Nitrogen dopant induced highly selective CO2 reduction over lotus-leaf shaped ZnO nanorods†
Fang
Lü‡
a,
Haihong
Bao‡
a,
Fei
He‡
b,
Gaocan
Qi
 *a,
Jiaqiang
Sun
*a,
Jiaqiang
Sun
 c,
Shusheng
Zhang
c,
Shusheng
Zhang
 d,
Longchao
Zhuo
e,
Hui
Yang
*a,
Guangzhi
Hu
d,
Longchao
Zhuo
e,
Hui
Yang
*a,
Guangzhi
Hu
 f,
Jun
Luo
f,
Jun
Luo
 a and
Xijun
Liu
a and
Xijun
Liu
 *ag
*ag
aTianjin Key Lab for Photoelectric Materials & Devices, School of Materials Science and Engineering, Tianjin University of Technology, Tianjin 300384, China. E-mail: xjliu@tjut.edu.cn; gaocanqi@tjut.edu.cn; y.hui1021@tjut.edu.cn
bKey Laboratory of Chemistry of Plant Resources in Arid Regions, State Key Laboratory Basis of Xinjiang Indigenous Medicinal Plants Resource Utilization, Xinjiang Technical Institute of Physics and Chemistry, Chinese Academy of Sciences, Urumqi, 830011, China
cState Key Laboratory of Coal Conversion, Institute of Coal Chemistry, Chinese Academy of Sciences, Taiyuan 030001, Shanxi, China
dCollege of Chemistry, Zhengzhou University, Zhengzhou 450000, China
eSchool of Materials Science and Engineering, Xi’an University of Technology, Xi’an 710048, Shanxi, China
fCollege of Biological, Chemical Sciences and Engineering, Jiaxing University, Jiaxing, Zhejiang 314001, China
gKey Laboratory of Civil Aviation Thermal Hazards Prevention and Emergency Response, Civil Aviation University of China, Tianjin, 300300, China
First published on 26th March 2021
Abstract
Heteroatom doping offers great promise for boosting the electrocatalytic activity for the CO2 reduction reaction (CO2RR). Herein, nitrogen-doped ZnO nanorods with a lotus-leaf shape were constructed as the active electrocatalysts, demonstrating enhanced CO2RR performance with a maximum CO faradaic efficiency of 76% at −0.7 V (vs. reversible hydrogen electrode, RHE) and excellent durable activity of more than 30 h. Density functional calculations reveal that doping nitrogen in ZnO induced the reduction of free-energy barrier for the key intermediate (*COOH) formation and the enhancement of electron exchange, thereby leading to a notable improvement in the CO2RR activity over these low-cost N-doped ZnO nanorods.
Introduction
The greenhouse effect due to CO2 has seriously influenced the carbon cycle in nature and posed a severe threat to human life.1,2 Electrocatalytic CO2 reduction reaction (CO2RR) is a promising technology that can convert CO2 to valuable feedstocks and chemical fuels.3–8 Thus far, great efforts have been devoted to searching for suitable materials for CO2 electroreduction. Among these materials, transition metal electrocatalysts such as Co,9 Ni,10 Bi,11 Cu,12,13 Ag14 and Zn15,16 are ideal candidates for converting CO2 to chemical fuels because their d bands contain valence electrons close to the Fermi level,17 thus beneficial for overcoming the activation barriers of the reduction reaction.In the past decades, earth-abundant and environmentally friendly Zn-based materials have attracted much attention as CO2RR electrocatalysts due to their high adsorption affinity towards CO2 molecules.18,19 However, bulk Zn catalysts require large overpotentials with low reaction activity, due to fewer active sites.20 To enhance the catalytic activity, significant efforts have focused on the structural design and morphology of Zn-based catalysts, including dendritic Zn,21 hexagonal Zn,22 nanoporous ZnO23 and ZnO nanosheets.24 Previous studies have confirmed heteroatom doping (such as B, N, P and S) to be an effective strategy for increasing the electrocatalytic activity. The introduction of dopants not only induces a great change in the electronic structure and enhances the conductivity, but also reduces the activation energy barrier of the electrocatalytic reaction.25–27 Recently, we have developed a facile approach to dope nitrogen into Ti3C2 MXene nanosheets for electrocatalytic CO2 reduction to produce CO.28
In this study, nitrogen-doped ZnO nanorods with lotus-leaf shape were fabricated as active electrocatalysts for CO2 conversion using a facile hydrothermal growth and subsequent plasma treatment process. Compared with undoped ones, N-doped ZnO nanorods exhibited a significant improvement in CO generation with a maximum faradaic efficiency (FECO) of 76% at −0.7 V (vs. reversible hydrogen electrode, RHE) and excellent durable activity of more than 30 h. Theoretical calculations confirm that the introduction of nitrogen dopants is beneficial for reducing the activation barrier for the key intermediate (*COOH) formation and enhancing the electron exchange between N dopants and coordinated Zn atoms, resulting in an outstanding CO2RR performance.
Results and discussion
The N-doped ZnO nanorods were fabricated according to the previously reported method29 and the schematic illustration is shown in Fig. 1a. ZnO nanorods were first grown on ITO conductive glass by the hydrothermal method, and the morphology of ZnO looks like lotus leaves as observed by scanning electron microscopy (SEM) (Fig. S1, ESI†). The N-doped ZnO nanorods were obtained by following the plasma treatment process, and these nanorods showed a similar morphology to that of lotus leaves in the undoped ones, represented in Fig. 1b (Fig. S2, ESI†) and transmission electron microscopy (TEM) (Fig. 1c) images. The corresponding high-resolution TEM (HRTEM) image (Fig. 1d) exhibits a lattice fringe distance of 0.26 nm, which corresponds to the (002) plane spacing of ZnO material. Additionally, N elements were distributed uniformly in the ZnO nanorods according to elemental mapping images from energy-dispersive X-ray spectroscopy (EDS) (Fig. 1e), and the content of nitrogen dopants in these N-doped ZnO nanorods was about 0.52 wt%.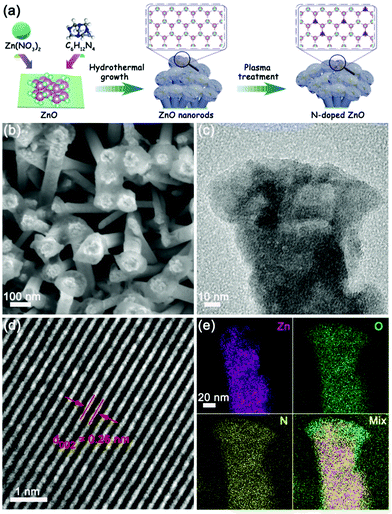 | ||
| Fig. 1 (a) Schematic illustration of the preparation, (b) SEM image, (c) TEM image, (d) high-magnification TEM image, and (e) EDS elemental mapping images of N-doped ZnO nanorods. | ||
The crystallographic structure was investigated by XRD patterns, (Fig. 2a) and all diffraction peaks for undoped and N-doped ZnO nanorods were approximately identical with no peak shifts, corresponding to the JCPDS files #75-1526 of ZnO. Nevertheless, the change in the surface chemical structure of N-doped ZnO nanorods was also measured by X-ray photoelectron spectroscopy (XPS) and is shown in Fig. 2. The binding energies of Zn 2p3/2 and Zn 2p1/2 peaks for N-doped ZnO nanorods are 1022.1 eV and 1045.1 eV, respectively. Obviously, the binding energy for Zn2+ showed a right shift of ∼0.2 eV for N-doped ZnO nanorods compared to the undoped ones (Fig. 2b). This indicates that a part of electrons have been transferred away from Zn, which may be caused by the introduction of N after the plasma treatment. The O 1s spectrum shown in Fig. 2c exhibits a very broad peak and can be deconvoluted into three peaks at 530.6 eV, 531.6 eV and 532.6 eV, which were assigned to O2− of lattice oxygen, deficient oxygen and chemically adsorbed oxygen, respectively.30 The binding energy of N 1s peak in N-doped ZnO (Fig. 2d) also can be deconvoluted into two peaks at 397.6 eV and 399.4 eV, which were assigned to Zn–N31 and O–N32 species (∼400 eV), indicating that N atoms have been successfully doped into ZnO nanorods.
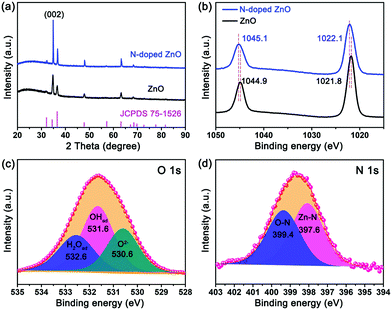 | ||
| Fig. 2 The structural characterization of ZnO and N-doped ZnO nanorods. (a) XRD patterns, (b) XPS spectra of Zn 2p, XPS fitting spectra of (c) O 1s and (d) N 1s of N-doped ZnO. | ||
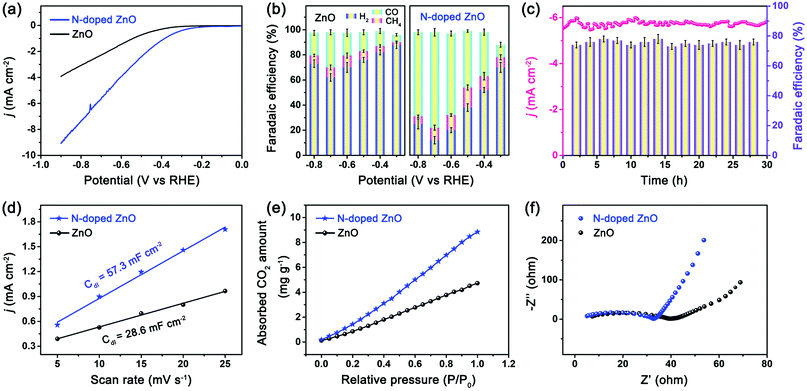
The electrocatalytic CO2 performance of the electrodes based on lotus leaf-shaped ZnO and N-doped ZnO nanorods were evaluated in a H-cell separated by a Nafion-115 proton exchange membrane with CO2-saturated 0.5 M KHCO3 (pH = 7.2), as previously reported by our group.33 The linear sweep voltammetry (LSV) curves were obtained using 90% iR-offset to compensate the drop of ohmic potential caused by electrolyte resistance. All potentials were reported with respect to the RHE, and each current density was normalized by the geometric area of the electrode. From LSV curves (Fig. S3 and S4, ESI†), the N-doped ZnO electrode showed lower overpotential and higher current density under CO2 atmosphere compared to N2 atmosphere, indicating better electrocatalytic activity for CO2RR. However, the undoped ZnO electrode showed lower current density under CO2 atmosphere compared to N2 (Ar) atmosphere, indicating better electrocatalytic activity for hydrogen evolution reaction. The N-doped ZnO electrode (Fig. 3a) exhibited a reduction current density of 5.6 mA cm−2 at −0.7 V vs. RHE; this value is 2.4 times greater than that of the undoped one, indicating a higher catalytic activity over the N-doped ZnO nanorods as compared to the undoped counterpart.
The products were quantitatively analyzed by gas chromatography (GC) and 1H nuclear magnetic resonance (NMR) spectrometry. Consequently, only CO, H2 and CH4 were detected and no liquid products were observed. The NMR spectrum of the N-doped ZnO electrode for CO2RR recorded at −0.7 V vs. RHE is shown in Fig. S5 (ESI†). The dependence of faradaic efficiency (FE) for different products on the applied potentials for both electrodes is shown in Fig. 3b. At a low applied potential of −0.3 V vs. RHE, low FE values for CO (11 ± 4%) and CH4 (7 ± 4%) production were observed over the N-doped ZnO electrode, while H2 (70 ± 4%) was the dominant product. With further increase in the applied potential, a maximum FECO of 76 ± 4% was achieved at −0.7 V vs. RHE, as well as a small amount of CH4 (∼8%) and H2 (∼14%) were detected. For observed products, the sum of FEs was above 90.0% for the syngas with a ratio of CO/H2 of 5.4, and also generates controllable ratios of syngas with varying potential during the CO2RR. For the reference ZnO electrode, the hydrogen evolution reaction (HER) dominated the entire potential in the range from −0.3 to −0.8 V vs. RHE, and it showed a maximum FECO of 28% at −0.7 V vs. RHE, indicating low activity for reducing CO2 to CO.
The long-term stability of electrocatalysts is another crucial indicator in evaluating the CO2 reduction performance. The current density of N-doped ZnO electrode was relatively stable with a value of 5.6 mA cm−2 at −0.7 V vs. RHE even after 30 h (Fig. 3c). Notably, the corresponding FE for CO production was constantly maintained to be greater than 75% for a duration of 30 h. Meanwhile, the structure and morphology of the N-doped ZnO after CO2RR are shown in Fig. S6 (ESI†). Also, the corresponding Nyquist plot after the long-term electrolysis of the N-doped ZnO electrode has been obtained and is shown in Fig. S7 (ESI†). After long-term electrolysis, the plot exhibited a little larger semicircle than before CO2 reduction, which indicates a higher interfacial charge-transfer resistance and a little decrease in the conductivity. The observed results showed that the N-doped ZnO nanorods showed a good structural and electrochemical stability.
The electrochemically active surface area of the electrodes was determined by double-layer capacitance (Cdl) to investigate the underlying kinetic mechanism for the enhanced CO2RR performance. The measured Cdl of the N-doped ZnO nanorods was 57.3 mF cm−2, approximately double that of the undoped counterpart (28.6 mF cm−2), which confirms an increased number of catalytically active sites because of N doping and subsequently improved electroreduction activity. Furthermore, the volumetric CO2 adsorption isotherms showed that N-doped ZnO nanorods exhibited much higher CO2 adsorption capacity (10.3 cm3 g−1) compared to that of the undoped counterpart, confirming that N-doped ZnO nanorods have much more abundant adsorption sites for CO2 on the surface due to the doping of nitrogen. The charge-transfer resistance values at the interfaces for the two electrodes were obtained by electrochemical impedance spectroscopy (EIS). The Nyquist plot of N-doped ZnO nanorods exhibited a much smaller semicircle and a steeper slope, which indicated a lower interfacial charge-transfer resistance and better conductivity, suggesting a much faster faradaic process. The Tafel plots (Fig. S8, ESI†) show that in the linear Tafel region, N-doped ZnO nanorods exhibited a lower Tafel slope (169 mV dec−1) than undoped ZnO nanorods (192 mV dec−1), indicating that the single-electron transfer to CO2 is the rate-determining step for CO2 reduction to CO34 on the two ZnO electrodes, and N-doping of ZnO improved the CO2 reduction thermodynamic kinetics, which is consistent with the LSV analysis (Fig. 3a).
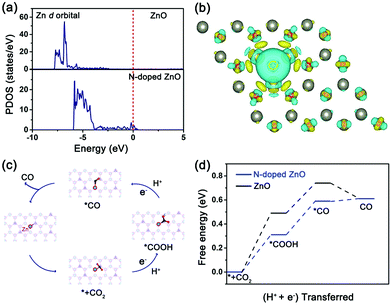
To further provide a theoretical insight into the underlying mechanism, we performed DFT calculations to calculate the density of states, differential charge density and the Gibbs free energy of the two nanorods. The projected density of states (PDOS) of Zn d-orbitals in the two nanorods are presented in Fig. 4a. N-Doped ZnO exhibits a significant increase in the density of states near the Fermi level compared to the undoped counterpart, which can enhance the electron exchange with the reactants and intermediates during the reaction of Zn atoms, thus improving the reactivity of CO2 electroreduction.35 The differential charge density of O–N–Zn in N-doped ZnO (Fig. 4b) revealed that the charge between N and active Zn atoms was accumulated, and the electron exchange between the N dopants and coordinated Zn atoms was much easier than that between the O and Zn atoms.
On the basis of above results and Tafel values, a possible mechanism for CO2 electroreduction to CO over N-doped ZnO (Fig. 4c) was proposed, where the overall path proceeds as CO2 → *CO2 → *COOH → *CO → CO, which is in agreement with the previous reports.19,36 Initially, a CO2 molecule gets adsorbed on the N site of ZnO surface and then generates *CO2. After that, the adsorbed *CO2 couples with a proton from the electrolyte to form *COOH intermediate, and this step is identified as the rate-limiting step (RLS). Finally, the *COOH intermediate leaves off the H+ + e− reactants to form H2O product and produce CO. DFT calculations were performed to gain the reaction free energy profile (Fig. 4d) and to analyze the kinetics of elementary steps in the CO2RR catalyzed by these two samples. It can be clearly seen that the activation energy barrier of the *COOH intermediate for N-doped ZnO (0.31 eV) is much lower than that of undoped ZnO (0.49 eV). Owing to the difference of 0.18 eV in activation barrier, the *COOH intermediate formation is much easier over N-doped ZnO and further promotes CO formation at lower overpotentials. Therefore, the reduction of the free-energy barrier and the enhanced electron exchange induced by N dopants provide superior CO2RR performance for N-doped ZnO nanorods with a lotus-leaf shape.
Conclusion
In summary, N-doped ZnO nanorods with a lotus-leaf shape were prepared by a simple hydrothermal synthesis and plasma treatment method. It exhibited an enhanced CO2RR performance with a maximum CO faradaic efficiency of 76% at −0.7 V vs. RHE. Further, more than 30 h durability test confirmed its excellent stability for the CO2RR. Theoretical calculations revealed that the nitrogen dopants enhance the electron exchange between the N dopants and coordinated Zn atoms, which induces the reduction of free-energy barriers and thus facilitates the formation of the intermediate (*COOH). Finally, these N-doped ZnO nanorods with a lotus-leaf shape show superb CO2RR activity.Author contributions
Xijun Liu, Hui Yang and Gaocan Qi designed the catalyst; Fang Lü, Haihong Bao and Fei He performed the experiments and DFT calculations; Jiaqiang Sun, Shusheng Zhang, Longchao Zhuo, and Jun Luo co-analyzed data; Gaocan Qi and Hui Yang co-wrote the manuscript; Xijun Liu revised the manuscript. All the authors discussed and commented on the data and contributed to the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by National Natural Science Foundation of China (22075211, 21601136, 21501132, 51971157, 12074017 and 51621003), Tianjin Science Fund for Distinguished Young Scholars (19JCJQJC61800), the Science and Technology Development Fund of Tianjin Education Commission for Higher Education (2018KJ126) and Tianjin Natural Science Foundation (18JCQNJC77100). The authors acknowledge Beijng PARATERA Tech Co., Ltd for providing HPC resources that have contributed to the research results reported within this paper.References
- B. A. Rosen, A. Salehi-Khojin, M. R. Thorson, W. Zhu, D. T. Whipple, P. J. A. Kenis and R. I. Masel, Ionic liquid-mediated selective conversion of CO to CO at low overpotentials, Science, 2011, 334, 643 CrossRef CAS PubMed.
- Y. Zheng, A. Vasileff, X. Zhou, Y. Jiao, M. Jaroniec and S. Z. Qiao, Understanding the roadmap for electrochemical reduction of CO2 to multi-carbon oxygenates and hydrocarbons on copper-based catalysts, J. Am. Chem. Soc., 2019, 141, 7646–7659 CrossRef CAS PubMed.
- X. Liu, H. Yang, J. He, H. Liu, L. Song, L. Li and J. Luo, Highly active, durable ultrathin MoTe2 layers for the electroreduction of CO2 to CH4, Small, 2018, 14, 1704049 CrossRef PubMed.
- J. Zhu, J. Fan, T. Cheng, M. Cao, Z. Sun, R. Zhou, L. Huang, D. Wang, Y. Li and Y. Wu, Bilayer nanosheets of unusual stoichiometric bismuth oxychloride for potassium ion storage and CO2 reduction, Nano Energy, 2020, 75, 104939 CrossRef CAS.
- F. Lü, H. Bao, Y. Mi, Y. Liu, J. Sun, X. Peng, Y. Qiu, L. Zhuo, X. Liu and J. Luo, Electrochemical CO2 reduction: from nanoclusters to single atom catalysts, Sustainable Energy Fuels, 2020, 4, 1012–1028 RSC.
- N. Han, P. Ding, L. He, Y. Li and Y. Li, Promises of main group metal–based nanostructured materials for electrochemical CO2 reduction to formate, Adv. Energy Mater., 2020, 10, 1902338 CrossRef CAS.
- W. Wang, L. Shang, G. Chang, C. Yan, R. Shi, Y. Zhao, G. I. N. Waterhouse, D. Yang and T. Zhang, Intrinsic carbon-defect-driven electrocatalytic reduction of carbon dioxide, Adv. Mater., 2019, 31, 1808276 CrossRef PubMed.
- P. Ding, H. Zhao, T. Li, Y. Luo, G. Chen, S. Gao, X. Shi, S. Lu and X. Sun, Metal-based electrocatalytic conversion of CO2 to formic acid/formate, J. Mater. Chem. A, 2020, 8, 21947–21960 RSC.
- C. Liu, T. R. Cundari and A. K. Wilson, CO2 reduction on transition metal (Fe, Co, Ni, and Cu) surfaces: in comparison with homogeneous catalysis, J. Phys. Chem. C, 2012, 116, 5681–5688 CrossRef CAS.
- J. Yang, Z. Qiu, C. Zhao, W. Wei, W. Chen, Z. Li, Y. Qu, J. Dong, J. Luo, Z. Li and Y. Wu, In situ thermal atomization to convert supported nickel nanoparticles into surface-bound nickel single-atom catalysts, Angew. Chem. Int. Ed., 2018, 57, 14095–14100 CrossRef CAS PubMed.
- J. Yang, X. Wang, Y. Qu, X. Wang, H. Huo, Q. Fan, J. Wang, L. Yang and Y. Wu, Bi-based metal-organic framework derived leafy bismuth nanosheets for carbon dioxide electroreduction, Adv. Energy Mater., 2020, 10, 2001709 CrossRef CAS.
- A. Guan, Z. Chen, Y. Quan, C. Peng, Z. Wang, T. Sham, C. Yang, Y. Ji, L. Qian, X. Xu and G. Zheng, Boosting CO2 electroreduction to CH4 via tuning neighboring single-copper sites, ACS Energy Lett., 2020, 5, 1044–1053 CrossRef CAS.
- S. Shen, J. He, X. Peng, W. Xi, L. Zhang, D. Xi, L. Wang, X. Liu and J. Luo, Stepped surface-rich copper fiber felt as an efficient electrocatalyst for the CO2RR to formate, J. Mater. Chem. A, 2018, 6, 18960 RSC.
- N. Zhang, X. Zhang, L. Tao, P. Jiang, C. Ye, R. Lin, Z. Huang, A. Li, D. Pang, H. Yan, Y. Wang, P. Xu, S. An, Q. Zhang, L. Liu, S. Du, X. Han, D. Wang and Y. Li, Silver single-atom catalyst for efficient electrochemical CO2 reduction synthesized from thermal transformation and surface reconstruction, Angew. Chem. Int. Ed., 2021, 60, 6170–6176 CrossRef CAS PubMed.
- L. Han, S. Song, M. Liu, S. Yao, Z. Liang, H. Cheng, Z. Ren, W. Liu, R. Lin, G. Qi, X. Liu, Q. Wu, J. Luo and H. L. Xin, Stable and efficient single-atom Zn catalyst for CO2 reduction to CH4, J. Am. Chem. Soc., 2020, 142, 12563 CrossRef CAS PubMed.
- B. Qin, Y. Li, H. Fu, H. Wang, S. Chen, Z. Liu and F. Peng, Electrochemical reduction of CO2 into tunable syngas production by regulating crystal facets of earth-abundant Zn catalyst, ACS Appl. Mater. Inter., 2018, 10, 20530–20539 CrossRef CAS PubMed.
- J. K. Nørskov, F. Abild-Pedersena, F. Studt and T. Bligaard, Density functional theory in surface chemistry and catalysis, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 937–943 CrossRef PubMed.
- Q. Tang and Q. Luo, Adsorption of CO2 at ZnO: A surface structure effect from DFT+U calculations, J. Phys. Chem. C, 2013, 117, 22954–22966 CrossRef CAS.
- T. Zhang, X. Li, Y. Qiu, P. Su, W. Xu, H. Zhong and H. Zhang, Multilayered Zn nanosheets as an electrocatalyst for efficient electrochemical reduction of CO2, J. Catal., 2018, 357, 154–162 CrossRef.
- Y. Hori, H. Wakebe, T. Tsukamoto and O. Koga, Electrocatalytic process of CO selectivity in electrochemical reduction of CO2 at metal electrodes in aqueous media, Electrochim. Acta, 1994, 39, 1833–1839 CrossRef CAS.
- J. Rosen, G. S. Hutchings, Q. Lu, R. V. Forest, A. Moore and F. Jiao, Electrodeposited Zn dendrites with enhanced CO selectivity for electrocatalytic CO2 reduction, ACS Catal., 2015, 5, 4586–4591 CrossRef CAS.
- D. H. Won, H. Shin, J. Koh, J. Chung, H. S. Lee, H. Kim and S. I. Woo, Highly efficient, selective, and stable CO2 electroreduction on a hexagonal Zn catalyst, Angew. Chem. Int. Ed., 2016, 55, 9297–9300 CrossRef CAS.
- X. Jiang, F. Cai, D. Gao, J. Dong, S. Miao, G. Wang and X. Bao, Electrocatalytic reduction of carbon dioxide over reduced nanoporous zinc oxide, Electrochem. Commun., 2016, 68, 67–70 CrossRef CAS.
- Z. Geng, X. Kong, W. Chen, H. Su, Y. Liu, F. Cai, G. Wang and J. Zeng, Oxygen vacancies in ZnO nanosheets enhance CO2 electrochemical reduction to CO, Angew. Chem. Int. Ed., 2018, 57, 6054–6059 CrossRef CAS.
- Y. Liu, S. Chen, X. Quan and H. Yu, Efficient electrochemical reduction of carbon dioxide to acetate on nitrogen-doped nanodiamond, J. Am. Chem. Soc., 2015, 137, 11631–11636 CrossRef CAS PubMed.
- C. Jia, W. Ren, X. Chen, W. Yang and C. Zhao, N, B) Dual heteroatom-doped hierarchical porous carbon framework for efficient electroreduction of carbon dioxide, ACS Sustainable Chem. Eng., 2020, 8, 6003–6010 CrossRef CAS.
- M. Kuang, A. Guan, Z. Gu, P. Han, L. Qian and G. Zheng, Enhanced N-doping in mesoporous carbon for efficient electrocatalytic CO2 conversion, Nano Res., 2019, 12, 2324–2329 CrossRef CAS.
- D. Qu, X. Peng, Y. Mi, H. Bao, S. Zhao, X. Liu and J. Luo, Nitrogen-doping and titanium vacancies synergistically promote CO2 fixation in seawater, Nanoscale, 2020, 12, 17191 RSC.
- G. Qi, S. Zhao and Z. Yuan, From function-guided assembly of a lotus leaf-like ZnO nanostructure to a formaldehyde gas-sensing application, Sens. Actuators B-Chem., 2013, 184, 143–149 CrossRef CAS.
- M. Chen, Z. Wang, D. Han, F. Gu and G. Guo, Porous ZnO polygonal nanoflakes: synthesis, use in high-sensitivity NO2 gas sensor, and proposed mechanism of gas sensing, Sens. Actuators B-Chem., 2011, 157, 565–574 CrossRef CAS.
- C. L. Perkins, S. Lee, X. Li, S. E. Asher and T. J. Coutts, Identification of nitrogen chemical states in N-doped ZnO via x-ray photoelectron spectroscopy, J. Appl. Phys., 2005, 97, 034907 CrossRef.
- J. A. Rengifo-Herrera, K. Pierzchala, A. Sienkiewicz, L. Forró, J. Kiwi, J. E. Moser and C. Pulgarin, Synthesis, characterization, and photocatalytic activities of nanoparticulate N, S-codoped TiO2 having different surface-to-volume ratios, J. Phys. Chem. C, 2010, 114, 2717–2723 CrossRef CAS.
- J. Fu, H. Bao, Y. Liu, Y. Mi, Y. Qiu, L. Zhuo, X. Liu and J. Luo, Oxygen doping induced by nitrogen vacancies in Nb4N5 enables highly selective CO2 reduction, Small, 2020, 16, 1905825 CrossRef CAS PubMed.
- Y. C. Hsieh, S. D. Senanayake, Y. Zhang, W. Xu and D. E. Polyansky, Effect of chloride anions on the synthesis and enhanced catalytic activity of silver nanocoral electrodes for CO2 electroreduction, ACS Catal., 2015, 5, 5349–5356 CrossRef CAS.
- S. Zhao, Y. Wen, X. Peng, Y. Mi, X. Liu, Y. Liu, L. Zhuo, G. Hu, J. Luo and X. Tang, Isolated single-atom Pt sites for highly selective electrocatalytic hydrogenation of formaldehyde to methanol, J. Mater. Chem. A, 2020, 8, 8913–8919 RSC.
- S. Gao, Y. Lin, X. Jiao, Y. Sun, Q. Luo, W. Zhang, D. Li, J. Yang and Y. Xie, Partially oxidized atomic cobalt layers for carbon dioxide electroreduction to liquid fuel, Nature, 2016, 529, 68–71 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1qm00344e |
| ‡ These authors contributed equally to this work. |
| This journal is © the Partner Organisations 2021 |