Open Access Article
Open Access ArticleFar-red/near-infrared emitting, two-photon absorbing, and bio-stable amino-Si-pyronin dyes†
Kyeong Hwan
Kim
a,
Subhankar
Singha
 *a,
Yong Woong
Jun
*a,
Yong Woong
Jun
 a,
Ye Jin
Reo
a,
Ye Jin
Reo
 a,
Hye Rim
Kim
a,
Hye Gun
Ryu
a,
Snehasis
Bhunia
b and
Kyo Han
Ahn
a,
Hye Rim
Kim
a,
Hye Gun
Ryu
a,
Snehasis
Bhunia
b and
Kyo Han
Ahn
 *a
*a
aDepartment of Chemistry, Pohang University of Science and Technology (POSTECH), 77 Cheongam-Ro, Nam-Gu, Pohang, Gyeongbuk 37673, Republic of Korea. E-mail: subhankar@postech.ac.kr; ahn@postech.ac.kr
bNational Institute for Nanomaterials Technology (NINT), Pohang University of Science and Technology, 77 Cheongam-Ro, Nam-Gu, Pohang, Gyeongbuk 37673, Republic of Korea
First published on 6th August 2019
Abstract
Organic fluorophores emitting in the far-red/near-infrared (NIR) wavelength region are in great demand for minimal autofluorescence and reduced light scattering in deep tissue or whole body imaging. Currently, only a few classes of far-red/NIR fluorophores are available including widely used cyanine dyes, which are susceptible to photobleaching and form nonfluorescent aggregates. Even rare are those far-red/NIR emitting dyes that have two-photon imaging capability. Here we report a new class of far-red/NIR-emitting dyes that are photo-stable, very bright, biocompatible, and also two-photon absorbing. The introduction of an electron-withdrawing group such as N-acyl or N-alkoxycarbonyl groups on the C-10-amino substituent of the new julolidine-derived amino-Si-pyronin dyes (ASiPj), which emit in the far-red region, causes large bathochromic shifts, leading to NIR-emitting amino-Si-pyronin dyes (NIR-ASiPj) having high cellular stability. Furthermore, the ASiPj–NIR-ASiPj couple offers a novel ratiometric bioimaging platform with a large spectral gap, as demonstrated here with a boronate-containing NIR-ASiPj derivative that is converted to the corresponding ASiPj dye upon reaction with hydrogen peroxide.
Introduction
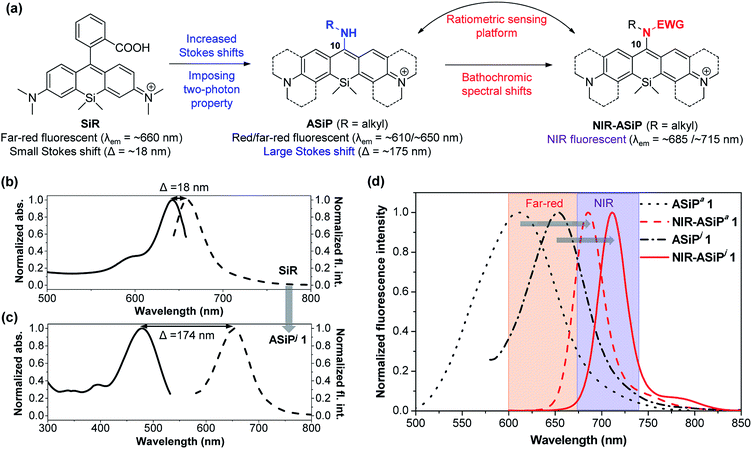
Fluorescence imaging offers a powerful means in modern science for studying and visualizing complex biological systems.1,2 The promising features of fluorescence imaging in turn have inspired a quest for novel fluorophores and molecular probes with desirable photophysical properties. A fluorescent molecule desirable for bioimaging application should be excitable and also emissive in the longer wavelength region, preferably from the far-red to near-infrared (NIR) region (650–950 nm), in order to minimize phototoxicity and background fluorescence from biomolecules (autofluorescence).3–5 NIR emitting cyanine dyes are widely used for bioimaging; however, they suffer from drawbacks such as photobleaching6 and also from susceptibility to form nonfluorescent aggregates.7 Therefore, the development of a new class of dyes with desirable photophysical properties is in great demand but remains a challenge. Furthermore, even rare are the far-red/NIR emitting dyes with two-photon imaging capability, which are in great demand for long-term monitoring of biological analytes with minimal autofluorescence.8,9Recently, Nagano and co-workers developed a family of far-red/NIR fluorescent rhodamine analogues including Si-rhodamine dyes (SiR),10 since the first report on the Si-pyronin (SiP) core by Xiao, Qian and co-workers.11 Silicon analogues still possess the optical properties of the original rhodamine dyes, such as high fluorescence quantum yields, photostability and cell permeability. Accordingly, SiR dyes have recently received significant attention for the development of fluorescent probes12 and fluorescence imaging techniques including super-resolution imaging and multicolor imaging.13–16 These conventional SiR dyes, which mostly emit in the far-red region, however, show very small Stokes shifts (Δλ = ∼18 nm),14 similar to BODIPY (Δλ = ∼10 nm)17 and rhodamine dyes (Δλ = ∼20 nm),18 which raises the light reabsorption issue in bioimaging. Also, they have been rarely used for two-photon microscopy imaging,19 a powerful means for obtaining high-resolution 3D images of living specimens (including animal tissues) down to the depth of a millimeter along with reduced photo-damage and photo-bleaching.20,21
The fact that the conversion of BODIPY and rhodamine to the corresponding amino-BODIPY22 and amino-pyronin23,24 dyes shows large Stokes shifts (∼60 and ∼105 nm, respectively) and good two-photon absorbing properties25,26 prompted us to investigate the corresponding amino-Si-pyronin (ASiP) dyes and their derivatives. Earlier, a structurally simple 10-amino-Si-xanthene precursor was used to synthesize N-acylated-iminoanthrone derivatives by Klán and co-workers.27 In the course of our study,28 Butkevich et al. reported alkylamino-Si-pyronin dyes and their application to super-resolution fluorescence microscopy.29 Also, Guo and co-workers subsequently reported ASiP dyes with two-photon imaging application.30 We noticed that even though it was possible to induce large Stokes shifts and two-photon imaging capability by converting the “conventional” SiR dye system into the corresponding ASiP system, unfortunately, the reported ASiP analogues showed hypsochromic shifts in their emission and absorption spectra (Δλ = ∼50 nm in a buffer solution) from the corresponding SiR dyes. Accordingly, the ASiP dyes known so far showed the maximum emission wavelength (λem) of around ∼610 nm, which belongs to the orange emission region, which would cause significant autofluorescence.8,9 To address this critical issue, it is necessary to develop fluorophores that emit in the far-red/NIR region. Currently, only a few classes of such dyes are available, which are mostly based on cyanines,31 BODIPYs,32 hemicyanine hybrids of rhodamines, fluoresceins and coumarins,33 silicon-based rhodamines,34 and benzocoumarins.8 To this end, we undertook a rational approach to develop a novel class of two-photon active, NIR-emitting ASiP derivatives (NIR-ASiP). At the same time, we also intended to modulate the photophysical properties of new ASiP dyes by implementing an “activable” functional group by an analyte or a stimulus, which would ultimately provide a ratiometric bioimaging platform (Fig. 1a). Although rhodamine and Si-rhodamine dyes have been widely used for the development of fluorescent probes, they mostly operate in the turn-on sensing mode through the lactam ring-opening process. For reliable and quantitative analysis, a ratiometric probe that shows signals at two different wavelengths with a large spectral separation is highly promising, as it avoids environmental and instrumental effects on fluorescence change.35 Herein, we disclose NIR-emitting ASiP dyes and their parent ASiP dyes, the couple of which also constitutes a promising ratiometric bioimaging platform with large spectral shifts.
Results and discussion
Design and development of amino-Si-pyronin based two-photon absorbing NIR emitting dyes
We reasoned that introducing an amine donor at C-10 of SiP dyes may impart electronic perturbation and intramolecular charge transfer (ICT) characteristics to the resulting dyes, which may endow them with two-photon absorbing properties as in the case of the amino-BODIPY system.25,26 In a further approach, modulating the electron-donating ability of the 10-amino group may induce bathochromic spectral shifts. Both approaches indeed ultimately have led to two-photon active, NIR-emitting Si-pyronin analogues.Thus, starting from a bis(dimethylamino)-Si-xanthone intermediate (for its synthesis, see the ESI†), we synthesized various amino-Si-pyronin compounds (ASiPa1–10; Tables 1 and S1, ESI†) by introducing different types of alkylamines at C-10 of the Si-pyronin core. Then, the electron-donating ability of the 10-amino group was further modulated by attaching an electron-withdrawing group (EWG) to it, which provided the NIR-emitting ASiPa analogues.
| R 1 (alkyl) | R 2 (EWG) | λ abs /nm | λ em /nm | Φ F | |
|---|---|---|---|---|---|
| a Maximum absorption wavelength. b Maximum emission wavelength in PBS (pH 7.4). c Fluorescence quantum yield. d Determined in methanol using fluorescein as the reference (ΦF = 0.95 in 0.1 M NaOH).18 e Determined in ethanol using rhodamine 101 (ΦF = 0.915 in EtOH) as the reference dye. f Determined in CH3CN using Nile blue (ΦF = 0.27 in ethanol) as the reference dye. | |||||
| ASiPa1 | Me | H | 452 | 612 | 0.33d |
| ASiPa2 | Bn | H | 469 | 623 | 0.34e |
| ASiPa3 | Propargyl | H | 473 | 628 | 0.25e |
| NIR-ASiPa1 | Me | CO2Bn | 669 | 685 | 0.42f |
| NIR-ASiPa2 | Propargyl | COCH3 | 676 | 694 | 0.31e |
| NIR-ASiPa3 | Propargyl | COCF3 | 684 | 707 | 0.27e |
| NIR-ASiPa4 | Propargyl | SO2CF3 | 694 | 713 | 0.25e |
| NIR-ASiPa5 | Bn | COCF3 | 684 | 704 | 0.29e |
| NIR-ASiPa6 | Cyclic urea | 674 | 690 | 0.22e | |
The photophysical properties of ASiPa1–10 measured in PBS (containing 1% DMSO) show the one-photon absorption maxima at around 450–475 nm while the corresponding emission maxima at around 600–625 nm (Tables 1 and S1, ESI†). We found that the propargylamine derivative ASiPa3 showed a little red-shift in the absorption and emission bands compared to those of the methylamine and benzylamine analogues, ASiPa1 and 2, which suggested that a further bathochromic shift would be induced by introducing a more electron-withdrawing substituent at C-10. Accordingly, we synthesized a carbamate derivative (NIR-ASiPa1) and measured its photophysical properties. Indeed, NIR-ASiPa1, which is the benzyl carbamate derivative of ASiPa1, exhibited large bathochromic shifts in the absorption and emission bands (Δλ = 218 nm and 75 nm, respectively) with the maximum intensity peaks at 669 nm and 685 nm, respectively (Table 1 and Fig. S1, ESI†).
Computational calculations (using Gaussian '09) performed for ASiPa1 and a simplified carbamate form NIR-ASiPa1 gave a HOMO–LUMO energy gap of 2.97 eV and 2.41 eV, respectively (Fig. S2, ESI†), supporting the bathochromic spectral shifts upon attaching an electron-withdrawing group to the C-10 amine site. The spectral tuning of such ASiPa dyes is thus readily realized by a simple transformation, avoiding laborious and complex synthetic operations.
Bio-stability issues of NIR emitting NIR-ASiPa dyes
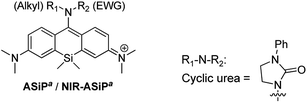
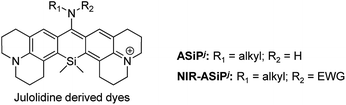
After characterizing the photophysical properties of the ASiPa dyes, we evaluated their cellular imaging capability toward HeLa cells under one-photon (λex = 488 nm) and two-photon excitation (λex = 900 nm) conditions. The selected dyes ASiPa2 and ASiPa3 provided very bright images by confocal laser-scanning microscopy (CLSM) (Fig. S3a and b, ESI†) as well as two-photon microscopy (TPM) (Fig. S3c and d, ESI†). The excellent imaging capability of the ASiPa dyes under two-photon excitation further confirms their two-photon absorbing properties, as also reported by Guo and co-workers.30Surprisingly, NIR-ASiPa1 showed very dim cellular images when the emission was collected in the far-red/NIR wavelength region of 650–800 nm upon excitation at 633 nm. In contrast, strong emission was observed in the shorter wavelength region of 500–630 nm upon excitation at 488 nm (Fig. 2a, panels A and G). The unexpected cellular imaging results of NIR-ASiPa1 indicated that the NIR-ASiPa type dyes are chemically unstable inside cells.
Indeed, NIR-ASiPa5 upon treatment with cysteine (Cys) or glutathione (GSH) in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 PBS–dioxane at 37 °C showed little fluorescence in the NIR region, whereas a new emission band at around 600 nm corresponding to ASiPa increased as time went (Fig. S4†). The new band was due to the ASiPa-type Cys-adduct, as confirmed by HPLC-MS and NMR analyses. The formation of the Cys-adduct also suggests that NIR-ASiPa type dyes also react with cellular proteins similarly through thiol addition followed by intramolecular amine substitution (Fig. 2b).36
1 PBS–dioxane at 37 °C showed little fluorescence in the NIR region, whereas a new emission band at around 600 nm corresponding to ASiPa increased as time went (Fig. S4†). The new band was due to the ASiPa-type Cys-adduct, as confirmed by HPLC-MS and NMR analyses. The formation of the Cys-adduct also suggests that NIR-ASiPa type dyes also react with cellular proteins similarly through thiol addition followed by intramolecular amine substitution (Fig. 2b).36
Although the carbamate NIR-ASiPa1 seems to be less reactive that the trifluoroacetyl analogue 5 towards biothiols (Cys and GSH) in the buffer solution (Fig. S5, ESI†), fluorescence from the shorter wavelength window was still observed in the cellular imaging data. This is likely due to the bioconjugation reactions with cellular proteins, as supported by a gel-electrophoresis analysis of A549 cells incubated with NIR-ASiPa1 (10 μM) for 1 h: a strong fluorescence band (broad) appeared, plausibly due to dye-labeled cellular proteins (Fig. S6, ESI†).
To secure the cellular stability of the NIR-ASiPa dyes, initially we varied the C-10 amino group. Changing the amino group into the corresponding carboxamide, sulfonamide, cyclic urea, and other functional groups, in all cases, was found to be ineffective in securing the desirable cellular stability (see Table 1 for the dye structures and Fig. 2a for the corresponding cellular images).
Thus, a different approach was necessary to address the cellular instability issue of such NIR-ASiPa dyes.
Securing bio-stability through developing julolidine-derived NIR-emitting amino-Si-pyronin dyes
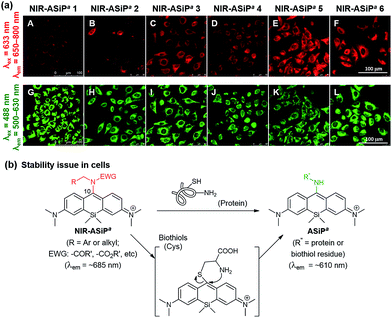
It is known that the C-10 of SiR is more susceptible to nucleophilic attack compared to that of rhodamine dyes. Indeed, the high electrophilicity at the C-10 of SiR was used for the development of a GSH probe37,38 and for super-resolution imaging.39–44 Importantly, the electrophilicity at the C-10 of SiR is also dependent on the polarity of the medium: for example, a 2′-carboxy-SiR compound exists mainly in the nonfluorescent spirolactone form in less polar media due to the nucleophilic attack of the nearby carboxylate to the C-10, whereas it exists in the fluorescent zwitterionic form in highly polar media (Fig. 3b).39–42 Similarly, polarity-dependent electrophilicity at C-10 would be also expected for NIR-ASiPa in the cellular matrix, the less polar media compared to the aqueous buffer solution, plausibly causing the nucleophilic attack by biomolecules (biothiols or amines).For electronic modulation at C-10, we also compared ASiPa derivatives with other amine substituents such as methoxyamine, phenylhydrazine or benzhydrazide (see Fig. S7 in the ESI† for the dye structures). Unfortunately, all these ASiPa analogues showed a tendency to exist in the non-fluorescent form. Introducing a sterically bulky amine such as benzhydrylamine provided a bright ASiPa analogue, but subsequent attempts to attach any electron-withdrawing substituent to the amine site were found to be difficult.
Toward cellular-stable NIR-ASiP dyes, finally we turned our attention to the dye's core structure. Eventually, we were able to overcome the bio-instability of the NIR-ASiPa dyes by changing the dye skeleton, from bis(dimethylamino)-SiP (ASiPa where a represents the acyclic amine donor) to julolidine-derived SiP (ASiPj), as shown in Fig. 3a. In the resultant ASiPj dyes, the four alkyl substituents on the Si-xanthene core are expected to increase the electron density at C-10, in addition to increasing the steric hindrance around it. Gratifyingly, these effects together are found to stabilize the corresponding NIR-emitting dyes (NIR-ASiPj) from possible nucleophilic attack by biothiols and amines inside cells.
Computational calculations using the Gaussian' 09 program (B3LYP/6-31+G(d)) for ASiPa1 and ASiPj1 showed that the Mulliken charge on the C-10 of ASiPa1 is +0.193 a.u., whereas that of ASiPj1 is −0.143 a.u. Thus, the electrophilicity at C-10 is significantly lowered in ASiPj1 compared to that in ASiPa1. Moreover, the corresponding HOMO–LUMO energy gaps obtained for ASiPa1 and ASiPj1 were −2.97 eV and −2.83 eV, respectively; hence, bathochromic spectral shifts are also expected upon introducing the alkyl substituents.11
The ASiP dyes' emission behavior is dependent on the medium polarity.29 By following the protocol established for carboxyphenyl-substituted Si-rhodamine dyes by Lukinavičius et al.39,41 and Butkevich et al.,40,42 we compared the equilibrium behavior of two selected dyes, ASiPa2 and ASiPj2, depending on the medium polarity. The ASiP dyes can exist in two forms, the highly conjugated enamine and less conjugated imine forms, of which equilibrium that involves protonation/deprotonation is dependent on the media (Fig. 3c).29 The enamine form with an extended π-conjugation feature is strongly emissive, whereas the imine form is poorly emissive plausibly due to imine bond isomerization and short π-conjugation. A series of absorption spectra were recorded for the two selected dyes in water–dioxane media by varying the water content and hence the polarity (Fig. S8 and S9, ESI†). The ratio of the enamine to the imine form for each of the ASiP dyes, as represented by the ratio of the corresponding absorbance, is plotted against the medium polarity (Fig. 3d). The data show that half of the ASiPa2 molecules exists in the fluorescent enamine form only in a medium of above 70% water–dioxane, whereas ASiPj2 is expected to remain in the fluorescent form over a wide range of polarity (>37% water–dioxane). Thus, we can conclude that the ASiPj skeleton is more electron-rich (that is, the enamine proton is less acidic) than the ASiPa skeleton. As a result, the ASiPj dyes are expected to show emission behavior even at cytosolic pH values. In contrast, the ASiPa dyes fluoresce only in acidic organelles such as lysosomes, but not in the cytosol (at neutral pH).30
Photophysical properties of julolidine derived ASiPj and NIR-ASiPj
Encouraged by the theoretical calculation and the medium-dependent equilibrium data of ASiPj, we synthesized several ASiPj compounds, by introducing methylamine (ASiPj1), benzylamine (ASiPj2) and propargylamine (ASiPj3) at C-10 of the common julolidine-derived Si-xanthone intermediate (Table 2; for the detailed synthetic procedure, see the ESI†).| R 1 (alkyl) | R 2 (EWG) | Solvent | λ abs /nm | ε /M−1 cm−1 | λ em /nm | Φ F | |
|---|---|---|---|---|---|---|---|
| a Maximum absorption wavelength. b Molar extinction coefficient. c Maximum emission wavelength. d Fluorescence quantum yields determined in CH3CN using e fluorescein (Φ = 0.95 in 0.1 M NaOH) or f Nile blue (Φ = 0.27 in ethanol) as reference dyes. | |||||||
| ASiPj1 | Me | H | PBS | 481 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
655 | 0.11e |
| EtOH | 487 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
628 | 0.28e | |||
| CH3CN | 488 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
640 | 0.26e | |||
| CH2Cl2 | 492 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
641 | 0.33e | |||
| ASiPj2 | Bn | H | PBS | 501 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
656 | 0.10e |
| EtOH | 502 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
632 | 0.31e | |||
| CH3CN | 506 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
642 | 0.30e | |||
| CH2Cl2 | 512 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
645 | 0.32e | |||
| ASiPj3 | Propargyl | H | PBS | 509 | 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
662 | 0.12e |
| EtOH | 510 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
644 | 0.19e | |||
| CH3CN | 515 | 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
654 | 0.18e | |||
| CH2Cl2 | 517 | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
657 | 0.23e | |||
| NIR-ASiPj1 | Me | CO2Bn | PBS | 702 | 109![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
715 | 0.05f |
| EtOH | 702 | 114![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
717 | 0.35f | |||
| CH3CN | 700 | 107![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
714 | 0.26f | |||
| CH2Cl2 | 702 | 102![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
718 | 0.40f | |||
| NIR-ASiPj2 | Me | COCH3 | PBS | 703 | 43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
714 | 0.06f |
| EtOH | 705 | 58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
717 | 0.29f | |||
| CH3CN | 700 | 52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
713 | 0.25f | |||
| CH2Cl2 | 706 | 53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
718 | 0.35f | |||
| NIR-ASiPj3 | Me | COCF3 | PBS | 711 | 79![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
724 | 0.04f |
| EtOH | 713 | 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
728 | 0.27f | |||
| CH3CN | 711 | 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
726 | 0.21f | |||
| CH2Cl2 | 715 | 87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
730 | 0.32f | |||
Photophysical properties of the new ASiPj dyes were measured in different solvents (PBS, ethanol, acetonitrile, and dichloromethane) (Table 2 and Fig. S10–S12, ESI†). In PBS, the absorption and emission maxima (λabs and λem) of the ASiPj dyes are in the range of 481–509 nm and 655–662 nm, respectively. It is notable that, in comparison with the corresponding ASiPa analogues (λabs ∼ 450 nm; λem ∼ 610 nm) that emit in the yellow to orange wavelength region, ASiPj dyes emit in the far-red region (λem ∼ 650–660 nm), similar to the SiR dyes (λem ∼ 660 nm). This bathochromic shift to the far-red emission is crucial to avoid the significant autofluorescence from biomolecules in the yellow emission channel in tissue imaging under two-photon excitation conditions.8,9 An added value of the ASiPj dyes is the large Stokes shift (Δλ = ∼175 nm), a contrasting feature from conventional SiR dyes (Δλ = ∼18 nm) (Fig. 1b and c).
Moreover, the ASiPj dyes also show bright one-photon emission (ΦF = 0.11–0.33) and good two-photon absorbing properties (TPACS value, δ = 113–122 GM) under excitation at 800 nm. Furthermore, the ASiPj dyes show strong fluorescence at acidic as well as neutral pH (pH 3–7); at basic pH (above pH 8), the fluorescence gradually diminishes due to the equilibrium shift to the non-fluorescent imine form (Fig. S13, ESI†).
Similarly to the case of ASiPa, finally we synthesized several NIR emitting dyes based on ASiPj: benzyl carbamate (NIR-ASiPj1), acetamide (NIR-ASiPj2) and trifluoroacetamide (NIR-ASiPj3) derivatives as the representative dyes (Table 2; for the detailed synthetic procedure, see the ESI†). Photophysical properties of the NIR-ASiPj dyes were measured in different solvents (PBS, ethanol, acetonitrile, and dichloromethane).
All the NIR-ASiPj dyes show large bathochromic shifts in the absorption and emission bands, emitting in the NIR region above 700 nm (Fig. 1c, Table 2 and Fig. S14–S16, ESI†). For example, NIR-ASiPj1 in PBS shows further bathochromic shifts of ∼30 nm in the absorption and emission bands (λabs = 702 nm; λem = 715 nm) compared to the corresponding acyclic analogue NIR-ASiPa1 (λabs = 669 nm; λem = 685 nm). Thus, NIR-ASiPj1 in PBS shows giant bathochromic shifts of ∼250 nm in the absorbance band and ∼105 nm in the emission band from those of the corresponding ASiPa1. Also, all the NIR-ASiPj dyes show good quantum yields (Φ = 0.05–0.40) (Table 2) and pH-insensitive NIR emission covering a wide range of pH (pH = 2–10) (Fig. S17, ESI†). The latter property is notable in comparison with the pH-dependent fluorescence emission behavior of the ASiPa dyes. An interesting observation is that compared to the ASiPj dyes (Δλ = 153–174 nm in PBS), the corresponding NIR-ASiPj dyes have much smaller Stokes shifts (Δλ = 11–13 nm in PBS). Similarly, the ASiPa dyes have large Stokes shifts (Δλ = 154–160 nm in PBS) but the corresponding NIR-ASiPa dyes have much smaller Stokes shifts (Δλ = 16–23 nm in PBS).
In comparison with the well-known NIR emitting cyanine dye (Cy 7: IR780) that undergoes fast photo-oxidation, all the new NIR-ASiPj dyes show excellent photostability under continuous irradiation at 633 nm (22 μW cm−2), usual CLSM imaging conditions or at 900 nm (145 mW cm−2), and harsh two-photon imaging conditions, both in PBS at pH 7.4 for 10 min (Fig. S19, ESI†).
Bioimaging capabilities of ASiPj and NIR-ASiPj
Above all, it was our keen concern whether the new NIR-ASiPj dyes would have cellular stability or not. We assessed their cellular imaging behavior along with their parent ASiPj dyes under one-photon excitation at 488 nm as well as two-photon excitation at 900 nm. Both the ASiPj and NIR-ASiPj dyes showed little cell toxicity from standard CCK assays (Fig. S20 and S21†). A549 cells incubated with each of the ASiPj dyes 1–3 at 1.0 μM show very bright images using CLSM and TPM (Fig. 4a and c). The bio-stability of the NIR-ASiPj dyes 1–3 was evaluated using CLSM, by collecting the emissions from two separate channels: (1) a far-red/NIR window (650–800 nm) under excitation at 633 nm, to collect emissions from NIR-ASiPj and (2) a shorter wavelength emission window (500–630 nm) under excitation at 488 nm, to collect emissions of any possible decomposed dyes from NIR-ASiPj. To our delight, unlike previous NIR-ASiPa1, the NIR-ASiPj dyes 1 and 2 show bright cellular images in the NIR emission window (650–800 nm) (in Fig. 4b, panels A and B) but negligible images in the shorter emission window (in Fig. 4b, panels D and E). Only in the case of the trifluoroacetamide derivative, NIR-ASiPj3, a slight interference was observed in the short emission window (in Fig. 4b, panel F).In solution, the julolidine-based NIR-ASiPj dyes also showed chemical stability toward Cys and GSH, in contrast to the corresponding NIR-ASiPa dyes (Fig. S4†). The results confirm that we have addressed the stability issue by modifying the dye skeleton.
Thus, we can conclude that such NIR-ASiPj dyes have bio-stability sufficient for cellular imaging applications. Furthermore, the new NIR-ASiPj dyes provide very bright TPM images under excitation at 900 nm (in Fig. 4c, panels D–F), a commonly accessible wavelength with commercial TPM, which also confirms their strong two-photon absorbing properties.
We also studied the intracellular distribution of the representative NIR-ASiPj1 by co-localization experiments using commercial reference dyes for the nucleus (Hoechst), lysosomes (LysoTracker green) and mitochondria (MitoTracker green), respectively. The results show that NIR-ASiPj1 does not localize in the nucleus (Pearson's correlation coefficient, PCC = −0.30) and lysosomes (PCC = 0.54) but mostly localizes in the mitochondria (PCC = 0.73), possibly due to the hydrophobic and cationic character (Fig. S22, ESI†).
In short, we have developed Si-pyronin based NIR-emitting dyes (NIR-ASiPj dyes) with secured cellular stability. Together with the parent amino-Si-pyronin (ASiPj) dyes, the new NIR emitting dyes are photostable, biocompatible, and also provide very bright cellular images under both one- and two-photon excitation conditions. These dyes emit in the far-red or higher wavelength regions, which is crucial for imaging of tissues with minimal autofluorescence interference by TPM.
Launching a unique ratiometric imaging platform through developing a ratiometric probe for H2O2
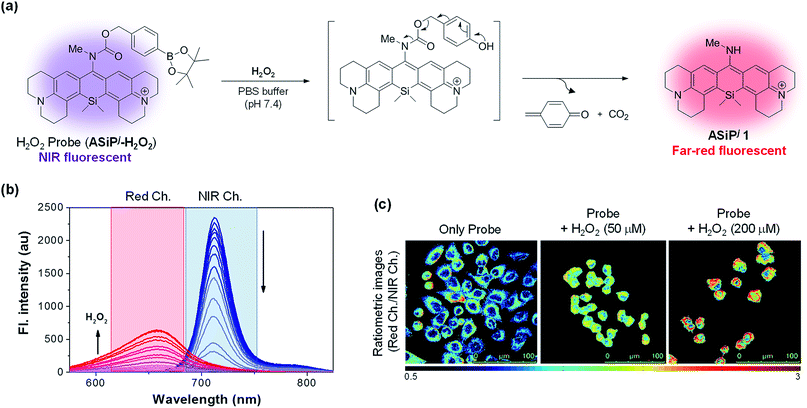
One of our aims in this study was to develop a ratiometric sensing platform based on the ASiPj–NIR-ASiPj couple. The dye couple would constitute a promising ratiometric sensing system if the latter could be converted into the former triggered by an analyte or other stimuli, which will be accompanied by a considerable spectral shift (Δλ > 60 nm). To demonstrate this scenario, we have prepared a fluorescent probe ASiPj-H2O2 (Fig. 5a) having a boronate group, which is well known to react with hydrogen peroxide.45Hydrogen peroxide (H2O2), the major reactive oxygen species, is vital to a certain level for normal cell functions such as cell signaling and defense.46 On the other hand, abnormal generation or accumulation of H2O2 can cause several human diseases including cancer and Alzheimer's and Parkinson's diseases.47,48 Therefore, efficient detection methods of H2O2 levels in biological systems are in high demand. In the presence of H2O2, the boronate moiety of the NIR emitting probe (ASiPj-H2O2) would undergo oxidative cleavage to generate a self-immolative carbamate intermediate, which in turn would undergo spontaneous decarboxylation to generate far-red fluorescent ASiPj1 as the product (Fig. 5a). As we expected, the probe emitted in the NIR region with the maximum peak at 712 nm when excited at 702 nm (the wavelength of maximum absorbance) (Fig. 5b). Upon the addition of H2O2 to a solution of the probe (10 μM in 10 mM PBS, pH 7.4), the probe peak gradually decreased, while a new emission peak at 655 nm started to increase when excited at 481 nm, the wavelength of maximum absorbance of ASiPj1. The corresponding absorption spectra also showed a gradual shift from the NIR (λabs = 702 nm) to visible region (λabs = 481 nm) (Fig. S23, ESI†). The fluorescence intensity ratio between the two peaks (I655/I712) showed a linear increase with time as well as with the concentrations of H2O2 (Fig. S24–S27, ESI†). The limit of detection was calculated to be as low as 9 nM (Fig. S27†). Thus, the probe is highly sensitive and can be used to detect low concentrations of endogenous H2O2 in cells. The probe also showed excellent selectivity towards H2O2 over various competing species such as ATP, NAD, metal ions (Fe(III), Fe(II), Zn(II), and Cu(II)), biothiols (GSH and Cys), reactive nitrogen species (NO) and other reactive oxygen species (tBuOOH, tBuOO˙, HO˙, and HOCl) (Fig. S28, ESI†). After confirming the excellent sensing properties of the probe in solution, we applied it for the ratiometric imaging of H2O2 in A549 cells. The cells were incubated with either the probe alone or with the probe and exogenously added H2O2 as a positive control. The images were captured in two separate emission channels: (1) an NIR channel (650–800 nm; λex = 633 nm) for the probe and (2) a red channel (500–650 nm; λex = 488 nm) for the product. The ratio (IRed/INIR) images were constructed from the pixel-to-pixel intensity ratios of the images collected in the red channel to those collected in the NIR channel. The data show the lowest intensity ratio for the cells treated with the probe alone, but a gradual increase in the intensity ratio as the exogenous H2O2 concentration was increased (Fig. 5c).
The results demonstrate that the ASiPj–NIR-ASiPj couple provides a novel sensing platform that enables ratiometric cellular imaging of a biological analyte, here H2O2, with a minimal spectral overlap through the collection of emissions from NIR to far-red channels, which is promising in that we can minimize the significant autofluorescence from the green and yellow emission channels. By introducing various analyte-specific reactive groups through a self-immolative linker at the 10-amino group of ASiPj dyes, similarly we could generate ratiometric imaging probes for other analytes of biological importance.
Conclusions
Organic dyes that absorb and emit in the biological optical window (650–950 nm) are in great demand for bioimaging with reduced light scattering and minimal autofluorescence interference. Only a few classes of such fluorophores are currently available, demanding a new type of far-red/NIR emitting dye. Even rare are those far-red/NIR emitting dyes with two-photon imaging capability. We have developed a novel class of such fluorophores based on a new Si-pyronin dye platform. The introduction of N-acyl or N-alkoxycarbonyl groups on the amine nitrogen of known amino-Si-pyronin dyes causes large bathochromic shifts, leading to the new NIR fluorophores. The promising in vitro photophysical properties of the dyes, however, deteriorated in cells, owing to unexpected chemical conversions. This cellular instability issue was eventually solved by changing the known Si-xanthene core into a new julolidine-derived one. The new julolidine-derived amino-Si-pyronin (ASiPj) and the corresponding NIR-emitting derivatives (NIR-ASiPj) emit in the far-red and NIR wavelength regions, respectively, afford very bright cellular images, and also have good two-photon absorbing properties. In addition, the new fluorophores are biocompatible, photostable, and have satisfactory water solubility. Furthermore, the NIR-ASiPj dyes, coupled with the corresponding ASiPj dyes, offer a unique ratiometric imaging platform that enables bioimaging with minimal spectral overlap, as demonstrated with a ratiometric hydrogen peroxide probe. The new dye systems thus hold great promise for bioimaging application and for the development of ratiometric imaging probes.Experimental section
The detailed experimental procedures for the measurement of one-photon and two-photon photophysical properties, cell viability assay, and synthesis and characterization data of the new dyes and probe (NMR and HR-mass spectra) are provided in the ESI.† Only the synthetic procedures of NIR-ASiPj1–3 are described here.Synthesis of NIR-ASiPj1
A solution of ASiPj1 (30 mg, 0.05 mmol) in anhydrous dichloromethane (5 mL) at 0 °C was treated with diisopropylethylamine (44 μL, 0.25 mmol) and pyridine (40 μL, 0.5 mmol), and the mixture was stirred for 10 min at 0 °C followed by the addition of benzyl chloroformate (71 μL, 0.5 mmol). The reaction mixture, after being stirred at room temperature for 3 h, was diluted with dichloromethane and washed with 1 N HCl. The organic layer was evaporated and the residue was subjected to flash column chromatography (eluent: CH2Cl2/MeOH = 95/5) to afford the product, which was further purified by preparative HPLC (XBD-C18; gradient 30/70–100/0 A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B over 40 min, A = acetonitrile, B = 0.1% TFA in water, flow rate = 20 mL min−1) to obtain the pure product (4 mg, 11%) as a green solid. 1H NMR (500 MHz, CD3OD, 298 K, δ): 7.25–7.21 (m, 3H), 7.10–7.05 (m, 4H), 5.04 (s, 2H), 3.61–3.57 (m, 8H), 3.22 (s, 3H), 2.96–2.94 (m, 4H), 2.72–2.68 (m, 2H), 2.62–2.58 (m, 2H), 2.06–2.05 (m, 4H), 1.97–1.96 (m, 4H), and 0.67 (d, J = 6.0 Hz, 6H); 13C NMR (125 MHz, CD3OD, 298 K, δ): 161.0, 160.5, 157.2, 157.1, 152.0, 142.9, 142.7, 138.3, 137.9, 135.2, 133.4, 129.9, 129.7, 129.6, 129.5, 129.2, 128.9, 126.6, 125.9, 125.7, 69.2, 68.8, 53.1, 52.5, 40.0, 39.8, 33.2, 30.9, 30.6, 30.1, 28.9, 28.8, 26.2, 22.1, 21.9, 14.6, −0.7, and −0.8; HRMS (ESI) m/z: [M]+ calcd for C36H42N3O2Si+, 576.3046; found, 576.3042.
B over 40 min, A = acetonitrile, B = 0.1% TFA in water, flow rate = 20 mL min−1) to obtain the pure product (4 mg, 11%) as a green solid. 1H NMR (500 MHz, CD3OD, 298 K, δ): 7.25–7.21 (m, 3H), 7.10–7.05 (m, 4H), 5.04 (s, 2H), 3.61–3.57 (m, 8H), 3.22 (s, 3H), 2.96–2.94 (m, 4H), 2.72–2.68 (m, 2H), 2.62–2.58 (m, 2H), 2.06–2.05 (m, 4H), 1.97–1.96 (m, 4H), and 0.67 (d, J = 6.0 Hz, 6H); 13C NMR (125 MHz, CD3OD, 298 K, δ): 161.0, 160.5, 157.2, 157.1, 152.0, 142.9, 142.7, 138.3, 137.9, 135.2, 133.4, 129.9, 129.7, 129.6, 129.5, 129.2, 128.9, 126.6, 125.9, 125.7, 69.2, 68.8, 53.1, 52.5, 40.0, 39.8, 33.2, 30.9, 30.6, 30.1, 28.9, 28.8, 26.2, 22.1, 21.9, 14.6, −0.7, and −0.8; HRMS (ESI) m/z: [M]+ calcd for C36H42N3O2Si+, 576.3046; found, 576.3042.
Synthesis of NIR-ASiPj2
A solution of ASiPj1 (30 mg, 0.05 mmol) in anhydrous dichloromethane (5 mL) at 0 °C was treated with 2,6-lutidine (87 μL, 0.75 mmol) and stirred for 10 min at 0 °C, followed by the addition of acetyl chloride (36 μL, 0.5 mmol). The resulting mixture, after being stirred at room temperature for 12 h, was diluted with dichloromethane and washed with 1 N HCl. The organic layer was evaporated and the residue was subjected to column chromatography (eluent: CH2Cl2/MeOH = 95/5) to afford the product, which was further purified by preparative HPLC (XBD-C18; gradient 30/70–100/0 A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B over 40 min, A = acetonitrile, B = 0.1% TFA in water, flow rate = 20 mL min−1) to obtain the pure product (5 mg, 15%) as a green solid. 1H NMR (500 MHz, CD3OD, 298 K, δ): 7.10 (s, 2H), 3.63–3.60 (m, 8H), 3.22 (s, 3H), 2.98–2.95 (m, 4H), 2.79–2.77 (m, 4H), 2.09–2.06 (m, 4H), 2.03–2.00 (m, 4H), 1.83 (s, 3H), and 0.68 (s, 6H); 13C NMR (125 MHz, CD3OD, 298 K, δ): 173.0, 160.2, 152.2, 142.6, 134.7, 134.0, 127.2, 125.4, 53.2, 53.1, 52.6, 39.1, 30.1, 28.9, 22.0, 21.9, and −0.8; HRMS (ESI) m/z: [M]+ calcd for C30H38N3OSi+, 484.2784; found, 484.2781.
B over 40 min, A = acetonitrile, B = 0.1% TFA in water, flow rate = 20 mL min−1) to obtain the pure product (5 mg, 15%) as a green solid. 1H NMR (500 MHz, CD3OD, 298 K, δ): 7.10 (s, 2H), 3.63–3.60 (m, 8H), 3.22 (s, 3H), 2.98–2.95 (m, 4H), 2.79–2.77 (m, 4H), 2.09–2.06 (m, 4H), 2.03–2.00 (m, 4H), 1.83 (s, 3H), and 0.68 (s, 6H); 13C NMR (125 MHz, CD3OD, 298 K, δ): 173.0, 160.2, 152.2, 142.6, 134.7, 134.0, 127.2, 125.4, 53.2, 53.1, 52.6, 39.1, 30.1, 28.9, 22.0, 21.9, and −0.8; HRMS (ESI) m/z: [M]+ calcd for C30H38N3OSi+, 484.2784; found, 484.2781.
Synthesis of NIR-ASiPj3
A solution of ASiPj1 (30 mg, 0.05 mmol) in anhydrous dichloromethane (10 mL) at 0 °C was treated with diisopropylethylamine (44 μL, 0.25 mmol) and stirred for 10 min at 0 °C, followed by the addition of trifluoroacetic anhydride (21 μL, 0.15 mmol). The resulting mixture, after being stirred at room temperature for 3 h, was diluted with dichloromethane and washed with 1 N HCl. The organic layer was evaporated and the residue was subjected to column chromatography (eluent: CH2Cl2/MeOH = 95/5) to afford the product, which was further purified by preparative HPLC (XBD-C18; gradient 30/70–100/0 A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B over 40 min, A = acetonitrile, B = 0.1% TFA in water, flow rate = 20 mL min−1) to obtain the pure product (7 mg, 21%) as a green solid. 1H NMR (500 MHz, CD3OD, 298 K, δ): 7.08 (s, 2H), 3.64–3.62 (m, 8H), 3.40 (s, 3H), 2.98–2.96 (m, 4H), 2.80–2.76 (m, 4H), 2.09–2.06 (m, 4H), 2.01–1.99 (m, 4H), and 0.69 (d, J = 15.0 Hz, 6H); 13C NMR (125 MHz, CD3OD, 298 K, δ): 156.5, 152.2, 142.2, 134.6, 134.2, 134.0, 133.9, 127.1, 127.1, 125.5, 123.6, 57.6, 53.3, 53.2, 52.6, 41.6, 30.9, 30.1, 28.9, 28.8, 22.0, 21.9, −0.7, and −1.0; HRMS (ESI) m/z: [M]+ calcd for C30H35F3N3OSi+, 538.2502; found, 538.2505.
B over 40 min, A = acetonitrile, B = 0.1% TFA in water, flow rate = 20 mL min−1) to obtain the pure product (7 mg, 21%) as a green solid. 1H NMR (500 MHz, CD3OD, 298 K, δ): 7.08 (s, 2H), 3.64–3.62 (m, 8H), 3.40 (s, 3H), 2.98–2.96 (m, 4H), 2.80–2.76 (m, 4H), 2.09–2.06 (m, 4H), 2.01–1.99 (m, 4H), and 0.69 (d, J = 15.0 Hz, 6H); 13C NMR (125 MHz, CD3OD, 298 K, δ): 156.5, 152.2, 142.2, 134.6, 134.2, 134.0, 133.9, 127.1, 127.1, 125.5, 123.6, 57.6, 53.3, 53.2, 52.6, 41.6, 30.9, 30.1, 28.9, 28.8, 22.0, 21.9, −0.7, and −1.0; HRMS (ESI) m/z: [M]+ calcd for C30H35F3N3OSi+, 538.2502; found, 538.2505.
Conflicts of interest
The authors are listed as co-inventors of a patent application related to the technology described in this work.Acknowledgements
This work is financially supported by the Global Research Laboratory Program (2014K1A1A2064569) through the National Research Foundation (NRF) funded by the Ministry of Science, ICT & Future Planning, Republic of Korea. S. Singha acknowledges support from the Basic Science Research Program (2018R1D1A1B07051403) through the National Research Foundation (NRF) of Korea funded by the Ministry of Education.Notes and references
- C. K. Rosenthal, et al. , Nat. Cell Biol., 2009, 11, 1165 CrossRef PubMed.
- J. Zhang, R. E. Campbell, A. Y. Ting and R. Y. Tsien, Nat. Rev. Mol. Cell Biol., 2002, 3, 906 CrossRef CAS PubMed.
- R. Weissleder, Nat. Biotechnol., 2001, 19, 316 CrossRef CAS PubMed.
- J. V. Frangioni, Curr. Opin. Chem. Biol., 2003, 7, 626 CrossRef CAS PubMed.
- L. D. Lavis and R. T. Raines, ACS Chem. Biol., 2014, 9, 855 CrossRef CAS PubMed.
- J. B. Pawley, Handbook of confocal microscopy, Plenum Press, NY, USA, 2nd edn, 1995 Search PubMed.
- A. H. Herz, Photogr. Sci. Eng., 1974, 18, 323 CAS.
- Y. W. Jun, H. R. Kim, Y. J. Reo, M. Dai and K. H. Ahn, Chem. Sci., 2017, 8, 7696 RSC.
- D. Kim, H. Moon, S. H. Baik, S. Singha, Y. W. Jun, T. Wang, K. H. Kim, B. S. Park, J. Jung, I. Mook-Jung and K. H. Ahn, J. Am. Chem. Soc., 2015, 137, 6781 CrossRef CAS PubMed.
- Y. Koide, Y. Urano, K. Hanaoka, T. Terai and T. Nagano, ACS Chem. Biol., 2011, 6, 600 CrossRef CAS PubMed.
- M. Fu, Y. Xiao, X. Qian, D. Zhao and Y. Xu, Chem. Commun., 2008, 1780 RSC.
- T. Ikeno, T. Nagano and K. Hanaoka, Chem.–Asian J., 2017, 12, 1435 CrossRef CAS PubMed.
- Y. Koide, Y. Urano, K. Hanaoka, W. Piao, M. Kusakabe, N. Saito, T. Terai, T. Okabe and T. Nagano, J. Am. Chem. Soc., 2012, 134, 5029 CrossRef CAS PubMed.
- T. Wang, Q.-J. Zhao, H.-G. Hu, S.-C. Yu, X. Liu, L. Liu and Q.-Y. Wu, Chem. Commun., 2012, 48, 8781 RSC.
- T. Egawa, K. Hanaoka, Y. Koide, S. Ujita, N. Takahashi, Y. Ikegaya, N. Matsuki, T. Terai, T. Ueno, T. Komatsu and T. Nagano, J. Am. Chem. Soc., 2011, 133, 14157 CrossRef CAS PubMed.
- Y. Kushida, T. Nagano and K. Hanaoka, Analyst, 2015, 140, 685 RSC.
- Y. Gabe, T. Ueno, Y. Urano, H. Kojima and T. Nagano, Anal. Biochem., 2006, 386, 621 CAS.
- M. Beija, C. A. M. Afonso and J. M. G. Martinho, Chem. Soc. Rev., 2009, 38, 2410 RSC.
- Z. Mao, H. Jiang, X. Song, W. Hu and Z. Liu, Anal. Chem., 2017, 89, 9620 CrossRef CAS PubMed.
- D. Kim, H. G. Ryu and K. H. Ahn, Org. Biomol. Chem., 2014, 12, 4550 RSC.
- H. M. Kim and B. R. Cho, Chem. Rev., 2015, 115, 5014 CrossRef CAS PubMed.
- J. Bañuelos, V. Martín, C. F. A. Gómez-Durán, I. J. A. Córdoba, E. Peña-Cabrera, I. García-Moreno, Á. Costela, M. E. Pérez-Ojeda, T. Arbeloa and Í. L. Arbeloa, Chem.–Eur. J., 2011, 17, 7261 CrossRef PubMed.
- H. Zhang, J. Liu, Y.-Q. Sun, Y. Huo, Y. Li, W. Liu, X. Wu, N. Zhu, Y. Shi and W. Guo, Chem. Commun., 2015, 51, 2721 RSC.
- X. Zhou, Y. Zeng, C. Liyan, X. Wu and J. Yoon, Angew. Chem., Int. Ed., 2016, 55, 4729 CrossRef CAS PubMed.
- K. N. Bobba, M. Won, I. Shim, N. Velusamy, Z. Yang, J. Qu, J. S. Kim and S. Bhuniya, Chem. Commun., 2017, 53, 11213 RSC.
- Y. W. Jun, T. Wang, S. Hwang, D. Kim, D. Ma, K. H. Kim, S. Kim, J. Jung and K. H. Ahn, Angew. Chem., Int. Ed., 2018, 57, 10142 CrossRef CAS PubMed.
- P. Horváth, P. Šebej, T. Šolomek and P. Klán, J. Org. Chem., 2015, 80, 1299 CrossRef PubMed.
- K. H. Ahn, H. G. Ryu, Y. W. Jun, K. H. Kim and S. Singha, One- or two-photon absorbing fluorescent dyes based on amino-Si-pyronin compound and the uses thereof, KOR 10-2017-0110708, 2017.
- A. N. Butkevich, G. Lukinavičius, E. D'Este and S. W. Hell, J. Am. Chem. Soc., 2017, 139, 12378 CrossRef CAS PubMed.
- H. Zhang, J. Liu, L. Wang, M. Sun, X. Yan, J. Wang, J.-P. Guo and W. Guo, Biomaterials, 2018, 158, 10 CrossRef CAS PubMed.
- W. Sun, S. Guo, C. Hu, J. Fan and X. Peng, Chem. Rev., 2016, 116, 7768 CrossRef CAS PubMed.
- H. Lu, J. Mack, Y. Yang and Z. Shen, Chem. Soc. Rev., 2014, 43, 4778 RSC.
- H. Chen, B. Dong, Y. Tang and W. Lin, Acc. Chem. Res., 2017, 50, 1410 CrossRef CAS PubMed.
- T. Ikeno, T. Nagano and K. Hanaoka, Chem.–Asian J., 2017, 12, 1435 CrossRef CAS PubMed.
- M. H. Lee, J. S. Kim and J. L. Sessler, Chem. Soc. Rev., 2015, 44, 4185 RSC.
- J. Tang, Z. Guo, Y. Zhang, B. Bai and W.-H. Zhu, Chem. Commun., 2017, 53, 10520 RSC.
- K. Umezawa, M. Yoshida, M. Kamiya, T. Yamasoba and Y. Urano, Nat. Chem., 2017, 9, 279 CrossRef CAS PubMed.
- H. Nie, L. Qiao, W. Yang, B. Guo, F. Xin, J. Jing and X. Zhang, J. Mater. Chem. B, 2016, 4, 4826 RSC.
- G. Lukinavičius, K. Umezawa, N. Olivier, A. Honigmann, G. Yang, T. Plass, V. Mueller, L. Reymond, I. R. Corrêa Jr, Z.-G. Luo, C. Schultz, E. A. Lemke, P. Heppenstall, C. Eggeling, S. Manley and K. Johnsson, Nat. Chem., 2013, 5, 132 CrossRef PubMed.
- A. N. Butkevich, G. Y. Mitronova, S. C. Sidenstein, J. L. Klocke, D. Kamin, D. N. H. Meineke, E. D'Este, P.-T. Kraemer, J. G. Danzl, V. N. Belov and S. W. Hell, Angew. Chem., Int. Ed., 2016, 55, 3290 CrossRef CAS PubMed.
- G. Lukinavičius, L. Reymond, K. Umezawa, O. Sallin, E. D'Este, F. Göttfert, H. Ta, S. W. Hell, Y. Urano and K. Johnsson, J. Am. Chem. Soc., 2016, 138, 9365 CrossRef PubMed.
- A. N. Butkevich, V. N. Belov, K. Kolmakov, V. V. Sokolov, H. Shojaei, S. C. Sidenstein, D. Kamin, J. Matthias, R. Vlijm, J. Engelhardt and S. W. Hell, Chem.–Eur. J., 2017, 23, 12114 CrossRef CAS PubMed.
- S.-N. Uno, M. Kamiya, T. Yoshihara, K. Sugawara, K. Okabe, M. C. Arhan, H. Fujita, T. Funatsu, Y. Okada, S. Tobita and Y. Urano, Nat. Chem., 2014, 6, 681 CrossRef CAS PubMed.
- E. Kozma, G. E. Girona, G. Paci, E. A. Lemke and P. Kele, Chem. Commun., 2017, 53, 6696 RSC.
- D. Srikun, E. W. Miller, D. W. Domaille and C. J. Chang, J. Am. Chem. Soc., 2008, 130, 4596 CrossRef CAS PubMed.
- E. A. Veal, A. M. Day and B. A. Morgan, Mol. Cell, 2007, 26, 1 CrossRef CAS PubMed.
- T. Finkel, M. Serrano and M. A. Blasco, Nature, 2007, 448, 767 CrossRef CAS PubMed.
- K. J. Barnham, C. L. Masters and A. I. Bush, Nat. Rev. Drug Discovery, 2004, 3, 205 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Supporting figures and schemes, experimental procedures for the measurement of one-photon and two-photon photophysical properties, cell viability assay, and detailed synthesis and characterization data of the new dyes and probe. See DOI: 10.1039/c9sc02287b |
| This journal is © The Royal Society of Chemistry 2019 |