TfOH-mediated [2 + 2 + 2] cycloadditions of ynamides with two discrete nitriles: synthesis of 4-aminopyrimidine derivatives†
Ping Chena,
Cai-xia Songa,
Wan-shu Wanga,
Xue-liang Yua and
Yu Tang*ab
aSchool of Pharmaceutical Science and Technology, Key Laboratory for Modern Drug Delivery & High-Efficiency, Tianjin University, Tianjin 300072, P. R. China
bKey Laboratory of Marine Drugs, Chinese Ministry of Education, School of Medicine and Pharmacy, Ocean University of China, 5 Yushan Road, Qingdao, China. E-mail: tangyu@ouc.edu.cn
First published on 16th August 2016
Abstract
In this study, a concise and atom-economical TfOH-mediated [2 + 2 + 2] cycloaddition of ynamides with two discrete nitriles is developed to synthesize multi-substituted 4-aminopyrimidine. A variety of functional groups and sterically and electronically diverse reaction partners are tolerated. The products were obtained in good to excellent yields.
Introduction
[2 + 2 + 2] cycloaddition reactions involve efficient methods in organic chemistry. These reactions aim to construct six-membered rings, such as pyridines, pyrimidines, benzenes, and other heterocyclic frameworks, in one step starting from commercially available materials possessing double- or triple- bonds, including alkenes, nitriles, and alkynes.1 Catalytic [2 + 2 + 2] cycloadditions between ynamides and nitriles or alkynes have been extensively examined by using many transition metal catalysts, such as Co, Ru, Rh, and Au.2 Liu et al.3 used ynamides to prepare 4-aminopyrimidine cores which appear in numerous active pharmaceutical ingredients, agrochemicals, and natural products. These cores have been also applied as versatile building blocks in organic synthesis (Fig. 1).4 However, these reactions require expensive catalysts to facilitate widespread applications. As such, a metal-free [2 + 2 + 2] cycloaddition has been applied to prevent loss of noble metals.Ynamides have been extensively investigated because of their unique activities5 including cyclizations,6a,6b,8 ring-closing metathesis,7 cycloadditions,9 and rearrangements.10
Our study focused on acid-mediated cycloaddition between ynamides and nitriles. In the presence of acetonitrile as a solvent, cycloaddition reaction afford good to excellent yields of 4-aminopyrimidine derivatives. Base on this finding, we explored flexible and practical synthetic routes to prepare multi-substituted 4-aminopyrimidines (Scheme 1).
Results and discussion
We first examined the cycloaddition between ynamide 1a and acetonitrile at −40 °C under nitrogen atmosphere. Various acids such as TfOH, HNTf2, HBF4 and HPF6 were screened. Among them, TfOH was found to be the optimal one and afforded product 3a in 92% yield (Table 1, entry 2). More than one equivalent of TfOH was needed to obtain the optimal yield. Lower temperatures benefited the reaction yields (Table 1, entries 4–6). This phenomenon might be attributed to the decomposition of ynamide at high temperatures. Ynamides are sensitive to moisture under acidic conditions.11 On the basis of our results, we then investigated the preventive effect of 4 Å molecular sieve (MS) on the by-product 6a. Notably, 4 Å MS facilitated the reaction to afford 3a in 97% yield. The reaction concentrations were also optimized and 0.075 mol L−1 of ynamide 1a was the optimal choice (Table 1, entry 7). The solvent could be replaced by dichloromethane. The reaction proceeded smoothly with 8 equiv. of acetonitrile in dichloromethane (Table 2) to provide the desired product 3a in 93% yield (Table 1, entry 10). Other solvents, such as toluene, were not superior to dichloromethane (Table 2, entry 5). Moreover, no cycloadduct was observed in THF; instead, some unknown products and 40% ynamide 1a were recovered at −40 °C for 1.5 h (Table 2, entry 4). Table 1 indicates that acetonitrile remains as the preferred solvent over dichloromethane because of its convenient operation and satisfactory yield.| Entrya | Acid (eq.) | T (°C) | C (mol L−1) | Yieldb (%) |
|---|---|---|---|---|
| a Unless noted otherwise, all reactions were conducted using 0.15 mmol ynamide 1a in MeCN in the presence of TfOH under N2 atmosphere.b Isolated yield.c 20 mg of 4 Å MS was added.d MeCN (8 equiv.) and 20 mg of 4 Å MS were added in CH2Cl2.e HBF4 (tetrafluoroboric acid–diethyl ether complex, 50–55% w/w).f HPF6 (60–65%wt% solution in water).g The desired product was not detected. | ||||
| 1 | TfOH (0.8) | −40 | 0.075 | 74 |
| 2 | TfOH (1.6) | −40 | 0.075 | 92 |
| 3 | TfOH (2.4) | −40 | 0.075 | 80 |
| 4 | TfOH (1.6) | −20 | 0.075 | 91 |
| 5 | TfOH (1.6) | −10 | 0.075 | 84 |
| 6 | TfOH (1.6) | rt | 0.075 | 66 |
| 7c | TfOH (1.6) | −40 | 0.075 | 97 |
| 8c | TfOH (1.6) | −40 | 0.038 | 95 |
| 9c | TfOH (1.6) | −40 | 0.150 | 94 |
| 10d | TfOH (1.6) | −40 | 0.075 | 93 |
| 11 | HNTf2 (1.6) | −10 | 0.075 | Trace |
| 12e | HBF4 (1.6) | −40 | 0.038 | 82 |
| 13f,g | HPF6 (1.6) | −40 | 0.150 | N.D |
| Entry | PhCN (eq.) | Solvent | C (mol L−1) | Yield (%) |
|---|---|---|---|---|
| a Unless noted otherwise, all reactions were conducted using 0.15 mmol of ynamide 1a in 2 mL of solvent in the presence of TfOH at −40 °C under N2 atmosphere.b Isolated yield.c 20 mg 4 Å MS was added.d The desired product was not detected. | ||||
| 1 | 4 | CH2Cl2 | 0.075 | 81 |
| 2 | 6 | CH2Cl2 | 0.075 | 87 |
| 3 | 8 | CH2Cl2 | 0.075 | 91 |
| 4d | 8 | THF | 0.075 | N.D |
| 5 | 8 | Toluene | 0.075 | 90 |
With the optimal reaction conditions established, various substituted ynamides and acetonitrile were investigated (Scheme 2). Various alkyl-substituted ynamides (R1 = n-butyl, cyclopropyl, and hydrogen) were examined. Compounds 3a–3c and obtained in 91–97% yields. Triisopropylsilyl (R1 = TIPS) was used instead of an alkyl group, which was removed in this reaction, to produce 3d in 87% yield. Using ynamides bearing oxazolidinone as an electron-withdrawing group with a large steric hindrance, the yields of compounds 3g and 3h were slightly decreased. Compounds 3e and 3f with a phenyl group bearing para-substituents (withdrawing or electron-donating groups, such as fluorine, -methyl), at the R1 position produced 82–93% yields. Carboxylic ester-substituted ynamide was also obtained the corresponding product 3n in 86% yield.
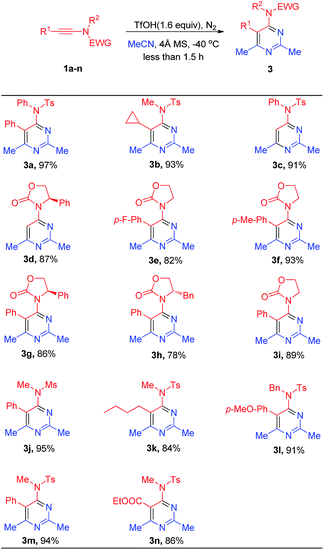 | ||
| Scheme 2 Cycloaddition of various ynamides with acetonitrile. aYnamides 1a–1n (0.15 mmol), TfOH (0.24 mmol), MeCN (2.0 mL), 20 mg 4 Å MS, −40 °C, N2. bIsolated yield. | ||
We extended the TfOH-mediated reaction to various nitriles with ynamides to afford desired products (Scheme 3). This protocol was also applicable to the ynamides comprising various substituents (R2 = phenyl or methyl, R1 = phenyl or carboxylic ester) and benzonitrile. Thus, compounds 4a–4c were produced in 83–91% yields. The yield of the reaction of benzonitrile with ortho-substituents was slightly lower (4d) than that with para-substituents (4e–4f). Alkyl nitriles containing electron-donating and electron-withdrawing substituents reacted well and produced the corresponding 4-aminopyrimidine derivatives in 74–78% yields. For instance, 2-thiophenecarbonitrile was used as a substrate and thus produced 4j in 93% yield. However, the protocol was inapplicable to other cases. For example, 4k could not be detected when 2-cyanopyrimidine was subjected to the optimized conditions. This phenomenon could be attributed to the strong electron deficiency or alkaline property. As such, the pyrimidine moiety of this nitrile trapped TfOH. Substituted benzyl cyanides were also applicable to this type of cycloadditions (4l–4s). 4m was obtained in 36% yield when a strong electron-withdrawing group was present at the para-position (R = NO2) in the aromatic ring.
 | ||
| Scheme 3 Cycloaddtion of various nitriles with ynamides. aYnamides 1 (0.15 mmol), TfOH (0.24 mmol), RCN (1.20 mmol), (2.0 mL), 20 mg of 4 Å MS, −40 °C, N2. bIsolated yield. | ||
We synthesized acceptable total yields of the corresponding products with different nitriles with moderate regioselectivity via a diversity-oriented approach to optimize their structures (Scheme 4). The reactivity of nitriles was an important factor affecting regioselectivity (ESI†).
A possible mechanism is illustrated in Scheme 5 on the basis of these results. Ynamide 1a in the presence of TfOH was activated by H+. As a result, a highly reactive keteniminium ion I was produced. Acetonitrile attacked keteniminium ion I to form intermediate II. Intermediate II was then attacked by another molecule of acetonitrile to produce iminium III. 3a was then obtained through intramolecular cyclization and aromatization.12
Conclusions
In summary, an efficient and practical approach involving TfOH-mediated [2 + 2 + 2] cycloaddition of ynamides with nitriles was described to synthesize 4-aminopyrimidine derivatives. This protocol provides several advantages, including using inexpensive and non-toxic TfOH. The method is applicable to various substrates to produce 4-aminopyrimidine derivatives with satisfactory to good yields via an atom-economical, environment-friendly transformation. This technique may be applied to synthesize useful complex molecules. Further investigations on TfOH-mediated cycloaddition of the corresponding derivatives are currently underway in our laboratories.Acknowledgements
This study is financially supported by the National Natural Science Foundation of China [grant no. 21172169 and 21572154] and the National Basic Research Project [grant no. 2014CB932201].References
- (a) A. Geny, N. Agenet, L. Lannazzo, M. Malacria, C. Aubert and V. Gandon, Angew. Chem., Int. Ed., 2009, 48, 1810 CrossRef CAS PubMed; (b) B. R. Galan and T. Rovis, Angew. Chem., Int. Ed., 2009, 48, 2830 CrossRef CAS PubMed; (c) J. A. Varela, L. Castedo and C. Saá, J. Am. Chem. Soc., 1998, 120, 12147 CrossRef CAS; (d) L. V. R. Boñaga, H. C. Zhang, A. F. Moretto, H. Ye, D. A. Gauthier, J. Li, G. C. Leo and B. E. Maryanoff, J. Am. Chem. Soc., 2005, 127, 3473 CrossRef PubMed; (e) C. García-Rubín, S. González-Rodríguez, C. García-Yebra, J. A. Varela, M. A. Esteruelas and C. Saá, Angew. Chem., Int. Ed., 2014, 53, 1841 CrossRef PubMed.
- (a) T. Stengel and B. Witulski, Angew. Chem., Int. Ed., 1999, 38, 2426 CrossRef; (b) M. R. Tracey, J. Oppenheimer and R. P. Hsung, J. Org. Chem., 2006, 71, 8629 CrossRef CAS PubMed; (c) P. G. Carcia, S. Moulin, Y. Miclo, D. Leboeuf, V. Gandon, C. Aubert and M. Malacria, Chem.–Eur. J., 2009, 15, 2129 CrossRef PubMed; (d) P. Garcia, Y. Evanno, P. George, M. Sevrin, G. Ricci, M. Malacria, C. Aubert and V. Gandon, Org. Lett., 2011, 13, 2030 CrossRef CAS PubMed; (e) B. Dassonneville, B. Witulshki and H. Detert, Eur. J. Org. Chem., 2011, 2836 CrossRef CAS; (f) P. Garcia, Y. Evanno, P. George, M. Sevrin, G. Ricci, M. Malacria, C. Aubert and V. Gandon, Chem.–Eur. J., 2012, 18, 4337 CrossRef CAS PubMed; (g) Y. Tokimizu, M. Wieteck, M. Rudolph, S. Oishi, N. Fujii, A. S. K. Hashmi and H. Ohno, Org. Lett., 2015, 17, 604 CrossRef CAS PubMed.
- S. N. Karad and R. S. Liu, Angew. Chem., Int. Ed., 2014, 53, 9072 CrossRef CAS PubMed.
- (a) M. E. Webb, A. Marquet, R. R. Mendel, F. Fébeillé and A. G. Smith, Nat. Prod. Rep., 2007, 24, 988 RSC; (b) T. R. Webb, T. Moran, C. Q. Huang, J. R. McCarthy, D. E. Grigoriadis and C. Chen, Bioorg. Med. Chem. Lett., 2004, 14, 3869 CrossRef CAS PubMed; (c) P. Chen, D. Norris, J. Das, S. H. Spergel, J. Wityak, L. Leith, R. Zhao, B. C. Chen, S. Pitt, S. Pang, D. R. Shen, R. Zhang, H. F. De Fex, A. M. Doweyko, K. W. McIntyre, D. J. Shuster, K. Behnia, G. L. Schieven and J. C. Barrish, Bioorg. Med. Chem. Lett., 2004, 14, 6061 CrossRef CAS PubMed; (d) L. C. W. Chang, R. F. Spanjersberg, J. K. von Frijtag Drabbe Künzel, T. Mulder-Krieger, G. van den Hout, M. W. Beukers, J. Brussee and A. P. IJzerman, J. Med. Chem., 2004, 47, 6529 CrossRef CAS PubMed; (e) V. Yaziji, D. Rodríquez, H. Gutiérrez-de-Terán, A. Coelho, O. Caamaño, X. García-Mera, J. Brea, M. I. Loza, M. I. Cadavid and E. Sotelo, J. Med. Chem., 2011, 54, 457 CrossRef CAS PubMed.
- For selected recent reviews on ynamides, see: (a) K. A. DeKorver, H. Y. Li, A. G. Lohse, R. Hayashi, Z. J. Lu, Y. Zhang and R. P. Hsung, Chem. Rev., 2010, 110, 5064 CrossRef CAS PubMed; (b) G. Evano, A. Coste and K. Jouvin, Angew. Chem., Int. Ed., 2010, 49, 2840 CrossRef CAS PubMed; (c) X. N. Wang, H. S. Yeom, L. C. Fang, S. Z. He, Z. X. Ma, B. L. Kedrowskl and R. P. Hsung, Acc. Chem. Res., 2014, 47, 560 CrossRef CAS PubMed.
- (a) S. Balieu, K. Toutah, L. Carro, L. M. Chamoreau, H. Rousselière and C. Courillon, Tetrahedron Lett., 2011, 52, 2876 CrossRef CAS; (b) F. Chemla, F. Dulong, F. Ferreira, M. P. Nüllen and A. Pérez-Luna, Synthesis, 2011, 9, 1347 CrossRef.
- H. Wakamatsu, M. Sakagami, M. Hanata, M. Takeshita and M. Mori, Macromol. Symp., 2010, 293, 5 CrossRef CAS.
- (a) W. Gati, M. M. Rammah, M. B. Rammah, F. Couty and G. Evano, J. Am. Chem. Soc., 2012, 134, 9078 CrossRef CAS PubMed; (b) Y. Kong, K. Jiang, J. Cao, L. Fu, L. Yu, G. Lai, Y. Cui, Z. Hu and G. Wang, Org. Lett., 2013, 15, 422 CrossRef CAS PubMed; (c) X. N. Wang, R. P. Hsung, R. Qi, S. K. Fox and M.-C. Lv, Org. Lett., 2013, 15, 2514 CrossRef CAS PubMed; (d) S. A. Gawade, D. B. Huple and R. S. Liu, J. Am. Chem. Soc., 2014, 136, 2978 CrossRef CAS PubMed; (e) C. Shu, Y. H. Wang, B. Zhou, X. L. Li, Y. F. Ping, X. Lu and L. W. Ye, J. Am. Chem. Soc., 2015, 137, 9567 CrossRef CAS PubMed; (f) S. J. Xu, J. Q. Liu, D. H. Hu and X. H. Bi, Green Chem., 2015, 17, 184 RSC; (g) Y. F. Yang, L. N. Wang, J. N. Zhang, Y. L. Jin and G. G. Zhu, Chem. Commun., 2014, 50, 2347 RSC.
- (a) H. Li, R. P. Hsung, K. A. DeKorver and Y. Wei, Org. Lett., 2010, 12, 3780 CrossRef CAS PubMed; (b) R. Yamasaki, N. Terashima, I. Sotome, S. Komagawa and S. Saito, J. Org. Chem., 2010, 75, 480 CrossRef CAS PubMed; (c) W. D. Mackay, M. Fistikci, R. M. Carris and J. S. Johnson, Org. Lett., 2014, 16, 1626 CrossRef CAS PubMed.
- (a) K. A. DeKorver, X. N. Wang, M. C. Walton and R. P. Hsung, Org. Lett., 2012, 14, 1768 CrossRef CAS PubMed; (b) J. Brioche, C. Meyer and J. Cossy, Org. Lett., 2013, 15, 1626 CrossRef CAS PubMed; (c) X. N. Wang, E. H. Krenske, R. C. Johnston, K. N. Houk and R. P. Hsung, J. Am. Chem. Soc., 2015, 137, 5596 CrossRef CAS PubMed.
- C. A. Zificsak, J. A. Mulder, R. P. Hsung, C. Rameshkumar and L. L. Wei, Tetrahedron, 2001, 57, 7575 CrossRef CAS.
- (a) A. G. Martínez, A. H. Fernández, R. M. Álvarez, M. D. Molero Vilchez, M. L. Laorden Gutiérrez and L. R. Subramanian, Tetrahedron, 1999, 55, 4825 CrossRef; (b) M. Radi, S. Schenone and M. Botta, Org. Biomol. Chem., 2009, 7, 2841 RSC; (c) A. Herrera, A. Riaño, R. Moreno, B. Caso, Z. D. Pardo, I. Fernández, E. Sáez, D. Molero, A. Sánchez-Vázquez and R. Martínez-Alvarez, J. Org. Chem., 2014, 79, 7012 CrossRef CAS PubMed; (d) C. Theunissen, B. Métayer, N. Henry, G. Compain, J. Marrot, A. Martin-Mingot, S. Thibaudeau and G. Evano, J. Am. Chem. Soc., 2014, 136, 12528 CrossRef CAS PubMed; (e) B. Peng, X. Huang, L.-G. Xie and N. Maulide, Angew. Chem., Int. Ed., 2014, 53, 8718 CrossRef CAS PubMed; (f) Y. Yamaoka, T. Yoshida, M. Shinozaki, K.-I. Yamada and K. Takasu, J. Org. Chem., 2015, 80, 957 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra11408c |
| This journal is © The Royal Society of Chemistry 2016 |






