Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A neutral dinuclear Ir(III) complex for anti-counterfeiting and data encryption†
Yang
Jiang
a,
Guangfu
Li
a,
Weilong
Che
a,
Yingjie
Liu
b,
Bin
Xu
b,
Guogang
Shan
a,
Dongxia
Zhu
*a,
Zhongmin
Su
 *a and
Martin R.
Bryce
*a and
Martin R.
Bryce
 *c
*c
aKey Laboratory of Nanobiosensing and Nanobioanalysis at Universities of Jilin Province, Department of Chemistry, Northeast Normal University, 5268 Renmin Street, Changchun, Jilin Province 130024, P. R. China. E-mail: zhudx047@nenu.edu.cn; zmsu@nenu.edu.cn
bKey Laboratory of Supramolecular Structure and Materials, Institute of Theoretical Chemistry, Jilin University, Changchun 130012, P. R. China
cDepartment of Chemistry, Durham University, Durham, DH1 3LE, UK. E-mail: m.r.bryce@durham.ac.uk
First published on 27th February 2017
Abstract
A neutral dinuclear Ir(III) Schiff base complex PIBIP has been synthesized and shown to exhibit both piezochromic luminescence (PCL) and aggregation induced emission (AIE) behaviour. An efficient second-level anti-counterfeit trademark and a data encryption device were fabricated using PIBIP as the active material.
Anti-counterfeiting and data security are important issues in the economy and military fields as well as in our everyday lives.1 This has led to the development of technologies such as infrared up-conversion,2 magnetic response3 and plasmonic security labels.4 However, these systems are presently facing various problems such as poor stability and high reaction temperature (the calcination temperatures usually reach 800 °C).5 Therefore, there is an urgent requirement for new advanced materials with high thermal stability and facile preparation to combat counterfeiting and information leakage.
Phosphorescent transition-metal complexes, such as Ir(III) systems, have great potential in this area due to their high luminescence quantum yields, easy handling, structural versatility and high photostability.6 However, traditional phosphorescent materials are usually used for first-level data encryption, with the disadvantage of security information being exposed immediately under ultraviolet (UV) light illumination. This means that encoded data are not secure and can be easily substituted by compounds with a similar emission colour. Therefore, the design of novel luminescent materials with more covert and reliable anti-counterfeit features is an appealing challenge. Piezochromic luminescent (PCL) compounds are a class of “smart” materials whose fluorescence properties change in response to external pressure or mechanical grinding.7 It has been shown that this process can be reversed and the original emission colour can be restored by altering the molecular packing mode in the solid state in response to external stimuli, such as heating or recrystallization.8 Taking advantage of this reversible property, PCL materials are widely used in sensors, memory chips, and security inks.9
However, PCL compounds generally require sophisticated synthesis (>10 steps) to introduce different components into one molecule.10 Moreover, similar to most conventional dyes, these luminophores suffer from aggregation caused quenching (ACQ), which results in low phosphorescence quantum yields in the solid state.11 All the aforementioned drawbacks significantly limit the real-world applications of PCL materials. In contrast, aggregation-induced emission (AIE), as coined by Tang et al. in 2001,12 is a property of compounds which emit weakly when dispersed in dilute solution but show strong emission when aggregated due to restricted intramolecular motions. This abnormal phenomenon has attracted considerable interest in the field of electroluminescent devices and chemical sensing.13 The motivation for the present work is to develop phosphorescent transition-metal complexes with combined AIE and PCL properties and to exploit them as candidates for applications in anti-counterfeiting and data encryption.
Herein, we designed and synthesized a new dinuclear Ir(III) complex (ppy)2Ir-(bsbd)-Ir(ppy)2 (PIBIP) with a Schiff base bridging ligand (bsbdH2) and phenylpyridine (ppy) cyclometalating ligands (Scheme S1, ESI†). The photophysical properties demonstrate that PIBIP is AIE active and simultaneously shows PCL properties. Moreover, the emission colour of PIBIP can be converted to the original colour upon simple treatment by an organic solvent. These results lead to the demonstration of a second-level anti-counterfeit trademark and data encryption device using PIBIP as the security ink. PIBIP is the first neutral dinuclear Ir(III) complex which exhibits both PCL and AIE characteristics, to the best of our knowledge. These combined properties are important for device applications.7 The flexible nature of the bsbd spacer allows the ligand to adopt optimal coordination geometries at the metal centres so as to facilitate the coexistence of PCL and AIE.
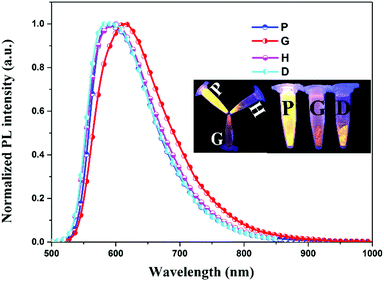
The structure of PIBIP was established by its 1H NMR and mass spectra, and single-crystal X-ray structure (ESI†). The pristine solid sample of PIBIP (hereafter abbreviated as P) shows yellow phosphorescence (λmax = 587 nm), as shown in Fig. 1. When powder P was thoroughly ground in an agate mortar for 5 min, a significant bathochromic shift (λmax = 613 nm) was observed in the orange-emitting ground sample G (Fig. 1). The emission intensity of G shows a significant weakening that is clearly visible to the naked eye. The photoluminescence quantum yield (PLQY) is decreased dramatically in the grinding process from 20% (P) to 7% (G) (Table S1, ESI†). To investigate the reversibility of this PCL behavior, G was wetted with dropwise addition of dichloromethane (DCM) solvent (sample D) and the emission colour reverted to the initial emission colour of P within a few seconds (Fig. 1). Notably, the emission could perfectly revert to G when D was further ground. This PCL behaviour of PIBIP was shown to be highly reversible for several grinding–wetting cycles (Fig. S4, ESI†).
In order to establish the origin and mechanism of this reversible piezochromic behaviour of PIBIP, the 1H NMR spectra of both P and G were obtained. The results show complete consistency of chemical shift values and peak shapes for both P and G (Fig. S1, ESI†). The changed phosphorescent colours in the grinding process are, therefore, attributed to physical processes.
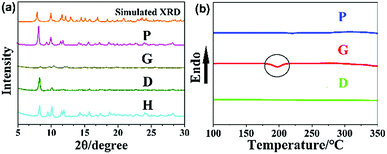
Powder X-ray diffraction (PXRD) was carried out to investigate the aggregation states of P and G. The sharp peaks in the XRD pattern (Fig. 2a) unambiguously indicate that the P sample has a well-ordered crystalline structure. In sharp contrast, the ground sample G exhibits weak and broad diffraction signals, which imply a crystalline to amorphous phase transition during the grinding process. The clear reflection peaks of pristine solid P reappeared after sample G was heated (sample H) or wetted with DCM solvent dropwise (sample D). In addition, upon heating G to 350 °C, the DSC traces exhibited a clear broad exothermic recrystallization peak at ca. 198 °C. This peak is at a similar temperature at which thermal recrystallization begins to take place (Fig. 2b). Therefore, when G was heated at 198 °C for 1 min, the crystalline state was recovered and consequently the emission colour reverted to the original one (Fig. 1). PIBIP emitted only weakly in amorphous G, but became strongly emissive in crystalline P. This is typical crystallization-induced emission enhancement (CIEE) behaviour.14 These results provide a rational explanation for the variation in PL intensity.
The single crystal X-ray structure analysis of PIBIP demonstrates that no intermolecular interactions exist in the molecular packing (Fig. S8, ESI†). By contrast, obvious intramolecular π–π interactions between the bridging phenyl ring and the adjacent phenyl rings of ppy are observed (Fig. 3). This structure might be easily modified when mechanical pressure is applied, resulting in a red-shift of the PL spectra.15 The excited-state lifetimes (τ) for PIBIP significantly decreased from P (0.97 μs) to G (0.49 μs) (Table S1, ESI†). Thus, it can be concluded that the changed τ in the PCL process is associated with alteration in the solid-state molecular packing and/or the intramolecular interactions. The similar τ values of both the P and D states are consistent with them having the same molecular arrangement.16
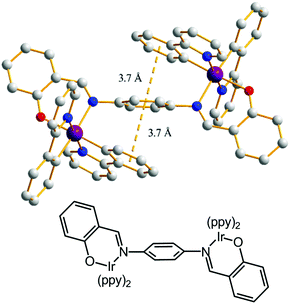 | ||
| Fig. 3 The X-ray molecular structure of PIBIP showing intramolecular π–π interactions (dashed line). Colour code: Ir purple; N blue; O red. | ||
Encouraged by the excellent PCL performance of PIBIP, anti-counterfeit trademark and data encryption devices were constructed (Fig. 4). MASK is a fluorescent emitter selected from the literature17 (for the structure see Fig. S2 in the ESI†), which shows a similar emission peak to G at around 610 nm (Fig. S3, ESI†). Thus, it is difficult to distinguish G from MASK due to their very similar emission colours and emission intensities. Furthermore, no colour change is observed when mechanical grinding is applied to MASK, which is an exclusive method to differentiate G and MASK (Fig. S3, ESI†). As shown in Fig. 4a and b, the anti-counterfeit trademark adopted the shape of a ‘flower’ comprising a central ‘stamen’ and a ‘petal’. The ‘stamen’ is the as-prepared powder of MASK, which emits orange fluorescence upon excitation with a 365 nm UV lamp and the ‘petal’ is the sample of G (the detailed method is given in the ESI†). This device could provide a second-level anti-counterfeit function. As shown in Fig. 4a, orange light was rapidly observed under the illumination of a standard UV lamp (at 254 and 365 nm), presenting a first-level anti-counterfeit system. When this ‘flower’ was sprayed with dichloromethane (DCM) solvent, the emission of the ‘petal’ changed immediately from orange (G) to yellow (D) (Fig. 4b). A second-level anti-counterfeit system was then successfully obtained as follows. Upon further grinding the ‘petal’, the original emission colour was regenerated and the first-level anti-counterfeit trademark reappeared (Fig. S5, ESI†).
Furthermore, a simple, convenient and efficient technology for data encryption and decryption was designed (Fig. 4c). G was used as a ‘cryptographic ink’, while MASK was used as a control reagent. In the encryption stage, the characters ‘NENU’ were written on a filter paper by using MASK, and then powder G was carefully spread on it as the letters ‘AIPE’. So G was hidden by MASK even under UV light, because G and MASK emitted unitary orange light. In the decryption stage, the yellow security letters ‘AIPE’ appeared clearly when DCM solvent was sprayed onto the as-prepared letters ‘NENU’. In contrast to the orange-emitting background, the letters ‘AIPE’ showed intense yellow emission, which is in agreement with the change in the emission colour from G to D. Moreover, ‘AIPE’ can be easily hidden again by grinding. This simple process demonstrates excellent encryption and decryption reversibility for several cycles. These data suggest that complex PIBIP has the potential to be employed in practical applications as an anti-counterfeit and security protection ink with simple optical authentication.
Since PIBIP exhibits strong luminescence in the solid state (Table S2, ESI†), the AIE property was probed by using different ratios of THF–water mixtures. As shown in Fig. 5, PIBIP exhibits very weak emission in pure THF solution, where it was well dissolved. Nevertheless, the PL intensity was dramatically enhanced when the water fraction reached 50%, increasing by a maximum of up to about 100-fold in comparison with the pure THF solution. The PL intensity then decreased with increasing water content >50% water fraction, but the PL intensity at 90% water fraction is still stronger than in the pure THF solution. There are two possible reasons for this behaviour. First, after aggregation, the molecules covered within the surface of the nanoparticles did not emit light, leading to a decrease in phosphorescence intensity. Second, crystalline particles and amorphous particles simultaneously form when the water fraction is increased. The former particles would enhance the emission intensity but the latter do not.8 Thus, the measured overall PL intensity depends on the combined actions of the two kinds of nanoparticles. Furthermore, the UV-visible absorption profile showed a Mie scattering effect for the mixtures of PIBIP with high water content (Fig. S6, ESI†).18 Transmission electron microscopy (TEM) and electron diffraction (ED) experiments indicated that amorphous molecular aggregates are formed in the mixtures (Fig. S7, ESI†).19 Evidently, PIBIP is an excellent AIE chromophore that could effectively suppress non-radiative decay to produce intense emission in the solid state.
In summary, a smart dinuclear Ir(III) Schiff base complex PIBIP shows simultaneous reversible PCL behaviour and AIE-activity. An obvious bathochromic shift occurs in the solid state upon mechanical grinding, and this new emission reverts to the original state after wetting with DCM solvent. Anti-counterfeiting trademark and data encryption devices have been successfully constructed by combining PIBIP and fluorescent MASK through the piezochromic and solvatochromic properties of PIBIP. Therefore, these findings indicate that PIBIP could expand the applications of PCL materials to anti-counterfeiting, information storage and data security protection.
The work was funded by the NSFC (No. 51473028), the Key Scientific and Technological Project of Jilin Province (20150204011GX, 20160307016GX), and the Development and Reform Commission of Jilin Province (20160058). Work in Durham was funded by EPSRC grant EP/K039423/1.
Notes and references
- (a) M. You, M. Lin, S. Wang, X. Wang, G. Zhang, Y. Hong, Y. Dong, G. Jin and F. Xu, Nanoscale, 2016, 8, 10096–10104 RSC; (b) M. You, J. Zhong, Y. Hong, Z. Duan, M. Lin and F. Xu, Nanoscale, 2015, 7, 4423–4431 RSC; (c) T. Zhang, L. Fu, Z. Chen, Y. Cui and X. Liu, Prog. Org. Coat., 2016, 100, 100–104 CrossRef CAS; (d) P. Kumar, S. Singh and B. K. Gupta, Nanoscale, 2016, 8, 14297–14340 RSC.
- J. F. Suyver, A. Aebischer, D. Biner, P. Gerner, J. Grimm, S. Heer, K. W. Krämer, C. Reinhard and H. U. Güdel, Opt. Mater., 2005, 27, 1111–1130 CrossRef CAS.
- R. Li, Y. Zhang, J. Tan, J. Wan, J. Guo and C. Wang, ACS Appl. Mater. Interfaces, 2016, 8, 9384–9394 CAS.
- Y. Cui, R. S. Hegde, I. Y. Phang, H. K. Lee and X. Y. Ling, Nanoscale, 2014, 6, 282–288 RSC.
- J. M. Meruga, W. M. Cross, P. Stanley May, Q. Luu, G. A. Crawford and J. J. Kellar, Nanotechnology, 2012, 23, 395201 CrossRef PubMed.
- (a) H. Sun, S. Liu, W. Lin, K. Y. Zhang, W. Lv, X. Huang, F. Huo, H. Yang, G. Jenkins, Q. Zhao and W. Huang, Nat. Commun., 2014, 5, 3601 Search PubMed; (b) H. Sasabe, J. Takamatsu, T. Motoyama, S. Watanabe, G. Wagenblast, N. Langer, O. Molt, E. Fuchs, C. Lennartz and J. Kido, Adv. Mater., 2010, 22, 5003–5007 CrossRef CAS PubMed; (c) M. Mydlak, C. Bizzarri, D. Hartmann, W. Sarfert, G. Schmid and L. De Cola, Adv. Funct. Mater., 2010, 20, 1812–1820 CrossRef CAS.
- Z. Chi, X. Zhang, B. Xu, X. Zhou, C. Ma, Y. Zhang, S. Liu and J. Xu, Chem. Soc. Rev., 2012, 41, 3878–3896 RSC.
- (a) M. Luo, X. Zhou, Z. Chi, S. Liu, Y. Zhang and J. Xu, Dyes Pigm., 2014, 101, 74–84 CrossRef CAS; (b) Q. Lu, X. Li, J. Li, Z. Yang, B. Xu, Z. Chi, J. Xu and Y. Zhang, J. Mater. Chem. C, 2015, 3, 1225–1234 RSC; (c) X. Zhang, Z. Ma, Y. Yang, X. Zhang, Z. Chi, S. Liu, J. Xu, X. Jia and Y. Wei, Tetrahedron, 2014, 70, 924–929 CrossRef CAS.
- (a) A. Pucci, F. Di Cuia, F. Signori and G. Ruggeri, J. Mater. Chem., 2007, 17, 783–790 RSC; (b) Y. Dong, J. W. Y. Lam, A. Qin, J. Liu, Z. Li, B. Z. Tang, J. Sun and H. S. Kwok, Appl. Phys. Lett., 2007, 91, 011111 CrossRef; (c) Z. Ning, Z. Chen, Q. Zhang, Y. Yan, S. Qian, Y. Cao and H. Tian, Adv. Funct. Mater., 2007, 17, 3799–3807 CrossRef CAS; (d) S. Hirata and T. Watanabe, Adv. Mater., 2006, 18, 2725–2729 CrossRef CAS; (e) A. Kishimura, T. Yamashita, K. Yamaguchi and T. Aida, Nat. Mater., 2005, 4, 546–549 CrossRef CAS PubMed.
- C. Ma, B. Xu, G. Xie, J. He, X. Zhou, B. Peng, L. Jiang, B. Xu, W. Tian, Z. Chi, S. Liu, Y. Zhang and J. Xu, Chem. Commun., 2014, 50, 7374–7377 RSC.
- Z. Zhang, Z. Wu, J. Sun, P. Xue and R. Lu, RSC Adv., 2016, 6, 43755–43766 RSC.
- J. Luo, Z. Xie, J. W. Y. Lam, L. Cheng, B. Z. Tang, H. Chen, C. Qiu, H. S. Kwok, X. Zhan, Y. Liu and D. Zhu, Chem. Commun., 2001, 1740–1741 RSC.
- J. Mei, N. L. Leung, R. T. Kwok, J. W. Lam and B. Z. Tang, Chem. Rev., 2015, 115, 11718–11940 CrossRef CAS PubMed.
- J. Tong, Y. J. Wang, Z. Wang, J. Z. Sun and B. Z. Tang, J. Phys. Chem. C, 2015, 119, 21875–21881 CAS.
- (a) Y. Dong, B. Xu, Y. J. Zhang, X. Tian, L. Wang, J. Chen, H. Lv, S. Wen, B. Li, L. Ye, B. Zou and W. Tian, Angew. Chem., Int. Ed., 2012, 51, 10782–10785 CrossRef CAS PubMed; (b) Q. Qi, J. Qian, X. Tan, L. Wang, B. Xu, B. Zou and W. Tian, Adv. Funct. Mater., 2015, 25, 4005–4010 CrossRef CAS.
- Y. Han, H. T. Cao, H. Z. Sun, Y. Wu, G. G. Shan, Z. M. Su, X. G. Hou and Y. Liao, J. Mater. Chem. C, 2014, 2, 7648–7655 RSC.
- R. K. Shah, K. S. Abou-Melha, F. A. Saad, T. Yousef, G. A. A. Al-Hazmi, M. G. Elghalban, A. M. Khedr and N. El-Metwaly, J. Therm. Anal. Calorim., 2015, 123, 731–743 CrossRef.
- B. Xu, W. Li, J. He, S. Wu, Q. Zhu, Z. Yang, Y.-C. Wu, Y. Zhang, C. Jin, P.-Y. Lu, Z. Chi, S. Liu, J. Xu and M. R. Bryce, Chem. Sci., 2016, 7, 5307–5312 RSC.
- G. G. Shan, H. B. Li, J. S. Qin, D. X. Zhu, Y. Liao and Z. M. Su, Dalton Trans., 2012, 41, 9590–9593 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, 1H NMR spectra, absorption and emission spectra, and crystallographic data. CCDC 1527325. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7cc00769h |
| This journal is © The Royal Society of Chemistry 2017 |