Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Recent progress in the development of fluorescent probes for imaging pathological oxidative stress
Yujie
Geng
a,
Zhuo
Wang
 *a,
Jiaying
Zhou
a,
Mingguang
Zhu
a,
Jiang
Liu
a and
Tony D.
James
*a,
Jiaying
Zhou
a,
Mingguang
Zhu
a,
Jiang
Liu
a and
Tony D.
James
 *bc
*bc
aState Key Laboratory of Chemical Resource Engineering, College of Chemistry, Beijing Advanced Innovation Center for Soft Matter Science and Engineering, Beijing University of Chemical Technology, Beijing, 100029, China. E-mail: wangzhuo77@mail.buct.edu.cn
bDepartment of Chemistry, University of Bath, Bath BA2 7AY, UK. E-mail: t.d.james@bath.ac.uk
cSchool of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang 453007, China
First published on 16th May 2023
Abstract
Oxidative stress is closely related to the physiopathology of numerous diseases. Reactive oxygen species (ROS), reactive nitrogen species (RNS), and reactive sulfur species (RSS) are direct participants and important biomarkers of oxidative stress. A comprehensive understanding of their changes can help us evaluate disease pathogenesis and progression and facilitate early diagnosis and drug development. In recent years, fluorescent probes have been developed for real-time monitoring of ROS, RNS and RSS levels in vitro and in vivo. In this review, conventional design strategies of fluorescent probes for ROS, RNS, and RSS detection are discussed from three aspects: fluorophores, linkers, and recognition groups. We introduce representative fluorescent probes for ROS, RNS, and RSS detection in cells, physiological/pathological processes (e.g., Inflammation, Drug Induced Organ Injury and Ischemia/Reperfusion Injury etc.), and specific diseases (e.g., neurodegenerative diseases, epilepsy, depression, diabetes and cancer, etc.). We then highlight the achievements, current challenges, and prospects for fluorescent probes in the pathophysiology of oxidative stress-related diseases.
1. Introduction
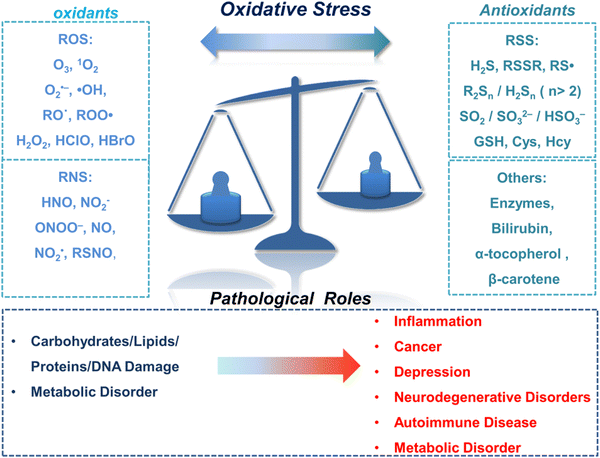
The oxidation–reduction process covers the basic process of almost all life functions from bioenergy to metabolism, so the oxidation–reduction homeostasis is very important for ensuring good health.1 In general, physiological levels of oxidants/reducing agents are important as redox signals, which are essential for maintaining a healthy body. However, excessive levels of oxidants can destroy biological molecules (such as deoxyribose, nucleic acids, proteins, lipids, etc.) causing oxidative damage and signal disturbances,2,3 which could potentially induce serious diseases (Fig. 1). Generally, the excessive production of oxidants and the serious imbalance of antioxidant consumption in organisms are collectively referred to as oxidative stress.4 Reactive oxygen species (ROS) and reactive nitrogen (RNS) are the two most important types of oxidants in the human body. They can be produced through a variety of endogenous and exogenous processes, and the negative effects are generally counteracted by reactive sulfur species (RSS), thereby forming a special redox homeostasis in the body (Fig. 1).5,6Normal physiological activities in organisms or external stimuli will induce the production of free radicals, which are highly active molecules or species.7 Free radicals possess a single or multiple unpaired electrons in the valence (outermost) electronic orbitals. When these unpaired electrons meet with oxygen molecules/nitric oxide in the organism, ROS/RNS will be generated. Therefore, ROS and RNS (RONS) refer to reactive radicals and non-radical derivatives of oxygen and nitrogen, respectively.8,9 It is generally believed that reactive oxygen species specifically include superoxide anion (O2˙−), hydrogen peroxide (H2O2), ozone (O3), singlet oxygen (1O2), alkoxyl (RO˙), lipid peroxy radical (ROO˙), carbonate (CO3˙−) radicals, hypochlorous acid (HClO), hypobromous acid (HBrO) and hydroxyl radicals (˙OH). Amongst them, O2˙− is one of the initial reactive oxygen species produced in cells, which is mainly produced by the reaction of electrons provided by nicotinamide adenine dinucleotide phosphate (NADPH) oxidase and oxygen during respiration (Scheme 1).10 At the same time, O2˙− can generate O2 and H2O2 through the disproportionation reaction in the presence of superoxide dismutase (SOD) and water (Scheme 1). H2O2 is an important signaling molecule and oxidant in the organism. However, abnormally high levels of H2O2 in the presence of redox active metal ions like Fe(II) or Cu(I) can generate ˙OH through the Fenton and Haber–Weiss reaction, which can cause damage to biomolecules (Scheme 1).11,12 In addition, due to the presence of chloride, bromide, and myeloperoxidase (MPO) in neutrophils, H2O2 can be converted into hypochlorous acid or hypobromous acid, which are extremely strong and destructive ROS (Scheme 1).13 RNS include peroxynitrite (ONOO−), nitric oxide (NO or NO˙), nitrogen dioxide radical (NO2˙), nitrite (NO2−), S-nitrosothiol (RSNO) and nitroxide (HNO), etc. Amongst them, NO is an important signaling molecule,14 and is irreplaceable in the collaborative work between cells in regulating human metabolism. ONOO− is produced by diffusion-controlled reaction of O2˙− and nitric oxide and has relatively strong oxidizing properties (Scheme 1).15 Active sulfur RSS refers to a species that contains sulfur atoms and has redox activity in the organism, and mainly includes biological thiols, hydrogen sulfide (H2S), disulfide (RSSR), polysulfides (R2Sn/H2Sn, n > 2), sulfur dioxide/sulfurous acid salt/bisulfite (SO2/SO32−/HSO3−), thienyl radical (RS˙) and sulfenic acid.16 Biological thiols (RSH) mainly includes glutathione (GSH), cysteine (Cys), and homocysteine (Hcy). RSH is an important reducing agent for the organism, and plays an important role in maintaining the redox balance. H2S is considered to be the third endogenous gaseous transmitter after NO and CO.17 Polysulfides are a class of sulfur-containing compounds that are widely found in prokaryotic and eukaryotic cells, and they are oxidation products of H2S (Scheme 1).18
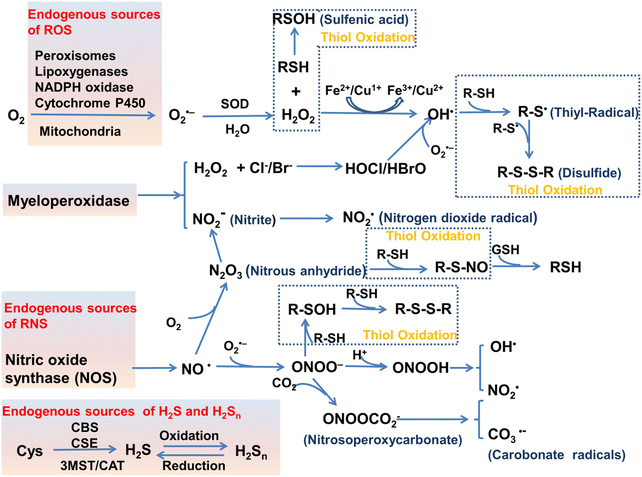 | ||
| Scheme 1 Various enzymatic and non-enzymatic processes that can generate ROS, RNS and RSS, as well as interactions between them (depletion and production). Abbreviations: cystathionine b-synthase (CBS), cystathionine c-lyase (CSE), 3-mercaptopyruvate sulfurtransferase (3MST), cysteine-aspartate aminotransferase (CAT). The schematic was drawn with reference to ref. 19–21. | ||
Meanwhile, biological and clinical methods have been used to prove that oxidative stress is closely related to the pathological process of a variety of diseases, such as inflammation, neurodegenerative diseases and cancer.22–25 Due to the short life time, high reactivity, and transient characteristics of RONSS (ROS, RNS, and RSS) in organisms, a deeper understanding and direct evidence of the link between RONSS and disease pathophysiology is not available. At present, the clinical judgment of oxidative stress in patients is mainly through the determination of the redox products produced by RONSS and the downstream functional markers of RONSS-induced damage. For example, a typical method is to determine the glutathione/GSSG and cysteine/cystine redox couple in the patient's plasma,26,27 as well as to determine the level of oxidized nucleosides in the urine (Judging the oxidative damage of DNA/RNA in cells).28,29 Unfortunately, these assays are susceptible to other pathological and physiological factors. At the same time, it is not possible to directly observe the dynamic process of oxidative stress in the patient's body in real time and determine which type of oxidant/reducing agent plays a decisive role in the pathophysiological process.30 Therefore, these methods cannot meet the requirements of biologists for the study of pathological oxidative stress in cells/tissues and in vivo. Fluorescence based imaging technologies that combine confocal imaging, two-photon imaging and in vivo imaging can provide non-invasive, real-time, and high-resolution images of cells and animals, and as such have gradually become powerful support tools for basic biomedical research.31–36
We and other researchers have reviewed fluorescent probes for RONSS detection over the last few years.37–41 These reviews focus mainly on probe design (selection of fluorophore and recognition groups), sensing mechanisms and performance evaluation, but provide little generalization or detail on biomedical applications. In 2019, Kim et al. discuss ROS and RNS fluorescence imaging in relation to pathophysiological processes, but ignore the essential role of RSS in redox homeostasis.42 In addition, RONSS fluorescent probes in the NIR II region and FL/PA dual-mode probes and dual-responsive probes are not included in the previous reviews. These three types of probes are receiving more and more attention due improved applicability. Notably, the field of fluorescent probes is facing a number of challenges. For example, the development of novel RONSS recognition groups is stalling, single-function fluorescent probes (non-NIR and unidirectional detection) are saturated, and many probes have similar biomedical applications (arthritis, peritonitis and liver injury). We believe that some guidance is urgently needed in this area to avoid stagnation in the development of improved probes.
As a commonly occurring pathological state, oxidative stress is closely related to human physiological and pathological activity, lifespan and many diseases. Fluorescent probes, a useful tool for biomedical research, can provide imaging information for the study of pathological oxidative stress. In this review we summarize representative RONSS fluorescent probes used for biomedical research published over the past six years. Based on the different pathological models, these probes are divided into three categories: (i) dual-response fluorescent probes for the monitoring of RONSS and flux of related active species in cells; (ii) fluorescent probes for RONSS imaging in pathological processes, including visualization of inflammation and organ damage, and evaluation of drug toxicity and safety of surgical procedures; (iii) fluorescent probes for RONSS imaging in oxidative stress-related disease models, involved in pathology studies, drug screening and evaluation of treatment efficacy. The links between oxidative stress and inflammation, organ damage, Alzheimer's disease, Parkinson's disease, epilepsy, depression, diabetes, and cancer are also introduced. At the same time, the design concepts, basic operational conditions for the probes and future development directions are outlined. Such research is a vital resource to provide the theoretical basis and intuitive evidence for the prevention of related diseases, drug design and postoperative diagnosis. Therefore, we anticipate that this review will provide appropriate guidance for chemical, biological, and medical researchers as well as drug discovery scientists.
2. Design of fluorescent probes for RONSS imaging
Fluorescent probes can detect target compounds using weak molecular interaction or chemical reactions.43 Molecular fluorescent probes are typically composed of three parts: a fluorophore, a linker and a recognition group. As far as the recognition mechanism is concerned, traditional design strategies provide optical recognition for detection through the interaction (including coordination and inclusion) of fluorescent probes with analytes (Fig. 2(a)).44 However, the design of lock-and-key molecular recognition and binding in the recognition of proteins is not suitable for most RONSS fluorescent probes.44,45 Most ROS and RNS molecules are similar in physical size, and most of them have the characteristics of short lifespan, low concentration, and high reactivity in biological systems. As such chemical reaction-based (activity-based) fluorescent probes are ideal tools for real-time imaging of RONSS (Fig. 2(b)).46 The sensing mechanisms used mainly include photoinduced electron transfer (PeT), intramolecular charge transfer (ICT), fluorescence resonance energy transfer (FRET), excited-state intramolecular proton transfer (ESIPT), and aggregation-induced emission (AIE). In this section, we will briefly introduce research progress for fluorophores, linkers and recognition groups related to RONSS.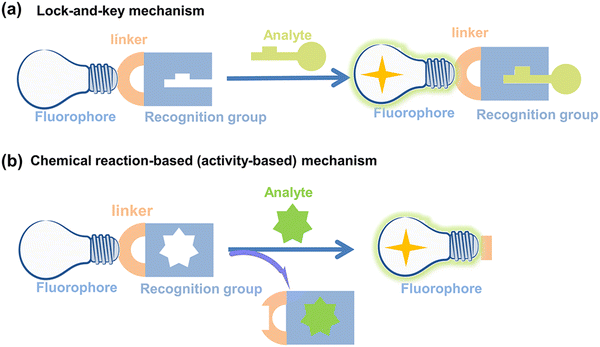 | ||
| Fig. 2 Schematic illustration of (a) lock-and-key mechanism and (b) chemical reaction-based (activity-based) mechanism for fluorescent probes. | ||
2.1 Fluorophores
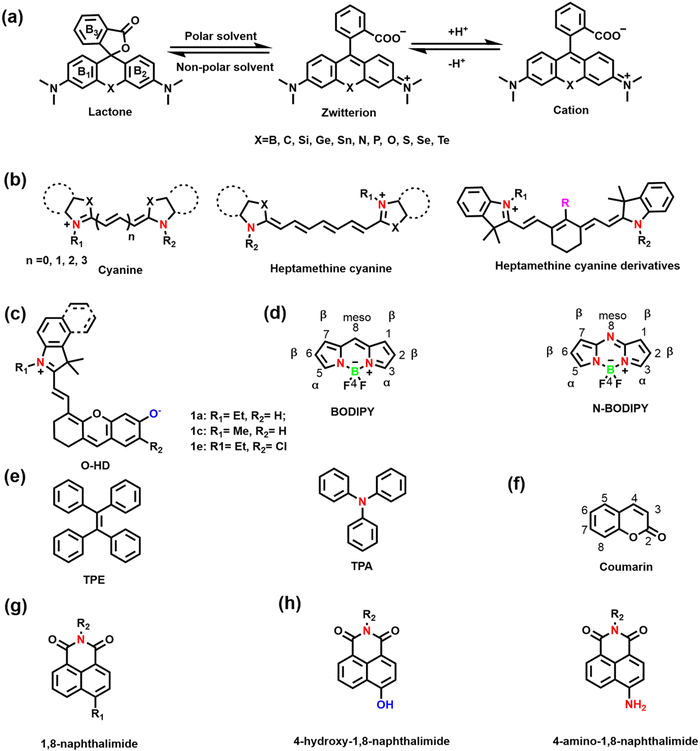
Hundreds of fluorescent probes have now been used to detect RONSS, almost all of which derive their primary fluorescent moieties from a number of classical fluorescent backbones and derivatives. They are chemically and photostable, while providing a wealth of modifiable structural sites. We selected six fluorophores that are widely used in the field of pathological RONSS imaging as examples.As an exceptional fluorophore over the past decade, hemicyanines are widely used to study the dynamic changes of RONSS levels in complex biological systems due to their easy structural modification and excellent biocompatibility.53,54 Generally, the hemicyanine structure consists of three parts: (i) a nitrogen-containing heterocycle with a positive charge as an electron acceptor; (ii) electron donor (e.g., hydroxyl, methoxy, amino or amine group); (iii) conjugated structure connecting the two parts.51 Hemicyanine dyes are donor–π–acceptor (D–π–A) systems. Significantly, many research groups have developed novel functionalized hemicyanine frameworks. In 2012, Lin et al. reported a new class of hemicyanine fluorescent dyes (O-HD) with NIR excitation and emission wavelengths and a modifiable terminal hydroxyl group (Fig. 3(c)).55
2.2 Linker
The linker is the bridge between the fluorophore and the recognition group, which is crucial for the optical properties of small molecule based fluorescent sensors. The linker used for fluorescent probes suitable for RONSS detection can be divided into four categories: (i) C–C/N/S single bonds or π bridges; (ii) ether bonds (such as O, S, Se, Te, etc.); (iii) ester bonds and amide bonds; (iv) pyridinium/quinolinium. In the presence of analytes, changes of the conjugated structure and the formation of residual groups, including hydroxyl, carboxyl, amino/amine, or pyridine/quinoline, etc., directly influences the spectral properties of the sensor.2.3 Recognition groups
Hydroxyl radicals have the strongest oxidative properties, short lifespans and low physiological concentrations of all the ROS.87 Compared with other ROS sensors, ˙OH still lacks a universal and specific recognition group.88 Several basic strategies can be used to design molecular fluorescence sensors for ˙OH, including aromatic hydroxylation strategies; oxidative dehydrogenation strategies; and oxidation strategies of sulfur atoms.89–91 HClO/ClO− is an endogenous ROS with strong oxidizing properties. Based on the reaction mechanism (such as oxidation of chalcogenides or oxidative cleavage reaction), common recognition groups can be roughly divided into three categories: (i) lactam/lactone, such as hydrazide, N,N-dimethylcarbamoyl; (ii) various double bonds, such as oxime, malononitrile and hemicyanine; (iii) sulfur/selenium-containing groups, such as thioethers, thioacetals, thioesters and selenolactones.92,93
Nitric oxide is a reactive free radical with oxidizing and reducing properties. o-diamino aromatics are the most commonly used recognition groups, which can form triazole derivatives with NO under aerobic conditions.94 In addition, N-nitrosation of aromatic amines is another common strategy.95 Peroxynitrite is a short-lived, low-concentration and highly reactive endogenous RNS.96 The common recognition groups for ONOO− can be divided into four categories: (i) the boronic ester/boronic acid and its benzyl derivatives (the reaction speed of boronic acids or boronates with ONOO− is much higher than for H2O2); (ii) hydrazide; (iii) carbon–carbon double bonds; (iv) aromatic phenols (these electron-rich phenol groups need to be bound to N-aryl-containing fluorophores).96,97
Furthermore, off-target activities of different probes should be treated carefully, as this is often a significant and yet commonly omitted aspect of the reliability of the RONSS detection by fluorescent probes. RONS can be divided into highly reactive h-RONS (e.g. ˙OH, ONOO−, and ClO−) and normally reactive n-RONS (e.g. H2O2, O2˙−, O3, NO). The difference in oxidation between the RONS is an important basis for the selection of the recognition group. In general, there is little mutual interference between n-RONS. At the same concentration and time scales, n-RONS hardly interfere with the recognition of h-RONS. However, as h-RONS are highly oxidative and nucleophilic, they not only increase the likelihood of off-target recognition of n-RONS (e.g. boronic esters and derivatives are more reactive with ONOO− than H2O2), but also interfere with each other. For example, the oxidation properties of ONOO− and HOCl are highly similar, but ONOO− is more oxidizing.98 Two recognition strategies are currently widely used in the field of fluorescence imaging of ONOO−, ˙OH and HOCl, which includes cleavage of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bonds and oxidation of chalcogenides. Therefore, the off-target activities of probes to identify h-ROS should be considered carefully in complex pathological environments.
C double bonds and oxidation of chalcogenides. Therefore, the off-target activities of probes to identify h-ROS should be considered carefully in complex pathological environments.
H2S is the simplest biological thiol in living systems and has strong nucleophilic and reducing properties.108 H2S can attack the electrophilic center of a conjugated structure through nucleophilic addition, so the positively charged indole salt of hemicyanine has been used as a recognition group.109,110 In addition reduction-based azide, nitro and thiolysis-based nitrobenzoxadiazole (NBD) ether, and 2,4-dinitrophenyl ether have been used as H2S-recognition groups.111–114 Hydrogen polysulfides (H2Sn, n > 1) are the oxidized form of H2S, which are more nucleophilic and reducing.115 The most commonly used recognition groups are divided into two categories according to the reaction mechanism. One is based on the aromatic nucleophilic substitution reaction, and the recognition groups are 2-fluoro-5-nitrobenzoic ester and phenyl 2-(benzoylthio) benzoate. The other one is based on the reduction reaction, and the recognition group has an aromatic nitro group.116–118 Sulfur dioxide (SO2) exists in aqueous solution as sulfite (SO32−) and hydrogen sulfite (HSO3−).119 Recognition groups designed for the strong nucleophilicity of SO2 can be divided into four categories: (i) levulinate; (ii) α,β-unsaturated ketones; (iii) aldehyde groups; (iv) carbon–carbon double bonds (nucleophilic addition to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds is a common strategy).120–123
C bonds is a common strategy).120–123
The RSS assay also requires vigilance for off-target activity of the probes. The nucleophilicity, reducibility and molecular structure of the different RSS are the basis for the selection of the recognition groups. Some biological thiols are generally less reactive than H2S/H2Sn, but they are very abundant in cells (e.g. 1–10 mM for GSH and 30–200 μM for Cys). Therefore, when assessing the off-target activity of RSS probes, physiological concentration levels of interferents need to be considered (Table 1).
| ROS/RNS/RSS | Recognition groups or strategies |
|---|---|
| H2O2 | • Boronic ester/boronic acid, nitrophenyldicarbonyl, pentafluorobenzenesulfonyl |
| O2˙− | • Benzothiazole, indoline, catecho, 2,4-dinitrobenzenesulfonyl, trifluoromethanesulfonate, diphenylphosphinate |
| O3 | • 3-Methylpyrazolone |
| ˙OH | • Aromatic hydroxylation strategies, oxidative dehydrogenation strategies, oxidation strategies of sulfur atoms |
| HClO/ClO− | • Lactam/lactone, such as hydrazide, N,N-dimethylcarbamoyl; various double bonds, such as oxime, malononitrile and hemicyanine; sulfur/selenium-containing groups, such as thioethers, thioacetals, thioesters and selenolactones |
| NO | • o-diamino aromatics, N-nitrosation of aromatic amines |
| ONOO− | • Boronic ester/boronic acid; hydrazide; carbon–carbon double bonds; aromatic phenols |
| Thiols | • Maleimide, 2,4-dinitrobenzenesulfonyl |
| Cys/Hcy | • Thioesters, acrylates, α,β-unsaturated ketones and aldehyde groups |
| H2S | • Indole salt, azide, nitrobenzoxadiazole (NBD) ether, 2,4-dinitrophenyl ether |
| H2Sn, n > 1 | • 2-Fluoro-5-nitrobenzoic ester, phenyl 2-(benzoylthio) benzoate |
| SO2 | • Levulinate, α,β-unsaturated ketones, aldehyde groups, carbon–carbon double bonds |
3. Fluorescent probes for oxidative stress imaging in cells
RONSS are ubiquitous in cells, and involved in numerous biological mechanisms including cell protection, apoptosis, signal transduction, inflammation and cancer.124 Physiological levels of RONSS are essential for the correct performance of cell functions, but long-term exposure to high levels of RONSS can damage organelles and ultimately induce cell apoptosis and various diseases. Importantly, these diseases are not caused by a single factor, and involve changes in the levels of multiple RONSS and related species. Therefore, the detection of multiple RONSS in cells is of significant interest for understanding the pathology of the disease. Over the past few decades, enzyme-linked immunosorbent assays (ELISA), electrochemical analysis, mass spectrometry (MS) and high-performance liquid chromatography (HPLC) methods have been used to detect intracellular RONSS.125–130 However, these methods have several disadvantages such as difficulty in sample preparation, require expensive instrumentation and are unsuitable for real-time analysis. In particular, the extraction of oxidative markers (such as deoxyribose, nucleic acids, proteins, lipids, etc.) often destroys the structure of cells and tissues making real-time analysis in vivo impossible. Compared with these detection technologies, fluorescent probes combined with a variety of imaging technologies can monitor the physiological and pathological conditions of cells in a non-invasive, real-time, highly-sensitivity and high-resolution manner.Many diseases are not typically caused by a single factor, and the combined use of multiple sensors can cause numerous problems such as spectral overlap, analyte crosstalk, increased biotoxicity, photobleaching and localization since two species are involved. A promising solution is to use a single probe that reacts with two analytes, called dual-responsive fluorescent probes.131 Based on the recognition logic, dual-responsive probes for RONSS detection are currently classified into the following types: (i) reversible probes, which mean two analytes interact with the probes in a reversible manner; in general, the two analytes are RONS and RSS which can proceed via a reversible redox reaction; (ii) sequence-specific reaction probes can react with different analytes through sequenced chemical reactions; (iii) competitive probes, which rely on competitive reactions between the two analytes and the probe, with varying optical properties of the reaction products; (iv) composite probes, which have two fluorophores and recognition groups, but no spectral overlap between the excitation and emission wavelengths. In this section, we have selected some recent examples describing multi-species imaging (RONSS and related species).
3.1 ROS +RSS
H2O2 and H2S are important redox signal molecules, and they jointly participate in many redox physiological and pathological processes.132 In order to further explore the relationship between the two signal molecules, Wang et al. developed a fluorescent probe (TCAB) that could detect H2S and H2O2 independently (Fig. 4(a)).133 The short-wavelength emitting coumarin HCB and the long-wavelength emitting fluorophore TQC together served as the fluorophore, while the benzyl boronic ester and azide group served as the respective sensing units of H2O2 and H2S, respectively. In the presence of H2O2, the benzyl boronic ester is removed to generate the cyan, fluorescent dye TCA. In the presence of H2S, the azido moiety was reduced to an amino derivative and undergoes 1,6-elimination, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double isomerization and subsequent spontaneous intramolecular cyclization to release two fluorophores simultaneously (HCB in the blue channel and TQC in the red channel). In addition, the probe was able to detect H2O2/H2S reversibly. The sequence of H2O2–H2S produced cyan and then red signals, while the reverse reaction sequence produced red and then cyan signals. This study facilitated the imaging of endogenous H2S and H2O2 in cells (Fig. 4(b)), which allowed the monitoring of H2O2 and H2S redox processes in living cells and organisms.
C double isomerization and subsequent spontaneous intramolecular cyclization to release two fluorophores simultaneously (HCB in the blue channel and TQC in the red channel). In addition, the probe was able to detect H2O2/H2S reversibly. The sequence of H2O2–H2S produced cyan and then red signals, while the reverse reaction sequence produced red and then cyan signals. This study facilitated the imaging of endogenous H2S and H2O2 in cells (Fig. 4(b)), which allowed the monitoring of H2O2 and H2S redox processes in living cells and organisms.
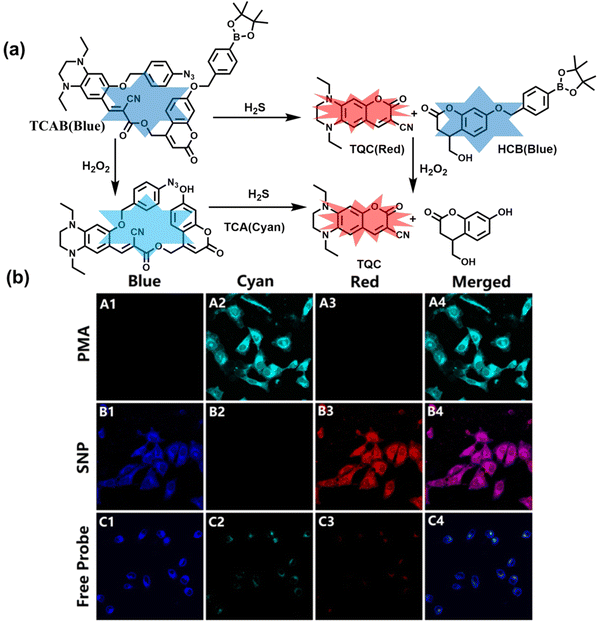 | ||
| Fig. 4 (a) Structure and sensing mechanism of TCAB for the discrimination of H2O2 and H2S. (b) Confocal fluorescence images of endogenous H2O2/H2S in living HeLa cells. SNP (sodium nitroprusside), Phorbol 12-myristate 13-acetate (PMA). Reproduced with permission from ref. 133. Copyright (2020) American Chemical Society. | ||
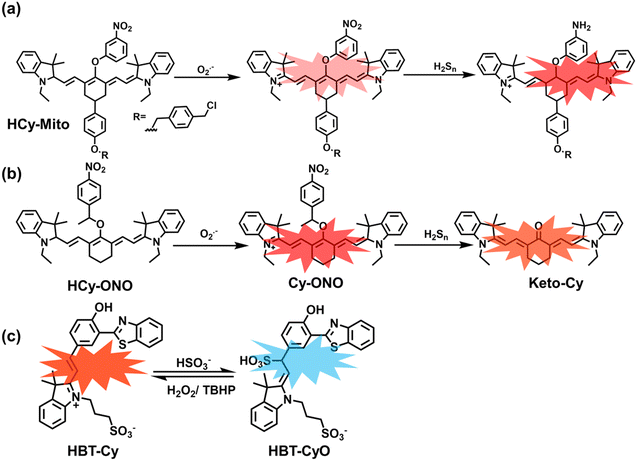
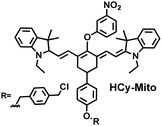
As a member of RSS, H2Sn is an important antioxidant in cells.134 The interdependence and mutual restriction between H2Sn and ROS promote redox homeostasis in cells. To better monitor the dynamic changes of redox in cells, Chen et al. developed a fluorescent probe (HCy-Mito) that could monitor the changes of H2Sn and O2˙− in cells (Fig. 5(a)).135 A heptamethine cyanine dye was chosen as the fluorophore. The oxidation of the N site in the cyanine platform was used to detect O2˙−, and meta-nitrophenol was used as a specific reaction site for H2Sn. After HCy-Mito reacts with O2˙−, the fluorescence intensity was partially restored and a positively charged intermediate was generated. This intermediate could further react with H2Sn in mitochondria to achieve complete recovery of the fluorescence intensity.
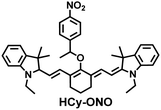
In addition, since HCy-Mito exhibited a degree of spectral overlap during imaging, Chen et al. developed an improved fluorescent probe (HCy-ONO) (Fig. 5(b)) and used it to explore the influence of intracellular H2Sn and O2˙− on redox homeostasis under hypoxic conditions.136 Cyanine was selected as the fluorophore and the recognition group for O2˙−, 1-(3-nitrophenyl) ethanol replaced the previously used meta-nitrophenol as the recognition group for H2Sn. In the presence of O2˙−, HCy-ONO formed Cy-ONO with low fluorescence quantum yield. This intermediate could further react with H2Sn to release a cyanine fluorophore with an enhanced Stokes shift. Cell imaging experiments indicated that cells with intermittent hypoxia exhibited a higher fluorescence signal in channel 1 (750 to 850 nm), but the fluorescence signal in channel 2 (600 to 700 nm) was significantly lower than that for continuous hypoxic cells. In addition, the apoptotic rate of intermittent hypoxic cells was higher than that of persistent hypoxic cells. These results indicated that reoxygenation during intermittent hypoxia could induce O2˙− bursts and consume high levels of over-expressed H2Sn, which is the main contributor for oxidative damage of cells.
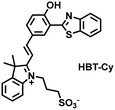
As an important antioxidant in organisms, SO2 plays an important role in regulating the redox balance. SO2 is produced by the oxidative decomposition of H2O2 and sulfur-containing amino acids in cells and exists as HSO3−.137 In order to clarify the complex role of H2O2/SO2 in regulating oxidative stress, Wang et al. designed a benzothiazole-based cyanine fluorescent probe HBT-Cy (Fig. 5(c)).138 The phenylthiophene and cyanine moieties resulted in the probe exhibiting dual emission bands at 450 nm and 590 nm under a single excitation of 390 nm. Interestingly, the fluorescence signal at 590 nm gradually disappears through the nucleophilic addition reaction between HBT-Cy and HSO3−, and the fluorescence signal was restored in the presence of H2O2. When HBT-Cy was co-cultured with human breast cancer cells, the cells exhibited bright red fluorescence and weak blue fluorescence. While the addition of an exogenous SO2 donor could increase the blue fluorescence and weaken the red fluorescence. This process was reversible with the changes of the dynamic levels of SO2 and H2O2 in the cells.
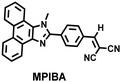
In addition to being an endogenous reducing agent, SO2 also has a certain oxidizing ability.139 In order to further understand the dual role of SO2 in oxidative stress, You et al. designed a dual response probe (MPIBA) for SO2/ClO− (Fig. 6(a)).140 MPIBA (red fluorescence, λem = 625 nm) was composed of a fluorophore modified with phenanthrimidazole and malononitrile, which could react with SO2/ClO− to produce compound DMPIA (blue fluorescence, λem = 410 nm) and MPIB (green fluorescence, λem = 500 nm). Interestingly, DMPIA could still be oxidized by HClO to produce MPIB (Fig. 6(a)). In addition, MPIBA also had ultra-fast response speed (SO2: <60 s; ClO−: within a few seconds) and high sensitivity (detection limit: SO2: 3.5 nM; ClO−: 12.5 nM). Finally, MPIBA was used to explore the dual role of SO2 in oxidative stress (Fig. 6(b–f)). Compared with the red fluorescence signal of the control group (Fig. 6(b) and (e)), bright blue fluorescence could be observed in HeLa cells treated with BTSA (an SO2 donor) (Fig. 6(c)). At the same time, an apoptosis rate of 6.5% indicated that excess SO2 results in cell oxidative damage. The cells treated with myeloperoxidase (MPO), BTSA, H2O2 and NaCl exhibited weak green fluorescence and weak blue fluorescence (Fig. 6(d)), indicating that SO2 could act as a reducing agent. Significantly, the cells treated with myeloperoxidase, H2O2 and NaCl exhibited only bright green fluorescence (Fig. 6(f)), but the apoptotic rate exceeded 45.8%, suggesting that ClO− could seriously damage the cells. These results confirmed the dual role of SO2 in cells under oxidative stress, where SO2 could exert both oxidative and anti-oxidative effects on cells.
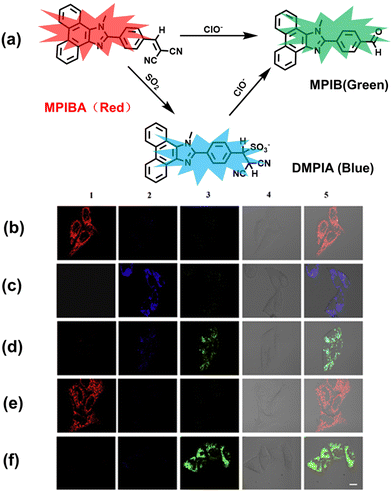 | ||
| Fig. 6 (a) MPIBA modified with phenanthrimidazole and malononitrile and reactions with ClO− and HSO3−. Confocal microscopic images were used to assess the dual role of intracellular SO2 in oxidative stress, HeLa cells were incubated with MPIBA (5 μM) for 30 min (b1–b5) and then incubated with 50 μM BTSA for 60 min (c1–c5). 0.01 Units of MPO, 100 μM H2O2 and 500 mM NaCl were added to cells and the mixture was further incubated for 1 h (d1–d5). HeLa cells were incubated with MPIBA (5 μM) for 30 min (e1–e5) and then with a media consisting of 0.01 Units of MPO, 100 μM H2O2 and 500 mM NaCl for 1 h (f1–f5). Reproduced with permission from ref. 140. Copyright (2017) Elsevier B.V. | ||
3.2 RNS + RSS
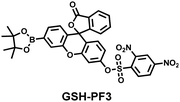
As the most abundant thiol in organisms, glutathione (GSH) exists in millimolar concentrations in most cells.141 While, ONOO− is a RNS with strong oxidizing ability, and high levels of expression are detrimental to cell health. To monitor the close relationship between ONOO− and GSH in cells, James et al. developed a fluorescent probe (GSH-PF3) using AND logic (Fig. 7(a)).142 Fluorescein was selected as the fluorophore, and 2,4-dinitrobenzenesulfonyl and the boronic ester were used as the specific sensing units for GSH and ONOO−, respectively. Since GSH-PF3 exhibited AND logic, there was no significant change in fluorescence intensity when ONOO− or GSH alone were present. However, when GSH-PF3 was exposed to both analytes, the fluorescence intensity increased significantly (40 times). Importantly, by co-cultivating the probe with macrophages stimulated under different conditions, it was found that cells co-stimulated with lipopolysaccharide (LPS, which mediates cellular production of RONS) and an appropriate amount of caffeic acid (CA, a drug that elicits endogenous GSH) displayed bright fluorescence (Fig. 7(c)). However, the fluorescence intensity for single-stimulated cells was significantly lower.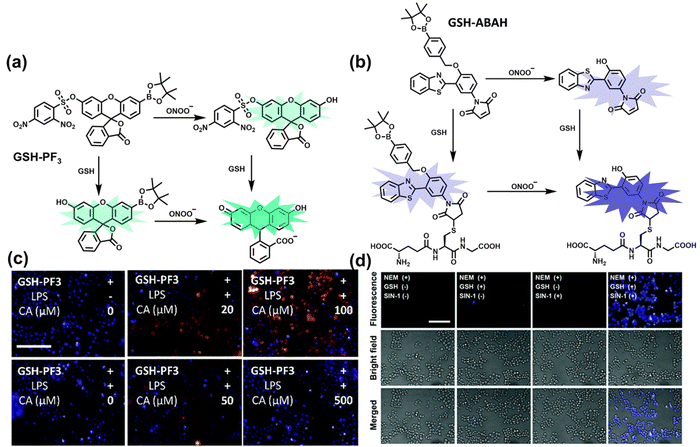 | ||
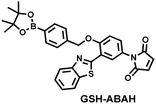
| Fig. 7 (a) and (b) Fluorescence turn ‘on’ mechanism of GSH-PF3 and GSH-ABAH in the presence of ONOO− and GSH. (c) Fluorescence imaging of RAW264.7 cells with GSH-PF3 in the absence and presence of LPS, which elicits ONOO− and increasing caffeic acid. Reproduced with permission from ref. 142. from the Royal Society of Chemistry. (d) Fluorescence imaging of RAW264.7 cells with GSH-ABAH in the presence of exogenously added GSH and/or SIN-1. Reproduced with permission from ref. 143. from the Royal Society of Chemistry. | ||
Probes based on excited state intramolecular proton transfer (ESIPT) exhibit the advantages of large Stokes shift, ratiometric fluorescence and environmental sensitivity, James et al. subsequently developed an (ESIPT)-based “AND” logic system probe (GSH-ABAH) for imaging thiols and ONOO− in cells (Fig. 7(b)).143 The probe consisted of three parts: (i) ESIPT-based fluorophore 4-amino-2-(benzo[d]thiazol-2-yl) phenol (ABAH); (ii) benzyl boronic ester was the recognition unit for ONOO− and blocked the ESIPT process; (iii) maleimide quenches the fluorescence by photoelectron transfer (PeT) and was selected as the GSH recognition unit. When co-cultured with macrophages supplemented with GSH or ONOO− alone, GSH-ABAH exhibited minimal fluorescence. However, when the cells were co-cultured with GSH and SIN-1 (ONOO−donor) a strong fluorescence response was observed (Fig. 7(d)).
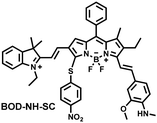
NO and H2S are two vital gas signaling molecules in mammals, and the interplay between them is of great significance for understanding the pathological process of oxidative stress. Zhao et al. designed an activatable NIR-II fluorescent probe (BOD-NH-SC), which was used to visualize the intracellular dynamics of cellular NO and H2S (Fig. 8).144 BOD-NH-SC contained three parts: (i) N-methyl-2-methoxyaniline moiety as the NO-recognition site; (ii) 4-nitrobenzenethiol as H2S-recognition site; (iii) boron dipyrromethene (BODIPY) was selected as the fluorophore. In the presence of NO, the N-nitroso product (BOD-NO-SC) generated a 214-fold increase in fluorescence at 655 nm. The product formed by the introduction of H2S (BOD-NO-SH) resulted in bright NIR-II fluorescence at 936 nm, accompanied by fluorescence quenching at 655 nm. The process could be cycled, so it could accurately monitor the dynamics of NO and H2S (Fig. 8(b)). Furthermore, the ability of BOD-NH-SC to detect endogenous NO/H2S in cells was evaluated. The presence of endogenous NO in macrophages caused BOD-NH-SC to exhibit bright red fluorescence, while the NIR-II fluorescence was weak. However, after incubating with fluvastatin-stimulated macrophages (fluvastatin can stimulate cells to produce endogenous H2S), the fluorescence signal from the NIR-II channel increased by 16.2 times compared with the untreated cells.
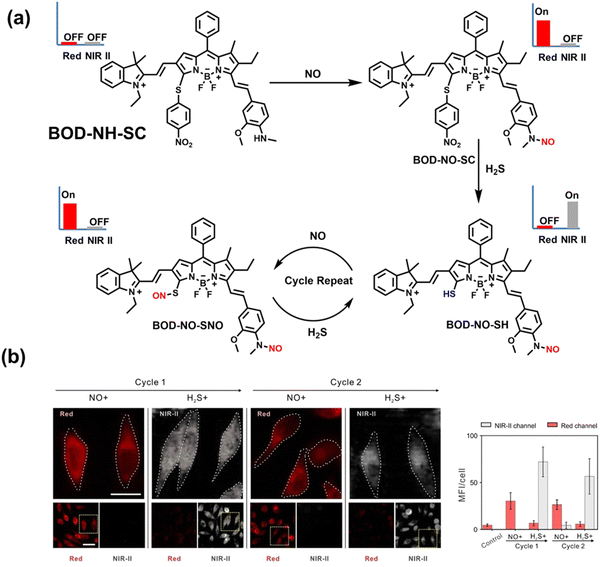 | ||
| Fig. 8 (a) The mechanism for reversible detection of NO and H2S by BOD-NH-SC. (b) Using BOD-NH-SC to alternately image NO and H2S in HepG2 cell. Reproduced with permission from ref. 144. Copyright (2021) Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
3.3 RONS + related species
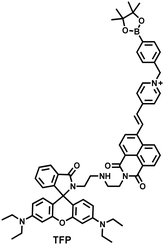
As the energy factory of life, oxidative stress and energy metabolism in mitochondria are vital biological events. Adenosine triphosphate is closely related to various physiological and pathological processes (apoptosis or necrosis).145 Tian et al. reported a two-photon fluorescence-lifetime-based probe (TFP) (Fig. 9(a)), which could simultaneously measure H2O2 and ATP levels in mitochondria.146 In order to achieve dual fluorescence channel detection, rhodamine (λex = 710 nm, λem = 550–650 nm) and naphthalimide derivatives (λex = 710 nm, λem = 430–530 nm) were selected as the fluorophores. A benzyl boronic ester and diethylenetriamine were used as the recognition units for H2O2 and ATP, respectively. Using fluorescence lifetime imaging, TFP exhibited a good linear relationship and selectivity for the detection of H2O2 (LOD = 68 ± 5 nM) and ATP (LOD = 33 ± 2 μM). With its excellent imaging capabilities, TFP was used to visualize the dynamic changes of H2O2 and ATP in neuronal mitochondria under different conditions (Fig. 9(b and c)). After being stimulated by O2˙− for a short period of time (8 min), the level of H2O2 in neurons increased about 4-fold, while ATP decreased to 86% of the initial level. After replacing with new culture medium, ATP and H2O2 in a parallel group returned to the initial levels within 16 min. However, long-term (50 minutes) O2˙− (100 μM) stimulation can cause permanent oxidative damage and energy deficiency in neurons. Importantly, after 50 min of H2O2 (100 μM) stimulation, the levels of mitochondrial H2O2 and ATP still exhibited recovery. These results were ascribed to the fact that exogenous H2O2 and O2˙− exhibit varying degrees of impact on mitochondrial function, whereas O2˙− displayed a more serious and negative impact.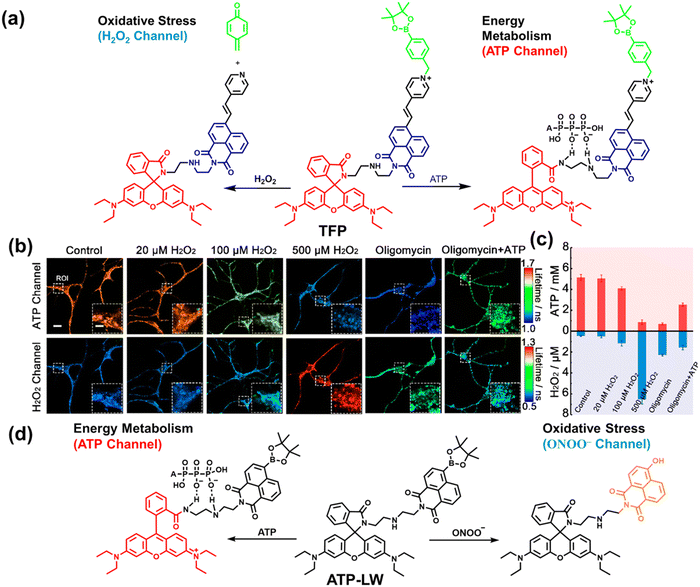 | ||
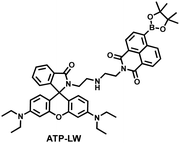
| Fig. 9 (a) Fluorescence turn ‘on’ mechanism of TFP in the presence of H2O2/ATP. (b) Fluorescence lifetime images of neurons with the addition of H2O2 (20, 100, and 500 μM) and oligomycin (An ATP synthase inhibitor) in the channels of H2O2 and ATP. (c) Summarized data for mitochondrial H2O2 and ATP changes in neurons toward different treatments. Reproduced with permission from ref. 146. Copyright (2020) American Chemical Society. (d) Fluorescence turn ‘on’ mechanism of ATP-LW in the presence of ONOO−/ATP. | ||
Recently, based on rhodamine and 1,8-naphthalimide fluorophores, James et al. reported a probe (ATP-LW) that can simultaneously monitor changes in the levels of ATP and ONOO− in cells (Fig. 9(d)).147 In the presence of ONOO−, the boronic ester was oxidatively removed to form 4-hydroxy-1,8-naphthalimide product NA-OH; in the presence of ATP, hydrogen bonding induced the opening of the spironolactone of rhodamine (Rh-Bpin). Due to the differences in emission between the two products, ATP-LW facilitated monitoring of the dynamic levels of ONOO− and ATP in the green (λex = 488 nm, λem = 500–575 nm) and red (λex = 514 nm, λem = 575–650 nm) channels, respectively. Moreover, ATP-LW was used to successfully visualize oxidative stress induced by oligomycin A (an ATP synthase inhibitor) in hepatocytes and an increase of ONOO− levels and reduction of ATP during APAP-induced hepatotoxicity. This research not only provided a general molecular design strategy for multi-species imaging, but also illustrated that mitochondrial oxidative stress was closely related to energy metabolism.
Simultaneous, and sequential detection of multiple analytes by a single fluorescent probe is an important and hot area for development. At present, there are relatively few dual-responsive probes. Almost all of them are based on cyanine, rhodamine, fluorescein and coumarin as the fluorophore skeleton, with RONSS recognition groups attached. Real-time monitoring of multiple target analytes in different disease models or designated areas of organisms is an important direction for development of this type of fluorescent probe in the future. For example, the simultaneous detection of Aβ protein/Tau protein and RONSS in the brain is beneficial for the early diagnosis of neurodegenerative diseases; the simultaneous detection of enzymes and RONSS is beneficial for the diagnosis of tumors and inflammation, as well as pathological research; different RONSS assays in liver/kidney are beneficial for evaluating drug-induced organ damage and pathological studies of related diseases. Therefore, such multi-species assays may help improve the clinical value of fluorescent probes (Table 2).
| Ref. | Chemical structure | Analyte | Response time | Detection limit (μM) | λ ex/λem (nm) | Application in cells |
|---|---|---|---|---|---|---|
| 133 |
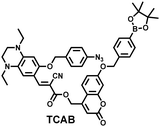
|
H2S | 80 min | 0.058 | 325/413, 475/627 | Monitor dynamic H2O2 and H2S redox processes in living cells |
| H2O2 | 120 min | 0.044 | 325; 486/413 | |||
| 135 |
|
O2˙− | 10 min | 0.05 | 730/780 | Monitor dynamic H2O2 and H2Sn redox processes in living cells |
| H2Sn | 10 min | 0.08 μ | 730/770 | |||
| 136 |
|
O2˙− | 150 s | 0.09 | 730/785 | Detection H2O2 and H2Sn in living cell models under continuous hypoxic and intermittent hypoxic conditions (IRI), |
| H2Sn | 5 min | 0.1 | 500/635 | |||
| 138 |
|
HSO3− | 15 min | 0.34 | 520/590 | Reversible monitoring of changes in dynamic levels of SO2 and H2O2 in cells. |
| H2O2 | 60 min | — | 520/590 | |||
| 140 |
|
SO2 | 60 s; | 0.035 | 440/625 | Explore the dual role of SO2 in cells (oxidative stress) |
| 363/410 | ||||||
| 142 |
|
ClO− | Seconds | 0.125 | 373/500 | Monitor the co-existence of metabolically produced ONOO− and GSH living cells |
| ONOO− | — | — | 488/512 | |||
| GSH | 5–10 min | — | 488/512 | |||
| 143 |
|
ONOO− | 30 s | — | 390/451 | Monitor the co-existence of metabolically produced ONOO− and GSH living cells |
| GSH | 30 s | — | 390/451 | |||
| 144 |
|
NO | 5 min | 0.031 | 570/655 | Reversible monitoring of changes in dynamic levels of NO and H2S in cells. |
| H2S | 10 min | 0.02 | 806/936 | |||
| 146 |
|
H2O2 | 8 min | 0.068 ± 0.005 | 380/470 | Visualization of the dynamic level changes of mitochondrial H2O2 and ATP induced by the superoxide anion (O2˙−). |
| TP710/470 | ||||||
| ATP | 2 min | 33 ± 2 | 562/590 | |||
| TP710/590 | ||||||
| 147 |
|
450/562 | Visualize an increase of ONOO− levels and depletion of ATP in cells during APAP-induced hepatotoxicity | |||
| ONOO− | 1 min | 0.026 | 488/568 | |||
| ATP | 100 min | 63 | 520/587 |
4. Fluorescent probes for imaging inflammation and organ oxidative damage
When subjected to external stimuli, an organism protectively initiates some physiological reactions related to oxidative stress. Usually these physiological processes are beneficial, but continuous high levels of RONSS stimulation can induce a variety of diseases. Given that inflammation and oxidative damage of organs are common physiological pathological processes, here we introduce some representative fluorescent probes for RONSS imaging of inflammation and organ injury induced by different conditions.4.1 Inflammation
Inflammation is a nonspecific immune reaction when the body suffers any type of injury.148 Inflammation cannot be regarded as a disease, but as a special biological process.149 According to the way induced, inflammation can be divided into two types: infectious inflammation and aseptic inflammation. Infectious inflammation is mainly caused by the invasion of microorganisms such as bacteria, fungi, and viruses, while aseptic inflammation is related to chronic diseases, trauma, radiation, and chemical damage.150,151 Importantly, both types of inflammation are closely related to oxidative stress.152 When damage is detected, a cascade of signals lead to the recruitment of inflammatory cells, such as neutrophils and macrophages. These cells will produce RONS, proteases and growth factors leading to tissue destruction, fibroblast proliferation and fibrosis.153 However, long-term exposure to high levels of oxidants inevitably causes oxidative damage to normal tissues and cells (such as protein oxidation and lipid peroxidation), and even cause chronic inflammatory diseases, such as cancer, diabetes, stroke, atherosclerosis and so on.154–159 Therefore, monitoring and understanding inflammation can help us better understand these diseases and develop better treatments. As a direct result, a significant number of fluorescent probes are being explored for the real-time imaging of oxidative stress in various inflammation models.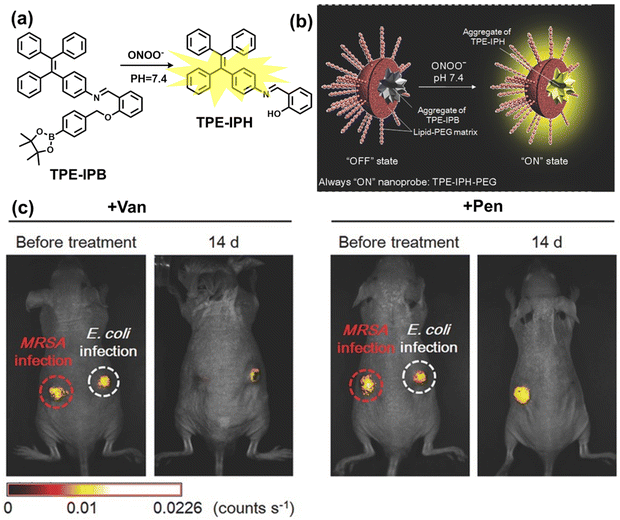 | ||
| Fig. 10 (a) Fluorescence turn ‘on’ mechanism of TPE–IPB towards ONOO−. (b) Schematic illustration of TPE–IPB–PEG and the performance after incubation with ONOO−. (c) In vivo fluorescence images of both MRSA and E. coli-infected mice before and after antibiotic treatment for 14 days. Reproduced with permission from ref. 160. Copyright (2016) Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
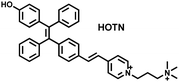
Wang et al. reported a fluorescent probe (PAM–BN–PB) for the rapid imaging of H2O2 in mice with peritonitis (Fig. 11).161 PAM–BN–PB consists of three parts: phenanthroimidazole, benzonitrile and benzyl boronic ester (Fig. 11(a)). The strong electron withdrawing effect of benzonitrile accelerated the nucleophilic reaction of the phenyl borate with H2O2. Compared with the usual probes for detecting H2O2, PAM–BN–PB could shorten the response time to within 10 minutes. Importantly, by using this probe in a rotenone-induced peritonitis model, the fluorescence intensity of the mouse abdomen was significantly enhanced, which indicated a positive correlation between peritonitis and H2O2 (Fig. 11(b)). Furthermore, Wang et al. reported a tetrastyrene-based AIE fluorescent probe (HOTN) (Fig. 11(c)), which was used to visualize HClO signals in mouse models for peritonitis, arthritis, and liver cancer.162 By attaching strong hydrophilic groups to the TPE backbone, the fluorescence intensity was almost completely quenched in an aqueous environment. In the presence of HClO, the pyridine salt and quaternary ammonium salt side chain undergoes oxidative cleavage to form a stable aldehyde group, the increase in hydrophobicity and the intermolecular hydrogen bond between the hydroxyl group and the aldehyde group significantly enhanced the AIE effect of HOTN, resulting in a significant increase in the fluorescence intensity (>1000 times). HOTN exhibited low detection limit (0.108 μM) and short response time (within a few seconds). Finally, this study successfully highlighted the use of fluorescent probes for monitoring the abdomen of peritonitis mice and the joints of the legs of arthritic mice (Fig. 11(d)).
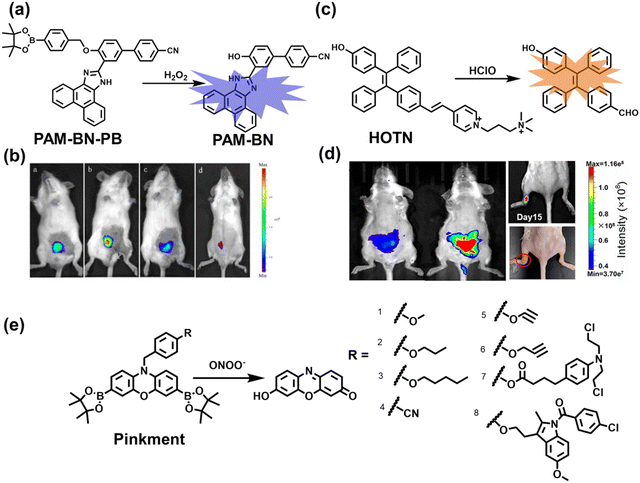 | ||
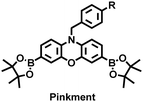
| Fig. 11 (a) Structures of PAM–BN–PB and sensing mechanism for discrimination of H2O2. (b) PAM–BN–PB was used for fluorescence imaging of rotenone-induced peritonitis in mice. Reproduced with permission from ref. 161. Copyright (2017) American Chemical Society. (c) Structures of HOTN and sensing mechanism for discrimination of ClO−. (d) Fluorescence imaging of HOTN in peritonitis and arthritis. Reproduced with permission from ref. 162. Copyright (2020) American Chemical Society. (e) Structures of Pinkment and sensing mechanism for discrimination of ONOO−. | ||
James et al. developed a fluorescent platform called Pinkment for the visualization of ONOO− in a LPS-induced peritonitis mouse model and the evaluation of the therapeutic effect of indomethacin.163 Resorufin was chosen as the fluorophore due to its red-shift fluorescence and the easy functionalization of the scaffold, while a boronic ester served as the ONOO− recognition group (Fig. 11(e)). Importantly, the benzyl unit could be functionalized with different functional units without affecting the selectivity towards ROS. Eight kinds of fluorescent probes with different substituents were prepared using this platform, all of which exhibited high selectivity and sensitivity for ONOO−. Among them, Pinkment-2, 5, and 6 (R = 2, 5, 6), were used to image exogenous and endogenous ONOO− in HeLa and RAW 264.7 macrophages. Significantly, the fluorescence intensity of Pinkment-8 (R = 8) conjugated with the indomethacin in inflammatory macrophages was significantly lower than the control group, indicating that this probe could reduce the inflammation by releasing indomethacin. In addition, the fluorescence intensity of alkyne-functionalized Pinkment-6 (R = 6) in an acute inflammation mouse model was significantly higher than that of healthy mice, which provided direct evidence for a link between acute inflammation and ONOO−.
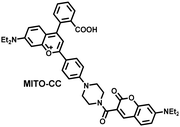
Ratiometric probes designed based on fluorescence resonance energy transfer (FRET) are ideal tools for the quantitative detection of biomolecules. The two emission bands facilitate calibration, and reduces false results caused by the dose, environment, and excitation intensity.164 Chang et al. reported a two-photon ratiometric fluorescence probe (MITO-CC), for the ratiometric detection of ONOO− in cells and for inflammation mouse models (Fig. 12(a)).165 MITO-CC was composed of two parts: a rhodamine derivative with good selectivity for ONOO− was selected as the energy acceptor, while coumarin with excellent optical properties was selected as the energy donor. When ONOO− was present, the emission peak of MITO-CC centered at 651 nm almost disappeared, while the emission peak at 451 nm was significantly enhanced. In addition, MITO-CC exhibited low detection limit (11.30 nM), rapid response time (within 20 s), good biocompatibility and excellent mitochondrial targeting ability. As such, MITO-CC was used for the ratiometric visualization of changes in the levels of ONOO− in LPS/IFN-γ-stimulated cells, living liver tissues, and inflammation mouse models (Fig. 12(b)).
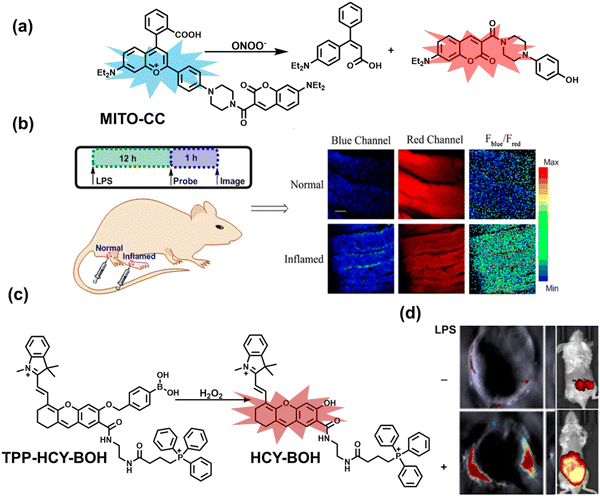 | ||
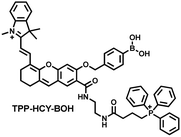
| Fig. 12 (a) Fluorescence turn ‘on’ mechanism of MITO-CC in the presence of ONOO−. (b) Fluorescence imaging of MITO-CC in mouse arthritis tissue and healthy tissue respectively. Reproduced with permission from ref. 165. Copyright (2017) American Chemical Society. (c) Fluorescence turn ‘on’ mechanism of TPP–HCY–BOH in the presence of H2O2. (d) TPP–HCY–BOH was used for photoacoustic and fluorescence imaging of peritonitis mice and healthy mice. Reproduced with permission from ref. 166. Copyright (2020) American Chemical Society. | ||
Although fluorescence imaging exhibits high sensitivity and contrast, most non-NIR fluorescent probes are unsuitable for in vivo imaging due to the limitation of the excitation and emission wavelengths, the depth of tissue penetration and the spatial resolution. Therefore, dual-modal probes with fluorescence and photoacoustic imaging capabilities can combine the high spatiotemporal resolution of ultrasound with the high contrast of optical imaging. Hai et al. reported a mitochondrial targeting probe (TPP–HCY–BOH), which was successfully used for fluorescence/photoacoustic (FL/PA) dual-mode imaging of H2O2 overproduction in vivo during inflammation.166 TPP–HCY–BOH was composed of three parts: (i) NIR dye HCY with excellent fluorescence and photoacoustic properties as the fluorophore; (ii) benzyl boronic acid the recognition group for H2O2; (iii) positively charged triphenylphosphine (TPP) as the mitochondrial targeting group (Fig. 12(c)). TPP–HCY–BOH exhibited a low limit of detection (LOD = 0.348 μM), good biocompatibility and excellent mitochondrial targeting (colocalization coefficient = 0.91). TPP–HCY–BOH was used to identify excess H2O2 produced in mitochondria and achieved a 2.4-fold increase in fluorescence signal and a 4.7-fold increase in photoacoustic signal. Moreover, TPP–HCY–BOH was used for FL/PA dual-modal imaging of H2O2 in LPS-induced acute abdominal inflammation of mice. Compared with the control group, the fluorescence signal was increased by 5.2-fold, while the photoacoustic signal was increased by 7.1-fold (Fig. 12(d)).
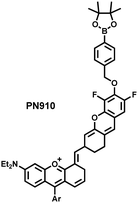
Additionally, due to the advantages of minimal background fluorescence and deep tissue penetration depth, fluorescence imaging in the second near-infrared window (NIR-II, 900–1700 nm) is a strong candidate for future diagnosis of pathological inflammation in the clinic. Zhang et al. recently reported a dual-activatable fluorescent probe (PN910) in the NIR-II window, which could respond to H2O2 and ONOO− with high selectivity in an alkaline inflammatory environment (Fig. 13).167 A series of hydroxyl-containing merocyanines with NIR-II fluorescence were prepared, which were named chrodol 1–3 according to the number of ortho-fluorines (0, 1, 2). Amongst them, chrodol 3 was selected as the fluorophore due to exhibiting the highest fluorescences quantum yield (0.34%) and excellent pKa (6.40). A benzyl boronic ester was used as the recognition group for H2O2/ONOO− (Fig. 13(a)). The probe could rapidly and selectively respond to low levels of H2O2 and ONOO− in an alkaline environment (pH > 7.4). During the real-time imaging of cystitis, PN910 exhibited a 1.5-fold increase in fluorescence signal in a physiological environment (pH = 8.0) (Fig. 13(b)). During the imaging of enteritis, the fluorescence signal in the colon of mice with acute colitis was almost 10 times higher than that of the control group (Fig. 13(c–e)). This research provides a unique method for real-time, high-contrast and non-invasive monitoring of RONS in an alkaline inflammatory environment in vivo.
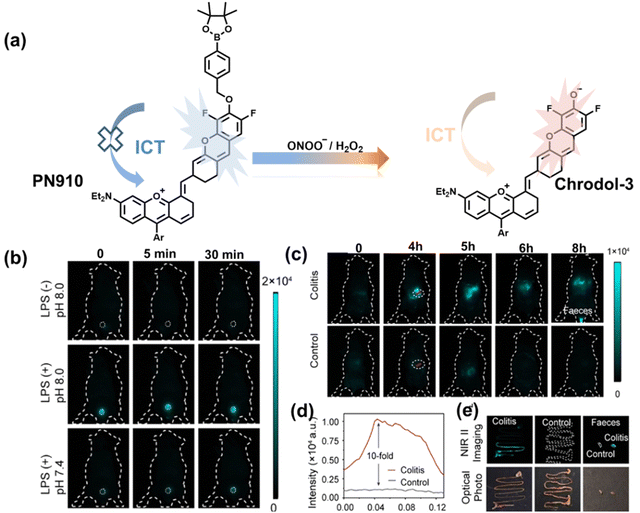 | ||
| Fig. 13 (a) Fluorescence turn ‘on’ mechanism of PN910 in an alkaline environment in the presence of H2O2/ONOO−. (b) NIR-II fluorescence imaging of H2O2/ONOO− in cystitis mice at different times and under different conditions. (c) NIR-II fluorescence imaging of H2O2/ONOO− in colitis mice at different times and under different conditions. (d) The fluorescence intensity profiles (red line RoI) of colitis mice and healthy mice at 4 h. (e) In vitro NIR II fluorescence images and optical photographs of colons and feces from colitis mice and healthy mice. Reproduced with permission from ref. 167. Copyright (2021) Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
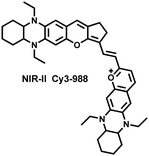
Yuan et al. recently reported a new class of trimethine skeleton NIR-II fluorophores (NIR-II Cy3s), which could serve as an effective platform for the reversible monitoring of the HClO/RSS-mediated redox process in a pathophysiology environment (Fig. 14a).168 NIR-II Cy3-988, modified with the 1,4-diethyl-decahydroquinoxaline group at both the donor and acceptor ends, exhibited the longest excitation and emission wavelengths (λex/λem = 988/1058 nm). In addition, the electron-rich nature of the 1,4-diethyl-decahydroquinoxaline group provides a reversible oxidation site within the probe. In the presence of HClO, the maximum absorption and emission peaks of NIR-II Cy3-988 are significantly reduced, while a new blue-shifted absorption band appeared at 824 nm. In the presence of H2S, the absorption (980 nm) and fluorescence signal (1040 nm) of NIR-II Cy3-988 was recovered. This process can be effectively cycled 4 times and was demonstrated by high resolution mass spectrometry (Fig. 14b). The researchers further used NIR-II Cy3-988 to assess oxidative stress and the therapeutic effects of three drugs (aspirin, ibuprofen and diclofenac) in a model of acute inflammation (Fig. 14c and d). Compared to the left leg of mouse (healthy), the fluorescence intensity of the right hind leg (carrageenan-induced acute inflammatory model) experienced a rapid decrease over the first 30 min (Fig. 14e–h). This result indicated the presence of high levels of HClO in areas of acute inflammation. Notably, diclofenac exhibited the best anti-inflammatory effect, significantly restoring the carrageenan-induced reduction in NIR-II fluorescence signal. A plausible explanation was that diclofenac rapidly inhibited the recruitment of neutrophils to reduce myeloperoxidase (MPO) and macrophage accumulation.
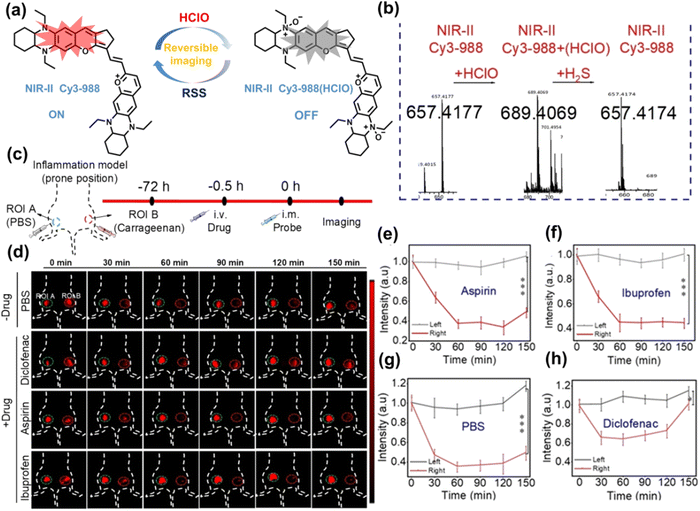 | ||
| Fig. 14 (a) The mechanism for reversible detection of HClO and H2S by NIR-II Cy3-988; (b) the reversible mechanism of NIR-II Cy3-988 for HClO and H2S was investigated by high-resolution mass spectrometry. (c) Schematic of fluorescence imaging of carrageenan-induced acute inflammation and drug-mediated repair using NIR-II Cy3-988. (d) After treatment with PBS, diclofenac, aspirin, or ibuprofen, NIR-II fluorescence imaging of hind legs is performed at representative time points of carrageenan-induced acute inflammatory models. The left hind leg is the control group and the right hind leg is the experimental group. (e–h) After treatment with aspirin (e), ibuprofen (f), PBS (g) diclofenac (h), the fluorescence intensity of NIR-II Cy3-988 in the acute inflammatory model was at representative time points. Reproduced with permission from ref. 168. Copyright (2022) Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
As a common pathological and physiological process, real-time monitoring of oxidative stress induced by inflammation is of great significance for investigating the pathology and early diagnosis of related diseases. Considering possible clinical needs in the future, we believe that NIR-II fluorescent probes, targeting specific areas or organs, drug safety evaluation, and application for more types of inflammation models are areas requiring additional research.
4.2 Organ injury
Oxidative stress can activate intracellular signals and destroy a variety of active components, thereby inducing cell over differentiation and apoptosis leading to organ dysfunction.169 Ischemia/Reperfusion Injury (IRI) and drug-induced organ injury are closely related to oxidative stress.170,171 IRI is a common clinical complication, which is caused by long-term ischemic injury of vital organs and secondary injury induced by blood perfusion recovery of ischemic organs.172 Although the pathogenesis of IRI includes many factors, IRI-induced bursts of reactive oxygen species in the mitochondria play a key role in destroying cellular components and triggering cell death.170 Clinical drugs for various diseases, including acetaminophen (APAP), cis-platinum (cis-diamminedichloroplatinum), and anthracyclines, have been shown to induce oxidative damage in different organs.171 In order to evaluate the side effects of IRI and drugs on organs, the detection of alanine aminotransferase in the blood and positron emission computed tomography (PET) imaging are commonly used.173,174 Compared with these methods, fluorescence imaging as a safe, non-invasive and sensitive imaging method can achieve precise imaging of specific oxidants and reductants at the injured site through the use of appropriately designed probes, therefore providing a new and effective technology to evaluate the drug toxicity and recovery after surgery. As such, many fluorescent probes are being developed to study IRI and drug-induced organ oxidative damage.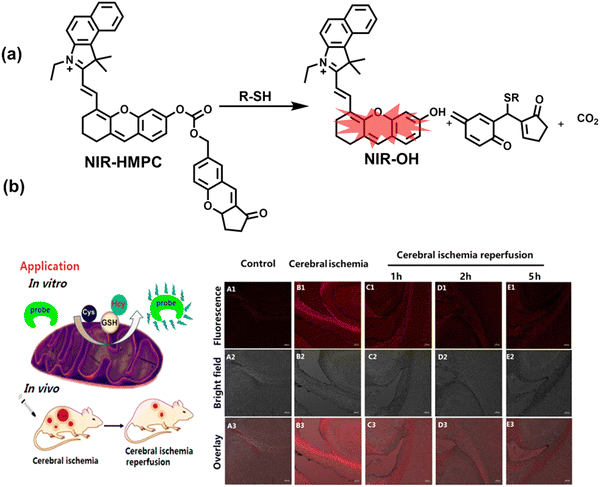 | ||
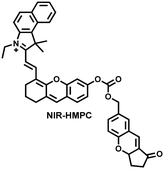
| Fig. 15 (a) Fluorescence turn ‘on’ mechanism of NIR-HMPC in the presence of thiols. (b) NIR-HMPC was used for fluorescence imaging of endogenous thiols in cerebral ischemia (2 h) and reperfusion brain tissue slices at different times (1–5 h). Reproduced with permission from ref. 176. Copyright (2020) American Chemical Society. | ||
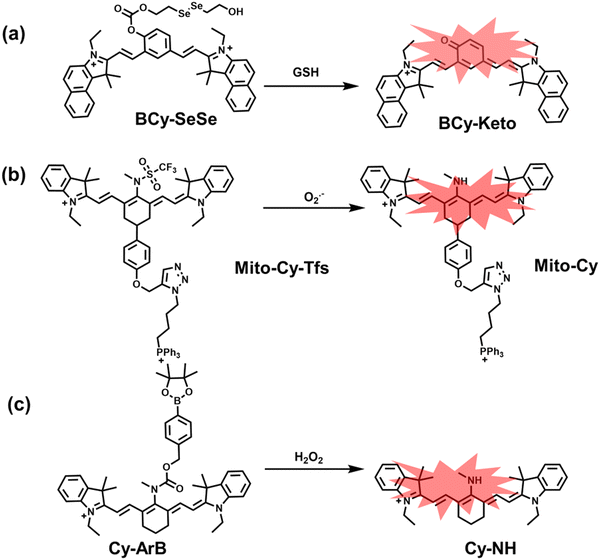
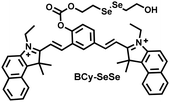
Glutathione is the most abundant thiol in cells. Significant reduction of GSH levels can lead to severe mitochondrial damage and induce a variety of diseases.177 Chen et al. designed and synthesized a novel NIR fluorescent probe (BCy-SeSe) based on BCy-Keto fluorophore and GSH-specific diselenide or disulfide recognition group (Fig. 16(a)).178 As the perfusion time was extended, the fluorescence intensity of BCy-SeSe in cells and (adult/aging) mouse brains decreased significantly. Similarly, the activity of GSH peroxidase in the brain was also reduced. Low levels of glutathione were positively correlated with aggravation of apoptosis and cerebral infarction, these observations were more significant in the aged group.
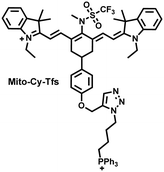
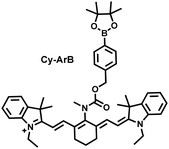
I/R injury often occurs in liver surgery and has become one of the important factors affecting the success rate of surgery and postoperative survival.179 In order to explore the relationship between liver I/R injury and O2˙− concentrations, Chen et al. developed a NIR fluorescent probe (Mito-Cy-Tfs) based on heptamethine cyanine fluorophore and trifluoromethanesulfonamide recognition group (Fig. 16(b)).180 Using the co-incubation of Mito-Cy-Tfs with cells subjected to different perfusion methods, it was found that compared with glucose or serum deprivation/reperfusion, hypoxia/reperfusion was the main factor that induces apoptosis. When Mito-Cy-Tfs was used to image O2˙− in a liver model of I/R mice, a significant increase in the fluorescence signal indicated that I/R could induce bursts of O2˙− in the liver and severe liver tissue damage. The fluorescence intensity in the liver region was significantly reduced after ischemic preconditioning (IPC) and postconditioning (IPTC), suggesting that these measures can protect the liver from oxidative damage. In addition to O2˙−, H2O2 is considered as an important oxidative stress marker in liver I/R. As such Chen et al. developed a NIR fluorescent probe (Cy-ArB) based on heptamethine cyanine fluorophore and benzyl boronic ester (Fig. 16(c)) and used the probe to image the changes in H2O2 levels in the mitochondria and mouse liver during I/R.181 Confocal imaging indicated that the fluorescence intensity of the cells in the glucose–serum–oxygen deprivation/reperfusion group was significantly higher than that of the control group. A significant increase in the rate of cell apoptosis indicated that the I/R process induces an increase in the level of H2O2, leading to cell oxidative damage. In addition, the fluorescence signal of the liver of I/R mice increases with the time of ischemia (0.5–2.5 h) and the perfusion time (3–6 h). Confirming that the process of liver ischemia and reperfusion in mice can induce H2O2 bursts and organ oxidative damage.
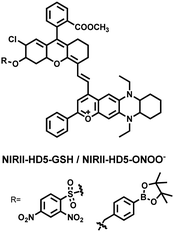
To achieve high-contrast real-time imaging of DILI in vivo, Yuan et al. developed an efficient activatable NIR-II platform.185 Compared with the previously reported hemicyanine dye O-HD, a 1,4-diethyl-decahydroquinoxaline (DQ) benzopyran group with electron-rich characteristics replaces the original indole heterocycle; the introduction of a chlorine atom at the ortho-position of the hydroxyl group reduces the pKa and the benzoic acid or methyl benzoate at the 9-position of the xanthene ring improves the stability of the fluorophore. Amongst the NIRII-HDs platforms prepared, NIRII-HD5 was selected for in vivo imaging due to its high molar absorptivity, excellent quantum yield (0.28%) and pKa (6.5) (Fig. 17(a)). Two NIR-II probes (NIRII-HD5-ONOO− and NIRII-HD5-GSH) responsive to ONOO− and GSH were developed by introducing benzyl boronic ester and 2,4-dinitrobenzenesulfonyl recognition groups at the hydroxyl position (Fig. 17(a)). In the liver of mice injected with excess APAP, the fluorescence signal of NIRII-HD5-ONOO− achieved a 2-fold enhancement, while the fluorescence signal of NIRII-HD5-GSH was only 60% of the control group (Fig. 17(b) and (c)). In addition, when mice were injected with excessive APAP and hepatoprotective drug N-acetylcysteine (NAC) at the same time, the fluorescence signals of the two probes in the liver were almost restored to the level of the control group (Fig. 17(c)). These results indicated that excessive use of APAP induces abnormal levels of ONOO−and depletion of GSH in the liver, while NAC can effectively improve DILI. This research provided a versatile NIR-II fluorescence platform for real-time imaging of DILI.
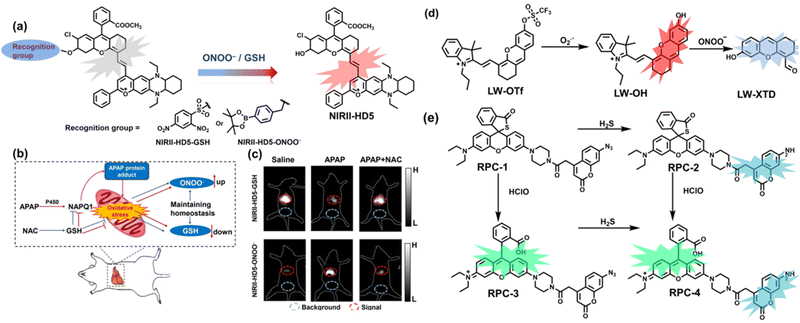 | ||
| Fig. 17 (a) Based on the NIRII-HD5 structure, two probes were designed for ONOO− and GSH detection, respectively. (b) Schematic illustration of APAP-induced liver injury and treatment with N-acetyl cysteine (NAC). (c) NIR-II fluorescence imaging of NIRII-HD5-GSH and NIRII-HD5-ONOO− in mouse liver regions under different stimulation conditions. (d) Structures of LW-OTf and sensing mechanism for discrimination of O2˙− and ONOO−. (e) Structures of RPC-1 and sensing mechanism for discrimination of HClO and H2S. Reproduced with permission from ref. 185. Copyright (2022) Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
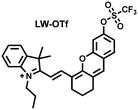
Considering that DILI is often involved in abnormally elevated levels of ROS and RNS oxidants, James et al. developed LW-OTf for monitoring the dynamic levels of O2˙− and ONOO− in the liver of DILI mice.186 LW-OTf consists of two parts: (i) hemicyanine-based NIR emitting fluorophore LW-OH; (ii) O2˙− reactive trifluoromethylsulfonyl (triflyl, Tf) recognition group. In the presence of O2˙−, the triflyl group was deprotected to release the NIR fluorophore LW-OH (Fig. 17(d)). Subsequently, ONOO− could be added to remove the indole heterocycle and formed the fluorophore LW-XTD which exhibited two-photon properties. Bright red and blue fluorescence could be observed in cells stimulated by cisplatin and APAP, respectively. In the liver area of DILI mice, both near-infrared fluorescence signals and two-photon fluorescence signals increased with an increase in the dose of APAP, which indicated that an overdose of APAP can cause abnormally high levels of ROS and RNS in the liver area. This study provided direct evidence for a positive correlation between APAP-induced DILI and oxidative stress.
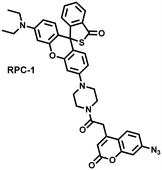
Unlike the low hepatotoxicity induced by a single administration, the wrong combination of some drugs can significantly increase liver injury. Tang et al. developed a two-photon fluorescent probe (RPC-1) that can simultaneously image HClO and H2S then evaluated the liver damage and relief caused by duloxetine and fluoxetine (antidepressants).187 RPC-1 consists of two parts: (i) thiorhodamine (λem = 580 nm) that specifically responds to HClO; (ii) 7-aminocoumarin with an azide group that specifically responds to H2S (λem = 445 nm) (Fig. 17(e)). Using RPC-1 to image cells and mouse liver regions, the use of a single drug (duloxetine or fluoxetine) resulted in a slight increase in fluorescence signal and did not cause significant liver damage. However, a combination of the two drugs (duloxetine and fluoxetine) could significantly increase the fluorescence intensity (λem = 580 nm) of the green channel, which indicated a significant up-regulation of HClO levels and significant liver injury. Furthermore, N-acetylcysteine (NAC) pretreatment could significantly increase the fluorescence intensity of the blue channel (λem = 445 nm) and decrease the fluorescence intensity of green channel and mouse liver area. This result indicated that NAC pretreatment could lead to an increase of endogenous H2S levels to alleviate liver injury.
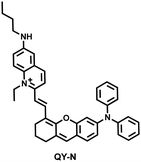
Herbs are widely used in health care and the treatment of chronic diseases, but excessive or incorrect use may cause severe liver injury.188 The overexpression of NO is closely related to liver inflammation caused by liver injury. Wu et al. designed a dual-modal activatable probe (QY-N) to detect triptolide (TP)-induced liver injury using photoacoustic imaging and NIR-II fluorescence imaging.189 QY-N was composed of three parts: (i) bismethoxyphenyl-amine-containing dihydroxanthene was selected as the electron donor; (ii) a quinolinium acted as an electron acceptor; (iii) butylamine acted as a NO recognition group and fluorescence quencher (Fig. 18(a)). When NO was present in the liver, QY-NO the nitrosation product of the imine, exhibited a red-shift in the absorption band (700–850 nm) for photoacoustic imaging and a strong emission (910–1110 nm) for NIR-II fluorescence imaging. Notably, the NIR-II fluorescence of QY-N was significantly enhanced in triptolide-induced liver injured mice and was positively correlated with the dosage of the drug (Fig. 18(b)). The spatio-temporal information provided by three-dimensional multispectral optoacoustic tomography (MSOT) images was able to size and locate the liver injury. In addition, after continuous use of the therapeutic drug N-acetylcysteine (NAC) (3 days, 6 days and 9 days), the fluorescence and photoacoustic signals in the liver area of the mice was significantly reduced (Fig. 18(c and d)).
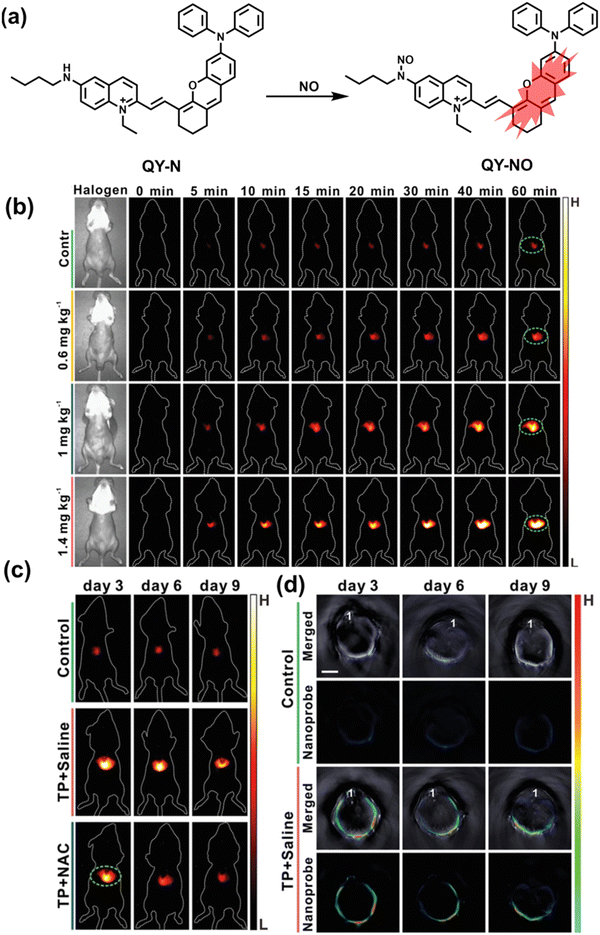 | ||
| Fig. 18 (a) Structures of QY-N and sensing mechanism for discrimination of NO. (b) Representative NIR-II fluorescence images at different time points for DILI mice from different groups. (c) Representative NIR-II fluorescent images of the mice from the control group, the “TP + Saline” group and the “TP + NAC” group at 3 days, 6 days and 9 days. (d) Representative cross-sectional MSOT images of the livers from the different groups of mice at 3 days, 6 days and 9 days. Reproduced with permission from ref. 189. Copyright (2021) Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
The kidney plays a vital role in removing wastes and toxins from the body. However, the kidney often suffers from drug induced injury. At present, the commonly used clinical methods for monitoring acute kidney injury (AKI), such as blood tests (serum creatinine (CREA) and blood urea nitrogen (BUN)), have problems of low sensitivity and are slow.190,191 As such, the emergence of FL/PA dual-modality imaging probes provides an effective way to solve these problems.
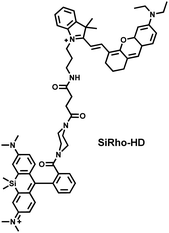
Cisplatin is a widely used anticancer drug. Tan et al. designed and synthesized a mitochondrial-targeted FL/PA dual-modal probe (SiRho-HD) based on hemicyanine dyes and Si-rhodamine dyes (Fig. 19(a)) and used the probe to image endogenous ONOO− of cisplatin-induced AKI mice.192 In the presence of ONOO−, SiRho-HD exhibited ratiometric fluorescence, based on the FRET principle. The specific oxidation of the hemicyanine dye resulted in a reduction of the PA signal and release of Si-rhodamine fluorescence. Confocal imaging indicated that the fluorescence intensity of SiRho-HD in the green channel (λem = 645–710 nm) was enhanced, and the fluorescence intensity of the red channel was significantly reduced (λem = 720–780 nm) for HK-2 cells in a cisplatin-caused nephrotoxicity model. The signal intensity was positively correlated with the concentration of cisplatin. In general, molecules with high hydrophilicity tend to be enriched in the kidney. The PA and fluorescence signals of the hemicyanine of SiRho-HD in the kidney area of the cisplatin-induced AKI mouse decreased, and the fluorescence signal intensity of SiR increased.
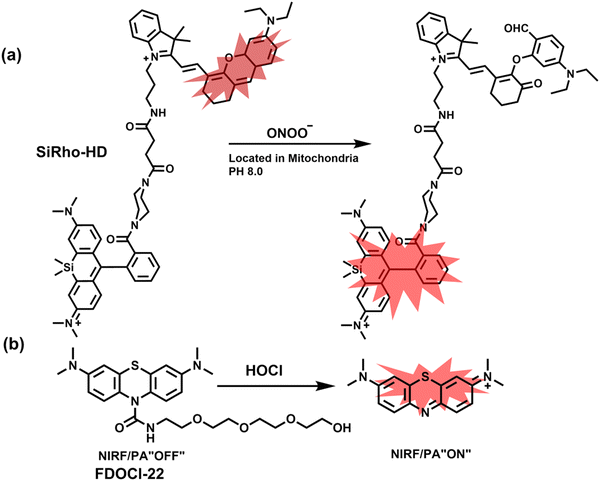 | ||
| Fig. 19 (a) Structures of SiRho-HD and sensing mechanism for discrimination of ONOO−. (b) Structures of FDOCl-22 and sensing mechanism for discrimination of HClO. | ||
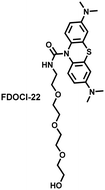
Hypochlorous acid is considered to be closely related to cisplatin-induced AKI. To monitor endogenous HClO in the kidney area of drug-induced AKI mice, Yi et al. developed a water-soluble fluorescent/photoacoustic dual-modal probe (FDOCl-22) based on methylene blue (MB) and urea derivatives with long glycol chains (Fig. 19(b)).193 In the presence of HClO, the cleavage of the amide bond releases MB, which significantly enhanced the absorption and emission signals. Combined with confocal imaging, the fluorescence intensity of FDOCl-22 in cisplatin-stimulated macrophages was significantly increased, and the apoptosis rate was positively correlated with the dosage of cisplatin. Moreover, FDOCl-22 was specifically enriched in the kidneys, and the fluorescence and photoacoustic signals of a cisplatin-induced AKI mouse model were significantly enhanced. Under the same dosage and incubation time, FDOCl-22 could detect AKI 24 h earlier than the standard clinical method (Table 3).
| Ref. | Chemical structure | Analyte | Response time | Detection limit (μM) | λ ex/λem (nm) | Application in pathological process |
|---|---|---|---|---|---|---|
| 160 |
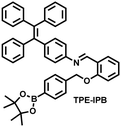
|
ONOO− | 30 min | 100 | 377/538 | Accumulate at the inflammation site of mice and visualize an increase of ONOO− levels |
| 161 |
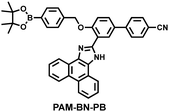
|
H2O2 | 8 min | 0.148 | 410/480 | Visualisation of H2O2 levels in ischemia-reperfusion injured cells and mice with peritonitis. |
| 162 |
|
ClO− | 30 s | 0.108 | 350/535 | Visualisation of HClO signalling in mouse models of peritonitis, arthritis and hepatocellular carcinoma. |
| 163 |
|
ONOO− | — | — | 550/590 | Visualization of ONOO− in a LPS-induced peritonitis mouse and the evaluation of the drug |
| 165 |
|
ONOO− | 20 s | 0.0113 | 420/651 | Visualization of changes in the levels of ONOO− in cells, liver tissues, and inflammation mouse model |
| 420/473 | ||||||
| 166 |
|
H2O2 | 30 min | 0.348 | 685/716 | FL/PA dual-modal imaging of H2O2 in LPS-induced acute abdominal inflammation of mice. |
| 167 |
|
H2O2/ONOO− | — | — | 870/908 | Visualization of changes in the levels of H2O2 and ONOO− in an alkaline inflammatory environment (cystitis and acute colitis) |
| 168 |
|
HClO | — | 0.056 | 988/1058 | Reversible monitoring of the HClO/RSS-mediated redox process in a model of acute inflammation |
| 824/No fluorescent signal | ||||||
| H2S | — | 0.046 | 980/1040 | |||
| 176 |
|
RSH | 711/731 | Real-time observation of fluctuations in thiol levels during apoptosis and cerebral ischaemia/reperfusion in the mouse brain | ||
| (Cys, Hcy, GSH) | 93 s | 0.39 | ||||
| 85 s | 0.54 | |||||
| 88 s | 0.59 | |||||
| 178 |
|
GSH | 80 s | 20 | 654/728 | Visualization of mitochondrial GSH changes during the cerebral I/R process. |
| 180 |
|
O2˙− | 180 s | 10 | 762/790 | Real-time imaging of O2˙− |
| 606/742 | Concentration in liver of I/R mice model | |||||
| 181 |
|
H2O2 | 70 min | 20 | 780/806 | Visualization of mitochondrial the fluctuations of H2O2 in cells and in vivo during the I/R process |
| 605/758 | ||||||
| 185 |
|
GSH | — | 15.8 | 854/895, 936 | Evaluated the redox potential of liver tissue during a liver injury in vivo |
| ONOO− | — | 0.08 | 854/895, 936 | |||
| 186 |
|
O2˙− | 10 min | 0.465 | 675/710 | Visualization of an increase in levels of both O2˙− and ONOO− in mouse livers during APAP-induced DILI |
| ONOO− | — | 0.382 | 360/461 | |||
| 187 |
|
HClO | Seconds | 0.0198 | 360/445 | Simultaneously image HClO and H2S then evaluated the liver damage and relief caused by duloxetine and fluoxetine |
| H2S | 900 s | 0.192 | 545/580 | |||
| 189 |
|
NO | 10 min | 0.023 | 780/935 | Visualization of herbal-medicine-induced liver injury by detecting hepatic NO |
| 192 |
|
ONOO− | 200 s | 0.36 | 635/750 | Mapping the fluctuation of ONOO− in cisplatin-induced acute kidney injury |
| 635/680 | ||||||
| 193 |
|
HOCl | 25 s | 0.0207 | 664/680 | Mapping the fluctuation of ONOO− in cisplatin-induced acute kidney injury |
5. Fluorescent probes for oxidative stress imaging in diseases
It is known that oxidative stress is closely related to the pathophysiological process of many diseases. However, there has been a lack of effective means for real-time imaging of oxidative stress in vivo. To better meet the requirements of medical and clinical research, real-time imaging for the pathological process using fluorescent probes for various diseases is an important area that needs additional development. Considering the biological environment and the background fluorescence of biological tissues, skin and blood, a limited number of fluorescent probes have been used for in vivo imaging of related diseases. Therefore, we selected several diseases closely related to oxidative stress, such as neurodegenerative diseases, epilepsy, depression, diabetes, and cancer, where the application of fluorescent probes in monitoring these diseases has been achieved.5.1 Alzheimer's disease
Alzheimer's disease (AD), as the most common neurodegenerative disease, poses a serious threat to human health and is a large economic burden on society.194 The most common clinical symptoms of AD patients are short-term and long-term memory loss, as well as the deterioration of progressive learning, reasoning, exercise, and communication skills as the disease develops.195 The extracellular amyloid plaques formed by abnormal accumulation of β-amyloid and neurofibrillary tangles formed by the deposition of tau protein in neurons are the main histopathological features of AD.196,197 In addition to the pathological brain aggregation of proteins, the homeostasis of metal ions, oxidative stress, and loss of cholinergic transmission are also considered as important pathogenic factors for AD.198 As a disease involving multiple pathological processes, the current treatment plans for a single pathological factor have all failed.199,200 However, significant evidence has pointed to the important role played by oxidative stress which may together with other pathological factors promote the emergence and progression of AD. Schubert et al. found that Aβ protein increased the levels of H2O2 and lipid peroxides, which gradually accumulated in cells.201 Butterfield et al. found that H2O2 and carbonyl protein levels increased in various AD transgenic mice (carrying APP and PS-1 mutants) models and were directly related to soluble Aβ levels.202 In addition, Markesbery et al. found abnormal levels of Cu, Zn, and Fe in the hippocampus and amygdala of AD patients, and these areas exhibited severe histopathological changes.203 In fact, abnormal levels of Fe/Cu in organisms can directly react with oxygen to produce superoxide anions, leading to the production of H2O2. At present, an explanation of the specific link between oxidative stress and pathological factors remains unknown. Therefore, fluorescent probes are urgently required to help provide new insight and facilitate new approaches for solving these problems.Aβ peptides activate microglia to produce high levels of ONOO− causing oxidative damage to neurons.204 Considering the complex biological environment in the AD brain, James et al. developed a class of 3-hydroxyflavone boronate-based fluorescent probes (Fig. 20(a)), in particular 3-HF could respond ratiometrically to ONOO− after binding to Aβ aggregates (I530/I420).205 In order to evaluate the potential for in vivo application, 3-HF was used for the fluorescence imaging of Aβ aggregates in AD brain slices (Fig. 20(b)). Aβ aggregates in brain slices exhibited blue fluorescence, while Aβ aggregates in brain slices treated with ONOO− exhibited green fluorescence. In general, 3-HF could accurately image Aβ aggregates in AD brain using two fluorescence channels in the absence/presence of ONOO−. Recently, Wang et al. reported a novel two-photon fluorescent probe (BTNPO) that could monitor the distribution and changes of Aβ aggregates and ONOO− through two independent fluorescence channels (Fig. 21).206 BTNPO consisted of two parts: (i) the benzothiazole-naphthalene derivative BTNPO was selected as the recognition group for Aβ aggregates due the structural similarity with thioflavin T; (ii) oxindole was selected as a recognition group for ONOO− (Fig. 21(a)). In the presence of Aβ aggregates/ONOO−, BTNPO produced significant fluorescence enhancement at 418 nm/506 nm (Fig. 21(a)). When the two analytes coexisted, the two reactions did not interfere with each other. As such using this probe, Aβ aggregate-induced ONOO− formation in neurons could be visualized and confirmed that ONOO− levels were positively correlated with the co-incubation time and concentration of Aβ aggregates (Fig. 21(b)). Furthermore, by co-imaging the dynamics of Aβ aggregates and ONOO− levels in brain slices at different ages (months), it was found that high levels of ONOO− were generated earlier than Aβ aggregates in AD mouse brains (Fig. 21(c)).
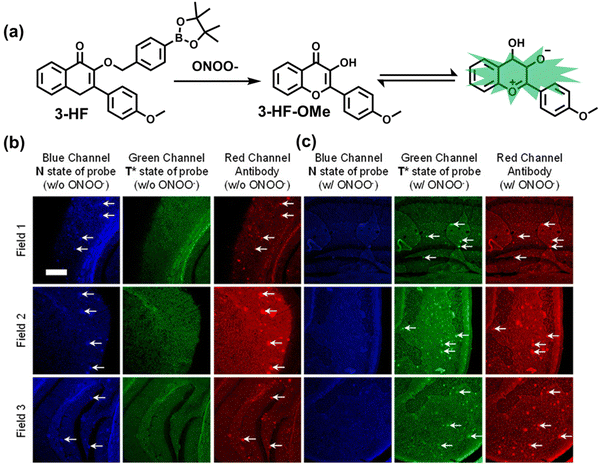 | ||
| Fig. 20 (a) Structure of 3-HF and reaction with ONOO−. (b) Fluorescence imaging of a brain section of a transgenic mouse treated with 3-HF (20 μM) without (w/o) (b) and with (w/) (c) ONOO− (30 μM). Reproduced with permission from ref. 205. Copyright (2018) American Chemical Society. | ||
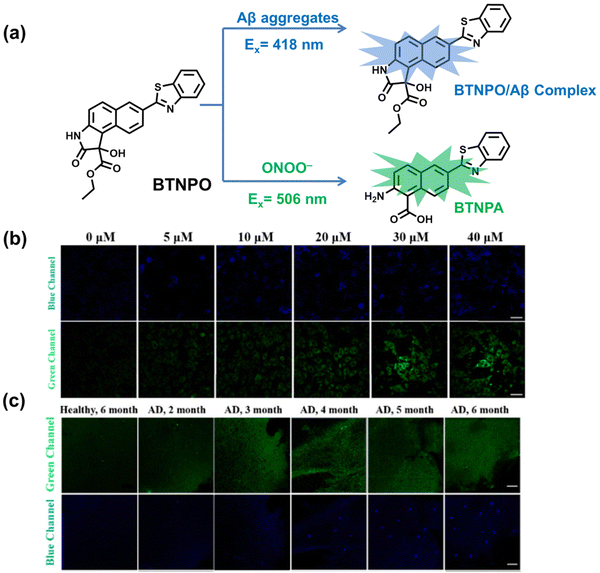 | ||
| Fig. 21 (a) Dual-Color fluorescence responses of BTNPO to Aβ aggregates and ONOO−. (b) PC12 cells were treated with Aβ aggregates (0, 5, 10, 20, 30, or 40 μM) for 12 h and then incubated with 10 μM BTNPO for 30 min. (c) Representative two-photon images of ONOO− and Aβ aggregates in brain tissues of AD mouse. Reproduced with permission from ref. 206. Copyright (2021) American Chemical Society. | ||
As an important signaling molecule and oxidant, H2O2 has been closely associated with the pathology of Alzheimer's disease. Ran et al. reported a cascade signal amplification fluorescent probe (CRANAD-88) that could be used to detect H2O2 and Aβ aggregates in the brain of AD mice.207 CRANAD-88 consisted of two parts: (i) an analog of curcumin-58 (Aβ aggregates recognition group) was used as the main fluorophore; (ii) benzyl boronic ester was used as the recognition group for H2O2 (Fig. 22(a)). The two parts were combined using a carbamate linkage. Due to the electron withdrawing effect of the carbamate, the excitation/emission wavelength of curcumin-58 underwent a hypsochromic shift and part of the fluorescence was quenched. In the presence of H2O2, CRANAD-88 exhibited a bathochromic shift of fluorescence excitation/emission and a 4-fold increase in fluorescence intensity. The reaction product associated with Aβ aggregates achieved a 16-fold increase in fluorescence intensity (Fig. 22(b)). This probe was then used for the in vitro microscopic imaging of AD mouse brain slices, and the signal with Aβ plaques and H2O2 was increased by about 2 times (Fig. 22(c)). Based on in vivo imaging experiments, CRANAD-88 successfully crossed the blood–brain barrier and exhibited stronger fluorescent signals in AD mouse brains. This was the first time that fluorescent probes were used to detect H2O2 in AD mouse brains.
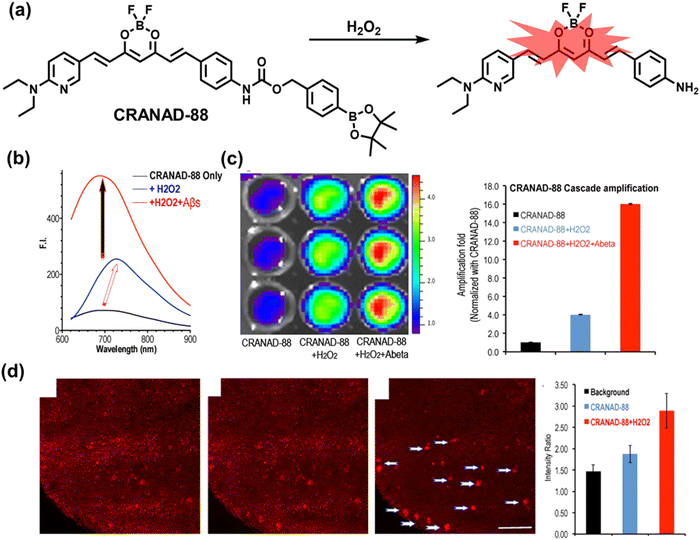 | ||
| Fig. 22 (a) Structures of CRANAD-88 and reaction with H2O2. (b) Fluorescence intensity changes of CRANAD-88 with H2O2 and with H2O2 + Aβ aggregates. (c) Cascade signal amplification validation of CRANAD-88 on a plate and quantitative analysis of the image. (d) H2O2-treated/untreated brain sections were evaluated for staining using CRANAD-88 and quantification histograms. Reproduced with permission from ref. 207. Copyright (2016) Springer Nature. | ||
The mitochondrial respiratory chain is the main pathway for the generation of ROS, and mitochondrial dysfunction induced by oxidative stress is an important factor in the pathogenesis of AD.208,209 Liu et al. designed a NIR fluorescent probe (Mito-NIRHV) (Fig. 23(a)), which was used to detect mitochondrial viscosity and H2O2 in the brain of AD mice.210 In the presence of H2O2, Mito-NIRHV exhibited a strong absorption peak at 440 nm, and the fluorescence signal at 700 nm was significantly enhanced. In addition, an increase of environmental viscosity enhanced the maximum absorption peak at 570 nm, and the fluorescence signal at 800 nm increased significantly. Mito-NIRHV exhibited low cytotoxicity, mitochondrial targeting (Pearson coefficient of 0.968) and a suitable lipid-water partition coefficient (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P = 1.57). Importantly, the fluorescence signal of Mito-NIRHV in an AD brain was stronger than that of healthy mice for both the 700 nm and 800 nm fluorescence channels. The results indicated that H2O2 levels and mitochondrial viscosity increased in AD brains.
P = 1.57). Importantly, the fluorescence signal of Mito-NIRHV in an AD brain was stronger than that of healthy mice for both the 700 nm and 800 nm fluorescence channels. The results indicated that H2O2 levels and mitochondrial viscosity increased in AD brains.
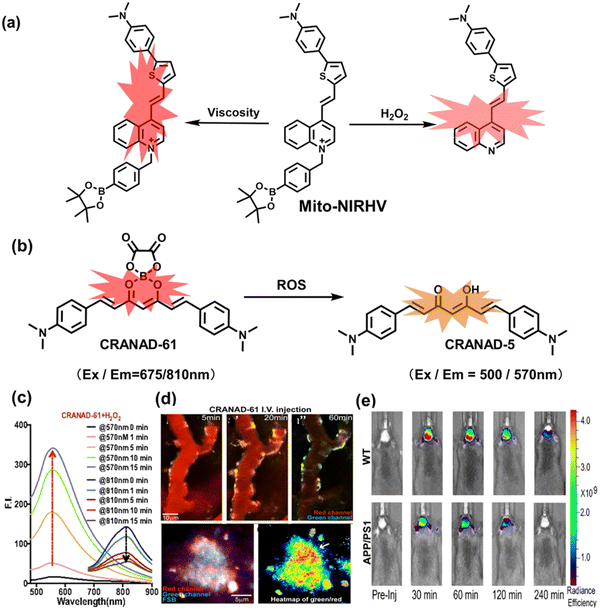 | ||
| Fig. 23 (a) Structure of Mito-NIRHV and sensing mechanism for the discrimination of H2O2 and viscosity. (b) Structure of CRANAD-61 and sensing mechanism for the discrimination of ROS. (c) The fluorescence intensity at 570 nm and 810nm of CRANAD-61 co-incubated with H2O2 at different time points. (d) CRANAD-61 was used for the fluorescence imaging of brain CAA (amyloid cerebrovascular disease) and amyloid plaques in AD mice. (e) CRANAD-61 was used for real-time imaging of AD mice and healthy mice at 30, 60, 120, and 240 min. Reproduced with permission from ref. 211. Copyright (2017) Published by National Academy of Sciences. | ||
There has been a lack of comprehensive biological evidence linking AD with oxidative stress. Ran et al. designed a NIR fluorescent probe (CRANAD-61) based on oxalate-curcumin, which could be used for macroscopic and microscopic detection of ROS levels in the brain of AD.211 CRANAD-61 was composed of two parts: (i) curcumin was selected as the host fluorophore; (ii) oxalate borate as a ROS recognition group which shifted the excitation and emission wavelength into the NIR region (λex/λem = 675/810 nm) (Fig. 23(b)). An appropriate lipid–water partition coefficient (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P = 2.4) enabled CRANAD-61 to efficiently pass through the BBB. In the presence of ROS, the cleavage of the oxalic acid caused a blue shift in the excitation and emission wavelengths (λex/λem = 500/570 nm) (Fig. 23(c)). In addition, active Aβ plaques produce a large amount of ROS during the growth process, which is expected to have stronger neurotoxicity than silent Aβ plaques.212 At the microscopic level, using CRANAD-61 and fluorostyrylbenzene (FSB, commercial Aβ plaque marker dye), the distinction between the two types of plaques and the local concentration of ROS around Aβ plaques could be evaluated. The areas with higher conversion efficiency were active Aβ plaques with higher ROS concentrations, while areas with bright red fluorescence and extremely low conversion rate were silent Aβ plaques (Fig. 23(d)). At the macroscopic level, the brain fluorescence intensity of AD mice was significantly lower than that of healthy mice at 30, 60, 120, and 240 minutes (Fig. 23(e)). This study provides important biological evidence for the relationship between Alzheimer's disease and oxidative stress.
P = 2.4) enabled CRANAD-61 to efficiently pass through the BBB. In the presence of ROS, the cleavage of the oxalic acid caused a blue shift in the excitation and emission wavelengths (λex/λem = 500/570 nm) (Fig. 23(c)). In addition, active Aβ plaques produce a large amount of ROS during the growth process, which is expected to have stronger neurotoxicity than silent Aβ plaques.212 At the microscopic level, using CRANAD-61 and fluorostyrylbenzene (FSB, commercial Aβ plaque marker dye), the distinction between the two types of plaques and the local concentration of ROS around Aβ plaques could be evaluated. The areas with higher conversion efficiency were active Aβ plaques with higher ROS concentrations, while areas with bright red fluorescence and extremely low conversion rate were silent Aβ plaques (Fig. 23(d)). At the macroscopic level, the brain fluorescence intensity of AD mice was significantly lower than that of healthy mice at 30, 60, 120, and 240 minutes (Fig. 23(e)). This study provides important biological evidence for the relationship between Alzheimer's disease and oxidative stress.
5.2 Parkinson's disease
Parkinson's disease (PD) is the second largest neurodegenerative disease, threatening the lives of millions of people worldwide. There are still a lack of effective therapeutic drugs and early diagnostic methods available for clinical practice.213 The loss of dopaminergic neurons in the substantia nigra (SN) area is the main histopathological feature of PD.214 The imbalance of redox homeostasis in neuronal cells often leads to the destruction of normal physiological processes and ultimately leads to cell apoptosis. To date, significant evidence for oxidative damage to numerous key cell components of PD patients has been accumulated (Such as protein, lipid, DNA and RNA oxidation and lower levels of reduced glutathione).215–220 In addition, a variety of toxins that can cause oxidative stress induce the recurrence of the important features of PD in laboratory animals.221,222 This evidence indicates that oxidative stress plays an important role in the degradation of dopaminergic neurons in PD. However, there is still a lack of methods that can detect oxidative stress in the brain of PD patients in real time, whether for clinical testing or the evaluation of drug efficacy. As such, the development of suitable fluorescent probes will provide appropriate methods to help improve the prognosis of PD patients.Mitochondrial dysfunction and oxidative stress play a vital role in the pathogenesis of PD, and the mitochondrial microenvironment and H2O2 levels are two key parameters for evaluating mitochondrial function.223 Yu et al. designed a two-photon (TP) fluorescent probe (Mito-LX), which could simultaneously monitor the dynamic changes of mitochondrial viscosity and H2O2 levels in the brain of PD animals.224 Mito-LX was composed of four parts: (i) N,N-dimethylamino as electron donor; (ii) pyridine salt cation as electron acceptor; (iii) naphthalene ring with extended conjugated structure; (iv) benzyl boronic ester as H2O2 recognition group (Fig. 24(a)). In the presence of H2O2, the cleavage of the benzyl boronic ester results in a significant increase in fluorescence intensity at 585 nm. In addition, with Mito-LX the fluorescence intensity at 730 nm increases as the viscosity (0.60–945 cp) increased. Moreover, by incubating Mito-LX with wild-type drosophila and PD drosophila respectively, the PD Drosophila brain exhibited a >2-fold enhanced fluorescence signal at 585 nm and a 1.5-fold enhanced fluorescence signal at 730 nm. The H2O2 levels and mitochondrial viscosity in PD brains were significantly higher than those for normal brains.
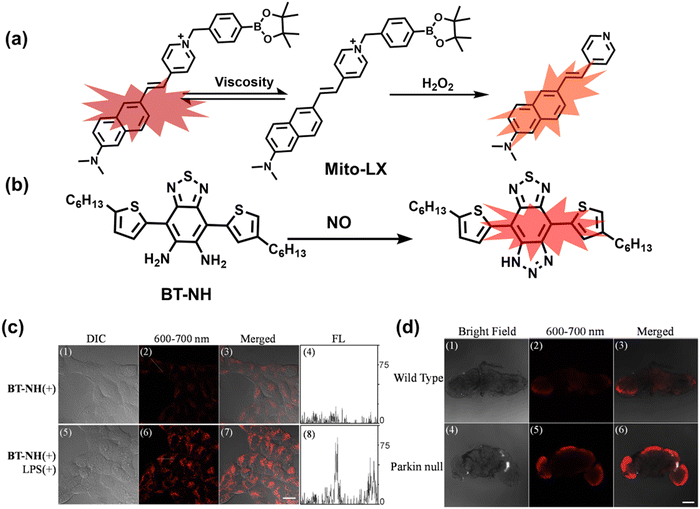 | ||
| Fig. 24 (a) Structures of Mito-LX and sensing mechanism for discrimination of H2O2 and viscosity. (b) Structures of BT-NH and sensing mechanism for the discrimination of NO. (c) Confocal fluorescence imaging of exogenous NO levels in living SH-SY5Y cells using BT-NH. (d) Fluorescence imaging of endogenous NO levels in drosophila brain tissues using BT-NH. Reproduced with permission from ref. 225. Copyright (2019) Elsevier B.V. | ||
Abnormally high levels of nitric oxide can react with reactive oxygen to generate a variety of reactive nitrogen species to induce oxidative stress in organisms. To achieve specific and highly sensitive detection of NO in the brain of PD animal models, Huang et al. designed and synthesized a fluorescent probe (BT-NH) (Fig. 24(b)).225 The structural design was derived from diaminofluorescein and benzobis (1,2,5-thiadiazole). The two ortho amino groups on the benzene ring can react with NO to increase the fluorescence intensity and generate an emission wavelength red shift (20 nm). Importantly, BT-NH had an extremely low detection limit (LOD = 6.96 nM), high selectivity for NO and exhibited pH insensitivity. To further evaluate the ability to detect NO, BN-TH was used to image endogenous NO in HepG2 cells (Fig. 24(c)) and PD Drosophila brains (Fig. 24(d)), respectively. The cells (LPS stimulated) and PD Drosophila brains all exhibited stronger fluorescence signals than the control groups, indicating that PD and inflammatory conditions can cause an abnormal increase in the NO levels. In addition, Huang et al. developed a ratiometric near-infrared fluorescent probe NIR-HP1 for exploring the dynamic changes of H2O2 flux in a PD model.226 NIR molecule 1 with ESIPT fluorescence mechanism was selected as the fluorophore, and benzyl boronic ester was used as the recognition site for H2O2 (Fig. 25(a)). NIR-HP1 exhibited significant ratiometric selectivity towards H2O2, and the ratio of fluorescence intensity at 650 and 500 nm (F650/F500) increased linearly with an increase in H2O2 concentrations. In vitro and in vivo experiments indicated that NIR-HP1 could accurately detect endogenous H2O2 in cells and zebrafish. Moreover, a higher fluorescence ratio in PD Drosophila brains was observed indicating high levels of H2O2.
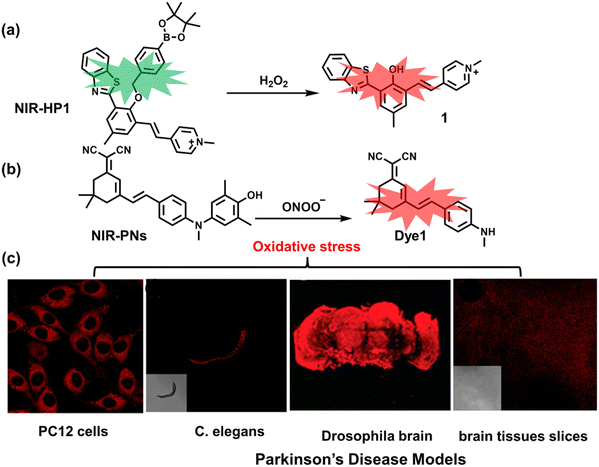 | ||
| Fig. 25 (a) Structures of NIR-HP1 and sensing mechanism for determination of H2O2. (b) Fluorescence turn ‘on’ mechanism of NIR-PNs in the presence of ONOO−. (c) NIR-PNs was used for the detection of ONOO− in PC12 cells and different PD models. Reproduced with permission from ref. 228. Copyright (2020) American Chemical Society. | ||
The overproduction of ONOO− is considered to be a key neurotoxic factor and potential biomarker of oxidative stress in the brain of PD patients.227 Liu et al. developed a series of near-infrared probes (NIR-PNs), which could be used for the ultra-fast (within a few seconds) and highly selective detection of ONOO− in a variety of PD animal models.228 A dicyanoisophorone with a donor (D)–π–acceptor(A) structure was selected as the host fluorophore, and methoxy-substituted p-aminophenol was used as the specific reaction site for ONOO− (Fig. 25(b)). In the presence of ONOO−, the p-aminophenol group can undergo a cleavage reaction to release p-benzoquinone. The disappearance of the PeT effect results in rapid recovery of the fluorescence signal (λex/λem = 520/670 nm). Cell imaging indicated that NIR-PNs could detect dynamic changes of endogenous ONOO−, with a 5.86-fold increase in fluorescence intensity. Moreover, NIR-PNs exhibited 4–7 fold fluorescence increase in various PD animal models and tissues (including parkin null drosophila, mice brain slices, and WLZ3 C. elegans) (Fig. 25(c)).
5.3 Epilepsy
Epilepsy is one of the most serious neurological diseases of the brain, and nearly 50 million people in the world suffer from this disease. Epilepsy is divided into three types according to the cause, namely idiopathic, induced, or symptomatic epilepsy. Symptomatic epilepsy is caused by disease or trauma; induced epilepsy is caused by drug stimulation or external environmental stimulation; and idiopathic epilepsy is linked to genetic factors.229 In addition, patients with epilepsy associated with these causes often have no neuroanatomical or neuropathological abnormalities.230,231 A significant amount of evidence indicates that the pathogenesis of epilepsy is closely related to oxidative stress.232 It has been reported that drug-induced epilepsy rats brain lipid and DNA exhibit significant oxidative damage, and blood antioxidants (such as cysteine, glutathione) levels are reduced.233–237 Compared with wild-type mice, transgenic mice lacking superoxide dismutase exhibited an increased incidence of spontaneous and handling-induced seizures.238 As such the development of fluorescent probes facilitating the early diagnosis of epilepsy will provide useful tools for understanding the pathological mechanisms linking epilepsy with oxidative stress.Myeloperoxidase is believed to play an important role in the pathological dysfunction of the epileptic brain.239 Qian et al. developed an efficient two-photon fluorescent probe (HCP), which could be used to detect endogenous HClO generated by myeloperoxidase (MPO) in the brain of epileptic mice.240 The quinoline skeleton was used as the host fluorophore and recognition site (Fig. 26(a)). The chlorination reaction with HClO resulted in a 100 nm blue-shifted emission wavelength and a 138-fold increase in fluorescence intensity. In addition, HCP also exhibited effective BBB permeability, high photostability, and excellent two-photon excitation characteristics. Through tail vein injection of HCP, enhanced fluorescence in the brain of epileptic mice could be directly observed, indicating that MPO-mediated oxidative stress was positively correlated with severe neuronal damage and epilepsy (Fig. 26(c and d)). Significantly, HCP was used to construct a simple high-throughput screening method to quickly screen potential anti-epileptic drugs (Fig. 26(b)). It was observed that the flavonoid compound apigenin can relieve MPO-mediated oxidative stress and inhibit neuronal cell hypertrophy (ferroptosis). This work not only provided a powerful fluorescent tool for understanding the pathological mechanism between hypochlorous acid-induced oxidative stress and epilepsy, but also elucidated a general strategy for screening anti-epileptic drugs.
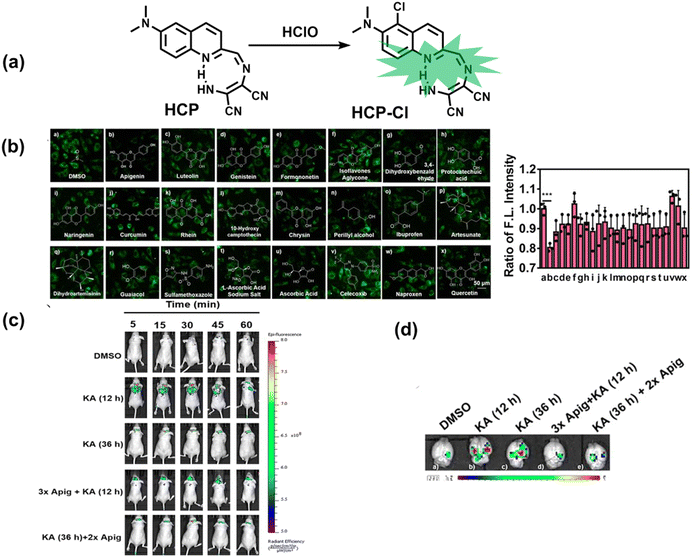 | ||
| Fig. 26 (a) Fluorescence turn ‘on’ mechanism of HCP in the presence of HClO. (b) HCP was used for the high-throughput screening of natural products in live H-SY5Y human neuroblastoma cells to control MPO-mediated oxidative stress. (c) HCP was used to detect hypochlorous acid in the brain of epileptic mice (kainic acid (KA)-induced epileptic seizure) and evaluate the therapeutic effect of apigenin. Reproduced with permission from ref. 240. Copyright (2020) Published by National Academy of Sciences. | ||
Abnormally high levels of ONOO− are considered to be a key neurotoxic factor and may serve as a potential biomarker for the early diagnosis of epilepsy and evaluation of drug efficacy.241,242 In order to monitor dynamic changes of the ONOO− signal in the brain of epileptic rats in real time, Yu et al. developed a two-photon fluorescent probe (DCM–ONOO) based on the DCM fluorophore and the ONOO− specific diphenylphosphonamide (Fig. 27(a)).243 Spectroscopic analyses indicated that DCM–ONOO exhibited high sensitivity, specificity, and rapid response. Combined with TP fluorescence imaging, the fluorescence intensity of DCM–ONOO in the brain of epileptic rats was significantly higher than that of healthy rats and epileptic rats treated using resveratrol. In addition, the brain tissue slices of the epileptic rats exhibited higher fluorescence intensity and a large amount of neuron loss, which confirmed that overexpression of ONOO− was positively correlated with seizures and neuronal damage.
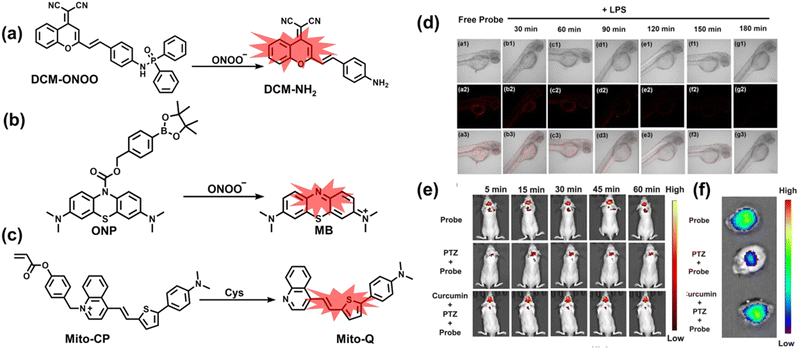 | ||
| Fig. 27 (a) Fluorescence turn ‘on’ mechanism of DCM–ONOO in the presence of ONOO−. (b) Fluorescence turn ‘on’ mechanism of ONP in the presence of ONOO−. (c) Fluorescence turn ‘on’ mechanism of Mito-CP in the presence of Cys. (d) Fluorescence images of Mito-CP responding to Cys in living zebrafish under oxidative stress (e) Mito-CP was used to image Cys in the brains of mice in epilepsy/treatment group. (f) Fluorescence imaging of dissected epilepsy/treated mouse brains. Reproduced with permission from ref. 245. Copyright (2020) American Chemical Society. | ||
To design potential screening tools for anti-epileptic drugs, Qian et al. designed an ONOO− responsive fluorescent probe (ONP) based on methylene blue and benzyl boronic ester group (Fig. 27(b)).244 ONP displays effective BBB permeability, extremely low cytotoxicity, and excellent temporal and spatial resolution. Using ONP, the dynamic changes of endogenous ONOO− flux in the epileptic brain of mice could be quickly and sensitively monitored. In vitro and in vivo imaging of the hippocampus of epileptic mice indicated that higher concentrations of ONOO− were positively correlated with severe neuronal damage and epilepsy. In addition, a high-throughput screening method for anti-epileptic inhibitors was constructed by combining high-content analysis (HCA) and ONP. In addition it was found that curcumin could effectively inhibit the overexpression of ONOO− in the brains of epileptic mice.
Cysteine (Cys) is an important free radical scavenger in organisms and plays an important role in regulating redox homeostasis. Liu et al. designed probe (Mito-CP) that could be used to monitor the dynamic changes of endogenous cysteine levels in the brain of mice with epilepsy.245 Mito-Q with near-infrared emission was selected as the host fluorophore, and acrylate was used as the recognition site for Cys (Fig. 27(c)). In addition, Mito-CP exhibited effective BBB permeability, mitochondrial targeting, and a large Stokes shift of approximately 260 nm. Using Mito-CP, Cys could be detected without interference from other thiols in PC12 cells and zebrafish (Fig. 27(d)). Importantly, changes in the dynamic flux of Cys in an epileptic brain was observed for the first time (Fig. 27(e and f)). This research confirmed that curcumin was beneficial for the recovery of Cys levels in epileptic brains.
5.4 Depression
Depression is a common mental illness. Significant and lasting depression is the main clinical feature. The manifestations of depressive episodes can be divided into core symptoms, psychological symptoms, and somatic symptoms.246 A depressed mood, slow thinking, and decreased volitional activity are typical symptoms of major depression.246 Global estimates indicated that there are approximately 322 million patients with depression in the world, with a prevalence rate of 4.4%.247 As such this disease has undoubtedly become one of the serious health problems that plague the world. However, there is still a lack of effective means for the rapid diagnosis of depression in clinical practice.248,249 Compared to other chronic diseases caused by a variety of pathological factors, the pathophysiology of depression is still in its infancy.250,251 In recent years, a large amount of evidence has shown that oxidative stress is closely related to the pathological process of depression.252 It has been found that the levels of peroxides and xanthine oxidase in the plasma of patients with depression is enhanced, while the plasma concentrations of many key antioxidants are significantly reduced (e.g., vitamin E, glutathione, zinc and coenzyme Q10).253–257 In addition, some key cellular components in the brain of depression (e.g., protein, fatty acid, DNA) exhibit varying degrees of oxidative damage.258–260 Although oxidative stress has attracted more and more attention in the investigation of the pathology of depression, there is still a lack of clinical tools that can be used for real-time imaging of RONSS in the brain of patients with depression. Recently, due to the excellent temporal and spatial resolution of fluorescence imaging technology, researchers have begun to use fluorescent probes for imaging oxidative stress in the brains of patients suffering from depression.As one of the reactive oxygen species with high reactivity and strong oxidizing properties, the accurate detection of ˙OH in the brain has always been a challenge. Li et al. developed a two-photon fluorescent probe (MD-B), which could be used for the imaging of ˙OH in the brains of depressed mice.261 Coumarin with excellent two-photon properties was selected as the host fluorophore, 3-methylpyrazolone was used as the specific recognition site and electron donor group for ˙OH (Fig. 28(a and b)). The introduction of the strong electron withdrawing group trifluoromethyl increased the push–pull electron effect of the conjugated system. MD-B exhibited rapid response, good BBB permeability and high sensitivity (detection limit was 2.4 nM). Using MD-B, dynamic changes of ˙OH in cells and the brains of mice with depression were imaged, and it was found that an increase of ˙OH flux in the brain was positively correlated with the severity of the depression phenotype (Fig. 28(c)). In addition, MD-B was used to demonstrate that the strong oxidizing properties of ˙OH could inactivate the deacetylase SIRT1, resulting in the development of the depressive phenotype.
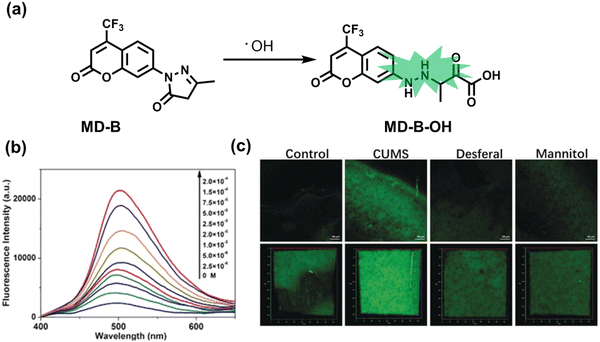 | ||
| Fig. 28 (a) Fluorescence turn ‘on’ mechanism of MD-B in the presence of ˙OH. (b) The fluorescence emission spectrum of MD-B changes with the addition of different concentrations of ˙OH (2.5–200 μM). (c) Two-photon fluorescence imaging of MD-B using a mouse cranial window. CUMS (Chronic unpredictable mild stress): mice exhibit depression-like behavior. Desferal: The susceptible mice injected desferrioxamine (iron scavenger). Mannitol: The susceptible mice injected with mannitol (˙OH scavenger). Reproduced with permission from ref. 261. Copyright (2019) Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
As a reactive oxygen species, ozone is believed to be closely related to the pathogenesis of many mental diseases including depression.262,263 Tang et al. designed and synthesized a cyanine dye-based probe (Acy-7) that can be used to visualize O3 in the brains of depressed mice.264 Acy-7 was constructed using a Cy7 fluorophore locked using a 3-butenyl group recognition group (Fig. 29(a)). Acy-7 exhibited good sensitivity (detection limit of 10 nM) and selectivity for O3. This study successfully visualized the overproduction of O3 in PC12 cells and macrophages under oxidative stress. Importantly, Acy-7 was used to monitor the increase in O3 in the brains of mice with depression for the first time (Fig. 29(b and c)). In addition, fluorescence imaging combined with enzyme-linked immunosorbent assay (ELISA) successfully confirmed that the overproduction of O3 in the mouse brain could promote the production of pro-inflammatory cytokine interleukin-8 (IL-8), which is one of the important factors leading to depression in mice.
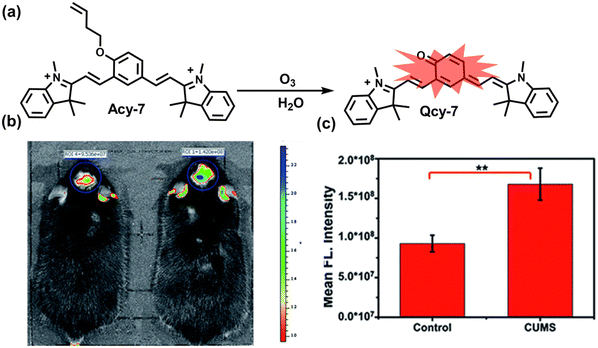 | ||
| Fig. 29 (a) Fluorescence turn ‘on’ mechanism of Acy-7 in the presence of O3. (b) Fluorescence imaging of O3 in the brains of depression mice. (c) Quantification of images in (b). Reproduced with permission from ref. 264. Copyright (2019) The Royal Society of Chemistry. | ||
As key antioxidants in the body against oxidative stress, thiols are closely related to the pathological process of depression. Li et al. designed and synthesized a two-photon fluorescent probe (ER-SH), which could be used to detect a variety of thiols in the brains of depressed mice.265 ER-SH was composed of three parts: (i) 1,8-naphthalene dimethylamine with good two-photon performance was selected as the fluorophore; (ii) 2,4-dinitrobenzene sulfonamide (DNBS) was used as the thiol specific recognition site; (iii) toluene sulfonamide served as the targeting group of the Golgi apparatus (Fig. 30(a)). ER-SH exhibited excellent sensitivity to three common thiols (Cys, GSH and Hcy), and was not affected by other biological species (including ROS, RNS and metal ions). This study successfully visualized endogenous thiols in the endoplasmic reticulum of PC12 cells and endogenous thiols in the brains of depressed mice. Meanwhile, it was found that the fluorescence signal of the brains of depressed mice was 2.6 times lower than normal mice.
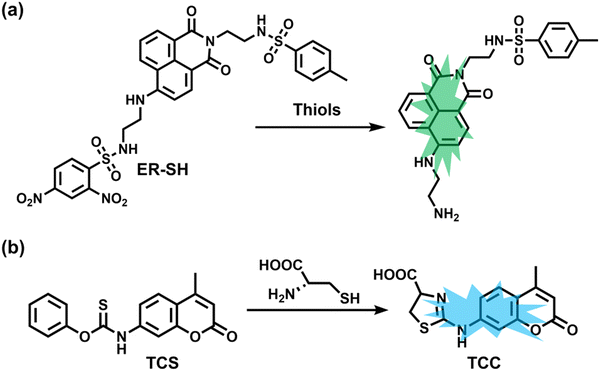 | ||
| Fig. 30 (a) Fluorescence turn ‘on’ mechanism of ER-SH in the presence of thiols. (b) Fluorescence turn ‘on’ mechanism of TCS in the presence of Cys. | ||
In addition, to accurately detect changes in the levels of a single thiol in a brain of model, Tang et al. recently reported a two-photon fluorescent probe (TCS) with high specificity for cysteine in the brain of depressed mice.266 Coumarin 120, which has excellent two-photon properties, was selected as the fluorophore. The thiobenzoate was used as a specific recognition site for cysteine (Fig. 30(b)). In the presence of Cys, a stable five-membered ring is formed through a selective nucleophilic addition reaction, thereby triggering a significant fluorescence enhancement. TCS exhibited effective BBB permeability, superior sensitivity, and biocompatibility. Importantly, TCS was used to directly observe the dynamic changes of Cys in the brains of depressed mice, and successfully revealed a negative correlation between Cys levels and the degree of depressive behavior. However, the emission wavelengths of ER-SH and TCS cannot reach the near-infrared region, which limited their application for in vivo imaging.
5.5 Diabetes
Diabetes is a metabolic disease characterized by hyperglycemia and insufficient endogenous insulin secretion or action.267 Defective or impaired insulin secretion can lead to elevated blood sugar levels in the body, while long-term hyperglycemia can induce complications including heart disease, stroke, vascular disease, and kidney disease.268 In particular, oxidative stress induced by hyperglycemia is thought to play an important role in the development of diabetic complications.269 Abnormally high levels of radicals can cause severe oxidative damage to a variety of biological components in cells, and ultimately lead to damage of cell function or death. Glucose oxidation is one of the main sources of radicals. The enediol radical anion is the main product of the glucose oxidation reaction, and this anion is rapidly converted into reactive ketoaldehydes and superoxide anion radicals due to its unstable structure.270 The radical is inherently highly oxidizing and easily decomposes to produce hydrogen peroxide or reacts with NO to produce peroxynitrite (ONOO−). In addition, by measuring the levels of oxidative stress markers in body fluids of patients and rodent disease models, it was found that lipids, proteins, enzymes, and DNA all exhibit varying degrees of oxidative damage, while the reductant levels of glutathione, α-lipoic acid, vitamin C and E and so on were significantly reduced.271–277 These results emphasized the link between oxidative stress and diabetes. In recent years, the development of fluorescent probes provides a promising means of revealing the link between oxidative stress and diabetes pathology.Guo et al. in 2016 reported a fluorescent probe (A2), which could be used to visualize the dynamic flux of ONOO− in the kidney tissue of diabetic rats.278 A BODIPY dye was selected as the fluorophore, and 4-methoxy-substituted N,N-dibenzylaniline was selected as the specific recognition group for ONOO− (Fig. 31(a)). A2 exhibited off–on fluorescence response (within seconds) and exhibited ultrasensitive detection (detection limit: <2 nM). Using A2 endogenous ONOO− in RAW264.7 mouse macrophages and oxygen glucose deprivation (OGD) endothelial cells could be visualized. Importantly, the fluorescence intensity of the organs and tissues of the diabetic mouse were significantly higher than for the healthy mouse, which indicated that the concentration of ONOO− was higher than the normal level in the kidney tissue of the diabetic mouse. Sadly, the excitation and emission wavelengths of A2 were both in the visible region, which was not conducive to in vivo imaging. Therefore, Guo et al. subsequently developed a near-infrared fluorescent probe (SiRTA) and achieved ONOO− specific imaging of the abdominal cavity of diabetic mice.279 SiRTA was obtained by the direct conjugation of a NIR fluorophore Si-rhodamine with an aromatic tertiary amine (Fig. 31(b)). In the presence of ONOO−, SiRTA reacts to produce the N-nitroso product, thereby rapidly turning on the fluorescence within 30 s due to inhibition of the PeT effect. This research was able to directly monitor high levels of endogenous ONOO− in streptozotocin (STZ)-induced pancreatic β cells (INS-1 cells), shedding light on the role of ONOO− in the pathophysiological processes of pancreatic β cell death and diabetes (Fig. 31(d)). In addition, SiRTA exhibited an enhanced fluorescence signal in the liver and kidney sections of diabetic mice than in healthy mice (Fig. 31(c)).
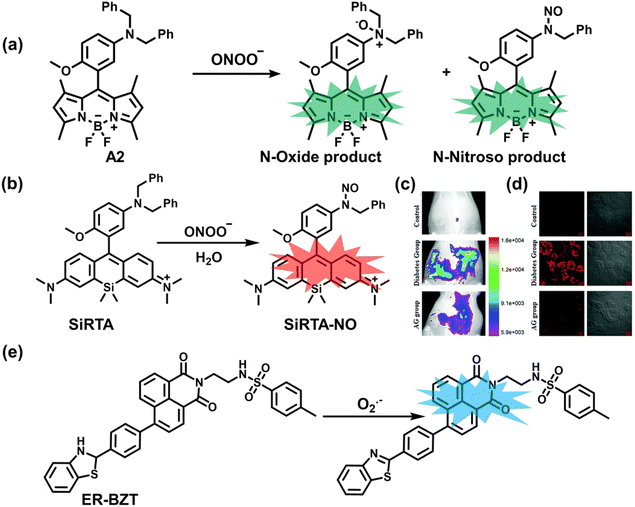 | ||
| Fig. 31 (a) Fluorescence turn ‘on’ mechanism for A2 in the presence of ONOO−. (b) Fluorescence turn ‘on’ mechanism for SiRTA in the presence of ONOO−. (c) Fluorescence imaging of ONOO− in the different groups of the peritoneal cavity of rats. AG: the NO synthase inhibitor aminoguanidine. (d) Representative confocal images of the kidney slices of rats in the control group, the diabetic group, and the AG group, respectively. Reproduced with permission from ref. 279. Copyright (2018) The Royal Society of Chemistry. (e) Fluorescence turn ‘on’ mechanism of ER-BZT in the presence of O2˙−. | ||
Superoxide anion (O2˙−) is the first ROS produced in organisms and as such plays an important role in oxidative stress and the pathological process of diabetes. Tang et al. designed and synthesized a two-photon fluorescent probe (ER-BZT) targeting the endoplasmic reticulum, which could be used to observe the differences in O2˙− levels in healthy/diabetic mice.280 ER-BZT was comprised of three parts: (i) 1,8-naphthalimide with excellent two-photon properties was selected as the main fluorophore; (ii) benzothiazoline was used as the recognition unit for O2˙−; (iii) methyl sulfonamide was chosen as the targeting group for the endoplasmic reticulum (Fig. 31(e)). Spectroscopic analysis indicated that ER-BZT exhibited a low detection limit (60 nM), rapid response time (within a few seconds), and good biocompatibility. Combined with in vivo fluorescence imaging, this probe highlighted the abnormally high levels of endogenous O2˙− in the abdominal cavity and liver tissue of diabetic mice. The use of the hypoglycemic drug metformin (MERF) resulted in a significant decrease in fluorescence intensity, indicating that the production of O2˙− was reduced.
As a metabolite of O2˙−, H2O2 is closely associated with the pathological process of diabetes. To explore the relationship between H2O2 and diabetes, Li et al. developed a NIR fluorescent probe (QX-B) based on quinolinium–xanthene dye and benzyl boronic ester recognition group for H2O2 (Fig. 32(a)).281 QX-B exhibited high sensitivity and excellent selectivity and could image endogenous H2O2 in cells and zebrafish (Fig. 32(b)). Furthermore, QX-B was used to monitor abnormally high levels of H2O2 in the kidney and liver of diabetic mice and used to evaluate the efficacy of the hypoglycemic drug MERF using dynamic changes in the levels of H2O2 (Fig. 32(c and d)).
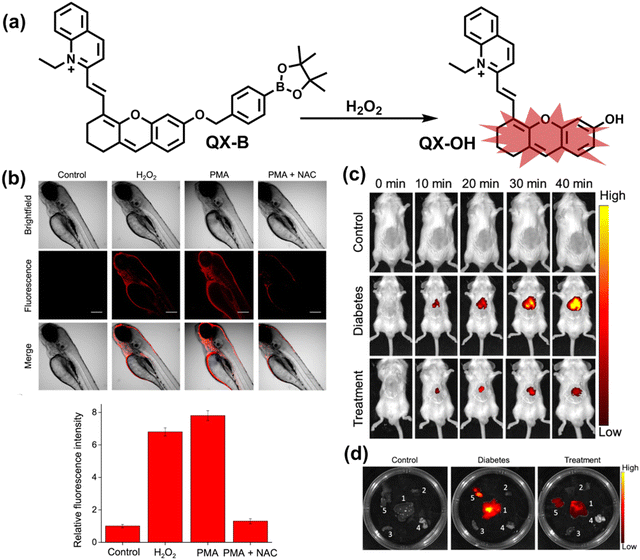 | ||
| Fig. 32 (a) Fluorescence turn ‘on’ mechanism of QX-B in the presence of H2O2. (b) QX-B was used for fluorescence imaging of endogenous and exogenous hydrogen peroxide in zebrafish. (c) QX-B was used for real-time imaging of hydrogen peroxide in healthy mice, diabetic mice and mice in the treatment group, respectively. (d) Fluorescence imaging in the main organs of the mice. Reproduced with permission from ref. 281. Copyright (2021) American Chemical Society. | ||
5.6 Cancer
Cancer is a ubiquitous fatal disease that threatens human health. Although the rapid diagnosis and treatment of cancer have developed greatly over the past 30 years, the mortality rate associated with cancer has not improved significantly.282 In recent years, the relationship between oxidative stress and cancer has been given more and more attention. In general, the pathogenesis of cancer is divided into at least three stages: initiation, promotion, and progression.283 Many studies indicate that oxidative stress can be involved with three stages of the process.284 At the initial stage, RONS attacks the DNA of the nucleus and mitochondria, leading to nucleoside oxidation and DNA damage. At the promotion stage, RONS induce abnormal gene expression, intercellular communication disruption and oxidative damage to the second messenger system, and then causes abnormal cell proliferation.283 Finally, RONS can increase cell proliferation, survival, and migration therefore promoting tumor cell survival and growth.285 However, due to the limitations of low abundance and short lifespan of RONS, limited of methods exits to observe the oxidative stress of tumors in the clinic. The continuous development of fluorescent probes and dual-mode probes is an area of research that offers significant promise for monitoring highly reactive oxygen species associated with cancer.Quinone oxidoreductase-1 (NQO1) is a key enzyme whose expression in cancer cells is enhanced when compared to normal cells (5–200 times).286 As a novel anticancer drug, β-Lap can rapidly produce a large amount of ROS by catalyzing NQO1 to induce cancer cell apoptosis. Guo et al. reported a novel near-infrared fluorescent probe (PSiR1) targeting lysosomes, which could image in vivo three highly reactive oxygen species (hROS: ˙OH, ONOO−, and ClO−) in mouse tumors.287 PSiR1 was composed of two parts: (i) the silanthracene dye was selected as the fluorophore; (ii) 2,4-dimethyl-3-ethylpyrrole for PeT was used as hROS recognition group (Fig. 33(a)). When hROS were present, PSiR1 reacted to generate N-hydroxyl products resulting in rapid fluorescence turn on in a few seconds (λex/λem = 650/680 nm). This probe was able to image endogenous hROS in cancer cells and indicated that β-Lap could induce hROS production in cellular lysosomes. In addition, Guo et al. developed PSiR3 with a higher signal-to-noise ratio to improve the differentiation between cancer cells and normal tissues.288 Replacing the Me2Si-unit of PSiR1 with the (Me2CH)2Si-unit, the aggregation induced fluorescence quenching in aqueous solution was partly inhibited (Fig. 33(a)). Using a combination of PSiR3 and β-Lap, high contrast differentiation was achieved for cancer cells/normal cells, and tumor tissues/healthy tissues, respectively (Fig. 33(b and c)). The lung tissue sections and thyroid tissue sections exhibited bright red fluorescence, while healthy tissue exhibited only a weak fluorescence. Notably, the combination of PSiR3 and β-Lap exhibited a weak fluorescent signal in inflammatory tissues, which indicated that the system could effectively distinguish tumor tissue from inflammatory tissue (Fig. 33(c)). These two systems provided an effective protocol for the study of the pathological mechanism of oxidative stress in cancer and the effective differentiation of cancer cells/healthy cells.
 | ||
| Fig. 33 (a) Fluorescence turn ‘on’ mechanism of PSiR1 and PSiR3 in the presence of h-ROS. (b) Confocal fluorescence images of normal cells (COS7 and HUVEC cells) and cancer cells (A549 and HepG2 cells) after co-incubation with PSiR3 (PSiR) and β-Lap. (c) Confocal fluorescence images of the benign uterine tissue sections, malignant lung tissue sections, and inflammatory thyroid tissue sections after co-incubation with PSiR3 (PSiR) and β-Lap. Reproduced with permission from ref. 288. Copyright (2021) Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
Two-photon fluorescence imaging technology has the advantage of deep tissue imaging, low background fluorescence and high three-dimensional (3D) resolution. Mao et al. reported a two-photon near-infrared fluorescent probe (DHQ-RD-PN), which could be used to accurately in vivo image ONOO− in mouse tumors.289 DHQ-RD-PN was composed of two parts: (i) rhodol was selected as the fluorophore; (ii) the 1-methylindoline-2,3-dione was selected as the recognition site (Fig. 34(a)). Furthermore, 1,4-diethylpiperazine extended the near-infrared emission, and the introduction of the ortho F atom to the phenol improved the pH-sensitivity of the rhodol fluorophore. In the presence of ONOO−, the fluorescence intensity of DHQ-RD-PN was significantly increased (F/F0 = 17). Cell imaging experiments indicated that DHQ-RD-PN could specifically detect changes of endogenous ONOO− in drug-stimulated RAW 264.7 cells. Notably, the fluorescence increased with the number of days of tumor growth (Fig. 34(b and c)).
 | ||
| Fig. 34 (a) Fluorescence turn ‘on’ mechanism of DHQ-RD-PN in the presence of ONOO−. (b) NIR fluorescence in vivo imaging of a tumor-bearing mouse at 0, 2, 6, and 8 days with intratumoral injection of DHQ-RD-PN. (c) Two-photon NIR in vivo imaging of a tumor-bearing mouse at 0, 2, 6, and 8 days with intratumoral injection of DHQ-RD-PN. Reproduced with permission from ref. 289. Copyright (2020) American Chemical Society. | ||
Dual-modal probes combining fluorescence and photoacoustic (PA) measurements result in high spatial and temporal resolution, satisfactory imaging depth and high contrast for optical imaging.290 Pu et al. reported probe (CySO3CF3), using NIR-Fluorescence and PA dual-modal imaging techniques to in vivo detect endogenous ONOO− in mouse tumors.291 CySO3CF3 was composed of two parts: (i) a caged NIR hemicyanine dye with a zwitterionic structure was used as the host fluorophore; (ii) trifluoromethyl ketone was used as the recognition group (Fig. 35(a)). In the presence of ONOO−, a new absorption peak at 686 nm and new fluorescence emission peak at 712 nm evolved. Meanwhile, the intensity of the PA at 680 nm was 5.1-fold higher than for the initial state. In addition, CySO3CF3 could accumulate in tumors after tail vein injection. In vivo real-time imaging data indicated that the fluorescence and photoacoustic signal intensity of tumor-bearing mice plateaued within 3 hours, the signal intensity was 2.1-fold and 5.3-fold higher than that of healthy mice, respectively (Fig. 35(b and c)).
 | ||
| Fig. 35 (a) Fluorescence and PA turn ‘on’ mechanism of CySO3CF3 in the presence of ONOO−. (b) NIR fluorescence in vivo imaging of a tumor-bearing mouse at 0, 3 and 4 h with systemic administration of CySO3CF3. (c) PA maximum imaging projection (MIP) of tumor at 0, 3 and 4 h with systemic administration of CySO3CF3. Reproduced with permission from ref. 291. Copyright (2018) American Chemical Society. | ||
As the most common ROS in organisms, the specific detection and quantification of H2O2 in tumors is necessary for understanding the relationship between oxidative stress and cancer. Bohndiek et al. designed a photoacoustic and fluorescent dual-modal probe (JW41) for detecting H2O2, that was successfully applied in vivo and for tumors.292 JW41 was composed of three parts: (i) heptamethine carbocyanine dye as the signal transmitter; (i) benzyl boronic ester connected to the dye backbone through a piperazine linker; (iii) a saccharide which acted as a targeting group for tumors (Fig. 36(a)). In the presence of H2O2, a significant increase in fluorescence signal at 825 nm was observed, and the photoacoustic emission peak moved from 705 nm to 785 nm (Fig. 36(c)). Real-time fluorescence (Fig. 36(d)) and photoacoustic imaging (Fig. 36(b)) of H2O2 mouse tumors and organs was demonstrated. Moreover, the specific accumulation and retention of JW41 in tumors and liver were confirmed by H&E staining (Fig. 36(e)) and mass spectrometry of tumor tissues (Fig. 36(f)). The total probe concentration in the liver was ∼4-fold higher than that in the tumor. The conversion of JW41 into JW35 in mice was calculated to be 36 ± 4% in tumor tissue, while the conversion of in the liver tissue was negligible (3 ± 3%). The data provided direct evidence for the presence of high levels of H2O2 in tumors (Table 4).
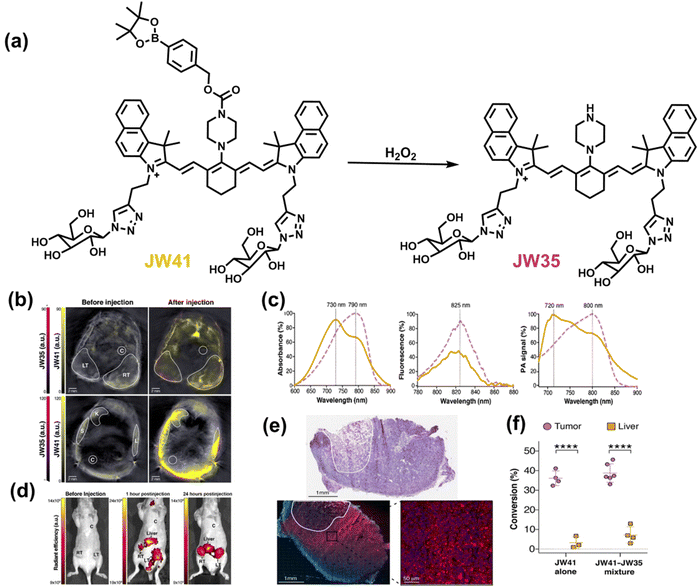 | ||
| Fig. 36 (a) Structures of JW41 dye and its reaction with H2O2. (b) Representative PAI sections of mouse subcutaneous tumors (top) and liver (bottom) before and 15 to 20 minutes after injection of JW41. (c) Absorption, fluorescence (λex = 740 nm) and photoacoustic spectra of JW41 and JW35 in aqueous solution. (d) Fluorescence images before, 1 and 24 hours post injection of JW41. (e) A representative H&E stained section of a mouse tumor injected with JW41, a serial tumor section (blue) after fixing with formaldehyde and staining with DAPI. The necrotic area is circled with a white line. (f) Quantification of the amount of JW41 present in the tumor and liver converted to JW35 by MRM-MS analysis based on LC/MS-MS (conversion rate, in mol%). Reproduced with permission from ref. 292. Copyright (2019) American Association for Cancer Research. | ||
| Ref. | Chemical structure | Analyte | Response time | Detection limit (μM) | λ ex/λem (nm) | Application in diseases |
|---|---|---|---|---|---|---|
| 205 |
|
ONOO− (Aβ-aggregates) | — | 0.0655 (variation with Aβ preparation time) | 370/530 | Visualization of Aβ42 aggregates in AD brain slices using two fluorescence channels in the absence/presence of ONOO−. |
| Aβ-Aggregates | — | — | 370/420 | |||
| 206 |
|
ONOO− | 10 s | — | 380/506 | Visualization of the distribution and variation of Aβ plaques and ONOO− through two independent fluorescence channels |
| Aβ-Aggregates | — | — | 340/418 | |||
| 207 |
|
H2O2 | 10 min | — | 630/730 | Detection of H2O2 and Aβ aggregates in brain slices and the brains of AD mice |
| Aβ-Aggregates | — | — | 640/700 | |||
| 210 |
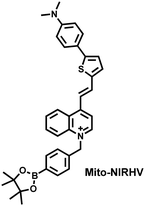
|
H2O2 | 40 min | 0.003 | 440/700 | Simultaneous imaging of mitochondrial viscosity and hydrogen peroxide in brains of AD mice |
| Viscosity | — | — | 570/800 | |||
| 211 |
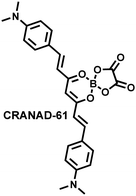
|
ROS | — | — | 675/810 | Visualization of high ROS concentrations in AD brains in animal studies at micro- and macrolevels. |
| 500/570 | ||||||
| Aβ-Monomer | — | 0.0032 | 675/740 | |||
| Aβ-Oligomer | — | 0.0038 | 675/740 | |||
| Aβ-Aggregates | — | 0.0381 | 675/740 | |||
| 224 |
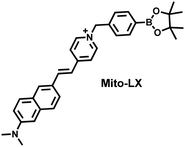
|
H2O2 | 30 min | 0.00498 | 380/585 | Simultaneous imaging of mitochondrial viscosity and hydrogen peroxide in cells and PD Drosophila brains |
| TP760/585 | ||||||
| Viscosity | — | — | 480/730 | |||
| TP800/730 | ||||||
| 225 |
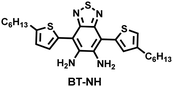
|
NO | 5 min | 0.00696 | 525/620 | Detection of NO in HepG2 cells and PD drosophila brains |
| 226 |
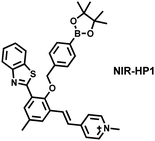
|
H2O2 | — | 0.27 | 405/500 | Visualization of the dynamic changes of H2O2 flux in a PD model |
| 405/650 | ||||||
| 228 |
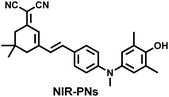
|
ONOO− | A few seconds | 0.00459 | 520/670 | Visualization of the dynamic changes of ONOO− flux in various PD animal models. |
| 240 |
|
HClO | 5 s | 0.104 | 390/581 | Detection of HClO generated by myeloperoxidase (MPO) in the brain of epileptic mice |
| TP800/581 | ||||||
| 243 |
|
ONOO− | 10 min | 0.151 | 512/685 | Visualization of dynamic changes of the ONOO− signal in the brain of epileptic rats |
| 244 |
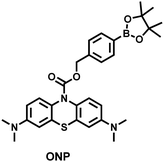
|
ONOO− | 15 min | 0.094 | 665/692 | Visualization of the dynamic changes of ONOO− flux in the epileptic brain of mice and screening of inhibitors |
| 245 |
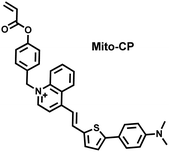
|
Cys | 15 min | 0.0208 | 440/700 | Detection of the dynamic changes of endogenous cysteine levels in the brain of mice with epilepsy |
| 261 |
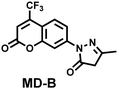
|
˙OH | A few seconds | 0.0024 | 380/500 | Visualization of the dynamic changes of ˙OH in the brains of depressed mice |
| TP800/500 | ||||||
| 264 |
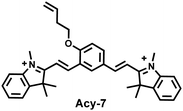
|
O3 | 40 min | 0.01 | 570/690 | Visualization of the dynamic changes of O3 in the brains of depressed mice |
| 265 |
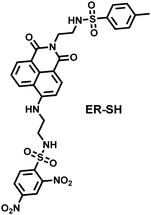
|
Thiols | 420/525 | Detection of various thiols in the brains of depressed mice. | ||
| Cys | — | 0.167 | TP800/525 | |||
| Hcy | — | 0.962 | ||||
| GSH | — | 4.7 | ||||
| 266 |
|
Cys | 14 min | 0.16 | 340/443 | Visualization of the dynamic changes of Cys in the brains of depressed mice |
| TP754/443 | ||||||
| 278 |
|
ONOO− | A few seconds | 0.002 | 517/485 | Visualization of the dynamic flux of ONOO− in the kidney tissue of diabetic rats |
| 279 |
|
ONOO− | 20 s | 0.003 | 650/680 | Visualization of the dynamic flux of ONOO− in the abdominal cavity of diabetic mice and kidney slices |
| 280 |
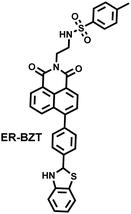
|
O2˙− | A few seconds | 0.06 | 375/450 | Visualization of abnormally high levels of endogenous O2˙− in the abdominal cavity and liver tissue of diabetic mice |
| TP700/450 | ||||||
| 281 |
|
H2O2 | 5 min | 0.17 | 725/772 | Visualization of abnormally high levels of H2O2 in the kidney and liver of diabetic mice and evaluate the efficacy of the drug(Merf) |
| 287 |
|
h-ROS | A few seconds | 650/680 | Imaging to discriminate cancer cells from normal cells, and tumors from healthy tissues. | |
| ONOO− | 0.0079 | |||||
| ˙OH | 0.14 | |||||
| HClO | 0.0106 | |||||
| 288 |
|
h-ROS | 10 s | 650/680 | Using β-Lap, high contrast differentiation was achieved for cancer cells from normal cells, and tumor tissues from healthy tissues, | |
| ONOO− | 0.00539 | |||||
| ˙OH | 0.12 | |||||
| HClO | 0.00686 | |||||
| 289 |
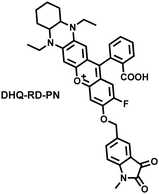
|
ONOO− | 240 s | 0.072 | 564/651 | Visualization of the dynamic flux of ONOO− in the in mouse tumors. |
| 291 |
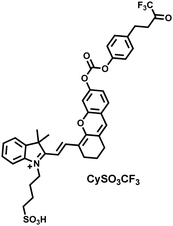
|
ONOO− | 100 s | 0.053 | 686/712 | NIRF and photoacoustic dual-modal imaging of ONOO− in the tumors of living mice. |
| 292 |
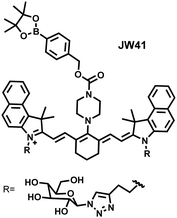
|
H2O2 | 10 min | — | 790/825 | NIRF and photoacoustic dual-modal imaging of H2O2 in the tumors of living mice. |
6. Conclusion and outlook
Considering that abnormal changes in the levels of oxidants and reductants are an immediate manifestation of oxidative stress, we have delineated the basic structure and design ideas of conventional RONSS fluorescent probes, as well as the need to be alert to problems associated with off-targeting. Furthermore, various fluorescent probes reported over past six years for detecting ROS, RNS and RSS in cells, pathophysiological processes, and diseases models have been comprehensively summarized. According to different applications, the probes introduced in this review are divided into the following categories:1. Fluorescent probes that simultaneously visualize the dynamic changes between oxidants, reductants, and related active species in cells. As such multi-species imaging can better reflect the dynamic changes of redox in physiological and pathological processes than single-species imaging techniques.
2. Fluorescent probes that are used for RONSS imaging in pathological processes including inflammation, drug-induced organ damage and IRI. By monitoring the dynamics of endogenous RONSS during different drug treatments, it is possible to understand the toxicity and therapeutic effects of the drugs. The safety and side effects of some surgical procedures can also be understood by monitoring the outbreak of RONS in different organs induced by IRI.
3. Fluorescent probes that are used for RONSS imaging in specific diseases including AD, PD, epilepsy, depression, diabetes and cancer. Evidence from fluorescence imaging facilitates understanding of the mechanisms of the onset and progression of oxidative stress-related diseases, as well as the therapeutic effects of the drugs used.
Compared with traditional methods used in clinical practice, fluorescent probes can image RONSS as biomarkers, providing researchers with important pathophysiological information related to oxidative stress through non-invasive, highly sensitive and real-time imaging. However, this imaging method is still limited by many factors. Here we summarize some common problems associated with most fluorescent probes:
1. The depth of fluorescence imaging in vivo is limited. Although the depth of fluorescence imaging in the near-infrared area is significantly increased, it still cannot meet the needs of clinical detection and imaging.
2. Multi-species detection using a single fluorescent probe remains a challenge. Accommodating two or more recognition groups with opposite/similar reactivity in a single chemical structure are the main challenges for the design of such probes.
3. Most fluorescent probes cannot target specific disease areas. In situ injection remains the most effective way for fluorescent probes to reach the diseased area, but this method is impractical for future clinical applications.
4. As tools for biological imaging, current probes are not suitable for in-depth biomedical applications. Since, they have only been used to draw conclusions in similar animal models, for example the association of RONS with peritonitis, arthritis and liver damage.
Considering the rapid development of fluorescent probes in the field of biological imaging in recent years, we end this review by proposing some areas where additional research is urgently required.
1. Responsive NIR-II fluorescent probe platforms or multi-modal probe platforms combined with other imaging methods. Compared with NIR-I fluorescence imaging, NIR-II fluorescence imaging exhibits much better performance in terms of tissue penetration depth, spatial resolution, and sensitivity. Currently, most NIR-II fluorescent probes are of the always-on type, which is not suitable for the detection of active molecules in vivo. Therefore, the development of more RONSS-responsive NIR-II fluorescent probes is an important direction for the future development of fluorescence imaging technology.
2. Fluorophores with multiple binding sites (such as coumarin, fluorescein, and cyanine), as well as recognition groups with specificity and stability are essential for the development of dual-responsive fluorescent probes.
3. According to the environment and metabolic mechanism of different organs, the selection of suitable targeting groups and adjusting the physical properties such as lipid solubility, molecular rigidity, molecular size and hydrogen bonding ability is required. For example, the kidney is more likely to be enriched in water-soluble molecules; while fat-soluble molecules are more likely to penetrate the blood–brain barrier; and the liver is enriched in various types of molecules.
4. The design of fluorescent probes should follow the needs of disease pathology, drug development, and post-operative evaluation, to further promote the interdisciplinarity and clinical transformation of the obtained results.
RONSS in cells and tissues are of great significance for early disease diagnosis, and research concerning dynamic change of pathological RONSS are particularly important. Real-time active molecular imaging capabilities are lacking with traditional clinical detection tools. Although fluorescent probes have made great progress in the field of oxidative stress marker detection, there is still a long way to go in the development of fluorescent probes for RONSS before they can be used for routine application in the diagnosis and monitoring of clinical diseases. The cooperation of chemists and researchers in different fields is becoming more important to discover molecular probes and expand their application. As such, guidance from biomedical specialists and experienced clinicians will be particularly beneficial to help develop fluorescent probes with significant clinical value.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are thankful for support from the National Key Research and Development Program of China (2021YFC2101500), Beijing Natural Science Foundation (no. 7232342), the Natural Science Foundation of China (no. 82131430174, 81961138011), the Academy of Medical Sciences Newton Advanced Fellowship (NAFR13\1015). TDJ wishes to thank the University of Bath and the Open Research Fund of the School of Chemistry and Chemical Engineering, Henan Normal University for support (2020ZD01).References
- H. Sies, C. Berndt and D. P. Jones, Annu. Rev. Biochem., 2017, 86, 715–748 CrossRef CAS PubMed.
- Q. Kong and Cl. G. Lin, Cell. Mol. Life Sci., 2010, 67, 1817–1829 CrossRef CAS PubMed.
- L. H. Sanders and J. T. Greenamyre, Free Radical Biol. Med., 2013, 62, 111–120 CrossRef CAS PubMed.
- D. P. Jones, Antioxid. Redox Signaling, 2006, 8, 1865–1879 CrossRef CAS PubMed.
- E. E. Battin and J. L. Brumaghim, Cell Biochem. Biophys., 2009, 55, 1–23, DOI:10.1007/s12013-009-9054-7.
- G. I. Giles, K. M. Tasker and C. Jacob, Free Radical Biol. Med., 2001, 31, 1279–1283 CrossRef CAS PubMed.
- A. Chandrasekaran, M. D. P. S. Idelchik and J. A. Melendez, Redox Biol., 2017, 11, 91–102 CrossRef CAS PubMed.
- L. C. D. Pomatto and K. J. A. Davies, Free Radical Biol. Med., 2018, 124, 420–430 CrossRef CAS PubMed.
- S. K. Powers, L. L. Ji, A. N. Kavazis and M. J. Jackson, Compr. Physiol., 2011, 1, 941–969, DOI:10.1002/cphy.c100054.
- Z. Jie, J. Liu, M. Shu, Y. Ying and H. Yang, Talanta, 2022, 236, 122892 CrossRef CAS PubMed.
- M. F. Beal, Curr. Opin. Neurobiol., 1996, 6, 661–666 CrossRef CAS PubMed.
- D. C. Malins, N. L. Polissar and S. J. Gunselman, Proc. Natl. Acad. Sci. U. S. A., 1996, 93, 2557–2563 CrossRef CAS PubMed.
- M. Genestra, Cell. Signaling, 2007, 19, 1807–1819 CrossRef CAS PubMed.
- L. Lamattina, C. G. Mata, M. Graziano and G. Pagnussat, Annu. Rev. Plant Biol., 2003, 54, 109–136 CrossRef CAS PubMed.
- B. Alvarez and R. Radi, Amino Acids, 2003, 25, 295–311 CrossRef CAS PubMed.
- M. C. H. Gruhlke and A. J. Slusarenko, Plant Physiol. Biochem., 2012, 59, 98–107 CrossRef CAS PubMed.
- B. Geng, J. Yang, Y. Qi, J. Zhao, Y. Pang, J. Du and C. Tang, Biochem. Biophys. Res. Commun., 2003, 313, 362–368 CrossRef PubMed.
- T. Mishanina, M. Libiad and R. Banerjee, Nat. Chem. Biol., 2015, 11, 457–464 CrossRef CAS PubMed.
- L. Adams, M. C. Franco and A. G. Estevez, Exp. Biol. Med., 2015, 240, 711–717 CrossRef CAS PubMed.
- P. C. Dedon and S. R. Tannenbaum, Arch. Biochem. Biophys., 2004, 423, 12–22 CrossRef CAS PubMed.
- H. Bayır, Crit. Care Med., 2005, 33, 498–501 CrossRef PubMed.
- D. A. Butterfield and B. Halliwell, Nat. Rev. Neurosci., 2019, 20, 148–160 CrossRef CAS PubMed.
- A. V. Ivanov, B. Bartosch, O. A. Smirnova, M. G. Isaguliants and S. N. Kochetkov, Viruses, 2013, 5, 439–469 CrossRef CAS PubMed.
- V. Sosa, T. Moliné, R. Somoza, R. Paciucci, H. Kondoh and M. E. LLeonart, Ageing Res. Rev., 2013, 12, 376–390 CrossRef CAS PubMed.
- A. Hald and J. Lotharius, Exp. Neurol., 2005, 193, 279–290 CrossRef CAS PubMed.
- R. Kinscherf, K. Cafaltzis, F. Röder, W. Hildebrandt, L. Edler, H.-P. Deigner, R. Breitkreutz, G. Feussner, J. Kreuzer, E. Werle, G. Michel, J. Metz and W. Dröge, Free Radical Biol. Med., 2003, 35, 1286–1292 CrossRef CAS PubMed.
- L. P. Liang and M. Patel, Redox Biol., 2016, 9, 45–49 CrossRef CAS PubMed.
- L. L. Wu, C. C. Chiou, P. Y. Chang and J. T. Wu, Clin. Chim. Acta, 2004, 339, 1–9 CrossRef CAS PubMed.
- J. L. Ravanat, P. Guicherd, Z. Tuce and J. Cadet, Chem. Res. Toxicol., 1999, 12, 802–808 Search PubMed.
- J. Frijhoff, P. G. Winyard, N. Zarkovic, S. S. Davies, R. Stocker, D. Cheng, A. R. Knight, E. L. Taylor, J. Oettrich, T. Ruskovska, A. C. Gasparovic, A. Cuadrado, D. Weber, H. E. Poulsen, T. Grune, H. H. H. W. Schmidt and P. Ghezzi, Antioxid. Redox Signaling, 2015, 23, 1144–1170 Search PubMed.
- L. Wu, J. Liu, P. Li, B. Tang and T. D. James, Chem. Soc. Rev., 2021, 50, 702–734 RSC.
- J. Yan, S. Lee, A. Zhang and J. Yoon, Chem. Soc. Rev., 2018, 47, 6900–6916 RSC.
- H. B. Cheng, Y. Li, B. Z. Tang and J. Yoon, Chem. Soc. Rev., 2020, 49, 21–31 RSC.
- H. Tian, A. C. Sedgwick, H. H. Han, S. Sen, G. R. Chen, Y. Zang, J. L. Sessler, T. D. James, J. Li and X. P. He, Coord. Chem. Rev., 2021, 427, 213577 CrossRef CAS.
- L. Wu, C. Huang, B. P. Emery, A. C. Sedgwick, S. D. Bull, X. P. He, H. Tian, J. Yoon, J. L. Sessler and T. D. James, Chem. Soc. Rev., 2020, 49, 5110–5139 RSC.
- Y. Geng, G. Zhang, Y. Chen, Y. Peng, X. Wang and Z. Wang, Anal. Chem., 2022, 94, 1813–1822 CrossRef CAS PubMed.
- X. Jiao, Y. Li, J. Niu, X. Xie, X. Wang and B. Tang, Anal. Chem., 2018, 90, 533–555 CrossRef CAS PubMed.
- X. Jiang, L. Wang, S. L. Carroll, J. Chen, M. C. Wang and J. Wang, Antioxid. Redox Signaling, 2018, 29, 518–540 CrossRef CAS PubMed.
- L. Wu, A. C. Sedgwick, X. Sun, S. D. Bull, X. P. He and T. D. James, Acc. Chem. Res., 2019, 52, 2582–2597 CrossRef CAS PubMed.
- X. Chen, X. Tian, I. Shin and J. Yoon, Chem. Soc. Rev., 2011, 40, 4783–4804 RSC.
- D. Wu, A. C. Sedgwick, T. Gunnlaugsson, E. U. Akkaya, J. Yoon and T. D. James, Chem. Soc. Rev., 2017, 46, 7105–7123 RSC.
- J. Hou, K. Yu, K. Sunwoo, W. Y. Kim, S. Koo, J. Wang, W. X. Ren, S. Wang, X. Yu and J. S. Kim, Chem., 2020, 6, 832–866 CAS.
- J. Zhou and H. Ma, Chem. Sci., 2016, 7, 6309–6315 RSC.
- D. J. Zheng, Y. S. Yang and H. L. Zhu, TrAC, Trends Anal. Chem., 2019, 118, 625–651 CrossRef CAS.
- C. J. Chang, T. D. James, E. J. New and B. Z. Tang, Acc. Chem. Res., 2020, 53(1), 1 CrossRef CAS PubMed.
- L. Wu, A. C. Sedgwick, X. Sun, S. D. Bull, X. P. He and T. D. James, Acc. Chem. Res., 2019, 52, 2582–2597 CrossRef CAS PubMed.
- L. D. Lavis and R. T. Raines, ACS Chem. Biol., 2008, 3, 142–155 CrossRef CAS PubMed.
- L. Wang, W. Du, Z. Hu, K. Uvdal, L. Li and W. Huang, Angew. Chem., Int. Ed., 2019, 131, 14164–14181 CrossRef.
- X. Chen, T. Pradhan, F. Wang, J. S. Kim and J. Yoon, Chem. Rev., 2012, 112, 1910–1956 CrossRef CAS PubMed.
- A. Mishra, R. K. Behera, P. K. Behera, B. K. Mishra and G. B. Behera, Chem. Rev., 2000, 100, 1973–2012 CrossRef CAS PubMed.
- W. Sun, S. Guo, C. Hu, J. Fan and X. Peng, Chem. Rev., 2016, 116, 7768–7817 CrossRef CAS PubMed.
- C. Sun, W. Du, B. Wang, B. Dong and B. Wang, BMC Chem., 2020, 14, 21 CrossRef PubMed.
- Z. Guo, S. Park, J. Yoon and I. Shin, Chem. Soc. Rev., 2014, 43, 16–29 RSC.
- D. A. Jose, R. Sakla, N. Sharma, S. Gadiyaram, R. Kaushik and A. Ghosh, ACS Sens., 2020, 5, 3365–3391 CrossRef CAS PubMed.
- L. Yuan, W. Lin, S. Zhao, W. Gao, B. Chen, L. He and S. Zhu, J. Am. Chem. Soc., 2012, 134, 13510–13523 CrossRef CAS PubMed.
- V. N. Nguyen, J. Ha, M. Cho, H. Li, K. M. K. Swamy and J. Yoon, Coord. Chem. Rev., 2021, 439, 213936 CrossRef CAS.
- Z. Shi, X. Han, W. Hu, H. Bai, B. Peng, L. Ji, Q. Fan, L. Li and W. Huang, Chem. Soc. Rev., 2020, 49, 7533–7567 RSC.
- J. Mei, Y. Huang and H. Tian, ACS Appl. Mater. Interfaces, 2018, 10, 12217–12261 CrossRef CAS PubMed.
- Y. Wang, B. Xia, Q. Huang, T. Luo, Y. Zhang, P. Timashev, W. Guo, F. Li and X. J. Liang, Adv. Healthcare Mater., 2021, 10, 2100945 CrossRef CAS PubMed.
- K. S. S. Kumar, Y. R. Girish, M. Ashrafizadeh, S. Mirzaei, K. P. Rakesh, M. H. Gholami, A. Zabolian, K. Hushmandi, G. Orive, F. B. Kadumudi, A. D. Pirouz, V. K. Thakur, A. Zarrabi, P. Makvandi and K. S. Rangappa, Coord. Chem. Rev., 2021, 447, 214135 CrossRef.
- N. L. C. Leung, N. Xie, W. Yuan, Y. Liu, Q. Wu, Q. Peng, Q. Miao, J. W. Y. Lam and B. Z. Tang, Chem. – Eur. J., 2014, 20, 15349–15353 CrossRef CAS PubMed.
- Y. Chen, W. Ai, X. Guo, Y. Li, Y. Ma, L. Chen, H. Zhang, T. Wang, X. Zhang and Z. Wang, Small, 2019, 15, 1902352 CrossRef PubMed.
- Y. Ma, W. Ai, J. Huang, L. Ma, Y. Geng, X. Liu, X. Wang, Z. Yang and Z. Wang, Anal. Chem., 2020, 92, 14444–14451 CrossRef CAS PubMed.
- W. Ai, Z. Yang, Y. Ma, X. Han, Y. Chen, K. Zhu and Z. Wang, Analyst, 2020, 145, 6435–6440 RSC.
- Y. Ma, H. Wang, S. Su, Y. Chen, Y. Li, X. Wang and Z. Wang, Analyst, 2019, 144, 3381–3388 RSC.
- Y. Song, Z. Chen and H. Li, Curr. Org. Chem., 2012, 16, 2690–2707 CrossRef CAS.
- D. Cao, Z. Liu, P. Verwilst, S. Koo, P. Jangjili, J. S. Kim and W. Lin, Chem. Rev., 2019, 119, 10403–10519 CrossRef CAS PubMed.
- L. Wu, Y. Shi, H. Yu, J. Zhang, Z. Li and X. F. Yang, Sens. Actuators, B, 2021, 337, 129790 CrossRef CAS.
- S. L. Shen, X. Zhao, X. F. Zhang, X. Li Liu, H. Wang, Y. Y. Dai, J. Y. Miaoc and B. X. Zhao, J. Mater. Chem. B, 2017, 5, 289–295 RSC.
- Y. Bai, M. X. Wu, Q. J. Ma, C. Y. Wang, J. G. Sun, M. J. Tiana and J. S. Li, New J. Chem., 2019, 43, 14763–14771 RSC.
- C. Geraghty, C. Wynne and R. B. P. Elmes, Coord. Chem. Rev., 2021, 437, 213713 CrossRef CAS.
- D. Jacquemin, E. A. Perpète, G. Scalmani, I. Ciofini, C. Peltier and C. Adamo, Chem. Phys., 2010, 372, 61–66 CrossRef CAS.
- H. Q. Dong, T. B. Wei, X. Q. Ma, Q. Y. Yang, Y. F. Zhang, Y. J. Sun, B. B. Shi, H. Yao, Y. M. Zhang and Q. Lin, J. Mater. Chem. C, 2020, 8, 13501–13529 RSC.
- H. Zhu, C. Liu, M. Su, X. Rong, Y. Zhang, X. Wang, K. Wang, X. Li, Y. Yu, X. Zhang and B. Zhu, Coord. Chem. Rev., 2021, 448, 214153 CrossRef CAS.
- L. Zhou, L. Xie, C. Liu and Y. Xiao, Chin. Chem. Lett., 2019, 30, 1799–1808 CrossRef CAS.
- A. R. Lippert, G. C. Van de Bittner and C. J. Chang, Acc. Chem. Res., 2011, 44, 793–804 CrossRef CAS PubMed.
- Y. Liu, C. Jiao, W. Lu, P. Zhang and Y. Wang, RSC Adv., 2019, 9, 18027–18041 RSC.
- H. Guo, G. Chen, M. Gao, R. Wang, Y. Liu and F. Yu, Anal. Chem., 2019, 91, 1203–1210 CrossRef CAS PubMed.
- M. Abo, Y. Urano, K. Hanaoka, T. Terai, T. Komatsu and T. Nagano, J. Am. Chem. Soc., 2011, 133, 10629–10637 CrossRef CAS PubMed.
- J. F. Turrens, J. Physiol., 2003, 552, 335–344 CrossRef CAS PubMed.
- R. Q. Li, Z. Q. Mao, L. Rong, N. Wu, Q. Lei, J. Y. Zhu, L. Zhuang, X. Z. Zhang and Z. H. Liu, Biosens. Bioelectron., 2017, 87, 73–80 CrossRef CAS PubMed.
- H. Xiao, W. Zhang, P. Li, W. Zhang, X. Wang and B. Tang, Angew. Chem., Int. Ed., 2020, 59, 4216–4230 CrossRef CAS PubMed.
- W. Zhang, P. Li, F. Yang, X. Hu, C. Sun, W. Zhang, D. Chen and B. Tang, J. Am. Chem. Soc., 2013, 135, 14956–14959 CrossRef CAS PubMed.
- H. Maeda, K. Yamamoto, Y. Nomura, I. Kohno, L. Hafsi, N. Ueda, S. Yoshida, M. Fukuda, Y. Fukuyasu, Y. Yamauchi and N. Itoh, J. Am. Chem. Soc., 2005, 127, 68–69 CrossRef CAS PubMed.
- J. J. Hu, N. K. Wong, S. Ye, X. Chen, M. Y. Lu, A. Q. Zhao, Y. Guo, A. C. H. Ma, A. Y. H. Leung, J. Shen and D. Yang, J. Am. Chem. Soc., 2015, 137, 6837–6843 CrossRef CAS PubMed.
- K. Xu, X. Liu, B. Tang, G. Yang, Y. Yang and L. An, Chem. – Eur. J., 2007, 13, 1411–1416 CrossRef CAS PubMed.
- C. C. Winterbourn, Nat. Chem. Biol., 2008, 4, 278–286 CrossRef CAS PubMed.
- J. T. Hou, M. Zhang, Y. Liu, X. Ma, R. Duan, X. Cao, F. Yuan, Y. X. Liao, S. Wang and W. X. Ren, Coord. Chem. Rev., 2020, 421, 213457 CrossRef CAS.
- L. Yuan, W. Lin and J. Song, Chem. Commun., 2010, 46, 7930–7932 RSC.
- H. Li, X. Li, W. Shi, Y. Xu and H. Ma, Angew. Chem., Int. Ed., 2018, 57, 12830–12834 CrossRef CAS PubMed.
- F. Liu, J. Du, D. Song, M. Xu and G. Sun, Chem. Commun., 2016, 52, 4636–4639 RSC.
- S. Dong, L. Zhang, Y. Lin, C. Ding and C. Lu, Analyst, 2020, 145, 5068–5089 RSC.
- J. T. Hou, N. Kwon, S. Wang, B. Wang, X. He, J. Yoon and J. Shen, Coord. Chem. Rev., 2022, 450, 214232 CrossRef CAS.
- Y. Chen, Nitric Oxide, 2020, 98, 1–19, DOI:10.1016/j.niox.2020.02.002.
- J. Miao, Y. Huo, X. Lv, Z. Li, H. Cao, H. Shi, Y. Shi and W. Guo, Biomaterials, 2016, 78, 11–19 CrossRef CAS PubMed.
- (a) S. Wang, L. Chen, P. Jangili, A. Sharma, W. Li, J. T. Hou, C. Qin, J. Yoon and J. S. Kim, Coord. Chem. Rev., 2018, 374, 36–54 CrossRef CAS; (b) H. Li, X. Li, X. Wu, W. Shi and H. Ma, Anal. Chem., 2017, 89, 5519–5525 CrossRef CAS PubMed.
- T. Peng, N. K. Wong, X. Chen, Y. K. Chan, D. H. H. Ho, Z. Sun, J. J. Hu, J. Shen, H. E. Nezami and D. Yang, J. Am. Chem. Soc., 2014, 136, 11728–11734 CrossRef CAS PubMed.
- C. Zhao, J. An, L. Zhou, Q. Fei, F. Wang, J. Tan, B. Shi, R. Wang, Z. Guo and W. H. Zhu, Chem. Commun., 2016, 52, 2075–2078 RSC.
- S. Wang, Y. Huang and X. Guan, Molecules, 2021, 26, 3575 CrossRef CAS PubMed.
- T. Matsumoto, Y. Urano, T. Shoda, H. Kojima and T. Nagano, Org. Lett., 2007, 9(17), 3375–3377 CrossRef CAS PubMed.
- H. Chen, Y. Tang, M. Ren and W. Lin, Chem. Sci., 2016, 7, 1896–1903 RSC.
- J. Wang, B. Li, W. Zhao, X. Zhang, X. Luo, M. E. Corkins, S. L. Cole, C. Wang, Y. Xiao, X. Bi, Y. Pang, C. A. McElroy, A. J. Bird and Y. Dong, ACS Sens., 2016, 1, 882–887 CrossRef CAS.
- L. Long, W. Lin, B. Chen, W. Gao and L. Yuan, Chem. Commun., 2011, 47, 893–895 RSC.
- L. Y. Niu, Y. Z. Chen, H. R. Zheng, L. Z. Wu, C. H. Tung and Q. Z. Yang, Chem. Soc. Rev., 2015, 44, 6143–6160 RSC.
- H. Chen, Y. Tang and W. Lin, TrAC, Trends Anal. Chem., 2016, 76, 166–181 CrossRef CAS.
- J. Liu, Y. Q. Sun, Y. Huo, H. Zhang, L. Wang, P. Zhang, D. Song, Y. Shi and W. Guo, J. Am. Chem. Soc., 2014, 136, 574–577 CrossRef CAS PubMed.
- S. Han, H. Zhang, X. Yue, J. Wang, L. Yang, B. Wang and X. Song, Anal. Chem., 2021, 93, 10934–10939 CrossRef CAS PubMed.
- V. S. Lin, W. Chen, M. Xian and C. J. Chang, Chem. Soc. Rev., 2015, 44, 4596–4618 RSC.
- L. He, X. Yang, K. Xu, X. Kong and W. Lin, Chem. Sci., 2017, 8, 6257–6265 RSC.
- M. Ren, B. Deng, X. Kong, K. Zhou, K. Liu, G. Xua and W. Lin, Chem. Commun., 2016, 52, 6415–6418 RSC.
- Z. Wu, D. Liang and X. Tang, Anal. Chem., 2016, 88, 9213–9218 CrossRef CAS PubMed.
- Q. Wu, C. Yin, Y. Wen, Y. Zhang and F. Huo, Sens. Actuators, B, 2019, 288, 507–511 CrossRef CAS.
- S. Gong, E. Zhou, J. Hong and G. Feng, Anal. Chem., 2019, 91, 13136–13142 CrossRef CAS PubMed.
- M. Qian, L. Zhang, Z. Pu, J. Xia, L. Chen, Y. Xia, H. Cui, J. Wang and X. Peng, J. Mater. Chem. B, 2018, 6, 7916–7925 RSC.
- Q. Yang, T. Lan and W. He, Dyes Pigm., 2021, 186, 108997 CrossRef CAS.
- Y. Fang, W. Chen, W. Shi, H. Li, M. Xian and H. Ma, Chem. Commun., 2017, 53, 8759–8762 RSC.
- Q. Han, Z. Mou, H. Wang, X. Tang, Z. Dong, L. Wang, X. Dong and W. Liu, Anal. Chem., 2016, 88, 7206–7212 CrossRef CAS PubMed.
- H. Zhou, J. Tang, L. Sun, J. Zhang, B. Chen, J. Kan, W. Zhang, J. Zhang and J. Zhou, Sens. Actuators, B, 2019, 278, 64–72 CrossRef CAS.
- Y. Q. Sun, J. Liu, J. Zhang, T. Yang and W. Guo, Chem. Commun., 2013, 49, 2637–2639 RSC.
- Y. Liu, J. Nie, J. Niu, W. Wang and W. Lin, J. Mater. Chem. B, 2018, 6, 1973–1983 RSC.
- Q. Sun, W. Zhang and J. Qian, Talanta, 2017, 162, 107–113 CrossRef CAS PubMed.
- X. Cheng, H. Jia, J. Feng, J. Qin and Z. Li, Sens. Actuators, B, 2013, 184, 274 CrossRef CAS.
- X. Kong, M. Li, B. Dong, N. Zhang, W. Song, Y. Lua and W. Lin, Analyst, 2019, 144, 4371–4379 RSC.
- S. D. Meo, T. T. Reed, P. Venditti and V. M. Victor, Oxid. Med. Cell. Longevity, 2016, 2016, e1245049, DOI:10.1155/2016/1245049.
- P. Monostori, G. Wittmann, E. Karg and S. Túri, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2009, 877, 3331–3346 CrossRef CAS PubMed.
- Y. Iwasaki, Y. Saito, Y. Nakano, K. Mochizuki, O. Sakata, R. Ito, K. Saito and H. Nakazawa, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2009, 877, 3309–3317 CrossRef CAS PubMed.
- D. G. Searcy and M. A. Peterson, Anal. Biochem., 2004, 324, 269–275 CrossRef CAS PubMed.
- P. R. Bérubé, P. D. Parkinson and E. R. Hall, J. Chromatogr. A, 1999, 830, 485–489 CrossRef.
- U. Pantke, T. Volk, M. Schmutzler, W. J. Kox, N. Sitte and T. Grune, Free Radical Biol. Med., 1999, 27, 1080–1086 CrossRef CAS PubMed.
- M. Tarvin, B. McCord, K. Mount, K. Sherlach and M. L. Miller, J. Chromatogr. A, 2010, 1217, 7564–7572 CrossRef CAS PubMed.
- J. L. Kolanowski, F. Liu and E. J. New, Chem. Soc. Rev., 2018, 47, 195–208 RSC.
- H. Kimura, Antioxid. Redox Signaling, 2010, 12, 1–13, DOI:10.1089/ars.2009.2919.
- L. Yang, Y. Zhang, X. Ren, B. Wang, Z. Yang, X. Song and W. Wang, Anal. Chem., 2020, 92, 4387–4394 CrossRef CAS PubMed.
- Y. Kimura, Y. Mikami, K. Osumi, M. Tsugane, J. Oka and H. Kimura, FASEB J., 2013, 27, 2451–2457 CrossRef CAS PubMed.
- Y. Huang, F. Yu, J. Wang and L. Chen, Anal. Chem., 2016, 88, 4122–4129 CrossRef CAS PubMed.
- M. Gao, X. Zhang, Y. Wang, Q. Liu, F. Yu, Y. Huang, C. Ding and L. Chen, Anal. Chem., 2019, 91, 7774–7781 CrossRef CAS PubMed.
- Y. Ma, Y. Tang, Y. Zhao and W. Lin, Anal. Chem., 2019, 91, 10723–10730 CrossRef CAS PubMed.
- Y. Zhang, L. Guan, H. Yu, Y. Yan, L. Du, Y. Liu, M. Sun, D. Huang and S. Wang, Anal. Chem., 2016, 88, 4426–4431 CrossRef CAS PubMed.
- A. Kubo, H. Saji, K. Tanaka and N. Kondo, Plant Mol. Biol., 1995, 29, 479–489 CrossRef CAS PubMed.
- K. Dou, Q. Fu, G. Chen, F. Yu, Y. Liu, Z. Cao, G. Li, X. Zhao, L. Xia, L. Chen, H. Wang and J. You, Biomaterials, 2017, 133, 82–93 CrossRef CAS PubMed.
- S. C. Lu, Mol. Aspects Med., 2009, 30, 42 CrossRef CAS PubMed.
- A. C. Sedgwick, H. H. Han, J. E. Gardiner, S. D. Bull, X. P. He and T. D. James, Chem. Sci., 2018, 9, 3672–3676 RSC.
- L. Wu, H. H. Han, L. Liu, J. E. Gardiner, A. C. Sedgwick, C. Huang, S. D. Bull, X. P. He and T. D. James, Chem. Commun., 2018, 54, 11336–11339 RSC.
- T. L. Zhu, N. Ren, X. Liu, Y. Dong, R. C. Wang, J. Z. Gao, J. Sun, Y. Zhu, L. H. Wang, C. H. Fan, H. Tian, J. Li and C. C. Zhao, Angew. Chem., Int. Ed., 2021, 60, 8450–8454 CrossRef CAS PubMed.
- S. Lee, E. Y. Tak, J. S. Lee, M. Rashid, M. P. Murphy, J. H. Ha and S. S. Kim, Cell Res., 2011, 21, 817–834 CrossRef CAS PubMed.
- Z. Wu, M. M. Liu, Z. C. Liu and Y. Tian, J. Am. Chem. Soc., 2020, 142, 7532–7541 CrossRef CAS PubMed.
- L. L. Wu, J. H. Liu, X. Tian, R. R. Groleau, B. D. Feng, Y. G. Yang, A. C. Sedgwick, H. H. Han, Y. Wang, H. M. Wang, F. Huang, P. Li, B. Tang, T. D. James and J. L. Sessler, J. Am. Chem. Soc., 2022, 144, 174–183 CrossRef CAS PubMed.
- L. Ferrero-Miliani, O. H. Nielsen, P. S. Andersen and S. E. Girardin, Clin. Exp. Immunol., 2007, 147, 227–235 CrossRef CAS PubMed.
- M. Fioranelli, M. G. Roccia, D. Flavin and L. Cota, Int. J. Mol. Sci., 2021, 22, 5277 CrossRef CAS PubMed.
- G. Y. Chen and G. Nuñe, Nat. Rev. Immunol., 2010, 10, 826–837 CrossRef CAS PubMed.
- N. Chiang, X. D. L. Rosa, S. Libreros, H. Pan, J. M. Dreyfuss and C. N. Serhan, Proc. Natl. Acad. Sci. U. S. A., 2021, 118, e2013374118 CrossRef CAS PubMed.
- (a) A. de Almeida, M. de Almeida Rezende, S. H. Dantas, S. de Lima Silva, J. de Oliveira, F. de Azevedo, R. F. R. Alves, G. de Menezes, P. Santos, T. A. F. Goncalves, V. B. Schini-Kerth and I. de Medeiros, Oxid. Med. Cell. Longevity, 2020, 2020, 1954398, DOI:10.1155/2020/1954398; (b) S. K. Biswas, Oxid. Med. Cell. Longevity, 2016, 2016, 5698931, DOI:10.1155/2016/5698931.
- G. Y. Chen and G. Nuñez, Nat. Rev. Immunol., 2010, 10, 826–837 CrossRef CAS PubMed.
- S. X. Liu, F. F. Hou, Z. J. Guo, R. Nagai, W. R. Zhang, Z. Q. Liu, Z. M. Zhou, M. Zhou, D. Xie, G. B. Wang and X. Zhang, Arterioscl. Throm. Vas., 2006, 26, 1156–1162 CrossRef CAS PubMed.
- A. Popa-Wagner, S. Mitran, S. Sivanesan, E. Chang and A. M. Buga, Oxid. Med. Cell. Longevity, 2013, 2013, 963520, DOI:10.1155/2013/963520.
- L. M. Coussens and Z. Werb, Nature, 2002, 420, 860–867 CrossRef CAS PubMed.
- S. Tsalamandris, A. S. Antonopoulos, E. Oikonomou, G. A. Papamikroulis, G. Vogiatzi, S. Papaioannou, S. Deftereos and D. Tousoulis, Eur. Cardiol. Rev., 2019, 14, 50–59 Search PubMed.
- J. Huang, U. M. Upadhyay and R. J. Tamargo, Surg. Neurol., 2006, 66, 232–245 CrossRef PubMed.
- P. Libby, Nature, 2002, 420, 868–874 CrossRef CAS PubMed.
- Z. G. Song, D. Mao, S. H. P. Sung, R. T. K. Kwork, J. W. Y. Lam, D. Kong, D. Ding and B. Z. Tang, Adv. Mater., 2016, 28, 7249–7256 CrossRef CAS PubMed.
- Y. Z. Chen, X. M. Shi, Z. L. Lu, X. F. Wang and Z. Wang, Anal. Chem., 2017, 89, 5278–5284 CrossRef CAS PubMed.
- X. M. Han, Y. F. Ma, Y. Z. Chen, X. F. Wang and Z. Wang, Anal. Chem., 2020, 92, 2830–2838 CrossRef CAS PubMed.
- M. Weber, H. H. Han, B. H. Li, M. L. Odyniec, C. E. F. Jarman, Y. Zang, S. D. Bull, A. B. Mackenzie, A. C. Sedgwick, J. Li, X. P. He and T. D. James, Chem. Sci., 2020, 11, 8567–8571 RSC.
- L. L. Wu, C. S. Huang, B. P. Emery, A. C. Sedgwick, S. D. Bull, X.-P. He, H. Tian, J. Y. Yoon, J. L. Sessler and T. D. James, Chem. Soc. Rev., 2020, 49, 5110–5139 RSC.
- D. Cheng, Y. Pan, L. Wang, Z. B. Zeng, L. Yuan, X. B. Zhang and Y. T. Chang, J. Am. Chem. Soc., 2017, 139, 285–292 CrossRef CAS PubMed.
- X. X. Chen, X. X. Ren, L. L. Zhang, Z. J. Liu and Z. J. Hai, Anal. Chem., 2020, 92, 14244–14250 CrossRef CAS PubMed.
- X. Zhang, Y. Chen, H. S. He, S. F. Wang, Z. H. Lei and F. Zhang, Angew. Chem., Int. Ed., 2021, 60, 26337–26341 CrossRef CAS PubMed.
- L. He, L. H. He, S. Xu, T. B. Ren, X. X. Zhang, Z. J. Qin, X. B. Zhang and L. Yuan, Angew. Chem., Int. Ed., 2022, 61, e202211409 CAS.
- S. Ogura and T. Shimosawa, Curr. Hypertens. Rep., 2014, 16, 452–456, DOI:10.1007/s11906-014-0452-x.
- T. Kalogeris, Y. Bao and R. J. Korthuis, Redox Biol., 2014, 2, 702–714 CrossRef CAS PubMed.
- K. Rashid, K. Sinha and P. C. Sil, Food Chem. Toxicol., 2013, 62, 584–600 CrossRef CAS PubMed.
- T. Kalogeris, C. P. Baines, M. Krenz and R. J. Korthuis, Int. Rev. Cell Mol. Biol., 2012, 298, 229–317 CAS.
- A. M. Larson, J. Polson, R. J. Fontana, T. J. Davern, E. Lalani, L. S. Hynan, J. S. Reisch, F. V. Schiødt, G. Ostapowicz, A. O. Shakil and W. M. Lee, Hepatology, 2005, 42, 1364–1372 CrossRef CAS PubMed.
- J. Salas, B. Chen, A. Wong, S. Duarte, S. Angarita, G. Lipshutz, O. Witte and P. Clark, J. Nucl. Med., 2018, 59, 1308–1315 CrossRef CAS PubMed.
- N. Foley, S. Marshall, J. Pikul, K. Salter and R. Teasell, J. Neurotraum., 2008, 25, 1415–1431 CrossRef PubMed.
- Y. T. Yang, T. T. Zhou, M. Jin, K. Y. Zhou, D. D. Liu, X. Li, F. J. Huo, W. Li and C. X. Yin, J. Am. Chem. Soc., 2020, 142, 1614–1620 CrossRef CAS PubMed.
- A. Jain, J. Mårtensson, E. Stole, P. A. M. Auld and A. Meister, Proc. Natl. Acad. Sci. U. S. A., 1991, 88, 1913–1917 CrossRef CAS PubMed.
- X. Zhang, Y. Huang, X. Y. Han, Y. Wang, L. W. Zhang and L. Chen, Anal. Chem., 2019, 91, 14728–14736 CrossRef CAS PubMed.
- Y. Zhai, H. Petrowsky, J. C. Hong, R. W. Busuttil and J. W. Kupiec-Weglinski, Nat. Rev. Gastroenterol. Hepatol., 2013, 10, 79–89 CrossRef CAS PubMed.
- X. Y. Han, R. Wang, X. Y. Song, F. B. Yu, C. J. Lv and L. X. Chen, Biomaterials, 2018, 156, 134–146 CrossRef CAS PubMed.
- R. F. Xu, Y. Wang, H. Y. You, L. W. Zhang, Y. Q. Wang and L. X. Chen, Analyst, 2019, 144, 2556–2564 RSC.
- R. J. Lu, Y. Zhang, F. L. Tang, Z. W. Zheng, Z. D. Fan, S. M. Zhu, X. F. Qian and N. N. Liu, Exp. Ther. Med., 2016, 12, 2606–2616, DOI:10.3892/etm.2016.3627.
- J. R. Senior, Drug Saf., 2014, 37, 9–17, DOI:10.1007/s40264-014-0182-7.
- (a) H. Jaeschke, G. J. Gores, A. I. Cederbaum, J. A. Hinson, D. Pessayre and J. J. Lemasters, Toxicol. Sci., 2002, 65, 166–176 CrossRef CAS PubMed; (b) J. S. Walsh and G. T. Miwa, Annu. Rev. Pharmacol., 2011, 51, 145–167 CrossRef CAS PubMed.
- Z. J. Qin, T. B. Ren, H. J. Zhou, X. X. Zhang, L. He, Z. Li, X. B. Zhang and L. Yuan, Angew. Chem., Int. Ed., 2022, 61, e202201541, DOI:10.1002/anie.202201541.
- L. L. Wu, J. H. Liu, X. Tian, R. R. Groleau, S. D. Bull, P. Li, B. Tang and T. D. James, Chem. Sci., 2021, 12, 3921–3928 RSC.
- X. Y. Jiao, Y. S. Xiao, Y. Li, M. W. Liang, X. L. Xie, X. Wang and B. Tang, Anal. Chem., 2018, 90, 7510–7516 CrossRef CAS PubMed.
- N. Sandhu and V. Navarro, Hepatol. Commun., 2020, 4, 631–645 CrossRef PubMed.
- L. H. Sun, J. Ouyang, Y. Q. Ma, Z. Zeng, C. Zeng, F. Zeng and S. Z. Wu, Adv. Healthcare Mater., 2021, 10, 2100867 CrossRef CAS PubMed.
- D. Sasaki, A. Yamada, H. Umeno, H. Kurihara, S. Nakatsuji, S. Fujihira, K. Tsubota, M. Ono, A. Moriguchi, K. Watanabe and J. Seki, Biomarkers, 2011, 16, 553–566 Search PubMed.
- X. L. Wang, J. Lewis, L. Appel, D. Cheek, G. Contreras, M. Faulkner, H. Feldman, J. Gassman, J. Lea, J. Kopple, M. Sika, R. Toto and T. Greene, J. Am. Soc. Nephrol., 2006, 17, 2900–2909 CrossRef CAS PubMed.
- H. W. Liu, H. Y. Zhang, X. F. Lou, L. L. Teng, J. Yuan, L. Yuan, X. B. Zhang and W. H. Tan, Chem. Commun., 2020, 56, 8103–8106 RSC.
- L. Y. Liu, L. P. Jiang, W. Yuan, Z. K. Liu, D. Y. Liu, P. Wei, X. Y. Zhang and T. Yi, ACS Sens., 2020, 5, 2457–2466 CrossRef CAS PubMed.
- A. Wimo, L. Jönsson, J. Bond, M. Prince and B. Winblad, Alzheimers Dement., 2013, 9, 1–11 CrossRef PubMed.
- R. L. Buckner, Neuron, 2004, 44, 195–208 CrossRef CAS PubMed.
- J. A. Hardy and G. A. Higgins, Science, 1992, 256, 184–185 CrossRef CAS PubMed.
- I. Grundke-Iqbal, K. Iqbal, Y. C. Tung, M. Quinlan, H. M. Wisniewski and L. I. Binder, Proc. Natl. Acad. Sci. U. S. A., 1986, 83, 4913–4917 CrossRef CAS PubMed.
- M. G. Savelieff, G. Nam, J. Kang, H. J. Lee, M. Lee and M. H. Lim, Chem. Rev., 2019, 119, 1221–1322 CrossRef CAS PubMed.
- K. P. Kepp, Chem. Rev., 2012, 112, 5193–5239 CrossRef CAS PubMed.
- D. J. Bonda, H. G. Lee, J. A. Blair, X. W. Zhu, G. Perry and M. A. Smith, Metallomics, 2011, 3, 267–270 CrossRef CAS PubMed.
- C. Behl, J. B. Davis, R. Lesley and D. Schubert, Cell, 1994, 77, 817–827 CrossRef CAS PubMed.
- H. M. Abdul, R. Sultana, J. N. Keller, D. K. S. Clair, W. R. Markesbery and D. A. Butterfield, J. Neurochem., 2006, 96, 1322–1335 CrossRef CAS PubMed.
- M. A. Deibel, W. D. Ehmann and W. R. Markesbery, J. Neurol. Sci., 1996, 143, 137–142 CrossRef CAS PubMed.
- K. M. Boje and P. K. Arora, Brain Res., 1992, 587, 250–256 CrossRef CAS PubMed.
- A. C. Sedgwick, W. T. Dou, J. B. Jiao, L. L. Wu, G. T. Williams, A. T. A. Jenkins, S. D. Bull, J. L. Sessler, X. P. He and T. D. James, J. Am. Chem. Soc., 2018, 140, 14267–14271 CrossRef CAS PubMed.
- X. L. Xie, G. Z. Liu, Y. X. Niu, C. H. Xu, Y. Li, J. Zhang, X. Y. Jiao, X. Wang and B. Tang, Anal. Chem., 2021, 93, 15088–15095 CrossRef CAS PubMed.
- J. Yang, J. Yang, S. H. Liang, Y. G. Xu, A. Moore and C. Z. Ran, Sci. Rep., 2016, 6, 35613 CrossRef CAS PubMed.
- A. M. Aleardi, G. Benard, O. Augereau, M. Malgat, J. C. Talbot, J. P. Mazat, T. Letellier, J. Dachary-Prigent, G. C. Solaini and R. J. Rossignol, J. Bioenerg. Biomembr., 2005, 37, 207–225, DOI:10.1007/s10863-005-6631-3.
- A. Bobba, G. Amadoro, D. Valenti, V. Corsetti, R. Lassandro and A. Atlante, Mitochondrion, 2013, 13, 298–311 CrossRef CAS PubMed.
- S. J. Li, P. P. Wang, W. Q. Feng, Y. H. Xiang, K. Dou and Z. H. Liu, Chem. Commun., 2020, 56, 1050–1053 RSC.
- J. Yang, X. L. Zhang, P. Yuan, J. Yang, Y. G. Xu, J. Grutzendler, Y. H. Shao, A. Moore and C. Z. Ran, Proc. Natl. Acad. Sci. U. S. A., 2017, 114, 12384–12389 CrossRef CAS PubMed.
- A. I. Bush and R. E. Tanzi, Neurotherapeutics, 2008, 5, 421–432 CrossRef CAS PubMed.
- L. V. Kalia and A. E. Lang, Lancet, 2015, 386, 896–912 CrossRef CAS PubMed.
- Y. H. Fu, G. Paxinos, C. Watson and G. M. Halliday, J. Chem. Neuroanat., 2016, 76, 98–107, DOI:10.1016/j.jchemneu.2016.02.001.
- A. Yoritaka, N. Hattori, K. Uchida, M. Tanaka, E. R. Stadtman and Y. Mizuno, Proc. Natl. Acad. Sci. U. S. A., 1996, 93, 2696–2701 CrossRef CAS PubMed.
- E. Floor and M. G. Wetzel, J. Neurochem., 1998, 70, 268–275 CrossRef CAS PubMed.
- D. T. Dexter, C. J. Carter, F. R. Wells, F. Javoy-Agid, Y. Agid, A. Lees, P. Jenner and C. D. Marsden, J. Neurochem., 1989, 52, 381–389 CrossRef CAS PubMed.
- Z. I. Alam, A. Jenner, S. E. Daniel, A. J. Lees, N. Cairns, C. D. Marsden, P. Jenner and B. Halliwell, J. Neurochem., 1997, 69, 1196–1203 CrossRef CAS PubMed.
- J. Zhang, G. Perry, M. A. Smith, D. Robertson, S. J. Olson, D. G. Graham and T. J. Montine, Am. J. Pathol., 1999, 154, 1423–1429 CrossRef CAS PubMed.
- J. Sian, D. T. Dexter, A. J. Lees, S. Daniel, Y. Agid, F. Javoy-Agid, P. Jenner and C. D. Marsden, Ann. Neurol., 1994, 36, 348–355 CrossRef CAS PubMed.
- J. R. Richardson, Y. Quan, T. B. Sherer, J. T. Greenamyre and G. W. Miller, Toxicol. Sci, 2005, 88, 193–201 CrossRef CAS PubMed.
- J. Callio, T. D. Oury and C. T. Chu, J. Biol. Chem., 2005, 280, 18536–18542 CrossRef CAS PubMed.
- A. Navarro and A. Boveris, Front. Aging Neurosci., 2010, 2, 34, DOI:10.3389/fnagi.2010.00034.
- H. Li, C. Xin, G. Zhang, X. Han, C. Yu, L. Li and W. Huang, J. Mater. Chem. B, 2019, 7, 4243–4251 RSC.
- M. Weng, X. Yang, Y. Ni, C. Xu, H. Zhang, J. Shao, C. Zhang and L. Li, Sens. Actuators, B, 2019, 283, 769–775 CrossRef CAS.
- Y. Liu, L. Bei, Y. Li, C. Xin, C. Zhang, J. Liu, Z. Liu, L. Li and W. Huang, Sens. Actuators, B, 2019, 279, 38–43 CrossRef CAS.
- H. C. Cubukcu, M. Yurtdas, Z. E. Durak, B. Aytac, H. N. Gunes, B. G. Cokal, T. K. Yoldas and I. Durak, Neurol. Sci., 2016, 37, 1793–1798 CrossRef PubMed.
- Q. Sun, J. Xu, C. Ji, Z. Li, K. Lim, C. Zhang, L. Li and Z. Liu, Anal. Chem., 2020, 92, 4038–4045 CrossRef CAS PubMed.
- C. C. T. Aguiar, A. B. Almeida, P. V. P. Araújo, R. N. D. C. Abreu, E. M. C. Chaves and O. C. Vale, Oxid. Med. Cell. Longevity, 2012, 2012, 12, DOI:10.1155/2012/795259.
- S. D. Shorvon, Epilepsia, 2011, 52, 1052–1057 CrossRef PubMed.
- A. K. Ngugi, C. Bottomley, I. Kleinschmidt, J. W. Sander and C. R. Newton, Epilepsia, 2010, 51, 883–890 CrossRef PubMed.
- E. J. Shin, J. H. Jeong, Y. H. Chung, W. K. Kim, K. H. Ko, J. H. Bach, J. S. Hong, Y. Yoneda and H. C. Kim, Neurochem. Int., 2011, 59, 122–137 CrossRef CAS PubMed.
- M. Patel, L. Liang and L. J. Roberts, J. Neurochem., 2001, 79, 1065–1069 CrossRef CAS PubMed.
- E. Candelario-Jalil, H. H. Ajamieh, S. Sam, G. Martínez and O. S. León Fernández, Eur. J. Pharmacol., 2000, 390, 295–298 CrossRef CAS PubMed.
- L. P. Liang, Y. Ho and M. Patel, Neuroscience, 2000, 101, 563–570 CrossRef CAS PubMed.
- M. Patel, Free Radical Biol. Med., 2004, 37, 1951–1962 CrossRef CAS PubMed.
- L. P. Liang and M. Patel, Free Radical Biol. Med., 2006, 40, 316–322 CrossRef CAS PubMed.
- L. P. Liang and M. Patel, Free Radical Biol. Med., 2004, 36, 542–554 CrossRef CAS PubMed.
- Y. Zhang, D. P. Seeburg, B. Pulli, G. R. Wojtkiewicz, L. Bure, W. Atkinson, S. Schob, Y. Iwamoto, M. Ali, W. Zhang, E. Rodriguez, A. Milewski, E. J. Keliher, C. Wang, Y. Pan, F. K. Swirski and J. W. Chen, Radiology, 2016, 278, 822–830 CrossRef PubMed.
- C. W. Shao, J. W. Yuan, Y. N. Liu, Y. J. Qin, X. A. Wang, J. Gu, J. Zhao, H. L. Zhu and Y. Qian, Proc. Natl. Acad. Sci. U. S. A., 2020, 117, 10155–10164 CrossRef CAS PubMed.
- J. Feng, X. Chen, B. Guan, C. Li, J. Qiu and J. Shen, Mol. Neurobiol., 2018, 55, 6369–6386 CrossRef CAS PubMed.
- Y. C. Chuang, S. D. Chen, C. W. Liou, T. K. Lin, W. N. Chang, S. H. Chan and A. Y. Chang, Epilepsia, 2009, 50, 731–746 CrossRef CAS PubMed.
- X. Luo, Z. Cheng, R. Wang and F. Yu, Anal. Chem., 2021, 93, 2490–2499 CrossRef CAS PubMed.
- J. S. Hu, C. Shao, X. Wang, X. Di, X. Xue, Z. Su, J. Zhao, H. L. Zhu, H. K. Liu and Y. Qian, Adv. Sci., 2019, 6, 1900341 CrossRef PubMed.
- S. Li, D. Song, W. Huang, Z. Li and Z. Liu, Anal. Chem., 2020, 92, 2802–2808 CrossRef CAS PubMed.
- (a) H. P. Kapfhammer, Dialogues. Clin. Neuro, 2006, 8, 227–239, DOI:10.31887/DCNS.2006.8.2/hpkapfhammer; (b) C. Mulholland and S. Cooper, Adv. Psychiatr. Treat., 2000, 6, 169–177, DOI:10.1192/apt.6.3.169.
- (a) B. Harerimana, C. Forchuk and T. O'Regan, Int. J. Mental. Health. Nurs., 2019, 28, 657–670 CrossRef PubMed; (b) M. J. Knol, J. W. R. Twisk, A. T. F. Beekman, R. J. Heine, F. J. Snoek and F. Pouwer, Diabetol., 2006, 49, 837–845 CrossRef CAS PubMed.
- M. D. Cabana, J. L. Rushton and A. J. Rush, Gen. Hosp. Psychiatry, 2022, 24, 35–42 CrossRef PubMed.
- E. A. Phelps and J. E. LeDoux, Neuron, 2005, 48, 175–187 CrossRef CAS PubMed.
- V. Krishnan and E. J. Nestler, Nature, 2008, 455, 894–902 CrossRef CAS PubMed.
- E. J. Nestler, M. Barrot, R. J. Dileone, A. J. Eisch, S. J. Gold and L. M. Monteggia, Neuron, 2002, 34, 13–25 CrossRef CAS PubMed.
- M. Maes, P. Galecki, Y. S. Chang and M. Berk, Prog. Neuropsychopharmacol. Biol. Psychiatry, 2011, 35, 676–692 CrossRef CAS PubMed.
- M. Maes, I. Mihaylova, M. Kubera, M. Uytterhoeven, N. Vrydags and E. Bosmans, J. Affective Disord., 2010, 125, 287–294 CrossRef CAS PubMed.
- T. M. Michel, S. Camara, T. Tatschner, S. Frangou, A. J. Sheldrick, P. Riederer and E. Grunblatt, World J. Biol. Psychiatry, 2010, 11, 314–320 CrossRef PubMed.
- M. Maes, N. D. Vos, R. Pioli, P. Demedts, A. Wauters, H. Neels and A. Christophe, J. Affective Disord., 2000, 58, 241–246 CrossRef CAS PubMed.
- J. Kodydková, L. Vávrová, M. Zeman, R. Jirák, J. Macásek and B. Stanková, Clin. Biochem., 2009, 42, 1368–1374 CrossRef PubMed.
- M. Maes, I. Mihaylova, M. Kubera, M. Uytterhoeven, N. Vrydags and E. Bosmans, Neuroendocrinol. Lett., 2009, 30, 470–476 CAS.
- A. C. Andreazza, F. Kapczinski, M. Kauer-Sant'Anna, J. C. Walz, D. J. Bond, C. A. Gonçalves and L. N. Yatham, J. Psychiatry Neurosci., 2009, 34, 263–271 Search PubMed.
- R. Edwards, M. Peet, J. Shay and D. Horrobin, J. Affective Disord., 1998, 48, 149–155 CrossRef CAS PubMed.
- M. J. Forlenza and G. E. Miller, Psychosom. Med., 2006, 68, 1–7 CrossRef CAS PubMed.
- X. Wang, P. Li, Q. Ding, C. Wu, W. Zhang and B. Tang, Angew. Chem., Int. Ed., 2019, 58, 4674–4678 CrossRef CAS PubMed.
- B. C. Dickinson and C. J. Chang, Nat. Chem. Biol., 2011, 7, 504–511 CrossRef CAS PubMed.
- J. C. Chen and J. M. Samet, Eur. J. Epidemiol., 2017, 32, 943–946 CrossRef PubMed.
- P. Li, J. Wang, X. Wang, Q. Ding, X. Bai, Y. Zhang, D. Su, W. Zhang, W. Zhang and B. Tang, Chem. Sci., 2019, 10, 2805–2810 RSC.
- P. Li, X. Shi, H. Xiao, Q. Ding, X. Bai, C. Wu, W. Zhang and B. Tang, Analyst, 2019, 144, 191–196 RSC.
- Y. Zhang, X. Wang, X. Bai, P. Li, D. Su, W. Zhang, W. Zhang and B. Tang, Anal. Chem., 2019, 91, 8591–8594 CrossRef CAS PubMed.
- M. J. Fowler, Clin. Diabetes Res., 2008, 26, 77–82, DOI:10.2337/diaclin.26.2.77.
- U. Asmat, K. Abad and K. Ismail, Saudi Pharm. J., 2016, 24, 547–553 CrossRef PubMed.
- M. Halim and A. Halim, Diabetes Metab. Syndr., 2019, 13, 1165–1172, DOI:10.1016/j.dsx.2019.01.040.
- A. C. Maritim, R. A. Sanders and J. B. Watkins, J. Biochem. Mol. Toxicol., 2003, 17, 24–38, DOI:10.1002/jbt.10058.
- L. A. Pham-Huy, H. He and C. Pham-Huy, Int. J. Biomed. Sci., 2008, 4, 89–96 CAS.
- J. L. Figarola, S. Scott, S. Loera, C. Tessler, P. Chu, L. Weiss, J. Hardy and S. Rahbar, Diabetologia, 2003, 46, 1140–1152, DOI:10.1007/s00125-003-1162-0.
- D. Prabakaran and N. Ashokkumar, Biochimie, 2013, 95, 366–373 CrossRef CAS PubMed.
- K. S. Park, J. H. Kim, M. S. Kim, J. M. Kim, S. K. Kim, J. Y. Choi, M. H. Chung, B. Han, S. Y. Kim and H. K. Lee, Diabetes, 2001, 50, 2837–2841 CrossRef CAS PubMed.
- R. V. Sekhar, S. V. McKay, S. G. Patel, A. P. Guthikonda, V. T. Reddy, A. Balasubramanyam and F. Jahoor, Diabetes Care, 2010, 34, 162–167, DOI:10.2337/dc10-1006.
- A. R. Smith, S. V. Shenvi, M. Widlansky, J. H. Suh and T. M. Hagen, Curr. Med. Chem., 2004, 11, 1135–1146 CrossRef CAS PubMed.
- F. Sun, K. Iwaguchi, R. Shudo, Y. Nagaki, K. Tanaka, K. Ikeda, S. Tokumaru and S. Kojo, Clin. Sci., 1999, 96, 185–190, DOI:10.1042/cs0960185.
- J. Miao, Y. Huo, Q. Liu, Z. Li, H. Shi, Y. Shi and W. Guo, Biomaterials, 2016, 107, 33–43 CrossRef CAS PubMed.
- J. Miao, Y. Huo, H. Shi, J. Fang, J. Wang and W. Guo, J. Mater. Chem. B, 2018, 6, 4466–4473 RSC.
- H. Xiao, X. Liu, C. Wu, Y. Wu, P. Li, X. Guo and B. Tang, Biosens. Bioelectron., 2017, 91, 449–455 CrossRef CAS PubMed.
- W. X. Wang, W. L. Jiang, G. J. Mao, M. Tan, J. Fei, Y. Li and C. Y. Li, Anal. Chem., 2021, 93, 3301–3307 CrossRef CAS PubMed.
- A. Jemal, R. Siegel, J. Xu and E. Ward, CA-Cancer J. Clin., 2010, 60, 277–300, DOI:10.3322/caac.20073.
- S. Reuter, S. C. Gupta, M. M. Chaturvedi and B. B. Aggarwal, Free Radical Biol. Med., 2010, 49, 1603–1616 CrossRef CAS PubMed.
- J. D. Hayes, A. T. Dinkova-Kostova and K. D. Tew, Cancer Cell, 2020, 38, 167–197 CrossRef CAS PubMed.
- T. Kamata, Cancer Sci., 2009, 100, 1382–1388 CrossRef CAS PubMed.
- S. Danson, T. H. Ward, J. Butler and M. Ranson, Cancer Treat. Rev., 2004, 30, 437–449 CrossRef CAS PubMed.
- H. Zhang, J. Liu, C. Liu, P. Yu, M. Sun, X. Yan, J. Guo and W. Guo, Biomaterials, 2017, 133, 60–69 CrossRef CAS PubMed.
- J. Liu, M. Liu, H. Zhang and W. Guo, Angew. Chem., Int. Ed., 2021, 60, 12992–12998 CrossRef CAS PubMed.
- W. Wang, J. Xiong, X. Song, Z. Wang, F. Zhang and Z. Mao, Anal. Chem., 2020, 92, 13305–13312 CrossRef CAS PubMed.
- V. Ntziachristos, Nat. Methods, 2010, 7, 603–614 CrossRef CAS PubMed.
- J. Zhang, X. Zhen, J. Zeng and K. Pu, Anal. Chem., 2018, 90, 9301–9307 CrossRef CAS PubMed.
- J. Weber, L. Bollepalli, A. M. Belenguer, M. D. Antonio, N. D. Mitri, J. Joseph, S. Balasubramanian, C. A. Hunter and S. E. Bohndiek, Cancer Res., 2019, 79, 5407–5417 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2023 |