Pressure and guest-mediated pore shape modification in a small pore MOF to 1200 bar†
Charles J.
McMonagle
a,
Gemma F.
Turner
b,
Isabelle
Jones
b,
David R.
Allan
 c,
Mark R.
Warren
c,
Konstantin V.
Kamenev
e,
Simon
Parsons
*f,
Paul A.
Wright
c,
Mark R.
Warren
c,
Konstantin V.
Kamenev
e,
Simon
Parsons
*f,
Paul A.
Wright
 *d and
Stephen A.
Moggach
*d and
Stephen A.
Moggach
 *b
*b
aSNBL at ESRF, Grenoble, France
bSchool of Molecular Sciences, The University of Western Australia, 35 Stirling Highway, Crawley, Perth, 6009, Western Australia, Australia. E-mail: stephen.moggach@uwa.edu.au
cDiamond Light Source, Rutherford Appleton Laboratory, Harwell Science and Innovation Campus, Fermi Avenue, Didcot, OX11 0DE, UK
dEastChem School of Chemistry, The University of St Andrews, Purdie Building, St Andrews, KY16 9ST, UK
eCentre for Science at Extreme Conditions and School of Engineering, The University of Edinburgh, King's Buildings, West Mains Road, Edinburgh EH9 3FJ, UK
fEastChem School of Chemistry, Joseph Black Building, The University of Edinburgh, David Brewster Road, Edinburgh EH9 3FJ, UK
First published on 16th September 2022
Abstract
Guest-mediated pore-shape modification of the metal–organic framework, Sc2BDC3 upon adsorption of n-pentane and isopentane is examined from 50–1200 bar. Rotation of the BDC linker responsible for the change in pore shape occurs at much lower pressures than previously reported, with distinct adsorption behaviour observed between pentane isomers.
Nanoporous metal–organic frameworks (MOFs) are structurally flexible under applied pressure.1–3 ‘Soft’ components of the framework architecture, such as distortable metal centres, flexible linkers, or compliant topologies, accommodate deformation of the framework without loss of crystallinity.4,5 The structural change is often dynamic and reversible, often resulting in a change in the functional or mechanical properties of the MOF, relevant to practical applications.6 In addition to direct pressure-stimulation of the framework, flexibility can be mediated by pressure-induced guest intrusion into the MOF pores.7–9 The guest species is used as the pressure-transmitting medium (PTM) in which the crystal or powder is suspended in the pressure cell.
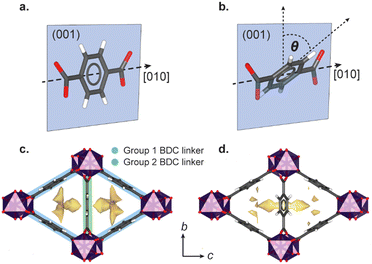
High-pressure single crystal X-ray diffraction using gas cell (<100 bar) or diamond anvil cell apparatus (DAC, 1000–100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 bar) has identified a variety of general flexible behaviour in MOFs, including negative linear compressibility (NLC),10–12 Jahn Teller switching,13 ‘gate-opening’ linker rotations,14,15 breathing,16–18 and pore-shape modification.8 The flexibility is often governed by the choice of PTM, which may directly compress the framework, or, if composed of molecules that are sufficiently small, infiltrate the pores. Previous high-pressure studies up to 8000 bar on the small pore scandium terephthalate MOF, Sc2BDC3 (where BDC = 1,4-benzenedicarboxylate), found that even ‘oversized’ guests can be incorporated into the framework pores. Sc2BDC3 crystallises in the orthorhombic space group, Fddd, with two symmetrically independent BDC linkers (labelled Group 1 and Group 2 in Fig. 1).19,20
000 bar) has identified a variety of general flexible behaviour in MOFs, including negative linear compressibility (NLC),10–12 Jahn Teller switching,13 ‘gate-opening’ linker rotations,14,15 breathing,16–18 and pore-shape modification.8 The flexibility is often governed by the choice of PTM, which may directly compress the framework, or, if composed of molecules that are sufficiently small, infiltrate the pores. Previous high-pressure studies up to 8000 bar on the small pore scandium terephthalate MOF, Sc2BDC3 (where BDC = 1,4-benzenedicarboxylate), found that even ‘oversized’ guests can be incorporated into the framework pores. Sc2BDC3 crystallises in the orthorhombic space group, Fddd, with two symmetrically independent BDC linkers (labelled Group 1 and Group 2 in Fig. 1).19,20
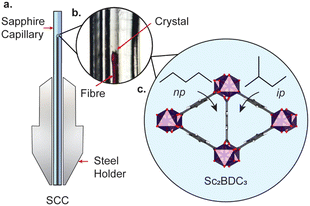
The framework is punctuated by triangular channels that are ∼4 Å in diameter. Upon compression of a single crystal of Sc2BDC3 in liquid hydrocarbons (C5–C8) with kinetic diameters exceeding 4 Å, the pores transformed from triangular to rhombic geometry.8 This pore-shape modification is driven by pressure-induced intrusion of alkanes into the framework, which causes the Group 2 BDC linker to rotate, merging adjacent pairs of triangular channels to form a single rhombus-shaped cavity. The degree of rotation of the BDC linker increases with the quantity of guest adsorbed, which is greater for linear alkanes over their branched isomers. For instance, intrusion of n-pentane into the channels caused a rotation of the Group 2 BDC linker by 52° at 4000 bar (quantified as the rotation of the phenyl group with respect to the c-axis, see Fig. 1b), whilst isopentane caused a rotation of 44° at a higher pressure of 5000 bar, due to its smaller kinetic diameter (ca. 4.5 Å vs. 5.0 Å). The guest-dependent response of Sc2BDC3 to pressure is promising for its application as a filtration material, as it uniquely responds to the size and geometry of the guest, thus differentiating between isomers of similar boiling points.21 To design effective filters or hosts, it is necessary to understand the structural mechanism governing this process. However, current pressure cells limit the accessible pressure ranges for in situ structural analysis below 2000 bar (the effective loading pressure inside a DAC), preventing the derivation of structure-pressure-guest relationships in the ‘moderate’ pressure region. This is a pressure regime within practical useability, with ultra-high-performance liquid chromatography systems often operating between ≈400 and 1500 bar, and therefore ripe for exploration. With this in mind, we recently developed a sapphire capillary cell (SCC, Fig. 2) which addresses this issue, and is designed for in situ single crystal X-ray diffraction using liquid PTM from ambient pressure to 1500 bar.22 Here, the SCC is used to extend previous work on the pore-shape modification of Sc2BDC3 upon adsorption of n-pentane or isopentane between 50 and 1200 bar, with the aim of clarifying the mechanism for the rotation of the Group 2 BDC linker at lower pressures. This is the first atomistic structural analysis of any metal–organic framework under these types of pressure conditions, using a cell capable of studying in detail, the uptake of a variety of guest species into the pores.
A single crystal of Sc2BDC3 was characterised under ambient pressure and temperature by single crystal X-ray diffraction (λ = 0.6889 Å), before being placed in a 0.6 mm ID × 1 mm OD SCC filled with either n-pentane or isopentane as the pressure-transmitting medium (ID = inner diameter, OD = outer diameter). The pressure was increased using a ENERPAC P2282 two-stage portable hand pump from 50 bar to 900 bar using a PTM of n-pentane, or from 100 bar to 1200 bar in isopentane. Structure refinements were performed in CRYSTALS.23 The solvent accessible volume and any residual electron density were calculated and modelled using the SQUEEZE routine in PLATON.24 Abridged crystallographic data and guest contents are summarised in ESI,† Tables S1 and S2.
According to the electron density in the pores, n-pentane or isopentane is incorporated into the channels of Sc2BDC3 upon initial compression in the SCC at 50 bar or 100 bar, respectively. Uptake of n-pentane occurs readily and is sufficient to induce a rotation of the Group 2 BDC linker by 400 bar. By contrast, significant twisting of the BDC phenyl group (causing static disorder of the BDC ligand) is not observed with isopentane until 1200 bar due to its branched geometry, which hinders intrusion into the pore.
A qualitative assessment of guest uptake into Sc2BDC3 can be derived from the compressibility of the unit cell parameters under applied pressure (ESI† S2 and S3). Compression of Sc2BDC3 in a PTM of n-pentane from ambient pressure to 900 bar caused the unit cell volume to increase by 7.6(4) Å3 (0.12%), as the n-pentane from the pressure medium is forced into the framework channels to form the guest-included Sc2BDC3(np) phase (Fig. S1 (ESI†), where (np) refers to n-pentane included framework). Swelling of the crystal upon pressure-induced guest adsorption is common for MOFs, as the pore volume expands to accommodate the incoming guest.25
By contrast, compression of the framework in a PTM of isopentane from ambient pressure to 1200 bar resulted in direct compression of the unit cell by 11(1) Å3 (−0.18%). The precision obtained in these measurements reflects that of the data collected previously on hexamethylenetetramine (or HMTH),22 making this cell ideal for studying very flexible systems under compression of a variety of fluids.
The compressibility of Sc2BDC3 in isopentane corresponds to a relatively high bulk modulus, K0, of 48(2) GPa, calculated using a third-order Birch–Murnaghan equation of state in the EoSFit7 program26 (ESI,† S3) and a comparison to the bulk moduli of other MOFs is shown in ESI,† Table S3. The relatively low compressibility of Sc2BDC3(ip) (where ip = isopentane included framework) compared with the more compressible native Sc2BDC3 framework (ca. K0 = 17.3 ± 1.8 GPa)27 from a previous study using Fluorinert FC-77 (a non-penetrating PTM), indicates at least a small degree of guest intrusion into the channels, supporting previously reported adsorption isotherms.8 Compression above 0.4 GPa in FC-77 also results in amorphisation of the framework, indicating that intrusion of the PTM helps to stabilise the crystalline phase.8
Compression of the unit cell in both Sc2BDC3(np) and Sc2BDC3(ip) is highly anisotropic, with negative linear compressibility (NLC) along the direction of the framework channels (a-axis). Extension of the a-axis is similar upon compression in either PTM, with a linear compressibility, ka, of −0.025(2) GPa−1 for Sc2BDC3(np) and −0.021(2) GPa−1 for Sc2BDC3(ip) (ESI,† S3). However, compression of the b- and c-axes is severely attenuated in Sc2BDC3(np) compared with Sc2BDC3(ip), leading to an overall increase in its unit cell volume. The channel is perpendicular to the bc plane. Facile uptake of n-pentane into the channels compared with isopentane decreases the compressibility of the channel diameter in the n-pentane loaded framework due to the internal pressure exerted by the guest.
A satisfactory atomistic model for the guest occupying the pores in Sc2BDC3(np) and Sc2BDC3(ip) could not be obtained over the measured pressure range, and so was modelled using the SQUEEZE algorithm in PLATON.24
This is unsurprising, as the data were collected at room temperature and the solvent diffuse, with the largest residual electron density peaks (prior to applying the SQUEEZE algorithm) measuring only 0.7 and 0.5 e Å−3 in n-pentane and isopentane at their highest reported pressures of 900 and 1200 bar respectively. A summary of the guest content in Sc2BDC3 during compression is provided in ESI,† S3–S5. Over the measured pressure range, the quantity of n-pentane steadily increased to a maximum of 1.1 molecules per channel at 800 bar, whilst the quantity of isopentane reaches a maximum of 0.8 molecules per channel at 1200 bar. Comparable guest uptakes were previously reported for Sc2BDC3 at pressures exceeding 2000 bar,8 indicating that the framework has reached its loading capacity for these guest species by 800–1200 bar.
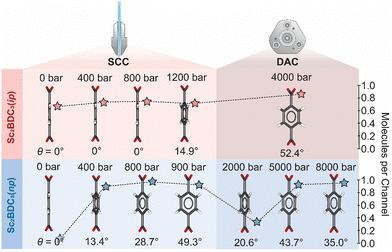
Comparison of the guest loading in the SCC to those reported in a DAC demonstrates distinct uptake and release behaviour between n-pentane and isopentane. In the SCC, the quantity of both n-pentane and isopentane adsorbed in the pores gradually increases with pressure. From comparison with previous DAC experiments,8 adsorption of isopentane plateaus at ∼0.8 molecules per pore at 1200 bar and is retained in the framework up to at least 4000 bar. By contrast, n-pentane appears to undergo a cycle of adsorption and desorption between the moderate and high-pressure regions. After reaching its adsorption capacity at 800 bar in the SCC, the quantity of n-pentane per channel is halved by 2000 bar in the DAC, before increasing to its previous maximum at 5000 bar.8
This discrepant guest-uptake behaviour is reflected in the degree of rotation of the Group 2 BDC linker (Fig. 3). Previous high-pressure analyses using a DAC reported static disordering of the BDC linker into two positions of equal occupancy and its rotation along [010] by 43.7° upon compression to 5000 bar in n-pentane, or by 52.4° at 4000 bar in isopentane.8 Similar structural behaviour is observed in the SCC, although at significantly lower pressure. Compression of Sc2BDC3 in a PTM of n-pentane from ambient pressure to 400 bar introduces 0.9 molecules of n-pentane into the channel, prompting a rotation of the Group 2 BDC linker by 13.4°. Further compression to 800 bar increases the guest content to 1.1 molecules per unit cell and the rotation angle to 28.7° (Fig. 3 and Fig. S4, ESI†). In both cases, the phenyl groups of the BDC are modelled as being disordered equally over two positions. At 900 bar, the rotation angle increases to 49.3° and the phenyl group of the BDC is disordered equally over four positions. The gradual ingress of guest into the channels of Sc2BDC3 therefore causes a gradual rotation of the Group 2 BDC linker. It is possible that the application of pressure to the guest-loaded phase, Sc2BDC3(np), also affects this rotation; between 800 bar and 900 bar, the rotation angle increases by 20.6° (to 49.3°) as the n-pentane content slightly decreases by 0.1 molecule. However, this effect is questionable since the rotation angle of the BDC ligand measured at 900 bar is approximately equivalent to that previously reported at 5000 bar (ca. 43.7°), at which the n-pentane storage capacity of Sc2BDC3 is reached. From comparison of the Group 2 BDC rotation angle during compression in the SCC (50–900 bar) to that previously reported in a DAC (2000–8000 bar),8 the degree of rotation is found to fluctuate with increasing pressure, in accord with the infiltration and ejection of the n-pentane guest (Fig. S2, ESI†). The framework reaches a maximum capacity of 1.0–1.1 molecules at 900 bar and 5000 bar, at which the rotation of BDC phenyl is most pronounced. Compression up to 900 bar results in gradual rotation of the BDC linker as n-pentane infiltrates the framework, whilst compression above these values decreases the rotation angle as n-pentane is ejected from the channels. Discrepancies here could be caused by several factors, including crystallite size and shape, which for ZIF-8 has previously been shown to significantly affect the N2 adsorption isotherm,28 and is an area worthy of further study.
 | ||
| Fig. 3 Guest-dependent rotation of the Group 2 BDC linker in a SCC (this work) and a DAC (McKellar et al.).8 Rotational disorder of the benzyl rings are omitted for clarity. Atoms are coloured as follows: C – grey, O – red. The guest content per channel for each pressure is shown as stars, coloured red for isopentane and blue for n-pentane (see Fig. S2, ESI†). | ||
Compression of Sc2BDC3 in a PTM of isopentane affords largely similar behaviour, with the exception that the isopentane is retained in the channels in the measured pressure regime and has a lower maximum adsorption capacity. From ambient pressure to 1200 bar, 0.8 molecules of isopentane are forced into the framework, prompting a rotation of the Group 2 BDC by 14.9°. The rotation is accompanied by disordering of the BDC phenyl group over two equally occupied positions. As for Sc2BDC3(np), the prolate geometry of the ADPs within the phenyl group indicate dynamic disorder (Fig. S6 and S7, ESI†). No structural evidence of significant uptake of isopentane (i.e. a measurable rotation of the Group 2 BDC angle) was observed below 1200 bar, although measurement of the diffuse guest content indicates that a maximum isopentane content of 0.8 molecules per channel is reached by 700 bar. This induces a small increase in the mean square displacement of the anisotropic displacement parameter (ADP) on the phenyl C-atoms. The fact this can be measured, is testament to the quality and precision of the data collected using the SCC.
Previous high-pressure measurements report a comparable maximum capacity of 0.87 isopentane molecules per channel at 4000 bar,8 albeit with much greater rotation of the Group 2 BDC linker (ca. 52°). The degree of rotation of the Group 2 BDC linker has a linear correlation with applied pressure, increasing threefold from 15° to 52° as the pressure is approximately tripled from 1200 bar to 4000 bar, with no significant change in the quantity of adsorbed isopentane to account for this. This implies that the twisting of the BDC ligand depends upon both the extent of guest-incorporation within the framework, and subsequent compression of the loaded material.
Using the SCC, it has been possible to observe the guest-mediated pore-shape modification in Sc2BDC3 with isomeric pentanes at considerably lower pressure (<1500 bar) than previously reported.8 Together with previous high-pressure results, a more thorough structure-pressure relationship has been derived, revealing discrepant adsorption/desorption behaviour between n-pentane and isopentane. The smaller kinetic diameter of linear n-pentane permits facile intrusion into the channels of Sc2BDC3, which occurs gradually from 50 to 900 bar. This, to the best of our knowledge, is the first structural analysis of a MOF at moderate pressure on intrusion of a liquid guest up to 1200 bar. Many MOFs demonstrate flexibility at gas loading pressure (<100 bar) and high-pressure (1000–100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 bar) but their structural response to ‘moderate’ pressure is unknown, limiting our understanding of their structure-pressure relationships. Understanding the structure-pressure-guest uptake relationships in MOFs is necessary for designing frameworks with predictable flexibility that may be optimised for a given application. The SCC used here demonstrates a versatile in-situ crystallographic tool to investigate flexible and soft molecular and porous systems below 2000 bar in liquid media, bridging the pressure gap between gas cells and diamond anvil cells. For Sc2BDC3, the distinct structural response to n-pentane and isopentane in the moderate pressure region is promising for the use of Sc2BDC3 as an absorbent in ultra-high pressure liquid chromatography (UHPLC), which uses pressure up to 1500 bar. In a broader context, the high precision of the SCC may facilitate the study of structural mechanisms guiding guest adsorption or desorption, guest or pressure-induced phase transitions, or topological flexibility in MOFs in a previously inaccessible pressure range, as well as detailed analyses of functional properties in molecular materials that occur below 2000 bar, such as spin crossover.
000 bar) but their structural response to ‘moderate’ pressure is unknown, limiting our understanding of their structure-pressure relationships. Understanding the structure-pressure-guest uptake relationships in MOFs is necessary for designing frameworks with predictable flexibility that may be optimised for a given application. The SCC used here demonstrates a versatile in-situ crystallographic tool to investigate flexible and soft molecular and porous systems below 2000 bar in liquid media, bridging the pressure gap between gas cells and diamond anvil cells. For Sc2BDC3, the distinct structural response to n-pentane and isopentane in the moderate pressure region is promising for the use of Sc2BDC3 as an absorbent in ultra-high pressure liquid chromatography (UHPLC), which uses pressure up to 1500 bar. In a broader context, the high precision of the SCC may facilitate the study of structural mechanisms guiding guest adsorption or desorption, guest or pressure-induced phase transitions, or topological flexibility in MOFs in a previously inaccessible pressure range, as well as detailed analyses of functional properties in molecular materials that occur below 2000 bar, such as spin crossover.
The authors acknowledge Diamond Light Source, Rutherford Appleton Laboratories, for beamtime on Station I19 and the staff on beamline I19 for assistance, and for providing funding for a PhD studentship to CJM. GFT acknowledges the Australian Government for the provision of an RTP scholarship. SAM thanks the Australian Research Council (ARC) for a Future Fellowship (FT200100243).
Conflicts of interest
There are no conflicts to declare.Notes and references
- J. M. Ogborn, I. E. Collings, S. A. Moggach, A. L. Thompson and A. L. Goodwin, Chem. Sci., 2012, 3, 3011–3017 RSC.
- S. C. McKellar and S. A. Moggach, Acta Crystallogr., Sect. B: Struct. Sci., Cryst. Eng. Mater., 2015, 71, 587–607 CrossRef CAS PubMed.
- F.-X. Coudert and J. D. Evans, Coord. Chem. Rev., 2019, 388, 48–62 CrossRef CAS.
- G. F. Turner, S. C. McKellar, D. R. Allan, A. K. Cheetham, S. Henke and S. A. Moggach, Chem. Sci., 2021, 12, 13793–13801 RSC.
- A. Schneemann, P. Vervoorts, I. Hante, M. Tu, S. Wannapaiboon, C. Sternemann, M. Paulus, D. C. F. Wieland, S. Henke and R. A. Fischer, Chem. Mater., 2018, 30, 1667–1676 CrossRef CAS.
- Z. Chang, D.-H. Yang, J. Xu, T.-L. Hu and X.-H. Bu, Adv. Mater., 2015, 27, 5432–5441 CrossRef CAS PubMed.
- A. J. Graham, A. M. Banu, T. Duren, A. Greenaway, S. C. McKellar, J. P. Mowat, K. Ward, P. A. Wright and S. A. Moggach, J. Am. Chem. Soc., 2014, 136, 8606–8613 CrossRef CAS PubMed.
- S. C. McKellar, J. Sotelo, A. Greenaway, J. P. S. Mowat, O. Kvam, C. A. Morrison, P. A. Wright and S. A. Moggach, Chem. Mater., 2016, 28, 466–473 CrossRef CAS.
- S. A. Moggach, A. Greenaway, C. Hobday, S. C. McKellar, J. P. S. Mowat, J. Sotelo, A. J. Warren, M. R. Warren and P. A. Wright, Conference Abstract, Diamond Light Source.
- W. Li, M. R. Probert, M. Kosa, T. D. Bennett, A. Thirumurugan, R. P. Burwood, M. Parinello, J. A. Howard and A. K. Cheetham, J. Am. Chem. Soc., 2012, 134, 11940–11943 CrossRef CAS PubMed.
- P. Serra-Crespo, A. Dikhtiarenko, E. Stavitski, J. Juan-Alcaniz, F. Kapteijn, F. X. Coudert and J. Gascon, CrystEngComm, 2015, 17, 276–280 RSC.
- K. J. Gagnon, C. M. Beavers and A. Clearfield, J. Am. Chem. Soc., 2013, 135, 1252–1255 CrossRef CAS PubMed.
- C. J. McMonagle, P. Comar, G. S. Nichol, D. R. Allan, J. González, J. A. Barreda-Argüeso, F. Rodríguez, R. Valiente, G. F. Turner, E. K. Brechin and S. A. Moggach, Chem. Sci., 2020, 11, 8793–8799 RSC.
- S. A. Moggach, T. D. Bennett and A. K. Cheetham, Angew. Chem., 2009, 121, 7221–7223 CrossRef.
- S. M. Hyun, J. H. Lee, G. Y. Jung, Y. K. Kim, T. K. Kim, S. Jeoung, S. K. Kwak, D. Moon and H. R. Moon, Inorg. Chem., 2016, 55, 1920–1925 CrossRef CAS PubMed.
- P. G. Yot, Q. Ma, J. Haines, Q. Yang, A. Ghoufi, T. Devic, C. Serre, V. Dmitriev, G. Férey, C. Zhong and G. Maurin, Chem. Sci., 2012, 3, 1100–1104 RSC.
- T. Kundu, M. Wahiduzzaman, B. B. Shah, G. Maurin and D. Zhao, Angew. Chem., 2019, 131, 8157–8161 CrossRef.
- L. Chen, J. P. Mowat, D. Fairen-Jimenez, C. A. Morrison, S. P. Thompson, P. A. Wright and T. Duren, J. Am. Chem. Soc., 2013, 135, 15763–15773 CrossRef CAS.
- S. R. Miller, P. A. Wright, C. Serre, T. Loiseau, J. Marrot and G. Férey, Chem. Commun., 2005, 3850–3852 RSC.
- S. R. Miller, P. A. Wright, T. Devic, C. Serre, G. Férey, P. L. Llewellyn, R. Denoyel, L. Gaberova and Y. Filinchuk, Langmuir, 2009, 25, 3618–3626 CrossRef CAS PubMed.
- J. P. S. Mowat, PhD thesis, University of St. Andrews, 2012.
- C. J. McMonagle, D. R. Allan, M. R. Warren, K. V. Kamenev, G. F. Turner and S. A. Moggach, J. Appl. Crystallogr., 2020, 53, 1519–1523 CrossRef CAS.
- P. W. Betteridge, J. R. Carruthers, R. I. Cooper, K. Prout and D. J. Watkin, J. Appl. Crystallogr., 2003, 36, 1487 CrossRef CAS.
- A. L. Spek, Acta Crystallogr., Sect. C: Struct. Chem., 2015, 71, 9–18 CrossRef CAS.
- A. J. Graham, D. R. Allan, A. Muszkiewicz, C. A. Morrison and S. A. Moggach, Angew. Chem., 2011, 123, 11334–11337 CrossRef.
- J. Gonzalez-Platas, M. Alvaro, F. Nestola and R. Angel, J. Appl. Crystallogr., 2016, 49, 1377–1382 CrossRef CAS.
- J. Sotelo, PhD thesis, University of Edinburgh, 2016.
- C. Zhang, J. A. Gee, D. S. Sholl and R. P. Lively, J. Phys. Chem. C, 2014, 118, 20727–20733 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2202161–2202178. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2cc04649k |
| This journal is © The Royal Society of Chemistry 2022 |