Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Topology- and wavelength-governed CO2 reduction photocatalysis in molecular catalyst-metal–organic framework assemblies†
Philip M.
Stanley‡
 a,
Karina
Hemmer‡
a,
Karina
Hemmer‡
 a,
Markus
Hegelmann
a,
Annika
Schulz
a,
Mihyun
Park
a,
Markus
Hegelmann
a,
Annika
Schulz
a,
Mihyun
Park
 a,
Martin
Elsner
a,
Martin
Elsner
 b,
Mirza
Cokoja
b,
Mirza
Cokoja
 a and
Julien
Warnan
a and
Julien
Warnan
 *a
*a
aChair of Inorganic and Metal–Organic Chemistry, Department of Chemistry, TUM School of Natural Sciences, Catalysis Research Center (CRC), Technical University of Munich, Garching, Germany. E-mail: julien.warnan@tum.de
bChair of Analytical Chemistry and Water Chemistry, Department of Chemistry, TUM School of Natural Sciences, Technical University of Munich, Garching, Germany
First published on 3rd October 2022
Abstract
Optimising catalyst materials for visible light-driven fuel production requires understanding complex and intertwined processes including light absorption and catalyst stability, as well as mass, charge, and energy transport. These phenomena can be uniquely combined (and ideally controlled) in porous host–guest systems. Towards this goal we designed model systems consisting of molecular complexes as catalysts and porphyrin metal–organic frameworks (MOFs) as light-harvesting and hosting porous matrices. Two MOF-rhenium molecule hybrids with identical building units but differing topologies (PCN-222 and PCN-224) were prepared including photosensitiser-catalyst dyad-like systems integrated via self-assembled molecular recognition. This allowed us to investigate the impact of MOF topology on solar fuel production, with PCN-222 assemblies yielding a 9-fold turnover number enhancement for solar CO2-to-CO reduction over PCN-224 hybrids as well as a 10-fold increase compared to the homogeneous catalyst-porphyrin dyad. Catalytic, spectroscopic and computational investigations identified larger pores and efficient exciton hopping as performance boosters, and further unveiled a MOF-specific, wavelength-dependent catalytic behaviour. Accordingly, CO2 reduction product selectivity is governed by selective activation of two independent, circumscribed or delocalised, energy/electron transfer channels from the porphyrin excited state to either formate-producing MOF nodes or the CO-producing molecular catalysts.
Introduction
Against the backdrop of increasing global energy requirements, photocatalysis has the potential to reduce the necessary energy for feedstock production and to transform waste into valuable feedstocks.1 Solar fuel production, merging solar energy harvesting with subsequent chemical energy conversion, is a pertinent route towards such artificial photosynthesis.2 The latter motivates bio-inspired molecular catalysts, fine-tuned to maximise selectivity and atom efficiency while minimising activation energy barriers.3,4 However, these are often mired by limited stability and device integrability, as well as modest light-harvesting efficiency for photocatalytic systems operating under broad-spectrum irradiation.1 Accordingly, current efforts synergise material and catalyst co-design to enable energy-intense reactions, such as selective CO2 reduction, in light-absorbing hybrids.3,4Here, metal–organic framework (MOF) materials show promise due to their highly controllable chemical and (photo)physical properties enabled by their modular assembly.5 This unlocks a breadth of differing porosities, topologies, pore sizes, guest inclusion opportunities, optoelectronic properties, and more, which intertwine and deeply condition reaction environments.6–8 Thus, combining molecular catalysts and MOFs to heterogeneous hybrids enables catalyst stabilisation and recyclability while also gaining control over its proximal and global environment.7,9,10 While previous reports on heterogeneous thermal MOF catalysis demonstrated that nanoreactors built by pore walls affect reaction activity, selectivity, and substrate diffusion to active sites,8,11 these findings are not generally transferable to systems with light-driven catalysis, cascade electroreductions or defined molecular complexes as the active species. Accordingly, challenges remain in the conceptual understanding of the intrinsic effects of a MOF pore environment on such molecular MOF hybrids towards rational design of effective materials for photocatalysis. Although a few studies have investigated pore size variation, topology-based substrate orientation, and active site distance optimisation, these parameters remain poorly understood and systematic studies on how MOF topology influences properties and solar fuel performances are needed.12–14
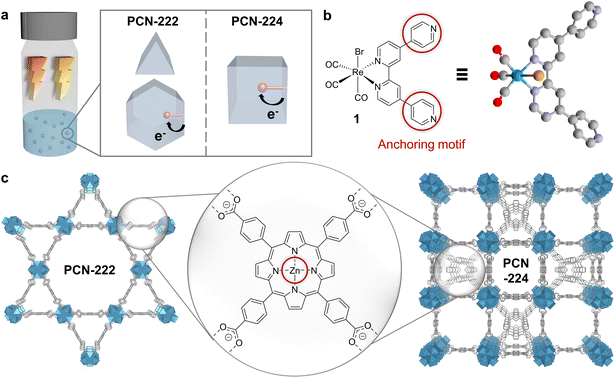
Herein, we designed two supramolecular MOF-catalyst hybrids constructed from identical building blocks but differing in topology (Fig. 1a). The assemblies consist of a fac-ReBr(CO)3(qtpy) (1, qtpy = 4,4′:2′,2′′:4,4′′′-quaterpyridine) CO2 reduction catalyst and Zn-metalated 5,10,15,20-tetrakis(4-carboxylphenyl)porphyrin (2-Zn) linker- and Zr6-oxo node-based MOFs, i.e., PCN-222 and PCN-224 (Fig. 1b and c). These MOFs were chosen as model systems for their broad visible-light absorption,15–18 and their reported directional exciton migration and energy funnelling to acceptors.19,20 While 1 has not been applied in photocatalytic CO2 reduction to date, its derivatives are known for selective CO formation under homogeneous and heterogeneous conditions, with turnover numbers (TONs) ranging from ∼10 to 4500 in the presence of sacrificial electron donors (SEDs).9,21–23
The MOF hosts, PCN-222 and PCN-224, were synthesised with high crystallinity, Zn metalated and then post-synthetically linker-functionalised with 1. In contrast to prior time-consuming multistep synthesis of dyadic porphyrin photosensitiser (PS) and Re-based catalyst assemblies (e.g., via alkyl chains, phenyl spacers, or amide group), here, interfacing was achieved in mild conditions through supramolecular Zn–N interactions.24–28 We took advantage of the penta-coordination preference of zinc in porphyrins and the strong affinity of azoarenes for Zn porphyrins to form a self-assembled dyadic system confined inside the porous network.29 Reminiscent of the Mg chlorophylls held by axial ligation of histidine on the natural light harvesting antennae protein scaffold, this approach enables the introduction of matching dyadic systems into two different MOF topologies towards monitoring the topology-catalysis relationships. Applying these Re-PCN colloids for photocatalytic CO2 reduction in acetonitrile (MeCN) with a SED showed a 9-fold TONCO enhancement for PCN-222 over PCN-224 hybrids as well as a 10-fold increase compared to the homogeneous catalyst-porphyrin dyad. Characterisations by powder X-ray diffraction (PXRD), solution and solid-state UV-vis, and attenuated total reflectance infrared (ATR-IR) spectroscopy; inductively coupled plasma mass spectrometry (ICP-MS); N2 adsorption; density functional theory (DFT) calculations and difference envelope density (DED) analysis uncovered mechanistic insights controlling reaction rate (via topology-guided mass and exciton transport) and selectivity (via wavelength-enabled electron/energy channel switching).
Results and discussion
Synthesis and characterisation
The quaterpyridine ligand and fac-ReBr(CO)3(qtpy) (1) were synthesised following literature known procedures,30,31 while the linker 5,10,15,20-tetrakis(4-carboxyphenyl)porphyrin (2) was prepared by saponification of 5,10,15,20-tetrakis(4-methoxycarbonylphenyl)-porphyrin (TPPCOOMe), attained from pyrrole and methyl 4-formylbenzoate.32 The Zn-metalated analogue of 2 (2-Zn) was prepared by reacting TPPCOOMe with ZnCl2, followed by saponification (details in the ESI) for benchmarking purposes. PCN-222 (222) and PCN-224 (224) syntheses were adapted from literature and the as-synthesised MOFs were treated with HCl in N,N-dimethylformamide (DMF) at 120 °C for modulator removal.14,33 Zinc metalation of the free base porphyrin linkers was performed in DMF at 100 °C for 24 h resulting in PCN-222(Zn) (222-Zn) and PCN-224(Zn) (224-Zn) (details and characterisation available in ESI†).34,351 was integrated into 222-Zn and 224-Zn, respectively, by soaking the MOFs in a 0.1 mM stock solution of 1 in MeCN resulting in 1@PCN-222(Zn) (Re-222) and 1@PCN-224(Zn) (Re-224), respectively, and isolated as powders. Loading of 1 was monitored by UV-vis measurements of the supernatant which displayed a strong decline in the intensity of the absorption bands of 1 after 24 h (Fig. S1†). After washing the composites with MeCN, no absorption bands of the catalyst were visible in the UV-vis spectrum of the washing solution, excluding strong leaching of the catalyst and underscoring robust Zn–N interactions (Fig. S2†). Control experiments performed by soaking the Zn-free MOF 222 or 224 in a 0.1 mM solution of 1 showed no decrease in supernatant absorption, confirming the specific dyadic anchoring site of 1 at Zn centres (Fig. S3†).
The unaltered PXRD data of both MOFs upon Zn metalation and Re catalyst incorporation revealed preserved phase purity and crystallinity while being in accordance with the simulated patterns (Fig. 2a). The ATR-IR spectra of both Re-222 and Re-224 display symmetric and asymmetric CO vibrations at 1926 and 2025 cm−1 and 1927 and 2024 cm−1, respectively (Fig. 2b and S4†), characteristic of 1, and suggesting successful entrapment as well as unaffected molecular structure after immobilisation in the MOFs.36 Furthermore, the shift of Zn–N bands at ∼398 cm−1 after loading of 1 hints at a comprehensive coordination of the Zn porphyrins (Fig. S5†).37
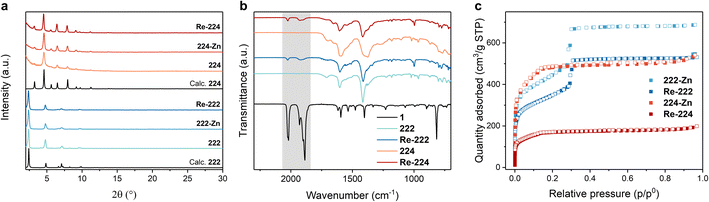 | ||
| Fig. 2 Material characterisation for pristine and loaded PCN-222 and PCN-224 assemblies. (a) Measured PXRD data. (b) ATR-IR spectra including the reference for pure catalyst 1 (black trace) and with the characteristic region for Re(CO)3 bands highlighted in grey. Full spectra in ESI.† (c) N2 sorption isotherms at 77 K. | ||
Solid-state UV-vis spectra showed comprehensive absorption in the visible-light region for both metalated MOFs, highlighting the suitability of these materials for broad range visible light harvesting (Fig. S6 and S7†). While 222 and 224 display broadened absorption features in line with 2's characteristic Soret band at 420 nm and the Q-bands in the range of 510–650 nm (Fig. S8†), hallmark symmetry-restoring changes were observed upon Zn metalation potentially indicating quantitative Zn metalation. In contrast, incorporation of 1 does not affect the absorption spectra suggesting that visible light irradiation will mainly result in MOF host excitation (Fig. S1, S6 and S7†).
ICP-MS performed after MOF digestion of 222-Zn and 224-Zn revealed Zr![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zn ratios of 1.8 and 1.9, respectively, indicating that every porphyrin is likely metalated (as Zr
Zn ratios of 1.8 and 1.9, respectively, indicating that every porphyrin is likely metalated (as Zr![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zntheoretical = 3 and 4, respectively, assuming reaction yield = 100%) and that additional Zn is physisorbed in the MOF pores (Table S1†). Together with the Zn-TCPP, these may act as additional anchoring sites for 1. Nevertheless, the Re content in Re-222 and Re-224 was found similar, ∼82 and ∼75 nmolRe mgMOF−1, respectively, further confirming the successful incorporation of 1 in 222-Zn and 224-Zn (∼0.1 catalyst per linker in both cases, see Table S1†). Loading values are comparable to other molecule-MOF hybrids which often use carboxylic or phosphonic acids for node- or linker-anchoring.9,12,19,38 Accordingly, our approach provides a synthetically straightforward alternative anchoring design to traditional acid groups (increasing the design space and assembly flexibility) while still affording stable tethering and similar loadings.
Zntheoretical = 3 and 4, respectively, assuming reaction yield = 100%) and that additional Zn is physisorbed in the MOF pores (Table S1†). Together with the Zn-TCPP, these may act as additional anchoring sites for 1. Nevertheless, the Re content in Re-222 and Re-224 was found similar, ∼82 and ∼75 nmolRe mgMOF−1, respectively, further confirming the successful incorporation of 1 in 222-Zn and 224-Zn (∼0.1 catalyst per linker in both cases, see Table S1†). Loading values are comparable to other molecule-MOF hybrids which often use carboxylic or phosphonic acids for node- or linker-anchoring.9,12,19,38 Accordingly, our approach provides a synthetically straightforward alternative anchoring design to traditional acid groups (increasing the design space and assembly flexibility) while still affording stable tethering and similar loadings.
Nitrogen adsorption measurements showed a reduction in the N2 quantity adsorbed for both 222-Zn and 224-Zn compared to the corresponding pristine MOFs. The Brunauer–Emmett–Teller (BET) surface area was reduced from ∼1990 to ∼1550 m2 g−1 for 222 and 222-Zn, and from ∼1980 to ∼1750 m2 g−1 for 224 and 224-Zn, respectively (Fig. S9 and S10†). Such a decrease upon metalation is common and has been observed for other PCN analogues.8,34 A further decrease of the N2 quantity adsorbed upon incorporating 1 is observable for both MOF topologies (Fig. 2c and S10†). The N2 quantity adsorbed in Re-224 is more dramatically reduced compared to Re-222, indicating a higher extent of pore blocking in the former case. This is further illustrated by the BET surface areas and calculated pore size distributions of ∼1219 m2 g−1 for Re-222, while a BET surface area of merely ∼618 m2 g−1 is obtained for Re-224. Scanning electron microscopy (SEM) images visualised rod-shaped ∼10 × 1 μm 222 crystals and cube-type ∼0.5 × 0.5 μm 224 crystals, in line with literature (Fig. S11 and S12†).14
Previous studies have reported on the measurements of conduction and valence band levels of PCN MOFs.39 Alternatively, a recent study on a PCN-molecular catalyst hybrid showed that complete isolation of the organic porphyrin linkers in a rigid structure with insulating metal nodes prevents an electronic coupling, concluding that excited energy levels of PCN MOFs can be regarded as those of the free linker.19 The oxidation potential of 2-Zn* (S1) was previously calculated at −1.24 V vs. saturated calomel electrode (VSCE).19 With E(1/1−) = −0.64 VSCE, photoinduced electron transfer from 2-Zn* to 1 in the PCN assemblies is thermodynamically possible, and was reported as the preferred first electron transfer step for Re-porphyrin dyads, as opposed to reductive quenching by the SED.24,25,31 In photocatalysis conditions, the corresponding oxidised PS at E = 0.88 VSCE will react with 1,3-dimethyl-2-phenyl-2,3-dihydro-1H-benzo[d]imidazole (BIH) employed as a benign SED to regenerate the PS ground state with E(BIH+/BIH) = 0.33 VSCE (Fig. S13†).39,40
In summary, assembly characterisation suggests similar catalyst loading and light-absorbing behaviour for both topologies, however, differing in permanent porosity and, accordingly, likely differing in substrate-catalyst accessibility.
Photocatalysis under broadband irradiation
Photocatalysis experiments were conducted under heterogeneous colloidal conditions, namely suspended MOF assembly (1.5 mg) in wet organic media (4 mL MeCN and 0.12 mL deionised H2O) and BIH (225 mg, 1 mmol) as the SED. The resulting suspension was saturated by bubbling CO2. Irradiation was performed with a Xenon Light Source (300 W, λ = 430–740 nm, ∼10 mW cm−2). CO and H2 evolution were analysed by gas chromatography of the reaction headspace, while formate formation was investigated by 1H NMR of the reaction solution.First, we examined the activity of the homogeneous dyadic PS-catalyst system as a benchmark for the Re-PCN assemblies towards CO2 reduction. To verify the dyadic system in solution, UV-vis spectra of 0.05 mM 2-Zn in MeCN before and after the addition of equimolar quantities of 1 were recorded (Fig. S14†). These displayed a 5 nm bathochromic shift of the Soret band from 414 to 419 nm indicating self-assembly of 1 and 2-Zn in solution.41
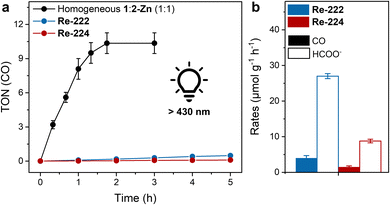
As ReX(CO)3(bpy)-based catalysts can photoreduce CO2 without an external photosensitiser,22,42 irradiation of 1 in the catalytic media (identical to heterogeneous conditions above) gave selective CO formation with a maximal TONCO of ∼5 after 2 h. Repeating the experiments in presence of an equimolar amount of 2-Zn yielded TONCO ∼10 (Fig. 3a and Table S2, see ESI† for TON calculation). While low stability under photocatalytic conditions is a common issue of Re catalysts, such a low activity compared to other reports on ReX(CO)3(bpy)-based catalysts and porphyrin–rhenium-catalyst dyads (with TONs in the low 100s) highlights the influence of the (pyridine) linking units.22,26–28,43
Remarkably, irradiating Re-222 colloids in photocatalytic media for 80 h conversely produced ∼27 μmolHCOO− g−1 h−1 (TONHCOO− per node ∼5) and subcatalytic amounts of CO (∼4 μmolCO g−1 h−1; TONCO per Re < 1) (Fig. 3a, b, Tables S3 and S4†). Incident photon conversion efficiency measurements and apparent quantum yield (AQY) calculations (details in ESI, Tables S5 and S6†) gave values of ∼0.01% (CO) and ∼0.10% (formate) at λ = 450 nm. AQYs in the range of 0.1–3.0% are common for colloidal visible light-driven CO2 reduction for comparable systems.19,44,45 Control photocatalysis experiments using Re-free 222-Zn showed no CO evolution but a comparable formate production rate, while experiments run without BIH or light yielded in all cases no detectable CO, H2 or formate (Table S3†). Product generation can be ascribed to light absorption by 2 followed by electron transfers to the CO-selective Re catalysts or to the Zr-oxo node-catalysts for formate production. The latter is in line with previous reports, and suggest two competing CO2 reduction mechanisms concomitantly occurring in Re-222.17,18,39,46 The corresponding Re-224-based experiments showed overall lower TONs and rates than for Re-222 but similar reactivity trends favouring formate over CO production, i.e., ∼2 μmolCO g−1 h−1 and ∼9 μmolHCOO− g−1 h−1 (Fig. 3a, b and Table S3†).
While interfacing the Re catalyst amid the MOF significantly increases its lifetime compared to when employed in homogenous conditions, its catalytic involvement is limited as formate evolution prevails. Together with impeded CO evolution, differing numerical performance between the two hybrid MOFs underlines a key influence of the host on the photocatalytic activity.
Post-catalysis PXRD analysis of Re-222 and Re-224 showed crystalline frameworks, while the Re(CO)3 moiety was no longer visible in the corresponding ATR-IR spectrum (Fig. S15 and S16†). This suggests that while the host is virtually stable under catalysis conditions, the catalyst is not, in line with its homogeneous instability.9,43,47
To examine the reasons for the differing product evolution rates in Re-222 and Re-224 samples, as well as the dramatic change of product selectivity between homogeneous and heterogeneous systems, the photocatalytic mechanism was investigated through spectroscopic and catalytic experiments.
Catalytic mechanism investigation and photocatalysis under selective irradiation
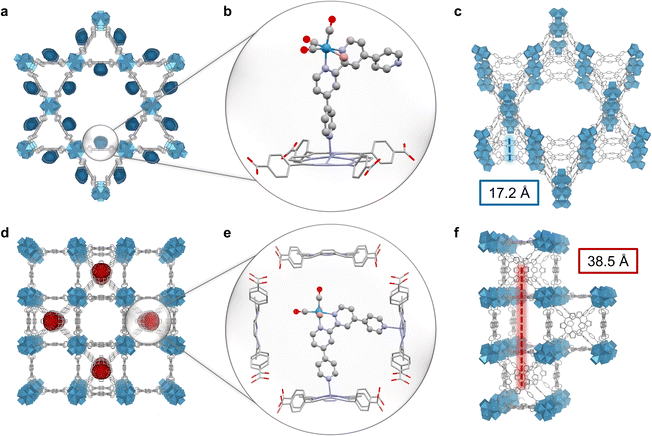
The topology of PCN-222 and -224 differs significantly. While 222 has hexagonal channels of ∼35 Å in size and trigonal channels of ∼10 Å, 224 has cubic pores/impeded channels with ∼20 Å diameter (Fig. 1c and S10†).8,13 The geometric structure of the catalyst optimised by DFT calculations yielded a maximum van-der-Waals sphere diameter of ∼13.5 Å, smaller than the MOFs' pore apertures (Fig. 1b, atomic coordinates in ESI†). Despite similar molecular loadings of 1, partial pore blocking is likely more prominent in Re-224 than Re-222 as shown by the former's lower N2 uptake (Fig. 2c and Table S1†). DED analysis was thus performed to map the electron density introduced by 1. Briefly, measured PXRD patterns were Le Bail-refined and the resulting structure factors were used to create structure envelopes for pristine and loaded MOFs, which were subtracted from each other (Fig. S17–S20, details in ESI†).48 For Re-222, 1 was found directed into the hexagonal channels leaving inner space for substrate diffusion (Fig. 4). In contrast, for Re-224, 1 fills almost the complete space of every second pore. These observations are supported by DFT calculations of 1 in a pore fragment of each MOF, confirming the catalyst positions suggested by DED analysis (Fig. 4a, b, d and e; details and atomic coordinates in ESI†). Specifically, for Re-224, this implies pore blocking potentially further rationalised by DFT calculations that also revealed the possibility of anchoring 1via two coordinating N–Zn bonds to neighbouring Zn-porphyrins in Re-224. Together with ATR-IR spectra (shift of Zn–N bands after 1 loading), steric pore restrictions (particularly for 224-Zn), and comparable catalyst loadings per linker for Re-222/Re-224, the DED analysis supports that anchoring of 1 occurs predominantly at the TCPP-bound Zn (Fig. 4, S5 and Table S1†). While not directly observed, partial coordination to physisorbed Zn cannot be ruled out.Overall, these analyses depict a more hindered nano-environment in Re-224 than in Re-222 potentially limiting reactant (e.g., BIH max. diameter = 10.6 Å),21 and product diffusion in line with a lower catalytic activity for the former.
Besides mass transport, transferring conclusions from recent reports on MOF-based exciton migration suggest that charge separation (CS) probability is significantly favoured for Re-222 over Re-224, due to topology-induced higher exciton hopping rates and a lower Förster radius R between chromophores (Förster resonance energy transfer scaling with R−6).20,49 Here, the translational hopping distance along the c-axis is ∼17 Å for 222, and ∼39 Å for 224 (Fig. 4c and f and non-translational distances in Fig. S21†). Additionally, 222's triangular motif with cofacial conformation translating along the lateral direction of the crystallographic c-axis further improves excited state delocalisation and hopping rates,50 due to head-to-tail coupling.51
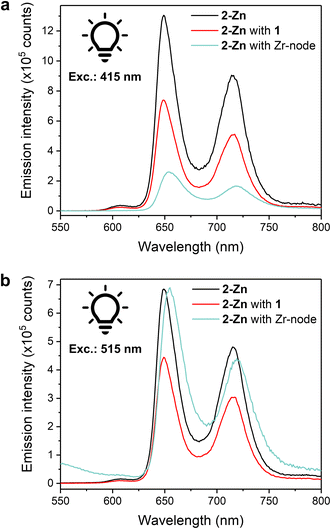
Steady-state luminescence measurements were conducted towards further understanding of photoinduced processes in our systems. Luminescence experiments on a 0.05 mM solution of 2-Zn in MeCN showed two prominent emission bands at λ = 649 and 714 nm upon excitation at either 415 or 515 nm (Soret or Q band, respectively) (Fig. 5a and b), matching literature reports ascribed to the de-excitation of the S1 states.52 The latter is produced by: internal conversion of the S2 state, via Soret region irradiation, or directly, via Q-band irradiation. Adding an equimolar amount of 1 to the solution to replicate homogeneous catalysis system resulted in a quenching of the luminescence intensity, comparable for both excitation wavelengths, and ascribed to photoinduced intramolecular electron transfers from 2-Zn to 1. Incomplete quenching possibly stems from dynamic self-assembly and unpaired 2-Zn as well as inefficient electron transfer. With fluorescence experiments on colloid MOFs being challenging due to high scattering and sedimentation propensity, fully soluble Zr-oxo-based assemblies were prepared as small-scale counterparts of the hybrid PCN systems to enable properties replication and photophysical investigations. Discrete Zr6-oxo-nodes (Zr6), featuring methacrylate ligands as capping agents to prevent aggregation, were synthesised and isolated following a literature procedure (see ESI†).53 Associating these with 2-Zn yielded the fully soluble CO2H-tethered composites Zr6|2-Zn (see ESI† for assembly conditions) and allowed solution fluorescence measurements without affecting 2-Zn's absorption properties (Fig. S22†). In stark contrast to the Zr6-free homogeneous systems, a lower luminescence intensity was recorded after excitation at 415 nm (Fig. 5a) whereas excitation at 515 nm afforded a spectrum comparable to the one of 2-Zn (Fig. 5b). These behaviours were also reproduced in the corresponding action spectra (λem = 720 nm) as Zr6|2-Zn displayed unchanged Q-band contributions compared to 2-Zn, and adding 1 resulted in lesser contributions (Fig. S23†).
Such wavelength-dependent luminescence behaviour hints at different energy relaxation pathways. Here, excitation into the Soret band in the Zr6|2-Zn assembly results in a high-energy S2 state (zero–zero transition energy, E00 ≈ 2.9 eV) and likely induces fast electron transfer to a Zr-oxo cluster.18 As no fluorescence quenching was observed upon Q-band irradiation, this pathway is disabled from the S1 state possibly due to its lower energy level (E00 ≈ 2.0 eV). This also implies that the kinetics of charge separation from S2 are significantly faster than the S2 → S1 internal conversion. While Zn porphyrins present various and complex relaxation mechanisms, this interpretation is coherent with the documented slow S2 → S1 kinetics in Zn porphyrins, and in line with hot electron transfers from S2 as previously observed in molecular dyads and in heterogeneous assemblies.25,54,55 As inferred by the wavelength-independent quenching of fluorescence in the 2-Zn with 1 system (Fig. 5), reduction of 1 from the S1 excited state of 2-Zn is anticipated and consistent with the significant associated exergonicity (change in Gibbs free energy value, ΔGCS = −0.6 eV).
To investigate whether this behaviour is reflected in the photocatalysis performance, we performed a series of wavelength-dependent investigations using lower energy-centred wavelengths λ = 490–740 nm (∼8 mW cm−2), thus omitting Soret band excitation and corresponding S2 excited state production.
For Re-222-based experiments, a dramatically increased reactivity, and a reversed selectivity from HCOO− to CO were observed with ∼370 μmolCO g−1 h−1 and <1 μmolHCOO− g−1 h−1 (Fig. 6a and Table S3†). Correspondingly, TONCO ∼100 were reached after 80 h compared to below 1 obtained with λ > 430 nm and a higher absolute light intensity (Fig. 3a, 6b, Tables S3 and S4†). Re-224-based experiments gave a similar trend with ∼24 μmolCO g−1 h−1 (TONsCO ∼ 12) and <1 μmolHCOO− g−1 h−1 after ∼60 h, albeit with the absolute performance remaining significantly inferior to Re-222 due to its topological limitations (Fig. 6a, b and Table S3†). With selective irradiation predominantly yielding formate or CO (Table S3†), electronic communication between nodes and Re catalysts within the pores is unlikely. After 80 h of >490 nm irradiation PXRD analysis of Re-222 and Re-224 showed crystalline framework retention, while the Re(CO)3 moiety was no longer visible in ATR-IR spectra, comparable to >430 nm irradiation (Fig. S15 and S16†). For Re-222 and Re-224, post-catalysis ICP-MS measurements revealed that 27 and 17% of immobilised Re leached into the supernatant, respectively (Table S7†). This is consistent with other reported photodegradations of Re catalysts and described complex decomposition and subsequent loss of activity.9,43,47,56 In addition, corresponding dynamic light scattering measurements revealed no Re nanoparticle formation (Fig. S24†).
Control experiments with 13C-labelled CO2 for both Re-PCN hybrids produced only 13CO, confirming that CO2 was the sole source of CO (Fig. S25†). Further control experiments conducted with the CO2H–analogue of 1, i.e., fac-ReBr(CO)3(4,4′-dicarboxy-2,2′-bipyridine), node-anchored in PCN-222 and PCN-224 (synthesis in ESI, Fig. S26–S28 and Table S1†) showed comparable wavelength-dependent performance trends to Re-222 and Re-224 under identical reaction conditions (Table S3†). This is consistent with a previously reported PCN-222-Re assembly displaying selective CO2-to-CO evolution upon >500 nm irradiation19 and thus excludes the catalyst and dyadic motif as the source of the observed trends.
These wavelength-dependent results suggest that excited state (S2vs. S1)-specific, selectivity-dictating catalytic channels are occurring in the MOF assemblies, with electron delivery to the CO evolution molecular catalyst or the formate producing node being selectively activated by low or high energy wavelength, respectively. This was confirmed by AQY measurements at λ = 520 nm, giving ∼0.35% (CO) and ∼0.01% (formate), showing a reverse trend compared to 450 nm values (Tables S5 and S6†).
Interestingly, the correlations between product selectivity and irradiation wavelength were found in both Re-PCN assemblies as well as in the homogeneous Zr6|2-Zn|1 system. The latter showed limited CO evolution (TONs ∼ 1) and traces of formate using full irradiation, while >490 nm irradiation experiments yielded TONs ∼10 (Table S2†). By contrast, homogeneous Zr6-free molecular 2-Zn with 1 delivered wavelength-independent performance. This further highlights the critical impact of the assembly/framework (MOF vs. molecular assemblies) on photocatalysis (Fig. 3a, b, 6a, b, Tables S2 and S3†).
Luminescence and catalytic experiments together provide consistent evidence that irradiation with λ > 430 nm during photocatalysis mainly results in excitation of the Soret-band (S0–S2 transition, Fig. S6–S8†) in PCN assemblies upon which the S2 state is rapidly quenched by electron transfer to the Zr6-oxo-node (Fig. 6c).17,18,39 Here, bimolecular reaction with the diffusing SED is likely to proceed subsequently to regenerate the ground state (Fig. S13†) or detrimental charge recombination occurs.19 Selective CO2-to-formate reduction proceeds via reduced Zr(III) centres upon collecting another electron.17,46 With each linker connected to four nodes and charge separation occurring at the light harvesting centre, this constitutes a localised electron channel (Fig. 6d). Shifting to irradiation with λ > 490 nm mainly results in excitation of the Q bands (S0–S1 transition),55 affording a lower energy excited state unable to trigger node-quenching but able to reduce 1. The latter effects selective CO2-to-CO reduction upon accumulating two electrons.9,19 Alternatively, in absence of a Re catalyst in direct vicinity, directional exciton migration proceeds within the MOF structure.19 As an exciton can visit 100+ linkers in its lifetime,19,51 this energy funnelling is highly delocalised and provides a long-range, antennae-like catalytic channel (Fig. 6d).
The minute, deactivated CO evolution when using full irradiation is noteworthy considering that Soret and Q bands are concomitantly excited. The rationale is likely intricated and may involve substrate diffusion, competing photophysical processes and slower CO catalysis kinetics. Tentatively, the high molar absorption of S2 together with an excess of nodes to catalysts (statistically each linker neighbours four Zr6 nodes and ∼0.1 Re catalysts, Table S1†) could result in a kinetic preference for available CO2 (with affinity to coordinate to Lewis–acidic Zr nodes) to be converted to formate, depleting local substrate concentrations.57 While future in-depth spectroscopic studies are needed to elucidate this phenomenon, this finding may hold important repercussions as most literature research focuses on full spectrum broadband irradiation.
Overall, the results show that the linker's excited states and quenching kinetics with the molecular catalyst vs. the node are critical for understanding and tailoring reactivity. As a perspective, the observed wavelength-dependency provides an edge over similar systems as these usually show a reverse trend, i.e., high-energy (near) UV irradiation achieves higher performance.45,58 Here, lower-energy visible light provides selective molecular CO2-to-CO reduction, allowing unusual control over product selectivity based on irradiation wavelength.
Experimental
For a comprehensive description of analytical methods and experimental procedures the reader is referred to the ESI.† An overview of the central syntheses is provided here.Synthetic procedures
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of THF/MeOH (70 mL). A solution of KOH (3.5 g, 62.4 mmol, 52 eq.) in H2O (30 mL) was added. The resulting mixture was refluxed for 15 h. After removing the organic solvents in vacuo, the solid was redissolved in H2O (150 mL) at 90 °C for 15 min. The solution was filtered and acidified with 1 M HCl (100 mL). The resulting green precipitate was filtered and dried to obtain the product (864 mg, 1.09 mmol, 92% yield), whose characterisations matched literature reports (see ESI†).
1 mixture of THF/MeOH (70 mL). A solution of KOH (3.5 g, 62.4 mmol, 52 eq.) in H2O (30 mL) was added. The resulting mixture was refluxed for 15 h. After removing the organic solvents in vacuo, the solid was redissolved in H2O (150 mL) at 90 °C for 15 min. The solution was filtered and acidified with 1 M HCl (100 mL). The resulting green precipitate was filtered and dried to obtain the product (864 mg, 1.09 mmol, 92% yield), whose characterisations matched literature reports (see ESI†).
This intermediate (214 mg, 235 μmol, 1.0 eq.) was dissolved in THF/MeOH 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (15 mL). KOH (691 mg, 12.3 mmol, 52 eq.) was dissolved in H2O (6 mL) and added to the prior solution. The reaction mixture was refluxed for 5 h under continuous stirring. After the mixture was cooled to room temperature, the organic solvents were removed by rotary evaporation. The crude product was dissolved in H2O (32 mL) and heated at 90 °C for 10 min. After cooling to room temperature, the aqueous mixture was acidified with 1 M HCl solution (21 mL) and 37% HCl (1 mL) in respective order. The resulting dark green precipitate was isolated by centrifugation and washed with H2O (8 × 30 mL). The dark green product was dried in vacuo overnight (137 mg, 161 μmol, 68% yield, see ESI†).
1 (15 mL). KOH (691 mg, 12.3 mmol, 52 eq.) was dissolved in H2O (6 mL) and added to the prior solution. The reaction mixture was refluxed for 5 h under continuous stirring. After the mixture was cooled to room temperature, the organic solvents were removed by rotary evaporation. The crude product was dissolved in H2O (32 mL) and heated at 90 °C for 10 min. After cooling to room temperature, the aqueous mixture was acidified with 1 M HCl solution (21 mL) and 37% HCl (1 mL) in respective order. The resulting dark green precipitate was isolated by centrifugation and washed with H2O (8 × 30 mL). The dark green product was dried in vacuo overnight (137 mg, 161 μmol, 68% yield, see ESI†).
The irradiation source was a heat-free white light generator Asahi Spectra MAX-303 Compact Xenon Light Source 300 W, with either a XVL0430-Longpass 430 nm filter (430–740 nm irradiation, ∼10 mW cm−2) or a XVL0490-Longpass 490 nm filter (490–740 nm irradiation, ∼8 mW cm−2). Reaction products were determined through headspace analysis by gas chromatography and 1H NMR of the reaction solution.
Conclusions
We successfully synthesised and characterised two different hybrids of fac-ReBr(CO)3(qtpy) in porphyrinic MOFs (PCN-222 and PCN-224) by post-synthetic self-assembly, allowing straightforward topology-independent catalyst anchoring. Corresponding smaller scale, molecular and supramolecular assemblies were also prepared for comparison and benchmarking purposes. Here, we demonstrated that material interfacing at various scales, i.e., molecule–molecule (1 + 2-Zn), molecule-cluster (Zr6|2-Zn|1), and molecule-particle (Re-PCNs) produces composites displaying unique and complex behaviours that are concomitantly reminiscent, diverging and unseen in their individual building blocks.Mainly, assembling Re catalyst-PCN composites afforded photocatalysts bearing two catalytic channels that are selectively activated by specific incident irradiation wavelengths and thus, ultimately govern the catalysis product selectivity. Shorter wavelengths resulted in localised charge transfers from the excited porphyrin linkers to the Zr6-oxo-nodes, producing formate. Alternatively, lower energy irradiation can be used to activate the CO-selective Re catalysts with exciton migration enabling long-range, delocalised catalysis. By contrast, the corresponding molecular and supramolecular systems yielded product selective and wavelength-independent catalysis.
While molecule-like localised S2 and S1 states more accurately describe the observed photoinduced processes in Re-PCNs over typical band-like description of MOFs, the hybrids also display material-like properties elusive to molecular species. Thus, a topology effect was shown with the PCN-222 hybrids displaying a higher photocatalytic activity over PCN-224, as small pore sizes hinder mass diffusion and short coupling distances promote antennae-effect. Further the nanoparticular hybrid porous systems enabled a 10-fold increase in CO2 reduction activity compared to their discrete counterparts, ascribed to stabilising pore confinement.
Although preliminary, this work constitutes the seminal investigation of the likely considerable influence of MOF topology on solar fuel production. Future efforts on controlled distances between catalyst and photosensitiser, understanding of underlying photoinduced processes, varied anchor principles, as well as optimised energy and mass transport will allow orthogonal component assembly and minimise energy loss for efficient solar fuel production. Finally, we show that while broadband light is often considered in its entirety in the broader literature, a finer differentiation may be needed to understand complex hybrid materials' behaviour and performance.
Data availability
The source data is available from the corresponding author upon reasonable request.Author contributions
P. M. Stanley: methodology, validation, formal analysis, investigation, data curation, writing – original draft, visualisation. K. Hemmer: methodology, validation, formal analysis, investigation, data curation, writing – original draft, visualisation. M. Hegelmann: investigation, data curation. A. Schulz: investigation, data curation. M. Park: investigation, data curation. M. Elsner: investigation. M. Cokoja: conceptualisation, writing – review and editing, supervision, resources, project administration. J. Warnan: conceptualisation, writing – review and editing, supervision, resources, project administration.Conflicts of interest
There are no conflicts to declare.Acknowledgements
P. M. S. and K. H. thank the Chemical Industry Fonds (FCI) for a PhD fellowship. The authors are grateful for the helpful discussions and the support from Roland Fischer and Bernhard Rieger, as well as Christine Benning for the ICP-MS measurements. Thanks to Sebastian Weishäupl and Simon Deger for luminescence measurements, and Katia Rodewald for SEM images. The help by the Central Analytics Facility of the TUM Catalysis Research Center, especially from Jürgen Kudermann, is gratefully acknowledged. Additional thanks to Silva Kronawitter and Johanna Haimerl for proof reading. This work was supported by the German Research Foundation (DFG) Priority Program 1928 ‘Coordination Networks: Building Blocks for Functional Systems’, the research project MOFMOX (grant number: FI 502/43-1), and by the Excellence Cluster 2089 ‘e-conversion’ (Fundamentals of Energy Conversion Processes).References
- H. J. Sayre, L. Tian, M. Son, S. M. Hart, X. Liu, D. M. Arias-Rotondo, B. P. Rand, G. S. Schlau-Cohen and G. D. Scholes, Energy Environ. Sci., 2021, 14, 1402 RSC.
- V. Kumaravel, J. Bartlett and S. C. Pillai, ACS Energy Lett., 2020, 5, 486 CrossRef CAS.
- K. E. Dalle, J. Warnan, J. J. Leung, B. Reuillard, I. S. Karmel and E. Reisner, Chem. Rev., 2019, 119, 2752 CrossRef CAS PubMed.
- A. Bachmeier and F. Armstrong, Curr. Opin. Chem. Biol., 2015, 25, 141 CrossRef CAS PubMed.
- R. Freund, O. Zaremba, G. Arnauts, R. Ameloot, G. Skorupskii, M. Dincă, A. Bavykina, J. Gascon, A. Ejsmont, J. Goscianska, M. Kalmutzki, U. Lächelt, E. Ploetz, C. S. Diercks and S. Wuttke, Angew. Chem., Int. Ed., 2021, 60, 23975 CrossRef CAS PubMed.
- (a) H. Furukawa, K. E. Cordova, M. O'Keeffe and O. M. Yaghi, Science, 2013, 341, 1230444 CrossRef PubMed; (b) G. A. Leith, C. R. Martin, J. M. Mayers, P. Kittikhunnatham, R. W. Larsen and N. B. Shustova, Chem. Soc. Rev., 2021, 50, 4382 RSC.
- K. Hemmer, M. Cokoja and R. A. Fischer, ChemCatChem, 2021, 13, 1683 CrossRef CAS.
- K. Epp, B. Bueken, B. J. Hofmann, M. Cokoja, K. Hemmer, D. Vos and R. A. Fischer, Catal. Sci. Technol., 2019, 9, 6452 RSC.
- P. M. Stanley, C. Thomas, E. Thyrhaug, A. Urstoeger, M. Schuster, J. Hauer, B. Rieger, J. Warnan and R. A. Fischer, ACS Catal., 2021, 11, 871 CrossRef CAS.
- X. Feng, Y. Pi, Y. Song, C. Brzezinski, Z. Xu, Z. Li and W. Lin, J. Am. Chem. Soc., 2020, 142, 690 CrossRef CAS PubMed.
- (a) J. Lyu, X. Zhang, K. Otake, X. Wang, P. Li, Z. Li, Z. Chen, Y. Zhang, M. C. Wasson, Y. Yang, P. Bai, X. Guo, T. Islamoglu and O. K. Farha, Chem. Sci., 2019, 10, 1186 RSC; (b) T.-Y. Zhou, B. Auer, S. J. Lee and S. G. Telfer, J. Am. Chem. Soc., 2019, 141, 1577 CrossRef CAS PubMed; (c) Y. Wang, H. Cui, Z.-W. Wei, H.-P. Wang, L. Zhang and C.-Y. Su, Chem. Sci., 2017, 8, 775 RSC; (d) D. J. Xiao, J. Oktawiec, P. J. Milner and J. R. Long, J. Am. Chem. Soc., 2016, 138, 14371 CrossRef CAS PubMed.
- P. M. Stanley, J. Haimerl, C. Thomas, A. Urstoeger, M. Schuster, N. B. Shustova, A. Casini, B. Rieger, J. Warnan and R. A. Fischer, Angew. Chem., Int. Ed., 2021, 60, 17854 CrossRef CAS PubMed.
- P. Deria, D. A. Gómez-Gualdrón, I. Hod, R. Q. Snurr, J. T. Hupp and O. K. Farha, J. Am. Chem. Soc., 2016, 138, 14449 CrossRef CAS PubMed.
- X. Gong, Y. Shu, Z. Jiang, L. Lu, X. Xu, C. Wang and H. Deng, Angew. Chem., Int. Ed., 2020, 59, 5326 CrossRef CAS PubMed.
- K. Sasan, Q. Lin, C. Mao and P. Feng, Chem. Commun., 2014, 50, 10390 RSC.
- Z.-B. Fang, T.-T. Liu, J. Liu, S. Jin, X.-P. Wu, X.-Q. Gong, K. Wang, Q. Yin, T.-F. Liu, R. Cao and H.-C. Zhou, J. Am. Chem. Soc., 2020, 142, 12515 CrossRef CAS PubMed.
- H.-Q. Xu, J. Hu, D. Wang, Z. Li, Q. Zhang, Y. Luo, S.-H. Yu and H.-L. Jiang, J. Am. Chem. Soc., 2015, 137, 13440 CrossRef CAS PubMed.
- P. Asselin and P. D. Harvey, ACS Appl. Nano Mater., 2022, 5, 6055 CrossRef CAS.
- S. Choi, W.-J. Jung, K. Park, S.-Y. Kim, J.-O. Baeg, C. H. Kim, H.-J. Son, C. Pac and S. O. Kang, ACS Appl. Mater. Interfaces, 2021, 13, 2710 CrossRef CAS PubMed.
- H.-J. Son, S. Jin, S. Patwardhan, S. J. Wezenberg, N. C. Jeong, M. So, C. E. Wilmer, A. A. Sarjeant, G. C. Schatz, R. Q. Snurr, O. K. Farha, G. P. Wiederrecht and J. T. Hupp, J. Am. Chem. Soc., 2013, 135, 862 CrossRef CAS PubMed.
- P. M. Stanley, M. Parkulab, B. Rieger, J. Warnan and R. A. Fischer, Faraday Discuss., 2021, 231, 281 RSC.
- G. Sahara and O. Ishitani, Inorg. Chem., 2015, 54, 5096 CrossRef CAS PubMed.
- (a) A. M. Cancelliere, F. Puntoriero, S. Serroni, S. Campagna, Y. Tamaki, D. Saito and O. Ishitani, Chem. Sci., 2020, 11, 1556 RSC; (b) P. M. Stanley and J. Warnan, Energies, 2021, 14, 4260 CrossRef CAS.
- A. Gabrielsson, F. Hartl, H. Zhang, J. R. Lindsay Smith, M. Towrie, A. Vlcek and R. N. Perutz, J. Am. Chem. Soc., 2006, 128, 4253 CrossRef CAS PubMed.
- K. Kiyosawa, N. Shiraishi, T. Shimada, D. Masui, H. Tachibana, S. Takagi, O. Ishitani, D. A. Tryk and H. Inoue, J. Phys. Chem. C, 2009, 113, 11667 CrossRef CAS.
- C. D. Windle, M. W. George, R. N. Perutz, P. A. Summers, X. Z. Sun and A. C. Whitwood, Chem. Sci., 2015, 6, 6847 RSC.
- P. Lang, M. Pfrunder, G. Quach, B. Braun-Cula, E. G. Moore and M. Schwalbe, Chem.–Eur. J., 2019, 25, 4509 CrossRef CAS PubMed.
- C. D. Windle, M. V. Câmpian, A.-K. Duhme-Klair, E. A. Gibson, R. N. Perutz and J. Schneider, Chem. Commun., 2012, 48, 8189 RSC.
- (a) Y. Kobuke, in Non-Covalent Multi-Porphyrin Assemblies, ed. E. Alessio, Springer-Verlag, Berlin/Heidelberg, 2006, vol. 121, pp. 49–104 Search PubMed; (b) A. Satake and Y. Kobuke, Tetrahedron, 2005, 61, 13 CrossRef CAS; (c) J. Warnan, Y. Pellegrin, E. Blart and F. Odobel, Chem. Commun., 2012, 48, 675 RSC.
- E. Rousset, D. Chartrand, I. Ciofini, V. Marvaud and G. S. Hanan, Chem. Commun., 2015, 51, 9261 RSC.
- P. Wolf, S. L. Heath and J. A. Thomas, Inorg. Chim. Acta, 2003, 355, 280 CrossRef.
- D. Feng, Z.-Y. Gu, J.-R. Li, H.-L. Jiang, Z. Wei and H.-C. Zhou, Angew. Chem., Int. Ed., 2012, 51, 10307 CrossRef CAS PubMed.
- K. Epp, A. L. Semrau, M. Cokoja and R. A. Fischer, ChemCatChem, 2018, 10, 3506 CrossRef CAS.
- J. Jin, React. Kinet., Mech. Catal., 2020, 131, 397 CrossRef CAS.
- J. Liang, Y.-Q. Xie, Q. Wu, X.-Y. Wang, T.-T. Liu, H.-F. Li, Y.-B. Huang and R. Cao, Inorg. Chem., 2018, 57, 2584 CrossRef CAS PubMed.
- A. Vlček, in Photophysics of Organometallics, ed. A. J. Lees, Springer Berlin/Heidelberg, Berlin, Heidelberg, 2010, pp. 115–158 Search PubMed.
- G. Lucazeau, L. Guemas and A. Novak, Inorg. Chim. Acta, 1976, 20, 11 CrossRef CAS.
- (a) Y. Benseghir, A. Lemarchand, M. Duguet, P. Mialane, M. Gomez-Mingot, C. Roch-Marchal, T. Pino, M.-H. Ha-Thi, M. Haouas, M. Fontecave, A. Dolbecq, C. Sassoye and C. Mellot-Draznieks, J. Am. Chem. Soc., 2020, 142, 9428 CrossRef CAS PubMed; (b) X. Wang, F. M. Wisser, J. Canivet, M. Fontecave and C. Mellot-Draznieks, ChemSusChem, 2018, 11, 3315 CrossRef CAS PubMed; (c) P. Deria, W. Bury, I. Hod, C.-W. Kung, O. Karagiaridi, J. T. Hupp and O. K. Farha, Inorg. Chem., 2015, 54, 2185 CrossRef CAS PubMed.
- J. Jin, New J. Chem., 2020, 44, 15362 RSC.
- Y. Pellegrin and F. Odobel, C. R. Chim., 2017, 20, 283 CrossRef CAS.
- (a) L. Favereau, A. Cnossen, J. B. Kelber, J. Q. Gong, R. M. Oetterli, J. Cremers, L. M. Herz and H. L. Anderson, J. Am. Chem. Soc., 2015, 137, 14256 CrossRef CAS PubMed; (b) M. A. Jinks, H. Sun and C. A. Hunter, Org. Biomol. Chem., 2014, 12, 1440 RSC.
- J. Hawecker, J.-M. Lehn and R. Ziessel, Helv. Chim. Acta, 1986, 69, 1990 CrossRef CAS.
- S. Meister, R. O. Reithmeier, M. Tschurl, U. Heiz and B. Rieger, ChemCatChem, 2015, 7, 690 CrossRef CAS.
- (a) M. Waki, M. Ikai, Y. Goto, Y. Maegawa and S. Inagaki, Eur. J. Inorg. Chem., 2021, 2021, 1624 CrossRef CAS; (b) D.-I. Won, J.-S. Lee, J.-M. Ji, W.-J. Jung, H.-J. Son, C. Pac and S. O. Kang, J. Am. Chem. Soc., 2015, 137, 13679 CrossRef CAS PubMed; (c) Z. Fu, X. Wang, A. M. Gardner, X. Wang, S. Y. Chong, G. Neri, A. J. Cowan, L. Liu, X. Li, A. Vogel, R. Clowes, M. Bilton, L. Chen, R. S. Sprick and A. I. Cooper, Chem. Sci., 2020, 11, 543 RSC; (d) B. Ma, G. Chen, C. Fave, L. Chen, R. Kuriki, K. Maeda, O. Ishitani, T.-C. Lau, J. Bonin and M. Robert, J. Am. Chem. Soc., 2020, 142, 6188 CrossRef CAS PubMed.
- D.-I. Won, J.-S. Lee, Q. Ba, Y.-J. Cho, H.-Y. Cheong, S. Choi, C. H. Kim, H.-J. Son, C. Pac and S. O. Kang, ACS Catal., 2018, 8, 1018 CrossRef CAS.
- R. Hariri and S. Dehghanpour, Appl. Organomet. Chem., 2021, 35, e6422 CrossRef CAS.
- E. E. Benson and C. P. Kubiak, Chem. Commun., 2012, 48, 7374 RSC.
- A. A. Yakovenko, J. H. Reibenspies, N. Bhuvanesh and H.-C. Zhou, J. Appl. Crystallogr., 2013, 46, 346 CrossRef CAS.
- S. M. Shaikh, S. Ilic, B. J. Gibbons, X. Yang, E. Jakubikova and A. J. Morris, J. Phys. Chem. C, 2021, 125, 22998 CrossRef CAS.
- (a) J. Yu, J. Park, A. van Wyk, G. Rumbles and P. Deria, J. Am. Chem. Soc., 2018, 140, 10488 CrossRef CAS PubMed; (b) S. Goswami, I. Hod, J. D. Duan, C.-W. Kung, M. Rimoldi, C. D. Malliakas, R. H. Palmer, O. K. Farha and J. T. Hupp, J. Am. Chem. Soc., 2019, 141, 17696 CrossRef CAS PubMed.
- S. S. Rajasree, J. Yu, S. M. Pratik, X. Li, R. Wang, A. S. Kumbhar, S. Goswami, C. J. Cramer and P. Deria, J. Am. Chem. Soc., 2022, 144, 1396 CrossRef CAS PubMed.
- (a) V. G. Rao, B. Dhital and H. P. Lu, Chem. Commun., 2015, 51, 16821 RSC; (b) S. Ye, A. Kathiravan, H. Hayashi, Y. Tong, Y. Infahsaeng, P. Chabera, T. Pascher, A. P. Yartsev, S. Isoda, H. Imahori and V. Sundström, J. Phys. Chem. C, 2013, 117, 6066 CrossRef CAS.
- G. Kickelbick and U. Schubert, Chem. Ber., 1997, 130, 473 CrossRef CAS.
- (a) R. T. Hayes, C. J. Walsh and M. R. Wasielewski, J. Phys. Chem. A, 2004, 108, 2375 CrossRef CAS; (b) J. Petersson, J. Henderson, A. Brown, L. Hammarström and C. P. Kubiak, J. Phys. Chem. C, 2015, 119, 4479 CrossRef CAS; (c) M. Morisue, N. Haruta, D. Kalita and Y. Kobuke, Chem.–Eur. J., 2006, 12, 8123 CrossRef CAS PubMed; (d) H. Chosrowjan, S. Tanigichi, T. Okada, S. Takagi, T. Arai and K. Tokumam, Chem. Phys. Lett., 1995, 242, 644 CrossRef CAS.
- J. Karolczak, D. Kowalska, A. Lukaszewicz, A. Maciejewski and R. P. Steer, J. Phys. Chem. A, 2004, 108, 4570 CrossRef CAS.
- (a) M. Pschenitza, S. Meister, A. von Weber, A. Kartouzian, U. Heiz and B. Rieger, ChemCatChem, 2016, 8, 2688 CrossRef CAS; (b) J. K. Nganga, C. R. Samanamu, J. M. Tanski, C. Pacheco, C. Saucedo, V. S. Batista, K. A. Grice, M. Z. Ertem and A. M. Angeles-Boza, Inorg. Chem., 2017, 56, 3214 CrossRef CAS PubMed; (c) Y. Kou, Y. Nabetani, D. Masui, T. Shimada, S. Takagi, H. Tachibana and H. Inoue, J. Am. Chem. Soc., 2014, 136, 6021 CrossRef CAS PubMed.
- (a) G. Jin, D. Sensharma, N. Zhu, S. Vaesen and W. Schmitt, Dalton Trans., 2019, 48, 15487 RSC; (b) A. Wagner, C. D. Sahm and E. Reisner, Nat. Catal., 2020, 3, 775 CrossRef CAS.
- C. Matlachowski, B. Braun, S. Tschierlei and M. Schwalbe, Inorg. Chem., 2015, 54, 10351 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Full experimental details, analysis method description, and supplementary figures and tables. See DOI: https://doi.org/10.1039/d2sc03097g |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2022 |