Open Access Article
Open Access ArticleA comprehensive overview of vaccines developed for pandemic viral pathogens over the past two decades including those in clinical trials for the current novel SARS-CoV-2
Kannan Damodharan
 ab,
Gandarvakottai Senthilkumar Arumugam
b,
Suresh Ganesanb,
Mukesh Doble
ab,
Gandarvakottai Senthilkumar Arumugam
b,
Suresh Ganesanb,
Mukesh Doble
 *b and
Sathiah Thennarasua
*b and
Sathiah Thennarasua
aDepartment of Organic and Bioorganic Chemistry, CSIR-Central Leather Research Institute (CLRI), Chennai, 600020, India
bBioengineering and Drug Design Lab, Department of Biotechnology, Indian Institute of Technology Madras (IITM), Chennai, 600032, India. E-mail: mukesh.doble0@gmail.com; mukeshd@iitm.ac.in
First published on 8th June 2021
Abstract
The unprecedented coronavirus disease 2019 (COVID-19) is triggered by a novel strain of coronavirus namely, Severe Acute Respiratory Syndrome-Coronavirus-2 (SARS-CoV-2). Researchers are working around the clock to control this pandemic and consequent waves of viral reproduction, through repurposing existing drugs as well as designing new vaccines. Several countries have hastened vaccine design and clinical trials to quickly address this outbreak. Currently, more than 250 aspirants against SARS-CoV-2 are in progress, including mRNA-replicating or non-replicating viral vectored-, DNA-, autologous dendritic cell-based-, and inactivated virus-vaccines. Vaccines work by prompting effector mechanisms such as cells/molecules, which target quickly replicating pathogens and neutralize their toxic constituents. Vaccine-stimulated immune effectors include adjuvant, affinity, avidity, affinity maturation, antibodies, antigen-presenting cells, B lymphocytes, carrier protein, CD4+ T-helper cells. In this review, we describe updated information on the various vaccines available over the last two decades, along with recent progress in the ongoing battle developing 63 diverse vaccines against SARS-CoV-2. The inspiration of our effort is to convey the current investigation focus on registered clinical trials (as of January 08, 2021) that satisfy the safety and efficacy criteria of international wide vaccine development.
Introduction
One of the most successful therapeutic strategies to prevent or control various diseases is by “vaccination” protocol.1,2 Millions of lives have been saved because of vaccinations, which cover a number of diseases including certain types of cancer, HIV and many viral infections.3 Vaccination was initially accomplished by Edward Jenner, who was the pioneer for smallpox in the late 18th century.4 In the 1980s, the development of vaccines to fight against pathogenic microorganisms was tentatively introduced. Vaccines are now employed to improve and increase the protection capability (immunity) of the body to fight severe infection and disease,5 and moreover, primarily intend to make immunity stronger and reduce resistivity of diseases by reducing the reproduction of target pathogens. The majority of vaccines actively exist in the immune system, since anti-bodies are constantly generated in the body to sustain a healthy immunity system.6,7General components of vaccines
A vaccine consists of an antigen, stabilizer, adjuvant, antibiotic, preservative, and chemical reagents such as formaldehyde.8 The advantages and disadvantages of vaccinations9a are listed in Table 1.| Advantages | Disadvantages |
|---|---|
| Protection of inhabitants against disease | Uncertainty about complete protection |
| Preventing epidemic and pandemic diseases | May have some possible side effects |
| Prevents diseases spreading to others | Requires NHS/individual outlay |
| Avoids large cost for the treatment of infected patients | Injections are not pleasant/more immune booster injections are inconvenient |
The MMR vaccine was developed by Maurice Hilleman in 1971. Mumps, like measles infections, are caused by an RNA based virus from the Paramyxoviridae family. Moreover, measles and mumps belong to the genus Rubulavirus, it is a human disease with no animal reservoirs. Generally, the MMR vaccine exhibits side effects of a painful arm from the shot, minor rashes, generally in teen/adult women who have no earlier immunity; then the rubella vaccine component can result in joint and tendon stiffness. This vaccine is also associated with the minor threat of seizures/jerking instigated by fever, but is not connected with any enduring effects. The threat of febrile seizures increases as infants get older, hence this vaccine is recommended at a young age. Some people may experience cheek/neck inflammation, impermanent low platelet counts that generally do not require treatment and are also not life threatening.9b
In 1955, Jonas Salk initiated an inactivated polio vaccine (IPV), after that, Albert Sabin further developed the live, OPV. Even though poliovirus has three serotypes, both vaccines are trivalent and offer good resistivity against poliomyelitis, a limited number of countries have continuously provided IPV. Sabin’s OPV vaccine is underused in most countries due to minimising oral management, but it advances immunity in the intestine, which is capable of spreading to others, and is associated with lower cost.9c
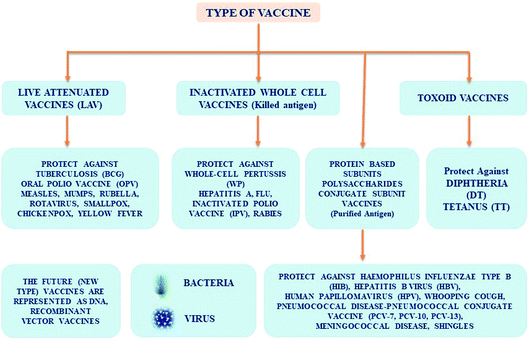
Types of vaccines
Various kinds of vaccines are available and those which are administered to infants and adults can be classified (Fig. 1) as follows:• Live-attenuated.
• Inactivated.
• Toxoid.
• Conjugate.
• Subunit.
Common characteristics of vaccines
Live attenuated vaccines are typically grown in animal cell lines under poor development conditions. The development of an inactivated vaccine involves use of thermal or chemical methods in the beginning, and its mode of action involved in conferring immunity is not fully known. However, the live attenuated or execution of entire organism-supported vaccines have shown a lot of success in the control and inhibition of severe transmittable diseases in human, including animal infectious cattle plague, classic swine fever, equine infectious anaemia, measles, mumps, polio, rubella, smallpox, and so on. In recent times, the use of LAVs, subunit and peptide based vaccines have become possible because of progressive technologies in molecular biology. LAVs are based on the mechanism of action associated with the immune response. Inactivated vaccines, based on antibodies have been mostly used to prevent and manage microbial infectious diseases. LAVs introduce stronger cell immune responses that are decisive to remove several intracellular viral pathogens. However, these pathogens sometimes bypass inactivated vaccines9d by mutating peripheral antigens. On the other hand, subunit and peptide based vaccines are less efficient in drawing a strong CD8+ immune response.Progressive vaccination involves the use of non-viral distributed nucleic acid-supported vaccines, which imitate live microorganism infection- or immunization. This leads to T-helper cellular immune responses. In addition, this vaccine development9e,f is harmless and consumes less. It does not require extreme infectious organisms, so is safe from infectivity through live transmittable agents and the discharge of harmful pathogenic organisms. These vaccines fill the gap between a virus outbreak and design of a desirable vaccine, wherein they are classified as DNA/RNA based pentose-carbon sugar motif. The remarkable growth of RNA-associated vaccines resulted in the growth of mRNA based vaccines. It is quite significant to note that mRNA vaccines provide many valuable benefits when compared to viral vectored- and DNA vaccines.10
Proceedings of vaccination against viral diseases
In 1970, to evade the prevalent spread of foot and mouth disease, scientists discovered a vaccine using a single protein from the virus. Despite their achievements in virology, in particular vaccine studies and their development, the lack of understanding of immunological mechanisms of action during induced defensive immunity, has prevented the use of existing vaccines during global pandemic outbreaks of related diseases with similar viral pathogens. The immune system protects against various pathogens, including distinctive units of T-helper cells that are useful to protect against various unusual pathogens. Besides, the follicular T-helper cells (TFH cells) generate interleukins (ILs) and support the partition of B cells (they are lymphocytes, and take part in the humoral immune response) and generation of Memory B cells. Furthermore, Memory T cells can be sub classified as CD4+/CD8+ T cells (Cluster of Differentiation) and their functionalities are (a) central memory and (b) effectors memory, which provide various responses upon vaccination against different pathogens.11 Different vaccines attempted for identical pathogens depend on the perceptions of the scientist,12 many healthcare professionals do not pay sufficient attention to vaccines, which may result in uncertainty in their efficacy, side effects and toxicity. Table 2 lists the various vaccines discovered that are available in the current market for protection against viral infections. They are listed on the basis of various factors,13,14 including the apparent protection level, plausible mechanism of action, possibility of usage for other diseases.15,16| Characteristic vaccines | |||||||
|---|---|---|---|---|---|---|---|
| S. no. | Generic name | Trade name | Type | Mode of action | Treatment for other diseases | Year of approval | Ref. |
| 1 | AJ vaccines | Picovax IPV vaccine, Danish Medicines Agency | Inactivated Polio Vaccine (IPV) | IPV provides serum immunity to each poliovirus (three types) and defence against any ensuing paralysis causative disease (poliomyelitis). The mucosal immunity level in the intestine is less than that afforded by OPV, this difference may slightly show in the pharyngeal mucosal lining. Prevents poliovirus in the nervous system; immunostimulant | Diphtheria, whooping cough, tuberculosis, tetanus, polio and bladder cancer | 2019 | 17 |
| 2 | Grippol plus | Quadrivalent AbbVie | Polymer supported inactivated influenza vaccine 3-valent, egg derived adjuvanted influenza vaccine, polyoxidonium as a distinct adjuvant | Vaccine primes advanced meticulous anti-influenza immunity formation; immunostimulant | To treat influenza virus infection (A/H1N1 + A/H3N2) | 2018 | 18 |
| 3 | Ys-On-001 | Yivyka polyinosinic-polycystidylic acid/inactivated rabies virus Yisheng Biopharma | Cancer vaccines anti-neoplastics | B-cell stimulant, cytokines stimulant, dendritic cell stimulant, immune-modulator, macrophage modulator, natural cell stimulant, regulatory T lymphocyte inhibitor, Th1 cell stimulant | Cancer vaccines pancreatic cancer | 2017 | 19 |
| 4 | Adimmune’s quadrivalent flu vaccine (4142) | Adimflu-S (QIS)BioB2B Taiwan | Monovalent vaccine, egg-based inactivated split virus, influenza H1N1 vaccine | Immunostimulants; inactivated virus from chick embryo culture split virus | Influenza A (H1N1) vaccine during pregnancy | 2017 | 20 |
| 5 | Quadrivalent influenza vaccine (J0&BB02) | Vaxigrip Tetra QIVSanofi Pasteur | Split influenza virus vaccine | Induces the humeral antibodies against hemagglutinins inhibition (HAI) within 2–3 weeks; antibodies neutralize the influenza viruses | Potent immunization against the four influenza virus strains (A and B types have two each) | 2017 | 21 |
| 6 | NBP-608 | SKY Zoster | Attenuated zoster-, varicella-type vaccine | Inducing both humoral and cellular immune response, which creates an IgG humoral immune response; varicella-zoster specifically activate CD4+ T-helper and CD8+ T-lymphocyte cells | Varicella-zoster virus (VZV), a human neurotropic alpha-herpes-virus. Major infection causes varicella (chickenpox) | 2017 | 22 |
| 7 | HBV-ISS (Dynavax) | Heplisav-B | Hepatitis B virus used for vaccine development | Immune-stimulatory DNA sequence (ISS) ISS-1018, adjuvant proceeds TLR-9 agonist, which is used for potential prevention and treatment of HIV infection | Treatment of HBV-, HIV viruses | 2017 | 23 |
| 8 | GSK-1437173A | Shingrix GSK | Non-live, recombinant subunit vaccine; varicella vaccine herpes zoster (Hz/su) 2q4vaccine candidates | Antigen IgE and adjuvant system AS01B develop VZV-specific immune response in a weakening immune system. Generates a long-term immune response, Shingrix helps to address age-related decline in shingles immunity | To prevent herpes zoster (HZ) infection | 2017 | 24 |
| 9 | DTPa-hepB-IPV-Hib | EasySix, Hexaxim Panacea Biotech | 6 in 1 combination Infanrix hexa whole cell pertussis antigen | The body produces its own antibodies to protect against bacteria and viruses causing different infections | Diphtheria, DTP, Hepatitis B, Hib, IPV, Pertussis, Polio, Tetanus vaccine | 2017 | 25 |
| 10 | Ad5-EBOVJ07BX02 | ErveboBIB, CanSinoBIO | Glycoprotein recombinant adenovirus type 5 Ebola virus vaccine | Recombinant replication deficient human Ad5 vector stimulating immune responses and defending against the Ebola virus | To treat Ebola virus | 2017 | 26 |
| 11 | Tetravalent inactivated influenza vaccine (TIV) | Vaxiflu-4 Zydus Cadila | Influenza vaccine | To increase immunity by generating antibody proteins, which defend against infection caused by the virus present in the vaccine | Treating influenza viruses-H1N1, H3N2, Type B (Brisbane and Phuket) | 2017 | 27 |
| 12 | Flu Vac Qs 2019–20 (4 Yr Up) CD,BL-125408 | Flucelvax Quadrivalent Seqirus, Inc | Cell based flu vaccines | Vaccine motivates the body to produce its own immunity (antibodies) against the virus | To prevent influenza A and B viruses | 2016 | 28 |
| 13 | Quadrivalent influenza vaccine (QIV) (GQM-10) | Vaxigrip TetraSanofi | Inactivated influenza vaccine (split version) | To enhance the protection from circulating influenza B viruses | To protect against influenza (flu) viruses | 2016 | 29 |
| 14 | Live attenuated influenza vaccine (LAIV) J07BB03 | FluMist Quadrivalent Influenza Tetra-Medimmune | Attenuated virus | LAIVs induce T cell antibody-reactions against the surface protein of HA and NA; LAIVs provide hetero-subtype protection in humans | To prevent common flu; influenza A-(H1N1), (H3N2), and B-viruses | 2016 | 30 |
| 15 | Enterovirus type 71 vaccine (Vero cells) | Inlive SINOVAC | Inactivated EV 71 virus antigen vaccine | Generate immune reactions against EV71 virus | To prevent hand-foot-mouth disease (HFMD) caused by EV-71 | 2016 | 31 |
| 16 | Inactivated influenza vaccine | Cadiflu-S CBL Biologicals Pvt. Ltd | Inactivated vaccine | To develop immunity against the disease by forming antibodies | To prevent influenza and protect against its effects | 2016 | 32 |
| 17 | Inactivated quadrivalent influenza vaccine (split version) | Afluria Quad 2020 Seqiruspty Ltd | Influenza quadrivalent vaccine 2020 | Against influenza-A and influenza-B type viruses | To prevent influenza A and B viruses | 2016 | 33 |
| 18 | Riv-4 (RIV4) | Flublok-QSonofi Pasteur | Quadrivalent recombinant influenza vaccine | Humoral immune response measured by hemagglutination inhibition antibodies | To prevent influenza A (H1N1) and B (H3N2) | 2016 | 34 |
| 19 | Gam Evac Combi | Combined Vector Based Vaccine | Heterologous VSV and adenovirus type-5 vectored Ebola virus | Heterologous prime-boost vaccine humoral immune response, cell facilitated immune response to Ebola | To prevent Ebola viral disease (EVD) | 2015 | 35 |
| 20 | In32 Activated Sabin polio vaccine | Ai Bi Wei IMB China | Inactivated Poliomyelitis (IPV) | Inactivated vaccines offer immunity by delivering an inactivated antigen. This vaccine cannot cause disease, thus, it may be administered to an immuno-compromised host | To prevent polio in new born babies | 2015 | 36 |
| 21 | Rotavirus Orv-116E | ROTAVAC 5D Vero cells Derived Bharath Biotech | Rotavirus vaccine live attenuated monovalent vaccine | Rotarix (vaccine prevents rotavirus infectivity) reproduces in the small intestine and induces immunity; the particular mechanistic action of immunology by rotarix against rotavirus gastroenteritis is unknown | To prevent rotavirus gastroenteritis | 2015 | 37 |
| 22 | NBP-607-QIV | SKY cell flu Quadrivalent SK Bioscience | Cell cultured quadrivalent inactivated subunit influenza vaccine | Immunity for influenza viruses A and B subtypes. Sero-protection is generally obtained within three weeks | To treat influenza virus infections | 2015 | 38 |
| 23 | GC-3110A, GC-3110B | GCflu Quad GC Pharma | Quadrivalent influenza virus vaccine | Hemagglutination inhibition antibody response | To treat influenza virus infections | 2015 | 39 |
| 24 | Chimerivax-dengue (CYD-TDV) | Dengvaxia Sanofi Pasteur | Live attenuated tetravalent chimeric vaccine | Antibody dependent improvement | To prevent Dengue virus-1, 2, 3 and 4 fever in humans | 2015 | 40 |
| 25 | H5N1 influenza (avian flu) vaccine (Rx) | Audenz Vn-101 Sonali Pasteur GSK | Egg based H5N1 vaccines, inactivated influenza virus vaccine | Induces immunity (antibodies), which act against viral HA in the vaccine, after interrupting viral attachment to human respiratory cells, provides immunity to influenza A virus subtype H5N1 | To treat influenza virus | 2014 | 41 |
| 26 | 9-Valent HPV vaccine [9v HPV] | GARDASIL 9 | Recombinant virus | Prevent HPV through humoral immune responses induced by the vaccine | Prevention of cervical, vuvar, vaginal, anal, or pharyngeal, head and neck cancer; by preventing papilloma virus (HPV) infection | 2014 | 42 |
| 27 | Cell cultured-H5N1 vaccine KD-295 | GSK | Emulsion cell culture influenza HA vaccine (Prototype) | Antibody titer calculated through hemagglutination inhibition (HI): high-titer virus generation led to suspension of growth of MDCK (Madin–Darby Canine Kidney) and Vero cells in a serum-free distribution | To prevent influenza A virus H5N1 | 2014 | 43 |
| 28 | Live modified vaccine virus Ankara Ankara-Bavarian Nordic (MVA-BN)-J07BX | IMVANEX/IMVAMUNE JYNNEOS | Non-replicating small pox vaccine | Non-replicating and capable of generating humoral and cellular immune response to orthopox-viruses | To prevent smallpox | 2013 | 44 |
| 29 | Japanese encephalitis vaccine BBIL/JEV | JENVAC Bharath Biotech | Inactivated Vero cell-derived viral vaccine | JENVAC is sufficient to elicit an immune response | To protect against Japanese encephalitis virus (JEV) | 2013 | 45 |
| 30 | Fluzone quadrivalent BL 103914/J07B B | FLUZONE Quadrivalent Sanofi Pasteur Inc. | Inactivated quadrivalent influenza virus vaccine type A and type B (split version) | Stimulates the production of specific antibodies | Prevents influenza diseases, type A and B | 2013 | 46 |
| 31 | FLU-Q-QIV flu laval quadrivalent GSK-2282511A | FluLaval™ Quadrivalent Glaxo Smith Kline (GSK) | Influenza virus vaccine | Vaccines which improve immunity against the viral pathogen leading to influenza: they induce the generation of antibodies | Prevents influenza diseases, type A and B | 2013 | 47 |
| 32 | DTa-IPV-HePB-HibHexavalent vaccine6-in-1 vaccine J07CA09 | Hexyon, Infanrix, Vaxelis | Hexavalent vaccine | Booster vaccination | To treat DTaP, hepatitis B, polio, haemophilus influenza diseases | 2013 | 48 |
| 33 | GSK-2282512a BL 125127 | Fluarix Quadrivalent (FLU Q-QIV) Glaxo Smith Kline Biologicals | Inactivated influenza vaccine, quadrivalent, seasonal | Increases immunity for treatment of disease originating from influenza-A subtype and type B viruses | Prevents disease triggered by influenza A subtype and type B viruses | 2012 | 49 |
| 34 | ChimeriVax™-JE | IMOJEV Sanofi | Live attenuated Japanese encephalitis vaccine (JEV), monovalent | IMOJEV is highly immunogenic and able to induce continuing immunity through both preclinical and clinical trials | To prevent yellow fever virus | 2012 | 50 |
| 35 | Prepandemic influenza vaccine (H5N1) J07BB01 | Vepacel | Inactivated flu strain known as A/Vietnam/1203/2004 (H5N1) whole virion, derived from inactivated Vero cells | Overall the vaccine primes the immune system | Protect against influenza H5N1 (bird flu) | 2012 | 51 |
| 36 | Medi-3250STN: 125020 | FLUMIST Quadrivalent Med-Immune | Influenza vaccine, live attenuated influenza vaccine (LAIV) | To provide immunity against influenza virus caused by subtypes A and B | Protects against influenza | 2012 | 52 |
| 37 | Hepatitis E hecolin (HEV-239) | Hecolin Xiamen Innovax Biotech | Non enveloped virus with positive sense HEV vaccine, a recombinant vaccine | HEV 239 treatment induces a strong anti-HEG IgG response, through early antibody mobilisation | Fights against hepatitis-E virus | 2012 | 53 |
| 38 | Measles/rubella vaccine | German measles | Live attenuated (weakened) viruses | Immunostimulant, produces antibodies (associated proteins fight and kill measles, mumps, and rubella (MMR) viruses | Prevent MMR viruses | 2011 | 54 |
| 39 | Human inactivated influenza vaccine (H1N1) 2009 vaccine. Pandemic influenza strain A/California/7/2009/nyMC X-179A | HNVAC (Bharath Biotech) | Inactivated Influenza A virus vaccine (H1N1). Cell culture derived vaccine | Active immunization agent, which acts against the influenza A (H1N1) 2009 virus | Activity against influenza A | 2010 | 55 |
| 40 | Influenza A virus (H1N1), monovalent vaccine | 2009 Influenza A (H1N1), Sonali Pasteur | Monovalent vaccine, adjuvant | Active immunization for preventing disease caused by influenza virus A (H5N1) | To prevent influenza A viral disease | 2010 | 56 |
| 41 | Quadrivalent flu vaccine | FLUCELVAX | Cellular influenza vaccine | Active immunization for preventing influenza subtypes A and B causative diseases | To defend against four different strains of influenza for both subtypes A and B | 2010 | 57 |
| 42 | Influenza vaccine (whole virion). Inactivated combination | Vaxiflu-S Fluzone Zydus Cadila Healthcare Ltd | Inactivated influenza vaccine (NZ) | Active immunization for preventing Vaxiflu-X | To assist in protection against influenza | 2010 | 58 |
| 43 | H1N1 pandemic influenza vaccine H5N1 strain of the flu virus A/Vietnam/1203/2004Flu strain/California/07/2009 (H1N1) virus | Celvapan Baxter International | Whole virion in Vero cell-based influenza vaccine, inactivated | Motivates the immune system to produce antibodies when exposed to the virus | To protect against the influenza strains of H5N1 virus | 2009 | 59 |
| 44 | Influenza A virus vaccine H5N1 | Fluval P-H5N1 Omninvest | Fluart innovative vaccine | Fluval affords active immunization against four virus strains; two for influenza A subtype, and two for B type | To prevent influenza a (H5N1) | 2009 | 60 |
| 45 | Non adjuvant influenza A (H1N1) 2009 monovalent vaccine. Influenza hemagglutinin (HA) A/California/07/2009 (H1N1) V-like virus | Panenza Sanofi-Pasteur | Non-adjuvanted pandemic vaccine | Induces a high immune (antibody) response, three weeks post-vaccination | To prevent influenza A (H5N1) | 2009 | 61 |
| 46 | Monovalent, cell culture-derived, inactivated subunit influenza vaccine. Produced from A/California/07/2009 (H1N1) with adjuvant MF-59 | Celtura | MF-59 adjuvanted cell cultured derivative A/H1N1 pandemic influenza vaccine | MF-59 induces a strong response in adults and substantially develops the response with growth of HA-specific Tfh (CD4+, ICOS+, CXCR5+, IL-21+) cells | Induces an immune response for protection against influenza virus | 2009 | 62 |
| 47 | Pandemic (H1N1) ASO3 adjuvanted influenza vaccine | Pandremix GSK | Combination of H1N1 virus antigen and adjuvant system of H1N1 | Enhances the natural immunity of the body | To prevent influenza A (H1N1), swine flu viral infections | 2009 | 63 |
| 48 | Influenza vaccine (H1N1) flu strain from A/California/7/2009 (H1N1) derived strain NYMC-181 J07BB02 | Focetria | Surface antigen (hemagglutinin and neuraminidase) inactivated adjuvant | Vaccine acts by priming the immune system | To protect against influenza type A (H1N1) 2009 virus | 2009 | 64 |
| 49 | Cell culture-derived adjuvanted influenza virus vaccine (Grippol TC | Grippol NeoSolvay pharmaceutical AbbVie | Cell based adjuvanted influenza vaccine | Activates the endosomal receptor, which leads non-specific activation of the surface TLRs, which induce the intracellular signals contributing to the antiviral mechanism | To prevent influenza virus infections | 2009 | 65 |
| 50 | Inactivated H5N1 influenza (avian flu) vaccine A/Vietnam/1194/2003/(H5N1) RG | Pan-flu (Sinovac Biotech) | Single shot vaccine against H1N1 influenza | Body reacts by creating antibodies | To prevent H5N1 pandemic influenza | 2008 | 66 |
| 51 | Pandemic influenza vaccine | Panvax H1N1 vaccine | Split virion inactivated vaccine | Panvax, is an inactive viral part of H1N1, the immune system responds by developing antibodies to the virus particle | Influenza virus (H1N1), swine flu infections | 2008 | 67 |
| 52 | Pre-pandemic influenza vaccine H5N1 (split virion, adjuvanted, inactivated) AS03-H5N1 vaccine A/Vietnam/1194/2004; or A/Indonesia/5/2005 GSK-1562902a | Prepandrix GSK | Split virion, inactivated, adjuvanted; hemagglutinin, antigen, adjuvanted | Potential immunization against influenza A subtype H5N1 virus | Influenza (H5N1) virus, swine flu infection | 2008 | 68 |
| 53 | Influenza vaccine (surface antigen, inactivated, prepared in cell cultures) J07BB02 | Optaflu Flucelvax TETRA Novartis | Influenza virus surface antigens, hemagglutinin and neuraminidase subunit vaccines | Optaflu comprising different strain surface regions of the flu virus. Vaccine causes immune system to recognize the unknown viral components and produce antibodies against them | Prevent influenza viral infection | 2008 | 69 |
| 54 | Smallpox (vaccinia) vaccine ACAM2000 | Acam-2000 Sanofi Pasteur Biologics Co. | The vaccine is made from a live virus, vaccinia | Possessing potent immunization against smallpox | Prevention of smallpox | 2007 | 70 |
| 55 | Inactivated quadrivalent influenza vaccine (split virion) | Afluria Seqirus Pty. Ltd. | Quadrivalent split virion, influenza virus hemagglutinin as the active ingredient. | Immunity against 3 (type A (2) and type B (1)) or 4 (type A (2) and type B (2)) microbial strains. Suitable for the annual flu season | To stop infection caused by influenza virus | 2007 | 71 |
| 56 | H5N1 avian flu vaccine, avian influenza or bird flu, vaccine is derived from A/Vietnam/1203/2004 influenza virus | Fluzone Sanofi Pasteur | Inactivated influenza virus vaccine | Provides protection against the H5N1 influenza virus stimulating the immune response | To prevent infection caused by influenza virus | 2007 | 72 |
| 57 | Adjuvanted H5N1 pre-pandemic vaccine | Daronix, GSK | Second generation pandemic vaccine | Prepare the body’s immune system to prevent a flu epidemic | Prevent common flu disease | 2007 | 73 |
| 58 | Influenza vaccine surface antigen, inactivated, prepared in cell culture: A/California/7/2009 (H1N1) pdm09-like strain, A/Switzerland/9715293/2013 (H3N2)-like strain, B/Phuket/3073/2013-like strain | Optaflu | Vaccine containing flu surface antigen | Enhances the body’s defence system, priming the immune system to make antibodies against the flu virus | To prevent infection caused by influenza virus | 2007 | 74 |
| 59 | Birch pollen allergy vaccine | Oralgen Birch Pollen ALK-Abello | Peptide based vaccine | Suppression of allergic reactions after immunization with fusion protein, which is caused by releasing the immune messenger interleukin-10 (IL-10), an autologous cytokine, functioning to decrease the overacting immune response | To prevent allergy | 2007 | 75 |
| 60 | Influenza vaccine, split virion, inactivated hemagglutinin of A/California/7/2009 Hemagglutinin of A/Victoria/210/2009 Hemagglutinin of B/Brisbane/60/2008 | Anflu Sinovac | Split virion, inactivated | Body generates immuno-reactions against influenza virus | To prevent infection caused by influenza virus | 2006 | 76 |
| 61 | Rec hepatitis B vaccine | Supervax | Hepatitis B vaccine, immunoglobulin (HBIG) | Active immunization of hepatitis B vaccine induces the immune system to create anti-HBs without an active infection risk | To prevent infection against hepatitis B virus | 2006 | 77 |
| 62 | Antirabies vaccine | RABIRIX Bharath Biotech | Vero cell based rabies vaccine (PVRV) | Inactivated virus vaccine boosts immunization against rabies | To prevent infection against rabies virus | 2006 | 78 |
| 63 | Live herpes zoster vaccine | Zostavax Merck | Live attenuated virus vaccine | Enhancing VZV – specific immunity against zoster | Treatment for herpes zoster/postherpetic neuralgia (PHN), a neuropathic pain | 2006 | 79 |
| 64 | Rotavirus vaccine, live rotavirus 116E strain derived in Vero cells | RotaTeq Bharath Biotech | Live attenuated, oral, monovalent | Active immunization for infants from the age of 6 weeks | To protect against rotavirus gastro-enteritis disease in infants and young children | 2006 | 80 |
| 65 | Human papillomavirus quadrivalent (type 6, 11, 16, and 18) vaccine (HPV vaccine) J07BM01 | Gardasil, Cervarix Merck | Protein subunit quadrivalent, recombinant | Body’s immune system identifies the viral proteins in Gardasil, develops antibodies against them | To protect against either two or four or nine types of HPV (cervical, vaginal and vulvar in females) | 2006 | 81 |
| 66 | Rotavirus vaccine with five strains of rotavirus, from both human and animal sources | ROTARIX | Live, attenuated, oral | Usually to develop immunity against rotavirus-based disease | Rotarix vaccine assists to prevent this disease in children | 2005 | 82 |
| 67 | Hepatitis B (r DNZ) vaccine, adjuvanted, absorbed J07BC01 | Fendrix GSK | Adjuvanted, absorbed, recombinant DNA technology | Vaccine works by priming the immune system | Prevents hepatitis B virus infection | 2005 | 83 |
| 68 | MR vaccine freeze-dried, live, attenuated, measles-rubella combined vaccine | Mearubik, Mitsubishi Tanabe Pharma Corporation | Live vaccine for measles, rubella | Immune system induced to produce antibodies (proteins that fight and also kills the rubella virus) | To prevent rubella virus | 2005 | 11 |
| 69 | Inactivated hepatitis A and hepatitis B (rDNA) – HAB adsorbed vaccine | Bilive Sinovac | Recombinant DNA technology | Suboptimal immune response to the vaccine | Prevents hepatitis B viral infection | 2005 | 84 |
| 70 | Virosomal influenza vaccine, Invivac influenza vaccine | Invivac Solvay Pharmaceuticals | Adjuvant | The virosome mechanism remains complex; it is the transporter as well as an immune stimulant | To treat influenza | 2004 | 85 |
| 71 | Influenza virus (live) (LAIV), inactivated influenza vaccine (IIV) | Flumist | Wild-type | Disease-causing viruses has been attenuated and inactivated, using the influence of heat/chemicals such as formaldehyde | Protects against infection from influenza viruses | 2003 | 86 |
| 72 | Hepatitis A and B Vac DB10989, DB11627 | Ambirix | Vaccine based | B-lymphocytes anti-HBs antibodies | Immunization against hepatitis A and B viral infectivity | 2003 | 87 |
Mode of action stimulated by vaccines
Normally antibodies prevent/minimize infections from extracellular pathogens:(a) use enzymatic active sites to fuse to toxins to break their diffusion;
(b) prophylactic action preventing viral replication;
(c) facilitate opsonophagocytosis of extracellular bacteria;
(d) inducing the complement cascade.
CD8+ T cells do not inhibit infection but work to minimize, regulate, and remove intracellular pathogens through:
(a) direct destruction of infected cells (discharge of perforin – a pore forming cytolytic protein present in cytotoxic T lymphocytes (CTLs) and natural killer (NK) cells), granzyme (serine proteases delivered through cytoplasmic granules inside cytotoxic T cells and NK cells);
(b) destroy infected cells with antimicrobial cytokine release.
CD4+ T cells do not inhibit infection, however they contribute in the minimization, regulation, and refinement of extra- and intra-cellular pathogens with their control and cytokine-development capabilities. The main examples of CD4+ T cells are:
(a) Follicular T-helper (Tfh) cells yielding predominantly interleukin (IL)-21 and providing assistance to B-cells;
(b) T-helper 1 (Th1) effector cells yield interferon (IFN)-γ, (TNF)-α/TNF-β, IL-2, and provide a major role in controlling intracellular pathogens e.g., viruses and bacteria such as M. tuberculosis;
(c) Th2 effector cells generate IL-4, IL-5, IL-13, and impact extracellular pathogens such as bacteria and helminths;
(d) Th9 effector cells generate IL-9 and also defend against extracellular pathogens;
(e) Th17 effector cells generate IL-17, IL-22, and IL-26 and participate in mucosal protection (for example against, S. pneumoniae, B. pertussis, M. tuberculosis).
Main effectors of vaccine responses
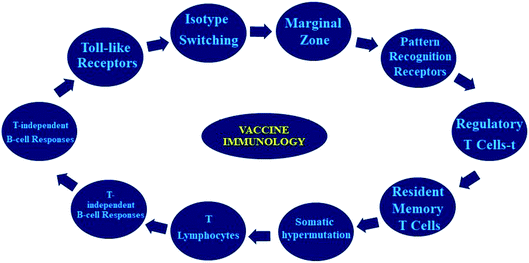
Vaccines prevent disease by inducing effector modes of action in cells/molecules to reduce the development of pathogens and deactivate their toxic effects. Vaccine-stimulating immune effectors are resourceful antibodies generated by B lymphocytes’ ability to interact to a particular toxin/pathogen. A pictographic representation of vaccine immunological function is shown in Fig. 2.The main target of immunization through vaccination is to inhibit specific infections and their unavoidable difficulties. The best vaccine is the one which concomitantly accomplishes the following criteria such as:
(a) Actively inhibit the infective disease or else minimize the adverse effects of the disease;
(b) Offer a strong and continuing defence against a specific disease;
(c) Improves immunity through a minimum quantity of administrations;
(d) Deliver abundant antigens to afford wide-ranging safety against infection;
(e) Never results in side effects, or keeps them to a minimum;
(f) Remains stable under storage conditions, preferably mild storage conditions, for its shelf-life;
(g) Can be produced on a huge scale;
(h) Should be economical and easily available.
Herein we consider the COVID-19 pandemic, the main resolution of vaccination against SARS-CoV-2 are:
(a) Inhibition of characteristic clinical symptoms so hospitalization is avoided, and reduces severe infectivity;
(b) Prevention of disease spreading before the corresponding antibodies are produced (sero conversion)
(c) Producing a strong neutralizing immune response able to link with the viral protein spike (S) that must prevent it from attaching to human cells.
From this perspective, the various immunological response effects which neutralize antibodies and CD8+ T cells are most significant.
The antibodies of anti-SARS-CoV-2 alert the host organism’s immune response to the presence of the virus; such antibodies are immunoglobulins, which are appropriately split into IgA, IgM, IgG, and less frequently IgD. Prior serological antibody model responses to viral infections have usually proven the subsequent sequence of these antibodies resulting from virus infection: the antibodies of IgA are primary, which are followed by IgM, IgG-type continues at high levels for a longer time than the preceding ones (IgA and IgM). For certain viruses, sometimes the antigen (the virus itself) co-occurs with antibodies, particularly with the antibodies of primary IgA and IgM. Further, viruses have a “serological window,” i.e., a period between the initial arrival of the antigen (in the blood) and the antibody response, thus phase intervals of infection occur. Eventually, IgG-type antibodies are specific to the novel coronavirus (SARS-CoV-2) and can be examined through chemiluminescence immunological routes, which is an automated laboratory process using enzyme-linked immunosorbent assays with higher arrangement. The examination mostly identifies the body’s immune response to SARS CoV-2 infection.
Significant characteristics of (corona) virus
Before 2019, there were two pandemics caused in the past two decades by coronavirus; namely SARS during 2002–2003 and Middle East Respiratory Syndrome (MERS) in 2011. According to the International Committee on Taxonomy of Viruses (ICTV), the coronaviruses (CoVs) are sub-classified as Othocoronavirinae, which in turn consist of four categories – alpha-CoV, beta-CoV, gamma-CoV, and delta-CoV. The alpha- and beta-CoVs transmit disease in mammals such as bats, pigs, cats and mice. Gamma- and delta-CoVs usually infect birds. In addition, the seven different types of human CoVs – which include HCoV-229E, HCoV-NL63 – belong to alpha-CoVs. The HCoV-OC43, HCoV-HKU1, SARS-, MERS- CoVs, and the current pandemic SARS-CoV-2 belong to beta-CoVs, and the genus of such beta coronavirus occurrences are zoonotic infections. The December 2019 outbreak coronavirus, which leads to the respiratory-associated syndrome, originated from Wuhan, China, and is called the novel corona virus disease 2019 or nCOVID-19, and its genome is fully sequenced.88 The genetic sequential arrangement of SARS-CoV-2 has an identical genomic array of SARS-/MERS-CoV.89,90Initially, the taxonomy of coronaviruses are split in to three groups, on account of genetic and serological interactions, the first set (Group-1) comprised several viruses including the porcine epidemic diarrhoea virus (PEDV), porcine transmissible gastroenteritis virus (TGEV), canine coronavirus (CCoV), feline infectious peritonitis virus (FIPV), the previously identified coronavirus of HCoV-229E, and HCoV-NL63. Whilst the second combination (Group-2) consists of murine hepatitis virus (MHV), bovine coronavirus (BCoV), human coronavirus OC43 (HCoV-OC43), rat sialodacryoadenitis virus (SDAV), porcine hemagglutinating encephalomyelitis virus (PHEV), canine respiratory coronavirus (CRCoV), and equine coronavirus (ECoV). In a similar pattern, the third group (Group-3) contains the avian infectious bronchitis virus (IBV) and Turkey coronavirus (TCoV). Now, the SARS coronaviruses (SARS-CoV) cannot be associated with any of these representative groups, however they possess some similarities along with the second group coronaviruses.91a
The battle between scientists and viral infections is a perpetual process and thus the identification of specific potential drugs with high efficiency and low toxicity is a continuous aim. Globally, this is the first time, that scientific researchers, diplomats, politicians, and capitalists have convened to work towards a common objective. The FDA approved drugs chloroquine and hydroxychloroquine to be utilized in critical illness cases, but clinical practices were still becoming overwhelmed by CoV cases. The suggestion to employ extensive use of these antiviral drugs was not sufficient. Certain polymerase nucleoside/nucleotide inhibitors are promising agents. Favipiravir a selective viral RdRp inhibitor, has been tested in clinical trials against COVID-19. Furthermore, the antiviral drugs lopinavir, ritonavir, remdesivir, nelfinavir, serine based protease inhibitors of nafamostat, camostat, efficient lipid reducing statin, rosovastatin, TNF alpha inhibitors, interleukin 1 receptor antagonists; Janus associated kinases (JAK) as well as monoclonal antibodies, tocilizumab, baricitinib and ruxolitinib etc., along with their combinations and other antiviral components, are also under investigation in clinical studies to combat COVID-19. In the future, researchers are requested to accumulate their results to provide more knowledge to repurpose significant drugs appropriately, and provide cheap drugs with the minimum toxicity profile.91b,c
The manufacture of SARS-CoV-2 vaccines through various approaches
Vaccines have to be approved based on sufficient evidence. Many scientific researchers have reviewed the current literature and worked towards the development of a specific treatment for COVID-19. Current studies have exposed several beneficial opportunities, a few of them are more established and are in preclinical trials in addition to some in clinical trials.92,93 Vaccines endeavor to represent the antibody to the antigen, and they should help the immune system (innate and adaptive immune responses, Fig. 3).Virus vaccines
A number of vaccines are being developed for SARS-CoV-2, including inactivated and weakened, replicating and non-replicating viral vectors, and the tentative use of nucleic acid in the form of DNA and RNA. Now, we can distinguish each vaccine characteristic as follows:Replicating viral vector (weakened measles). One of the approved Ebola vaccines is a typical paradigm for a viral-vector vaccine. Even if replication occurs inside cells, this vaccine is safe and will intensify the immune response.
Non-replicating viral vector (adenovirus variety). In general, viral vectors are genetically transformed to generate defective replications which are termed non-replicating vectors. Numerous viruses like adenovirus, adeno-linked, measles, and human parainfluenza viruses has been extensively utilized as viral vectors. Ultimately, the virus attains an attenuated state in which they can activate the anticipated human immune responses, however, they are unable to reproduce in human cells.
Table 3 and 4 provide a comprehensive overview of the current development of vaccines for SARS-CoV-2, their mode of action, their utility and the name of the manufacturer.99 More than 10 vaccines have almost reached the approval stage, while many are in the preclinical stage. Fig. 4 portrays the number of vaccine aspirants which are under clinical (∼63 drug aspirants) and preclinical (∼172 drug aspirants) stages against nCOVID-19.99a Protein-based techniques seem to be the most popular among the various mechanisms (see https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines).
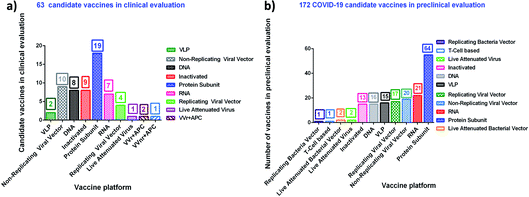 | ||
| Fig. 4 nCOVID-19 vaccine aspirants at the clinical (63 drug aspirants) and preclinical (172 drug aspirants) stage. | ||
| a Vaccine candidate list and relevant information shown according to the World Health Organization (WHO) statistical data for COVID-19 during 2019–2020. Clinical research data (last updated on January 08, 2021) may be variable. |
|---|
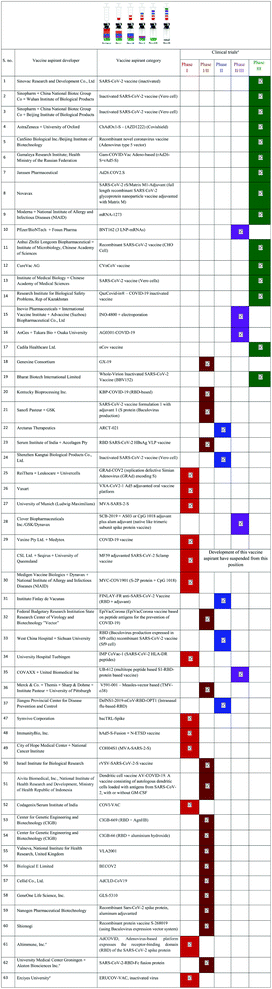 |
| ID | Vaccine division acronym | Vaccine sector description | Vaccine category and composition | Characteristic nature of vaccination | Producers | Clinical phases | Vaccine utility for other viruses | Ref. |
|---|---|---|---|---|---|---|---|---|
| a Globally, vaccine producers have the ability to achieve rapid development of highly efficient, safe – minimum toxicity – vaccines. | ||||||||
| 1 | IV | Inactivated virus | Vaccines of CoronaVac, PiCoVaccine, based on vaccine cultured in Vero cells, inactivated pathogen | Based on an inactivated pathogen, body generates a varied immune response against several viral antigens, producing neutralizing antibodies | Sinovac R&D Co. Ltd | Phase III NCT04456595, Phase 1/2 NCT04383574, NCT04352608 | Vaccine being developed for treatment of SARS-CoV-2 | 101 |
| 2 | IV | Inactivated virus | Inactivated vaccine, Vero cell based | Vaccine from non-living viral particles, bacteria, and other pathogens which are developed in a culture medium. No potential for infection, but induces an immune system response | Sinopharm + WIBP | Phase 3, Phase-I/II/III ChiCTR2000031809 | Vaccine being developed for treatment of SARS-CoV-2 | 102 |
| 3 | IV | Inactivated virus | Inactivated and similar to virus vaccine based on Vero cells | The foremost vaccine, does not exhibit any adverse side effects with favored immunogenicity and safety; also an inactivated new crown vaccine which completely neutralizes the antibodies in 28 days | Sinopharm + BIBP | Phase 3 ChiCTR2000032459 | Vaccine being developed for treatment of similar viruses | 92d |
| 4 | VVnr | Viral vector (non-replicating) | Covishield ChAdOx1-S-(AZD1222) | Adenovirus vector based on chimpanzee adenovirus | AstraZeneca + Oxford University | Phase 3 ISRCTN89951424, Phase2b/3 2020-001228-32, Phase 1/2 PACTR2020069221651322020-001072-15 | MERS, influenza, TB, Chikungunya, Zika, MenB, plague | 103 |
| 5 | VVnr | Viral vector (non-replicating) | Ad5-nCoV recombinant vaccine for CoV | Recombinant adenovirus type 5 vector based vaccine aspirant, which is genetically modified with replication-deficient groups, mimics SARS-CoV-2 spike protein | Beijing Biotechnology Institute/CanSino Biological Inc. | Phase 2 ChiCTR2000031781, Phase 1 ChiCTR2000030906 | EBOV (Ebola virus) | 104 |
| 6 | VVnr | Viral vector (non-replicating) | Gam-COVID-Vac adeno-based (rad26-S+rAd5-S), Russian COVID-19 vaccine, adenovirus based, and non-replicating | Develop immunity against the coronavirus, and strengthens the immune system | Gamaleya Research Institute; Ministry of Health, Russian Federation | Phase-I/III NCT04436471, NCT04437875 | Vaccine being developed for treatment of SARS-CoV-2 | 105 |
| 7 | VVnr | Viral vector (non-replicating) | Ad26.COV2.S recombinant serotype 26, adenovirus vectors, multivalent vaccine | Activates specific acquired immunity for Ebola virus | Janssen Pharmaceutical | Phase 3 NCT04505722, NCT04614948 | Vaccine being developed for treatment of similar viruses | 106 |
| 8 | PS | Protein subunit | SARS-CoV-2 rS/Matrix M1-adjuvant (full size recombinant SARS CoV-2 GP nanoparticle based vaccine with Matrix Mas adjuvant) | Matrix-M, an adjuvant which improves the immune response and induces advanced neutralizing antibodies | Novavax | Phase 3 NCT04611802, NCT04583995 | Ebola, Lassa, MERS, Nipah, Rift Valley Fever and Chikungunya | 107 |
| 9 | RNA | RNA based vaccine | Moderna mRNA-1273 vaccine, LNP encapsulated cell bank; mRNA, VAX (non-replicating viral vector) | A vaccine with mRNA encapsulated in LNP, encoding for perfusion stabilized spike (S) protein. Host generates an immune response against the spike protein on SARS-CoV-2 | Moderna + NIAID | Phase 3 NCT04470427, Phase 2 NCT04405076, Phase 1 NCT04283461 | Multiple agents | 108 |
| 10 | RNA | mRNA based vaccine | BNT162 (3 LNP-mRNAs) | BNT162 contains a nucleoside modified mRNA (modRNA) encoding the viral spike (S) glycoprotein | BioNTech + Fosun Pharma; Jiangsu Provincial CDC + Pfizer | Phase 2/3 NCT04368728 | To be implemented | 109 |
| 11 | PS | Protein subunit | Recombinant COVID-19 vaccine, CHO (Chinese hamster ovary) cell system, prevents CoVs | Increases rate of new coronavirus neutralizing S protein antibody (IgG) and RBD protein antibody (IgG) | Anhui Zhifei Longcom Biopharmaceutical + IMCAS | Phase-I NCT04453852, NCTo4445194, Phase 3 NCT04646590 | Vaccine being developed for treatment of SARS-CoV-2 | 110 |
| 12 | RNA | mRNA based vaccine | CVnCoV vaccine | Nucleotides without chemical modifications in the mRNA | CureVac AG | NCT04449276 Phase 1, NCT04515147 Phase 2, NCT04674189 Phase 3 | CureVac’s vaccine candidate against SARS-CoV-2 | 111 |
| 13 | IV | Inactivated virus | Vaccine for SARS-CoV-2 based on Vero cells | ELISA derived antibodies (IgGs) and neutralizing antibodies, target the spike protein, N protein virion and the specific positive CTL responses against N, S and virion antigens | IMB + CAMS | Phase 3 NCT04659239, Phase 1/2 NCT04470609, NCT04412538 | To be implemented | 112 |
| 14 | IV | Inactivated virus | QazCovid-in®-COVID-19 inactivated vaccine | There are specific antibodies that activate upon receiving small dilutions given in the vaccine, they constantly counterbalance the novel coronavirus with a virulence dose of 3000 TCD50 | RIBSP, Republic of Kazakhstan | Phase 1/2 NCT04530357 | Vaccine being developed for treatment of SARS-CoV-2 | 113 |
| 15 | DNA | DNA based vaccine | INO-4800 + electroporation vaccine with DNA–plasmid based connecting electroporation device | DNA plasmids distribute by electro-permeabilization (electro-transfer), a computationally sequenced design to produce a specific immune response | Inovio Pharmaceuticals + IVI | Phase 1 NCT04336410, NCT04447781, Phase 2 ChiCTR2000040146, Phase 2/3 NCT04642638 | Multiple agents, Cancer, HBV, HIV, HPV, Lassa, Nipah, Zika, | 114 |
| 16 | DNA | DNA based vaccine | AG0301-COVID19 | Plasmid DNA vaccine developed through an intradermal gene transfer targeting the S protein, which increases the efficiency of gene expression and antibody production | AnGes + Takara Bio + Osaka University | Phase 2/3 NCT04655625, Phase 1/2 NCT04463472, NCT04527081 | To be implemented | 115 |
| 17 | DNA | DNA based vaccine | nCov vaccine viral vector, membrane protein based vaccine | Progression of DNA vaccine with the viral membrane protein liable for CoV cell entry; plasmid DNA incorporated into the host cell changes the viral protein, leading to a strong immune response intervened by cellular and humoral immunity. Also used to produce live attenuated recombinant measles viral vector vaccine | Cadila Healthcare Ltd. | Phase 1/2 CTRI/2020/07/026352 | To be implemented | 116 |
| 18 | DNA | DNA based vaccine | GX-19 | Designed to make antigens by adding nucleic acids into the body, the antigens then produce an immune response | Genexine Consortium | Phase-1/2 NCT04445389 | To be implemented | 117 |
| 19 | IV | Inactivated virus | Whole-virion inactivated vaccine (BBV152), Covaxin for SARS-CoV-2, India’s trials on nCoV-19 vaccine | Isolated from asymptomatic COVID-19 patient at NIV Pune, India | Bharat Biotech International Limited | Phase 1/2 NCT04471519, Phase 3 NCT04641481; CTRI/2020/11/028976 | Vaccine being developed for treatment of SARS-CoV-2 | 118 |
| 20 | PS | Protein subunit | KBP-COVID-19 (RBD-based), plant based technology | A distinctive plant-based vaccine technology for the generation of antigens to various diseases | KBP., Inc. | Phase 1/2 NCT04473690 | To be implemented | 119 |
| 21 | PS | Protein subunit | Adjuvanted vaccine of SARS-CoV-2 formulation spike protein (baculovirus production) | — | Sanofi Pasteur + Glaxo SmithKline plc. | Phase 1/2 NCT04537208 | 120 | |
| 22 | RNA | RNA based vaccine | ARCT-021 | Powerful single dose, vaccine is based upon self-replicating mRNA, self-transcribing and replicating RNA along with its liquid-enabling and resolving nucleo-monomer facilitator altered RNA | Arcturus Therapeutics | Phase 1/2 NCT0448095 | Vaccine being developed for treatment of SARS-CoV-2 | 121 |
| 23 | VLP | Virus like particle | RBD SARS-CoV-2 HBsAg VLP vaccine | Neutralizing antibodies response in pigs, treated together with pseudo type lentivirus and live SARS-CoV-2 viruses. Immunoglobulin (IgG) response observed after booster immunization | SII + Accelagen Pty | Phase 1/2 ACTRN12620000817943 | Vaccine being developed for treatment of SARS-CoV-2 | 122 |
| 24 | VVnr + APC | Viral vector (non-replicating) + APC IV inactivated virus | SARS-CoV-2 vaccine (inactivated) | Shenzhen Geno-Immune Medical Institute | Phase 1/2 NCT04276896 | Vaccine for SARS-CoV-2 | 123 | |
| 25 | VVnr | Viral vector (non-replicating) | GRAd-COV2 replication using defective simian adenoviral vector (GRAd), encodes full length CoV spike protein | Able to generate immune response (antibodies and T cells) | ReiThera + Leukocare + Univercells | Phase 2 ChiCTR2000039462 | Ebola and RSV (respiratory syncytial virus) | 124 |
| 26 | VVnr | Viral vector (non-replicating) | VXA-CoV2-1 Ad5 adjuvanted oral vaccine platform | Vaccine mainly provides mucosal immunity, this vital factor targets mucosal pathogens, including the current coronavirus. Stimulates antigen-specific CD4+ and CD8+ T cells at low and high dosage levels | Vaxart | Phase 1 NCT04563702 | Influenza infection H1N1 | 125 |
| 27 | VVnr | Viral vector (non-replicating) | MVA-SARS-2-S | Immune responses and neutralizing antibodies directing the S antigen used to defend against infectivity. The double recombinant sMVA-CoV-2 vectors give S-specific CD8+ and CD4+ T cells to both S and N antigens in Balb/c mice, respectively | University of Munich (Ludwig-Maximilians) | Phase 1 NCT04569383 | Infectious diseases and cancer | 126 |
| 28 | PS | Protein subunit | SCB-2019 + AS03/CpG 1018 adjuvant plus alum adjuvant (similar to native trimeric subunit spike protein vaccine) | S-Trimer, antigen used to target the antibodies of novel CoV spike protein and is in ACE2-viable human convalescent sera samples, obtained from recovered COVID-19 patients | Clover Biopharmaceuticals Inc./GSK/Dynavax | Phase 1 NCT04405908 | Vaccine being developed for treatment of SARS-CoV-2 | 127 |
| 29 | PS | Protein subunit | COVID19 vaccine | Effective T cells and neutralizing antibody response | Vaxine Pty Ltd. + Medytox | Phase 1 NCT04453852 | Vaccine being developed for treatment of SARS-CoV-2 | 128 |
| Protein subunit | MF59 adjuvanted SARS-CoV-2 S clamp vaccine | Advanced antibodies are able to neutralize infectivity through live virus in the cell culture | CSL Ltd. + Seqirus + University of Queensland | Phase 1, development was suspended and the candidate vaccine was removed from the summary analysis | Vaccine production against similar viruses is on hold | |||
| 30 | PS | Protein subunit | MVC-COV1901 (S-2P protein + CpG 1018) | High titer of neutralizing antibodies are prompted against pseudo type novel CoV in sera of immunized mice | Medigen Vaccine Biologics Corp + NIAID + Dynavax | Phase 1 NCT04487210 | Vaccine being developed for treatment of SARS-CoV-2 | 129 |
| 31 | PS | Protein subunit | FINLAY-FR anti-SARS-CoV-2 vaccine (RBD + adjuvant) | It has the capability to generate a substantial immune reaction | Instituto Finlay de Vacunas | Phase 2 RPCEC00000347, Phase 1/2 RPCEC00000332, Phase 1 RPCEC00000338 | Vaccine being developed for treatment of SARS-CoV-2 | 130 |
| 32 | PS | Protein subunit | EpiVacCorona, based on peptide antigens | This antigens-based preparation stimulates the immune reaction | Federal Budgetary Research Institution SRC VB VECTOR | Phase 1/2 NCT04527575 | Vaccine being developed for treatment of SARS-CoV-2 | 131 |
| 33 | PS | Protein subunit | RBD, baculovirus production expressed in Sf9 cells, recombinant SARS-CoV-2 vaccine (Sf9 cell) | Specific immune blotting RBD, S-WT and S-2P, motivates high neutralization titers | WCH, Sichuan University | Phase 2 NCT04640402, ChiCTR2000039994, Phase 1 NCT04530656 | Vaccine being developed for treatment of SARS-CoV-2 | 132 |
| 34 | PS | Protein subunit | IMP CoVac-1 (SARS-CoV-2 HLA-DR peptides) | University Hospital Tuebingen (UKT) | Phase 1 | Vaccine being developed for treatment of SARS-CoV-2 | 133 | |
| 35 | PS | Protein subunit | UB-612, multitope peptide based S1-RBD-protein based vaccine | This vaccine has the immunity potential to reduce future pandemic rates | COVAXX + United Biomedical Inc | Phase 1 NCT04545749 | Vaccine being developed for treatment of SARS-CoV-2 | 134 |
| 36 | VVr | Viral vector (replicating) | V591-001-measles-vector based (TMV-o38) | Attains the target immune response in humans | Merck & Co., Themis + Sharp & Dohme + Pasteur Institute + Pittsburgh University | Phase 1 CT04498247, Phase1/2 NCT04497298NCT04569786 | Vaccine being developed for treatment of SARS-CoV-2 | 135 |
| 37 | VVr | Viral vector (replicating) | DelNS1-2019-nCoV-RBD-OPT1, intranasal flu-based-RBD | SARS-CoV-2 RBD protein based vaccine used to motivate cross-reactivity or cross-neutralizing antibodies; moreover it blocks previous CoV pseudovirus and the advanced novel CoV pseudovirus into hACE2 expressing 293 T cells (IC50 = 4.1 and 11.63 μg ml−1) | Jiangsu Provincial CDC | Phase 2 ChiCTR2000039715, Phase 2 ChiCTR2000037782 | Vaccine being developed for treatment of SARS-CoV-2 | 136 |
| 38 | RNA | RNA based vaccine | LNP-nCoVsaRNA | The saRNA vaccine can activate a strong immune response; it is suggested that the IM vaccination antigen is expressed in muscle cells, then moves to antigen presenting cells (APC), representing a cross priming mode of potentiality to prominent CD8+ T cells | Imperial College London | Phase 1 ISRCTN17072692 | Vaccine being developed for treatment of SARS-CoV-2 | 137 |
| 39 | RNA | RNA based vaccine | SARS-CoV-2 mRNA vaccine | — | Shulan (Hangzhou) Hospital and CDC at Guangxi Zhuang Autonomous Region | Phase 1 | Vaccine being developed for treatment of SARS-CoV-2 | 138 |
| 40 | VLP | Virus-like particle | Coronavirus-like particle COVID-19 (CoVLP) | Provides immunity by generating a harmless spike protein member, reduces severe infection effects | Medicago Inc. | Phase 2/3 | Vaccine being developed for treatment of similar viruses | 139 |
| 41 | VVr + APC | Viral vector (replicating) + APC | COVID-19/aAPC vaccine produced from a variation of lentivirus with immune modulatory genes and viral minigenes to the aAPCs | Artificial antigen-pathogen-specific vaccine, which uses the spike protein binding to the ACE2 receptor; the vaccine utilizes the modified minigenes to direct viral proteins, vary aAPC, and trigger T-cells | Shenzhen Genoimmune Medical Institute | Phase 1 NCT04299724 | Vaccine being developed for treatment of SARS-CoV-2 | 140 |
| 42 | VVnr + APC | Viral vector (non-replicating) + APC | LV-SMENP-DC vaccine. Dendritic cells are modified with lentivirus vectors transmitting COVID-19 minigene SMENP and immune modulatory genes. Cytotoxic T lymphocytes (CTLs) are activated by LV-DC offering COVID-19 specific antigens. | Vaccine directed with antigen-specific CTLs | Same medical institute | Phase 1/2 NCT04276896 | Vaccine being developed for treatment of similar viruses | 141 |
| 43 | PS | Protein subunit | AdimrSC-2f (recombinant RBD ± aluminium) | — | Adimmune Corporation | Phase 1 NCT04522089 | Vaccine being developed for treatment of SARS-CoV-2 | 142 |
| 44 | DNA | DNA based vaccine | Covigenix VAX-001 | Induces neutralizing antibody levels and stable T helper cell immunity | Entos Pharmaceuticals Inc. | Phase 1 NCT04591184 | Vaccine being developed for treatment of SARS-CoV-2 | 143 |
| 45 | DNA | DNA based vaccine | CORVax | CORVax12 initiates a coordinated vaccine response, able to expose the innate adaptive humoral and cellular arms. These cellular immune responses have the potential to generate a strong antiviral response | Providence Health & Services | Phase 1 NCT04627675 | Vaccine being developed for treatment of similar viruses | 144 |
| 46 | RNA | RNA based vaccine | ChulaCov19 mRNA vaccine | The mRNA based vaccine encodes a protein antigen, while RNA is considered to be unstable; the design and development of this novel vaccine is improving its constancy and protein translation efficacy, so it efficiently enhances immune response | Chulalongkorn University | Phase 1 NCT04566276 | Vaccine being developed for treatment of similar viruses | 145 |
| 47 | DNA | DNA based vaccine | bacTRL-Spike | Symvivo’s bacTRL gene therapy platform associates an advanced gene-expression plasmid with a probiotic bacterium to resolve restrictions and distribute the DNA vaccine directly to the gut. The bacterium triggers the immune response | Symvivo Corporation | Phase 1 NCT04334980 | Vaccine being developed for treatment of similar viruses | 146 |
| 48 | VVnr | Viral vector (non-replicating) | hAd5-S-Fusion + N-ETSD vaccine | A viral spike protein. Virus enters the host cells through the ACE2 receptor with a fusion linker, S is antigenic, and generates an efficient immune response | ImmunityBio, Inc. | Phase 1 NCT04591717, NCT04710303, NCT04732468 | Vaccine being developed for treatment of similar viruses | 147 |
| 49 | VVnr | Viral vector (non-replicating) | COH04S1 (MVA-SARS-2-S) | Vaccine holds the SARS-CoV-2 spike and nucleocapsid proteins inserted into the MVA platform that can replicate DNA within cells. Thus it generates novel CoV protein expression to trigger host immunity against the virus | City of Hope National Medical Center | Phase 1 NCT04639466 | Vaccine in development | 148 |
| 50 | VVr | Viral vector (replicating) | rVSV-SARS-CoV-2-S vaccine | rVSV-ΔG-spike stimulated a safe, efficient and adequate neutralizing antibody. Vaccination leads to lower morbidity, protects lungs, and provides fast viral clearance | IIBR, Israel | Phase 1/2 NCT04608305 | Vaccine in development | 149 |
| 51 | VVr + APC | Viral vector (replicating) + APC | Dendritic cell vaccine AV-COVID-19: contains autologous dendritic cells load with antigens from SARS-CoV-2, with/without GM-CSF | Produced from isolated peripheral blood monocytes from patients. Monocytes are then distinguished into dendritic cells with GM-CSF and IL=4 | Aivita Biomedical, Inc. | Phase 1/2 NCT04690387 NCT04386252 | Vaccine in development | 150 |
| 52 | LAV | Live attenuated virus | COVI-VAC | Codagenix’s synthetic attenuated virus engineering (SAVE) platform utilizes synthetic biology to re-code the virus genes into the vaccine. Since this design involves distributing a benign, live attenuated version of SARS-CoV-2 it may stimulate a strong and enduring immune response | Codagenix/Serum Institute of India | Phase 1 NCT04619628 | Vaccine in development | 151 |
| 53 | PS | Protein subunit | CIGB-669 (RBD + AgnHB) | Stimulates the generation of antibodies that enable a strong immune response to the pathogen | CIGB | Phase 1/2 RPCEC00000345 | Vaccine in development | 130 |
| 54 | PS | Protein subunit | CIGB-66 (RBD + aluminium hydroxide) | Stimulates the generation of antibodies that boost the targeted immune response to the virus | CIGB | Phase 1/2 RPCEC00000346 | Vaccine in development | 130 |
| 55 | IV | Inactivated virus | VLA2001 | Vero-cell supported the refined inactivated candidate, based on Valneva’s JE vaccine, uses the spike protein normal structural array with CpG 1018 and can stimulate an immune response with a high titer of neutralizing antibodies | Valneva, NIHR, United Kingdom | Phase 1/2 NCT04671017 | Vaccine in development | 130 |
| 56 | PS | Protein subunit | BECOV2 | Candidate based on inactivated SARS-CoV-2 virus components | Biological E Limited | Phase 1/2 CTRI/2020/11/029032 | Vaccine in development | 130 |
| 57 | VVr | Viral vector (replicating) | AdCLD-CoV19 | Immunotherapeutic vaccine based on cells | Cellid Co., Ltd. | Phase 1/2 NCT04666012 | Vaccine in development | 152 |
| 58 | DNA | DNA based vaccine | GLS-5310 | Nucleic acid-based vaccine platform | GeneOne Life Science, Inc. | Phase 1/2 NCT04673149 | Vaccine in development | 152 |
| 59 | PS | Protein subunit | Recombinant SARS-CoV-2 spike protein, aluminum adjuvanted | Protein subunit containing the recombinant SARS-CoV-2 S1 domain of the spike protein, also integrates either CoVaccine HT™ or alhydrogel. CoVaccine HT™, a single dose stimulated high titers of antigen-binding IgG. This accelerates the affinity maturation and switching of class to higher values, enhancing cell-induced immunity and virus antibodies | Nanogen Pharmaceutical Biotechnology | Phase 1/2 NCT04683484 | Vaccine in development | 153 |
| 60 | PS | Protein subunit | Recombinant protein vaccine S-268019 (based on Baculovirus expression vector system) | Vaccine is based on recombinant protein units together with GSK; development of mRNA vaccine by Sanofi, in collaboration with Translate Bio. Preclinical data revealed that the two immunizations of the mRNA based vaccine stimulated high neutralizing antibody levels equal to those produced in infected humans | Shionogi | Phase 1/2 jRCT2051200092 | Vaccine in development | 130 |
Comparison between previous vaccines and COVID-19 vaccines
Comparing COVID-19 vaccine novelty may seem challenging, but it is normal for queries to arise on a vaccine’s overall potential, safety, toxicity and its side effects etc.,100a and some of the efficacy, efficiency and safety of the COVID-19 frontrunner vaccines can be determined by comparison with other vaccines, like flu vaccines. For example, Pfizer/BioNTech released their COVID-19 vaccine initially in the UK and USA, and the rest of the world could see their effectiveness. However, prior to this demonstration the three prominent COVID-19 vaccines – Pfizer/BioNTech claims an efficacy of 95%, the Oxford/AstraZeneca provides 70%, and the Moderna is stated as 94.1% efficacy – were compared with other available vaccines such as the flu, polio, and measles, which helped provide their effectiveness and efficiency predictions.It is important to discuss here that “effectiveness” and “efficacy” are different.100b Efficacy states how a vaccine performs in ideal lab circumstances, like those present in clinical trials. Whereas, effectiveness means how the vaccine works in normal, non-controlled conditions. For example, in a clinical trial, 90% efficacy refers to 90% lower disease rates in the group getting the vaccine compared with the sample group. But, the members in a group selected for a clinical trial have to be in good health and young and they usually have no underlying health conditions. Besides, medical researchers will not generally consider some demographics in these clinical research studies like children/pregnant women. Thus, once a vaccine is able to prevent disease in clinical trials, we may observe the effectiveness drop when directed to different demographics.
Vaccines do not necessarily need high effectiveness to protect several thousands of lives from disease. For instance, the vaccine for flu100c has 40–60% effectiveness according to CDC data. During 2018–19, this vaccine prevented millions of influenza cases and its associated illness, but determining the exact effectiveness rate is challenging. Dosages can also increase effectiveness for some vaccines. The two doses of a vaccine can give a protection boost, nevertheless this advantage is sometimes limited to only certain groups like children/organ transplanted people. The booster dosage may not provide an advantage in people aged 65 years.
Through comparing vaccines, like the ones for polio and measles, we see heavy dosages are needed to realize effectiveness.
Polio vaccines100d should be up to 100% effective. According to the CDC, “Two inactivated polio vaccine (IPV) dosages have 90% effectiveness; three dosages are 99–100% effective.” The IPV vaccine prevents poliomyelitis (poliovirus), which can activate infection in the brain and spinal cord leading to paralysis.
The MMR vaccine100e defends against measles, mumps, and rubella, which tends to have up to 97% effectiveness at inhibiting measles once directed in two dosages. A single dose is around 93% effective, as reported by the CDC. They suggest to give the initial dose at “12–15 months of age, followed by the second dose at 4–6 years.”
In order to determine the similarities and differences between previously available vaccines and COVID-19 vaccines, the Bacillus Calmette–Guérin (BCG) vaccine against tuberculosis is provided as one more example; it confers a wide range of immunity against other infections, and it also may minimize the intensity of COVID-19. Moreover one epidemiological analyses provided universal connections between the vaccinations of BCG and COVID-19 mortality: the suggestion of BCG vaccination results on COVID-19 fatality are dominated by socio-economical and demographical variations between countries. In the wake of reducing the manifold distracting factors, many substantial connections between the BCG vaccination and decreased COVID-19 fatalities were perceived. Obviously this investigation emphasizes the necessity for an intrinsic mechanism of studies supporting BCG vaccination effects on COVID-19, and also for clinical evaluation to control the COVID-19 pandemic.99b
COVID-19 is a serious respiratory related disease, so the scientific and medicinal community are working hard across the globe to develop a vaccine. Presently, around sixty vaccine candidates are on trial in many countries. Now, nearing twenty candidate vaccines are in phase 3 clinical trials. Gratifyingly, seven vaccines have been approved in many countries.
Conclusions
Globally, as of January 2021, there have been around 88 million COVID-19 cases, including nearly 1.9 million fatalities, WHO have registered (see WHO Covid-19 case report).99d These numbers are expected to increase further, so there is an emergency requirement to produce vaccines to protect people. Several candidates vaccines are being developed which are in pre-clinical and clinical trials. The mechanism of action of these candidates varies significantly, as better knowledge becomes available154a about this virus, researchers can adapt their design so if one candidate shows low efficacy, another one may be more active.Similarly, different demographics may necessitate the need for designing vaccines with different mechanisms of action. The time required for the various stages of clinical trials needs to be shortened (without compromising on the ethics and safety) to achieve the desired goal in a short period of time.154b Finally, manufacturing such large quantities of the vaccine or vaccines, quickly (without compromising on the quality, purity and efficacy) and distributing them to all parts of the world is another problem which the present planners have not faced before. This requires sufficient manufacturing capacity, availability of raw materials, logistics and several other factors. Also, as all resources are diverted towards SARS-CoV-2, epidemiologists and public health organizations should not lose track of their fight against other viruses.155
The current assemblage of vaccine developers156a may reward researchers with increased capability, as numerous basic, transformational and preclinical statistical data have become available during coronavirus exploration. These factors combine together as a substantial promising source for rapid vaccine development.156b
Since, December 2020, some new vaccines have emerged and also have been approved by certain national regulatory authorities for use against COVID-19. Amongst these, as per the universal expectations of the WHO EUL/PQ assessment, the Pfizer vaccine and some other candidates have been approved. More studies on vaccine aspirant efficacy and safety results,157 including on the Moderna and AstraZeneca vaccine, therein have been widely reported, and AstraZeneca have published their results in well reputed journals. Thus we are eagerly expecting more potential COVID-19 vaccine candidates will be offered to governing authorities for approval in the coming years. Gratifyingly, the growth of many efficient COVID-19 vaccine aspirants under clinical trials is fascinating. When the vaccine candidates are proven to be benign and efficient, they need to be acknowledged by the governing authorities, produced to the necessary standard, and distributed. WHO is collaborating all over the world to assist with the roles in this process, which includes facilitating reasonable access to safe and effective COVID-19 vaccines for everyone.
Author contributions
KD and SA equally contributed to the collection of data and developed the entire manuscript. MD corrected the manuscript. SG and ST assisted in drafting.Conflicts of interest
We have no conflicts of interest.Acknowledgements
KD thanks the Science and Engineering Research Board (SERB), New Delhi, India for the National Post-Doctoral Fellow (NPDF) [Project No. PDF/2017/001743].References
- J. M. Ryan, R. L. Jonathan and V. Olivia, Chem. Rev., 2020, 120, 3210–3229 CrossRef PubMed.
- Y. Tingting, Z. Zifu, G.-S. Adolfo, S. Michael and G. D. G. Bruno, Angew. Chem., Int. Ed., 2020, 59, 18885–18897 CrossRef PubMed.
- B. Greenwood, Philos. Trans. R. Soc., B, 2014, 369, 20130433 CrossRef PubMed.
- K. A. Smith, Front. Immunol., 2011, 2, 1–6 Search PubMed.
- A. S. Clem, J. Global Infect. Dis., 2011, 3, 73–78 CrossRef CAS PubMed.
- L. B. Nicholson, Essays Biochem., 2016, 60, 275–301 CrossRef PubMed.
- J. Lu, G. Lu, S. Tan, J. Xia, H. Xiong, X. Yu, Q. Qi, X. Yu, L. Li, H. Yu, N. Xia, T. Zhang, Y. Xu and J. Lin, Cell Res., 2020, 30, 936–939 CrossRef CAS PubMed.
- B. E. Eldred, A. J. Dean, T. M. McGuire and A. L Nash, Med. J. Aust., 2006, 184, 170–175 CrossRef PubMed.
- (a) W. Shang, Y. Yang, Y. Rao and X. Rao, npj Vaccines, 2020, 5, 18 CrossRef CAS PubMed; (b) C. Edwards, West. J. Med., 2001, 174, 197–198 CrossRef CAS PubMed; (c) K. Chumakov, E. Ehrenfeld, E. Wimmer and V. I. Agol, Nat. Rev. Microbiol., 2007, 5, 952–958 CrossRef CAS PubMed; (d) H. Wang, Y. Zhang, B. Huang, W. Deng, Y. Quan, W. Wang, W. Xu, Y. Zhao, N. Li, J. Zhang, H. Liang, L. Bao, Y. Xu, L. Ding, W. Zhou, H. Gao, J. Liu, P. Niu, L. Zhao, W. Zhen, H. Fu, S. Yu, Z. Zhang, G. Xu, C. Li, Z. Lou, M. Xu, C. Qin, G. Wu, G. F. Gao, W. Tan and X. Yang, Cell, 2020, 82, 713–721 CrossRef PubMed; (e) N. Chauhan, S. Soni, A. Gupta, M. Aslam and U. Jain, J. Med. Virol., 2021, 93, 1967–1982 CrossRef CAS PubMed; (f) N. Lurie, M. Saville, R. Hatchett and J. Halton, N. Engl. J. Med., 2020, 382, 1969–1973 CrossRef CAS PubMed.
- C. Zhang, G. Maruggi, H. Shan and J. Li, Front. Immunol., 2019, 10, 594–606 CrossRef CAS PubMed.
- B. Pulendran and R. Ahmed, Nat. Immunol., 2011, 12, 509–517 CrossRef CAS PubMed.
- V. Vetter, G. Denizer, L. R. Friedland, J. Krishnan and M. Shapiro, Ann. Med., 2018, 50, 110–120 CrossRef PubMed.
- A. H. Ellebedy and R. Ahmed, The Vaccine Book, 2nd edn, 2016, pp. 283–310. DOI:10.1016/B978-0-12-802174-3.00015-1.
- E. De Clercq and G. Li, Clin. Microbiol. Rev., 2016, 29, 695–747 CrossRef PubMed.
- K. Bharati and S. Vrati, Proc. Natl. Acad. Sci., India, Sect. B, 2012, 82, 181–198 CrossRef PubMed.
- (a) https://clinicaltrials.gov; (b) https://www.drugbank.ca; (c) D. E. Speiser and M. F. Bachmann, Vaccines, 2020, 8, 404 CrossRef CAS PubMed; (d) S. Tavakol, M. S. Alavijeh and A. M. Seifalian, Curr. Pharm. Des., 2021, 27, 1553–1563 CrossRef CAS PubMed; (e) S. P. Kaur and V. Gupta, Virus Res., 2020, 288, 198114 CrossRef CAS PubMed.
- J. P. Fox, Rev. Infect. Dis., 1984, 6, S352–S355 CrossRef PubMed.
- M. P. Kostinov, A. P. Cherdantsev, A. I. Kuselman, N. K. Akhmatova, A. M. Kostinova, E. Viktorovna, D. E. Olegonva and A. M. Kostinov, Hum. Vaccines Immunother., 2018, 14, 2971 CrossRef PubMed.
- ClinicalTrials.govIdentifier: NCT03131765, April 27, 2017.
- (a) J. S. Tregoning, R. F. Russell and E. Kinnear, Hum. Vaccines Immunother., 2018, 14, 550–564 CrossRef PubMed; (b) https://www.prnewswire.com/news-releases/adimmunes-quadrivalent-flu-vaccine-approved-by-tfda-300455062.html.
- Quadrivalent-inactivated-influenza-vaccine-adimmune-corporation, Reactions Weekly, 2021, 1837, 611 Search PubMed.
- (a) ClinicalTrials.gov Identifier: NCT03120364, April 19, 2017; (b) S. J. Lee, H. J. Park, H. L. Ko, J. E. Lee, H. J. Lee, H. Kim and J. H. Nam, Immun., Inflammation Dis., 2020, 8, 216–227 CrossRef PubMed.
- J. J. Y. Sung and H. L. Yuen, Curr. Opin. Mol. Ther., 2006, 8, 150–155 CAS.
- ClinicalTrials.govIdentifier: NCT00920218 December 12, 2017.
- K. Mahmood, S. Pelkowski and J. J. Donnelly, Hum. Vaccines Immunother., 2013, 9, 1894–1902 CrossRef CAS PubMed.
- A. K. Mc Elory, R. S. Akondy, C. W. Davis, A. H. Ellebedy, A. K. Mehta, C. S. Kraft, G. M. Lyon, B. S. Ribner, J. Varkey and J. Sidney, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 4719–4724 CrossRef PubMed.
- (a) www.adisinsight.springer.com; (b) http://zyduscadila.com/research.
- (a) www.rxlist.com/flucelvax-drug.html; (b) http://europa.eu/en/documents/product-information/flucelvax-tetra-epar-product-information_en.pdf.
- (a) www.medbroadcast.com/drug/getdrug/vaxigrip; (b) http://products.sanofi.com.au/vaccines/VAXIGRIP_NZ_CMI.pdf.
- (a) B. Brandenburg, W. K. Klaren, C. Tang, M. V. Bujny, H. J. W. Korse, T. Kwaks, J. J. Otterstrom, J. Juraszek, A. M. Van Oijen and R. Vogels, PLoS One, 2013, 8, 80034 CrossRef PubMed; (b) C. Yamazaki, M. Sugiyama, T. Ohta, H. Hemmi, E. Hamada, I. Sasaki, Y. Fukuda, T. Yano, M. Nobuoka, T. Hirashima, A. Iizuka, K. Sato, T. Tanaka, K. Hoshino and T. Kaisho, J. Immunol., 2013, 190, 296–306 CrossRef PubMed.
- H. Juin, H. Lim, J. A. Lee, H. J. Kim, J. W. Kim, J. Y. Hyeon, S. G. Yeo, J. W. Lee, J. S. Yoo, Y. K. Choi and S. W. Lee, PLoS One, 2017, 12, 0178259 Search PubMed.
- http://www.lybrate.com/amp/medicine/cadiflu-s-vaccine.
- P. T. Boer, J. K. Kelso, N. Halder, T. P. L. Nguyen, J. Modyes, C. Cohen, I. G. Barr, M. J. Postma and G. J. Milne, Vaccine, 2018, 836, 997–1007 CrossRef PubMed.
- Influenza virus RIV4 vaccine (Flublok Quadrivalent): VAERS reports, Reactions Weekly, 2021, 1847, 8 Search PubMed.
- I. V. Dolzhikova, O. V. Zubkova, A. I. Tukhvatulin, A. S. Dzharullaeva, Z. M. Yukhvatulina, D. V. Shcheblyakov, M. M. Shmarov, E. A. Tokarskaya, Y. V. Simakova, D. A. Egorova, D. N. Scherbinin, I. L. Tutykhina, A. A. Lysenko, A. V. Lostranoy, P. G. Gancheva, T. A. Ozharovskaya, B. V. Belugin, L. V. Kolobukhina, V. B. Pantyukhov, S. I. Syromyatnikova, I. V. Shatokhina, T. V. Sizikova, I. G. Rumyantseva, A. F. Andrus, N. V. Boyarskaya, A. N. Voytyuk, V. F. Babira, S. V. Volchikhina, D. A. Kutaev, A. N. Bel’skih, K. V. Zhdanov, S. M. Zakharenko, S. V. Borisevich, D. Y. Logunov, B. S. Naroditsky and A. L. Gintsburg, Hum. Vaccines Immunother., 2017, 13, 613–620 CrossRef CAS PubMed.
- (a) https://www.who.int/teams/health/vaccines-quality/poliomyelitis; (b) A. R. Hinman, J. P. Koplan, W. A. Orenstein, E. W. Brink and B. M. N. Kowane, Am. J. Public Health, 1998, 78, 291–295 CrossRef PubMed.
- N. Bhandari, T. R. Chandola, A. Bavdekar, J. John, K. Antony, S. Taneja, N. Goyal, A. Kawade, G. Kang, S. Singh Rathore, S. Juvekar, J. Muliyil, A. Arya, H. Shaikh, V. Abraham, S. Vrati, M. Proschan, R. Kohberger, G. Thiry, R. G. Harry, B. Greenberg, G. Curlin, K. Mohan, G. V. J. A. Harshavardhan, S. Prasad, T. S. Rao, J. Boslego and M. K. Bhan, Lancet, 2014, 383, 2136–2143 CrossRef CAS.
- W. S. Choi, J. Y. Noh, J. Y. Song, H. J. Cheong, S. H. Wie, J. S. Lee, J. Lee, S. W. Kim, H. W. Jeong, S. I. Jung, Y. S. Kim, H. J. Woo, K. H. Kim, H. Kim and W. J. Kim, Hum. Vaccines Immunother., 2017, 13, 1653–1660 CrossRef PubMed.
- J. Lee, K. Y. Lee, J. H. Kim, C. S. Kim, B. W. Eun, H. M. Kim, D. H. Kim, Y. J. Hong, Y. Y. Choi, D. S. Jo, S. H. Ma and J. H. Kang, J. Korean Med. Sci., 2018, 26, 100 CrossRef PubMed.
- J. Arroyo, C. Miller, J. Catalan, G. A Myers, M. S. Ratterree, D. W Trent and T. P Monath, J. Virol., 2004, 78, 12497 CrossRef CAS PubMed.
- (a) J. Schon, W. R. M. Gorka, M. Schwemmle, M. Beer and D. Hoffmann, npj Vaccines, 2020, 5, 40 CrossRef PubMed; (b) http://www.who.int/influenza/resources/avin_influenza/en.
- A. Luxembourg, D. Brown, C. Bouchard, A. R. Giuliano, O. E. Lversen, E. A. Joura, M. E. Penny, J. A. Restrepo, J. Romaguera, R. Maansson, E. Moeller, M. Ritter and J. Chen, Hum. Vaccines Immunother., 2015, 11, 1313–1322 CrossRef PubMed.
- M. Endo, M. Tanishima, K. Lbarai, K. Hayashida, T. Fukuda, T. Tanable, T. Naruse, Y. Kino and K. Ueda, Influenza Other Respir. Viruses, 2020, 14, 551–563 CrossRef CAS PubMed.
- ClinicalTrials.govIdentifier: NCT02977715, September, 23, 2020.
- L. Turtle and T. Solomon, Nat. Rev. Neurol., 2018, 14, 298–313 CrossRef PubMed.
- http://www.who.int/immunizati_standards/vaccine_quality/fluzone_sanofi_pasteur_product_insert.pdf.
- (a) http://www.fda.gov/media/115785/download; (b) http://www.immunizationinfo.com/flulaval-vaccine/amp.
- Y. Y. Syed, Paediatr. Drugs, 2019, 21, 501 CrossRef PubMed.
- ClinicalsTrials.govIdentifier: NCT02148211, May 28, 2014.
- (a) J. Arroyo, C. Miller, J. Catalan, G. A Myers, M. S Ratterree, D. W. Trent and T. P. Monath, J. Virol., 2004, 78, 12497–12507 CrossRef CAS PubMed; (b) M. G. Moloney, A. P. Goncalvez, J. Catalan, V. Lecouturier, Y. G. Chambaz, F. Diaz, F. M. Arocho, R. C. Gomila, M. C. Bernard, R. Oomen, S. Delagrave, N. Burdin, H. Kleanthous, N. Jackson, J. Heinrichs and K. V. Pugachev, Sci. Rep., 2018, 8, 13206 CrossRef PubMed.
- G. M. Keating, G. L Plosker and K. A. L. Williamson, Biodrugs, 2012, 26, 425–430 CrossRef CAS PubMed.
- (a) J. H. C. M. Kreijtz, R. A. M. Fouchier and G. F. Rimmelzwaan, Virus Res., 2011, 162, 19–30 CrossRef CAS PubMed; (b) S. S. Wong and R. J. Webby, Clin. Microbiol. Rev., 2013, 26, 476–492 CrossRef CAS PubMed.
- X. Wu, P. Chen, H. Lin, X. Hao and Z. Liang, Hum. Vaccines Immunother., 2016, 12, 2603–2610 CrossRef PubMed.
- B. M. Laksono, R. D. Vries, R. J. Verburgh, E. G. Visser, A. deong, P. L. A. Fraaij, W. L. M. Ruijs, D. F. Nieuwenhuijse, H. J. Ham, M. P. G. Koopmans, M. C. van Zelm, A. D. M. E. Osterhaus and R. L. de Swart, Nat. Commun., 2018, 9, 4944 CrossRef PubMed.
- N. R. Hegde, D. Kumar, P. P. Rao, P. K. Kumari, Y. Kaushik, R. Ravikrishnan, S. D. Prasad and K. M. Ella, Vaccine, 2014, 32, 3636 CrossRef CAS PubMed.
- A. Choudhry, S. Singh, S. Khare, A. Rai, D. S. Rawat, R. K. Aggarwal and L. S. Chauhan, Indian J. Med. Res., 2012, 135, 534–537 Search PubMed.
- Y. N. Lamb, Drugs, 2019, 79, 1337–1348 CrossRef CAS PubMed.
- C. M. Trombetta, E. Gianchecchi and E. Montomoli, Hum. Vaccines Immunother., 2018, 14, 657–670 CrossRef PubMed.
- K. J. Kallen, R. Heidenreich, M. Schnee, B. Petsch, T. Schlake, A. P. Baumh, B. Scheel, S. D. Koch and M. F. Mleczek, Hum. Vaccines Immunother., 2013, 9, 2263–2276 CrossRef CAS PubMed.
- J. S. Tregoning, R. F. Russell and E. Kinnear, Hum. Vaccines Immunother., 2018, 14, 550–564 CrossRef PubMed.
- N. Chotirosniramit, P. Sugandhavesa, L. Aurpibul, S. Thetket, N. Kosashunhanan, T. Supindham, P. Wongkulab, Q. Kaewpoowat, K. Chaiklang, O. Kaewthip, P. Sroysuwan, A. Wongthanee, H. Lerdsamran, P. Puthavathana and K. Suparatpinyo, Hum. Vaccines Immunother., 2012, 8, 1854–1859 CrossRef CAS PubMed.
- H. Reynales, P. Astudillo, S. de Vallière, C. Hatz, P. Schlagenhauf, B. Rath, P. Velentgas, A. Fariña, V. Sales Carmona and N. Groth, Vaccine, 2012, 30, 6436–6443 CrossRef CAS PubMed.
- P. Hallberg, H. Smedje, N. Eriksson, H. Kohnke, M. Daniilidou, I. Öhman, Q. Y. Yue, M. Cavalli, C. Wadelius, P. K. E. Magnusson, A. M. Landtblom and M. Wadelius, EBioMedicine, 2019, 40, 595–604 CrossRef PubMed.
- N. A. T. van der Maas, S. Godefrooij, P. E. Vermeer-de Bondt, H. E. de Melker and J. Kemmeren, Hum. Vaccines Immunother., 2016, 12, 1027–1032 CrossRef CAS PubMed.
- V. Talayev, I. Zaichenko, M. Svetlova, A. Matveichev, O. Babaykina, E. Voronina and A. Mironov, Vaccine, 2020, 38, 6645–6655 CrossRef PubMed.
- (a) https://www.sciencedirect.com/topics/medicine-and-dentistry/hong-kong-influenza; (b) W.-S. Ryu, Molecular Virology of Human Pathogenic Viruses, 2017 Search PubMed.
- J. M. Vernon and T. Nolan, Expert Rev. Vaccines, 2011, 10, 35–43 CrossRef PubMed.
- N. J. Carter and G. L. Plosker, BioDrugs, 2008, 22, 279–292 CrossRef CAS PubMed.
- I. Manini, A. Domnich, D. Amicizia, S. Rossi, T. Pozzi, R. Gasparini, D. Panatto and E. Montomoli, Expert Rev. Vaccines, 2015, 14, 789–804 CrossRef CAS PubMed.
- A. Nalca and E. E. Zumbrun, Drug Des., Dev. Ther., 2010, 4, 71–79 CrossRef CAS PubMed.
- (a) https://www.immunizationinfo.com/afluria-vaccine; (b) V. A. Statler, F. R. Albano, J. Airey, D. C. Sawlwin, A. Graves, J. V. Matassa, E. H. J. Edelman and G. S. Marshall, Vaccine, 2019, 37, 343–351 CrossRef CAS PubMed.
- C. A. Robertson, C. A. D. Granados, M. D. Decker, A. Chit, M. Mercer and D. P. Greenberg, Expert Rev. Vaccines, 2016, 15, 1495–1505 CrossRef CAS PubMed.
- (a) https://www.ema.europa.eu/en/documents/product-information/covid-19-vaccine%20moderna-epar-product-information_en.pdf; (b) N. Pardi, M. J. Hogan, F. W. Porter and D. Weissman, Nat. Rev. Drug Discovery, 2018, 17, 261 CrossRef CAS PubMed.
- I. Manini, A. Domnich, D. Amicizia, S. Rossi, T. Pozzi, R. Gasparini, D. Panatto and E. Montomoli, Expert Rev. Vaccines, 2015, 14, 789–804 CrossRef CAS PubMed.
- G. Pauli, T. H. Larsen, S. Rak, F. Horak, E. Pastorello, R. Valenta, A. Purohit, M. Arvidsson, A. Kavina, J. W. Schroeder, N. Mothes, S. Spitzauer, A. Montagut, S. Galvain, M. Melac, C. André, L. K. Poulsen and H. J. Malling, J. Allergy Clin. Immunol., 2008, 122, 951–960 CrossRef CAS PubMed.
- M. Margarita, G. Lorenzo and M. J. Fenton, Chest, 2013, 143, 502–510 CrossRef PubMed.
- D. T. O. Hagan, L. R. Friedland, E. Hanon and A. M. Didierlaurent, Curr. Opin. Immunol., 2017, 47, 93–102 CrossRef PubMed.
- J. P. Mc Gettigan, Expert Rev. Vaccines, 2010, 9, 1177–1186 CrossRef CAS PubMed.
- M. Sanford and G. M. Keating, Drugs Aging, 2010, 27, 159–176 CrossRef CAS PubMed.
- G. M. Keating, BioDrugs, 2016, 30, 243–254 CrossRef CAS PubMed.
- D. M. H. Leslie and R. De Mars, Gynecol. Oncol., 2017, 146, 196–204 CrossRef PubMed.
- J. M. Hyser and M. K. Estes, Curr. Opin. Gastroenterol., 2009, 25, 36–43 CrossRef PubMed.
- G. L. Roels, Med. Microbiol. Immunol., 2015, 204, 69–78 CrossRef PubMed.
- Y. L. Zhao, Y. G. Chen, J. Li, G. X. Han, C. Tian, J. L. Liang, Z. G. Wang, Y. G. Zhu, Z. N. Tian, H. Y. Zhang, Z. J. Wan, Z. L. Liang, S. L. Bi, Z. L. X. Bing and X. Z. Zhi, Zhonghua Liuxingbingxue Zazhi, 2004, 25, 470–473 Search PubMed.
- I. A. de Bruijn, J. Nauta, L. Gerez and A. M. Palache, Vaccine, 2006, 24, 6629–6631 CrossRef CAS PubMed.
- S. S. Wong and R. J. Webby, Clin. Microbiol. Rev., 2013, 26, 476–492 CrossRef CAS PubMed.
- B. Jarvis and D. P. Figgitt, Drugs, 2003, 63, 214–215 CrossRef PubMed.
- F. Wu, S. Zhao, B. Yu, Y.-M. Chen, W. Wang, Z.-G. Song, Y. Hu, Z.-W. Tao, J.-H. Tian, Y.-Y. Pei, M.-L. Yuan, Y.-L. Zhang, F.-H. Dai, Y. Liu, Q.-M. Wang, J.-J. Zheng, L. Xu, E. C. Holmes and Y.-Z. Zhang, Nature, 2020, 579, 265–269 CrossRef CAS PubMed.
- (a) H. Wang, X. Li, T. Li, S. Zhang, L. Wang, X. Wu and J. Liu, Eur. J. Clin. Microbiol. Infect. Dis., 2020, 39, 1629–1635 CrossRef CAS PubMed; (b) N. Wang, J. Shang, S. Jiang and L. Du, Front. Microbiol., 2020, 11, 298 CrossRef PubMed; (c) Y. D. Li, W. Y. Chi, J. H. Su, L. Ferrall, C. F. Hung and T. C. Wu, J. Biomed. Sci., 2020, 27, 104 CrossRef PubMed.
- P. Zhou, X.-L. Yang, X.-G. Wang, B. Hu, L. Zhang, W. Zhang, H.-R. Si, Y. Zhu, B. Li, C.-L. Huang, H.-D. Chen, J. Chen, Y. Luo, H. Guo, R.-D. Jiang, M.-Q. Liu, Y. Chen, X.-R. Shen, X. Wang, X.-S. Zheng, K. Zhao, Q.-J. Chen, F. Deng, L-L Liu, B. Yan, F.-X. Zhan, Y.-Y. Wang, G.-F. Xiao and Z.-L. Shi, Nature, 2020, 579, 270–273 CrossRef CAS PubMed.
- (a) L. Vijgen, E. Keyaerts, E. Moës, I. Thoelen, E. Wollants, P. Lemey, A.-M. Vandamme and M. V. Ranset, J. Virol., 2005, 79, 1595–1604 CrossRef CAS PubMed; (b) J. Sultana, S. Crisafulli, F. Gabbay, E. Lynn, S. Shakir and G. Trifirò, Front. Pharmacol., 2020, 11, 588654 CrossRef CAS PubMed; (c) S. Dotolo, A. Marabotti, A. Facchiano and R. Tagliaferri, Brief. Bioinform., 2020 Search PubMed; (d) Y. Zhou, F. Wang, J. Tang, R. Nussinov and F. Cheng, Lancet Glob. Health, 2020, 2, e667–e676 CrossRef.
- (a) L. Cynthia, Q. Zhou, Y. Li, V. L. V. Garner, S. P. Watkins, L. J. Carter, J. Smoot, A. C. Gregg, A. D. Daniels, S. Jervey and D. Albaiu, ACS Cent. Sci., 2020, 6, 315–331 CrossRef PubMed; (b) D. Calina, C. Sarkar, A. L. Arsene, B. Salehi, A. Docea, M. Mondal, M. T. Islam, A. Zaliand and J. S. Rad, Immunol. Res., 2020, 68, 315–324 CrossRef PubMed; (c) Y. Dong, T. Dai, Y. Wei, L. Zhang, M. Zheng and F. Zhou, Signal Transduction Targeted Ther., 2020, 5, 237 CrossRef CAS PubMed; (d) G. N. A. Rego, M. P. Nucci, A. H. Alves, F. A. Oliveira, L. C. Marti, L. P. Nucci, J. B. Mamani and L. F. Gamarra, Vaccines, 2020, 8, 474 CrossRef CAS PubMed; (e) D. D. Li and Q. H. Li, Mil. Med. Res., 2021, 8, 1–15 CAS.
- H. Li, Y. Zhou, M. Zhang, H. Wang, Q. Zhao and J. Liu, Antimicrob. Agents Chemother., 2020, 64, e00483 Search PubMed.
- (a) R.-G. Marjorie, Curr Opin Biotechnol., 2007, 18, 546–556 CrossRef PubMed; (b) G. N. A. Rego, M. P. Nucci, A. H. Alves, F. A. Oliveira, L. C. Marti, L. P. Nucci, J. B. Mamani and L. F. Gamarra, Vaccines, 2020, 8, 474 CrossRef CAS PubMed.
- W. H. Chen, U. Strych, P. J. Hotez and M. E. Bottazzi, Curr. Trop. Med. Rep., 2020, 7, 61–64 CrossRef PubMed.
- W. Ning, S. Jian, J. Shibo and D. Lanying, Front. Microbiol., 2020, 11, 298 CrossRef PubMed.
- R. António, C. M. M. Maria, R. C. Leda, J. T. C. Manuel and M. A. Paula, Expert Rev. Vaccines, 2010, 9, 1149–1176 CrossRef PubMed.
- L. Jie, U. Laura, S. Erica, R. T. Deborah and V. Raphael, Viral Immunol., 2013, 126–132 Search PubMed.
- (a) https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines; (b) L. E. Escobara, A. M. Cruz and C. B. Mury, Proc Natl. Acad. Sci., 2020, 117, 27741–27742 CrossRef PubMed; (c) https://www.clinicaltrials.gov; (d) https://covid19.who.int/.
- (a) S. Geoghegan, K. P. O’Callaghan and P. A. Offit, Front. Microbiol., 2020, 11, 372 CrossRef PubMed; (b) T. H. T. Quach, N. A. Mallis and J. F. Cordero, Matern. Child Health J., 2020, 24, 229–240 CrossRef PubMed; (c) L. K. Boerner, ACS Cent. Sci., 2020, 6, 89–92 CrossRef PubMed; (d) M. Famulare, C. Selinger, K. A. McCarthy, P. A. Eckhoff and G. C. Couture, PLoS Biol., 2018, 16, e2002468 CrossRef PubMed; (e) D. A. Geier, J. K. Kern and M. R. Geier, BMC Pediatr., 2019, 19, 325 CrossRef PubMed.
- R. Palacios, E. G. Patiño, R. de Oliveira Piorelli, M. Tilli Reis Pessoa Conde, A. Paula Batista, G. Zeng, Q. Xin, E. G. Kallas, J. Flores, C. F. Ockenhouse and C. Gast, Trials, 2020, 21, 853 CrossRef CAS PubMed.
- S. Xia, K. Duan, Y. Zhang, D. Zhao, H. Zhang, Z. Xie, X. Li, C. Peng, Y. Zhang, W. Zhang, Y. Yang, W. Chen, X. Gao, W. You, X. Wang, Z. Wang, Z. Shi, Y. Wang, X. Yang, L. Zhang, L. Huang, Q. Wang, J. Lu, Y. Yang, J. Guo, W. Zhou, X. Wan, C. Wu, W. Wang, S. Huang, J. Du, Z. Meng, A. Pan, Z. Yuan, S. Shen, W. Guo and X. Yang, JAMA, J. Am. Med. Assoc., 2020, 324, 951–960 CrossRef CAS PubMed.
- M. Oysey, S. A. C. Clemens and S. A. Madhi, et al., Lancet, 2021, 397, 99–111 CrossRef.
- S. Ahamad, S. Branch, S. Harrelson, M. K. Hussain, M. Saquib and S. Khan, Eur. J. Med. Chem., 2021, 209, 112862 CrossRef CAS PubMed.
- D. Y. Logunov, I. V. Dolzhikova, O. V. Zubkova, A. I. Tukhvatulin, D. V. Shcheblyakov, A. S. Dzharullaeva, D. M. Grousova, A. S. Erokhova, A. V. Kovyrshina, A. G. Botikov, F. M. Izhaeva, O. Popova, T. A. Ozharovskaya, I. B. Esmagambetov, I. A. Favorskaya, D. I. Zrelkin, D. V. Voronina, D. N. Shcherbinin, A. S. Semikhin, Y. V. Simakova, E. A. Tokarskaya, N. L. Lubenets, D. A. Egorova, M. M. Shmarov, N. A. Nikitenko, L. F. Morozova, E. A. Smolyarchuk, E. V. Kryukov, V. F. Babira, S. V. Borisevich, B. S. Naroditsky and A. L. Gintsburg, Lancet, 2020, 396, 887 CrossRef CAS.
- J. Sadoff, M. Le Gars, G. Shukarev, D. Heerwegh, C. Truyers, A. M. de Groot, J. Stoop, S. Tete, W. Van Damme, I. Leroux-Roels, P.-J. Berghmans, M. Kimmel, P. Van Damme, J. de Hoon, W. Smith, K. E. Stephenson, S. C. De Rosa, K. W. Cohen, M. J. McElrath, E. Cormier, G. Scheper, D. H. Barouch, J. Hendriks, F. Struyf, M. Douoguih, J. Van Hoof and H. Schuitemaker, N. Engl. J. Med., 2021, 384, 1824–1835 CrossRef CAS PubMed.
- E. Tumban, Viruses, 2021, 13, 54 CrossRef CAS PubMed.
- L. R. Baden, H. M. El Sahly, B. Essink, K. Kotloff, S. Frey, R. Novak, D. Diemert, S. A. Spector, N. Rouphael, C. B. Creech, J. McGettigan, S. Khetan, N. Segall, J. Solis, A. Brosz, C. Fierro, H. Schwartz, K. Neuzil, L. Corey, P. Gilbert, H. Janes, D. Follmann, M. Marovich, J. Mascola, L. Polakowski, J. Ledgerwood, B. S. Graham, H. Bennett, R. Pajon, C. Knightly, B. Leav, W. Deng, H. Zhou, S. Han, M. Ivarsson, J. Miller and T. Zaks, N. Engl. J. Med., 2021, 384, 403–416 CrossRef CAS PubMed.
- F. P. Polack, S. J. Thomas, N. Kitchin, J. Absalon, A. Gurtman, S. Lockhart, J. L. Perez, G. P. Marc, E. D. Moreira, C. Zerbini, R. Bailey, K. A. Swanson, S. Roychoudhury, K. Koury, P. Li, W. V. Kalina, D. Cooper, R. W. Frenck, L. L. Hammitt, Ö. Türeci, H. Nell, A. Schaefer, S. Ünal, D. B. Tresnan, S. Mather, P. R. Dormitzer, U. Şahin, K. U. Jansen and W. C. Gruber, N. Engl. J. Med., 2020, 383, 2603–2615 CrossRef CAS PubMed.
- R. Chakraborty and S. Parvez, Biochem. Pharmacol., 2020, 180, 114184 CrossRef CAS PubMed.
- G. A. Poland, I. G. Ovsyannikova and R. B. Kennedy, Lancet, 2020, 396, 1595–1606 CrossRef CAS.
- T. Li, T. Zhang, Y. Gu, S. Li and N. Xi, Fundam. Res., 2021, 1, 139–150 CrossRef.
- J. Y. Chung, M. N. Thone and Y. J. Kwon, Adv. Drug Delivery Rev., 2021, 170, 1–25 CrossRef CAS PubMed.
- L. Calzetta, B. L. Ritondo, A. Coppola, M. G. Matera, N. D. Daniele and P. Rogliani, Vaccines, 2021, 9, 341 CrossRef PubMed.
- C. Corti and G. Curigliano, Ann. Oncol., 2021, 32, 569–571 CrossRef CAS PubMed.
- A. K. Yadav, S. Ghosh and A. Kotwal, J. Mar. Med. Soc., 2020, 22, 110 CrossRef.
- E. U. Haq, J. Yu and J. Guo, Exp. Hematol. Oncol., 2020, 9, 24 CrossRef CAS PubMed.
- P. Yadav, R. Ella, S. Kumar, D. Patil, S. Mohandas, A. Shete, G. Bhati, G. Sapkal, H. Kaushal, S. Patil, R. Jain, G. R. Deshpande, N. Gupta, K. Agarwal, M. Gokhale, B. Mathapati, S. Metkari, C. Mote, D. D. Patil, B. S. S. Prasad, A. Suryawanshi, M. Kadam, A. Kumar, S. Daigude, S. Gopale, T. Majumdar, D. Mali, P. Sarkale, S. Baradkar, P. Gawande, Y. Joshi, S. Fulari, H. Dighe, S. Sharma, R. Gunjikar, A. Kumar, K. Kalele, V. K. Srinivas, K. Mohan, R. Gangakhedkar, K. Ella, P. Abraham, S. Panda and B. Bhargava, Nat. Commun., 2021, 12, 1386–1394 CrossRef CAS PubMed.
- M. F. Haidere, Z. A. Ratan, S. Nowroz, S. B. Zaman, Y.-J. Jung, H. Hosseinzadeh and J. Y. Cho, Biomol. Ther., 2021, 29, 1 CrossRef PubMed.
- Y.-D. Li, W.-Y. Chi, J.-H. Su, L. Ferrall, C.-F. Hung and T.-C. Wu, J. Biomed. Sci., 2020, 27, 104 CrossRef PubMed.
- C. Chakraborty, A. R. Sharma, M. Bhattacharya, G. Sharma, R. P. Saha and S.-S. Lee, Immune Network, 2021, 21, e5 CrossRef PubMed.
- L. Scarabel, M. Guardascione, M. D. Bo and G. Toffoli, Int. J. Infect. Dis., 2021, 104, 441 CrossRef CAS PubMed.
- R. Simoneaux and S. L. Shafer, ASA Monitor., 2020, 84, 17–18 CrossRef.
- T. M. Karpiński, M. Ożarowski, A. S. Mrozikiewicz, H. Wolski and D. Wlodkowic, Theranostics, 2021, 11, 1690 CrossRef PubMed.
- Q. Huang and J. Yana, Fundam. Res., 2021, 1, 131 CrossRef.
- S. M. Vrba, N. M. Kirk, M. E. Brisse, Y. Liang and H. Ly, Vaccines, 2020, 8, 680 CrossRef CAS PubMed.
- J. G. Liang, D. Su, T. Z. Song, Y. Zeng, W. Huang, J. Wu, R. Xu, P. Luo, X. Yang, X. Zhang, S. Luo, Y. Liang, X. Li, J. Huang, Q. Wang, X. Huang, Q. Xu, M. Luo, A. Huang, D. Luo, C. Zhao, F. Yang, J. B. Han, Y. T. Zheng and P. Liang, Nat. Commun., 2021, 12, 1346 CrossRef CAS PubMed.
- R. Chakraborty and S. Parvez, Biochem. Pharmacol., 2020, 180, 114184 CrossRef CAS PubMed.
- K. Kucukoglu, N. Faydalı and D. Bul, Med. Chem. Res., 2020, 10, 1 Search PubMed.
- S. Kashte, A. Gulbake, S. F. El-Amin and A. Gupta, Hum. Cell, 2021, 34, 711–733 CrossRef CAS PubMed.
- N. Chauhan, S. Soni, A. Gupta, M. Aslam and U. Jain, J Med Virol, 2021, 93, 1967 CrossRef CAS PubMed.
- L. Scarabel, M. Guardascione, M. D. Bo and G. Toffoli, Int. J. Infect. Dis., 2021, 104, 441 CrossRef CAS PubMed.
- I. Jatoi and J. Fan, Biomater. Transl. Med., 2021, 2, 30 Search PubMed.
- J. S. Tregoning, E. S. Brown, H. M. Cheeseman, K. E. Flight, S. L. Higham, N.-M. Lemm, B. F. Pierce, D. C. Stirling, Z. Wang and K. M. Pollock, Clin. Exp. Immunol., 2020, 202, 162 CrossRef CAS PubMed.
- Z. Strizova, J. Smetanova, J. Bartunkova and T. Milotaa, Int. Arch. Allergy Immunol., 2021, 182, 339 CrossRef CAS PubMed.
- M. Galdiero, M. Galdiero, V. Folliero, C. Zannella, A. De Filippis, A. Mali, L. Rinaldi and G. Franci, Eur. Rev. Med. Pharmacol. Sci., 2021, 25, 2752 CAS.
- S. P. Kaur and V. Gupta, Virus Res., 2020, 288, 198114 CrossRef CAS PubMed.
- B. Stav, B. Tal, P. S. Cederna and R. J. Rohrich, Plast. Reconstr. Surg., 2020, 8, e3206 Search PubMed.
- T. M. Belete, Infect. Drug Resist., 2021, 14, 151 CrossRef PubMed.
- M. P. Lythgoe and P. Middleton, Trends Pharmacol. Sci., 2020, 41, 363 CrossRef CAS PubMed.
- A. Muacevic and J. R. Adler, Cureus, 2020, 12, e8342 Search PubMed.
- J. Pollet, W.-H. Chen and U. Strycha, Adv. Drug Delivery Rev., 2021, 170, 71 CrossRef CAS PubMed.
- M. M. Silveira, G. M. S. G. Moreira and M. Mendonç, Life Sci., 2021, 267, 118919 CrossRef CAS PubMed.
- D. Pushparajah, S. Jimenez, S. Wonga, H. Alattas, N. Nafissi and R. A. Slavcev, Adv. Drug Delivery Rev., 2021, 170, 113 CrossRef CAS PubMed.
- J. Kim, Y. Eygeris, M. Gupta and G. Sahaya, Adv. Drug Delivery Rev., 2021, 170, 83 CrossRef CAS PubMed.
- M. Bhatta, S. Nandi, S. Dutta and M. K. Saha, Hum. Vaccines Immunother., 2021, 1 Search PubMed.
- Y. Li, R. Tenchov, J. Smoot, C. Liu, S. Watkins and Q. Zhou, ACS Cent. Sci., 2021, 7, 512–533 CrossRef CAS PubMed.
- K. Lundstrom, Viruses, 2021, 13, 317 CrossRef CAS PubMed.
- M. E. Dieterle, D. Haslwanter, R. H. Bortz III, A. S. Herbert, K. Chandran and R. K. Jangra, Cell Host Microbe, 2020, 28, 486 CrossRef CAS PubMed.
- M. K. Saadeldin, A. K. Abdel-Aziz and A. Abdellatif, Med. Hypotheses, 2021, 146, 110365 CrossRef CAS PubMed.
- P. McIntyre, Y. J. Joo, C. Chiu, K. Flanagan and K. Macartney, Aust. Prescr., 2021, 44, 19 CrossRef PubMed.
- J.-H. Yoo, J. Korean Med. Sci., 2021, 36, e54 CrossRef CAS PubMed.
- M. Kool, T. Soullie, M. van Nimwegen, M. A. M. Willart, F. Muskens, S. Jung, H. C. Hoogsteden, H. Hammad and B. N. Lambrecht, J. Exp. Med., 2008, 205, 869 CrossRef CAS PubMed.
- (a) M. Peiris and G. M. Leung, Lancet, 2020, 396, 1467–1469 CrossRef CAS; (b) D. B. Fogel, Contemp. Clin. Trials Commun., 2018, 156–164 CrossRef PubMed.
- K. Goodarz, A. Mohammad, M. S. Hossein, T. Niloufar, R. Sajjad, I. Neda and S. H. N. Seyed, Arch. Acad. Emerg. Med., 2020, 8, e41 Search PubMed.
- (a) A. Awadasseid, Y. Wu, Y. Tanaka and W. Zhang, Int. J. Biol. Sci., 2021, 17, 8–19 CrossRef CAS PubMed; (b) L. Dai and G. F. Gao, Nat. Rev. Immunol., 2021, 21, 73–82, DOI:10.1038/s41577-020-00480-0.
- M. C. Castells and E. J. Phillips, N. Engl. J. Med., 2021, 384, 643–649 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2021 |