Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
What does it mean that “something is green”? The fundamentals of a Unified Greenness Theory
Paweł Mateusz
Nowak

Jagiellonian University in Kraków, Faculty of Chemistry, Department of Analytical Chemistry, Laboratory of Forensic Chemistry, Gronostajowa St. 2, 30-387 Kraków, Poland. E-mail: pm.nowak@uj.edu.pl
First published on 31st May 2023
Abstract
Can the exact science of chemistry use such an abstract concept as “greenness”? What does “something is green” or “greener” even mean? Is there only one correct interpretation of being green? Where is the line between science and philosophy? Is the current theory still sufficient? The answer to these and other questions is the proposed Unified Greenness Theory (UG-theory). This concept is intended to be a solid foundation for all activities undertaken in the field of green chemistry, regardless of whether the ultimate goal is synthesis or analysis, and even to reach beyond chemistry. One of its assumptions is the unification of the 12 Principles of Green Chemistry, 12 Principles of Green Analytical Chemistry, and other collections of principles, into a set of new cardinal statements of more primary and universal nature (Unified Principles of Greenness). For this purpose, new terminological solutions were proposed, the most primal components of greenness were identified, and a way to describe basic concepts using the language of mathematics was proposed. The idea of green chemistry is presented as an element of a larger whole, the idea of “white chemistry”, which combines greenness with red and blue colours indicating functionality.
1. Introduction
Each natural science needs a solid and constantly improving theoretical foundation for its development. A good theory should set the goal pursued by a given science and the means it uses, strictly describe the cause-and-effect relationships between the objects of interest, if possible using the language of mathematics, and use transparent terminology. Green Chemistry (GC) is no exception to this rule.In the literature dedicated to GC, there are many theoretical considerations, but the fact is that these are mainly theories underlying specific techniques and methodologies that we define as green,1–4 and not theories of greenness in the literal sense. The theoretical basis of GC was embodied in the famous set of “12 Principles of Green Chemistry” proposed by Anastas and Warner in 1998.5 Subsequently, these principles inspired the development and formulation of the “12 Principles of Green Engineering”,6 which referred to the technological aspects of chemistry on a large scale. To facilitate the transfer of information and remembering the most important rules, both collections have been reformulated into a mnemonic known as “IMPROVEMENTS PRODUCTIVELY”.7,8 It is noteworthy that all these principles are focused on the processes of product synthesis, treating chemistry as “the art of conducting chemical reactions”.
At the same time, it was noted that the idea of GC should be treated more broadly and also refer to analytical processes, i.e., testing the chemical composition of samples.9–12 The fact is that carrying out analyses also has a negative impact on the environment through the use of chemicals, energy, and waste generation. Although the scale of the impact of “analytical chemistry” may appear insignificant compared to large-scale production processes, the number of laboratories around the world focused on analytics is enormous. In addition, production processes also require constant analytical control. As a result, the idea of Green Analytical Chemistry (GAC) was born.9–12 In 2012, the “12 Principles of Green Analytical Chemistry” were published.13
Worth mentioning are other attempts to verbalize the idea of GC of a more specific nature, such as the “12 more Principles of Green Chemistry”,14 “13 Principles of Green Chemistry and Engineering for a Greener Africa”,15 and “10 Principles of Green Sample Preparation”.16
Undoubtedly, the formulation of all these principles had and still has a huge impact on the development of the field, contributing to the achievements on many levels. Nevertheless, these are only general rules, patterns of behaviour, indications of goals that we should strive for. One may ask, is this a sufficient theoretical basis for GC as a science?
2. Analysis of the 12 Principles of GC and GAC
Let's take a closer look at the wording of the particular GC and GAC principles (Tables 1 and 2, respectively), by asking the following questions:• What audience is the principle aimed at?
• Should its character be defined as primary – clearly indicating the elementary criterion of greenness, or secondary – indicating only the way to the implementation of the primary principles?
• What is the scope of application of a given principle?
| Principle | To whom? | Character | Scope |
|---|---|---|---|
| #1. Prevention. Preventing waste is better than treating or cleaning up waste after it is created | Method developers and users (applied research) | Primary | Broad (whole chemistry) |
| #2. Atom economy. Synthetic methods should try to maximize the incorporation of all materials used in the process into the final product. This means that less waste will be generated as a result | Method developers and users (applied research) | Secondary (it refers to #1 concerning waste) | Narrow (synthesis) |
| #3. Less hazardous chemical syntheses. Synthetic methods should avoid using or generating substances toxic to humans and/or the environment | Method developers and users (applied research) | Primary | Narrow (synthesis) |
| #4. Designing safer chemicals. Chemical products should be designed to achieve their desired function while being as non-toxic as possible | Designers (basic research) | Secondary (it refers to #3 concerning toxicity of chemicals) | Broad (whole chemistry) |
| #5. Safer solvents and auxiliaries. Auxiliary substances should be avoided wherever possible, and as non-hazardous as possible when they must be used | Method developers and users (applied research) | Secondary (it refers to #1 concerning waste and #3 concerning toxicity of chemicals) | Broad (whole chemistry) |
| #6. Design for energy efficiency. Energy requirements should be minimized, and processes should be conducted at ambient temperature and pressure whenever possible | Designers (basic research) | Primary | Broad (whole chemistry) |
| #7. Use of renewable feedstocks. Whenever it is practical to do so, renewable feedstocks or raw materials are preferable to non-renewable ones | Method developers and users (applied research) | Primary | Broad (whole chemistry) |
| #8. Reduce derivatives. Unnecessary generation of derivatives—such as the use of protecting groups—should be minimized or avoided if possible; such steps require additional reagents and may generate additional waste | Method developers and users (applied research) | Secondary (it refers to #1 concerning waste and #3 concerning toxicity of chemicals) | Narrow (synthesis) |
| #9. Catalysis. Catalytic reagents that can be used in small quantities to repeat a reaction are superior to stoichiometric reagents (ones that are consumed in a reaction) | Method developers and users (applied research) | Secondary (it refers to #1 concerning waste) | Narrow (synthesis) |
| #10. Design for degradation. Chemical products should be designed so that they do not pollute the environment; when their function is complete, they should break down into non-harmful products | Designers (basic research) | Primary | Broad (whole chemistry) |
| #11. Real-time analysis for pollution prevention. Analytical methodologies need to be further developed to permit real-time, in-process monitoring and control before hazardous substances form | Method developers and users (applied research) | Secondary (it refers to #3 concerning toxicity of chemicals) | Narrow (synthesis) |
| #12. Inherently safer chemistry for accident prevention. Whenever possible, the substances in a process, and the forms of those substances, should be chosen to minimize risks such as explosions, fires, and accidental releases | Method developers and users (applied research) | Primary | Broad (whole chemistry) |
| Principle | To whom? | Character | Scope | Analogy to principles of GC |
|---|---|---|---|---|
| #1. Direct analytical techniques should be applied to avoid sample treatment | Method developers and users (applied research) | Secondary (it refers to #7 concerning waste, #9 concerning energy, #11 concerning toxicity of chemicals, and #12 concerning safety of the operator | Narrow (analysis) | No |
| #2. Minimal sample size and minimal number of samples are goals | Method developers and users (applied research) | Secondary (it refers to #7 concerning waste, #9 concerning energy, #11 concerning toxicity of chemicals, and #12 concerning safety of the operator | Narrow (analysis) | No |
| #3. In situ measurements should be performed | Method developers and users (applied research) | Secondary (it refers to #7 concerning waste, #9 concerning energy, #11 concerning toxicity of chemicals, and #12 concerning safety of the operator | Narrow (analysis) | No |
| #4. Integration of analytical processes and operations saves energy and reduces the use of reagents | Method developers and users (applied research) | Secondary (it refers to #7 concerning waste, #9 concerning energy, #11 concerning toxicity of chemicals, and #12 concerning safety of the operator | Narrow (analysis) | No |
| #5. Automated and miniaturized methods should be selected | Method developers and users (applied research) | Secondary (it refers to #7 concerning waste, #9 concerning energy, #11 concerning toxicity of chemicals, and #12 concerning safety of the operator | Broad (whole chemistry) | No |
| #6. Derivatization should be avoided | Method developers and users (applied research) | Secondary (it refers to #7 concerning waste, #9 concerning energy, #11 concerning toxicity of chemicals, and #12 concerning safety of the operator | Broad (whole chemistry) | Yes, #8 of GC |
| #7. Generation of a large volume of (analytical) waste should be avoided and proper management of analytical waste should be provided | Method developers and users (applied research) | Primary | Broad (whole chemistry) | Yes, #1 of GC |
| #8. Multi-analyte or multi-parameter methods are preferred versus methods using one analyte at a time | Method developers and users (applied research) | Secondary (it refers to #7 concerning waste, #9 concerning energy, #11 concerning toxicity of chemicals, and #12 concerning safety of the operator | Narrow (analysis) | No |
| #9. The use of energy should be minimized | Method developers and users (applied research) | Primary | Broad (whole chemistry) | Yes, #6 of GC |
| #10. Reagents obtained from renewable source should be preferred | Method developers and users (applied research) | Primary | Broad (whole chemistry) | Yes, #7 of GC |
| #11. Toxic reagents should be eliminated or replaced | Method developers and users (applied research) | Primary | Broad (whole chemistry) | Yes, #3 of GC |
| #12. The safety of the operator should be increased | Method developers and users (applied research) | Primary | Broad (whole chemistry) | Yes, #12 of GC |
At the beginning, it is noteworthy that most of the GC rules seem to be addressed to the creators and users of methods representing applied research, while three of them (#4, #6 and #10) are directed towards designers of new techniques, methodologies and chemical structures representing basic research. Nevertheless, the more important issue seems to be their internally inconsistent nature. The principles #1, #3, #6, #7, #10 and #12 seem to address elementary greenness criteria such as waste, reagent toxicity, energy use, use of renewable materials, creation of degradable products, and user safety, respectively. The remaining principles, on the other hand, indicate the means to implement the primary principles, so they are of a secondary nature. For example, the principle #2 on the “atom economy” is an indication of the course of action, the ultimate goal of which will be to reduce waste, i.e., the implementation of the principle #1. In addition, some of the principles are formulated in such a way that they have a wide range of applicability across various chemical disciplines, while others are strictly related to chemical synthesis. Some of them, such as #3, would be more universal if formulated in a more general way.
Regarding the GAC rules (Table 2), the situation is quite similar. The difference is that all principles seem to have the same intended audience: method creators and users. None of them directly relates to the design stage, which was the case with the three GC principles. The nature of the GAC rules is also not uniform. Principles #7, #9, #10, #11 and #12 are of a primary nature, while all the others are clearly secondary in nature and constitute recommendations for actions whose final positive effect will be the implementation of the primary principles. In addition, the inconsistency seems to be greater here because the primary principles, which should intuitively be indicated as superior, are listed at the end of the list. It is also worth mentioning that the rules indicated as primary, unlike the secondary ones, have a wide range of applicability and are in fact a copy of the primary GC rules shown in Table 1.
What are the consequences of the above analysis? Placing primary and secondary principles in one set, without specifying their hierarchy, may lead to problematic situations. In particular, recognizing the equal importance of the primary principle and the secondary principle (describing the means to implement the primary principle), may lead to counterproductive conclusions and paradoxes. This is because the way indicated by the secondary principle may simply be ineffective.
For example, principle #1 of GAC states that direct measurement techniques should be used, avoiding the need for additional sample preparation.13 It is true that the choice of such a technique eliminates the need to use, for example, extraction solvents, which account for a large part of the waste generated. This does not mean, however, that the total amount of waste will always be smaller if a technique that avoids sample preparation is chosen. Compliance with the secondary principle may require the use of a different technique in the next step, such as chromatographic separation, which will necessitate the use of additional solvents. The overall waste balance is likely to be favourable in most cases, but not always.
However, if the superiority of the primary principle was clearly stated, i.e., always striving to minimize the amount of waste, there would be no such risk. The secondary principle could serve as a proposal worth considering minimizing waste generation, the effectiveness of which needs to be always verified.
Similar conclusions can also be reached by analysing other popular sets of the principles relating to the idea of GC, such as the “12 Principles of Green Engineering”,6 “12 more Principles of Green Chemistry”,14 “13 Principles of Green Chemistry and Engineering for a Greener Africa”,15 and “10 Principles of Green Sample Preparation”.16 Their detailed analysis would significantly extend the length of this article without, however, bringing no significant added value. Therefore, they have been omitted here.
To conclude, all these collections are clearly aimed at a certain audience: synthetic chemists, analytical chemists, chemical engineers, sample preparation specialists, etc. No set of rules is truly universal, i.e., applicable to all disciplines at the same time. Each branch has actually its own cardinal guidelines and a recipe for “how to be green”. In particular, distinguishing GAC from GC creates an unnecessary appearance of separateness, presenting GC and GAC as parallel ideas addressed to different goals. In fact, however, analytical chemistry is a part of chemistry (one of its disciplines), and the parallel concepts are “green synthesis” and “green analysis”. The general rules of GC, to be really “general”, should be formulated in such a way that they apply to any chemical discipline, both synthesis and analysis. Otherwise, they should be referred to as e.g., “green synthesis guidelines”, etc. Therefore, the following questions arise: is it a good direction? Shouldn't the pursuit of greenness unite us instead of dividing us?
Secondly, placing in one set the statements of a different nature, for example indicating the aims and the ways of their achieving, requires the clear indication of these differences and the mutual hierarchy. There can be no doubt which principle is more important. When hierarchy is not indicated, it is logical and reasonable to give equal importance to each of them.
It should be clearly emphasized that my goal is not to criticize the current sets of principles because their great merit for the propagation of ideas and stimulation of the development of new, more environmentally and human-friendly methods of synthesis and analysis is undeniable. The goal, however, is to indicate potential areas for improving the theoretical basis of GC, and one of them is the possibility of formulating new, unified, and universal guidelines. The proposal for such a “yet another set of principles” is presented and discussed in section 9.
Before that, however, it is necessary to begin with an attempt to address more fundamental issues. Indeed, after 25 years of continuous development of GC, after the publication of thousands of articles on various green solutions in chemistry, we need something more than only “yet another collection of principles”. The first burning issue is solving the age-old terminological dilemma.
3. Green beyond doubt
The definition of GC presented by Anastas is “Green Chemistry – design of chemical products and processes that minimize or eliminate the use or generation of substances hazardous to humans, animals, plants, and the environment”.5 In later studies, this definition was extended and became more general. According to the United States Environmental Protection Agency (EPA), “Green chemistry applies across the life cycle of a chemical product, including its design, manufacture, use, and ultimate disposal”.17 This definition, together with the previously mentioned sets of principles, clearly indicate what GC is and what is its object of interest, but they do not specify the details important for stating and quantifying the greenness of a specific object.“Greenness” is a central concept for GC and has been used many thousands of times in the scientific literature over the last 25 years in a variety of contexts, so it is a real foundation. The overarching problem is the lack of clear guidelines indicating when a given technique/methodology, method/procedure or synthesis/analysis process could, beyond any doubt, be given the tag “green”. This problem also concerns when and how we can objectively say that “something is greener than something else”.
This issue is extremely relevant today. As already noted in the GC community, the tag “green” is now more and more often used, and thus, in many cases abused.1,18 Since greenness is now a mainstream topic, it can be assumed that presenting something as green can achieve some benefit, for example, facilitate the publication of a method in a recognized journal. However, abuse can be both conscious and unconscious because as humans, we tend to follow the mainstream and unintentionally copy common patterns of behaviour. Disputes about whether something is green or not were even the leitmotiv of separate articles, where each side presented its rational arguments.19–21 Do we really have the solid basis to clearly state when we can talk about abuse?
There is no doubt that relying on the intuitive and contextual understanding of “being green” is highly unfavourable for GC as a science. As a result, it loses its original exactness characteristic, bringing its specificity closer to the humanities, where there is much greater freedom of verbal expression.
4. Definition of greenness and the state of being green
4.1. Greenness as a parameter
At the beginning, it is necessary to distinguish greenness understood as a state, i.e., the state of being green, from greenness understood as a scale of colour saturation, i.e., a parameter. It is worth noting that we can talk about the greenness of an object understood as colour saturation even when the considered object is not green at all. Then its greenness is simply zero. In order not to cause confusion, from now on, the former meaning of the word “greenness” will be simply “the state of being green”. As “greenness” we will understand only a parameter/scale. What does this parameter actually measure? Let me propose the following definition:“Greenness is a measure of the destructive impact that humans have on the environment and themselves. The greater this impact is, the lesser the greenness of actions leading to this impact.”
Identification and analysis of the elemental effects that we might describe as a “destructive impact” is presented in section 6. At the moment, let us focus on greenness. Upon accepting the above definition, it seems logical that:
“The expression of greenness are the parameters showing the probable strength of the destructive impact of a specific nature.”
Such a parameter may be, for example, the amount of energy needed to carry out the reaction, and the impact may be the emission of carbon dioxide contributing to global warming. So, we can say, for example, “process A is greener than process B in terms of energy requirements” if we know exactly the energy consumption of process A and B. If we assume this to be the case without having hard data, we should conclude that “process A appears greener in terms of…”, alternatively “process A is probably/hypothetically greener in terms of…”, etc.
However, it is problematic to talk about greenness in the overall sense, i.e., to say that, for example, “process A is (as a whole) greener than process B”. It must be clearly emphasized that certain elemental effects cannot be objectively compared and unified. For instance, it is impossible to say which is more destructive and less green, emitting 1 ton of carbon dioxide into the atmosphere, releasing 1 kg of pesticides into a river, or starting a fire that threatens the life of the user of the method (more on this in the section 6). All we can do is make some arbitrary assumptions, develop a model, and then apply it. The modelling result could then indicate which process is overall greener, but this would be purely theoretical greenness. Therefore, it remains that
“We should never say that something is overall more or less green in an absolute way.”
Instead, we can indicate which object (product, process, method) seems greener according to the overall evaluation model used, by indicating exactly this model and replacing the verb “to be” with “to appear” or another one with a similar meaning. For example,
“We can conclude that object A appears (overall) greener than B according to the X model, etc.”
4.2. The state of being green
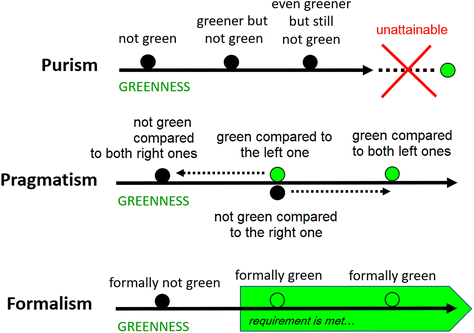
Now, let us consider three interpretations of the state of being green, according to three different linguistic philosophies: purism, pragmatism, and formalism. This will allow us to propose general rules for the use of the terms “green product”, “green process”, “green method”, etc.“A green (product, process, method) is one that does not cause any destructive impact on the environment and human.”
It must be realized and openly admitted that in practice all activities undertaken in the field of chemistry will ultimately have some destructive effect. To illustrate, let us consider energy consumed to synthesize a product or analyse a sample. Even if there is no need to power any device in a certain process, its implementation requires the supply of materials, reagents and instruments, the production and transport of which will always require energy. Since there are no ways to provide energy that is completely clean, i.e., devoid of any carbon footprint, any synthesis and analysis will contribute directly or indirectly to carbon dioxide emissions and climate change. So, according to the definition presented earlier:
“The state of being green is practically unattainable. In fact, the implementation of any method leads directly or indirectly to unavoidable impacts of a destructive nature, therefore no method, no process, no product of this process, can be considered green.”
The linguistic implications of adopting a purist interpretation of the state of being green, relevant to the evaluation of chemical methods, processes, and products, would be as follows:
“We should never say that object (product, process, method) is or appears to be green – because nothing is green, and nothing can be green.”
At first glance, it may seem that the purist approach is overkill, and its consistent use will limit freedom of expression. Indeed, changing the current narrative found in GC and GAC literature to a purist one would require a change of habits and a more careful choice of words.
What is worth emphasizing, however, is that consistent application of these rules could bring a number of positive effects. Overuse of terms such as a “new green method” should in theory be completely eliminated; in addition, discussions would become more transparent. This would facilitate the full transmission and reception of information, and the price for this would be a less colourful language (due to the inability to tell that something is green). Nonetheless, in the case of natural science, this should not be a problem, and perhaps even desirable.
“A green (product, process, method) is one that is likely to cause a small destructive impact of a specific nature compared to other commonly used alternatives (competing products, processes, methods). Being green simply means being ahead of the competition at the moment. So green objects are common.”
It is worth noting here that a green object may cease to be green over time, when all competing objects in relation to which it could be described as green at the beginning fall out of use. In other words:
“Being green all the time requires constant development.”
So, we can say that “method A is green in terms of waste amount” if there is an alternative method B in common use that generates more waste than method A, assuming we know the amount of waste exactly and the difference is not negligibly small. In a situation wherein we do not know this amount, but can only predict it, for example, based on our experience and intuition, we should conclude that “method A (only) seems green in terms of waste amount”.
To sum up:
“It is possible to say that object A is green in terms of a given criterion against the competing object B or a group of objects, as long as it is concluded on the basis of objective data.”
“When making a subjective judgment, we should state that object A appears green in relation to object B or a group of objects in terms of a given criterion (do not use the verb “to be”).”
As we can easily see, the pragmatic interpretation allows for more freedom of expression, the use of more colourful language, and treats being green as something common. The diversity of objects (products, processes, methods) will always allow to indicate some of them as green, i.e., simply “more friendly to people and the environment at the moment”.
More important is that the correct use of terminology and the above rules allows the potential reader to recognize the situation and distinguish facts (supported by data) from conjectures. It is also positive that being green is not unchangeable, which can stimulate the continuous development of the field.
The disadvantage of the pragmatic interpretation is that the state of being green is something “too common”. In practice, the frequent use of the words “green”, “potentially green”, or “hypothetically green” may give rise to some room for abuse and overinterpretation and negatively affect the precision of the language. Another disadvantage is that it is not entirely clear how to consider certain objects as “commonly used alternatives”. The choice of objects to compare can be a debatable matter and another opportunity for abuse. Thus, it is the responsibility of the person making a claim of greenness to specify the alternatives being included in the comparison.
“A green (product, process, method) is one that meets the formal requirement addressed to the specific criterion. The state of being green is achievable when there are objects capable of meeting the imposed formal requirements. Being green depends not only on the influence on the environment and humans but also on the severity of requirements adopted.”
It follows that while the purist and pragmatic interpretations presented the state of being green as empirically determined, this interpretation gives it a formal character (hence the name).
With regard to linguistic implications,
“It is possible to say that object A is green in terms of a given criterion if it meets the formal requirements addressed to the specificity of the object. This fact must be established on the basis of objective data.”
“It is possible to say that object A appears green with respect to a given criterion if we have reason to believe that it would be able to meet the formal requirements.”
One can get the impression that the formal interpretation combines the advantages of both previous ones. On the one hand, the introduction of formal rules allows us to eliminate the dilemmas of a pragmatic approach, e.g., which objects to choose for comparison so that they do not raise doubts, and on the other hand, it does not limit the freedom of expression as much as purism. The biggest problem of formalism is hidden in the statement “meets the formal requirements addressed to the specificity of the object”. The fact is that developing formal norms in a reliable manner, taking into account the huge variety of objects subjected to assessment, is an extremely difficult challenge. It must be emphasized that adopting a single measure that does not consider this diversity is a dead end. With this approach, some types of objects would always be green, and others would never be, which is clearly not our goal.
According to my opinion,
“Clear indication and consistent application of a chosen interpretation of being green is more important than this choice itself.”
The purist interpretation seems to be the safest solution if we do not want to be accused of terminological misuse. The resulting language of discussion is the most restrained and precise and closest to the ideal of exact science. It requires abandonment of habits and careful choice of words.
The pragmatic interpretation seems to be the easiest to use. Its implementation would also increase the substantive value of the discussion but still with a certain risk of abuse. The most debatable issue is the choice of reference objects (common alternative solutions) against which the state of being green is concluded. Undoubtedly, this issue would require discussion and justification by the authors in the text of the articles describing “green solutions”. The article's reviewers should take care of it as well.
Unlike the purist and pragmatic interpretation, the formalistic approach cannot be applied immediately, and it requires the prior establishment of norms. These guidelines must consider the diverse specificities of assessment objects, and their creation will be a difficult, long-term process, and require the involvement of many environments. However, the potential benefits of developing a “green formalism” are significant and worth considering. Ideas on what such formal standards could potentially look like should be the subject of separate articles published in the area of GC.
At the moment, the most important thing is to stick to the rule:
“Whenever something is stated to be green or to appear to be green, the appropriate definition should be quoted, and then the terminology used consistently.”
Fig. 1 and Table 3 may be helpful in understanding the possible approaches and their implications.
| What is known? | Purist interpretation | Pragmatic interpretation | Formal interpretation |
|---|---|---|---|
| We know from empirical data that process A uses less energy than competing process B | Process A is greener than process B in terms of energy consumption | ||
| Neither process A nor process B can be described as green by any means (their colour may become greener and greener, but never fully green) | Process A is green in terms of energy consumption relative to process B | Both process A and process B can be considered green in terms of energy consumption if they meet the formal requirement | |
| We suspect that process A uses less energy than competing process B | Process A appears greener than process B in terms of energy consumption | ||
| Neither process A nor process B can appear to be green by any means (their colour may become greener and greener, but never fully green) | Process A appears green in terms of energy consumption relative to process B | Both process A and process B can appear to be green in terms of energy consumption, if we can suspect that they meet the formal requirement | |
| We know from the adopted model X that the greenness score of process A is higher than that of process B | Process A appears (overall) greener than process B according to the model X | ||
| Neither process A nor process B can appear to be green by any means (their colour may become greener and greener, but never fully green) | Process A appears to be (overall) green compared to process B according to the adopted model X | Both process A and process B may appear to be (overall) green if the results of model X meet the formal requirements | |
Importantly, Table 3 points some useful possibilities of the formulation of statements in a correct way. To preserve the purity of the message, other less strict constructions should not be used. In particular,
“Even according to the pragmatic and formal interpretation, we should not assume or conclude that anything is or seems green other than by reference to a specific parameter or outcome of an appropriate model.”
It should be realized that in practice, it may be difficult to comply with the above rules and to find appropriate alternative terms. For example, the use of the term “green” in titles should be avoided. The title itself does not ensure the necessary context. In this case, instead of writing, for instance: “New green method of...”, it would be better to write “New method of... assessed for greenness”, or “Greenness of a new approach to… assessed with…” etc. However, these alternative titles may seem simply too long or less eye-catching.
Sometimes the discussion can also be more general and colloquial, and “being green” can be used as a certain shorthand. For instance, GC specialists in their portfolio may write that they deal with “green technologies” or “green solvents”, not having any specific objects in mind but a general area of interest. In such cases, referring to greenness as a general concept seems to be fully acceptable and there is no point in forcibly changing one's habits. The most important thing is to ensure transparency and unambiguity of statements in the case of scientific discussions, characteristics of new products and their comparison with competing solutions, in particular when we focus on describing the criteria that relate to greenness. Therefore, the optimal approach is a commonsense approach.
5. Green, sustainable, or… white?
Another pivotal issue is using of appropriate words when we refer to the criteria other than green, which prove the functionality and overall value of an object of interest. The GC definition quoted earlier (“design of chemical products and processes that minimize or eliminate the use or generation of substances hazardous to humans, animals, plants, and the environment”) says nothing about functional features, focusing on reducing hazards as the basic determinant of greenness. Nevertheless, it would be irrational to assume a blind focus on green criteria in isolation from usability. Indeed, principle #4 of GC says that “Chemical products should be designed to achieve their desired function while being as non-toxic as possible”, suggesting that the priority should be to ensure the functionality of the object (here the product), and subsequently limiting its impact as much as possible. However, this still does not allow us to unambiguously state what is the relationship between greenness and functionality, and what terms to use in the discussion.One may simply assume that greenness contains functionality as an integral part. This would mean that a more usable object of a similar impact on the environment and humans should be described as greener beyond doubt. Hence, greener would mean overall better. It contradicts however our previous assumptions and intuition which suggests that functional criteria, not related to the environment and safety, are something separate.
Another approach is to use the term “sustainable” when we consider the object from a broader perspective. The original meaning of “sustainable” proposed by von Carlowitz (1645–1714) indicates the act of using renewable resources at a rate no greater than the rate at which nature can replenish those resources. More recently the “sustainability” concept is primarily associated with the “17 Sustainable Development Goals”,22 which deal with topics such as poverty, education, health security, food production, female empowerment, water access, justice, etc. The term “sustainable” should therefore be understood in the context of whether a given product meets the idea of “sustainable development” defined by these 17 points. When it comes to chemistry, there is a problem with transferring all these 17 goals. “Sustainable chemistry” is commonly interpreted as the concept combining three components: aspects of care for the natural environment, economy, and society (people).23–25 In other words, it merges greenness, rational management of financial resources, and easy accessibility of products/methods for everyone. However, it is difficult to find where functionality is located, including such parameters as yield, performance, output, etc. The situation is complicated by yet another quite common interpretation, according to which sustainability is simply a synonym for greenness.26
The solution to this dilemma has already been proposed in GAC. In 2021, I proposed the idea of “white analytical chemistry (WAC)”,27 which has already met with a positive reception in the GAC environment.28–32 This concept joins greenness, sustainability and performance-related criteria:
“Green is a colour, the obtaining of colour by a method, process or product is an abstraction, but still appealing to the imagination and memorable. Perhaps the reference to the colour, to which we as humans are sensitive, explains the enthusiasm with which the idea of GC was received and propagated. Nevertheless, green is not the only colour. The world appears to us as a spectrum of different colours, it is colourful, so we do not have to limit our interpretation of the world of chemistry to only one of them.”
WAC refers directly to the red–green–blue colour model (RGB),33 which describes the colour of light that we perceive. There are three primary colours of light: red, green, and blue, and their combination gives the impression of whiteness. When we consider an object holistically, we consider all primary colours simultaneously. Redness represents the effectiveness of achieving the goal (in analytical chemistry the performance criteria like accuracy, precision, limit of detection, etc.; in synthesis, they could be yield, purity, repeatability, etc.), greenness represents the care for environment and safety, and blueness the practical and economic utility (cost-effectiveness, time-effectiveness, requirements, user-friendliness, etc.). Therefore, WAC is an extension of GAC, and not a competing idea.
It can also be easily seen that the blue attribute combines the two pillars of sustainable development, i.e., economy and society because it values cost-effective, simple, and accessible solutions. In other words, sustainability is included in whiteness. Concurrently, the red attribute adds functional criteria which are required to ensure completeness, but vaguely addressed by the sustainability concept. Functionality can therefore be understood as a combination of redness and blueness because even the most effective object in achieving the intended goal will not perform well if it is not practical, for example too expensive and time consuming.
The idea of WAC can be and should be understood more broadly, as a general idea of “white chemistry (WC)”, connecting all chemical disciplines. Regardless of the specificity of the laboratory, each process and method can be considered in terms of all three primary colours. As shown in Fig. 2, the main goal according to WC is the pursuit of whiteness, i.e., reconciling the idea of greenness with the other two attributes. It requires searching for the best possible and fair compromise between the three components (to be “fit-for-purpose”). The pursuit of redness, greenness and blueness should occur in parallel, through the cooperation of various environments. Some of us will focus our efforts on red, others on green, and yet others on the blue attribute, but the WC idea should be superior and unite us all.
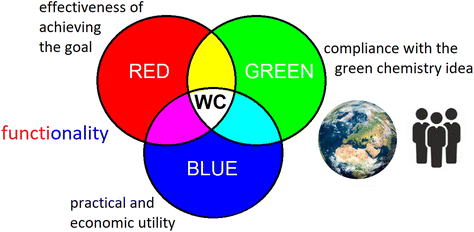 | ||
| Fig. 2 White chemistry (WC) as a pursuit of the best possible compromise, combining three parallel ideas: red chemistry, green chemistry, and blue chemistry. | ||
Is there a solution that does not raise any doubts that each of us can accept? In practice, the matter may not be simple because, as before, each of us may have our own beliefs and habits. The following rule, in my opinion, suggests a certain consensus:
“The greenness of the object is evidenced by the parameters directly related to the destructive impact on the environment and humans. However, it makes sense to talk about the greenness of the object only on the assumption that it meets a certain minimum of utility. If some features exclude the use of the object in practice, the assessment of its greenness will be a dead end and irrational. When we use the concept of sustainability, we should clearly define it because this word currently has too many meanings. However, sustainability should not be used as a full synonym for functionality. The concept based on the RGB model (whiteness) unambiguously indicates that we consider the object holistically, looking for the best compromise in a given situation. Whiteness combines greenness with functionality, in general, whiter means globally better.”
6. Searching for the greenness elements
In section 2, the nature of the 12 Principles GC and GAC was analysed (Tables 1 and 2). It has been concluded that some principles are primary, and some are secondary. The primary ones were those relating to criteria such as amount of waste, energy consumption, toxicity of reagents, user safety, renewability of the materials used and their degradability. Now consider whether these criteria are really the most primal possible. In other words, can these parameters, in analogy to chemical elements, be called “greenness elements”?Let us first consider two hypothetical methods, A and B, both dedicated to the same purpose. Suppose that the method A uses twice as much electricity as the method B. In that case, the method A should be considered less green in terms of the primary principle concerning the need to reduce energy consumption. Let us assume, however, that the method A is implemented in a country where the share of renewable energy sources in total production is very high, e.g., in Sweden.34,35 In turn, method B is implemented in a country where the vast majority of energy comes from fossil fuels, e.g., in Poland (in 2022, the emissivity of energy in Poland was as much as 60 times higher than in Sweden).34,35 Let us ask ourselves, the implementation of which method will be associated with a larger carbon footprint in this particular case? The answer is – method B, which however was stated before a moment to be greener.
The current wording of the principles indicates energy consumption as the criterion, not the actual impact, i.e., the carbon footprint (GC principle #6 and GAC principle #9). Therefore, the realization of the method A always should be stated less green because it uses more energy. In practice, however, it causes less destructive impact on the planet. Similar examples of ambiguities arising from the current wording of the 12 Principles of GC and GAC can easily be found. The cause of the problem would always be the same. This inconsistency comes from the fact that the principle we have considered primary is in fact also secondary. “Truly” primary principles must refer directly to the elemental effects that can be caused by applying a particular method in specific circumstances. Otherwise, we cannot avoid such paradoxes.
Now let us try to think about what these effects are and how we can name them appropriately. Let us also try to define them in such a way as not to be limited to greenness understood in relation to chemistry, but as broadly as possible. My subjective proposal is as follows:
The first elemental effect is the emission of greenhouse gases causing changes in the climate of the Earth, which is expressed in the carbon footprint. Reducing this effect is an urgent need for all mankind, and the effects of the progressive warming of the climate can be felt by each of us almost every day.
The second effect should include the consequences associated with the use of chemical reagents that are dangerous to the environment and/or human health. It is of particular importance in the case of chemistry and related fields. Chemical impact can occur on several levels: during the production of reagents, their transport, storage, and use in synthesis and analysis processes, but also during waste disposal and their uncontrolled release into the environment. It is worth noting that this effect combines three principles indicated in section 2 as seemingly primal.
The potential impact of a substance depends not only on its chemical characteristics (the degree of hazards it poses), but also on its quantity and the possibility of spontaneous decomposition to safe products (biodegradability). The principle relating to the amount of chemical (waste) concerns the same elemental effect as the principle relating to its properties (hazards), with the difference that the former one emphasizes the quantitative aspect and the latter qualitative. It is also worth emphasizing that if a given process generates a lot of waste that is safe, or uses a very toxic substance but in a minimal amount, the risk of adverse impact is small. Regarding degradability, its absence will in fact mean an increased risk of chemical impact. All these aspects are intrinsically connected. Therefore, the element of greenness associated with the use and generation of chemicals should be seen as their combination.
The third effect, the nature of which is different from the two previously mentioned, is related to the use of the Earth's natural resources and the real possibility of their recycling. Although the primary principles discussed earlier indicate the need to use renewable materials and reagents produced from renewable sources (GC principle #7 and GAC principle #10), they do not identify other significant natural wealth. Natural resources of the Earth exploited by mankind include both raw materials, minerals, fossil fuels, water, and air, but also living organisms (e.g., animals, plants), soil used for plant cultivation, and even the naturalness of the genetic pool (use of GMOs, extinction of species).
One can say that obtaining the reagent from plant crops instead of fossil raw materials is, after all, an approach in line with the GC idea. In fact, not entirely, large-scale industrial cultivation requires the use of fertilizers and pesticides, and in addition excludes the possibility of using the soil for “greener” purposes, i.e., eliminating malnutrition in Third World countries. The effect of using natural resources is of great importance in the case of chemistry. It should be highlighted that most chemical reagents are obtained from petroleum. In addition, water is used in chemical processes on a grand scale.
At this point, it should be noted that the production of a large amount of waste may therefore indicate the risk of two elemental effects: chemical impact and use of (water) resources. Similarly, the production of energy from fossil fuels, which affects the climate, at the same time irretrievably devastates natural resources of coal and natural gas. We can minimize some effects by recycling waste and running processes in a closed loop. However, this is not feasible for all resource types.
The fourth effect focuses on the physical factors. Firstly, it refers to the safety of the method user (operator) and the injuries that may be incurred when exposed to physical factors that pose a direct threat to life and health. User safety and risk of accidents are mentioned in principles #12 GC and #12 GAC. However, the content of these rules indicates varied types of risk, and it is worth breaking them down into primary factors. The workplace hazards resulting from the chemical characteristics of the reagents used, e.g., the risk of acid burns, are a part of the effect previously discussed as second in sequence, i.e., the chemical impact. Physical hazards resulting not from the chemical nature of the substance, but from the form of its storage, are a separate issue. For example, helium itself is not dangerous (chemically), but its high compression in the cylinder creates a risk of explosion, which is a physical hazard. Other effects and hazards worth mentioning here are heat and cold damage, the possibility of injury with sharp objects, noise, radiation, high voltage, magnetic field, vibration, etc.
In addition, this effect, as the other ones, should also be considered with regard to the natural environment. It includes contamination of the biosphere with radioactive materials as the consequences of exploiting nuclear energy, as well as pollution with light and noise, which can also disturb the life of plants and animals. In addition, it includes physical transformation of the natural landscape, deforestation, pollution of outer space by debris orbiting the Earth, posing the risk of physical collisions, and any physical damage caused by warfare.
Finally, the fifth elemental effect comprises the consequences of exposure to biological hazards. This type of hazard may occur when dangerous organisms are used in laboratory, e.g., viruses and bacteria, and when the operator is exposed to potential infection, e.g., when working with biological material. These issues seem to be especially significant in the case of medical and pharmaceutical laboratories. In a broader perspective, this effect includes man-made epidemics and a number of negative consequences resulting from them (we have recently been able to observe them directly in the case of Covid-19).
To sum up:
“Effects of the most primal nature, i.e., the so-called greenness elements, seem to be”
• Carbon footprint (CF), i.e., the emission of greenhouse gases that cause climate change.
• Chemical contamination (CC), i.e., the entire chemical impact of substances used on the environment and process operator.
• Earth exploitation (EE), i.e., irreversible devastation of physical resources (raw materials, fossil fuels, water, soil, air) and biological resources (animals, plants, genomic pool).
• Physical impact (PI), i.e., injuries sustained by exposure to physical factors (temperature, radiation, high pressure, high voltage, cuts, light, noise, etc.).
• Infections and epidemics (IE), i.e., infections with pathogens that are dangerous to health and life, and resulting epidemics.
These effects are elemental because they are of a separate nature, and it is never possible to objectively determine which are more “destructive” and which are less. There are no more or less important among them, and each of them is an integral part of the idea of greenness.
The most primal components of greenness have been identified and named above. However, this is only a proposal that may give rise to discussion. Distinguishing these effects is more of a pragmatic than empirical nature, and it helps to order our theory and perspective of future actions. The boundary between the elements is by definition conventional, and it is not always sharp (for example, global warming results from air pollution with greenhouse gases). On the other hand, some effects included in the same element have a quite different nature and could be treated as separate elements in a different approach. Therefore, the presented proposal is a compromise. In addition, it is possible to indicate further elements that have been omitted here.
Currently, we are probably on the verge of a revolution related to the development of artificial intelligence (AI). As with the industrial revolution, the potential outcomes are hard to predict. Although technological progress can improve achieving of the intended goals, including limiting the adverse impact of chemical laboratories on the environment and improving safety, it is still unknown whether and when AI algorithms can get out of our control. Already, the available tools are smart enough to manipulate and predict our (human) emotions. In the dark scenario, we will soon have to introduce the sixth element to the collection – “the AI effect”.
7. Mathematical description
At the very beginning it was stated that “a good theory of natural science should use the language of mathematics”. Thus, is it possible to apply this language to GC? Is it possible to define greenness with an equation?In the previous section, it was stated that greenness is elemental in nature, which means that it is expressed in elemental effects that cannot be objectively unified, i.e., summed up. This excludes the possibility of defining greenness as the result of an algebraic equation referring to these effects.
“Assuming that greenness is inversely proportional to the sum of elemental effects (destructive impacts) would be a similar mistake to trying to add the mass of an object to its length. Although both quantities describe the same object, they are mutually non-addable.”
However, there is another way to define greenness:
“Greenness can be represented mathematically as a set of elemental effects because the elements of the set need not be of the same nature.”
Therefore, we can write:
| G = {E1,E2,…,En} | (1) |
Assuming we can currently identify five elemental effects discussed in section 6:
| G = {CF,CC,EE,PI,IE} | (2) |
Another question now arises. How can we mathematically define an elemental effect?
“Elemental effects, i.e., the so-called elements of greenness, are functions of three basic variables: the severity of hazard, the amount of the factor posing a hazard, and the effectiveness of counteracting the hazard.”
| E = f(h,q,p) | (3) |
This definition is reflected in reality.
With regard to the CF element, h is the energy intensity, i.e., the electrical power of devices determining the instantaneous demand for energy, q is the working time of devices powered by electricity, and p is the share of renewable energy sources in the total production of energy taken from the grid (energy decarbonisation).
With regard to the CC element, h is the toxicity of chemical reagents (understood as a set of all adverse effects on health and the environment resulting from the chemical nature of a given substance), q is the mass of these reagents, and p is the effectiveness of protection against contact with reagents and release into the environment.
With regard to the EE element, h is the degree of depletion and related consequences (Pt is e.g. more at risk of depletion than Si), and the degree of renewability of a given raw material/resource, q is the required amount of raw material/resource, and p is the efficiency of its recycling and replenishing.
With regard to the PI and IE elements, h is the potential health and environmental consequences of contact with a hazardous factor, q is the time of exposure to this factor and its intensity, and p is the effectiveness of protection against this factor.
It is worth noting that we can control all these variables, but in different ways and at different levels.
The severity of hazard (h) is influenced by the designers and creators of new techniques, methodologies, instruments, materials, and reagents, as well as the creators of methods through the skilful selection of these components during method development.
The amount of factor q is influenced by the creators of methods (because each procedure is different), as well as the users of these methods, who are responsible for the strict implementation of the method according to the protocol.
The prevention efficiency (p) is influenced by the users of methods, who should take care of all the necessary protection measures and comply with health and safety regulations, but also by an unspecified group of other people, namely decision makers. They can be building designers, administrators, and even people in power (who, for example, decide on the development of renewable energy).
Therefore, combining efforts and cooperation of various groups of people play the key role in effectively achieving the intended goals. It includes the appropriate education and shaping the awareness of the need for cooperation. Admittedly, there will always be people with different views, who are sure they are right. They should not be condemned, but listened to, tried to be understood, and re-convinced using better and better arguments. The activities aimed at deepening the productive exchange of information and experience between the scientific and commercial sectors are also necessary. See Fig. 3 for visualization.
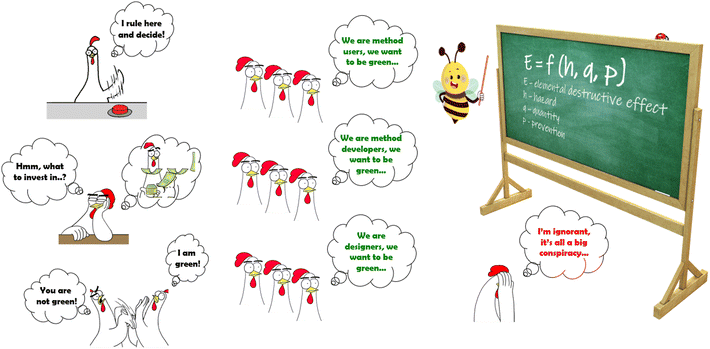 | ||
| Fig. 3 An illustration of the need to integrate various groups of people, educate, cooperate at different levels, and look for agreement in difficult and conflict situations. | ||
Therefore, it seems that
“The full implementation of the GC idea requires the cooperation of many different environments, from designers of new technologies, method creators, users, and teachers, to investors, administrators, and people in power. Simultaneous cooperation on three distinct levels is crucial: elimination of hazards, reduction of the amount of hazardous factors, and effective prevention.”
8. Greenness assessment and evaluation
8.1. Current status
Over the last 25 years, since the GC idea was formulated, many attempts have been made to develop tools to measure and compare how green are the objects of interest. First, simple indicators relating to the mass of substances used and waste generated were introduced.36–39 These parameters, in particular, the E-factor (the ratio of the mass of waste per mass of product),25,40 and atom economy (the conversion efficiency of a chemical process in terms of all atoms involved and the desired products produced),37 largely contributed to the propagation of the GC idea in science and industry.Meanwhile, a number of more complex approaches were developed, taking into account multiple factors simultaneously. Among them, the approach referred to as life cycle assessment (LCA) should be mentioned, according to which the greenness of a given product is assessed holistically, from design, through production, and use to disposal (in other words: from the cradle to the grave).41,42 Another idea was Eco-Scale,43 a simple and useful scoring system, which over time was implemented also in the GAC.44 The recently published metric system developed by a commercial entity (Merck) is DOZN™, an advanced model fully aligned with the 12 Principles of GC.45
The other metrics focused on analytical methods are NEMI,46 GAPI,47,48 AGREE,49,50 AMGS,51 HEXAGON,52 MCDA,53 RGB,33 RGB12,27etc.54 They differ in structure, form of presentation of results and degree of sophistication. In addition, some of them, such as HEXAGON, MCDA, RGB and RGB 12, allow the evaluation of a method from various angles, greenness as well as additional criteria determining the usefulness of the method.27,57
A thorough description of these and other tools used to assess greenness of synthetic and analytical methods and processes is available in many review articles.54–58 The interested reader is referred to them.
8.2. Method component vs. method vs. process
An important issue in the assessment of greenness is to ask ourselves the key question – what object do we want to assess and what is its nature? An often-overlooked fact is that we should approach differently, for example, the assessment of a new “potentially green” reagent, the assessment of the method that uses this reagent, and the assessment of the synthesis or analysis process carried out according to this method.In the first case, when we are talking about “method components” such as reagents, materials, instruments, technologies, etc., we can assess the severity of hazard associated with their potential use, i.e., the variable h in eqn (3). We do not know the amount of the hazardous factor (variable q), because this parameter appears only when a particular method is developed, using a given method component (hazardous factor).
In the case of assessing a method (procedure), we can consider the variables h and q, but we still cannot consider the third variable appearing in eqn (3) related to prevention (p). The methods published in the GC literature are de facto recipes for successfully performing a particular chemical reaction or analysis. These recipes indicate the necessary instruments, reagents, materials, and how they are interconnected into a functional whole (methodology). However, they do not specify aspects such as personal protective equipment, measures to prevent the release of reagents into the environment, required procedures for waste management and recycling of materials. They also do not indicate the source of energy used to power the instruments (it is hard to even imagine).
The situation however is different in the case of assessing a process, i.e., the event of using a given method in practice, in the specific circumstances. These circumstances actually determine the p variable, i.e., the degree of prevention. A simple example is energy consumption. As mentioned earlier, in this case, the p variable should be understood as energy decarbonization, i.e., the share of clean energy sources such as the sun and wind. This share in unknown when looking from the method perspective.
It is also crucial to realize that in the case of processes we are always dealing with a chain of interrelated events. The process of synthesis or analysis is always preceded by the processes of production, preparation, and delivery of materials, including energy, and is always followed by processes of utilization and processing of products and waste. Therefore, in the case of process assessment, we should always try to use the LCA approach,41,42i.e., consider the entire chain of events, not limited to the event-of-interest. With regard to energy, this means that when calculating the carbon footprint of the product of a given process, we should consider emissions related to the production of materials, as well as their disposal and processing after the end of the synthesis of the target product.
However, when assessing a method, not the process of applying it, we do not know exactly what happens before and after hypothetical method application, because the method's protocol usually does not describe it. For example, if a given synthesis or analysis protocol requires the use of a particular reagent or material, it usually says nothing about how it is to be produced and delivered to the laboratory. Similarly, the method does not indicate exactly how materials should be disposed after synthesis and analysis. These additional processes are regulated according to distinct methods, addressed specifically to them. Of course, it can be assumed that this additional information must be included in the protocol, but in practice this will turn out difficult or even impossible. In other words:
“The method itself, understood as a recipe, is an isolated and theoretical object, effective application of the LCA approach to method assessment is by definition impossible. However, the process of applying the method is a sequence of events happening in reality, in the specific circumstances. When assessing a process or the product of that process, we should always use the LCA approach.”
Does this mean that in the case of method the greenness assessment is unfeasible or useless?
In section 4.1 it was stated that “the expression of greenness are the parameters showing the probable strength of the destructive impact.” Since this definition refers to probability, not certainty, it is possible to refer to greenness also when we have access to inherently limited information. In other words, methods and method components can also be classified in terms of greenness by referring to parameters that are adequate to their nature.
A full assessment according to the LCA approach, taking into account all three variables (h, q, p), should be a priority when some process is used routinely, in specific and well-established circumstances. This applies in particular to the industrial and service sectors, wherein, additionally, the “achieving greenness” by the product often plays a marketing role. A partial assessment of a method, against the LCA idea, taking into account only two variables (h and q), should be a routine in basic science, i.e. in R&D and academic sectors. However, one should be aware that the assessment of the method is de facto the assessment of the risk of its use, not the assessment of its actual impact.
Continuing along this line,
“The interpretation of greenness of various objects (processes, methods, and method components) should have a hierarchical structure. The greenness of the process is always superior to the greenness of the method, and the greenness of the method is always superior to the greenness of the method component.”
To illustrate this, let us reconsider once more the issue of energy consumption and carbon footprint. It can be assumed that in some process the instrument with a very high energy intensity is used (highly unfavourable value of h), additionally, the method assumes its continuous operation for a long time (highly unfavourable value of q). However, the instrument is powered entirely by a renewable energy source, for example wind (highly favourable value of p). In this case, it is obvious that the actual impact (carbon footprint) will be low, and the process could be greener than many alternatives, although the instrument (method component) and the method itself are far from this.
8.3. Examples of method assessment
It is worth reminding that,“Methods can be assessed and evaluated in terms of only two of the three essential variables: the severity of hazard and the quantity of factor posing that hazard. The degree of prevention cannot be assessed objectively as it depends on the individual circumstances of the method application process.”
The correlation between the hazard (h) and its quantity (q) can be defined as “the risk of a destructive effect caused by the use of a particular method, under unspecified circumstances”:
| R = f(h,q) | (4) |
We can also assume that, approximately, the unit risk associated with a single factor will be the product of these two variables:
| R′ = h·q | (5) |
Eqn (5) works very well for the CF element. In this case, h is the electrical power (temporary energy consumption) of the given device, q is the working time of this device, and R′ will simply be the amount of electricity used to power the device during the method implementation.
An interesting idea of assessing the risk of chemical impact of waste, taking into account the qualitative (h) and quantitative (q) aspects, was the EQ factor developed by Sheldon and co-workers,59i.e., a parameter linking the amount of waste with its chemical characteristics.
Another proposal for a direct indicator relating to the CC element is the recently proposed “ChlorTox Scale”.60 This is a new approach aimed at estimating the chemical risk of any laboratory method/procedure in a comprehensive but still quick and simple way. The basis of this approach is to refer hazards related to the substance-of-interest to the hazards identified for the standard substance – chloroform (it indicates the h variable), and to consider the precisely known mass of the substance used in the method (q variable). The results are expressed in equivalent mass of chloroform, indicating the degree of estimated chemical risk, see Fig. 4. Although the usability of this scale has been demonstrated by examples of analytical methods,60 there is nothing to prevent its transfer to the area of synthesis.
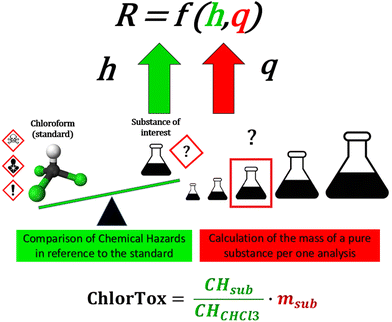 | ||
| Fig. 4 The idea of assessing the chemical risk of a laboratory method (R) using the ChlorTox Scale, based on the variables h (chemical hazard) and q (mass). | ||
To conclude, the purpose of this article is neither to discuss current greenness assessment tools nor to propose new approaches. The goal, however, is to prepare a right theoretical ground for their future improvement and development. The mathematical basis for assessing the greenness of processes, methods and method components should be constantly developed and improved. The above considerations should be treated as a proposal for a starting point, which still requires discussion and validation.
9. Unified Principles of Greenness
Finally, the time has come to propose a set of new updated principles that would precisely and clearly define the most important assumptions of the GC idea. My proposal is to perform a synthesis of statements that have already been made in the previous sections, and which seem to be of the greatest importance. These rules will be common to all chemical disciplines, and moreover, to other fields of human activity. Hence, they have been called “Unified Principles of Greenness”.The Unified Principles of Greenness have been formulated in a hierarchical order. The first principle (#1) is of the utmost importance because it refers to actual impact of our actions; the second (#2) indicates how to increase the effectiveness of implementing the first principle; the third (#3) indicates the pitfall to be avoided when striving for greenness; the fourth (#4) indicates how to ensure that we are doing the right thing; and the fifth and last (#5) indicates how to communicate with others:
#1. The principle of limitation:
“In order to strive for greenness, limit all destructive impacts on the environment and humans caused by your actions directly or indirectly, in particular:
- Limit greenhouse gas emissions.
- Limit chemical impact of the substances used at every possible stage.
- Limit irreversible processing and devastation of the Earth's natural resources.
- Limit injuries and impacts caused by physical factors.
- Limit infections caused by hazardous pathogens.”
Admittedly, this is only the foundation of a much larger construction. The secondary rules addressed to specific disciplines, such as those indicated in Tables 1 and 2, may create the next levels of this construction and indicate the ways to implement the general primary principle. The principles #2, #4, #5, #8, #9 and #11 from the set of 12 Principles of GC would refer to the field of chemical synthesis, and the principles #1, #2, #3, #4, #5, #6 and #8 from the set of 12 Principles of GAC would refer to the field of analysis. Thanks to this, the current sets of rules could be integrated with the proposed UG-theory. The wording of individual secondary rules could also be improved. In addition, it is possible to expand these sets and add more rules. The most important thing is their clear embedding on a common foundation – the primary principle #1 being of the utmost importance.
The other principles presented below refer to aspects that have been overlooked so far (by the currently known sets of rules), but which are important for the achievement of the assumed goals. They give an additional dimension to the space of possibilities, and complying with them will be the key to the dynamic progress in all kinds of “green areas”. They apply to all disciplines simultaneously:
#2. The principle of cooperation:
“Three types of actions lead to greenness: elimination of hazards, reduction of the quantity of hazardous factors, and increase in the effectiveness of counteracting (prevention). It is important to merge efforts and act simultaneously at all these levels. Do not act alone, cooperate with others. Listen to your colleagues, competitors, and people looking from a different perspective, be open, unify instead of divide, talk to people in power using convincing arguments, educate others.”
#3. The principle of compromise:
“Do not strive to achieve your goals at any cost. Improving one greenness criterion may inevitably worsen the others. Search for the optimal compromise in a specific situation and circumstances, guided by common sense. Remember that the ultimate goal is a reasonable balance of green and functional criteria. Don't waste your effort and creative energy.”
#4. The principle of verification:
“Before you say something is green or greener, verify it. Whenever possible, rely on hard empirical data, avoid conjectures and subjective assumptions. Use models with care, don't treat them like oracles. Be critical of yourself and your beliefs.”
#5. The principle of clarity:
“Use strict and transparent language. Define the terms you use and use the terminology consistently. Avoid saying “something is green or greener” unless the context clearly indicates what you mean. Don't call your solution green to achieve the desired benefits faster and with less effort, always play fair.”
10. Summary
The concept of the Unified Greenness Theory (UG-theory) was presented. As the name suggests, it is just a theory, and theories should be critically verified, discussed, improved, and replaced with better ones over time. This is only a proposal. The UG-theory tries to unify the GC, GAC, and other areas so that we can all simply refer to ourselves as “green chemists”, regardless of which chemical discipline we are closer to. Its aim is also to identify and solve the key problems and dilemmas regarding the concept of greenness. Although it is in fact an abstract concept, an attempt has been made to describe it mathematically. Moreover, new terminological rules have been proposed to keep the exact character of GC as a natural science. The hitherto guidelines, 12 Principles of GC, 12 Principles of GAC, and other sets of principles, have been merged and reformulated into a novel set of five hierarchal and universal statements that reflect the key challenges of today – the Unified Principles of Greenness. Their meticulous application will sustain the dynamic development of GC and keep it on the right track.Anyone willing to share a critical opinion on the considerations presented here, ask in case of doubt or cooperate in the future, is asked to contact me directly by e-mail.
Abbreviations
| AI | Artificial intelligence |
| GAC | Green analytical chemistry |
| GC | Green chemistry |
| UG-theory | Unified Greenness Theory |
| WAC | White analytical chemistry |
| WC | White chemistry |
Conflicts of interest
The author declares no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This research contributing to the development of the UG-theory was funded by the National Science Centre, Poland (P. M. N.; OPUS, 2020–2024, Grant No. 2019/35/B/ST4/01022).References
- R. A. Sheldon, Chem. Soc. Rev., 2012, 41, 1437–1451 RSC.
- A. D. Curzons, D. J. C. Constable, D. N. Mortimer and V. L. Cunningham, Green Chem., 2001, 3, 1–6 RSC.
- C. K. Z. Andrade and A. R. Dar, Tetrahedron, 2016, 72, 7375–7391 CrossRef CAS.
- M. Poliakoff, J. M. Fitzpatrick, T. R. Farren and P. T. Anastas, Science, 2002, 297, 807–810 CrossRef CAS PubMed.
- Green Chemistry: Theory and Practice, ed. P. T. Anastas and J. C. Warner, Oxford University Press, Oxford, 1998 Search PubMed.
- P. T. Anastas and J. B. Zimmerman, in Sustainability Science and Engineering Defining Principles, ed. M. A. Abrahams, Elsevier, 2006, pp. 11–32 Search PubMed.
- S. L. Y. Tang, R. L. Smith and M. Poliakoff, Green Chem., 2005, 7, 761 RSC.
- S. Y. Tang, R. A. Bourne, R. L. Smith and M. Poliakoff, Green Chem., 2008, 10, 268–269 RSC.
- P. T. Anastas, Crit. Rev. Anal. Chem., 1999, 29, 167–175 CrossRef CAS.
- M. Koel and M. Kaljurand, Pure Appl. Chem., 2006, 78, 1993–2002 CAS.
- S. Armenta, S. Garrigues and M. de la Guardia, TrAC, Trends Anal. Chem., 2008, 27, 497–511 CrossRef CAS.
- M. Koel, Green Chem., 2016, 18, 923–931 RSC.
- A. Gałuszka, Z. Migaszewski and J. Namieśnik, TrAC, Trends Anal. Chem., 2013, 50, 78–84 CrossRef.
- Views News, Green Chem., 2001, 3, G73–G81, 10.1039/B110187K.
- N. Asfaw, Y. Chebude, A. Ejigu, B. B. Hurisso, P. Licence, R. L. Smith, S. L. Y. Tang and M. Poliakoff, Green Chem., 2011, 13, 1059–1060 RSC.
- A. I. Lopez-Lorente, F. Pena-Pereira, S. Pedersen-Bjergaard, V. G. Zuin, S. A. Ozkan and E. Psillakis, TrAC, Trends Anal. Chem., 2022, 148, 116530 CrossRef CAS.
- https://www.epa.gov/greenchemistry/basics-green-chemistry (accessed on 7 March 2023).
- K. J. M. Matus, W. C. Clark, P. T. Anastas and J. B. Zimmerman, Environ. Sci. Technol., 2012, 46, 10892–10899 CrossRef CAS PubMed.
- P. Cintas, Ultrason. Sonochem., 2016, 28, 257–258 CrossRef CAS PubMed.
- S. L. Fegade and J. P. Trembly, Ultrason. Sonochem., 2017, 37, 686–687 CrossRef CAS PubMed.
- S. L. Fegade, Mater. Chem. Phys., 2015, 154, 176 CrossRef CAS.
- P. T. Anastas and J. B. Zimmerman, Chem, 2016, 1, 10–12 CAS.
- R. Marcinkowska, J. Namieśnik and M. Tobiszewski, Curr. Opin. Green Sustainable Chem., 2019, 19, 19–23 CrossRef.
- E. J. Yanarella, R. S. Levine and R. W. Lancaster, Sustainability, 2009, 2, 296–302 CrossRef.
- R. A. Sheldon, Green Chem., 2017, 19, 18–43 RSC.
- H. Mutlu and L. Barner, Macromol. Chem. Phys., 2022, 223, 2200111 CrossRef CAS.
- P. M. Nowak, R. Wietecha-Posłuszny and J. Pawliszyn, TrAC, Trends Anal. Chem., 2021, 138, 116223 CrossRef CAS.
- V. Mazzaracchio, A. Sassolini, K. Y. Mitra, D. Mitra, G. M. Stojanović, A. Willert, E. Sowade, R. R. Baumann, R. Zichner, D. Moscone and F. Arduini, Green Anal. Chem., 2022, 1, 100006 CrossRef.
- J. Pawliszyn, D. Barceló, F. Arduini, L. Mondello, Z. Ouyang, P. M. Nowak and R. Wietecha-Posłuszny, Green Anal. Chem., 2022, 1, 100001 CrossRef.
- Ch. M. Hussain, Ch. G. Hussain and R. Keçili, TrAC, Trends Anal. Chem., 2023, 159, 116905 CrossRef CAS.
- P. Kościelniak, TrAC, Trends Anal. Chem., 2022, 157, 116758 CrossRef.
- K. A. Schug, The LCGC Blog: An RGB Additive Color Model for Analytical Method Evaluation, 2019, https://www.chromatographyonline.com/view/lcgc-blog-rgb-additive-color-model-analytical-method-evaluation Search PubMed.
- P. M. Nowak and P. Kościelniak, Anal. Chem., 2019, 91, 10343–10352 CrossRef CAS PubMed.
- P. M. Nowak, A. Bis, M. Rusin and M. Woźniakiewicz, Green Anal. Chem., 2023, 4, 100051 CrossRef.
- Our World in Data, Global Change Data Lab, 2022, https://ourworldindata.org/grapher/carbon-intensity-electricity (accessed on 3 November 2022).
- R. A. Sheldon, Chem. Ind., 1992, 903–906 CAS.
- B. M. Trost, Science, 1991, 254, 1471–1477 CrossRef CAS PubMed.
- C. Jimenez-Gonzalez, C. S. Ponder, Q. B. Broxterman and J. B. Manley, Org. Process Res. Dev., 2011, 15, 912–917 CrossRef CAS.
- C. Jiménez-González, D. J. C. Constable and C. S. Ponder, Chem. Soc. Rev., 2012, 41, 1485–1498 RSC.
- R. A. Sheldon, Green Chem., 2023, 25, 1704–1728 RSC.
- C. Jiménez-González, A. D. Curzons, D. J. C. Constable and V. L. Cunningham, Int. J. Life Cycle Assess., 2004, 9, 114–121 CrossRef.
- A. D. Curzons, C. Jiménez-González, A. L. Duncan, D. J. C. Constable and V. L. Cunningham, Int. J. Life Cycle Assess., 2007, 12, 272–280 CrossRef CAS.
- K. Van Aken, L. Strekowski and L. Patiny, Beilstein J. Org. Chem., 2006, 2, 3 Search PubMed.
- A. Gałuszka, Z. M. Migaszewski, P. Konieczka and J. Namieśnik, TrAC, Trends Anal. Chem., 2012, 37, 61–72 CrossRef.
- DOZN™ Quantitative Green Chemistry Evaluator, Merck, https://www.sigmaaldrich.com/PL/pl/services/software-and-digital-platforms/dozn-tool?utm_source=redirect&utm_medium=promotional&utm_campaign=dozn (accessed on 7 March 2023).
- L. H. Keith, L. U. Gron and J. L. Young, Chem. Rev., 2007, 107, 2695–2708 CrossRef CAS PubMed.
- J. Płotka-Wasylka, Talanta, 2018, 181, 204–209 CrossRef PubMed.
- J. Płotka-Wasylka and W. Wojnowski, Green Chem., 2021, 23, 8657–8665 RSC.
- F. Pena-Pereira, W. Wojnowski and M. Tobiszewski, Anal. Chem., 2020, 92, 10076–10082 CrossRef CAS PubMed.
- W. Wojnowski, M. Tobiszewski, F. Pena-Pereira and E. Psillakis, TrAC, Trends Anal. Chem., 2022, 149, 116553 CrossRef CAS.
- M. B. Hicks, W. Farrell, Ch. Aurigemma, L. Lehmann, L. Weisel, K. Nadeau, H. Lee, C. Moraff, M. Wong, Y. Huang and P. Ferguson, Green Chem., 2019, 21, 1816–1826 RSC.
- A. Ballester-Caudet, P. Campíns-Falcó, B. Pérez, R. Sancho, M. Lorente, G. Sastre and C. González, TrAC, Trends Anal. Chem., 2019, 118, 538–547 CrossRef CAS.
- K. Steele, Y. Carmel, J. Cross and C. Wilcox, Risk Anal., Int. J., 2009, 29, 26–33 CrossRef PubMed.
- M. Sajid and J. Płotka-Wasylka, Talanta, 2022, 238, 123046 CrossRef CAS PubMed.
- J. Martínez, J. F. Cortés and R. Miranda, Processes, 2022, 10, 1274 CrossRef.
- D. J. C. Constable, A. D. Curzons and V. L. Cunningham, Green Chem., 2002, 4, 521–527 RSC.
- F. Roschangar, R. A. Sheldon and C. H. Senanayake, Green Chem., 2015, 17, 752–768 RSC.
- P. M. Nowak, P. Kościelniak, M. Tobiszewski, A. Ballester-Caudet and P. Campíns-Falcó, TrAC, Trends Anal. Chem., 2020, 133, 116065 CrossRef CAS.
- R. A. Sheldon, CHEMTECH, 1994, 24, 38–47 CAS.
- P. M. Nowak, R. Wietecha-Posłuszny, J. Płotka-Wasylka and M. Tobiszewski, Green Anal. Chem., 2023, 5, 100056 CrossRef.
| This journal is © The Royal Society of Chemistry 2023 |