 Open Access Article
Open Access ArticlePreparation and applications of electrochemical chemosensors based on carbon-nanomaterial-modified molecularly imprinted polymers
Rijun
Gui†
 *a,
Huijun
Guo†
b and
Hui
Jin
*a
*a,
Huijun
Guo†
b and
Hui
Jin
*a
aCollege of Chemistry and Chemical Engineering, Intellectual Property Research Institute, Qingdao University, Shandong 266071, PR China. E-mail: guirijun@qdu.edu.cn; jinhui@qdu.edu.cn; Fax: +86 532 85953981; Tel: +86 532 85953981
bAdvanced Fiber and Composites Research Institute, Jilin Institute of Chemical Technology, Jilin 132022, PR China
First published on 29th July 2019
Abstract
The past few decades have witnessed a rapid development in electrochemical chemosensors (ECCSs). The integration of carbon nanomaterials (CNMs) and molecularly imprinted polymers (MIPs) has endowed ECCSs with high selectivity and sensitivity toward target detection. Due to the integrated merits of MIPs and CNMs, CNM-modified MIPs as ECCSs have been widely reported and have excellent detection applications. This review systematically summarized the general categories, preparation strategies, and applications of ECCSs based on CNM-modified MIPs. The categories include CNM-modified MIPs often hybridized with various materials and CNM-encapsulated or CNM-combined imprinting silica and polymers on working electrodes or other substrates. The preparation strategies include the polymerization of MIPs on CNM-modified substrates, co-polymerization of MIPs and CNMs on substrates, drop-casting of MIPs on CNM-modified substrates, self-assembly of CNMs/MIP complexes on substrates, and so forth. We discussed the in situ polymerization, electro-polymerization, and engineering structures of CNM-modified MIPs. With regard to potential applications, we elaborated the detection mechanisms, signal transducer modes, target types, and electrochemical sensing of targets in real samples. In addition, this review discussed the present status, challenges, and prospects of CNM-modified MIP-based ECCSs. This comprehensive review is desirable for scientists from broad research fields and can promote the further development of MIP-based functional materials, CNM-based hybrid materials, advanced composites, and hybrid materials.
1. Introduction
1.1. Development process and preparation of MIPs
Before discussing molecularly imprinted polymers (MIPs), we review the development process of the molecular imprinting technology (MIT). The history of MIT seems to be somewhat older. The first report on MIT dates back to the 1930s. As early as 1931, Polyakov first interpreted the selective template effects of polymers.1 Templates and additives were included in template-participated polymerization. In 1940, Pauling postulated molecular recognition and involved antigens as the template in the self-assembly of protein antibodies.2 During the subsequent few decades, there was no significant progress in MIT. Thereafter, Wulff et al. prepared organic polymers via reversible chemical bonds.3 Vlatakis et al. prepared MIP based on noncovalent MIT.4 After the pioneering studies, numerous scientists have paid close attention to MIT. Since then, MIT-related studies have started to grow rapidly.5The general preparation of MIP involves covalence, noncovalence, or semi-covalence binding interactions between the templates and monomers. Wulff et al. first proposed a covalent approach to fabricate reversible covalent bonds between the templates and monomers before polymerization.3 A marked merit of the covalent approach is the high stability of the template–monomer interactions, which yield considerable homogenous binding sites.6 However, intense actions result in difficulty to thoroughly remove the templates. Arshady et al. employed a noncovalent approach to combine templates with monomers through in situ noncovalent interactions including van der Waals forces, hydrogen binding, and electrostatic interactions.7 Under weak interactions, the preparation procedure of MIPs is relatively facile, and most of the monomers can interact with most of the templates. This approach has been widely used for MIP preparation,8–12 but certain drawbacks inevitably exist because of the low selectivity of the binding sites. A semi-covalent approach has the combined features of both covalent and noncovalent approaches. MIPs prepared from covalent interactions can re-recognize templates via the noncovalent molecular imprinting process and reversible bonds.13,14 Due to the advantages of two binding interactions, a semi-covalent imprinting approach can be used to fabricate stable, stoichiometric complexes in covalent imprinting, realizing fast guest binding in noncovalent imprinting.15
Various strategies involve the preparation of MIP. One of the most frequently used strategies is bulk polymerization. MIPs serve as the receptors to recognize the target molecules or templates. A general procedure for MIP preparation includes three steps. Step (I) is the prepolymerization of the functional monomers. Step (II) is the preparation of molecular recognition sites on high cross-linked polymers via noncovalent or covalent interactions. Step (III) is the generation of recognition cavities after template removal. These cavities are complementary to the target molecules with specific shapes, sizes, structures, and functional groups.16–19 Bulk polymerization is simple and universal, but it is time-consuming and labor-intensive. The resulting MIP particles are often accompanied by irregular shapes and have low recovery as useable particles.20 With regard to suspension polymerization, water as a dispersing agent reduces the number and strength of binding sites between the functional monomers and templates.21 Precipitation polymerization belongs to a well-suited strategy to synthesize MIP microspheres with desirable features. During the polymerization process, the growing polymer chains are insoluble in porogens and tend to precipitate. The noticeable advantage of this strategy is the convenient achievement of MIP in the micron-size range, without surfactants and the control of polymerization conditions.22,23 A two-step or multiple swelling polymerization process can be used to fabricate MIP beads via laborious steps. The uniformed-sized seed particles remain suspended in water. After the addition of suitable organic solvents, the initial particles swell to their final size with the desired performance.5,24 In addition, the surface imprinting technique attracts considerable interest and serves as a new alternative for the preparation of MIP with improved performance. The imprinted binding sites are located on or near the surface of polymers. These characteristics conquer some limitations, such as limited mass transfer, small binding capacity, and incomplete removal of templates.25,26 Two principal strategies are used for the preparation of surface-imprinted polymers. One involves the synthesis of a thin polymer film with the bulk imprinting technique. The other comprises the attachment of templates on the surface of flat or spherical substrates through polymerization.
1.2. Characteristics of MIP and applications as electrochemical chemosensors (ECCSs)
MIP is similar to an antibody or bioreceptor. With regard to synthetic polymers, MIP has predetermined selectivity toward recognizing particular targets or other species with structural similarities to the targets or templates. Due to the existence of artificially created recognition sites, MIP is complementary to the sizes, shapes, molecular structures, or functional groups of the templates. MIP can specifically bind targets and imitate natural receptor systems, such as enzymes, antibodies, and hormones.27,28 The significant characteristics of MIPs are facile preparation, specific identification, high stability and selectivity, wide practicability, etc. MIPs have attracted considerable attention in current research fields, such as extraction, separation, optical elements, electronic and optoelectronic devices, sensors, catalysis, etc. The prepared MIPs often lack electrocatalytic and conductive activities. Other drawbacks are low sensitivity toward target recognition, large sizes, heterogeneous size distribution, partial embedding of binding sites, poor site accessibility for templates, etc. In this context, a proper modification of MIP with active substances is essential and can improve the performance of MIP applications. During the past few decades, numerous studies have reported using MIPs as ECCSs. There are more than 1000 target molecules and imprinting structures using different templates, such as inorganic ions, drugs, nucleic acids, proteins, viruses, and even cells.11 MIP-based ECCSs integrate the merits of MIP and ECCS, exhibiting high selectivity, sensitivity, stability, facile preparation, low cost, reusability, and miniaturization.29In recent years, MIP-based ECCSs have attracted tremendous interest in promising applications, such as biomedical diagnosis, biochemical analysis, environmental monitoring, and food safety evaluation. In particular, they have been widely used for the accurate analysis of important biomolecules, such as proteins, hormones, drugs, nucleic acids, etc. Mechanical and thermal stabilities as well as high specificity and sensitivity of MIP-based ECCSs toward targets endow them with promising potential toward high-performance sensing applications over the traditional instruments, techniques, and sensors. As tailor-made biomimetic materials, MIPs have obvious priority over other recognition elements. MIP-based ECCSs use MIPs as the specific recognition elements of templates (targets) and act as smart devices for signal outputs. High selectivity and sensitivity are two major requirements in the design of efficient sensors. To improve the sensitivity of MIP-based ECCSs, the principal routes involve enlarging the effective sensing surface areas and the modification of sensing surfaces with electroactive substances. To enlarge the sensing surface areas, commercial electrodes and other electrochemical substrates are widely used to fabricate ECCSs, such as nickel foam (NF), conducting glass, Cu wire, Au film, indium tin oxide, etc. Sensitive electrochemical sensing devices can be prepared by constructing ECCSs on various substrates with higher surface areas. The modification of MIP with electroactive substances is a superior route to improve the sensitivity of MIP-based ECCSs.
1.3. Carbon nanomaterials (CNMs)-modified MIPs as ECCSs and introduction of this review
In earlier reports, various electroactive substances have been used to construct MIP-based ECCSs, such as CNMs; noble metal nanoparticles (NPs); alloyed, bimetal, and metal oxide NPs; nanospheres and nanosheets; ionic liquids (ILs); polymers; organic–inorganic hybrid materials; and composite materials. Among these electroactive substances, CNMs have attracted considerable attention because of their unique electrical, optical, thermal, mechanical, and chemical properties; low cost; easy availability of raw materials; high biocompatibility; and deformability. Currently, CNMs have been widely used in electronics, optoelectronics, photovoltaics, flexible sensors, advanced devices, and bioassay technology (biosensors). Their excellent properties majorly depend on the functional atomic structures of CNMs and the synergistic interactions of CNMs with other materials. Typical CNMs include graphene (GR), graphene oxide (GO), reduced graphene oxide (rGO), single- and multiwalled carbon nanotubes (SWCNTs and MWCNTs), mesoporous/porous/three-dimensional (3D) carbon structures, GR, and carbon quantum dots (QDs). In this review, we systematically summarize numerous earlier studies regarding ECCSs based on CNM-modified MIPs. Prior to this current review, recent reviews involving MIP or MIP-based sensors have been introduced.11,29–42 Whitcombe et al. reviewed the rational development of MIP-based sensors for protein detection.38 Huynh et al. reviewed MIP as the recognition material for use in electronic tongues.39 Ashley et al. summarized MIPs for sample preparation and biosensing in food analyses.40 Dabrowski et al. reported a nanostructured MIP for protein chemosensing.41During the past decade, numerous studies have investigated ECCSs based on CNM-modified MIPs due to the integrated merits of MIPs, ECCSs, and CNMs. Such a type of ECCS was largely prepared and widely used for the electrochemical sensing of various targets, with obvious superiority over other analytical techniques. Earlier reviews have referred to MIP-based sensors, but a timely and comprehensive review that summarizes the currently significant topic of CNM-modified MIPs as ECCSs is still lacking. In this review (Scheme 1), we systematically summarize the recent advances in CNM-modified-MIP-based ECCSs, focusing on the general categories, preparation strategies, and analytical applications. With regard to categories, we review the CNM-modified MIPs without or with the hybridization of other materials, and ECCSs fabricated on various work electrodes and substrates. With regard to preparation strategies, we firstly introduce CNM-encapsulated or CNM-combined imprinted silica and polymers. Then, we summarize the various preparation strategies, including the polymerization of MIPs on CNM-modified substrates, copolymerization of MIPs and CNMs on substrates, dropcasting of MIPs on CNM-modified substrates, dropcasting of MIP/CNMs on substrates, self-assembly of CNMs/MIP/hybrids on substrates, dropcasting of self-assembled MIP/CNMs/hybrids on substrates. Further, we discuss the in situ chemical polymerization of monomers, electropolymerization of electroactive monomers, dummy templates, and engineering structures of CNM-modified MIPs. With regard to analytical applications, we elaborate the detection mechanisms of nonelectroactive and electroactive targets, signal transducer modes, target types, electrochemical sensing of one and two types of targets, and real samples for detection. This review discusses the present status, challenges, and perspectives in CNM-modified-MIP-based ECCSs. This review is timely, comprehensive, in-depth, and desirable for scientists in broad fields. It will promote the further development of MIP-based functional materials, CNM-based hybrid materials, and other functionalized composites or hybrid materials.
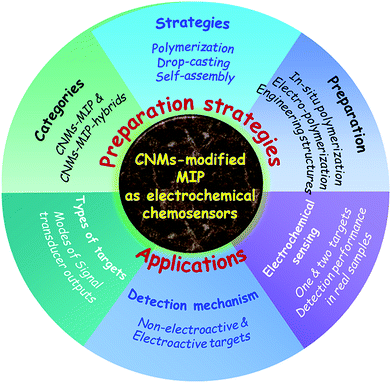 | ||
| Scheme 1 Total architecture of this review, involving the preparation strategies and applications of CNM-modified MIP-based ECCSs. | ||
2. Categories of ECCSs based on CNM-modified MIPs
2.1. MIPs modified with CNMs without hybridization
In this review, we summarize the general categories of ECCSs based on CNM-modified MIPs. On the surfaces of work electrodes and other sensing substrates, CNMs usually are dropcasted or grown in situ to form CNM-modified electrodes or substrates, followed by the in situ polymerization of MIPs or dropcasting of MIPs to fabricate CNM-modified MIPs. We have regularly summarized studies involving CNM-modified-MIP-based ECCSs,43–92 often fabricated onto the surface of commercial glassy carbon electrodes (GCE, Table 1), other work electrodes, and electrochemical substrates (Table 2). There are two general categories to prepare such ECCSs. One is to prepare CNM-modified MIPs on the surface of the work electrodes or other substrates without the hybridization of other materials. The other is to prepare CNM-modified MIPs with the hybridization of inorganic, organic, mixed, hybrid, or composite materials,93–197 as summarized in Tables 3–6.| Sensing materials | Targets | Modes | LOD | Detection ranges | Detected samples | Ref. |
|---|---|---|---|---|---|---|
| a Abbreviation carbon nanotubes, CNTs; glassy carbon electrode, GCE; multiwalled carbon nanotubes, MWCNTs; 3-(3-aminopropyl)-1-vinylimidazole tetrafluoroborate, APVIMBF4; graphene oxide, GO; reduced graphene oxide, rGO; 2-amino-5-mercapto-1,3,4-thiadiazole, AMT; graphene quantum dots, GQDs; single-walled carbon nanotubes, SWCNTs; 3-hexadecyl-1-vinylimidazolium chloride, C16VimCl; bovine serum albumin, BSA; 4,4-methylene diphenylamine, MDA; differential pulse voltammetry, DPV; cyclic voltammetry, CV; square wave voltammetry, SWV; linear sweep voltammograms, LSV; electrochemical impedance spectroscopy, EIS. | ||||||
| Polypyrrole/CNTs/GCE | Dopamine | DPV | 1 × 10−11 M | 5 × 10−11 to 5 × 10−6 M | Human serum, urine | 43 |
| Polydopamine/MWCNTs/GCE | Sunset yellow | DPV | 1.4 nM | 2.2 nM to 4.64 μM | Jelly, drink, chocolate, juice, ice cream, candy | 44 |
| Poly(o-aminophenol)/MWCNTs/GCE | Levofloxacin | DPV | 1 μM | 3–200 μM | Pharmaceuticals | 45 |
| Polypyrrole/MWCNTs–COOH/GCE | Dopamine | DPV | 6 × 10−8 M | 6.25 × 10−7 to 1 × 10−4 M | — | 46 |
| Poly(APVIMBF4)/MWCNTs–COOH/GCE | BSA | CV | 3.91 × 10−10 M | 1.5 × 10−9 to 1.5 × 10−6 M | Liquid milk | 47 |
| Chitosan/graphene/GCE | L-Dopa | DPV | 0.012 μM | 0.4–100 μM | Tablet, blood serum | 48 |
| Chitosan/graphene/GCE | Dopamine | DPV | 1 × 10−11 M | 1 × 10−9 to 8 × 10−8 M, 1 × 10−7 to 1 × 10−4 M | Human blood serum | 49 |
| Polyaniline/graphene/GCE | 2,4,6-Trinitrotoluene (picric acid) | DPV | — | 0.73–31.55 μM | — | 50 |
| Polypyrrole/graphene/GCE | Trimethoprim | SWV | 1.3 × 10−7 M | 1 × 10−6 to 1 × 10−4 M | Human urine | 51 |
| Polyacrylamide/graphene/GCE | Artemisinin | DPV | 2 × 10−9 M | 1 × 10−8 to 4 × 10−5 M | Extract of Artemisia annua L. | 52 |
| Poly(4-vinylpyridine)/graphene/GCE | MDA, aniline | DPV | — | 1–15 μM | White spoon, black spatula, black spoon | 53 |
| Poly(methacrylic acid)/graphene/GCE | 4-Nitrophenol | DPV | 5 nM | 0.01–100, 200–1000 μM | Lake, tap water | 54 |
| Poly(acrylamide)/graphene/GCE | Phoxim | DPV | 2 × 10−8 M | 8 × 10−7 to 1.4 × 10−4 M | Cucumber | 55 |
| Poly(4-vinylbenzoic acid)/graphene/GCE | Thiamethoxam | LSV | 0.04 μM | 0.5–20 μM | Brown rice | 56 |
| Poly(p-vinylbenzoic acid)/graphene/GCE | Imidacloprid | LSV | 0.1 μM | 0.5–15 μM | Brown rice | 57 |
| SiO2/GO/GCE | Paracetamol | DPV | 20 nM | 0.1–80 μM | Urine, tablets | 58 |
| Polypyrrole/GO/GCE | Quercetin | DPV | 4.8 × 10−8 M | 6 × 10−7 to 1.5 × 10−5 M | Apple juices | 59 |
| Polydopamine/GO/GCE | Bovine hemoglobin | DPV | 2 × 10−10 mg mL−1 | 1 × 10−9 to 1 × 10−1 mg mL−1 | Bovine blood plasma | 60 |
| Poly(β-cydodextrin)/GO/GCE | Epigallocatechin gallate | DPV | 8.78 × 10−9 M | 3 × 10−8∼1 × 10−5 M | Tea samples | 61 |
| Poly(methacrylic acid)/GO/GCE | 2,4-Dichlorophenol | DPV | 0.5 nM | 0.004–10 μM | Lake water | 62 |
| Imprinted silica sol–gel/GO/GCE | Mesalazine | DPV | 0.97 μM | 2–20, 20–150 μM | Drug tablet | 63 |
| Polypyrrole/rGO/GCE | Melamine | EIS | 0.83 nM | 4–240 nM | Liquid milk | 64 |
| Poly(o-phenylenediamine)/rGO/GCE | Imidacloprid | LSV | 4 × 10−7 M | 7.5 × 10−7 to 7 × 10−5 M | Pears | 65 |
| Poly(methacrylic acid)/rGO/GCE | 4-Nitrophenol | DPV | 0.005 μM | 0.01–100 μM | Tap/river water | 66 |
| Poly(AMT)/rGO/GCE | Uric acid, tyrosine | DPV | 3.2 nM, 0.046 μM | 0.01–100, 0.1–400 μM | Human serum, urine | 67 |
| Poly(5-amino-8-hydroxyquinoline)/rGO/GCE | Dopamine | DPV | 32.7 nM | 1 × 10−7 to 14 × 10−7 M | Human blood plasma | 68 |
| Poly(o-phenylenediamine)/Ag–N-doped rGO/GCE | Salbutamol | DPV | 7 nM | 0.03–20 μM | Human urine, pork | 69 |
| Polypyrrole/GQDs/GCE | Bisphenol A | DPV | 0.04 μM | 0.1–50 μM | Sea, bottled water | 70 |
| Poly(3,4-ethylenedioxythiophene)/graphene nanoribbons/GCE | Octylphenol | LSV | 6 nM | 0.04–8 μM | River, bottled mineral water, urine | 71 |
| Poly(phenyl trimethoxysilane)/SWCNTs/graphene/GCE | Propyl gallate | DPV | 5 × 10−8 M | 8 × 10−8 to 2.6 × 10−3 M | Edible oil, instant noodles, cookies | 72 |
| Polypyrrole/rGO–carbon dots/GCE | Rutoside | DPV | 3 nM | 0.01–6.5 μM | Human serum | 73 |
| Poly(3-aminopropyltriethoxysilane)/GQDs/graphene nanoplatelets/GCE | Metronidazole | DPV | 0.52 nM | 0.005–0.75, 0.75–10 μM | Human blood plasma | 74 |
| Poly(C16VimCl)/MWCNTs/mesoporous carbon/porous rGO/GCE | Chloramphenicol | DPV | 1 × 10−10 M | 5 × 10−9 to 5 × 10−7 M, 5 × 10−7 to 4 × 10−6 M | Milk, honey | 75 |
| Sensing materials | Targets | Modes | LOD | Detection ranges | Detected samples | Ref. |
|---|---|---|---|---|---|---|
| a Abbreviation: multiwalled carbon nanotubes, MWCNTs; tetraethylene glycol-3-morpholine propionate acrylate, TEGMPA; reduced graphene oxide, rGO; N-acryloyl-4-aminobenzamide, N-ABA; graphene quantum dots, GQDs; carbon nanotubes, CNTs; lindane (γ-hexachlorocyclohexane), γ-HCCH; bovine serum albumin, BSA; differential pulse voltammetry, DPV; linear sweep voltammograms, LSV; differential pulse cathodic stripping voltammetry, DPCSV; square wave voltammetry, SWV; cyclic voltammetry, CV; differential pulse anodic stripping voltammetry, DPASV. | ||||||
| Poly(itaconic acid)/MWCNTs/carbon paste electrode | Bi3+ | DPV | 8.9 nM | 0.2–2 μM | Bi substrate, sea, river water, human plasma | 76 |
| Poly(methacrylic acid)/MWCNTs/Cu electrode | γ-HCCH | LSV | 1 × 10−10 M | 1 × 10−10 to 1 × 10−3 M | Fruits, vegetables, water | 77 |
| Polybenzidine/MWCNTs–COOH/pencil graphite electrode | L-Methionine | DPCSV | 2.4–3 ng L−1 | 11.7–206.3 ng L−1 | Tablet, human blood serum | 78 |
| Poly(TEGMPA)/MWCNTs–COOH/ceramic electrode | BSA | DPV | 0.42 ng mL−1 | 1.99–30.91 ng mL−1 | Aqueous, human serum, milk, pharmaceutics | 79 |
| Polyacrylamide/MWCNTs–COOH/Pt electrode | Pb(II) ions | DPV | 0.02 μM | 1–5 ppm | Mining effluent, lake water, food, cosmetics | 80 |
| Chitosan/graphene/acetylene black paste electrode | Bisphenol A | LSV | 6 nM | 8 nM to 1 μM, 1–20 μM | Plastic bottled drinking water, canned beverages | 81 |
| Poly(methacrylic acid)/graphene/carbon paste electrode | Chlordiazepoxide | SWV | 2.61 × 10−10 M | 6 × 10−10 to 7.5 × 10−8 M | Tablets, human serum, urine | 82 |
| Polypyrrole/graphene/carbon electrode | 4-Nonylphenol | DPV | 3.5 × 10−12 g mL−1 | 1 × 10−11 to 1 × 10−8 g mL−1 | Rain water, lake water | 83 |
| Polypyrrole/NH2–graphene/screen-printed electrode | Methcathinone, cathinone | DPV | 3.3, 8.9 pg mL−1 | 4.9 × 10−6 to 9.8 × 10−3, 1.5 × 10−5 to 1.1 × 10−2 μg mL−1, | Practical serum samples | 84 |
| Poly(o-aminophenol)/N,S-activated graphene/glassy electrode | Cyclo-phosphamide | CV | 3.4 × 10−12 M | 8 × 10−12 to 8 × 10−7 M | Male rabbit blood plasma | 85 |
| Polyaniline/rGO/carbon paste electrode | Diclofenac | DPV | 1.1 mg L−1 | 5–80 mg L−1 | Injection, tablet, urine | 86 |
| Poly(pyrrole–COOH)/rGO/screen-printed electrode | Human cardiac troponin T | DPV | 0.006 ng mL−1 | 0.01–0.1 ng mL−1 | Blood serum | 87 |
| Poly(N-ABA)/GQDs/screen-printed carbon electrode | Ifosfamide | DPASV | 0.08–0.11 ng mL−1 | 0.25–121.35 ng mL−1 | Aqueous, blood plasma, urine, pharmaceutical | 88 |
| Polyaniline/CNTs/graphene/carbon electrode | BSA | DPV | 6.2 × 10−11 g mL−1 | 1 × 10−4 to 1 × 10−10 g mL−1 | Human serum | 89 |
| Polypyrrole/graphene/CNTs/carbon electrode | Tetrabromo bisphenol A | DPV | 3.7 × 10−12 M | 1 × 10−11 to 1 × 10−8 M | Catfish, chub, carp | 90 |
| Polyacrylamide/rGO–C60/pencil graphite electrode | D-serine, L-serine | DPASV | 0.24, 0.25 ng mL−1 | 0.83–20.63, 0.87–20.45 ng mL−1 | Serum, cerebrospinal fluid, pharmaceutics | 91 |
| Poly(o-phenylenediamine)/CNTs/graphene/nickel foam | Dopamine | CV | 6.67 × 10−16 M | 2 × 10−15 to 1 × 10−12 M | — | 92 |
| Sensing materials | Targets | Modes | LOD | Detection ranges | Detected samples | Ref. |
|---|---|---|---|---|---|---|
| a Abbreviation: gold nanoparticles, AuNPs, multiwalled carbon nanotubes, MWCNTs; glassy carbon electrode, GCE; N-acryloyl-pyrrolidine-2,5-dione, NAPD; nanoparticles, NPs; 2-aminoethanethiol, 2AET; polymethyl methacrylate, PMMA; binuclear phthalocyanine cobalt(ii) sulfonate, BiCoPc; glycidoxypropyl trimethoxysilane, GPTS; 1-vinyl-3-octylimidazole hexafluoride phosphorus, VOIm PF6; single-walled carbon nanotubes, SWCNTs; human immunodeficiency virus, HIV; linear sweep stripping voltammetry, LSSV; differential pulse voltammetry, DPV; anodic stripping differential pulse voltammograms, ASDPV; differential pulse anodic stripping voltammetry, DPASV; differential pulse and square wave stripping voltammetry, DPSV/SWSV; square wave voltammetry, SWV; cyclic voltammetry, CV. | ||||||
| Polyacrylamide/AuNPs/MWCNTs/GCE | Isoniazid | LSSV | 0.3 × 10−9 M, 5 × 10−8 M | 1 × 10−9 to 1.4 × 10−8 M, 2 × 10−8∼1 × 10−7 M | Body fluid, pharmaceutics | 93 |
| Poly(aminothiophenol)/AuNPs/MWCNTs/GCE | Cholesterol | DPV | 3.3 × 10−14 M | 1 × 10−13–1 × 10−9 M | — | 94 |
| Poly(2,2′-dithiodianiline)/AuNPs/MWCNTs/GCE | Valganciclovir | ASDPV | 0.3 nM | 1–500, 500–2000 nM | Tablet, human blood serum | 95 |
| Poly(o-phenylenediamine)/AuNPs/MWCNTs–COOH/GCE | Olaquindox | DPV | 2.7 nM | 10–200 nM | Pork, fish | 96 |
| Poly(methacrylic acid)/MWCNTs–COOH/AuNPs/Pt electrode | Ampicillin | DPV | 1 × 10−9 M | 1 × 10−8 to 5 × 10−6 M | Milk, animal feed, fresh egg | 97 |
| Poly(triaminotriazine)/AuNPs/MWCNTs–COOH/pencil graphite electrode | Norepinephrine, uric acid | DPASV | 0.62, 0.43 ng mL−1 | 2.98–40.69, 1.94–43.59 ng mL−1 | Aqueous, serum, urine, pharmaceutics | 98 |
| Poly(NAPD)/TiO2 NPs/MWCNTs/pencil graphite electrode | L-Aspartic acid | DPASV | 1.73–179 ng mL−1 | 9.98–532.72 ng mL−1 | Serum, cerebrospinal fluid, pharmaceutics | 99 |
| Poly(itaconic acid)/Mn NPs/MWCNTs/pencil graphite electrode | PSA | SWSV, DPSV | 0.25 pg L−1, 3.04 pg L−1 | 0.99–5.99 pg L−1, 9.99 pg L−1 to 5.99 ng L−1 | Blood serum, urine, forensic samples | 100 |
| Poly(4-vinylpyridine)/IL@PtNPs/MWCNTs/GCE | Tartrazine | DPV | 8 nM | 0.03–5, 5–20 μM | Fanta, mirinda drink, orange powder | 101 |
| Polypyrrole/Fe@AuNPs/2AET–MWCNTs/p-aminothiophenol/GCE | Cefexime | SWV | 2.2 × 10−11 M | 1 × 10−10 to 1 × 10−8 M | Human plasma | 102 |
| Poly(methacrylic acid)/PMMA/Fe3O4/MWCNTs/ceramic electrode | Kanamycin | DPV | 2.3 × 10−11 M | 1 × 10−10 to 1 × 10−6 M | Chicken, pig liver, milk | 103 |
| Poly(o-aminophenol)/polypyrrole/MWCNTs–BiCoPc/GCE | Metolcarb | CV | 7.88 × 10−9 M | 1 × 10−8 to 0.6 × 10−6 M | Cucumber, cabbage | 104 |
| Poly(4-vinylpyridine-methacrylic acid)/GPTS-vinyl/MWCNTs/GCE | Bisphenol A | DPV | 0.2 × 10−11 M | 1 × 10−10 to 4 × 10−4 M | Baby feeding bottle | 105 |
| Polyarginine/sodium alginate–MWCNTs/GCE | Theophylline | DPV | 3.2 nM | 0.01–60 μM | Tablet, oral solution, human blood serum | 106 |
| Poly(3-aminophenylboronic acid)/MWCNTs/chitosan/GCE | Epinephrine | DPV | 35 nM | 0.2–800 μM | Human serum, injection | 107 |
| Polydopamine/glutaraldehyde/chitosan/MWCNTs/GCE | Chorionic gonadotropin | DPV | 0.035 pg mL−1 | 0.5 pg mL−1 to 250 ng mL−1 | Human serum | 108 |
| Polyacrylamide/glutaraldehyde/chitosan/MWCNTs/GCE | HIV-p24 | DPV | 0.083 pg cm−3 | 1 × 10−4 to 2 ng cm−3 | Human serum | 109 |
| Poly(VOIm PF6)/MWCNTs@polydopamine–SWCNTs–COOH/GCE | Vanillin | DPV | 0.1 μM | 0.2–10 μM | Biscuit, cake, milk tea | 110 |
| Imprinted SiO2/sol–gel/MWCNTs/carbon paste electrode | Bisphenol A | DPV | 4.4 × 10−9 M | 4 × 10−9 to 1 × 10−7, 5 × 10−7 to 5 × 10−5 M | Polycarbonate bottled drinking water | 111 |
| Imprinted SiO2/polytyramine/sol–gel/MWCNTs/AuNPs/pencil graphite electrode | Ketamine | SWV | 0.7 nM | 1–50, 50–1000 nM | Drug-free urine, human plasma | 112 |
| Imprinted SiO2 film/sol–gel/MWCNTs–chitosan/GCE | Quinoxaline-2-carboxylic acid | DPV | 4.4 × 10−7 M | 2 × 10−6 to 1 × 10−3 M | Pork samples | 113 |
| Sensing materials | Targets | Modes | LOD | Detection ranges | Detected samples | Ref. |
|---|---|---|---|---|---|---|
| a Abbreviation: F-doped SnO2 conducting glass, FTO; ionic liquid, IL; glassy carbon electrode, GCE; poly(diallyldimethylammonium chloride), PDDA; gold nanoparticles, AuNPs, nanoparticles NPs; AuNPs-supported amino-functionalized Ti-benzenedicarboxylate porous metal–organic frameworks, Au/NH2–MIL–125Ti; bovine serum albumin, BSA; alpha-fetoprotein, AFP; carcinoembryonic antigen, CEA; apoferritin-encoded metallic labels, rApo; photoelectrochemical, PEC; differential pulse voltammetry, DPV; cyclic voltammetry, CV; chronoamperometry, CA; linear sweep voltammograms, LSV; square wave voltammetry, SWV. | ||||||
| Polypyrrole/CdS–graphene/FTO | 4-Aminophenol | PEC | 2.3 × 10−8 M | 5 × 10−8 to 3.5 × 10−6 M | Lake water | 114 |
| Polypyrrole/IL/graphene/GCE | Bovine hemoglobin | DPV | 3.09 × 10−11 g L−1 | 1 × 10−1 to 1 × 10−3 g L−1 | Bovine blood | 115 |
| Poly(methacrylic acid)/PDDA–graphene/GCE | 4-Chlorophenol | DPV | 0.3 μM | 0.8–100 μM | Tap, lake water | 116 |
| Poly(methacrylic acid)/hemin–graphene/GCE | p-Aminophenol | DPV | 0.06 μM | 0.3–25 μM | Tap, lake water | 117 |
| Polypyrrole/graphene–AuNPs/GCE | Levofloxacin | DPV | 0.53 μM | 1–100 μM | Capsule samples | 118 |
| Poly(methacrylic acid)/graphene–AuNPs/GCE | Colchicine | DPV | 4.8 × 10−9 M | 1.2 × 10−8 to 1.2 × 10−6, 1.2 × 10−6 to 1 × 10−4 M | Colchicine tablets, human serum | 119 |
| Polypyrrole/graphene–Prussian blue/GCE | Butylated hydroxytoluene | CV | 0.02 ppb | 0.02–1 ppb | — | 120 |
| Polypyrrole/graphene–Prussian blue/GCE | Butylated hydroxyanisole | CA | 7.63 × 10−8 M | 9 × 10−8 to 7 × 10−5 M | Potato chip | 121 |
| Poly(o-phenylenediamine)/HSO3–graphene/Au electrode | Dopamine | CV | 0.11 mg L−1 | 0.5–7 mg L−1 | Human serum | 122 |
| Polypyrrole/graphene/benzenediazonium/carbon electrode | Tetrabromo bisphenol A | DPV | 0.23 nM | 0.5–4.5 nM | Rain, lake water | 123 |
| Poly(methacrylic acid)/graphene sheets/Congo red/GCE | Dopamine | LSV | 20 μM | 1 × 10−7 to 8.3 × 10−4 M | — | 124 |
| Poly(vinyl ferrocene)/cyclodextrin-graphene nanosheet/GCE | 1-Naphthylamine | DPV | 0.1 μM | 0.3–100 μM | Lake water | 125 |
| Polypyrrole/β-cydodextrin/AuNPs/graphene/GCE | Quercetin | DPV | 1 × 10−10 M | 1 × 10−9 to 1 × 10−6 M | Compound tablets | 126 |
| AuNPs/over-oxidized polypyrrole/graphene/GCE | Dopamine | CV | 0.1 μM | 0.5–8 μM | Rabbit serum, rabbit urine | 127 |
| Polypyrrole/Co–Ni NPs/graphene/carbon electrode | Octylphenol | DPV | 3.6 × 10−11 M | 1 × 10−10 to 1 × 10−7 M | Plastic, metal bottle, food packaging bags | 128 |
| Poly(Zn-porphyrin)/chitosan/AuNPs/graphene/GCE | Methyl parathion | DPV | 3.16 × 10−10 M | 1 × 10−6 to 8 × 10−9 M | Apple samples | 129 |
| Polypyrrole/chitosan/IL/graphene/GCE | BSA | DPV | 2 × 10−11 g L−1 | 1 × 10−10 to 1 × 10−4 g L−1 | Bovine plasma | 130 |
| Poly(methacrylic acid)/IL–graphene/chitosan/GCE | 6-Benzylamino purine | DPV | 0.2 μM | 0.5–50 μM | Bean sprout, potato, tomato, lake water | 131 |
| Poly(methacrylic acid)/SiO2/IL/graphene/GCE | 2,6-Diamino pyridine | CV | 0.0275 mg kg−1 | 0.05–35 mg kg−1 | Hair dyes, L'Oréal, HUYO, Zhanghua | 132 |
| Poly(o-phenylenediamine)/AuNPs/IL/graphene/GCE | Ractopamine | DPV | 0.46 μg L−1 | 10–5000 μg L−1 | Swine urine | 133 |
| Poly(p-aminothiophenol)/AuNPs/IL–graphene/GCE | 4-Nonylphenol | DPV | 0.01 ng mL−1 | 50–500 ng mL−1 | Boxes, plastic bag, milk package bottle | 134 |
| Poly(o-aminophenol-resorcinol)/Au–Prussian blue/graphene/AuNPs/GCE | Tebuconazole | DPV | 1.25 × 10−8 M | 5 × 10−8 to 4 × 10−4 M | Cucumber, green vegetable, strawberry | 135 |
| Imprinted SiO2/sol–gel/boronic acid-graphene/GCE | Glycoprotein | DPV | 2 × 10−11 mg mL−1 | 1 × 10−10–1 × 10−4 mg mL−1 | Chicken, quail eggs | 136 |
| Poly(L-cysteine)/graphene/Ag@NH2–MIL–125Ti/GCE | BSA | DPV | 4.147 × 10−19 g mL−1 | 1 × 10−18 to 1 × 10−12 g mL−1 | Liquid milk | 137 |
| Poly(aminothiophenol)/AuNPs–bionic poly dopamine film/dopamine@graphene/GCE | Cholesterol | DPV | 3.3 × 10−19 M | 1 × 10−18 to 1 × 10−13 M | Human serum | 138 |
| Poly(p-aminothiophenol)/graphene-IL-nano Au/chitosan- AuPt alloy NPs/GCE | Carbaryl | DPV | 8 nM | 0.03–6 μM | Cabbage, apple peel | 139 |
| Poly(6-mercaptonicotinic acid)–AuNPs/graphene–AuNPs/chitosan–PtNPs/gold electrode | Erythromycin | CV | 2.3 × 10−8 M | 7 × 10−8 to 9 × 10−5 M | Milk, honey | 140 |
| Graphene–Au/BSA/anti-AFP@rApo–Cd, anti-CEA@rApo-Pb/polydopamine/Fe3O4/magnetic electrode | AFP, CEA | SWV | 0.3 pg mL−1, 0.35 ng mL−1 | 0.001–5 ng mL−1 | Human serum | 141 |
| Sensing materials | Targets | Modes | LOD | Detection ranges | Detected samples | Ref. |
|---|---|---|---|---|---|---|
| a Abbreviation graphene oxide, GO; ionic liquid, IL; glassy carbon electrode, GCE; nanoparticles, NPs; ethylene glycol dimethacrylate, EGDMA; gold nanoparticles, AuNPs; 2-aminoethanethiol, 2-AET; binuclear phthalocyanine cobalt(II) sulfonate, BiCoPc; reduced graphene oxide, rGO; 1-(α-methyl acrylate)-3-allylimidazolium bromide, 1 MA-3AI-Br; poly(3,4-ethylenedioxythiophene), PEDOT; three-dimensional, 3D; ordered mesoporous carbon material, CMK-3 or OMC; graphene quantum dots, GQDs; breast cancer susceptibility gene, BRCA-1; differential pulse voltammetry, DPV; cyclic voltammetry, CV; square wave voltammetry, SWV; electrochemical impedance spectroscopy, EIS; linear sweep voltammograms, LSV; photoelectrochemical, PEC. | ||||||
| Poly(methacrylic acid)/SiO2/GO | Dopamine | DPV | 3 × 10−8 M | 5 × 10−8 to 1.6 × 10−4 M | HCl injections, urine | 142 |
| Poly(methacrylic acid)/IL–GO/GCE | Methyl parathion | DPV | 6 nM | 0.01–7 μM | Cabbage, apple peel | 143 |
| Polypyrrole/NiNPs/GO/carbon electrode | Tetrabromo bisphenol A | DPV | 1.3 × 10−10 M | 5 × 10−10 to 1 × 10−5 M | Tap, rain, lake water | 144 |
| Poly(3-aminophenylboronic acid)/Fe3O4@GO/Au film electrode | Interleukin-8 | CV | 0.04 pM | 0.1–10 pM | Human saliva | 145 |
| Poly(o-phenylenediamine)/SiO2/GO/GCE | 2,4-Dinitrophenol | DPV | 0.4 μM | 1–150 μM | Tap water, artificial wastewater | 146 |
| EGDMA-vinyl/NH2–SiO2/GO/GCE | Bisphenol A | DPV | 0.003 μM | 0.006–0.1/0.2–20 μM | Milk, mineral water | 147 |
| Polyphenol/cubic AuNPs/2AET–GO/GCE | Tyrosine | DPV | 1.5 × 10−10 M | 1 × 10−9 to 2 × 10−8 M | Milk samples | 148 |
| Poly(methacrylic acid)/AuNPs/Fe3O4/GO/GCE | Dibutyl phthalate | DPV | 8 × 10−10 M | 2.5 × 10−9 to 5 × 10−6 M | Brand wine drinks | 149 |
| Poly(o-phenylenediamine)/polypyrrole/BiCoPc/GO/GCE | Quinoxaline-2-carboxylic acid | SWV | 2.1 nM | 1 × 10−8 to 1 × 10−4, 1 × 10−4 to 5 × 10−4 M | Pork, chicken muscle | 150 |
| Polyvinyl acetate/MnO2/GO/CuO/Cu wire | Glucose | CV | 53 μM | 0.5–4.4 mM | — | 151 |
| Polypyrrole/AuNPs/β-cydodextrin/Fe3O4/GO/GCE | Chrysoidine | DPV | 1.7 × 10−8 M | 5 × 10−8 to 5 × 10−6 M | Tap water | 152 |
| Poly(methacrylic acid)/AuNPs/β-cydodextrin–IL/Fe3O4/GO/GCE | Sunset yellow | DPV | 2 × 10−9 M | 5 × 10−9 to 2 × 10−6 M | Water, mirinda drink, minute maid | 153 |
| SiO2@Ag/rhodamine B-labeled/DNA/poly(methacrylic acid)/AuNPs–GO/chitosan/GCE | BRCA-1 | DPV | 2.53 fM | 10 fM to 100 nM | Clinical human serum | 154 |
| Poly(methacrylic acid)/Fe3O4@rGO/GCE | 17β-Estradiol | DPV | 0.819 nM | 0.05–10 μM | Water environment | 155 |
| Poly(o-phenylenediamine)/AuNPs/rGO/GCE | D-Mannitol | DPV | 7.7 × 10−13 M | 1 × 10−12 to 2 × 10−11, 2 × 10−11 to 3 × 10−10 M | Sugarcane vinasse samples | 156 |
| Poly(methacrylic acid)/rGO@Au/GCE | Carbofuran | DPV | 2 × 10−8 M | 5 × 10−8 to 2 × 10−5 M | Cabbage, cucumber | 157 |
| Poly(o-phenylenediamine)/poly(sodium 4-styrenesulfonate)–rGO/GCE | Daidzein | CV | 0.5 nM | 1–20 nM | Human serum, pueraria extraction | 158 |
| Poly(carboxymethyl-β-cydodextrin)/TiO2/rGO/Pt electrode | Toltrazuril | DPV | 0.21 μg L−1 | 0.43–42.54 μg L−1 | Egg, chicken muscle | 159 |
| Polypyrrole/rGO–AuNPs/nickel foam | Gastrodin | DPV | 1 nM | 0.01–1 μM | Human serum | 160 |
| Poly(methacrylic acid, 4-vinylpyridine, 1 MA-3AI-Br)/rGO–IL/GCE | Sunset yellow | DPV | 4 nM | 0.01–1.4, 1.4–16 μM | Fruit juice, mirinda drink, orange juice | 161 |
| Polyaniline/Fe3O4/rGO/magnetic GCE | Amaranth | DPV | 50 nM | 0.05–0.5, 0.5–50 μM | Grapes, watermelon, peach flavor | 162 |
| Polyaniline@Fe3O4/rGO/magnetic GCE | Glutathione | DPV | 3 nM | 0.05–50 μM | Human whole blood | 163 |
| Poly(methacrylic acid)/AuNPs/Fe3O4@rGO/GCE | Ractopamine | DPV | 0.02 nM | 0.002–0.1 μM | — | 164 |
| Polyacrylamide/AgNPs–Fe3O4–rGO/screen-printed electrode | Quercetin | DPV | 13 nM | 20 nM to 250 μM | Medicinal tablets | 165 |
| Polyphenol/AgNPs/H3PW12O40/rGO/GCE | Tert-Butyl hydroquinone | DPV | 1.48 × 10−11 M | 0.05–1.5 nM | Soybean, blend oil, beef tallow | 166 |
| Polypyrrole/PtNPs/polyoxometalate/rGO/GCE | Citrinin | DPV | 2 × 10−13 M | 1 × 10−12 to 1 × 10−10 M | Rye samples | 167 |
| Poly(p-aminothiophenol)/AuNPs/polyaniline/rGO/GCE | 5-Hydroxy tryptamine | DPV | 11.7 nM | 0.2–10 μM | Human blood serum | 168 |
| Poly(2-mercaptonicotinic acid)/PtNPs/rGO–poly(sodium 4-styrenesulfonate)/PtNPs/GCE | 17β-Estradiol | DPV | 0.002 μM | 0.004–0.06, 0.06–50 μM | Hand cream, facial cleanser | 169 |
| Poly(o-phenylenediamine)/gold networks@IL/porous PtNPs/COOH-rGO/GCE | Cefotaxime | DPV | 1 × 10−10 M | 3.9 × 10−9 to 8.9 × 10−6 M | Human serum, urine | 170 |
| Poly(eriochrome black T)/PEDOT/laser-induced graphene electrode/polyimide | Chloramphenicol | EIS | 0.62 nM | 1 nM to 10 mM | — | 171 |
| Polypyrrole/NiCo2O4 nanoneedle arrays/3D graphene electrode | Sulfadimidine | DPV | 0.169 ng mL−1 | 0.2–1000 ng mL−1 | Commercial milk | 172 |
| Poly(para-aminobenzoic acid)/porous PdCu allow NPs/3D porous graphene/GCE | Melamine | DPV | 2 nM | 0.01–1 μM | Raw milk, milk powder | 173 |
| Poly(o-phenylenediamine-co-o-toluidine)/IL–N-doped graphene nanoribbons/GCE | 4-Nonyl-phenol | LSV | 8 nM | 0.04–6 μM | Lake, river, tap water | 174 |
| Polyphenol/PtNPs/C3N4 nanotubes/GCE | Atrazine | SWV | 1.5 × 10−13 M | 1 × 10−12 to 1 × 10−10 M | Wastewater | 175 |
| Poly(o-phenylenediamine)/g–C3N4–AuNPs/indium tin oxide | Triclosan | PEC | 6.01 × 10−13 M | 2 × 10−12 to 8 × 10−10 M | Toothpastes | 176 |
| Poly(p-aminothiophenol)/AuNs/OMC/screen-printed electrode | Ractopamine | DPV | 4.23 × 10−11 M | 5 × 10−11 to 1 × 10−9 M | Swine urine | 177 |
| Poly(methacrylic acid)/N-carbon nanosheet Fe-frameworks/poly(vinyl pyrrolidone)/GCE | Mebendazole, catechol | DPV | 0.004, 0.06 μM | 0.01–1.5, 0.5–25 μM | Tap, river water | 178 |
| Poly(3,4-ethylenedioxythiophene)/AuNPs/graphene nanoribbons/GCE | Octylphenol | LSV | 1 nM | 0.02–8 μM | River, bottled mineral water, urine | 71 |
| Poly(para-aminobenzoic acid)/Prussian blue/CMK-3/GCE | Metolcarb | LSV | 9.3 × 10−11 M | 5 × 10−10 to 1 × 10−4 M | Cucumber, cabbage, apple juice | 179 |
| Poly(p-aminothiophenol)/AuNPs/chitosan–carbon dots/GCE | Patulin, 2-oxindole | DPV | 7.57 × 10−13 M | 1 × 10−12 to 1 × 10−9 M | Fresh apple juice | 180 |
| Polypyrrole/GQDs/hollow Ni nanospheres/GCE | Bisphenol S | DPV | 0.03 μM | 0.1–50 μM | Mineral water, plastic samples | 181 |
| Polypyrrole/ZnO@GQDs/pencil graphite electrode | 6-Mercaptopurine | DPV | 5.72 nM | 0.01–50, 50–700 μM | Tablet, serum, urine | 182 |
| Sensing materials | Targets | Modes | LOD | Detection ranges | Detected samples | Ref. |
|---|---|---|---|---|---|---|
| a Abbreviation: carbon nanotubes, CNTs; glassy carbon electrode, GCE; graphene oxide, GO; single-walled carbon nanotubes, SWCNTs; multiwalled carbon nanotubes, MWCNTs; three-dimensional, 3D; ionic liquid, IL; gold nanoparticles, AuNPs; reduced graphene oxide, rGO; 3-propyl-1-vinylimidazolium bromide, C3VimBr; ordered mesoporous carbon material, CMK-3; poly(glycerol monomethacrylate), p(GMMA); nanoparticles, NPs; silylated graphene oxide-grafted chemically modified nanocellulose, Si-GO-g-CMNC; linear sweep voltammograms, LSV; differential pulse voltammetry, DPV; cyclic voltammetry, CV; cathodic stripping different pulse voltammetry, CSDPV; square wave stripping voltammetry, SWSV. | ||||||
| Polycarbazole/MoS2/graphene–CNTs/GCE | Luteolin | LSV | 9 nM | 0.04–2 μM | Carrot, chrysanthemum tea | 183 |
| WS2–poly(3,4-ethylenedioxythiophene)/GO–SWCNTs/GCE | Vitamin B2 | LSV | 0.7 nM | 0.002–0.9 μM | Vitamin B2 tablets | 184 |
| Poly(methacrylic acid)/MWCNTs/graphite/paraffin/carbon paste electrode | Gallic acid | DPV | 47 nM | 0.12–380 μM | Apple, orange, green tea, pineapple juice | 185 |
| Poly(p-aminothiophenol-co-p-aminobenzoic acid)/3D graphene–CNTs–IL/GCE | Eugenol | LSV | 1 × 10−7 M | 5 × 10−7∼2 × 10−5 M | Curry powder, perfume, capsule | 186 |
| Poly(p-aminobenzoic acid)/3D PdNPs–porous graphene–CNTs/GCE | Quercetin | DPV | 5 nM | 0.01–0.5 μM | Pule'an tablet, red wine, honeysuckle juice | 187 |
| Silicon alkoxide/graphite powder/cholesteryl chitin carbonate/MWCNTs/ceramic carbon electrode | Cholesterol | LSV | 1 nM | 10–300 nM | — | 188 |
| Poly(p-aminothiophenol)/AuNPs/MWCNTs@rGO nanoribbons/GCE | p-Nonylphenol | CV, CSDPV | 0.73 pM, 4.8 fM | 1 pM–1 μM, 10 fM to 1 nM | Tap water, flesh fish, sewage water | 189 |
| Poly(C3VimBr)/AuNPs/CMK-3/3D porous graphene/GCE | Dimetridazole | DPV | 5 × 10−10 M | 2 × 10−9 to 2.5 × 10−7 M, 2.5 × 10−7 to 3 × 10−6 M | Milk, honey, porcine muscle | 190 |
| p(GMMA)/poly(4-vinylpyridine)/MWCNTs/graphene–IL/GCE | Brucine | DPV | 2 nM | 0.006–0.6, 0.6–5 μM | Gujinwan/yaotongning capsule, human serum | 191 |
| Poly(o-phenylenediamine-o-toluidine)/CNTs-AuNPs/boron-doped ordered mesoporous carbon/GCE | Bisphenol A | DPV | 5 nM | 0.01–10 μM | Milk samples | 192 |
| Poly(o-phenylenediamine)/PtAu NPs/CNTs–COOH/GO/GCE | Propyl gallate | CV | 2.51 × 10−8 M | 7 × 10−8 to 1 × 10−5 M | Vegetable oils | 193 |
| Polypyrrole/AuNPs/activated MWCNTs@GO nanoribbons/GCE | 3-Nitrotyrosine | SWSV | 50 nM | 0.2–50 μM | Human serum, urine | 194 |
| Poly(allylamine-co-itaconic acid)/Si-GO-g-CMNC-ZnO particles/GCE | Cholesterol | CV, DPV | 7.4 μM, 98.6 μM | 5.18–25.9 μM, 0.6475–10.36 mM | Blood serum of normal person, a patient | 195 |
| Polypyrrole/MWCNTs–COOH/IL/AuNPs/GO/GCE | Vanillin | DPV | 6.23 × 10−10 M | 1 × 10−8 to 2.5 × 10−6 M | Tap water | 196 |
| Poly(p-aminobenzoic acid)/hyaluronic acid–MWCNTs/polypyrrole–graphene/GCE | Tryptamine | CV | 7.4 × 10−8 M | 9 × 10−8 to 7 × 10−5 M | Cheese, lactobacillus beverage | 197 |
The fabrication of CNM-modified MIPs widely involves different CNMs, including zero-, one-, two-, and three-dimensional (0D, 1D, 2D, and 3D, respectively), or micro-scale CNMs. 0D CNMs comprise GR quantum dots (GQDs), carbon dots (CDs), and C60, while 1D CNMs majorly contain CNTs, CNT–COOH, SWCNTs, MWCNTs, SWCNT-/MWCNT–COOH, and C3N4 nanotubes. Typical 2D CNMs comprise GR, GO, rGO, GR/N-doped GR/GO/rGO nanoribbons, NH2–GR, N,S-activated GR, rGO–COOH, Ag–N-doped rGO, or GR nanoplatelets. Principally, 3D CNMs include 3D porous GR, porous rGO, and boron-doped/ordered mesoporous carbon (CKM-3). As a type of microscale CNM, graphite powder participates in the modification of MIPs with CNMs and other materials.
With regard to the use of “MIP modified with CNMs without hybridization” to fabricate ECCSs, GCE is widely used as the work electrode (Table 1). One or more types of CNMs are prepared and used for the modification of electrodes or substrates, followed by the preparation of MIPs on CNM-modified electrodes or substrates. Bai et al. designed an electrochemical artemisinin (ART) sensor by the in situ polymerization of ART-imprinted membrane on GR-modified GCE surfaces.52 Acrylamide (AM) and ethylene glycol dimethacrylate (EGDMA) acted as the monomer and cross-linking agent, respectively (Fig. 1a). In addition to GCE, other commercial work electrodes often serve as the substrates to fabricate CNM-modified MIPs (Table 2), such as carbon paste electrode, Cu electrode, Pt electrode, Au electrode, magnetic electrode, pencil graphite electrode (PGE), ceramic electrode, acetylene black paste electrode, carbon electrode (CE), screen-printed/ceramic CE, glassy electrode, etc. Huang et al. synthesized N,S-doped activated GR (N,S-AGR) that was dropcasted on a glassy electrode.85 The MIP layer was in situ electropolymerized on an N,S-AGR-modified electrode to form a platform for cyclophosphamide (CPA) detection (Fig. 1b).
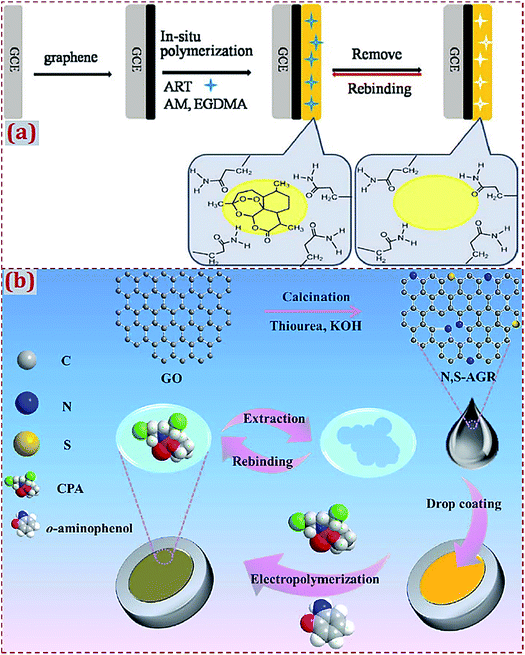 | ||
| Fig. 1 (a) Detailed procedure diagrams for the fabrication of artemisinin-molecularly imprinted membranes/graphene-modified glassy carbon electrode (ART-MIM/G/GCE) sensor. Reproduced with permission from ref. 52. Copyright 2015, Elsevier. (b) Schematic representation for preparing molecularly imprinted polymer decorated on N,S-co-doped activated graphene on glassy electrode (MIP/N,S-AGR/GE). Reproduced with permission from ref. 85. Copyright 2017, Elsevier. | ||
Moreover, different types of CNMs can simultaneously participate in the surface modification of electrodes and substrates. Thereafter, MIP can be prepared on multiple CNM-modified electrodes and substrates,72–75,90–92 which have improved electron transfer capabilities and enhanced surface areas. Based on a composite of GR and SWCNT–COOH, an imprinted sol–gel electrochemical sensor was developed for the detection of propyl gallate (PG).72 GR–SWCNTs composite was synthesized by the in situ hydrothermal reduction of exfoliated GO in the presence of hydrazine and ammonia solution. The dropping of GR–SWCNTs on GCE aims to prepare a modified electrode. By the in situ electropolymerization of phenyltrimethoxysilane (PTMOS as a functional monomer), a sol–gel MIP was grown on GR–SWCNTs-modified GCE to form MIP/GR–SWCNTs/GCE. The modification of MIP with GR–SWCNTs obviously improves the sensitivity of this sensor, which originates from the synergistic interactions between GR and SWCNTs to accelerate the electron transfer during the reaction process.
2.2. MIPs modified with CNMs and hybridized with other materials
In addition to the use of CNMs to prepare CNM-modified MIPs, in earlier studies, researchers have mostly used other materials to hybridize with CNMs in order to fabricate ECCSs based on MIPs modified with CNMs and hybridized with other materials. As summarized in Tables 3–6, various materials have been widely used in the hybridization with CNMs, including noble metal NPs, alloyed/bimetal NPs, metal oxide NPs, nanosheets, small molecules, biomolecules, polymers, organic–inorganic hybrid materials, and composite materials. One or more types of these materials can be prepared and used for hybridization with CNMs, followed by the preparation of MIPs on CNMs and other materials in the form of dual-modified electrodes or substrates. Ji et al. constructed an electrochemical sensor for cholesterol (CHO) detection94 through the MIP membrane on MWCNTs and gold NPs (AuNPs)-modified GCE. CHO and p-aminothiophenol (p-ATP) were assembled on the modified surface of GCE by Au–S bonds and hydrogen bonds, followed by the generation of an MIP membrane via the electropolymerization in a prepolymer solution containing p-ATP, HAuCl4, tetrabutyl ammonium perchlorate (TBAP), and CHO (Fig. 2a). The copolymerization of poly(p-ATP) and AuNPs on MWCNT-modified GCE can maximize the amount of effective imprinted sites and enhance conductivity.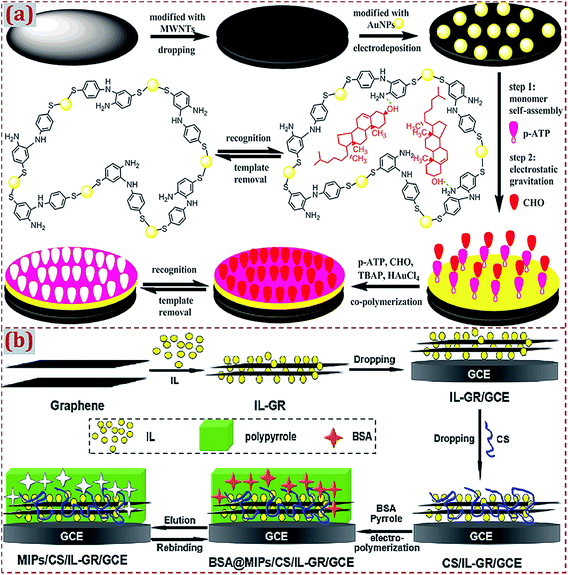 | ||
| Fig. 2 (a) Preparation of a molecularly imprinted membrane based on a MIP film on AuNP-CNTs-modified GCE. Reproduced with permission from ref. 94. Copyright 2015, Elsevier. (b) Schematic diagram for the preparation procedures of a molecularly imprinted electrochemical sensor of BSA based on chitosan/ionic liquid–graphene (CS/IL–GR)-modified GCE. Reproduced with permission from ref. 130. Copyright 2016, Elsevier. | ||
Xia et al. fabricated a molecularly imprinted electrochemical sensor for bovine serum albumin (BSA) detection based on chitosan/IL–GR (CS/IL–GR)-modified GCE.130 IL is suitable for use as a supporting electrolyte due to its high ionic conductivity and chemical stability. As compared to GR, the GR/IL composite has an improved electrocatalytic ability for electrochemical sensing. Chitosan (CS) has –OH and –NH– active groups and can serve as a matrix to immobilize protein molecules containing –COOH groups. To produce MIPs on the surface of CS/IL–GR/GCE, a polypyrrole was electropolymerized by using BSA as the template molecule (Fig. 2b). As typical GR-based CNMs, CS/IL–GR nanocomposites contain the hybridization of GR with CS and IL. Synergistic interactions among CS, IL, and GR in nanocomposites improve the electrochemical responses and detection sensitivity of this sensor. Most of the relevant studies mention two or more types of materials that can hybridize with CNMs during the preparation process of CNM-modified MIPs. The synergistic effects between CNMs and other materials during hybridization further improve the sensitivity and electrochemical responses of ECCSs for efficient target detection. Xu et al. prepared MoS2/GR–CNTs nanocomposites via the hydrothermal method.183 MoS2/GR–CNTs combined the high catalysis of MoS2 and superior electronic conductivity of GR–CNTs, yielding high electrochemical sensitivity toward luteolin. A MIP film was deposited through electropolymerization using the carbazole (Cz) monomer and luteolin template. The electrochemical sensor of MIP/MoS2/GR–CNTs/GCE had high performance for luteolin detection.
2.3. ECCSs fabricated on work electrodes or other substrates
In the fabrication of ECCSs based on CNM-modified MIPs, various commercial work electrodes have frequently served as substrates to support CNM-modified MIPs. In addition, certain functionalized materials can serve as electrochemical sensing substrates to construct CNM-modified-MIP-based ECCSs, such as NF,92,160 F-doped SnO2 conducting glass (FTO),114 GO,142 Au film electrode,145 Cu wire,151 laser-induced GR electrode,171 3D GR electrode,172 and indium tin oxide (ITO).176 Jin et al. reported an electrochemical coreduction method to synthesize rGO and silver NPs (AgNPs) on the surface of NF.160 After the in situ electropolymerization of polypyrrole in the presence of the gastrodin (GAS) template, MIP was prepared on rGO–AgNPs-modified NF. After modification with electroactive substances, NF exhibits abundant active sites on its surface and high electronic conductivity because of the presence of desirable porous and 3D nanostructures. The modified NF acts as an excellent electrode material for GAS sensing (Fig. 3a). Feng et al. designed a photoelectrochemical (PEC) sensor of triclosan.176 A MIP layer of poly(o-phenylenediamine, o-PD) was electropolymerized on GR-like carbon nitride (g-C3N4) and AuNPs dual-modified ITO electrode (Fig. 3b). The MIP/g-C3N4–AuNPs/ITO sensor had highly sensitive and selective PEC response toward triclosan detection.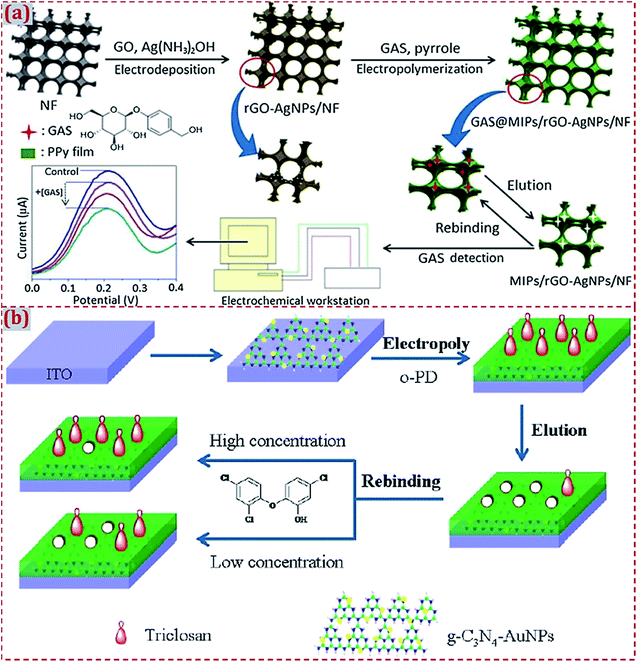 | ||
| Fig. 3 (a) Schematic illustration of the preparation and application of MIP/rGO–AgNPs/NF sensing system. The control is MIP/rGO–AgNPs/NF without the addition of GAS. Reproduced with permission from ref. 160. Copyright 2018, Elsevier. (b) Schematic of the fabrication of a MIP photoelectrochemical sensor based on g–C3N4–AuNP-modified ITO electrode. Reproduced with permission from ref. 176. Copyright 2018, Wiley. | ||
3. Preparation strategies of ECCSs based on CNM-modified MIPs
3.1. CNM-encapsulated or CNM-combined imprinting of silica and polymers
There are three major routes to achieve CNM-modified MIPs. Route I is to prepare CNM-encapsulated imprinting of silica (hard inorganic matrix). Route II is to prepare CNM-encapsulated imprinting of polymers (soft organic matrix). Route III is to prepare MIPs combined with CNMs. Silica as a promising inorganic material has high acid, thermostability, excellent permeability to templates, and biocompatibility. The classic Stöber method is often employed to synthesize silica NPs,58,111 and it involves the hydrolysis of tetraethoxysilane (TEOS) under basic conditions. Luo et al. reported the one-pot synthesis of GO that was coated with molecularly imprinted sol–gel SiO2 for the electrochemical sensing of paracetamol (PR).58 A GO/MIP complex was synthesized by mixing GO with phenyltriethoxysilane (PTEOS), tetramethoxysilane (TMOS) monomers, and PR template, followed by sol–gel copolymerization and extraction (Fig. 4). By depositing a GO/SiO2–MIP thin film on GCE, a molecular recognition element was constructed and used as an electrochemical sensor of PR. This work described a facile imprinting complex that combined the merits of surface molecular imprinting, CNMs, SiO2, and sol–gel technology, which enabled the specific recognition and detection of PR.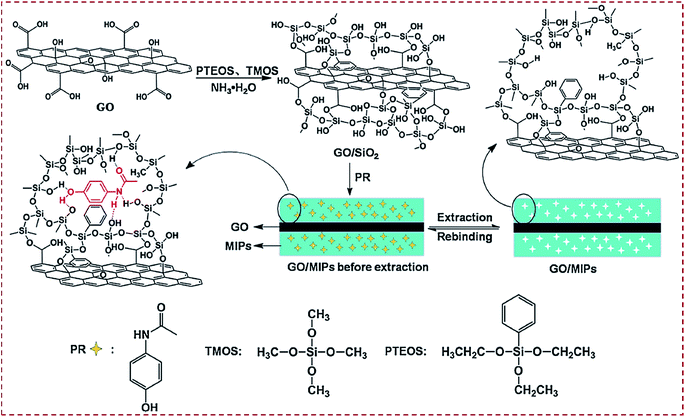 | ||
| Fig. 4 Preparation routes for molecularly imprinted sol–gel polymer (GO/SiO2 MIP) via a one-pot room-temperature polymerization reaction in an aqueous solution. Reproduced with permission from ref. 58. Copyright 2014, Springer. | ||
Patra et al. developed an electrochemical sensor of the prostate-specific antigen (PSA) based on surface imprinting nanotechnology.100 Through the decoration of MWCNTs with MnNPs and further functionalization with thiol groups, a nano-iniferter was prepared and served as the platform to synthesize 3D MIP matrixes for PSA based on a controlled radical polymerization technique. With regard to polymerization on a PGE surface, a prepolymer solution containing itaconic acid as the monomer, PSA as the template, and EGDMA as the cross-linking agent was dropcasted on the tip of the nano-iniferter-modified PGE and heated at 50 °C for 2 h. The adduct-modified PGE suffered from the template extraction of PSA from the polymer matrix to yield MIP.
The combination of MIP with CNMs is a common route to obtain CNM-modified MIPs. Liang et al. designed an electrochemical sensor based on MIP/GO-modified GCE for 2,4-dichlorophenol (2,4-DCP) determination.62 MIP was synthesized via precipitation polymerization using 2,4-DCP as the template, methacrylic acid (MAA) as the functional monomer, and EGDMA as the cross-linking agent in the presence of azodiisobutyronitrile (AIBN) initiator. GO was dropcasted on GCE and MIP was dropped on GO/GCE surface to prepare the MIP/GO/GCE sensing platform (Fig. 5a). Due to the high binding affinity and π–π interactions, MIP/GO/GCE yielded high recognition capability and electrochemical activity toward 2,4-DCP. Liu et al. reported a MIP sensor for dopamine (DA) detection based on CS dispersed with GR as the functional matrix and DA as the template molecule.49 Through the co-electrodeposition of a polymer film on GCE and the potential elution of DA, CS-based MIP–GR/GCE was prepared and had high sensitivity, selectivity, and rapid responses to DA oxidation (Fig. 5b). As natural polymers, CS mixed with GR and DA form a functional matrix. GR–CS–DA composite was co-electrodeposited to generate a MIP film on GCE, aiming to construct a MIP electrochemical sensor for DA sensing.
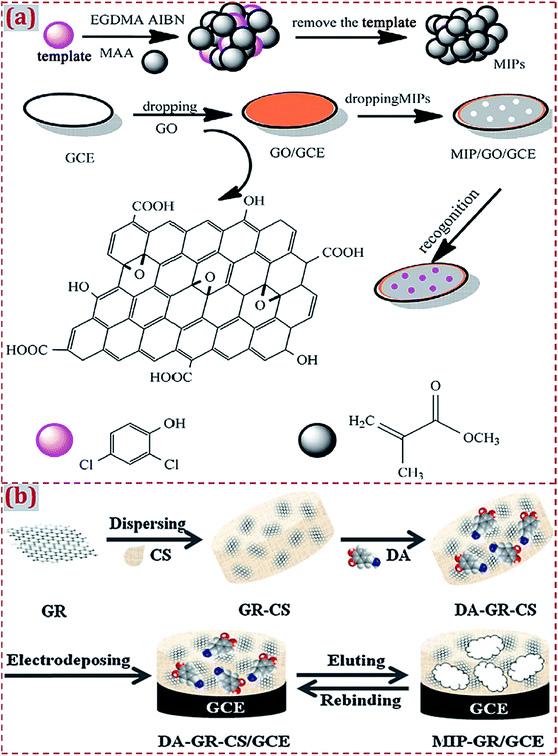 | ||
| Fig. 5 (a) Schematic illustration for the synthesis procedures of GO/MIP and construction process of a modified electrode. Reproduced with permission from ref. 62. Copyright 2017, Elsevier. (b) Preparation of MIP–GR/GCE and its recognition for DA. Reproduced with permission from ref. 49. Copyright 2012, Elsevier. | ||
3.2. Polymerization of MIP on CNM-modified substrates
Different strategies have discussed the preparation of CNM-modified-MIPs as ECCS decorated on various work electrodes or other substrates. Based on earlier studies, we have systematically summarized the major preparation strategies. Strategy I involves the polymerization of MIP on CNM-modified substrates. Strategy II involves the copolymerization of MIP and CNMs on substrates. Strategy III involves the dropcasting of MIPs on CNM-modified substrates. Strategy IV involves the dropcasting of a MIP/CNMs complex on substrates. Strategy V involves the self-assembly of MIP/CNMs/hybrids complex on substrates. Strategy VI involves the dropcasting of the self-assembled complex of MIP/CNMs/hybrids on substrates. In this section, we introduce some typical studies to summarize MIP polymerization on CNM-modified substrates (Strategy I). This strategy implies MIP preparation on the surface of CNM-modified substrates. The preparation strategies of MIPs focus on electropolymerization and in situ chemical polymerization.Yola et al. prepared a platinum NPs/carbon nitride nanotubes (PtNPs/C3N4 NTs) complex through a hydrothermal reaction.175 By a cyclic voltammetry (CV) scan, atrazine-imprinted polyphenol was electropolymerized on the complex-modified GCE to develop a MIP electrochemical sensor of atrazine. Chen et al. prepared electro-reduced GR (GP) that covalently modified the surface of the CE.83 Polypyrrole was electrodeposited on a GP-modified CE to prepare an imprinted sensor of 4-nonylphenol (Fig. 6a). NH2-terminated GO was covalently modified on a CE surface with diazonium salt reactions to improve the stability and reproducibility of this sensor. GP prepared by the electroreduction of GO enhanced the reactivity of 4-nonylphenol and sensitivity of this sensor. Yola et al. prepared 2-aminoethanethiol (2-AET)-functionalized GO (2-AETGO) via an amide coupling reaction; 2-AETGO was self-assembled with cubic AuNPs to generate a cAuNPs/2-AETGO mixture.148 The mixture was dropped on the GCE surface to construct cAuNPs/2-AETGO-modified GCE, followed by the electropolymerization of polyphenol in the presence of tyrosine as the template (Fig. 6b). A tyrosine-imprinted electrochemical sensor of MIP/cAuNPs/2-AETGO/GCE facilitated the sensitive detection of tyrosine.
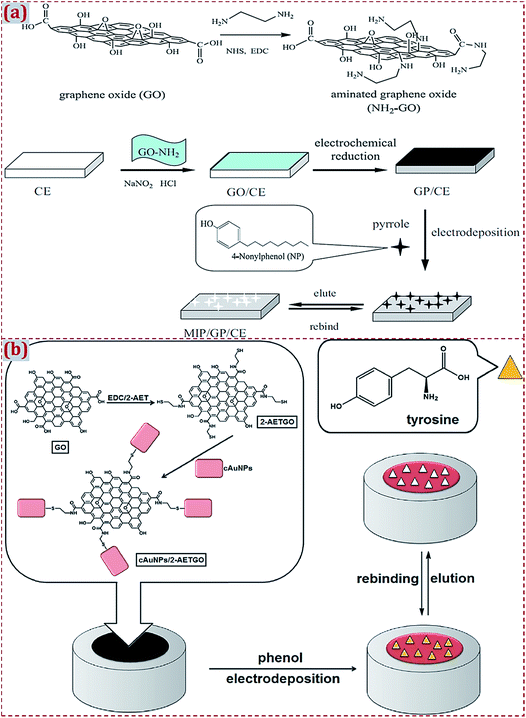 | ||
| Fig. 6 (a) Detailed procedure diagrams for the fabrication of MIP/GP/CE sensor for 4-nonylphenol. Reproduced with permission from ref. 83. Copyright 2013, Elsevier. (b) Procedures for fabrication of MIP/cAuNPs/2-AETGO/GC sensor for tyrosine detection. Reproduced with permission from ref. 148. Copyright 2015, Elsevier. | ||
In earlier reports, the electropolymerization strategy is widely used to prepare MIPs on substrates that have already been modified with CNMs and CNM-based hybrids. GO was prepared from a graphite powder by the classic Hummers' method. GO often suffers from electroreduction to yield GR. GP has enhanced reactivity and sensitivity during the detection of targets.54,64,68,83,118,123,128,144 Wang et al. synthesized a reduced GR–AuNPs nanocomposite via the one-step coreduction reaction of GO and HAuCl4 as the precursors.118 The GCE surface was dropcasted with GR–AuNPs suspension to generate GR–AuNPs/GCE, followed by the electropolymerization of polypyrrole on modified GCE under a CV scan. Prior to the electropolymerization of MIP-adduct,61,64,73,87,106,107,113,118,122,137,148,152,167,173,175,178,187,193,196 the direct dropcasting of CNM-complex on the substrates was considered to be a typical strategy to obtain CNM-modified substrates. CNMs or CNM-based hybrids can be electrodeposited on substrates to form CNM-modified substrates.46,68,156 Kan et al. developed an imprinted electrochemical sensor of DA.46 After the electrodeposition of MWCNT–COOH on GCE, a MIP film was formed by the electropolymerization of polypyrrole in the presence of DA. Beluomini et al. designed an electrochemical sensor of D-mannitol based on MIP on GCE modified with AuNP-decorated rGO.156 rGO was prepared by the electrodeposition of GO on GCE. AuNPs were deposited on the rGO/GCE surface by chronoamperometry. The electropolymerization of MIP was performed on AuNPs/rGO/GCE by a CV scan, using D-mannitol as the template and o-PD as the functional monomer.
Except for electropolymerization, MIP is prepared on CNM-modified substrates by in situ chemical polymerization.79,99 Prasad et al. fabricated a MWCNT–ceramic electrode (CE) covered with a substrate-selective imprinted polymer for BSA determination.79 MWCNTs were dispersed in a ceramic sol–gel matrix to improve the stability of CE. The MWCNT–CE surface is amenable to a “surface grafting’’ route for growing nanometer-thick MIP thin films. Vinyl-exposed MWCNT–CE was obtained by chemical reactions and physical treatments. This work reported a new way to realize a combination of CNMs with substrates in order to fabricate CNM-modified substrates, which is different from the dropcasting and electrodeposition of CNMs or CNM-based hybrids on substrates. To graft MIPs on vinyl-exposed MWCNT–CE, an in situ chemical polymerization reaction was performed in the prepolymer mixture containing BSA as the template, tetraethylene glycol 3-morpholin propionate acrylate (TEGMPA) as the functional monomer, diacryloyl urea (DAU) as the cross-linker, and ammonium persulfate (APS) as the initiator. MIP-modified MWCNT–CE as an electrochemical sensor enabled the ultra-trace detection of BSA.
3.3. Copolymerization of MIP and CNMs on substrates
CNMs or CNM-based hybrids are copolymerized with MIPs to generate a MIP/CNMs complex on substrates. This strategy avoids the modification of substrates with CNMs before the preparation of a MIP on CNM-modified substrates. Prasad et al. prepared a nanocomposite of functional GQDs and imprinted a polymer on a screen-printed carbon electrode (SPCE) by using N-acryloyl-4-aminobenzamide (N-ABA) as the monomer and the anticancer drug ifosfamide (IFO) as the template.88 GQDs were functionalized to GQD–COCl, followed by covalent bonding with N-ABA to form m-GQDs. The authors used a surface grafting route for the coating of m-GQD–MIP on SPCE. The prepolymer mixture consisted of m-GQDs, IFO as the template, AIBN as the initiator, and EGDMA as the cross-linker. The mixture was spin-coated on the SPCE surface and free-radical polymerization on the SPCE surface was initiated to obtain the MIP-adduct@SPCE. The IFO template was retrieved from the MIP-adduct by stirring the modified SPCE in an ammonia solution to obtain m-GQD–MIP@SPCE (Fig. 7). The firm coating of m-GQDs can be attributed to the interactions of m-GQDs with the SPCE surface via the cumulative effects of physisorption and π–π interactions. The covalent binding of GQDs with the monomeric units of N-ABA helps to obtain an electrochemical sensing platform that has a large surface area for the fast ingress and egress of analytes.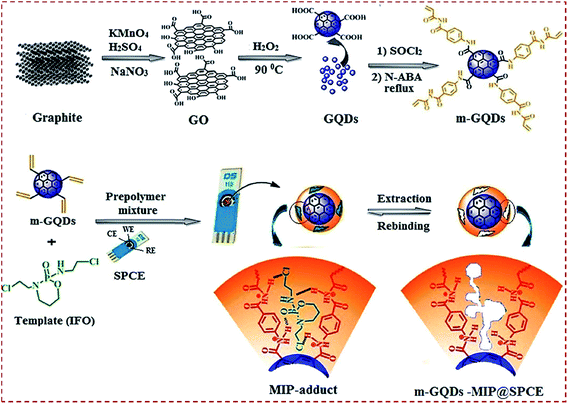 | ||
| Fig. 7 (a) Schematic illustration for the preparation of monomeric graphene quantum dots (m-GQDs) and fabrication of screen-printed carbon electrode (SPCE) modified with m-GQD–MIP. Reproduced with permission from ref. 88. Copyright 2017, Elsevier. | ||
Hatamluyi et al. reported an electrochemical biosensor platform using MIP reinforced by ZnO–GR-capped QDs for 6-mercaptopurine (6-MP) detection.182 ZnO@GQDs core–shell NPs were synthesized by the solvothermal method using GO and Zn(CH3COO)2 as the precursors. In a prepolymer solution containing ZnO@GQDs, pyrrole monomer, and 6-MP sol, the 6-MP-imprinted sol–gel film was electrodeposited on a PGE surface by performing CV runs. After template elimination by differential pulse voltammetry scanning, a polypyrrole/sol–gel/ZnO@GQDs/MIP/PGE sensor was obtained. The sensor integrated the features of the conducting polymer, sol–gel, and ZnO@GQDs core–shell QDs for the sensitive and selective sensing of 6-MP. As typical 0D CNMs, GQDs or CDs frequently hybridize with other CNMs and electroactive materials to form GQDs and CD-based hybrid materials. The use of hybrid materials to modify the substrates can facilitate the subsequent preparation of MIPs on the modified substrates.70,73,74,88,180–182 With regard to CNM-modified MIPs as ECCSs involving 0D CNMs, GQDs or CDs with high conductivity improve the electron transfer rate, sensitivity, and electrochemical responses toward analytes. Lin et al. fabricated a MIP-based electrochemical sensor of L-3,4-dihydroxy phenylalanine (L-dopa).48 A mixture was prepared by dispersing GR in a CS solution containing L-dopa. A pretreated GCE was immersed in the mixture solution, followed by electrodeposition under CV scans. This one-step electrodeposition method, namely, the copolymerization of MIP and CNMs on substrates, yielded an L-dopa-imprinted CS/GR/GCE sensing platform.
3.4. Dropcasting of MIPs on CNM-modified substrates
As a facile strategy to form CNM-modified-MIP-based ECCSs, Strategy III involves the dropcasting of already formed MIPs on substrates modified by CNMs or CNM-based complexes.62,97,116,117,129,132,190,191 Liang et al. synthesized MIPs via precipitation polymerization using 2,4-DCP as the template, MAA as the functional monomer, EGDMA as the cross-linker, and AIBN as the initiator.62 GO-based GCE was made by dropping GO dispersion on GCE. MIP suspension was dropped on GO/GCE to formulate MIP/GO/GCE for 2,4-DCP detection (Fig. 5a). Wang et al. prepared MIP via bulk polymerization using 4-chlorophenol as the template, as well as MAA, EGDMA, and AIBN.116 Poly(diallyldimethylammonium chloride)-functionalized GR (PDDA-G) was prepared through hydrazine hydride reduction. PDDA-G was used to modify GCE to obtain PDDA-G/GCE. MIP suspension was transferred to PDDA-G/GCE to fabricate MIP/PDDA-G/GCE for the electrochemical detection of 4-chlorophenol. Wei et al. prepared MIP, using ampicillin as the template, as well as MAA, EGDMA, and AIBN.97 AuNPs were dropped on a Pt electrode. MWCNT–COOH was added on AuNP-modified electrodes, followed by the dropcasting of a MIP solution on AuNPs/MWCNTs-modified electrode to develop an ampicillin-imprinted sensor.He et al. reported a biomimetic sensor based on Zn–porphyrin MIP microspheres (MIPM–Zn), AuNPs, and carboxyl-GR (CG).129 MIPM–Zn was prepared via the precipitation polymerization of a prepolymer mixture containing methyl parathion as the template, as well as Zn–porphyrin, EGDMA, and AIBN. AuNPs/CG complex was prepared through the reduction of HAuCl4 with NaBH4. AuNPs/CG suspension was transferred on GCE. MIPM–Zn suspension was dropped on AuNPs/CG/GCE. MIPM–Zn/AuNPs/CG/GCE was constructed and acted as a porphyrin-imprinted electrochemical sensor of methyl parathion. Yang et al. reported the preparation of AuNPs@MIP on CKM-3 and 3D porous GR (P-rGO)-modified GCE.190 IL was added into AuNPs solution to obtain AuNPs@IL via self-assembly. AuNPs@MIP was prepared by polymerization using AuNPs@IL as the monomer, dimetridazole as the template, as well as EGDMA and AIBN. P-rGO suspension was dropped on GCE, followed by the coating of CKM-3 suspension on P-rGO-modified GCE. AuNPs@MIP was then dropcasted to obtain AuNPs@MIP/CKM-3/P-rGO/GCE as a sensing platform for the electrochemical detection of dimetridazole. Zhao et al. synthesized IL-functionalized GR (GR–IL) dropcasted on GCE.191 The authors reported the fabrication of surface-imprinted polymer-coated MWCNTs that were grafted with a water-compatible external layer (MWCNTs@GMIP) through reversible addition–fragmentation chain-transfer precipitation polymerization. MWCNTs@GMIP was dropped onto GR–IL/GCE to obtain MWCNTs@GMIP/GR–IL/GCE as an electrochemical sensor for the detection of brucine.
3.5. Dropcasting of MIP/CNMs complex on substrates
Earlier studies have widely involved the strategy of “the dropcasting of MIP/CNMs complex on substrates” to design ECCSs.44,54,56,57,63,66,103,105,111,124,125,131,142,147,155,164,165,185,188 With regard to this strategy, MIP/CNMs complex, MIP/CNMs complex mixed with other materials, and MIP mixed with CNMs and other materials were dropcasted on the substrates to yield MIP/CNMs-complex-based hybrids-modified substrates. Yin et al. synthesized the MWCNTs@MIP–DA complex that was dropped on GCE to design a DA-imprinted film coated on GCE through self-assembly during solvent evaporation in air.44 Li et al. dispersed the Fe3O4–MIP@rGO complex in water under sonication.155 The solution was dropcasted on GCE and dried at room temperature. Kim et al. prepared GO/MIP aqueous suspension under ultrasonication.63 The suspension was transferred on GCE, followed by drying with an infrared lamp. Other studies have reported the dropcasting of MIP/CNMs complexes on substrates to fabricate ECCSs.54Usually, the MIP/CNMs complex is ultrasonically mixed with an acetic acid solution containing CS or Nafion (as a coupling or adhesive agent).103,105,124,125,131,142,147,165 The suspension is dropped on the surface of the substrates, followed by drying at room temperature in air or irradiation with an infrared lamp. In addition, a CS solution is dropped on MIP/CNMs-modified substrates for immobilization.56,57 Long et al. developed an imprinted electrochemical sensor by using magnetic MIP (MMIP).103 MMIP was prepared on MWCNT-decorated Fe3O4 NPs using kanamycin as the template and MAA as the monomer (Fig. 8a). CS was dissolved into an acetic acid solution under ultrasonication. MMIP was added to form a MMIP/CS mixture and the mixture was coated on a CE surface. A layer of the imprinted film was coated on the electrode to form a MMIP sensor of kanamycin. Zhu et al. prepared a composite of GR and MIP using IL as both the monomer and cross-linker for the electrochemical sensing of 6-benzylaminopurine.131 IL–GR–MIP was dispersed in an acetic acid solution containing CS to form a suspension that was dropped on GCE (Fig. 8b). The solvent was evaporated under an infrared lamp.
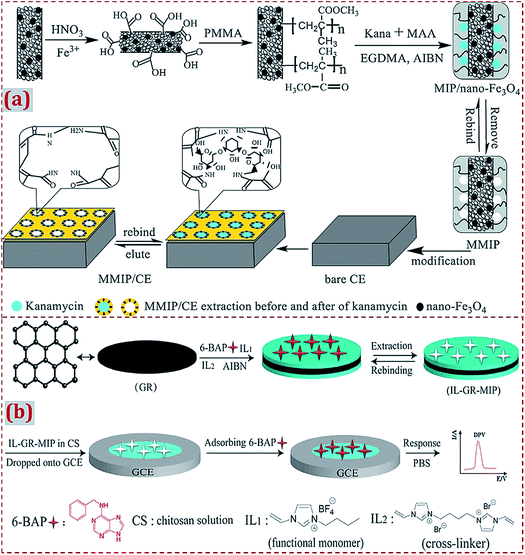 | ||
| Fig. 8 (a) Schematic of the preparation process of magnetism imprinted electrochemical sensor based on MWCNTs decorated on Fe3O4 NPs. Reproduced with permission from ref. 103. Copyright 2015, Elsevier. (b) Schematic illustration for the synthesis of IL–GR–MIP composite dropped on GCE and the detection process for the electrochemical sensor. Reproduced with permission from ref. 131. Copyright 2018, Elsevier. | ||
Before the dropcasting of the already prepared MIP/CNMs complex on the electrode surface, the electrode is often polished with alumina slurry, followed by rinsing with a water–ethanol mixture and ultrasonic treatments in nitric acid, NaOH, acetone, and deionized water. The pretreatments aim to achieve a clean and smooth surface on the electrode, which is propitious toward the surface modification of the electrode with other materials in subsequent experiments. Zeng et al. prepared a rGO–MIP composite by free-radical polymerization.66 rGO–MIP was dispersed under ultrasonication in an acetic acid solution containing CS. The suspension was dropped on the pretreated GCE surface together with drying at room temperature (Fig. 9a). Zhang et al. employed p-vinylbenzoic acid (p-VBA) as a monomer to immobilize on GR via π–π interactions and the direct polymerization of a uniform MIP layer on the surfaces of GR sheets.57 The GR/MIP composite was dropped on a well-polished GCE, followed by drying at room temperature. The acetic acid solution containing CS was dropped onto GR/MIP/GCE for the immobilization of GR/MIP on GCE followed by drying in air (Fig. 9b).
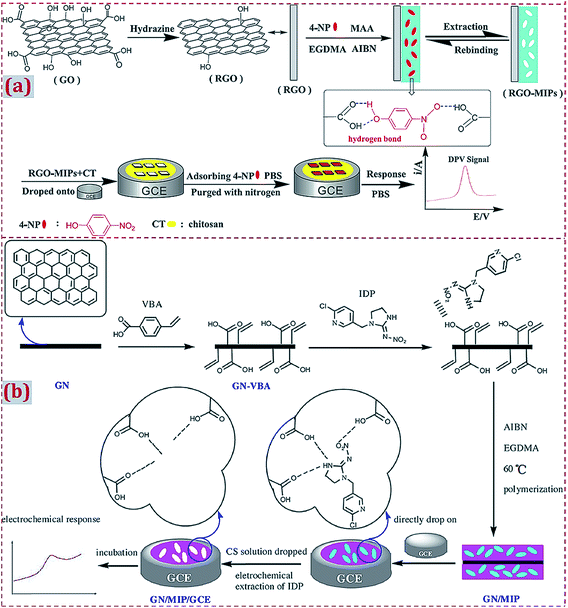 | ||
| Fig. 9 (a) Illustration of the preparation procedures for rGO-MIPs and the detection process for the electrochemical sensor based on a composite of rGO and MIPs. Reproduced with permission from ref. 66. Copyright 2014, Elsevier. (b) Schematic procedures for the preparation of GN/MIPs/GCE and a concept for the selective electrochemical detection of imidacloprid. Reproduced with permission from ref. 57. Copyright 2017, Elsevier. | ||
Different from dropcasting of the already fabricated MIP/CNMs complex, the mixture of MIP/CNMs and other materials can be poured on substrates to fabricate MIP/CNMs-complex-modified substrates under mechanical pressure. Tong et al. investigated an electrochemical cholesterol sensor on ceramic CE modified with the MWCNTs@MIP complex.188 MWCNTs@MIP, graphite powder, and MIP were added in the solution containing ethyl trimethoxysilane (as a coupling agent), ethanol, and HCl. The mixture was pestled and was firmly packed into the electrode cavity of a Teflon sleeve, followed by drying at room temperature. Güney et al. fabricated a carbon paste electrode modified with bisphenol-A-imprinted polymer (BPA-IP), sol–gel, and MWCNTs by mixing BPA-IP, carbon-powder–MWCNTs mixture, and paraffin oil in a mortar until a homogeneous paste was formed.111 The paste was pressed into a glass tube and an electrical contact was achieved by inserting a Cu wire into the glass tube. A new electrode surface was obtained by pushing an excess of the paste out of the glass tube and polishing it on fine smooth paper. Shojaei et al. constructed MIP–MWCNTs that were used to modify the carbon paste electrode.185 Graphite powder was homogenized with MIP and MWCNTs. Paraffin was added to the MIP–MWCNT–graphite blend and mixed with a stainless steel spatula to obtain a homogenous paste. The paste was used to fill a hole at the end of electrode body. MIP–MWCNT–electrode was rinsed with a water–ethanol solution. An excess of the solidified material coming out of the hole was removed with a sheet of paper.
3.6. Self-assembly of MIP/CNMs/hybrids complex on substrates
To prepare CNM-modified-MIP-based ECCSs, earlier studies have reported the preparation of various types of MIPs through in situ chemical polymerization and electropolymerization strategies. The resulting MIP is often modified with CNMs, accompanied by hybridization with other materials, to form a MIP/CNMs/hybrids complex. The complex is frequently prepared on work electrodes or other substrates via layer-by-layer self-assembly.75,93,96,108,109,127,128,133–135,138,177,180,181,192 Shen et al. reported an imprinted electrochemical sensor of human chorionic gonadotropin (hCG).108 GCE was modified through a layer-by-layer coating of MWCNTs, CS, and glutaraldehyde. Further, hCG was covalently bonded on the modified GCE followed by the electropolymerization of DA. After template elution, a MIP film was formed on the complex-modified GCE. Guo et al. modified the GCE with CDs and CS, followed by the electropolymerization of AuNPs on CS/CDs/GCE, self-assembly of p-ATP monomer on AuNPs/CS/CDs/GCE, and H-bonding adsorption of 2-oxindole as a dummy template on p-ATP. The authors performed the copolymerization of p-ATP and 2-oxindole to generate a polymer film on AuNPs/CS/CDs/GCE and then removed the template to form MIP/AuNPs/CS/CDs/GCE for the electrochemical sensing of patulin.180The layer-by-layer self-assembly for preparing MIP/CNMs/hybrids complex on work electrodes and other substrates was largely reported.93,96,109,128,133–135,138,177,192 Wen et al. reported a sandwich-type MIP electrochemical sensor of 17β-estradiol (E2).169 By using electrodeposition and cast-coating methods, PtNPs and rGO were fabricated on GCE. 6-Mercaptonicotinic acid (MNA) was self-assembled on the modified GCE with the formation of a Pt–S bond. E2 was assembled by forming a hydrogen bond with MNA. A polymer film was formed by the electropolymerization of MNA. Specific recognition cavities were formed after template removal. Nguyen et al. designed a sensitive GCE modified with GR, AuNPs, molecularly imprinted overoxidized polypyrrole for the detection of DA.127 The authors prepared a GR film by the chemical vapor deposition method. The film was transferred to GCE by chemical etching. An imprinted polypyrrole was electropolymerized on GR/GCE. After template removal, the overoxidization of a MIP film (OPPy) was conducted by CV scans. AuNPs were electrodeposited on the OPPy surface to form AuNPs/OPPy/GR/GCE. The OPPy film exhibited better cation exchange and molecular sieve capability when compared with those of polypyrrole, further enhancing the selectivity and sensitivity for DA detection.
The substrates can be modified with CNMs using Nafion as a coupling or adhesive agent.93 Moreover, the surface of the substrates can be electroactivated beforehand with functional molecules to facilitate the self-assembly of MIP/CNMs/hybrids complexes on electroactivated substrates. Yola et al. reported a MIP sensor of cefixime on GCE.102 GCE combined with p-nitrophenyl via a chemical reaction under CV followed by the reduction of a nitro group to amine to prepare p-aminophenyl (AP)-modified GCE. COOH-functionalized f-MWCNTs were combined with 2-AET and AP via amide coupling. Fe@AuNPs and 2-AET combined with f-MWCNTs were self-assembled on GCE, followed by the electropolymerization of polypyrrole. NH2-terminated benzene diazonium (NBD) was employed to modify the CE.123,144 4-Nitrobenzenediazonium tetrafluoroborate salt was electrodeposited on CE. The nitro group was electroreduced to amine by CV scans. The layer-by-layer self-assembly of CNMs, other materials, and MIP was conducted on NBD-functionalized CE. Chen et al. reported a sensor of 3,3′,5,5′-tetrabromo bisphenol A (TBBPA) on CE modified with NiNP–GR complex.144 GO was covalently combined with NBD-modified CE. GO was electroreduced to GR. NiNPs were electrodeposited on GR/CE, followed by forming a MIP via electropolymerization (Fig. 10). Yang et al. designed an electrochemical sensor of chloramphenicol through the layer-by-layer self-assembly of MWCNTs@MIP, CKM-3, and P-rGO on GCE.75 P-rGO was coated on GCE. CKM-3 was coated on P-rGO/GCE followed by the dropcasting of MWCNTs@MIP to fabricate MWCNTs@MIP/CKM-3/P-rGO/GCE.
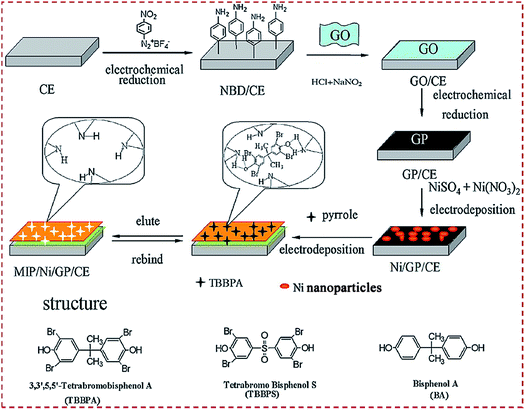 | ||
| Fig. 10 Detailed procedure diagrams for fabricating a MIP/Ni/GP/CE sensor. The chemical structures of TBBPA, TBBPS, and BPA. Reproduced with permission from ref. 144. Copyright 2014, Elsevier. | ||
The fabrication of the MIP/CNMs/hybrids self-assembled complex on substrates is often accompanied by functional and multiple polymerization steps. Xue et al. developed a double-layer membrane interface for 5-hydroxytryptamine (5-HT) detection.168 A polyaniline-coated rGO composite was prepared by a one-step electrodeposition process on GCE. AuNPs@MIP was formed on the modified GCE via electropolymerization using 5-HT as the template, functionalized AuNPs as the functional monomer, and p-ATP as the cross-linker (Fig. 11a). Kong et al. designed a bilayer membrane that consisted of polypyrrole, functionalized MWCNTs, and binuclear phthalocyanine cobalt(II) sulfonate (BiCoPc) on GCE.104 The polypyrrole–MWCNT–BiCoPc complex was one-step electrodeposited on GCE via chronoamperometry. A MIP membrane was produced on the complex-modified GCE via electropolymerization using o-aminophenol (o-AP) as the monomer and metolcarb (MTMC) as the template (Fig. 11b). Yang et al. prepared a bilayer of polypyrrole composite and MIP.150 A polypyrrole–GO–BiCoPc composite was formed on GCE by CV scans, followed by the electropolymerization of MIP on the composite-modified GCE using o-PD as the functional monomer and quinoxaline-2-carboxylic acid as the template. Jaiswal et al. fabricated a layer-by-layer imprinted sensor.91 A PGE was spin-coated with rGO-functionalized D-serine-imprinted polyacrylamide (PAA). The electrode was further modified with fullerene (0D C60)-functionalized L-serine-imprinted PAA. This bilayer assembly enabled the enantioselective analyses of D- and L-serine.
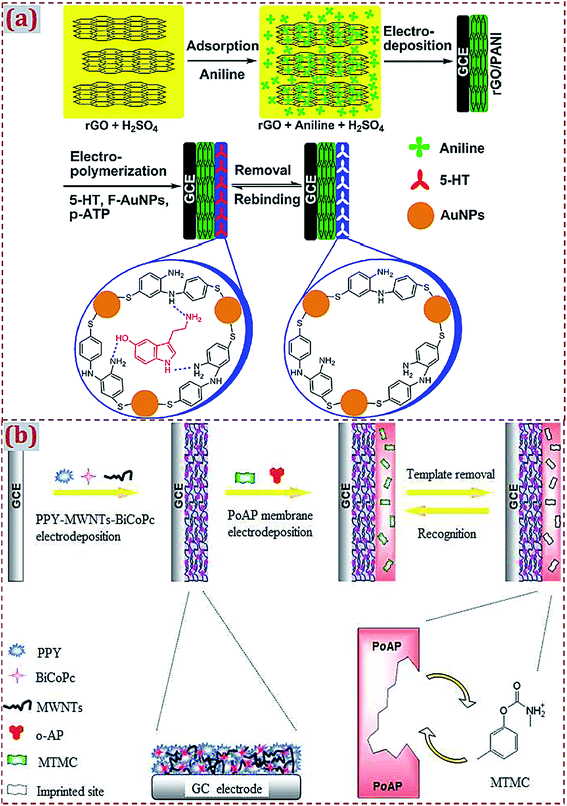 | ||
| Fig. 11 (a) Schematic representation for the preparation of AuNPs@molecularly imprinted electrochemical sensor (MIES) based on a double-layered membrane of rGO/polyaniline nanocomposites and MIPs embedded with AuNPs. Reproduced with permission from ref. 168. Copyright 2014, Elsevier. (b) Preparation procedures for imprinted PPY–MWCNT–BiCoPc–GCE based on composites consisting of polypyrrole (PPY), functionalized MWCNTs, and binuclear phthalocyanine cobalt(II) sulfonate (BiCoPc) on a GCE surface. Reproduced with permission from ref. 104. Copyright 2015, the Royal Society of Chemistry. | ||
3.7. Dropcasting of self-assembled complex of MIP/CNMs/hybrids on substrates
As an alternative strategy to fabricate CNM-modified-MIP-based ECCSs, a facile and effective strategy is the dropcasting of already prepared self-assembled MIP/CNMs/hybrids complexes on substrates. Li et al. developed an electrochemical sensor of dibutyl phthalate (DBP).149 A complex of AuNPs and GO decorated with Fe3O4 NPs (MGO) was synthesized by coprecipitation and self-assembly. DBP, as the template, was absorbed on the complex and then the copolymerization of MAA and EGDMA was conducted. After template extraction via potential scans, MIP/MGO@AuNPs complex was prepared and dispersed under ultrasonication in an acetic acid solution containing CS. The suspension was dropped on Au electrodes to generate MIP/MGO@AuNPs/Au-sensing platform. Li et al. reported an imprinted sensor of Sunset Yellow (SY).153 MGO was prepared by the coprecipitation of iron ions with an ammonia solution. Fe3O4 NPs were decorated on GO. MGO and β-cyclodextrin (β-CD) were self-assembled in an ammonia and hydrazine solution. With the addition of ILs, the MGO/β-CD/IL complex was formed under sonication. AuNPs were mixed with MGO/β-CD/IL followed by the in situ precipitation polymerization using MAA as the monomer, SY as the template, EGDMA as the cross-linker, and AIBN as the initiator. The products were washed with methanol–acetic acid solution to yield the MIP/AuNPs/MGO/β-CD/IL complex. The complex was dispersed under sonication in an acetic acid solution containing CS and was dropped on GCE. After solvent evaporation under an infrared lamp, the self-assembled complex-modified GCE was obtained.Magnetic-field-directed self-assembly is an effective strategy to implement the dropcasting of MIP/CNMs/hybrids complex on substrates. Han et al. prepared the Fe3O4@rGO-complex-doped MIP membrane for amaranth detection.162 The Fe3O4@rGO composite was obtained by the initial intercalating of iron ions between the GO layers via electrostatic interactions followed by reduction with hydrazine hydrate to deposit Fe3O4 NPs on rGO nanosheets. The mixture, containing aniline as the monomer, amaranth as the template, and Fe3O4@rGO, was preassembled through π–π stacking and hydrogen bonding interactions and was then self-assembled on magnetic GCE with magnetic field induction before electropolymerization (Fig. 12a). Tang et al. fabricated a sensor for interleukin-8 (IL-8) detection.145 IL-8 surface MIP NPs were synthesized by using GO-modified Fe3O4 NPs as the core and IL-8-imprinted polymer as the shell. Fe3O4@GO@MIP NPs were self-assembled on Au film electrodes under a magnetic field, which realized the direct dropcasting of self-assembled MIP/CNMs-complex-based materials on the Au film electrode to form ECCSs (Fig. 12b). Zhu et al. reported the one-step fabrication of MIP membranes by magnetic-field-directed self-assembly for glutathione sensing.163 Ternary Fe3O4@polyaniline/rGO was prepared by the chemical oxidative polymerization and intercalation of Fe3O4@polyaniline into GO layers via π–π stacking interactions, followed by the reduction of GO with hydrazine hydrate. The prepolymer mixture, containing glutathione as the template, ternary composite as the monomer, and pyrrole as the cross-linker and comonomer, was assembled via N–H hydrogen bonds and electrostatic interactions and was rapidly oriented on magnetic GCE under magnetic field induction. By electropolymerization and template reveal, the self-assembled MIP/Fe3O4@polyaniline/rGO–complex-modified magnetic GCE was achieved.
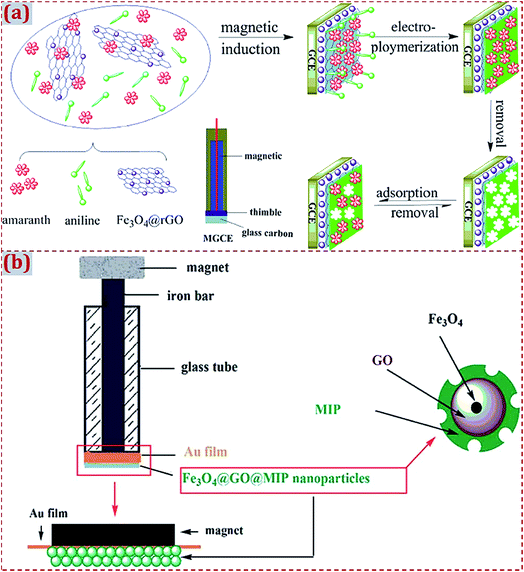 | ||
| Fig. 12 (a) Schematic representation for the preparation of magnetic molecularly imprinted electrochemical sensor (MIES) based on magnetic GCE modified with MIPs@Fe3O4@rGO. Reproduced with permission from ref. 162. Copyright 2014, Elsevier. (b) Schematic representation for preparing a work electrode: full view of Fe3O4@GO@MIP-nanoparticle-modified electrode; partially enlarged drawing of the electrode surface; schematic presentation of the nanoparticles. Reproduced with permission from ref. 145. Copyright 2015, the Royal Society of Chemistry. | ||
3.8. In situ chemical polymerization of functional monomers
The in situ chemical polymerization of functional monomers is a classic strategy for the synthesis of MIPs, which is often used to synthesize MIPs used in CNM-modified-MIP-based ECCSs. The prepolymer mixture principally contains monomers, templates, cross-linkers, initiators, catalysts, solvents, and other auxiliary substances. There are noncovalent and covalent interactions among the monomers, templates, and cross-linkers. Driven by initiators, catalysts, and auxiliary substances, the highly cross-linked polymers are prepared and formed as MIP-adduct. The polymerization process is usually undertaken as hydrothermal, solvothermal, or room-temperature reactions. To generate molecular recognition cavities in the MIP-adduct, template molecules need to be removed by rinsing with water or other solvents. MIPs with molecular cavities are obtained by template removal. The cavities are complementary to analytes and have specific shapes, sizes, structures, and functional groups, which are similar to those for template molecules.With regard to CNM-modified-MIP-based ECCSs (Tables 1–6), the in situ chemical polymerization of functional monomers has been popular to prepare MIPs in earlier studies.52,57,62,66,79,100,103,161 The frequently used functional monomers include AM and MAA. The cross-linkers majorly involve EGDMA and N,N-methylene-bis-acrylamide (MBA). AIBN and APS are popular initiators. Zhao et al. reported SY-imprinted IL polymers and IL-functionalized rGO complex films coated on GCE.161 Water-compatible MIPs were prepared by free-radical polymerization in a water–methanol system using SY as the template and 1-(α-methylacrylate)-3-allylimidazolium bromide (1-MA-3AI-Br) IL as the monomer. The IL interacted with SY through π–π stacking, hydrogen bonding, and electrostatic interactions. MIPs were prepared by a noncovalent method. In the mixture containing SY, IL, AIBN, EGDMA, and methanol–water as the solvents, prepolymerization was performed at room temperature followed by the hydrothermal treatment of the mixture to trigger polymerization. The product was washed with methanol–ammonia, followed by centrifugation collection. IL–rGO suspension was dropcasted on GCE. Solvents were evaporated under an infrared lamp. MIP suspension was dropped on IL–rGO/GCE and was evaporated in air. MIP–IL–rGO/GCE was used as a sensor for the detection of SY.
Anirudhan et al. reported the in situ chemical copolymerization of dual-functional monomers to prepare MIPs.105 In the prepolymerization mixture comprising bisphenol A (BPA) as the template, MAA and vinylpyridine as the co-functional monomers, EGDMA as the cross-linker, and AIBN as the initiator, the polymerization reaction was performed at 70 °C for 16 h. The product was centrifuged and then washed with ethanol to remove the unreacted reagents. The BPA templates were removed by Soxhlet extraction with methanol and acetic acid. BPA-imprinted MIPs were obtained on the surface of glycidoxypropyl-trimethoxysilane-functionalized MWCNT–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2. MIP/MWCNTs composite was mixed with acetic acid and CS solution. The mixture was dropped on bare GCE to form MIP/MWCNTs/GCE for the electrochemical sensing of BPA (Fig. 13). Anirudhan et al. prepared silylated GO-grafted chemically modified nanocellulose (Si-GO-g-CMNC) for the electrochemical sensing of cholesterol.195 CMNC was modified by grafting allyl amine on ZnO NCs, followed by the polymerization of itaconic acid to generate –COOH. Si–GO was polymerized with the prepolymerization mixture using MBA as the cross-linker and AIBN as the free-radical initiator in a polymeric chain reaction. Si-GO-g-CMNC was dispersed under ultrasonication in acetic acid–CS solution, followed by dropping on GCE and solvent evaporation at 50 °C.
CH2. MIP/MWCNTs composite was mixed with acetic acid and CS solution. The mixture was dropped on bare GCE to form MIP/MWCNTs/GCE for the electrochemical sensing of BPA (Fig. 13). Anirudhan et al. prepared silylated GO-grafted chemically modified nanocellulose (Si-GO-g-CMNC) for the electrochemical sensing of cholesterol.195 CMNC was modified by grafting allyl amine on ZnO NCs, followed by the polymerization of itaconic acid to generate –COOH. Si–GO was polymerized with the prepolymerization mixture using MBA as the cross-linker and AIBN as the free-radical initiator in a polymeric chain reaction. Si-GO-g-CMNC was dispersed under ultrasonication in acetic acid–CS solution, followed by dropping on GCE and solvent evaporation at 50 °C.
 | ||
| Fig. 13 Schematic illustration of MIP/MWCNTs/GCE for the electrochemical sensing of BPA. Reproduced with permission from ref. 105. Copyright 2018, Elsevier. | ||
3.9. Electrochemical polymerization of electroactive functional monomers
As a facile strategy to prepare MIPs, electropolymerization is popularly applied in the preparation of MIPs to fabricate CNM-modified-MIP-based ECCSs.44,45,67,72,78,85,88,93,94,102,104,114,130,138,144,152,160,168,169,172–174,176,179,181,183,186 The electropolymerization of MIP requires the presynthesis of a polymer solution containing template molecules, functional monomers, solvents, electrolyte solution, etc. In contrast to in situ chemical polymerization, electropolymerization can avoid the use of cross-linkers, initiators, or catalysts during the preparation of MIPs, thereby simplifying the experimental procedures. The electropolymerization of MIPs involves CV scan cycles in a polymer solution together with the potential sweeping within proper scan ranges and rates.Wen et al. developed the one-step fabrication of poly(o-AP)/MWCNTs-film-modified electrode for the detection of levofloxacin.45 Under sonication, a mixture containing o-AP, MWCNTs, sodium dodecyl sulfate (SDS), and H2SO4 was prepared. GCE was dipped in this mixture solution. By potential scanning in the range of −0.20 to 0.84 V at 0.1 V s−1 for 10 cycles, poly(o-AP)/MWCNTs/GCE was constructed. SDS increases the conductivity of poly(o-AP) and o-AP can be easily oxidized to form more monocation radicals on GCE. At the first cycle, a distinct irreversible anodic peak was found at 0.71 V. Therefore, o-AP was irreversibly oxidized at the electrode surface. Two pairs of redox peaks appeared. Redox and redox pairs were respectively ascribed to the redox process of phenoxazine units and intermediate generation in the oxidation process of o-AP. Yin et al. reported MIP–polydopamine (PDA)-coated MWCNTs for the detection of SY.44 SY was mixed with DA to induce molecular self-assembly through intermolecular forces, such as hydrogen bonding, π–π, and electrostatic interactions. DPA was formed by the in situ spontaneous oxidative polymerization of DA in an alkaline solution and at room temperature. DA adhered on MWCNTs to produce a DPA film. The SY template was embedded in a DPA film through intermolecular forces. After template removal, SY-imprinted MWCNTs@MIP–DPA was obtained and dropped on GCE to fabricate an electrochemical sensor of SY.
In particular, different functional monomers with electroactivity can simultaneously participate in the preparation process of MIPs174,186,192 with the features of highly cross-linking copolymers. Hu et al. explored a BPA sensor based on MIP/CNT–AuNPs/boron-doped ordered CKM-3-composite-modified GCE.192 MIP was prepared by cycling the potential of 0–0.65 V at 50 mV s−1 in an acetate buffer solution using o-phenylenediamine (o-PD) and o-toluidine (o-TD) as the co-functional monomers and BPA as the template. Yang et al. reported a sensor of eugenol using 3D molecularly imprinted poly(p-aminothiophenol-co-p-aminobenzoic acid), namely, poly(p-ATP-co-p-ABA)-film-modified GCE.186 The sensor was composed of MIP and IL-functionalized GR–CNTs composite prepared by a one-step hydrothermal method. Porous molecularly imprinted copolymers were prepared on GR–CNT–IL/GCE by CV scans (Fig. 14a). Pan et al. presented a sensor of 4-nonyl-phenol (NP) based on molecularly imprinted poly(o-phenylenediamine-co-o-toluidine), i.e., poly(o-PD-co-o-TD)-N-doped GR nanoribbons (NGRRs)–IL film.174 NGRRs were prepared by unzipping MWCNTs, followed by hydrothermal treatment with ammonium–NaOH solution. MIP was prepared by electropolymerization on NGRR–IL-modified GCE using NP as the template and o-PD and o-TD as comonomers (Fig. 14b).
 | ||
| Fig. 14 (a) Preparation routes of MIP/GN–CNT–IL/GCE for the electrochemical determination of eugenol using a 3D molecularly imprinted poly(p-ATP-co-p-ABA)-film-modified electrode. Reproduced with permission from ref. 186. Copyright 2016, Elsevier. (b) Preparation routes for MIP/NGNR–IL/GCE as an electrochemical sensor of 4-nonyl-phenol (NP) based on molecularly imprinted poly(o-PD-co-o-TD)-nitrogen-doped graphene nanoribbons (NGNRs)–ionic liquid (IL) composite film. Reproduced with permission from ref. 174. Copyright 2015, Elsevier. | ||
3.10. Dummy templates and engineering structures of CNM-modified MIPs
With regard to the preparation of MIPs, certain target molecules are difficult to obtain or harmful. Dummy molecules with similar molecular structures as those of the targets often serve as alternative templates. Patulin (4-hydroxy-4H-furo[3,2-c]pyran-2(6H)-one) as a type of polyketide lactone is a toxic secondary metabolite produced by numerous species of filamentous fungi belonging to the genera. Patulin is toxic to animals. Guo et al. adopted 2-oxindole as a dummy template to replace patulin with the purpose of forming a MIP cavity at a lower cost.180 The authors fabricated a MIP sensor for patulin detection based on GCE modified with CDs, AuNPs, CS, and electropolymerized MIP. Shi et al. reported a sensor using MIPs for the recognition of 2,4,6-trinitrotoluene (TNT).50 The safe detection of TNT cannot be easily achieved. Picric acid has a molecular structure similar to that of TNT and acts as a dummy template of TNT. GR–polyaniline (GR–PANI) composite was prepared by the electropolymerization of aniline on GR. GR–PANI–MIP was formed by in situ polymerization using picric acid as the template, AM as the monomer, EGDMA as the cross-linker, and AIBN as the initiator (Fig. 15). After the reaction of the mixture at 70 °C for 12 h, the product was collected by centrifugation, followed by washing with ethanol and water, and eluting with ethanol and acetic acid. GR–PANI–MIP was dropped on GCE. The modified electrode was used for TNT detection. | ||
| Fig. 15 Schematic of fabricating molecularly imprinted sites for TNT recognition based on GN–PANI–MIP/GCE. Reproduced with permission from ref. 50. Copyright 2015, Wiley. | ||
There are various engineering structures of CNM-modified MIPs to fabricate ECCSs. Category I involves a core–shell-structured composite that uses CNMs or CNM-based hybrids as the core and MIP as the shell. Category II comprises composite NPs or particles containing CNMs, MIPs and hybrids. Category III involves a layer-by-layer self-assembled complex of CNMs/MIP/hybrids on electrodes. Category IV comprises bulk material containing CNMs, MIP, and hybrids on bulk substrates. Category V includes the surface-imprinted material of CNM-modified MIPs on bulk substrates. Category VI comprises a 3D structural complex of CNMs/MIP/hybrids on electrodes. Wang et al. reported a sensor of 4-aminophenol using CdS QD–GR composite and MIP.114 FTO was treated with poly(diallyldimethyl ammonium chloride) to improve the immobilization of CdS and GO on FTO through electrostatic attraction. CdS–GO-modified FTO was thermally heated for the reduction of GO to GR. A polypyrrole layer with surface imprinting of 4-aminophenol was electropolymerized on CdS–GO-modified FTO (Fig. 16). The template was removed by electrochemical treatments in Na2HP4 solution. MIP with surface imprinting was electrodeposited on this modified FTO to fabricate MIP/CdS–GO/FTO sensing platform.
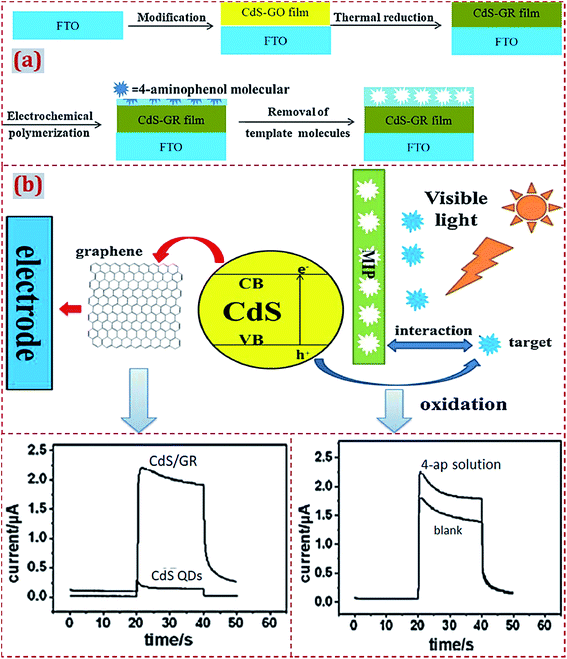 | ||
| Fig. 16 (a) Schematic illustration for the fabrication of MIP/CdS–GR/FTO-based photoelectrochemical sensor. (b) A proposed mechanism for the photoelectrochemical sensing of 4-aminophenol based on MIP/CdS–GR-modified electrode. Reproduced with permission from ref. 114. Copyright 2014, Elsevier. | ||
With regard to CNM-modified-MIP-based ECCSs, electrodes or substrates are frequently modified with CNMs/MIP/hybrids complexes with 3D structures.172,173,186,187,190 3D-Structured CNMs/MIP/hybrids complexes are synthesized and dropped on substrates or they are directly fabricated on substrates via self-assembly. Shang et al. fabricated a 3D hybrid film with in- and out-of-plane pores by using porous GR as the framework structure and porous Pd–Cu alloy NPs as the building blocks.173 A 3D porous hybrid was dropped on GCE, followed by the electropolymerization of p-ABA to prepare MIPs. A 3D porous MIP hybrid film was formed on GCE and enabled the electrochemical sensing of melamine. 3D porous GR–CNT–IL composite was prepared by the one-step hydrothermal method and was used to modify the GCE.186 Eugenol-imprinted poly(p-ATP-co-p-ABA) was electrodeposited on the modified GCE via copolymerization to form a MIP electrochemical sensor. 3D PdNP–porous GR–CNTs composite was prepared via a one-step hydrothermal method. Quercetin-imprinted poly(p-ABA) was deposited on this modified GCE via the electropolymerization of p-ABA.187 Yang et al. reported a sensor of dimetridazole by using AuNPs@MIP, CKM-3, and P-rGO.190 AuNPs@MIP was coated on CKM-3- and P-rGO-modified GCE to fabricate a 3D porous MIP hybrid film.
Other engineering structures of CNM-modified MIP have been reported. Wei et al. prepared MIP/NiCo2O4 nanoneedle arrays on 3D GR electrodes to detect sulfadimidine (SM2).172 NiCo2O4 nanoneedle arrays were decorated on free-standing and conductive 3D GR via a hydrothermal process. Polypyrrole was coated on these nanoneedle arrays via electropolymerization by using SM2 as the template. SM2-imprinted MIP/nanoneedle arrays/3D GR complex electrodes were thus formed. Cardoso et al. reported a sensor of chloramphenicol (CAP) based on CO2 laser-induced GR technique for electrode patterning.171 GR produced on a polyimide substrate showed a porous multilayer structure. The sensor was designed as a three-electrode system. Auxiliary and working electrodes were made of GR by laser patterning. The reference electrode was handmade by casting silver ink. MIP was produced at the working electrode through the direct electropolymerization of Eriochrome Black T (EBT) using CAP as the template. GR-based electrodes obtained from the laser-induced GR technique are simple and quick for onsite sensing.
4. Applications of ECCSs based on CNM-modified MIPs
4.1. Targets, signal transducer modes, and real samples for detection
In earlier studies, various template molecules and targets (analytes) have been used to prepare MIPs in CNM-modified-MIP-based ECCSs (Tables 1–6). The targets majorly involve metal ions (Bi3+), small molecules, and biomacromolecules. Small target molecules include various drug molecules used for treating human diseases, biological small molecules from human bodies, molecules that are toxic and harmful toward human health, pesticides for agricultural products, etc. The examples of biomacromolecules are proteins, nucleic acids, enzymes, disease biomarkers, etc. Other targets focus on industrial raw materials, environmental pollutants, food additives, explosives, etc. With regard to CNM-modified-MIP-based ECCSs, different modes of signal transducer outputs are used for the electrochemical sensing of various targets. Different signal transducer modes are used, such as differential pulse voltammetry (DPV), CV, square wave voltammetry (SWV), linear sweep voltammograms (LSV), electrochemical impedance spectroscopy (EIS), differential pulse cathodic stripping voltammetry (DPCSV), differential pulse anodic stripping voltammetry (DPASV), linear sweep stripping voltammetry (LSSV), anodic stripping differential pulse voltammograms (ASDPV), differential pulse stripping voltammetry (DPSV), square wave stripping voltammetry (SWSV), PEC and chronoamperometry (CA), cathodic stripping different pulse voltammetry (CSDPV), etc.CNM-modified-MIP-based ECCSs are widely applied in the electrochemical detection of various targets in real samples. Various samples are involved, including questionable samples containing targets as well as healthy and safe samples without targets. The contents of the targets in questionable samples are determined directly using ECCSs. In addition, the targets are externally introduced into healthy and safe samples and are determined based on a standard addition method. The types of real samples for determination are several and varied (Tables 1–6), including biological and biomedical samples of human bodies or small animals, pharmaceuticals, food samples, agricultural products, industrial and environmental samples, etc. In different real samples, various targets were effectively detected by using CNM-modified-MIP-based ECCSs. As confirmed in earlier studies, this type of ECCS exhibited high detection sensitivity and selectivity, stability, and repeatability, as well as high detection recoveries.
4.2. Detection mechanisms of nonelectroactive and electroactive targets
With regard to the electrochemical sensing applications of CNM-modified-MIP-based ECCSs, there are two principal detection mechanisms for nonelectroactive and electroactive targets (Scheme 2). When the targets are nonelectroactive, the redox electrochemical probes from CNM-modified MIPs or MIP/CNMs/hybrids complexes on the substrates or those already added into electrolyte solutions can act as the signal outputs for the sensing of nonelectroactive targets. When the targets are electroactive, redox of the targets acts as the signal outputs. In this case, the targets frequently combine with other electrochemical probes from the ECCS sensing platform or electrolyte solutions to yield dual, triple, and multiple signal outputs, which facilitate the development of dual-signal ratiometric and simultaneous sensing of dual, triple, or multiple targets. | ||
| Scheme 2 Scheme illustration of the electrochemical sensing applications of CNM-modified MIP-based ECCSs using two principal detection mechanisms for nonelectroactive and electroactive targets. | ||
Qian et al. proposed a MIP sensor of DA based on molecularly imprinted oxygen-containing polypyrrole coated on CNTs.43 Polypyrrole/CNT–MIP complex was dropped on GCE to fabricate a sensing platform. DA served as the target and its redox characteristics were used as the signal outputs for DA sensing without the introduction of other electrochemical probes from the sensing platform and electrolyte solutions (Fig. 17). In this case, the sensing platform can produce enhanced electrochemical signal responses to electroactive targets. The targets enter into the MIP cavities, pass through the cavities, and reach the electrode (substrate) surface to undergo redox reactions, which finally yield redox electrochemical signal outputs. This detection mechanism is widely used for the electrochemical sensing of nonelectroactive targets in ECCSs based on CNM-modified MIPs and MIP/CNMs/hybrids complexes.
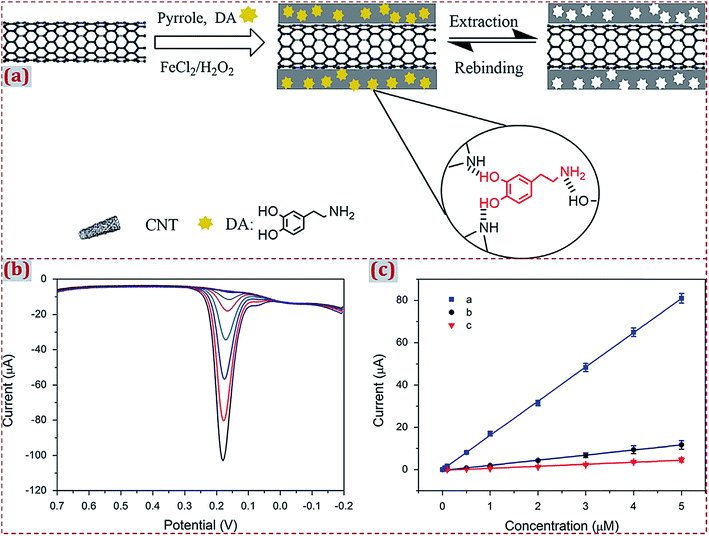 | ||
| Fig. 17 (a) Chemical routes for the preparation of polypyrrole/CNT–MIP. (b) DPV curves with an increase in DA concentrations in 0.1 M PBS (pH 6.5). DA concentration was 0.05–50 nM and 0.1–5 μM (from top to bottom), respectively. (c) Calibration curves of DA obtained with (blue) polypyrrole/CNT–MIP, (black) polypyrrole/CNT–NIP and (red) CNT-modified GCE. Reproduced with permission from ref. 43. Copyright 2014, Elsevier. | ||
With regard to the electrochemical sensing of nonelectroactive targets, redox probes are often used for signal outputs. Redox probes include the frequently used [Fe(CN)6]3−/2−,136,138,158,159 Prussian blue (PB),120,121,135,179 N2H4·H2O,173etc. Wang et al. reported a 3D MIP electrochemical sensor based on PB-mediated amplification together with signal enhancement of ordered CKM-3 for the quantification of MTMC.179 PB has high stability and excellent electrocatalytic activity. A PB film is often prepared by electrochemical methods and is widely used as an electronic mediator in ECCSs. Upon modification with a PB film, the performance of CNMs as transducers gets enhanced. As an inorganic conductive film, a PB film directly produces an electrochemical signal that improves sensitivity. In Fig. 18a, PB–CMK-3 hybrid with a 3D structure is shown, which acts as a low-potential redox mediator and electron transfer carrier. The hybrid has a desirable hosting structure, efficient facilitation effect on electron transfer of CMK-3, and effective low-potential electron transfer mediation of PB. With regard to MIP/PB–CMK-3/GCE, the current reduction of the PB signal indicates the MTMC content.
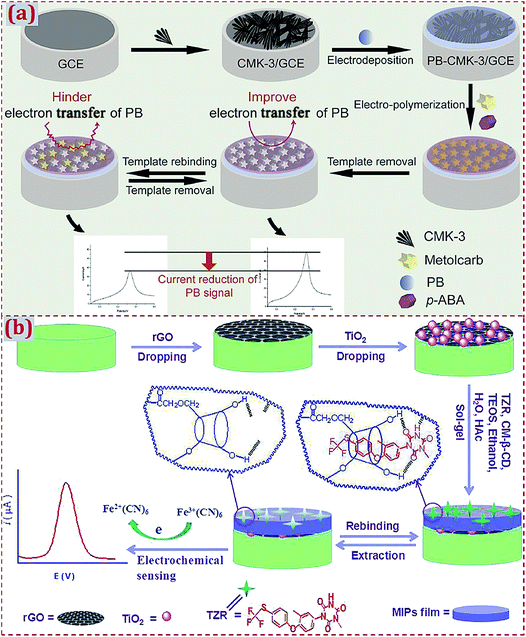 | ||
| Fig. 18 (a) Schematic representation of the fabrication process of MIP/PB–CMK-3/GCE. Reproduced with permission from ref. 179. Copyright 2015, Elsevier. (b) Schematic illustration for the fabrication of an electrochemical sensor based on MIPs/TiO2/rGO/Pt electrode. Reproduced with permission from ref. 159. Copyright 2019, Elsevier. | ||
Huang et al. developed a sensor of toltrazuril (TZR) using MIP/TiO2/rGO-complex-modified Pt electrode.159 The modified electrode was incubated in a TZR solution and measured in a K3[Fe(CN)6] solution containing KCl by DPV (Fig. 18b). The MIP-adduct film was insulating and it hindered the movement of [Fe(CN)6]3−/4− probe ions through the polymer network for redox reactions. After template removal, a TZR-imprinted polymer had an increased peak current. Upon the incubation of the modified electrode in a TZR solution, the peak current significantly reduced because the partially imprinted sites were occupied by TZR, leading to fewer channels for the diffusion of [Fe(CN)6]3−/4− on the electrode surface. The current response of [Fe(CN)6]3−/4− was proportional to the amount of unoccupied imprinted sites and was indirectly correlated to the TZR concentration. Shang et al. fabricated 3D porous GR/PdCu alloy NPs complex decorated on MIP for the electrocatalytic assay of melamine.173 Melamine was detected by DPV using hydrazine as the electrochemical probe. Hydrazine had an oxidation peak at the modified electrode. The detection signal was amplified due to the catalytic oxidation of hydrazine. After the rebinding of melamine, the peak current decreased because electroinactive melamine deterred the access of hydrazine on the surface of the modified electrode. The detection signal was generated from the peak current deduction of hydrazine.
4.3. Electrochemical sensing of different types of targets
CNM-modified-MIP-based ECCSs are popularly used for the electrochemical sensing of various targets (analytes) in the liquid (solution) phase, such as pharmaceuticals, drugs, biomarkers, proteins, nucleic acids, enzymes, food additives, industrial raw materials, environment pollutants, pesticides, explosives, etc. The electrochemical sensing applications of ions in solution and gas molecules have been undertaken. By introducing one typical work, we elaborated the applications of CNM-modified-MIP-based ECCSs for the electrochemical sensing of molecules and ions in a solution, as well as the electrochemical sensing of gases. You et al. designed an electrochemical sensor to determine the breast cancer susceptibility gene BRCA-1 amplified by SiO2@AgNPs.154 AuNPs/GO-composite-modified GCE was covered with a prepared MIP layer using rhodamine B (RhB) as the template, MAA as the monomer, and Nafion as the additive. The signal amplification trace tag SiO2@AgNPs was prepared by covering AgNPs on SiO2 NP surface in situ. DNA probes were modified on AgNPs by the Ag–S bond to form the SiO2@Ag/DNA complex. In the presence of the target DNA, homogeneous hybridization was performed with SiO2@Ag/DNA and RhB-labeled DNA (Fig. 19). SiO2@Ag/dsDNA/RhB was specifically recognized with MIP via interactions between imprinting cavities and RhB. This biosensor had a wide linear range from 10 fM to 100 nM, low detection limit of 2.53 fM, excellent selectivity, reproducibility, stability, and feasibility in serum analyses.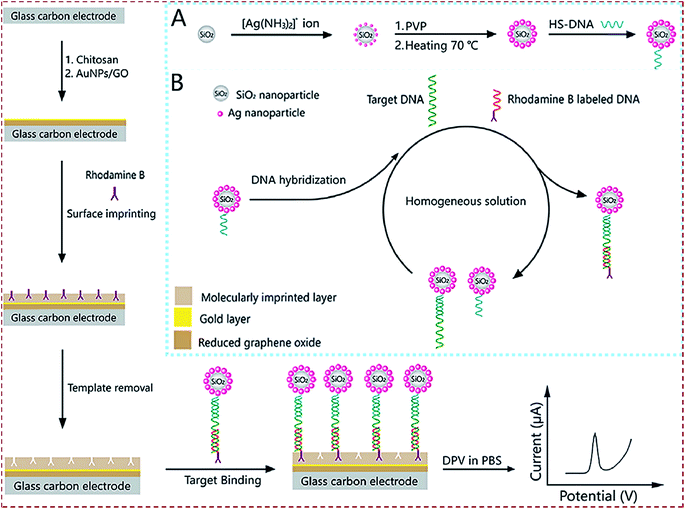 | ||
| Fig. 19 Schematic illustration of a MIP-based electrochemical DNA biosensor for the determination of breast cancer susceptibility gene (BRCA-1) amplified by SiO2@Ag. Insets of (A) and (B) show the preparation process of SiO2@Ag/DNA and homogeneous DNA hybridization. Reproduced with permission from ref. 154. Copyright 2018, Elsevier. | ||
Emam et al. reported a MIP electrochemical sensor of gases to detect butylated hydroxytoluene (BHT) in air.120 A three-layer sensor was formed by depositing a thin layer of GR on a GCE substrate. In the prepolymer solution containing BHT as the template (target) and pyrrole as the monomer, electropolymerization was initiated to form polypyrrole on GR/GCE. After template removal, a MIP layer as the conductive polymer film was generated. The sensor contained molecularly imprinted cavities for the selective recognition of target molecules (Fig. 20). Two sets of sensors were designed. First, a GR sensor was fabricated with a layer of rGO and tested over 5–100 parts per million (ppm). In the second batch, PB was added to GR before polymerization, which enhanced the electrochemical properties. The sensor was tested over 0.02–1 parts per billion (ppb) level of the target. The sensor resistance was monitored. Alizadeh et al. designed a sensor for Bi3+ detection using nanostructured Bi3+-imprinted polymer-modified carbon/CNTs paste electrode.76 Bi3+–MIP was prepared by the copolymerization of Bi3+–methylene succinate complex and EGDMA in acetonitrile through precipitation polymerization (Fig. 21). Polymeric NPs were used as Bi3+-selective modifier of carbon/CNTs paste electrode. Bi3+ ions were accumulated on the electrode surface in a Bi3+ solution. ASDPV signals of the modified electrode were recorded as the analytical signal, which were significantly higher than those of nonimprinted polymer-based electrodes. The electrode had a dynamic linear response range of 0.2–2 μM and detection limit of 8.9 μM for Bi3+. The sensitivity was high and reached 112.25 μA μM−1. This sensor was used for the electrochemical detection of Bi3+ in environmental, pharmaceutical, and biological samples.
 | ||
| Fig. 20 (a, b) Schematic diagrams of both sensors: a layer of MIP is deposited on graphene (GR) or a GR–Prussian blue (PB)-modified surface of GCE. (c) Mechanism of the sensor among different molecules in environment; only the template can be absorbed on the MIP layer. When the template molecule is trapped on the MIP layer, an extra electron is transferred to the GR layer, which induces a resistance change. Test results of GR sensors in the range of 5–100 ppm in red (d) and GR–PB sensor in the range of 0.02–1 ppb in green (e) along with the normalized resistance change. At a concentration of 0.3 ppb, the vacuum was purged to the sealed chamber; therefore, the resistance increase occurs with a lower slope. Reproduced with permission from ref. 120. Copyright 2018, Hindawi. | ||
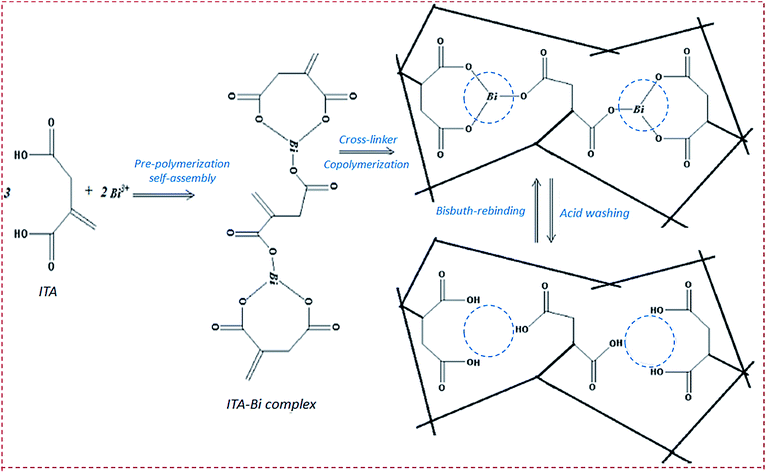 | ||
| Fig. 21 Schematic representation of Bi3+-imprinted polymer synthesis including Bi3+ interaction with itaconic acid (ITA) molecules (prepolymerization reaction) and copolymerization reaction. Reproduced with permission from ref. 76. Copyright 2017, Elsevier. | ||
4.4. Respective and simultaneous electrochemical sensing of two targets
Most of the earlier reported ECCSs based on CNM-modified MIPs can be used to determine only a single type of targets, with high detection sensitivity and selectivity. Moreover, scientists have investigated different CNM-modified-MIP-based ECCSs for the respective and simultaneous electrochemical determination of two types of targets.53,67,84,91,98,141 Jaiswal et al. presented the enantioselective analysis of D- and L-serine on a layer-by-layer imprinted electrochemical sensor.91 The authors employed AM-functionalized rGO–fullerene layer-by-layer assembled dual-imprinted polymers to quantify D- and L-serine at the ultra-trace level in real aqueous samples. The sensor exhibited better electronic properties with improved synergism and had a lower detection limit (0.24 ng mL−1) for both the isomers. Zang et al. formulated a disposable simultaneous electrochemical sensor array using a MIP film on NH2–GR-modified screen-printed electrode for the detection of psychotropic drugs.84 The MIP film was prepared by electropolymerization using methcathinone and cathinone as dual templates and pyrrole as the monomer. This multiplexed immunoassay method had wide linear ranges over 3 orders of magnitude together with detection limits down to 3.3 and 8.9 pg mL−1 for methcathinone and cathinone, respectively. This sensor array was applied to detect methcathinone and cathinone in practical serum samples.Chen et al. prepared MIP-grafted GR (MIP-G) for the simultaneous sensing of 4,4-methylene diphenyl amine (MDA) and aniline.53 MIP-G was prepared through a free-radical polymerization reaction using MDA as the template and 4-vinylpyridine as the monomer. MIP-G had high binding affinity toward MDA and underwent π–π interactions with aniline. The oxidation potentials for MDA at 0.62 V and aniline at 0.72 V were distinguishable. MIP-G-modified GCE had a linear response to MDA and aniline over the range of 1.0–15 μM. MIP-G-based sensors were applied in plastic tableware. Prasad et al. adopted Monomer dual-imprinted dendrimer nanofilm-modified electrode for the simultaneous detection of uric acid and norepinephrine.98 AuNP-functionalized MWCNTs composite was used for growing a thin nanofilm based on the surface grafting route on PGE. This sensor enabled the simultaneous analysis of one target in the presence of the other, without any cross-reactivity involving interferences and false positives. The detection limits were calculated to be 0.62 ng mL−1 for norepinephrine and 0.43 ng mL−1 for uric acid in aqueous, biological, and pharmaceutical samples.
Wang et al. reported simultaneous immunoassay using GR–AuNPs-grafted recombinant apoferritin-encoded metallic NP labels as the signal tags and dual-template magnetic MIP as the capture probes.141 This multiplexed immunoassay enabled the simultaneous detection of alpha-fetoprotein (AFP) and carcinoembryonic antigen (CEA). The labels were prepared by loading recombinant apoferritin and separately immobilized primary antibodies via AuNP growth on GR. The capture probes were prepared by the self-polymerization of DA on Fe3O4 NPs using AFP and CEA as the template proteins. The analysis of metal components in an immune complex provided a route to quantify the targets based on the peak currents of Cd and Pb. The immunoassay enabled the simultaneous detection of AFP and CEA, showing broad dynamic ranges of 0.001–5 ng mL−1 and low detection limits of 0.3 for AFP and 0.35 pg mL−1 for CEA. Zheng et al. designed a sensor based on the MIP/rGO composite for the simultaneous determination of uric acid and tyrosine.67 A MIP layer was electropolymerized on a rGO-modified electrode using dual templates of uric acid and tyrosine (Fig. 22). The sensor had broad linear ranges for uric acid (0.01–100 μM) and tyrosine (0.1–400 μM) with low detection limits of 32 and 46 nM, respectively. The sensor was used to detect uric acid and tyrosine in serum and urine samples.
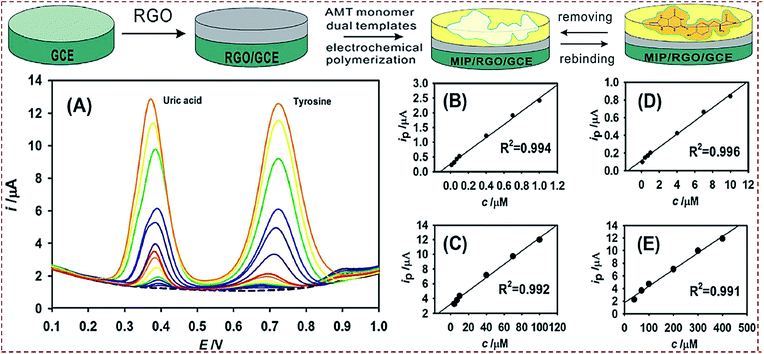 | ||
| Fig. 22 Preparation process of MIP–rGO composites. (A) DPV responses of a MIP/rGO sensor for different concentrations of uric acid and tyrosine. From bottom to top, the concentrations of uric acid and tyrosine are 0.01 to 100 μM and 0.1 to 400 μM, respectively. The dashed line at the bottom denotes the data obtained from blank PBS. Calibration curves corresponding to uric acid in the concentration ranges of 0.01–1.0 μM (B) and 4–100 μM (C). Calibration curves corresponding to tyrosine in the concentration ranges of 0.1–10 μM (D) and 40–400 μM (E). Reproduced with permission from ref. 67. Copyright 2018, Elsevier. | ||
5. Conclusions and perspectives
5.1. Summary of general categories and preparation strategies
This review systematically summarizes the recent advances in ECCSs based on CNM-modified MIPs, categorized into general categories, preparation strategies, and analytical applications. We introduce the development process and preparation principles of MIPs. Based on the structural characteristics of MIPs, we describe the potential applications of ECCSs. Owing to the integrated merits of MIPs, ECCSs, CNMs, or CNM-based hybrids, numerous studies have been devoted toward CNM-modified-MIP-based ECCSs. During the past decade, ECCSs have been widely investigated toward the electrochemical detection of various targets in real samples, exhibiting high sensitivity and selectivity toward target detection. ECCSs are classified into two general categories: (I) MIP modified with CNMs without hybridization and (II) MIP modified with CNMs and hybridized with other materials. ECCSs can be constructed on commercial work electrodes or other sensing substrates. There are three major routes to achieve CNM-modified MIPs. Route I is to prepare CNM-encapsulated imprinting of silica. Route II is to prepare CNM-encapsulated imprinting of polymers. Route III is to prepare MIP combined with CNMs.To obtain CNM-modified-MIP-based ECCSs, various preparation strategies have been widely reported. They include the (a) polymerization of MIP on CNM-modified substrates, (b) copolymerization of MIP and CNMs on substrates, (c) dropcasting of already fabricated MIPs on CNM-modified substrates, (d) dropcasting of already made MIP/CNMs complexes on substrates, (e) dropcasting of already made self-assembled complexes of MIP/CNMs/hybrids on substrates, and (f) self-assembly of MIP/CNMs/hybrids complexes on substrates. With regard to the polymerization process of MIPs, the frequently used strategies are the in situ chemical polymerization of functional monomers and electrochemical polymerization of electroactive functional monomers. Because some templates are expensive, toxic, and harmful to organisms and the environment, dummy molecules often act as alternative templates to prepare MIPs. There are various engineering structures of CNM-modified MIPs to fabricate ECCSs, majorly involving (i) core–shell-structured composites, (ii) composite NPs or particles, (iii) layer-by-layer self-assembled complexes, (iv) bulk macromaterials, (v) surface-imprinted materials, and (vi) 3D structural complexes.
5.2. Summary of potential applications
With regard to CNM-modified-MIP-based ECCSs, different modes of electrochemical signal from transducer outputs can be employed for the sensing of various targets, focusing on metal ions, small molecules, and macromolecules. Small target molecules include drug molecules used for treating human diseases, biological small molecules from human bodies, molecules that are toxic and harmful toward human health, pesticides for agricultural products, etc. Different macromolecules are proteins, nucleic acids, enzymes, disease biomarkers, etc. Other targets focus on industrial raw materials, environmental pollutants, food additives, explosives, etc. Various practical samples are involved and include questionable samples containing targets and healthy and safe samples without targets. There are two major detection mechanisms for nonelectroactive and electroactive targets. For nonelectroactive targets, redox probes serve as the signal outputs for electrochemical sensing. When the targets are electroactive, redox of the targets can act as signal outputs. Most of the CNM-modified-MIP-based ECCSs enable the determination of only a single type of target. Scientists have explored this type of ECCS for respective and simultaneous electrochemical determination of two types of targets.5.3. Improvements in preparation strategies
Numerous studies have referred to CNM-modified-MIP-based ECCSs during the past decade, but certain potential challenges inevitably remain. In subsequent studies, scientists should pay close attention to the rational design and optimal preparation of CNM-modified MIPs with functional structures, simplified preparation steps, and high electrical activity, aiming to develop excellent ECCSs for the highly sensitive and selective sensing of various targets. Novel engineering structures of CNM-modified MIPs will be further explored toward multiple functions and applications of ECCSs. Versatile core–shell-engineered MIP1/MIP2 hybrids with multi-imprinting cavities from different templates can yield versatile detection applications, such as high-performance detection of dual, triple, or multiple targets. This is imperative for the accurate diagnosis of complex diseases and even cancers. Electropolymerization is considerably suitable for the controllable preparation of MIPs on substrates modified with CNMs or CNM-based hybrids, facilitating the fabrication of various engineering structures of CNM-modified MIPs. The integration of different preparation strategies can be used to design new and multifunctional structures of ECCSs. Different from the in situ copolymerization of MIP and CNMs on substrates, magnetic MIP (CNM-modified MIP) already prepared in the solution phase can be absorbed on the magnetic electrode surface. The use of a magnetic electrode avoids the requirements of complicated modification procedures and time-consuming and tedious polymerization operations necessitated in order to prepare MIPs on electrode surfaces. The combination of magnetic MIP complexes with a magnetic electrode is efficient and ensures high stability of MIP-complex-based ECCSs. Most of the CNM-modified-MIP-based ECCSs involve hybridization with other electroactive materials, which synergistically improves the sensitivity of the responses of the electrochemical signal toward the targets. Several types of materials have been prepared and used for hybridization with CNMs, followed by preparing MIPs on CNMs/other materials dual-modified electrodes or substrates. In the subsequent studies, certain aspects of electroactive materials should be addressed, particularly with respect to low toxicity, cost-effectiveness, facile preparation, colloidal stability, and electrical activity.1985.4. Further exploration of potential applications
With regard to the detection applications of CNM-modified-MIP-based ECCSs, targets frequently combine with other electrochemical probes from the ECCS platforms or electrolyte solutions to yield dual, triple, or multiple signal outputs, which can facilitate the development of dual-signal ratiometric and simultaneous sensing of dual, triple, or multiple targets. Earlier reports have prominently referred to single-signal outputs for the electrochemical sensing of a single target. A single-signal output corresponds to only one type of target. With regard to the detection applications of ECCSs, the dual-signal-ratiometric output is superior to a single-signal output.199–202 The ratiometry of dual signals is independent of the contents of the sensors and reagents, providing an intrinsic built-in correction to conquer the potentially adverse effects of the sensing systems and background signals.203–206 Generally, to acquire the accurate intensity of a single signal is difficult in the sensing process of targets. The ratiometry of dual signals can efficiently improve the accuracy and sensitivity of ECCSs for target detection in real samples. Scientists need to rationally design new CNM-modified MIPs and develop versatile ECCSs that have layer-by-layer assembled or core–shell-engineered structures. Different types of electroactive probe molecules can be loaded into different layers and core–shell structures to yield ratiometric ECCSs. Except for commercial work electrodes, other solid sensing substrates have been widely used to develop CNM-modified MIPs. ECCSs constructed on solid substrates show promising potential for various applications, particularly electrochemical sensing elements, as well as flexible and wearable intelligent devices.207 Except for electrochemical signal from transducer outputs reported in earlier studies, other advanced electrochemical signals from other transducer output modes, such as electrochemiluminescence and photoelectrochemical signals,208–212 can be introduced in CNM-modified-MIP-based ECCSs. The introduction of advanced electrochemical signal transducer outputs can endow ECCSs with newer opportunities for applications in the visual detection of targets in real samples and electrochemical imaging detection in vivo/in vivo. These studies demonstrate the promising prospects of CNM-modified-MIP-based ECCSs in current research fields, such as targeting chemo/bioanalysis as well as the accurate diagnosis and therapy of serious diseases and cancers in clinics.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the Natural Science Foundation of Shandong Province (ZR2019MB026), and the Source Innovation Plan Application Basic Research Project of Qingdao (18-2-2-26 jch, 17-1-1-72-jch).Notes and references
- M. V. Polyakov, Zh. Fiz. Khim., 1931, 2, 799–805 Search PubMed.
- L. Pauling, J. Am. Chem. Soc., 1940, 62, 2643–2657 CrossRef CAS.
- G. Wulff and A. Sarhan, Angew. Chem., Int. Ed., 1972, 11, 341–344 CAS.
- G. Vlatakis, L. I. Andersson, R. Müller and K. Mosbach, Nature, 1993, 361, 645–647 CrossRef CAS PubMed.
- X. Hu, Y. Hu and G. Li, J. Chromatogr. A, 2007, 1147, 1–9 CrossRef CAS PubMed.
- A. Martín-Esteban, TrAC, Trends Anal. Chem., 2013, 45, 169–181 CrossRef.
- R. Arshady and K. Mosbach, Makromol. Chem., 1981, 182, 687–692 CrossRef CAS.
- F. G. Tamayo, E. Turiel and A. Martín-Esteban, J. Chromatogr. A, 2007, 1152, 32–40 CrossRef CAS PubMed.
- N. M. Bergmann and N. A. Peppas, Prog. Polym. Sci., 2008, 33, 271–288 CrossRef CAS.
- C. Malitesta, E. Mazzotta and R. A. Picca, Anal. Bioanal. Chem., 2012, 402, 1827–1846 CrossRef CAS PubMed.
- O. S. Ahmad, T. S. Bedwell, C. Esen, A. Garcia-Cruz and S. A. Piletsky, Trends Biotechnol., 2019, 37, 294–309 CrossRef CAS PubMed.
- J. J. BelBruno, Chem. Rev., 2019, 119, 94–119 CrossRef CAS PubMed.
- Y. Fuchs, O. Soppera and K. Haupt, Anal. Chim. Acta, 2012, 717, 7–20 CrossRef CAS PubMed.
- J. Zhan, G. Z. Fang, Z. Yan, M. F. Pan, C. C. Liu and S. Wang, Anal. Bioanal. Chem., 2013, 405, 6353–6363 CrossRef CAS PubMed.
- D. L. Huang, R. Z. Wang, Y. G. Liu, G. M. Zeng, C. Lai, P. Xu, B. A. Lu, J. J. Xu, C. Wang and C. Huang, Environ. Sci. Pollut. Res., 2015, 22, 963–977 CrossRef CAS PubMed.
- S. Wu, L. Tan, G. Wang, G. Peng, C. Kang and Y. Tang, J. Chromatogr. A, 2013, 1285, 124–131 CrossRef CAS PubMed.
- A. A. Volkert and A. J. Haes, Analyst, 2014, 139, 21–31 RSC.
- Y. Ma, S. Xu, S. Wang and L. Wang, TrAC, Trends Anal. Chem., 2015, 67, 209–216 CrossRef CAS.
- N. Pérez-Moral and A. G. Mayes, Anal. Chim. Acta, 2004, 504, 15–21 CrossRef.
- C. Zheng, Y. P. Huang and Z. S. Liu, Anal. Bioanal. Chem., 2013, 405, 2147–2161 CrossRef CAS PubMed.
- A. G. Mayes and K. Mosbach, Anal. Chem., 1996, 68, 3769–3774 CrossRef CAS PubMed.
- C. He, Y. Long, J. K. Pan and F. Liu, J. Biochem. Biophys. Methods, 2007, 70, 133–150 CrossRef CAS PubMed.
- S. Yan, Z. Gao, Y. Fang, Y. Cheng, H. Zhou and H. Wang, Dyes Pigm., 2007, 74, 572–577 CrossRef CAS.
- E. Turiel and A. Martin-Esteban, Anal. Bioanal. Chem., 2004, 378, 1876–1886 CrossRef CAS PubMed.
- D. Gao, Z. Zhang, M. Wu, C. Xie, G. Guan and D. Wang, J. Am. Chem. Soc., 2007, 129, 7859–7866 CrossRef CAS PubMed.
- B. Gao, J. Wang, F. An and Q. Liu, Polymer, 2008, 49, 1230–1238 CrossRef CAS.
- Z. X. Xu, G. Z. Fang and S. Wang, Food Chem., 2010, 119, 845–850 CrossRef CAS.
- S. Ansari, TrAC, Trends Anal. Chem., 2017, 93, 134–151 CrossRef CAS.
- R. Gui, H. Jin, H. Guo and Z. Wang, Biosens. Bioelectron., 2018, 100, 56–70 CrossRef CAS PubMed.
- B. Yang, C. Fu, J. Li and G. Xu, TrAC, Trends Anal. Chem., 2018, 105, 52–67 CrossRef CAS.
- L. Uzun and A. P. F. Turner, Biosens. Bioelectron., 2016, 76, 131–144 CrossRef CAS PubMed.
- J. Wackerlig and P. A. Lieberzeit, Sens. Actuators, B, 2015, 207, 144–157 CrossRef CAS.
- P. S. Sharma, M. Dabrowski, F. D'Souza and W. Kutner, TrAC, Trends Anal. Chem., 2013, 51, 146–157 CrossRef CAS.
- S. Ansari, TrAC, Trends Anal. Chem., 2017, 93, 134–151 CrossRef CAS.
- L. Chen, S. Xu and J. Li, Chem. Soc. Rev., 2011, 40, 2922–2942 RSC.
- L. Chen, X. Wang, W. Lu, X. Wu and J. Li, Chem. Soc. Rev., 2016, 45, 2137–2211 RSC.
- S. Beyazit, B. T. S. Bui, K. Haupt and C. Gonzato, Prog. Polym. Sci., 2016, 62, 1–21 CrossRef CAS.
- M. J. Whitcombe, I. Chianella, L. Larcombe, S. A. Piletsky, J. Noble, R. Porter and A. Horgan, Chem. Soc. Rev., 2011, 40, 1547–1571 RSC.
- T. P. Huynh and W. Kutner, Biosens. Bioelectron., 2015, 74, 856–864 CrossRef CAS PubMed.
- J. Ashley, M. A. Shahbazi, K. Kant, V. A. Chidambara, A. Wolff, D. D. Bang and Y. Sun, Biosens. Bioelectron., 2017, 91, 606–615 CrossRef CAS PubMed.
- M. Dabrowski, P. Lach, M. Cieplak and W. Kutner, Biosens. Bioelectron., 2018, 102, 17–26 CrossRef CAS PubMed.
- J. Pan, W. Chen, Y. Ma and G. Pan, Chem. Soc. Rev., 2018, 47, 5574–5587 RSC.
- T. Qian, C. Yu, X. Zhou, P. Ma, S. Wu, L. Xu and J. Shen, Biosens. Bioelectron., 2014, 58, 237–241 CrossRef CAS PubMed.
- Z. Z. Yin, S. W. Cheng, L. B. Xu, H. Y. Liu, K. Huang, L. Li, Y. Y. Zhai, Y. B. Zeng, H. Q. Liu, Y. Shao, Z. L. Zhang and Y. X. Lu, Biosens. Bioelectron., 2018, 100, 565–570 CrossRef CAS PubMed.
- W. Wen, D. M. Zhao, X. H. Zhang, H. Y. Xiong, S. F. Wang, W. Chen and Y. D. Zhao, Sens. Actuators, B, 2012, 174, 202–209 CrossRef CAS.
- X. Kan, H. Zhou, C. Li, A. Zhu, Z. Xing and Z. Zhao, Electrochim. Acta, 2012, 63, 69–75 CrossRef CAS.
- Y. Wang, M. Han, G. Liu, X. Hou, Y. Huang, K. Wu and C. Li, Biosens. Bioelectron., 2015, 74, 792–798 CrossRef CAS PubMed.
- L. Lin, H. T. Lian, X. Y. Sun, Y. M. Yu and B. Liu, Anal. Methods, 2015, 7, 1387–1394 RSC.
- B. Liu, H. T. Lian, J. F. Yin and X. Y. Sun, Electrochim. Acta, 2012, 75, 108–114 CrossRef CAS.
- L. Shi, A. G. Hou, L. Y. Chen and Z. F. Wang, Polym. Compos., 2015, 36, 1280–1285 CrossRef CAS.
- H. da Silva, J. G. Pacheco, J. M. Magalhães, S. Viswanathan and C. Delerue-Matos, Biosens. Bioelectron., 2014, 52, 56–61 CrossRef CAS PubMed.
- H. Bai, C. Wang, J. Chen, J. Peng and Q. Cao, Biosens. Bioelectron., 2015, 64, 352–358 CrossRef CAS PubMed.
- N. Chen, L. Chen, Y. Cheng, K. Zhao, X. Wu and Y. Xian, Talanta, 2015, 132, 155–161 CrossRef CAS PubMed.
- J. Luo, J. Cong, J. Liu, Y. Gao and X. Liu, Anal. Chim. Acta, 2015, 864, 74–84 CrossRef CAS PubMed.
- X. Tan, J. Wu, Q. Hu, X. Li, P. Li, H. Yu, X. Li and F. Lei, Anal. Methods, 2015, 7, 4786–4792 RSC.
- T. Xie, M. Zhang, P. Chen, H. Zhao, X. Yang, L. Yao, H. Zhang, A. Dong, J. Wang and Z. Wang, RSC Adv., 2017, 7, 38884–38894 RSC.
- M. Zhang, H. T. Zhao, T. J. Xie, X. Yang, A. J. Dong, H. Zhang, J. Wang and Z. Y. Wang, Sens. Actuators, B, 2017, 252, 991–1002 CrossRef CAS.
- J. Luo, J. Cong, R. Fang, X. Fei and X. Liu, Microchim. Acta, 2014, 181, 1257–1266 CrossRef CAS.
- S. Sun, M. Zhang, Y. Li and X. He, Sensors, 2013, 13, 5493–5506 CrossRef CAS PubMed.
- J. Luo, S. Jiang and X. Liu, Sens. Actuators, B, 2014, 203, 782–789 CrossRef CAS.
- Y. Liu, L. Zhu, Y. Hu, X. Peng and J. Du, Food Chem., 2017, 221, 1128–1134 CrossRef CAS PubMed.
- Y. Liang, L. Yu, R. Yang, X. Li, L. Qu and J. Li, Sens. Actuators, B, 2017, 240, 1330–1335 CrossRef CAS.
- S. Kim, N. Wang, Y. Li and X. He, Anal. Methods, 2016, 8, 7780–7788 RSC.
- M. Shamsipur, N. Moradi and A. Pashabadi, J. Solid State Electrochem., 2018, 22, 169–180 CrossRef CAS.
- L. Kong, X. Jiang, Y. Zeng, T. Zhou and G. Shi, Sens. Actuators, B, 2013, 185, 424–431 CrossRef CAS.
- Y. Zeng, Y. Zhou, T. Zhou and G. Shi, Electrochim. Acta, 2014, 130, 504–511 CrossRef CAS.
- W. Zheng, M. Zhao, W. Liu, S. Yu, L. Niu, G. Li, H. Li and W. Liu, J. Electroanal. Chem., 2018, 813, 75–82 CrossRef CAS.
- V. M. A. Mohanan, A. K. Kunnummal and V. M. N. Biju, J. Mater. Sci., 2018, 53, 10627–10639 CrossRef CAS.
- J. Li, Z. Xu, M. Liu, P. Deng, S. Tang, J. Jiang, H. Feng, D. Qian and L. He, Biosens. Bioelectron., 2017, 90, 210–216 CrossRef CAS PubMed.
- F. Tan, L. Cong, X. Li, Q. Zhao, H. Zhao, X. Quan and J. Chen, Sens. Actuators, B, 2016, 233, 599–606 CrossRef CAS.
- Y. Pan, F. Zhao and B. Zeng, RSC Adv., 2015, 5, 57671–57677 RSC.
- G. Xu, Y. Chi, L. Li, S. Liu and X. Kan, Food Chem., 2015, 177, 37–42 CrossRef CAS PubMed.
- H. Guo, R. Gui, H. Jin and Z. Wang, New J. Chem., 2017, 41, 9977–9983 RSC.
- A. A. Ensafi, P. Nasr-Esfahani and B. Rezaei, Sens. Actuators, B, 2018, 270, 192–199 CrossRef CAS.
- G. Yang and F. Zhao, Biosens. Bioelectron., 2015, 64, 416–422 CrossRef CAS PubMed.
- T. Alizadeh, N. Hamidi, M. R. Ganjali and P. Nourozi, Sens. Actuators, B, 2017, 245, 605–614 CrossRef CAS.
- T. S. Anirudhan and S. Alexander, Biosens. Bioelectron., 2015, 64, 586–593 CrossRef CAS PubMed.
- B. B. Prasad, I. Pandey, A. Srivastava, D. Kumar and M. P. Tiwari, Sens. Actuators, B, 2013, 176, 863–874 CrossRef CAS.
- B. B. Prasad, A. Prasad and M. P. Tiwari, Biosens. Bioelectron., 2013, 39, 236–243 CrossRef CAS PubMed.
- M. Sebastian and B. Mathew, J. Mater. Sci., 2018, 53, 3557–3572 CrossRef CAS.
- P. Deng, Z. Xu and Y. Kuang, Food Chem., 2014, 157, 490–497 CrossRef CAS PubMed.
- A. Motaharian and M. R. M. Hosseini, Anal. Methods, 2016, 8, 6305–6312 RSC.
- H. J. Chen, Z. H. Zhang, R. Cai, X. Chen, Y. N. Liu, W. Rao and S. Z. Yao, Talanta, 2013, 115, 222–227 CrossRef CAS PubMed.
- D. Zang, M. Yan, S. Ge, L. Ge and J. Yu, Analyst, 2013, 138, 2704–2711 RSC.
- B. Huang, L. Xiao, H. Dong, X. Zhang, W. Gan, S. Mahboob, K. A. Al-Ghanim, Q. Yuan and Y. Li, Talanta, 2017, 164, 601–607 CrossRef CAS PubMed.
- M. Mostafavi, M. R. Yaftian, F. Piri and H. Shayani-Jam, Biosens. Bioelectron., 2018, 122, 160–167 CrossRef CAS PubMed.
- B. V. M. Silva, B. A. G. Rodríguez, G. F. Sales, M. D. P. T. Sotomayor and R. F. Dutra, Biosens. Bioelectron., 2016, 77, 978–985 CrossRef CAS PubMed.
- B. B. Prasad, A. Kumar and R. Singh, Biosens. Bioelectron., 2017, 94, 1–9 CrossRef PubMed.
- H. J. Chen, Z. H. Zhang, D. Xie, R. Cai, X. Chen, Y. N. Liu and S. Z. Yao, Electroanalysis, 2012, 24, 2109–2116 CrossRef CAS.
- Z. Zhang, R. Cai, F. Long and J. Wang, Talanta, 2015, 124, 435–442 CrossRef PubMed.
- S. Jaiswal, R. Singh, K. Singh, S. Fatma and B. B. Prasad, Biosens. Bioelectron., 2019, 124–125, 176–183 CrossRef CAS PubMed.
- Y. Li, J. Liu, M. Liu, F. Yu, L. Zhang, H. Tang, B. C. Ye and L. Lai, Electrochem. Commun., 2016, 64, 42–45 CrossRef CAS.
- B. Wu, L. Hou, T. Zhang, Y. Han and C. Kong, Anal. Methods, 2015, 7, 9121–9129 RSC.
- J. Ji, Z. Zhou, X. Zhao, J. Sun and X. Sun, Biosens. Bioelectron., 2015, 66, 590–595 CrossRef CAS PubMed.
- M. B. Gholivand and M. Torkashvand, Mater. Sci. Eng., C, 2016, 59, 594–603 CrossRef CAS PubMed.
- H. Wang, S. Yao, Y. Liu, S. Wei, J. Su and G. Hu, Biosens. Bioelectron., 2017, 87, 417–421 CrossRef CAS PubMed.
- S. Wei, Y. Liu, T. Hua, L. Liu and H. Wang, J. Appl. Polym. Sci., 2014, 131, 40613 CrossRef.
- B. B. Prasad and S. Fatma, Electrochim. Acta, 2017, 232, 474–483 CrossRef CAS.
- B. B. Prasad, A. Srivastava and M. P. Tiwari, Mater. Sci. Eng., C, 2013, 33, 4071–4080 CrossRef CAS PubMed.
- S. Patra, E. Roy, R. Madhuri and P. K. Sharma, Biosens. Bioelectron., 2015, 66, 1–10 CrossRef CAS PubMed.
- L. Zhao, B. Zeng and F. Zhao, Electrochim. Acta, 2014, 146, 611–617 CrossRef CAS.
- M. L. Yola, T. Eren and N. Atar, Biosens. Bioelectron., 2014, 60, 277–285 CrossRef CAS PubMed.
- F. Long, Z. Zhang, Z. Yang, J. Zeng and Y. Jiang, J. Electroanal. Chem., 2015, 755, 7–14 CrossRef CAS.
- L. J. Kong, M. F. Pan, G. Z. Fang, X. L. He, Y. Q. Xia and S. Wang, RSC Adv., 2015, 5, 11498–11505 RSC.
- T. S. Anirudhan, V. S. Athira and V. Chithra Skhar, Polymer, 2018, 146, 312–320 CrossRef CAS.
- M. Salajegheh, M. Ansari, M. M. Foroghi and M. Kazemipour, J. Pharm. Biomed. Anal., 2019, 162, 215–224 CrossRef CAS PubMed.
- J. Zhang, X. T. Guo, J. P. Zhou, G. Z. Liu and S. Y. Zhang, Mater. Sci. Eng., C, 2018, 91, 696–704 CrossRef CAS PubMed.
- X. Shen, Y. Ma, Q. Zeng, J. Huang, J. Tao and L. Wang, ChemistrySelect, 2017, 2, 6549–6555 CrossRef CAS.
- Y. Ma, X. L. Shen, Q. Zeng, H. S. Wang and L. S. Wang, Talanta, 2017, 164, 121–127 CrossRef CAS PubMed.
- W. Wu, L. Yang, F. Zhao and B. Zeng, Sens. Actuators, B, 2017, 239, 481–487 CrossRef CAS.
- S. Güney and O. Güney, Electroanalysis, 2017, 29, 2579–2590 CrossRef.
- B. Deiminiat and G. H. Rounaghi, Sens. Actuators, B, 2018, 259, 133–141 CrossRef CAS.
- Y. Yang, G. Fang, G. Liu, M. Pan, X. Wang, L. Kong, X. He and S. Wang, Biosens. Bioelectron., 2013, 47, 475–481 CrossRef CAS PubMed.
- R. Wang, K. Yan, F. Wang and J. Zhang, Electrochim. Acta, 2014, 121, 102–108 CrossRef CAS.
- Z. Wang, F. Li, J. Xia, L. Xia, F. Zhang, S. Bi, G. Shi, Y. Xia, J. Liu, Y. Li and L. Xia, Biosens. Bioelectron., 2014, 61, 391–396 CrossRef CAS PubMed.
- B. Wang, O. K. Okoth, K. Yan and J. Zhang, Sens. Actuators, B, 2016, 236, 294–303 CrossRef CAS.
- Y. Liu, K. Yan, B. Wang, C. Yang and J. Zhang, J. Electrochem. Soc., 2017, 164, B776–B780 CrossRef CAS.
- F. Wang, L. Zhu and J. Zhang, Sens. Actuators, B, 2014, 192, 642–647 CrossRef CAS.
- H. Bai, C. Wang, J. Chen, Z. Li, K. Fu and Q. Cao, J. Electroanal. Chem., 2018, 816, 7–13 CrossRef CAS.
- S. Emam, A. Adedoyin, X. Geng, M. Zaeimbashi, J. Adams, A. Ekenseair, E. Podlaha-Murphy and N. X. Sun, J. Sensors, 2018, 2018, 1–9 CrossRef.
- M. Cui, S. Liu, W. Lian, J. Li, W. Xu and J. Huang, Analyst, 2013, 138, 5949–5955 RSC.
- D. Wu, H. Li, X. Xue, H. Fan, Q. Xin and Q. Wei, Anal. Methods, 2013, 5, 1469–1473 RSC.
- H. J. Chen, Z. H. Zhang, R. Cai, X. Q. Kong, X. Chen, Y. N. Liu and S. Z. Yao, Analyst, 2013, 138, 2769–2776 RSC.
- Y. Mao, Y. Bao, S. Gan, F. Li and L. Niu, Biosens. Bioelectron., 2011, 28, 291–297 CrossRef CAS PubMed.
- H. Tang, J. Chen, Y. Zeng, Z. Li, H. Huang and L. Li, Anal. Methods, 2016, 8, 1681–1689 RSC.
- B. Lu, J. Xia, Z. Wang, F. Zhang, M. Yang, Y. Li and Y. Xia, RSC Adv., 2015, 5, 82930–82935 RSC.
- P. T. Do, P. Q. Do, H. B. Nguyen, C. N. Van, D. L. Tran, T. H. Le, H. N. Le, H. V. Pham, T. L. Nguyen and Q. H. Tran, J. Mol. Liq., 2014, 198, 307–312 CrossRef CAS.
- F. Long, Z. Zhang, J. Wang, L. Yan and B. Zhou, Electrochim. Acta, 2015, 168, 337–345 CrossRef CAS.
- B. He, Y. L. Mao, Y. Zhang, W. Yin, C. J. Hou, D. Q. Huo and H. B. Fa, NANO, 2017, 12, 1750046 CrossRef CAS.
- J. Xia, X. Cao, Z. Wang, M. Yang, F. Zhang, B. Lu, F. Li, L. Xia, Y. Li and Y. Xia, Sens. Actuators, B, 2016, 225, 305–311 CrossRef CAS.
- X. Zhu, Y. Zeng, Z. Zhang, Y. Yang, Y. Zhai, H. Wang, L. Liu, J. Hu and L. Li, Biosens. Bioelectron., 2018, 108, 38–45 CrossRef CAS PubMed.
- P. Zhao and J. Hao, Biosens. Bioelectron., 2015, 64, 277–284 CrossRef CAS PubMed.
- T. Li, T. Yao, C. Zhang, G. Liu, Y. She, M. Jin, F. Jin, S. Wang, H. Shao and J. Wang, RSC Adv., 2016, 6, 66949–66956 RSC.
- L. Zheng, C. Zhang, J. Ma, S. Hong, Y. She, A. M. Abd El-Aty, Y. He, H. Yu, H. Liu and J. Wang, Anal. Biochem., 2018, 559, 44–50 CrossRef CAS PubMed.
- P. Qi, J. Wang, Z. Wang, X. Wang, X. Wang, X. Xu, H. Xu, S. Di, H. Zhang, Q. Wang and X. Wang, Electrochim. Acta, 2018, 274, 406–414 CrossRef CAS.
- J. Huang, Y. Wu, J. Cong, J. Luo and X. Liu, Sens. Actuators, B, 2018, 259, 1–9 CrossRef CAS.
- D. Duan, H. Yang, Y. Ding, D. Ye, L. Li and G. Ma, Electrochim. Acta, 2018, 261, 160–166 CrossRef CAS.
- H. Yang, L. Li, Y. Ding, D. Ye, Y. Wang, S. Cui and L. Liao, Biosens. Bioelectron., 2017, 92, 748–754 CrossRef CAS PubMed.
- L. Zhao, F. Zhao and B. Zeng, Int. J. Electrochem. Sci., 2014, 9, 1366–1377 Search PubMed.
- W. Lian, S. Liu, J. Yu, X. Xing, J. Li, M. Cui and J. Huang, Biosens. Bioelectron., 2012, 38, 163–169 CrossRef CAS PubMed.
- D. Wang, N. Gan, H. Zhang, T. Li, L. Qiao, Y. Cao, X. Su and S. Jiang, Biosens. Bioelectron., 2015, 65, 78–82 CrossRef CAS PubMed.
- Y. Zeng, Y. Zhou, L. Kong, T. Zhou and G. Shi, Biosens. Bioelectron., 2013, 45, 25–33 CrossRef CAS PubMed.
- L. Zhao, F. Zhao and B. Zeng, Sens. Actuators, B, 2013, 176, 818–824 CrossRef CAS.
- H. Chen, Z. Zhang, R. Cai, W. Rao and F. Long, Electrochim. Acta, 2014, 117, 385–392 CrossRef CAS.
- P. Tang, H. Zhang, J. Huo and X. Lin, Anal. Methods, 2015, 7, 7784–7791 RSC.
- Y. Liu, L. Zhu, Y. Zhang and H. Tang, Sens. Actuators, B, 2012, 171–172, 1151–1158 CrossRef CAS.
- S. Dadkhah, E. Ziaei, A. Mehdinia, T. B. Kayyal and A. Jabbari, Microchim. Acta, 2016, 183, 1933–1941 CrossRef CAS.
- M. L. Yola, T. Eren and N. Atar, Sens. Actuators, B, 2015, 210, 149–157 CrossRef CAS.
- X. Li, X. Wang, L. Li, H. Duan and C. Luo, Talanta, 2015, 131, 354–360 CrossRef CAS PubMed.
- Y. Yang, G. Fang, X. Wang, M. Pan, H. Qian, H. Liu and S. Wang, Anal. Chim. Acta, 2014, 806, 136–143 CrossRef CAS PubMed.
- M. M. Farid, L. Goudini, F. Piri, A. Zamani and F. Saadati, Food Chem., 2016, 194, 61–67 CrossRef CAS PubMed.
- X. Wang, X. Li, C. Luo, M. Sun, L. Li and H. Duan, Electrochim. Acta, 2014, 130, 519–525 CrossRef CAS.
- J. Li, X. Wang, H. Duan, Y. Wang, Y. Bu and C. Luo, Talanta, 2016, 147, 169–176 CrossRef CAS PubMed.
- M. You, S. Yang, W. Tang, F. Zhang and P. He, Biosens. Bioelectron., 2018, 112, 72–78 CrossRef CAS PubMed.
- Y. Li, X. Zhao, P. Li, Y. Huang, J. Wang and J. Zhang, Anal. Chim. Acta, 2015, 884, 106–113 CrossRef CAS PubMed.
- M. A. Beluomini, J. L. da Silva, G. C. Sedenho and N. R. Stradiotto, Talanta, 2017, 165, 231–239 CrossRef CAS PubMed.
- X. Tan, Q. Hu, J. Wu, X. Li, P. Li, H. Yu, X. Li and F. Lei, Sens. Actuators, B, 2015, 220, 216–221 CrossRef CAS.
- Y. Liang, C. Qu, R. Yang, L. Qu and J. Li, Sens. Actuators, B, 2017, 251, 542–550 CrossRef CAS.
- X. Huang, S. Wei, S. Yao, H. Zhang, C. He and J. Cao, J. Pharm. Biomed. Anal., 2019, 164, 607–614 CrossRef CAS PubMed.
- H. Jin, H. Guo, X. Gao and R. Gui, Sens. Actuators, B, 2018, 277, 14–21 CrossRef CAS.
- L. Zhao, F. Zhao and B. Zeng, Electrochim. Acta, 2014, 115, 247–254 CrossRef CAS.
- Q. Han, X. Wang, Z. Yang, W. Zhu, X. Zhou and H. Jiang, Talanta, 2014, 123, 101–108 CrossRef CAS PubMed.
- W. Zhu, G. Jiang, L. Xu, B. Li, Q. Cai, H. Jiang and X. Zhou, Anal. Chim. Acta, 2015, 886, 37–47 CrossRef CAS PubMed.
- Y. Li, W. Xu, X. Zhao, Y. Huang, J. Kang, Q. Qi and C. Zhong, Analyst, 2018, 143, 5094–5102 RSC.
- Z. Yao, X. Yang, X. Liu, Y. Yang, Y. Hu and Z. Zhao, Microchim. Acta, 2018, 185, 70 CrossRef PubMed.
- R. Qin, Q. Wang, C. Ren, X. Dai and H. Han, Int. J. Electrochem. Sci., 2017, 12, 8953–8962 CrossRef CAS.
- N. Atar, M. L. Yola and T. Eren, Appl. Surf. Sci., 2016, 362, 315–322 CrossRef CAS.
- C. Xue, X. Wang, W. Zhu, Q. Han, C. Zhu, J. Hong, X. Zhou and H. Jiang, Sens. Actuators, B, 2014, 196, 57–63 CrossRef CAS.
- T. Wen, C. Xue, Y. Li, Y. Wang, R. Wang, J. Hong, X. Zhou and H. Jiang, J. Electronanal. Chem., 2012, 682, 121–127 CrossRef CAS.
- G. Yang, F. Zhao and B. Zeng, Biosens. Bioelectron., 2014, 53, 447–452 CrossRef CAS PubMed.
- A. R. Cardoso, A. C. Marques, L. Santos, A. F. Carvalho, F. M. Costa, R. Martins, M. G. F. Sales and E. Fortunato, Biosens. Bioelectron., 2019, 124–125, 167–175 CrossRef CAS PubMed.
- X. Wei, Z. Zhang, L. Zhang and X. Xu, J. Mater. Sci., 2019, 54, 2066–2078 CrossRef CAS.
- L. Shang, F. Zhao and B. Zeng, ACS Appl. Mater. Interfaces, 2014, 6, 18721–18727 CrossRef CAS PubMed.
- Y. Pan, L. Shang, F. Zhao and B. Zeng, Electrochim. Acta, 2015, 151, 423–428 CrossRef CAS.
- M. L. Yola and N. Atar, Ind. Eng. Chem. Res., 2017, 56, 7631–7639 CrossRef CAS.
- S. Feng, X. Wei, L. Zhong and J. Li, Electroanalysis, 2018, 30, 320–327 CrossRef CAS.
- M. Ma, P. Zhu, F. Pi, J. Ji and X. Sun, J. Electroanal. Chem., 2016, 775, 171–178 CrossRef CAS.
- H. Rao, X. Liu, F. Ding, Y. Wan, X. Zhao, R. Liang, P. Zou, Y. Wang, X. Wang and Q. Zhao, Chem. Eng. J., 2018, 338, 478–487 CrossRef CAS.
- Y. Yang, Y. Cao, X. Wang, G. Z. Fang and S. Wang, Biosens. Bioelectron., 2015, 64, 247–254 CrossRef CAS PubMed.
- W. Guo, F. Pi, H. Zhang, J. Sun, Y. Zhang and X. Sun, Biosens. Bioelectron., 2017, 98, 299–304 CrossRef CAS PubMed.
- H. Rao, X. Zhao, X. Liu, J. Zhong, Z. Zhang, P. Zou, Y. Jiang, X. Wang and Y. Wang, Biosens. Bioelectron., 2018, 100, 341–347 CrossRef CAS PubMed.
- B. Hatamluyi and Z. Es'haghi, Electrochim. Acta, 2018, 283, 1170–1177 CrossRef CAS.
- B. Xu, B. Zhang, L. Yang, F. Zhao and B. Zeng, Electrochim. Acta, 2017, 258, 1413–1420 CrossRef CAS.
- Z. Zhang, J. Xu, Y. Wen and T. Wang, Mater. Sci. Eng., C, 2018, 92, 77–87 CrossRef CAS PubMed.
- S. Shojaei, N. Nasirizadeh, M. Entezam, M. Koosha and M. Azimzadeh, Food Anal. Methods, 2016, 9, 2721–2731 CrossRef.
- L. Yang, F. Zhao and B. Zeng, Electrochim. Acta, 2016, 210, 293–300 CrossRef CAS.
- L. Yang, B. Xu, H. Ye, F. Zhao and B. Zeng, Sens. Actuators, B, 2017, 251, 601–608 CrossRef CAS.
- Y. Tong, H. Li, H. Guan, J. Zhao, S. Majeed, S. Anjum, F. Liang and G. Xu, Biosens. Bioelectron., 2013, 47, 553–558 CrossRef CAS PubMed.
- J. Ai, H. Guo, R. Xue, X. Wang, X. Lei and W. Yang, Electrochem. Commun., 2018, 89, 1–5 CrossRef CAS.
- G. Yang and F. Zhao, Sens. Actuators, B, 2015, 220, 1017–1022 CrossRef CAS.
- L. Zhao, F. Zhao and B. Zeng, Biosens. Bioelectron., 2014, 60, 71–76 CrossRef CAS PubMed.
- X. Hu, Y. Feng, H. Wang, F. Zhao and B. Zeng, Anal. Methods, 2018, 10, 4543–4548 RSC.
- M. Cui, J. Huang, Y. Wang, Y. Wu and X. Luo, Biosens. Bioelectron., 2015, 68, 563–569 CrossRef CAS PubMed.
- S. Wang, G. Sun, Z. Chen, Y. Liang, Q. Zhou, Y. Pan and H. Zhai, Electrochim. Acta, 2018, 259, 893–902 CrossRef CAS.
- T. S. Anirudhan, J. R. Deepa and Binussreejayan, Mater. Sci. Eng., C, 2018, 92, 942–956 CrossRef CAS PubMed.
- X. Wang, C. Luo, L. Li and H. Duan, RSC Adv., 2015, 5, 92932–92939 RSC.
- X. Xing, S. Liu, J. Yu, W. Lian and J. Huang, Biosens. Bioelectron., 2012, 31, 277–283 CrossRef CAS PubMed.
- Z. Wang, J. Yu, R. Gui, H. Jin and Y. Xia, Biosens. Bioelectron., 2016, 79, 136–149 CrossRef CAS PubMed.
- H. Jin, R. Gui, J. Yu, W. Lv and Z. Wang, Biosens. Bioelectron., 2017, 91, 523–537 CrossRef CAS PubMed.
- X. Gao, R. Gui, H. Guo, Z. Wang and Q. Liu, Sens. Actuators, B, 2019, 285, 201–208 CrossRef CAS.
- H. Jin, C. Zhao, R. Gui, X. Gao and Z. Wang, Anal. Chim. Acta, 2018, 1025, 154–162 CrossRef CAS PubMed.
- C. Zhao, H. Jin, R. Gui and Z. Wang, Sens. Actuators, B, 2017, 242, 71–78 CrossRef CAS.
- R. Gui, H. Jin, X. Bu, Y. Fu, Z. Wang and Q. Liu, Coord. Chem. Rev., 2019, 383, 82–103 CrossRef CAS.
- Y. Fu, H. Jin, X. Bu and R. Gui, J. Agric. Food Chem., 2018, 66, 9819–9827 CrossRef CAS PubMed.
- R. Gui, X. Bu, W. He and H. Jin, New J. Chem., 2018, 42, 16217–16225 RSC.
- X. Bu, Y. Fu, H. Jin and R. Gui, New J. Chem., 2018, 42, 17323–17330 RSC.
- R. Gui, H. Jin, Z. Wang and J. Li, Chem. Soc. Rev., 2018, 47, 6795–6823 RSC.
- Y. Wang, D. Zang, S. Ge, L. Ge, J. Yu and M. Yan, Electrochim. Acta, 2013, 107, 147–154 CrossRef CAS.
- B. Babamiri, A. Salimi and R. Hallaj, Biosens. Bioelectron., 2018, 117, 332–339 CrossRef CAS PubMed.
- B. Babamiri, A. Salimi, R. Hallaj and M. Hasanzadeh, Biosens. Bioelectron., 2018, 107, 272–279 CrossRef CAS PubMed.
- X. Jin, G. Fang, M. Pan, Y. Yang, X. Bai and S. Wang, Biosens. Bioelectron., 2018, 102, 357–364 CrossRef CAS PubMed.
- W. Zhang, H. Xiong, M. Chen, X. Zhang and S. Wang, Biosens. Bioelectron., 2017, 96, 55–61 CrossRef CAS PubMed.
Footnote |
| † These authors have the equivalent contribution to this work. |
| This journal is © The Royal Society of Chemistry 2019 |



