A simple/green process for the preparation of composite carbon nanotube fibers/yarns
Abstract
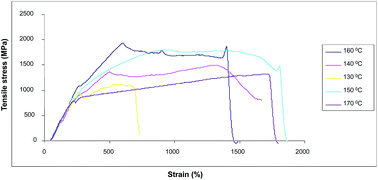
We report a simple and green process to prepare poly(vinyl alcohol)/carbon nanotube (PVA/CNT) composite fibers having high mechanical properties. This process, an environmentally friendly one with no use of acid or hazardous solvent, produces the composite fibers utilizing a PVA layer pre-coated on a PET film. SEM micrographs indicated that the CNTs are well dispersed in the PVA matrix, and the diameter of the fiber is around 50 μm. Mechanical properties of the composite fiber treated at different thermal annealing conditions are also reported.

 Please wait while we load your content...
Please wait while we load your content...