Importance and synthesis of benzannulated medium-sized and macrocyclic rings (BMRs)
Altaf Hussainab,
S. K. Yousufb and
Debaraj Mukherjee*ab
aAcedemy of Scientific and Innovative Research (AcSIR), New Delhi, India. E-mail: dmukherjee@iiim.ac.in; Fax: +91-191-2569111; Tel: +91-191-2569000
bIndian Institute of Integrative Medicine (CSIR-IIIM), Canal Road, Jammu (J&K), India. E-mail: dmukherjee@iiim.ac.in; Fax: +91-191-2569111; Tel: +91-191-2569000
First published on 15th August 2014
Abstract
Cyclic molecular frameworks, especially the benzannulated medium-sized and macrocyclic ring (BMR) systems, constitute an integral component of a large number of biologically significant natural or synthetic molecules. Many of these BMR compounds are either approved as drugs or have reached the late developmental stages in clinical trials. Such cyclic systems have been shown to possess great potential, especially in the discovery of new anticancer leads. Efforts from synthetic chemists have led to the development of elegant new strategies for the construction of BMR scaffolds of medicinal importance. This review intends to highlight the importance of benzannulated medium-sized and macrocyclic rings (BMRs) and the strategies developed over the years for their synthesis.
1. Introduction
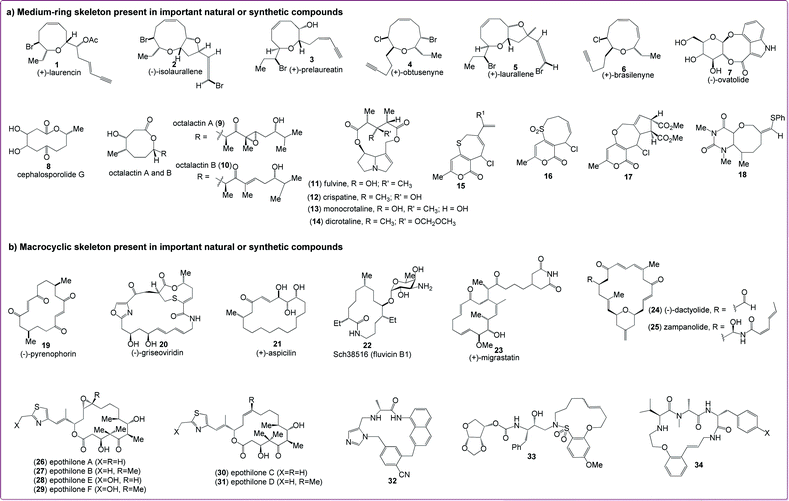
Cyclic molecular frameworks occupy a unique segment of chemical space. Cyclic scaffolds, particularly medium-sized and macrocyclic rings, are useful for organizing the overall presentation of functional groups to biological targets.1 The conformational constraint provided by cyclic scaffolds can afford enhanced binding affinity compared to corresponding linear structures.2 Conformational restriction has also been correlated with improved bioavailability3 and, in some cases, enhanced cell permeability.4,5 Thus, cyclic scaffolds enable molecules to achieve a high degree of structural pre-organization such that key functional groups can interact across extended binding sites in proteins without a major entropic loss in binding. These cyclic systems can therefore be highly potent as well as selective. In the past decade, their chemical diversity has expanded significantly, supported by advances in bioinformatics and synthetic methodology. As a consequence, this structural type has now been successfully tested on most biological target classes.Medium-sized (7- to 11-membered) rings constitute an important class of cyclic frameworks. Medium-ring carbo- and hetero-cycles are quite significant in organic chemistry as they form the structural core of a large number of biologically active natural products6 and medicinally important synthetic compounds that address diverse and challenging biological targets.7 Marine organisms have produced various nonterpenoid acetogenenins containing halogenated medium-ring ethers. These natural metabolites contain a number of different ring sizes such as (+)-laurencin (1), (−)-isolaurallene (2), (+)-prelaureatin (3), (+)-obtusenyne (4), (+)-laurallene (5), and (+)-brasilenyne (6), as shown in Fig. 1a.8 The importance of medium-ring compounds can also be seen from their existence in many of the bioactive natural products shown in Fig. 1a such as (−)-ovatolide (7),9 cephalosporolide G (8),10 octalactins A (9) and B (10),11 fulvine (11),12 crispatine (12),13 monocrotaline (13),14 and dicrotaline (14).14 Although there are synthetic challenges associated with the synthesis of medium-sized rings, efforts from synthetic chemists have led to the generation of unnatural medium ring compounds 15–18 (Fig. 1a).15 However, despite their occurrence in many important natural products, medium-sized rings are absent among the current top 200 brand name and top 200 generic drugs, perhaps due to the limited methods for their synthesis. While synthetic approaches to 5- and 6-membered rings are common via cyclization and cycloaddition reactions, strategies to form medium-sized rings are often inhibited due to entropic factors and transannular interactions, which can pose unique challenges for the synthesis of such molecular frameworks.16
 | ||
| Fig. 1 Abundance of medium-sized and macrocyclic ring skeleton in bioactive natural and synthetic molecules. | ||
Macrocycles, on the other hand, have been defined as ring systems consisting of 12 or more atoms7 and constitute the skeletal framework of a diverse range of bioactive natural products such as (−)-pyrenophorin (19), (−)-griseoviridin (20), (+)-aspicilin (21), fluvicin B1 (22), (+)-migrastatin (23), (−)-dactyolide (24), zampanolide (25), epothilones A–F (26–31), and synthetic molecules 32–34, as shown in Fig. 1b. Many natural products possess a macrocyclic core, suggesting that an evolutionary advantage may be associated with the production of secondary metabolites based upon these scaffolds.17,18 Macrocyclic compounds are attractive targets when searching for novel structures with biological activity due to the fact that naturally occurring macrocycles often display diverse and interesting biological activities such as antibiotic, anticancer, antifungal, and immunosuppressive activities as seen for erythromycin,19 epothilone B20 amphotericin B,21 and rapamycin,22 respectively. Macrocyclic drug candidates have originated either from natural sources or synthetic macrocycles. Exploitation of natural product macrocycles has yielded several drugs that are either approved for clinical use or have reached late-stage clinical development, such as the mTOR inhibitor Torisel® (temsirolimus),23,24 the microtubulin stabilizer Ixempra® (ixabepilone),25,26 the Hsp90 inhibitor 17-allylamino-geldanamycin,27 vancomycin,28 and cyclosporine.29 The medicinal chemistry of macrocyclic natural products usually involved direct use as therapeutic agents or the functionalization of the natural product scaffold by semisynthesis. This parallels significant advances in the total synthesis of macrocyclic natural products during the past two decades.30,31 Thus, the potential for macrocycles as drugs is evident from the above facts.
Macrocycles inherently possess a lower number of rotatable bonds and hence, are conformationally more restricted than their acyclic analogues, which can potentially impart greater target binding and selectivity with improved oral bioavailability.3 Topologically, macrocycles have the unique ability to span large surface areas, which makes them especially suited for targets displaying shallow surfaces, which can prove to be quite challenging for acyclic small molecules. Thus, macrocyclic compounds have long been clinically useful and attention is now being focused on the wider use of macrocyclic scaffolds in medicinal chemistry in the search for new drugs for increasingly challenging targets.
2. Benzannulated medium-sized and macrocyclic rings (BMRs)
The medium-sized and macrocyclic ring scaffolds annulated to an aromatic system are termed benzannulated medium-sized rings and benzannulated macrocycles, respectively. Furthermore, 1,3- or 1,4-linkages with benzene rings have been named in this review as bridged benzannulated medium-sized or macrocyclic rings (e.g. cyclophanes). The abbreviation BMR has been used for all such cyclic systems in this review. BMRs are of particular interest owing to their presence in an innumerable number of bioactive natural molecules and pharmacologically important synthetic molecules.2.1. Biological significance of benzannulated medium-sized rings
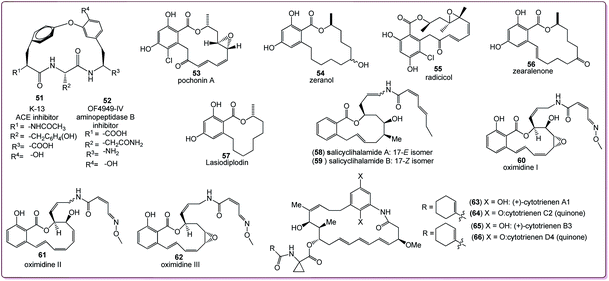
Benzannulated medium-sized rings are of great interest due to their presence in a number of biologically significant natural molecules, which include aryl ethers such as heliannuol A (35, allelopathic activity)32 and brazilone (36, anticoagulant),33 diaryl ethers such as aspercyclide A (37, IgE receptor inhibitor, prevention of allergic rhinitis or asthma),34 aryl esters such as plural A (38, Chinese traditional medicine component)35 and kurzichalcone (39, anticancer)36 and biaryls such as pterocayanin C (40, anticancer, antiviral),37 steganacin (41, antileukemic)38 and rhazinilin (42, anticancer),39 as shown in Fig. 2. Benzannulated medium-rings also constitute the core of coleophomones B (43) and C (44) (which show anti-fungal and serine protease enzyme inhibition activities),40 purpactin A (45, acyl-CoA-cholesterol acyl transferase inhibitor),41 and penicillide (46, antagonist of the peptide hormone oxytocin and inhibitor of cholesterol ester transfer protein).42,43 Many synthetically produced benzannulated medium-ring compounds (47–50) have also appeared in the literature (listed in Fig. 2). The presence of the medium-ring skeleton in bioactive natural products clearly indicates that the benzannulated medium-ring framework is a biologically significant system.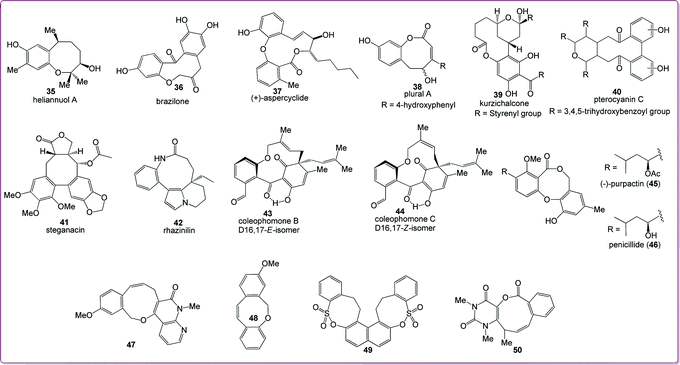 | ||
| Fig. 2 Benzannulated medium-sized rings in biologically significant natural products and synthetic molecules. | ||
2.2. Biological significance of benzannulated macrocycles
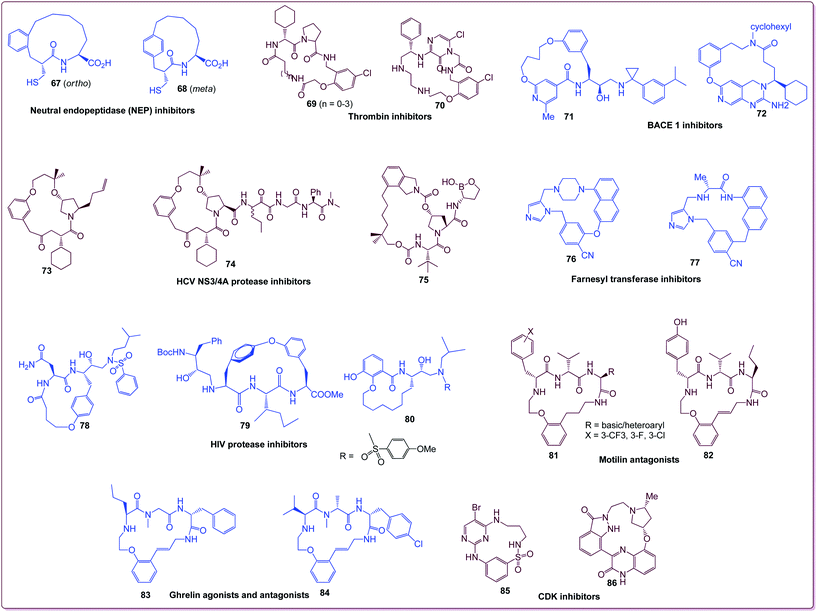
Benzannulated macrocycles are of considerable interest in drug discovery in light of their therapeutic value. They are appealing for drug discovery as they provide diverse functionality in a conformationally pre-organized ring structure, often resulting in high affinity and selectivity for protein targets. The majority of current macrocyclic drugs are derived from natural sources, and several synthetic macrocycles are now in pre-clinical and clinical development.(a) Neutral endopeptidase (NEP) inhibition. Ksander et al. reported that ortho- and meta-substituted benzannulated macrocycles 67 and 68 (bridged benzannulated) displayed potent neutral endopeptidase (NEP) inhibition (IC50 of 67 = 3 nM and that of 68 = 8 nM) with a high selectivity for angiotensin-converting enzyme (ACE, IC50 of 67 = 8 nM and that of 68 = 4 nM).48,49
(b) Thrombin inhibitors. Benzannulated macrocycles 69 (Ki 0.4–2.9 nM) and 70 (Ki = 0.09 nM) are potent and selective thrombin inhibitors.
(c) BACE-1 inhibitors. The bridged benzannulated macrocycles 71 and 72 are highly potent BACE-1 inhibitors with IC50 values of 70 nM (cell) and 7 nM (cell), respectively.50,51
(d) Hepatitis C virus (HCV) NS3 protease inhibitors. Bridged benzannulated macrocyclic scaffolds 73 (Ki = 530 and IC50 = 400 nM) and 74 (Ki = 6 nM and IC50 = 130 nM) are potent hepatitis C virus (HCV) NS3 protease inhibitors with nanomolar activities. Finally, Li et al. recently reported novel NS3 inhibitor 75 with submicromolar cellular inhibition of the NS3 protease (Ki = 43 nM and IC50 = 780 nM).52
(e) Farnesyl transferase inhibitors (FTase). The inhibition of FTase has been pursued as an approach to treat cancer, leading to several clinical candidates. The scaffolds 76 and 77 possess excellent FTase inhibitory activities (IC50 = 0.1 and 1.3 nM respectively).53,54
(f) HIV protease inhibition. The bridged benzannulated macrocyclic compounds 78 and 79 and the benzannulated macrocycle 80 displayed nanomolar potency towards HIV protease inhibition with Ki values of 0.6, 15 and 0.7 nM, respectively.55
(g) Motilin antagonists. Benzannulated macrocyclic motilin antagonist 81 was identified from an HTS campaign, and it possesses a high potency (IC50 = 137 nM). Lead optimization studies led to multiple analogues with low nanomolar potency, including analogue 82 (IC50 = 1–20 nM).56
(h) Ghrelin agonists. Hoveyda et al. identified the benzannulated macrocycle 83 as a ghrelin agonist possessing an EC50 of 68 nM, a high level of potency.57 The analogue 84 has been found to be more active with an EC50 value of 14 nM.58
(i) CDK inhibitors. Deregulation of kinase signaling pathways can be useful in the treatment of cancer. The benzannulated macrocyclic compounds 85 and 86 are highly potent antiproliferative agents. Compound 85 showed IC50 values of 20 nM (CDK1), 140 nM (CDK2), 40 nM (VEGFR-R2), and 200 nM (MCF7), while 86 showed IC50 values of 1 nM (CDK1), 3.4 nM (CDK2), 6.4 nM (CDK4), and 12 nM (CDK6).59,60
3. Benzannulated macrocyclic rings as drugs and clinical candidates
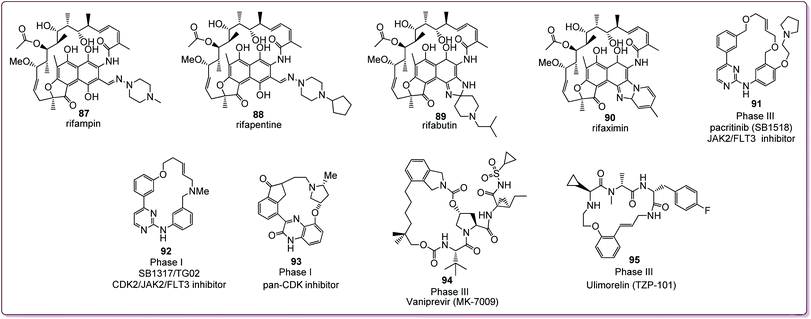
Macrocycles, particularly benzannulated macrocycles, have broad applications in drug discovery and development. Numerous benzannulated macrocyclic compounds present exceptional therapeutic potential and unrivalled biological activities17,59 and many of these molecules have been developed into approved drugs (Fig. 5).17,18 The benzannulated macrocyclic rifamycin drug family includes rifampin (87), rifapentine (88), rifabutin (89) and rifaximin (90), which constitute a notable class of antibiotic drugs.61Moreover, a number of benzannulated or bridged benzannulated macrocycles have entered clinical development (Fig. 5) such as the dual JAK2/FLT3 inhibitor pacritinib (91), now in advanced Phase III trials,62,63 and the CDK2/JAK2/FLT3 inhibitor 92 (SB1317) in Phase I trials.64 The pan-CDK inhibitor 93 is another example of a synthetic benzannulated macrocyclic ring, proposed as a development candidate65 (Fig. 5). The efforts from synthetic chemists delivered a clinical candidate, vaniprevir (94, MK-7009). It possessed outstanding in vitro (IC50 = 50 pM) and excellent in cellulo potency (IC50 = 3–20 nM), combined with advantageous selectivity compared to several other HCV NS3/4A inhibitors.66 Ulimorelin (95, TZP-101) is another example of a benzannulated macrocycle, which demonstrated efficacy in Phase II clinical studies when administered intravenously for the treatment of postoperative ileus and acute gastroparesis.67 Note that ulimorelin has finally entered Phase III clinical trials.
4. General strategies for the synthesis of benzannulated medium-sized and macrocyclic rings (BMRs)
One of the challenges associated with the exploration of the medium-ring or macrocyclic framework for drug discovery is the difficulty in synthesizing such structures. Fascinated by their intriguing biological activity and inspired by the intractable synthetic complexity of naturally occurring medium-sized or macrocyclic rings, much effort has been devoted to explore highly efficient and superior synthetic methods for the preparation of medium-sized rings or macrocycles.68–71 Among the cyclization methodologies, lactonization, lactamization, transition metal catalyzed coupling reactions, ring-closing metathesis, and click chemistry represent the most efficient and commonly used synthetic approaches for the construction of BMR frameworks.4.1. Lactonization and lactamization
Lactones constitute a major part of synthetic or naturally occurring BMRs, which mediate diverse biological activities.72 For their synthesis, many reports regarding efficient cyclizations have been published. In general, the most frequently used and attractive cyclic approaches still involve the direct lactonization of acids and alcohols using various activation schemes.73Porco et al. reported the synthesis of 8-membered benzolactone (97) from 96 in a Boeckman-type lactonization (Scheme 1a).74 A methodology related to Boeckman's lactonization has also been used in the synthesis of salicylihalamide 100 (Scheme 1b).75 Panek et al. used a cyanomethyl ester previously described in intermolecular transesterifications76 in the synthesis of apicularen (102, Scheme 1c).77 S. S. Palimkar et al. reported the synthesis of the pyran-based macrocyclic benzolactone (−)-apicularen (104) from compound 103, where the key cyclization step was a Yamaguchi coupling (Scheme 1).78
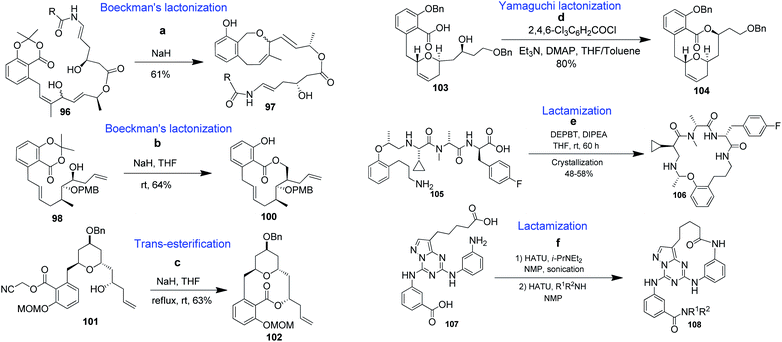 | ||
| Scheme 1 Lactonization and lactamization strategies for the synthesis of benzannulated medium-sized and macrocyclic rings. | ||
Among the many methods for synthesizing lactams, the most common and efficient approach is lactamization. Lactamization of 105 followed by purification by crystallization provided 106 in very good overall yield (Scheme 1e).79 Nie et al. reported the formation of bridged benzannulated macrocycle 108 via a lactamization strategy in the presence of two carboxylic acids (107), using a one-pot, two-step diamide synthesis (Scheme 1f).80
4.2. Transition metal catalyzed coupling reactions
Over the past few decades, transition metal catalyzed cross coupling reactions have become a prominent tool for creating new C–C, C–O or C–N bonds.81 Among transition metals, the most versatile metal is palladium, which has huge applications in the syntheses of BMR scaffolds as depicted in Scheme 2.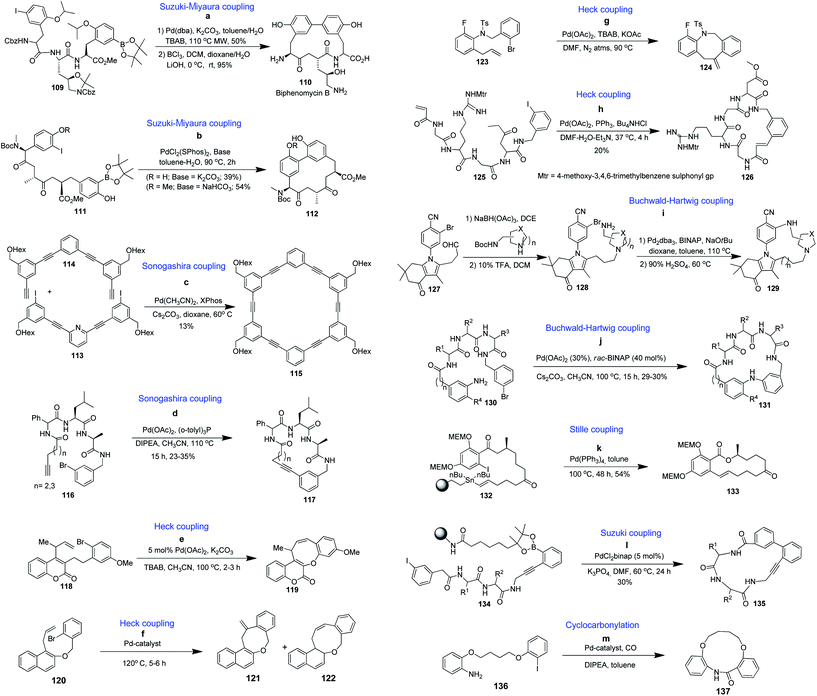 | ||
| Scheme 2 Synthesis of benzannulated medium-sized and macrocyclic rings via Pd-catalyzed coupling reactions. | ||
Zhu and coworkers reported the total synthesis of bridged benzannulated macrocycle 110 by applying microwave-assisted intramolecular Suzuki–Miyaura cross coupling as a key step for macrocyclization (Scheme 2a).82 An intramolecular Suzuki–Miyaura reaction of tripeptidomimetic 111 yielded the strained bridged benzannulated 14-membered macrocycle 112 (Scheme 2b).83 Saito and coworkers applied the Sonogashira reaction to prepare benzannulated macrocycles by treatment with catalytic Pd(CH3CN)2Cl2, ligand XPhos, and Cs2CO3 in dioxane. The terminal alkyne 114 was added dropwise to the diluted aryl iodide 113 for 5 h to obtain the final product 115 in 13% yield (Scheme 2c).84 The Sonogashira reaction has also been successfully utilized for a series of bridged benzannulated macrocyclic peptide mimetics as illustrated in Scheme 2d for the synthesis of tripeptidomimetic 117.85
Intramolecular Heck coupling has also found an application in the synthesis of a large number of biologically significant macrocycles, but there is only one report of it being utilized for the synthesis of benzannulated ring ethers. The Heck reaction to synthesize 118 was carried out using 5 mol% Pd(OAc)2 as catalyst, K2CO3 as base, and TBAB as an additive in CH3CN at 80 °C for 2 hours, and the benzannulated medium-ring compound 119 was obtained in 70% yield (Scheme 2e).86
K. C. Majumdar et al. reported an efficient and high yielding method for the synthesis of benzannulated 8- and 9-membered ring ethers (121 and 122) via a palladium-catalyzed intramolecular Heck reaction (Scheme 2f).87
When the intramolecular Heck reaction was performed with the substrate 123 applying the concept of Jeffrey's two-phase protocol in the presence of Pd(OAc)2, KOAc and TBAB in dry DMF under a nitrogen atmosphere for 6 h, the benzannulated eight-membered exo-Heck product 124 was obtained in 72–79% yield (Scheme 2g).88 Heck methodology was also employed in the solid phase construction of cyclic RGD peptidomimetic 126 (Scheme 2h).89
Zapf and coworkers designed and synthesized the amino-based bridged benzannulated macrocyclic structure 129 via the Buchwald–Hartwig reaction (Scheme 2i).90 Balraju and Iqbal also employed the Buchwald–Hartwig C–N coupling reaction for macrocyclization in the construction of bridged benzannulated macrocycle 131 constrained with a diphenylamine linker (Scheme 2j).91
Stille coupling provided the key step in a cyclization release solid phase synthesis strategy directed toward macrocyclic natural product 133 (Scheme 2k).92 Li and Burgess reported solid phase linker 134 specifically for enabling a simultaneous Suzuki coupling, macrocyclization, and resin release process giving an alternative method for the construction of benzannulated macrocycles of the type 135 (Scheme 2l).93 Shui-Ming Lu et al. reported an intramolecular cyclocarbonylation method with palladium-complexed dendrimers on silica gel as catalysts for the synthesis of 12 to 18-membered benzannulated macrocyclic ethers like 137 from iodoamine 136, as depicted in Scheme 2m.94 This process can tolerate a wide variety of functional groups, including halide, ether, ketone, and ester.
Apart from Pd, other transition metals, like Ni, Cu, and Cr, have also found applications in the synthesis of benzannulated cyclic systems. Thus, microwave-assisted cyclization by different strategies provides an efficient route to synthesize diverse medium-sized heterocycles95 and macrocycles.96 Recently, Sun's group reported a microwave-assisted intramolecular Ullmann reaction to yield bridged benzannulated macrocyclic diaryl ether analogues (139) from 138 (Scheme 3a).97 P. Mestichelli et al. reported the synthesis of compounds such as 141 in the presence of catalytic quantities of Cu(I). Readily accessible acyclic precursors like 140 can undergo an intramolecular C–O bond-forming reaction (Scheme 3b).98
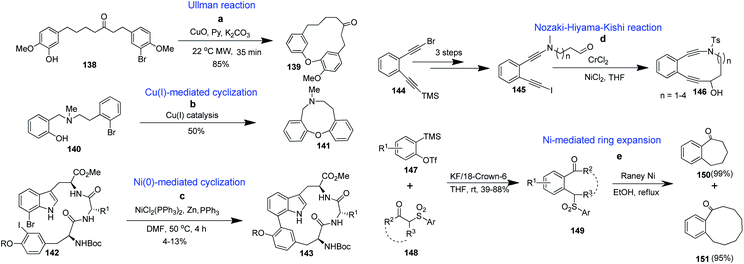 | ||
| Scheme 3 Synthesis of benzannulated medium-sized and macrocyclic rings via Cu(II), Ni(II) and Cr(II) catalyzed reactions. | ||
Nickel-mediated cyclization of 142 provided the target molecule 143 (Scheme 3c).99 Poloukhtine et al. reported that Nozaki–Hiyama–Kishi conditions gave benzannulated medium-sized rings or macrocycles of the type 146 (Scheme 3d).100 Zhang et al. reported that by ring expansion and desulfonylation reactions, medium- and large-sized benzannulated rings of the type 150 and 151 were obtained in excellent yields (Scheme 3e).101
4.3. Biomimetic and oxidative ring expansions
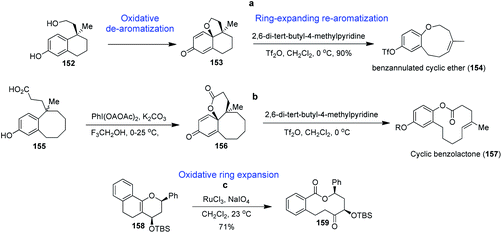
Owing to the limitations of conventional cyclization-based approaches to medium-ring synthesis, these structures remain severely underrepresented in current probe and drug discovery efforts. R. A. Bauer et al. established an alternative biomimetic ring expansion approach to the diversity-oriented synthesis of medium-ring libraries. As depicted in Scheme 4, the oxidative de-aromatization of bicyclic phenols (152) affords polycyclic cyclohexadienones (153) that undergo efficient ring expanding re-aromatization to form benzannulated medium-ring scaffolds (154), as depicted in Scheme 4a.102 The ring expansion reaction can be induced using three complementary reagents that avoid competing dienone-phenol rearrangements and is driven by re-aromatization of a phenol ring adjacent to the scissile bond. This method was also successfully applied to bicyclic phenols (155) to afford polycyclic cyclohexadienones (156) that undergo efficient ring expansion to form benzolactones of the type 157 (Scheme 4b). Cheminformatic analysis of the resulting first-generation library confirms that these molecules occupy chemical space overlapping with medium-ring natural products and distinct from that of synthetic drugs and drug-like libraries.F. Kopp et al. have developed a concise, modular oxidative ring expansion approach for the synthesis of macrolactones involving oxidative cleavage of a bridging double bond in polycyclic enol ethers (158). These substrates undergo ring expansion to afford highly functionalized medium-ring and macrocyclic benzolactones of the type 159 (Scheme 4c).103
4.4. Nucleophilic substitutions
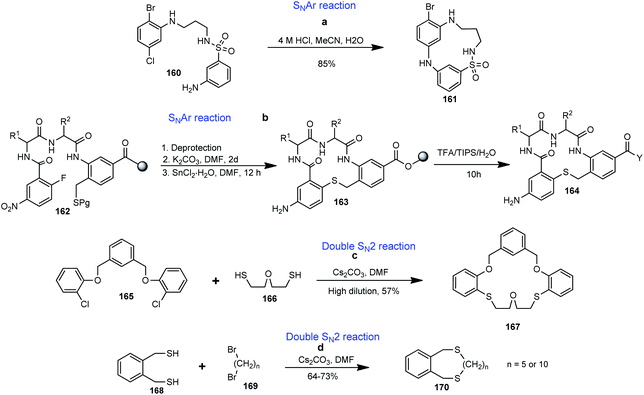
SN2 and SNAr reactions have found application in the synthesis of benzannulated macrocycles as exemplified in the synthesis of bridged benzannulated macrocycle 161 depicted in Scheme 5a.58 SNAr methodology was utilized as a key step in the solid phase synthesis of benzannulated macrocyclic thio-ether-based dipeptidomimetic 164 designed as a mimic of the β-turn structure of neurotrophin growth factors (Scheme 5b).104Eun-Ju Kang et al. reported the formation of benzannulated macrocyclic thio-ethers by the SN2 reaction of dichloride (165) and 2-mercaptoethyl ether (166), leading to the formation of macrocyclic thio-ether 167 (Scheme 5c).105 Buter et al. also reported the synthesis of benzannulated macrocyclic thio-ether 170 via the SN2 reaction of a cesium thiolate (generated in situ from dithiol 168 and Cs2CO3 in DMF) with dibromide (169) (Scheme 5d).106
4.5. Ring-closing metathesis (RCM)
The construction of medium-sized rings and macrocycles by ring-closing metathesis (RCM) is often used as the key step in the synthesis of natural products containing large rings. This reaction is attractive because of its high functional group compatibility and the possibility for further transformations. The determination of suitable reaction conditions is critical for the success of the synthesis. There are numerous examples of benzannulated medium-sized or macrocyclic rings synthesized by RCM.R. Mamouni et al. reported that bis-allyl ether derivative 171 directly led to the corresponding benzannulated medium-ring compound 172 in good to excellent yield under RCM conditions (Scheme 6a).107 S. K. Chattopadhyay et al. reported that when a dichloromethane solution of 173 and commercially available Grubbs' catalyst I was stirred at room temperature under nitrogen atmosphere, the reaction proceeded smoothly to afford the bis-benzoxepine 174 in 90% yield (Scheme 6b).108 Fürstner et al. developed an RCM-based approach to synthesize benzannulated macrocyclic intermediate 176 which was then converted to (R)-(+)-lasiodiplodin (Scheme 6c).109 Evano et al. reported the synthesis of bridged benzannulated macrocycle 178 with excellent selectivity and yield (Scheme 6d).110 The highly strained benzannulated medium ring of coleophomones B and C (180 and 181) was constructed using an impressive olefin metathesis reaction to build the bond between C-16 and C-17 (Scheme 6e).111
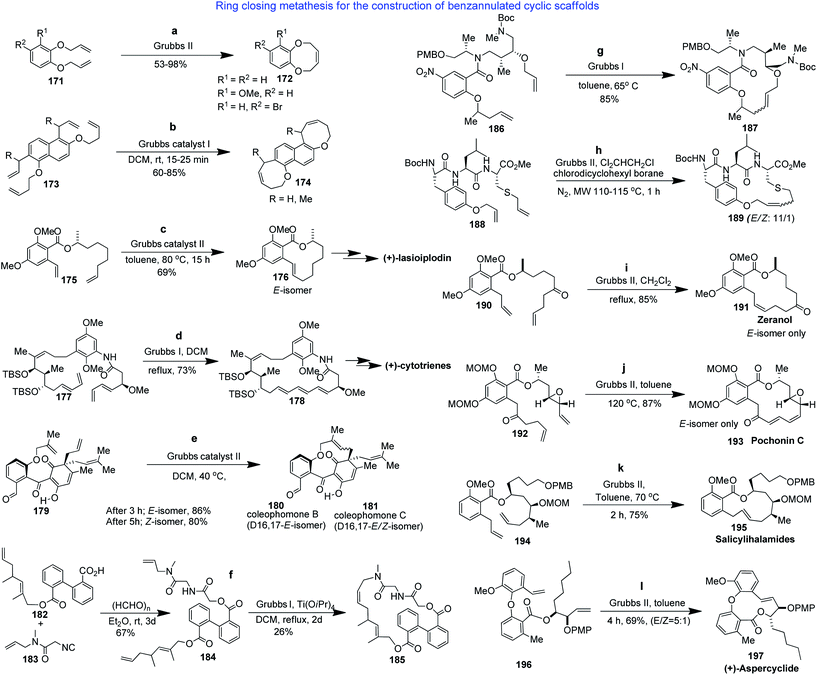 | ||
| Scheme 6 Application of ring closing metathesis in the construction of medium- and large-sized benzannulated rings. | ||
Beck et al. reported a combination of Ugi and Passerini type MCR with RCM and described the potential applicability of this sequence for generating many diverse benzannulated macrocycles.112 The approach for a representative compound is outlined in Scheme 6f, wherein 22-membered benzannulated macrocycle 185 was produced after a tandem sequence beginning from acid 182, isocyanide 183 and paraformaldehyde.
Dandapani et al. demonstrated the use of RCM for the generation of skeletally diverse benzannulated macrocyclic ethers such as 187 by a diversity-oriented synthesis approach (Scheme 6g).113
Abell et al. demonstrated the ring closing metathesis approach in the synthesis of S-containing bridged benzannulated macrocycle 189 (E/Z ratio: 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) from scaffold 188 using MW irradiation combined with Lewis acid conditions (Scheme 6h).114
1) from scaffold 188 using MW irradiation combined with Lewis acid conditions (Scheme 6h).114
Ring-closing metathesis (RCM) has been extensively used for the synthesis of resorcylic acid lactones (RALs).115 There are numerous parameters that influence metathesis reactions; therefore, there are no general conditions that can be given that will guarantee the success of the process.116 Fürstner et al. developed an RCM-based approach to zeranol (191) using Grubbs' second generation catalyst under reflux conditions and selectively synthesized the E-isomer in 85% yield as depicted in Scheme 6i.117 S. Barluenga et al. reported the synthesis of pochocin C via polyene RCM. They treated the diene 192 at 120 °C leading exclusively to macrocyclic benzolactone 193 in 10 minutes, as shown in Scheme 6j.118 C. Herb et al. cyclized the ester 194 to produce the macrocyclic benzolactone core structure of salicylihalamide (195) through ring closing metathesis (Scheme 6k).119 A. Fürstner and C. Müller reported the total synthesis of (+)-aspercyclide,120 in which the key step was a kinetically controlled RCM reaction of cyclization precursor 196 to form the medium-ring benzolactone core of (+)-aspercyclide (197), as depicted in Scheme 6l.
4.6. Mitsunobu reaction
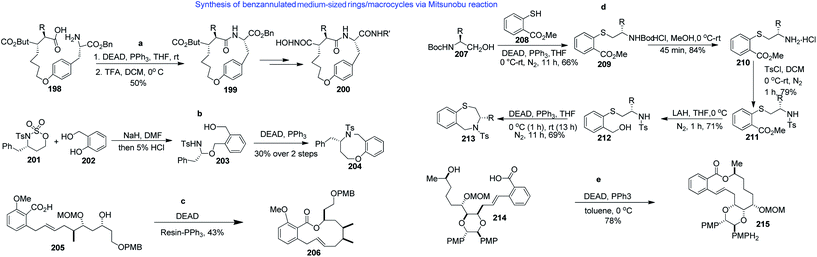
The Mitsunobu reaction and its variants are among the most useful reactions in synthetic chemistry. Not surprisingly, therefore, this mild and versatile chemistry has also found some utility for the construction of BMR scaffolds. For example, the bridged benzannulated macrocycle 200 was synthesized starting from amino acid 198 (Scheme 7a)121 while the benzannulated medium-ring ether (48) was prepared via nucleophilic cleavage of enantiomerically pure 1,2-cyclic sulfamidate 201 with phenol derivative 202, followed by Mitsunobu reaction of 203, which led to the formation of scaffold 204 in 30% overall yield, as shown in Scheme 7b.122 Another variant of the Mitsunobu reaction was reported to affect the closure of a 13-membered lactone in the presence of supported triphenylphosphine in low yield (10%) but was recently more successful in the formal synthesis of salicylihalamides (206, Scheme 7c).119,123The intramolecular Mitsunobu cyclization between the sulphonamide and benzylic hydroxyl in 212 (derived from amino acid 207 through a series of reactions, as depicted in Scheme 7d) furnished enantiomerically pure benzannulated medium-ring thio-ethers of the type 213 in 69% yield, as depicted in Scheme 7d.124
Macrocyclization of cyclization precursor 214 under Mitsunobu conditions (DEAD, PPh3, toluene, 0 °C) gave rise to benzolactone 215 in 78% yield (Scheme 7e). Most likely, conformational constraints on the backbone, imposed by the dioxane ring, facilitated the formation of the macrocycle.125
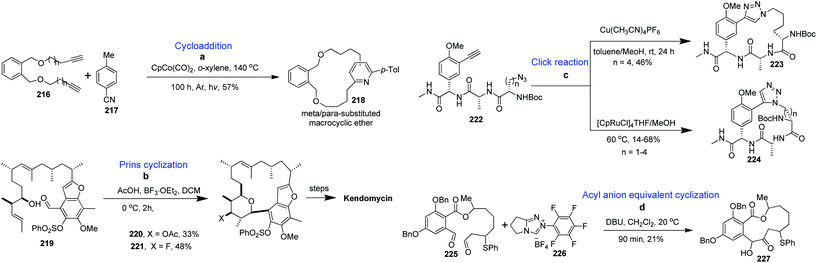
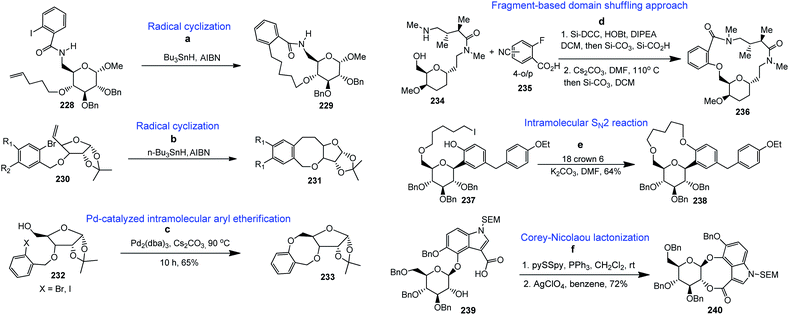
4.7. Other cyclization methods
4.8. Synthesis of medium-ring and macrocyclic scaffolds from carbohydrates
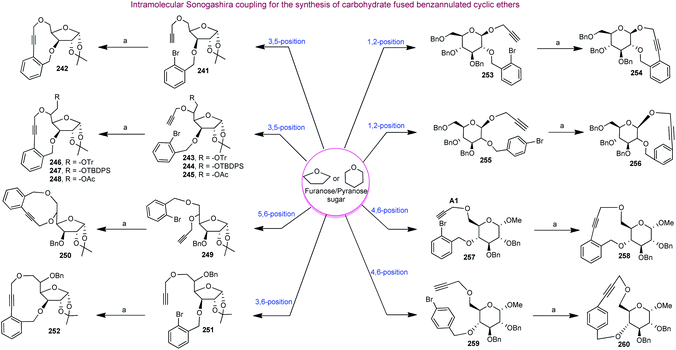
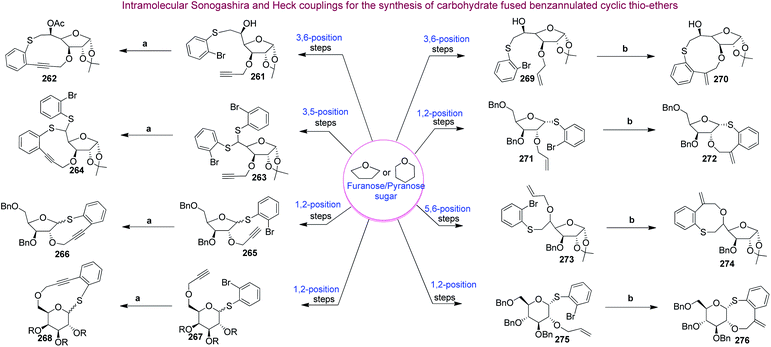
Carbohydrates along with their derivatives, particularly 1,2;5,6-di-O-isopropylidene-α-D-glucofuranoside, unsaturated sugars (glycals), peracetylated sugars, have long been recognised as extremely useful starting materials in organic synthesis. Among the various chiral pool molecules existing in nature, carbohydrates occupy an exceptional space since they are easily accessible in nature, cheap and enantiomerically pure. Furthermore, they have a very unique arrangement of functional groups, and hence they are useful as a chiral pool in organic synthesis. Moreover, carbohydrates can act as templates on which complex medium-sized or macrocyclic ring scaffolds can be constructed. Following are the literature reports on the synthesis of biologically useful BMR frameworks from carbohydrates.Our next target was to synthesize a series of BMR thio-ethers. In general, the synthesis of different building blocks involves acetonide deprotection, alkylation/arylation, epoxidation, epoxide opening, sugar aldehyde generation and glycosylation reactions. It is noteworthy that using glycosylation reactions, we managed to create both cis- and trans-stereochemistry, which enables us to generate cis/trans fused benzannulated medium-ring thio-ethers on carbohydrate chiral templates. All the building blocks containing the required moieties (aryl halide and alkene or alkyne) were then cyclised via Sonogashira and Heck coupling reactions to produce the BMR thio-ethers fused with carbohydrates (Scheme 11).137
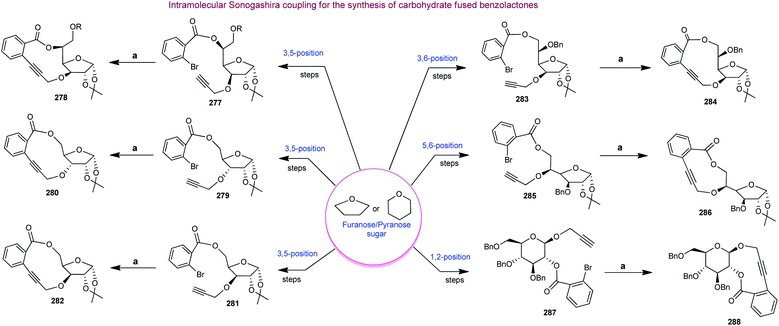
Finally, our group reported the synthesis of 10-, 11- and 12-membered chiral benzolactones fused to furanose/pyranose sugars in good to excellent yields using carbohydrates as chiral templates under similar coupling conditions, as shown in Scheme 12.138 The 1-2, 3-5, 3-6 and 5-6 positions of the sugars were utilized for the construction of the medium-ring or macrocyclic skeletons. The requisite furanose/pyranose scaffolds were synthesized utilising conventional protection–deprotection strategies like benzylation, tritylation, detritylation, EDC coupling, and propargylation.
5. Conclusions
Benzannulated medium-ring and macrocyclic ring (BMR) scaffolds have been found to be of immense potential in contemporary medicinal chemistry and drug discovery programs due to their unique structural properties. Many BMR compounds have either been approved as drugs or reached the late developmental stages in clinical trials. Although efforts from synthetic and medicinal chemists have led to the development of a large number of new elegant synthetic strategies for the construction of highly potent benzannulated macrocyclic rings (12-membered or above), methods for the construction of benzannulated medium-sized rings (7–11 membered) need to be further explored. Furthermore, it has been shown that carbohydrates could be an excellent starting point for the construction of chiral BMRs due to their inherent chirality and the presence of different types of hydroxyl groups.Acknowledgements
The authors are highly grateful to director CSIR-IIIM-Jammu, Dr Ram Vishwakarma, for necessary facilities and A. Hussain is grateful to University Grants Commission for Senior Research Fellowship. CSIR-IIIM Publication No - IIIM/1689/2014.Notes and references
- T. P. Majhi, B. Achari and P. Chattopadhyay, Heterocycles, 2007, 71, 1011–1052 CrossRef CAS PubMed.
- A. R. Khan, J. C. Parrish, M. E. Fraser, W. W. Smith, P. A. Bartlett and M. N. James, Biochemistry, 1998, 37, 16839–16845 CrossRef CAS PubMed.
- D. F. Veber, S. R. Johnson, H. Y. Cheng, B. R. Smith, K. W. Ward and K. D. Kopple, J. Med. Chem., 2002, 45, 2615–2623 CrossRef CAS PubMed.
- T. Rezai, B. Yu, G. L. Millhauser, M. P. Jacobson and R. S. Lokey, J. Am. Chem. Soc., 2006, 128, 2510–2511 CrossRef CAS PubMed.
- Y. U. Kwon and T. Kodadek, Chem. Biol., 2007, 14, 671–677 CrossRef CAS PubMed.
- (a) T. K. Devon and A. I. Scott, Handbook of Naturally Occurring Compounds, Academic Press, New York and London, 1972, vol. 2 Search PubMed; (b) D. J. Faulkner, Nat. Prod. Rep., 1984, 1, 251–280 RSC.
- J. Mallinson and I. Collins, Future Med. Chem., 2012, 4, 1409–1438 CrossRef CAS PubMed.
- S. E. Denmark and S. M. Yang, J. Am. Chem. Soc., 2004, 126, 12432–12440 CrossRef CAS PubMed.
- A. Delgado and J. Clardy, J. Org. Chem., 1993, 58, 2862–2866 CrossRef CAS , and references cited therein.
- A. Farooq, J. Gordon, J. R. Hanson and J. A. Takahashi, Phytochemistry, 1995, 38, 557 CrossRef CAS.
- D. M. Tapiolas, M. Roman, W. Fenical, T. J. Stout and J. Clardy, J. Am. Chem. Soc., 1991, 113, 4682–4683 CrossRef.
- C. C. J. Culvenor and L. W. Smith, Aust. J. Chem., 1963, 16, 239–245 CrossRef CAS.
- O. P. Suri and C. K. Atal, Curr. Sci., 1967, 36, 614–615 CAS.
- R. Adams and B. L. Van Duuren, J. Am. Chem. Soc., 1953, 75, 2377–2379 CrossRef CAS.
- K. C. Majumdar, RSC Adv., 2011, 1, 1152–1170 RSC.
- G. Illuminati and L. Mandolini, Acc. Chem. Res., 1981, 14, 95–102 CrossRef CAS.
- E. M. Driggers, S. P. Hale, J. Lee and N. K. Terrett, Nat. Rev. Drug Discovery, 2008, 7, 608–624 CrossRef CAS PubMed.
- L. A. Wessjohann, E. Ruijter, D. Garcia-Rivera and W. Brandt, Mol. Diversity, 2005, 9, 171–186 CrossRef CAS.
- (a) S. Pal, Tetrahedron, 2006, 62, 3171–3200 CrossRef CAS PubMed; (b) L. Katz and G. W. Ashley, Chem. Rev., 2005, 105, 499–527 CrossRef CAS PubMed.
- D. S. Su, D. Meng, P. Bertinato, A. Balog, E. J. Sorensen, S. J. Danishefsky, Y. H. Zheng, T. C. Chou, L. He and S. B. Horwitz, Angew. Chem., Int. Ed. Engl., 1997, 36, 757–759 CrossRef CAS PubMed.
- K. C. Nicolaou, R. A. Daines, T. K. Chakraborty and Y. Ogawa, J. Am. Chem. Soc., 1987, 109, 2821–2822 CrossRef CAS.
- K. C. Nicolaou, T. K. Chakraborty, A. D. Piscopio, N. Minowa and P. Bertinato, J. Am. Chem. Soc., 1993, 115, 4419–4420 CrossRef CAS.
- V. E. Kwitkowski, T. M. Prowell, A. Ibrahim, A. T. Farrell, R. Justice, S. S. Mitchell and R. S. R. Pazdur, Oncologist, 2010, 15, 428–435 CrossRef CAS PubMed.
- E. Raymond, J. Alexandre, S. Faivre, K. Vera, E. Materman, J. Boni, C. Leister, J. Korth-Bradley, A. Hanauske and J. P. Armand, J. Clin. Oncol., 2004, 22, 2336–2347 CrossRef CAS PubMed.
- A. Conlin, M. Fornier, C. Hudis, S. Kar and P. Kirkpatrick, Nat. Rev. Drug Discovery, 2007, 6, 953–954 CrossRef CAS.
- S. Goodin, Am. J. Health-Syst. Pharm., 2008, 65, S10–S15 CrossRef CAS PubMed.
- E. Mcdonald, P. Workman and K. Jones, Curr. Top. Med. Chem., 2006, 6, 1091–1107 CrossRef CAS.
- G. Sakoulas, P. A. Moise-Broder, J. Schentag, A. Forrest, R. C. Moellering Jr and G. M. Eliopoulos, J. Clin. Microbiol., 2004, 42, 2398–2402 CrossRef CAS PubMed.
- D. A. Cantrell and K. A. Smith, Science, 1984, 224, 1312–1316 CAS.
- A. Gradillas and J. Perez-Castells, Angew. Chem., Int. Ed., 2006, 45, 6086–6101 CrossRef CAS PubMed.
- A. Parenty, X. Moreau and J. M. Campagne, Chem. Rev., 2006, 106, 911–939 CrossRef CAS PubMed.
- F. A. Macías, R. M. Varela, A. Torres, J. M. G. Molinillo and F. R. Fronczek, Tetrahedron Lett., 1993, 34, 1999–2002 CrossRef.
- T. Shimokawa, J. Kinjo, J. Yamahara, M. Yamasaki and T. Nohara, Chem. Pharm. Bull., 1985, 33, 3545–3547 CrossRef CAS.
- S. B. Singh, H. Jayasuriya, D. L. Zink, J. D. Polishook, A. W. Dombrowski and H. Zweerink, Tetrahedron Lett., 2004, 45, 7605–7608 CrossRef CAS PubMed.
- Y. Shirataki, Y. Tagaya, I. Yokoe and M. Komatsu, Chem. Pharm. Bull., 1987, 35, 1637–1640 CrossRef CAS.
- X. Fu, T. Sévenet, F. Remy, M. Païs, A. Hamid, A. Hadi and L. M. Zeng, J. Nat. Prod., 1993, 56, 1153–1163 CrossRef CAS.
- S. Quideau and K. S. Feldman, Ellagitannin chemistry, Chem. Rev., 1996, 96, 475–504 CrossRef CAS PubMed.
- S. M. Kupchan, R. W. Britton, M. F. Ziegler, C. J. Gilmore, R. J. Restivo and R. F. Bryan, J. Am. Chem. Soc., 1973, 95, 1335–1336 CrossRef CAS.
- D. J. Abraham, R. D. Rosenstein, R. L. Lyon and H. H. S. Fong, Tetrahedron Lett., 1972, 13, 909–912 CrossRef.
- H. Urata, A. Kinoshita, K. S. Misono, F. M. Bumpus and A. Husain, J. Biol. Chem., 1990, 265, 22348–22357 CAS.
- H. Tomoda, H. Nishida, R. Masuma, J. Cao, S. Okuda and S. Omura, J. Antibiot., 1991, 44, 136–143 CrossRef CAS.
- G. M. Salituro, D. J. Pettibone, B. V. Clineschmidt, J. M. Williamson and D. L. Zink, Bioorg. Med. Chem. Lett., 1993, 3, 337–340 CrossRef CAS.
- D. Brückner, F. T. Hafner, V. Li, C. Schmeck, J. Telser, A. Vakalopoulos and G. Wirtz, Bioorg. Med. Chem. Lett., 2005, 15, 3611–3614 CrossRef PubMed.
- (a) H. Kase, M. Kaneko and K. Yamada, J. Antibiot., 1987, 40, 450–454 CrossRef CAS; (b) S. Sano, K. Ikai, K. Katayama, K. Takesako and T. Nakamura, J. Antibiot., 1986, 39, 1685–1696 CrossRef CAS.
- E. Moulin, S. Barluenga and N. Winssinger, Org. Lett., 2005, 7, 5637–5639 CrossRef CAS PubMed.
- (a) A. S. Khartulyari, M. Kapur and M. E. Maier, Org. Lett., 2006, 8, 5833–5836 CrossRef CAS PubMed; (b) A. Fiirstner, G. Seklel and N. Kindler, Tetrahedron, 1999, 55, 8215–8230 CrossRef.
- (a) S. S. Palimkar, J. Uenishi and H. Ii, J. Org. Chem., 2012, 77, 388–399 CrossRef CAS PubMed; (b) M. Huss, O. Vitavska, A. Albertmelcher, S. Boeckelmann, C. Nardmann, K. Tabke, F. Tiburcy and H. Wieczorek, Eur. J. Cell Biol., 2011, 90, 688–695 CrossRef CAS PubMed.
- G. M. Ksander, R. de Jesus, A. Yuan, R. D. Ghai, C. McMartin and R. Bohacek, J. Med. Chem., 1997, 40, 506–514 CrossRef CAS PubMed.
- G. M. Ksander, R. de Jesus, A. Yuan, R. D. Ghai, A. Trapani, C. McMartin and R. Bohacek, J. Med. Chem., 2011, 54, 1961–2004 CrossRef PubMed.
- A. Lerchner, R. Machauer, C. Betschart, S. Veenstra, H. Rueeger, C. McCarthy, M. Tintelnot-Blomley, A. L. Jaton, S. Rabe, S. Desrayaud, A. Enz, M. Staufenbiel, P. Paganetti, J. M. Rondeau and U. Neumann, Bioorg. Med. Chem. Lett., 2010, 20, 603–607 CrossRef CAS PubMed.
- Y. Huang, E. D. Strobel, C. Y. Ho, C. H. Reynolds, K. A. Conway, J. A. Piesvaux, D. E. Brenneman, G. J. Yohrling, H. M. Arnold, D. Rosenthal, R. S. Alexander, B. A. Tounge, M. Mercken, M. Vandermeeren, M. H. Parker, A. B. Reitz and E. W. Baxter, Bioorg. Med. Chem. Lett., 2010, 20, 3158–3160 CrossRef CAS PubMed.
- X. Li, Y. K. Zhang, Y. Liu, C. Z. Ding, Y. Zhou, Q. Li, J. J. Plattner, S. J. Baker, S. Zhang, W. M. Kazmierski, L. L. Wright, G. K. Smith, R. M. Grimes, R. M. Crosby, K. L. Creech, L. H. Carballo, M. J. Slater, R. L. Jarvest, P. Thommes, J. A. Hubbard, M. A. Convery, P. M. Nassau, W. McDowell, T. J. Skarzynski, X. Qian, D. Fan, L. Liao, Z. J. Ni, L. E. Pennicott, W. Zou and J. Wright, Bioorg. Med. Chem. Lett., 2010, 20, 5695–5700 CrossRef CAS PubMed.
- C. J. Dinsmore, M. J. Bogusky, J. C. Culberson, J. M. Bergman, C. F. Homnick, C. B. Zartman, S. D. Mosser, M. D. Schaber, R. G. Robinson, K. S. Koblan, H. E. Huber, S. L. Graham, G. D. Hartman, J. R. Huff and T. M. Williams, J. Am. Chem. Soc., 2001, 123, 2107–2108 CrossRef CAS.
- C. J. Dinsmore, C. B. Zartman, J. M. Bergman, M. T. Abrams, C. A. Buser, J. C. Culberson, J. P. Davide, M. Ellis-Hutchings, C. Fernandes, S. L. Graham, G. D. Hartman, H. E. Huber, R. B. Lobell, S. D. Mosser, R. G. Robinson and T. M. Williams, Bioorg. Med. Chem. Lett., 2004, 14, 639–643 CrossRef CAS PubMed.
- P. G. Nantermet, J. C. Barrow, C. L. Newton, J. M. Pellicore, M. Young, S. D. Lewis, B. J. Lucas, J. A. Krueger, D. R. McMasters, Y. Yan, L. C. Kuo, J. P. Vacca and H. G. Selnick, Bioorg. Med. Chem. Lett., 2003, 13, 2781–2784 CrossRef CAS.
- E. Marsault, K. Benakli, S. Beaubien, C. Saint-Louis, R. Deziel and G. Fraser, Bioorg. Med. Chem. Lett., 2007, 17, 4187–4190 CrossRef CAS PubMed.
- E. Marsault and M. L. Peterson, J. Med. Chem., 2011, 54, 1961–2004 CrossRef CAS PubMed.
- G. L. Fraser and H. R. Hoveyda, PCT Intl Appl. WO 06/046977, 2005.
- U. Lucking, G. Siemeister, M. Schafer, H. Briem, M. Kruger, P. Lienau and R. Jautelat, ChemMedChem, 2007, 2, 63–77 CrossRef PubMed.
- N. Kawanishi, T. Sugimoto, J. Shibata, K. Nakamura, K. Masutani, M. Ikuta and H. Hirai, Bioorg. Med. Chem. Lett., 2006, 16, 5122–5126 CrossRef CAS PubMed.
- X. Yu and D. Sun, Molecules, 2013, 18, 6230–6268 CrossRef CAS PubMed.
- S. Hart, K. C. Goh, V. Novotny-Diermayr, C. Y. Hu, H. Hentze, Y. C. Tan, B. Madan, C. Amalini, Y. K. Loh, L. C. Ong, A. D. William, A. Lee, A. Poulsen, R. Jayaraman, K. H. Ong, K. Ethirajulu, B. W. Dymock and J. W. Wood, Leukemia, 2011, 25, 1751–1759 CrossRef CAS PubMed.
- R. S. Komrojki, M. Wadleigh and J. F. Seymour, presented at: 53rd ASH Annual Meeting and Exposition, San Diego Convention Center, San Diego, CA, USA, pp. 10–13, December 2011 Search PubMed.
- A. D. William, A. C. H. Lee and K. C. Goh, J. Med. Chem., 2012, 55, 169–196 CrossRef CAS PubMed.
- H. Hirai, I. Takahashi-Suziki, T. Shimomura, K. Fukasawa, T. Machida, T. Takaki, M. Kobayashi and T. Eguchi, Invest. New Drugs, 2011, 29, 534–543 CrossRef CAS PubMed.
- N. J. Liverton, S. S. Carroll, J. Dimuzio, C. Fandozzi, D. J. Graham, D. Hazuda, M. K. Holloway, S. W. Ludmerer, J. A. McCauley, C. J. McIntyre, D. B. Olsen, M. T. Rudd, M. Stahlhut and J. P. Vacca, Antimicrob. Agents Chemother., 2010, 54, 305–311 CrossRef CAS PubMed.
- K. C. Lasseter, L. Shaughnessy, D. Cummings, J. C. Pezzullo, W. Wargin, R. Gagnon, J. Oliva and G. Kosutic, J. Clin. Pharmacol., 2008, 48, 193–202 CrossRef CAS PubMed.
- J. C. Collins and K. James, Med. Chem. Commun., 2012, 3, 1489–1495 RSC.
- D. C. Harrowven and S. L. Kostiuk, Nat. Prod. Rep., 2012, 29, 223–242 RSC.
- N. K. Terrett, Drug Discovery Today: Technol., 2010, 7, e97–e104 CrossRef CAS PubMed.
- L. Wessjohann and E. Ruijter, in Natural Product Synthesis I, Springer Berlin Heidelberg, Berlin, Germany, 2005, vol. 243, pp. 137–184 Search PubMed.
- A. E. Horton, O. S. May, M. R. J. Elsegood and M. C. Kimber, Synlett, 2011, 22, 797–800 Search PubMed.
- A. Parenty, X. Moreau, G. Niel and J. M. Campagne, Chem. Rev., 2013, 113, PR1–PR40 CrossRef CAS PubMed.
- R. Shen, C. T. Lin, E. J. Bowman, B. J. Bowman and J. A. Porco Jr, J. Am. Chem. Soc., 2003, 125, 7889–7901 CrossRef CAS PubMed.
- G. A. Holloway, H. M. Hugel and M. A. Rizzacasa, J. Org. Chem., 2003, 68, 2200–2204 CrossRef CAS PubMed.
- R. Shen, C. T. Lin and J. A. Porco Jr, J. Am. Chem. Soc., 2002, 124, 5650–5651 CrossRef CAS PubMed.
- Q. Su and J. S. Panek, J. Am. Chem. Soc., 2004, 126, 2425–2430 CrossRef CAS PubMed.
- S. S. Palimkar, J. Uenishi and H. Ii, J. Org. Chem., 2012, 77, 388–399 CrossRef CAS PubMed.
- E. Marsault, L. Ouelet, C. Saint-Louis, S. Beaubien, K. Benakli, H. R. Hoveyda, M. L. Peterson and S. Bhat, U.S. Pat., Appl. 2009/0198050, 2008.
- Z. Nie, C. Perretta, P. Erickson, S. Margosiak, J. Lu, A. Averill, R. Almassy and S. Chu, Bioorg. Med. Chem. Lett., 2008, 18, 619–623 CrossRef CAS PubMed.
- W. Zhang and J. S. Moore, Angew. Chem., Int. Ed., 2006, 45, 4416–4439 CrossRef CAS PubMed.
- R. Lépine and J. Zhu, Org. Lett., 2005, 7, 2981–2984 CrossRef PubMed.
- J. N. L. Dufour and J. Zhu, Chem.–Eur. J., 2010, 16, 10523–10534 CrossRef CAS PubMed.
- R. Yamasaki, A. Shigeto and S. Saito, J. Org. Chem., 2011, 76, 10299–10305 CrossRef CAS PubMed.
- V. Balraju, D. S. Reddy, M. Periasamy and J. Iqbal, J. Org. Chem., 2005, 70, 9626–9628 CrossRef CAS PubMed.
- K. C. Majumdar, B. Chattopadhyay and K. Ray, Tetrahedron Lett., 2007, 48, 7633–7636 CrossRef CAS PubMed.
- (a) K. C. Majumdar and B. Chattopadhyay, Synthesis, 2009, 2385 CrossRef CAS PubMed; (b) K. C. Majumdar and B. Chattopadhyay, Synlett, 2008, 0979–0982 CrossRef CAS PubMed.
- K. C. Majumdar, B. Chattopadhyay and S. Samanta, Tetrahedron Lett., 2009, 50, 3178–3181 CrossRef CAS PubMed.
- K. Akaji, K. Teruya, M. Akaji and S. Aimoto, Tetrahedron, 2001, 57, 2293–2303 CrossRef CAS.
- C. W. Zapf, J. D. Bloom, J. L. McBean, R. G. Dushin, J. M. Golas, H. Liu, J. Lucas, F. Boschelli, E. Vogan and J. I. Levin, Bioorg. Med. Chem. Lett., 2011, 21, 3627–3631 CrossRef CAS PubMed.
- V. Balraju and J. Iqbal, J. Org. Chem., 2006, 71, 8954–8956 CrossRef CAS PubMed.
- K. C. Nicolaou, N. Winssinger, J. Pastor and F. Murphy, Angew. Chem., Int. Ed., 1998, 37, 2537–2547 Search PubMed.
- W. Li and K. Burgess, Tetrahedron Lett., 1999, 40, 6527–6530 CrossRef CAS.
- S. M. Lu and H. Alper, Chem.–Eur. J., 2007, 13, 5908–5916 CrossRef CAS PubMed.
- A. Sharma, P. Appukkuttan and E. van der Eycken, Chem. Commun., 2012, 48, 1623–1637 RSC.
- Q. Su, A. B. Beeler, E. Lobkovsky, J. A. Porco and J. S. Panek, Org. Lett., 2003, 5, 2149–2152 CrossRef CAS PubMed.
- L. Shen, C. J. Simmons and D. Sun, Tetrahedron Lett., 2012, 53, 4173–4178 CrossRef CAS PubMed.
- P. Mestichelli, M. J. Scott, W. R. J. D. Galloway, J. Selwyn, J. S. Parker and D. R. Spring, Org. Lett., 2013, 15, 5448–5451 CrossRef CAS PubMed.
- A. Berthelot, S. Piguel, G. Le Dour and J. Vidal, J. Org. Chem., 2003, 68, 9835–9838 CrossRef CAS PubMed.
- A. Poloukhtine, V. Rassadin, A. Kuzmin and V. V. Popik, J. Org. Chem., 2010, 75, 5953–5962 CrossRef CAS PubMed.
- T. Zhang, X. Huang, J. Xue and S. Sun, Tetrahedron Lett., 2009, 50, 1290–1294 CrossRef CAS PubMed.
- R. A. Bauer, T. A. Wenderski and D. S. Tan, Nat. Chem. Biol., 2013, 9, 21–30 CrossRef CAS PubMed.
- F. Kopp, C. F. Stratton, L. B. Akella and D. S. Tan, Nat. Chem. Biol., 2012, 8, 358–365 CrossRef CAS PubMed.
- M. C. Zaccaro, H. B. Lee, M. Pattarawarapan, Z. Xia, A. Caron, P. J. L. Heureux, Y. Bengio, K. Burgess and H. U. Saragovi, Chem. Biol., 2005, 12, 1015–1028 CrossRef CAS PubMed.
- E. J. Kang, S. Y. Lee, H. Lee and S. S. Lee, Inorg. Chem., 2010, 49, 7510–7520 CrossRef CAS PubMed.
- J. Buter and R. M. Kellogg, J. Org. Chem., 1981, 46, 4481–4485 CrossRef CAS.
- R. Mamouni, M. Soukri, S. Lazar, M. Akssira and G. Guillaumet, Tetrahedron Lett., 2004, 45, 2631–2633 CrossRef CAS PubMed.
- S. K. Chattopadhyay, T. Biswas and S. Maity, Synlett, 2006, 2211 CrossRef CAS PubMed.
- A. Fürstner, G. Seidel and N. Kindler, Tetrahedron, 1999, 55, 8215–8230 CrossRef.
- G. Evano, J. V. Schaus and J. S. Panek, Org. Lett., 2004, 6, 525–528 CrossRef CAS PubMed.
- K. C. Nicolaou, T. Montagnon, G. Vassilikogiannkabis and C. J. N. Mathison, J. Am. Chem. Soc., 2005, 127, 8872–8888 CrossRef CAS PubMed.
- B. Beck, G. Larbig, B. Mejat, M. Magnin-Lachaux, A. Picard, E. Herdtweck and A. Domling, Org. Lett., 2003, 5, 1047–1050 CrossRef CAS PubMed.
- S. Dandapani and L. A. Marcaurelle, Nat. Chem. Biol., 2010, 6, 861–863 CrossRef CAS PubMed.
- A. D. Abell, N. A. Alexander, S. G. Aitken, H. Chen, J. M. Coxon, M. A. Jones, S. B. Mc Nabb and A. Muscroft-Taylor, J. Org. Chem., 2009, 74, 4354–4356 CrossRef CAS PubMed.
- J. Pérez-Castells and A. Gradillas, Angew. Chem., Int. Ed., 2006, 45, 6086–6101 CrossRef PubMed.
- S. Y. Hong, M. W. Day and R. H. Grubbs, J. Am. Chem. Soc., 2004, 126, 7414–7415 CrossRef CAS PubMed.
- A. Fürstner, G. Seidel and N. Kindler, Tetrahedron, 1999, 55, 821–8230 CrossRef.
- S. Barluenga, P. L. Spez, E. Moulin and N. Winssinger, Angew. Chem., 2004, 116, 3549–3552 (Angew. Chem., Int. Ed., 2004, 43, 3467–3470) CrossRef PubMed.
- C. Herb, A. Bayer and M. E. Maier, Chem.–Eur. J., 2004, 10, 5649–5660 CrossRef CAS PubMed.
- A. Fürstner and C. Müller, Chem. Commun., 2005, 5583–5585 RSC.
- K. C. Swamy, N. N. Kumar, E. Balaraman and K. V. Kumar, Chem. Rev., 2009, 109, 2551–2651 CrossRef CAS PubMed.
- J. Rujirawanich and T. Gallagher, Org. Lett., 2009, 11, 5494–5496 CrossRef CAS PubMed.
- C. Herb and M. E. Maier, J. Org. Chem., 2003, 68, 8129–8135 CrossRef CAS PubMed.
- J. K. Mishra and G. Panda, J. Comb. Chem., 2007, 9, 321–338 CrossRef CAS PubMed.
- A. Delgado and J. Clardy, J. Org. Chem., 1993, 58, 2862 CrossRef CAS , and references cited therein.
- L. V. R. Boñaga, H. C. Zhang, A. F. Moretto, H. Ye, D. A. Gauthier, J. Li, G. C. Leo and B. E. Maryanoff, J. Am. Chem. Soc., 2005, 127, 3473–3485 CrossRef PubMed.
- B. Kevin Bahnck and S. D. Rychnovsky, J. Am. Chem. Soc., 2008, 130, 13177–13181 CrossRef PubMed.
- J. Zhang, J. Kemmink, D. T. S. Rijkers and R. M. J. Liskamp, Org. Lett., 2011, 13, 3438–3441 CrossRef CAS PubMed.
- S. M. Mennen and S. J. Miller, J. Org. Chem., 2007, 72, 5260–5269 CrossRef CAS PubMed.
- R. F. P. Faraco, G. C. B. de Oliveira, G. D. Pinto, A. P. C. Rocha, R. J. Alves, R. B. Alves, P. V. Abdelnur, M. N. Eberlin and M. A. F. Prado, J. Braz. Chem. Soc., 2009, 20, 1504–1514 CrossRef CAS PubMed.
- (a) P. Chattopadhyay, M. Mukherjee and S. Ghosh, Chem. Commun., 1997, 2139–2140 RSC; (b) A. Neogi, T. P. Majhi, N. Ghoshal and P. Chattopadhyay, Tetrahedron, 2005, 61, 9368–9374 CrossRef CAS PubMed.
- A. Neogi, T. P. Majhi, B. Achari and P. Chattopadhyay, Eur. J. Org. Chem., 2008, 330–336 CrossRef CAS PubMed.
- E. Comer, H. Liu, A. Joliton, A. Clabaut, C. Johnson, L. B. Akella and L. A. Marcaurelle, PNAS, 2011, 108, 6751–6756 CrossRef CAS PubMed.
- M. J. Kim, S. H. Lee, S. O. Park, H. Kang, J. S. Lee, K. N. Lee, M. E. Jung, J. Kim and J. Lee, Bioorg. Med. Chem., 2011, 19, 5468–5479 CrossRef CAS PubMed.
- E. J. Corey and K. C. Nicolaou, J. Am. Chem. Soc., 1974, 96, 5614 CrossRef CAS.
- A. Hussain, S. K. Yousuf, D. Kumar, L. Mallikharjuna, S. Maity, B. Singh and D. Mukherjee, Adv. Synth. Catal., 2012, 354, 1933–1940 CrossRef CAS PubMed.
- A. Hussain, S. K. Yousuf, D. Kumar, L. Mallikharjuna, B. Singh and D. Mukherjee, Tetrahedron, 2013, 69, 5517–5524 CrossRef CAS PubMed.
- A. Hussain, L. Mallikharjuna, D. K. Sharma, A. K. Tripathi, B. Singh and D. Mukherjee, RSC Adv., 2013, 3, 19899–19904 RSC.
| This journal is © The Royal Society of Chemistry 2014 |