Discovery of superconductivity in technetium borides at moderate pressures†
Xiangru
Tao‡
a,
Aiqin
Yang‡
a,
Yundi
Quan
a,
Biao
Wan
b,
Shuxiang
Yang
*c and
Peng
Zhang
 *a
*a
aMOE Key Laboratory for Non-equilibrium Synthesis and Modulation of Condensed Matter, Shaanxi Province Key Laboratory of Advanced Functional Materials and Mesoscopic Physics, School of Physics, Xi'an Jiaotong University, Xi'an, Shaanxi 710049, P.R. China. E-mail: zpantz@mail.xjtu.edu.cn
bKey Laboratory of Material Physics of Ministry of Education, School of Physics and Microelectronics, Zhengzhou University, Zhengzhou 450052, Henan, P.R. China
cZhejiang Laboratory, Hangzhou, Zhejiang, P.R. China. E-mail: yang_shuxiang@zhejianglab.com
First published on 25th April 2024
Abstract
Advances in theoretical calculations have boosted the search for high-temperature superconductors, such as sulfur hydrides and rare-earth polyhydrides. However, the required extremely high pressures for stabilizing these superconductors has handicapped further implementation. Based upon thorough structural searches, we identified a series of unprecedented superconducting technetium borides at moderate pressures, including TcB (P63/mmc) with a superconducting transition temperature of Tc = 20.2 K at ambient pressure and TcB2 (P6/mmm) with Tc = 23.1 K at 20 GPa. Superconductivity in these technetium borides mainly originates from the coupling between the low-frequency vibrations of technetium atoms and the dominant technetium-4d electrons at the Fermi level. Our work therefore presents a fresh group in the family of superconducting borides, whose diversified crystal structures suggest rich possibilities in the discovery of other superconducting transition-metal borides.
1 Introduction
The discovery of superconductivity in mercury1 motivated a century-long race for higher-temperature superconductors. Owing to the progress in theoretical calculations,2–5 numerous high-temperature superconducting hydrides have been discovered in the past decade, including H3S (Tc ≈ 191–204 K at 200 GPa)6,7 and LaH10 (Tc ≈ 274–286 K at 210 GPa) with record-high superconducting transition temperatures.8–11 However, the stable presence of these superconducting hydrides requires very high pressures, which largely limits their potential implementations.12–14Among all Bardeen–Cooper–Schrieffer (BCS) superconductors, borides represent a unique category with superconductivity at relatively low pressures. MgB2 has the highest superconducting transition temperature, Tc = 39 K, among all BCS-type superconductors at ambient pressure.15 At present, the discovered bulk superconducting borides with the same stoichiometry as MgB2 include CaB2 (Tc ∼ 50 K![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 or 9.4–28.6 K
16 or 9.4–28.6 K![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17 at ambient pressure, theory), NbB2 (Tc ∼ 9.2 K at ambient pressure, experiment18–20), OsB2 (Tc = 2.1 K at ambient pressure, experiment21), RuB2 (Tc = 1.6 K at ambient pressure, experiment21), ScB2 (Tc = 1.5 K at ambient pressure, experiment22), WB2 (maximum Tc = 15 K at 100 GPa, experiment23), ZrB2 (Tc = 5.5 K at ambient pressure, experiment24), SiB2 (Tc = 21 K at ambient pressure, theory25) and MoB2 (Tc = 32 K at 100 GPa, experiment26). Superconducting borides with other stoichiometries include X7B3 (X = Re and Ru with Tc = 3.3 and 2.6 K, respectively, at ambient pressure, experiment27,28), Re3B (Tc = 4.8 K at ambient pressure, experiment28), X2B (X = Mo, Re, Ta and W with Tc = 5.1, 2.8, 3.1 and 3.2 K, respectively, at ambient pressure, experiment27), XB (X = Hf, Nb, Mo, Ta and Zr with Tc = 3.1, 8.3, 0.5, 4.0 and 2.8–3.4 K, respectively, at ambient pressure, experiment27), FeB4 (Tc = 2.9 K at ambient pressure, theory and experiment29,30), XB5 (X = Na, K, Rb, Ca, Sr, Ba, Sc and Y with Tc = 17.5, 14.7, 18.6, 6.6, 6.8, 16.3, 14.2 and 12.3 K, respectively, at ambient pressure, theory31), BeB6 (Tc = 24 K at 4 GPa, theory32), CB6 (Tc = 12.5 K at ambient pressure, theory33), MgB6 (Tc = 9.5 K at 32.6 GPa, theory34), ScB6 (in P21/m-, C2/m- and Cmcm-structure with Tc = 5.8 K at ambient pressure, 2.2 K at 500 GPa, and 2.6 K at 800 GPa, respectively, theory35), XB6 (X = Nb, La, Th and Y with Tc = 3.0, 5.7, 0.74 and 7.1 K, respectively, at ambient pressure, experiment27), XB7 (X = Li, Na, K, Mg, Ca and Sr with Tc = 21.6, 18.3, 26.2, 29.3, 7.7 and 12.7 K, respectively, at ambient pressure, theory36), RbB6 and RbB8 (Tc = 7.3–11.6 K and 4.8–7.5 K at ambient pressure, respectively, theory37), YB6 (Tc = 7.2 K at ambient pressure, experiment38), LaB8 (Tc = 14 K
17 at ambient pressure, theory), NbB2 (Tc ∼ 9.2 K at ambient pressure, experiment18–20), OsB2 (Tc = 2.1 K at ambient pressure, experiment21), RuB2 (Tc = 1.6 K at ambient pressure, experiment21), ScB2 (Tc = 1.5 K at ambient pressure, experiment22), WB2 (maximum Tc = 15 K at 100 GPa, experiment23), ZrB2 (Tc = 5.5 K at ambient pressure, experiment24), SiB2 (Tc = 21 K at ambient pressure, theory25) and MoB2 (Tc = 32 K at 100 GPa, experiment26). Superconducting borides with other stoichiometries include X7B3 (X = Re and Ru with Tc = 3.3 and 2.6 K, respectively, at ambient pressure, experiment27,28), Re3B (Tc = 4.8 K at ambient pressure, experiment28), X2B (X = Mo, Re, Ta and W with Tc = 5.1, 2.8, 3.1 and 3.2 K, respectively, at ambient pressure, experiment27), XB (X = Hf, Nb, Mo, Ta and Zr with Tc = 3.1, 8.3, 0.5, 4.0 and 2.8–3.4 K, respectively, at ambient pressure, experiment27), FeB4 (Tc = 2.9 K at ambient pressure, theory and experiment29,30), XB5 (X = Na, K, Rb, Ca, Sr, Ba, Sc and Y with Tc = 17.5, 14.7, 18.6, 6.6, 6.8, 16.3, 14.2 and 12.3 K, respectively, at ambient pressure, theory31), BeB6 (Tc = 24 K at 4 GPa, theory32), CB6 (Tc = 12.5 K at ambient pressure, theory33), MgB6 (Tc = 9.5 K at 32.6 GPa, theory34), ScB6 (in P21/m-, C2/m- and Cmcm-structure with Tc = 5.8 K at ambient pressure, 2.2 K at 500 GPa, and 2.6 K at 800 GPa, respectively, theory35), XB6 (X = Nb, La, Th and Y with Tc = 3.0, 5.7, 0.74 and 7.1 K, respectively, at ambient pressure, experiment27), XB7 (X = Li, Na, K, Mg, Ca and Sr with Tc = 21.6, 18.3, 26.2, 29.3, 7.7 and 12.7 K, respectively, at ambient pressure, theory36), RbB6 and RbB8 (Tc = 7.3–11.6 K and 4.8–7.5 K at ambient pressure, respectively, theory37), YB6 (Tc = 7.2 K at ambient pressure, experiment38), LaB8 (Tc = 14 K![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 39 or 20 K
39 or 20 K![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 at ambient pressure, theory), XB12 (X = Nb, La, Th, Y and Zr with Tc = 3.0, 5.7, 0.74, 7.1 and 5.8 K, respectively, at ambient pressure, experiment27,41–43), ternary borides like SrB3C3 (Tc = 22 K at 23 GPa, theory and experiment44), or even quaternary borides RbYbB6C6 and RbBaB6C6 (both with Tc ≈ 71 K at ambient pressure, theory45).
40 at ambient pressure, theory), XB12 (X = Nb, La, Th, Y and Zr with Tc = 3.0, 5.7, 0.74, 7.1 and 5.8 K, respectively, at ambient pressure, experiment27,41–43), ternary borides like SrB3C3 (Tc = 22 K at 23 GPa, theory and experiment44), or even quaternary borides RbYbB6C6 and RbBaB6C6 (both with Tc ≈ 71 K at ambient pressure, theory45).
There have been extensive efforts in searching for similar metal-boride superconductors since the discovery of MgB2 as presented above. Unfortunately, the outcomes have been discouraging in that the Tc values of most metal borides are much lower than that of MgB2. However, the recent experimental discovery of superconducting MoB2 with Tc = 32 K at 100 GPa![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 26 has ignited further enthusiasm in looking for superconducting transition-metal borides at lower pressures or even at ambient pressure. In addition, the superconducting mechanism of MoB2 is suggested to be very different from MgB2. In MgB2 the B-p electrons play a dominant role in its superconductivity,46,47 while in MoB2 its Mo-4d electrons contribute majorly.26,48 This raises two essential questions: (1) Can we find other superconducting transition-metal borides neighboring to MoB2, with Tc at least above 10 K and at moderate pressures? (2) Does the superconducting mechanism of MoB2 apply to other superconducting transition-metal borides? To answer these two questions, we decided to work on borides of technetium, which comes immediately after molybdenum in the periodic table.
26 has ignited further enthusiasm in looking for superconducting transition-metal borides at lower pressures or even at ambient pressure. In addition, the superconducting mechanism of MoB2 is suggested to be very different from MgB2. In MgB2 the B-p electrons play a dominant role in its superconductivity,46,47 while in MoB2 its Mo-4d electrons contribute majorly.26,48 This raises two essential questions: (1) Can we find other superconducting transition-metal borides neighboring to MoB2, with Tc at least above 10 K and at moderate pressures? (2) Does the superconducting mechanism of MoB2 apply to other superconducting transition-metal borides? To answer these two questions, we decided to work on borides of technetium, which comes immediately after molybdenum in the periodic table.
Although technetium is rare in nature, technetium-based compounds have been investigated in multiple disciplines in the past. ATcO3 perovskites (where A = Ca, Sr, Ba) have attracted extensive interest due to their extremely high antiferromagnetic Néel temperatures (750–1200 K).49–52 First-principle calculations have predicted a stable structure of ternary compound Tc2AlB2 with Cmcm symmetry at ambient pressure.53 Recently, technetium hydrides were theoretically predicted and then experimentally synthesized under high pressure.54,55
Technetium borides have been extensively investigated due to their outstanding mechanical properties.56–76 Three technetium borides have long been synthesized experimentally at ambient pressure,56 Tc3B (Cmcm) with an orthorhombic structure, and Tc7B3 (P63/mmc) and TcB2 (P63/mmc, Vickers hardness 38.4 GPa![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 76 or 39.4 GPa
76 or 39.4 GPa![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 75) with hexagonal structures. Later theoretical calculations also proposed three stoichiometries of TcB, TcB3 and TcB4.62,70–75 First-principle DFT calculations by Li et al.62 suggested that hexagonal TcB (P
75) with hexagonal structures. Later theoretical calculations also proposed three stoichiometries of TcB, TcB3 and TcB4.62,70–75 First-principle DFT calculations by Li et al.62 suggested that hexagonal TcB (P![[6 with combining macron]](https://www.rsc.org/images/entities/char_0036_0304.gif) m2) could be energetically stable. Structural searches by Wu et al.71 found a thermodynamically stable TcB (Cmcm) structure above 8 GPa. Later structural searches by Zhang et al.72 led to the argument that TcB (P
m2) could be energetically stable. Structural searches by Wu et al.71 found a thermodynamically stable TcB (Cmcm) structure above 8 GPa. Later structural searches by Zhang et al.72 led to the argument that TcB (P![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m1, Vickers hardness 30.3 GPa) could be energetically more stable than the above two structures. Structural predictions by Van Der Geest et al.70 suggest there are two thermodynamically stable structures, TcB (Pnma) and TcB4 (P63/mmc), at 30 GPa. First-principle DFT calculations by Miao et al.74 reported a thermodynamically stable TcB3 structure (P
m1, Vickers hardness 30.3 GPa) could be energetically more stable than the above two structures. Structural predictions by Van Der Geest et al.70 suggest there are two thermodynamically stable structures, TcB (Pnma) and TcB4 (P63/mmc), at 30 GPa. First-principle DFT calculations by Miao et al.74 reported a thermodynamically stable TcB3 structure (P![[6 with combining macron]](https://www.rsc.org/images/entities/char_0036_0304.gif) m2, Vickers hardness 29 GPa) above 4 GPa. Structural searches by Ying et al.73,75 suggested two structures, TcB3 (P
m2, Vickers hardness 29 GPa) above 4 GPa. Structural searches by Ying et al.73,75 suggested two structures, TcB3 (P![[6 with combining macron]](https://www.rsc.org/images/entities/char_0036_0304.gif) m2, Vickers hardness 30.7 GPa) and TcB4 (P63/mmc, Vickers hardness 32.4 GPa), that are thermodynamically stable at 0 and 100 GPa, respectively. However, the discussions of possible superconductivity in technetium borides are totally absent.
m2, Vickers hardness 30.7 GPa) and TcB4 (P63/mmc, Vickers hardness 32.4 GPa), that are thermodynamically stable at 0 and 100 GPa, respectively. However, the discussions of possible superconductivity in technetium borides are totally absent.
In this paper, we choose to search the technetium–boron binary system for new superconductors. A comprehensive phase diagram of all thermodynamically stable technetium borides up to 180 GPa has been derived. We also found five superconducting technetium borides possessing metastable states for the first time, including TcB (P63/mmc), TcB2 (P6/mmm), Tc2B (I4/mcm), Tc3B (P4/mmm) and TcB (Cmcm), that remain dynamically stable at low or even ambient pressures. The mechanical properties of these superconducting technetium borides have been investigated as well.
2 Methods
The structure prediction for technetium–boron binary crystals is performed by the CALYPSO package.77 The crystal structures and the X-ray diffraction (XRD) patterns are generated by the VESTA package. The electronic structures and the phonon properties are calculated using the QUANTUM-ESPRESSO (QE) package.78 The plane-wave kinetic-energy cutoff and the charge density energy cutoff are 100 Ry and 400 Ry, respectively. An optimized norm-conversing pseudopotential with valence electron configurations of Tc-4p6 4d5 5s2 and B-2s2 2p1 and a Methfessel–Paxton smearing79 width of 0.02 Ry is used.The dynamic matrix and the electron–phonon coupling (EPC) constant λ are calculated using the density functional perturbation theory.80 The superconducting transition temperature is estimated following the Allen-Dynes modified McMillan equation,81
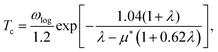 | (1) |
3 Results and discussion
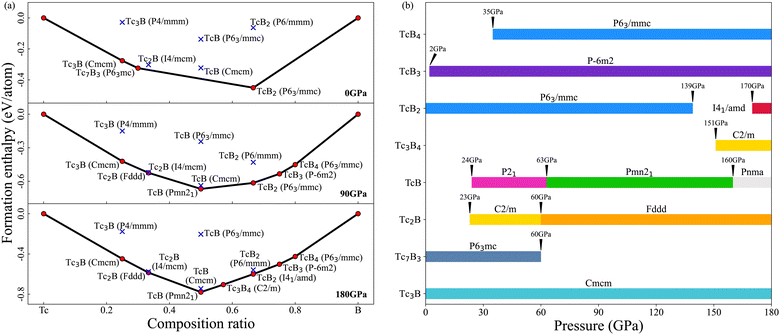
3.1 Convex hull and phase diagram
We have done variable-composition and fixed-composition structure searches in the Tc–B system at pressures of 0, 90 and 180 GPa. Thermodynamically stable structures and the derived composition–pressure phase diagram are presented in Fig. 1. Three existing technetium borides at ambient pressure, Tc3B (Cmcm), Tc7B3 (P63/mmc) and TcB2 (P63/mmc), have been successfully identified. Tc3B (Cmcm) is thermodynamically stable up to 180 GPa in our study. In contrast, Tc7B3 (P63/mmc) and TcB2 (P63/mmc) stop being energetically favorable above 60 GPa and 139 GPa, respectively, and a new TcB2 (I41/amd) thermodynamically stable phase shows up above 170 GPa. Two previously predicted structures, TcB3 (P![[6 with combining macron]](https://www.rsc.org/images/entities/char_0036_0304.gif) m2)73,74 and TcB4 (P63/mmc),70,75 also have been found in our calculations, and these structures are thermodynamically stable above 2 and 35 GPa, respectively. We found a TcB (P21) structure that is thermodynamically stable above 24 GPa, then transfers into a Pmn21 structure at 63 GPa, and finally into the previously predicted Pnma structure70 at 160 GPa. We also discovered technetium borides with two new stoichiometries, Tc3B4 and Tc2B. Tc3B4 (C2/m) is thermodynamically stable above 151 GPa. The Tc2B (C2/m) structure is thermodynamically stable above 23 GPa, then transfers into an Fddd structure at 60 GPa. The crystal structure information and the XRD patterns of all thermodynamically stable phases are presented in Table S4 and Fig. S4 of the ESI.†
m2)73,74 and TcB4 (P63/mmc),70,75 also have been found in our calculations, and these structures are thermodynamically stable above 2 and 35 GPa, respectively. We found a TcB (P21) structure that is thermodynamically stable above 24 GPa, then transfers into a Pmn21 structure at 63 GPa, and finally into the previously predicted Pnma structure70 at 160 GPa. We also discovered technetium borides with two new stoichiometries, Tc3B4 and Tc2B. Tc3B4 (C2/m) is thermodynamically stable above 151 GPa. The Tc2B (C2/m) structure is thermodynamically stable above 23 GPa, then transfers into an Fddd structure at 60 GPa. The crystal structure information and the XRD patterns of all thermodynamically stable phases are presented in Table S4 and Fig. S4 of the ESI.†
3.2 Superconductivity of thermodynamically metastable technetium borides
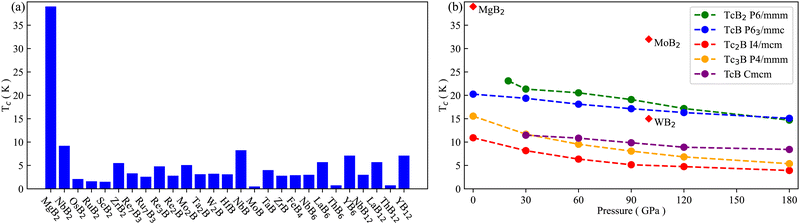
We have examined potential superconductivity in the predicted technetium borides, including all thermodynamically stable structures and the thermodynamically metastable structures within a range of 300 meV above the convex hull. A total of five thermodynamically metastable technetium borides have been found to be superconducting at 180 GPa, including TcB2 (P6/mmm, 42 meV per atom above the hull), TcB (P63/mmc, 255 meV per atom above the hull), Tc2B (I4/mcm, 2 meV per atom above the hull), Tc3B (P4/mmm, 248 meV per atom above the hull) and TcB (Cmcm, 25 meV per atom above the hull). These five superconducting technetium borides stay dynamically stable at decreased pressures. The minimum dynamically stable pressures of TcB2 (P6/mmm) and TcB (Cmcm) are 20 and 30 GPa, respectively, while TcB (P63/mmc), Tc2B (I4/mcm) and Tc3B (P4/mmm) are dynamically stable even at ambient pressure.The superconducting transition temperatures of the technetium borides are presented in Fig. 2, together with the transition temperatures of other known superconducting metal borides that have been measured experimentally for benchmarking. As shown in Fig. 2b, the superconducting transition temperatures of the technetium borides in our study increase at decreased pressure. Although the superconducting transition temperatures of all five technetium borides are always lower than these of MgB2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 and MoB2,26 they are higher than the superconducting transition temperatures of other metal borides in Fig. 2a at their lowest dynamically stable pressures. In addition, TcB2 (P6/mmm) and TcB (P63/mmc) have higher superconducting transition temperatures at 100 GPa than WB2 with Tc = 15 K.23
15 and MoB2,26 they are higher than the superconducting transition temperatures of other metal borides in Fig. 2a at their lowest dynamically stable pressures. In addition, TcB2 (P6/mmm) and TcB (P63/mmc) have higher superconducting transition temperatures at 100 GPa than WB2 with Tc = 15 K.23
We also present the superconducting transition temperatures of the five technetium borides at their lowest dynamically stable pressures, together with their total electronic DOS at the Fermi level N(EF), the EPC parameter λ and the logarithmic average frequency ωlog in Table 1. TcB2 (P6/mmm) has the highest superconducting transition temperature of 23.1 K at 20 GPa, which comes from its largest EPC parameter of λ = 1.85. In contrast, TcB (Cmcm) has a much lower superconducting transition temperature of 11.5 K at 30 GPa due to its small EPC parameter of λ = 0.96. The superconducting transition temperatures of TcB (P63/mmc), Tc2B (I4/mcm) and Tc3B (P4/mmm) at 0 GPa are 20.2, 10.9, and 12.9 K, respectively. Although the EPC parameters λ of these metastable technetium borides are not small, their logarithmic average frequencies ωlog are rather low with a maximum of 165.1 cm−1, which limits their superconducting transition temperature. This is in sharp contrast with MgB2, which has a smaller λ value of 0.87 but a much larger ωlog value of 504 cm−1, and the highest BCS-type superconducting transition temperature of 39 K at ambient pressure.85
| Formula | Space group | P (GPa) | N(EF) (states per eV per f.u.) | λ | ω log (cm−1) | T c (K) |
|---|---|---|---|---|---|---|
| TcB2 | P6/mmm | 20 | 1.41 | 1.85 | 125.1 | 23.1 |
| TcB | P63/mmc | 0 | 1.63 | 1.56 | 126.1 | 20.2 |
| Tc2B | I4/mcm | 0 | 1.75 | 0.85 | 165.1 | 10.9 |
| Tc3B | P4/mmm | 0 | 2.85 | 0.92 | 162.9 | 12.9 |
| TcB | Cmcm | 30 | 1.05 | 0.96 | 135.5 | 11.5 |
The thermodynamically metastable nature of the discovered superconducting technetium borides doesn't necessarily exclude their experimental synthesis. Metastable materials have long been synthesized and implemented,86 typically like fullerene C60. As for superconductors, DFT calculations predict NdH9 (P63/mmc) has a formation enthalpy that is 35 meV per atom above the convex hull at 150 GPa, yet it has been successfully synthesized with Tc ≈ 4.5 K.87 Several metastable borides have been predicted to be superconducting in recent structural searches. Xia et al. discovered thermodynamically metastable CB6 with a superconducting transition temperature of 12.5 K at ambient pressure.33 Zhang et al. also found thermodynamically metastable RbB6 (Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m) and RbB8 (Immm) with superconducting transition temperatures of 7.3–11.6 and 4.8–7.5 K at ambient pressure, respectively.37 These works further validate the importance and necessity of our discoveries of superconducting technetium borides.
m) and RbB8 (Immm) with superconducting transition temperatures of 7.3–11.6 and 4.8–7.5 K at ambient pressure, respectively.37 These works further validate the importance and necessity of our discoveries of superconducting technetium borides.
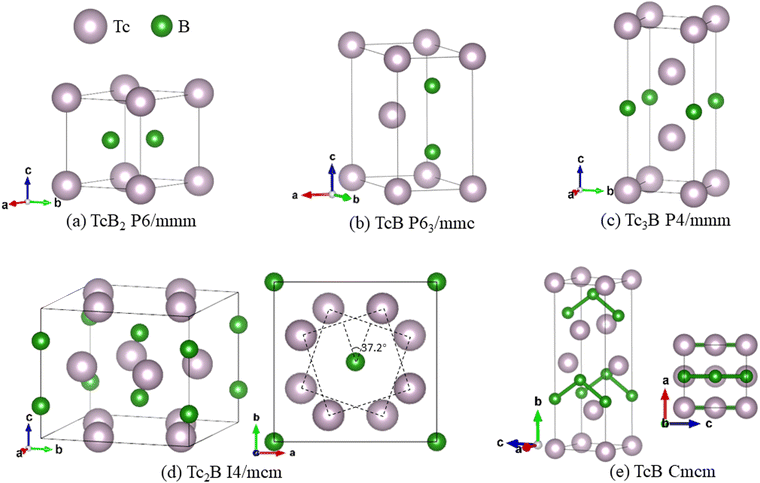
3.3 Crystal structures
The crystal structures of the five superconducting technetium borides in our study are presented in Fig. 3. TcB2 (P6/mmm) shares exactly the same crystal structure with MgB2 and MoB2. TcB (P63/mmc) has a TiAs-type structure, in which the rhombus Tc-layers are AB-stacking along the c-axis and the rhombus B-layers are sandwiched between the neighbouring Tc-layers. Tc3B (P4/mmm) has square Tc-layers stacking in an ABB-pattern along the c-axis, and the square B-layers are located between the two Tc-layers of the BB-pattern. Tc2B (I4/mcm) consists of square Tc-layers AB-stacking along the c-axis, where the neighbouring Tc-layers are twisted by 37.2 degrees. The B-layers in Tc2B (I4/mcm) are sandwiched between the neighbouring Tc-layers as well. In TcB (Cmcm), the square Tc-layers stack in an ABCD-pattern along the b-axis, and the B atoms form zig-zag chains along the c-axis between the AB and CD Tc-layers. The angle of the zig-zag chain of the B atoms is around 108.8 degrees. Crystal structure information and the XRD patterns of the superconducting technetium borides are presented in Table S3 and Fig. S3 of the ESI.†3.4 Electronic structures
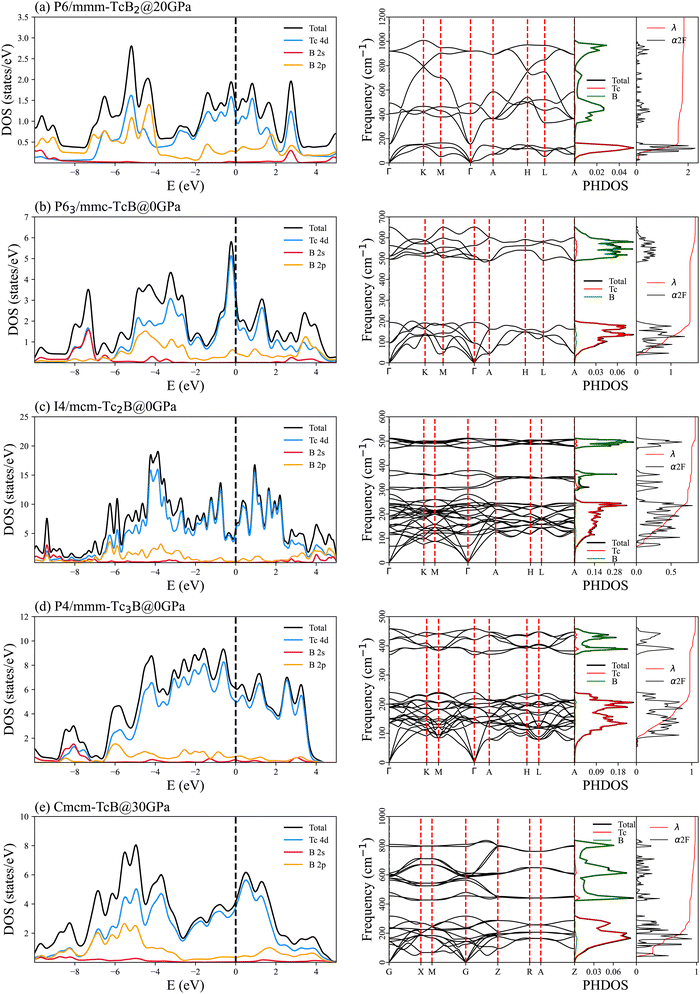
The electronic DOS of superconducting technetium borides at their lowest stabilizing pressure are presented in the left column of Fig. 4. The electronic DOS of all technetium borides share certain features. The total DOS at the Fermi level are dominated by the states of the Tc-4d bands. Although the B-2p DOS have considerable weight away the Fermi level, its contribution is minor at the Fermi level, if not zero. The B-2s DOS almost vanish around the Fermi level, which makes the B-2s bands almost irrelevant for electronic conduction. Our DOS results for technetium borides have a close resemblance to those for the transition-metal boride MoB2,26 while they are in obvious contrast to results for the alkali-earth-metal boride MgB2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 46,47 and the alkali-metal boride RbB6.37 In both MgB2
46,47 and the alkali-metal boride RbB6.37 In both MgB2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 46,47 and RbB6,37 the major DOS at the Fermi level are contributed by the B-p bands. But in both the superconducting technetium borides of our study and MoB2,26 the 4d electronic states play dominant roles around the Fermi level.
46,47 and RbB6,37 the major DOS at the Fermi level are contributed by the B-p bands. But in both the superconducting technetium borides of our study and MoB2,26 the 4d electronic states play dominant roles around the Fermi level.
The electronic band structures, the partial DOS of the Tc-4d orbitals and the Fermi surfaces of the superconducting technetium borides are presented in Fig. S2 of the ESI.† For all five superconducting technetium borides, either their band structure or their Fermi surface show obvious electronic dispersion in three dimensions. Typically, for example, TcB2, which shares the same crystal structure as MgB2 and MoB2, has a three-dimensional Fermi surface like MoB2,26,88 while being distinct from the quasi-two-dimensional Fermi surface of MgB2.47,89
3.5 Dynamic stability and electron–phonon coupling
The phonon spectra, the PHDOS, the Eliashberg functional α2F(ω) and the corresponding integrated EPC constant λ of the superconducting technetium borides at their lowest dynamically stable pressures are presented in the right column of Fig. 4. There is no sign of an imaginary frequency in the phonon spectra of all five superconducting technetium borides, which proves the dynamic stability of these structures at the corresponding pressures. The distribution of the PHDOS and the Eliashberg spectral functional α2F(ω) of the superconducting technetium borides show clear separation between the low-frequency phonon modes of the heavier Tc atoms and the high frequency phonon modes of the lighter B atoms. This enables us to separate the integrated EPC constant λ into two parts, the EPC from the Tc atoms λTc, and the EPC from the B atoms λB. The ratio of EPC from oscillation of the Tc atoms relative to the total EPC, λTc/λ, are 0.883, 0.910, 0.873, 0.907 and 0.911 for TcB2 (P6/mmm), TcB (P63/mmc), Tc2B (I4/mcm), Tc3B (P4/mmm) and TcB (Cmcm), respectively. It indicates that superconductivity in these five technetium borides mainly originates from the coupling between the Tc-4d electrons and the low frequency phonon modes of the Tc atoms.The superconducting mechanism of our predicted technetium borides is similar to that in the transition-metal boride MoB2, whose superconductivity mainly originates from the coupling between the Mo-4d electrons and the low frequency Mo-phonon modes.26 However, the superconducting scenarios in the alkali-earth-metal boride MgB2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 46,47 and the alkali-metal boride RbB6
46,47 and the alkali-metal boride RbB6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 37 are very different in that the couplings between the B-2p electrons and the high-frequency B-phonon modes play dominant roles. At least three isotopes of technetium have reasonably long half-lives (Tc-97, Tc-98 and Tc-99 at 4.2 × 106, 6.6 × 106 and 2.13 × 105 years, respectively). Therefore, we suggest experiments on the isotope effects of technetium to examine our prediction.
37 are very different in that the couplings between the B-2p electrons and the high-frequency B-phonon modes play dominant roles. At least three isotopes of technetium have reasonably long half-lives (Tc-97, Tc-98 and Tc-99 at 4.2 × 106, 6.6 × 106 and 2.13 × 105 years, respectively). Therefore, we suggest experiments on the isotope effects of technetium to examine our prediction.
Observations in the phonon spectra, PHDOS and EPC of the superconducting technetium borides are consistent with their relatively smaller logarithmic average frequency ωlog as listed in Table 1, since Tc atoms are much heavier than B atoms. The enhanced λ plus small ωlog characteristics of TcB2 have also been seen in the iso-structural superconductor TlBi2 of heavy atomic mass,90 with λ = 1.4, ωlog = 37 cm−1 and rather low Tc = 5.5 K. Recent work suggests that the introduction of hydrogen atoms into the non-superconducting transition-metal boride Ti2B2 will result in superconducting Ti2B2H4 (Tc = 48.6 K at ambient pressure), through expansion of the frequency range of the phonon spectrum and consequently enlarged electron–phonon coupling.91 Similar hydrogenation probably helps in elevating the superconducting transition temperatures of technetium borides by the enlarged ωlog.
Another interesting observation on the discovered superconducting technetium borides is that the Fermi levels of TcB2 and TcB fall closely above the peak positions of their DOS, as shown in Fig. 4. Since the EPC in technetium borides is controlled by the coupling between the Tc-4d electrons and the oscillation of the Tc atoms, slight hole-doping could lower the Fermi level, thus enhancing the effective number of electrons participating in the superconducting pairing and therefore increasing the superconducting transition temperatures.
3.6 Hardness
We also calculated the Vickers hardness of the discovered superconducting technetium borides as presented in Table 2. At ambient pressure, TcB (P63/mmc), Tc2B (I4/mcm) and Tc3B (P4/mmm) have Vickers hardness values of 2.8–4.8, 11.8–13.0 and 10.0–11.3 GPa, respectively. The Vickers hardness values of TcB2 (P6/mmm) and TcB (Cmcm) are 9.8–11.3 GPa and 12.2–13.7 GPa at pressures of 20 and 30 GPa, respectively. The superconducting technetium borides in our study have lower hardness values than those previously stated for superconducting superhard borides, for example RbB6 (Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m, Vickers hardness of 19.7 GPa at ambient pressure) and RbB8 (Immm, Vickers hardness of 36.9 GPa at ambient pressure).37 Other mechanical parameters including elastic constants Cij, bulk modulus B, and shear modulus G at their lowest dynamically stable pressures were also calculated. Mechanical stability criteria92 related to the elastic constants of these superconducting technetium borides are fulfilled as presented in the ESI.†
m, Vickers hardness of 19.7 GPa at ambient pressure) and RbB8 (Immm, Vickers hardness of 36.9 GPa at ambient pressure).37 Other mechanical parameters including elastic constants Cij, bulk modulus B, and shear modulus G at their lowest dynamically stable pressures were also calculated. Mechanical stability criteria92 related to the elastic constants of these superconducting technetium borides are fulfilled as presented in the ESI.†
4 Conclusions
In summary, we have conducted thorough structural searches in the technetium–boron binary system. An updated composition–pressure phase diagram for technetium borides at up to 180 GPa have been derived, including two new stoichiometries as Tc3B4 and Tc2B. More importantly, we also found five unprecedented superconducting technetium borides that remain dynamically stable at moderate or even ambient pressures. Among these thermodynamically metastable superconducting technetium borides, TcB2 (P6/mmm) has the highest superconducting transition temperature of 23.1 K at 20 GPa, and TcB (P63/mmc) has the highest superconducting transition temperature of 20.2 K at ambient pressure. The superconductivity in these technetium borides mainly originates from the coupling between the dominant presence of Tc-4d electronic states around the Fermi level and the low-frequency vibrational modes of the technetium atoms, which are closely analogous to another transition-metal-boride MoB2. Our calculations not only identified that superconducting TcB2 (P6/mmm) has the same crystal structure as MgB2 and MoB2, but also led to the discovery of a series of superconducting technetium borides with diversified crystal structures. This work proves the rich structures and stoichiometries in superconducting technetium borides at high pressures, thus indicating the necessity for extended research in the discovery of new superconducting transition-metal borides.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
P. Z., Y. D. Q. and S. X. Y. designed the project; X. R. T. conducted the structure searches; P. Z. and A. Q. Y. calculated the electronic structures, the phonon spectra and the superconducting transition temperatures; all authors prepared the manuscript together. We would like to thank Jianjun Ying, Defang Duan and Zihao Huo for the important discussions in preparing this manuscript. This work is supported by the National Natural Science Foundation of China (Grants No. 11604255), the Fundamental Research Funds for the Central Universities (Grants No. xzy022023011 and xhj032021014-04), and the Natural Science Basic Research Program of Shaanxi (Grants No. 2021JM-001). Shuxiang Yang is supported by the Key Research Projects of Zhejiang Lab (Grant No. 2021PB0AC02). The computations were performed at the TianHe-2 national supercomputing center in Guangzhou and the HPC platform of Xian Jiaotong University.References
- H. K. Onnes, Proceedings Koninklijke Akademie van Wetenschappen te Amsterdam, 1911, pp. 1274–1276 Search PubMed.
- C. J. Pickard and R. Needs, J. Phys.: Condens. Matter, 2011, 23, 053201 CrossRef PubMed.
- Y. Wang, J. Lv, L. Zhu and Y. Ma, Comput. Phys. Commun., 2012, 183, 2063–2070 CrossRef CAS.
- A. R. Oganov and C. W. Glass, J. Chem. Phys., 2006, 124, 244704 CrossRef PubMed.
- F. Giustino, Rev. Mod. Phys., 2017, 89, 015003 CrossRef.
- D. Duan, Y. Liu, F. Tian, D. Li, X. Huang, Z. Zhao, H. Yu, B. Liu, W. Tian and T. Cui, Sci. Rep., 2014, 4, 6968 CrossRef CAS PubMed.
- A. Drozdov, M. Eremets, I. Troyan, V. Ksenofontov and S. I. Shylin, Nature, 2015, 525, 73–76 CrossRef CAS PubMed.
- H. Liu, I. I. Naumov, R. Hoffmann, N. Ashcroft and R. J. Hemley, Proc. Natl. Acad. Sci. U. S. A., 2017, 114, 6990–6995 CrossRef CAS PubMed.
- Z. M. Geballe, H. Liu, A. K. Mishra, M. Ahart, M. Somayazulu, Y. Meng, M. Baldini and R. J. Hemley, Angew. Chem., Int. Ed., 2018, 57, 688–692 CrossRef CAS PubMed.
- M. Somayazulu, M. Ahart, A. K. Mishra, Z. M. Geballe, M. Baldini, Y. Meng, V. V. Struzhkin and R. J. Hemley, Phys. Rev. Lett., 2019, 122, 027001 CrossRef CAS PubMed.
- A. Drozdov, P. Kong, V. Minkov, S. Besedin, M. Kuzovnikov, S. Mozaffari, L. Balicas, F. Balakirev, D. Graf and V. Prakapenka, Nature, 2019, 569, 528–531 CrossRef CAS PubMed.
- X. Tao, A. Yang, S. Yang, Y. Quan and P. Zhang, Sci. Bull., 2023, 68, 1372–1378 CrossRef CAS PubMed.
- A. Yang, X. Tao, Y. Quan and P. Zhang, Phys. B, 2024, 677, 415706 CrossRef CAS.
- W. Sun, B. Chen, X. Li, F. Peng, A. Hermann and C. Lu, Phys. Rev. B, 2023, 107, 214511 CrossRef CAS.
- J. Nagamatsu, N. Nakagawa, T. Muranaka, Y. Zenitani and J. Akimitsu, Nature, 2001, 410, 63–64 CrossRef CAS PubMed.
- H. J. Choi, S. G. Louie and M. L. Cohen, Phys. Rev. B: Condens. Matter Mater. Phys., 2009, 80, 064503 CrossRef.
- Z. Yu, T. Bo, B. Liu, Z. Fu, H. Wang, S. Xu, T. Xia, S. Li, S. Meng and M. Liu, Phys. Rev. B, 2022, 105, 214517 CrossRef CAS.
- J. Schirber, D. Overmyer, B. Morosin, E. Venturini, R. Baughman, D. Emin, H. Klesnar and T. Aselage, Phys. Rev. B: Condens. Matter Mater. Phys., 1992, 45, 10787 CrossRef CAS PubMed.
- A. Yamamoto, C. Takao, T. Masui, M. Izumi and S. Tajima, Phys. C, 2002, 383, 197–206 CrossRef CAS.
- H. Takeya, K. Togano, Y. S. Sung, T. Mochiku and K. Hirata, Phys. C, 2004, 408–410, 144–145 CrossRef CAS.
- Y. Singh, A. Niazi, M. D. Vannette, R. Prozorov and D. C. Johnston, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 76, 214510 CrossRef.
- G. V. Samsonov, Handbook of Refractory Compounds, Springer, New York, 1980 Search PubMed.
- C. Pei, J. Zhang, C. Gong, Q. Wang, L. Gao, Y. Zhao, S. Tian, W. Cao, C. Li, Z.-Y. Lu, H. Lei, K. Liu and Y. Qi, Sci. China: Phys., Mech. Astron., 2022, 65, 287412 CAS.
- L. Leyarovska and E. Leyarovski, J. Less-Common Met., 1979, 67, 249–255 CrossRef CAS.
- X. Liang, A. Bergara, Y. Xie, L. Wang, R. Sun, Y. Gao, X.-F. Zhou, B. Xu, J. He, D. Yu, G. Gao and Y. Tian, Phys. Rev. B, 2020, 101, 014112 CrossRef CAS.
- C. Pei, J. Zhang, Q. Wang, Y. Zhao, L. Gao, C. Gong, S. Tian, R. Luo, M. Li, W. Yang, Z.-Y. Lu, H. Lei, K. Liu and Y. Qi, Natl. Sci. Rev., 2023, 10, nwad034 CrossRef CAS PubMed.
- C. Buzea and T. Yamashita, Supercond. Sci. Technol., 2001, 14, R115 CrossRef CAS.
- A. Kawano, Y. Mizuta, H. Takagiwa, T. Muranaka and J. Akimitsu, J. Phys. Soc. Jpn., 2003, 72, 1724–1728 CrossRef CAS.
- A. N. Kolmogorov, S. Shah, E. R. Margine, A. F. Bialon, T. Hammerschmidt and R. Drautz, Phys. Rev. Lett., 2010, 105, 217003 CrossRef CAS PubMed.
- H. Gou, N. Dubrovinskaia, E. Bykova, A. A. Tsirlin, D. Kasinathan, W. Schnelle, A. Richter, M. Merlini, M. Hanfland, A. M. Abakumov, D. Batuk, G. Van Tendeloo, Y. Nakajima, A. N. Kolmogorov and L. Dubrovinsky, Phys. Rev. Lett., 2013, 111, 157002 CrossRef PubMed.
- H. Xie, H. Wang, F. Qin, W. Han, S. Wang, Y. Wang, F. Tian and D. Duan, Matter Radiat. Extremes, 2023, 8, 058404 CrossRef CAS.
- L. Wu, B. Wan, H. Liu, H. Gou, Y. Yao, Z. Li, J. Zhang, F. Gao and H.-K. Mao, J. Phys. Chem. Lett., 2016, 7, 4898–4904 CrossRef CAS PubMed.
- K. Xia, M. Ma, C. Liu, H. Gao, Q. Chen, J. He, J. Sun, H.-T. Wang, Y. Tian and D. Xing, Mater. Today Phys., 2017, 3, 76–84 CrossRef.
- L. Duan, J. Su, N. Gong, B. Wan, P. Chen, P. Zhou, Z. Wang, Z. Li and L. Wu, Dalton Trans., 2019, 48, 14299–14305 RSC.
- J. Du, X. Li and F. Peng, Phys. Chem. Chem. Phys., 2022, 24, 10079–10084 RSC.
- S. Han, L. Yu, Y. Liu, B. Zhao, C. Wang, X. Chen, Y. Zhang, R. Yu and X. Liu, Adv. Funct. Mater., 2023, 33, 2213377 CrossRef CAS.
- P. Zhang, Y. Tian, Y. Yang, H. Liu and G. Liu, Phys. Rev. Res., 2023, 5, 013130 CrossRef CAS.
- R. Lortz, Y. Wang, U. Tutsch, S. Abe, C. Meingast, P. Popovich, W. Knafo, N. Shitsevalova, Y. B. Paderno and A. Junod, Phys. Rev. B: Condens. Matter Mater. Phys., 2006, 73, 024512 CrossRef.
- L. Ma, X. Yang, G. Liu, H. Liu, G. Yang, H. Wang, J. Cai, M. Zhou and H. Wang, Phys. Rev. B, 2021, 104, 174112 CrossRef CAS.
- Y. Liang, M. Xu, S. Lin, X. Yuan, Z. Qu, J. Hao and Y. Li, J. Mater. Chem. C, 2021, 9, 13782–13788 RSC.
- G. Akopov, W. H. Mak, D. Koumoulis, H. Yin, B. Owens-Baird, M. T. Yeung, M. H. Muni, S. Lee, I. Roh, Z. C. Sobell, P. L. Diaconescu, R. Mohammadi, K. Kovnir and R. B. Kaner, J. Am. Chem. Soc., 2019, 141, 9047–9062 CrossRef CAS PubMed.
- J. Teyssier, A. B. Kuzmenko, D. van der Marel, F. Marsiglio, A. B. Liashchenko, N. Shitsevalova and V. Filippov, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 75, 134503 CrossRef.
- B. T. Matthias, T. H. Geballe, K. Andres, E. Corenzwit, G. W. Hull and J. P. Maita, Science, 1968, 159, 530 CrossRef CAS PubMed.
- L. Zhu, H. Liu, M. Somayazulu, Y. Meng, P. A. Guńka, T. B. Shiell, C. Kenney-Benson, S. Chariton, V. B. Prakapenka, H. Yoon, J. A. Horn, J. Paglione, R. Hoffmann, R. E. Cohen and T. A. Strobel, Phys. Rev. Res., 2023, 5, 013012 CrossRef CAS.
- Q. Duan, L. Zhan, J. Shen, X. Zhong and C. Lu, Phys. Rev. B, 2024, 109, 054505 CrossRef CAS.
- J. M. An and W. E. Pickett, Phys. Rev. Lett., 2001, 86, 4366–4369 CrossRef CAS PubMed.
- J. Kortus, I. I. Mazin, K. D. Belashchenko, V. P. Antropov and L. L. Boyer, Phys. Rev. Lett., 2001, 86, 4656–4659 CrossRef CAS PubMed.
- C. Zhou, H. Yu, Z. Zhang, Z. Yu, J. Zhu, K. Bao and T. Cui, Phys. Rev. B, 2024, 109, 064502 CrossRef CAS.
- M. Avdeev, G. J. Thorogood, M. L. Carter, B. J. Kennedy, J. Ting, D. J. Singh and K. S. Wallwork, J. Am. Chem. Soc., 2011, 133, 1654–1657 CrossRef CAS PubMed.
- C. Franchini, T. Archer, J. He, X.-Q. Chen, A. Filippetti and S. Sanvito, Phys. Rev. B: Condens. Matter Mater. Phys., 2011, 83, 220402 CrossRef.
- E. E. Rodriguez, F. Poineau, A. Llobet, B. J. Kennedy, M. Avdeev, G. J. Thorogood, M. L. Carter, R. Seshadri, D. J. Singh and A. K. Cheetham, Phys. Rev. Lett., 2011, 106, 067201 CrossRef PubMed.
- J. Mravlje, M. Aichhorn and A. Georges, Phys. Rev. Lett., 2012, 108, 197202 CrossRef PubMed.
- H. Zhang, J. Wang, M. Khazaei, F. Guegan and G. Frapper, Phys. Chem. Chem. Phys., 2021, 23, 22086–22095 RSC.
- X. Li, H. Liu and F. Peng, Phys. Chem. Chem. Phys., 2016, 18, 28791–28796 RSC.
- D. Zhou, D. V. Semenok, M. A. Volkov, I. A. Troyan, A. Y. Seregin, I. V. Chepkasov, D. A. Sannikov, P. G. Lagoudakis, A. R. Oganov and K. E. German, Phys. Rev. B, 2023, 107, 064102 CrossRef CAS.
- W. Trzebiatowski and J. Rudzinski, J. Less-Common Met., 1964, 6, 244–245 CrossRef CAS.
- D. Armstrong, J. Less-Common Met., 1979, 67, 191–203 CrossRef CAS.
- A. Pallas and K. Larsson, J. Phys. Chem. B, 2006, 110, 5367–5371 CrossRef CAS PubMed.
- Y. Wang, Appl. Phys. Lett., 2007, 91, 101904 CrossRef.
- M. Wang, Y. Li, T. Cui, Y. Ma and G. Zou, Appl. Phys. Lett., 2008, 93, 101905 CrossRef.
- S. Aydin and M. Simsek, Phys. Rev. B: Condens. Matter Mater. Phys., 2009, 80, 134107 CrossRef.
- J. Li, X. Wang, K. Liu, Y. Sun, L. Chen and H. Yang, Phys. B, 2010, 405, 4659–4663 CrossRef CAS.
- W. Chen and J. Jiang, Solid State Commun., 2010, 150, 2093–2096 CrossRef CAS.
- W. Jie Zhao and B. Xu, Comput. Mater. Sci., 2012, 65, 372–376 CrossRef.
- E. Deligoz, K. Çolakoğlu, H. B. Ozisik and Y. O. Ciftci, Solid State Sci., 2012, 14, 794–800 CrossRef CAS.
- M. Zhong, X. Yu Kuang, Z.-H. Wang, P. Shao, L.-P. Ding and X.-F. Huang, J. Phys. Chem. C, 2013, 117, 10643–10652 CrossRef CAS.
- M. Zhang, H. Yan, Q. Wei and H. Wang, Comput. Mater. Sci., 2013, 68, 371–378 CrossRef CAS.
- C. Ying, E. Zhao, L. Lin and Q. Hou, Mod. Phys. Lett. B, 2014, 28, 1450213 CrossRef CAS.
- F.-G. Kuang, X.-Y. Kuang, S.-Y. Kang and X.-F. Huang, Curr. Inorg. Chem., 2015, 5, 143–150 CrossRef CAS.
- A. Van Der Geest and A. Kolmogorov, CALPHAD: Comput. Coupling Phase Diagrams Thermochem., 2014, 46, 184–204 CrossRef CAS.
- J. Wu and G. Yang, Comput. Mater. Sci., 2014, 82, 86–91 CrossRef CAS.
- G. Zhang, T. Bai, H. Yan and Y.-R. Zhao, Chin. Phys. B, 2015, 24, 106104 CrossRef.
- C. Ying, X. Bai, Y. Du, E. Zhao, L. Lin and Q. Hou, Int. J. Mod. Phys. B, 2016, 30, 1650131 CrossRef CAS.
- X. Miao, W. Xing, F. Meng and R. Yu, Solid State Commun., 2017, 252, 40–45 CrossRef CAS.
- C. Ying, T. Liu, L. Lin, E. Zhao and Q. Hou, Comput. Mater. Sci., 2018, 144, 154–160 CrossRef CAS.
- H. Wu, Y. X. Wang, Z. Xin Yan, W. Liu, Z. Q. Wang and J. B. Gu, Appl. Phys. A: Mater. Sci. Process., 2023, 129, 175 CrossRef CAS.
- Y. Wang, J. Lv, L. Zhu and Y. Ma, Comput. Phys. Commun., 2012, 183, 2063–2070 CrossRef CAS.
- P. Giannozzi, S. Baroni, N. Bonini, M. Calandra, R. Car, C. Cavazzoni, D. Ceresoli, G. L. Chiarotti, M. Cococcioni and I. Dabo, J. Phys.: Condens. Matter, 2009, 21, 395502 CrossRef PubMed.
- M. Methfessel and A. Paxton, Phys. Rev. B: Condens. Matter Mater. Phys., 1989, 40, 3616 CrossRef CAS PubMed.
- S. Baroni, S. De Gironcoli, A. Dal Corso and P. Giannozzi, Rev. Mod. Phys., 2001, 73, 515 CrossRef CAS.
- P. B. Allen and R. C. Dynes, Phys. Rev. B: Condens. Matter Mater. Phys., 1975, 12, 905–922 CrossRef CAS.
- P. Morel and P. W. Anderson, Phys. Rev., 1962, 125, 1263–1271 CrossRef.
- X.-Q. Chen, H. Niu, D. Li and Y. Li, Intermetallics, 2011, 19, 1275–1281 CrossRef CAS.
- Y. Tian, B. Xu and Z. Zhao, Int. J. Refract. Met. Hard Mater., 2012, 33, 93–106 CrossRef CAS.
- Y. Kong, O. V. Dolgov, O. Jepsen and O. K. Andersen, Phys. Rev. B: Condens. Matter Mater. Phys., 2001, 64, 020501 CrossRef.
- M. Aykol, S. S. Dwaraknath, W. Sun and K. A. Persson, Sci. Adv., 2018, 4, eaaq0148 CrossRef PubMed.
- D. Zhou, D. V. Semenok, H. Xie, X. Huang, D. Duan, A. Aperis, P. M. Oppeneer, M. Galasso, A. I. Kartsev, A. G. Kvashnin, A. R. Oganov and T. Cui, J. Am. Chem. Soc., 2020, 142, 2803–2811 CrossRef CAS PubMed.
- Y. Quan, K.-W. Lee and W. E. Pickett, Phys. Rev. B, 2021, 104, 224504 CrossRef CAS.
- H. J. Choi, D. Roundy, H. Sun, M. L. Cohen and S. G. Louie, Nature, 2002, 418, 758–760 CrossRef CAS PubMed.
- A. Yang, X. Tao, Y. Quan and P. Zhang, Phys. Rev. B, 2023, 108, 075203 CrossRef CAS.
- Y.-L. Han, Y.-P. Li, L. Yang, H.-D. Liu, N. Jiao, B.-T. Wang, H.-Y. Lu and P. Zhang, Mater. Today Phys., 2023, 30, 100954 CrossRef CAS.
- F. Mouhat and F. Coudert, Phys. Rev. B: Condens. Matter Mater. Phys., 2014, 90, 224104 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4cp00191e |
| ‡ These authors contributed equally to this work. |
| This journal is © the Owner Societies 2024 |