Enhancement of volumetric adsorption capacities on a 2D layered MOF by controlling crystal morphology
Yuna
Kawanami
a,
Wataru
Nakanishi
a and
Atsushi
Kondo
 *abc
*abc
aGraduate School of Engineering, Oita University, Oita 870-1192, Japan. E-mail: kondoa@oita-u.ac.jp
bFaculty of Science and Technology, Oita University, Oita 870-1192, Japan
cResearch Center for Advanced Technology and GX, Oita University, Dannoharu 700, Oita 870-1192, Japan
First published on 14th November 2025
Abstract
By investigation of synthetic conditions of Zr-MOF, the morphology of the MOF was controlled to be hexagonal-shaped plates with high uniformity. The hexagonal-shaped platelet crystals of the MOF showed an apparent high packing density, leading to an enhanced volumetric adsorption capacity.
Control of crystal morphology and size is critically important, as these parameters govern bulk density, compressibility, mechanical strength, optical properties, electrical properties, photoelectron properties, catalytic properties, and adsorption behavior.1–7 For inorganic materials such as metal oxides and noble metals, research on morphology and size control has been intensively conducted as an important and pursued topic. In contrast, although extensive studies have focused on structural design in metal–organic frameworks (MOFs) or porous coordination polymers, the control of MOF crystal morphology has largely been overlooked. In recent years, however, the importance of morphological control in MOFs has been increasingly recognized across several research fields,8–13 and it is now emerging as a significant area of study.
For example, morphological control of MOF crystals enables the regulation of pore length, thereby influencing molecular adsorption kinetics. Colwell et al. showed the increment of adsorbate flux with decreased MOF crystal thickness and suggested that the shorter crystals were the most promising candidates for separation of molecules.10 It has also been demonstrated that facet regulation enhances catalytic properties of MOFs. Cheng et al. indicated that NH2-MIL-125(Ti) with high {111} facets exhibits photocatalytic CO2 reduction properties several times higher than NH2-MIL-125(Ti) with {001} facets.11 Wan et al. showed ZIF-67 crystals with a {002} facet showed higher oxygen evolution reaction (OER), an important process in water splitting for hydrogen production, compared to nanocrystals with {011} and/or {111} facets.13 These findings clearly underscore the importance of developing reliable methodologies for morphology control in MOFs.
Greenhouse gases have gathered much attention due to their substantial impact on the environment. One technology to capture greenhouse gases is adsorption/separation by porous materials. A variety of MOF materials have been synthesized and investigated to clarify their adsorption/separation properties of greenhouse gases such as CO2 and N2O. However, investigations of volumetric adsorption capacity on MOFs by morphology control are scarce compared to researches related to gravimetric adsorption. It is important to develop the methodology for high volumetric capacity of MOFs for efficient adsorption/separation in real system. Fig. 1 shows schematic representations of packing states of different-shaped particles. It is known that the uniform spherical particles have the limitation of packing density up to 74% (Fig. 1(a)). Recently, Matzger's group showed the optimization of hydrogen storage by using bimodal crystal size mixture technique (Fig. 1(b)).12 Smaller crystals filled the interstitial voids which were formed by larger crystals, leading to high packing density. On the other hand, hexagonal-shaped plates inherently have a potential to realize packing density to overcome the limitation in the uniform spherical particle system because of the decrease of interstitial void among the plates (Fig. 1(c)).
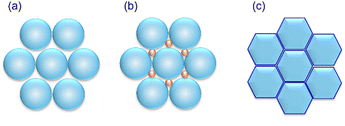 | ||
| Fig. 1 Schematic representation of packing states of (a) uniform spherical particles, (b) bimodal mixture of spherical particles, and (c) uniform hexagonal-shaped plates. | ||
Herein, we report a drastic enhancement of volumetric capacity on a MOF by morphology control. Without the mixture technique, hexagonal platelet crystals of the MOF showed high packing density, leading to enhancement of adsorption capacities of greenhouse gases such as CO2 and N2O per unit volume of adsorbent materials. This is a good and facile example to enhance apparent densities and volumetric adsorption capacities of porous materials.
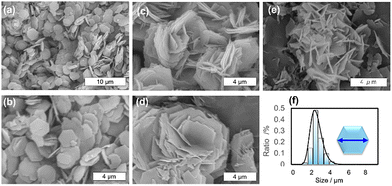
The reaction of a mixture containing zirconium tetrachloride and 4,4′,4″-s-triazine-2,4,6-tryil-tribenzoic acid (H3TATB) with acid additives in DMF gives crystals of the Zr-MOF (named Zr-TATB). The detail synthetic procedure was shown in SI. The reaction with small amounts of hydrochloric acid tended to yield a higher amount of the material with lower crystal uniformity (Fig. S1). In contrast, sufficient amounts of hydrochloric acid in a reaction mixture gives well defined uniform hexagonal MOF plates (Fig. 2(a) and (b)). The uniformity of the hexagonal plates was evaluated by statistical processing of SEM images. The distribution of the plate sizes is well followed by a log-normal distribution, and the averaged plate size is 2.4 µm with standard deviation of 1.2 µm (Fig. 2(f)). The thickness is approximately 100 nm. Using formic acid except hydrochloric acid gives stacked-shaped plates with moderate uniformity of crystal morphology (Fig. 2(c) and Fig. S2), and the usage of acetic acid failed to obtain Zr-TATB. Syntheses using mixed acids such as HCl/HCOOH or HCl/CH3COOH provide Zr-TATB particles with different morphologies (Fig. 2(d), (e) and Fig. S3).
PXRD of the samples synthesized with the different conditions were compared with each other (Fig. S4). All the samples indicated similar XRD patterns, showing basically the same crystal structure. All the TG curves of the samples showed a large weight loss at around 800 K, implying high thermal stability (Fig. S5). XRD measurements of the MOFs after thermal treatments showed high thermal stability of Zr-TATB in air (Fig. S6). Sometimes, a subphase was observed as shown in Fig. S4, which should be from the derivatives with the protonated organic ligands observed by FT-IT (Fig. S7). However, thermal treatment higher than 473 K can remove the subphase as shown in Fig. S6. Through the thermal treatment at 588 K, the morphology of the MOF was not largely changed (Fig. S8) and the averaged plate size is 2.4 µm with an approximately 100 nm thickness, which is similar to those of samples before the thermal treatment (Fig. S9).
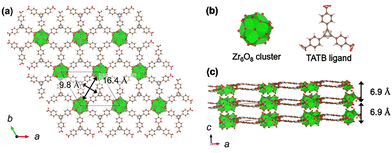
Crystal structural analysis revealed that Zr-TATB is a two-dimensional material containing nanoscale channels (Fig. 3 and Fig. S10). In the crystal, Zr6O8 clusters are aligned hexagonally through TATB ligands to form diamond-shaped pores with the size of 9.8 × 16.4 Å2, resulting in a 2D layered structure. The 2D layers are stacked with an interlayer distance of 6.9 Å in an AA fashion along c axis. Between the layers, there is no apparent chemical bonds, implying exfoliatable nature of the material.
Zr-TATB is considered as an analogue material to Zr-MOFs reported previously.14–16 Metal–organic layers, which are composed of Zr clusters with TATB ligands and have 2D networks with Kagome lattices, indicate similar layer structures to Zr-TATB.14,15 Ma et al. showed an isoreticular material UMCM-309a which is composed of 4,4′,4″-benzene-1,3,5-triyl-trisbenzoic acids (H3BTB) and Zr clusters. Because of the similarity of the crystal structure of Zr-TATB and those Zr-MOFs synthesized in similar preparation procedures, a chemical formula of [Zr6(μ3-O)4(μ3-OH)4(TATB)2(OH)6(H2O)6]14–16 is proposed, which is in accord with TGA data of Zr-TATB synthesized with HCl of 2.5 mL. On the other hand, the MOFs with HCl of 5 mL or the mixed acids indicated relatively smaller amount of inorganic residues in TG measurements, suggesting defect formation (Table S1).
To investigate the porosity of Zr-TATB, N2 adsorption isotherms were measured at 77 K (Fig. S11). All the isotherms showed type I in IUPAC classification with a steep uptake at low relative pressure followed by a plateau in higher pressure region. The BET analysis gives the surface area of 360 m2 g−1. Dubinin–Radushkevich analysis shows that the micropore volume and isosteric heat of adsorption qst were 0.14–0.15 mL g−1 and 15.2 kJ mol−1, respectively (Fig. S12 and S13).
TEM analysis of the MOF directly showed the ordered crystal structure. By ultrasonication of a Zr-TATB ethanol solution, Tyndall phenomenon was observed by laser irradiation, indicating exfoliation of the MOF.17–19 Then, the supernatant was dropped on a carbon-coated Cu mesh and TEM observation was performed. Fig. 4 shows TEM images of the MOF dispersed in ethanol solvent. Thin hexagonal crystal plates were observed and an ordered structure was observed. The FFT pattern of a selected area of the TEM image clearly indicates C6 symmetry ordering. High-resolution dark-field STEM image directly indicates the six-fold symmetry location of Zr6O8 clusters with distances of 1.9–2.0 nm, which corresponds to the distance between Zr6O8 clusters in the crystallographic structure. It also should be noted that the pore channel orientation is perpendicular to the crystallographic planes of the crystal plates.
 | ||
| Fig. 4 (a) TEM image of Zr-TATB and inserted image is a FFT pattern of a selected area of the TEM image shown in red square, (b) enlarged TEM image, and (c) high resolution STEM image of Zr-TATB. | ||
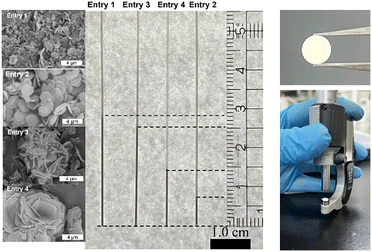
The packing densities of the materials were compared among the samples with different morphologies. Here, the samples are named entry 1–4 which have different morphology depending on the synthetic conditions (Table 1). Each sample with the same weight (approximately 1.1 mg) was put in a glass capillary with a diameter of 0.5 mm by just taps until no apparent volume change was observed, and the sample volumes were compared (Fig. 5). It is clear that the volumes occupied by the powders were largely influenced by the morphology. The most uniform of entry 2 indicated the highest calculated packing density of 0.65 g cm−3 based on a calculated weight of a dried sample, which is approximately 4 times higher than that of entry 1. The entries with moderate morphologies showed middle apparent density between entry 1 and entry 2. To further enhancement of packing density, the sample of entry 2 was pelletized with a pressure at 10 MPa (SI). Surprisingly, the calculated packing density of entry 2 based on the sample without guest was 1.2 g cm−3 which is almost the same to the crystallographic density of Zr-TATB (1.22 g cm−3), although the crystal structure was maintained by the compaction treatment, which is confirmed by XRD measurements of the pellet. It is said that uniform spherical particles has the limitation of packing density of 74%, and recently Song et al. showed that the random packing of hard spheres in three dimensions cannot exceed a density limit of 63.4%.20 On the other hand, Matzger et al. reported an extremely high packing density of powders by using bimodal crystal size mixture technique.12 This our work suggests that the morphology control of hexagonal-shaped crystal plates is a good way to realize very high packing density without any proactive mixture of particles with different sizes/morphologies. It should also worth mentioning that high packing density of hexagonal-shaped plates was realized not in a perfectly uniform system but in a real particle system with different sizes of particles. These results could pave the way to new strategies to realize high packing density of small particles and related applications such as high volumetric adsorption and efficient molecular separation due to the reduction of void space among particles.
| Entry | Acid added | Densitya (g cm−3) |
|---|---|---|
| a Apparent density was calculated by using sample weight measured under air. The value in parenthesis is the calculated density of material without guest. | ||
| 1 | HCl 2.5 mL | 0.20 (0.17) |
| 2 | HCl 5.0 mL | 0.80 (0.65) |
| 3 | HCl 1.25 mL + CH3COOH 1.25 mL | 0.22 (0.17) |
| 4 | HCl 1.25 mL + HCOOH 1.25 mL | 0.40 (0.30) |
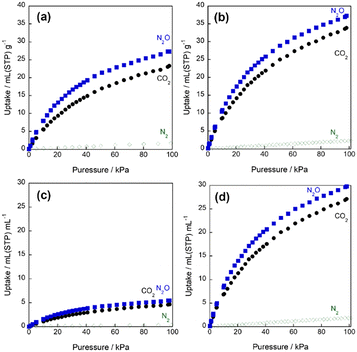
Fig. 6 shows gas adsorption isotherms on the samples entry 1 and 2. On both samples, CO2 and N2O adsorption isotherms indicate type I adsorption isotherm in IUPAC classification, and on the other hand, N2 adsorption isotherms were in Henry type. Although similar molecular properties of CO2 and N2O, both samples indicate larger N2O uptake than CO2, implying N2O selective adsorption properties of the material. The difference of packing density leads to drastic changes in volumetric adsorption capacities per a unit volume of adsorbents. Although the N2O adsorption uptakes on entry 1 and entry 2 per a unit weight of adsorbents are 27 mL (STP) g−1 and 37 mL (STP) g−1 at around 100 kPa, respectively. The difference of adsorptivities between entry 1 and 2 should come from defect formed in the materials. On the other hand, the N2O volumetric adsorption capacity of entry 2 is 30 mL (STP) mL−1 which is approximately 5.5 times higher than that of entry 1 (Fig. 6(c) and (d)). This tendency is not specific in N2O adsorption, but observed in CO2 and N2 adsorption. Therefore, it could be considered that volumetric adsorption capacity is generally controlled by the morphology of adsorbents in the real system.
The gas selectivities on Zr-TATB were evaluated by ideal adsorbed solution theory (IAST) analyses.21 IAST theory was firstly proposed by A. Myers and J. Prausnitz, and molecular separation properties of materials from a gaseous mixture could be predicted by using pure-component adsorption isotherms at the same temperature and on the same materials. The adsorption isotherms on entry 2 were fitted by Langmuir equations and adsorption selectivities at 50 kPa were calculated based on the IAST theory (SI). With an assumption of an equimolar mixture of N2O and CO2 as input gases, the selectivity of S(N2O/CO2) on Zr-TATB was calculated to be 1.35, which is similar to that on an ionic liquid22 and much higher than that on the MOF NU-1000-PhTz.23 Other combinations of equimolar gases were also evaluated. The resultant gas selectivity of S(N2O/N2) was calculated to be about 399, which are extremely higher than the reported values of 20–30 on DMOF.24 Furthermore, the selectivity of S(CO2/N2) on Zr-TATB was calculated to be 167, which is higher than those results reported in the literatures for some MOFs25–27 and other adsorbents such as zeolite 13×,28 porous polyaminals,29 and Mg-exchanged gismondine,30 implying that Zr-TATB is a good candidate to remove N2O and CO2 from N2O/N2 and/or CO2/N2 mixtures. The investigation to clarify the reason of the high selectivity is the next research topic.
In conclusion, we showed the synthesis, characterization and adsorption/separation properties of a 2D layered Zr-TATB. The MOF Zr-TATB is thermally stable up to 588 K in air and has uniform micropores. The crystal morphology of the MOF can be controllable by changing synthetic conditions. Hexagonal-shaped platelet crystals of the MOF showed apparent high packing density, leading to an enhanced volumetric adsorption capacity. The MOF also shows high selectivities of N2O and CO2 over N2. These results highlight the importance of morphology control in MOFs and provide new opportunities for the development of gas adsorption/separation and related applications.
A. K. designed the project. A. K. and Y. K. wrote the original manuscript. Y. K. synthesized and characterized the materials by PXRD, TGA, FT-IR, and SEM. TEM observation was performed by W. N. and A. K performed the analysis. The packing density evaluation was performed by Y. K. with supervision from A. K. Structural model was obtained by A. K. Adsorption isotherms were obtained and analysed by Y. K. with supervision from A. K.
Authors thanks to Professor K. Urita at Nagasaki University Japan for the support of TEM observation and thanks to the Nichia corporation for the preparation of the organic ligand H3TATB.
Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information: synthetic procedure, characterization details, scanning electron microscopy, TG, FT-IR, XRD, structural model, gas adsorption isotherms and characterization, and thermal properties. See DOI: https://doi.org/10.1039/d5cc06142c.Notes and references
- S. C. Davis and K. J. Klabunde, Chem. Rev., 1982, 82, 153–208 CrossRef CAS.
- A. Y. Lee and A. S. Myerson, MRS Bull., 2006, 31, 881–886 CrossRef CAS.
- K. Byrappa and T. Adschiri, Prog. Cryst. Growth Charact. Mater., 2007, 53, 117–166 CrossRef CAS.
- C.-H. Kuo and M. H. Huang, Nano Today, 2010, 5, 106–116 CrossRef CAS.
- P. Strasser, M. Gliech, S. Kuehl and T. Moeller, Chem. Soc. Rev., 2018, 47, 715–735 RSC.
- J. Joo, T. Kim, J. Lee, S.-I. Choi and K. Lee, Adv. Mater., 2019, 1806682 CrossRef.
- Z. Wang, C. Li and K. Domen, Chem. Soc. Rev., 2019, 48, 2109–2125 RSC.
- M. Sindoro, N. Yanai, A.-Y. Jee and S. Granick, Acc. Chem. Res., 2014, 47, 459–469 CrossRef CAS PubMed.
- M. Y. Masoomi, S. Beheshti and A. Morsali, Cryst. Growth Des., 2015, 15, 2533–2538 CrossRef CAS.
- Q. Wen, S. Tenenholtz, L. J. W. Shimon, O. Bar-Elli, L. M. Beck, L. Houben, S. R. Cohen, Y. Feldman, D. Oron, M. Lahav and M. E. van der Boom, J. Am. Chem. Soc., 2020, 142, 14210–14221 CrossRef CAS PubMed; K. A. Colwell, M. N. Jackson, R. M. Torres-Gavosto, S. Jawahery, B. Vlaisavljevich, J. M. Falkowski, B. Smit, S. C. Weston and J. R. Long, J. Am. Chem. Soc., 2021, 143, 5044–5052 CrossRef.
- X.-M. Cheng, X.-Y. Dao, S.-Q. Wang, J. Zhao and W.-Y. Sun, ACS Catal., 2021, 11, 650–658 CrossRef CAS.
- K. Suresh, D. Aulakh, J. Purewal, D. J. Sigel, M. Veenstra and A. J. Matzger, J. Am. Chem. Soc., 2021, 143, 10727–10734 CrossRef CAS PubMed.
- J. Wan, D. Liu, H. Xiao, H. Rong, S. Guan, F. Xie, D. Wang and Y. Li, Chem. Commun., 2020, 56, 4316–4319 RSC.
- E. Liu, J. Zhu, W. Yang, F. Liu, C. Huang and S. Yin, ACS Appl. Nano Mater., 2020, 3, 3578–3584 CrossRef CAS.
- H. Liu, H. Wang, Q. Song, K. Küster, U. Starke, P. A. van Aken and E. Klemm, Angew. Chem., Int. Ed., 2022, 61, e202117058 CrossRef CAS PubMed.
- J. Ma, A. G. Wong-Foy and A. J. Matzger, Inorg. Chem., 2015, 54, 4591–4593 CrossRef CAS.
- J. C. Tan, P. J. Saines, E. G. Bithell and A. K. Cheetham, ACS Nano, 2012, 6, 615–621 CrossRef CAS.
- A. Kondo, C. C. Tiew, F. Moriguchi and K. Maeda, Dalton Trans., 2013, 42, 15267–15270 RSC.
- T. Araki, A. Kondo and K. Maeda, Chem. Commun., 2013, 49, 552–554 RSC.
- C. Song, P. Wang and H. A. Makse, Nature, 2008, 453, 629–632 CrossRef CAS PubMed.
- A. L. Myers and J. M. Prausnitz, AIChE J., 1965, 11, 121–127 CrossRef CAS.
- M. B. Shiflett, A. M. S. Niehaus and A. Yokozeki, J. Phys. Chem. B, 2011, 115, 3478–3487 CrossRef CAS.
- G. Mercuri, M. Moroni, S. Galli, G. Tuci, G. Giambastiani, T. Yan, D. Liu and A. Rossin, ACS Appl. Mater. Interfaces, 2021, 13, 58982–58993 CrossRef CAS PubMed.
- L. Wang, Z. Ye, M. Wang, Z. Liu, J. Li and J. Yang, Inorg. Chem., 2024, 3, 11501–11505 CrossRef.
- J. Liu, J. Tian, P. K. Thallapally and B. P. McGrail, J. Phys. Chem. C, 2012, 116, 9575–9581 CrossRef CAS.
- J.-H. Wang, D. Luo, M. Li and D. Li, Cryst. Growth Des., 2017, 17, 3387–3394 CrossRef CAS.
- C.-N. Li, S.-M. Wang, Z.-P. Tao, L. Liu, W.-G. Xu, X.-J. Gu and Z.-B. Han, Inorg. Chem., 2023, 62, 7853–7860 CrossRef CAS PubMed.
- S. Cavenati, C. A. Grande and A. E. Rodrigues, J. Chem. Eng. Data, 2004, 49, 1095–1101 CrossRef CAS.
- B. Zhang, J. Yan, G. Li and Z. Wang, ACS Appl. Polym. Mater., 2019, 1, 1524–1531 CrossRef CAS.
- J. A. Atrach, I. E. Golub, E. B. Clatworthy, J. Rey, Y. Xiong, A. Daouli, M. Desmurs, M. Badawi, R. Guillet-Nicolas and V. Valtchev, Chem. Mater., 2024, 36, 1556–1570 CrossRef.
| This journal is © The Royal Society of Chemistry 2026 |