Highly oxidized U(VI) within the smallest fullerene: gas-phase synthesis and computational study of boron-doped U@C27B†
Antonio
Moreno-Vicente
a,
Marc
Alías-Rodríguez
ad,
Paul W.
Dunk
b,
Coen
de Graaf
 *ac,
Josep M.
Poblet
*ac,
Josep M.
Poblet
 a and
Antonio
Rodríguez-Fortea
a and
Antonio
Rodríguez-Fortea
 *a
*a
aDepartament Química Física i Inorgànica, Universitat Rovira i Virgili, C/Marcel·lí Domingo 1, 43007, Tarragona, Spain. E-mail: antonio.rodriguezf@urv.cat
bNational High Magnetic Field Laboratory, Florida State University, 1800 East Paul Dirac Drive, Tallahassee, FL 32310, USA
cICREA, Pg Lluis Companys 23, 08010, Barcelona, Spain
dAix-Marseille Univ, CNRS, ICR, Marseille, France
First published on 7th December 2022
Abstract
The smallest borafullerene U@C27B has been synthesized using a laser vaporization cluster source and detected as the most abundant species in the U@C2n−1B family. Density functional theory (DFT) calculations show that the U atom is placed just at the center of the cage interacting almost in the same way with all its atoms as if the cage were a 28-hapto ligand. Besides, the spin density is not at the metal, but distributed over the cage indicating that U is formally U(VI), even though it is not bonded to very electronegative atoms like O, F, N or Cl. All in all, we have synthesized a rather exotic radical that is another example of the distinctive host–guest chemistry that appears in endohedral metallofullerenes.
1. Introduction
Replacing a carbon atom in the fullerene cage by a heteroatom modifies the electronic structure of the fullerenes and hence their properties. The first heterofullerenes, detected only six years after the discovery of C60,1 were obtained as boron-doped carbon cages C60−xBx by laser vaporization of graphite and boron nitride.2 Indeed, boron and nitrogen are the preferred elements to dope fullerenes since they resemble carbon in size and electronegativity and are therefore most likely to form covalent bonds within the cage structure. Nitrogen-containing fullerenes have received more investigation than boron-doped cages because rational synthetic routes for the former were devised.3–7 Production of macroscopic quantities of boron-doped C60−xBx by arc burning was achieved in the late 90′s.8,9 In 2013, Kroto and co-workers reported the formation of borafullerenes by direct exposure of pristine C60 (and C70) to boron vapor by means of pulsed laser vaporization cluster source.10 The C59B radical, with the unpaired electron delocalized over the carbon cage, was predicted to have a high electron affinity so as to get reduced and become a closed-shell anion. A year before, the same group had synthesized and detected the smallest fullerene known so far, the C28 cage, which needs to be stabilized by the encapsulation of electropositive metals that donate four electrons to the cage, M@C28 (with M = Ti, Zr, Hf and also U).11 In fact, they also detected other “magic” peaks for the U@C2n series at C36 and C44 and concluded that the formation of endohedral mono-metallofullerenes M@C2n is directed by charge transfer, i.e. the smaller the carbon cage the larger the formal transfer to get the M@C2n stabilized.12Other gas-phase detections of uranofullerenes13,14 and computational studies on the U-cage interaction in small cages15,16 or U–U bond in U2@C60 have been reported.17 However, it was not until 2017 that the first actinidofullerene was synthesized by an arc-discharge method and fully characterized.18 In Th@C3v(8)-C82, there is a formal transfer of four electrons from Th to the cage, in contrast to the three electrons transferred in lanthanide counterparts as La@C2v(9)-C82. The same year, three mono-uranofullerenes, U@D3h-C74, U@C2(5)-C82 and U@C2v(9)-C82 were also obtained and characterized, which provided the first observation of cage isomer dependent charge transfer states for U.19 Since then, many other mono- and di-actinidofullerenes have been obtained, as for example U@C1(17418)-C76, U@C1(28324)-C80 and Th@C1(28324)-C80 in cages that do not obey the isolated pentagon rule (IPR),20 U2@Ih(7)-C80,21 or more recently U@Cs(6)-C82, U@C2(8)-C84, U@Cs(15)-C84, U@C1(12)-C86, Th@D5h(6)-C80 and Th2@Ih(7)-C80, among others.22–24 The carbon cages can be used as effective nanocontainers to study the elusive actinide-actinide bond,25,26 as for example in Th2@Ih(7)-C80,24 or to encapsulate reactive or rare actinide clusters as in U2N@Ih(7)-C80, U2C@Ih(7)-C80, U2C2@Ih(7)-C80, U2C2@D3h(5)-C78 and UCN@Cs(6)-C82.27–30 The encapsulated actinides show a wide range of oxidation states, especially uranium, which is present as (i) U(V) in U2C@Ih(7)-C80; (ii) U(IV) in U2C2@Ih(7)-C80 or U@C1(28324)-C80 and most of the mono-uranofullerenes; and (iii) U(III) in U2@Ih(7)-C80, U@C2v(9)-C82 or UCN@Cs(6)-C82, for which the authors claimed an ambiguous oxidation state, U(III) vs. U(I).28 The shortest U⋯C distances in all these uranofullerenes range between 2.40 and 2.55 Å, slightly shorter than in uranocene U(η8-C8H8)2, U(η5-C5H5)4 or in arene-bridged diuranium complexes.31,32
Herein, using a laser vaporization cluster source, we aim to prepare and characterize boron-doped U@C2n−1B endohedrals that can be scaled up for macroscopic production as well as have new properties and are attractive for applications. First, we describe the gas-phase synthesis and detection of boron-doped uranium endohedral fullerenes, along with the computational details of the calculations, and then we discuss the results. Two remarkable features are found in the smallest U@C27B: (i) highly-oxidized U(VI) formal state; and (ii) large hapticity of the nanocontainer ligand.
2. Experimental
2.1. Gas-phase synthesis of U@C2n−1B
The starting materials, graphite (99.9999%), boron powder (96%) and UO2, are thoroughly mixed and then molded into a composite rod by compression. The optimal carbon target composition for boron-doped uranofullerene formation is found to be 0.8% U (atomic percent) and 10% B (atomic percent). U@C2n−1B are formed in situ by use of a pulsed supersonic cluster source by a single laser pulse of a Nd:YAG laser (532 nm, 5 mj per pulse) under a flow of helium.12,33 The gas-phase reaction products were analysed by a custom-built 9.4 T FT-ICR mass spectrometer directly coupled to the cluster source and are conducted with positive ions.34 Ions produced by 10 individual vaporization events were accumulated and transferred by octopoles to an open cylindrical trap ICR cell. The ions are then accelerated to a detectable radius by a broadband frequency sweep excitation, and detected as the differential current induced between two opposed electrodes of the ICR cell. The positively charged molecular ions are expected to be representative of the neutral abundance distribution generated by laser vaporization. Although, we note that the corresponding neutrals may exhibit different stabilities.2.2. Computational details
The geometry optimizations were performed at density functional theory (DFT) level with the ADF 2018 program using different functionals (see ESI†).35,36 The exchange–correlation hybrid functional PBE0 and the Slater TZP basis sets were used (PBE0/TZP) for the analysis.37–39 Frozen cores consisting of (i) the 1s shell for C and B; (ii) the 1s to 5d shells for U were used. Relativistic corrections were included by means of the ZORA formalism. In addition, the Grimme Dispersion D3 method was considered.40CASSCF/CASPT2 calculations were carried out using OpenMolcas 20.10.41 The basis sets used were taken from the ano-rcc internal basis set library using the following contractions: (9s8p7d5f2g1h) for U and (4s3p1d) for C and B. No symmetry restrictions were imposed and Cholesky decomposition was employed to speed up the handling of two-electron integrals. Scalar relativistic effects were included through the Douglas–Kroll–Hess Hamiltonian. The molecular orbitals were optimized for the lowest 10 roots of each multiplicity in a state-average (SA-CASSCF) calculation. The active space contains 7 electrons distributed in all the possible ways over 13 molecular orbitals, including all the f-type U orbitals and six orbitals of the cage (Fig. S1†). Some of the active orbitals are clearly localized on U or the cage, but others are delocalized over the cage and U, which hampers the electron count on U, and makes it difficult to assign an oxidation state to the metal ion. To accurately determine the oxidation of each electronic state, we have localized the orbitals performing an orthonormal transformation of the MO in the active space.42 The new set of orbitals (Fig. S2†) are localized on U or the cage with small orthogonalization tails onto the metal. Re-expressing the multiconfigurational wave function in this set of localized orbitals allows a direct determination of the number of electrons associated to U. The number of electrons in each localized orbital, the total number of electrons over U atom and the CASSCF energy can be found in Table S1.† Despite the accurate analysis that can be performed using a CASSCF wave function, the energies are commonly far from being correct because of the lack of dynamic electron correlation. These effects were added applying the second order perturbative correction to all electrons except the deep core ones to the CASSCF reference wave function, in the so-called single-state (SS-CASPT2) calculation. The standard 0.25 Eh IPEA shift was used and the imaginary level shift was fixed at 0.05 Eh to avoid the presence of weak intruder states. The influence of the imaginary level shift in the relative energies has also been tested (Fig. S3†).
3. Results and discussion
Fig. 1 shows that U@C27B forms as the smallest and most abundant species in the U@C2n−1B borafullerene family under our conditions, which is in agreement with the known smallest cage all-carbon analogue.11 Other much less abundant peaks are also observed for U@C35B, U@C43B and U@C49B. To better understand this result, we have analysed the geometry and the electronic structure of U@C27B. Due to the high symmetry of the cage (Td), this fullerene shows only two positions for the substitution of a carbon atom by boron. It can be placed in the centre of three pentagon rings (also called 555 position) or in the centre of two pentagons and one hexagon (556). The energy difference found for these two isomers is 11.7 kcal mol−1, with the 556 isomer lower in energy.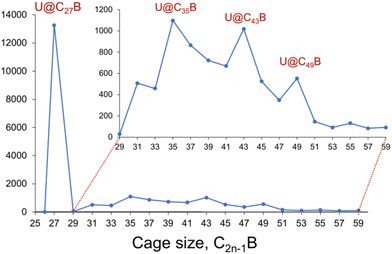 | ||
| Fig. 1 Formation distribution (positive ions) for U@C2n−1B products formed by laser vaporization of U-containing graphite starting material. Inset: the region 2n−1 = 29 to 59 is expanded for clarity. | ||
To confirm the endohedral position of U within the C27B cage, we have computed some isomers with U coordinated to B and/or C atoms outside the cage (Table S5†). Those exohedral U-C27B systems considered are more than 150 kcal mol−1 higher in energy than U@C27B. Interestingly, in U@C27B the U atom is placed at the centre of the fullerene void interacting with all the 28 atoms of the cage in a very similar manner due to the small inner void and the large size of uranium, as compared for example with Ti@C28, where Ti is off-centered.11 All the C–U distances range between 2.41 and 2.53 Å, with the B–U distance being 2.51 Å (Fig. 2).
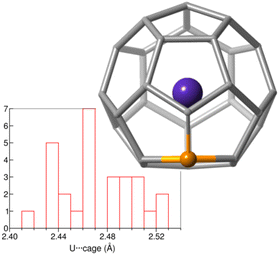 | ||
| Fig. 2 Histogram of the U-cage distances in the optimized structure of U@C27B. Besides, the optimized structure for the isomer 556 is shown. | ||
Neutral U@C27B shows an unpaired electron, which is not located on the B atom, but mainly delocalized on the fullerene cage with a residual contribution on the internal U atom (see spin density in Fig. S4†). The oxidation potential is low in line with the high energy of the singly-occupied molecular orbital (SOMO, Fig. 3). Besides, it is remarkable that uranium atom formally transfers all its six valence electrons to the fullerene cage, as can be seen from an analysis of the electronic structure where all 5f orbitals of uranium are empty (Fig. 3). The doublet with the U(V) f1 configuration is at 3.8 kcal mol−1. It is then one very rare example in which the formal oxidation state +6 of uranium is stabilized without the presence of highly electronegative atoms as oxygen, nitrogen, chlorine or fluorine.
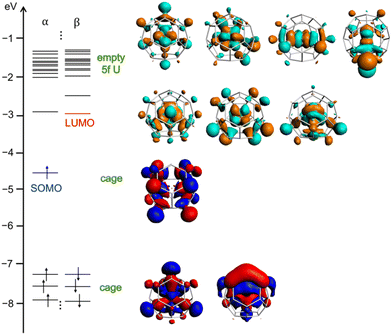 | ||
| Fig. 3 Frontier molecular orbitals of U@C27B at PBE0/TZP level. SOMO and LUMO are essentially cage orbitals and all U-5f orbitals are empty. | ||
We have confirmed this result by using multi-configurational wave-function based methods, CASSCF/CASPT2 (see Computational methods for details). The lowest-energy doublet, with formally 5f0 U(VI), is 7.3 kcal mol−1 lower in energy than the lowest doublet with 5f1 U(V) electronic configuration (Table S1†). The charge distribution on the cage atoms, according to the Bader's Quantum Theory of Atoms in Molecules (QTAIM),43 is found to be highly unequal, particularly around the heteroatom, as a consequence of their different electronegativities. The B atom bears a significant positive charge (+1.79) while the three C atoms directly bonded to it accumulate each an important negative charge (−0.65, −0.63 and −0.62) as compared to the low charge in the remaining 24 C atoms of the cage (around −0.05), see Fig. 4. Localization index (LI), a QTAIM-based index that defines the population of an atomic basin minus the shared electrons, has been used recently to measure the oxidation state of electropositive atoms.44,45 The difference between the LI and atomic number provides a measure of the charge of an atom after the ionic approximation for heteroatomic bonds, which corresponds to the IUPAC definition of oxidation state. Measure of LIU in U@C27B also confirms the U(VI) oxidation state. Furthermore, QTAIM was used to characterize the U-cage interactions. The bond critical point (bcp) postulated by Bader between two atoms is a necessary and sufficient condition for the atoms to be bonded. We have found 28 U-cage bcp, one for each of the 27 U–C interactions as well as for U–B (Fig. 4b). At these bcp, the corresponding values of electron density ρbcp and Laplacian of the electron density ∇2ρbcp are displayed in Table S3.†
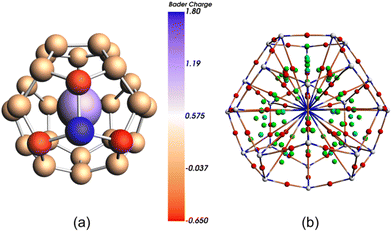 | ||
| Fig. 4 Bader charges (a) and AIM critical points (CP) and bond paths (b) for U@C27B. Atom CP in white, bond CP in red, ring CP in green and cage CP in light blue. | ||
As found for M-cage interactions in larger cages,46 densities ρbcp are around 0.5 eÅ−3 and values of the Laplacian ∇2ρbcp are positive about 4–5 eÅ−5. Ring critical points (rcp) in the middle of the triangle formed by two contiguous U–C bcp and a C–C bcp are also found (Fig. S7†). Delocalization indices, δ(U-cage), which provide a quantitative measure of the degree of electron sharing between the U atom and the cage and have been proposed as a measure of bond order,47 are found to be around 0.3 for U–C interactions (Table S6†) and somewhat smaller, albeit non negligible, for the U–B interaction (0.097, Table S6†). From this topological analysis of the electron density and the histogram of the U–C distances, we can see the C27B cage that encapsulates U as if it were a ligand with a unusual very large hapticity (η28).
Besides, we have analysed the electronic structure of U@C27B+ cation, since this is the species detected in the mass spectrometer when working in the positive mode. This cation, still with U(VI) oxidation state, is a rather stable closed-shell species with a considerably high HOMO–LUMO gap (4.92 eV at PBE0 level). CASPT2 calculations confirm that the lowest-energy singlet, by more than 80 kcal mol−1, is essentially described (67%) by a configuration with formally U(VI) (see Table S2 and Fig. S5†).
Electronic structure and U–C bond lengths suggest a strong interaction between the actinide and the carbon cage, with the metal acting very likely as a template in the formation of the endohedral fullerene, as it is presumed for other endofullerenes or clusterfullerenes as Sc3N@C80.33,34,48 Indeed, the importance of the metal–ligand interaction is clearly shown in the larger monometallic EMFs, where the U ion never remains in the centre of the cavity, but moves to the wall of the carbon cage.19,20,23 This behaviour is general and does not depend on the metal type. The nature of the interaction is not very different from that observed in classical organometallic ligands.31,32,49–51
Finally, we have analysed the hypothetical system Pa@C27B, isoelectronic to U@C27B+ with a closed-shell structure. Protactinium, with a very similar size as U, has the same spatial constraints and it is placed at the centre of the cage interacting with boron and all the carbon atoms in a quite similar way. The distance between the protactinium and the boron atom is 2.59 Å while the carbon–protactinium distances range between 2.45 and 2.58 Å (Fig. S6†). From an analysis of the electronic structure, we find that there is a huge gap between the HOMO and LUMO energy levels (3.93 eV, see Fig. S7†). As for U@C27B, the metal transfers all its valence electrons, now five of them, to the fullerene cage, as shown by the seven empty 5f orbitals of protactinium with the HOMO belonging to the carbon cage. So, in this case the system can be seen, within the ionic model, as Pa5+@C27B5−. Analysis of the critical points show that there is one bcp for each Pa–C interaction, but no bcp is found now for Pa–B (Table S4†). Delocalization indices, δ(Pa–C), are around 0.25 and δ(Pa–B) = 0.073.
4. Conclusions
The smallest fullerene observed so far, M@C28 (M = Ti, Zr and U), is boron-doped in the form U@C27B using laser vaporization techniques and detected by high resolution FT-ICR mass spectrometry as the most abundant borafullerene in the U@C2n−1B family. The large size of the actinide combined with the small inner void inside C27B enforces the U atom to be placed in the middle of the cage in such a way that the interaction with all 28 atoms is optimized and the encapsulation can be seen as a 28-hapto interaction with the cage. Significant asymmetry in charges is observed around the heteroatom, which is positively charged, with the three C atoms directly bonded to it that keep most of the negative charge. From a detailed analysis of the electronic structure uranium can be formally described as highly oxidized U(VI), U6+@C27B6−, even though no bond to very electronegative atoms like O, F, N or Cl exists. This inorganic radical that is formed in the gas phase, remains very abundant when detected as U@C27B+ in the positive mode of the mass spectrometer, once one electron is lost and a large-gap closed shell structure is obtained. Stabilization by forming charge-transfer complexes with electron acceptors would be expected for this U@C27B bizarre radical, which is another example of the special host–guest interaction in endohedral metallofullerenes.Author contributions
A.M.-V. investigation, formal analysis, methodology, visualization, writing – original draft; M.A.-R. investigation, formal analysis, methodology; P.W.D. conceptualization, investigation, methodology, validation, writing – review & editing; C.d.G. conceptualization, funding acquisition, supervision, writing – review & editing; J.M.P. conceptualization, funding acquisition, supervision, writing – review & editing; A.R.-F. conceptualization, investigation, formal analysis, funding acquisition, supervision, writing – original draft; writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
A.R.-F., J.M.P. and C.d.G. thank the Spanish Ministry of Science (grants PID2020-112762GB-I00 and PID2020-113187GB-I00), the Generalitat de Catalunya (grant 2017SGR629) and the URV for support.References
- H. W. Kroto, J. R. Heath, S. C. O'Brien, R. F. Curl and R. E. Smalley, C60 - Buckminsterfullerene, Nature, 1985, 318, 162–163 CrossRef CAS.
- T. Guo, C. M. Jin and R. E. Smalley, Doping Bucky - Formation and Properties of Boron—Doped Buckminsterfullerene, J. Phys. Chem., 1991, 95, 4948–4950 CrossRef CAS.
- J. C. Hummelen, B. Knight, J. Pavlovich, R. Gonzalez and F. Wudl, Isolation of the Heterofullerene C59N As Its Dimer (C59N)2, Science, 1995, 269, 1554–1556 CrossRef CAS PubMed.
- M. Keshavarz-K, R. Gonzalez, R. G. Hicks, G. Srdanov, V. I. Srdanov, T. G. Collins, J. C. Hummelen, C. Bellavia-Lund, J. Pavlovich, F. Wudl and K. Holczer, Synthesis of Hydroazafullerene C59HN, the Parent Hydroheterofullerene, Nature, 1996, 383, 147–150 CrossRef CAS.
- I. Lamparth, B. Nuber, G. Schick, A. Skiebe, T. Grosser and A. Hirsch, C59N+ and C69N+ - Isoelectronic Heteroanalogues of C60 and C70, Angew. Chem., Int. Ed. Engl., 1995, 34, 2257–2259 CrossRef CAS.
- B. Nuber and A. Hirsch, A New Route to Nitrogen Heterofullerenes and the First Synthesis of (C69N)2, Chem. Commun., 1996, 1421–1422 RSC.
- N. N. Xin, H. Huang, J. X. Zhang, Z. F. Dai and L. B. Gan, Fullerene Doping: Preparation of Azafullerene C59NH and Oxafulleroids C59O3 and C60O4, Angew. Chem., Int. Ed., 2012, 51, 6163–6166 CrossRef CAS PubMed.
- B. P. Cao, X. H. Zhou, Z. J. Shi, Z. X. Jin, Z. N. Gu, H. Z. Xiao and J. Z. Wang, Synthesis and Characterization of Boron-Doped Fullerenes, Acta Phys.–Chim. Sin., 1997, 13, 204–206 CAS.
- H. J. Muhr, R. Nesper, B. Schnyder and R. Kotz, The Boron Heterofullerenes C59B and C69B: Generation, Extraction, Mass Spectrometric and XPS Characterization, Chem. Phys. Lett., 1996, 249, 399–405 CrossRef CAS.
- P. W. Dunk, A. Rodriguez-Fortea, N. K. Kaiser, H. Shinohara, J. M. Poblet and H. W. Kroto, Formation of Heterofullerenes by Direct Exposure of C60 to Boron Vapor, Angew. Chem., Int. Ed., 2013, 52, 315–319 CrossRef CAS PubMed.
- P. W. Dunk, N. K. Kaiser, M. Mulet-Gas, A. Rodriguez-Fortea, J. M. Poblet, H. Shinohara, C. L. Hendrickson, A. G. Marshall and H. W. Kroto, The Smallest Stable Fullerene, M@C28 (M=Ti, Zr, U): Stabilization and Growth from Carbon Vapor, J. Am. Chem. Soc., 2012, 134, 9380–9389 CrossRef CAS PubMed.
- P. W. Dunk, M. Mulet-Gas, Y. Nakanishi, N. K. Kaiser, A. Rodriguez-Fortea, H. Shinohara, J. M. Poblet, A. G. Marshall and H. W. Kroto, Bottom-up Formation of Endohedral Mono-Metallofullerenes is Directed by Charge Transfer, Nat. Commun., 2014, 5, 5844 CrossRef CAS PubMed.
- M. D. Diener, C. A. Smith and D. K. Veirs, Anaerobic Preparation and Solvent-Free Separation of Uranium Endohedral Metallofullerenes, Chem. Mater., 1997, 9, 1773–1777 CrossRef CAS.
- A. Gomez-Torres, R. Esper, P. W. Dunk, R. Morales-Martinez, A. Rodriguez-Fortea, L. Echegoyen and J. M. Poblet, Small Cage Uranofullerenes: 27 Years after Their First Observation, Helv. Chim. Acta, 2019, 102, e1900046 CrossRef.
- X. Dai, Y. Gao, W. Jiang, Y. Lei and Z. Wang, U@C28: The Electronic Structure Induced by the 32-Electron Principle, Phys. Chem. Chem. Phys., 2015, 17, 23308–23311 RSC.
- J.-P. Dognon, C. Clavaguera and P. Pyykko, A Predicted Organometallic Series Following a 32-Electron Principle: An@C28 (An=Th, Pa+, U2+, Pu4+), J. Am. Chem. Soc., 2009, 131, 238–243 CrossRef CAS PubMed.
- X. Wu and X. Lu, Dimetalloendofullerene U2@C60 Has a U-U Multiple Bond Consisting of Sixfold One-Electron-Two-Center Bonds, J. Am. Chem. Soc., 2007, 129, 2171–2177 CrossRef CAS PubMed.
- Y. Wang, R. Morales-Martinez, X. Zhan, W. Yang, Y. Wang, A. Rodriguez-Fortea, J. M. Poblet, L. Feng, S. Wang and N. Chen, Unique Four-Electron Metal-to-Cage Charge Transfer of Th to a C82 Fullerene Cage: Complete Structural Characterization of Th@C3V(8)-C82, J. Am. Chem. Soc., 2017, 139, 5110–5116 CrossRef CAS PubMed.
- W. Cai, R. Morales-Martinez, X. Zhang, D. Najera, E. L. Romero, A. Metta-Magana, A. Rodriguez-Fortea, S. Fortier, N. Chen, J. M. Poblet and L. Echegoyen, Single Crystal Structures and Theoretical Calculations of Uranium Endohedral Metallofullerenes (U@C2n, 2n=74, 82) Show Cage Isomer Dependent Oxidation States for U, Chem. Sci., 2017, 8, 5282–5290 RSC.
- W. Cai, L. Abella, J. Zhuang, X. Zhang, L. Feng, Y. Wang, R. Morales-Martinez, R. Esper, M. Boero, A. Metta-Magana, A. Rodriguez-Fortea, J. M. Poblet, L. Echegoyen and N. Chen, Synthesis and Characterization of Non-Isolated-Pentagon-Rule Actinide Endohedral Metallofullerenes U@C1(17418)-C76, U@C1(28324)-C80, and Th@C1(28324)-C80: Low-Symmetry Cage Selection Directed by a Tetravalent Ion, J. Am. Chem. Soc., 2018, 140, 18039–18050 CrossRef CAS PubMed.
- X. Zhang, Y. Wang, R. Morales-Martinez, J. Zhong, C. de Graaf, A. Rodriguez-Fortea, J. M. Poblet, L. Echegoyen, L. Feng and N. Chen, U2@Ih(7)-C80: Crystallographic Characterization of a Long-Sought Dimetallic Actinide Endohedral Fullerene, J. Am. Chem. Soc., 2018, 140, 3907–3915 CrossRef CAS PubMed.
- Y. Yan, R. Morales-Martinez, J. Zhuang, Y.-R. Yao, X. Li, J. M. Poblet, A. Rodriguez-Fortea and N. Chen, Th@D5h(6)-C80: A Highly Symmetric Fullerene Cage Stabilized by a Single Metal Ion, Chem. Commun., 2021, 57, 6624–6627 RSC.
- Y.-R. Yao, Y. Rosello, L. Ma, A. R. P. Santiago, A. Metta-Magana, N. Chen, A. Rodriguez-Fortea, J. M. Poblet and L. Echegoyen, Crystallographic Characterization of U@C2n (2n=82-86): Insights about Metal-Cage Interactions for Mono-metallofullerenes, J. Am. Chem. Soc., 2021, 143, 15309–15318 CrossRef CAS PubMed.
- J. Zhuang, R. Morales-Martinez, J. Zhang, Y. Wang, Y.-R. Yao, C. Pei, A. Rodriguez-Fortea, S. Wang, L. Echegoyen, C. de Graaf, J. M. Poblet and N. Chen, Characterization of a Strong Covalent Th3+-Th3+ Bond Inside an Ih(7)-C80 Fullerene Cage, Nat. Commun., 2021, 12, 2372 CrossRef CAS PubMed.
- C. Foroutan-Nejad, J. Vicha, R. Marek, M. Patzschke and M. Straka, Unwilling U-U Bonding in U2@C80: Cage-Driven Metal-Metal Bonds in Di-Uranium Fullerenes, Phys. Chem. Chem. Phys., 2015, 17, 24182–24192 RSC.
- A. Jaros, C. Foroutan-Nejad and M. Straka, From pi Bonds without sigma Bonds to the Longest Metal-Metal Bond Ever: A Survey on Actinide-Actinide Bonding in Fullerenes, Inorg. Chem., 2020, 59, 12608–12615 CrossRef CAS PubMed.
- X. Li, Y. Rosello, Y.-R. Yao, J. Zhuang, X. Zhang, A. Rodriguez-Fortea, C. de Graaf, L. Echegoyen, J. M. Poblet and N. Chen, U2N@Ih(7)-C80: Fullerene Cage Encapsulating an Unsymmetrical U(IV)=N=U(V) Cluster, Chem. Sci., 2021, 12, 282–292 RSC.
- Q. Meng, L. Abella, W. Yang, Y.-R. Yao, X. Liu, J. Zhuang, X. Li, L. Echegoyen, J. Autschbach and N. Chen, UCN@Cs(6)-C82: An Encapsulated Triangular UCN Cluster with Ambiguous U Oxidation State U(III) versus U(I), J. Am. Chem. Soc., 2021, 143, 16226–16234 CrossRef CAS PubMed.
- X. Zhang, W. Li, L. Feng, X. Chen, A. Hansen, S. Grimme, S. Fortier, D.-C. Sergentu, T. J. Duignan, J. Autschbach, S. Wang, Y. Wang, G. Velkos, A. A. Popov, N. Aghdassi, S. Duhm, X. Li, J. Li, L. Echegoyen, W. H. E. Schwarz and N. Chen, A Diuranium Carbide Cluster Stabilized Inside a C80 Fullerene Cage, Nat. Commun., 2018, 9, 2753 CrossRef PubMed.
- J. Zhuang, L. Abella, D.-C. Sergentu, Y.-R. Yao, M. Jin, W. Yang, X. Zhang, X. Li, D. Zhang, Y. Zhao, X. Li, S. Wang, L. Echegoyen, J. Autschbach and N. Chen, Diuranium(IV) Carbide Cluster U2C2 Stabilized Inside Fullerene Cages, J. Am. Chem. Soc., 2019, 141, 20249–20260 CrossRef CAS PubMed.
- P. L. Diaconescu, P. L. Arnold, T. A. Baker, D. J. Mindiola and C. C. Cummins, Arene-Bridged Diuranium Complexes: Inverted Sandwiches Supported by Delta Backbonding, J. Am. Chem. Soc., 2000, 122, 6108–6109 CrossRef CAS.
- B. Vlaisayljevich, P. L. Diaconescu, W. L. Lukens Jr., L. Gagliardi and C. C. Cummins, Investigations of the Electronic Structure of Arene-Bridged Diuranium Complexes, Organometallics, 2013, 32, 1341–1352 CrossRef.
- M. Mulet-Gas, L. Abella, M. R. Ceron, E. Castro, A. G. Marshall, A. Rodriguez-Fortea, L. Echegoyen, J. M. Poblet and P. W. Dunk, Transformation of Doped Graphite into Cluster-Encapsulated Fullerene Cages, Nat. Commun., 2017, 8, 1222 CrossRef PubMed.
- P. W. Dunk, N. K. Kaiser, C. L. Hendrickson, J. P. Quinn, C. P. Ewels, Y. Nakanishi, Y. Sasaki, H. Shinohara, A. G. Marshall and H. W. Kroto, Closed Network Growth of Fullerenes, Nat. Commun., 2012, 3, 855 CrossRef PubMed.
- ADF 2018, SCM, Theoretical Chemistry, Vrije Universiteit, Amsterdam, The Netherlands, https://www.scm.com.
- G. T. te Velde, F. M. Bickelhaupt, E. J. Baerends, C. F. Guerra, S. J. A. Van Gisbergen, J. G. Snijders and T. Ziegler, Chemistry with ADF, J. Comput. Chem., 2001, 22, 931–967 CrossRef CAS.
- C. Adamo and V. Barone, Toward Reliable Density Functional Methods without Adjustable Parameters: The PBE0 Model, J. Chem. Phys., 1999, 110, 6158–6170 CrossRef CAS.
- M. Ernzerhof and G. E. Scuseria, Assessment of the Perdew-Burke-Ernzerhof Exchange-Correlation Functional, J. Chem. Phys., 1999, 110, 5029–5036 CrossRef CAS.
- E. Van Lenthe and E. J. Baerends, Optimized Slater-Type Basis Sets for the Elements 1-118, J. Comput. Chem., 2003, 24, 1142–1156 CrossRef CAS PubMed.
- S. Grimme, S. Ehrlich and L. Goerigk, Effect of the Damping Function in Dispersion Corrected Density Functional Theory, J. Comput. Chem., 2011, 32, 1456–1465 CrossRef CAS PubMed.
- I. Fdez. Galván, M. Vacher, A. Alavi, C. Angeli, F. Aquilante, J. Autschbach, J. J. Bao, S. I. Bokarev, N. A. Bogdanov, R. K. Carlson, L. F. Chibotaru, J. Creutzberg, N. Dattani, M. G. Delcey, S. S. Dong, A. Dreuw, L. Freitag, L. M. Frutos, L. Gagliardi, F. Gendron, A. Giussani, L. González, G. Grell, M. Guo, C. E. Hoyer, M. Johansson, S. Keller, S. Knecht, G. Kovačević, E. Källman, G. Li Manni, M. Lundberg, Y. Ma, S. Mai, J. P. Malhado, P. Å. Malmqvist, P. Marquetand, S. A. Mewes, J. Norell, M. Olivucci, M. Oppel, Q. M. Phung, K. Pierloot, F. Plasser, M. Reiher, A. M. Sand, I. Schapiro, P. Sharma, C. J. Stein, L. K. Sørensen, D. G. Truhlar, M. Ugandi, L. Ungur, A. Valentini, S. Vancoillie, V. Veryazov, O. Weser, T. A. Wesołowski, P.-O. Widmark, S. Wouters, A. Zech, J. P. Zobel and R. Lindh, OpenMolcas: From Source Code to Insight, J. Chem. Theory Comput., 2019, 15, 5925–5964 CrossRef PubMed.
- A. Sadoc, R. Broer and C. de Graaf, Role of Charge Transfer Configurations in LaMnO3, CaMnO3, and CaFeO3, J. Chem. Phys., 2007, 126, 134709 CrossRef PubMed.
- R. F. W. Bader, Atoms in Molecules: a Quantum Theory, Clarendon Press, Oxford, 1990 Search PubMed.
- C. S. Day, C. D. Do, C. Odena, J. Benet-Buchholz, L. Xu, C. Foroutan-Nejad, K. H. Hopmann and R. Martin, Room-Temperature-Stable Magnesium Electride via Ni(II) Reduction, J. Am. Chem. Soc., 2022, 144, 13109–13117 CrossRef CAS PubMed.
- J. Mehara, A. K. Surendran, T. van Wieringen, D. Setia, C. Foroutan-Nejad, M. Straka, L. Rulisek and J. Roithova, Cationic Gold(II) Complexes: Experimental and Theoretical Study, Chem. – Eur. J., 2022, 28, e202201794 CAS.
- A. A. Popov and L. Dunsch, Bonding in Endohedral Metallofullerenes as Studied by Quantum Theory of Atoms in Molecules, Chem. – Eur. J., 2009, 15, 9707–9729 CrossRef CAS PubMed.
- C. Outeiral, M. A. Vincent, A. Martín Pendás and P. L. A. Popelier, Revitalizing the Concept of Bond Order through Delocalization Measures in Real Space, Chem. Sci., 2018, 9, 5517–5529 RSC.
- S. Stevenson, G. Rice, T. Glass, K. Harich, F. Cromer, M. R. Jordan, J. Craft, E. Hadju, R. Bible, M. M. Olmstead, K. Maitra, A. J. Fisher, A. L. Balch and H. C. Dorn, Small-Bandgap Endohedral Metallofullerenes in High Yield and Purity, Nature, 1999, 401, 55–57 CrossRef CAS.
- W. Fang, Q. Zhu and C. Zhu, Recent Advances in Heterometallic Clusters with f-Block Metal–Metal Bonds: Synthesis, Reactivity and Applications, Chem. Soc. Rev., 2022, 51, 8434–8449 RSC.
- J. K. Pagano, J. Xie, K. A. Erickson, S. K. Cope, B. L. Scott, R. Wu, R. Waterman, D. E. Morris, P. Yang, L. Gagliardi and J. L. Kiplinger, Actinide 2-Metallabiphenylenes That Satisfy Hückel’s Rule, Nature, 2020, 578, 563–567 CrossRef CAS PubMed.
- I. A. Popov, B. S. Billow, S. H. Carpenter, E. R. Batista, J. M. Boncella, A. M. Tondreau and P. Yang, An Allyl Uranium(IV) Sandwich Complex: Are ϕ Bonding Interactions Possible?, Chem. – Eur. J., 2022, 28, e202200114 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2qi02160a |
| This journal is © the Partner Organisations 2023 |
