Bright NUV mechanofluorescence from a terpyridine-based pure organic crystal†
Qikun
Sun
a,
Liangliang
Tang
a,
Zhenzhen
Zhang
a,
Kai
Zhang
a,
Zongliang
Xie
b,
Zhenguo
Chi
 b,
Haichang
Zhang
*a and
Wenjun
Yang
b,
Haichang
Zhang
*a and
Wenjun
Yang
 *a
*a
aKey Laboratory of Rubber-Plastics of Ministry of Education/Shandong Province (QUST), School of Polymer Science & Engineering, Qingdao University of Science & Technology, 53-Zhengzhou Road, Qingdao 266042, P. R. China. E-mail: haichangzhang@hotmail.com; ywjph2004@qust.edu.cn
bState Key Laboratory of Optoelectronic Material and Technologies, School of Chemistry and Chemical Engineering, Sun Yat-sen University Guangzhou, 510275, P. R. China. E-mail: chizhg@mail.sysu.edu.cn
First published on 29th November 2017
Abstract
A bright NUV mechanofluorescent terpyridine-based material with a piezoelectric space group was obtained. The single crystal analysis and quantum chemical calculations indicated the existence of enhanced intermolecular and intramolecular interactions and intersystem crossing, but which did not facilitate the room temperature phosphorescence.
Mechanoluminescence (ML) was first pointed out by Francis Bacon in 1605 based on his attentive observation on hard sugar fracture.1 This type of luminescence could be excited by various external mechanical actions, such as stretching, cleaving, cutting, rubbing, grinding, shaking or scratching, sonication, etc.2 It was predicted that nearly half of all solids probably showed the ML phenomenon.3 However, for centuries, inorganic salts and metal–organic complexes have been the mainstay of ML materials, and only about 400 papers were published before the 21st century.2–4 Recently, an increasing interest in ML has emerged as evidenced by more than 400 papers published within the past 17 years, owing to the re-recognition of its potential applications in display, lighting, bioimaging, and stress sensing5 and its academic significance in understanding fundamental photo-physical processes.
Conjugated organic luminophores might be promising ML candidates because of their structure diversity, intrinsic luminescence, easy accessibility and modification. However, their ML properties started to draw serious research attention only within the past few years.6 Generally, crystals with piezoelectric space groups (dipolar structures and noncentrosymmetric molecular arrangements), strong intermolecular and intramolecular interactions and crystalline fluorescence are expected to be promising ML candidates. For example, recently, some aggregation-induced emission (AIE) molecules have been found to be ML-active materials emitting blue and green light.7,8 However, bright organic ML luminogens are still limited in number and kinds, and the understanding of the crystal structure-ML activity relationship and the luminescence mechanism are not comprehensive and clear. Moreover, there are only a few reports on organic ML luminogens with bight NUV emission and no AIE effect at present.8 We considered that NUV organic ML emitters could not only help one to further understand the ML process but also serve as the energy donor (host) to generate various visible MLs and expand the color gamut. Therefore, to broaden the ML family and deepen the understanding of the ML mechanism, it is necessary to exploit other kinds of bright ML organic materials with non-AIE effect and NUV emission.
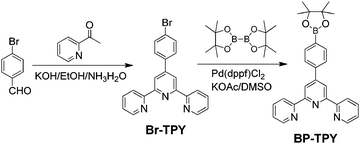
Based on the above considerations, in the current work, we have designed and synthesized a terpyridine derivative (BP-TPY, Scheme 1) to investigate its mechanoluminescence (ML), photoluminescence (PL), and crystal structure. The details for the synthesis and characterization are shown in the ESI.† As BP-TPY has a short conjugation and a branched configuration, efficient NUV emission could be expected. The polar boric acid ester and lone pair electron-containing pyridyl units should render the crystal with strong intermolecular and intramolecular interactions. Moreover, this noncentrosymmetric tapered molecule could be prone to stack in the piezoelectric space group. Thus, a bright NUV emission could be expected under light and mechanical stimuli.
Fig. S1 (ESI†) shows the absorption and PL spectra of BP-TPY in THF solution and solid states. The light absorption for both the solution and the solid film mainly appeared before 330 nm with the peak at 300 nm. Under 300 nm light excitation, BP-TPY emitted NUV light with the emission peaks at 358 and 368 nm for the solution and the solid, respectively, and a good color purity is observed, evidenced by the narrow full width at half-maximum of 35 nm. The corresponding fluorescence efficiencies were 15% and 13%, respectively, which are commendable in view of its large band gap, small size, and low conjugation degree. BP-TPY could be regarded as a non-AIE but multi-state emission organic dye although its fluorescence efficiency is decreased by 50% in aqueous media (Fig. S2, ESI†).
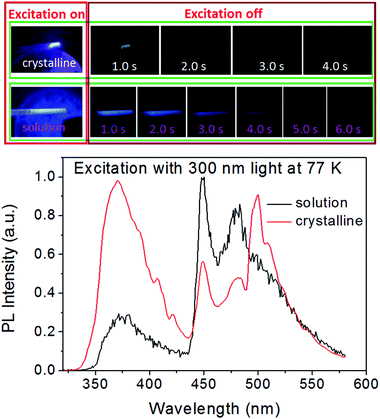
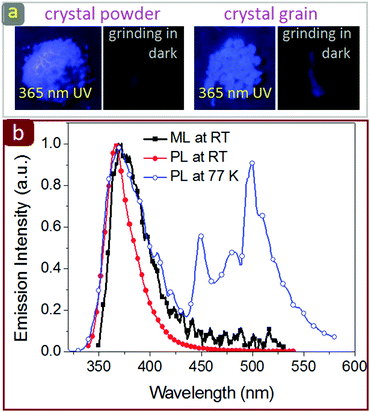
Under UV light excitation, the BP-TPY crystal and solution all exhibited remarkable fluorescence–phosphorescence dual emission at 77 K (Fig. 1), but only the fluorescence emission was observed at room temperature (Fig. S1, ESI†). The BP-TPY crystal had a short (2.4 ns) and a long (1.53 s) luminescence lifetimes at 77 K, but only a short fluorescence lifetime of 1.9 ns at room temperature (Fig. S3, ESI†). When the crystalline powder of BP-TPY was ground in a glass bottle using a metal or plastic spade in dark room, an NUV light emission could be observed by the naked eye and a video was recorded using a digital camera (iPhone 7, Fig. 2 and Fig. S4, ESI†). Unfortunately, the emission spectrum was not able to be analyzed using a CCD spectrometer, probably due to that the light signal was not strong enough. In order to enhance the brightness, the bulky crystal grains were prepared by slowly evaporating its chloroform/methanol solution and grinding as described for the crystalline powder. As expected, a bright deep blue light could be seen and it was analyzed well using a CCD spectrometer, which afforded a UV emission spectrum with the peak at 372 nm. Since the ML spectrum matched very well with the room-temperature photofluorescence spectra (Fig. 2), these light emissions should occur from the same excited states, regardless of the mode of excitation.
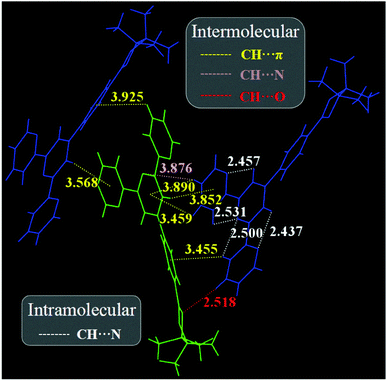
To gain insights into the effect of the crystal structure on the ML effect, the single crystal of BP-TPY was cultured and analyzed. The result revealed that BP-TPY adopted two different twisted conformations to pack each other and form a monoclinic crystal system with noncentrosymmetric packing and a piezoelectric space group Pc (Fig. S5 and Table S1, ESI†). The twisted backbone and small-size conjugation without any electron donor moiety contributed to the NUV emission. As expected, there were indeed strong intermolecular and intramolecular interactions in the BP-TPY crystal (Fig. 3), which should largely reduce the possible energy loss through non-radiative relaxation under mechanical stimulus. These results could explain the bright mechanoluminescence of the BP-TPY crystal. On the other hand, it was known that the crystal fracture could separate electrical charges and ionized air (such as nitrogen), and the discharge of the ionized nitrogen could emit a multiple-peak spectrum in the range of 310–430 nm with a fine six-finger structure.4a Since the strong absorption of BP-TPY was before 310 nm, together with the spectral identity between light and mechanical excitation, the structure-less ML should result from the direct excitation of the crystal fracture-induced piezoelectric field, rather than the secondary excitation of the nitrogen discharge emission on the BP-TPY crystal. It was noticed that the intermediate Br-TPY crystal exhibited unique photoluminescence but no ML activity (Fig. S6, ESI†), which was not surprising since each identified ML compound present in it is still an isolated event and its ML activity was impossible to predict in advance.
Recently, Li et al. have found a unique example of the fluorescence–phosphorescence dual mechanoluminescence at room temperature from m-terphenyl boric acid ester (DPP-BO).6f By comparing the optical properties of BP-TPY and DPP-BO, we could find that they have the same photoluminescence behaviors, such as fluorescence–phosphorescence dual photo-luminescence at low temperature and only photofluorescence at room temperature. However, further investigations are required to understand why BP-TPY only exhibits the mechanofluorescence.
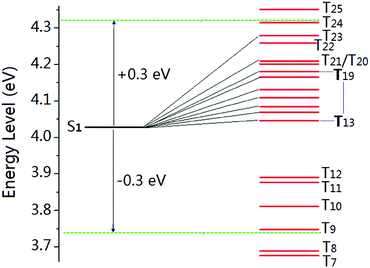
Generally, luminophores containing multiple heteroatoms like N, P, and O are prone to phosphorescence emission since the lone pair electrons should favour the n–π* transition and populate triplet excitons through facilitating the spin-forbidden transition of singlet-to-triplet excited states (inter-system crossing, ISC). Moreover, by analyzing the packing structure of the BP-TPY crystal, we found that the intermolecular and intramolecular interactions in the BP-TPY crystal were significantly stronger than those in the DPP-BO one, in which the additional C–H⋯N interactions were observed besides a shorter C–H⋯O interaction distance (2.518 Å versus 3.184 Å) for the former. These interactions were considered to be much beneficial to the n–π* transition and the ISC enhancement. To further understand the ISC feature, TD-DFT calculations were carried out based on a pair of strong interacting BP-TPY derived from a single crystal structure (Fig. 4). It was found that there were 9 Tn states containing 19 possible channels for ISC based on the same transition orbital compositions between Tn and S1 within the ±0.3 eV energy level9 (Table S2 in the ESI†). The highly increased number of ISC transitions for BP-TPY compared to that of DPP-BO should be much more favourable for populating triplet excitons. Thus, the absence of room-temperature phosphorescence in the BP-TPY crystal should result from the energy loss possibly through non-radiative relaxation channels from T1 to S0 as well as the endothermicity of the ISC process from S1 to Tn, which could significantly decrease the phosphorescence emission probability. In other words, the ML features and mechanisms still remain complicated and ambiguous and are dependent on the studied compound structures. Enhancing the ISC transitions was only a necessary but not sufficient condition for the spin-forbidden triplet-to-singlet phosphorescence emission.
In summary, a new terpyridine-based ML material (BP-TPY) with bright NUV emission has been obtained. BP-TPY is a multi-state emission and non-AIE-active luminophore, and its ML spectrum is almost the same as the PL spectra. There are strong intermolecular and intramolecular interactions and highly increased number of ISC transitions in BP-TPY crystals with multiple heteroatoms, but room-temperature photo- and mechano-phosphorescence are not observed. Therefore, for spin-forbidden phosphorescence emission, enhancing the ISC transitions was only a necessary but not sufficient condition, and ML features and mechanisms still remain ambiguous and seem to be dependent on the studied molecular structures. NUV ML emissions are very meaningful because they could not only help in understanding the ML process but also serve as the energy donor to generate various visible ML emissions by in situ exciting other fluorescent dopants. The investigation on “BP-TPY as a host function” is another interesting subject and is underway in our laboratory.
Financial support for this research was provided by the National Natural Science Foundation of China (No. 51673105, 51573081, and 51473084). We thank the Open Project of the State Key Laboratory of Supramolecular Structure and Materials of Jilin University (SKLSSM-201728).
Conflicts of interest
There are no conflicts to declare.Notes and references
- L. Picard, Science, 1939, 89, 460 Search PubMed.
- (a) P. Jha and B. P. Chandra, Luminescence, 2014, 29, 977 CrossRef CAS PubMed; (b) N. C. Eddingsaas and K. S. Suslick, Nature, 2006, 444, 163 CrossRef CAS PubMed; (c) N. C. Eddingsaas and K. S. Suslick, J. Am. Chem. Soc., 2007, 129, 6718 CrossRef CAS PubMed.
- (a) L. M. Sweeting, Chem. Mater., 2001, 13, 854 CrossRef CAS; (b) Y. Tsuboi, T. Seto and N. Kitamura, J. Phys. Chem. A, 2008, 112, 6517 CrossRef CAS PubMed; (c) L. S. McCarty and G. M. Whitesides, Angew. Chem., Int. Ed., 2008, 47, 2188 CrossRef CAS PubMed.
- (a) A. Walton, J. Adv. Phys., 1977, 26, 887 CrossRef CAS; (b) J. I. Zink, Acc. Chem. Res., 1978, 11, 289 CrossRef CAS; (c) B. P. Chandra and J. I. Zink, Chem. Phys., 1980, 73, 5933 CAS; (d) J. I. Zink, Naturwissenschaften, 1981, 68, 507 CrossRef CAS; (e) B. P. Chandra and J. I. Zink, Phys. Rev. B: Condens. Matter Mater. Phys., 1980, 21, 816 CrossRef CAS.
- (a) S. M. Jeong, S. Song, K.-I. Joo, J. Kim, S.-H. Hwang, J. Jeong and H. Kim, Energy Environ. Sci., 2014, 7, 3338 RSC; (b) Y. Chen, Y. Zhang, D. Karnaushenko, L. Chen, J. Hao, F. Ding and O. G. Schmidt, Adv. Mater., 2017, 29, 1605165 CrossRef PubMed; (c) I. Sage, R. Badcock, L. Humberstone, N. Geddes, M. Kemp and G. Bourhill, Smart Mater. Struct., 1999, 8, 504 CrossRef; (d) D. O. Olawale, K. Kliewer, A. Okoye, T. J. Dickens, M. J. Uddin and O. I. Okoli, J. Lumin., 2014, 147, 235 CrossRef CAS; (e) C. N. Xu, T. Watanabe, M. Akiyama and X. G. Zheng, Appl. Phys. Lett., 1999, 74, 1236 CrossRef CAS; (f) N. Terasaki, H. Zhang, H. Yamadaa and C. N. Xu, Chem. Commun., 2011, 47, 8034 RSC.
- (a) H. Nakayama, J. I. Nishida, N. Takada, H. Sato and Y. Yamashita, Chem. Mater., 2012, 24, 671 CrossRef CAS; (b) J. Nishida, H. Ohura, Y. Kita, H. Hasegawa, T. Kawase, N. Takada, H. Sato, Y. Sei and Y. Yamashita, J. Org. Chem., 2016, 81, 433 CrossRef CAS PubMed; (c) Y. J. Xie, J. Tu, T. Q. Zhang, J. Q. Wang, Z. L. Xie, Z. G. Chi, Q. Peng and Z. Li, Chem. Commun., 2017, 53, 11330 RSC; (d) B. J. Xu, W. L. Li, J. J. He, S. K. Wu, Z. G. Chi, S. W. Liu, J. R. Xua and R. B. Martin, Chem. Sci., 2016, 7, 5307 RSC; (e) S. D. Xu, T. T. Liu, Y. X. Mu, Y. F. Wang, Z. G. Chi, S. W. Liu and J. R. Xu, Angew. Chem., Int. Ed., 2015, 54, 874 CrossRef CAS PubMed; (f) J. Yang, Z. C. Ren, Z. L. Xie, Y. J. Liu, C. Wang, W. J. Tian, F. Zhang, Z. G. Chi and Z. Li, Angew. Chem., Int. Ed., 2017, 56, 880 CrossRef CAS PubMed.
- (a) B. Xu, J. He, Y. Mu, Q. Zhu, S. Wu, Y. Wang, Y. Zhang, C. Jin, C. Lo, Z. Chi, A. Lien, S. Liu and J. Xu, Chem. Sci., 2015, 6, 3236 RSC; (b) C. Wang, B. Xu, M. Li, Z. Chi, Y. Xie, Q. Li and Z. Li, Mater. Horiz., 2016, 3, 220 RSC.
- (a) J. I. Zink, J. Am. Chem. Soc., 1974, 96, 6775 CrossRef CAS; (b) G. E. Hardy, J. C. Baldwin, J. I. Zink, W. C. Kaska, P. Liu and L. Duboisi, J. Am. Chem. Soc., 1977, 99, 3552 CrossRef CAS; (c) J. I. Zink and W. C. Kaska, J. Am. Chem. Soc., 1973, 95, 7510 CrossRef CAS; (d) W. Wu, T. Narisawa and S. Hayashi, Jpn. J. Appl. Phys., 2001, 40, 1294 CrossRef CAS; (e) K. K. Neena, P. Sudhakar, K. Dipak and P. Thilagar, Chem. Commun., 2017, 53, 3641 RSC.
- (a) G. Zhang, J. Chen, S. J. Payne, S. E. Kooi, J. N. Demas and C. L. Fraser, J. Am. Chem. Soc., 2007, 129, 8942 CrossRef CAS PubMed; (b) S. Menning, M. Kr-mer, B. A. Coombs, F. Rominger, A. Beeby, A. Dreuw and U. H. F. Bunz, J. Am. Chem. Soc., 2013, 135, 2160 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Details for synthesis and characterization Fig. S1–S6. CCDC 1580060. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7cc08064f |
| This journal is © The Royal Society of Chemistry 2018 |