Surface modifications of gold nanorods for applications in nanomedicine
E. Locatelli
,
I. Monaco
and
M. Comes Franchini
*
Department of Industrial Chemistry “Toso Montanari”, University of Bologna, Viale Risorgimento 4, 40136 Bologna, Italia. E-mail: mauro.comesfranchini@unibo.it; Fax: +39 051 2093654; Tel: +39 051 2093626
First published on 9th February 2015
Abstract
Gold nanorods (GNRs) are appealing nanostructures for a wide variety of nanomedicine-based diagnostic and therapeutic approaches against untreatable diseases. Indeed, they possess unique and extraordinary optical features, which if conveniently stressed, would bring several benefits to non-invasive theranostic treatments. Major concerns regarding their practical application are derived not directly from GNRs but from molecules linked onto their surface, which could be source of toxicity as well as powerful allies for treatments' enhancement. Thus, specific and tailored surface modification of GNRs with several active moieties has become a crucial factor for their development. In this review, crucial breakthroughs and major possibilities deriving from the surface decoration of GNRs for final nanomedicine applications, as well as progress in therapy and diagnosis relying on the functionalization of these nanosystems will be summarized and discussed.
1. Introduction
Unlike other metal nanostructures, gold nanorods (GNRs) have appeared fascinating in many scientific fields, among which nanomedicine is certainly one of the most important.The success of GNRs for medical purposes is partially related to the fact that gold has been demonstrated to be a highly biocompatible material because it presents very low toxicity. Even at high concentrations, organs are not damaged after prolonged exposure to it, and it can be excreted via the hepatobiliary system.1,2 Moreover, GNRs longitudinal plasmon resonance (LPR) can be finely “moved” towards high wavelengths up to 1200 nm by simply increasing their aspect ratio (ratio between length and width). In this way its LPR can fall in the range of 800–1200 nm (Fig. 1), where the so-called near infrared (NIR) window exists, which is particularly attractive for medical applications due to the high transmittance (low absorbance) of water, deoxygenated haemoglobin and oxygenated haemoglobin, which allows the use of lasers without interfering with or burning healthy tissues and organs.3
 | ||
| Fig. 1 GNRs: variation in optical properties by increasing their aspect ratio. From C. J. Murphy et al., J. Phys. Chem., 2005, 109, 13857. | ||
GNRs represent an innovative contrast agent for non-invasive diagnostic techniques, such as optoacoustic imaging and X-ray computed tomography because they present considerable advantages in comparison to other common contrast agents such as molecular dyes, fluorophores or quantum dots, which present poor stability, photo bleaching under common imaging conditions, high toxicity and frequently insufficient absorption cross sections and scattering signals.4
In addition, GNRs have been investigated for their use as a therapeutic tool because more than 96% of the absorbed radiation is converted into heat due to the higher absorption cross section of GNRs than of other nanostructures: this means that though GNRs rapidly absorb light, their relaxation process is slow, resulting in the energy release in the form of heat. The generated local hyperthermia could easily reach several degrees.5 Such a strong localized increment in temperature can be exploited to selectively destroy cancer cells or diseased tissues under laser irradiation as a powerful alternative to medical surgery or invasive therapies, making GNRs a real and appealing therapeutic agent.
Due to these reasons, GNRs are nowadays finding applications in nanomedicine as the most promising theranostic (therapeutic + diagnostic) agent.
Despite the attractive possibilities opened up by GNRs, their synthesis and surface modification still present opportunities for their wider applications. Mostly, the GNRs are synthesised in aqueous medium with the assistance of various surfactants, which act as both template and stabilizer for the growing nanoparticles against aggregation phenomena.6 These surfactants remain adsorbed or deposited onto the surface of the nanoparticles once the process is completed, avoiding the post-synthesis collapse of the created nanoparticles. Unfortunately, most of these surfactants are strongly toxic or simply not suitable for the desired final application because they do not allow further synthetic modifications.
The removal of surfactants requires the development of specific ligands that would be able to replace them, to prevent the aggregation phenomena, and at the same time ensure that the specific desired final properties of GNRs are retained.
In this review, after a due paragraph concerning the synthesis of GNRs, the possibilities of surface modification with several moieties will be covered: organic molecules, synthetic polymers, natural biopolymers, peptides or proteins, oligonucleotides, DNA and RNA have all been exploited for the coating of GNRs and their subsequent application in nanomedicine. Finally, the most significant successes derived from surface-modified GNRs in the field of theranostics will be discussed.
2. Synthesis of GNRs
Nowadays, several methodologies ranging from chemistry to physics have been exploited for the preparation of GNRs with different aspect ratios; however, the most popular synthetic method is the so-called “seed-mediated growth method”, which allows reproducible results and an easily tunable final aspect ratio. This methodology was firstly optimized for the synthesis of GNRs by Nikoobakht and El-Sayed in 2003.7 In a general procedure, a high concentration of the cationic surfactant cetyltrimethylammonium bromide (CTAB) is used to simultaneously permit GNRs formation and avoid GNRs aggregation and precipitation once they are synthesized.The method involves preparing “seeds”, which can work as nucleation sites, by reducing a small amount of tetrachloroauric acid (HAuCl4) in aqueous solution in the presence of the surfactant with a strong reducing agent such as sodium borohydride (NaBH4). A small amount of these seeds is then introduced into the real growth solution containing Ag+ ions, excess of surfactant and a large amount of HAuCl4 that was already partially reduced from Au3+ to Au+ by ascorbic acid to facilitate the deposition of gold onto the seeds during the growth stage. The entire reaction occurs in 24 hours at room temperature (around 30 °C) and in an aqueous environment, thus allowing its application in general, and not-particularly equipped laboratories.
Several studies have confirmed that CTAB is arranged in a double layer (bilayer) around the GNRs in the growth phase: in the first layer the head-group of CTAB is oriented towards the NPs surface, while in the second layer is directed towards water, leaving the two hydrophobic tails in the centre, in a fashion similar to a cellular membrane.8
Positively charged silver ions appear to be intercalated between the negatively charged bromine head groups on the surface of the nanostructure, thus limiting the tendency of the negative charged heads to repel each other, and promoting the elongation of the GNRs. In fact it has been shown that within certain limits, the greater the amount of silver ions is present in the solution, the greater the aspect ratio of the GNRs is obtained, thus making it very easy to tune the size or shape of the GNRs, therefore facilitating control over their properties.
The fact that the growth preferentially occurs in one direction rather than in all the possible directions is attributed to the role of CTAB, which during the nucleation step creates a preliminary facial differentiation of the seeds. Once immersed in the growth solution, the seeds undergo a preferential attack by the surfactant on the more accessible face {100}, while the face {111} remained free from CTAB can grow, thus leading to the elongated cylindrical structure. For the same reason, the {111} face remains more reactive in each stage than the {100} face, which is the fact that influences all the surface chemistry of these nanostructures (Fig. 2).9
It has been demonstrated that free CTAB molecules, desorbing from the surface of the GNRs when in physiological conditions, have a strong cytotoxic effect on healthy cells.4 This is due to their ionic nature, which leads to a strong interaction with DNA and RNA molecules; in addition, their complete removal from the surface of the GNRs always gives an immediate irreversible aggregation. The replacement of CTAB appears to be the only alternative but it represents a great challenge due to the different reactivity of the {111} and {100} faces of the nanorods.10
3. Surface modification of GNRs
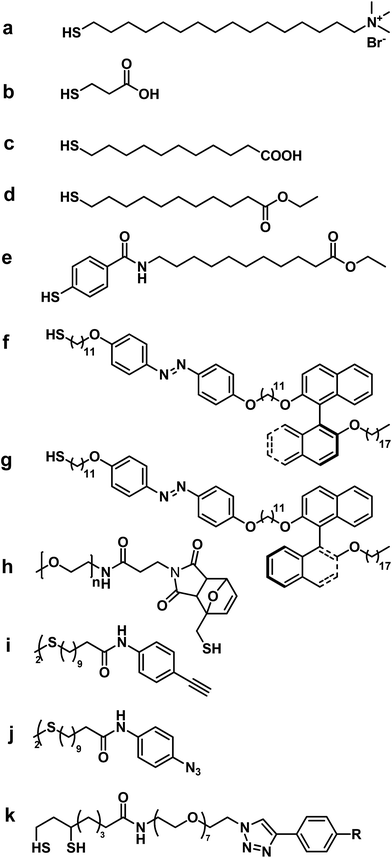
In the last decade, many efforts were made to address this issue, especially by developing a plethora of molecules or moieties that can substitute toxic surfactants in their role and simultaneously allow, reinforce or modify GNRs theranostic properties (Table 1). Moreover, several protocols assisting in these replacements have been described in the literature and many other studies are entering into the scientific landscape, highlighting the urgent need for a review summarizing the various options currently available (Fig. 3).3.1 Organic molecules
In recent years, many studies reported in the literature have showed the possibility to remove CTAB from the surface of GNRs and replace it with various organic molecules.In some cases, organic molecules have been directly linked to the surface of the GNRs, exploiting the presence of functional groups with high affinity for gold. These molecules can bind to the gold surface leading to the formation of a self-assembled monolayer (SAM) and to the displacement of the double layer of CTAB (Fig. 4). In other cases, organic molecules are previously modified by binding with other molecules such as a linker, which constitute the real coating layer on the surface of GNRs.
The thiol group is the most used functional group to bind with gold surface. Indeed, sulphur is characterized by a great affinity to the transition and noble metals.11 For this reason, thiol groups can bind strongly to gold surfaces to form a self-assembled monolayer. In this way, it is possible to form an organic protective layer on the surface of the GNRs by the replacement of CTAB, and thus it is possible to obtain GNRs with greater stability, better biocompatibility and hydrophilic or lipophilic properties, depending on the organic molecules that are used.
Thiolated CTAB and different thiol ligands have been used in ligand exchange reactions to modify the surface of GNRs. In particular, Vigderman et al.12 reported a strategy to coat GNRs with a thiolated CTAB analogue, namely, (16-mercaptohexadecyl)trimethylammonium bromide (MTAB) (Fig. 5a). The MTAB ligand contains an entire CTAB moiety and a pendant thiol group, which can be used to anchor the molecule on to the gold surface. In this way, a compact monolayer on the surface of the GNRs is formed.
In their study, Garabagiu et al.13 reported the ligand exchange reaction with 3-mercaptopropionic acid (Fig. 5b). In this case, 3-mercaptopropionic acid can be used as a linker thanks to the presence of the carboxylic group that could be exploited for any further functionalization. In addition, Dai et al.14 showed that it was possible to obtain GNRs that are soluble in both polar and non-polar organic solvents. In this case, the authors used 11-mercaptoundecanoic acid (MUA) (Fig. 5c) in a ligand exchange reaction through ion exchange resin to obtain GNRs–MUA, which were stable and soluble in both chloroform and methanol.
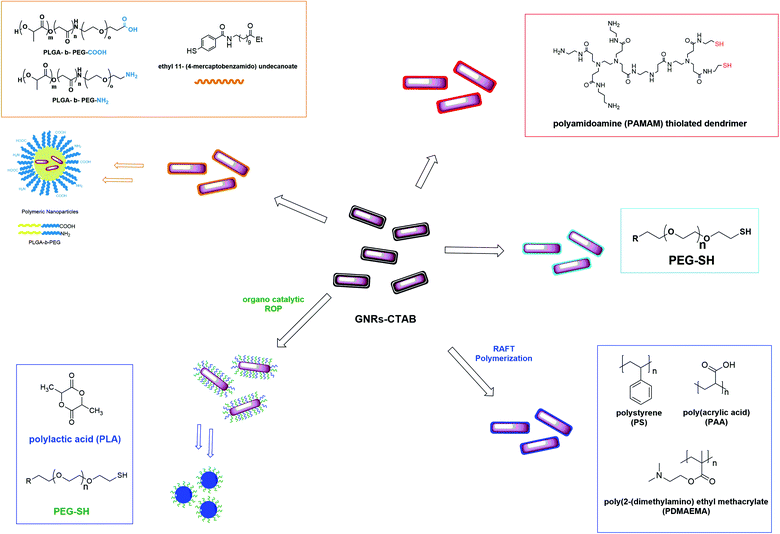
However, in the ligand exchange reaction on GNRs surface, all organic thiol ligands could not be used. We described the synthesis of lipophilic GNRs by ligand exchange reactions using two different organic thiols: ethyl 11-mercaptoundecanoate and ethyl 11-(4-mercaptobenzamido) undecanoate (Fig. 5d–e).15 The study showed that the exchange reaction and the consequent formation of lipophilic GNRs occured only with ethyl 11-(4-mercaptobenzamido) undecanoate. The different behaviour could be explained by the different structural properties of the two ligands, which confer different robustness and stability to the obtained GNRs. The presence of aromatic and hydrogen-bonding moieties, such as amides, allow adjacent molecules (ligands) to form multiple interactions, providing the formation of stable and robust monolayer on the surface of the GNRs.
Usually the use of organic molecules is not limited to the coating of the GNRs surfaces in order to improve biocompatibility. In some cases, specific organic molecules have been used to give specific chemical properties to the GNRs. An example for this is the study reported by Li et al.,16 who described the coating of GNRs with two opposite chiral azo thiol enantiomers synthesized starting from (R)-(+)-1,1′-bi-(2-naphthol) (Fig. 5f) and (S)-(−)-1,1′-bi-(2-naphthol) (Fig. 5g). After surface modification with the two different enantiomers of the chiral thiol, a protective organic monolayer on the surface is formed and the obtained GNRs acquired lipophilic and optical properties.
Moreover, GNRs can be used to impart specific properties to other materials. For example, Ori et al.17 used the thiol ligand ethyl 11-(4-mercaptobenzamido) undecanoate (Fig. 5e) to form lipophilic GNRs for the functionalization of a glass surface to constitute a powerful tool in areas ranging from electronics to biosensors. This system has been achieved by the immobilization of lipophilic GNRs on a thiol functionalized glass surface and can confer optical properties to the modified solid support.
When the organic thiol bears an additional functional group at the end of the alkyl chain, several interesting features can be exploited. Yamashita et al.18 described the synthesis of GNRs coated with a PEG-linked Diels–Alder cycloadduct (Fig. 5h) in which the organic molecules anchored on the surface become substrates of the chemical processes. In this reaction, GNRs are modified by insertion on the surface of the cycloadducts via Au–S linkages. The as obtained GNRs are irradiated by NIR light, which generates a photothermal effect inducing a retro Diels–Alder reaction that releases PEG chains bound to the cycloadducts.
Another example is represented by a click chemistry reaction on the surface of the GNRs coated by thiol organic ligands characterized by acetylene groups. We have described the development of a novel nanosystem consisting of GNRs assembled with silver nanoparticles, which presents both therapeutic and diagnostic capabilities.19 Lipophilic GNRs were synthesized using simultaneously two ω-functionalized-disulfides characterized by the presence of esters and acetylenes, while for silver nanoparticles a terminal azide was chosen (Fig. 5i–j). In this way, it was possible to covalently link the two nanostructures, which reacted by 1,3-dipolar click cycloaddition between an acetylene on GNRs and an azide group on the silver nanoparticles (Fig. 6).
However, polyethylene glycol thiolate (PEG–SH) is the organic thiol that is mostly used in ligand exchange reactions with GNRs thanks to the well-known biocompatibility of the PEG substrate. The use of synthetic polymers for these purposes will be discussed extensively in the subsequent section. We briefly describe here the use of thiol–PEG as a binding ligand on GNRs for the deposition of other functional molecules. Dreaden et al.20 published a study describing a novel delivery system to tumor associated macrophages (TAM). In this case, macrolides, a class of antibodies used for the treatment of microbial infections, were firstly covalently linked via click chemistry to thiol–PEG chains (Fig. 5k) and then the thiol groups of PEG were used to bind it to the surface of GNRs.
The thiol–PEG has also been used as linker by Son et al.21 to immobilize mannose on the surface of gold/nickel nanorods to obtain nanobrigdes for immune cell recognition.
The coating with organic thiols is not the only way to remove CTAB and modify the surface of the GNRs. Huang et al.22 described the coating of GNRs with silica using APTES, a reagent commonly used to form SiO2–NH2 in core–shell systems. The layer of silica is applied with the Strober method to remove the CTAB and improve the biocompatibility of the obtained GNRs. Moreover, the presence of amino groups on the surface provides the possibility to functionalize the surface covalently by conjugating targeting agents such as, in this case, folic acid.
3.2 Synthetic Polymers
Different synthetic polymers have been used to improve the biocompatibility of GNRs but PEG is the most utilized polymer in the literature. It is well-known that PEG gives biocompatibility, stealth characteristics and resistance for protein adsorption, providing a long plasma circulation time.In particular, the study reported in the literature by Grabinski et al.23 related to an in-depth analysis on the toxicity, comparing organic molecules and polymer coating surface of GNRs; two different nanosystems were synthesized from GNRs–CTAB by a ligand exchange reaction using two organic molecules, namely, mercaptohexadecanoic acid (MHDA) and thiol–PEG. The toxicity of these different systems has been investigated by the study of consequent gene expression in the cell lines. The in vitro studies showed that GNRs–MHDA have a dramatic effect on gene expression, more than PEG–GNRs. This phenomenon could be explained because of their enhanced interaction with cell membranes compared to PEG–GNRs, which also led to a greater uptake.
Recently, studies showed the use of not only “already formed” synthetic polymers but also different approaches to coat GNRs depending on the polymer used.
In this view, studies describing the synthesis of polymeric GNRs via radical polymerization are the most promising. Hotchkiss et al.24 used reversible addition–fragmentation chain transfer polymerization (RAFT) to coat GNRs with synthetic polymers. The authors investigated three different polymers, namely, PDMAEMA (poly(2-(dimethylamino) ethyl methacrylate), PAA (poly(acrylic acid)) and PS (polystyrene), and tested two different methods for the surface coating. In each case, they obtained GNRs completely surrounded by a relatively high polymer layer, the thickness of which depended on the polymer and grafting technique used.
In addition, the study of Song et al.25 reported the first example of surface-initiated living ring-opening polymerization (ROP) of biodegradable polymers on GNRs to obtain nanosystems for plasmonic theranostic applications. They described the synthesis of amphiphilic GNRs coated with polylactic acid (PLA), via surface initiated organocatalytic living ROP, and PEG, via a ligand exchange reaction. In the presence of water, the so-obtained GNRs formed well-defined vesicles consisting of a PEG corona and a GNRs-embedded PLA shell, characterized by a unique combination of structural and optical properties.
Another interesting polymeric material used for coating GNRs are dendrimers, which are a class of polymers with a highly ordered branched structure. Dendrimer coatings are used to modify the surface because of their capability to alter charge surface, functionality, and reactivity, as well as to enhance the stability and dispersion of the nanosystems. Li et al.26 demonstrated the use of polyamidoamine (PAMAM) thiolated dendrimer to synthesize dendrimer-modified GNRs, removing CTAB from the surface of the GNRs and improving their stability and biocompatibility. Indeed, dendritic nanocomposites are characterized by different properties, such as large amount of functional groups, perfect symmetry, and internal cavities, which make them excellent tools for applications in nanomedicine. In addition, the dendrimer-modified GNRs were further modified on the surface with a targeting agent, which confers high selectivity to the synthesized nanosystems.
As shown by these examples (Fig. 7), in most cases, synthetic polymers are used to coat the surface of the GNRs by direct linkages of end-functionalized polymers on the surface via a ligand exchange reaction. However, the synthetic polymers can also be used to form polymeric nanocarriers to entrap lipophilic GNRs and to form targetable biocompatible nanosystems. We15,27 showed the synthesis of polymeric nanoparticles, consisting of the amphiphilic copolymer poly(lactic-co-glycolic)-co-poly(ethylene glycol) (PLGA-b-PEG–COOH). This polymer consists of both PEG and a low-molecular-weight hydrophobic core-forming block such as PLGA. Using this copolymer, it is possible to form GNRs containing polymeric nanoparticles, presenting a hydrophobic core to entrap lipophilic GNRs and a hydrophilic shell to allow the stabilization of the nanosystem in aqueous solution as well as further surface conjugation.
3.3 Polyelectrolytes
A widely used method to coat nanosystems is the electrostatic physisorption of polyelectrolytes (Fig. 8). This method improves the dispersion stability and provides support for the immobilization of targeted agents (antibiotics and proteins). However, nowadays, it is still necessary to improve the stability and biocompatibility of nanosystems coated with polyelectrolytes because they are generally cytotoxic. Indeed, physisorbed polyelectrolytes, under certain physiological conditions, are easily desorbed from the surface of the nanosystem because of their variable and labile surface binding energies.One of the first examples regarding the use of polyelectrolytes to modify the surface of GNRs is reported by Gole et al.28 The authors used the layer-by-layer method to form multilayers of polyelectrolytes on positively charged GNRs–CTAB by the sequential deposition of anionic and cationic polyelectrolytes. Initially, GNRs–CTAB are coated with the anionic poly(sodium-4-styrenesulfonate) (PSS) and then with the cationic poly(diallyldimethylammonium chloride) (PDADMAC). This process is continued until multiple layers of polyelectrolytes are formed on the surface of the GNRs. In a study reported by Parab et al.,29 the same method was used to coat GNRs–CTAB with poly(sodium-4-styrenesulfonate) (PSS). The PSS modification allowed the conjugation of the IgG antibody on the surface of the GNRs, which facilitated the investigation of their cytotoxicity, cellular uptake and physiological detection of proteins. This study showed that PSS significantly increases the cell viability and internalization of GNRs, and the presence of the antibody promotes the assembly of GNRs with preferential lateral orientation (side-to-side and end-to-end).
However, polyelectrolyte multilayers prepared with the layer-by-layer method are characterized by a poorly defined interface between the various layers and also by non-covalent bonds between organic moieties and the surface. These features confer instability to these modified nanosystems. Leonov et al.30 thoroughly discussed the importance of CTAB replacement and developed a novel scalable protocol for surfactant exchange based on polyelectrolytes-coated GNRs. However, in this work, it was shown that the PSS-coated GNRs are highly cytotoxic due to the presence of residual CTAB–PSS complexes, which are gradually desorbed from the surface of GNRs, and therefore it is necessary to carefully purify GNRs to obtain the complete replacement of CTAB. In addition, it has been demonstrated that cellular uptake is strongly influenced by different superficial charges that characterize the synthesized polyelectrolytes–GNRs. The studies reported by Hauck et al.31,32 assessed the relationship between surface coating and cellular uptake. GNRs were covered by the layer-by-layer method with various polyelectrolytes, resulting in nanorods characterized with different surface charges. The study showed that the lowest cellular uptake was exhibited by negatively surface charged GNRs, while the highest cellular uptake was exhibited by the positively charged system.
The same concept has been reported by Xu et al.33 The study described the use of GNRs as possible vehicles for gene delivery, in particular as DNA vaccine adjuvants. Two different cationic molecules, namely, poly(diallyldimethyl ammonium chloride) (PDDAC) and polyethyleneimine (PEI), were used to modify the surface of the GNRs on which HIV Env plasmid DNA was conjugated. Biological assays showed that PDDAC- and PEI-modified GNRs can significantly improve cellular and tumoral immunity due to the surface chemistry on the adjuvant activity.
Another interesting method for the surface modification of GNRs involves the use of phospholipids, which form a double layer on the surface. The study by Takahashi et al.34 described the formation of phospholipids–GNRs by extraction using a chloroform phase. They showed that CTAB was successfully removed from the GNRs solution by simple extraction using chloroform containing phosphatidylcholine (PC), as an additional stabilizing agent. Indeed, PC is a possible candidate for suppressing the aggregation of GNRs after CTAB extraction and for reducing cytotoxicity.
Finally, Orendoff et al.35 developed a method to synthesize phospholipids–GNRs using the lipid vesicle fusion approach. In this way, it was possible to obtain GNRs with phopholipid vesicles consisting of phosphatidylcholine lipids by immobilizing liposomes at the surface of the nanorods. Although this polyelectrolyte differs from CTAB surfactant in net electrostatic charge at neutral pH, both are terminated with trimethylammonium head groups that may interact similarly with the surface of the GNRs. This work showed that it is possible to modify the surface of the GNRs with different functional groups by coating them with a single component ligand or bilayers.
3.4 Peptides, proteins and natural sugars
Natural materials, such as natural polysaccharides, natural polymers, peptides and natural products, have been used extensively as possible materials in the development of biocompatible drug delivery systems. Indeed, they have important features, such as high stability, biodegradability, and biocompatibility that make them potential tools for biomedicine applications.Chitosan is a non-toxic, biocompatible, biodegradable and natural polysaccharide, which is produced by the deacetylation of chitin, and it is found in the exoskeleton of some crustaceans and insects. This natural polysaccharide is used successfully in nanomedicine applications for the delivery of drugs, genes and proteins. In particular, GNRs have also been coated with chitosan using different techniques and materials to develop a novel biocompatible theranostic nanosystem. Indeed, chitosan can be covalently bound to the surface of GNRs in different ways. It is possible to covalently bind chitosan to the organic molecules presented on the surface of GNRs, or to chemically modify chitosan chains with organic molecules characterized by functional groups, which are able to coordinate to the thiol groups on the surface of the GNRs. In both the cases, the formation of covalent bonds involves a coupling reaction to form a stable amide bond between the carboxylic group on the organic molecules, activated in some cases by 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) chemistry, and the amine groups present over the surface of chitosan.
In the study reported by Charan et al.,36 chitosan oligosaccharide-modified gold nanorods were synthesized using 11-mercaptoundecanoic acid (MUA). Chitosan was linked to the carboxylic groups of MUA on the GNRs. The study showed that chitosan–MUA–GNRs exhibited the least toxicity in comparison to other synthesized surface-modified gold nanorods. The resulting chitosan–GNRs were conjugated with a tumor targeting monoclonal antibody against EGFR, which showed good cellular uptake and biodistribution.
In the work of Garabagiu et al.,37 GNRs have been coated with chitosan via a cross-linker, namely, 3-mercaptopropionic acid (MPA). The chitosan–MPA–GNRs were synthesized by not using EDC chemistry but using magnetic stirring for 2 days.
The organic molecules utilized to modify chitosan are characterized by features that improve the chemical properties and functional groups of chitosan which, in turn, is able to coordinate to the surface of the gold. In the study of Wang et al.38 chitosan was covalently grafted with mercaptoacetic acid (MAA). The obtained modified chitosan–MAA was characterized by the presence of thiol groups that bind with the surface of the GNRs. In addition, the targeting agent folic acid was also conjugated on the surface of the GNRs.
R. Duan et al.39 in their work modified chitosan with polyethylene glycol (PEG) and thiolated polyethylenimine (PEI). The introduction of these hydrophilic polymer chains enhanced the water solubility of chitosan, which is normally soluble only in acid conditions (pH < 5). Doxorubicin (DOX) was then chemically conjugated to thiol-modified chitosan and the as-synthesized chitosan–polymer–DOX was used to coat GNRs to obtain novel nanocarriers with good biocompatibility and optical properties.
In addition, Choi et al.40 synthesized nanocarriers for GNRs using photopolymerization with chitosan chemically conjugated to Pluronic F68. The obtained GNRs showed increased cellular uptake in vitro and a photothermal effect for a cancer cell line, suggesting a promising feature for clinical phototherapeutic applications.
Moreover, peptides and other natural materials have been used to modify the surface of GNRs to improve cellular uptake. For example, in the study of Khan et al.,41 GNRs have been coated with three different amphiphilic ligands to study how different ligand properties could influence protein crown formation and consequently cellular uptake. Three different amphiphilic ligands were obtained from GNRs–CTAB by ligand exchange reaction: neutrally charged GNRs–polyoxyethylene (10) cetyl ether, cationic GNRs–phospholipid oligofectamine and anionic GNRs IPID–phosphatidylserine (PS). The study showed that the protein crown formation and their physical properties were influenced by the nature of the amphiphilic ligands.
In the study of Murakami et al.,42 GNRs were modified with natural molecules, in particular (Z)-9-octadecenoate (oleate) and high density lipoprotein (HDL) (a mediator of reverse cholesterol transport), which can interact with tissues and cells. The results showed that the as-synthesized cpHD–GNRs were 80 times more efficiently internalized than poly-(ethylene glycol)-conjugated GNRs and were able to elicit cancer cell photoablation.
In other examples, the surface of the GNRs were decorated with different peptides, which may act as targeting agents such as in the study reported by Alkilani et al.43 GNRs were functionalized with the EphA2 homing peptide, YSA, using a layer-by-layer polypeptide wrapping approach. The peptide was linked to a polyelectrolyte chain (polyaspartate) via a PEG linker to enable polyelectrolyte wrapping around the starting cationic GNRs. The obtained peptide-functionalized GNRs were used to explore how the presence and orientation of the YSA peptide can influence the stability of GNRs on biological media, cellular uptake, and proliferation in cancer cells.
Moreover, Park et al.44 described a method to functionalize GNRs with an engineered fusion protein in which gold-binding polypeptide (GBP) was fused with staphylococcal protein A (SpA). The replacement of free CTAB during the functionalization step prevents the CTAB-induced aggregation of GNRs and GBP–SpA complexes. The resulting nanosystems can be easily functionalized and conjugated to form a potential tool for theranostic applications.
Finally, in the study of Jang et al.,45 a multifunctional nanomedicine platform consisting of a gold nanorods–photosentizer complex was developed for non-invasive near-infrared fluorescence imaging and cancer therapy. The synthesis of this nanosystem consisted of sequential conjugation of thiol-terminated monomethoxy poly(ethylene glycol) (mPEG–SH) and a targeting ligand consisting of a positively charged oligopeptide made of arginine (R), leucine (L) and cysteine (C) (RRLAC) on the surface of GNRs. Then, the negatively charged photosensitizer Au(III) phthalocyanine chloride tetrasulfonic acid (AlPcS4) was incorporated onto positively charged PEG–GNRs–RRLAC. This study showed that it is possible to combine a photosensitzer agent with GNRs to combine photothermal therapy (PTT) with photodynamic therapy (PDT) in cancer treatments.
3.5 Monoclonal antibodies
Monoclonal antibodies are widely recognized as one of the most selective and promising agents to target cancer cells. They are engineered to exploit their natural ability to recognize a single specific antigen, making them potentially suitable for active drug delivery of nanoparticles against any disease.46 Moreover, monoclonal antibodies have advanced significantly over the past two decades as therapeutics for cancer therapy, showing the appealing possibility to act both as a targeting agent and as a therapeutic selective drug.47It is clear that their linking onto GNRs could lead to important advantages due to the combination of these important monoclonal antibody features and the characteristics of the nanostructures.
Firstly, in 2006, El-Sayed's research was focused on this topic.48 In this study, an anti-epidermal growth factor receptor (anti-EGFR) monoclonal antibody was conjugated onto the surface of GNRs that were previously capped with poly(styrenesulfonate) through electrostatic physisorption interactions. The aim was to demonstrate the in vitro efficacy of GNRs on malignant oral epithelial cell lines as a contrast agent for both molecular imaging and photothermal cancer therapy. The antibody-GNRs were able to selectively bind to the malignant cells with a much higher affinity due to the overexpressed EGFR on their cytoplasmatic membrane. In this case, only electrostatic bonds for the interaction between GNRs and the monoclonal antibody were exploited. Another example of GNRs modified with monoclonal antibodies is found in the work of Park et al.49 In 2009, they reported the layer-by-layer deposition of anti-rabbit IgGs onto the surface of GNRs by electrostatic interactions to enhance the targeting and imaging of biomarkers expressed on the surface membrane of cancer cells. In this case, mercaptopyridine was attached to the surface of GNRs and their surface charge was modified using a layer of poly-(sodium 4-styrene-sulfonate) switching it from positive to negative to enable antibody electrostatic absorption.
More recently, Choi et al.50 covalently linked GNRs and cetuximab, an anti-epidermal growth factor receptor, in a two-step reaction: first, GNRs were coated with a hetero-bifunctional PEG, bearing both a thiol and a carboxylic acid group (HS–PEG–COOH), thus removing CTAB molecules and increasing biocompatibility; then, the carboxylic groups remained exposed on the surface were covalently linked with an amino group present on the internal structure of cetuximab. The as-obtained functionalized GNRs showed excellent tumor targeting ability and a promising effect in imaging and photothermal therapy of epithelial cancer cells.
Similarly, Liao et al.51 and Puvanakrishnan et al.52 covalently conjugated monoclonal antibodies onto the surface of GNRs exploiting a mixed coating with methoxy–PEG–thiol and thiol–PEG–thiol to dilute the reactive groups on the surface of GNRs, thus avoiding cross-linking issues. The remaining terminal thiol group at one end of the bifunctional PEG was reacted with the antibody previously functionalized with a maleimide-containing crosslinker agent.
Despite the improvement in the efficacy of GNRs that can be achieved with the decoration of antibodies, many approaches still suffer several drawbacks including low stability and potential cytotoxicity of bioconjugates that are produced by electrostatic interactions, as well as lack of control over antibody orientation during covalent conjugation. Due to this reason, Joshi et al.53 investigated a “directional” antibody conjugation onto the surface of GNRs. In their work, the directional conjugation was achieved by oxidizing the carbohydrate moiety, specifically present on the heavy chain of the fragment crystallizable (Fc) region of most antibodies, to an aldehyde group and then by attaching it with a hetero-functional linker with hydrazide and dithiol groups on the surface of GNRs. The obtained modified GNRs were tested both for stability and cancer cell recognition with satisfactory results.
3.6 Oligonucleotides
Gene therapy has been studied intensively during the last few years due to its appealing features. Using RNA, DNA or chemically-modified oligonucleotides, the protein-based treatment issues, such as in vivo immunogenicity, lack of thermal stability and large scale synthesis, were overcome. Nucleic acid-based moieties can be divided in several categories based on their composition and structure (such as, single strand oligonucleotides, antisense oligonucleotides, DNA decoy, and RNA decoy) or their target (such as, proteins, genes, and micro RNA).54 Oligonucleotides have shown promising ability both as therapeutic agents because they can interact in cell gene-expression and life cycle, and as a targeting species due to their almost unique specificity against a target receptor.55,56Despite the many studies already available concerning the modification of nanoparticles with oligonucleotides,57 there are still only a few examples of such types of modifications onto GNRs.
One of the first examples of oligonucleotide-conjugated GNRs for nanomedicine applications was reported in 2005 by Takahashi et al.58 They employed phosphatidylcholine modified GNRs for plasmid DNA anchoring and release under NIR stimuli. Then, in 2006, Chen et al.59 reported the first preparation of a DNA-fragment covalently attached onto GNRs via a thiol group, previously inserted in the DNA sequence. In this case, DNA was selectively released from GNRs upon ultrafast NIR laser irradiation to induce specific gene expression in target cells. In 2008, Wijaya et al. exploited a similar procedure60 for the conjugation and selective release of two different DNA oligonucleotides from two gold nanorods with different aspect ratios just triggering laser wavelength. A particularly appealing innovation was proposed by Xu et al.,33 who investigated novel surface-engineered GNRs used as a promising carrier for a DNA vaccine against HIV; three different types of molecules were placed onto GNRs (namely, CTAB, poly(diallydimethylammonium) chloride and polyethyleneimine), and their transfection capability, internalization, cellular trafficking and DNA releasing ability were all related to the primary surface modification, thus casting light on the rational design of nanomaterials as a versatile platform for vaccine adjuvants/delivery systems. Recently, Shanmugam et al.61 successfully developed a hybrid double stranded DNA that ended with a thiol group for conjugation with GNRs, which worked as an intercalating binding site for doxorubicin and as a tethering agent for platinum [Pt(IV)] prodrugs. In this case, cancer cells were targeted with folic acid that was covalently conjugated to the acid group in the axial ligand of the platinum pro-drug. This complex architecture could release two chemotherapeutics under NIR laser exposure as result of GNRs generated hyperthermia, thus limiting the toxicity of common cancer treatments.
Small interfering RNA (siRNA) was coupled with GNRs in 2009 in the work of Bonoiu et al.62 They developed GNRs–siRNA complexes (called nanoplexes) that targeted the dopaminergic signalling pathway in the brain. In particular, GNRs were used to show the effective interaction with siRNA through shifts in their longitudinal plasmon resonance and to visualize neurons in vitro. The same authors also released a patent in 2011 regarding the methods of using GNRs–siRNA complexes for gene therapy.63 One last example of GNRs–siRNA complex could be found in the work of Tahmasebifara et al.64 They investigated the various phenomena occurring during the formation of GNR–siRNA nanoplexes. The authors, using several analytical methodologies, gave an important insight into the nature of the interaction between the metal surface and such biomolecules.
Aptamers, another class of potent and very modern oligonucleotides, were also exploited for conjugation with GNRs. Indeed, in 2012 (ref. 65) and with improvements in 2013,66 Wang et al. interestingly developed an aptamer switch probe linked to a photosensitizer molecule and covalently attached it to the surface of GNRs to combine photodynamic therapy (PDT) and photothermal therapy (PTT). They were able to modify its conformation, and the photosensitizer molecule was released only in the presence of target cancer cells. A different aptamer was used by the same group to target and kill cancer cells and cancer stem cells upon photothermal activation of GNRs.67
This overview suggests that, with time, oligonucleotides-conjugated GNRs have been attracting increasing attention, and an increasing number of publications are appearing in scientific journals, even if the total amount remains scarce and requires more studies (Table 1).
| Entry | Surface modification | Test | Cell line/tumour | Results | Ref. |
|---|---|---|---|---|---|
| 1 | SiO2–NH2/folic acid | CCK-8 assay | MGC803 cells | GNRs–SiO2–FA possesses non-cytotoxicity and excellent biocompatibility | 22 |
| 2 | Mercaptohexadecanoic acid (MHDA) and thiol–PEG | MTS test | HaCaT (keratinocyte cells) | Ligand exchange to MHDA and PEG resulted in improved viability and lower toxicity | 23 |
| 3 | Vesicles consisting of PEG corona and GNRs-embedded PLA shell | Combined dual-modality chemo-photothermal therapy with CCK-8 assay | In vitro: Hep 3B cells | GNR@PEG/PLA vesicles showed no toxicity till 24 h after uptake by cells | 25 |
| 4 | Dendrimer-modified GNRs (dGNRs) | Cytotoxicity studies with Kit-8 assay and selective photothermal therapy of dGNRs | In vitro: HUVEC (non-malignant cells) A375 (melanoma cell lines) | RGD-conjugated gGNRs are not cytotoxic | 26 |
| 5 | GNRs-(11-(4-mercaptobenzamido) undecanoate) entrapped in polymeric nanoparticles | Cytotoxicity studies with CFE assay | In vitro: Balb/3T3 | IC50 = 20.3 μM | 27 |
| 6 | poly(sodium-4-styrenesulfonate) (PSS) | Cytotoxicity and cellular uptake studies with MTS assay | In vitro: S-G (normal gingival epithelioid cells) TW 2.6 (oral cancer cells) | PSS significantly increased the cell viability and showed easy intracellular uptake of the nanorods | 29 |
| 7 | poly(sodium-4-styrenesulfonate) (PSS) | Cytotoxicity studies with MTT and LDH assays | In vitro: LLC-PK1 (porcine kidney cells) HepG2 (human liver carcinoma cells) KB (human nasopharyngeal carcinoma cells) | Cytotoxicity assays of PSS-coated GNRs exhibited IC50 values in the low to submicromolar range | 30 |
| 8 | Differently charged polyelectrolytes (layer-by-layer method) | Cell uptake and transmission electron microscopy (TEM) | In vitro: OCI AML3 (myeloid leukemia cells) Jurkat T-cells MCF-7 (breast cancer cells) | The study showed that the lowest cellular uptake was exhibited by negatively charged surface of GNRs, while the highest cellular uptake was exhibited by the positively charged system | 31 and 32 |
| 9 | Phosphatidylcholine (PC) (phospholipids–GNRs) | Cytotoxicity studies | In vitro: HeLa cells | GNRs–PC show lower cytotoxicity than GNRs–CTAB | 34 |
| 10 | Chitosan–MUA (11-mercaptoundecanoic acid) | Cytotoxicity studies with MTT assay and study of toxicity in vivo | In vitro: CAL27 (carcinoma cells) | Chitosan modification exhibited low toxicity and rapid excretion | 36 |
| In vivo: BALB/c nude mice model | |||||
| 11 | Chitosan–mercaptoacetic acid (MAA) | Cytotoxicity studies with MTT assay | In vitro: HT-29 (colorectal carcinoma cells) NHI 3T3 (embryonic fibroblast) | Cell viability of CTAB-passivated GNRs was improved by thiolated chitosan capped GNRs | 38 |
| 12 | Chitosan–polyethilene glycol (PEG)/thiolated polyethylenimine (PEI)/doxorubicin (DOX) | Cytotoxicity test CCK-8 assay | In vitro: MCF-7 (breast cancer cells) A549 (lung cancer cells) HeLa (cervical cancer cells) and L929 (fibroblast cells) | Chitosan modified GNRs have been demonstrated to have good biocompatibility and stability | 39 |
| 13 | Chitosan–pluronic F68 | Cytotoxicity test and cellular uptake | In vitro: SCC7 (tumor cells) NIH/3T3 (fibroblast cells) | Chitosan conjugated GNRs have not shown any acute cytotoxicity | 40 |
| 14 | YSA, EphA2 homing peptide | Cytotoxicity studies with MTS assay | In vitro: PC-3 cells | Functionalized GNRs induced no acute cytotoxicity under the concentrations tested | 43 |
| 15 | PSS and anti-EGFR monoclonal antibody | Light scattering images | In vitro: non-malignant epithelial cell line (HaCat) and two malignant oral epithelial cell lines (HOC313 clone8 and HSC 3) | No cytotoxicity evidence after 30 minutes incubation | 48 |
| 16 | Mercaptopyridine, poly-(sodium 4-styrene–sulfonate) and anti-rabbit IgGs | Dark-field images | MCF7 breast cancer cells | No cytotoxicity evidence after 2 hours incubation | 49 |
| 17 | COOH–PEG–SH and cetuximab | MTT assay | A-431 and MCF7 cell line | 10-fold Lower cytotoxicity after 24 hours incubation for conjugated GNRs in comparison with non-conjugated GNRs | 50 |
| 18 | Methoxy–PEG–thiol and thiolated antibody | MTS assay | A431, MDAMB-435 cancer cells and J774A.1 macrophage cells | No cytotoxicity of surface modified-GNRs. Strong cytotoxicity for CTAB coated GNRs | 53 |
| 19 | pEGFP–N1 template DNA–SH | Trypan blue assay | HeLa cells | No induced cytotoxicity. After NIR exposure, gene expression was revealed after 1–2 day only for GNRs antibody-conjugated | 59 |
| 20 | Double stranded DNA with doxorubicin or platinum prodrugs plus folic acid | MTT assay | HeLa cells | Enhanced toxicity and drug release under NIR exposure of surface modified GNRs in comparison to components alone | 61 |
| 21 | siRNA (nanoplexes) | MTT assay | DAN cells | 98% viability one week post treatment | 62 |
| 22 | mPEG–SH, ASP and Ce6 photosensitizer | MTS assay | CCRF-CEM cells | Cell viability decreased to about 80% after combined PTT and PDT therapy | 65 and 66 |
| 23 | CSC1 aptamer and CSC13 aptamer | MTS assay | DU145 cells | Strong cytotoxicity after exposure to aptamer-conjugated GNRs and NIR irradiation | 67 |
4. Nanomedicine applications of GNRs
As discussed above, the great success of GNRs during the last few years is mainly due to their various exploitable and tunable properties for nanomedicine applications. The abovementioned features of GNRs make them suitable as contrast agents for the early diagnosis of diseases as well as a therapy mediator, allowing the use of non-invasive techniques in both the cases. Indeed, the possibility to combine together diagnosis and therapy is at the base of the innovative field of theranostics (therapeutic + diagnostic), which is now leading the most innovative worldwide investigations (Fig. 9).68Strong light absorption and scattering of GNRs allow their use in several imaging techniques, such as dark-field microscopy,69,70 optical coherence tomography (OCT),71–73 two-photon luminescence (TPL),74,75 photoacoustics (PA),27,76 X-ray computed tomography (CT) imaging,77 and ultrasound (US), which enable early pre-symptomatic diagnosis of various diseases.78 With regards to therapy, great ability of GNRs to convert light into heat is mostly exploited through the hyperthermia effect for photothermal therapy (PTT);79–81 moreover it can also be used to trigger the thermo-sensitive release of active moieties in drug delivery systems.60,82 Recently, as mentioned earlier, GNRs–photosensitizer composites have been developed and exploited in photodynamic therapy (PDT).66
The development and improvement of an appropriate coating of GNRs have gained increasing attention, and in some cases, these investigations have been successful. Because various reviews have already satisfactorily described all the appealing possibilities to use GNRs in nanomedicine, both for diagnosis and for therapy, the subsequent paragraphs will focus mainly on the progress made in these research fields using GNRs decorated with several organic molecules and active components.
4.1 Imaging with surface-modified GNRs
When discussing imaging techniques assisted by the use of GNRs, in vitro imaging and in vivo applicable imaging must be distinguished. Dark field microscopy and two-photon luminescence are mostly related to a cellular environment, while optical coherence tomography, photoacoustic imaging and X-ray computed tomography are applicable also pre-clinically and to living beings.4Dark field microscopy is widely used to localize GNRs in the cellular environmental and to demonstrate the selective targeting of these nanostructures against specific target sites when coated with selective active moieties such as proteins, peptides, monoclonal antibodies and oligonucleotides, as already discussed above. A perfect example of this technique, which is at the heart of the first papers concerning imaging using GNRs, is represented by the work of El-Sayed.48 They not only investigated the variation in the different colored scattered light by changing the aspect ratio of GNRs, but also showed that GNRs conjugated to anti EGFR scattered differently after binding to malignant or nonmalignant cells. Indeed, cancerous cells showed a red to orange scattered light, while individual noncancerous cells were hardly identifiable due to the non-specific interactions between the nanoparticles and the cells. Following this research, several other investigations, both practical and theoretical, have appeared in the scientific literature.83–85
Two-photon luminescence using GNRs followed a similar pathway. Indeed, it was observed that when molecules are adsorbed on the surface of noble metal particles, a huge field enhancement occurs, which gives rise not only to the well-known surface enhanced Raman scattering (SERS) signals of molecules but also to a spectrally broad background photoluminescence (PL).86 Even if studies of PL from metal nanoparticles were limited, due to their low quantum efficiencies, the strong enhancement of PL from GNRs upon single photon excitation was reported probably due to their unique SPR.87 Two-photon optical processes, which involve an additional field enhancement, and thus a greater enhancement of PL efficiency, has been recently applied in GNRs imaging.88 Moreover, for dark-field microscopy, TPL has also been exploited many a times for the in vitro detection of cancer cells after binding it with targeting agents that are conjugated to GNRs. Durr et al.,75 in 2007, demonstrated with phantom experiments that the TPL intensity from GNRs-labeled cancer cells was 3 orders of magnitude brighter than the two-photon auto-fluorescence (TPAF) emission intensity from unlabeled cancer cells at 760 nm excitation light, showing that GNRs can be an attractive contrast agent for the two-photon imaging of epithelial cancer. More recently, the first application of TPL in in vivo systems has been also reported. Wang et al.89 demonstrated the possibility to use GNRs as TPL imaging agents by the in vivo monitoring of single nanorods flowing in the blood vessels of mouse ear due to the intrinsic 3D spatial resolution of TPL, which can be useful for monitoring biological processes in real time even if it lacks deep tissue penetration. Unfortunately, in this work, no particular attention was paid to the functionalization of GNRs because they were coated with the original CTAB molecules, but the results achieved are still worth mentioning, and surely an appropriate coating will help improving this imaging modality.
More appealing possibilities were derived from the use of well-established imaging techniques, which were already suitable for living systems. Optical coherence tomography (OCT), an interferometric imaging technique based on the detection of backscattered NIR light, can provide an innovative, non-invasive diagnostic methodology due to good penetration depth as well as high spatial resolution, which are both superior to the present clinical methods of non-invasive imaging, such as ultrasound or magnetic resonance imaging. The use of enhancer contrast agents for this technique is strongly encouraged, and GNRs have been recently discovered to be excellent nanostructures for this task.90 GNRs have been shown to produce a detectable OCT signal at the minimal concentration of 25 μg of Au per ml in intralipid suspension. Moreover, Troutman et al.90 showed that GNRs with plasmon resonance wavelengths overlapping the OCT source yielded a signal-to-background ratio of 4.5 dB in tissue phantoms. Despite the great potential of this methodology, still only a few papers appear to report an in vivo diagnostic application. One of the first attempts was made by Tucker-Schwartz et al.,91 who applied photothermal-optical coherence tomography (PT-OCT) for in vivo imaging using GNRs as a contrast agent. In vivo PT-OCT images were acquired after subcutaneously injecting 400 pM GNRs embedded in a gel matrix into mice, which revealed an appreciable increase in signal in the presence of GNRs compared to controls, demonstrating the possible translation of PT-OCT from in vitro to in vivo imaging.
Probably the most widely used modality to visualize GNRs is photoacoustic imaging (also known as optoacoustic imaging). The intrinsic conversion of light into heat in GNRs is exploited in this technique, which involves the use of laser pulse irradiation and the detection of the resulting acoustic waves generated from the temperature gradient and expansion of the illuminated contrast agent. The technique itself already found large applications for tumor detection92 or blood oxygenation monitoring,93 but GNRs can provide an increasing detection capability, thus widening the applications of this field.
Agarwal et al.94 showed that GNRs, with peak absorption in the range of 700–840 nm, conjugated with an antibody specifically designed for Her-2/neu antigen, which is overexpressed in LNCaP prostate cancer lines, can be used to enhance optical absorption and photoacoustic signals in targeted prostate cancer tissue, thus providing high contrast for non-invasive cancer imaging of a single layer of cells. Moreover, Li et al.95 demonstrated the possibility to exploit GNRs with different aspect ratios for the combined PA imaging and simultaneously measuring the expression levels of different oncogenes in cancer cells. Interestingly, in this study, an antibody for HEGR2 antigen, expressed in MBT2 (murine bladder cancer) cells, and another one for CXCR4 antigen, expressed in HepG2 (human hepatocellular carcinoma) cells, were used as target molecules. These two monoclonal antibodies were conjugated to the surface of two types of GNRs with different aspect ratios (5.9 and 3.7) and different optical absorption peaks (1000 and 785 nm, respectively). Appropriate selection of laser irradiation wavelength allows PA signals only from GNRs corresponding to specific bindings, thus making them distinguishable.
In vivo applications of GNRs as PA contrast agents have also been reported since 2007. Eghtedari et al.96 investigated the detection limit of GNRs in vivo with PA; in this investigation, GNRs were coated with mPEG–thiol chains or alternatively with PSS to render them suitable for in vivo injection. The results showed that 25 μL of GNRs at a concentration of 1.25 pM can be detected in mice after subcutaneous injection using a single-channel acoustic transducer, confirming that these nanostructures are powerful contrast agents for PA imaging. More recently, we27 demonstrated that the ability to detect surface-modified GNRs entrapped in biodegradable polymeric micelles can be highly suitable for advanced drug delivery applications. As already explained, with a double phase transfer protocol, lipophilic GNRs can be encapsulated into physiologically stable, biocompatible and targetable micelles, while remaining detectable with PA imaging until the concentration of 11 μM. Latterly, Li et al.97 continued their studies regarding discernible cancer cells using GNRs with different aspect ratios and demonstrated the same possibility also in vivo. In addition, in this case, two different GNRs were coated with two monoclonal antibodies and PEG to use them in vivo and to avoid non-specific interaction between antibodies and GNRs, and then they were injected subcutaneously in tumor-bearing mice. The results clearly showed the presence of GNRs at the tumor site, and increase in PA signals only when the corresponding antibody was conjugated onto the surface, allowing not only easy tumor detection but also the possibility to distinguish different types of cancer.
On top of the most applied clinical imaging techniques, ultrasound (US) presents unique features in terms of low cost, manageability, non-invasiveness and real-time imaging. Even if US is already widely used by clinicians, the sensitivity of this diagnostic method could be greatly improved by exploiting ultrasound contrast agents (UCA).78 GNRs, especially when entrapped into soft-materials such as polymeric capsules or micro-bubbles, have been demonstrated to be appropriate contrast agents. In one of our papers, we demonstrated27 by phantom study the possibility to visualize GNRs previously entrapped in polymeric micelles using only US imaging. Gel spheres with concentrations of GNRs ranging from 550 μM to 11 μM were imaged with a single laser pulse, delivering energy of approximately 10 mJ cm−2, which were clearly detected by US.
This technique was also recently applied in vivo by Ke et al.,98 who deposited GNRs modified with PSS via electrostatic interaction onto a surface of microcapsules made from polylactic acid and modified with a layer of poly(allylamine hydrochloride) (PAH). The as-obtained nanosystem was evaluated for acoustic enhancement; in vivo imaging of the kidney of rabbits post injection provided a clear and detailed view of renal vascularity with excellent enhancement compared to the same investigation without any contrast agent.
However, because the same instrumentation is used both for US and PA techniques, they are frequently coupled to create dual methodologies that provide complementary information. This combination is described as photoacoustic ultrasound (PAUS) imaging.99
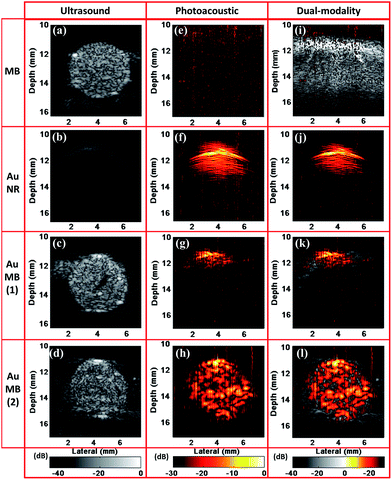
Wang et al.100 prepared cystamine modified GNRs to increase biocompatibility and to enable their entrapment into human serum albumin microbubbles (MB), which can be detected under both US and PA imaging. Dual-modality contrast enhancement obtained with PAUS was demonstrated in polyacrylic acid gel spheres loaded with the hybrid nanosystem, and the enhancement obtained in signal and resolution was evident (Fig. 10).
In vivo application of PAUS has also been developed. For instance, Kim et al.101 produced biocompatible GNRs by coating their surface with mPEG–thiol polymer and using it as a silane coupling agent for silica coating. The silica-coated GNRs were chosen due to their enhanced thermal stability and photoacoustic signal response. Ultrasound-guided photoacoustic imaging in xenograft tumor-bearing mouse was exploited to obtain an image of the tumor and to guide the therapy as well as for monitoring temperature changes during the treatment. After intravenous injection of silica-coated GNRs and sufficient circulation time, the delivery of the nanoparticles and their spatial distribution was evaluated with three-dimensional PAUS, and then PTT was performed. During PTT, photoacoustic images were acquired continuously and used to measure the temperature changes within the tissue.
X-ray computed tomography being one of the most exploited diagnostic techniques in hospitals, nowadays, has been considered as a powerful instrument for molecular imaging. Due to their high atomic weight and strong X-ray absorption, GNRs have been evaluated as novel contrast agents for this technique; in addition, because iodine, the widely used contrast agent today, presents severe side effects, such as kidney toxicity and fast body excretion, GNRs can be used as a suitable alternative. Moreover, the ability to guide GNRs toward the desired site of action, taking advantage of the surface conjugation of target molecules, represents another appealing strategy for the most effective diagnosis.
Because most of the basic investigations are already carried out on gold nanoparticles, GNRs were directly evaluated for advanced studies, such as the one reported by Luo et al.,102 who proposed silica coated-indocyanine green embedded-GNRs for a dual-mode X-ray CT and NIR fluorescence imaging. 12 hours post-intratumoral injection of 200 μL of GNRs coated solution at a concentration of 1.5 mg mL−1, X-ray CT scanning showed that this system could provide significant contrast enhancement, while NIR fluorescence generated by the dye was still present; thus, GNRs can be used as a promising contrast agent for dual mode imaging. Equally, Huang et al.22 developed folic acid conjugated silica-modified GNRs for X-ray CT and photothermal therapy, and tested them in vivo, which showed promising results in a perfect example of real theranostics (Fig. 11).
4.2 Therapy and theranostics with surface-modified GNRs
As discussed above, most of the therapeutic strategies relying on GNRs can be remotely controlled by NIR light, which can penetrate deep into human tissues with minimal collateral effects.Among these strategies, the most widely investigated treatment has been those based on hyperthermia, which is generated during the so-called photothermal therapy (PTT), gaining advantages by the powerful conversion of light into heat that takes place in GNRs. Due to the longitudinal plasmon resonance falling in the NIR window, where the interaction between light and tissues is minimal, GNRs have been selected among all the other nanoparticles to be employed for this application.103 Even if the local temperature increase could reach hundreds of degrees using GNRs, such extreme temperatures are often not desired for in vivo treatments because it would burn and destroy malignant cells; thus, the power of the laser can be reduced to more tolerable values.104
Several papers appeared when searching the literature for keywords such as “GNRs photothermal therapy”; therefore, only the most innovative reports and only those that clearly show an advantage given by the surface modification of GNRs will be summarized in this review.
In regards to in vitro results, the mechanism of the induced damages by hyperthermia in malignant cells, which causes death of the cells, has been thoroughly investigated. It is concluded that the disruption of the cytoplasmic membrane and the influx of the large amounts of Ca2+ ions into the cell compartment from the plasma, as a consequence of the heating of GNRs during laser exposure is the primary cause of the death of the cells.105 Similarly, Cabada et al. demonstrated the effectiveness of continuous wave laser irradiation of GNRs in glioblastoma cell lines, analyzing the mechanism which leads to cells death after photothermal therapy, reaching the same conclusion as reported above.106
The surface-targeting of GNRs for the selective destruction of malignant cells have also been tested. Chlorotoxin-targeted polymeric micelles-entrapped GNRs were reported by us, which showed the death of glioblastoma cells, while the untargeted analogs showed very low toxicity after laser exposure, confirming the necessity for an effective targeting of these nanostructures into cells for better efficacy.80 Equally, GNRs conjugated with both the targeting agents, namely, transferrin and PEG, were used for combining TPL and PTT onto HeLa cells. Although TPL allowed the imaging of GNRs–transferrin incubated cells, no uptake was observed when the targeting agent was not present; equally, clear cell death was observed only when GNRs–transferrin was used with a laser power of 25 mW after 20 scans (1.05 s per scan).107
Concerning in vivo application of this technique, Dickerson et al. reported the use of PEG-modified GNRs with prolonged blood circulation for in vivo PTT, after both intratumoral and intravenous injections in subcutaneous tumor-bearing mice, obtaining significant results in terms of tumor reduction. An inhibition of average tumor growth for both delivery methods over a 13 day period was observed with a specific tumor re-absorption of >57% of the directly-injected tumors and 25% of the intravenously-treated tumors.81 Li et al.26 improved PTT with GNRs by developing thiolated polyamidoamine (PAMAM) dendrimers as a replacement for the CTAB molecules onto the surface of GNRs and by conjugating arginine–glycine–aspartic acid (RGD) peptides, for the selective targeting of the melanoma A375 cell line with overexpression of αvβ3. The as-obtained system showed highly selective targeting and destructive effects on both the cancer cells and xenograft solid tumors implanted in mice under NIR laser irradiation, leading to the complete disappearance of the tumors in some of the treated animals.
More recently, various therapeutic techniques have been combined to enhance the efficacy of the treatment, often taking advantages from the increasing number of surface conjugation strategies. For example, PDT therapy, which requires the use of a photosensitizer molecule that is able to generate the toxic singlet oxygen species, has been coupled with PTT due to the possibility to conjugate these photosensitizer agents onto the surface of GNRs. Wang et al.108 conjugated Rose Bengal (RB) molecules onto GNRs; the as-obtained GNRs–RB system exhibited efficient singlet oxygen generation when illuminated by 532 nm green light due to the presence of RB, and it can be used for PTT using 810 nm NIR irradiation due to the presence of GNRs, thus presenting two different mechanisms for the death of cancer cells. In vitro tests also showed that RB could improve the uptake of GNRs by cancer cells. In vivo experiments on hamster cheek pouches (a model for human oral cancer) demonstrated that combined PDT-PTT capabilities provide better therapeutic effects against oral cancer in comparison to single strategy treatments.
With a similar strategy, Terentyuk et al.109 fabricated GNRs with a silica shell doped with hematoporphyrin (HP), which can be used for combining PDT and PTT in vivo because they present absorbance peaks at both 633 nm (HP) and 820 nm (GNRs). Large solid tumors in xenograft tumor rat model were treated in vivo with simultaneous irradiation at the two reported wavelengths after intratumor injection. Moreover, the efficiency of the combined therapy was evaluated by OCT, perfectly demonstrating the concept of a theranostic approach. Tumor volume was also monitored during a 21 day period, and the combined PDT and PTT treatments resulted in the large-area tumor necrosis and led to a dramatic decrease in the tumor volume.
The same strategy was applied at the base of the work proposed by Wang et al.66 and has already been described in the paragraph regarding oligonucleotides conjugation. Indeed, the system consisting of the aptamer switch probe (ASP) linking the photosensitizer molecule chlorin e6 conjugated onto the surface of GNRs was exploited to target cancer cells, for both PDT and PTT, showing once again that combining various strategies is the key for a real improvement of the therapeutic effect.
Finally, as another example of theranostics, Jang et al.110 developed a GNRs–photosensitizer complex for NIR fluorescence imaging and PTT/PDT cancer therapy (Fig. 12).
 | ||
| Fig. 12 Combined therapy and theranostic applications of GNRs photosensitizer system. Adapted from B. Jang, ACS Nano, 2011, 5, 1086. | ||
This system was non-toxic while in circulation due to the quenched fluorescence emission and non-singlet oxygen generation by the photosensitizer when close to the surface of the GNRs, but it became strongly toxic when it entered cancer cells, where the photosensitizer can be detached from the metallic surface. Thus, after the intravenous injection of the GNRs complex, the tumor sites were clearly identified on NIR fluorescence images, and PDT as well as PTT can be activated. Tumor growth reduced by 95% with dual PTT and PDT, demonstrating the interesting possibilities to effectively treat several types of cancer and with minimum side effects (Table 2).
| Entry | Surface modification | Imaging | Therapy | Target | Remarkable results | Ref. |
|---|---|---|---|---|---|---|
| 1 | Anti-EGFR monoclonal antibodies | DFM | PTT | In vitro: epithelial cell line HaCat (non-malignant) HOC313 clone8, HSC3 (malignant) | Twice uptake in malignant cells | 48 |
| 2 | PSS and anti-EGFR monoclonal antibody | TPL | — | In vitro: A431 skin cancer cells | TPL intensity from the cells with GNRs was 3 orders of magnitude brighter than the one from the cells alone | 75 |
| 3 | mPEG–SH and matrigel | PT-OCT | — | In vivo: healthy mice-ear blood vessel | Imaging possible at depths approaching 1 mm in vivo | 91 |
| 4 | Polyacrylic acid and anti-Her-2/neu monoclonal antibody | PA | — | In vitro: LNCaP prostate cancer lines | GNRs–antibody concentration in cells was an order of magnitude higher than the GNRs alone | 94 |
| 5 | HER2 and CXCR4 monoclonal antibodies | PA | — | In vitro: MBT2 (murine bladder cancer) and HepG2 (human epathoma) cell lines | PA signals enhanced by 7–12 dB and good correlation to specific bindings | 95 |
| 6 | mPEG–SH or PSS | PA | — | In vivo: nude mice | Ability to detect and localize GNRs at low concentration deep within tissue | 96 |
| 7 | Organic thiol ligand (Fig. 5e), PLGA-b-PEG and chlorotoxin | PA and US | — | In vitro: Balb/3T3 mouse fibroblasts | Optical detectability of GNRs at 11 μM with no cytotoxicity below 20 μM | 27 |
| 8 | Anti-HER2 and anti-EFGR monoclonal antibodies | PA | — | In vitro: oral cancer OECM1 and Cal27 | Imaging with multiple selective targeting demonstrated | 97 |
| In vivo: subcutaneous tumor-bearing mice | ||||||
| 9 | PSS, PHA and PLA | US | PTT | In vitro: HeLa cells | Low toxicity, in vivo high resolution imaging ability and PTT on cells | 98 |
| In vivo: rabbit kidney | ||||||
| 10 | mPEG–SH and silica | PAUS | PTT | In vivo: epithelial subcutaneous tumor-bearing mice | PAUS images acquired continuously during PTT. 53 °C reached in tumor | 101 |
| 11 | Indocyanine green and silica | X-ray CT NIR fluorescence | — | In vivo: gastric cancer subcutaneous tumor-bearing mice | Dual mode imaging capability of a single nanoparticle probe using CT and NIR fluorescence | 102 |
| 12 | Silica and folic acid | X-ray CT | PTT | In vitro: MGC803 gastric cancer cells | Three times higher uptake with folic acid. Excellent PTT effects on cells and strong X-ray attenuation for in vivo X-ray CT imaging | 22 |
| In vivo: gastric cancer subcutaneous tumor-bearing mice | ||||||
| 13 | Organic thiol ligand (Fig. 5e), PLGA-b-PEG and chlorotoxin | PA | PTT | In vitro: U87MG glioblastoma cells | Enhanced binding affinity toward GBM cells. Cells damage after laser irradiation. Higher tumor retention with targeted GNRs | 80 |
| In vivo: glioblastoma cancer subcutaneous tumor-bearing mice | ||||||
| 14 | Transferrin and PEG | TPL | PTT | In vitro: HeLa cells | Pronounced difference of the cellular uptake between targeted and non-targeted GNRs | 107 |
| 15 | mPEG–SH | — | PTT | In vivo: squamous cell carcinoma xenograft tumor-bearing mice | Accumulation in tumor due to EPR effect. Selective hyperthermia of malignant tissues reduced tumor growth | 81 |
| 16 | PAMAM dendrimers and RGD peptide | — | PTT | In vitro: A375 melanoma cells | Highly selective targeting and destructive effects. Disappearance of the tumor | 26 |
| In vivo: xenograft tumor bearing mice | ||||||
| 17 | PAH and RB molecules | — | PTT, PDT | In vitro: Cal-27 human oral squamous cell carcinoma cell line | Improved photodynamic efficacy due to enhanced uptake of RB by cancer cells | 108 |
| In vivo: hamster cheek pouches | ||||||
| 18 | Silica and hematoporphirin | OCT | PTT, PDT | In vivo: alveolaris liver cancer xenograft tumor rat model | Large area of tumor necrosis and decrease in tumor volume | 109 |
| 19 | mPEG–SH, ASP and Ce6 photosensitizer | — | PTT | In vitro: CCRF-CEM acute lymphoblastic leukaemia cell line | High specific internalization by the target cancer cells. Selective PTT and PDT upon laser irradiation | 66 |
| PDT | ||||||
| 20 | mPEG–SH, RRLAC peptide and AlPcS4 photosensitizer | NIR fluorescence | PTT, PDT | In vitro: SCC7 squamous cell carcinoma | Intracellular uptake of AlPcS4 improved by about 4-fold. Highly effective PTT/PDT dual therapy proved in vivo | 110 |
| In vivo: SCC7 cancer xenograft tumor-bearing mice |
5 Conclusion
There are plenty of fascinating features in the surface properties of GNRs, and in this review, we demonstrated the tremendous advantages that can be achieved using surface modified GNRs for nanomedicine applications. Several of the most important results achieved relied on well-fashioned, highly-selective and specifically-designed surface chemistry modifications of these nanostructures. Without putting efforts in discovering and engineering novel surface coatings most of these outcomes would not have been possible. Scientific literature within this field has increased enormously in the last several years and GNRs are continuing to gain interest on a yearly basis. Imaging and therapy are clearly clinically transferable with GNRs, and thus surface engineered GNRs are likely to become the weapon of choice in the next years to treat cancer via the theranostic approach.Acknowledgements
University of Bologna is gratefully acknowledged.Notes and references
- C. Lasagna-Reeves, D. Gonzalez-Romero, M. A. Barria, I. Olmedo, A. Clos, V. M. Sadagopa Ramanujam, A. Urayama, L. Vergara, M. J. Kogan and C. Soto, Biochem. Biophys. Res. Commun., 2010, 393, 649 CrossRef CAS PubMed.
- C. J. Murphy, A. M. Gole, J. W. Stone, P. N. Sisco, A. M. Alkilany, E. C. Goldsmith and S. C. Baxter, Acc. Chem. Res., 2008, 41, 1721 CrossRef CAS PubMed.
- R. Weissleder, Nat. Biotechnol., 2001, 19, 316 CrossRef CAS PubMed.
- L. Tong, Q. Wei, A. Wei and J. X. Cheng, Photochem. Photobiol., 2009, 85, 21 CrossRef CAS PubMed.
- P. K. Jain, K. S. Lee, I. H. El-Sayed and M. A. El-Sayed, J. Phys. Chem. B, 2006, 110, 7238 CrossRef CAS PubMed.
- J. Turkevich, P. C. Stevenson and J. Hiller, Discuss. Faraday Soc., 1951, 11, 55 RSC.
- B. Nikoobakht and M. A. El-Sayed, Chem. Mater., 2003, 15, 1957 CrossRef CAS.
- B. Nikoobakht and M. A. El-Sayed, Langmuir, 2001, 17, 6368 CrossRef CAS.
- C. J. Murphy, T. K. Sau, A. M. Gole, C. J. Orendorff, J. Gao, L. Gou, S. E. Hunyadi and T. Li, J. Phys. Chem., 2005, 109, 13857 CrossRef CAS PubMed.
- J. Y. Chang, H. Wu, H. Chen, Y. C. Ling and W. Tan, Chem. Commun., 2005, 1092 RSC.
- J. C. Love, L. A. Estroff, J. K. Kriebel, R. G. Nuzzo and G. M. Whitesides, Chem. Rev., 2005, 105, 1103 CrossRef CAS PubMed.
- L. Vigderman, P. Manna and E. R. Zubarev, Angew. Chem., Int. Ed., 2012, 51, 636 CrossRef CAS PubMed.
- S. Garabagiu and I. Bratu, Appl. Surf. Sci., 2013, 284, 780 CrossRef CAS PubMed.
- Q. Dai, J. Coutts, J. Zou and Q. Huo, Chem. Commun., 2008, 2858 RSC.
- D. Gentili, G. Ori and M. Comes Franchini, Chem. Commun., 2009, 5874 RSC.
- Y. Li, D. Yu, L. Dai, A. Urbas and Q. Li, Langmuir, 2010, 27, 98 CrossRef PubMed.
- G. Ori, D. Gentili, M. Cavallini, M. C. Franchini, M. Zapparoli, M. Montorsi and C. Siligardi, Nanotechnology, 2012, 23, 055605 CrossRef PubMed.
- S. Yamashita, H. Fukushima, Y. Niidome, T. Mori, Y. Katayama and T. Niidome, Langmuir, 2011, 27, 14621 CrossRef CAS PubMed.
- E. Locatelli, G. Ori, M. Fournelle, R. Lemor, M. Montorsi and M. Comes Franchini, Chem.–Eur. J., 2011, 17, 9052 CrossRef CAS PubMed.
- E. C. Dreaden, S. C. Mwakwari, L. A. Austin, M. J. Kieffer, A. K. Oyelere and M. A. El-Sayed, Small, 2012, 18, 2819 CrossRef PubMed.
- Y. J. Son, H. Kim, K. W. Leong and H. S. Yoo, ACS Nano, 2013, 7, 9771 CrossRef CAS PubMed.
- P. Huang, L. Bao, C. Zhang, J. Lin, T. Luo, D. Yang, M. He, Z. Li, G. Gao, B. Gao, S. Fu and D. Cui, Biomaterials, 2011, 32, 9796 CrossRef CAS PubMed.
- C. Grabinski, N. Schaeublin, A. Wijaya, H. D'Couto, S. H. Baxamusa, K. Hamad-Schifferli and S. M. Hussain, ACS Nano, 2011, 5, 2870 CrossRef CAS PubMed.
- J. W. Hotchkiss, A. B. Lowe and S. G. Boyes, Chem. Mater., 2007, 19, 6 CrossRef CAS.
- J. Song, L. Pu, J. Zhou, B. Duan and H. Duan, ACS Nano, 2013, 7, 9947 CrossRef CAS PubMed.
- Z. Li, P. Huang, X. Zhang, J. Lin, S. Yang, B. Liu, F. Gao, P. Xi, Q. Ren and D. Cui, Mol. Pharm., 2009, 7, 94 CrossRef PubMed.
- M. Comes Franchini, J. Ponti, R. Lemor, M. Fournelle, F. Broggi and E. Locatelli, J. Mater. Chem., 2010, 20, 10908 RSC.
- A. Gole and C. J. Murphy, Chem. Mater., 2005, 17, 1325 CrossRef CAS.
- H. J. Parab, H. M. Chen, T. C. Lai, J. H. Huang, P. H. Chen, R. S. Liu, M. Hsiao, C. H. Chen, D. P. Tsai and Y. K. Hwu, J. Phys. Chem. C, 2009, 113, 7574 CAS.
- A. P. Leonov, J. Zheng, J. D. Clogston, S. T. Stern, A. K. Patri and A. Wei, ACS Nano, 2008, 2, 2481 CrossRef CAS PubMed.
- T. S. Hauck, A. A. Ghazani and W. C. W. Chan, Small, 2008, 4, 153 CrossRef CAS PubMed.
- T. S. Hauck, T. L. Jennings, T. Yatsenko, J. C. Kumaradas and W. C. W. Chan, Adv. Mater., 2008, 20, 1 CrossRef.
- L. Y. Liu, Z. Chen, W. Li, Y. Liu, L. Wang, Y. Liu, X. Wu, Y. Ji, Y. Zhao, L. Ma, Y. Shao and C. Chen, Nano Lett., 2012, 12, 2003 CrossRef PubMed.
- H. Takahashi, Y. Niidome, T. Niidome, K. Kaneko, H. Kawasaki and S. Yamada, Langmuir, 2006, 22, 2 CrossRef CAS PubMed.
- C. J. Orendorff, T. M. Alarm, D. Y. Sasaki, B. C. Bunker and J. A. Voigt, ACS Nano, 2009, 3, 971 CrossRef CAS PubMed.
- S. Charan, K. Sanjiv, N. Singh, F. C. Chien, Y. F. Chen, N. N. Nergui, S. H. Huang, C. H. Kuo, T. C. Lee and P. Chen, Bioconjugate Chem., 2012, 23, 2173 CrossRef CAS PubMed.
- S. Garabagiu, C. Pestean and R. Stefan, J. Lumin., 2013, 143, 271 CrossRef CAS PubMed.
- C. H. Wang, C. W. Chang and C. A. Peng, J. Nanopart. Res., 2011, 13, 2749 CrossRef CAS.
- R. Duan, Z. Zhou, G. Su, L. Liu, M. Guan, B. Du and Q. Zhang, Macromol. Biosci., 2014, 14, 1160 CrossRef CAS PubMed.
- W. Choin, J. Y. Kim, C. Kang, C. C. Byeon, Y. H. Kim and G. Tae, ACS Nano, 2011, 5, 1995 CrossRef PubMed.
- J. C. Y. Kah, C. Grabinski, E. Untener, J. Chen, D. Zhu, S. M. Hussain and K. Hamad-Schifferli, ACS Nano, 2014, 8, 4608 CrossRef CAS PubMed.
- T. Murakami, H. Nakatsuji, N. Morone, J. E. Heuser, F. Ishidate, M. Hashida and H. Imahori, ACS Nano, 2014, 8, 7370 CrossRef CAS PubMed.
- A. Alkilany, S. Boulos, S. E. Lohse, L. B. Thompson and C. J. Murphy, Bioconjugate Chem., 2014, 25, 1162 CrossRef CAS PubMed.
- W. M. Park, B. G. Choi, Y. S. Huh, S. Y. Lee and T. J. Park, ChemPlusChem, 2013, 78, 48 CrossRef CAS.
- B. Jang, J. Y. Park, C. H. Tung, I. H. Kim and Y. Choi, ACS Nano, 2011, 5, 1086 CrossRef CAS PubMed.
- V. V. Ranade, J. Clin. Pharmacol., 1989, 29, 873 CrossRef CAS.
- M. A. Firer and G. Gellerman, J. Hematol. Oncol., 2012, 9, 5 Search PubMed.
- X. Huang, I. H. El-Sayed, W. Qian and M. El-Sayed, J. Am. Chem. Soc., 2006, 128, 2115 CrossRef CAS PubMed.
- H. Park, S. Lee, L. Chen, E. K. Lee, S. Y. Shin, Y. H. Lee, S. W. Son, C. H. Oh, J. M. Song, S. H. Kang and J. Choo, Phys. Chem. Chem. Phys., 2009, 11, 7444 RSC.
- J. Choi, J. Yang, D. Bang, J. Park, J. S. Suh, Y. M. Huh and S. Haam, Small, 2012, 8, 746 CrossRef CAS PubMed.
- H. Liao and J. H. Hafner, Chem. Mater., 2005, 17, 4636 CrossRef CAS.
- P. Puvanakrishnan, P. Diagaradjane, S. M. Kazmi, A. K. Dunn, S. Krishnan and J. W. Tunnell, Lasers Surg. Med., 2012, 44, 310 CrossRef PubMed.
- P. P. Joshi, S. J. Yoon, W. G. Hardin, S. Emelianov and K. V. Sokolov, Bioconjugate Chem., 2013, 24, 878 CrossRef CAS PubMed.
- H. Sun, X. Zhu, P. Y. Lu, R. R. Rosato, W. Tan and Y. Zu, Mol. Ther.--Nucleic Acids, 2014, 3, e182 CrossRef CAS PubMed.
- C. A. Stein and Y. C. Cheng, Science, 1993, 261, 1004 CAS.
- U. Galderisi, A. Cascino and A. Giordano, J. Cell. Physiol., 1999, 181, 251 CrossRef CAS.
- H. Wang, R. Yang, L. Yang and W. Tan, ACS Nano, 2009, 3, 2451 CrossRef CAS PubMed.
- H. Takahashi, Y. Niidome and S. Yamada, Chem. Commun., 2005, 2247 RSC.
- C. C. Chen, Y. P. Lin, C. W. Wang, H. C. Tzeng, C. H. Wu, Y. C. Chen, C. P. Chen, L. C. Chen and Y. C. Wu, J. Am. Chem. Soc., 2006, 128, 3709 CrossRef CAS PubMed.
- A. Wijaya, S. B. Schaffer, I. G. Pallares and K. Hamad-Schifferli, ACS Nano, 2008, 3, 80 CrossRef PubMed.
- V. Shanmugam, Y. H. Chien, Y. S. Cheng, T. Y. Liu, C. C. Huang, C. H. Su, Y. S. Chen, U. Kumar, H. F. Hsu and C. S. Yeh, ACS Appl. Mater. Interfaces, 2014, 6, 4382 CAS.
- A. C. Bonoiu, S. D. Mahajan, H. Ding, I. Roy, K. T. Yong, R. Kumar, R. Hu, E. J. Bergey, S. A. Schwartz and P. N. Prasad, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 5546 CrossRef CAS PubMed.
- E. J. Bergey, A. Bonoiu, S. Mahajan, P. N. Prasad, I. Roy, S. A. Schwartz and K. T. Yong, U.S. Pat. no. 8,035,016, U.S. Patent and Trademark Office, Washington, DC, 2011.
- A. Tahmasebifar, Y. Yazdani and M. Shahbazi, Am. J. Adv. Drug Delivery, 2014, 2, 254 Search PubMed.
- J. Wang, M. You, G. Zhu, M. I. Shukoor, Z. Chen, Z. Zhao, M. B. Altman, Q. Yuan, Z. Zhu, Y. Chen, C. Z. Huang and W. Tan, Small, 2013, 9, 3678 CrossRef CAS PubMed.
- J. Wang, G. Zhu, M. You, E. Song, M. I. Shukoor, K. Zhang, M. B. Altman, Y. Chen, Z. Zhu, C. Z. Huang and W. Tan, ACS Nano, 2012, 6, 5070 CrossRef CAS PubMed.
- J. Wang, K. Sefah, M. B. Altman, T. Chen, M. You, Z. Zhao, C. Z. Huang and W. Tan, Chem.–Asian J., 2013, 8, 2417 CrossRef CAS PubMed.
- J. A. Webb and R. Bardhan, Nanoscale, 2014, 6, 2502 RSC.
- C. Ungureanu, R. Kroes, W. Petersen, T. A. M. Groothuis, F. Ungureanu, H. Janssen, F. W. B. van Leeuwen, R. P. H. Kooyman, S. Manohar and T. G. van Leeuwen, Nano Lett., 2011, 11, 1887 CrossRef CAS PubMed.
- T. K. Sau and C. J. Murphy, Langmuir, 2004, 20, 6414 CrossRef CAS PubMed.
- A. L. Oldenburg, M. N. Hansen, T. S. Ralston, A. Wei and S. A. Boppart, J. Mater. Chem., 2009, 19, 6407 RSC.
- R. K. Chhetri, K. A. Kozek, A. C. Johnston-Peck, J. B. Tracy and A. L. Oldenburg, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2011, 83, 040903 CrossRef.
- Y. Jung, R. Reif, Y. Zeng and R. K. Wang, Nano Lett., 2011, 11, 2938 CrossRef CAS PubMed.
- H. Wang, T. B. Huff, D. A. Zweifel, W. He, P. S. Low, A. Wei and J. X. Cheng, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 15752 CrossRef CAS PubMed.
- N. J. Durr, T. Larson, D. K. Smith, B. A. Korgel, K. Sokolov and A. Ben-Yakar, Nano Lett., 2007, 7, 941 CrossRef CAS PubMed.
- Y. Sun, H. Jiang and B. E. O'Neill, J. Biosens. Bioelectron., 2011, 2, 1000108 Search PubMed.
- S. Manohar, C. Ungureanu and T. G. Van Leeuwen, Contrast Media Mol. Imaging, 2011, 6, 389 CrossRef CAS PubMed.
- D. Cosgrove, Eur. J. Radiol., 2006, 60, 324 CrossRef PubMed.
- X. Huang, I. H. El-Sayed and M. A. El-Sayed, Methods Mol. Biol., 2010, 624, 343 CAS.
- E. Locatelli, W. Bost, M. Fournelle, J. Llop, L. Gil, F. Arena, V. Lorusso and M. C. Franchini, J. Nanopart. Res., 2014, 16, 2304 CrossRef.
- E. B. Dickerson, E. C. Dreaden, X. Huang, I. H. El-Sayed, H. Chu, S. Pushpanketh, J. F. McDonald and M. A. El-Sayed, Cancer Lett., 2008, 269, 57 CrossRef CAS PubMed.
- P. Rai, S. Mallidi, X. Zheng, R. Rahmanzadeh, Y. Mir, S. Elrington, A. Khurshid and T. Hasan, Adv. Drug Delivery Rev., 2010, 62, 1094 CrossRef CAS PubMed.
- M. Hu, C. Novo, A. Funston, H. Wang, H. Staleva, S. Zou, P. Mulvaney, Y. Xia and G. V. Hartland, J. Mater. Chem., 2008, 18, 1949 RSC.
- G. Raschke, S. Kowarik, T. Franzl, C. Sönnichsen, T. A. Klar and J. Feldmann, Nano Lett., 2003, 3, 935 CrossRef CAS.
- J. W. Stone, P. N. Sisco, E. C. Goldsmith, S. C. Baxter and C. J. Murphy, Nano Lett., 2007, 7, 116 CrossRef CAS PubMed.
- A. M. Michaels, J. Jiang and L. Brus, J. Phys. Chem. B, 2000, 104, 11965 CrossRef CAS.
- M. B. Mohamed, V. Volkov, S. Link and M. A. El-Sayed, Chem. Phys. Lett., 2000, 317, 517 CrossRef CAS.
- K. Imura, T. Nagahara and H. Okamoto, J. Phys. Chem. B, 2005, 109, 13214 CrossRef CAS PubMed.
- H. Wang, T. B. Huff, D. A. Zweifel, W. He, P. S. Low, A. Wei and J. X. Cheng, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 15752 CrossRef CAS PubMed.
- T. S. Troutman, J. K. Barton and M. Romanowski, Opt. Lett., 2007, 32, 1438 CrossRef.
- J. M. Tucker-Schwartz, T. A. Meyer, C. A. Patil, C. L. Duvall and M. C. Skala, Biomed. Opt. Express, 2012, 3, 2881 CrossRef CAS PubMed.
- R. O. Esenaliev, A. A. Karabutov and A. A. Oraevsky, IEEE J. Sel. Top. Quantum Electron., 1999, 5, 981 CrossRef CAS.
- R. O. Esenaliev, I. V. Larina, K. V. Larin, D. J. Deyo, M. Motamedi and D. S. Prough, Appl. Opt., 2002, 41, 4722 CrossRef.
- A. Agarwal, S. W. Huang, M. O'Donnell, K. C. Day, M. Day, N. Kotov and S. Ashkenazi, J. Appl. Phys., 2007, 102, 064701 CrossRef PubMed.
- P. C. Li, C. W. Wei, C. K. Liao, C. D. Chen, K. C. Pao, C. R. C. Wang, Y. N. Wu and D. B. Shieh, IEEE Trans. Ultrason. Ferroelectrics Freq. Contr., 2007, 54, 1642 CrossRef.
- M. Eghtedari, A. Oraevsky, J. A. Copland, N. A. Kotov, A. Conjusteau and M. Motamedi, Nano Lett., 2007, 7, 1914 CrossRef CAS PubMed.
- P. C. Li, C. R. C. Wang, D. B. Shieh, C. W. Wei, C. K. Liao, C. Poe, S. Jhan, A. A. Ding and Y. N. Wu, Opt. Express, 2008, 16, 18605 CrossRef CAS.
- H. Ke, J. Wang, Z. Dai, Y. Jin, E. Qu, Z. Xing, C. Guo, J. Liu and X. Yue, J. Mater. Chem., 2011, 21, 5561 RSC.
- J. Yao and L. V. Wang, Contrast Media Mol. Imaging, 2011, 6, 332 CrossRef CAS PubMed.
- Y. H. Wang, A. H. Liao, J. H. Chen, C. R. C. Wang and P. C. Li, J. Biomed. Opt., 2012, 17, 045001 CrossRef PubMed.
- S. Kim, Y. S. Chen, G. P. Luke and S. Y. Emelianov, IEEE Trans. Ultrason. Ferroelectrics Freq. Contr., 2014, 61, 891 CrossRef.
- T. Luo, P. Huang, G. Gao, G. Shen, S. Fu, D. Cui, C. Zhou and Q. Ren, Opt. Express, 2011, 19, 17030 CrossRef CAS PubMed.
- X. Huang, P. K. Jain, I. H. El-Sayed and M. A. El-Sayed, Laser Med. Sci., 2008, 23, 217 CrossRef PubMed.
- D. Jaque, L. Martínez Maestro, B. del Rosal, P. Haro-Gonzalez, A. Benayas, J. L. Plaza, E. Martín Rodríguez and J. García Solé, Nanoscale, 2014, 6, 9494 RSC.
- L. Tong, Y. Zhao, T. B. Huff, M. N. Hansen, A. Wei and J. X. Cheng, Adv. Mater., 2007, 19, 3136 CrossRef CAS PubMed.
- T. F. Cabada, C. S. L. de Pablo, A. M. Serrano, F. del Pozo Guerrero, J. J. S. Olmedo and M. R. Gomez, Int. J. Nanomed., 2011, 7, 1511 Search PubMed.
- J. L. Li, D. Day and M. Gu, Adv. Mater., 2008, 20, 3866 CrossRef CAS.
- B. Wang, J. H. Wang, Q. Liu, H. Huang, M. Chen, K. Li, C. Li, X. F. Yu and P. K. Chu, Biomaterials, 2014, 35, 1954 CrossRef CAS PubMed.
- G. Terentyuk, E. Panfilova, V. Khanadeev, D. Chumakov, E. Genina, A. Bashkatov, V. Tuchin, A. Bucharskaya, G. Maslyakova, N. Khlebtsov and B. Khlebtsov, Nano Res., 2014, 7, 325 CrossRef CAS.
- B. Jang, J. Y. Park, C. H. Tung, I. H. Kim and Y. Choi, ACS Nano, 2011, 5, 1086 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |