Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis, structure, and reactivity of crystalline molecular complexes of the {[C5H3(SiMe3)2]3Th}1− anion containing thorium in the formal +2 oxidation state†
Ryan R.
Langeslay
,
Megan E.
Fieser
,
Joseph W.
Ziller
,
Filipp
Furche
* and
William J.
Evans
*
Department of Chemistry, University of California, Irvine, California 92697-2025, USA. E-mail: wevans@uci.edu; filipp.furche@uci.edu; Fax: +1-949-824-2210; Tel: +1-949-824-5174
First published on 3rd November 2014
Abstract
Reduction of the Th3+ complex Cp′′3Th, 1 [Cp′′ = C5H3(SiMe3)2], with potassium graphite in THF in the presence of 2.2.2-cryptand generates [K(2.2.2-cryptand)][Cp′′3Th], 2, a complex containing thorium in the formal +2 oxidation state. Reaction of 1 with KC8 in the presence of 18-crown-6 generates the analogous Th2+ compound, [K(18-crown-6)(THF)2][Cp′′3Th], 3. Complexes 2 and 3 form dark green solutions in THF with ε = 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 cm−1, but crystallize as dichroic dark blue/red crystals. X-ray crystallography revealed that the anions in 2 and 3 have trigonal planar coordination geometries, with 2.521 and 2.525 Å Th–(Cp′′ ring centroid) distances, respectively, equivalent to the 2.520 Å distance measured in 1. Density functional theory analysis of (Cp′′3Th)1− is consistent with a 6d2 ground state, the first example of this transition metal electron configuration. Complex 3 reacts as a two-electron reductant with cyclooctatetraene to make Cp′′2Th(C8H8), 4, and [K(18-crown-6)]Cp′′.
000 M−1 cm−1, but crystallize as dichroic dark blue/red crystals. X-ray crystallography revealed that the anions in 2 and 3 have trigonal planar coordination geometries, with 2.521 and 2.525 Å Th–(Cp′′ ring centroid) distances, respectively, equivalent to the 2.520 Å distance measured in 1. Density functional theory analysis of (Cp′′3Th)1− is consistent with a 6d2 ground state, the first example of this transition metal electron configuration. Complex 3 reacts as a two-electron reductant with cyclooctatetraene to make Cp′′2Th(C8H8), 4, and [K(18-crown-6)]Cp′′.
One of the fundamental characteristics of any metal is the extent to which it loses electrons to form charged species in different formal oxidation states. This ionization can occur in the gas phase to form short-lived species in a wide range of oxidation states, but the number of oxidation states available in solution in molecular metal complexes for productive chemistry is smaller. Chemists have tested the limits of oxidation states of all the elements for over 100 years and the boundaries of oxidation states accessible in solution are well established.
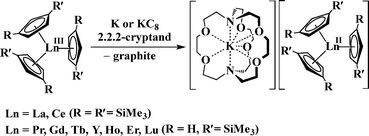
Nevertheless, it was recently discovered that the +2 oxidation state is accessible in soluble molecular complexes for all the elements in the lanthanide series except promethium, eqn (1).1 Previously, it was thought that only the traditional six Ln2+ ions of Eu, Yb, Sm, Tm, Dy, and Nd were obtainable in solution on the basis of calculated reduction potentials2 and solid state chemistry.3
 | (1) |
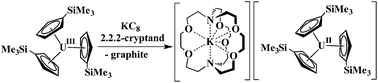
Extension of this reductive chemistry to uranium was not initially tried since it is well known that the redox chemistry of uranium, which includes multiple oxidation states, +3, +4, +5, and +6, is quite different from that of the rare earths. Although it was likely that uranium would be different, an analogous synthesis was eventually attempted and the first fully characterizable U2+ complex, [K(2.2.2-cryptand)][Cp′3U] (Cp′ = C5H4SiMe3), was isolated according to eqn (2).4
 | (2) |
Synthesis of a Th2+ complex viaeqn (1) or (2) seemed even more unlikely for several reasons. Complexes of Th3+ are already difficult to obtain. The Th4+/Th3+ redox potential is estimated to be −3.0 and −3.8 V vs. NHE5 and a Th3+/Th2+ redox potential of −4.9 V vs. NHE is in the literature.6 Reduction to metallic thorium would be predicted to be favored before formation of a Th2+ species.6 Many studies have been reported to find oxidation states lower than +4 for thorium,7 but only five Th3+ complexes have ever been structurally characterized.7k–o An analog of eqn (2) was not possible since Cp′3Th has not yet been synthesized. Despite these issues, thorium reduction chemistry was examined using Cp′′3Th [Cp′′ = C5H3(SiMe3)2-1,3],7k prepared by Lappert et al. in 1986, and the results are described here.
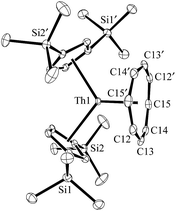
Addition of potassium graphite to a dark blue solution of Cp′′3Th, 1, and 2.2.2-cryptand in THF immediately forms a green solution from which dichroic dark blue/red crystals of [K(2.2.2-cryptand)][Cp′′3Th], 2, can be isolated and crystallographically characterized, Fig. 1, eqn (3). The analogous reaction with 18-crown-6 instead of 2.2.2-cryptand as the potassium chelator provides [K(18-crown-6)(THF)2][Cp′′3Th], 3, which was also crystallographically characterized [see (ESI†)], eqn (3). Elemental analysis was consistent with the structures determined crystallographically. The 1H and 13C NMR spectra of 2 and 3 gave resonances in the diamagnetic region with a Me3Si 1H NMR resonance shifted about 0.4 ppm from that of KCp′′. A resonance was observed in the 29Si NMR spectrum of 3 at −6 ppm in the region close to the −8 and −15.5 ppm signals of diamagnetic Cp′′3ThBr and KCp′′, respectively. Evans method measurements8 on both 2 and 3 and SQUID analysis9 at low temperature suggest the [Cp′′3Th]1− anion is diamagnetic. No EPR spectra were observed for 2 and 3. Decomposed samples showed the EPR spectrum of Cp′′3Th.7m
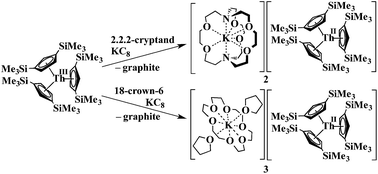 | (3) |
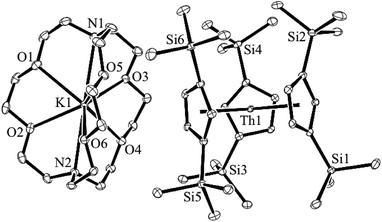 | ||
| Fig. 1 Molecular structure of [K(2.2.2-cryptand)][Cp′′3Th], 2. Thermal ellipsoids are drawn at the 50% probability level and hydrogen atoms are omitted for clarity. | ||
The structures of the anions in 2 and 3 are very similar to the structure of Cp′′3Th. All three structures have a trigonal planar arrangement of the three Cp′′ rings around thorium with a sum of (ring centroid)–Th–(ring centroid) angles of 360°. The structure of 2, however, is not isomorphous with the lanthanum complex of the same formula, [K(2.2.2-cryptand)][Cp′′3La].1a The average Th–(Cp′′ ring centroid) distances of 2.521 Å in 2 and 2.525 Å in 3 are equivalent to the 2.520 Å distance in Cp′′3Th. The negligible differences in the Th–(ring centroid) distances between the Th3+ precursor and the formally Th2+ complexes 2 and 3 are similar to the small differences between the Cp′3Ln and Cp′′3Ln Ln3+ complexes and the (Cp′3Ln)1− and (Cp′′3Ln)1− complexes, respectively, of all the new Ln2+ ions that have 4fn5d1 ground states1 instead of the 4fn+1 configurations expected by reduction of a 4fn Ln3+ ion. Similarly, the 2.521 Å U–(ring centroid) distance in the U2+ complex, [K(2.2.2-cryptand)][Cp′3U], which appears to have a 5f36d1 ground state, is only slightly larger than the 2.508 Å value in the U3+ analog, Cp′3U.4 These small changes in M–(ring centroid) distances match the small changes in radial size commonly seen in transition metal complexes,10 but contrast with the 0.10–0.20 Å differences generally seen for complexes of 4fn+1 Ln2+ complexes compared to their 4fn Ln3+ counterparts.11
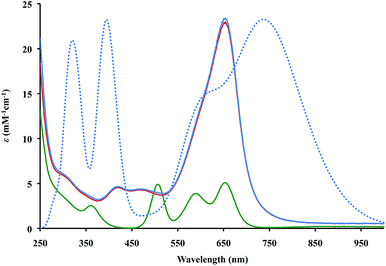
The UV-Vis spectra of 2 and 3 in THF, Fig. 2, contain absorptions at 650 nm with extinction coefficients of 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 cm−1, that are significantly larger than those of Cp′′3Th, 5000 M−1 cm−1. This is similar to the larger intensities observed for the +2 complexes, [K(2.2.2-cryptand)][Cp′3Ln]1c,d and [K(2.2.2-cryptand)][Cp′3U],4 compared to their +3 analogs, Cp′3Ln and Cp′3U, respectively. However, the absorptions of the Th2+ complexes are even more intense and the solutions look like ink.
000 M−1 cm−1, that are significantly larger than those of Cp′′3Th, 5000 M−1 cm−1. This is similar to the larger intensities observed for the +2 complexes, [K(2.2.2-cryptand)][Cp′3Ln]1c,d and [K(2.2.2-cryptand)][Cp′3U],4 compared to their +3 analogs, Cp′3Ln and Cp′3U, respectively. However, the absorptions of the Th2+ complexes are even more intense and the solutions look like ink.
Density functional theory (DFT) using the TPSSh functional12 was used to examine the (Cp′′3Th)1− anion in 2 and 3. Calculations using scalar-relativistic effective core potentials13 and triple-zeta valence basis sets, def-TZVP, for thorium14 predicted trigonal planar structures for Cp′′3Th and (Cp′′3Th)1− that match the crystallographic data. The calculated Th–Cp′′(centroid) lengths of 2.538 Å for Cp′′3Th and 2.526 Å for (Cp′′3Th)1− are similar to the experimentally determined distances of 2.52 Å. It is interesting to note that the calculations for the Th3+ complex show a slightly longer metal ligand distance than for the Th2+ complex. The calculations indicate a spin-paired ground state of 6d2 for (Cp′′3Th)1− and a 6d1 ground state for Cp′′3Th; the latter is consistent with previous analyses of Cp′′3Th,7g,m (C5Me5)2[iPrNC(Me)NiPr]Th7n and [K(DME)2]{[C8H6(SitBuMe2)2]2Th}.7l Gas-phase studies of Th2+ indicate a ground state of 5f16d1, but the 6d2 configuration is just 63 cm−1 higher and the 5f17s1 is 2527 cm−1 higher than the ground state.15 For (Cp3Th)1− the triplet 5f16d1 state is computed to be 9–14 kcal mol−1 higher in energy than the singlet 6d2 ground state.
The 6d2 singlet ground state can arise in this case due to stabilization of a dz2 orbital by the trigonal ligand environment as found in DFT calculations on (Cp′3Ln)1− and (Cp′3U)1− complexes1c,d,4 and noted earlier in the literature for tris(cyclopentadienyl) metal complexes.7g,m,16 Indeed, both the lowest unoccupied molecular orbital (LUMO) of Cp′′3Th and the highest occupied molecular orbital (HOMO) of (Cp′′3Th)1− have dz2 character, Fig. 3. Complexes 2 and 3 provide the first examples of the 6d2 configuration since stable transition metal ions are only known with the 5dn configurations of the third row transition metals. The 6d2 configuration is that predicted for ions like Rf2+ and Db3+.17
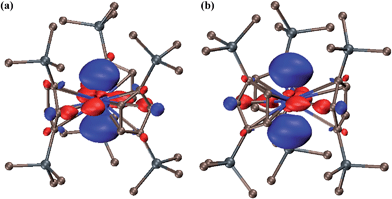 | ||
| Fig. 3 Contour plots of (a) the LUMO of Cp′′3Th and (b) the HOMO of the (Cp′′3Th)1− anion in 3. Contour value is 0.05. | ||
Time-dependent density functional theory was used to simulate the UV-Vis spectra for the (Cp′′3Th)1− anion as shown in Fig. 2 (see ESI† for a description of the predicted excitations). The maxima in the calculated spectra are lower in energy than those observed experimentally, but this is often the case with such calculations.18 Analysis of the calculated low energy peak shows that it arises from metal-to-metal transitions that have d → f and d → p character. The high energy peaks arise from metal-to-ligand charge transfer transitions similar to those found in the spectral analysis of (Cp′3Ln)1−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1b–d and (Cp′3U)1−.4 However, the d → f transitions found for (Cp′′3Th)1− were not apparent in the analysis of the spectra of (Cp′3Ln)1b–d and (Cp′3U)1−.4
1b–d and (Cp′3U)1−.4 However, the d → f transitions found for (Cp′′3Th)1− were not apparent in the analysis of the spectra of (Cp′3Ln)1b–d and (Cp′3U)1−.4
The rate of decomposition of [K(18-crown-6)(THF)2][Cp′′3Th], 3, at room temperature was studied by 1H NMR spectroscopy since monitoring by UV-Vis spectroscopy is complicated by the formation of highly colored Cp′′3Th, as identified by X-ray crystallography.7k The rate of decomposition of 3 is much slower than that of the U2+ complex, [K(2.2.2-cryptand)][Cp′3U], which has a half-life of 1.5 h in THF at room temperature.4 Complex 3 decomposed only 8% after 8 days at 298 K and a sample kept in the dark showed even less decomposition. This suggests that the formally Th2+ species are significantly more stable than the other newly discovered +2 ions.1d,4
Complexes 2 and 3 were treated with H2 to determine if a Th3+ hydride complex such as “[K(2.2.2-cryptand)][Cp′′3ThH]” would form in analogy to the complex formed by reaction of [K(2.2.2-cryptand)][Cp′3U] with H2.4 Analogous chemistry is not observed with either H2 or KH. Complexes 2 and 3 react in solution within minutes with 1 atm of H2 and also over several hours at 60 psi in the solid state19 to make EPR active new crystalline complexes that appear to be bimetallic, but suitable models for the crystallographic data on the products have not been obtainable. The reactivity of 2 and 3 with H2 contrasts with that of the Th3+ complex, Cp′′3Th, which does not react under analogous conditions.
The (Cp′′3Th)1− anion displays net two-electron reduction chemistry in its reaction with 1,3,5,7-cyclooctatetraene (C8H8). The Th4+ complex Cp′′2Th(C8H8), 4, is formed as shown in eqn (4) and was characterized by X-ray crystallography, Fig. 4. The (C8H8)2− ring in 4, like that of (C5Me4H)2U(C8H8),20 displays considerable distortion from the normal planar geometry with several atoms 0.095 Å out of the best plane of the eight carbon atoms. This is reflected by a large range of Th–C(C8H8) distances: 2.736(4) to 2.841(4) Å. This 0.105 Å range is similar to the 0.123 Å range in (C5Me4H)2U(C8H8).20
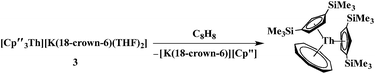 | (4) |
The isolation of the formally Th2+ ion in (Cp′′3Th)1− is likely aided by the stabilization of the potassium counter-cation by the 18-crown-6 and 2.2.2-cryptand ligands. This was also observed with U2+ in the (Cp′3U)1− anion4 and in the (Cp′3Ln)1− complexes of the new Ln2+ ions.1 In the absence of these potassium-stabilizing chelates, isolation of Th2+ appears to be more difficult as described in a 2001 paper by Lappert and co-workers on the formation of Cp′′3Th by Na–K reduction of Cp′′3ThCl.7m In that paper, Lappert reports that treatment of Cp′′3ThCl with excess Na–K alloy caused the initially blue solution (presumably Cp′′3Th) to change to dark green. They isolated a diamagnetic green compound they postulated to be “[K(THF)x][ThCp′′3] and/or ThCp′′2(THF)x” but they could not characterize it or obtain reproducible analytical results. Hence, the (Cp′′3Th)1− anion was probably generated over 10 years ago, but could not be isolated in pure form as a simple [K(THF)x]1+ salt.
In summary, although it is difficult to obtain Th3+ complexes, further reduction is still possible with thorium: the +2 formal oxidation state of this metal is accessible in soluble molecular complexes. The Th2+ complexes provide the first examples of an isolable ion with a 6d2 electron configuration, the configuration possible for fourth row transition metal congeners of Hf2+ or Ta3+. The synthesis of these complexes demonstrates the power of specific ligand fields to generate new ground states with actinides. The identification of Th2+ is more evidence that the oxidation state diversity for the f elements is still increasing. Stabilization of higher-lying d orbitals by the ligand field appears to be a key factor in isolating these new ions and provides a new option in expanding the oxidation state chemistry of these elements. This approach should be pursued further as attempts are made to synthesize soluble molecular complexes of +1 ions of these metals.
Acknowledgements
We thank the Chemical Sciences, Geosciences, and Biosciences Division of the Office of Basic Energy Sciences of the Department of Energy (DE-SC0004739, W.J.E.) for support of the experimental studies and the U.S. National Science Foundation (CHE-1213382, F.F.) for support of the theoretical studies. We also thank Jordan F. Corbey for assistance with X-ray crystallography and K. R. Meihaus and J. R. Long for the SQUID measurements under NSF grant CHE-111190.Notes and references
- (a) P. B. Hitchcock, M. F. Lappert, L. Maron and A. V. Protchenko, Angew. Chem., Int. Ed., 2008, 47, 1488 CrossRef CAS PubMed; (b) M. R. MacDonald, J. W. Ziller and W. J. Evans, J. Am. Chem. Soc., 2011, 133, 15914 CrossRef CAS PubMed; (c) M. R. MacDonald, J. E. Bates, M. E. Fieser, J. W. Ziller, F. Furche and W. J. Evans, J. Am. Chem. Soc., 2012, 134, 8420 CrossRef CAS PubMed; (d) M. R. MacDonald, J. E. Bates, J. W. Ziller, F. Furche and W. J. Evans, J. Am. Chem. Soc., 2013, 135, 9857 CrossRef CAS PubMed.
- L. R. Morss, Chem. Rev., 1976, 76, 827 CrossRef CAS.
- (a) G. Meyer, Chem. Rev., 1988, 88, 93 CrossRef CAS; (b) G. Meyer and H. Meyer, J. Chem. Mater., 1992, 4, 1157 CrossRef CAS; (c) G. Meyer and M. S. Wickleder, in Handbook on the Physics and Chemistry of Rare Earths, Elsevier Science B. V., Amsterdam, 2000, vol. 28 Search PubMed; (d) G. Meyer, in The Rare Earth Elements, ed. D. A. Atwood, Wiley, 2012 Search PubMed.
- M. R. MacDonald, M. E. Fieser, J. E. Bates, J. W. Ziller, F. Furche and W. J. Evans, J. Am. Chem. Soc., 2013, 135, 13310 CrossRef CAS PubMed.
- (a) S. G. Bratsch and J. J. Lagowski, J. Phys. Chem., 1986, 90, 307 CrossRef CAS; (b) G. Ionova, C. Madic and R. Guillaumont, Polyhedron, 1998, 17, 1991 CrossRef CAS; (c) L. J. Nugent, R. D. Baybarz, J. L. Burnett and J. L. Ryan, J. Phys. Chem., 1973, 77, 1528 CrossRef CAS.
- R. J. M. Konings, L. R. Morss and J. Fuger, in The Chemistry of the Actinide and Transactinide Elements, ed. L. R. Morss, N. M. Edelstein and J. Fuger, Springer, The Netherlands, 4th edn, 2010 Search PubMed.
- (a) R. J. Clark and J. D. Corbett, Inorg. Chem., 1963, 2, 460 CrossRef CAS; (b) D. E. Scaife and A. W. Wylie, J. Chem. Soc., 1964, 5450 RSC; (c) L. J. Guggenberger and R. A. Jacobson, Inorg. Chem., 1968, 7, 2257 CrossRef CAS; (d) B. Kanellakopulos, E. Dornberger and F. Baumgaertner, Inorg. Nucl. Chem. Lett., 1974, 10, 155 CrossRef CAS; (e) E. Dornberger, R. Klenze and B. Kanellakopulos, Inorg. Nucl. Chem. Lett., 1978, 14, 319 CrossRef; (f) J. W. Bruno, D. G. Kalina, E. A. Mintz and T. J. Marks, J. Am. Chem. Soc., 1982, 104, 1860 CrossRef CAS; (g) W. K. Kot, G. V. Shalimoff, N. M. Edelstein, M. A. Edelman and M. F. Lappert, J. Am. Chem. Soc., 1988, 110, 986 CrossRef CAS; (h) I. Korobkov, S. Gambarotta and G. P. A. Yap, Angew. Chem., Int. Ed., 2003, 42, 814 CrossRef CAS PubMed; (i) I. Korobkov, S. Gambarotta and G. P. A. Yap, Angew. Chem., Int. Ed., 2003, 42, 4958 CrossRef CAS PubMed; (j) A. Arunachalampillai, P. Crewdson, I. Korobkov and S. Gambarotta, Organometallics, 2006, 25, 3856 CrossRef CAS; (k) P. C. Blake, M. F. Lappert, J. L. Atwood and H. Zhang, J. Chem. Soc., Chem. Commun., 1986, 1148 RSC; (l) J. S. Parry, F. G. N. Cloke, S. J. Coles and M. B. Hursthouse, J. Am. Chem. Soc., 1999, 121, 6867 CrossRef CAS; (m) P. C. Blake, N. M. Edelstein, P. B. Hitchcock, W. K. Kot, M. F. Lappert, G. V. Shalimoff and S. Tian, J. Organomet. Chem., 2001, 636, 124 CrossRef CAS; (n) J. R. Walensky, R. L. Martin, J. W. Ziller and W. J. Evans, Inorg. Chem., 2010, 49, 10007 CrossRef CAS PubMed; (o) N. A. Siladke, C. L. Webster, J. R. Walensky, M. K. Takase, J. W. Ziller, D. J. Grant, L. Gagliardi and W. J. Evans, Organometallics, 2013, 32, 6522 CrossRef CAS.
- (a) D. F. Evans, J. Chem. Soc., 1959, 2003 RSC; (b) J. K. Becconsall, Mol. Phys., 1968, 15, 129 CrossRef.
- K. R. Meihaus and J. R. Long, Unpublished results, Univ. of California, Berkeley.
- For example, Cp2TiCl2: (a) A. Clearfield, D. K. Warner, C. H. Saldarriaga-Molina, R. Ropal and I. Bernal, Can. J. Chem., 1975, 53, 1622 CrossRef CAS PubMed and [Cp2Ti(μ-Cl)]2: (b) R. Jungst, D. Sekutowski, J. Davis, M. Luly and G. Stucky, Inorg. Chem., 1977, 16, 1645 CrossRef CAS have average C(η5-Cp) bond lengths of 2.370 and 2.350 Å, respectively. Similarly, Cp4Zr: (c) R. D. Rogers, R. V. Bynum and J. L. Atwood, J. Am. Chem. Soc., 1978, 100, 5238 CrossRef CAS and Cp3Zr: (d) W. L. Lukens Jr and R. A. Andersen, Organometallics, 1995, 14, 3435 CrossRef have the same average Zr–C(η5-Cp) bond length of 2.58 Å.
- (a) M. N. Bochkarev, Coord. Chem. Rev., 2004, 248, 835 CrossRef CAS PubMed; (b) W. J. Evans, N. T. Allen and J. W. Ziller, J. Am. Chem. Soc., 2000, 122, 11749 CrossRef CAS; (c) M. N. Bochkarev, I. L. Fedushkin, S. Dechert, A. A. Fagin and H. Schumann, Angew. Chem., Int. Ed., 2001, 40, 3176 CrossRef CAS; (d) W. J. Evans and S. E. Foster, J. Organomet. Chem., 1992, 433, 79 CrossRef CAS; (e) Y. K. Gun'ko, P. B. Hitchcock and M. F. Lappert, Chem. Commun., 1998, 1843 RSC; (f) L. Huebner, A. Kornienko, T. J. Emge and J. G. Brennan, Inorg. Chem., 2004, 43, 5659 CrossRef CAS PubMed; (g) R. D. Shannon, Acta Crystallogr., Sect. A: Cryst. Phys., Diffr., Theor. Gen. Crystallogr., 1976, 32, 751 CrossRef.
- V. N. Staroverov, G. E. Scuseria, J. Tao and J. P. Perdew, J. Chem. Phys., 2003, 119, 12129 CrossRef CAS PubMed.
- W. Küchle, M. Dolg, H. Stoll and H. Preuss, J. Chem. Phys., 1994, 100, 7535 CrossRef PubMed.
- X. Cao and M. Dolg, J. Mol. Struct., 2004, 673, 203 CrossRef CAS PubMed.
- W. S. Wickleder, B. Fourest and P. K. Dorhout, in The Chemistry of the Actinide and Transactinide Elements, ed. L. R. Morss, N. M. Edelstein and J. Fuger, Springer, The Netherlands, 4th edn, 2010 Search PubMed.
- (a) R. J. Strittmatter and B. E. Bursten, J. Am. Chem. Soc., 1991, 113, 552 CrossRef CAS; (b) B. E. Bursten and R. J. Strittmatter, J. Am. Chem. Soc., 1987, 109, 6606 CrossRef CAS; (c) B. E. Bursten, L. F. Rhodes and R. J. Strittmatter, J. Am. Chem. Soc., 1989, 111, 2758 CrossRef CAS; (d) J. W. Lauher and R. Hoffmann, J. Am. Chem. Soc., 1976, 98, 1729 CrossRef CAS; (e) W. W. Lukens Jr and R. A. Andersen, Organometallics, 1995, 14, 3435 CrossRef; (f) R. G. Denning, J. Harmer, J. C. Green and M. Irwin, J. Am. Chem. Soc., 2011, 133, 20644 CrossRef CAS PubMed.
- P. Pyykkö, Phys. Chem. Chem. Phys., 2011, 13, 161 RSC.
- R. Send, M. Kühn and F. Furche, J. Chem. Theory Comput., 2011, 7, 2376 CrossRef CAS.
- C. L. Webster, J. W. Ziller and W. J. Evans, Organometallics, 2013, 33, 433 CrossRef.
- M. K. Takase, J. W. Ziller and W. J. Evans, Chem.–Eur. J., 2011, 17, 4871 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental and computational details; crystallographic data collection, structure solution, and refinement; and crystallographic data and complete bond distances and angles for compounds 1–4. CCDC 1018011–1018014. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4sc03033h |
| This journal is © The Royal Society of Chemistry 2015 |