Engineering hierarchical porous carbon-supported Fe-doped cobalt phosphides from MOF templates for alkaline water oxidation
Linchao
Xu
a,
Junliang
Chen
c,
Qipeng
Li
 *b and
Jinjie
Qian
*b and
Jinjie
Qian
 *c
*c
aCollege of Optoelectronic Manufacturing, Zhejiang Industry and Trade Vocational College, Wenzhou 325003, Zhejiang, P. R. China
bCollege of Chemistry and Chemical Engineering, Zhaotong University, Zhaotong 657000, Yunnan, P. R. China. E-mail: qpli@ztu.edu.cn
cCollege of Chemistry and Materials Engineering, Wenzhou University, Wenzhou 325035, Zhejiang, P. R. China. E-mail: jinjieqian@wzu.edu.cn
First published on 17th November 2025
Abstract
A MOF-on-MOF strategy is utilized to synthesize Fe-doped cobalt phosphide nanoparticles within a porous N-doped carbon matrix (S-CoFeP). Detailed characterization confirms the successful formation of Co2P as the primary phase, with Fe incorporated as a beneficial dopant. S-CoFeP exhibits exceptional OER performance in alkaline media, achieving a small overpotential of 234 mV at 10 mA cm−2 and satisfactory durability for over 70 hours.
In recent years, the global energy crisis and environmental pollution have become increasingly prominent, primarily due to the excessive consumption of fossil fuels.1 The urgent need for sustainable energy conversion technologies has positioned electrocatalytic water splitting as a cornerstone for the production of clean hydrogen fuel.2 The efficiency of this process, however, is severely hampered by the kinetically sluggish oxygen evolution reaction (OER) at the anode.3,4 Intrinsically, it involves a complicated four-electron transfer reaction with high energy barrier, which severely limits the efficiency of overall water electrolysis.5,6 Although state-of-the-art noble metal-based (Ru, Ir) electrocatalysts exhibit high catalytic activity, their widespread application is highly restricted by their high cost and limited operational stability.7,8 This has spurred extensive research into developing non-precious metal alternatives with cost-efficiency, high activity and stability in the field of energy conversion.
Transition metal phosphides (TMPs), especially those from the iron group, have emerged as promising candidates.9 Possessing unique electronic structures and chemical properties, versatile TMPs are endowed with desirable catalytic activity for the OER process.10,11 However, their relatively poor conductivity and limited number of active sites restrict further improvement in catalytic performance.12,13 Metal–organic frameworks (MOFs), which consist of inorganic metal ions or clusters and bridging organic ligands, have garnered significant attention in the field of materials science thanks to their highly ordered porous structures and adjustable pore sizes.14–18 MOFs have proven to be exceptional precursors for the synthesis of such nanomaterials,19,20 as their pyrolysis yields tailored carbon scaffolds with high surface areas while preserving metal sites.21,22 The morphology and structure of MOF precursors can influence the morphology, composition, structure, and performance of the resultant materials.23,24 However, achieving precise control over the final catalyst's morphology, porosity, and heteroatom doping remains a significant synthetic challenge.
In this study, we report a rational and stepwise strategy for preparing a highly active and stable TMP-based OER electrocatalyst, denoted as S-CoFeP. Our approach begins with the meticulous design of bimetallic ZnCo-MOF precursors where Zn2+ acts as a morphology modulator, and subsequent pyrolysis results in a sheet-like, high-surface-area carbon framework. This optimized substrate then enables the uniform growth of a cobalt-iron Prussian blue analogue (CoFePBA), which serves as a self-sacrificing source for Fe, Co, and N. The final phosphatization step converts the intermediate into active Fe-doped cobalt phosphide nanoparticles embedded within a hierarchically porous, N-doped carbon matrix. The resulting S-CoFeP catalyst exhibits exceptional OER performance, achieving an ultralow overpotential of 234 mV at 10 mA cm−2 and satisfactory electrocatalytic stability over 70 hours, significantly surpassing benchmark RuO2. This work provides a comprehensive blueprint for designing advanced OER catalysts through synergistic morphology control and heteroatom doping, highlighting a promising path toward efficient and durable energy conversion systems (Scheme 1).
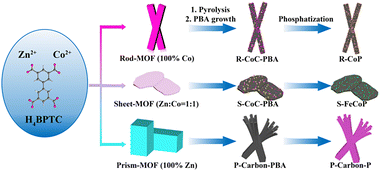 | ||
| Scheme 1 Stepwise synthesis of a series of MOF-derived hierarchical carbon-supported TMP nanomaterials. | ||
The MOF precursors were synthesized via a solvothermal reaction in a mixed solution containing inorganic Co2+/Zn2+ salts and organic ligand (H4BPTC, see the SI for full experimental details). As observed by scanning electron microscopy (SEM) in Fig. 1a–c, their morphology evolved significantly with the introduction of Zn2+. The CoOF-1 precursor exhibited a rod-like shape, which transitioned to a sheet-like structure (ZnCoOF-1) and finally to a prism-like morphology (ZnOF-1) as the concentration of Zn2+ was increased. The powder X-ray diffraction (PXRD) pattern of CoOF-1 (Fig. 1d) corresponded well with the simulated pattern (CCDC #1912147), with two main diffraction peaks at 8.2° and 15.2° indexed to the (110) and (211) planes, respectively, and the (110) plane was the dominant facet. For the bimetallic ZnCoOF-1, the diffraction intensity of the (110) plane was attenuated, while that of the (211) plane intensified. This trend culminated in pure ZnOF-1, where the (110) peak disappeared entirely and the (211) plane became predominant. This systematic shift in diffraction intensities suggested that the morphological transformation was attributable to the incorporation of Zn2+ and its differential coordination behavior with the H4BPTC linker compared to Co2+.25 These precursors were subsequently pyrolyzed, yielding carbon materials that retained the morphological features of their respective precursors: R-CoC and S-CoC maintained the rod-like and sheet-like shapes, while P-carbon resembled bundles of branches (Fig. S1–S3). Furthermore, the phase composition of the MOF-derived carbon materials was characterized by PXRD analysis in Fig. S4. This revealed that R-CoC and S-CoC exhibited identical crystalline phases, with two prominent peaks at 44.2° and 51.5° corresponding to the (111) and (200) crystal planes of metallic cobalt (PDF#97-005-2934), respectively. In contrast, the pattern for the Zn-based MOF-derived P-carbon showed no distinct peaks, which was attributed to the evaporation of zinc species during pyrolysis. The porous architecture of the carbon materials was probed using N2 sorption measurements (Fig. 1e). All samples displayed typical Type-IV isotherms with hysteresis loops, characteristic of mesoporous materials. Pore size distribution (PSD) analysis (Fig. 1f) further detailed that while mesopores dominated in R-CoC and S-CoC, these materials also contained a distribution of micropores and macropores. Notably, in P-carbon, the volume of micropores was comparable to that of mesopores, a finding consistent with previous reports.26,27 As anticipated, the BET specific surface areas increased substantially from 138.2 m2 g−1 for R-CoC to 354.6 m2 g−1 for S-CoC and 1019.3 m2 g−1 for P-carbon (Table S2). This suggested that the introduction of zinc species served as a beneficial strategy for constructing highly porous carbon frameworks.
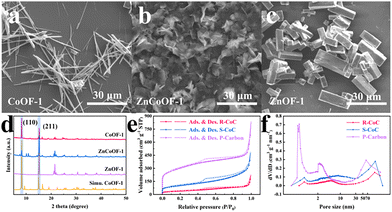 | ||
| Fig. 1 (a)–(c) SEM images, (d) PXRD patterns of Co/ZnCo/ZnOF-1 precursors. (e) N2 sorption isotherms, and (f) the corresponding PSD curves of Co/ZnCo/ZnOF-1 derived carbon materials. | ||
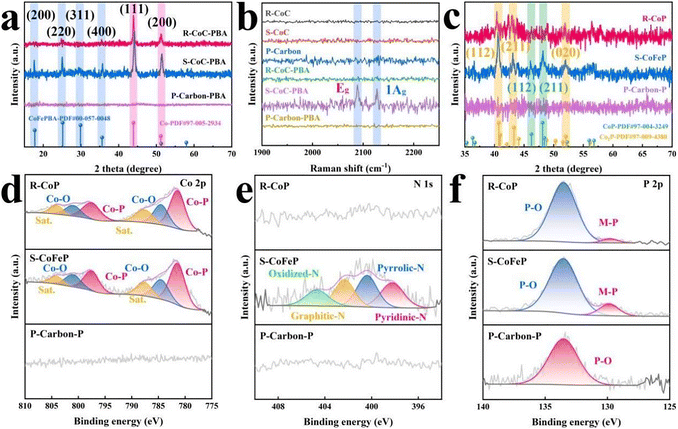
Thereafter, a hydrothermal process was employed to grow Prussian blue analogue (PBA) crystals on the carbon substrates. SEM images revealed a stark contrast in PBA growth in Fig. S5–S7. While few PBA cubes were observed on R-CoC-PBA, abundant and well-dispersed cubes formed on S-CoC-PBA. This divergence was attributed to the difference in substrate porosity; the larger specific surface area of S-CoC enhanced the accessibility for PBA nucleation and growth. Although P-carbon possessed the highest surface area, the absence of the cobalt source precluded effective PBA formation. Phase identification corroborated these findings (Fig. 2a), where R-CoC-PBA showed only the diffraction peaks of metallic cobalt, indicating suppressed PBA growth. In contrast, the pattern for S-CoC-PBA featured four additional peaks at 17.7°, 25.1°, 29.9°, and 35.6°, indexed to the (200), (220), (311), and (400) planes of CoFePBA, respectively. The PXRD pattern for P-carbon-PBA showed no signals for either PBA or cobalt. Moreover, two distinct Raman peaks at 2088 and 2127 cm−1, corresponding to the Eg and 1Ag stretching vibrations of the C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N bond in CoFePBA, were observed exclusively in the S-CoC-PBA spectrum (Fig. 2b). Following phosphorization, carbon substrates were covered with nanoparticles for R-CoP and S-CoFeP, whereas P-carbon-P retained a smooth surface (Fig. S8–S10). The PXRD patterns of R-CoP and S-CoFeP constituted two groups of peaks in Fig. 2c: one set matched the (112), (211), and (020) planes of Co2P (PDF#97-009-4380
N bond in CoFePBA, were observed exclusively in the S-CoC-PBA spectrum (Fig. 2b). Following phosphorization, carbon substrates were covered with nanoparticles for R-CoP and S-CoFeP, whereas P-carbon-P retained a smooth surface (Fig. S8–S10). The PXRD patterns of R-CoP and S-CoFeP constituted two groups of peaks in Fig. 2c: one set matched the (112), (211), and (020) planes of Co2P (PDF#97-009-4380![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40.7°, 43.3°, 52.1°), while the other corresponded to the (112) and (211) planes of CoP (PDF#97-004-3249
40.7°, 43.3°, 52.1°), while the other corresponded to the (112) and (211) planes of CoP (PDF#97-004-3249![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 46.2°, 48.1°). Furthermore, X-ray photoelectron spectroscopy (XPS) was employed to reveal the surface chemical states. The high-resolution Co 2p spectra were deconvoluted into three pairs of doublets (Fig. 2d), corresponding to Co-P (781.4/797.6 eV), Co-O (784.5/801.0 eV), and satellite peaks (787.6/804.1 eV). It should be noted that P-carbon showed no signals in either the Co 2p or Zn 2p regions (Fig. S11), consistent with the absence of Co and the evaporation of Zn during pyrolysis. Notably, a distinct N 1s signal was observed only for S-CoFeP (Fig. 2e), which was fitted with four components: oxidized-N (404.7 eV), graphitic-N (402.3 eV), pyrrolic-N (400.4 eV), and pyridinic-N (398.2 eV), confirming the successful incorporation of nitrogen species from CoFePBA. The presence of Fe in S-CoFeP was further verified by the Fe 2p spectrum (Fig. S12), which exhibited peaks for Fe-P (711.3/719.3 eV), Fe-O (713.8/722.1 eV), and a Co LMM Auger peak at 716.5 eV. A weak apparent Fe signal was also detected in R-CoP, as PBA growth was inhibited on this substrate.28,29 Finally, the P 2p spectra for R-CoP and S-CoFeP could be divided into two parts: M-P (metal-phosphide, 129.9 eV) and P-O (133.5 eV) in Fig. 2f. For P-carbon-P, only the P-O peak was observed, indicating that there was no metal phosphide formation owing to the lack of a metallic source.
46.2°, 48.1°). Furthermore, X-ray photoelectron spectroscopy (XPS) was employed to reveal the surface chemical states. The high-resolution Co 2p spectra were deconvoluted into three pairs of doublets (Fig. 2d), corresponding to Co-P (781.4/797.6 eV), Co-O (784.5/801.0 eV), and satellite peaks (787.6/804.1 eV). It should be noted that P-carbon showed no signals in either the Co 2p or Zn 2p regions (Fig. S11), consistent with the absence of Co and the evaporation of Zn during pyrolysis. Notably, a distinct N 1s signal was observed only for S-CoFeP (Fig. 2e), which was fitted with four components: oxidized-N (404.7 eV), graphitic-N (402.3 eV), pyrrolic-N (400.4 eV), and pyridinic-N (398.2 eV), confirming the successful incorporation of nitrogen species from CoFePBA. The presence of Fe in S-CoFeP was further verified by the Fe 2p spectrum (Fig. S12), which exhibited peaks for Fe-P (711.3/719.3 eV), Fe-O (713.8/722.1 eV), and a Co LMM Auger peak at 716.5 eV. A weak apparent Fe signal was also detected in R-CoP, as PBA growth was inhibited on this substrate.28,29 Finally, the P 2p spectra for R-CoP and S-CoFeP could be divided into two parts: M-P (metal-phosphide, 129.9 eV) and P-O (133.5 eV) in Fig. 2f. For P-carbon-P, only the P-O peak was observed, indicating that there was no metal phosphide formation owing to the lack of a metallic source.
The morphology and elemental distribution of these MOF-derived metal-carbon nanomaterials were further characterized by transmission electron microscopy (TEM) and energy-dispersive X-ray spectroscopy (EDS) mapping. As shown in Fig. 3a, the sample of S-CoFeP retained a sheet-like architecture decorated with abundant nanoparticles. Magnified TEM images revealed a hierarchically porous nanostructure, a feature attributed to the complete vaporization of Zn species during pyrolysis, with Fig. S13 clearly showing interior cavities indicative of a spongy, highly porous carbon matrix. High-resolution TEM (HR-TEM) imaging identified distinct lattice fringes with a spacing of 0.22 nm (Fig. 3b), corresponding to the (112) crystallographic plane of Co2P. This finding aligned with the PXRD results and confirms Co2P as the primary active phase in the as-obtained S-CoFeP. Elemental mapping (Fig. 3c and d) illustrated the spatial distribution of cobalt, iron, carbon, nitrogen, and phosphorous. In this instance, the C signal was uniformly dispersed, originating from the MOF-derived porous carbon substrate. In contrast, the Co, Fe, N, and P signals were co-located within the nanoparticles, confirming that they were the products of the phosphorized CoFePBA. It should be noted that the Fe signal intensity was significantly weaker than that of Co, indicating its successful incorporation as a minor dopant within the dominant Co2P phase, a modification critical for enhancing subsequent electrochemical performance toward the OER. The physicochemical characterizations unveiled a hierarchically porous carbon embedded with abundant cobalt phosphide nanoparticles, which exhibited multiple positive effects on the OER in terms of both activity and stability.
 | ||
| Fig. 3 (a) TEM image, (b) HR-TEM image, (c) HAADF-STEM image, and (d) the corresponding element mapping of S-CoFeP. | ||
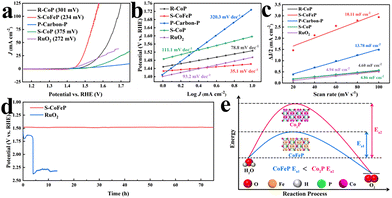
The OER performance of all MOF-derived carbon catalysts was assessed using linear sweep voltammetry (LSV). As illustrated in Fig. 4a, S-CoFeP exhibited superior activity with an overpotential (η10) of 234 mV to achieve a current density of 10 mA cm−2, significantly lower than that of R-CoP (η10 = 301 mV) and P-carbon-P (η10 > 500 mV). This performance enhancement underscored the critical role of successful PBA growth via introducing Fe species to boost catalytic activity. This conclusion was further supported by the inferior performance of a control sample of S-CoP (synthesized identically to S-CoFeP but without the PBA growth step), which yielded a high η10 value of 375 mV. Notably, S-CoFeP also outperformed commercial RuO2 (η10 = 272 mV), demonstrating its potential as a non-noble metal alternative. The OER kinetics, evaluated by Tafel slopes (Fig. 4b), revealed that S-CoFeP possessed the smallest value (35.1 mV dec−1), indicating the most favorable reaction kinetics and further confirming the beneficial impact of Fe incorporation. To probe the origin of this enhanced activity, the electrochemically active surface area (ECSA) was estimated from the double-layer capacitance (Cdl) measured via cyclic voltammetry (Fig. 4c and Fig. S14, S15). S-CoFeP displayed the highest Cdl value (18.11 mF cm−2), suggesting the largest exposure of active sites. Furthermore, electrochemical impedance spectroscopy (EIS) showed that the optimal S-CoFeP had the lowest charge transfer resistance of 2.6 Ω (Fig. S17 and Table S3), signifying superior electronic conductivity and more efficient charge transfer. Finally, the electrochemical stability of S-CoFeP was evaluated against RuO2 using chronopotentiometry (CP) at a constant current density. It showed negligible potential decay over 70 hours, while RuO2 degraded rapidly within 5 hours (Fig. 4d). This exceptional stability, coupled with the preserved crystallographic phase and morphology after the OER (Fig. S18 and S19), confirmed the structural robustness of the S-CoFeP catalyst.
In summary, we have successfully demonstrated a strategic synthesis of a series of MOF-derived hierarchically porous carbon nanomaterials for the electrocatalytic OER. The methodology hinged on the rational design of MOF precursors, where the introduction of Zn2+ was shown to be a critical factor. It not only induced a morphological evolution from nanorods to nanosheets but also served as a sacrificial template, creating a high-surface-area porous carbon substrate, which was essential for the subsequent uniform growth of CoFePBA. After carbonization, comprehensive characterization confirmed the successful formation of Co2P as the primary active phase, with Fe incorporated as a beneficial dopant, and the retention of nitrogen species from the PBA. The synergistic interplay between the porous carbon matrix and the bimetallic FeCo phosphide particles endowed S-CoFeP with exceptional OER performance. The obtained sheet-like catalyst exhibited a small η10 value of 234 mV and a Tafel slope of 35.1 mV dec−1 with desired durability, significantly outperforming its monometallic counterparts and even benchmark RuO2. Therefore, this work provides a feasible and effective synthetic route for designing non-precious TMPs as OER electrocatalysts, highlighting the importance of precursor morphology control and heteroatom doping for enhancing both activity and durability in energy conversion applications.
This work was financially supported by the Special Basic Cooperative Research Programs of Yunnan Provincial Undergraduate Universities Association (202301BA070001-093) and Deep Learning-Based Online Intelligent Inspection & Packaging System for Zippers (ZG2023016).
Conflicts of interest
There are no conflicts to declare.Data availability
The data that support the findings of this study are available in the supplementary information (SI), and more details are available from the corresponding author upon reasonable request. Supplementary information is available. See DOI: https://doi.org/10.1039/d5cc05689f.Notes and references
- Q. Dang, H. Lin, Z. Fan, L. Ma, Q. Shao, Y. Ji, F. Zheng, S. Geng, S.-Z. Yang, N. Kong, W. Zhu, Y. Li, F. Liao, X. Huang and M. Shao, Nat. Commun., 2021, 12, 6007 CrossRef CAS.
- L. Bai, C.-S. Hsu, D. T. L. Alexander, H. M. Chen and X. Hu, Nat. Energy, 2021, 6, 1054–1066 CrossRef CAS.
- B. Zhang, Y. Zheng, T. Ma, C. Yang, Y. Peng, Z. Zhou, M. Zhou, S. Li, Y. Wang and C. Cheng, Adv. Mater., 2021, 33, 2006042 CrossRef CAS PubMed.
- Q. Qian, Y. Zhu, N. Ahmad, Y. Feng, H. Zhang, M. Cheng, H. Liu, C. Xiao, G. Zhang and Y. Xie, Adv. Mater., 2024, 36, 2306108 CrossRef CAS.
- A. Govind Rajan, J. M. P. Martirez and E. A. Carter, J. Am. Chem. Soc., 2020, 142, 3600–3612 CrossRef CAS PubMed.
- K. Shah, R. Dai, M. Mateen, Z. Hassan, Z. Zhuang, C. Liu, M. Israr, W.-C. Cheong, B. Hu, R. Tu, C. Zhang, X. Chen, Q. Peng, C. Chen and Y. Li, Angew. Chem., Int. Ed., 2022, 61, e202114951 CAS.
- J. Quílez-Bermejo, S. García-Dalí, A. Daouli, A. Zitolo, R. L. S. Canevesi, M. Emo, M. T. Izquierdo, M. Badawi, A. Celzard and V. Fierro, Adv. Funct. Mater., 2023, 33, 2300405 CrossRef.
- L. Huo, M. Lv, M. Li, X. Ni, J. Guan, J. Liu, S. Mei, Y. Yang, M. Zhu, Q. Feng, P. Geng, J. Hou, N. Huang, W. Liu, X. Y. Kong, Y. Zheng and L. Ye, Adv. Mater., 2024, 36, 2312868 CrossRef CAS.
- Z. Pu, T. Liu, I. S. Amiinu, R. Cheng, P. Wang, C. Zhang, P. Ji, W. Hu, J. Liu and S. Mu, Adv. Funct. Mater., 2020, 30, 2004009 CrossRef CAS.
- Y. Li, Z. Dong and L. Jiao, Adv. Energy Mater., 2020, 10, 1902104 CrossRef CAS.
- Y. Shi, M. Li, Y. Yu and B. Zhang, Energy Environ. Sci., 2020, 13, 4564–4582 RSC.
- P. M. Bodhankar, P. B. Sarawade, P. Kumar, A. Vinu, A. P. Kulkarni, C. D. Lokhande and D. S. Dhawale, Small, 2022, 18, 2107572 CrossRef CAS PubMed.
- C.-J. Huang, H.-M. Xu, T.-Y. Shuai, Q.-N. Zhan, Z.-J. Zhang and G.-R. Li, Appl. Catal., B, 2023, 325, 122313 CrossRef CAS.
- J.-R. Li, J. Sculley and H.-C. Zhou, Chem. Rev., 2012, 112, 869–932 CrossRef CAS.
- O. M. Yaghi, M. O'Keeffe, N. W. Ockwig, H. K. Chae, M. Eddaoudi and J. Kim, Nature, 2003, 423, 705–714 CrossRef CAS PubMed.
- H. Furukawa, K. E. Cordova, M. O’Keeffe and O. M. Yaghi, Science, 2013, 341, 1230444 CrossRef.
- Z. Zhang, S. Tang, L. Xu, J. Wang, A. Li, M. Jing, X. Yang and F. Song, Int. J. Hydrogen Energy, 2024, 74, 10–16 CrossRef CAS.
- S. Tang, L. Xu, X. Ding, Q. Lv, H. Qin, A. Li, X. Yang, J. Han and F. Song, Fuel, 2025, 381, 133424 CrossRef CAS.
- L. Chai, Z. Hu, X. Wang, Y. Xu, L. Zhang, T.-T. Li, Y. Hu, J. Qian and S. Huang, Adv. Sci., 2020, 7, 1903195 CrossRef CAS PubMed.
- S. Li, Y. Gao, N. Li, L. Ge, X. Bu and P. Feng, Energy Environ. Sci., 2021, 14, 1897–1927 RSC.
- J. Du, F. Li and L. Sun, Chem. Soc. Rev., 2021, 50, 2663–2695 RSC.
- Q. Wang and D. Astruc, Chem. Rev., 2020, 120, 1438–1511 CrossRef CAS PubMed.
- X. Wang, L. Chai, J. Ding, L. Zhong, Y. Du, T.-T. Li, Y. Hu, J. Qian and S. Huang, Nano Energy, 2019, 62, 745–753 CrossRef CAS.
- E. Vijayakumar, S. Ramakrishnan, C. Sathiskumar, D. J. Yoo, J. Balamurugan, H. S. Noh, D. Kwon, Y. H. Kim and H. Lee, Chem. Eng. J., 2022, 428, 131115 CrossRef CAS.
- J. Troyano, A. Carné-Sánchez, C. Avci, I. Imaz and D. Maspoch, Chem. Soc. Rev., 2019, 48, 5534–5546 RSC.
- Y. Xiao, H. Yang, X. Bu and P. Feng, Carbon, 2021, 176, 421–430 CrossRef CAS.
- Q. Lai, Y. Zhao, Y. Liang, J. He and J. Chen, Adv. Funct. Mater., 2016, 26, 8334–8344 CrossRef CAS.
- P. Xue, C. Guo, L. Li, H. Li, D. Luo, L. Tan and Z. Chen, Adv. Mater., 2022, 34, 2110047 CrossRef PubMed.
- J. Liu, G. Li, D. Luo, J. Li, X. Zhang, Q. Li, H. Li, Y. Zhang and Z. Chen, Adv. Funct. Mater., 2024, 34, 2303357 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2026 |