Use of herbs, spices and their bioactive compounds in active food packaging
A. Valdés
,
A. C. Mellinas
,
M. Ramos
,
N. Burgos
,
A. Jiménez
and
M. C. Garrigós
*
University of Alicante, Dpt. Analytical Chemistry, Nutrition & Food Sciences, 03690, San Vicente del Raspeig, Spain. E-mail: mc.garrigos@ua.es
First published on 21st April 2015
Abstract
The interest and societal demand on the use of natural, biodegradable and renewable resources has increased in the last few years. In addition, food producers and consumers have improved their requirements for the quality of processed food, particularly in the field of increasing shelf-life while preserving organoleptic and nutritional properties. Active packaging technologies have greatly developed in the last decade by trying to satisfy the need for long-life processed food in addition to antioxidant/antimicrobial components in the packaging material. These components are intended to be released in a controlled way to food. These rising trends have been reflected in the field of food packaging by the use of chemicals extracted and obtained from plants in active packaging formulations. Herbs and spices have shown great potential to be used as renewable, biodegradable and valuable sources of chemicals, such as polyphenols, with high antioxidant/antimicrobial performance. This review aims to present the latest published work in this area.
1. Introduction
It is well known that antioxidants are present in many plants as a defence towards oxidative degradation coming from the environment. These bio-based and natural antioxidants have improved their use in food-related applications through their positive contributions to human health. Many plants have been considered as potential antioxidant sources and some of them have been studied in recent years. In this context, numerous herbs and spices are the most important and studied targets in the search for natural antioxidants.1 The terms ‘herbs’ and ‘spices’ have more than one definition in common languages, but the most commonly used are those which consider herbs to be obtained from the green parts of plants, such as the stem and leaves, while spices are usually obtained from the bark, roots, seeds or flowers. Most of the common spices are usually extracted from plants grown in warm or tropical climates while herbal plants can be grown in nearly all conditions and geographical areas around the world. In addition, spices have a more intense taste and are used in small amounts.2 Based on data reported by the Food and Agriculture Organization of the United Nations (FAO), the worldwide spice production has increased significantly in the last few years3 whereas the global production of herbs has not been reported in the literature.Since ancient times, herbs and spices have been extensively used to enhance or improve the flavour of food and by their preservative properties.4 Several popular herbs and spices are known to have beneficial effects for human health, including digestive stimulant, anti-inflammatory, antimicrobial, antioxidant and anti-carcinogenic activities, which are mostly attributed to polyphenols present in their chemical composition. Moreover, other volatile constituents in herbs and spices (particularly essential oils) can also contribute to the plant's biological activity, resulting in benefits for human health. In general terms, essential oils are chemically constituted by more than 70 different compounds, mostly polyphenols, terpenes, monoterpenes and sesquiterpenes, some of them representing more than 85% of the total oil content.5 Essential oils and their components are gaining attention by their wide acceptance by consumers and the possibility of exploitation for potential multi-purpose functional uses.
Phytochemicals extracted from various herbs and spices, including polyphenols, flavonoids, flavonols, tannins and terpenoids, could help to minimise tumour development by inhibiting the initiation and promotion of carcinogenesis, inducing tumour cell differentiation and apoptosis, while suppressing tumour angiogenesis. They are also able to prevent and reverse many of the processes that underlie chronic diseases. These phytochemicals can be broadly classified as carotenoids, phenolics, alkaloids, nitrogen-containing compounds and organosulfur compounds.6 Some studies have been recently reported to state the presence and activity of antioxidants in herbs, to determine their total phenolic contents and to relate the total antioxidant and antimicrobial activities of methanolic extracts, infusions and decoctions of several plants; such as peppermint, thyme, lemon balm, basil, rosemary and sage.6,7
On the other hand, antioxidants present in spices have gained importance by their role in the prevention of atherogenesis. Although spices have been recognized by their beneficial health effects and have been used in traditional medicine since ancient times, their attributes in health protection have been experimentally verified only in the last three decades. In this sense, the antioxidant effects of turmeric/curcumin, clove/eugenol, onion, garlic, ginger/gingerol, red pepper/capsaicin, black pepper/piperine and fenugreek, have been recently reviewed.8
Beneficial effects of chemicals obtained from herbs and spices can be used not only directly into food but also in packaging materials to increase food shelf-life and quality. In recent years, the increase in consumer's demand for minimally processed foods, the change in retail and distribution practices associated with global markets, new product logistics and distribution trends (such as on-line shopping), automatic handling systems and strict requirements regarding consumer's health and safety have been the major driving force for innovation in food packaging technologies.9 In this context, packaging of fat food is a particular issue, since lipid oxidation after microbial growth is the main cause of food spoilage.10 In particular, food with high content in poly-unsaturated fatty acids is more prone to oxidative deterioration resulting in the development of off-flavours, such as toxic aldehydes, and rendering products unacceptable for human consumption by their loss in nutritional quality.11 The use of active packaging systems with antioxidant activity is a promising approach to improve food quality by the incorporation of antioxidant agents to the packaging material increasing the stability of oxidation-sensitive food. However, the use of synthetic antioxidants in these active systems is questioned due to potential toxicological risks, resulting in requirements for strict controls. The alternative based on the use of natural antioxidants, particularly polyphenols, extracted from plants and essential oils from herbs and spices12 and from agricultural by-products13 is one of the current trending topics in food packaging research. In this sense, natural extracts have been incorporated into plastic packaging materials in order to achieve antioxidant properties. For instance, Li et al.14 developed active gelatin films containing natural antioxidants extracted from different sources (green tea, grape seed polyphenols, grape seed proanthocyanidins, ginger and gingko leaf) for food packaging applications, showing different effects on the food packaged depending on the incorporated extract. Among them, the gingko leaf extract induced the highest radical-scavenging activity whereas the green tea extract resulted in increased protection against moisture. Quince seed mucilage films containing oregano essential oils were prepared and their antioxidant properties were evaluated by Jouki et al.15 These films exhibited high level of radical-scavenging activity (45, 57 and 61% for 1, 1.5 and 2 wt% of essential oils, respectively). In an interesting work recently reported by Castro et al.,16 good feasibility of catechins and quercetin released from green tea natural extracts incorporated into polypropylene films was observed.
Several different methods have been proposed to determine the antioxidant performance of active packaging systems. Some of them are based on measuring the degree of lipid oxidation of food in direct contact with packaging materials. Several parameters, such as peroxide index, conjugated dienes, conjugated triene hydroperoxides, free fatty acids, thiobarbituric acid index and p-anisidine value (AV) can be determined to assess lipid hydrolysis and primary and secondary lipid oxidation in fat food. In addition, the antioxidant activity provided by films for food packaging can be evaluated by measuring the radical scavenging ability in food simulants. Several procedures have been proposed, most of them based on producing and determining oxidative free-radicals, to evaluate the antioxidant properties of additives used in packaging materials. This is the case of oxygen radical absorbance capacities (ORAC), ferric reducing antioxidant power (FRAP), ABTS and DPPH tests.17
Some studies have been published on the antimicrobial performance of essential oils extracted from plants against food-borne pathogens. Since essential oils are rich in volatile terpenoids and phenolics, they show high potential to inhibit a wide spectrum of microorganisms. The active components of plant essential oils are able to inhibit microorganisms' proliferation through disturbance of the cytoplasmic membrane, disrupting the proton motive force, electron flow, active transport and inhibition of protein synthesis.18
Antimicrobial and/or antioxidant active packaging systems are getting attention from food and packaging industries due to the increasing consumer demands for minimally processed and preservative-free food products. Some plastic films allow the controlled release of additives onto food to cover the whole storage and distribution operations, while limiting undesirable effects in flavour and taste. A raising trend in the use of antimicrobial additives, such as those derived from essential oils, to minimise contamination in different types of food, such as meat, fruits and vegetables, has been reported and will be discussed in this review article. For instance, Ramos et al. reported the development of antimicrobial active films based on polypropylene by incorporating thymol and carvacrol at three different concentrations (4, 6 and 8 wt%) demonstrating their potential to be used as active additives.19 Pereira de Abreu et al.20 reported the potential use of films containing antioxidant extracts from barley husks for the commercialization of frozen cod fillets and the effect on lipid oxidation during prolonged frozen storage. This increasing interest in the use of natural extracts obtained from plants in active packaging technologies is the main reason of this review, where the current situation in the potential use of herbs, spices and their bioactive compounds in antioxidant and antimicrobial active food packaging systems is discussed.
2. Herbs
The antioxidant/antimicrobial properties of herbs have been known since ancient times and they have been used in many applications, such as traditional medicine, food preservation and others. But the raising trend for the use of active packaging in processed food has resulted in new applications of herbs and/or extracted chemicals in food technology. It is known that antimicrobial/antioxidant agents can be incorporated directly into the active packaging systems (either as aqueous or ethanolic extracts). Some examples are summarized in Table 1.| Herb extract | Polymer | Effect on food packaging | References |
|---|---|---|---|
| Ginseng | LDPE, PVC | Antimicrobial | 22 |
| Alginate-based | Antioxidant | 41 | |
| Rosemary | LDPE | Antioxidant | 23 |
| LDPE | Lipid oxidation | 24 and 25 | |
| PCL, methylcellulose | Antimicrobial | 42 | |
| Murta | Carboxymethylcellulose | Antioxidant/antimicrobial | 12, 43 and 44 |
| Green tea | Fish skin gelatin | Antioxidant | 30 |
| Silver carp skin gelatin | Antioxidant | 31 | |
| Multilayer PET/PE/EVOH/PE | Antioxidant/antimicrobial | 45 | |
| EVOH | Antioxidant | 46 | |
| Fish skin gelatin | Antioxidant | 30 | |
| Agar–fish gelatin | Antioxidant | 32 | |
| EVOH | Lipid oxidation | 47 | |
| Agar with glycerol | Antimicrobial | 33 | |
| Chitosan | Lipid oxidation | 35 and 36 | |
| Cassava starch with glycerol | Antioxidant | 48 | |
| Gingko leaf | Fish skin gelatin | Antioxidant | 30 |
| Mint | Chitosan and polyvinyl alcohol | Antioxidant/antimicrobial | 21 |
Among them, green tea has been considered for years as a good source of polyphenolic compounds. Catechins (also known as flavanols), including epicatechin, epigallocatechin, epicatechin gallate, epigallocatechin gallate, catechin, gallocatechin gallate, catechin gallate and gallocatechin, are the dominant phenolic compounds in tea leaves and most of them have been extracted from green tea leaves and other parts of the plant.21
Some commodity polymers such as low density polyethylene (LDPE),22–26 poly(vinyl chloride) (PVC)22 and polypropylene (PP)27–29 have been tested as adequate matrices to host different herb extracts. But in recent years biopolymers based on starch, cellulose derivatives, chitosan/chitin, gums, proteins obtained from animal or plant-sources and lipids have emerged as environmentally-friendly alternatives to synthetic polymers as carriers of herb extracts (Table 1). Biopolymers could be processed by wet methods using water and other solvents, avoiding the degradation and loss of volatile active compounds in the final material due to the severe thermo-mechanical conditions applied in conventional techniques for polymer processing (extrusion, injection, blow molding and heat pressing). These biomaterials show additional advantages, such as their biocompatibility, edibility, barrier to moisture and/or gases, non-toxicity, mechanical integrity and relatively low cost.12 Fish gelatin30–34 and chitosan35–40 have been widely used as biopolymer matrices by their own intrinsic antimicrobial activity getting synergies with natural additives.
Different methods have been proposed to evaluate the antioxidant effectiveness of active materials17 directly in food or food simulants. The antioxidant activity provided by polymer films can be evaluated by measuring the radical scavenging ability in food simulants, by using the DPPH (2,2-diphenyl-1-picrylhydrazyl) test.21,23–41,46,47 Other similar tests were based on the ferric reducing antioxidant power (FRAP)32 and the antioxidant activity evaluated by complexation of 2,2-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS).32–46
Other test methods are based on the measurement of the degree of lipid oxidation, particularly by the determination of the initial autoxidation products, i.e. hydroperoxides, which decompose to low molecular-weight compounds resulting in the development of rancid flavours and odours.49 Frequently, both the primary and secondary oxidation products are monitored by the peroxide and TBAR indices22–24,47,48 in shelf-life assays directly applied to food.
In a recent report, authors have not only incorporated aqueous extracts obtained from herbs to polymer matrices but also in the form of volatile essential oils (EOs), normally found in special cells or groups of cells present in leaves and stems, and commonly concentrated in leaves, bark or fruits.50 Some results in the use of EOs extracted from herbs are presented in Table 2.
| Essential oil | Polymer | Effect on food packaging | References |
|---|---|---|---|
| Zataria multiflora | Kappa-carrageenan (KC) | Antimicrobial | 56 and 57 |
| Soluble soybean polysaccharide | Antioxidant/antimicrobial | 58 | |
| Satureja hortensis | KC | Antioxidant/antimicrobial | 59 |
| Mentha pulegium | KC | Antioxidant/antimicrobial | 56 |
| Soluble soybean polysaccharide | Antioxidant/antimicrobial | 58 | |
| Oregano | Ethylene vinyl alcohol copolymer | Antioxidant/antimicrobial | 51 |
| PET | Antimicrobial | 27 | |
| Mucilage | Antioxidant/antimicrobial | 60 | |
| Thermoplastic starch with poly(butylene adipate-co-terephthalate) | Antioxidant/antimicrobial | 61 | |
| Chitosan | Antioxidant/antimicrobial | 54 | |
| PP | Antimicrobial | 27 | |
| Methylcellulose | Antimicrobial | 62 | |
| PP | Insect-repellent | 28 | |
| Polyvinyl alcohol (PVA) | Antimicrobial | 55 | |
| PP/EVOH | Antimicrobial | 52–63 | |
| Multilayer PET/PE/EVOH/PE | Antioxidant/antimicrobial | 45 | |
| Isolated soy protein | Antioxidant | 40 | |
| PP | Antifungal | 29 | |
| LDPE | Antimicrobial | 26 | |
| PLA-CNC | Antimicrobial | 53 | |
| Cellulose and protein isolate | Antimicrobial | 64 | |
| Green tea | Ethylene vinyl alcohol copolymer (EVOH) | Antioxidant/antimicrobial | 51 |
| Rosemary | PP | Insect-repellent | 28 |
| Cellulose acetate | Antimicrobial | 65 | |
| Chitosan | Antioxidant/antimicrobial | 37 and 38 | |
| Sage | Cellulose and protein isolate | Antimicrobial | 64 |
| Basil | Isolated fish skin gelatin | Antimicrobial | 34 |
| Citronella | PP | Insect-repellent | 28 |
| Thyme | Isolated soy protein | Antioxidant | 40 |
| Pectin | Antimicrobial | 66 | |
| Chitosan | Antimicrobial | 39 |
Oregano is one of the most studied plants by its high antioxidant and antimicrobial properties.40,51 Oregano is a popular culinary herb with high content in EOs containing terpenoids, mainly the monoterpenoid phenols of thymol(5-methyl-2-[1-methylethyl]phenol) and carvacrol(5-isopropyl-2-methyl phenol).50 One important feature of the EOs obtained from oregano is their high antimicrobial activity, which is usually determined by the agar plate diffusion method, where the inhibition on solid media was used to determine the antimicrobial effects of active films against different bacteria.26 Other studies have highlighted the antimicrobial activity of EOs directly on food, such as meat or fish.52,53 Oregano and other herbs have shown good antimicrobial performance depending on the type of food bacteria. For example, oregano EOs have been reported as effective against Gram-positive bacteria, such as Staphylococcus aureus, Bacillus cereus, Streptococcus mutans, L. monocytogenes and Enterococcus faecalis; as well as against Gram-negative bacteria, such as Escherichia coli and Salmonella enterica subsp.54,55 Results showed positive inhibition for EOs concentrations higher than 5% (w/w) for all bacteria.27,52–55
3. Spices
Spices have been traditionally used as food additives to improve their organoleptic characteristics as well as to extend shelf-life by the inhibition of pathogenic bacteria, yeast and moulds and the reduction of lipid oxidation, which is associated to the presence of terpenoids, flavonoids and phenolic compounds.67,68 Therefore, the use of spice extracts as active agents in food packaging has been suggested and studied since they provide antioxidant and antimicrobial properties to packaging materials69 acting as potential alternatives to food synthetic preservatives.70 The bioactive effects of the main spice extracts incorporated to food packaging films or coatings in the last few years are summarized in Table 3.| Spice extract | Polymer | Effect on food packaging | References |
|---|---|---|---|
| Cinnamon EO | Cellulose acetate | Alteration of microstructures and mechanical properties | 72 |
| PP | Sensory evaluation: increase in shelf-life from 3–10 days | 81 | |
| Cassava starch | Antimicrobial | 76 and 77 | |
| Self-adhesive PP active label inside a PET tray | Antioxidant, antifungal and inhibition of oxidative enzymes | 80 | |
| Chitosan | Antimicrobial | 82 | |
| PP coated with an organic-base formulation with EO | Antimicrobial, antifungal | 29 | |
| Cinnamon EO fortified | PP | Antifungal, antimycotoxigenic | 83 |
| Cinnamon EO microencapsulated | LDPE-PP | Insect-repelling agent to protect food from Indian meal moth (Plodia interpunctella) | 78 and 79 |
| Cinnamon EO nanoliposomes | Fish gelatin | Antimicrobial stability and decrease of release rate | 84 |
| Ginger extract | Fish skin gelatin/glycerol 30% (w/w) | Antioxidant, physical and mechanical changes | 34 |
| Turmeric oleoresin encapsulated | Gelatin–gum Arabic | Improve stability to light | 85 |
| Clove EO | Chicken feather protein/gelatin | Antimicrobial and antioxidant activity on smoked salmon | 86 |
| Fish protein | Antioxidant/antimicrobial | 74 | |
| Cassava bagasse/PVA/glycerol (trays) | Antimicrobial | 55 | |
| Sunflower protein | Antioxidant, antimicrobial and lipid oxidation on sardine patties | 87 | |
| Gelatin/chitosan | Antimicrobial on fish during chilled storage | 88 | |
| Clove EO (coarse and nanoemulsion) | Methylcellulose/PEG | Antimicrobial activity on sliced bread | 62 |
| Cinnamon and lavender (EOs) | NaAlg/glycerol | Antimicrobial, antifungal | 73 |
| Cinnamon and cumin (EOs) | Whey protein | Antimicrobial activity on fresh beef | 75 |
| Cinnamon, clove and ginger (EOs) | PP | Antioxidant | 89 |
| Cinnamon, clove and red pepper powders | Cassava starch | Antimicrobial effect on bread slices | 71 |
| Allspice, cinnamon and clove bud EOs | Edible apple films | Antimicrobial activity | 90 |
| Asian (nutmeg, lemongrass and citral) and Italian spice EOs (oregano and lemongrass) | Methylcellulose (MC), PCL/ALG | Antibacterial effect on pre-cut broccoli | 42 |
| PCL/MC (EO)/PCL | Antimicrobial activity on pre-cut broccoli | 91 |
Spice extracts have been usually incorporated as EOs into natural or synthetic polymers in food packaging applications. Some authors evaluated the direct incorporation of spice powders or their aqueous extracts into natural polymer matrices for food protective packaging, but no satisfactory results were reported up to now. For instance, Li et al.14 studied the effect of ginger extract (water soluble) into fish gelatins with no improvement in their antioxidant properties (measured by the DPPH radical scavenging method). In other study, Kechichian et al.71 evaluated the antimicrobial effect of cassava starch films with clove and cinnamon powders in contact with bread slices. They concluded that the antimicrobial effect of this combination of spices could not be clearly assessed since water activity of the bread slices increased considerably during storage, resulting in good conditions for microorganisms' proliferation.
As in the case of herbs, biopolymers such as starch, cellulose derivatives, chitosan, proteins or gums have been proposed as carrier matrices for spice extracts (Table 3). EOs from spices have been usually incorporated into polymer matrices by casting72–77 or surface coating.29,55,78,79 New active packaging materials consisting of self-adhesive PP labels with cinnamon EOs inside PET trays were used to extend the shelf-life in late-maturing peach fruits.80 Authors reported the high decrease in the number of infected fruits after 12 days at room temperature when the active label was used (13%), in comparison with the non-active packaged peaches (86%).
The extraction method selected to obtain EOs from spices plays a key role in their composition and final quality,92 modifying their antimicrobial activity.93 Nevertheless, the current use of EOs extracted from spices in food packaging is still limited by their volatility and low stability against oxygen and light during processing and storage. Microencapsulation technologies have emerged as a promising alternative to control the release of EOs onto food surfaces and to increase stability against environmental factors. Kim et al.78,79 developed laminated films based on PP and LDPE coated with a printing ink containing microencapsulated cinnamon EO to protect food from the Indian meal moth (Plodia interpunctella). Authors reported that these films effectively inhibited the invasion of moth larvae in cookies, milk, chocolate and caramel, acting as insect-resistant films. They observed that microencapsulation decreased the release rate of cinnamaldehyde EO and increased their thermal stability, with no effect on the tensile and moisture barrier properties of the active film. Zuanon et al.85 observed that the complex coacervation of turmeric oleoresin (a natural pigment) using gelatin and Arabic gum as wall materials was a feasible process to produce microcapsules with suitable colour, physical attributes and high curcumin retention. However, this process did not preserve the pigment under natural radiation.
Other strategies to incorporate EOs into water-soluble polymers to form antimicrobial films have been reported. Otoni et al.62 prepared coarse emulsions (1.3–1.9 μm diameter) and nanoemulsions (180–250 nm diameter) of clove bud EOs through low-speed mixing and ultrasonication, respectively, to be incorporated into methylcellulose matrices. They observed that droplet size reduction provided further improvement in antimicrobial properties against yeasts and moulds in sliced bread. In addition, low EOs contents might be used if encapsulated in smaller particles to keep antimicrobial efficiency. However, the EO emulsions reduced the rigidity and increased the extensibility of films and these effects were more pronounced for nanodroplets. Some increase in the antimicrobial stability with the decrease in cinnamon EOs release rate was observed for gelatin films incorporated with cinnamon EO nanoliposomes.84 In order to minimize organoleptic effects, Bentayeb et al.89 prepared films with higher thickness and low concentration of EOs.
EOs extracted from cinnamon and cloves were studied with successful incorporation into natural and synthetic polymers, providing bioactive properties to films (Table 3). Cinnamon oil is mainly composed of cinnamaldehyde (60.4%), with eugenol, linalool and 1,8-cineole as minor compounds.82 These EOs have been reported to show antimicrobial, antibacterial, antifungal, insect-repellent and antioxidant activities.69,94
In the case of clove EOs, the major component is eugenol, with eugenyl-acetate, β-caryophyllene and 2-heptanone as minor compounds, with variable proportions depending on the type and origin of the plant and the extraction method.95 Clove oil exhibited antimicrobial activity against moulds, yeasts and bacteria, and also antifungal and antioxidant properties when incorporated into natural polymers (Table 3).
The addition of spice extracts with antioxidant and/or antimicrobial properties into a polymer matrix could affect the physical, morphological, mechanical, thermal and gas barrier properties of the film. In this sense, significant decrease in tensile strength and elongation at break of films with spice extracts were reported.34,73,74,76 However, Wu et al.84 studied the incorporation of cinnamon EOs to fish gelatin resulting in more flexible films. On the other hand, some positive effects related with the decrease in water solubility and water vapour permeability of films were observed by some authors.74,76,84,87
4. Active compounds
As previously reported, essential oils have been thoroughly evaluated in their antimicrobial, antifungal, antiviral, insecticidal and antioxidant properties, but the most innovative approaches in this area come from the use of their bioactive compounds.12,69,96 For this reason, current trends in food packaging research include the development of active systems based on materials able to host a variety of bioactive compounds, extracted from herbs and spices.69,98 The addition of bioactive compounds with antioxidant/antimicrobial properties directly to food or packaging materials depends primarily on their activity against the targeted microorganisms and oxidative degradation, and their ability to be released at controlled rate during storage and distribution, resulting in extension of food shelf-life.99 Nevertheless, other factors, including specific activity of bioactive chemicals, resistance of microorganisms to their action, controlled release mechanisms, chemical nature of the packaged food, physical and mechanical properties of packaging materials, organoleptic properties and potential toxicity of additives should be considered to design packaging systems with bioactive compounds.17,69 The mechanical, gas barrier, thermal and morphological properties of films can be altered by the incorporation of bioactive additives. In consequence, the evaluation of these properties will help to understand the interaction of polymer matrices with additives as well as to set processing parameters for fabrication of bioactive films.Bastarrachea et al.100 reported a significant change in tensile properties of polymer films after the incorporation of some antimicrobials. Barrier properties were evaluated with different gases with significant effect of additives, mostly but not always negative. Authors discussed the addition of antimicrobials and their influence in permeability to gases by changing their solubility or due to the creation of pinholes in the packaging structure depending on the chemical structure of the antimicrobial agent.100 In addition, some modifications in crystallinity and glass transition temperatures were observed.19 Scanning electron microscopy studies helped to explain structural modifications induced by the incorporation of additives to polymer matrices, by detecting formation of pores, thereby influencing the tensile and gas barrier properties of the film.101
Diffusion of additive molecules through films has been also studied and mathematical models were proposed to describe the mass transfer of additive molecules and other substances through the polymer bulk to the surface. Most of these models were based on Fick's laws helping in the assessment of compliance with specific migration limits and describing the effective transport of migrating species with time.102 Modifications in diffusion coefficients of additive molecules through the polymer structure can be related with formation/rupture of hydrogen bonds, hydrophobic and/or electrostatic interactions. Some authors proposed kinetic models where high initial rates were observed with further observation of a plateau at different times and temperatures.103–105 This behaviour was attributed to hydrogen bonding effects between additives and polymer matrices and/or to tortuosity effects within polymer macromolecular structures.106
Many active packaging materials have been developed in the last few years with bioactive compounds extracted from spices and herbs. Typically, in antimicrobial and antioxidant packaging systems, additives are incorporated in different ways:107 (a) incorporation by melt-blending before extrusion where a masterbatch is obtained with further injection to obtain films; (b) solvent-compounding and solution coating to prevent the loss of volatile additives107 where solutions can be incorporated to the polymer matrix as thin layers or coatings by spraying, casting or lamination after extrusion;17,108 and (c) encapsulation109 to permit the formation of a physical barrier between the external media and sensitive active compounds to be protected against oxidation and changes in moisture and pH. Encapsulated microparticles can be produced by spray-drying, spray-cooling/chilling, coacervation, gelation, solvent evaporation, extrusion methods and supercritical fluid expansion;79 (d), grafting,110 to permit the covalent binding of active compounds to natural and synthetic polymers.
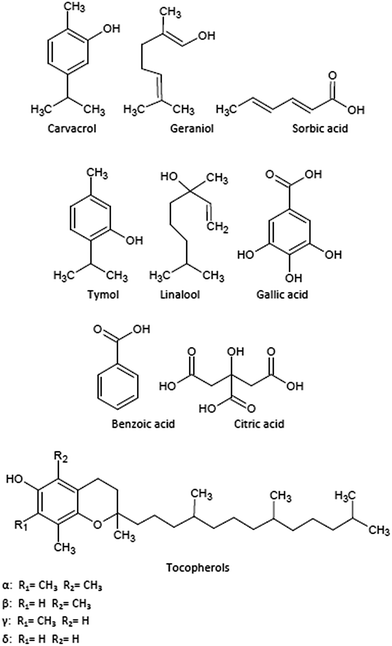
Fig. 1 summarizes the chemical structures of the main bioactive compounds incorporated into or coated onto packaging films in the last years. The most common bioactive additives are those with antioxidant performance, particularly phenols, such as carvacrol, tocopherols and thymol. These natural compounds can be found in fruits, vegetables, cereals, tea, oils, and many herbs and spices.111 Their action against the lipidic auto-oxidation is based on the phenols' ability to release H-atoms from their hydroxyl groups and further reaction with peroxyl radicals to produce stable phenoxyl radicals to terminate lipid peroxidation chain reactions.112,113 The efficiency of the extracted chemicals in their antioxidant activity depends on the electronic and steric effects of the phenol ring, their substituents and the strength of the hydrogen-bonding interactions between phenol and solvents.98
Manzanarez-López et al. developed new films for food packaging based on PLA with 2.58 wt% of α-tocopherol.99 They evaluated the optical and thermal properties of these films as well as the kinetics of the antioxidant diffusion from the PLA matrix to ethanol and vegetable oil. Results showed that the diffusion of α-tocopherol into oil was slower than to ethanol with 5.1 and 12.9%, respectively, after 10 days of contact time. They also studied the release of α-tocopherol from films to soybean oil with the aim to delay the induction of oxidation in food stored at 20 and 30 °C.99
Another interesting alternative in the use of PLA in active packaging formulations by using casting processing was reported by Jamshidian et al.114 who obtained films with natural antioxidants including α-tocopherol and synthetic commercial phenols, such as butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), propyl gallate or tert-butylhydroquinone (TBHQ). Their release in different food simulants was studied to evaluate the kinetic coefficients to support the continuous control of the oxidation reactions in diverse foodstuff.
Other biopolymer matrices have been tested as host for active components. For example, edible polymer films comprise polysaccharides, lipids, proteins or blends of these macromolecules. Cellulose-based edible films, such as methylcellulose or chitosan,115 were recently proposed to develop antioxidant active packaging materials based on the incorporation of α-tocopherol to this blend by encapsulation of this additive to the chitosan matrix.116 Other authors have proposed poly (ε-caprolactone) (PCL) nanocapsule suspensions to permit a longer permanence of the additive into the polymer matrix.117
As previously stated, carvacrol and thymol are two phenolic monoterpenes obtained from oregano and with high potential as antioxidant active compounds.118 They have received attention due to their natural origin and broad antimicrobial and antifungal spectra against different microorganisms, high antioxidant activity and heat stability when incorporated into packaging materials.50,119,120 A wide variety of polymers were used to host carvacrol and/or thymol, and different properties in these active materials were evaluated. Thermal, mechanical and optical performance in these blends showed to be competitive in comparison with common polymers used in food packaging, while the release of these additives from polymer matrices showed adequate rate to understand the transport mechanisms of these molecules to go through polymer matrices and to reach food. LDPE in combination with an organo-modified montmorillonite (MMT) and carvacrol has been proposed as a potential antimicrobial active packaging material.121 These blends proved to be more effective against E. coli than LDPE with just carvacrol without the addition of MMT. This effect was ascribed to the significantly higher carvacrol content permitted by the presence of the nanoclay. Similar results were obtained by Efrati et al. for nanocomposites based on LDPE with thymol and different organo-modified montmorillonites.122 They found a dependence between the concentration of clay in the film and the desorption mechanism of thymol. Ramos et al. prepared PP-based nanocomposites with thymol and carvacrol, reporting that both additives were more effective against gram negative (E. coli) than against gram positive (S. aureus)19 bacteria.
A wide variety of antimicrobial materials based on biopolymer matrices has been developed. Some authors based their studies in chitosan with carvacrol as antimicrobial or antioxidant agent.123–126 Different procedures were proposed to determine the antimicrobial and antioxidant activities and release in different simulants to determine their performance as materials for active food packaging. Kavoosi et al. used gelatin solutions with carvacrol at different concentrations (1–5 wt%) to develop antioxidant and antimicrobial films.127 They stated that these films exhibited excellent antioxidant and antibacterial properties against both gram positive and gram negative bacteria.
Thymol has been also incorporated into different matrices to enhance their antimicrobial and antioxidant performance to increase biosafety of the packed food.128 Wu et al. used PLA/PCL blends prepared by solvent casting,130 while Ramos et al. prepared PLA-based films by melt-blending and hot-pressing with improved thermal, mechanical and gas barrier properties.98,101,129 In addition, these films showed enhanced inhibition to different food borne bacteria and excellent antioxidant capacity.
Geraniol and linalool are monoterpenes traditionally used as fragrance/flavour additives in food industry with properties to tackle infectious diseases and/or to preserve food due to their remarkable properties as insect repellent, antimicrobial, antioxidant, anti-inflammatory and anticancer.130,131 These are natural components of EOs of citronella and coriander, respectively, and they have been used to develop antimicrobial active packaging materials with evaluation of their stability and physico-mechanical and optical properties. Kayaci et al. based their studies in the development of encapsulation strategies with cyclodextrins to extend the product shelf-life by improving the additives stability.123 Thermogravimetric analysis results showed higher thermal stability in geraniol complexed with cyclodextrins than in pure geraniol permitting an easier incorporation into the polymer matrices. Linalool at relatively low (0.75 ± 0.08 wt%), medium (1.08 ± 0.04 wt%) and high concentrations (3.20 ± 0.29 wt%) were incorporated into thermoplastic starch (TPS).132 Authors reported that the direct incorporation of this additive into TPS films did not adversely affect the film properties, particularly at low linalool concentration. These studies need to go deeper into the antimicrobial effect of these films, but some preliminary studies showed their potential application in functional food packaging and other food or medical related applications.
Organic acids, such as benzoic, gallic, citric and sorbic, also showed strong antimicrobial and/or antioxidant activities and they have been used as food preservatives.133 They have been also proposed as active additives in food packaging materials, since they have been included in a positive list by the Food and Drug Administration (FDA) and they are compounds generally recognized as safe (GRAS).134,135 A recent study showed that the incorporation of citric acid to linear LDPE, besides showing enhanced antimicrobial and antioxidant properties in meat samples stored under refrigeration conditions, could also act as plasticizer in films.135 Sorbic and benzoic acids and their salts are also active against yeast, moulds and many bacteria.115 Da Rocha et al. found that protein-based edible films with 1.50 wt% of sorbic or benzoic acids inhibited the proliferation of E. coli O157:H7, Listeria monocytogenes and Salmonella enteritidis; but they did not inhibit the growing of S. aureus.134 Manab et al. showed that benzoic acid incorporated to whey protein-based films significantly inhibited E. coli and Salmonellas.136
5. Final remarks
The rising number of studies presented in the last years on the incorporation of natural active additives to polymer and more specifically to biopolymer matrices has shown the great potential of these formulations to improve selected properties in food packaging materials and to protect the packaged food in a more efficient way. This review focused on studies with natural additives obtained from herbs and spices, either getting directly some of the valuable components from plants or, more commonly, by extraction of chemicals with antioxidant/antimicrobial properties. As a conclusion of this exhaustive study, it should be highlighted that herbs and spices have shown great potential as sources of highly valuable compounds to be further used in different applications, particularly in active food packaging. Nevertheless deeper insight in the interactions of these additives with biopolymer matrices is necessary and further studies are currently on-going.Acknowledgements
Authors would like to thank the Spanish Ministry of Economy and Competitiveness (MINECO) by their financial support (Project MAT2011-28648-C02-01). Moreover, Marina Ramos would like to thank University of Alicante (Spain) for UAFPU2011-48539721S predoctoral research grant.References
- M. Viuda-Martos, E. S. Zapata, J. Fernández-López and J. A. Pérez-Álvarez, Flavour Fragrance J., 2010, 25, 13–19 CrossRef CAS.
- B. Ranton, Handbook of herbs and spices, CRC Press, 2004 Search PubMed.
- FAO, Last access December 2014.
- A. Vallverdú-Queralt, J. Regueiro, M. Martínez-Huélamo, J. F. Rinaldi Alvarenga, L. N. Leal and R. M. Lamuela-Raventos, Food Chem., 2014, 154, 299–307 CrossRef PubMed.
- T. Kulisic, A. Radonic, V. Katalinic and M. Milos, Food Chem., 2004, 85, 633–640 CrossRef CAS.
- R. I. Shobha, C. U. Rajeshwari and B. Andallu, in Cancer, ed. V. Preedy, Academic Press, San Diego, 2014, pp. 91–100 Search PubMed.
- S. Kamiloglu, E. Capanoglu, O. Yilmaz, A. F. Duran and D. Boyacioglu, Qual. Assur. Saf. Crops Foods, 2014, 6, 151–158 CrossRef.
- K. Srinivasan, Crit. Rev. Food Sci. Nutr., 2014, 54, 352–372 CrossRef CAS PubMed.
- P. Singh, A. A. Wani and S. Saengerlaub, Nutr. Food Sci., 2011, 41, 249–260 CrossRef.
- P. Sanchez-Bel, I. Egea, M. T. Pretel, F. B. Flores, F. Romojaro and M. C. Martínez-Madrid, Food Sci. Technol. Int., 2011, 17, 529–540 CrossRef CAS PubMed.
- A. Valdés, A. Beltrán and M. C. Garrigós, J. Food Sci., 2013, 78, C138–C144 CrossRef PubMed.
- A. Silva-Weiss, M. Ihl, P. J. A. Sobral, M. C. Gómez-Guillén and V. Bifani, Food Eng. Rev., 2013, 5, 200–216 CrossRef CAS.
- A. Valdés, A. C. Mellinas, M. Ramos, M. C. Garrigós and A. Jiménez, Front. Chem., 2014, 2, 1–10 Search PubMed.
- J. H. Li, J. Miao, J. L. Wu, S. F. Chen and Q. Q. Zhang, Food Hydrocolloids, 2014, 37, 166–173 CrossRef CAS.
- M. Jouki, F. T. Yazdi, S. A. Mortazavi and A. Koocheki, Food Hydrocolloids, 2014, 36, 9–19 CrossRef CAS.
- M. M. Castro, J. M. López and M. V. González, Food Chem., 2014, 150, 119–127 CrossRef PubMed.
- J. Gómez-Estaca, C. López-de-Dicastillo, P. Hernández-Muñoz, R. Catalá and R. Gavara, Trends Food Sci. Technol., 2014, 35, 42–51 CrossRef.
- S.-Y. Sung, L. T. Sin, T.-T. Tee, S.-T. Bee, A. R. Rahmat, W. A. W. A. Rahman, A.-C. Tan and M. Vikhraman, Trends Food Sci. Technol., 2013, 33, 110–123 CrossRef CAS.
- M. Ramos, A. Jiménez, M. Peltzer and M. C. Garrigós, J. Food Eng., 2012, 109, 513–519 CrossRef CAS.
- D. A. Pereira de Abreu, J. Maroto, K. Villalba Rodríguez and J. M. Cruz, J. Sci. Food Agric., 2012, 92, 427–432 CrossRef CAS PubMed.
- S. R. Kanatt, M. S. Rao, S. P. Chawla and A. Sharma, Food Hydrocolloids, 2012, 29, 290–297 CrossRef CAS.
- W. Hu, P. Xu and T. Uchino, J. Sci. Food Agric., 2005, 85, 2475–2481 CrossRef CAS.
- L. Barbosa-Pereira, G. P. Aurrekoetxea, I. Angulo, P. Paseiro-Losada and J. M. Cruz, Meat Sci., 2014, 97, 249–254 CrossRef CAS PubMed.
- T. Bolumar, M. L. Andersen and V. Orlien, Food Chem., 2011, 129, 1406–1412 CrossRef CAS.
- O. E. Çoban and O. P. Can, J. Aquat. Food Prod. Technol., 2013, 22, 361–370 CrossRef.
- A. C. V. Solano and C. R. de Gante, Food Bioprocess Technol., 2012, 5, 2522–2528 CrossRef.
- V. Otero, R. Becerril, J. A. Santos, J. M. Rodríguez-Calleja, C. Nerín and M.-L. García-López, Food Control, 2014, 42, 296–302 CrossRef CAS.
- F. Licciardello, G. Muratore, P. Suma, A. Russo and C. Nerín, Innovative Food Sci. Emerging Technol., 2013, 19, 173–180 CrossRef CAS.
- S. Manso, R. Becerril, C. Nerín and R. Gómez-Lus, Food Control, 2015, 47, 20–26 CrossRef CAS.
- J.-H. Li, J. Miao, J.-L. Wu, S.-F. Chen and Q.-Q. Zhang, Food Hydrocolloids, 2014, 37, 166–173 CrossRef CAS.
- J. Wu, S. Chen, S. Ge, J. Miao, J. Li and Q. Zhang, Food Hydrocolloids, 2013, 32, 42–51 CrossRef CAS.
- B. Giménez, A. López de Lacey, E. Pérez-Santín, M. E. López-Caballero and P. Montero, Food Hydrocolloids, 2013, 30, 264–271 CrossRef.
- A. M. López de Lacey, M. E. López-Caballero and P. Montero, LWT--Food Sci. Technol., 2014, 55, 559–564 CrossRef.
- Y. A. Arfat, S. Benjakul, T. Prodpran, P. Sumpavapol and P. Songtipya, Food Hydrocolloids, 2014, 41, 265–273 CrossRef CAS.
- U. Siripatrawan and S. Noipha, Food Hydrocolloids, 2012, 27, 102–108 CrossRef CAS.
- U. Siripatrawan and B. R. Harte, Food Hydrocolloids, 2010, 24, 770–775 CrossRef CAS.
- M. Abdollahi, M. Rezaei and G. Farzi, Int. J. Food Sci. Technol., 2012, 47, 847–853 CrossRef CAS.
- M. Abdollahi, M. Rezaei and G. Farzi, J. Food Eng., 2012, 111, 343–350 CrossRef CAS.
- M. H. Hosseini, S. H. Razavi, S. M. A. Mousavi, S. A. S. Yasaghi and A. G. Hasansaraei, J. Appl. Sci., 2008, 8, 2895–2900 CrossRef CAS.
- B. Kodal Coşkun, E. Çalikoǧlu, Z. Karagöz Emiroǧlu and K. Candoǧan, J. Food Qual., 2014, 37, 203–212 CrossRef.
- K. Norajit, K. M. Kim and G. H. Ryu, J. Food Eng., 2010, 98, 377–384 CrossRef CAS.
- P. N. Takala, K. D. Vu, S. Salmieri, R. A. Khan and M. Lacroix, LWT--Food Sci. Technol., 2013, 53, 499–506 CrossRef CAS.
- C. Ramírez, I. Gallegos, M. Ihl and V. Bifani, J. Food Eng., 2012, 109, 424–429 CrossRef.
- M. Quilaqueo Gutiérrez, I. Echeverría, M. Ihl, V. Bifani and A. N. Mauri, Carbohydr. Polym., 2012, 87, 1495–1502 CrossRef.
- J. M. Lorenzo, R. Batlle and M. Gómez, LWT--Food Sci. Technol., 2014, 59, 181–188 CrossRef CAS.
- C. López De Dicastillo, C. Nerín, P. Alfaro, R. Catalá, R. Gavara and P. Hernández-Muñoz, J. Agric. Food Chem., 2011, 59, 7832–7840 CrossRef PubMed.
- C. López-de-Dicastillo, J. Gómez-Estaca, R. Catalá, R. Gavara and P. Hernández-Muñoz, Food Chem., 2012, 131, 1376–1384 CrossRef.
- K. K. N. L. C. L. Perazzo, A. C. V. Conceição, J. C. P. Dos Santos, D. D. J. Assis, C. O. Souza and J. I. Druzian, PLoS One, 2014, 9 DOI:10.1371/journal.pone.0105199.
- F. Tian, E. A. Decker and J. M. Goddard, Food Funct., 2013, 4, 669–680 CAS.
- K. K. Kuorwel, M. J. Cran, K. Sonneveld, J. Miltz and S. W. Bigger, J. Food Sci., 2011, 76, R164–R177 CrossRef CAS PubMed.
- V. Muriel-Galet, M. J. Cran, S. W. Bigger, P. Hernández-Muñoz and R. Gavara, J. Food Eng., 2015, 149, 9–16 CrossRef CAS.
- V. Muriel-Galet, J. P. Cerisuelo, G. López-Carballo, S. Aucejo, R. Gavara and P. Hernández-Muñoz, Food Control, 2013, 30, 137–143 CrossRef CAS.
- S. Salmieri, F. Islam, R. A. Khan, F. M. Hossain, H. M. M. Ibrahim, C. Miao, W. Y. Hamad and M. Lacroix, Cellulose, 2014, 21, 4271–4285 CrossRef CAS.
- N. M. Hromiš, V. L. Lazić, S. L. Markov, Ž. G. Vaštag, S. Z. Popović, D. Z. Šuput and N. R. Džinić, Acta Period. Technol., 2014, 45, 33–43 CrossRef.
- F. Debiagi, R. K. T. Kobayashi, G. Nakazato, L. A. Panagio and S. Mali, Ind. Crops Prod., 2014, 52, 664–670 CrossRef CAS.
- S. Shojaee-Aliabadi, H. Hosseini, M. A. Mohammadifar, A. Mohammadi, M. Ghasemlou, S. M. Hosseini and R. Khaksar, Carbohydr. Polym., 2014, 101, 582–591 CrossRef CAS PubMed.
- S. Shojaee-Aliabadi, M. A. Mohammadifar, H. Hosseini, A. Mohammadi, M. Ghasemlou, S. M. Hosseini, M. Haghshenas and R. Khaksar, Int. J. Biol. Macromol., 2014, 69, 282–289 CrossRef CAS PubMed.
- D. Salarbashi, S. Tajik, S. Shojaee-Aliabadi, M. Ghasemlou, H. Moayyed, R. Khaksar and M. S. Noghabi, Food Chem., 2014, 146, 614–622 CrossRef CAS PubMed.
- S. Shojaee-Aliabadi, H. Hosseini, M. A. Mohammadifar, A. Mohammadi, M. Ghasemlou, S. M. Ojagh, S. M. Hosseini and R. Khaksar, Int. J. Biol. Macromol., 2013, 52, 116–124 CrossRef CAS PubMed.
- M. Jouki, F. T. Yazdi, S. A. Mortazavi and A. Koocheki, Food Hydrocolloids, 2014, 36, 9–19 CrossRef CAS.
- L. A. Cestari, R. C. Gaiotto, J. L. Antigo, M. R. S. Scapim, G. S. Madrona, F. Yamashita, M. S. S. Pozza and I. N. Prado, J. Food Sci. Technol., 2014 DOI:10.1007/s13197-014-1357-z.
- C. G. Otoni, S. F. O. Pontes, E. A. A. Medeiros and N. d. F. F. Soares, J. Agric. Food Chem., 2014, 62, 5214–5219 CrossRef CAS PubMed.
- V. Muriel-Galet, J. P. Cerisuelo, G. López-Carballo, M. Lara, R. Gavara and P. Hernández-Muñoz, Int. J. Food Microbiol., 2012, 157, 195–201 CrossRef CAS PubMed.
- M. Royo, I. Fernández-Pan and J. I. Maté, J. Sci. Food Agric., 2010, 90, 1513–1519 CrossRef CAS PubMed.
- A. A. M. de Melo, R. M. Geraldine, M. F. A. Silveira, M. C. L. Torres, C. S. M. e. Rezende, T. H. Fernandes and A. N. de Oliveira, Braz. J. Microbiol., 2012, 43, 1419–1427 CrossRef PubMed.
- P. J. P. Espitia, R. J. Avena-Bustillos, W.-X. Du, R. F. Teófilo, N. F. F. Soares and T. H. McHugh, Food Packaging and Shelf Life, 2014, 2, pp. 38–49 Search PubMed.
- P. S. Negi, Int. J. Food Microbiol., 2012, 156, 7–17 CrossRef PubMed.
- M. M. Tajkarimi, S. A. Ibrahim and D. O. Cliver, Food Control, 2010, 21, 1199–1218 CrossRef CAS.
- F. Sadaka, C. Nguimjeu, C. H. Brachais, I. Vroman, L. Tighzert and J. P. Couvercelle, Innovative Food Sci. Emerging Technol., 2014 DOI:10.1016/j.ifset.2014.03.002.
- J. Gómez-Estaca, C. López-de-Dicastillo, P. Hernández-Muñoz, R. Catalá and R. Gavara, Trends Food Sci. Technol., 2014, 35, 42–51 CrossRef.
- V. Kechichian, C. Ditchfield, P. Veiga-Santos and C. C. Tadini, LWT--Food Sci. Technol., 2010, 43, 1088–1094 CrossRef CAS.
- P. J. P. Espitia, N. D. F. F. Soares, L. C. M. Botti and W. A. Silva, Macromol. Symp., 2011, 299–300, 199–205 CrossRef.
- I. Liakos, L. Rizzello, D. J. Scurr, P. P. Pompa, I. S. Bayer and A. Athanassiou, Int. J. Pharm., 2014, 463, 137–145 CrossRef CAS PubMed.
- B. Teixeira, A. Marques, C. Pires, C. Ramos, I. Batista, J. A. Saraiva and M. L. Nunes, LWT--Food Sci. Technol., 2014, 59, 533–539 CrossRef CAS.
- K. R. Badr, Z. S. Ahmed and M. S. El Gamal, Int. J. Agric. Res., 2014, 9, 242–250 CrossRef.
- S. Bahram, M. Rezaei, M. Soltani, A. Kamali, S. M. Ojagh and M. Abdollahi, J. Food Process. Preserv., 2014, 38, 1251–1258 CrossRef CAS.
- A. C. Souza, G. E. O. Goto, J. A. Mainardi, A. C. V. Coelho and C. C. Tadini, LWT--Food Sci. Technol., 2013, 54, 346–352 CrossRef CAS.
- I.-H. Kim, J. Han, J. H. Na, P.-S. Chang, M. S. Chung, K. H. Park and S. C. Min, J. Food Sci., 2013, 78, E229–E237 CrossRef CAS PubMed.
- I.-H. Kim, A. Y. Song, J. Han, K. H. Park and S. C. Min, J. Food Sci., 2014, 79, E2023–E2030 CrossRef CAS PubMed.
- P. Montero-Prado, A. Rodriguez-Lafuente and C. Nerin, Postharvest Biol. Technol., 2011, 60, 211–219 CrossRef CAS.
- L. Gutiérrez, C. Sánchez, R. Batlle and C. Nerín, Trends Food Sci. Technol., 2009, 20, 92–99 CrossRef.
- S. M. Ojagh, M. Rezaei, S. H. Razavi and S. M. H. Hosseini, Food Chem., 2010, 122, 161–166 CrossRef CAS.
- S. Manso, D. Pezo, R. Gómez-Lus and C. Nerín, Food Control, 2014, 45, 101–108 CrossRef CAS.
- J. Wu, H. Liu, S. Ge, S. Wang, Z. Qin, L. Chen, Q. Zheng, Q. Liu and Q. Zhang, Food Hydrocolloids, 2015, 43, 427–435 CrossRef CAS.
- L. A. C. Zuanon, C. R. Malacrida and V. R. N. Telis, J. Food Process Eng., 2013, 36, 364–373 CrossRef CAS.
- N.-B. Song, J.-H. Lee, M. Al Mijan and K. B. Song, LWT--Food Sci. Technol., 2014, 57, 453–460 CrossRef CAS.
- P. R. Salgado, M. E. López-Caballero, M. C. Gómez-Guillén, A. N. Mauri and M. P. Montero, Food Hydrocolloids, 2013, 33, 74–84 CrossRef CAS.
- J. Gómez-Estaca, A. López de Lacey, M. E. López-Caballero, M. C. Gómez-Guillén and P. Montero, Food Microbiol., 2010, 27, 889–896 CrossRef PubMed.
- K. Bentayeb, P. Vera, C. Rubio and C. Nerin, Anal. Bioanal. Chem., 2009, 394, 903–910 CrossRef CAS PubMed.
- W. X. Du, C. W. Olsen, R. J. Avena-Bustillos, T. H. McHugh, C. E. Levin and M. Friedman, J. Food Sci., 2009, 74, M372–M378 CrossRef CAS PubMed.
- P. N. Takala, S. Salmieri, A. Boumail, R. A. Khan, K. D. Vu, G. Chauve, J. Bouchard and M. Lacroix, J. Food Eng., 2013, 116, 648–655 CrossRef CAS.
- M. Bendahou, A. Muselli, M. Grignon-Dubois, M. Benyoucef, J.-M. Desjobert, A.-F. Bernardini and J. Costa, Food Chem., 2008, 106, 132–139 CrossRef CAS.
- M. Lahlou, Phytother. Res., 2004, 18, 435–448 CrossRef CAS PubMed.
- H. J. Jo, K. M. Park, S. C. Min, J. H. Na, K. H. Park and J. Han, J. Food Sci., 2013, 78, E1713–E1720 CrossRef CAS PubMed.
- K. Chaieb, H. Hajlaoui, T. Zmantar, A. B. Kahla-Nakbi, M. Rouabhia, K. Mahdouani and A. Bakhrouf, Phytother. Res., 2007, 21, 501–506 CrossRef CAS PubMed.
- S. Burt, Int. J. Food Microbiol., 2004, 94, 223–253 CrossRef CAS PubMed.
- S. Kordali, A. Cakir, H. Ozer, R. Cakmakci, M. Kesdek and E. Mete, Bioresour. Technol., 2008, 99, 8788–8795 CrossRef CAS PubMed.
- M. Ramos, A. Jiménez, M. Peltzer and M. C. Garrigós, Food Chem., 2014, 162, 149–155 CrossRef CAS PubMed.
- F. Manzanarez-López, H. Soto-Valdez, R. Auras and E. Peralta, J. Food Eng., 2011, 104, 508–517 CrossRef.
- L. Bastarrachea, S. Dhawan and S. Sablani, Food Eng. Rev., 2011, 3, 79–93 CrossRef.
- M. Ramos, E. Fortunati, M. Peltzer, F. Dominici, A. Jiménez, M. d. C. Garrigós and J. M. Kenny, Polym. Degrad. Stab., 2014, 108, 158–165 CrossRef CAS.
- M. F. Poças, J. C. Oliveira, F. A. R. Oliveira and T. Hogg, Crit. Rev. Food Sci. Nutr., 2008, 48, 913–928 CrossRef PubMed.
- A. Z. Graciano-Verdugo, H. Soto-Valdez, E. Peralta, P. Cruz-Zárate, A. R. Islas-Rubio, S. Sánchez-Valdes, A. Sánchez-Escalante, N. González-Méndez and H. González-Ríos, Food Res. Int., 2010, 43, 1073–1078 CrossRef CAS.
- F. Iñiguez-Franco, H. Soto-Valdez, E. Peralta, J. F. Ayala-Zavala, R. Auras and N. Gámez-Meza, J. Agric. Food Chem., 2012, 60, 6515–6523 CrossRef PubMed.
- M. Ramos, A. Beltrán, M. Peltzer, A. J. M. Valente and M. d. C. Garrigós, LWT--Food Sci. Technol., 2014, 58, 470–477 CrossRef CAS.
- K. K. Kuorwel, M. J. Cran, K. Sonneveld, J. Miltz and S. W. Bigger, LWT--Food Sci. Technol., 2013, 50, 432–438 CrossRef CAS.
- Innovations in Food Packaging, ed. J. H. Han, Elsevier Academic Press, Canada, 2005 Search PubMed.
- M. Ramos, A. Beltran, A. Valdes, M. A. Peltzer, A. Jimenez and M. C. Garrigos, J. Bioequivalence Bioavailability, 2013, 5, 154–160 CAS.
- H. M. C. Marques, Flavour Fragrance J., 2010, 25, 313–326 CrossRef.
- S. B. Schreiber, J. J. Bozell, D. G. Hayes and S. Zivanovic, Food Hydrocolloids, 2013, 33, 207–214 CrossRef CAS.
- J. Mastelic, I. Jerkovic, I. Blazevic, M. Poljak-Blazi, S. Borovic, I. Ivancic-Bace, V. Smrecki, N. Zarkovic, K. Brcic-Kostic, D. Vikic-Topic and N. Müller, J. Agric. Food Chem., 2008, 56, 3989–3996 CrossRef CAS PubMed.
- L. Barbosa-Pereira, J. M. Cruz, R. Sendón, A. Rodríguez Bernaldo de Quirós, A. Ares, M. Castro-López, M. J. Abad, J. Maroto and P. Paseiro-Losada, Food Control, 2013, 31, 236–243 CrossRef CAS.
- J. Hughes, R. Thomas, Y. Byun and S. Whiteside, Carbohydr. Polym., 2012, 88, 165–172 CrossRef CAS.
- M. Jamshidian, E. Tehrany and S. Desobry, Food Bioprocess Technol., 2013, 6, 1450–1463 CrossRef CAS.
- M. C. Cruz-Romero, T. Murphy, M. Morris, E. Cummins and J. P. Kerry, Food Control, 2013, 34, 393–397 CrossRef CAS.
- M. V. Dias, V. Machado Azevedo, S. V. Borges, N. d. F. F. Soares, R. V. de Barros Fernandes, J. J. Marques and É. A. A. Medeiros, Food Chem., 2014, 165, 323–329 CrossRef CAS PubMed.
- C. M. Noronha, S. M. de Carvalho, R. C. Lino and P. L. M. Barreto, Food Chem., 2014, 159, 529–535 CrossRef CAS PubMed.
- G. Al-Bandak and V. Oreopoulou, Eur. J. Lipid Sci. Technol., 2007, 109, 247–255 CrossRef CAS.
- N. Didry, L. Dubreuil and M. Pinkas, Pharm. Acta Helv., 1994, 69, 25–28 CrossRef CAS PubMed.
- H. J. D. Dorman and S. G. Deans, J. Appl. Microbiol., 2000, 88, 308–316 CrossRef CAS PubMed.
- R. Shemesh, D. Goldman, M. Krepker, Y. Danin-Poleg, Y. Kashi, A. Vaxman and E. Segal, J. Appl. Polym. Sci., 2015, 132, n/a-n/a CrossRef.
- R. Efrati, M. Natan, A. Pelah, A. Haberer, E. Banin, A. Dotan and A. Ophir, J. Appl. Polym. Sci., 2014, 131, 40564 Search PubMed.
- L. Higueras, G. López-Carballo, P. Hernández-Muñoz, R. Catalá and R. Gavara, Int. J. Food Microbiol., 2014, 188, 53–59 CrossRef CAS PubMed.
- M. Kurek, A. Guinault, A. Voilley, K. Galić and F. Debeaufort, Food Chem., 2014, 144, 9–17 CrossRef CAS PubMed.
- M. López-Mata, S. Ruiz-Cruz, N. Silva-Beltrán, J. Ornelas-Paz, P. Zamudio-Flores and S. Burruel-Ibarra, Molecules, 2013, 18, 13735–13753 CrossRef PubMed.
- M. Kurek, S. Moundanga, C. Favier, K. Galić and F. Debeaufort, Food Control, 2013, 32, 168–175 CrossRef CAS.
- G. Kavoosi, S. M. M. Dadfar, A. Mohammadi Purfard and R. Mehrabi, J. Food Saf., 2013, 33, 423–432 CrossRef.
- N. Khairuddin and I. I. Muhamad, Sciences and Engineering, 2013, 62, 31–34 Search PubMed.
- Y. Wu, Y. Qin, M. Yuan, L. Li, H. Chen, J. Cao and J. Yang, Polym. Adv. Technol., 2014, 25, 948–954 CrossRef CAS.
- N. G. Sahib, F. Anwar, A.-H. Gilani, A. A. Hamid, N. Saari and K. M. Alkharfy, Phytother. Res., 2013, 27, 1439–1456 CAS.
- F. Kayaci, H. S. Sen, E. Durgun and T. Uyar, Food Res. Int., 2014, 62, 424–431 CrossRef CAS.
- K. K. Kuorwel, M. J. Cran, K. Sonneveld, J. Miltz and S. W. Bigger, Packag. Technol. Sci., 2014, 27, 149–159 CrossRef CAS.
- K. A. Hammer, C. F. Carson and T. V. Riley, J. Appl. Microbiol., 1999, 86, 985–990 CrossRef CAS PubMed.
- M. d. Rocha, M. R. Loiko, E. C. Tondo and C. Prentice, Food Hydrocolloids, 2014, 37, 213–220 CrossRef.
- A. Júnior, N. Fronza, F. Foralosso, D. Dezen, E. Huber, J. dos Santos, R. Machado and M. Quadri, Food Bioprocess Technol., 2014, 1–13 Search PubMed.
- A. Manab, M. E. Sawitri, K. U. Al Awwaly and H. Purnomo, Afr. J. Food Sci., 2011, 1, 6–11 Search PubMed.
| This journal is © The Royal Society of Chemistry 2015 |