks, L. J. Lauhon and M. C. Hersam, Appl. Phys. Lett., 2013, 102, 173107 CrossRef PubMed
 .
. - H. S. S. R. Matte, A. Gomathi, A. K. Manna, D. J. Late, R. Datta, S. K. Pati and C. N. R. Rao, Angew. Chem., 2010, 122, 4153 CrossRef
 .
. - D. J. Late, B. Liu, H. S. S. R. Matte, C. N. R. Rao and V. P. Dravid, Adv. Funct. Mater., 2012, 22, 1894 CrossRef CAS
 .
. - Z. Yin, H. Li, H. Li, L. Jiang, Y. Shi, Y. Sun, G. Lu, Q. Zhang, X. Chen and H. Zhang, ACS Nano, 2012, 6, 74 CrossRef CAS PubMed
 .
. - A. Castellanos-Gomez, N. Agrat and G. Rubio-Bollinger, Appl. Phys. Lett., 2010, 96, 213116 CrossRef PubMed
 .
. - R. V. Kashid, D. J. Late, S. S. Chou, Y. Huang, M. De, D. S. Joag, M. A. More and V. P. Dravid, Small, 2013, 9, 2730 CrossRef CAS PubMed
 .
. - Q. Zhang and H. Zhang, Small, 2012, 8, 63 CrossRef PubMed
 .
. - D. J. Late, Y. Huang, B. Liu, J. Luo, J. Acharya, S. N. Shirodkar, J. Luo, A. Yan, D. Charles, U. V. Waghmare, V. P. Dravid and C. N. R. Rao, ACS Nano, 2013, 7, 4879 CrossRef CAS PubMed
 .
. - F. K. Perkins, A. L. Friedman, E. Cobas, P. M. Campbell, G. G. Jernigan and B. T. Jonker, Nano Lett., 2013, 13, 668 CrossRef CAS PubMed
 .
. - C. Lee, H. Yan, L. E. Brus, T. F. Heinz, J. Hone and S. Ryu, ACS Nano, 2010, 4, 2695 CrossRef CAS PubMed
 .
. - J. D. Lin, C. Han, F. Wang, R. Wang, D. Xiang, S. Qin, X. Zhang, L. Wang, H. Zhang, A. T. S. Wee and W. Chen, ACS Nano, 2014, 8, 5323 CrossRef CAS PubMed
 .
. - Y. Shi, J. Huang, L. Jin, Y. Hsu, S. F. Yu, L. Li and H. Y. Yang, Sci. Rep., 2013, 3, 1839 Search PubMed
 .
. - D. Braga, I. G. Lezama, H. Berger and A. F. Morpurgo, Nano Lett., 2012, 12, 5218 CrossRef CAS PubMed
 .
. - T. Georgiou, R. Jalil, B. D. Belle, L. Britnell, R. V. Gorbachev, S. V. Morozov, Y. J. Kim, A. Gholinia, S. J. Haigh, O. Makarovsky, L. Eaves, L. A. Ponomarenko, A. K. Geim, K. S. Novoselov and A. Mishchenko, Nat. Nanotechnol., 2013, 8, 100 CrossRef CAS PubMed
 .
. - A. Berkdemir, H. R. Gutiérrez, A. R. Botello-Mendez, N. Perea-López, A. L. Elías, C.-I. Chia, B. Wang, V. H. Crespi, F. López-Urías, J. C. Charlier, H. Terrones and M. Terrones, Sci. Rep., 2013, 3, 1755 Search PubMed
 .
. - N. Perea-Lopez, A. L. Elías, A. Berkdemir, A. Castro-Beltran, H. R. Gutiérrez, S. Feng, R. Lv, T. Hayashi, F. López-Urías, S. Ghosh, B. Muchharla, S. Talapatra, H. Terrones and M. Terrones, Adv. Funct. Mater., 2013, 23, 5511 CrossRef CAS
 .
. - S. Tongay, J. Zhou, C. Ataca, K. Lo, T. S. Matthews, J. Li, J. C. Grossman and J. Wu, Nano Lett., 2012, 12, 5576 CrossRef CAS PubMed
 .
. - J. S. Ross, S. Wu, H. Yu, N. J. Ghimire, A. M. Jones, G. Aivazian, J. Yan, D. G. Mandrus, D. Xiao, W. Yao and X. Xu, Nat. Commun., 2013, 4, 1474 CrossRef PubMed
 .
. - S. Larentis, B. Fallahazad and E. Tutuc, Appl. Phys. Lett., 2012, 101, 223104 CrossRef PubMed
 .
. - D. J. Late, T. Doneux and M. Bougouma, Appl. Phys. Lett., 2014, 105, 233103 CrossRef PubMed
 .
. - H. Fang, S. Chuang, T. C. Chang, K. Takei, T. Takahashi and A. Javey, Nano Lett., 2012, 12, 3788 CrossRef CAS PubMed
 .
. - W. Liu, J. Kang, D. Sarkar, Y. Khatami, D. Jena and K. Banerjee, Nano Lett., 2013, 13, 1983 CrossRef CAS PubMed
 .
. - H. Sahin, S. Tongay, S. Horzum, W. Fan, J. Zhou, J. Li, J. Wu and F. M. Peeters, Phys. Rev. B: Condens. Matter Mater. Phys., 2013, 87, 165409 CrossRef
 .
. - Y. Zhao, X. Luo, H. Li, J. Zhang, P. T. Araujo, C. K. Gan, J. Wu, H. Zhang, S. Y. Quek, M. S. Dresselhaus and Q. Xiong, Nano Lett., 2013, 13, 1007 CrossRef CAS PubMed
 .
. - A. M. Jones, H. Yu, N. J. Ghimire, S. Wu, G. Aivazian, J. S. Ross, B. Zhao, J. Yan, D. G. Mandrus, D. Xiao, Y. Yao and X. Xu, Nat. Nanotechnol., 2013, 8, 634 CrossRef CAS PubMed
 .
. - H. Yuan, M. S. Bahramy, K. Morimoto, S. Wu, K. Nomura, B. Yang, H. Shimotani, R. Suzuki, M. Toh, C. Kloc, X. Xu, R. Arita, N. Nagaosa and Y. Iwasa, Nat. Phys., 2013, 9, 563 CrossRef CAS
 .
. - J. K. Huang, J. Pu, C.-L. Hsu, M. H. Chiu, Z.-Y. Juang, Y.-H. Chang, W.-H. Chang, Y. Iwasa, T. Takenobu and L.-J. Li, ACS Nano, 2014, 8, 923 CrossRef CAS PubMed
 .
. - D. J. Late, B. Liu, J. Luo, A. Yan, H. S. S. R. Matte, M. Grayson, C. N. R. Rao and V. P. Dravid, Adv. Mater., 2012, 24, 3549 CrossRef CAS PubMed
 .
. - A. Pan, Y. Chen and J. Li, CrystEngComm, 2015, 17, 1098 RSC
 .
. - C. R. Dean, A. F. Young, I. Meric, C. Lee, L. Wang, S. Sorgenfrei, K. Watanabe, T. Taniguchi, P. Kim, K. L. Shepard and J. Hone, Nat. Nanotechnol., 2010, 5, 722 CrossRef CAS PubMed
 .
. - H. Zeng, C. Zhi, Z. Zhang, X. Wei, X. Wang, W. Guo, Y. Bando and D. Golberg, Nano Lett., 2010, 10, 5049 CrossRef CAS PubMed
 .
. - L. Song, L. Ci, H. Lu, P. B. Sorokin, C. Jin, J. Ni, A. G. Kvashnin, D. G. Kvashnin, J. Lou, B. I. Yakobson and P. M. Ajayan, Nano Lett., 2010, 10, 3209 CrossRef CAS PubMed
 .
. - S. Balendhran, S. Walia, M. Alsaif, E. P. Nguyen, J. Z. Ou, S. Zhuiykov, S. Sriram, M. Bhaskaran and K. Kalantar-zadeh, ACS Nano, 2013, 7, 9753 CrossRef CAS PubMed
 .
. - S. Balendhran, S. Walia, M. Alsaif, E. P. Nguyen, J. Z. Ou, S. Zhuiykov, S. Sriram, M. Bhaskaran and K. Kalantar-zadeh, ACS Nano, 2013, 7, 9753 CrossRef CAS PubMed
 .
. - Y. Li, Y.-L. Li, C. M. Araujo, W. Luo and R. Ahuja, Catal. Sci. Technol., 2013, 3, 2214 CAS
 .
. - M. Shanmugam, C. A. Durcan and B. Yu, Nanoscale, 2012, 4, 7399 RSC
 .
. - M. Bernardi, M. Palummo and J. C. Grossman, Nano Lett., 2013, 13, 3664 CrossRef CAS PubMed
 .
. - X. Gu, W. Cui, H. Li, Z. Wu, Z. Zeng, S. Lee, H. Zhang and B. Sun, Adv. Energy Mater., 2013, 3, 1262 CrossRef CAS
 .
. - R. Coehoorn, C. Haas and R. A. de Groot, Phys. Rev. B: Condens. Matter Mater. Phys., 1987, 35, 6203 CrossRef CAS
 .
. - H. Terrones, F. López-Urías and M. Terrones, Sci. Rep., 2013, 3, 1549 Search PubMed
 .
. - L. Cao, S. Yang, W. Gao, Z. Liu, Y. Gong, L. Ma, G. Shi, S. Lei, Y. Zhang, S. Zhang, R. Vajtai and P. M. Ajayan, Small, 2013, 9, 2905 CrossRef CAS PubMed
 .
. - D. Hanlon, C. Backes, T. M. Higgins, M. Hughes, A. O’Neill and P. King, et al., Chem. Mater., 2014, 26, 1751 CrossRef CAS
 .
. - S. Ratha and C. S. Rout, ACS Appl. Mater. Interfaces, 2013, 5, 11427 CAS
 .
. - D. J. Late, S. N. Shirodkar, U. V. Waghmare, V. P. Dravid and C. N. R. Rao, ChemPhysChem, 2014, 15, 1592 CrossRef CAS PubMed
 .
. - C. S. Rout, P. D. Joshi, R. V. Kashid, D. S. Joag, M. A. More, A. J. Simbeck, M. Washington, S. K. Nayak and D. J. Late, Sci. Rep., 2013, 3, 3282 Search PubMed
 .
. - H. X. Chang and H. K. Wu, Energy Environ. Sci., 2013, 6, 3483 CAS
 .
. - H. T. Zhang, X. Zhang, X. Z. Sun, D. C. Zhang, H. Lin, C. H. Wang, H. Wang and Y. Ma, ChemSusChem, 2013, 6, 1084 CrossRef CAS PubMed
 .
. - B. Zhang, R. Shi, Y. P. Zhang and C. X. Pan, Prog. Nat. Sci.-Mater, 2013, 23, 164 CrossRef PubMed
 .
. - D. Yu, K. Goh, L. Wei, H. Wang, Q. Zhang, W. Jiang, R. Si and Y. Chen, J. Mater. Chem. A, 2013, 1, 11061 CAS
 .
. - Q. Wang, J. E. Moser and M. Gratzel, J. Phys. Chem. B, 2005, 109, 14945–14953 CrossRef CAS PubMed
 .
.
|
| This journal is © The Royal Society of Chemistry 2015 |
Click here to see how this site uses Cookies. View our privacy policy here. 






.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.







