A uracil nitroso amine based colorimetric sensor for the detection of Cu2+ ions from aqueous environment and its practical applications†
Samadhan R. Patila,
Jitendra P. Nandrea,
Prashant A. Patilb,
Suban K. Sahooc,
Manisha Devid,
Chullikkattil P. Pradeepd,
Yu Fabiaoe,
Lingxin Chen e,
Carl Redshawf and
Umesh D. Patil*a
e,
Carl Redshawf and
Umesh D. Patil*a
aSchool of Chemical Sciences, North Maharashtra University, Jalgaon-425 001, M.S., India. E-mail: udpatil.nmu@gmail.com; Tel: +91-9273060210
bS.S.V.P.S's L. K. Dr P. R. Ghogrey Science College, Dhule-424 001, India
cDepartment of Applied Chemistry, S. V. National Institute Technology, Surat-395 007, Gujarat, India
dSchool of Basic Sciences, Indian Institute of Technology, Mandi, Himachal Pradesh-175001, India
eKey Laboratory of Coastal Zone Environmental Processes and Ecological Remediation, Yantai Institute of Coastal Zone Research, Chinese Academy of Sciences, Yantai 264003, China
fDepartment of Chemistry, University of Hull, Cottingham Road, Hull, HU6 7RX, UK
First published on 9th February 2015
Abstract
A simple uracil nitroso amine based colorimetric chemosensor (UNA-1) has been synthesized and screened for its cation recognition ability. Sensor UNA-1 exhibited a high sensitivity and selectivity towards Cu2+ ions in aqueous medium in the presence of a wide range of other competing cations (Ag+, Al3+, Ba2+, Ca2+, Cd2+, Co2+, Cr3+, Cs+, Fe2+, Fe3+, Li+, Mg2+, Mn2+, Na+, Ni2+, Pb2+, Zn2+, Hg2+ and Sr2+). With Cu2+, the sensor UNA-1 gave a distinct color change from colorless to dark yellow by forming a complex of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry. Furthermore, sensor UNA-1 was successfully utilized in the preparation of test strips and supported silica for the detection of Cu2+ ions from aqueous environment.
1 stoichiometry. Furthermore, sensor UNA-1 was successfully utilized in the preparation of test strips and supported silica for the detection of Cu2+ ions from aqueous environment.
Introduction
Copper, the third-most abundant transition element in the body, plays an important role in various physiological processes such as hemoglobin biosynthesis, bone development, dopamine production, nerve function regulation, gene expression, and the functional and structural enhancement of proteins.1–6 Due to its redox-active nature, copper serves as an essential co-factor for a variety of metalloenzymes in living organisms such as cytochrome c oxidase, lysyl oxidase, copper–zinc superoxide dismutase and tyrosinase, which have a significant role in the enzymatic defense against oxygen toxicity.7–9 However, at high concentration, its redox properties turn into biologically hazardous materials because of its ability to generate reactive species, which create problems in the cellular metabolism.8,9 Apart from the biological and environmental importance, other advantages are that copper is relatively abundant, of low cost, and possess good malleability, electrical conductivity, thermal conductivity, chemical stability as well as germicidal efficiency. All of these properties make copper central to the pharmaceutical and industrial sectors for making alloys, electrical wires, machine parts, batteries, drugs and fertilizers etc.10,11 However, with excessive loading, Cu2+ is highly toxic to living organisms. For example, it's over accumulation in human being leads to various diseases including neurodegenerative diseases such as Alzheimer's disease, Wilson's disease, Menkes disease, prion disease, gastrointestinal disorders, kidney damage, amyotrophic sclerosis, lipid metabolism and inflammatory disorders.12–15The World Health Organization (WHO) have reported that the maximum limit of copper in drinking water should be 2 ppm (30 μM).16 Under normal conditions, the average concentration of copper in the blood should not exceed 100–150 μg dL−1.17 However, due to the widespread use of copper in household appliances, industry, agricultural and water-pipes, Cu2+ pollution has increased immensely throughout the world. Therefore, it is necessary to develop fast, convenient and reliable analytical methods for the qualitative and quantitative detection of copper, particularly in drinking water and in biological samples. Several analytical techniques such as atomic absorption spectrometry (AAS), inductively coupled plasma mass spectroscopy (ICP-MS), inductively coupled plasma atomic emission spectrometry (ICP-AES) and voltammetry, quantum-dot-based assay have been developed for the qualitative and quantitative detection of Cu2+ ions at trace levels. These technologies can detect Cu2+ ion selectively with high sensitivity, but tend to need highly sophisticated and expensive instrumentation, and require tedious sample preparation and highly trained operators.18–21 By contrast, naked-eye detection methods permit detection of the target analyte at the micro/submicromolar levels without any need for expensive/sophisticated instrumentation.22,23 Therefore, given the importance of and the hazardous roles played by copper, we were encouraged to develop a colorimetric sensor with naked-eye capability for detecting Cu2+ from pure aqueous media.
On surveying the literature, we have noted that most of the reported Cu2+ selective colorimetric sensors have a number of drawbacks, viz. long response times, poor detection limits, tedious synthetic procedures, use of organic solvents, and interference from other transition metal ions (Table S1†).22–32 Herein, as a part of our ongoing research on chemosensors,32–35 we have developed a simple and easy to prepare colorimetric chemosensor, namely the uracil nitroso amine derivative UNA-1 (Scheme 1), which can be used for the highly selective and sensitive recognition of Cu2+ ions. This chemosensor gives a visual color change from colorless to clear dark yellow allowing for the naked-eye detection of Cu2+.
Results and discussion
Naked-eye detection of Cu2+
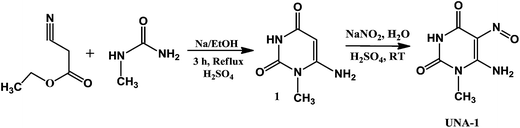
The synthesis of the uracil nitroso amine derivative (UNA-1) was achieved in two steps by the reaction of ethyl-cyanoacetate and N-methylurea in the presence of sodium ethoxide as a catalyst under acidic medium to give pyrimidine 1, which further undergoes nitrosation with sodium nitrite to afford UNA-1 (Scheme 1). Then, the colorimetric sensing ability of UNA-1 (5 × 10−5 M, in CH3OH–H2O, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
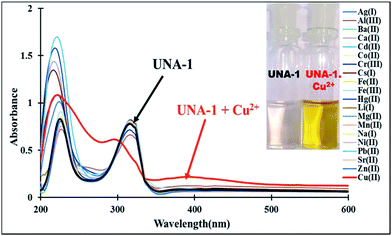
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90, v/v) was tested via the addition of 5 equivalents of various cations (1 × 10−2 M, in H2O). As shown in Fig. 1, UNA-1 exhibits a selective, sensitive and qualitative recognition of Cu2+ ions in day light/sunlight through a distinct visual color change from colorless to dark yellow. No noticeable color change was observed in the presence of any other cations screened herein.
90, v/v) was tested via the addition of 5 equivalents of various cations (1 × 10−2 M, in H2O). As shown in Fig. 1, UNA-1 exhibits a selective, sensitive and qualitative recognition of Cu2+ ions in day light/sunlight through a distinct visual color change from colorless to dark yellow. No noticeable color change was observed in the presence of any other cations screened herein.
 | ||
| Fig. 1 Naked-eye detectable color change of ions with UNA-1 in the presence of 5 equivalents of different cations. | ||
The concentration dependent naked-eye study was performed (Fig. S1a†) by addition of various concentrations of UNA-1 (A = 1 × 10−3 M, B = 1 × 10−4 M, C = 1 × 10−5 M, D = 5 × 10−5, E = 1 × 10−6 M, F = 5 × 10−6 M and G = 1 × 10−7 M) to a fixed concentration of Cu2+ ions (1 × 10−3 M, in H2O). The observed color change clearly suggested that the sensor was quite sensitive up to a concentration of 5 × 10−5 M for the detection of Cu2+ ions. Next, we investigated the effect of changing the concentration of Cu2+ ions from 1 × 10−3 M to 1 × 10−7 M to a fixed concentration of UNA-1 (1 × 10−4 M), which inferred that our sensor was able to detect Cu2+ up to the concentration of 1 × 10−5 M (Fig. S1b†).
Cation sensing studies
The cation recognition behavior of sensor UNA-1 with group I, II and III metal ions (Ba2+, Ca2+, Cs+, Li+, Mg2+, Na+, Al3+ and Sr2+) and transition and heavy metal ions (Co2+, Cu2+, Cr3+, Fe2+, Cd2+, Fe3+, Mn2+, Ni2+, Pb2+, Zn2+ and Hg2+) was investigated using UV-Vis absorption spectroscopy. The absorption spectrum of sensor UNA-1 in CH3OH–H2O (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90, v/v) solvent system exhibited two absorption bands centered at 227 nm and 315 nm due to π–π* and n–π* electronic transitions, respectively. Upon addition of 5 equivalents of different cations (50 μL, 1 × 10−2 M, in water) to a 5 × 10−5 M solution of UNA-1 in CH3OH–H2O (10
90, v/v) solvent system exhibited two absorption bands centered at 227 nm and 315 nm due to π–π* and n–π* electronic transitions, respectively. Upon addition of 5 equivalents of different cations (50 μL, 1 × 10−2 M, in water) to a 5 × 10−5 M solution of UNA-1 in CH3OH–H2O (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90, v/v), only the Cu2+ ions was able to perturb the absorption spectrum of UNA-1 effectively. The addition of aqueous Cu2+ ions to the UNA-1 solution led to the disappearance of the absorption band at 315 nm and the appearance of a new broad band between 335–500 nm due to the interaction of the paramagnetic Cu2+ with UNA-1 (Fig. 2). The appearance of a new charge transfer band was responsible for the naked-eye detectable color change of UNA-1.
90, v/v), only the Cu2+ ions was able to perturb the absorption spectrum of UNA-1 effectively. The addition of aqueous Cu2+ ions to the UNA-1 solution led to the disappearance of the absorption band at 315 nm and the appearance of a new broad band between 335–500 nm due to the interaction of the paramagnetic Cu2+ with UNA-1 (Fig. 2). The appearance of a new charge transfer band was responsible for the naked-eye detectable color change of UNA-1.
The UV-Vis absorption titration was next performed upon successive addition of 1–10 equivalents of Cu2+ ions to the solution of UNA-1 to determine the binding ability and the limit of detection. With the incremental addition of Cu2+, the absorbance at wavelength 315 nm decreased continuously with the appearance of the new broad peak between 335–500 nm (Fig. 3). The titration resulted in the formation of an isosbestic point at 300 nm, which suggested the formation of a complex between UNA-1 and copper ions in solution.
The association constant (Ka) was estimated graphically by plotting 1/ΔA against 1/[Cu2+] (Fig. 4). The data was linear (fitted according to the Benesi–Hilderbrand equation) and the Ka value was obtained from the slope and intercept of the line. The Ka value for the UNA-1 copper complex was found to be 2.8 × 104 M−1 (R2 = 0.9933). The value suggested that the sensor UNA-1 has high affinity towards Cu2+ ions. The limit of detection (LOD) and limit of quantification (LOQ) of UNA-1 were also calculated from the absorption titration data. According to the IUPAC definition, the LOD and LOQ were calculated using the relationship LOD = (3.3 × standard deviation)/slope and LOQ = (10 × standard deviation)/slope. To calculate the relative standard deviation, the absorption measurements of ten blank samples were taken. As shown in Fig. S2,† the absorbance calibration values were normalized between the minimum intensity and the maximum intensity and then a linear regression curve was fitted to these normalized data to get the slope. With this approach, the LOD and LOQ were found to be 10 μM and 33 μM, respectively.
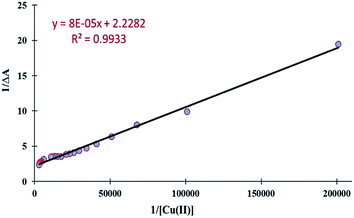 | ||
| Fig. 4 Benesi–Hilderbrand plot of chemosensor UNA-1 with Cu2+ ion for evaluation of association constant or binding constant (where, ΔA is at λmax = 315 nm). | ||
The 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding stoichiometry for the complexation between UNA-1 and Cu2+ was determined using a Job's plot experiment (Fig. 5) and a mole ratio plot (Fig. 3, inset). Furthermore, more direct evidence for the formation of this 1
1 binding stoichiometry for the complexation between UNA-1 and Cu2+ was determined using a Job's plot experiment (Fig. 5) and a mole ratio plot (Fig. 3, inset). Furthermore, more direct evidence for the formation of this 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex was obtained from the ESI-MS spectra of UNA-1 in the presence of 1.0 equivalent of Cu2+ in methanol–water (10
1 complex was obtained from the ESI-MS spectra of UNA-1 in the presence of 1.0 equivalent of Cu2+ in methanol–water (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90, v/v) (Fig. S3†). For pure UNA-1, a characteristic peak at m/z = 207.0405 was obtained which corresponds to the species [(UNA-1)·2H2O + H], whilst on addition of copper perchlorate, the peak at 207.0405 disappeared and a new peak appeared at m/z = 287.0051 corresponding to the species [(UNA-1–H)·Cu·3H2O]+ (Fig. S3†). MS-MS of 287.0051 peak corresponding to the hydrated copper complex of UNA-1 shows fragmentation giving peaks at 251.94 and 233.97 corresponding to the species [(UNA-1)–H·Cu·H2O]+ and [(UNA-1)–H·Cu]+ respectively (Fig. S3†).
90, v/v) (Fig. S3†). For pure UNA-1, a characteristic peak at m/z = 207.0405 was obtained which corresponds to the species [(UNA-1)·2H2O + H], whilst on addition of copper perchlorate, the peak at 207.0405 disappeared and a new peak appeared at m/z = 287.0051 corresponding to the species [(UNA-1–H)·Cu·3H2O]+ (Fig. S3†). MS-MS of 287.0051 peak corresponding to the hydrated copper complex of UNA-1 shows fragmentation giving peaks at 251.94 and 233.97 corresponding to the species [(UNA-1)–H·Cu·H2O]+ and [(UNA-1)–H·Cu]+ respectively (Fig. S3†).
 | ||
Fig. 5 Job's plot for the determination of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry upon complexation of UNA-1 with Cu2+ ions (where, ΔA is at λmax = 315 nm). 1 stoichiometry upon complexation of UNA-1 with Cu2+ ions (where, ΔA is at λmax = 315 nm). | ||
The chemosensor UNA-1 can bind to the Cu2+ ion via binding sites consisting of an amino and a nitroso group. All the crystal structures reported for complexes with similar ligands show the coordination through amino nitrogen and nitrogen of nitroso group.36–41 Thus, the lone pair of electrons on the nitrogen atoms of the amino and nitroso groups of the sensor UNA-1 are delocalized to the vacant orbital localized on the Cu2+ as shown in the Fig. 6. This electron donation or charge transfer gave rise to a color change from colorless to clear yellow. The charge of the copper is +2 and hence there should be two negative charges in our proposed structure for charge neutrality. Therefore, we propose the deprotonation of –NH2 group and the inclusion of ClO4− counter ion in the complex formula. Further, for more evidence of the binding of the Cu2+, we carried out 1H NMR titration studies on UNA-1 by adding Cu2+ solutions (Fig. S4†). It was observed that the peak at δ 12.91 corresponding to the –NH2 protons showed an up-field shift from 12.91 to 12.52 ppm accompanied by a broadening of the peak, while the peak at δ 9.08 corresponding to the –NH proton shows a small downfield shift from 9.08 to 9.28 ppm with broadening of the peak on addition of 1.0 equivalent of copper perchlorate. These observed shifts could be due to the complexation as proposed earlier. The possible 3D structure and the charge transfer processes occurring during the encapsulation of Cu2+ by UNA-1 was investigated by density functional theory (DFT) calculations. The optimized structure of UNA-1 and its complex with Cu2+ are shown in Fig. 7. On complexation of UNA-1 with Cu2+, a lowering in the interaction energy by −145.16 kcal mol−1 was observed, which indicates the formation of a stable complex with the calculated average Cu–N bond length of 2.062 Å. Further, the analysis of the frontier molecular orbitals (FMOs) plots (Fig. 7b and c) of the UNA-1·Cu2+ complex indicates that the intramolecular charge transfer (ICT) occurred between the receptor UNA-1 and Cu2+. Also, the band gap between the beta HOMO and LUMO of UNA-1·Cu2+ complex was lowered than the receptor UNA-1, which caused the experimentally observed red-shift in the absorption band.
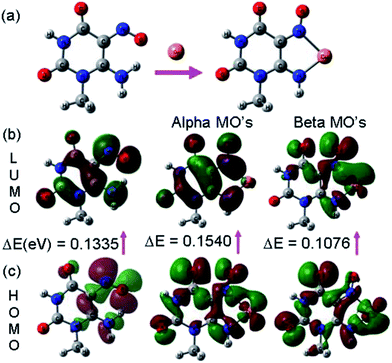 | ||
| Fig. 7 DFT computed (a) optimized structure of UNA-1 and its complex with Cu2+, and the (b) LUMO and (c) HOMO diagrams of UNA-1 and its UNA-1·Cu2+ complex. | ||
Practical applications of UNA-1
The analytical applicability of UNA-1 was first tested by performing competitive experiments. The absorption and color changes caused by the mixture of Cu2+ with the other metal ions was similar to that caused by Cu2+ alone (Fig. S5†), which indicates that the other metal ions did not interfere with the binding of the chemosensor UNA-1 with Cu2+. Secondly, the reversibility of UNA-1 for the detection of Cu2+ was examined. To perform the reversibility test, a stock solution of UNA-1 (1 × 10−3 M) was first treated with 1 equivalent of Cu2+. The color of the solution changes from colorless to yellow. To the same solution, the reverse color change from yellow to colorless was observed upon addition of 4 equivalents of aqueous EDTA solution (Fig. S6 and ESI Video†). This result demonstrated the reversibility of the sensor UNA-1.The sensing of Cu2+ by UNA-1 worked very well on a solid support (Fig. 8 and ESI Video†). In this experiment, the silica gel (60–120 mesh, 10.0 g, colorless) was soaked with UNA-1 (in methanol, 50 mL, 1 × 10−2 M) and then dried to afford a faint pink color silica gel due to the adsorption of the sensor on the surface. When the treated silica gel was added to a 10 mL aqueous solution of Cu2+ (1 × 10−3 M), the faint pink color promptly turned to a dark greenish/yellow color (ESI Video†). The instantaneous color change of the solid silica gel in aqueous solution clearly inferred the practical application of UNA-1 for the qualitative detection of Cu2+ in aqueous medium. Then, the UNA-1 supported silica gel was treated with different concentrations of Cu2+ (B = 1 × 10−3 M, C = 1 × 10−4 M, D = 1 × 10−5 M, E = 1 × 10−6 M), which indicated that the silica gel can be used to detect Cu2+ up to 1 × 10−5 M by a visually detectable color change (Fig. 9). The results indicate that we can use this silica supported method not only in the determination of Cu2+ ions from water but also in the extraction/separation of Cu2+ ions from water.
 | ||
| Fig. 8 Application of sensor UNA-1 on supported silica, and color changes of silica gel with/without UNA-1 and Cu2+ solution (before and after the addition). | ||
 | ||
| Fig. 9 UNA-1 supported silica gel was treated with different concentrations of Cu2+. (A) Silica without UNA-1 + 1 × 10−3 M Cu2+ as control. | ||
In another approach, the practical utility of UNA-1 for the detection of Cu2+ was studied by developing a test paper strip. The cellulose paper (Whatman no. 42) was dipped in the methanolic solution of UNA-1 (1 × 10−2 M) followed by drying in air to prepare the desire test strip. When this strip was dipped into an aqueous solution of Cu2+ (1 × 10−3 M), the colorless strip sharply turned to a yellow color (Fig. S7 and ESI Video†). The rapid color change of the test strip in solution clearly inferred the practical application of UNA-1 for the qualitative detection of Cu2+ in aqueous medium.
Conclusion
In summary, we have developed a simple uracil nitroso amine derivative UNA-1 for the colorimetric detection of Cu2+ in aqueous media. The sensor exhibited excellent selectivity and high sensitivity towards Cu2+ ions. The recognition of Cu2+ induced a clearly distinct color change of UNA-1 from colorless to yellow allowing for naked-eye detection. Moreover, UNA-1 can be applied to the detection of Cu2+ in aqueous media by a test paper strip and silica support method. These methods offer a very simple and quick detection for Cu2+ in aqueous media with a detection limit down to 10 μM. The good selectivity of sensor UNA-1 towards Cu2+ coupled with the use of pure water as the sole solvent in the detection process makes UNA-1 a promising candidate for the qualitative and quantitative detection of Cu2+ in various chemical, environmental and biological systems.Experimental
Chemicals and instrumentations
Unless otherwise stated, all chemicals used for the synthesis of UNA-1 were of AR grade and were purchased either from S.D. Fine chemicals or Sigma Aldrich. All solvents were of spectroscopic grade and were used without further treatment. The aqueous stock solutions of cations (1 × 10−2 M) such as Ag+, Al3+, Ba2+, Cd2+, Co2+, Cu2+, Cr3+, Fe2+, Fe3+, Mg2+, Mn2+, Ni2+, Pb2+, and Zn2+ were prepared from their perchlorates salts; Ca2+, Na+, Sr2+ and Cs+ were prepared from their nitrate salts; Hg2+ from its chloride salt and Li+ from its bromide salt. The stock solution of UNA-1 (1 × 10−3 M) was prepared in methanol and then diluted to 5 × 10−5 mol L−1 with methanol–water (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90, v/v).
90, v/v).
The 1H and 13C NMR spectra were recorded on a Jeol JNM-ECX 500 MHz multinuclear probe NMR spectrometer at ambient temperature in DMSO-d6 with TMS as internal standard and chemical shifts reported in ppm. Mass spectra were recorded on a Bruker Compact HD mass spectrometer. The IR spectra were recorded on a Perkin Elmer FTIR spectrophotometer by using KBr discs and the IR bands are expressed in frequency (cm−1). Absorption spectra were recorded on a Perkin Elmer U 3900 Co, USA UV/visible double beam spectrophotometer. The purity of the compound and progress of the reaction was monitored by means of a thin layer chromatography (TLC). Pre-coated silica gel 60 F254 (Merck) on alumina plate (7 × 3 cm2) was used and visualized by using either an iodine or a short UV/visible lamp. Melting points were recorded on the Celsius scale by open capillary method and are uncorrected.
Synthesis of UNA-1
The chemosensor UNA-1 was synthesized by following the reported method42 in two steps as depicted in Scheme 1. In the first step, ethyl cyanoacetate (2.1 mL, 0.019 mol) was added from a dropping funnel under vigorous stirring to a solution of sodium ethoxide prepared from 0.92 g of metallic sodium and 20 mL of ethanol. A white solid was separated from the solution. After the complete addition of ethyl cyanoacetate, the mixture was stirred for 20 min at room temperature. N-Methylurea (1.20 g, 0.016 mol) was added and the mixture was heated for 3 h under reflux conditions on a water bath. The obtained white precipitate was filtered off, washed with ethanol, and then dissolved in 10 mL of water. The pH of the solution was adjusted to 7 by adding dilute sulphuric acid (2 N), and the mixture was stirred for 2 h to afford the pyrimidine 1 as product in 95% yield, m.p > 300 °C.In the second step, a solution of 1.50 g of sodium nitrite in 4.0 mL of water was added to a mixture of 2.41 g of 5-amino-2,4-dihydroxypyrimidine (1) and 12 mL of water. Then, 1.70 g of conc. H2SO4 was added dropwise under vigorous stirring. A solid precipitated, which was stirred for 6 h at room temperature, and the obtained product was filtered and washed with ethanol and water. Yield 86%. IR spectrum, ν, cm−1: 3550, 3320, 3157, 3040, 2968, 2851, 1722, 1712, 1666, 1630, 1513, 1594, 1513, 1462, 1436, 1385, 1288, 1237, 1140, 1079, 1053, 512, 491. HRMS: m/z 207.0405 corresponding to the species (UNA-1)·2H2O + H. 1H NMR (500 MHz, DMSO-d6, δ ppm): 12.91 (s, 2H, –NH2), 9.08 (s, 1H, –NH), 3.25 (s, 3H, –CH3).13C-NMR (125 MHz, DMSO-d6, δ ppm): 27.95 (–CH3), 139.23 (–CN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 146.25 (C–NH2), 149.50 (C
O), 146.25 (C–NH2), 149.50 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 160.40 (C
O), 160.40 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) (Fig. S8–S11†).
O) (Fig. S8–S11†).
Computational study
The structural optimization of UNA-1 and its host–guest complexes with Cu2+ was performed using the computer program Gaussian 09W43 by applying the density functional theory (DFT) method. All the DFT calculations were performed in the gas phase with a hybrid functional B3LYP (Becke's three parameter hybrid functional using the LYP correlation functional) using the basis sets 6-31G (d,p) for C, H, N, O atoms and LANL2DZ for Cu atom.Acknowledgements
S. R. Patil is thankful to DST, New Delhi, India, for financial assistant under INSPIRE fellowship scheme and Dr U. D. Patil is grateful for the financial support from the DST, New Delhi, INDIA, under FAST TRACK scheme for Young Scientists, (Reg. no. CS-088/2013).References
- E. Gaggelli, H. Kozlowski, D. Valensin and G. Valensin, Chem. Rev., 2006, 106, 1995 CrossRef CAS PubMed.
- E. Crabb, E. Moore and L. E. Smart, Concepts in Transition Metal Chemistry, RSC Publishing, Cambridge, UK, 1st edn, 2010 Search PubMed.
- E. D. Harris, J. Trace Elem. Exp. Med., 2001, 14, 207 CrossRef.
- C. Andreini, L. Banci, I. Bertini and A. Rosato, J. Proteome Res., 2008, 7, 209 CrossRef CAS PubMed.
- E. L. Que, D. W. Domaille and C. J. Chang, Chem. Rev., 2008, 108, 1517 CrossRef CAS PubMed.
- Z. Xu, K.-H. Baek, H. Kim, J. Cui, X. Qian, D. R. Spring, I. Shin and J. Yoon, J. Am. Chem. Soc., 2010, 132, 601 CrossRef CAS PubMed.
- K. D. Karlin, Science, 1993, 261, 701 CAS.
- B. Halliwell and J. M. C. Gutteridge, Biochem. J., 1984, 219, 1 CAS.
- M. C. Linder and M. Hazegh-Azam, Am. J. Clin. Nutr., 1996, 63, 797S CAS.
- A. K. Jain, R. K. Singh, S. Jain and J. Raisoni, Transition Met. Chem., 2008, 33, 243 CrossRef CAS.
- P. G. Georgopoulos, A. Roy, M. J. Yonone-Lioy, R. E. Opiekun and P. J. Lioy, J. Toxicol. Environ. Health, Part B, 2001, 4, 341 CAS.
- D. Strausak, J. F. B. Mercer, H. H. Dieter, W. Stremmel and G. Multhaup, Brain Res. Bull., 2001, 55, 175 CrossRef CAS.
- E. Gaggelli, H. Kozlowski, D. Valensin and G. Valensin, Chem. Rev., 2006, 106, 1995 CrossRef CAS PubMed.
- G. Muthaup, A. Schlicksupp, L. Hess, D. Beher, T. Ruppert, C. L. Masters and K. Beyreuther, Science, 1996, 271, 1406 Search PubMed.
- R. A. Løvstad, BioMetals, 2004, 17, 111 CrossRef.
- WHO, WHO Guidelines Values for Chemicals that are of Health Significance in Drinking Water, Guidelines for Drinking Water Quality, Geneva, 3rd edn, 2008 Search PubMed.
- R. B. Jonas, Appl. Environ. Microbiol., 1989, 55, 43 CAS.
- N. Pourreza and R. Hoveizavi, Anal. Chim. Acta, 2005, 549, 124 CrossRef CAS PubMed.
- J. Becker, S. Zoriy, M. V. Pickhardt, C. N. Palomero-Gallagher and K. Zilles, Anal. Chem., 2005, 77, 3208 CrossRef CAS PubMed.
- Y. Liu, P. Liang and L. Guo, Talanta, 2005, 68, 25 CrossRef CAS PubMed.
- J. Otero-Romani, A. Moreda-Pineiro, A. Bermejo-Barrera and P. Bermejo-Barrera, Anal. Chim. Acta, 2005, 536, 213 CrossRef CAS PubMed.
- X. Ma, Z. Tan, G. Wei, D. Wei and Y. Du, Analyst, 2012, 137, 1436 RSC.
- Z. Xu, L. Zhang, R. Guo, T. Xiang, C. Wu, Z. Zheng and F. Yang, Sens. Actuators, B, 2011, 156, 546 CrossRef CAS PubMed.
- X. Chen, M. J. Jou, H. Lee, S. Kou, J. Lim, S.-W. Nam, S. Parka, K.-M. Kim and J. Yoon, Sens. Actuators, B, 2009, 137, 597 CrossRef CAS PubMed.
- T. Gunnlaugsson, J. P. Leonard and N. S. Murray, Org. Lett., 2004, 6, 1557 CrossRef CAS PubMed.
- B. N. Ahamed and P. Ghosh, Dalton Trans., 2011, 40, 6411 RSC.
- E. Hrishikesan, C. Saravanan and P. Kannan, Ind. Eng. Chem. Res., 2011, 50, 8225 CrossRef CAS.
- Y. Liu, Y. Sun, J. Du, X. Lv, Y. Zhao, M. Chen, P. Wang and W. Guo, Org. Biomol. Chem., 2011, 9, 432 CAS.
- R. Sheng, P. Wang, Y. Gao, Y. Wu, W. Liu, J. Ma, H. Li and S. Wu, Org. Lett., 2008, 10, 5015 CrossRef CAS PubMed.
- H. S. Jung, P. S. Kwon, J. W. Lee, J. Kim, C. S. Hong, J. W. Kim, S. Yan, J. Y. Lee, J. H. Lee and T. Joo, J. Am. Chem. Soc., 2009, 131, 2008 CrossRef CAS PubMed.
- C. Zong, K. Ai, G. Zhang, H. Li and L. Lu, Anal. Chem., 2011, 83, 3126 CrossRef CAS PubMed.
- S. R. Patil, J. P. Nandre, D. Jadhav, S. Bothra, S. K. Sahoo, M. Devi, C. P. Pradeep, P. P. Mahulikar and U. D. Patil, Dalton Trans., 2014, 43, 13299 RSC.
- J. P. Nandre, S. R. Patil, V. S. Patil, F. Yu, L. Chen, S. K. Sahoo, T. Prior, C. Redshaw, P. P. Mahulikar and U. D. Patil, Biosens. Bioelectron., 2014, 61, 612 CrossRef CAS PubMed.
- S. K. Sahoo, D. Sharma, R. K. Bera, G. Crisponi and J. F. Callan, Chem. Soc. Rev., 2012, 41, 7195 RSC.
- A. K. Gupta, A. Dhir and C. P. Pradeep, Dalton Trans., 2013, 42, 12819 RSC.
- R. Kivekas, A. Pajunen, E. Colacio, J. M. Dominguez-Vera, J. M. Moreno and A. Romerosa, Acta Chem. Scand., 1997, 51, 1051 CrossRef CAS PubMed.
- E. Colacio, J. M. Dominguez-Vera, A. Escuer, R. Kivekas and A. Romerosa, Inorg. Chem., 1994, 33, 3914 CrossRef CAS.
- E. Colacio, J. M. Dominguez-Vera, A. Romerosa, R. Kivekas, M. Klinga and A. Escuer, Inorg. Chim. Acta, 1995, 234, 61 CrossRef CAS.
- G. Ferguson, J. N. Low, M. Q. Olozabal, J. M. S. Peregrin, F. H. Urena and M. N. M. Carretero, Polyhedron, 1996, 15, 3233 CrossRef CAS.
- E. Colacio, J. M. Dominguez-Vera, A. Escuer, M. Klinga, R. Kivekas and A. Romerosa, J. Chem. Soc., Dalton Trans., 1995, 343 RSC.
- J. M. Salas, M. A. Romero, M. P. Sanchez, M. N. Moreno and M. Q. M. Faure, Polyhedron, 1992, 11, 2217 CrossRef CAS.
- I. Fatima, M. A. Munawar and A. Tasneem, J. Mex. Chem. Soc., 2010, 54, 227 CAS.
- A. H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, Ö. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Revision A.1, Gaussian, Inc., Wallingford CT, 2009 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra10419f |
| This journal is © The Royal Society of Chemistry 2015 |