Cobalt-catalyzed acceptorless dehydrogenative coupling of aminoalcohols with alcohols: direct access to pyrrole, pyridine and pyrazine derivatives†
Siba P.
Midya
,
Vinod G.
Landge
,
Manoj K.
Sahoo
,
Jagannath
Rana
and
Ekambaram
Balaraman
 *
*
Catalysis Division, Dr. Homi Bhabha Road, CSIR-National Chemical Laboratory (CSIR-NCL), Pune - 411008, India. E-mail: eb.raman@ncl.res.in
First published on 27th November 2017
Abstract
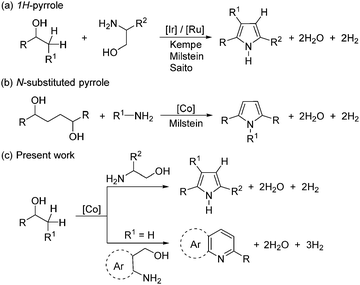
Here, the first example is reported of a new, molecularly defined SNS-cobalt(II) catalyst for the acceptorless dehydrogenative coupling (ADC) of unprotected amino alcohols with secondary alcohols leading to pyrrole and pyridine derivatives.
Transition-metal-catalyzed acceptorless dehydrogenation (AD) and hydrogen autotransfer (HA) reactions have been attracting much interest in recent times, in large part due to their excellent step-economy and high atom-efficiency.1,2 These strategies play a crucial role in activating inert chemical bonds, such as the O–H bond of alcohols and the N–H bond of amines without pre-functionalization. In particular, catalytic acceptorless dehydrogenative coupling (ADC) reactions provide a direct approach for the construction of diverse heterocyclic compounds from easily available starting materials such as alcohols, amines, and unsaturated systems with the liberation of H2 and H2O.
Pyrrole constitutes one of the most important N-heterocyclic motifs and is ubiquitous in natural products, drug intermediates, agrochemicals, dyes, and functional materials.3,4 Given their importance, the development of efficient strategies for the synthesis of pyrroles from simple feedstock chemicals is a prime focus in contemporary science. The classical approach to pyrrole synthesis involves the well-established Knorr,5 Paal–Knorr,6 and Hantzsch7 methods. However, direct and sustainable access to pyrroles from simple alcohols is appealing, since alcohols can be derived from abundantly available lignocellulosic biomass by hydrogenolysis.8
In 2013, Kempe and co-workers demonstrated the first direct synthesis of pyrroles from amino alcohols and secondary alcohols efficiently catalyzed by iridium(III)-complexes.9 Subsequently, Milstein10 and Saito11 reported a conceptually similar strategy using a ruthenium(II)-catalyst. A ruthenium-catalyzed three-component reaction of benzylic ketones, vicinal diols, and amines to access pyrroles has been reported by Beller and co-workers.12 However, the majority of the ADC reactions depend on the precious and less-abundant 4d and 5d transition-metals.1,2 In recent times, intensive efforts have been devoted to the design of novel catalysts based on non-precious metals,13–15 in particular, cobalt(II)-complexes in various AD/HA reactions.15 Based on Crabtree's original work with a Ru catalyst,16 Milstein and co-workers reported the synthesis of N-substituted pyrroles from aliphatic diols and amines using a moleculary defined PNN-Co(II) pincer complex (Scheme 1).15h
Importantly, it should be noted that all of the catalysts in AD/HA reactions possess (electron-rich) phosphine ligands. Despite the tremendous success of phosphine ligands in homogeneous catalysis, their preparation is often non-trivial, requiring handling under an inert atmosphere, needing multi-step syntheses, etc. As a consequence, the phosphine ligands are expensive and can be challenging to make on a large scale, therby hindering sustainable development.17 Of late, Gusev and co-workers showed that a sulfur-based ligand is a possible extension to replace the phosphine ligand and worked efficiently in the hydrogenation of esters.17a
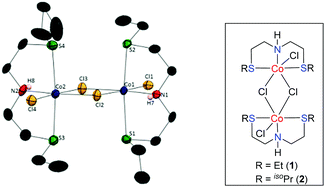
Intrigued by these findings, we began to explore the potential of a simple, phosphine ligand-free Co(II)-complex as a precatalyst for the preparation of diverse N-heterocycles. Initially, new sulphur-based SNS-cobalt(II) pincer complexes 1–2 have been prepared. The reaction of L1 and L2 with CoCl2 in MeOH at room temperature results in the formation of the corresponding Co-complexes 1–2, in 77% and 72% yields, respectively.18 Complexes 1–2 were characterized by elemental analysis, MALDI-TOF, EPR, IR and magnetic measurements.
A structural determination of 1 indicated a dimeric structure.19 The dimeric molecules made up of hexacoordinated Co(II) ions are joined by two bridging chloride ligands. The neutral EtSNS ligand is coordinated to the cobalt center in the typical tridentate mode, with a S1–Co1–S2 angle of 161.29(9)°. Selected bond distances and angles are given in the caption of Fig. 1. Remarkably, complexes 1 and 2 catalyze the dehydrogenative coupling of unprotected 1,2- and 1,3-amino alcohols with secondary alcohols in an efficient manner that enables the direct synthesis of 1H-pyrroles, and pyridines (or quinolines), respectively.20 This reaction involves the consecutive C–N and C–C bond formation with the liberation of hydrogen gas and water.
The reaction of 1-amino-3-methylbutan-2-ol (3c) with 1-phenylethanol was chosen as a model system for the acceptorless dehydrogenative coupling to form 1H-pyrroles (see ESI,† for optimization studies). Thus, refluxing an equimolar amount of 1,2-amino alcohol (1 mmol), 1-phenylethanol (1 mmol) and KOtBu (1 mmol) in m-xylene in the presence of 2.5 mol% complex 1 gave 69% yield of 2-isopropyl-5-phenyl-1H-pyrrole (5c). In the same reactions, complex 2 yielded 67% of 5c. Analysis of the gas phase by gas chromatography (GC) revealed the formation of dihydrogen. Indeed, increasing the ratio of 1,2-amino alcohol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1-phenylethanol to 1
1-phenylethanol to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 slightly enhanced the yield of the desired 1H-pyrrole to 77%. Next, the solvent effect on the reaction was examined. Thus, the yield of 5c in m-xylene (77%) was higher than in toluene (47%) or THF (32%) or in n-octane (52%). Various bases such as KHMDS (67%), KH (71%), and NaOtBu (63%) were also employed under similar catalytic conditions and gave relatively decent yields of 5c. Notably, under similar reaction conditions, a Ru(II)-catalyst RuCl2(PPh3)[HN(C2H4SEt)2]18 derived from the EtSNS ligand did not perform better than the present cobalt(II) catalysts.
2 slightly enhanced the yield of the desired 1H-pyrrole to 77%. Next, the solvent effect on the reaction was examined. Thus, the yield of 5c in m-xylene (77%) was higher than in toluene (47%) or THF (32%) or in n-octane (52%). Various bases such as KHMDS (67%), KH (71%), and NaOtBu (63%) were also employed under similar catalytic conditions and gave relatively decent yields of 5c. Notably, under similar reaction conditions, a Ru(II)-catalyst RuCl2(PPh3)[HN(C2H4SEt)2]18 derived from the EtSNS ligand did not perform better than the present cobalt(II) catalysts.
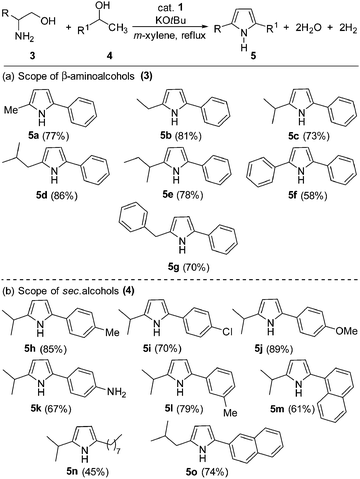
With the optimized reaction conditions in hand, the present cobalt-catalyzed dehydrogenative annulation of amino alcohols with secondary alcohols was then deployed in the synthesis of diverse 1H-pyrroles. The scope of the present cobalt catalysis was probed with 1-phenylethan-1-ol as the benchmark substrate and different β-aminoalcohols. As shown in Scheme 2, our strategy is compatible with various β-aminoalcohols and secondary alcohols, affording unsymmetrical 2,5-disubstituted 1H-pyrroles in excellent yields (up to 89% yield).
Interestingly, the reaction proceeded successfully with both aliphatic and aromatic unprotected β-aminoalcohols and gave the desired 1H-pyrroles in moderate to good yields (58–86%). The impact of varying the substituents on the secondary alcohol coupling partner was assessed using L-valinol as a benchmark substrate. The straightforward 1H-pyrrole formation reaction proceeded in excellent yields with electron-donating substituents on the 1-phenylethan-1-ol and led to the desired dehydrogenated products 5h, 5j and 5l in good yields. On the other hand, the presence of an electron-withdrawing substituent (–Cl) on the phenyl ring resulted in a moderate yield of the corresponding 1H-pyrrole (product 5i in 70%). Interestingly, the reaction of 1-(4-nitrophenyl)ethan-1-ol with 2-amino-3-methylbutan-1-ol gave 5k in 67% isolated yield, where hydrogenation of the nitro group (by the in situ generation of hydrogen gas) was observed. Notably, an aliphatic alcohol (e.g. 2-decanol) underwent dehydrogenative coupling successfully and gave the expected product 5n in 45% isolated yield. Under the optimal conditions, 1-phenylethan-1-ol reacted with 2-amino-4-methylpentan-1-ol and led to 5o in good yield (74%).
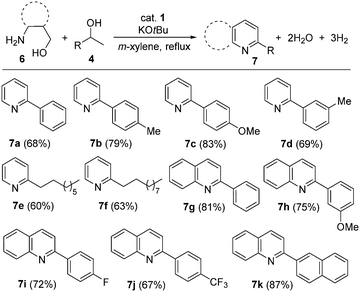
After having established that β-aminoalcohols and secondary alcohols have been successfully coupled via ADC to form 1H-pyrroles under our cobalt catalytic process, we explored the use of γ-aminoalcohols as coupling partners to access pyridine and/or quinoline derivatives. Pyridines and quinolines are other important classes of N-heteroarenes with interesting bioactivity and their synthesis is of industrial significance.21 Notable progress has been made in the direct synthesis of pyridine and quinoline derivatives based on the ADC strategy using noble metal (Ir and Ru) based complexes.10,22,23 Very recently, Kirchner and co-workers reported the manganese-catalyzed efficient synthesis of quinolines by reaction of 2-aminobenzyl alcohols and secondary alcohols.24 Indeed, the ADC approach marks a substantial departure from the classical Hantzch-like dihydropyridine synthesis25 and offers a concise and regiospecific access to pyridine or quinoline derivatives. Interestingly, a series of secondary alcohols have suitably assembled with γ-aminoalcohols to afford excellent product selectivity and good isolated yields of the desired pyridine derivatives under optimal conditions. This acceptorless dehydrogenation leads to aromatization and the condensation step deoxygenates the alcohol component.
As shown in Scheme 3, different aryl systems with varying electronic properties and alkyl groups have been regioselectively introduced, giving rise to the C-2 substituted pyridines. All of the pyridine derivatives (7a–f) were isolated in moderate to good yields (60–83%). More pleasingly, C-2 substituted quinolines (7g–k) were also prepared involving dehydrogenative cyclization of 2-aminobenzyl alcohol with various secondary alcohols using our established protocol in good yields (up to 87%). Thus, the present phosphine-free cobalt(II) complex displayed remarkable activity in the direct synthesis of various 2-substituted pyridines and quinolines.
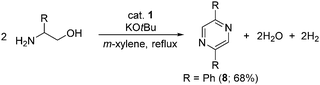
Pyrazines are biologically important organic compounds and have potential applications in the chemical industry.26,27 Gratifyingly, treatment of β-aminoalcohol with an equivalent amount of KOtBu in the presence of a catalytic amount of 1 selectively led to pyrazine (8) with the liberation of hydrogen gas and water (Scheme 4). The present cobalt-catalyzed direct pyrazine synthesis was tested for gram-scale synthesis, and it worked smoothly and gave 8 in 68% (1.02 g) isolated yield. This result implies that our elegant cobalt-based catalytic system has potential for the large-scale production of pyrazine under operationally simple, benign conditions.
In summary, the first phosphine ligand-free, non-precious base-metal catalyst28 that enables the direct synthesis of N-heterocyclic compounds (1H-pyrroles, pyridines, quinolines, and pyrazine) from amino alcohols and alcohols as key starting materials with the liberation of hydrogen gas and water as the sole by-products has been described. The present expedient strategy makes use of a new, air-stable molecularly defined SNS-cobalt(II) complex.
This research is supported by the EMR/2015/30 and SERB (SB/FT/CS-065/2013). SPM, VGL, MKS and JR thank CSIR for the fellowship. We thank Mr Saibal Bera for X-ray analysis and Dr Kumar Vanka for helpful suggestions.
Conflicts of interest
There are no conflicts to declare.Notes and references
- For recent reviews on AD and HA reactions, see: (a) A. J. Watson and J. M. Williams, Science, 2010, 329, 635 CrossRef CAS PubMed; (b) G. Guillena, D. J. Ramon and M. Yus, Chem. Rev., 2010, 110, 1611 CrossRef CAS PubMed; (c) R. Yamaguchi, K. Fujita and M. Zhu, Heterocycles, 2010, 81, 1093 CrossRef CAS; (d) J. F. Bower and M. J. Krische, Top. Organomet. Chem., 2011, 34, 107 CrossRef CAS PubMed; (e) C. Gunanathan and D. Milstein, Acc. Chem. Res., 2011, 44, 588 CrossRef CAS PubMed; (f) J. Choi, A. H. MacArthur, M. Brookhart and A. S. Goldman, Chem. Rev., 2011, 111, 1761 CrossRef CAS PubMed; (g) S. Bähn, S. Imm, L. Neubert, M. Zhang, H. Neumann and M. Beller, ChemCatChem, 2011, 3, 1853 CrossRef; (h) R. H. Crabtree, Organometallics, 2011, 30, 17 CrossRef CAS; (i) A. C. Marr, Catal. Sci. Technol., 2012, 2, 279 RSC; (j) C. Gunanathan and D. Milstein, Science, 2013, 341, 249 CrossRef CAS PubMed; (k) S. Pan and T. Shibata, ACS Catal., 2013, 3, 704 CrossRef CAS; (l) Y. Obora, ACS Catal., 2014, 4, 3972 CrossRef CAS; (m) K.-I. Shimizu, Catal. Sci. Technol., 2015, 5, 1412 RSC; (n) Q. Yang, Q. Wang and Z. Yu, Chem. Soc. Rev., 2015, 44, 2305 RSC; (o) A. Quintard and J. Rodriguez, ChemSusChem, 2016, 9, 28 CrossRef CAS PubMed.
- For selected reviews and pioneering examples, see: (a) A. Dobson and S. D. Robinson, Inorg. Chem., 1977, 16, 137 CrossRef CAS (the first report on acceptorless alcohol dehydrogenation); (b) J. F. Bower, E. Skucas, R. L. Patman and M. J. Krische, J. Am. Chem. Soc., 2007, 129, 15134 CrossRef CAS PubMed (the first use of dehydrogenation to promote formal alcohol C–H functionalization); (c) C. Gunanathan and D. Milstein, Science, 2013, 341, 1229712 CrossRef PubMed; (d) J. M. Ketcham, I. Shin, T. P. Montgomery and M. J. Krische, Angew. Chem., Int. Ed., 2014, 53, 9142 CrossRef CAS PubMed; (e) C. Gunanathan and D. Milstein, Chem. Rev., 2014, 114, 12024 CrossRef CAS PubMed; (f) A. Nandakumar, S. P. Midya, V. G. Landge and E. Balaraman, Angew. Chem., Int. Ed., 2015, 54, 11022 CrossRef CAS PubMed; (g) Y. Obora, Top. Curr. Chem., 2016, 374 Search PubMed.
- (a) C. T. Walsh, S. Garneau-Tsodikova and A. R. Howard-Jones, Nat. Prod. Rep., 2006, 23, 517 RSC; (b) A. Loudet and K. Burgess, Chem. Rev., 2007, 107, 4891 CrossRef CAS PubMed; (c) V. Bhardwaj, D. Gumber, V. Abbot, S. Dhiman and P. Sharma, RSC Adv., 2015, 5, 15233 RSC.
- (a) H. Nishide and K. Oyaizu, Science, 2008, 319, 737 CrossRef CAS PubMed; (b) A. Hagfeldt, G. Boschloo, L. Sun, L. Kloo and H. Pettersson, Chem. Rev., 2010, 110, 6595 CrossRef CAS PubMed.
- L. Knorr, Ber. Dtsch. Chem. Ges., 1884, 17, 1635 CrossRef.
- C. Paal, Ber. Dtsch. Chem. Ges., 1885, 18, 367 CrossRef.
- A. Hantzsch, Ber. Dtsch. Chem. Ges., 1890, 23, 1474 CrossRef.
- (a) T. P. Vispute, H. Zhang, A. Sanna, R. Xiao and G. W. Huber, Science, 2010, 330, 1222 CrossRef CAS PubMed; (b) C. O. Tuck, E. Pérez, I. T. Horváth, R. A. Sheldon and M. Poliakoff, Science, 2012, 337, 695 CrossRef CAS PubMed; (c) K. Barta and P. C. Ford, Acc. Chem. Res., 2014, 47, 1503 CrossRef CAS PubMed; (d) M. Besson, P. Gallezot and C. Pinel, Chem. Rev., 2014, 114, 1827 CrossRef CAS PubMed.
- S. Michlik and R. Kempe, Nat. Chem., 2013, 5, 140 CrossRef CAS PubMed.
- D. Srimani, Y. Ben-David and D. Milstein, Angew. Chem., Int. Ed., 2013, 52, 4012 CrossRef CAS PubMed.
- K. Iida, T. Miura, J. Ando and S. Saito, Org. Lett., 2013, 15, 1436 CrossRef CAS PubMed.
- (a) M. Zhang, X. Fang, H. Neumann and M. Beller, J. Am. Chem. Soc., 2013, 135, 11384 CrossRef CAS PubMed; (b) M. Zhang, H. Neumann and M. Beller, Angew. Chem., Int. Ed., 2013, 52, 597 CrossRef CAS PubMed.
- Recent reviews on catalysis by non-precious metals, see: (a) M. Albrecht, R. Bedford and B. Plietker, Organometallics, 2014, 33, 5619 CrossRef CAS; (b) Catalysis without Precious Metals, ed. R. M. Bullock, Wiley-VCH, Weinheim, Germany, 2010 Search PubMed; (c) I. Bauer and H.-J. Knölker, Chem. Rev., 2015, 115, 3170 CrossRef CAS PubMed; (d) B. Su, Z.-C. Cao and Z.-J. Shi, Acc. Chem. Res., 2015, 48, 886 CrossRef CAS PubMed; (e) S. J. ConnellyáRobinson and D. M. Heinekey, Chem. Commun., 2017, 53, 669 RSC.
- (a) T. Yan, B. L. Feringa and K. Barta, Nat. Commun., 2014, 5, 5602 CrossRef CAS PubMed; (b) A. J. Rawlings, L. J. Diorazio and M. Wills, Org. Lett., 2015, 17, 1086 CrossRef CAS PubMed; (c) S. Elangovan, J.-B. Sortais, M. Beller and C. Darcel, Angew. Chem., Int. Ed., 2015, 54, 14483 CrossRef CAS PubMed; (d) S. Chakraborty, P. E. Piszel, W. W. Brennessel and W. D. Jones, Organometallics, 2015, 34, 5203 CrossRef CAS; (e) A. Mukherjee, A. Nerush, G. Leitus, L. J. W. Shimon, Y. Ben-David, N. A. E. Jalapa and D. Milstein, J. Am. Chem. Soc., 2016, 138, 4298 CrossRef CAS PubMed; (f) A. Nerush, M. Vogt, U. Gellrich, G. Leitus, Y. Ben-David and D. Milstein, J. Am. Chem. Soc., 2016, 138, 6985 CrossRef CAS PubMed; (g) S. Elangovan, J. Neumann, J.-P. Sortais, K. Junge, C. Darcel and M. Beller, Nat. Commun., 2016, 7, 12641 CrossRef PubMed; (h) M. Mastalir, M. Glatz, N. Gorgas, B. Stöger, E. Pittenauer, G. Allmaier, L. F. Veiros and K. Kirchner, Chem. – Eur. J., 2016, 22, 12316 CrossRef CAS PubMed; (i) T. Yan, B. L. Feringa and K. Barta, ACS Catal., 2016, 6, 381 CrossRef CAS; (j) T. Yan and K. Barta, ChemSusChem, 2016, 9, 2321 CrossRef CAS PubMed; (k) B. Emayavaramban, M. Sen and B. Sundararaju, Org. Lett., 2017, 19, 6 CrossRef CAS PubMed.
- For a cobalt-catalyzed dehydrogenation reaction, see: (a) G. Zhang and S. K. Hanson, Org. Lett., 2013, 15, 650 CrossRef CAS PubMed; (b) R. Xu, S. Chakraborty, H. Yuan and W. D. Jones, ACS Catal., 2015, 5, 6350 CrossRef CAS; (c) S. Rösler, M. Ertl, T. Irrgang and R. Kempe, Angew. Chem., Int. Ed., 2015, 54, 15046 CrossRef PubMed; (d) G. Zhang, Z. Yin and S. Zheng, Org. Lett., 2016, 18, 300 CrossRef CAS PubMed; (e) M. Mastalir, G. Tomsu, E. Pittenauer, G. Allmaier and K. Kirchner, Org. Lett., 2016, 18, 3462 CrossRef CAS PubMed; (f) Z. Yin, H. Zeng, J. Wu, S. Zheng and G. Zhang, ACS Catal., 2016, 6, 6546 CrossRef CAS; (g) N. Deibl and R. Kempe, J. Am. Chem. Soc., 2016, 138, 10786 CrossRef CAS PubMed; (h) P. Daw, S. Chakraborty, J. A. Garg, Y. Ben-David and D. Milstein, Angew. Chem., Int. Ed., 2016, 55, 14373 CrossRef CAS PubMed; (i) S. P. Midya, A. Mondal, A. Begum and E. Balaraman, Synthesis, 2017, 3957 CAS.
- N. D. Schley, G. E. Dobereiner and R. H. Crabtree, Organometallics, 2011, 30, 4174 CrossRef CAS.
- (a) D. Spasyuk, S. Smith and D. G. Gusev, Angew. Chem., Int. Ed., 2013, 52, 2538 CrossRef CAS PubMed; (b) E. C. Hansen, D. J. Pedro, A. C. Wotal, N. J. Gower, J. D. Nelson, S. Caron and D. J. Weix, Nat. Chem., 2016, 8, 1126 CrossRef CAS PubMed.
- See the ESI†.
- To avoid the disorder in the structure, the chemical occupancy of C16A and C16B has been assigned as 0.5 and the CCDC No of complex 1 is 1555768.
- Complex 1 also catalyzes N-alkylation of amines with alcohols by hydrogen autotransfer strategy. S. P. Midya and E. Balaraman, to be published.
- J. A. Joule and K. Mills, Heterocyclic Chemistry, Blackwell, Oxford, 4th edn, 2000 Search PubMed.
- Pyridine synthesis via an ADC strategy: (a) S. Michlik and R. Kempe, Angew. Chem., Int. Ed., 2013, 52, 6326 CrossRef CAS PubMed; (b) D. Srimani, Y. Ben-David and D. Milstein, Chem. Commun., 2013, 49, 6632 RSC; (c) S. Ruch, T. Irrgang and R. Kempe, Chem. – Eur. J., 2014, 20, 13279 CrossRef CAS PubMed; (d) N. Deibl, K. Ament and R. Kempe, J. Am. Chem. Soc., 2015, 137, 12804 CrossRef CAS PubMed; (e) T. Hille, T. Irrgang and R. Kempe, Angew. Chem., Int. Ed., 2017, 56, 371 CrossRef CAS PubMed.
- Quinoline synthesis via an ADC strategy, see: (a) C. C. Cho, B. T. Kim, T.-J. Kim and S. C. Shim, Chem. Commun., 2001, 2576 RSC; (b) H. Vander Mierde, N. Ledoux, B. Allaert, P. Vander Voort, R. Drozdzak, D. De Vos and F. Verpoort, New J. Chem., 2007, 31, 1572 RSC; (c) R. Martinez, D. J. Ramon and M. Yus, Eur. J. Org. Chem., 2007, 1599 CrossRef CAS; (d) H. V. Vander Mierde, P. Vander Voort, D. De Vos and F. Verpoort, Eur. J. Org. Chem., 2008, 1625 CrossRef CAS; (e) B. Pan, B. Liu, E. Yue, Q. Liu, X. Yang, Z. Wang and W. H. Sun, ACS Catal., 2016, 6, 1247 CrossRef CAS.
- M. Mastalir, M. Glatz, E. Pittenauer, G. Allmaier and K. Kirchner, J. Am. Chem. Soc., 2016, 138, 15543 CrossRef CAS PubMed.
- Name Reactions in Heterocyclic Chemistry, ed. J.-J. Li and E. J. Corey, Wiley-Interscience, Hoboken, NJ, 2005 Search PubMed.
- (a) D. F. Taber, P. W. DeMatteo and K. V. Taluskie, J. Org. Chem., 2007, 72, 1492 CrossRef CAS PubMed; (b) M. Chaignaud, I. Gillaizeau, N. Ouhamou and G. Coudert, Tetrahedron, 2008, 64, 8059 CrossRef CAS.
- B. Gnanaprakasam, E. Balaraman, Y. Ben-David and D. Milstein, Angew. Chem., Int. Ed., 2011, 50, 12240 CrossRef CAS PubMed.
- A triazine-based electron-rich PN5P-Mn(I)-complex catalyzed synthesis of pyrrole has been reported. However, it is noteworthy to mention that the relative Co-complex is inactive and failed to yield the 1H-pyrrole, see: F. Kallmeier, B. Dudziec, T. Irrgang and R. Kempe, Angew. Chem., Int. Ed., 2017, 56, 7261 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1555768. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7cc07427a |
| This journal is © The Royal Society of Chemistry 2018 |