MXene and MXene-based composites: synthesis, properties and environment-related applications
Xiaoxue
Zhan
a,
Chen
Si
 *ab,
Jian
Zhou
*ab,
Jian
Zhou
 a and
Zhimei
Sun
a and
Zhimei
Sun
 *ab
*ab
aSchool of Materials Science and Engineering, Beihang University, Beijing 100191, China. E-mail: sichen@buaa.edu.cn; zmsun@buaa.edu.cn
bCenter for Integrated Computational Materials Engineering, International Research Institute for Multidisciplinary Science, Beihang University, Beijing 100191, China
First published on 5th November 2019
Abstract
In recent years, a new large family of two dimensional transition metal carbides, carbonitrides, and nitrides, so-called MXenes, have grabbed considerable attention, owing to their many fascinating physical and chemical properties that are closely related to the rich diversity of their elemental compositions and surface terminations. In particular, it is easy for MXenes to form composites with other materials such as polymers, oxides, and carbon nanotubes, which further provides an effective way to tune the properties of MXenes for various applications. Not only have MXenes and MXene-based composites come into prominence as electrode materials in the energy storage field as is widely known, but they have also shown great potential in environment-related applications including electro/photocatalytic water splitting, photocatalytic reduction of carbon dioxide, water purification and sensors, thanks to their high conductivity, reducibility and biocompatibility. In this review, we summarize the synthesis and properties of MXenes and MXene-based composites and highlight their recent advances in environment-related applications. Challenges and perspectives for future research are also outlined.
1 Introduction
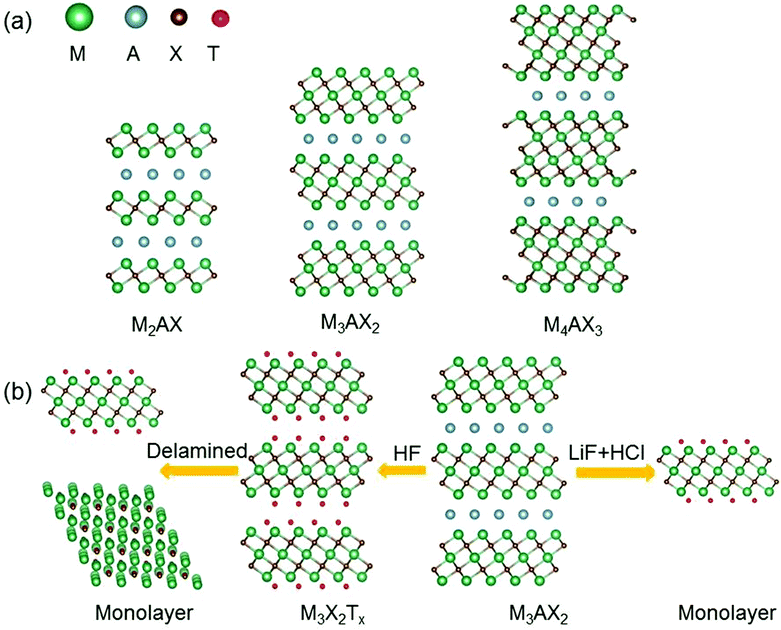
Since the discovery of graphene in 2004, two-dimensional (2D) materials have attracted great interest.1–4 Owing to the reduction of the dimension and size, 2D materials have exhibited many intriguing properties that are not found in their bulk counterparts, holding tremendous promise for a host of applications ranging from electronic and optoelectronic devices to electrochemical catalysis.5–9 In recent years, with great advances in synthetic techniques, more and more 2D materials beyond graphene have been successfully produced.10 Among them, a newly discovered large family of 2D early transition metal carbides and/or nitrides, named “MXenes”, is a rapidly rising star.11–18 MXenes are synthesized mainly by etching the A layers from the MAX phases19–21 which are ternary carbides or nitrides with a general chemical formula Mn+1AXn, where M stands for an early transition metal, A is a group IIIA or IVA element, X is C and/or N, and n = 1, 2, 3.22 MAX phases possess layered hexagonal structures in which the Mn+1Xn units and the A layers are alternately stacked (see Fig. 1a).23 Because the M–X bonds are much stronger than the M–A bonds, the A layers can be selectively chemically etched without destroying the M–X bonds, resulting in weakly bonded Mn+1Xn layers that can be readily separated by sonication.24 The formed 2D materials are called MXenes to stress the loss of A layers from the parent MAX phase and their 2D nature similar to that of graphene. It should be mentioned that in the etching process, the surfaces of the Mn+1Xn units are always covered with functional groups such as oxygen (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), hydroxyl (–OH) or/and fluorine (–F).25,26 So the chemical formula of MXenes is summarized as Mn+1XnTx, where Tx represents the surface functional groups. Experimentally, the proportions of different functional groups on the MXene surfaces are uncertain and change with the etching condition.
O), hydroxyl (–OH) or/and fluorine (–F).25,26 So the chemical formula of MXenes is summarized as Mn+1XnTx, where Tx represents the surface functional groups. Experimentally, the proportions of different functional groups on the MXene surfaces are uncertain and change with the etching condition.
The versatile chemistries of MXenes endow them with plenty of intriguing mechanical, electronic, magnetic and electrochemical properties. In particular, the good flexibility of MXenes, combined with their 2D morphology and layered structures, makes it easy for MXenes to form composites with other materials, which provides an opportunity of integrating the outstanding properties of different materials in a complementary way. Therefore, not only MXenes but MXene-based composites also have attracted considerable research interest, holding great promise for many applications. Due to their high conductivity and excellent electrochemical activity, MXenes and MXene-based composites initially find applications in energy storage as high-performance electrode materials for lithium–sulfur batteries,27–30 sodium-ion batteries27,31,32 and supercapacitors.33 It is remarkable that recently they have further risen to prominence in the environment-related fields. Specifically speaking, (i) they have been used as efficient catalysts or co-catalysts for electro/photocatalytic water splitting34,35 and photocatalytic reduction of carbon dioxide (CO2);36 (ii) they can remove contaminants in water including heavy metal ions, organic dyes, eutrophic substances and nuclear waste;37–39 (iii) they have also been applied to biosensors and gas sensors,40,41 exhibiting excellent performances. Although there have been a few reviews13,14,18 about MXenes, these works are dedicated extensively to MXenes and pay little attention to MXene-based composites. Moreover, while the applications of MXenes in energy storage have been highlighted and carefully discussed in previous reviews,11,12,15–17 the latest encouraging progress in environment-related applications of MXenes has not been summarized in detail. As environmental pollution is constantly intensified in many parts of the world, environmental protection and remediation has been a task that brooks no delay. In this context, it is highly desirable to summarize the progress of MXenes and MXene-based composites in environment-related applications.
In the following sections, we will give an overview of the most recent advances of MXenes and their composites, covering both experimental and theoretical studies. We begin with a brief review of the synthesis and processing of MXenes, then we summarize and classify the synthesized various MXene-based composites. After that, we move on to the properties and applications of MXenes and MXene-based composites. We discuss their mechanical, electronic and magnetic properties and highlight their tremendous promise in environment-related applications including catalysis, water remediation and sensors. Finally, we conclude this review with a description of the challenges and an outlook for future research in the MXene field, so as to promote more explorations of this new, but promising and quickly expanding family of materials.
2 Synthesis of MXenes
As summarized in Table 1, more than 20 different MXenes have been synthesized by chemically selectively etching certain atomic layers from the layered carbide, nitride, or carbonitride precursors. The etchants can be mainly divided into two categories: acidic solutions containing fluoride ions (HF, a mixture of LiF and HCl, or NH4HF2) and salts containing fluorine ions (NH3F, KF, LiF or NaF).| Precursors | Mxene | Etchants | Conditions | Yield (%) | Ref. | |
|---|---|---|---|---|---|---|
| T (°C) | Time (h) | |||||
| Ti2AlC | Ti2CTx | 10% HF | RT | 10 | 80 | 43 |
| V2AlC | V2CTx | 50% HF | RT | 90 | 60 | 45 |
| Nb2AlC | Nb2CTx | 50% HF | RT | 90 | 100 | 44 |
| Ti2AlN | Ti2NTx | 5% HF | RT | 24 | NA | 46 |
| Mo2Ga2C | Mo2CTx | 50% HF | 50 | 3 | NA | |
| (Ti0.5Nb0.5)2AlC | (Ti0.5Nb0.5)2CTx | 51% HF | RT | 28 | 80 | 47 |
| Ti3AlC2 | Ti3C2Tx | 50% HF | RT | 2 | 100 | 24 |
| (V0.5Cr0.5)3AlC2 | (V0.5Cr0.5)3C2Tx | 50% HF | RT | 69 | NA | 47 |
| Ta4AlC3 | Ta4C3Tx | 50% HF | RT | 72 | 90 | 47 |
| Nb4AlC3 | Nb4C3Tx | 50% HF | RT | 96 | 77 | 53 |
| V4AlC3 | V4C3Tx | 40% HF | RT | 165 | NA | 54 |
| Ti3AlCN | Ti3CNTx | 30% HF | RT | 18 | 80 | 47 |
| Mo2TiAlC2 | Mo2TiC2Tx | 50% HF | RT | 48 | 100 | 55 |
| Mo2Ti2AlC3 | Mo2Ti2C3Tx | 50% HF | 55 | 90 | 100 | 55 |
| (Mo2/3Y1/3)2AlC | Mo4/3CTx | 48% HF | RT | 60 | NA | 48 |
| 10% HF | RT | 72 | ||||
| (Nb2/3Sc1/3)2AlC | Nb4/3CTx | 48% HF | RT | 30 | NA | 49 |
| (W2/3Sc1/3)2AlC | W4/3CTx | 48% HF | RT | 30 | NA | 50 |
| Zr3Al3C5 | Zr3C2Tx | 50% HF | RT | 60 | NA | 68 |
| Hf3[Al(Si)]4C6 | Hf3C2Tx | 35% HF | RT | 60 | NA | 69 |
| Ti2AlC | Ti2CTx | 0.9 M LiF + 6 M HCl | 40 | 15 | NA | 57 |
| Mo2Ga2C | Mo2CTx | 3 M LiF + 12 M HCl | 35 | 384 | NA | 58 |
| V2AlC | V2CTx | 2 g LiF + 40 M HCl | 90 | 48 | NA | 59 |
| Ti3AlC2 | Ti3C2Tx | 3 M LiF + 6 M HCl | 40 | 45 | 100 | 56 |
| Ti3AlCN | Ti3CNTx | 0.66 g LiF + 6 M HCl | 35 | 12 | NA | 60 |
| Cr2TiAlC2 | Cr2TiC2Tx | 5 M LiF + 6 M HCl | 55 | 42 | 80 | 61 |
| (Nb0.8Zr0.2)4AlC3 | (Nb0.8Zr0.2)4C3Tx | LiF + 12 M HCl | 50 | 168 | NA | 62 |
| (W2/3Sc1/3)2AlC | W4/3CTx | 4 g LiF + 12 M HCl | 35 | 48 | NA | 50 |
| Ti3AlC2 | Ti3C2Tx | 1 M NH4HF2 | 80 | 12 | NA | 63 |
| Ti3AlC2 | Ti3C2Tx | NH3F | 150 | 24 | NA | 65 |
| Ti4AlN3 | Ti4N3Tx | 59% KF + 29% LiF + 12% NaF | 550 | 0.5 | NA | 66 |
Initially, MXenes are separated from MAX phases by soaking MAX phases in specific acids and destroying the M–A bonds. In this process, a certain corrosion time and full agitation are needed.
Naguib et al. synthesized the first MXene, Ti3C2Tx, by immersing Ti3AlC242 powders in 50% concentrated hydrofluoric acid (HF) at room temperature (RT) for 2 h.24Fig. 2a shows the X-ray diffraction (XRD) pattern before and after Ti3AlC2 is etched. It is clearly seen that after HF treatment the position of the main peak in the XRD pattern shifts from around 40° to around 10°, indicating that Ti3AlC2 is replaced by Ti3C2Tx. Note that the experimental XRD pattern after HF etching is similar to the simulated XRD patterns of Ti3C2F2 and Ti3C2(OH)2 (green and red curves in Fig. 2a), suggesting the presence of functional groups. Subsequently, this method of HF etching is successfully applied to other MAX phases, yielding many new MXenes including Ti2CTx,43 V2CTx,44,45 Nb2CTx,44 Ti2NTx,46 (V0.5Cr0.5)3C2Tx,47 (Ti0.5Nb0.5)2CTx,47 Mo4/3CTx,48 Nb4/3CTx,49 W4/3CTx,50 Ti3C2Tx,51,52 Ti3CNTx,47 Nb4C3Tx,53 Ta4C3Tx,47 V4C3Tx,54 Mo2TiC2Tx,55 Mo2Ti2C3Tx55 and Cr2TiC2Tx.55
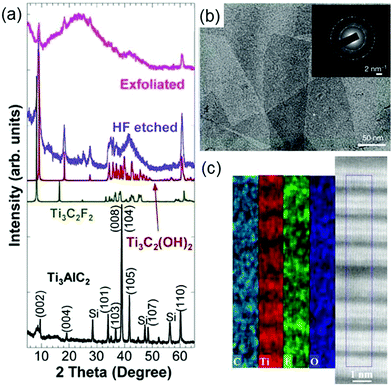 | ||
| Fig. 2 (a) XRD pattern of Ti3AlC2 before and after HF etching. Reprinted with permission from ref. 24 Copyright (2011) Wiley Online Library. (b) TEM image of lateral Ti3C2Tx. The whole selected area electron diffraction pattern is shown as the inset. Reprinted with permission from ref. 56 Copyright (2014) Nature. (c) STEM image (right panel) and EDX maps (left panel) of Ti3C2Tx. Reprinted with permission from ref. 63 Copyright (2014) American Chemical Society. | ||
Considering that HF is harmful to the human body and environment, it is a must to develop other harmless etchants. Ghidiu et al. used a safer mixture solution of hydrochloric acid (HCl) and lithium fluoride (LiF) in which Ti3AlC2 powders were soaked at 35 °C for 24 h to obtain Ti3C2Tx.56 It can be seen from the transmission electron microscopy (TEM) image of Ti3C2Tx (Fig. 2b) that the synthesized sample has fewer defects. Because the HCl and LiF mixture solution also has hydrogen and fluoride ions, we can infer that it has the same etching mechanism for the MAX phases with the HF solution. In fact, other combinations of acids (H2SO4) and salts containing fluorine ions (NaF, FeF3, KF, CsF, CaF2) have also been used to synthesize MXenes.56 So far, many MXenes including Ti2CTx,57 Mo2CTx,58 V2CTx,59 W4/3CTx,50 Ti3C2Tx,56 Ti3CNTx,60 Cr2TiC2Tx61 and (Nb0.8Zr0.2)4C3Tx62 and (Nb0.8Ti0.2)4C3Tx62 have been successfully produced by using mixture solutions of acids and salts containing fluorine ions. However, in the etching process these mixture solutions still release a few harmful HF gases.
Interestingly, Hliam et al. find that the weakly acidic and environmentally friendly fluoride-containing ammonium hydroxide (NH4HF2) can synthesize MXense but does not produce harmful gases.63Fig. 2c displays the scanning transmission electron microscopy (STEM) image and the energy dispersive X-ray spectroscopy (EDX) maps of the synthesized Ti3C2Tx. The EDX maps show the distribution of C, Ti, F, and O atoms over the STEM image, confirming the existence of terminal hydroxyl and fluoride groups on the surface of Ti3C2Tx. Moreover, it is observed that the c lattice constant of Ti3C2Tx etched by NH4HF is 25% larger than that of Ti3C2Tx etched by HF. Subsequently, Feng et al. further find that the presence of surface functional groups makes the Ti3C2Tx surface negative, hence the cations (NH4+) are attracted by the negative surface and anchored onto the surface, and the c lattice parameter of Ti3C2Tx is enlarged accordingly.64
It is noteworthy that in the etching process, a complete conversion of the MAX phase to MXene is required, which depends on a careful adjustment of the etching conditions. Usually, the etching conditions should be changed with the M atom and the n in the chemical formula Mn+1AXn. When the atomic number of M increases, the M–A bond energies increase and thus both the fluorine ion concentration in the etchant solution and the etching time should be increased. A larger n vaule also requires a longer etching time and a larger fluorine ion concentration. Furthermore, if the etchant becomes less acidic, a higher temperature is needed. Therefore, not only the acid solutions but the salts containing fluorine ions are also able to serve as etchants for making MXenes, although the latter should be associated with a much higher etching temperature. For example, by immersing NH4F and Ti3AlC2 powders in deionized water, fully stirring, and heating isothermally at a high temperature (150 °C) for 24 h, Wang et al. have synthesized Ti3C2Tx.65 In addition, Urbankowski et al. successfully produce the first nitride-based MXene (Ti4N3Tx) by heating a mixture of Ti4AlN3 powders and molten fluoride salts at 550 °C in an argon atmosphere.66 The synthesis of Ti4N3Tx provides a good example for the fabrication of other 2D transition metal nitrides.
Besides MAX phases, Zhou et al. discover that a new family of layered ternary and quaternary compounds with the formula of MnAl3C2 or Mn[Al(Si)]4C3 can also be used as precursors to synthesize MXenes. The structure of MnAl3C2 or Mn[Al(Si)]4C3 can be described as 2D Mn+1Cn layers “glued” together with (AlC)x or [Al(Si)C]x untis.67 Using this new family of compounds, they have successfully produced Zr3C2Tx and Hf3C2Tx. Specifically, Zr3C2Tx is obtained by removing the (AlC)x units from Zr3Al3C5 in 50% concentrated HF solution.68 As disclosed by the XRD patterns (Fig. 3a), after Zr3Al3C5 is treated with HF, the intensities of the peaks originating from Zr3Al3C5 substantially decrease and the position of the highest peak changes from (103) to (111). Moreover, the scanning electron microscopy (SEM) image (Fig. 3b) of Zr3Al3C5 after the HF treatment shows the exfoliation of individual Al–C layers along the basal plane, which also verifies the formation of Zr3C2Tx. The chemical reactions occurring during the etching process of Zr3Al3C5 can be reasonably described by the following simplified equation:
| Zr3Al3C5 + HF = AlF3 + CH4 + Zr3C2 | (1) |
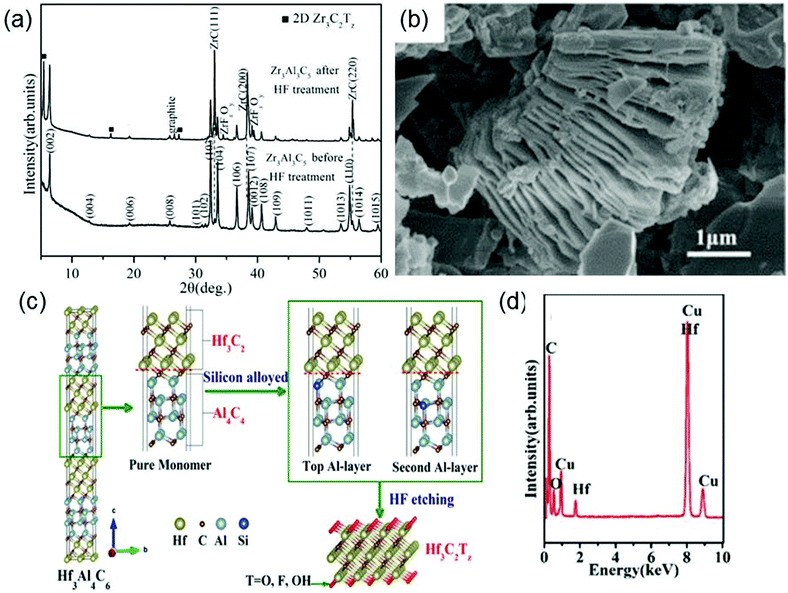 | ||
| Fig. 3 (a) XRD patterns of Zr3Al3C5 before and after HF etching. (b) SEM images of the Zr3Al3C5 powders after HF treatment. Reprinted with permission from ref. 68 Copyright (2016) Wiley Online Library. (c) Schematic of the synthesis process of the Hf3C2Tx. (d) EDS results of the HF-etched Hf3[Al(Si)]4C6 powders. Reprinted with permission from ref. 69 Copyright (2017) American Chemical Society. | ||
However, different from Zr3C2Tx, Hf3C2Tx cannot be synthesized by removing (AlC)x units from Hf3Al3C5 by HF etching, because the interfacial bonds between Hf–C and Al–C units are very strong in Hf3Al3C5. Fortunately, by doping Si atom in Hf3Al4C6, the interfacial bond strength between Hf–C and Al–C units is effectively reduced, enabling the synthesis of Hf3C2Tx from Hf3[Al(Si)]4C6 by removing [Al(Si)]4C4 units using HF (Fig. 3c).69 The energy dispersive spectrometer (EDS) analysis of the HF-treated Hf3[Al(Si)]4C6 powders is shown in Fig. 3d, where not only the Hf and C signals but also the O signal appears, proving the presence of surface functional groups.
Another non-MAX phase, Mo2Ga2C, has also been reported to be able to serve as the precursor to synthesize Mo2CTx.58,70 The structure of Mo2Ga2C is composed of stacks of Mo2C layers interleaved with the Ga bilayers. At present, both HF70 and LiF + HCl58 can be used to synthesize Mo2CTx by etching the Ga bilayers from Mo2Ga2C. This also suggests that the scope of MXene precursors could be further expanded to Ga-containing carbides and nitrides.
In general, the synthesized MXenes are usually multi-layered and need further processing to obtain a single-layer MXene.71 Currently, there are two methods (mechanical delamination and delamination by intercalation) for delaminating multi-layered MXenes.56 By the first method, the yield of monolayer MXenes is very low, because multi-layered MXenes have strong interlayer interactions. So far, only two research groups have successfully achieved the delamination of multi-layered Ti3C2Tx by the mechanical layering method.72,73 Most single-layer MXene flakes are synthesized via intercalation, since multi-layered MXenes can accommodate various ions and molecules between their layers.74 The interlayer space of multi-layered MXenes is increased by intercalation, therefore, the intercalated MXenes can be converted into individual sheets by sonication in deaerated water.71
The intercalants can be divided into organic compounds and ionic compounds. Usually, the delamination of HF-synthesized MXenes needs organic compounds as intercalants. Organic compounds mainly include polar organic molecules, such as hydrazine, urea, isopropylamine, tetrabutylammonium hydroxide (TBAOH) and dimethyl sulfoxide (DMSO). To date, TBAOH has been successfully applied to the delamination of Ti3CNTx,75 Mo2CTx,58 Ti4N3Tx66 and V2CTx,75 and DMSO and isopropylamine have been used to delaminate Ti3C2Tx71,76 and Nb2CTx,29 respectively.
For multi-layered MXenes synthesized by the LiF + HCl or NH4F etchant, Li+ or NH4+ ions can insert between the MXene layers.77 Hence, these MXenes have longer c lattice-parameters than HF-produced MXenes, and the corresponding monolayer MXenes can be directly obtained by sonication76 without additional intercalation (see Fig. 1b). What's more, monolayer MXenes produced by this method have fewer defects compared to those obtained by other methods.58
3 Synthesis of MXene-based composites
In recent years, fabricating composites is an attractive strategy to develop stable and versatile materials. Due to their 2D morphology, layered structures and good flexibility, MXenes are considered as outstanding candidates for the synthesis of multifunctional composites, which has spurred a surge in the study of MXene-based composites. So far, many novel composites have been synthesized by combining MXenes with different materials including polymers, metal oxides and carbon nanotubes.3.1 MXene–polymer composites
When forming composites with polymers, MXenes with excellent mechanical properties, hydrophilic surfaces, and metallic conductivity can improve the mechanical and thermal properties of polymers. In contrast to multi-layered MXenes, single-layer MXenes have higher accessible surface hydrophilicity and better compatibility with polymers. Thus, MXenes are usually delaminated before combining with polymers.Ling et al. report the synthesis of a single-layered Ti3C2Tx–polyvinyl alcohol (PVA) composite by mixing the colloidal solution of Ti3C2Tx films with a PVA aqueous solution.78 The Ti3C2Tx–PVA composite is a layered structure where PVA is distributed between Ti3C2Tx films (the synthesis schematic is shown in Fig. 4a). It possesses high thermostability because highly hydrophilic PVA can form strong hydrogen bonds with Ti3C2Tx. Compared with isolated Ti3C2Tx and PVA, Ti3C2Tx–PVA has more excellent flexibility and tensile and compressive strengths. In particular, the tensile strength of the Ti3C2Tx–PVA composite with 40 wt% Ti3C2Tx reaches 91 ± 10 MPa, which is about four times larger than that of the Ti3C2Tx film (see Fig. 4b). In addition, Ti3C2Tx endows this composite with high conductivity.
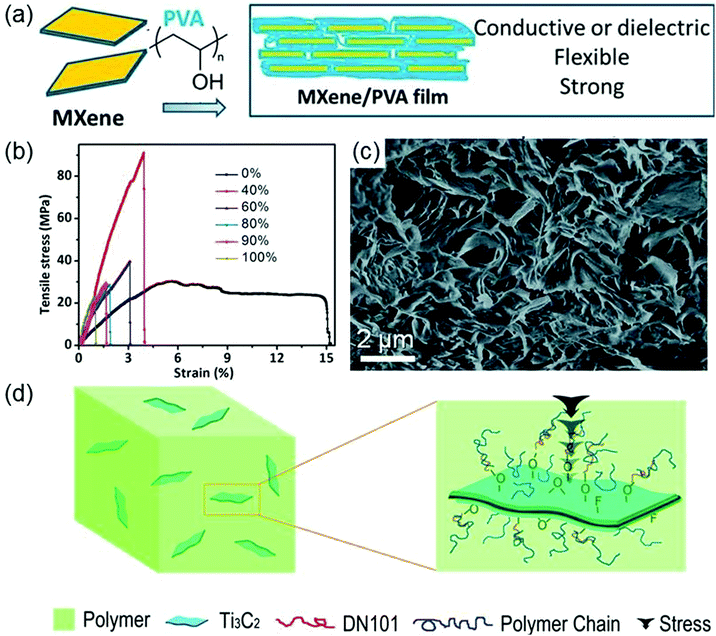 | ||
| Fig. 4 (a) Schematic for the synthesis of the Ti3C2Tx–PVA composite. (b) Stress–strain curves for Ti3C2Tx–PVA films with different Ti3C2Tx weights. Reprinted with permission from ref. 78 Copyright (2014) PNAS. (c) SEM fracture surface morphology images of the 6 wt% Ti3C2Tx–PAM sample. Reprinted with permission from ref. 79 Copyright (2016) Royal Society of Chemistry. (d) Schematic representation of forces on the Ti3C2Tx–UHMWPE composite. Reprinted with permission from ref. 80 Copyright (2015) Elsevier. | ||
Naguib et al. synthesize a Ti3C2Tx–polyacrylamide (PAM) composite by using a two-step method.79 First, the DMSO is introduced in the interlayers of Ti3C2Tx to increase the layer spacing of Ti3C2Tx and to achieve full delamination of individual Ti3C2Tx layers. Then the prepared Ti3C2Tx and PAM solutions are uniformly mixed and dried at ambient temperature for 4–5 days. Different Ti3C2Tx–PAM composites are prepared by varying the mass ratio of Ti3C2Tx to PAM. When Ti3C2Tx accounts for 6 wt%, the composite has the best mechanical properties and the highest electrical conductivity of 3.3 × 10−2 S m−1. As shown in the SEM fracture surface morphology image of the 6 wt% Ti3C2Tx–PAM sample (Fig. 4c), there are a large number of dimples, indicating that the fracture surface is a typical ductile fracture and this sample has good toughness.
UHMWPE (ultrahigh molecular weight polyethylene) is also reported to be able to form composites with Ti3C2Tx. Before the Ti3C2Tx–UHMWPE composite is fabricated, the surface-modified Ti3C2Tx powders need to be prepared first, and the modified surface can improve the compatibility and dispersion stability of Ti3C2Tx in UHMWPE.80 The mixtures of surface-modified Ti3C2Tx and UHMWPE are molded on a press vulcanizer, heated at a rate of 10 °C min−1 to 220 °C and then held for 30 min under 10 MPa. The formed Ti3C2Tx–UHMWPE composites with different weights of Ti3C2Tx have a higher strength than UHMWPE. Among them, the 10 wt% Ti3C2Tx–UHMWPE has the highest yield strength, ultimate tensile strength and elongation.80 The mechanism for the enhancement of mechanical properties of UHMWPE by Ti3C2Tx is displayed in Fig. 4d. As the strain is applied to the Ti3C2Tx–UHMWPE composite, the stress on UHMWPE can be transferred to Ti3C2Tx along the polymer chains, and the flexible Ti3C2Tx can bear more stress than UHMWPE and effectively prevent the generation and development of cracks, accounting for the outstanding mechanical performances of this composite.
In addition to the polymers mentioned above, poly(acrylic acid),81 polyvinylpyrrolidone (PVP),82 poly(ethylene oxide) (PEO)81 and alginate/PEO81 can also form composites with MXenes. Generally, the MXene–polymer composites have better mechanical performances than the isolated MXenes and polymers. Moreover, many of them show good electric conductivity and thus are promising for applications in wearable electronic devices.
3.2 MXene–oxide composites
Owing to their high electrical conductivity and good electrochemical stability, MXene–oxide composites are potential electrode materials in supercapacitors and sodium- or lithium-ion batteries (LIB).16 Zhu et al. synthesize TiO2–Ti3C2Tx nanocomposites in a hydrothermal environment and use them as electrodes for electrochemical supercapacitors (Fig. 5a). The synthesis process is as follows: first, tetrabutyl titanate (TBOT) reacts with Ti3C2Tx to form the precipitates of TiO2 particles that adhere to the surface of Ti3C2Tx; then the TiO2–Ti3C2Tx nanocomposite forms by heating the sample at 500 °C for 4 hours.83 As expected, the synthesized TiO2–Ti3C2Tx electrode exhibits a high specific capacitance and excellent cycling stability. The high electrochemical performance of the TiO2–Ti3C2Tx electrode is due to the introduction of TiO2. On the one hand, TiO2 particles spreading in the interlayers of Ti3C2Tx can increase the distances between Ti3C2Tx nanosheets to facilitate the insertion of cations; on the other hand, the presence of TiO2 provides extra paths for the diffusion of electrolyte ions.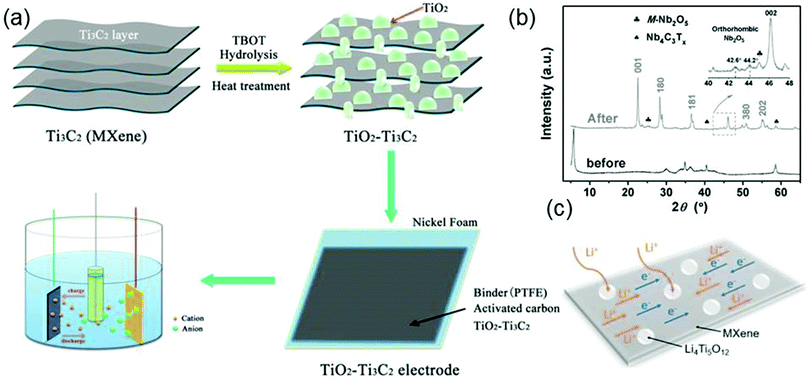 | ||
| Fig. 5 (a) Procedures of hydrothermal synthesis of the TiO2–Ti3C2Tx nanocomposite. Reproduced with permission from ref. 83 Copyright (2016) ECS. (b) XRD patterns of Nb4C3Tx before (black curves) and after (gray curves) oxidation. Reprinted with permission from ref. 84 Copyright (2016) Wiley Online Library. (c) Schematic for the ion and electron transfer in the Li4Ti5O12–Ti3C2Tx electrode. Reprinted with permission from ref. 85 Copyright (2017) Elsevier. | ||
Zhang et al. report a layered orthorhombic Nb2O5–Nb4C3Tx hierarchical composite which can be facilely synthesized by a one-step oxidation of Nb4C3Tx powders in flowing CO2 at 850 °C for 0.5 h.84 A comparison of the XRD patterns of Nb4C3Tx before and after oxidation confirms that Nb2O5 is generated on the Nb4C3Tx surfaces after oxidation (see Fig. 5b). In the Nb2O5–Nb4C3Tx composite, Nb2O5 is uniformly distributed at the edges and in the interlayers of the Nb4C3Tx sheets, effectively shortening the ion diffusion paths. Meanwhile, the internal unoxidized Nb4C3Tx promotes the overall electronic conductivity of the composite. The above two factors are responsible for the high electrochemical and cycling performances of the Nb2O5–Nb4C3Tx composite.
Wang et al. propose a simple strategy to obtain a Li4Ti5O12–Ti3C2Tx composite. The corresponding synthetic process is composed of two procedures. First, by mixing Ti3C2Tx, various hydrogen peroxides and LiOH·H2O together in deionized (DI) water, the lithium ions can enter into the interlamination of Ti3C2Tx to enlarge its interlayer spacing. Then the obtained products are washed using DI water several times and dried at 40 °C for 24 h, followed by calcination at 550 °C for 4 h under N2 to obtain the final products.85 The synthesized Li4Ti5O12–Ti3C2Tx composite also has excellent electrochemical properties, being considered as a promising anode material for lithium ion batteries (LIBs). Fig. 5c displays the schematic of the transfer of electrons and ions in the layered-stacked Li4Ti5O12–Ti3C2Tx composite. When the Li4Ti5O12–Ti3C2Tx electrode is soaked in the electrolyte, Li4Ti5O12 with low lithium ion diffusion barriers grows on the surface of the MXene, resulting in the substantial shortening of the migration paths of lithium ions. Moreover, the high electrical conductivity of Ti3C2Tx ensures the rapid electron transfer from the electrolyte to electrode. Consequently, the Li4Ti5O12–Ti3C2Tx electrode has fast electronic and ionic diffusion, leading to the improvement of the lithium-storage performance.
Huang et al. introduce metal oxide nanobelts into MXene to synthesize a sandwich-structured Ti3C2Tx–Na0.23TiO2 nanocomposite which is made of interwoven amorphous Na0.23TiO2 nanobelts growing on layered Ti3C2Tx nanosheets (Fig. 6a), via a hot aqueous method.86 This nanocomposite as an LIB anode not only exhibits an impressive cycling performance but also shows a remarkable rate capability, as shown in Fig. 6b and d. The cycling stability of Na0.23TiO2–Ti3C2Tx is evaluated by performing a long cycling test on the Na0.23TiO2–Ti3C2Tx electrode at 5 A g−1 (see Fig. 6b). The discharge capacity exhibits an upward trend with an increase in the number of cycles and is finally stabilized at a high value after 4000 cycles. On the other hand, as shown in Fig. 6d, as the current density varies from 0.1 to 5 A g−1, the reversible capacity is quite high all along, and it can still reach a large value of 288 mA h g−1 even when the current density is suddenly returned to 0.1 A g−1 from 5 A g−1. This directly verifies the high rate capability of Na0.23TiO2–Ti3C2Tx. The above high cycling stability and rate capability of Na0.23TiO2–Ti3C2Tx can be attributed to its sandwich-like structure that reduces the Li+/Na+ transfer paths and effectively releases the strain of the electrode upon cycling (see Fig. 6c).
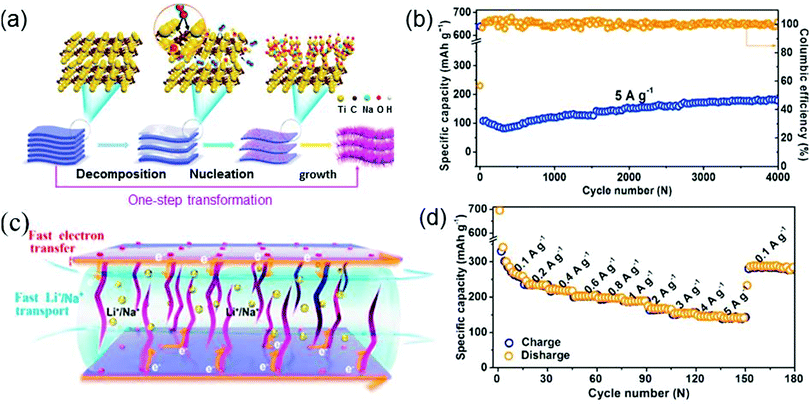 | ||
| Fig. 6 (a) Schematic diagram for the synthesis of Na0.23TiO2–Ti3C2Tx composites by an in situ transformation reaction. (b) Cycling performances of Na0.23TiO2–Ti3C2Tx composites as LIB anodes. (c) Energy-storage mechanism of Na0.23TiO2–Ti3C2Tx in the discharge process. (d) Rate capability of Na0.23TiO2–Ti3C2Tx. Reprinted with permission from ref. 86 Copyright (2018) Elsevier. | ||
So far, the metal oxides which have been reported to form composites with MXenes include TiO2,83 Nb2O5,84 Li4Ti5O12,85 Na0.23TiO2,86 SnO2,87 Co3O4,88 NiCo2O4,89 Sb2O3,89 Fe3O4,90 and Cu2O.91 It is clearly seen that these oxides are basically transition metal oxides (TMOs). The formed TMO–MXene composites working as electrodes of sodium- or lithium-ion batteries and supercapacitors all exhibit good performances. Among them, a spray-coated Ti3C2Tx–NiCo2O4 hybrid film electrode has the best reversible capacity (1330 mA h g−1 at 0.1C) and cycling stability (1330 mA h g−1 at 1C after 100 cycles).88
3.3 MXene–carbon nanotube (CNT) composites.
Zhao et al. fabricate Ti3C2Tx–CNT composites and employ them in electrochemical capacitors. Fig. 7a shows the schematic for the preparation of the Ti3C2Tx–CNT composite, where Ti3C2Tx layers and CNT layers are alternately deposited on top of each other until the total layer number reaches 6–10.92 In the microscopic morphology of the synthesized Ti3C2Tx–CNT composite (Fig. 7b), one can clearly see the sandwich-like superposition of the MXene and CNT layers. When acting as the electrode of the supercapacitor, the Ti3C2Tx–CNT exhibits significantly higher volumetric capacitance and rate performance than isolated MXene. As shown in Fig. 7c, at 10 A g−1, the volumetric capacitance of the Ti3C2Tx–CNT electrode increases from 340 to 370 F cm−3 after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles, which is about 55% higher than that of the Ti3C2Tx electrode. The mechanism responsible for the excellent electrochemical performance of the Ti3C2Tx–CNT composite is that the incorporation of CNT into Ti3C2Tx enlarges the interlayer space between the Ti3C2Tx flakes for cation intercalations and increases the diffusion paths of electrolyte ions.
000 cycles, which is about 55% higher than that of the Ti3C2Tx electrode. The mechanism responsible for the excellent electrochemical performance of the Ti3C2Tx–CNT composite is that the incorporation of CNT into Ti3C2Tx enlarges the interlayer space between the Ti3C2Tx flakes for cation intercalations and increases the diffusion paths of electrolyte ions.
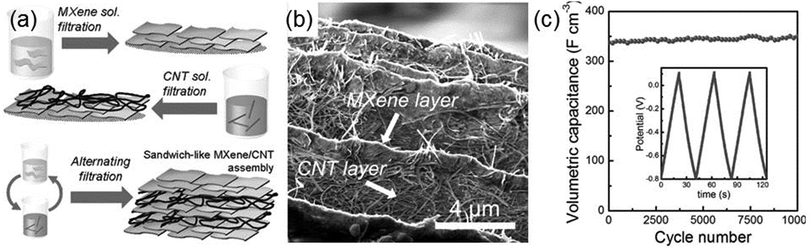 | ||
| Fig. 7 (a) Preparation of the sandwich-like Ti3C2Tx–CNT composite. (b) Cross-sectional SEM images of Ti3C2Tx–CNT papers. (c) Cycling stability of the Ti3C2Tx–CNT electrode at 10 A g−1. Reprinted with permission from ref. 92 Copyright (2015) Wiley Online Library. | ||
Later, the Ti3C2Tx–CNT composites are further used in sodium- or lithium-ion batteries.28,32 Moreover, a Ti3C2Tx–CNT based strain sensor has also been developed, exhibiting outstanding performances including extraordinary sensitivity, high stretchability and a tunable sensing range.93
4 Properties
4.1 Mechanical properties
MXenes’ mechanical properties strongly depend on their surface terminations. It is predicted that the O terminated MXenes have very high stiffness, but MXenes terminated by other groups (F and OH) show lower elastic stiffness than their O-terminated counterparts.94 This may be related to the different lattice constants of MXenes with different terminations: usually, the O-terminated MXenes have smaller lattice parameters than the F or OH terminated MXenes.95 Compared with bare MXenes, the surface-functionalized MXenes have larger flexibility. Using Ti2C as an example, Guo et al. find that the functionalization would reduce the Young's modulus of Ti2C, but the functionalized Ti2C can withstand a larger strain than bare Ti2C and even graphene.96 The surface terminated groups act as a buffer layer for Ti2C under tensile deformation, slowing down the collapse of Ti layers and increasing the value of critical strain where Ti2C fractures.The number of atomic layers in MXene, determined by n in its chemical formula Mn+1Xn, also influences the mechanical properties of MXene. Yorulmaz et al. study the elastic constant and Young's moduli of bare MXenes by classical molecular dynamics. They find that among all Tin+1Cn (n = 1, 2, 3), the thinnest Ti2C has the highest Young's modulus and the elastic constant of Ti2C is almost twice higher than that of MoS2.97 For the functionalized MXene, Borysiuk et al. show that with the decrease of n, the strength and hardness of Mn+1XnTx gradually increase.98
Experimentally, it is found that the mechanical properties of MXenes are enhanced by forming composites with polymers or carbon nanotubes. By combining different types of polymers, the flexibility, toughness, and tensile and compressive strengths of MXenes can be enhanced to different degrees. For example, the Ti3C2Tx–polyvinyl alcohol (PVA) composite has excellent flexibility and large tensile and compressive strengths. In particular, the tensile strength of Ti3C2Tx–PVA composites is about four times larger than that of isolated Ti3C2Tx.79 Moreover, Ti3C2Tx–PAM80 and Ti3C2Tx–UHMWPE81 are also reported to show high toughness and yield strength.
4.2 Electronic properties
MXenes cover various electronic properties ranging from metallicity and semiconductivity to topological insulativity, due to their high compositional diversity, various surface functionalization possibilities, and flexible thickness controllability.99,100 Among them, all the bare MXenes and the majority of surface-functionalized MXenes are metallic. Interestingly, the calculated work functions (WFs) of metallic MXenes can vary in a very wide range, from 1.8 eV to 8 eV, as shown in Fig. 8a.100 It is seen that the WFs of MXenes are sensitive to their surface chemistry: for a given MXene, compared with the bare surface, the OH (O) decoration always decreases (increases) its WF, whereas the F decoration displays either trend relying on the specific material. It is worth mentioning that all the OH-terminated MXenes show ultralow WFs (<2.8 eV), lower than that of Sc which is nearly the lowest among those of elemental metals. On the other hand, the WFs of some O-terminated MXenes are even larger than that of Pt which has the highest WF of all elemental metals. The WFs of F-terminated MXenes always fall between those of OH- and O-terminated counterparts. The change of WF after surface functionalization comes from the change of surface dipole moment induced by functionalization.100 The OH (O) termination always leads to a negative (positive) surface dipole moment and thus decreases (increases) WF, while F can introduce either a negative or positive surface dipole moment depending on the specific MXene material. However, it is noted that in current experiments, the mix of F, O and OH groups on the MXene surfaces usually brings the WFs of MXenes back into an intermediate value. For example, by performing Kelvin probe force microscopy analysis, Xu et al. determine the work function of Ti2C(OH)xFy, which is estimated to be ≈4.98 eV.101 Therefore, to achieve the ultralow (or ultrahigh) work function of OH (or O)-terminated MXenes, a fine control of the types of the surface functional groups is indispensable.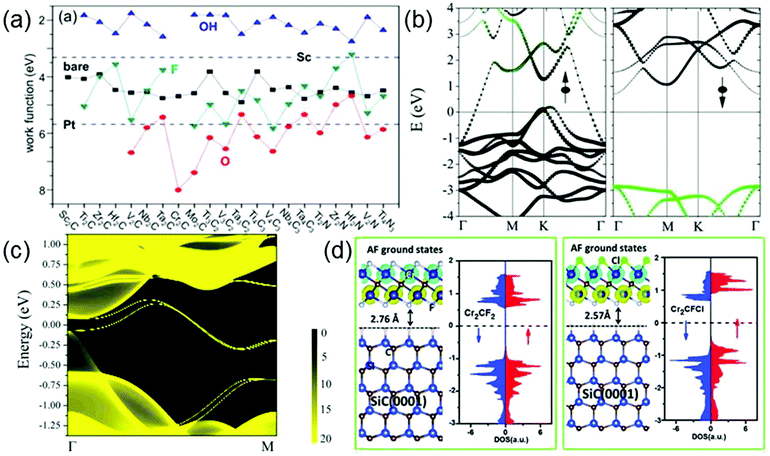 | ||
| Fig. 8 (a) Work functions of MXenes with various terminations. Black squares, red circles, blue up-triangles, and cyan down-triangles are for bare, O-terminated, OH-terminated, and F-terminated MXenes, respectively. For comparison, work functions of Sc and Pt are also shown by dashed lines. Reprinted with permission from ref. 100 Copyright (2016) American Chemical Society. (b) Band structure of Cr2C. Reprinted with permission from ref. 126 Copyright (2015) American Chemical Society. (c) Local density of states for Mo2HfC2O2 on the zigzag edge. The edge states connecting the bulk valence and conduction bands form a single Dirac cone at the M point. Reprinted with permission from ref. 122 Copyright (2016) Royal Society of Chemistry. (d) Density of states of Cr2CF2 (left panel) and Cr2CFCl (right panel) supported on the SiC(0001) substrate. Reprinted with permission from ref. 130 Copyright (2016) Royal Society of Chemistry. | ||
Metallic MXenes with high/low WFs show great potential as Schottky-barrier (SB)-free metal contacts to 2D semiconductors. When MXene forms a van der Waals (vdW) heterostructure with a given 2D semiconductor, the weak interfacial vdW interaction ensures weak Fermi level pinning, which together with the high (low) work function of MXene can enable the SB-free hole (electron) injection into the 2D semiconductor. Based on first-principles calculations, it has been predicted that the OH-terminated MXenes with ultralow WFs can form n-type Schottky barrier-free contacts with common 2D semiconductors such as transition metal dichalcogenides100,102 and blue phosphorene.103 Among all known 2D semiconductors, MoS2 has attracted the most attention, holding tremendous promise for substituting silicon in future nanoelectronics.104 However, because the ionization energy of MoS2 is as high as ∼6.0 eV, it is still a great challenge to make a SB-free p-type contact to MoS2. Experimentally MoS2 usually displays n-type behavior when in contact with metals.105 It is worth mentioning that in the whole MXene family, You et al. find six materials (V2CO2, Cr2CO2, Mo2CO2, V4C3O2, Cr2NO2, and V2NO2) that can be used as metal contacts to MoS2 with disappearing p-type Schottky barriers at MoS2–MXene interfaces, leading to highly efficient hole injections into MoS2.106 This will facilitate the realization of high-performance MoS2 field-effect transistors with both polarities.
Moreover, the metallicity endows many MXenes with high electronic conductivity, making them promising in many applications, particularly, as electrode materials in batteries and supercapacitors. The conductivity of MXenes ranges from smaller than 1 S cm−1 to thousands of S cm−1, which depends strongly on their synthesis and delamination methods. For instance, the HF-etched Ti3C2Tx possesses a low conductivity of 2 S cm−1,107 but the conductivity of LiF + HCl-etched Ti3C2Tx can reach up to 1500 S cm−1.108–110 Such a large difference is possibly because the former has more defects and higher F-terminations. If the LiF + HCl-etched Ti3C2Tx is delaminated, its conductivity will be further improved.111 In practical application, MXenes are usually combined with other promising electrode materials (e.g. TMOs) to form composites which exhibit extraordinary electrochemical characteristics in lithium and sodium ion batteries and lithium–sulfur batteries. The extraordinary electrochemical performances of MXene–TMO composites are attributed to the synergistic effects of MXenes and TMO: a highly conductive MXene facilitates fast electron transport between composite materials and electrolytes; meanwhile, TMOs have rich redox reactions involving different ions that lead to a significantly higher capacity/capacitance of MXene–TMO composites than that of carbon-based electrode materials. In dozens of TMO-composites that have been synthesized, four materials which involve three distinct lithium storage mechanisms are worth mentioning. Among them, TiO2–Ti3C2Tx is based on an intercalation/deintercalation mechanism112 and SnO2–Ti3C2Tx relies on an alloying/dealloying mechanism,112 while Fe3O4–Ti3C2Tx and Co3O4–Ti3C2Tx both utilize a unique “conversion reaction” mechanism113–115 in which the oxidation state of high-valence Co/Fe can effectively store Li-ions.
Some MXenes with specific surface functionalizations, such as Sc2CO2,116 Sc2CF2,116 Sc2C(OH)2,116 Ti2CO2,117 Zr2CO2,118 Hf2CO2,118 Cr2CF2119 and Cr2C(OH)2,119 are semiconductors, and a handful of MXenes, including W2CO2,120 M′2M′′C2O2 (M′ = Mo, W and M′′ = Ti, Zr, Hf)121–123 and Ti3N2F2124 are even predicted to be 2D topological insulators (TIs). 2D TIs are a new class of materials with an insulating gap in the bulk and gapless states at the edge. Remarkably, the scatterings of the edge states are completely forbidden, leading to a dissipationless charge or spin transport. Fig. 8c shows the local density of states of Mo2HfC2O2 on its zigzag edge, where one can clearly see that a pair of topological edge states connects the bulk conduction and valence bands and forms a single Dirac cone at the M point.122 However, so far neither semiconducting nor topological insulating states in MXenes are realized experimentally. This is because by the present synthesis procedures, the obtained MXenes all have mixed terminations; however, the predicted superconducting and topological states basically require a single type of termination on the MXene surfaces. A control over surface chemistry of MXenes would be a key to obtaining semiconducting or topological insulating MXenes.
4.3 Magnetic properties
Although many 2D materials have been found, the majority of them are nonmagnetic, which limits their applications in spintronics. Therefore, the pursuit of controlled magnetism in 2D material has been a long-sought goal. Interestingly, some pristine MXenes, such as Ti2C, Ti2N, Cr2C, Cr2N and Mn2C, are predicted to be intrinsic magnetic materials. Among them, Ti2C,127 Ti2N125 and Cr2C126 are ferromagnetic, while Cr2N127 and Mn2C128 are antiferromagnetic. In particular, recently increasing attention has been paid to magnetic MXenes with half-metallicity. In half-metals, one spin-channel is metallic, while another spin channel is semiconducting, resulting in completely spin-polarized electrons at the Fermi level. Cr2C is the first predicted half metal in the MXene family.126 Its band structure is shown Fig. 8b, where one can obviously observe a metallic spin-up channel and an insulating spin-down channel. Moreover, its half-metallic gap, the difference between the Fermi level and the maximum of the occupied spin-down band, is as large as about 2.9 eV, indicating that the 100% spin-filter efficiency can be preserved in a broad bias range. Subsequently, the half metallicity has also been predicted in Ti2C and Ti2N.125The magnetic and electronic properties of bare MXenes can be easily modulated by surface functionalization. Because the magnetisms of bare MXenes usually come from the unpaired electrons of the surface transition metal atoms, upon surface passivation, the magnetisms of most MXenes will be suppressed. It is remarkable that Cr- and Mn-containing MXenes are exceptions whose magnetic moments don’t disappear after surface functionalization. However, their magnetic couplings and electronic structures would be affected by functional groups. For example, when Cr2C is functionalized by F, H, OH or Cl groups, it will be transformed from a ferromagnetic half metal into an antiferromagnetic semiconductor with a sizeable band gap.115 In contrast to Cr2C, after surface passivation by O,127 Cr2N changes from an antiferromagnetic metal to a ferromagnetic half metal. Given that Cr2NO2 displays half-metallicity in its surface-passivated form, its half-metallicity may be achieved relatively easily from the experimental point of view. For Mn-based MXenes, unterminated Mn2C is an antiferromagnetic metal, whereas Mn2CCl2 is an antiferromagnetic insulator and Mn2CF2 even becomes a ferromagnetic half metal with a high Curie temperature, a large half metallic gap and a sizeable magnetic anisotropy.129
Besides the symmetrical surface terminations mentioned above, the influences of the asymmetrical functionalization on MXenes have also been extensively investigated. It is predicted that Cr2CXX′ (X, X′ = H, F, Cl, Br, OH) including Cr2CFCl, Cr2CClBr, Cr2CHCl, Cr2CHF, and Cr2CFOH (corresponding to Cr2C with top and bottom surfaces asymmetrically terminated by different functional groups) are all antiferromagnetic semiconductors with Néel temperatures of about 400 K.130 Apparently, asymmetrical functionalization induces the same ferromagnetic–antiferromagnetic transition in Cr2C as the symmetrical functionalization. However, it will introduce some additional changes on the electronic structure of Cr2C, compared with the symmetrical functionalization. Fig. 8d shows the density of states (DOS) of Cr2CF2 (left panel) and Cr2CFCl (right panel). Different from the DOS of Cr2CF2 with symmetrical distribution, Cr2CFCl presents the feature of bipolar magnetic semiconductors, i.e., the VBM and CBM possess opposite spin polarizations. Similar bipolar magnetic properties are also found in other Cr2CXX′, making them good candidates for spintronic applications.130
5 Environment-related applications
5.1 Catalysis
As the global air pollution is constantly increasing and the energy crises continue to be aggravated,131 efficient clean energy conversion technologies such as electrochemical water splitting,132 photocatalytic water splitting and photocatalytic reduction of CO2 are becoming of great significance. High-performance catalysts are crucial for the successful implementation of these technologies. Given that the applications of traditional catalysts including precious metals and precious-metal-based oxides (Pt,133 RuO2134 and IrO2135) are limited by their scarcity and high cost, developing efficient catalysts which not only have high performances but also are cost-effective is indispensable for clean energy conversion technologies. As a class of novel materials with high electronic conductivity, good surface hydrophilicity and abundant exposed metal sites, MXenes and their composites have been used in electrochemical water splitting as highly active electrode materials (electrocatalysts) as well as in photocatalytic water splitting and photocatalytic reduction of CO2 as catalysts or co-catalysts, exhibiting tremendous possibilities for replacing precious metal materials.The electrocatalytic activity of g-C3N4 is significantly increased by combining g-C3N4 with Ti3C2Tx to form a composite film Ti3C2Tx–g-C3N4 (TCCN) with hydrophilic surfaces and conductive frameworks.148 The highly flexible TCCN film has a size of a few centimeters and a thickness of 8–10 μm (see the inset of Fig. 9a), with plenty of large pores ranging from tens to hundreds of nanometers distributed in the interlayers of the film (Fig. 9a). Ti3C2Tx not only provides a high conductivity but also forms Ti–Nx units with g-C3N4 in the TCCN composite. Fig. 9b shows the polarization curves of TCCN, Ir2O/C (IrO2 supported on carbon powders), Ti3C2Tx and g-C3N4. It is seen that the anodic current recorded on TCCN has a sharp increase at 1.44 V and even exceeds that of IrO2/C at potentials beyond 1.54 V, which indicates that the TCCN has a very high OER response and catalytic activity. Moreover, after 5000 cycles, the polarization curve of TCCN is almost unchanged, revealing the good stability of TCCN.148 Note that in addition to the high conductivity of TCCN, the hierarchical pores and the specific Ti–Nx units in TCCN also make significant contributions to the excellent catalytic performance of TCCN. The hierarchical pores can achieve rapid transport of reactants and reaction products, and the Ti–Nx units act as electroactive sites for reactants.
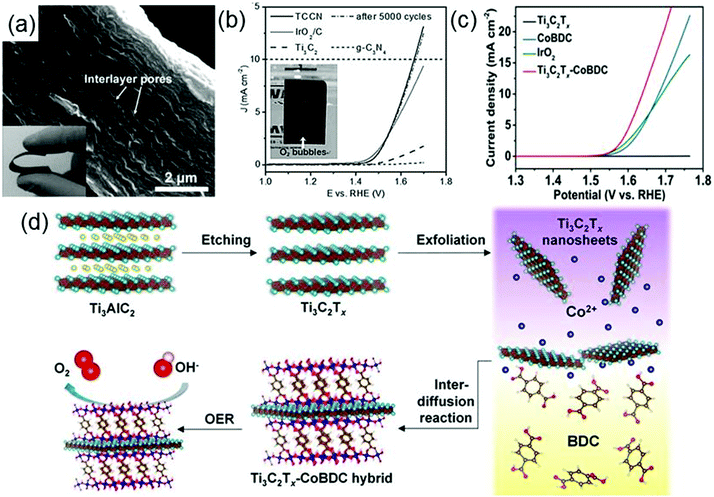 | ||
| Fig. 9 (a) SEM and optical (inset) images of TCCN. (b) Polarization curves of TCCN, IrO2/C, Ti3C2Tx, and g-C3N4. The inset is the optical image of TCCN directly used as the OER electrode with generated bubbles on the surface suggesting the formation of O2. Reprinted with permission from ref. 148 Copyright (2016) Wiley Online Library. (c) OER polarization curves of glassy carbon electrodes modified with Ti3C2Tx, CoBDC, IrO2, and the Ti3C2Tx–CoBDC hybrid. (d) Schematic illustration of the preparation process of the Ti3C2Tx–CoBDC hybrid for the OER. Reprinted with permission from ref. 149 Copyright (2017) American Chemical Society. | ||
Designing metal–organic frameworks, MOFs, which involves the assembly of organic ligands and metal ions or clusters, is a potential approach to develop new excellent electrocatalysts for the OER. Generally, MOFs have porous structures, abundant exposed active metal centers but poor electrical conductivity. Hence, the combination of MOFs with a conductive material is anticipated to improve the electrocatalytic performance of MOFs in the OER. Based on this idea, a novel MXene–MOF composite, Ti3C2Tx–CoBDC (cobalt 1,4-benzenedicarboxylate), is synthesized via an interdiffusion-mediated strategy (see Fig. 9d).149 It exhibits remarkably strong electrocatalytic activity for the OER. As indicated by the linear-sweep voltammetry measurements of different catalysts deposited on glassy carbon (GC) electrodes (Fig. 9c), the current density of Ti3C2Tx–CoBDC can reach 10 mA cm−2 at a potential of 1.64 V (vs. reversible hydrogen electrode, RHE), higher than those of both IrO2 and CoBDC at the same potential. Similarly, a hybrid of a MOF derivative NiCoS and Ti3C2Tx is also reported to have strong electrocatalytic activity for the OER. Strong interactions are formed between NiCoS and Ti3C2Tx, responsible for the high conductivity and efficient electron transfer in the NiCoS–Ti3C2Tx composite.150
Due to the large redox activity, LDHs containing positively charged metal hydroxide layers and charge-compensating anions (e.g., NO3−, CO32−, etc.) hold great promise as alternatives of noble metal electrocatalysts for the OER. However, intrinsically poor conductivity and limited electrochemically active surfaces restrict the application of LDH in the OER. Yu et al. attempt to solve this problem by combining highly conductive MXenes with LDH. They fabricate a FeNi-LDH/Ti3C2Tx composite with a structure of interconnecting porous networks as a new OER electrocatalyst that exhibits large activity for the OER.151 Strong electronic coupling and interfacial interaction between FeNi-LDH and Ti3C2Tx are the main factors guaranteeing the good electrocatalytic performance of this composite. Moreover, the OER catalytic performance of FeNi-LDH/Ti3C2Tx electrocatalysts can be further improved by coating the electrode with nickel foam (NF).
Besides being used as conductive materials to improve the activity of existing catalysts, MXenes have also shown potential as new electrocatalysts for the HER. MoS2 is the first 2D material identified as a promising HER catalyst. However, only the exposed edge sites of MoS2 are catalytically active but its basal plane is inert, thus complex design is required to maximize the edge sites. Seh et al. report that MXenes can also be used as electrocatalysts for the HER based on both theoretical calculations and experiments.152 By calculating the expected overpotential and hydrogen adsorption free energy (ΔGH) of a series of M2XTx (M = Mo, Sc, Ti, Zr, Hf, and V) materials, they predict that Mo2CTx is the most effective electrocatalyst. Moreover, unlike MoS2, Mo2CTx has a large number of catalytic sites on its basal plane. To evaluate the real electrocatalytic performances of MXenes for the HER, a linear-sweep voltammetry measurement is performed by using glassy carbon as the substrate electrode. As shown in Fig. 10a, Mo2CTx requires a much lower overpotential (283 mV) to reach the current density of 10 mA cm−2geo during the initial sweep, compared with Ti2CTx that needs a 609 mV overpotential, indicating that Mo2CTx has a higher electrocatalytic activity than Ti2CTx. On the other hand, the Mo2CTx curve exhibits a smaller change after continuing cycling than the Ti2CTx curve, indicating that Mo2CTx is a rather stable catalyst for the HER.152
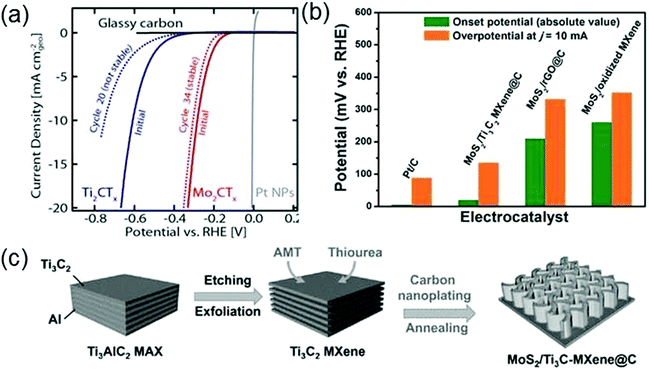 | ||
| Fig. 10 (a) Anodic-going iR-LSVs of Ti2CTx and Mo2CTx (mass loading: 0.1 mg cm−2geo on glassy carbon), compared with that of bare glassy carbon. Reprinted with permission from ref. 152 Copyright (2016) American Chemical Society. (b) Comparison of Pt/C, MoS2–Ti3C2Tx@C, MoS2–rGO@C, and MoS2/oxidized Ti3C2Tx catalysts in terms of onset potential and overpotential at 10 mA cm−2 (c) The synthesis procedures for MoS2–Ti3C2Tx@C nanohybrids. Reprinted with permission from ref. 153 Copyright (2017) Wiley Online Library. | ||
As metal atoms cover a large proportion of the MXene surfaces, MXenes suffer poor oxygen resistance in oxygen-containing electrolytic solution, which dramatically influences the stability of MXenes as electrocatalysts and thus restricts the applications of MXenes. In order to enhance the oxygen resistance, Wu et al. introduce a nanoplating strategy to successfully synthesize hierarchical MoS2–Ti3C2Tx@C (Fig. 10c).153 The carbon cover on the surface of Ti3C2Tx (Ti3C2Tx@C) can effectively suppress the surface oxidation, thus strengthening the stability of Ti3C2Tx. Moreover, the bifunctional activity of MoS2 towards both Li storage and HER increases the possibility of multi-field applications of MoS2–Ti3C2Tx@C. It has been found that this hierarchical structure composed of MoS2 and Ti3C2Tx@C shows an excellent electrocatalytic performance for the HER. Fig. 10b shows that Ti3C2Tx@C only requires a small overpotential of 135 mV to reach the current density of 10 mA cm−2, which is lower than those for MoS2–rGO@C and Ti3C2Tx@C. Such a low value of overpotential verifies the high electrocatalytic activity of MoS2–Ti3C2Tx@C. In addition, after 2000 cycles of cyclic voltammetry, there is no significant shift in the polarization curve of the MoS2–Ti3C2Tx@C electrode, indicating that MoS2–Ti3C2Tx@C also has good stability.
Recently, researchers have further confirmed that MXenes composites (Ni2P–Cr2CTx,34 CoP@3D-Ti3C2Tx154 and Ni0.7Fe0.3PS3–Ti3C2Tx155) can act as bifunctional electrocatalysts toward OER and HER by both theoretical predictions and experimental verifications. Moreover, the synthesized CoP@3D–Ti3C2Tx154 and Ni0.7Fe0.3PS3–Ti3C2Tx155 both have high electrocatalytic activity which even surpasses those of the traditional bifunctional electrocatalysts RuO2 and Pt.
MXenes have been regarded as promising candidate catalysts for photocatalytic hydrogen production reactions. By ab initio calculations, Guo et al. have found two suitable photocatalysts (Zr2CO2 and Hf2CO2) in the MXene family for the photocatalytic hydrogen production reaction.35 The electrical structure features of a material, including the band gap size, the positions of the conduction band minimum (CBM) and the valence band maximum (VBM), are usually used as the descriptors characterizing the feasibility of this material as a photocatalyst. Specifically, for the photocatalytic hydrogen production reactions, the band gap value of the photocatalyst should be between 1.55 and 3.0 eV. Moreover, its CBM should be higher than the hydrogen reduction potential of H+/H2 and its VBM should be lower than the water oxidation potential of H2O/O2. As shown in Fig. 11a, Zr2CO2 and Hf2CO2 meet these criteria. Additionally, a favorable photocatalyst should be able to efficiently utilize the solar energy. In Fig. 11b, one can see that both 2D Zr2CO2 and Hf2CO2 display large optical absorption in the visible light region, guaranteeing the feasibility of Zr2CO2 and Hf2CO2 used as photocatalysts in water splitting reactions for producing hydrogen.35
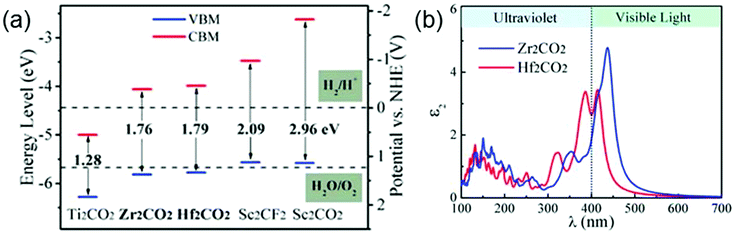 | ||
| Fig. 11 (a) Electronic band edge positions of various MXenes relative to the water redox potential levels. (b) Optical adsorption of Zr2CO2 and Hf2CO2. Reprinted with permission from ref. 35 Copyright (2016) Royal Society of Chemistry. | ||
Besides developing new catalysts, considerable attention has been paid to explore new ways to enhance the catalytic performances of existing photocatalysts. In recent years, co-catalysts have shown great success in boosting both the activity and the stability of photocatalysts. An outstanding co-catalyst should have the following characteristics: (i) plentiful surface functionalities to establish strong connections with the photocatalyst for perennial stability and rapid interfacial charge transfer; (ii) sufficient contacts with water molecules; (iii) a high conductivity to ensure efficient charge transport. Remarkably, MXenes can meet all the above requirements for co-catalysts. First, MXenes possess hydrophilic functional groups (–OH and –O) on their surfaces, enabling them to easily form strong interactions with various semiconductor photocatalysts and water molecules. Second, excellent electrical conductivities of MXenes guarantee efficient electron shuttling. Therefore, there has been increasing interest in exploring MXenes as co-catalysts for photocatalysts.
Ran et al. reveal the potential of Ti3C2Tx as a highly efficient co-catalyst for H2 evolution.156 By rationally integrating Ti3C2Tx nanoparticles with a chosen photocatalyst CdS via a hydrothermal strategy, extremely large visible-light photocatalytic H2 production activity of 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 342 μmol h−1 g−1 is achieved, exceeding that of CdS by an incredible factor of 137. By contrast, isolated Ti3C2Tx nanoparticles display no activity towards photocatalytic hydrogen production, indicating its role as a co-catalyst rather than as a photocatalyst. It is remarkable that Ti3C2Tx-loaded CdS also shows a higher quantum efficiency of 40.1% at 420 nm than other noble-metal free CdS-based photocatalysts reported to date including Ni–CdS, Ni(OH)2–CdS, graphene-oxide–CdS and so on. The high performance of Ti3C2Tx-loaded CdS can be mainly attributed to the following two points. First, the deposition of Ti3C2Tx on CdS enhances charge separation and suppresses charge recombination. Under light irradiation, the photo-excited electrons on the conduction band of CdS can immediately migrate to Ti3C2Tx because the Fermi level of Ti3C2Tx is energetically lower than the conduction band of CdS. Then the electrons can further rapidly move to the surface active sites of Ti3C2Tx, thanks to the excellent conductivity of Ti3C2Tx. Second, the number of O terminations on the Ti3C2Tx surface, as the effective active sites, is greatly increased by the hydrothermal treatment which makes a large number of F terminations exchanged into O terminations. Due to the similar mechanisms, Ti3C2Tx nanoparticles can as also act as an efficient HER co-catalyst on ZnxCd1−xS or ZnS.156
342 μmol h−1 g−1 is achieved, exceeding that of CdS by an incredible factor of 137. By contrast, isolated Ti3C2Tx nanoparticles display no activity towards photocatalytic hydrogen production, indicating its role as a co-catalyst rather than as a photocatalyst. It is remarkable that Ti3C2Tx-loaded CdS also shows a higher quantum efficiency of 40.1% at 420 nm than other noble-metal free CdS-based photocatalysts reported to date including Ni–CdS, Ni(OH)2–CdS, graphene-oxide–CdS and so on. The high performance of Ti3C2Tx-loaded CdS can be mainly attributed to the following two points. First, the deposition of Ti3C2Tx on CdS enhances charge separation and suppresses charge recombination. Under light irradiation, the photo-excited electrons on the conduction band of CdS can immediately migrate to Ti3C2Tx because the Fermi level of Ti3C2Tx is energetically lower than the conduction band of CdS. Then the electrons can further rapidly move to the surface active sites of Ti3C2Tx, thanks to the excellent conductivity of Ti3C2Tx. Second, the number of O terminations on the Ti3C2Tx surface, as the effective active sites, is greatly increased by the hydrothermal treatment which makes a large number of F terminations exchanged into O terminations. Due to the similar mechanisms, Ti3C2Tx nanoparticles can as also act as an efficient HER co-catalyst on ZnxCd1−xS or ZnS.156
Considering the synergistic effects of different co-catalysts, two or more co-catalysts have been combined to explore more efficient co-catalytic systems. For example, using Ti3C2Tx nanoparticles and Pt nanoclusters as dual co-catalysts for photocatalyst g-C3N4,157 the photocatalytic hydrogen production activity of g-C3N4 is increased to 5100 mol h−1g−1, far higher than that of g-C3N4 with Pt or Ti3C2Tx used separately as the co-catalyst. This exceptional activity in the hybrid of g-C3N4, Ti3C2Tx and Pt is ascribed to the fast interfacial charge transfer from g-C3N4 to Ti3C2Tx and to Pt, leading to the effective separation of photo-generated carriers.
The photocatalytic CO2 reduction reaction can produce hydrocarbon solar fuels that are advantageous to the alleviation of energy crisis and air pollution. MXene can also serve as a co-catalyst in photocatalytic CO2 reduction. For instance, the surface alkalinized Ti3C2Tx (Ti3C2Tx–OH) as a co-catalyst has been employed to enhance the photoactivity of titania (P25) in photocatalytic CO2 reduction.36 As the mass ratio of Ti3C2Tx–OH to P25 varies to 5%, the evolution rates of CO and CH4 reach the maximum values, 11.74 and 16.61 μmol g−1 h−1, respectively (see Fig. 12a), which are apparently higher than those of P25, Pt–P25 and Ti3C2Tx–P25, indicating that the photocatalytic activity of P25 has been considerably boosted by using Ti3C2Tx–OH as the co-catalyst. The excellent performance of Ti3C2Tx–OH can be ascribed to the increase of the number of hydroxyl groups on Ti3C2Tx surfaces providing sufficient sites for CO2 adsorption.
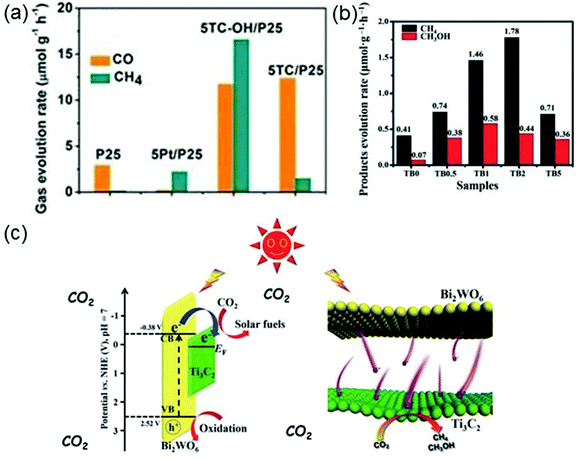 | ||
| Fig. 12 (a) Evolution rates of CO and CH4 over P25, 5Pt/P25 (containing 5 wt% Pt), 5Ti3C2Tx/P25 (containing 5 wt% Ti3C2Tx) and 5TCOH/P25 (containing 5 wt% Ti3C2Tx–OH) under the irradiation of a 300 W Xe lamp. Reprinted with permission from ref. 36 Copyright (2018) Wiley Online Library. (b) Photocatalytic activity (products evolution rates) of Ti3C2Tx–Bi2WO6 (TB) with different weights of Ti3C2Tx (0, 0.5, 1, 2 and 5 wt%). (c) Energy level structure of the TB hybrid (left panel) and the photo-induced electron transfer process at the TB interface (right panel). Reprinted with permission from ref. 158 Copyright (2018) Advanced Science News. | ||
Cao et al. successfully prepare 2D heterojunctions of ultrathin Ti3C2Tx–Bi2WO6 nanosheets (Bi2WO6 is the photocatalyst, and Ti3C2Tx works as the co-catalyst) which are found to have much higher CO2 adsorption capability and more efficient interfacial charge transfer ability than isolated Bi2WO6.158 The excellent performance of Ti3C2Tx–Bi2WO6 (TB) originates from its porous structure, large interface contact area and short-distance charge transport. Fig. 12b shows the evolution rates of products (methane (CH4) and methanol (CH3OH)) for different wt% (0–5 wt%) Ti3C2Tx-modified Bi2WO6 nanosheets used for photocatalytic CO2 reduction. Among all considered samples, the TB2 where Ti3C2Tx accounts for 2 wt% has the highest evolution rate for CH4 and the second highest evolution rate for CH3OH, which are 1.78 μmol g−1 h−1 and 0.44 μmol g−1 h−1, respectively. It is noted that the yield of CH4 and CH3OH over TB2 is 4.6 times that over the isolated Bi2WO6. The photocatalytic mechanism of ultrathin TB is described in Fig. 12c. Under solar irradiation, photo-induced electrons are generated on Bi2WO6, then the electrons move through the ultrathin layered heterojunction to the Ti3C2Tx surface and react with the adsorbed CO2 molecules.
5.2 Water remediation
Water pollution is a severe ecological and environmental issue.159 The contaminants include heavy metal ions, organic substances (dyes), eutrophic substances and nuclear waste. Various methods such as adsorption, biological treatment, chemical oxidation and catalytic degradation have been developed for wastewater treatment. Among those methods, adsorption and catalytic degradation are ideal methods for removing harmful substances from water. Owing to their advantages of easy preparation, large specific surface area and good reducibility, MXenes, as new adsorbents, are expected to play an important role in water remediation.Considering that Ti has a high adsorption capacity for heavy metal ions, Ti-containing substances are considered as promising candidates to remove heavy metal ions from water.160 By chemical exfoliation and alkalization treatment, Peng et al. have prepared hydroxyl-terminated Ti3C2Tx (alk-Ti3C2Tx) which presents efficient lead ion (Pb(II)) uptake performance.37 The purpose of alkalization is to form more hydroxyl groups on the surface of Ti3C2Tx, which will contribute to the adsorption of Pb(II). Significantly, each kilogram (kg) of Ti3C2Tx can treat 4500 kg water containing Pb(II), and the content of remanent Pb(II) in water can successfully reach the drinking water standard recommended by the WHO (10 μg L−1). In the actual situation, there are various kinds of metal ions in wastewater, thus it is necessary to determine whether common metal ions (Ca and Mg cations) in wastewater affect the adsorption selectivity of alk-Ti3C2Tx toward Pb(II). Fig. 13a and b demonstrate that alk-Ti3C2Tx still exhibits a distinctly selective adsorption for Pb(II) in a solution containing Ca(II) or Mg(II). By increasing the ratio of Ca(II)/Pb(II) (or Ca(II)/Mg(II)) in the solution, the selectivity of the alk-Ti3C2Tx to Pb(II) can exceed that of the traditional adsorbent 001×7. It is noticed that the Pb(II) adsorption is a pH-dependent process (Ti3C2Tx exhibits the best Pb(II) sorption performance after the pH value is changed from 5 to 7) and the used Ti3C2Tx can be regenerated by acidic solutions.
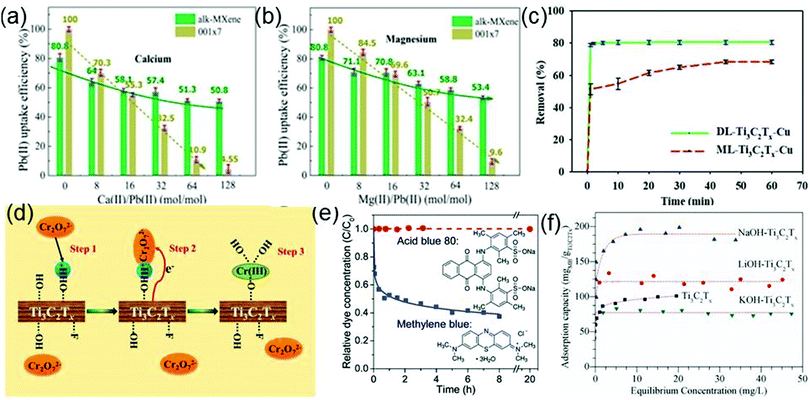 | ||
| Fig. 13 Effects of the coexisting calcium (a) or magnesium (b) ions in water on the selective adsorption of lead ions onto alk-Ti3C2Tx and 001×7. Reprinted with permission from ref. 37 Copyright (2014) American Chemical Society. (c) Comparison of Cu ion removal by DL-Ti3C2Tx and by ML-Ti3C2Tx. Reprinted with permission from ref. 163 Copyright (2017) American Chemical Society. (d) Schematic diagrams of the mechanism responsible for Cr(VI) removal by the Ti3C2Tx nanosheets. Reprinted with permission from ref. 161 Copyright (2015) American Chemical Society. (e) Evolutions of concentrations of MB and AB80 in aqueous solutions containing suspended Ti3C2Tx particles as a function of time in the dark. Reproduced with permission from ref. 38 Copyright (2014) Royal Society of Chemistry. (f) Adsorption isotherms of MB on Ti3C2Tx, LiOH–Ti3C2T and NaOH–Ti3C2Tx and KOH–Ti3C2Tx. Reprinted with permission from ref. 164 Copyright (2017) Elsevier. | ||
As a kind of heavy metal ion that widely exists in industrial wastewater, Cr(VI) is highly toxic to plants, animals, and the human body.161 Hence, the industrial wastewater must undergo Cr(VI) adsorption treatment. Since Ti3C2Tx has been successfully used for the adsorption of heavy metal Pb ions, it is expected that Ti3C2Tx also has the potential for removing Cr(VI) from wastewater. Ying et al. find that the Ti3C2Tx indeed shows an outstanding removal capacity for Cr(VI) as large as 250 mg g−1. After treatment by Ti3C2Tx, the residual Cr(VI) concentration in the water becomes lower than the standard concentration of Cr(VI) stipulated by the World Health Organization in drinking water.162 As shown in Fig. 13d, the removal process of Cr(VI) can be mainly divided into three steps: (1) Cr(VI) is attracted to the surface of Ti3C2Tx with abundant protonated hydroxyl groups and adhered on the surface; (2) the absorbed Cr(VI) is reduced to Cr(III) by Ti3C2Tx; (3) the produced Cr(III) form Ti–O–Cr(III) links with the surface Ti–O bonds via covalent bonding. The Cr(VI) removal is also a pH-dependent process where the performance of Ti3C2Tx towards the Cr(VI) removal is the best at the pH of 5.
Shahzad et al. confirm that Ti3C2Tx nanosheets also have a strong adsorption capacity for Cu ions and delaminated (DL)-Ti3C2Tx exhibits a more excellent adsorption capacity than multilayered-Ti3C2Tx (Fig. 13c).163 The highest adsorption capacity of DL-Ti3C2Tx toward Cu ions is 78.45 mg g−1, which is 1.17 and 2.7 times those of the multilayered-Ti3C2Tx and commonly used activated carbon, respectively. The adsorption mechanism is uncovered by the XRD analysis of DL-Ti3C2Tx. After the adsorption of Cu ions, the XRD pattern shows the emergence of the precipitates Cu2O and CuO, suggesting that Cu ions react with the surface functional groups of DL-Ti3C2Tx, generating Cu2O and CuO. To present the actual adsorption capacity of DL-Ti3C2Tx for Cu ions, it is immersed in the simulated electroplating wastewater containing four kinds of heavy metal ions (Cu2+, Pb2+, Cd2+, and Cr3+). After 5 hours, the removal efficiencies of four metal ions are 98.29%, 90.04%, 52.90%, and 73.64%, respectively, which demonstrates that the Cu2+ adsorption capacity of DL-Ti3C2Tx is not affected by common cations in wastewater. The above result also shows the potential of Ti3C2Tx for treating multiple heavy metal ions in water.
As potential adsorbents, MXenes are also applied to dye sewage treatment. Mashtalir et al. investigate the adsorption capacity of Ti3C2Tx for AB80 (an anionic dye) and MB (a cationic dye).38Fig. 13e shows the time dependence of the removal of AB80 and MB from solution in the dark by Ti3C2Tx. After 20 h, the concentration of AB80 has no change, but the MB concentration displays a dramatic decrease within 8 h. Apparently, Ti3C2Tx preferentially captures the cationic dye, which is closely related to the negatively charged surface of Ti3C2Tx that can bind strongly with MB.
In order to enhance the dye adsorption capacity and the stability of Ti3C2Tx in water, Wei et al. adjust the surface functional groups and the internal structure of Ti3C2Tx by alkalization.164 Experimentally, they use three alkaline solutions (LiOH, NaOH and KOH) respectively to treat Ti3C2Tx and obtain LiOH–Ti3C2Tx, NaOH–Ti3C2Tx and KOH–Ti3C2Tx which have distinctly different adsorption isotherms for MB (Fig. 13f). In particular, the MB adsorption capacity of NaOH–Ti3C2Tx reaches 189 mg g−1, which is substantially higher than those of pristine Ti3C2Tx, LiOH–Ti3C2Tx and KOH–Ti3C2Tx. During the adsorption process, the MB molecules intercalate into alkalized Ti3C2Tx, leading to the increase of the adsorption capacity of Ti3C2Tx. NaOH–Ti3C2Tx has more stacking gaps than LiOH–Ti3C2Tx and KOH–Ti3C2Tx, which provides more positions for the MB intercalation adsorption.
In addition to heavy metal ions and dyes, MXenes and their composites can also treat eutrophic substances in wastewater. The synthesis of a sandwich-like Ti3C2Tx/Fe3O4/Fe2O3 nanocomposite (MXI) makes it possible to use MXenes for water eutrophication (phosphate) remediation. In the MXI, the nano-Fe2O3 is distributed in the interior layers of the MXenes and increases the number of available activated Ti–OH layers, and Fe3O4 inserts into the gaps between the multiple layers of MXene, forming the Fe–OH species that facilitate the preferential adsorption of phosphate and improve the sorption capability.165 In practical application, each kilogram of MXI can handle 2300/2400 kilograms of phosphorus-containing wastewater, indicating that MXI can be used for wastewater treatment on a large scale. Moreover, the used Ti3C2Tx/Fe3O4/Fe2O3 can be regenerated repeatedly by soaking the sample in the mixture solution of alkaline and salt.
With increasing utilization of nuclear energy, nuclear waste is becoming a worldwide environmental problem. To remedy nuclear contamination, the adsorption technique is widely used for the removal and separation of radionuclides. Therefore, the development of effective adsorbents for nuclear waste is very important. MXenes with a large surface area and abundant active sites are considered as ideal adsorbents for nuclear waste. In the MXene family, Ti3C2(OH)2 is firstly studied as a promising adsorbent for the hydrated uranyl cation [UO2(H2O)5]2+ by using density functional theory (DFT).166 Soon afterwards, Wang et al. experimentally report that V2CTx has a strong adsorption performance for uranium ions (U(VI)).167 The adsorption capacity of V2CTx can be up to 174 mg g−1 which is even larger than that of the conventional inorganic nanomaterials. Moreover, they show that V2CTx is the most stable at the pH of 5 and uranium-adsorbed V2CTx can be reused after soaking in acidic solution (pH < 3). These above findings suggest the great promise of MXenes for applications in nuclear waste treatment.
In addition, researchers have also explored the antibacterial properties of MXenes for their potential use as biocides in water treatment.168,169 As a representative material in the MXene family, Ti3C2Tx has been verified by Rassol et al. to have a higher antibacterial efficiency toward both Bacillus subtilis (B. subtilis) and Escherichia coli (E. coli), compared with graphene oxide (GO)168,169 that is widely reported as an antibacterial agent, which opens a door for MXenes’ applications in antimicrobial coatings and water purification membranes. The excellent antibacterial activity of MXenes should be related to their hydrophilicity surfaces which may promote the inactivation of bacteria via direct contact interactions. The high-density defects and the functional groups on MXenes could also strengthen the antibacterial activity. In the bactericidal process, a variety of chemical reactions of MXenes with bacterial membranes are possible, however, they need further investigations.
5.3 Sensor
Due to their large specific surface area, excellent conductive properties, and good biocompatibility, 2D materials have been used in sensors for more than ten years.170–179 Not surprisingly, MXenes have exhibited excellent performances in biosensors and gas sensors.In a biosensor, the active protein usually loses its bioactivity when it directly contacts the electrode surface, hence specific materials are selected to immobilize active proteins and maintain their activity. Notably, MXenes, as protein immobilization matrixes, can not only help to protect active proteins but also facilitate the direct electron transfer between the enzyme and the electrode, yielding a possibility of fabricating mediator-free biosensors.
Ti3C2Tx has firstly been chosen to immobilize hemoglobin (Hb) in the biosensor (Nafion/Hb/Ti3C2Tx/GCE) for detecting nitrites.180 Because of the relatively high conductivity of Ti3C2Tx, the Nafion/Hb/Ti3C2Tx/GCE biosensor shows an excellent biosensor performance with an enlarged detection range and a reduced detection limit. Specifically speaking, the nitrite detection range of the biosensor reaches 0.5–11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 μM and the lowest detection limit is lowered to 0.12 μM.
800 μM and the lowest detection limit is lowered to 0.12 μM.
Recently, it is found that a TiO2-modified Ti3C2Tx nanocomposite synthesized by a hydrothermal process can also be used as the immobilization matrix for hemoglobin in the biosensor (Nafion/Hb/Ti3C2Tx–TiO2/GCE) detecting hydrogen peroxide.40 The microstructure of this nanocomposite is shown in Fig. 14a where one can see that the Ti3C2Tx nano-layers exhibit an organ-like structure with one end closing and the other end opening, and numerous white TiO2 nanoparticles are loaded on the Ti3C2Tx layers. The organ-like structure is beneficial to the enzyme immobilization on the inner surfaces of Ti3C2Tx. Moreover, TiO2 possesses excellent biocompatibility and chemical stability, which can provide a protective microenvironment for the enzymes. Fig. 14b displays the schematic illustration for the TiO2–Ti3C2Tx nanocomposite encapsulating hemoglobin. The enzyme is immobilized on the inner surfaces of the organ-like structured Ti3C2Tx nano-layers and adjacent to TiO2, which ensures the stability and activity of the enzyme for a long time. Compared with the biosensor Nafion/Hb/Ti3C2Tx/GCE where Ti3C2Tx acts as the immobilization matrix, the Nafion/Hb/TiO2–Ti3C2Tx/GCE biosensor has a faster response (a response time of less than 3 s) and a wider linear range from 0.1 to 380 μM.
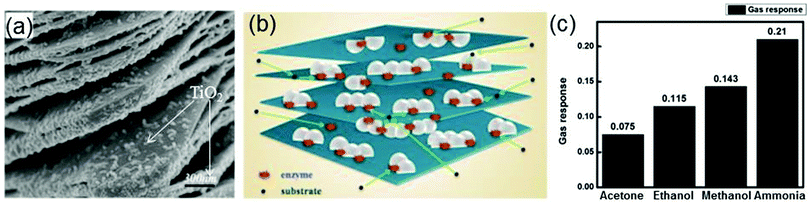 | ||
| Fig. 14 (a) SEM image of the TiO2–Ti3C2 nanocomposite. (b) Schematic illustration of the TiO2–Ti3C2 nanocomposite. Reprinted with permission from ref. 40 Copyright (2015) Elsevier. (c) Response of the Ti3C2Tx sensor to four different gases. Reprinted with permission from ref. 191 Copyright (2017) American Chemical Society. | ||
In the biosensor for glucose detection, Au nanostructures are widely used to retain the biological activity of enzymes and reduce the insulating effect of the protein shell for direct electron transfer.181–187 In order to make MXene have an excellent performance in the enzymatic glucose biosensor, a MXene–Au nanocomposite with a structure similar to that of TiO2–Ti3C2Tx mentioned above is used as the immobilization matrix in a GOx/Au/MXene/Nafion/GC biosensor.188 It is amazing that the fabricated biosensor shows a wide detection range for the glucose concentration from 0.1 mM to 18 mM, a low detection limit of 5.9 μM, excellent stability and good reproducibility. The Au nanoparticles distributed on the surfaces of MXene nanosheets further improve the electrical conductivity of MXene, which will strongly promote the electron exchange between the active center of enzymes and the electrode.
MXenes can be used in not only biosensors but also gas sensors.189–191 The utilization of MXenes in gas sensors overcomes conventional gas sensors’ drawback of a lack of selectivity for particular gas NH3. Based on first-principles calculations, Yu et al. firstly investigate the adsorption of gas molecules (NH3, H2, CH4, CO, CO2, N2, NO2, and O2) on monolayer Ti2CO2.41 It is found that NH3 is preferentially adsorbed on Ti2CO2, compared with other molecules. After NH3 adsorption, the calculated current–voltage relation curve of Ti2CO2 shows a large fluctuation, in contrast to the weak fluctuations of the corresponding curves of MoS2 and phosphorene in the same case, indicating the high sensitivity of Ti2CO2 to NH3.41 Inspired by this theoretical study, the gas sensor based on MXenes–Ti3C2Tx is fabricated subsequently. It is found that this sensor can successfully measure various gases (ethanol, methanol, acetone, and NH3) at room temperature and shows p-type sensing behavior. The average gas responses of Ti3C2Tx for ethanol, methanol, acetone, and ammonia are shown in Fig. 14c. Obviously, the Ti3C2Tx sensor shows the highest response to NH3 and the lowest response to acetone. The sensing mechanism of the Ti3C2Tx sensor involves absorption/desorption of gases by both defects and functional groups of Ti3C2Tx.191
6 Conclusions and outlook
This review summarizes recent progress of MXenes and MXene-based composites in their synthesis, properties and applications. More than 20 different MXenes have been produced in the past few years by selectively etching and exfoliation of layered ternary metal carbides, nitrides or carbonitrides. This synthetic process leads to the surface decorations of MXenes by various functional groups including O, F and OH, where the proportions of different functional groups are arbitrary, depending on the etching time and temperature. Due to this versatile surface chemistry, MXenes have exhibited many fascinating electronic, magnetic, electrochemical and mechanical properties. Several novel quantum phenomena including half-metallicity and topological insulativity are also predicted for some MXenes, which is appealing for 2D spintronics. Thanks to their excellent flexibility, 2D morphology and layered structures, MXenes easily form multifarious composites with other materials, which offers an effective way to tune the properties and performances of MXenes for various applications. For example, using metal oxides and CNTs as fillers in MXenes can not only improve the specific capacity of MXenes but also provide additional electron conductive pathways and promote electrolyte ion transportation, which will lead to the great enhancement of the overall electrochemical performances of MXenes.Due to the unique morphologies, layered structures and excellent properties, MXenes and their composites have good application prospects in catalysis, water remediation and sensors. As catalysts or co-catalysts, MXenes and their composites are widely used in electro/photocatalytic water splitting and photocatalytic reduction of CO2, where the excellent performances of certain MXene and MXene-based materials suggest that they have great potential to replace traditional precious metal catalysts. In sewage treatment, large surface areas and specific surface groups have endowed Ti3C2Tx and its composites with strong adsorption abilities for heavy metal ions, dyes and eutrophic substances, enabling that they can be used as adsorbents for water remediation. Additionally, MXenes and their composites with high conductivity and good biocompatibility can immobilize enzyme and facilitate the direct electron transfer between the enzyme and the electrode in various biosensors. It is also amazing to find that some MXenes, e.g., Ti3C2Tx, are sensitive to NH3 and can convert the NH3 concentration into electrical signals, making it possible to fabricate MXene-based NH3 sensors which will overcome conventional gas sensors’ drawback of a lack of selectivity for NH3.
Note that compared with graphene, MXenes’ study is still at an early stage. Opportunities and challenges coexist in the future research of MXenes. In the aspect of synthesis, (i) many MXenes such as Sc2C, Hf2C, W2C and so on are predicted to be able to stably exist, but their precursors have not yet been produced. Therefore, the synthesis of new MAX phases or other layered carbide and nitride precursors will be of significance for expanding the MXene family. (ii) It is highly desirable to develop new methods for synthesizing high-quality MXenes with large lateral dimensions, fewer defects and controlled surface terminations. In particular, achieving uniform terminations with only a single type of functional group is the most challenging task for experimentalists. Ultimately, bare MXenes without surface terminations must be synthesized, by chemical vapor deposition (CVD) or physical methods. In the aspect of property exploration, most of the known properties are predicted by theoretical calculations and await experimental verification. Particularly, semiconductivity has not been observed in MXenes, in spite of theoretical predictions. Moreover, there have been no reports on experimental measurements of the band structures of MXenes which are important to understand the fundamental properties of MXenes. It is also necessary to understand the structures and properties of MXenes intercalated with various ions or compounds. In the aspect of applications, although MXenes have shown excellent performances in many application fields, many underlying physical mechanisms still need to be intensively explored. For example, a deep understanding of catalytic and electrocatalytic properties of various MXene-based composites is essential for designing new high-efficient catalysts and may open new avenues in clean-energy conversion. Moreover, the mechanism for the high sensitivity of MXenes to NH3 remains elusive, which limits the practical applications of MXene-based gas sensors.
Finally, it is highly recommended to combine experimental and computational studies in the future explorations of MXenes. Considering different surface terminations and elemental compositions, there are hundreds of MXene members. The prior computational predictions of the properties and application potentials of MXenes could guide experimentalists to synthesize and investigate the most promising compounds, which will greatly reduce the research cost. Recently, it has been shown that machine learning can accurately predict the band gaps of MXenes.192 It is reasonable to expect that machine learning will play a significant role in predicting the properties of MXenes in the foreseeable future.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Research and Development Program of China (Grant No. 2017YFB0701700) and the National Natural Science Foundation of China (11874079, 11504015 and 51871009).References
- Y. Shao, J. Wang, H. Wu, J. Liu, I. A. Aksay and Y. Lin, Electroanalysis, 2010, 22, 1027–1036 CrossRef CAS.
- M. J. Allen, V. C. Tung and R. B. Kaner, Chem. Rev., 2009, 110, 132 CrossRef.
- C. Mattevi, H. Kim and M. Chhowalla, J. Mater. Chem., 2011, 21, 3324–3334 RSC.
- H. Wang, T. Maiyalagan and X. Wang, ACS Catal., 2012, 2, 781–794 CrossRef CAS.
- L. Li, Y. Yu, G. J. Ye, Q. Ge, X. Ou, H. Wu, D. Feng, X. H. Chen and Y. Zhang, Nat. Nanotechnol., 2014, 9, 372–377 CrossRef CAS.
- B. Lalmi, H. Oughaddou, H. Enriquez, A. Kara, S. Vizzini, B. Ealet and B. Aufray, Appl. Phys. Lett., 2010, 97, 223109 CrossRef.
- M. E. Dávila, L. Xian, S. Cahangirov, A. Rubio and G. Le Lay, New J. Phys., 2014, 16, 095002 CrossRef.
- H. Liu, A. T. Neal, Z. Zhu, Z. Luo, X. Xu, D. Tománek and P. D. Ye, ACS Nano, 2014, 8, 4033–4041 CrossRef CAS PubMed.
- S. Cahangirov, M. Topsakal, E. Aktürk, H. Şahin and S. Ciraci, Phys. Rev. Lett., 2009, 102, 236804 CrossRef CAS PubMed.
- G. R. Bhimanapati, Z. Lin, V. Meunier, Y. Jung, J. Cha, S. Das, D. Xiao, Y. Son, M. S. Strano, V. R. Cooper and L. Liang, ACS Nano, 2015, 9, 11509–11539 CrossRef CAS PubMed.
- X. Tang, X. Guo, W. Wu and G. Wang, Adv. Energy Mater., 2018, 8, 1801897 CrossRef.
- B. Anasori, M. R. Lukatskaya and Y. Gogotsi, Nat. Rev. Mater., 2017, 2, 16098 CrossRef CAS.
- M. Naguib, V. N. Mochalin, M. W. Barsoum and Y. Gogotsi, Adv. Mater., 2014, 26, 992–1005 CrossRef CAS.
- K. Hantanasirisakul and Y. Gogotsi, Adv. Mater., 2018, 30, e1804779 CrossRef.
- M. Khazaei, A. Ranjbar, M. Arai, T. Sasaki and S. Yunoki, J. Mater. Chem. C, 2017, 5, 2488–2503 RSC.
- Y. T. Liu, X. D. Zhu and L. Pan, Small, 2018, 14, e1803632 CrossRef.
- J. Nan, X. Guo, J. Xiao, X. Li, W. Chen, W. Wu, H. Liu, Y. Wang, M. Wu and G. Wang, Small, 2019, e1902085 CrossRef.
- V. M. H. Ng, H. Huang, K. Zhou, P. S. Lee, W. Que, J. Z. Xu and L. B. Kong, J. Mater. Chem. A, 2017, 5, 3039–3068 RSC.
- M. W. Barsoum and T. El-Raghy, Am. Sci., 2001, 89, 334–343 CrossRef.
- M. W. Barsoum, Prog. Solid State Chem., 2000, 28, 201–281 CrossRef CAS.
- Z. M. Sun, Int. Mater. Rev., 2013, 56, 143–166 CrossRef.
- H. Högberg, L. Hultman, J. Emmerlich, T. Joelsson, P. Eklund, J. M. Molina-Aldareguia, J.-P. Palmquistc and U. Wilhelmsson, Surf. Coat. Technol., 2005, 193, 6–10 CrossRef.
- X. Zhang, J. Xu, H. Wang, J. Zhang, H. Yan, B. Pan, J. Zhou and Y. Xie, Angew. Chem., Int. Ed., 2013, 52, 4361–4365 CrossRef CAS.
- M. Naguib, M. Kurtoglu, V. Presser, J. Lu, J. Niu, M. Heon, L. Hultman, Y. Gogotsi and M. W. Barsoum, Adv. Mater., 2011, 23, 4248–4253 CrossRef CAS.
- G. R. Berdiyorov and K. A. Mahmoud, Appl. Surf. Sci., 2017, 416, 725–730 CrossRef CAS.
- S. Li, P. Tuo, J. Xie, X. Zhang, J. Xu, J. Bao, B. Pan and Y. Xie, Nano Energy, 2018, 47, 512–518 CrossRef CAS.
- X. Liang, A. Garsuch and L. F. Nazar, Angew. Chem., Int. Ed., 2015, 54, 3907–3911 CrossRef CAS.
- X. Liang, Y. Rangom, C. Y. Kwok, Q. Pang and L. F. Nazar, Adv. Mater., 2017, 29, 1603040 CrossRef.
- O. Mashtalir, M. R. Lukatskaya, M. Q. Zhao, M. W. Barsoum and Y. Gogotsi, Adv. Mater., 2015, 27, 3501–3506 CrossRef CAS.
- Y. Xie, M. Naguib, V. N. Mochalin, M. W. Barsoum, Y. Gogotsi, X. Yu, K. W. Nam, X. Q. Yang, A. I. Kolesnikov and P. R. Kent, J. Am. Chem. Soc., 2014, 136, 6385–6394 CrossRef CAS.
- X. Wang, S. Kajiyama, H. Iinuma, E. Hosono, S. Oro, I. Moriguchi, M. Okubo and A. Yamada, Nat. Commun., 2015, 6, 6544 CrossRef CAS.
- X. Xie, M. Q. Zhao, B. Anasori, K. Maleski, C. E. Ren, J. Li, B. W. Byles, E. Pomerantseva, G. Wang and Y. Gogotsi, Nano Energy, 2016, 26, 513–523 CrossRef CAS.
- J. Yan, C. E. Ren, K. Maleski, C. B. Hatter, B. Anasori, P. Urbankowski, A. Sarycheva and Y. Gogotsi, Adv. Funct. Mater., 2017, 27, 1701264 CrossRef.
- Y. Cheng, Y. Zhang, Y. Li, J. Dai and Y. Song, J. Mater. Chem. A, 2019, 7, 9324–9334 RSC.
- Z. Guo, J. Zhou, L. Zhu and Z. Sun, J. Mater. Chem. A, 2016, 4, 11446–11452 RSC.
- M. Ye, X. Wang, E. Liu, J. Ye and D. Wang, ChemSusChem, 2018, 11, 1606–1611 CrossRef CAS.
- Q. Peng, J. Guo, Q. Zhang, J. Xiang, B. Liu, A. Zhou, R. Liu and Y. Tian, J. Am. Chem. Soc., 2014, 136, 4113–4116 CrossRef CAS.
- O. Mashtalir, K. M. Cook, V. N. Mochalin, M. Crowe, M. W. Barsoum and Y. Gogotsi, J. Mater. Chem. A, 2014, 2, 14334–14338 RSC.
- Q. Zhang, J. Teng, G. Zou, Q. Peng, Q. Du, T. Jiao and J. Xiang, Nanoscale, 2016, 8, 7085–7093 RSC.
- F. Wang, C. Yang, M. Duan, Y. Tang and J. Zhu, Biosens. Bioelectron., 2015, 74, 1022–1028 CrossRef CAS.
- X. F. Yu, Y. C. Li, J. B. Cheng, Z. B. Liu, Q. Z. Li, W. Z. Li, X. Yang and B. Xiao, ACS Appl. Mater. Interfaces, 2015, 7, 13707–13713 CrossRef CAS.
- X. H. Wang and Y. C. Zhou, J. Mater. Sci. Technol., 2010, 26, 385–416 CrossRef CAS.
- K. Zhu, Y. Jin, F. Du, S. Gao, Z. Gao, X. Meng, G. Chen, Y. Wei and Y. Gao, J. Mater. Chem., 2018, 31, 1–8 Search PubMed.
- M. Naguib, J. Halim, J. Lu, K. M. Cook, L. Hultman, Y. Gogotsi and M. W. Barsoum, J. Am. Chem. Soc., 2013, 135, 15966–15969 CrossRef CAS.
- A. VahidMohammadi, A. Hadjikhani, S. Shahbazmohamadi and M. Beidaghi, ACS Nano, 2017, 11, 11135–11144 CrossRef CAS.
- B. Soundiraraju and B. K. George, ACS Nano, 2017, 11, 8892–8900 CrossRef CAS.
- M. Naguib, O. Mashtalir, J. Carle, V. Presser, J. Lu, L. Hultman, Y. Gogotsi and M. W. Barsoum, ACS Nano, 2012, 6, 1322–1331 CrossRef CAS.
- Q. Tao, M. Dahlqvist, J. Lu, S. Kota, R. Meshkian, J. Halim, J. Palisaitis, L. Hultman, M. W. Barsoum, P. O. A. Persson and J. Rosen, Nat. Commun., 2017, 8, 14949 CrossRef PubMed.
- J. Halim, J. Palisaitis, J. Lu, J. Thörnberg, E. J. Moon, M. Precner, P. Eklund, P. O. Å. Persson, M. W. Barsoum and J. Rosen, ACS Appl. Nano Mater., 2018, 1, 2455–2460 CrossRef CAS.
- R. Meshkian, M. Dahlqvist, J. Lu, B. Wickman, J. Halim, J. Thornberg, Q. Tao, S. Li, S. Intikhab, J. Snyder, M. W. Barsoum, M. Yildizhan, J. Palisaitis, L. Hultman, P. O. A. Persson and J. Rosen, Adv. Mater., 2018, 30, e1706409 CrossRef.
- X. Wang, X. Shen, Y. Gao, Z. Wang, R. Yu and L. Chen, J. Am. Chem. Soc., 2015, 137, 2715–2721 CrossRef CAS PubMed.
- H. W. Wang, M. Naguib, K. Page, D. J. Wesolowski and Y. Gogotsi, J. Am. Chem. Soc., 2016, 28, 349–359 CAS.
- S. Zhao, X. Menga, K. Zhu, F. Du, G. Chen, Y. Wei, Y. Gogotsi and Y. Gao, Energy Storage Mater., 2017, 8, 42–48 CrossRef.
- M. H. Tran, T. Schäfer, A. Shahraei, M. Dürrschnabel, L. Molina-Luna, U. I. Kramm and C. S. Birkel, ACS Appl. Energy Mater., 2018, 1, 3908–3914 CrossRef CAS.
- B. Anasori, Y. Xie, M. Beidaghi, J. Lu and M. W. Barsoum, ACS Nano, 2015, 9, 9507–9516 CrossRef CAS PubMed.
- M. Ghidiu, M. R. Lukatskaya, M. Q. Zhao, Y. Gogotsi and M. W. Barsoum, Nature, 2014, 516, 78–81 CrossRef CAS PubMed.
- S. Kajiyama, L. Szabova, H. Iinuma, A. Sugahara, K. Gotoh, K. Sodeyama, Y. Tateyama, M. Okubo and A. Yamada, Adv. Sci. News, 2016, 7, 1601873 Search PubMed.
- J. Halim, S. Kota, M. R. Lukatskaya, M. Naguib, M. Q. Zhao, E. J. Moon, J. Pitock, J. Nanda, S. J. May, Y. Gogotsi and M. W. Barsoum, Adv. Funct. Mater., 2016, 26, 3118–3127 CrossRef CAS.
- F. Liu, J. Zhou, S. Wang, B. Wang, C. Shen, L. Wang, Q. Hu, Q. Huang and A. Zhou, J. Electrochem. Soc., 2017, 164, A709–A713 CrossRef CAS.
- F. Du, H. Tang, L. Pan, T. Zhang, H. Lu, J. Xiong, J. Yang and C. J. Zhang, Electrochim. Acta, 2017, 235, 690–699 CrossRef CAS.
- B. Anasori, Y. Xie, M. Beidaghi, J. Lu, B. C. Hosler, L. Hultman, P. R. C. Kent, Y. Gogotsi and M. W. Barsoum, ACS Nano, 2015, 9, 9507–9516 CrossRef CAS.
- J. Yang, M. Naguib, M. Ghidiu, L.-M. Pan, J. Gu, J. Nanda, J. Halim, Y. Gogotsi and M. W. Barsoum, J. Am. Ceram. Soc., 2015, 99, 660–666 CrossRef.
- J. Halim, M. R. Lukatskaya, K. M. Cook, J. Lu, C. R. Smith, L. A. Naslund, S. J. May, L. Hultman, Y. Gogotsi, P. Eklund and M. W. Barsoum, Chem. Mater., 2014, 26, 2374–2381 CrossRef CAS.
- A. Feng, Y. Yu, F. Jiang, Y. Wang, L. Mi, Y. Yu and L. Song, Ceram. Int., 2017, 43, 6322–6328 CrossRef CAS.
- L. Wang, H. Zhang, B. Wang, C. Shen, C. Zhang, Q. Hu, A. Zhou and B. Liu, Electron. Mater. Lett., 2016, 12, 702–710 CrossRef CAS.
- P. Urbankowski, B. Anasori, T. Makaryan, D. Er, S. Kota, P. L. Walsh, M. Zhao, V. B. Shenoy, M. W. Barsoum and Y. Gogotsi, Nanoscale, 2016, 8, 11385–11391 RSC.
- Y. C. Zhou, L. F. He, Z. J. Lin and J. Y. Wang, J. Eur. Ceram. Soc., 2013, 33, 2831–2865 CrossRef CAS.
- J. Zhou, X. Zha, F. Y. Chen, Q. Ye, P. Eklund, S. Du and Q. Huang, Angew. Chem., Int. Ed., 2016, 55, 5008–5013 CrossRef CAS PubMed.
- J. Zhou, X. Zha, X. Zhou, F. Chen, G. Gao, S. Wang, C. Shen, T. Chen, C. Zhi, P. Eklund, S. Du, J. Xue, W. Shi, Z. Chai and Q. Huang, ACS Nano, 2017, 11, 3841–3850 CrossRef CAS PubMed.
- R. Meshkian, L.-Å. Näslund, J. Halim, J. Lu, M. W. Barsoum and J. J. S. M. Rosen, Scr. Mater., 2015, 108, 147–150 CrossRef CAS.
- O. Mashtalir, M. Naguib, V. N. Mochalin, Y. Dall'Agnese, M. Heon, M. W. Barsoum and Y. Gogotsi, Nat. Commun., 2013, 4, 1716 CrossRef PubMed.
- Y. Wu, P. Nie, J. Wang, H. Dou and X. Zhang, ACS Appl. Mater. Interfaces, 2017, 9, 39610–39617 CrossRef CAS PubMed.
- C. E. Ren, K. B. Hatzell, M. Alhabeb, Z. Ling, K. A. Mahmoud and Y. Gogotsi, J. Phys. Chem. Lett., 2015, 6, 4026–4031 CrossRef CAS PubMed.
- H. Wang, J. Zhang, Y. Wu, H. Huang, G. Li, X. Zhang and Z. Wang, Appl. Surf. Sci., 2016, 384, 287–293 CrossRef CAS.
- M. Naguib, R. R. Unocic, B. L. Armstrong and J. Nanda, Dalton Trans., 2015, 44, 9353–9358 RSC.
- T. Zhang, L. Pan, H. Tang, F. Du, Y. Guo, T. Qiu and J. Yang, J. Alloys Compd., 2016, 695, 818–826 CrossRef.
- A. Lipatov, M. Alhabeb, M. R. Lukatskaya, A. Boson, Y. Gogotsi and A. Sinitskii, Adv. Electron. Mater., 2016, 2, 1600255 CrossRef.
- Z. Ling, C. E. Ren, M. Q. Zhao, J. Yang, J. M. Giammarco, J. Qiu, M. W. Barsoum and Y. Gogotsi, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 16676–16681 CrossRef CAS PubMed.
- M. Naguib, T. Saito, S. Lai, M. S. Rager, T. Aytug, M. P. Paranthaman, M. Q. Zhao and Y. Gogotsi, RSC Adv., 2016, 6, 72069–72073 RSC.
- H. Zhang, L. Wang, Q. Chen, P. Li, A. Zhou, X. Cao and Q. Hu, Mater. Des., 2015, 92, 682–689 CrossRef.
- E. A. Mayerberger, O. Urbanek, R. M. McDaniel, R. M. Street, M. W. Barsoum and C. L. Schauer, J. Appl. Polym. Sci., 2017, 134, 45295 CrossRef.
- J. Luo, X. Tao, J. Zhang, Y. Xia, H. Huang, L. Zhang, Y. Gan, C. Liang and W. Zhang, ACS Nano, 2016, 10, 2491–2499 CrossRef CAS PubMed.
- J. Zhu, Y. Tang, C. Yang, F. Wang and M. Cao, J. Electrochem. Soc., 2016, 163, A785–A791 CrossRef CAS.
- C. Zhang, S. J. Kim, M. Ghidiu, M. Q. Zhao, M. W. Barsoum, V. Nicolosi and Y. Gogotsi, Adv. Funct. Mater., 2016, 26, 4143–4151 CrossRef CAS.
- J. Wang, S. Dong, H. Li, Z. Chen, S. Jiang, L. Wu and X. Zhang, J. Electroanal. Chem., 2017, 810, 27–33 CrossRef.
- J. Huang, R. Meng, L. Zu, Z. Wang, N. Feng, Z. Yang, Y. Yu and J. Yang, Nano Energy, 2018, 46, 20–28 CrossRef CAS.
- B. Ahmed, D. H. Anjum, Y. Gogotsi and H. N. Alshareef, Electrochim. Acta, 2017, 202, 24–31 Search PubMed.
- M. Q. Zhao, M. Torelli, C. E. Ren, M. Ghidiu, Z. Ling, B. Anasori, M. W. Barsoum and Y. Gogotsi, Nano Energy, 2015, 30, 603–613 CrossRef.
- X. Guo, X. Xie, S. Choi, Y. Zhao, H. Liu, C. Wang, S. Chang and G. Wang, J. Mater. Chem. A, 2017, 5, 12445–12452 RSC.
- Y. Wang, Y. Li, Z. Qiu, X. Wu, P. Zhou, T. Zhou, J. Zhao, Z. Miao, J. Zhou and S. Zhuo, J. Mater. Chem. A, 2018, 6, 11189–11197 RSC.
- H. Zhang, H. Dong, X. Zhang, Y. Xu and J. Fransaer, Electrochim. Acta, 2016, 202, 24–31 CrossRef CAS.
- M. Q. Zhao, C. E. Ren, Z. Ling, M. R. Lukatskaya, C. Zhang, K. L. Van Aken, M. W. Barsoum and Y. Gogotsi, Adv. Mater., 2015, 27, 339–345 CrossRef CAS PubMed.
- Y. Cai, J. Shen, G. Ge, Y. Zhang, W. Jin, W. Huang, J. Shao, J. Yang and X. Dong, ACS Nano, 2018, 12, 56–62 CrossRef CAS PubMed.
- Y. Bai, K. Zhou, N. Srikanth, J. H. Pang, X. He and R. Wang, RSC Adv., 2016, 6, 35731–35739 RSC.
- X. H. Zha, K. Luo, Q. Li, Q. Huang, J. He, X. Wen and S. Du, EPL, 2015, 111, 26007 CrossRef.
- Z. Guo, J. Zhou, C. Si and Z. Sun, Phys. Chem. Chem. Phys., 2015, 17, 15348–15354 RSC.
- U. Yorulmaz, A. Özden, N. K. Perkgöz, F. Ay and C. Sevik, Nanotechnology, 2016, 27, 335702 CrossRef PubMed.
- V. N. Borysiuk, V. N. Mochalin and Y. Gogotsi, Nanotechnology, 2015, 26, 265705 CrossRef PubMed.
- M. Khazaei, M. Arai, T. Sasaki, A. Ranjbar, Y. Liang and S. Yunoki, Phys. Rev. B: Condens. Matter Mater. Phys., 2015, 92, 075411 CrossRef.
- Y. Liu, H. Xiao and W. A. Goddard, J. Am. Chem. Soc., 2016, 138, 15853–15856 CrossRef CAS.
- J. Xu, J. Shim, J. H. Park and S. J. A. F. M. Lee, Adv. Funct. Mater., 2016, 26, 5328–5334 CrossRef CAS.
- Q. Peng, C. Si, J. Zhou and Z. Sun, Appl. Surf. Sci., 2019, 480, 199–204 CrossRef CAS.
- H. Wang, C. Si, J. Zhou and Z. Sun, J. Phys. Chem. C, 2017, 121, 25164–25171 CrossRef CAS.
- Q. H. Wang, K. Kalantar-Zadeh, A. Kis, J. N. Coleman and M. S. Strano, Nat. Nanotechnol., 2012, 7, 699–712 CrossRef CAS PubMed.
- A. Allain, J. Kang, K. Banerjee and A. Kis, Nat. Mater., 2015, 14, 1195–1205 CrossRef CAS PubMed.
- J. You, C. Si, J. Zhou and Z. Sun, J. Phys. Chem. C, 2019, 123, 3719–3726 CrossRef CAS.
- T. Hu, H. Zhang, J. Wang, Z. Li, M. Hu, J. Tan, P. Hou, F. Li and X. Wang, Sci. Rep., 2015, 5, 16329 CrossRef CAS PubMed.
- C. E. Ren, M. Q. Zhao, T. Makaryan, J. Halim, M. Boota, S. Kota, B. Anasori, M. W. Barsoum and Y. Gogotsi, ChemElectroChem, 2016, 3, 689 CrossRef CAS.
- M.-Q. Zhao, M. Torelli, C. E. Ren, M. Ghidiu, Z. Ling, B. Anasori, M. W. Barsoum and Y. Gogotsi, Nano Energy, 2016, 30, 603 CrossRef CAS.
- T. Zhang, L. Pan, H. Tang, F. Du, Y. Guo, T. Qiu and J. Yang, J. Alloys Compd., 2017, 695, 818 CrossRef CAS.
- F. Shahzad, M. Alhabeb, C. B. Hatter, B. Anasori, S. Man Hong, C. M. Koo and Y. Gogotsi, Science, 2016, 353, 1137 CrossRef CAS PubMed.
- H. B. Wu, J. S. Chen, H. H. Hng and X. W. Lou, Nanoscale, 2012, 4, 2526 RSC.
- J. Jiang, Y. Li, J. Liu, X. Huang, C. Yuan and X. W. Lou, Adv. Mater., 2012, 24, 5166 CrossRef CAS PubMed.
- Z. Wang, L. Zhou and X. W. Lou, Adv. Mater., 2012, 24, 1903 CrossRef CAS PubMed.
- D. Xiong, X. Li, Z. Bai and S. Lu, Small, 2018, 14, 1703419 CrossRef PubMed.
- S. Kumar and U. Schwingenschlögl, Phys. Rev. B, 2016, 94, 035405 CrossRef.
- X. Zhang, X. Zhao, D. Wu, Y. Jing and Z. Zhou, Nanoscale, 2015, 7, 16020–16025 RSC.
- Y. He, M. Zhang, J. Shi, Y. Cen and M. Wu, J. Phys. Chem. C, 2019, 123, 12781–12790 CAS.
- Y. Cheng, L. Wang, Y. Li, Y. Song and Y. Zhang, J. Phys. Chem. C, 2019, 123, 15629–15636 CrossRef CAS.
- H. Weng, A. Ranjbar, Y. Liang, Z. Song, M. Khazaei, S. Yunoki, M. Arai, Y. Kawazoe, Z. Fang and X. Dai, Phys. Rev. B: Condens. Matter Mater. Phys., 2015, 92, 075436 CrossRef.
- M. Khazaei, A. Ranjbar, M. Arai and S. Yunoki, Phys. Rev. B, 2016, 94, 125152 CrossRef.
- C. Si, K. H. Jin, J. Zhou, Z. M. Sun and F. Liu, Nano Lett., 2016, 16, 6584–6591 CrossRef CAS PubMed.
- C. Si, J. You, W. Shi, J. Zhou and Z. Sun, J. Mater. Chem. C, 2016, 4, 11524–11529 RSC.
- Y. Liang, M. Khazaei, A. Ranjbar, M. Arai, S. Yunoki, Y. Kawazoe, H. Weng and Z. Fang, Phys. Rev. B, 2017, 96, 195414 CrossRef.
- G. Gao, G. Ding, J. Li, K. Yao, M. Wu and M. Qian, Nanoscale, 2016, 8, 8986–8994 RSC.
- C. Si, J. Zhou and Z. Sun, ACS Appl. Mater. Interfaces, 2015, 7, 17510–17515 CrossRef CAS PubMed.
- G. Wang, J. Phys. Chem. C, 2016, 120, 18850–18857 CrossRef CAS.
- L. Hu, X. Wu and J. Yang, Nanoscale, 2016, 8, 12939–12945 RSC.
- J. He, P. Lyu and P. Nachtigall, J. Mater. Chem. C, 2016, 4, 11143–11149 RSC.
- J. He, P. Lyu, L. Z. Sun, A. M. Garcia and P. Nachtigall, J. Mater. Chem. C, 2016, 4, 6500–6509 RSC.
- G. Centi and S. Perathoner, Catal. Today, 2010, 150, 151–162 CrossRef CAS.
- A. P. Upadhyay, D. K. Behara, G. P. Sharma, A. Bajpai, N. Sharac, R. Ragan, R. G. Pala and S. Sivakumar, ACS Appl. Mater. Interfaces, 2013, 5, 9554–9562 CrossRef CAS PubMed.
- S. Cheng, H. Liu and B. E. Logan, Environ. Sci. Technol., 2006, 40, 364–369 CrossRef CAS PubMed.
- B. Z. Zhan, M. A. White, T. K. Sham, J. A. Pincock, R. J. Doucet, K. R. Rao, K. N. Robertson and T. S. Cameron, J. Am. Chem. Soc., 2003, 125, 2195–2199 CrossRef CAS PubMed.
- M. Yagi, E. Tomita, S. Sakita, T. Kuwabara and K. Nagai, J. Phys. Chem. B, 2005, 109, 21489–21491 CrossRef CAS PubMed.
- Y. Zheng, Y. Jiao, Y. Zhu, Q. Cai, A. Vasileff, L. H. Li, Y. Han, Y. Chen and S. Z. Qiao, J. Am. Chem. Soc., 2017, 139, 3336–3339 CrossRef CAS.
- H. Yu, L. Shang, T. Bian, R. Shi, G. I. Waterhouse, Y. Zhao, C. Zhou, L. Z. Wu, C. H. Tung and T. Zhang, Adv. Mater., 2016, 28, 5080–5086 CrossRef CAS.
- J. Tian, R. Ning, Q. Liu, A. M. Asiri, A. O. Al-Youbi and X. Sun, ACS Appl. Mater. Interfaces, 2014, 6, 1011–1017 CrossRef CAS.
- S. Zhao, H. Yin, L. Du, L. He, K. Zhao, L. Chang, G. Yin, H. Zhao, S. Liu and Z. Tang, ACS Nano, 2014, 8, 12660–12668 CrossRef CAS PubMed.
- A. Aijaz, N. Fujiwara and Q. Xu, J. Am. Chem. Soc., 2014, 136, 6790–6793 CrossRef CAS.
- T. Y. Ma, S. Dai, M. Jaroniec and S. Z. Qiao, J. Am. Chem. Soc., 2014, 136, 13925–13931 CrossRef CAS PubMed.
- B. Y. Xia, Y. Yan, N. Li, H. B. Wu, X. W. Lou and X. Wang, Nat. Energy, 2016, 1, 15006 CrossRef CAS.
- S. Zhao, Y. Wang, J. Dong, C. T. He, H. Yin, P. An, K. Zhao, X. Zhang, C. Gao, L. Zhang, J. Lv, J. Wang, J. Zhang, A. M. Khattak, N. A. Khan, Z. Wei, J. Zhang, S. Liu, H. Zhao and Z. Tang, Nat. Energy, 2016, 1, 16184 CrossRef CAS.
- F. Song and X. Hu, Nat. Commun., 2014, 5, 4477 CrossRef CAS.
- J. Jiang, A. Zhang, L. Li and L. Ai, J. Power Sources, 2015, 278, 445–451 CrossRef CAS.
- Z. Lu, W. Xu, W. Zhu, Q. Yang, X. Lei, J. Liu, Y. Li, X. Sun and X. Duan, Chem. Commun., 2014, 50, 6479–6482 RSC.
- F. Song and X. Hu, J. Am. Chem. Soc., 2014, 136, 16481–16484 CrossRef CAS.
- T. Y. Ma, J. L. Cao, M. Jaroniec and S. Z. Qiao, Angew. Chem., Int. Ed., 2016, 55, 1138–1142 CrossRef CAS.
- L. Zhao, B. Dong, S. Li, L. Zhou, L. Lai, Z. Wang, S. Zhao, M. Han, K. Gao, M. Lu, X. Xie, B. Chen, Z. Liu, X. Wang, H. Zhang, H. Li, J. Liu, H. Zhang, X. Huang and W. Huang, ACS Nano, 2017, 11, 5800–5807 CrossRef CAS.
- H. Zou, B. He, P. Kuang, J. Yu and K. Fan, ACS Appl. Mater. Interfaces, 2018, 10, 22311–22319 CrossRef CAS PubMed.
- M. Yu, S. Zhou, Z. Wang, J. Zhao and J. Qiu, Nano Energy, 2018, 44, 181–190 CrossRef CAS.
- Z. W. Seh, K. D. Fredrickson, B. Anasori, J. Kibsgaard, A. L. Strickler, M. R. Lukatskaya, Y. Gogotsi, T. F. Jaramillo and A. Vojvodic, ACS Energy Lett., 2016, 1, 589–594 CrossRef CAS.
- X. Wu, Z. Wang, M. Yu, L. Xiu and J. Qiu, Adv. Energy Mater., 2017, 29, 1607017 CrossRef PubMed.
- L. Xiu, Z. Wang, M. Yu, X. Wu and J. Qiu, ACS Nano, 2018, 12, 8017–8028 CrossRef CAS PubMed.
- C. F. Du, K. N. Dinh, Q. Liang, Y. Zheng, Y. Luo, J. Zhang and Q. Yan, Adv. Energy Mater., 2018, 8, 1801127 CrossRef.
- J. Ran, G. Gao, F. T. Li, T. Y. Ma, A. Du and S. Z. Qiao, Nat. Commun., 2017, 8, 13907 CrossRef CAS PubMed.
- X. An, W. Wang, J. Wang, H. Duan, J. Shi and X. Yu, Phys. Chem. Chem. Phys., 2018, 20, 11405–11411 RSC.
- S. Cao, B. Shen, T. Tong, J. Fu and J. Yu, Adv. Sci. News, 2018, 28, 1800136 Search PubMed.
- H. Brix and H. H. Schierup, Ambio, 1989, 18, 100–107 Search PubMed.
- Y. C. Lee and J. W. Yang, J. Ind. Eng. Chem., 2012, 18, 1178–1185 CrossRef CAS.
- Q. Zhang, Y. Li, Q. Yang, H. Chen, X. Chen, T. Jiao and Q. Peng, J. Hazard. Mater., 2018, 342, 732–740 CrossRef CAS PubMed.
- Y. Ying, Y. Liu, X. Wang, Y. Mao, W. Cao, P. Hu and X. Peng, ACS Appl. Mater. Interfaces, 2015, 7, 1795–1803 CrossRef CAS PubMed.
- A. Shahzad, K. Rasool, W. Miran, M. Nawaz, J. Jang, K. A. Mahmoud and D. S. Lee, ACS Sustainable Chem. Eng., 2017, 5, 11481–11488 CrossRef CAS.
- Z. Wei, Z. Peigen, T. Wubian, Q. Xia, Z. Yamei and M. Zheng, Mater. Chem. Phys., 2017, 206, 270–276 CrossRef.
- Q. Zhang, J. Teng, G. Zou, Q. Peng, Q. Du, T. Jiao and J. Xiang, Nanoscale, 2016, 8, 7085–7093 RSC.
- Y.-J. Zhang, J.-H. Lan, L. Wang, Q.-Y. Wu, C.-Z. Wang, T. Bo, Z.-F. Chai and W.-Q. Shi, J. Hazard. Mater., 2016, 308, 402–410 CrossRef CAS PubMed.
- L. Wang, L. Yuan, K. Chen, Y. Zhang, Q. Deng, S. Du, Q. Huang, L. Zheng, J. Zhang and Z. Chai, ACS Appl. Mater. Interfaces, 2016, 8, 16396–16403 CrossRef CAS PubMed.
- K. Rasool, M. Helal, A. Ali, C. E. Ren, Y. Gogotsi and K. A. Mahmoud, ACS Nano, 2016, 10, 3674–3684 CrossRef CAS PubMed.
- K. Rasool, K. A. Mahmoud, D. J. Johnson, M. Helal, G. R. Berdiyorov and Y. Gogotsi, Sci. Rep., 2017, 7, 1598 CrossRef PubMed.
- M. Yousef Elahi, A. A. Khodadadi and Y. Mortazavi, J. Electrochem. Soc., 2014, 161, B81–B87 CrossRef.
- Y. Wang, H. Li and J. Kong, Sens. Actuators, B, 2014, 193, 708–714 CrossRef CAS.
- K.-A. N. Duerloo, M. T. Ong and E. J. Reed, J. Phys. Chem. Lett., 2012, 3, 2871–2876 CrossRef CAS.
- C. N. Rao, K. Gopalakrishnan and U. Maitra, ACS Appl. Mater. Interfaces, 2015, 7, 7809–7832 CrossRef CAS PubMed.
- J. F. Wu, M. Q. Xu and G. C. Zhao, Electrochem. Commun., 2010, 12, 175–177 CrossRef CAS.
- G. X. Wang, W. J. Bao, J. Wang, Q. Q. Lu and X. H. Xia, Electrochem. Commun., 2013, 35, 146–148 CrossRef.
- Y. H. Zhang, Y. B. Chen, K. G. Zhou, C. H. Liu, J. Zeng, H. L. Zhang and Y. Peng, Nanotechnology, 2009, 20, 185504 CrossRef PubMed.
- C. C. Mayorga-Martinez, Z. Sofer and M. Pumera, Angew. Chem., Int. Ed., 2015, 54, 14317–14320 CrossRef CAS PubMed.
- M. Gautam and A. H. Jayatissa, Solid-State Electron., 2012, 78, 159–165 CrossRef CAS.
- R. Jain, R. Sharma, R. K. Yadav and R. Shrivastava, J. Electrochem. Soc., 2013, 160, H179–H184 CrossRef CAS.
- H. Liu, C. Duan, C. Yang, W. Shen, F. Wang and Z. Zhu, Sens. Actuators, B, 2015, 218, 60–66 CrossRef CAS.
- C. Li, O. Dag, T. D. Dao, T. Nagao, Y. Sakamoto, T. Kimura, O. Terasaki and Y. Yamauchi, Nat. Commun., 2015, 6, 6608 CrossRef CAS PubMed.
- A. Samphao, P. Butmee, J. Jitcharoen, L. Svorc, G. Raber and K. Kalcher, Talanta, 2015, 142, 35–42 CrossRef CAS PubMed.
- R. Devasenathipathy, V. Mani, S. M. Chen, S. T. Huang, T. T. Huang, C. M. Lin, K. Y. Hwa, T. Y. Chen and B. J. Chen, Enzyme Microb. Technol., 2015, 78, 40–45 CrossRef CAS PubMed.
- Q. Xu, S. X. Gu, L. Jin, Y. Zhou, Z. Yang, W. Wang and X. Hu, Sens. Actuators, B, 2014, 190, 562–569 CrossRef CAS.
- A. D. Chowdhury, R. Gangopadhyay and A. De, Sens. Actuators, B, 2014, 190, 348–356 CrossRef CAS.
- G. X. Zhong, W. X. Zhang, Y. M. Sun, Y. Q. Wei, Y. Lei, H. P. Peng, A. L. Liu, Y. Z. Chen and X. H. Lin, Sens. Actuators, B, 2015, 212, 72–77 CrossRef CAS.
- Q. Wang, F. Min and J. Zhu, Mater. Lett., 2013, 91, 9–11 CrossRef CAS.
- R. B. Rakhi, P. Nayak, C. Xia and H. N. Alshareef, Sci. Rep., 2016, 6, 36422 CrossRef CAS PubMed.
- B. Xiao, Y. Li, X. Yu and J. Cheng, Sens. Actuators, B, 2016, 235, 103–109 CrossRef CAS.
- S. J. Kim, H. J. Koh, C. E. Ren, O. Kwon, K. Maleski, S. Y. Cho, B. Anasori, C. K. Kim, Y. K. Choi, J. Kim, Y. Gogotsi and H. T. Jung, ACS Nano, 2018, 12, 986–993 CrossRef CAS PubMed.
- E. Lee, A. VahidMohammadi, B. C. Prorok, Y. S. Yoon, M. Beidaghi and D. J. Kim, ACS Appl. Mater. Interfaces, 2017, 9, 37184–37190 CrossRef CAS PubMed.
- A. C. Rajan, A. Mishra, S. Satsangi, R. Vaish, H. Mizuseki, K.-R. Lee and A. K. Singh, Chem. Mater., 2018, 30, 4031–4038 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2020 |