A new composite thixotropic hydrogel composed of a low-molecular-weight hydrogelator and a nanosheet†
Yutaka Ohsedo*a,
Masashi Oonob,
Kowichiro Saruhashib,
Hisayuki Watanabeab and
Nobuyoshi Miyamotoc
aAdvanced Materials Research Laboratory, Collaborative Research Division, Art, Science and Technology Center for Cooperative Research, Kyushu University, 4-1 Kyudaishinmachi Nishi-ku, Fukuoka 819-0388, Japan. E-mail: ohsedo@astec.kyushu-u.ac.jp; Fax: +81-92-400-4382; Tel: +81-92-400-4381
bNissan Chemical Industries, Ltd, 2-10-1 Tsuboinishi Funabashi, Chiba 274-8507, Japan
cDepartment of Life, Environment and Materials Science, Fukuoka Institute of Technology, 3-30-1, Wajiro-Higashi, Higashi-ku, Fukuoka 811-0295, Japan
First published on 9th September 2014
Abstract
A two-component composite comprised of a low-molecular-weight hydrogelator and the nanosheet Laponite®, a synthetic layered silicate, showed thixotropic behaviour, whereas each single component did not show thixotropic behaviour at the same concentration. This mixing enhancement effect may be attributed to the improved quality of the gel network.
Molecular hydrogels composed of crystalline, fibrous networks of low-molecular-weight gelators (LMWGs)1,2 and water have received considerable attention as new soft materials due to their resemblance to biological compounds that enable formation of molecular hydrogels. In particular, they are considered to have potential application as biocompatible drug delivery materials.3 Moreover, the multi-responsiveness4 and thixotropy (shear thinning)5,6 of molecular hydrogels and organogels have attracted much attention, both as new aspects of soft matter and as clues to enhancing the poor mechanical properties of known molecular gels and increase their use in medical, electroactive and photoactive applications.4 Although, LMWGs feature well-defined molecular structures and various molecular designs similar to those of polymer hydrogel materials, the available list of LMWGs for molecular hydrogels remains limited at present. Therefore, to broaden the scope of known molecular hydrogels, new methods for adding enhanced functionality to assembled networks of LMWGs must be investigated and new LMWG's must be explored. We discovered that mixing-induced thixotropy of LMWGs with different alkyl chains (commercially available alkylhydrazides, alkylamides and alkylureas) can serve as a new method for enhancing the functionality of molecular organogels.7
Herein, we report a new method for functionalization of molecular hydrogels by the formation of a composite of an LMWG and Laponite®, a commercially available inorganic silicate rheology modifier, which is a type of inorganic nanosheet that exists in the form of nanodiscs (diameter: 30 mm, thickness: 1 nm).8 Although several systems that involve the mixing-induced enhancement of gel properties or mixed gel formulations,9,10 including organic/inorganic composite hydrogels, have been studied,11–13 our route involves mixing Laponite with a hydrogelator, i.e. palmitoyl-glycine-histidine hydrogelator (Scheme 1).14 Moreover, no thixotropic behaviour has yet been reported for LMWG/nanosheet composite gels.12
Initially, the hydrogelation ability of PalGH/Laponite composites was tested. For ease of handling, a non-viscous 1 wt% Laponite aqueous solution was used. The composites were prepared by heating and cooling a mixture of PalGH powder and Laponite aqueous solution. As shown in Fig. 1(a) and (c), respectively, only PalGH in water formed a hydrogel when incorporated at a concentration above 0.5 wt%, while even a 2 wt% Laponite solution remained a liquid. For the PalGH/Laponite composites (Fig. 1(b)), a hydrogel with slightly increased clarity compared to that of the PalGH gel at 0.5 wt% was formed, possibly due to an increase in the homogeneity of the hydrogel. Moreover, rheometry measurements for the PalGH 1 wt%/Laponite 1 wt% composite confirmed the formation of a softer hydrogel than the corresponding PalGH hydrogel (Fig. S1, ESI†). Notably, the composite gels maintained their gel states for at least six months.
In the two-component composite hydrogel comprising 1 wt% of PalGH and Laponite aqueous solution, respectively, thixotropic behaviour was observed. The individual components did not exhibit such behaviour at the same concentration (Fig. 1(d) and (e)). Moreover, a 2 wt% PalGH hydrogel exhibited thixotropic behaviour but required 2 h for recovery to the gel state after shaking (Fig. S2, ESI†). Furthermore, 1 and 2 wt% Laponite aqueous solutions remained in the liquid state. These results suggested that a new network structure that is different from the network structures of individual components may form upon mixing.
To evaluate the thixotropic behaviour of the composite hydrogels, the rheometry of the gels was investigated using step-shear measurements (Fig. 2). The PalGH 1 wt%/Laponite 1 wt% composite hydrogel exhibited a large recovery of G′ and G′′ and reverted to the gel-like state (G′ > G′′) after repeated application of a large deformation shear. Although repeated recovery of the PalGH gel was also observed, unlike in the thixotropic test (Fig. 2(b)), this recovery did not directly affect the macro-scale reconstruction of the hydrogel. Moreover, the Laponite solution showed liquid-like behaviour (G′ < G′′) at this concentration. Thus, these results clearly demonstrate qualitatively the mixing enhancement effect on the thixotropic properties of the composite.
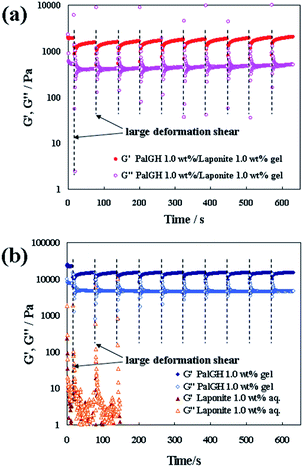 | ||
| Fig. 2 Periodic step-shear test results for (a) the PalGH 1 wt%/Laponite 1 wt% composite hydrogel and (b) the PalGH gel and Laponite solution. | ||
To obtain information about the network that provides the enhanced mechanical properties of the composite hydrogels, differential scanning calorimetry (DSC) measurements were performed (Table S1 and Fig. S3, ESI†). The DSC curves of the PalGH 1 wt%/Laponite 1 wt% composite hydrogel underwent ordinal sol-to-gel and gel-to-sol changes with a similar ΔH. Compared to the peaks for the pure PalGH solution, the peaks for the composite hydrogel were shifted to lower temperature. Aqueous 1–3 wt% Laponite solutions showed no peaks, probably due to the dominant nature of the liquid. This result suggests that the composite hydrogel forms a new, possibly finer network structure than that of the pure PalGH.
To investigate the microstructure of the composite hydrogels, scanning electron microscopy (SEM) images of the corresponding xerogels and a dried Laponite solution were obtained (Fig. 3). The PalGH (1 wt%)/Laponite (1 wt%) composite xerogel exhibited a sub-micrometer-scale network fibre structure with a micrometer-scale network sheet structure. These structures appeared to originate from the PalGH fibre network and aggregated Laponite silicate discs, respectively, as determined by comparing the SEM images of the single components with that of the composite. Notably, the composite xerogel contained finer fibres than the Laponite xerogel (Fig. 3(b) and (c)), possibly because of the presence of PalGH, which might prevent aggregation of the silicate nanodiscs.
To obtain structural information on the crystalline fibres in the hydrogels, small-angle X-ray scattering (SAXS) results were obtained, and the corresponding Kratky-type plots11d,15 are shown in Fig. 4. The slope of −2 for the Laponite confirms the homogeneous dispersion of flat discs in this solution (see Fig. S4, ESI†).15,16 For the composite and PalGH hydrogels, the same peaks corresponding to rigid PalGH moieties and an interdigitated packing structure were observed (Fig. S5, ESI†). The interdigitated packing structure of PalGH derivatives has been extensively covered in the literature.14c These results suggest that the composite hydrogel is composed of a network of PalGH fibre-like crystals (such as those in the single-component PalGH hydrogel) and well-dispersed Laponite.
 | ||
| Fig. 4 Kratky-type plots of SAXS data of hydrogels and Laponite aqueous solution (see also Fig. S3 and S4, ESI†). | ||
The mixing enhancement effect on the thixotropic behaviour may be explained by assuming the formation of an improved network of the PalGH in the presence of Laponite. Note that reduced aggregation of Laponite in the presence of PalGH was observed (Fig. 3(b)). Moreover, both in the gel and solution states, Laponite existed as colloidal particles.17 Note that no interactions between the PalGH and Laponite were detected via FT-IR analysis (no significant peaks were observed in the spectrum). Given that the PalGH and Laponite do not interact with each other, it is possible that the bundle formation of PalGH fibre bundles may be reduced in the homogeneous presence of Laponite resulting in the formation of a high density network of finer PalGH fibres (In Fig. 3, it is possible that the finer fibres readily aggregated during the freeze-dry process). Subsequently, this new improved network may exhibit thixotropic behaviour, unlike the individual 1 wt% components.
In conclusion, we demonstrated that a new composite composed of a hydrogelator and Laponite enabled induction of thixotropic behaviour. The mixing-induced thixotropy may be attributed to the formation of an extended network with crosslinking between the fibres of each component. We are currently investigating the application of this mixed strategy to other systems.
Notes and references
- Molecular Gels: Materials With Self-Assembled Fibrillar Networks, ed. R. G. Weiss and P. Terech, Kluwer Academic Pub, 2006 Search PubMed.
- For reviews: (a) A. Dawn, T. Shiraki, S. Haraguchi, S.-I. Tamaru and S. Shinkai, Chem.–Asian J., 2011, 6, 266 CrossRef CAS PubMed; (b) J. W. Steed, Chem. Commun., 2011, 47, 1379 RSC; (c) X.-Y. Yang, G.-X. Zhang and D.-Q. Zhang, J. Mater. Chem., 2012, 22, 38 RSC; (d) A. J. Jonker, D. W. P. M. Löwik and J. C. M. Van Hest, Chem. Mater., 2012, 24, 759 CrossRef CAS; (e) M. Yamanaka, J. Inclusion Phenom. Macrocyclic Chem., 2013, 77, 33 CrossRef CAS; (f) M. D. Segarra-Maset, V. J. Nebot, J. F. Miravet and B. Escuder, Chem. Soc. Rev., 2013, 42, 7086 RSC; (g) R. G. Weiss, J. Am. Chem. Soc., 2014, 136, 7519 CrossRef CAS PubMed; (h) M. Cametti and Z. Džolić, Chem. Commun., 2014, 50, 8273 RSC.
- (a) A. R. Hirst, B. Escuder, J. F. Miravet and D. K. Smith, Angew. Chem., Int. Ed., 2008, 47, 8002 CrossRef CAS PubMed; (b) F. Zhao, M. L. Ma and B. Xu, Chem. Soc. Rev., 2009, 38, 883 RSC; (c) J. B. Matson and S. I. Stupp, Chem. Commun., 2012, 48, 26 RSC.
- For recent examples: (a) A. Dawn, T. Shiraki, H. Ichikawa, A. Takada, Y. Tahakashi, Y. Tsuchiya, L. T. N. Lien and S. Shinkai, J. Am. Chem. Soc., 2012, 134, 2161 CrossRef CAS PubMed; (b) S.-Y. Dong, B. Zheng, D.-H. Xu, X.-H. Yan, M.-M. Zhang and F.-H. Huang, Adv. Mater., 2012, 24, 3191 CrossRef CAS PubMed; (c) Z.-X. Liu, Y. Feng, Z.-C. Yan, Y.-M. He, C.-Y. Liu and Q.-H. Fan, Chem. Mater., 2012, 24, 3751 CrossRef CAS; (d) X.-Z. Yan, D.-H. Xu, X.-D. Chi, J.-Z. Chen, S.-Y. Dong, X. Ding, Y.-H. Yu and F.-H. Huang, Adv. Mater., 2012, 24, 362 CrossRef CAS PubMed; (e) S. K. Samanta and S. Bhattacharya, J. Mater. Chem., 2012, 22, 25277 RSC; (f) P.-C. Xue, J.-B. Sun, Q.-X. Xu, R. Lu, M. Takafuji and H. Ihara, Org. Biomol. Chem., 2013, 11, 1840 RSC; (g) R. Ochi, K. Kurotani, M. Ikeda, S. Kiyonaka and I. Hamachi, Chem. Commun., 2013, 49, 2115 RSC; (h) Y. Ohsedo, M. Miyamoto, A. Tanaka and H. Watanabe, New J. Chem., 2013, 37, 2874 RSC; (i) Y. Jiang, F. Zeng, R.-Y. Gong, Z.-X. Guo, C.-F. Chen and X.-B. Wan, Soft Matter, 2013, 9, 7538 RSC; (j) J. T. van Herpt, M. C. A. Stuart, W. R. Browne and B. L. Feringa, Langmuir, 2013, 29, 8763 CrossRef CAS PubMed; (k) P. Fatás, J. Bachl, S. Oehm, A. I. Jiménez, C. Cativiela and D. D. Díaz, Chem.–Eur. J., 2013, 19, 8861 CrossRef PubMed; (l) M. Ikeda, T. Tanida, T. Yoshii, K. Kurotani, S. Onogi, K. Urayama and I. Hamachi, Nat. Chem., 2014, 6, 511 CrossRef CAS PubMed; (m) R. Yang, S. Peng and T. C. Hughes, Soft Matter, 2014, 10, 2188 RSC; (n) S. Basak, J. Nanda and A. Banerjee, Chem. Commun., 2014, 50, 3004 RSC.
- J. W. Goodwin, R. W. Hughes, Rheology for Chemists: An Introduction, RSC Publishing, 2nd edn, 2008 Search PubMed.
- For recent examples of thixotropic LMWG gels: (a) A. A. Sobczuk, Y. Tsuchiya, T. Shiraki, S.-I. Tamaru and S. Shinkai, Chem.–Eur. J., 2012, 18, 2832 CrossRef CAS PubMed; (b) N. Yan, Z.-Y. Xu, K. K. Diehn, S. R. Raghavan, Y. Fang and R. G. Weiss, Langmuir, 2013, 29, 793 CrossRef CAS PubMed; (c) Z.-Y. Xu, J.-X. Peng, N. Yan, H. Yu, S.-S. Zhang, K.-Q. Liu and Y. Fang, Soft Matter, 2013, 9, 1091 RSC; (d) D. Higashi, M. Yoshida and M. Yamanaka, Chem.–Asian J., 2013, 8, 2548 CrossRef PubMed; (e) J. T. van Herpt, M. C. A. Stuart, W. R. Browne and B. L. Feringa, Langmuir, 2013, 29, 8763 CrossRef CAS PubMed; (f) Z.-F. Sun, Z.-Y. Li, Y.-H. He, R.-J. Shen, L. Deng, M.-H. Yang, Y.-Z. Liang and Y. Zhang, J. Am. Chem. Soc., 2013, 135, 13379 CrossRef CAS PubMed; (g) H. Hoshizawa, Y. Minemura, K. Yoshikawa, M. Suzuki and K. Hanabusa, Langmuir, 2013, 29, 14666 CrossRef CAS PubMed.
- (a) Y. Ohsedo, H. Watanabe, M. Oono and A. Tanaka, Chem. Lett., 2013, 42, 363 CrossRef CAS; (b) Y. Ohsedo, H. Watanabe, M. Oono and A. Tanaka, RSC Adv., 2013, 3, 5803 RSC; (c) Y. Ohsedo, M. Oono, A. Tanaka and H. Watanabe, New J. Chem., 2013, 37, 2250 RSC; (d) Y. Ohsedo, M. Taniguchi, M. Oono, K. Saruhashi and H. Watanabe, RSC Adv., 2014, 4, 35484 RSC.
- N. Miyamoto and T. Nakato, Isr. J. Chem., 2012, 52, 881 CrossRef CAS.
- For reviews: (a) A. R. Hirst and D. K. Smith, Chem.–Eur. J., 2005, 11, 5496 CrossRef CAS PubMed; (b) C. A. Dreiss, Soft Matter, 2007, 3, 95 RSC; (c) L. E. Buerkle and S. J. Rowan, Chem. Soc. Rev., 2012, 41, 6089 RSC.
- For recent examples: (a) L. Meazza, J. A. Foster, K. Fucke, P. Metrangolo, G. Resnati and J. W. Steed, Nat. Chem., 2013, 5, 42 CrossRef CAS PubMed; (b) P.-C. Xue, R. Lu, R. Zhang, J.-H. Jia, Q.-X. Xu, T.-R. Zhang, M. Takafuji and H. Ihara, Langmuir, 2013, 29, 417 CrossRef CAS PubMed; (c) M. M. Smith, W. Edwards and D. K. Smith, Chem. Sci., 2013, 4, 671 RSC; (d) S. K. Samanta and S. Bhattacharya, Chem. Commun., 2013, 49, 1425 RSC; (e) W. Edwards and D. K. Smith, J. Am. Chem. Soc., 2013, 135, 5911 CrossRef CAS PubMed; (f) A. J. Kleinsmann and B. J. Nachtsheim, Chem. Commun., 2013, 49, 7818 RSC; (g) K.-Q. Liu and J. W. Steed, Soft Matter, 2013, 9, 11699 RSC; (h) S. Bhattacharjee, S. Datta and S. Bhattacharya, Chem.–Eur. J., 2013, 19, 16672 CrossRef CAS PubMed; (i) J. A. Foster, R. M. Edkins, G. J. Cameron, N. Colgin, K. Fucke, S. Ridgeway, A. G. Crawford, T. B. Marder, A. Beeby, S. L. Cobb and J. W. Steed, Chem.–Eur. J., 2014, 20, 279 CrossRef CAS PubMed; (j) H. Kar and S. Ghosh, Chem. Commun., 2014, 50, 1064 RSC; (k) W. Edwards and D. K. Smith, J. Am. Chem. Soc., 2014, 136, 1116 CrossRef CAS PubMed.
- (a) K. Haraguchi and T. Takehisa, Adv. Mater., 2002, 16, 1120 CrossRef; (b) P. Shexnailder and G. Schmidt, Colloid Polym. Sci., 2009, 287, 1 CrossRef; (c) Q.-G. Wang, J. L. Mynar, M. Yoshida, E. lee, M.-S. Lee, K. Okuro, K. Kinbara and T. Aida, Nature, 2010, 463, 339 CrossRef CAS PubMed; (d) N. Miyamoto, M. Shintate, Y. Hoshida, R. Motokawa and M. Annaka, Chem. Commun., 2013, 49, 1082 RSC.
- Example of composite of LMWG and laponite; F. Song, L.-M. Zhang, J.-F. Shi and N.-N. Li, Colloids Surf., B, 2010, 81, 486 CrossRef CAS PubMed.
- Example of composite of LMWG and montmorillonite for biosensing; M. Ikeda, T. Yoshii, T. Matsui, T. Tanida, H. Komatsu and I. Hamachi, J. Am. Chem. Soc., 2011, 133, 1670 CrossRef CAS PubMed.
- Derivative of PalGH have been studied: (a) D. Koda, T. Maruyama, N. Minakuchi, K. Nakashima and M. Goto, Chem. Commun., 2010, 46, 979 RSC; (b) A. Shundo, K. Mizuguchi, M. Miyamoto, M. Goto and K. Tanaka, Chem. Commun., 2011, 47, 8844 RSC; (c) D. P. Penaloza Jr, A. Shundo, K. Matsumoto, M. Ohno, K. Miyaji, M. Goto and K. Tanaka, Soft Matter, 2013, 9, 5166 RSC; (d) M. Miyamoto, N. Miyachi, T. Iwama, WO2010/013555, 2010.
- Small Angle X-ray Scattering, ed. O. Glatter and O. Kratky, Academic Press, 1982 Search PubMed.
- D. Bonn, H. Kellay, H. Tanaka, G. Wegdam and J. Meunier, Langmuir, 1999, 15, 7534 CrossRef CAS.
- A. Mourchid, E. Lécolier, H. Van Damme and P. Levits, Langmuir, 1998, 14, 4718 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra08542f |
| This journal is © The Royal Society of Chemistry 2014 |



