Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Recent advances in thin film nanocomposite membranes containing an interlayer (TFNi): fabrication, applications, characterization and perspectives
Jiaqi Wang ab,
Lei Wang
ab,
Lei Wang *ab,
Miaolu Heab,
Xudong Wangab,
Yongtao Lvab,
Danxi Huangab,
Jin Wangab,
Rui Miao
*ab,
Miaolu Heab,
Xudong Wangab,
Yongtao Lvab,
Danxi Huangab,
Jin Wangab,
Rui Miao ab,
Lujie Nieab,
Jiajin Haoab and
Jianmin Wangc
ab,
Lujie Nieab,
Jiajin Haoab and
Jianmin Wangc
aResearch Institute of Membrane Separation Technology of Shaanxi Province, Key Laboratory of Membrane Separation of Shaanxi Province, Key Laboratory of Northwest Water Resources, Environmental and Ecology, Ministry of Education, Key Laboratory of Environmental Engineering, No. 13 Yan Ta Road, Shaanxi Province, Xi'an 710055, China. E-mail: wl0178@126.com
bSchool of Environmental & Municipal Engineering, Xi'an University of Architecture and Technology, No. 13 Yan Ta Road, Xi'an 710055, China
cZhongfan International Engineering Design Co., Lian Hu Road, No. 6 Courtyard, Xi'an 710082, China
First published on 29th November 2022
Abstract
Polyamide (PA) reverse osmosis and nanofiltration membranes have been applied widely for desalination and wastewater reuse in the last 5–10 years. A novel thin-film nanocomposite (TFN) membrane featuring a nanomaterial interlayer (TFNi) has emerged in recent years and attracted the attention of researchers. The novel TFNi membranes are prepared from different nanomaterials and with different loading methods. The choices of intercalated nanomaterials, substrate layers and loading methods are based on the object to be treated. The introduction of nanostructured interlayers improves the formation of the PA separation layer and provides ultrafast water molecule transport channels. In this manner, the TFNi membrane mitigates the trade-off between permeability and selectivity reported for polyamide composite membranes. In addition, TFNi membranes enhance the removal of metal ions and organics and the recovery of organic solvents during nanofiltration and reverse osmosis, which is critical for environmental ecology and industrial applications. This review provides statistics and analyzes the developments in TFNi membranes over the last 5–10 years. The latest research results are reviewed, including the selection of the substrate and interlayer materials, preparation methods, specific application areas and more advanced characterization methods. Mechanistic aspects are analyzed to encourage future research, and potential mechanisms for industrialization are discussed.
1. Introduction
Data indicate that half of the global population is expected to live in water-scarce areas by 2025.1 Therefore, water treatment technologies based on membrane separation, such as reverse osmosis and nanofiltration, are playing increasingly important roles in the efficient treatment, recycling and desalination of produced and domestic water.2–4 (Reverse osmosis) RO and (Nanofiltration) NF membranes for desalination and recycled water reuse usually have a thin film composite (TFC) structure4,5 that consists of two parts: a porous substrate and an ultrathin PA separation layer.4 TFC membrane separation layers are usually formed by interfacial polymerization (IP) reactions in aqueous solutions of m-phenylenediamine (MPD), p-phenylenediamine or piperazine (PIP), which serve as the aqueous phase, and hexane solutions containing trimesoyl chloride (TMC), which serves as the organic phase.However, the “trade-off” effect between permeate flux and selective separation by TFC membranes has limited their development.6–8 In addition, contamination of the PA separation layer on the membrane surface and corrosion by organic solvents have reduced the lifetimes and stabilities of TFC membranes. Researchers have been seeking to develop novel TFC membranes that solve these problems. Therefore, TFN membranes that form nanoscale selective channels utilizing the internal channels of porous nanomaterials and the interfacial channels of PA separation layers have been developed. Conventional TFN membranes are prepared by adding nanomaterials to an MPD aqueous solution or TMC organic phase solution through an IP reaction. Alternatively, a mixed matrix membrane is obtained by adding nanomaterials to the casting solution to modify the substrate.
Different types of nanomaterials, such as SiO2,9 metal–organic frameworks (MOFs),10,11 Ag,12 graphene oxide (GO),13 and clay,14 have been used to prepare TFN membranes. However, the selectivities of many TFN membranes are not substantially increased, and even the retention of the target contaminants is reduced as a result of the irregular distribution or agglomeration of nanomaterials, leading to impaired membrane integrity.
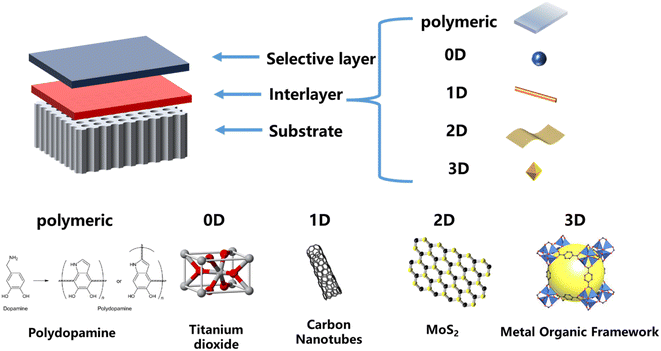
As shown in Fig. 1, the preparation of TFNi membranes using pressure filtration, predeposition or in situ growth of nanomaterials on substrates prior to the formation of PA layers has become a focus of interest. This method consists of 0D (e.g., graphene quantum dots (GQDs),16 TiO2 (ref. 17) and Ag18), 1D (e.g., SWCNTs19 and dopamine20 and polyethyleneimine12), 2D (e.g., MXene, MOFs of the tetrakis(4-carboxyphenyl)porphyrin (TCPP) series and covalent organic frameworks (COFs) of 2D TpPa, etc.) and 3D (e.g., UiO-66 and ZIF-8,21 etc. MOFs) nanomaterials in combination. Surface coating,20 predeposition,22 in situ growth23 and sacrificial intercalation nanomaterials17 are used to prepare TFNi membranes.
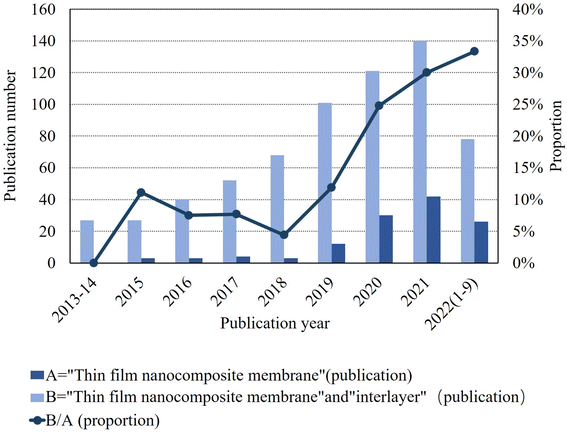
As shown in Fig. 2, a 2022 literature search using the Web of Science search system with interlayers as the subject keyword identified approximately 35% of all TFN membranes, and the trend has been increasing annually over the past 10 years. To date, several review articles related to the preparation of polyamide composite membranes through intercalation have been published. Among them, Liao et al.24 targeted the selection of different types of nanofillers based on TFN membranes in application scenarios such as gas separation, nanofiltration, organic solvent nanofiltration, reverse osmosis, forward osmosis, and osmotic evaporation. Seah et al.25 and Bhaskar, V. V. et al.26 reviewed the IP processes of TFN and TFC membranes, including support-free IP, filtration-based IP, spin-based IP, ultrasound-based IP, and spray-based IP. The intent was to compare which IP process is more likely to be used in real production and is environmentally friendly. Zhao et al.27 reviewed the application of TFN membranes in RO with the intention of investigating the effect of nanofillers on the polyamide layer and the improvement of membrane contamination resistance in practical water treatment processes. Zhang et al.28 reviewed organic nanomaterial-modified TFN membranes and their research progress in various water/organic separation processes. The intention was to explore the possible applications of organic nanomaterial-modified TFN membranes in practical water treatment projects. Yang et al.29 intended to investigate the advantages of TFNi membranes compared to TFN and TFC membranes through basic theory and to determine the effect of different nanomaterials on the polyamide layer.
Compared to the aforementioned reviews, this paper mainly focuses on the preparation methods and categorization of TFNi membranes with the intention of exploring the possibility of scaling up their production toward practical water treatment projects and summarizes several conventional techniques for the characterization of TFNi membranes.
2. Properties of different substrate materials
TFNi membranes usually consist of a substrate, a PA layer and a nanomaterial sandwich. The physicochemical properties of the substrate directly influence the selection of the interlayer nanomaterial and the properties of the PA layer. Therefore, a suitable substrate should be selected according to the different physicochemical properties of the target contamination.Polyimide (PI) membranes have been used in membrane technology for many years and have a wide range of applications. PI membranes are excellent polymers for the preparation of substrates because of their excellent heat resistance and good mechanical strength, as well as chemical resistance to a variety of solvents.30 PI membranes were initially used for gas separation31 and permeate evaporation32 due to their high mechanical strengths. In addition, due to their high organic solvent resistance, they have become an important part of organic solvent nanofiltration processes, and these PI membranes are currently used to recover various organic solvents.33–36
Polysulfone (PSf) has good chemical resistance to alkaline solutions, acids, and chlorine, and it has high mechanical strength and is easily processed. Therefore, PSf is used as a raw material for the preparation of microfiltration and ultrafiltration membranes, as well as a substrate material for the preparation of reverse osmosis and nanofiltration membranes.37 However, the hydrophobicity of PSf tends to make the membrane surface susceptible to contamination.38,39 Therefore, the surface of the membrane is modified to enhance its resistance to contaminants by increasing its hydrophilicity to improve its long-term stability in water treatment processes.40
Polyethersulfone (PES) is a typical industrial water treatment membrane. One of its major applications is the removal of various organic and inorganic heavy metals and dyes from wastewater.41 It has excellent mechanical properties and heat resistance.42 Therefore, membranes synthesized from PES can be fabricated into structures suitable for various wastewater treatment methods. Despite the remarkable excellence of PES as a substrate material, it is, like PSf, susceptible to contamination, which reduces its treatment efficiency.43
Polyvinylidene fluoride (PVDF) has received increasing attention as a membrane material with a high mechanical strength, thermal stability and corrosion resistance comparable to those of other commercial polymer materials. PVDF membranes have been widely used in membrane separation applications such as ultrafiltration and microfiltration.44 However, PVDF is less commonly used for the recovery of organic solvents because it is readily soluble in organic solvents such as ethanol and N,N-dimethylformamide (DMF).45
In addition to these polymers, others, such as Nylon, PSU,46 PPS,47 SPEEK12 and CTA,48 have been used as TFNi membrane substrates for water treatment. Notably, each substrate has its own unique advantages and characteristics and should be selected appropriately according to the practical operating environment. In the context of the in situ growth method, a substrate is needed as a support during synthesis. Therefore, the mechanical strength, acid resistance and temperature range to which the substrate are subjected during the growth process are considered. All these factors limit the selection of substrates and nanomaterials.
3. Selection of interlayer materials
The physicochemical properties of the intercalated material can be used to control the diffusion concentrations of amine monomers in the aqueous phase during the IP reaction. In addition, if the intercalated material is a two- or three-dimensional nanomaterial, the target material can be processed using its own formed Å-sized layer spacings or microwindows. Therefore, the choice of interlayer nanomaterial exerts a substantial effect on the performance of a TFNi membrane.Zero-dimensional nanomaterials, such as graphene quantum dots (GQDs), constitute a new class of graphene oxide materials consisting of single-atom (<2 nm) nanosheet structures with high specific surface areas and excellent chemical stabilities. Deposition of an appropriate amount of GQDs on the surface of the substrate may subsequently reduce the thickness of the PA layer, which helps to improve the permeabilities of TFN membranes.15,16
Tannic acid (TA), a plant processing product, easily modifies the surfaces of substrates via complexation reactions with metal ions and organic substances because of its abundant hydroxyl groups and hydrogen bonds. For example, complexes formed from tannic acid (TA) and Cu2+,49 Fe3+,50 polyethyleneimine (PEI),51 polydopamine (PDA),20 and diethylenetriamine (DETA)52 were used as intercalation nanomaterials to modify TFNi membranes.
Polyethyleneimine (PEI), a cationic polyelectrolyte with a high charge density and easy protonation, has been widely used to prepare positively charged TFC membranes. Polyphenols composed of PEI and TA complexes have been deposited on the surface of the substrate, and the formation of a PA separation layer on the surface of the substrate was analyzed by determining the diffusion kinetics of fluorescence-labeled piperazine (FITC-PIP), the diffusion behavior of an n-hexane solution containing chlorinated chloride, and with in situ Fourier transform infrared spectroscopic (FT-IR) studies.51 PEI deposition of a carboxylated polyacrylonitrile (PAN) substrate was used to control the subsequent IP reaction by modulating the diffusion rate of the –NH2 monomer with PEI.53,54 In addition, the hydrophilic interlayer formed by PEI and PDA codeposition also controls the subsequent IP reaction.47,48,55,56
Dopamine self-polymerizes into PDA under weakly alkaline conditions at pH = 8, and the surface of the substrate forms a stable surface coating. PDA provides a versatile platform for the subsequent modification of nanomaterials with physical and chemical interactions.57 Using PDA as a representative adhesive material, the mechanism of action for intercalation materials deposited on TFNi membranes can be explored.58
MOFs, also known as porous coordination polymers, are widely used in adsorption and membrane separations due to their adjustable porosities, dimensionalities, and internal pore channels.59 Various methods have been proposed to improve interfacial compatibility issues with MOFs and substrates. These methods include an in situ growth strategy, in which the zeolite-imidazolite framework (ZIF-8) and the in situ growth of ZIF-8 form a uniformly distributed intercalation material on the surface of the substrate.60 Similar to this method, PI was used as a substrate, and PI was preimmersed in a Cu2+ solution; subsequently, HKUST-1 nanomaterials were grown in situ on the PI surface using a low-temperature solvothermal method.61 The photocatalytic properties of specific MOF nanomaterials have improved the anti-pollution properties of membranes. If copper-triazole62 or Fe-TCPP63 was pressure filter loaded onto the substrate surface, the resulting TFNi membranes were self-cleaned by applying light during the subsequent dye wastewater treatment, which improved the water treatment stability and enabled good flux recovery.
COFs are emerging as porous crystals. COFs are two- or three-dimensional polymers connected by covalent bonds, and they have unique properties, such as tunable pore channels, high specific surface areas, and chemical stability.64,65 TpPa nanomaterials are often used as COFs in membrane separations because they are synthesized at ambient temperature and pressure, grown in situ and loaded on the surface of the substrate without damaging the membrane structure.66–71
GO is a frequently applied two-dimensional material that has been used in TFNi membranes, but GO is less stable in aqueous solutions.72 GO is usually modified with hydrophilic polymers such as PDA and PEI to solve this problem.12,47,73 Alternatively, carbon nanotubes (CNTs) are blended with GO74 and pressure filtered onto the surface of the substrate to serve as an intercalation material. In addition, two-dimensional MXene materials are more frequently used as interlayer-modified TFNi membranes due to the large number of hydroxyl hydrophilic functional groups on the surface. However, the disadvantage of MXenes is that they will be oxidized to TiO2 when exposed to air for a long time,75 and this property can be used to prepare sandwich material-free modified TFC membranes by flushing the sandwich material TiO2 with water.17
Different preparation methods correspond to the selection of different intercalation materials, and the preparation methods are divided into three categories: predeposition, pressure filtration and in situ growth. The strengths and weaknesses of these three loading methods and the materials used with each preparation method are indicated and summarized in each subsequent section.
4. Different methods for preparing TFNi membranes
Three categories are summarized here, depending on the loading method of the interlayer material, namely, predeposition, pressure filter loading and in situ growth. Each preparation method corresponds to the specific properties of interlayer nanomaterials, and the researcher should select the appropriate interlayer material according to the actual application scenario (e.g., NF, RO, or FO). (1) The pressure filtration method has recently attracted increasing attention from researchers due to its convenient and controlled preparation process and low material consumption. The preparation process uses one-dimensional or two-dimensional materials as interlayer materials, and the interlayer materials are uniformly distributed on the substrate surface in a regular shape by applying an external force. The physical and chemical properties of the intercalated material are mainly used to control the subsequent formation of the PA layer on its surface. (2) The representative material of the TFNi membrane prepared using the predeposition method is a polymer, which regulates the subsequent formation of the PA layer on the substrate surface through the adhesion of the polymer. (3) In situ growth typically uses the substrate as a support and occurs directly on the substrate surface during the synthesis of the material. The applicable water treatment scenarios for each of the three preparation methods and the respective representative materials are discussed in detail in the subsequent sections.4.1 Pressure filtration
The pressure filtration method is performed by applying pressure to filter the nanomaterial dispersion onto the substrate surface, followed by an IP reaction on its surface to obtain the TFNi membrane. The preparation method may be inspired by laminated membranes of 2D materials because the process used to prepare TFNi membranes with the pressure filter loading method is similar to that of laminated membranes, except for the IP reaction, as the nanofolds are similar to those formed by laminated membranes composed of 2D materials.Due to limitations on the thickness of the interlayer material in the preparation of TFNi membranes using the pressure filter loading method, an excessively thick interlayer may decrease the stability of the PA separation layer, and thus a high separation performance is impossible to maintain for a long time. Therefore, the thickness of the sandwich material of a TFNi membrane is usually approximately 5–10 nm.
Furthermore, water molecule transport through the channels of laminated membranes involves interlayer transport, and the resulting wrinkles help to accelerate transport to a certain extent. Similarly, TFNi membranes have been constructed by intercalating materials to create a selective layer with folds that accelerate water transport. For example, Guo et al. prepared TFNi membranes by pressure filter loading sulfonated polyaniline SPANI nanocellulose as an intercalation material. After grafting sulfonic acid groups on polyaniline, the electronegativity of the whole membrane was significantly increased, which increased the repulsive force for divalent ions.76 Excellent water permeability and selectivity for mono- and divalent ions were observed. Second, SPANI nanofiber interlayers provide hydrophilic porous surfaces that contribute to the formation of defect-free and thin PA selective layers with pleated nanostructures. Doping ions into the interior of the polymer forms stable six-membered rings in the self-doped structures, decreasing the susceptibility of the polymer to damage by weakly acidic and weakly basic solutions. The stability of the TFNi membrane is improved.77
Removal of target contaminants has been enhanced by changing the charge of the intercalated material. For example, Li et al. mixed positively charged PEI and negatively charged MXene nanosheets onto the surface of a PAN substrate for filtration and applied the obtained TFNi membrane to dye retention.78 As shown in Fig. 3(a), Chen et al. used PEI mixed with CNTs and loaded it onto the surface of a PVDF substrate by filtration. CNT TFNi membranes were obtained and applied to dye retention, and the retention performance was adjusted by changing the pH (2.12% pH = 12, 99.38% pH = 7).79
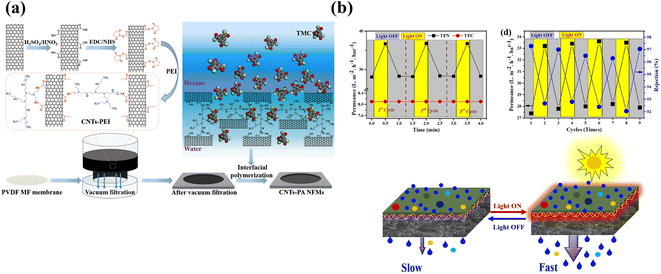 | ||
| Fig. 3 (a) PEI was grafted onto the surface of CNTs and deposited on a PVDF substrate. The grafting of PEI resulted in an amine-rich interlayer [reprinted with permission from ref. 79. Copyright 2021, Elsevier]. (b) Relative pure water permeance of TFC and TFN membranes as a function of the illumination time, effect of light illumination for 0.5 min on pure water permeance and schematic illustrating the photothermal effect on the TFN NF membrane after lights were switched ON and OFF [reprinted with permission from ref. 63. Copyright 2022, Elsevier]. | ||
The “ridge and valley” feature on the surface of the PA layer is caused by the release of nanobubbles encapsulated in the PA layer during the IP process.80,81 This phenomenon may be attributed to two factors: (1) the heat generated reduces the solubility of dissolved gases in the aqueous solution because the IP process requires a thermal cross-linking reaction; and (2) the protons generated during the IP process form H2CO3 from HCO3−, which accelerates the formation of CO2 in the thermal cross-linking environment at 60 °C. Sun et al. prepared MXene/CNTs TFNi membranes to increase the adsorption of amine monomers in the aqueous phase solution onto the intercalated layer and to limit bubble generation during IP reactions.82 Similar to the process shown in Fig. 3(b), Wen et al. prepared two-dimensional MOF (Zn-TCPP) TFNi membranes to enhance the confinement of degassing nanobubbles, which reduced the diffusion rate of –NH2 monomers.83 In addition, two-dimensional MOFs of the porphyrin family constitute a class of photothermal sensitive devices84 that were used as tunable photoresponsive TFNi membranes by Hussain et al. The ultrafast photothermal responses of Fe-TCPP (2 s of light irradiation increased the temperature by 88.4 °C) resulted in a 30% increase in water flux through the membrane during visible light irradiation for 30 s.63
Although the leaching of nanomaterials from TFNi membranes prepared using pressure filtration has been substantially reduced during the treatment process compared to predeposition and in situ growth methods, the leaching of nanomaterials is still a concern that requires a solution. As shown in Fig. 4(a), Xu et al. converted the PES substrate after filtering MXene into TiO2 using mild oxidation of H2O2 and flushed it away after the IP reaction to form a PA layer as an approach to solve this problem. The resulting TFC membrane conferred a high rejection rate >96% for Na2SO4 and an excellent permeance of 45.7 L·m−2 h−1 bar−1, which was approximately 4.5 times higher than that of the control membrane (10.2 L m−2 h−1 bar−1). This method fully uses the physicochemical properties of the MXene nanomaterial, eliminates the additional hydraulic resistance defects of the intercalated material, and solves the negative effect on environmental water after leaching.17
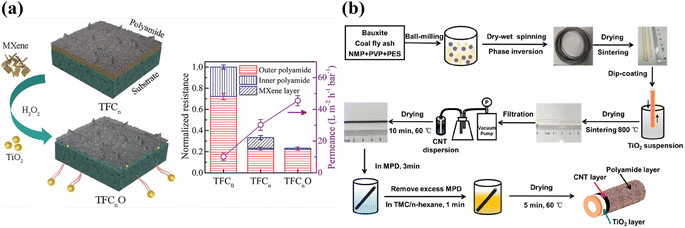 | ||
| Fig. 4 (a) The MXene lamellar layer was eliminated by mild oxidation after IP to avoid imparting additional hydraulic resistance [reprinted with permission from ref. 17. Copyright 2021, American Chemical Society]. (b) Schematic diagram of the preparation process for a ceramic-based FO membrane with a TiO2/CNT nanocomposite interlayer [reprinted with permission from ref. 87. Copyright 2020, American Chemical Society]. | ||
Most of the intercalation materials that can be used in pressure filtration preparation processes are nanomaterials with one- or two-dimensional morphology. Zhu et al. used cellulose nanocrystals (CNCs) and GO, both alone and combined, as intercalation materials to investigate the performance of TFNi membranes. The experimental results showed an effective reduction in the surface roughness of the interlayer and syntheses of 10–15 nm ultrathin PA layers with a low nanomaterial concentration of 0.025 wt%.85
Tian et al. filtered GO onto the outer surface of a PSf hollow fiber (HF) substrate to prepare HF nanofiltration membranes, and the surface morphology and cross-sectional structure of the prepared TFNi nanofiltration membrane were investigated extensively.86 As shown in Fig. 4(b), Jin et al. obtained mechanically strong TFNi membranes by growing TiO2 on the surface of a PES HF substrate and subsequently loading CNTs on the TiO2 surface via filtration. The structural morphologies and properties of the nanomaterial-free interlayers, TiO2 interlayers and TiO2/CNT interlayered TFNi membranes were characterized. The introduction of nanocomposite interlayers with lower roughness resulted in TFNi membranes with better surface properties and facilitated the formation of defect-free PA separation layers with higher cross-linking.87
Although membrane separation technology has great potential in dye wastewater treatment, a common problem is substantial decreases in separation effectiveness and lifetime due to membrane contamination. Zhou et al. exploited the photocatalytic properties of the copper triazolate (CuTz-1) MOF to degrade organic molecules with visible light.88 The MOFs (CuTz-1) and GO were filtered and chemically cross-linked to construct (CuTz-1)/GO intercalated TFNi membranes. The presence of CuTz-1 led to an increase in the GO interlayer distance, thus promoting enhanced water flux without affecting dye retention. Moreover, after visible light irradiation, the permeation and separation properties of the TFNi membrane recovered well due to effective photocatalytic removal of the dye attached to the membrane surface.62
Compared to the internal and interfacial channels of typical TFN membranes, which contain channels measuring in the subnanometer to low nanometer range, the nanochannels generated by sacrificial nanomaterials usually have larger sizes (>10 nm). For example, Yang et al. incorporated sacrificial Cu NPs into a PA rejection layer.89 The nanocavities created by subsequent acid etching resulted in a 3-fold increase in water flux at the cost of a slight reduction in NaCl retention.
The aforementioned representative cases of TFNi membranes prepared using the pressure filtration method are selected from Table 1 according to their preparation methods and target contaminants. Table 1 also provides a summary of TFNi membranes prepared using the pressure filtration method.
| Substrate material | Interlayer nanomaterials | Membrane type | Operating conditions (applied pressure and feed solution) | Permeability (L m−2 h−1 bar−1) | Rejection (%) | Reference |
|---|---|---|---|---|---|---|
| PAN | PEI/MXene | NF | Na2SO4 and 1000 mg L−1, dyes 200 mg L−1 | 20.9 L m−2 h−1 bar−1 | RNa2SO4 = 65.7%, RNaCl = 23.9%, RCongored = 99.42%, Rreactiveblue = 99.02%, Rmethylblue = 98.84% | 78 |
| PES | β-Cyclodextrin (β-CD)/GO | NF | Crystal violet 10 ppm, Congo red 100 ppm, methylene blue 10 ppm, Na2SO4, NaCl and MgCl2 1000 ppm | 82 L m−2 h−1 bar−1 | Rcrystalviolet = 99.98% | 92 |
| PVDF | CNTs-PEI | NF | Na2SO4 and NaCl 1000 ppm, Congo red 100 ppm | 49.8 L m−2 h−1 bar−1 | RCongored = 99.47%, separation α (NaCl/CR) = 150.32 | 79 |
| PSf | Hydroxyapatite (HAP) | NF | Na2SO4 and NaCl 1000 ppm | 177 L m−2 h−1 6 bar | RNa2SO4 = 98.2%, separation α (NaCl/Na2SO4) = 19 | 94 |
| PAN | CuTz-1 | Self-cleaning NF | Na2SO4 and NaCl 1000 ppm, crystal violet 500 ppm, Congo red 500 ppm, methylene blue 500 ppm | 40.2 L m−2 h−1 bar | RNa2SO4 = 19.6%, RNaCl = 0.3%, RCongored = 99.4%, Rdirectred = 98.2%, Rmethylblue = 94.9% | 62 |
| PES | CNCs/PDA | NF | Na2SO4 and NaCl sodium, lignosulfonate, alkaline lignin, and BSA 500 ppm, Congo red 500 ppm, rhodamine B 500 ppm | 19.0 L m−2 h−1 bar | RNa2SO4 = 98.2% | 95 |
| PSf | GO | NF | Na2SO4 2000 ppm | 80 L m−2 h−1 at 0.6 MPa | RNa2SO4 = 96.1% | 86 |
| PSf | CNCs/GO | NF | Na2SO4 1000 ppm | 45.9 L m−2 h−1 bar | RNa2SO4 = 97.2% | 85 |
| PSf | g-C3N4/halloysite nanotubes (HNTs)/PIP | NF | Na2SO4 1000 ppm | 20.5 L m−2 h−1 bar | RNa2SO4 = 94.5% | 96 |
| PES | Carboxylated CNCs (CNC–COOH) | NF | LiCl and MgCl2 2000 ppm | 4.17 L m−2 h−1 bar | Separation α (Mg2+/Li+) = 12.5 | 97 |
| PES | CNS | NF | Na2SO4 1000 ppm | 204 g m−2 h−1 at 0.6 MPa | RNa2SO4 = 97% | 98 |
| PES | MXene/TiO2 | NF | Na2SO4 1000 ppm | 45.7 L m−2 h−1 bar | RNa2SO4 = 96% | 17 |
| PES | Fe-TCPP | NF | Methyl orange, indocyanine green, brilliant blue and direct red 30 ppm | 28.1 L m−2 h−1 bar | Rmethylorange = 84.5%, Rindocyaninegreen = 97.2%, Rbrilliantblue = 98.3%, Rdirectred = 99.1% | 63 |
| PPS | NGO | NF | NaCl, CuSO4, ZnSO4 and AlCl3 1000 ppm | 40.80 L m−2 h−1 at 0.6 MPa | RNaCl = 48.24% RCuSO4 = 92.14%, RZnSO4 = 92.93% RAlCl3 = 91.23% | 47 |
| Polytetrafluoroethylene (PTFE) | PDA/SWCNT | OSN | Congo red 20 ppm | 13.2 L m−2 h−1 bar | RHNSA = 89.6% | 99 |
| PES | PDA/NMG/SiO2 | OSN | Acid red 18 | 2.18 L m−2 h−1 bar | Racidred18 = 99.9% | 100 |
| PAN | Ethylenediamine modified graphene oxide (eGO) | OSN | Water/isopropanol separation | 4150 g m−2 h−1 | Separation α (water/isopropanol) = 1866 | 101 |
| PES | Zn-TCPP | RO | NaCl 2000 ppm | 4.82 L m−2 h−1 bar | RNaCl = 97.4% | 83 |
| PSf | PEI/GO | RO | NaCl 2000 ppm | 2.24 L m−2 h−1 bar−1 | RNaCl = 96.6% | 91 |
| PES | MXene/CNT/PDA | FO | 0.5, 1, 2 and 3 mol per L NaCl | 31 L m−2 h−1 | Js NaCl 0.07 g m−2 h−1 | 82 |
| PES | GO quantum dots (GOQDs)/Ag | FO | 1 mol per L NaCl | 65.8 L m−2 h−1 | Js NaCl 1.4 g m−2 h−1 | 102 |
| PES | GO | FO | 2 mol per L NaCl | 22 L m−2 h−1 | Js NaCl 5.8 g m−2 h−1 | 93 |
| PAN | PDA | FO | 2 mol per L NaCl, Cr3+, Cu2+ and Ni2+ 20 mg L−1 | 29.2 L m−2 h−1 | RCr3+ = 99.5%, RCu2+ = 99.1%, RNi2+ = 98.1% | 103 |
| PVDF | GO/CNTs | FO | 0.6 mol per L NaCl | 305.89 L m−2 h−1 | Js NaCl 0.37 g m−2 h−1 | 74 |
| PES | TiO2/CNT | FO | 0.5, 1 and 2 mol per L NaCl | 2.0 L m−2 h−1 bar | RNaCl = 97.2% | 87 |
| Nylon | MXene | FO | 5 mol per L monoethanolamine (MEA) solution | 30.8 L m−2 h−1 bar | CO2 capture | 90 |
| PES | CNT | FO | 1 mol per L NaCl | 35 L m−2 h−1 bar | Js NaCl 0.55 g m−2 h−1 | 104 |
The intercalation materials used in the pressure filtration method are mostly 1D or 2D materials or their modified composites, with representative 2D materials including MXenes,17,78,82,90 metal-TCPP63,83 and GO.47,74,85,86,91–93 This phenomenon may be attributed to the three factors listed below. (1) The pressure filtration method enables orderly loading onto the substrate surface, which maximizes the advantages of the physicochemical properties of 2D materials, as well as the Å level layer spacing. (2) The exfoliated monolayer 2D materials are generally uniformly dispersed in deionized water or organic solvents. Therefore, if the TFNi membranes composed of 2D materials are prepared using pressure filtration, the experimental reproducibility is high, and other experimental conditions can be easily investigated. (3) Compared to predeposition and in situ growth, pressure filtration exhibits close to one hundred percent utilization of the material. Therefore, the preparation process is also the least polluting to the environment.
Currently, due to its limited preparation device requirements, most of the TFNi membranes prepared using the pressure filtration method have an area of approximately 10–100 cm2, and thus further industrialization is challenging. Second, although the material utilization rate of the pressure filtration method is relatively efficient, the cost of currently used materials remains expensive if we want to further expand production. Moreover, the environmental pollution caused by the secondary leaching of the intercalated material in the treatment process is also a problem that we must consider.
4.2 Predeposition
The predeposition method usually consists of covering the material on the substrate surface by deposition using its physicochemical properties to control the diffusion rate of the amine monomer to the organic phase, thereby regulating the subsequent IP reaction. Compared to Table 1, which summarizes the pressure filtration method for preparing TFNi membranes, as shown in Table 2, the predeposition method uses a wide range of polymers, such as PDA,58 PIPA,105 PEI56,106 and SA (sodium alginate),107 as additional intercalation materials. Using this approach, the cost of the preparation process and the secondary pollution to the environment in water treatment are substantially reduced. Second, it provides the possibility to further expand the production of TFNi membranes.| Substrate material | Interlayer nanomaterials | Membrane type | Operating conditions (applied pressure and feed solution) | Permeability (L m−2 h−1 bar−1) | Rejection (%) | Reference |
|---|---|---|---|---|---|---|
| PES | 3-Aminobenzenesulfonamide (ABSA) | NF | NaCl, MgSO4, Na2SO4 and MgCl2 1000 ppm, dye solutions 0.1 g L−1 | 12.4 L m−2 h−1 bar−1 | RNa2SO4 = 95%, Rdyes = 99.3% | 120 |
| PES | GO/PDA | NF | MgSO4, NaCl, Na2SO4 and MgCl2 1000 ppm | 11.4 L m−2 h−1 bar−1 | RNaCl = 66.99% RMgSO4 = 97.76%, RMgCl2 = 84.05% RNa2SO4 = 98.22% | 73 |
| PSf hollow fiber | TpPa (COFs) | NF | ZnSO4, CuSO4, Cr2(SO4)3, Na2SO4 and MnSO4 2000 ppm | 86.6 L m−2 h−1 Mbar−1 | RZnSO4 = 91.7%, RMnSO4 = 91.0%, RCr2(SO4)3 = 95.4% RNa2SO4 = 96.6%, RCuSO4 = 94.3% | 68 |
| PES | MXenes | NF | NaCl and Na2SO4 1000 ppm | 27.8 L m−2 h−1 bar−1 | Separation α (NaCl/Na2SO4) = 480 | 121 |
| PSf | PEI-Noria | NF | MgSO4, NaCl, Na2SO4 and MgCl2 2000 ppm | 110.4 L m−2 h−1 Mbar−1 | RMgCl2 = 95.4% | 106 |
| PSf | Catechol/sodium periodate | NF | Na2SO4, NaCl, CaCl2 NaCl and MgCl2 2000 ppm | 15.4 L m−2 h−1 bar−1 | RNa2SO4 = 98.42% | 122 |
| PAN | TpPa (COFs) | NF | MgSO4, NaCl 2000 ppm, acid fuchsia, Congo red 100 ppm | 104.8 L m−2 h−1 (0.6 MPa) | Separation α (NaCl/acid fuchsia = 402.5, NaCl/Congo red = 398.0) | 69 |
| PSf | PVA/GO | NF | Na2SO4 and NaCl 2000 ppm | 158 L m−2 h−1 MPa−1 | RNa2SO4 = 99.7% separation α (NaCl/Na2SO4) = 230 | 116 |
| PSf | Homogeneous gelatin (GT) | NF | Na2SO4, CaCl2, NaCl and MgSO4 2000 ppm | 16.95 L m−2 h−1 bar−1 | RNa2SO4 = 99.3% separation α (NaCl/Na2SO4) = 97.73 | 123 |
| PES | ZIF-8 | NF | Na2SO4, NaCl 500 ppm, acid fuchsia, Congo red 100 ppm | 9.6 L m−2 h−1 bar−1 | 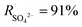 |
124 |
| PES | SWCNT | NF | Na2SO4, NaCl, MgSO4 and MgCl2 1000 ppm | 18.24 L m−2 h−1 bar−1 | RNa2SO4 = 97.88% | 19 |
| PES | Iron oxohydroxide (FeOOH) nanorods | NF | Na2SO4, NaCl, MgSO4 and MgCl2 2000 ppm | 34.1 L m−2 h−1 bar−1 | RNa2SO4 = 95.0% separation α (Cl−/SO42−) = 97.73 | 125 |
| PSf | PDA | NF | Na2SO4, MgSO4 and MgCl2 1000 ppm | 14.8 L m−2 h−1 bar−1 | Separation α (Cl−/SO42−) = 34.4 | 58 |
| PES | PVA | NF | Na2SO4 2000 ppm | 31.4 L m−2 h−1 bar−1 | RNa2SO4 = 99.4% | 115 |
| PES | TpPa (COFs) | NF | Na2SO4 1000 ppm | 171.35 L m−2 h−1 MPa−1 | RNa2SO4 = 90% | 126 |
| Polyphenylene | PDA/PEI/GO | NF | AlCl3, NaCl, ZnSO4 and CuSO4 1000 ppm | 40.8 L m−2 h−1 MPa−1 | RZnSO4 = 92.93%, RAlCl3 = 91.23%, RNaCl = 48.24%, RCuSO4 = 92.14% | 47 |
| Ceramic | Poly(4-styrenesulfonic acid) (PSS)/ZIF-8 | NF | Water–ethanol | 4.47 kg m−2 h−1 | Pw/Pe = 318–127 | 127 |
| PAN | PDA/COF | NF | Orange GII 100 mg L−1, Na2SO4 1000 mg L−1 | 207.07 L m−2 h−1 MPa−1 | RNa2SO4 = 93.4%, RorangeGII = 94.5% | 71 |
| PES | CNT | NF | Methyl violet 100 mg L−1, Na2SO4, NaCl, MgSO4 2000 ppm | 21 L m−2 h−1 bar−1 | RNa2SO4 = 98.3%, RMgSO4 = 98.3%, Rmethylviolet = 99.5%, separation α (Cl−/SO42−) = 85.5, (NaCl/methyl violet) = 123.5 | 128 |
| PSf | PEI/PDA | NF | Na2SO4 and MgSO4 1000 ppm | 45 L m−2 h−1 at 0.6 MPa | RNa2SO4 = 97% | 56 |
| PSf | TA/DETA | NF | Na2SO4 and MgSO4 2000 ppm | 63 L m−2 h−1 at 0.6 MPa | RNa2SO4 = 98% | 52 |
| Polyimide (PI) | TpPa (COFs) | OSN | Rhodamine B 100 mg L−1 | 60 L m−2 h−1 Mbar−1 | RRhodamineb = 99.2% | 67 |
| Polyketone (PK) | PDA/GQDNH2 | OSN | Rhodamine B 20 ppm | 11.1 L m−2 h−1 bar−1 | RRhodamineb = 99% | 15 |
| Polyamide 6 hollow fiber | Silicate sheets | OSN | Vitamin B12 100 mg L−1 | 0.1 L m−2 h−1 bar−1 (MeOH) | RVB = 99% | 129 |
| PI | ZIF-8 | OSN | Rhodamine B | 28 L m−2 h−1 MPa−1 (ethanol) | RRhodamineb = 99.1% | 130 |
| PI | TA–Cu | OSN | Rhodamine B 100 mg L−1 | 150.05 L m−2 h−1 MPa−1 | RRhodamineb = 97.8% | 49 |
| Polyimide polymer (P48) | GQDs/PEI | OSN | Rhodamine B | 40.3 L m−2 h−1 MPa−1 | RRhodamineb = 98.7% | 16 |
| PI | GO | OSN | Rhodamine B | 41.47 L m−2 h−1 MPa−1 | RRhodamineb = 99.4% | 131 |
| PSf | Polyamide@NH2_MIL-88B | RO | NaCl 2000 ppm | 35.8 L m−2 h−1 15.5 bar | RNaCl = 92% | 132 |
| PSf | Sodium alginate (SA) | RO | NaCl 2000 ppm | 27.8 L m−2 h−1 Mbar−1 | RNaCl = 99.2% | 107 |
| PAN | PEI/PPA | RO | NaCl 2000 ppm | 20.7 L m−2 h−1 0.6 MPa | RNaCl = 98.7% | 54 |
| PSf | PPA | RO | Na2SO4 and NaCl 2000 ppm | 2.86 L m−2 h−1 bar−1 | RNaCl = 97.3% | 109 |
| PSf | TpPa (COFs) | RO | NaCl 2000 ppm | 16.78 L m−2 h−1 MPa−1 | RNaCl = 99.2% | 70 |
| Polyethylene (PE) | PDA/TA-Fe3+ | FO | 1 mol per L NaCl | 71.2 L m−2 h−1 | Js NaCl 0.03 g m−2 h−1 | 117 |
| CTA | PDA/PEI | FO | 1 mol per L NaCl | 7.1 L m−2 h−1 | Js NaCl 0.2 g L−1 | 48 |
| PSf/TPU | UiO-66-NH2 | FO | Cu2+ (500 μg L−1), tetracycline TC (CFU 103–1011) | 65 L m−2 h−1 bar−1 | RCu2+ = 99.53%, RTC = 99.53% | 133 |
| PAN | Electrospun CNTs | FO | 2 mol per L NaCl | 49.2 L m−2 h−1 | Js NaCl 7.2 g m−2 h−1 | 118 |
| PVDF | TA–Fe3+ | FO | 2 mol per L NaCl | 36.9 L m−2 h−1 | Js NaCl 2.7 g m−2 h−1 | 50 |
| Nylon | MXene | FO | 1 mol per L LiCl | 9.5 L m−2 h−1 | Js LiCl 0.4 g m−2 h−1 | 119 |
| Mixed cellulose ester | SWCNTs/PDA-PEI | FO | 1 mol per L LiCl | 51.3 L m−2 h−1 | Js NaCl 7.9 g m−2 h−1 | 55 |
| PSf | PDA/halloysite nanotubes | FO | 1 mol per L NaCl | 26.9 L m−2 h−1 | Js NaCl 3.35 g m−2 h−1 | 134 |
| Ceramic hollow fiber (CHF) | TiO2 | Prevaporation | Dehydration | 6.44 kg m−2 h−1 | — | 135 |
| PAN | PEI/PPA | FO | 0.5 mol per L NaCl | 24.6 L m−2 h−1 | Js NaCl 2.36 g m−2 h−1 | 105 |
Similar to the pressure filter loading method, the predeposition method can also produce wrinkles. For example, Gui et al. predeposited g-C3N4 nanofibers on the surfaces of PES membranes and obtained a high-quality PA layer with a folded structure using the ultrahigh hydrophilicity of the g-C3N4 nanofiber network intermediate layer. Due to the ultrahigh specific surface area of this special structure, the prepared NF membranes showed an ultrahigh water flux of 15.2 L m−2 h−1, a Na2SO4 retention efficiency of 98.9% (4 bar), and a significantly higher SO42−/Cl− selectivity.108
Since the PA layer formed by cross-linking the amine monomers with TMC in conventional TFC membranes is thick and has uneven pores, it is not conducive to the transport of water molecules. As shown in Fig. 5(a), Gan et al. solved this problem by introducing a sparser poly(piperazine amide) (PPA) interlayer between the PA layer and the PSf substrate. The obtained TFNi membrane showed a 2–2.5 times higher water flux than the TFC membrane while maintaining a higher retention rate.109 In addition, Yang et al. used PDA as an interlayer nanomaterial to investigate its effects on the selective permeabilities of TFNi membranes. The membrane separation performance was enhanced due to the combined effects of grooves formed by the PDA interlayer and indirect adhesion, with the grooves playing a more dominant role in increasing the roughness and cross-linking of the PA layer.58 The deposition time was substantially reduced.110 Yang et al. compensated for the oxidation of PDA in air and the long predeposition time by reacting PDA with PEI via a Michael addition reaction and used predeposition of a PDA/PEI sandwich on the PSf substrate to regulate the IP reaction of PIP and TMC and prepare TFNi membranes.56 Wang et al. similarly used PDA/PEI predeposition on a CTA substrate followed by IP to prepare TFNi membranes.48 However, the PDA predeposition process usually takes a long time. Therefore, Wang et al. studied the macrocyclic polyphenols of Noria and performed PEI predeposition on the surface of the substrate in only a few seconds.111 Positively charged TFNi membranes were prepared via the interaction of macrocyclic polyphenols of Noria and PEI.106
 | ||
| Fig. 5 Predeposition of TFNi membranes with different nanomaterials. (a) The plain blue arrows represent water flow through a loose region (PPA). The blue arrows glowing red represent water flow through a dense region (PA) with higher hydraulic resistance [reprinted with permission from ref. 109. Copyright 2020, Elsevier]. (b) A high-performance NF membrane (TFC-P) was fabricated via IP on a PVA interlayered PES substrate [reprinted with permission from ref. 115. Copyright 2020, American Chemical Society]. (c) Schematic illustration of the modification of the PE substrate and the fabrication of PDA-t, Fe-y, and TA-x membranes [reprinted with permission from ref. 117. Copyright 2022, Elsevier]. (d) A high-performance MXene TFNi FO membrane was fabricated via a combination of a facile and scalable brush coating of MXene on nylon substrates and the IP process [reprinted with permission from ref. 119. Copyright 2020, American Chemical Society]. | ||
GO nanomaterials have received increasing attention in the field of membrane separation.112 Graphene quantum dots (GQDs) are zero-dimensional nanomaterials in the graphene oxide family.113 Liang et al. used GQDs and PEI predeposited on a PI substrate and subsequently obtained organic solvent nanofiltration membranes by performing IP reactions with low concentrations of aqueous and organic solutions.16
Polyvinyl alcohol (PVA) is a polymer with a linear molecular structure that can be used as an organic binder with good adhesion when dissolved in water.114 As shown in Fig. 5(b), Zhu et al. deposited PVA on the surface of a PES substrate, and a dense PA layer with a thickness of 9.6 nm formed on the PES-PVA surface due to its large specific surface area, good hydrophilicity, and high porosity. In addition, the TFNi membranes formed via PVA intercalation had smaller pore sizes and larger specific surface areas. Importantly, the PVA intercalation strategy was further advanced for possible application in NF membrane pilot lines by preparing membranes exhibiting stable water flux and high separation coefficients comparable to those of laboratory-scale TFNi membranes.115 Liang et al. used polyvinyl alcohol (PVA)-modified GO and subsequent glutaraldehyde postcrosslinking to control the IP reaction, which significantly reduced the separation layer thickness (to 15 nm) and roughness (to 5 nm). The authors obtained TFNi membranes with considerably increased chloride resistance.116
TA, a plant processing product, is susceptible to complexation reactions with metal ions and organic matter used to modify the surface of the substrate due to its abundant hydroxyl groups and hydrogen bonds. Zhang et al. prepared polyphenol interlayers using TA and DETA predeposition.52 As shown in Fig. 5(c), Xiao et al. introduced a TA–Fe3+ interlayer with a thickness of 7 μm on a PDA-modified polyethylene (PE) substrate and used the TA–Fe3+ interlayer to control the diffusion of MPD, leading to the formation of a unique worm-like morphology in the PA layer during the IP reaction. The substrate and the PA layer were closely connected by the TA–Fe3+ interlayer, which improved the stability of the TFNi membrane.117
Yao et al. formed PA layers by predepositing TA–Cu complexes on the surface of a PI substrate as interlayers to reduce the concentration of amine monomers in the organic solution during the IP reaction. The prepared TFNi membranes showed high permeability to organic solvents because of an activation process using DMF during fabrication.49 In addition, S. R. Razavi et al. prepared polyphenol interlayers using TA as a ligand on a PVDF substrate and crosslinking with Fe3+ ions. The effects of the TA–Fe3+ interlayer on the hydrophilicity and roughness of the membrane were investigated.50
Electrospun nanofibrous mats are widely used as substrates for forward osmosis membranes because they are unlikely to cause internal concentration polarization. Yang et al. prepared high-performance FO membranes by adding carbon nanotubes (CNTs) between a PA layer and an electrostatically spun polyacrylonitrile (PAN) substrate. The larger pore sizes of the substrate with the CNT interlayer provided a better platform for subsequent growth of the PA layer and maintained a stable osmotic pressure difference between the solutions on both sides of the membrane during operation.118
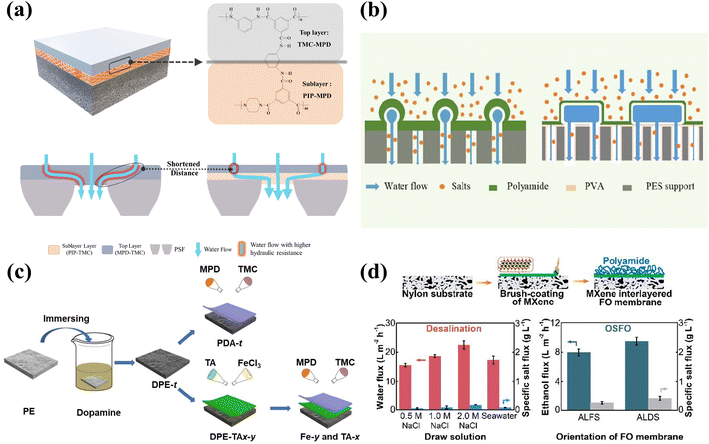
Since stable and large-scale production of two-dimensional nanosheets is difficult to achieve, Wu et al. developed a brush coating method similar to the spraying process used for large-scale preparation of MXene TFNi membranes. As shown in Fig. 5(d), the optimal permeate flux and retention rate of the FO membrane were investigated by controlling the number of brushing cycles used to place MXene on the nylon substrate and the concentration of the MXene solution. The as-prepared FO membrane showed a high water permeability of 31.8 L m−2 h−1 and a low specific salt flux of 0.27 g L−1 when using 2.0 mol L−1 sodium chloride as the draw solution. The obtained FO membranes were used for organic solvent recovery.119
The inkjet printing process facilitates rapid deposition of inks with precise quantities and positions. The process may be automated with precise control of the spraying process, which facilitates mass production. As shown in Fig. 6, Wang et al. took advantage of the inkjet printing process and applied it to syntheses of nanofiltration membranes, in which single-walled carbon nanotubes (SWCNTs) were deposited by the inkjet printing process. The SWCNTs served as an interlayer between the PA layer and the PES substrate. The effect of the SWCNT interlayer thickness on the formation of the PA layer was investigated by controlling the number of times SWCNTs were printed on the PES substrate. Ultimately, the best membrane properties were obtained for an NF membrane synthesized by printing the SWCNTs 15 times.19
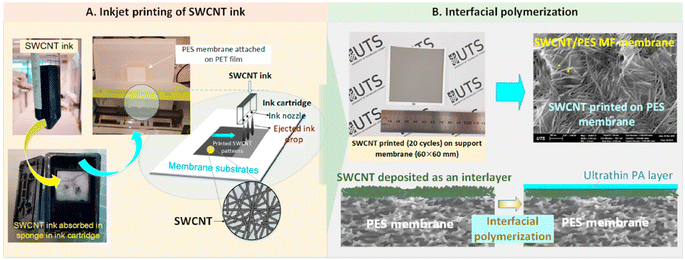 | ||
| Fig. 6 Schematic of (A) SWCNT predeposition on the PES membrane via inkjet printing and (B) deposition of an ultrathin PA layer onto the SWCNT-coated PES membrane via the IP reaction [reprinted with permission from ref. 19. Copyright 2021, Elsevier]. | ||
Future industrialization with the predeposition method is likely. On the one hand, most of the interlayer materials are metal compounds due to the use of the filtration loading method, which causes secondary leaching of metal ions in the subsequent water treatment process and secondary pollution of the environment. Second, if the separation layer of the NF membrane, PIPA, is used as the interlayer material and the PA separation layer of the RO membrane is subsequently formed on its surface, the preparation process may be expanded to enable rapid production. Therefore, industrial applications are more likely when producing TFNi membranes where the compound is predeposited on the surface of the substrate using methods such as spraying or brushing.
4.3 In situ growth
The shortcoming resulting from loading a PA layer onto the surface of the substrate by filtration is weak interfacial bonding between the substrate and the PA layer, which may lead to detachment of the PA layer and leaching of the nanomaterials during long-term water treatment.136 Nanomaterials were grown directly on the surface of the substrate during synthesis to solve this problem.The choice of material and substrate is considered. In situ growth is usually performed directly on the surface of the material, using the substrate as a support during the synthesis process. Most of the materials are synthesized at high temperatures and under acidic conditions. Therefore, the choice of substrates and nanomaterials is limited considering the mechanical strength, acid resistance and temperature tolerance range of the substrate.
For example, Song et al. grew TiO2 on the surface of a PSf substrate via an in situ growth method. Since the synthesis process requires the substrates to be immersed in the organic solvent ethanol for a long time, good resistance to organic solvents is needed. The TiO2 interlayer prepared using the in situ growth method significantly improved the surface hydrophilicity of the PSf substrate and promoted aggregation of the amine monomers in the aqueous solution. TiO2 also slowed the polymerization of amine monomers in the organic phase with the TMC interface and promoted the formation of ultrathin PA layers (13 nm).137
MOF porous nanomaterials have attracted extensive research interest in recent years.138,139 The current methods used to prepare MOF interlayers are coating,140 filtration141 and layer-by-layer self-assembly.142 However, the disadvantage of these preparation methods is weak interfacial compatibility due to poor adhesion between MOFs and the substrate. Crosslinking with the polymer is enhanced by heating the PI substrate, which does not affect the stability and flux of the membrane.143 As shown in Fig. 7, Wei et al. cross-linked PI with hexamethylene diamine and grew ZIF-8 nanomaterials in situ on its surface, which effectively prevented agglomeration of ZIF-8 on the PI surface.60 In addition, Chen et al. synthesized HKUST-1 on the PI surface using a similar in situ growth method. The hydrophilicity and porosity of the substrate were improved by intercalating HKUST-1 nanomaterials, and the obtained TFNi membrane was subsequently used for the recovery of bright blue g250 dye from organic solvents.61
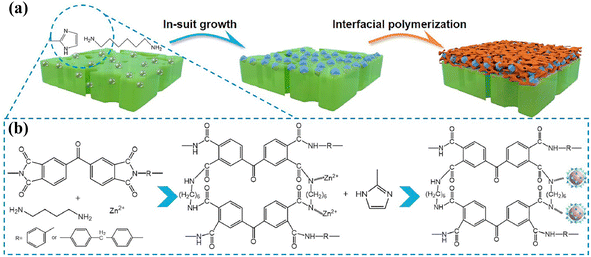 | ||
| Fig. 7 (a) Schematic illustrating the fabrication of TFNi membranes featuring an interlayer of ZIF-8 nanocrystals and (b) mechanism, including the crosslinking reaction and nucleation of the ZIF-8 nanocrystals [reprinted with permission from ref. 60. Copyright 2021, Elsevier]. | ||
Methods for the chemical modification of substrates, such as nylon, PSf, PAN and materials grown on their surfaces, are also emerging. For example, Hu et al. grafted a PAP-like molecule onto the surface of a substrate with a diazonium-induced anchoring process (DIAP).144 The PAP layer significantly increased the porosity and hydrophilicity of the substrate surface but had little effect on its surface pore size structure. The effects of PAP layers on the distribution, release and uptake of aqueous phase piperazine (PIP) monomers during the IP reaction were also systematically investigated. He et al. used nylon membranes as the substrate, which were first hydrolyzed to carry free amino groups on their surfaces and then reacted them with TMC to obtain negatively charged nylon membranes. Subsequently, TFNi membranes were prepared using the in situ growth of sulfonated COF intercalated nanomaterials.66 Chen et al. enhanced the surface cross-linking of PEI with the carboxylated PAN substrate, and the PEI intercalation layer improved the hydrophilicity of the substrate. This approach enabled the substrate to adsorb more amine monomers from aqueous solution and control the IP reaction with the release rate.53
TpPa is a COF, and it is often used in membrane separations because it can be synthesized at normal temperature and pressure and loaded onto the surface of the substrate through in situ growth without affecting the membrane structure.66–71 Wang et al. constructed structurally complete and stable TpPa interlayers on a PI substrate in a manner similar to that reported by Yang et al. to control the IP process. The COF interlayer was grown in situ using a low concentration of precursor solution, and the PA separation layer was prepared using low-concentration aqueous and organic solutions. The obtained membranes were used for organic solvent nanofiltration due to the inherently high stability of COFs toward organic solvents and the low roughness of the obtained PA layer.67,145
As shown in Fig. 8, Zhang et al. introduced TpPa on the surface of a PAN substrate and performed an IP reaction on its surface to prepare a TFNi membrane with a thin PA layer.69 Li et al. assembled COF interlayers on the surfaces of a PSf substrate and then generated a PA layer with the IP reaction, and the prepared TFNi membranes were used in a reverse osmosis process.70 In addition to the flat PSf substrate, Jiang et al. grew COFs in situ on the surface of a PSf HF substrate and constructed HF membranes exhibiting significantly improved separation performance.68 Overall, COF intercalation modulates the pore structure of the substrate and controls the uptake and release of amine monomers during the IP reaction, resulting in a thinner and more highly cross-linked PA separation layer.
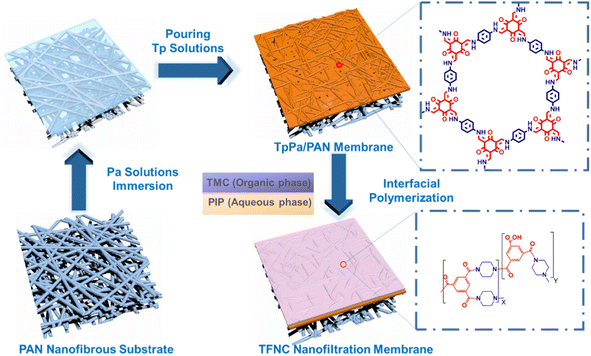 | ||
| Fig. 8 Schematic diagram illustrating the method for preparing the PA/TpPa/PAN TFNC membrane [reprinted with permission from ref. 69. Copyright 2021, Elsevier]. | ||
TFNi membranes were prepared using the electrospray IP (EIP) method, which enhanced the separation performance by increasing the crosslinking of PA layers through electrospraying aqueous and organic solutions.146,147 Yang et al. prepared Span 80 intercalated TFNi membranes by electrospraying and improved both the water treatment stability and separation performance of the TFNi membranes.148 Combining electrospraying and sandwiching methods represents a useful approach to prepare high-performance TFNi membranes.
The aforementioned in situ growth method has fewer options available due to the high requirements for substrate and interlayer materials. As shown in Table 3, fewer studies have used this approach compared to pressure filtration and predeposition. In addition, the in situ growth method has a lower material utilization rate during the TFNi membrane preparation process. The process is more complicated, resulting in a lower experimental reproducibility. Therefore, its industrialization potential is relatively limited.
| Substrate material | Interlayer nanomaterials | Membrane type | Operating conditions (applied pressure and feed solution) | Permeability (L m−2 h−1 bar−1) | Rejection (%) | Reference |
|---|---|---|---|---|---|---|
| Nylon | SCOF | NF | Na2SO4 1000 ppm | 36.6 L m−2 h−1 bar−1 | RNa2SO4 = 99.6% | 149 |
| PSf | SiO2 | NF | Na2SO4 1000 ppm | 14.5 L m−2 h−1 bar−1 | RNa2SO4 = 98.7% | 137 |
| PI | HKUST-1 | NF | Brilliant blue G 250 (858.05 g mol−1) | 9.59 L m−2 h−1 bar−1 | Rdyes = 98.8% | 61 |
| PES/SPSf | Chitosan nanoparticles (CSPs) | NF | NaCl, MgSO4, Na2SO4 and MgCl2 1000 ppm | 45.2 L m−2 h−1 bar−1 | RNa2SO4 = 99.3% | 150 |
| PSf | PDA/PEI or TA/PEI or ZIF-8/PEI | NF | NaCl, MgSO4, Na2SO4 and MgCl2 1000 ppm | 141.238 L m−2 h−1 bar−1 | RNa2SO4 = 98.4% | 151 |
| CA (cellulose acetate) | PEI-MWCNT | NF | MgCl2 (Mg2+), Co(NO3)2 (Co2+), Zn(NO3)2 (Zn2+), Pb(NO3)2 (Pb2+), CuCl2 (Cu2+), Ni(NO3)2 (Ni2+), and CdCl2 (Cd2+)1000 ppm | 16.5 L m−2 h−1 bar−1 | RMgCl2 = 95% RCo(NO3)2 = 80%, RZn(NO3)2 = 73% RNi(NO3)2, CuCl2, Pb(NO3)2 < 20% | 152 |
| PSf | ZIF-8 | NF | Congo red | 27.1 L m−2 h−1 bar−1 | Rdyes = 99.8% | 21 |
| PSf | PDA/UiO-66-NH2 | NF | NaCl, MgSO4, Na2SO4 and MgCl2 1000 ppm | 12.2 L m−2 h−1 bar−1 | RNa2SO4 = 98.1% | 57 |
| PSf | PEI/TA | NF | Na2SO4 1000 ppm | 65 L m−2 h−1 bar−1 | RNa2SO4 = 99% | 51 |
| PSf | PAP | NF | MgSO4, Na2SO4 and MgCl2 2000 ppm | 6.5 L m−2 h−1 bar−1 | R = 98% | 144 |
| SPEEK | GO/PEI/GA | OSN | AF (585.54 g mol−1), CR (696.68 g mol−1), MB (799.8 g mol−1), RB (1017 g mol−1) | Water 30 L m−2 h−1 bar−1, acetone 27.16 L m−2 h−1 bar−1, methanol 13.88 L m−2 h−1 bar−1, ethanol 9.94 L m−2 h−1 bar−1, IPA 7.42 L m−2 h−1 bar−1 | Rdyes = 99% | 12 |
| PSf | Fe(BTC) | RO | NaCl | 2.93 L m−2 h−1 bar−1 | RNaCl = 96.8% | 153 |
| PI | ZIF-8 | OSFO | Ethanol | Ethanol 2.10 L m−2 h−1 | Rpolystyrene = 89.8% | 60 |
| PAN | PEI | FO | MgCl2 500 ppm | 16.1 L m−2 h−1 bar−1 | Js NaCl 1.25 g m−2 h−1 | 53 |
| Nylon | SCOF | FO | 1 mol per L NaCl | 26.7 L m−2 h−1 | Js NaCl 1.08 g m−2 h−1 | 66 |
5. TFNi membrane characterization technology
The properties of TFN membranes are determined by the polyamide layer on the support surface and therefore require qualitative analyses, quantitative analyses and surface observations. Several characterization methods are presented below to determine the thickness of the polyamide layer formed on the membrane surface, its denseness, and the effect of the interlayer material interfacial polymerization on the IP process.5.1 Spectroscopic techniques
Spectroscopy is a general term for a class of characterization techniques that use electromagnetic radiation to obtain relevant information. The process of PA layer formation in TFNi membranes has been analyzed with various spectroscopic techniques.Among them, Fourier transform infrared (FT-IR) spectroscopy relies on the detection of molecular bond vibrations to determine the functional groups of interest but differs because it provides more timely feedback on the detection results than conventional infrared spectroscopy. In situ FT-IR spectroscopy is an effective tool for studying the IP reactions between solid and liquid phases154 that provides information on the mechanism and kinetics of IP reactions.155
For example, Ren et al. and Han et al. used a homemade FT-IR cuvette to monitor the IP reactions of alicyclic methacrylate hydrogels used to control drug release.156,157 In addition, the thickness of the PA layer was detected in real time by fitting the absorption intensities of characteristic bands in the FT-IR spectrum with a mathematical equation relating them to the thickness of the PA layer.158
As shown in Fig. 9(a), Yang et al. used in situ infrared spectroscopy to study the IP reaction in real time and successfully established a relationship between the thickness of the PA layer and the rate of the IP reaction.151 IP reactions on the PSf substrate were monitored using FT-IR spectroscopy. Usually, FT-IR spectroscopy can monitor a reaction for a few hours. However, because of the short duration of the IP reaction, the monitoring time is usually fixed between 0–300 s with a minimum interval of 15 s. The response of the IP reaction rate to the addition of nanomaterials or polymer additives to the surface of the substrate was observed.
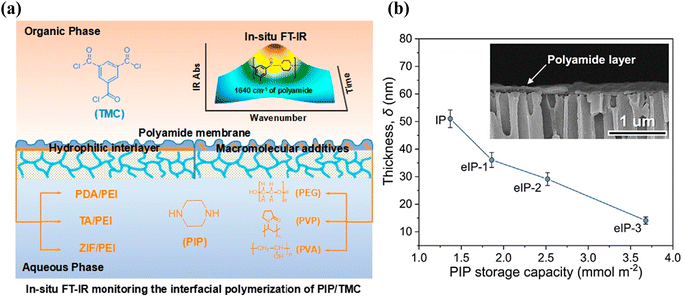 | ||
| Fig. 9 (a) In situ FT-IR spectroscopic monitoring of the IP reaction of PIP and TMC for the introduction of hydrophilic interlayers on the pristine PSf substrate or macromolecular additives in the aqueous solution composed of PIP [Reprinted with permission from ref. 151. Copyright 2020, Molecular Diversity Preservation International]. (b) Thicknesses of PA membranes prepared using conventional IP and eIP. (Inset) Cross-sectional morphology of the ultrathin polyamide membrane on the AAO substrate [reprinted with permission from ref. 162. Copyright 2021, Elsevier]. | ||
The effect of nanomaterial intercalation on the rate of amine monomer adsorption and diffusion in aqueous solution was determined.
Song et al. studied the diffusion of PIP from the aqueous phase to the organic phase with UV–vis spectroscopy. Specifically, the aqueous solution containing dispersed SiO2 was poured into a beaker, and the organic solution was added slowly. After 30 s of the reaction, 2 mL of the organic solution were poured into a quartz cuvette. The concentration of amine monomer in hexane (diffusion rate) was determined by observing the intensity of the absorption peak for PIP at 212.5 nm.159–161
The concentration of PIP in the organic phase was determined with UV–vis absorption spectrometry, as shown in Fig. 9(b), and the amount of PIP adsorbed by the intercalated nanomaterials was determined based on the diffusion kinetics of PIP. The effects of different substrates on the diffusion rate (Dr) of PIP were calculated using eqn (1):162
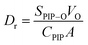 | (1) |
The kinetic model proposed by Freger for PA layer formation by IP was used to evaluate the PA layer thickness. At the same diffusion time, the thickness of the PA layer is inversely proportional to the amine monomer concentration at reaction equilibrium and proportional to the one-third power of the diffusion coefficient for transfer of the amine monomer into the organic phase. The Freger kinetic model for the formation of the separation layer has been used to calculate the thickness of the separation layer with eqn (2):163
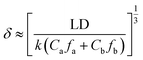 | (2) |
The experimental and simulation results showed that when the IP reaction was performed on the surface of the substrate with a high loading of the nanomaterial interlayers, the rate for diffusion of the amine monomers from the aqueous phase to the organic phase decreased; accordingly, a thinner and more cross-linked PA layer was formed.137
5.2 X-ray spectroscopy
X-ray photoelectron spectroscopy (XPS) uses electromagnetic radiation to quantify elemental compositions. XPS characterization plays an important role in studying TFNi membranes by analyzing the elemental composition to determine whether the nanomaterial was successfully intercalated. In addition, the elemental composition and chemical bonding state of the membrane surface can also be analyzed. As shown in Fig. 10, You et al. enhanced crosslinking of the PA layer by electrically driving the IP reaction, which increased the N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O content from 66.8% to 79.6% and decreased the O–C
O content from 66.8% to 79.6% and decreased the O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O content from 33.2% to 20.4%.162
O content from 33.2% to 20.4%.162
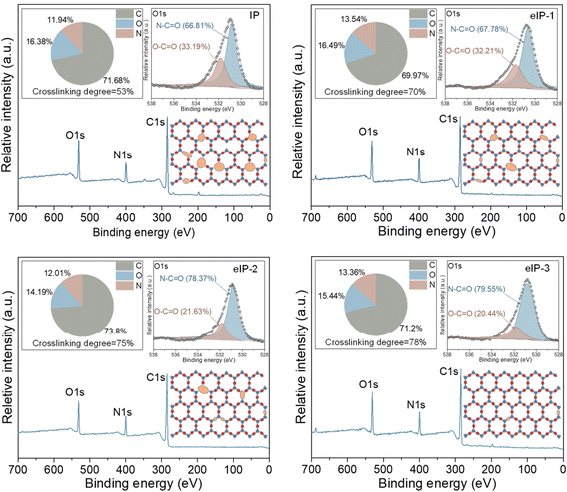 | ||
| Fig. 10 Elemental compositions (top left panel), high-resolution O 1s core spectrum (top right panel) and crosslinking structure (bottom right panel) of polyamide membranes [reprinted with permission from ref. 162. Copyright 2021, Elsevier]. | ||
The crosslinking degree of the TFNi membrane was calculated using XPS to compare the elemental concentrations of the different membranes with eqn (3):164
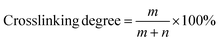 | (3) |
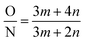 | (4) |
Microscopy is a technique enabling direct visualization and determination of the physical characteristics of a PA layer on a membrane surface. The different microscopy techniques, including atomic force microscopy (AFM), transmission electron microscopy (TEM) and scanning electron microscopy (SEM), are discussed below.
AFM, which is also referred to as scanning probe microscopy (SPM), uses physical interactions of the probe tip with the sample surface to obtain the corresponding image. This method does not require a vacuum, can be used in both gaseous and liquid environments, and does not require electrically charged or metal-coated samples. As a result, the measurements are convenient and less destructive to the sample. In addition, AFM provides additional advantages for analyzing the surfaces and microstructures of objects and quantifying the thicknesses of PA layers.
You et al. immersed a TFN membrane in DMF, dissolved the substrate with an organic solvent, and then washed it with ethanol to obtain a PA layer without substrate to characterize the thickness of the PA layer. This material was transferred to an anodic aluminum oxide (AAO) substrate to observe the complete structure of the PA layer, which enabled the measurement of the membrane thickness using AFM. As shown in Fig. 11, the thicknesses of eIP-1, eIP-2, and eIP-3 were ∼36 nm, ∼29 nm, and ∼14 nm, respectively, according to the height distributions of the AFM images.162
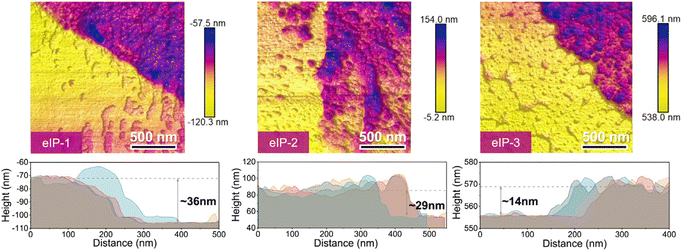 | ||
| Fig. 11 AFM images and corresponding height profiles for substrate-free eIP polyamide membranes [reprinted with permission from ref. 162. Copyright 2021, Elsevier]. | ||
In addition to measuring the thickness of the PA layer, the magnitude of the interaction force between the amine monomer and the surface of the substrate can also be measured with a solution test. Shen et al. performed AFM in probe tapping mode.165 As shown in Fig. 12, the amine monomer was adsorbed by the probe tip when the AFM probe was immersed in the aqueous solution. When the measurement started, the amine monomer adsorbed on the probe tip established an interaction force with the substrate. Subsequently, the probe tip with the adsorbed amine monomer was retracted from the surface of the substrate to determine the resistance of the interaction forces established between the amine monomer and the substrate. This amine monomer substrate interaction force can be characterized quantitatively at the atomic scale by constructing a force–distance curve.
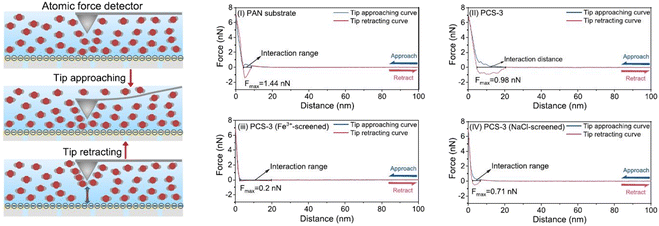 | ||
| Fig. 12 Schematic diagrams showing measurements of the interaction between PIP and the substrate with an atomic force detector and force–distance curves for wetting of substrate surfaces. The atom-scale PIP-monomer interactions with PAN(i), PCS-3 (ii), PCS-3 (Fe3+-screened) (iii), and PCS-3 (NaCl-screened) (iv) were measured [reprinted with permission from ref. 162. Copyright 2021, Elsevier]. | ||
5.3 SEM and TEM
SEM provides an image of the surface topography of the test object by focusing an electron beam on the sample surface and detecting secondary electrons, backscattered electrons and X-rays emitted by the sample.166 SEM has the advantage of shorter image acquisition and capture time than AFM. In addition, sample preparation for SEM is easier than for TEM. Therefore, SEM is widely used by researchers.Song et al. used SEM to observe the nodular structure of a TFC membrane surface with closely dispersed granular bumps. In addition, the morphological characteristics of the SiO2 TFNi membrane were observed, and the surface microstructure was noticeable and contained abundant ridge-like nanoribbons. With increases in the amount of nanomaterial added, the surface of the substrate in the TFNi membrane showed more structural features of a nanoribbon PA layer.137
TEM provides images with a resolution close to the atomic scale and provides two different types of images: dark field and bright field. Dark-field images are generated by diffracted electrons, and thus only the details of the crystal structure are reflected. In bright-field images, beam attenuation at different sample densities is caused by transmitted electrons. TEM techniques have been used to characterize the surface properties of films. The specific distribution of nanomaterials in a PA layer have been observed using TEM. For example, Yang et al.18 used TEM to show that hydrophilic AgNP nanomaterials attract surrounding aqueous phase amine monomers and subsequently block the formation of IP reactions around them to reduce the thickness of the PA layer and increase cross-linking.
5.4 Quartz crystal microscale dissipation (QCMD)
The QCMD technique has been widely used to study the thicknesses of materials deposited on solid surfaces.167 Song et al.168 investigated the deposition rates of ultrathin PA layers measuring 8, 15, and 25 nm with the QCMD technique. The authors first washed the SiO2 chip sensor with deionized water and then soaked the sensor in an aqueous solution. An organic solution was then applied to the sensor surface. The amount of PA deposited was calculating by recording the adsorbed mass Δms (ng cm−2) on the sensor surface, and Δms was determined using eqn (5):169
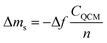 | (5) |
| Thickness = Δms/ρ | (6) |
In addition, as shown in Fig. 13, Gan et al. used QCMD to monitor changes in quality during the formation of PA layers.109 The process was divided into two stages. In the first stage, a PPA sublayer was generated via the reaction of an aqueous solution of PIP (0.05 wt%) with TMC/hexane (0.01 wt%). In the second stage, the MPD aqueous solution (0.1 wt%) reacted with the TMC/hexane (0.02 wt%) solution directly on top of the sublayer to form the PA layer. The change in sensor mass due to the formation of the PA layer was quantified by analyzing the frequency change with the Q-Tool software using the Sauerbrey equation.
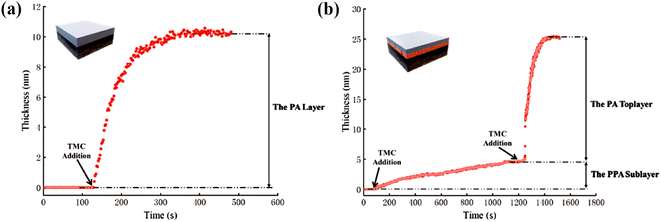 | ||
| Fig. 13 QCMD characterization of the evolution process of selected layers in the (a) uPA layer and (b) A-uPA sublayer and a top PA layer [reprinted with permission from ref. 109. Copyright 2020, Elsevier]. | ||
Surface wettability depends on the interfacial tension of three interfaces, solid–liquid, solid–gas and liquid–gas, which reflects the interactions between liquid and solid phase surfaces. During the operation of the membrane, the contact angle of the membrane is closely related to the permeability, anti-pollution and other properties of the membrane and is the main index used to judge the hydrophobicity of the membrane (when the contact angle >90°, the membrane surface is superhydrophobic). The main method for measuring the contact angle is the static drop method, which is widely used because it is quick and easy to apply.
6. Conclusions and outlook
This review presents three methods for the preparation of TFNi membranes, namely, pressure filtration, deposition and in situ growth, as well as several common methods for characterizing TFNi membranes. Each preparation method has its own advantages and disadvantages. As shown in Fig. 14, the number of articles using different methods to fabricate TFNi membranes that were published from 2015–2022 is summarized, and the total number of papers is increasing annually.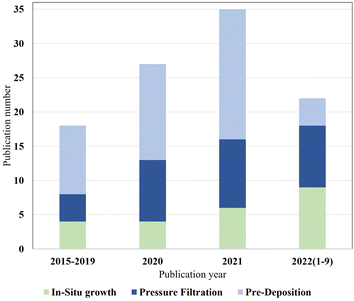 | ||
| Fig. 14 Statistical data on in situ growth, pressure filtration and predeposition methods are based on the ratio of different preparation methods to total publications. | ||
The proportion of TFNi membranes prepared using pressure filtration loading methods has likewise increased. This phenomenon may be attributed to the fact that the pressure filtration process is more controllable than the other two methods, and the variety of substrate and interlayer materials available makes it more appropriate for laboratory-scale studies. However, the pressure filtration method of loading the interlayer material with a limited effective filtration area and the cost prevent its production from being scaled for industrial applications.
For the predeposition method, a broader selection of intercalation materials is available, including some polymers such as PDA or PPA. The advantage is that the effective filtration area of TFNi membranes prepared using predeposition can be quickly scaled up compared to pressure filtration. The preparation process is equally convenient to the industry as it is controlled.
The in situ growth method is the technique that has received the least attention from researchers. This phenomenon may be attributed to the choice of materials and substrates. In situ growth is generally conducted directly on the material surface using the substrate as a support during synthesis. Most materials are synthesized under high-temperature and acidic conditions. Therefore, the selection of substrates and nanomaterials is limited. Moreover, TFNi membranes prepared using both in situ growth and pressure filtration methods are subject to the leaching of materials during water treatment, causing secondary environmental contamination.
Based on the summary provided above, we will discuss several potential considerations for future research on TFNi membranes and their development trends.
Regarding the selection of materials, we should consider the leaching of nanomaterials and cost, regardless of the method used to prepare TFNi membranes. Therefore, we should opt for environmentally friendly and low-cost intercalation materials. Since TFNi membranes are more suitable for RO/NF due to their smaller structural parameters and denser polyamide layer than TFN membranes and commercialized TFN membranes are already on the market,170,171 the commercialization of TFNi membranes is very promising.
Several potential methods for the industrial preparation of TFNi membranes are worth noting. By sacrificing the intercalation material, the modified polyamide layer is maintained, and the concern of secondary leaching in the water treatment process is avoided. Furthermore, when using the PPA layer of the NF membrane as the interlayer material, we are able to reduce the structural parameters and further improve the RO membrane performance. In addition, this method can be quickly scaled up for manufacturing. In addition, the spraying of the interlayer material with mechanical equipment enables the precise control and rapid scale up of production.
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This research was supported by the Research program of Tibet Province (ID XZ202101ZY0006G), the National Natural Science Foundation of China (52204321), the Key R&D Programs (2022YFC2904302), the Beilin District Science and Technology Plan Project (GX2222), Key R&D program of Tibet Province (ID XZ202101ZY0006G) and China Postdoctoral Science Foundation (2022MD713781). The authors would like to extend their deepest gratitude to Professor Zhiying Wang for his constant encouragement and guidance.References
- F. R. Rijsberman, Agricultural Water Management, 2006, vol. 80, pp. 5–22 Search PubMed.
- C. Y. Tang, Z. Yang, H. Guo, J. J. Wen, L. D. Nghiem and E. Cornelissen, Environ. Sci. Technol., 2018, 52, 10215–10223 CrossRef CAS PubMed.
- M. Elimelech and A. Phillip William, Science, 2011, 333, 712–717 CrossRef CAS PubMed.
- K. P. Lee, T. C. Arnot and D. Mattia, J. Membr. Sci., 2011, 370, 1–22 CrossRef CAS.
- Z. Yang, X.-H. Ma and C. Y. Tang, Desalination, 2018, 434, 37–59 CrossRef CAS.
- Z. Yang, H. Guo and C. Y. Tang, J. Membr. Sci., 2019, 590, 117297 CrossRef.
- G. M. Geise, H. B. Park, A. C. Sagle, B. D. Freeman and J. E. McGrath, J. Membr. Sci., 2011, 369, 130–138 CrossRef CAS.
- J. R. Werber, C. O. Osuji and M. Elimelech, Nat. Rev. Mater., 2016, 1, 16018 CrossRef CAS.
- N. Niksefat, M. Jahanshahi and A. Rahimpour, Desalination, 2014, 343, 140–146 CrossRef CAS.
- M. He, L. Wang, Y. Lv, X. Wang, J. Zhu, Y. Zhang and T. Liu, Chem. Eng. J., 2020, 389, 124452 CrossRef CAS.
- R. Dai, X. Zhang, M. Liu, Z. Wu and Z. Wang, J. Membr. Sci., 2019, 573, 46–54 CrossRef CAS.
- N. Cao, Z. Y. Lin, R. Y. Sun, L. Y. Chen, J. H. Pang and Z. H. Jiang, Carbon, 2021, 185, 39–47 CrossRef CAS.
- H. Dong, L. Wu, L. Zhang, H. Chen and C. Gao, J. Membr. Sci., 2015, 494, 92–103 CrossRef CAS.
- A. Zirehpour, A. Rahimpour and M. Ulbricht, J. Membr. Sci., 2017, 531, 59–67 CrossRef CAS.
- Q. Shen, Y. Q. Lin, P. F. Zhang, J. Segawa, Y. D. Jia, T. Istirokhatun, X. Z. Cao, K. C. Guan and H. Matsuyama, J. Membr. Sci., 2021, 635, 119498 CrossRef CAS.
- Y. Z. Liang, C. Li, S. X. Li, B. W. Su, M. Z. Hu, X. L. Gao and C. J. Gao, Chem. Eng. J., 2020, 380, 122462 CrossRef.
- D. L. Xu, X. W. Zhu, X. S. Luo, Y. Q. Guo, Y. T. Liu, L. Yang, X. B. Tang, G. B. Li and H. Liang, Environ. Sci. Technol., 2021, 55, 1270–1278 CrossRef CAS PubMed.
- Z. Yang, H. Guo, Z.-k. Yao, Y. Mei and C. Y. Tang, Environ. Sci. Technol., 2019, 53, 5301–5308 CrossRef CAS PubMed.
- M. J. Park, C. Wang, D. H. Seo, R. R. Gonzales, H. Matsuyama and H. K. Shon, J. Membr. Sci., 2021, 620, 118901 CrossRef CAS.
- L. X. Xie, Y. Liu, W. Zhang and S. C. Xu, Membranes, 2021, 11, 342 CrossRef CAS PubMed.
- L. Y. Wang, M. Q. Fang, J. Liu, J. He, J. D. Li and J. D. Lei, ACS Appl. Mater. Interfaces, 2015, 7, 24082–24093 CrossRef CAS PubMed.
- Z. Yang, Y. Wu, H. Guo, X.-H. Ma, C.-E. Lin, Y. Zhou, B. Cao, B.-K. Zhu, K. Shih and C. Y. Tang, J. Membr. Sci., 2017, 544, 351–358 CrossRef CAS.
- Z. Zhai, N. Zhao, W. Dong, P. Li, H. Sun and Q. J. Niu, ACS Appl. Mater. Interfaces, 2019, 11, 12871–12879 CrossRef CAS PubMed.
- Z. Liao, J. Zhu, X. Li and B. Van der Bruggen, Sep. Purif. Technol., 2021, 266, 118567 CrossRef CAS.
- M. Q. Seah, W. J. Lau, P. S. Goh, H.-H. Tseng, R. A. Wahab and A. F. Ismail, Polymers, 2020, 12, 2817 CrossRef CAS PubMed.
- V. V. Bhaskar and N. J. Kaleekkal, Emergent Mater., 2021, 5(5), 1373–1390 CrossRef.
- D. L. Zhao, S. Japip, Y. Zhang, M. Weber, C. Maletzko and T.-S. Chung, Water Res., 2020, 173, 115557 CrossRef CAS PubMed.
- N. Zhang, X. Song, H. Jiang and C. Y. Tang, Sep. Purif. Technol., 2021, 269, 118719 CrossRef CAS.
- Z. Yang, P.-F. Sun, X. Li, B. Gan, L. Wang, X. Song, H.-D. Park and C. Y. Tang, Environ. Sci. Technol., 2020, 54, 15563–15583 CrossRef CAS PubMed.
- P. Vandezande, L. E. M. Gevers and I. F. J. Vankelecom, Chem. Soc. Rev., 2008, 37, 365–405 RSC.
- H. Kita, T. Inada, K. Tanaka and K.-i. Okamoto, J. Membr. Sci., 1994, 87, 139–147 CrossRef CAS.
- K.-i. Okamoto, H. Wang, T. Ijyuin, S. Fujiwara, K. Tanaka and H. Kita, J. Membr. Sci., 1999, 157, 97–105 CrossRef CAS.
- Y. H. See-Toh, M. Silva and A. Livingston, J. Membr. Sci., 2008, 324, 220–232 CrossRef CAS.
- I. Soroko and A. Livingston, J. Membr. Sci., 2009, 343, 189–198 CrossRef CAS.
- K. Vanherck, A. Cano-Odena, G. Koeckelberghs, T. Dedroog and I. Vankelecom, J. Membr. Sci., 2010, 353, 135–143 CrossRef CAS.
- M. E. Rezac, E. Todd Sorensen and H. W. Beckham, J. Membr. Sci., 1997, 136, 249–259 CrossRef CAS.
- S. Kheirieh, M. Asghari and M. Afsari, Rev. Chem. Eng., 2018, 34, 657–693 CAS.
- M. Mulder, Basic Principles of Membrane Technology, 1999 Search PubMed.
- L. Wang, J. Chen, Y.-T. Hung and N. Shammas, Membrane and Desalination Technologies, 2011 Search PubMed.
- D. Rana and T. Matsuura, Chem. Rev., 2010, 110, 2448–2471 CrossRef CAS PubMed.
- D. D. Al-Araji, F. H. Al-Ani and Q. F. Alsalhy, Int. J. Environ. Anal. Chem., 2021, 1–25, DOI:10.1080/03067319.2021.1931163.
- A. Rahimpour, M. Jahanshahi, S. Khalili, A. Mollahosseini, A. Zirepour and B. Rajaeian, Desalination, 2012, 286, 99–107 CrossRef CAS.
- Q. Shi, Y. Su, S. Zhu, C. Li, Y. Zhao and Z. Jiang, J. Membr. Sci., 2007, 303, 204–212 CrossRef CAS.
- A. Bottino, G. Capannelli and A. Comite, Desalination, 2005, 183, 375–382 CrossRef CAS.
- F. Liu, N. A. Hashim, Y. Liu, M. R. M. Abed and K. Li, J. Membr. Sci., 2011, 375, 1–27 CrossRef CAS.
- N. M. Justino, D. S. Vicentini, K. Ranjbari, M. Bellier, D. J. Nogueira, W. G. Matias and F. Perreault, Environ. Sci.: Nano, 2021, 8, 565–579 RSC.
- Y. Gao, K. M. Su, X. T. Wang, M. L. Zhang, Z. H. Li and K. Jia, Desalination, 2020, 479, 114211 CrossRef CAS.
- S. F. Wang, Y. Yu and Q. Y. Wu, Acta Polym. Sin., 2020, 51, 385–392 CAS.
- Q. S. Yao, S. X. Li, R. R. Zhang, L. H. Han and B. W. Su, Sep. Purif. Technol., 2021, 258(2), 118027 CrossRef CAS.
- S. R. Razavi, A. Shakeri, S. M. M. Babaheydari, H. Salehi and R. G. H. Lammertink, Chem. Eng. Res. Des., 2020, 161, 232–239 CrossRef CAS.
- X. Yang, ACS Omega, 2019, 4, 13824–13833 CrossRef CAS PubMed.
- X. Zhang, Y. Lv, H. C. Yang, Y. Du and Z. K. Xu, ACS Appl. Mater. Interfaces, 2016, 8, 32512–32519 CrossRef CAS PubMed.
- Y. Q. Chen, X. J. Song, N. Zhang, X. Q. Zhang, G. Su, M. H. Huang and H. Q. Jiang, Macromol. Mater. Eng., 2021, 306, 2000818 CrossRef CAS.
- J. E. Gu, J. S. Lee, S. H. Park, I. T. Kim, E. P. Chan, Y. N. Kwon and J. H. Lee, Appl. Surf. Sci., 2015, 356, 659–667 CrossRef CAS.
- Y. Y. Tang, H. Y. Yu, Y. L. Xing, C. J. Gao and J. Xu, Desalination, 2020, 482, 114408 CrossRef CAS.
- X. Yang, Y. Du, X. Zhang, A. He and Z. K. Xu, Langmuir, 2017, 33, 2318–2324 CrossRef CAS PubMed.
- X. Zhang, Y. F. Zhang, T. C. Wang, Z. Fan and G. L. Zhang, RSC Adv., 2019, 9, 24802–24810 RSC.
- Z. Yang, F. Wang, H. Guo, L. E. Peng, X. H. Ma, X. X. Song, Z. W. Wang and C. Y. Tang, Environ. Sci. Technol., 2020, 54, 11611–11621 CrossRef CAS PubMed.
- D. Banerjee, H. Wang, Q. Gong, A. M. Plonka, J. Jagiello, H. Wu, W. R. Woerner, T. J. Emge, D. H. Olson, J. B. Parise and J. Li, Chem. Sci., 2016, 7, 759–765 RSC.
- Y. Y. Wei, Z. Yang, L. Wang, Y. F. Yu, H. Yang, H. Jin, P. Lu, Y. Wang, D. P. Wu, Y. S. Li and C. Y. Tang, J. Membr. Sci., 2021, 636, 119586 CrossRef CAS.
- K. Chen, P. Li, H. Y. Zhang, H. X. Sun, X. J. Yang, D. H. Yao, X. J. Pang, X. L. Han and Q. J. Niu, Sep. Purif. Technol., 2020, 251, 117387 CrossRef CAS.
- S. Y. Zhou, X. Q. Feng, J. Y. Zhu, Q. Q. Song, G. Yang, Y. T. Zhang and B. B. Van der, J. Membr. Sci., 2021, 623, 119058 CrossRef CAS.
- S. Hussain and X. S. Peng, Sep. Purif. Technol., 2022, 278, 119528 CrossRef CAS.
- S.-Y. Ding and W. Wang, Chem. Soc. Rev., 2013, 42, 548–568 RSC.
- S. Diercks Christian and M. Yaghi Omar, Science, 2017, 355, eaal1585 CrossRef PubMed.
- Y. S. He, X. G. Lin, J. H. Chen and H. B. Zhan, J. Membr. Sci., 2021, 635, 119476 CrossRef CAS.
- C. R. Yang, S. X. Li, X. H. Lv, H. H. Li, L. H. Han and B. W. Su, J. Membr. Sci., 2021, 637, 119618 CrossRef CAS.
- Y. X. Jiang, S. X. Li, J. H. Su, X. H. Lv, S. X. Liu and B. W. Su, J. Membr. Sci., 2021, 635, 119523 CrossRef CAS.
- T. H. Zhang, P. Y. Li, S. P. Ding and X. F. Wang, Sep. Purif. Technol., 2021, 270, 118802 CrossRef CAS.
- C. Li, S. X. Li, J. M. Zhang, C. R. Yang, B. W. Su, L. H. Han and X. L. Gao, J. Membr. Sci., 2020, 604, 118065 CrossRef CAS.
- M. Y. Wu, J. Q. Yuan, H. Wu, Y. L. Su, H. Yang, X. D. You, R. N. Zhang, X. Y. He, N. A. Khan, R. Kasher and Z. Y. Jiang, J. Membr. Sci., 2019, 576, 131–141 CrossRef CAS.
- C.-N. Yeh, K. Raidongia, J. Shao, Q.-H. Yang and J. Huang, Nat. Chem., 2015, 7, 166–170 CrossRef CAS PubMed.
- T. Wang, J. Wang, Z. Z. Zhao, X. Zheng, J. D. Li, H. L. Liu and Z. P. Zhao, Ind. Eng. Chem. Res., 2021, 60, 14868–14883 CrossRef CAS.
- F. Y. Yu, H. T. Shi, J. Shi, K. Y. Teng, Z. W. Xu and X. M. Qian, J. Membr. Sci., 2020, 616, 118611 CrossRef CAS.
- H. Ghassemi, W. Harlow, O. Mashtalir, M. Beidaghi, M. R. Lukatskaya, Y. Gogotsi and M. L. Taheri, J. Mater. Chem. A, 2014, 2, 14339–14343 RSC.
- Y. L. Guo, C. H. Ji, Y. D. Ye, Y. Chen, Z. Yang, S. M. Xue and Q. J. Niu, J. Membr. Sci., 2022, 652, 120441 CrossRef CAS.
- Q. Wu, Z. Qi and F. Wang, Synth. Met., 1999, 105, 191–194 CrossRef CAS.
- J. Li, L. Li, Y. L. Xu, J. Y. Zhu, F. Liu, J. N. Shen, Z. Y. Wang and J. Y. Lin, Chem. Eng. J., 2022, 427, 132070 CrossRef CAS.
- L. Y. Chen, M. Y. Jiang, Q. Zou, S. W. Xiong, Z. G. Wang, L. S. Cui, H. Guo, T. Zhou and J. G. Gai, Chem. Eng. J., 2021, 425, 130684 CrossRef CAS.
- L. E. Peng, Z. Yao, X. Liu, B. Deng, H. Guo and C. Y. Tang, Environ. Sci. Technol., 2019, 53, 9764–9770 CrossRef CAS PubMed.
- X.-H. Ma, Z.-K. Yao, Z. Yang, H. Guo, Z.-L. Xu, C. Y. Tang and M. Elimelech, Environ. Sci. Technol. Lett., 2018, 5, 123–130 CrossRef CAS.
- P. F. Sun, Z. Yang, X. X. Song, J. H. Lee, C. Y. Y. Tang and H. D. Park, Environ. Sci. Technol., 2021, 55, 13219–13230 CAS.
- Y. Wen, X. R. Zhang, X. S. Li, Z. W. Wang and C. Y. Tang, ACS Appl. Nano Mater., 2020, 3, 9238–9248 CrossRef.
- Y.-Z. Chen, Z. U. Wang, H. Wang, J. Lu, S.-H. Yu and H.-L. Jiang, J. Am. Chem. Soc., 2017, 139, 2035–2044 CrossRef CAS PubMed.
- C. Y. Zhu, X. Zhang and Z. K. Xu, J. Appl. Polym. Sci., 2021, 138(9), e49940 CrossRef.
- L. Tian, Y. X. Jiang, S. X. Li, L. H. Han and B. W. Su, Sep. Purif. Technol., 2020, 248, 117153 CrossRef CAS.
- M. M. Zhang, W. B. Jin, F. L. Yang, M. Duke, Y. C. Dong and C. Y. Y. Tang, Environ. Sci. Technol., 2020, 54, 7715–7724 CrossRef CAS PubMed.
- X. Cai, Z. Xie, B. Ding, S. Shao, S. Liang, M. Pang and J. Lin, Adv. Sci., 2019, 6, 1900848 CrossRef PubMed.
- Z. Yang, X. Huang, X.-h. Ma, Z.-w. Zhou, H. Guo, Z. Yao, S.-P. Feng and C. Y. Tang, J. Membr. Sci., 2019, 570–571, 314–321 CrossRef CAS.
- X. Wu, D. Fernandes, P. Feron, M. M. Ding, H. Xu and Z. L. Xie, J. Membr. Sci., 2022, 641, 119877 CrossRef CAS.
- Z. C. Ng, W. J. Lau, K. C. Wong, M. A. Al-Ghouti and A. F. Ismail, Sep. Purif. Technol., 2021, 274, 119035 CrossRef CAS.
- J. Pang, X. L. Cui, Y. Feng, Z. J. Guo, G. D. Kong, L. T. Yu, C. Y. Zhang, R. M. Wang, Z. X. Kang and D. F. Sun, Sep. Purif. Technol., 2022, 278, 119504 CrossRef CAS.
- X. J. Song, Y. J. Zhang, H. M. Abdel-Ghafar, E. S. A. Abdel-Aal, M. H. Huang, S. Gul and H. Q. Jiang, Chem. Eng. J., 2021, 412, 128607 CrossRef CAS.
- L. L. Chen, C. X. Zhang, A. L. Gao, J. Cui and Y. A. Yan, Colloids Surf., A, 2021, 626, 127001 CrossRef CAS.
- L. M. Bai, J. W. Ding, H. R. Wang, N. Q. Ren, G. B. Li and H. Liang, Sci. Total Environ., 2020, 743, 140766 CrossRef CAS PubMed.
- Y. L. Liu, X. M. Wang, X. Q. Gao, J. F. Zheng, J. Wang, A. Volodin, Y. F. F. Xie, X. Huang, B. Van der Bruggen and J. Y. Zhu, J. Membr. Sci., 2020, 596, 117717 CrossRef CAS.
- C. S. Guo, N. Li, X. M. Qian, J. Shi, M. L. Jing, K. Y. Teng and Z. W. Xu, Sep. Purif. Technol., 2020, 230, 115567 CrossRef.
- J. J. Wang, H. C. Yang, M. B. Wu, X. Zhang and Z. K. Xu, J. Mater. Chem. A, 2017, 5, 16289–16295 RSC.
- Y. Lu, Z. Y. Wang, W. X. Fang, Y. Z. Zhu, Y. T. Zhang and J. Jin, Ind. Eng. Chem. Res., 2020, 59, 22533–22540 CrossRef CAS.
- Y. L. Chen, M. Toth and C. J. He, Appl. Surf. Sci., 2019, 496, 143483 CrossRef.
- C. Cheng, P. Y. Li, K. Shen, T. H. Zhang, X. Z. Cao, B. Y. Wang, X. F. Wang and B. S. Hsiao, J. Membr. Sci., 2018, 553, 70–81 CrossRef CAS.
- D. Wang, S. Y. Li, F. L. Li, J. M. Li, N. Li and Z. N. Wang, Sci. Total Environ., 2021, 770, 145370 CrossRef CAS PubMed.
- F. Luo, J. Wang, Z. K. Yao, L. Zhang and H. L. Chen, J. Membr. Sci., 2021, 618, 118673 CrossRef CAS.
- Z. Y. Zhou, Y. X. Hu, C. Boo, Z. Y. Liu, J. Q. Li, L. Y. Deng and X. C. An, Environ. Sci. Technol. Lett., 2018, 5, 243–248 CrossRef CAS.
- S. B. Kwon, J. S. Lee, S. J. Kwon, S. T. Yun, S. Lee and J. H. Lee, J. Membr. Sci., 2015, 488, 111–120 CrossRef CAS.
- M. Wang, W. J. Dong, Y. L. Guo, Z. Zhai, Z. X. Feng, Y. F. Hou, P. Li and Q. J. Niu, Desalination, 2021, 513, 111–120 CrossRef.
- X. J. Cheng, Y. Peng, S. X. Li and B. W. Su, J. Membr. Sci., 2021, 638, 119680 CrossRef CAS.
- L. L. Gui, Y. Q. Cui, Y. Z. Zhu, X. Q. An, H. C. Lan and J. Jin, Sep. Purif. Technol., 2022, 293, 121125 CrossRef CAS.
- B. W. Gan, S. R. Qi, X. X. Song, Z. Yang, C. Y. Y. Tang, X. Z. Cao, Y. Zhou and C. J. Gao, J. Membr. Sci., 2020, 612, 118402 CrossRef CAS.
- J. Jiang, L. Zhu, L. Zhu, B. Zhu and Y. Xu, Langmuir, 2011, 27, 14180–14187 CrossRef CAS PubMed.
- Z. Zhai, C. Jiang, N. Zhao, W. Dong, H. Lan, M. Wang and Q. J. Niu, J. Mater. Chem. A, 2018, 6, 21207–21215 RSC.
- D. Ji, C. Xiao, S. An, J. Zhao, J. Hao and K. Chen, Chem. Eng. J., 2019, 363, 33–42 CrossRef CAS.
- X. Li, M. Rui, J. Song, Z. Shen and H. Zeng, Adv. Funct. Mater., 2015, 25, 4929–4947 CrossRef CAS.
- C. Cheng, L. Shen, X. Yu, Y. Yang, X. Li and X. Wang, J. Mater. Chem. A, 2017, 5, 3558–3568 RSC.
- X. W. Zhu, X. X. Cheng, X. S. Luo, Y. T. Liu, D. L. Xu, X. B. Tang, Z. D. Gan, L. Yang, G. B. Li and H. Liang, Environ. Sci. Technol., 2020, 54, 6365–6374 CrossRef CAS PubMed.
- J. M. Zhang, S. X. Li, D. C. Ren, H. H. Li, X. H. Lv, L. H. Han and B. W. Su, Sep. Purif. Technol., 2021, 268, 118649 CrossRef CAS.
- F. K. Xiao, H. C. Ge, Y. Y. Wang, S. J. Bian, Y. B. Tong, C. J. Gao and G. R. Zhu, J. Membr. Sci., 2022, 642, 119976 CrossRef CAS.
- Y. Yang, Y. L. Xu, Z. J. Liu, H. Y. Huang, X. F. Fan, Y. Wang, Y. X. Song and C. W. Song, J. Membr. Sci., 2020, 616, 118563 CrossRef CAS.
- X. Wu, M. M. Ding, H. Xu, W. Yang, K. S. Zhang, H. L. Tian, H. T. Wang and Z. L. Xie, ACS Nano, 2020, 14, 9125–9135 CrossRef CAS PubMed.
- Y. Cao, J. Q. Luo, C. L. Chen and Y. H. Wan, Chem. Eng. J., 2021, 425, 131791 CrossRef CAS.
- X. W. Zhu, X. Y. Zhang, J. Y. Li, X. S. Luo, D. L. Xu, D. J. Wu, W. Q. Wang, X. X. Cheng, G. B. Li and H. Liang, J. Membr. Sci., 2021, 635, 119536 CrossRef CAS.
- B. Z. Tian, P. Hu, S. C. Zhao, M. Wang, Y. F. Hou, Q. J. S. Niu and P. Li, Desalination, 2021, 512, 115118 CrossRef CAS.
- H. L. Lan, P. F. Li, H. Wang, M. Wang, C. Jiang, Y. F. Hou, P. Li and Q. J. Niu, Sep. Purif. Technol., 2021, 264, 118391 CrossRef CAS.
- B. Zhao, Z. Q. Guo, H. L. Wang, L. Wang, Y. R. Qian, X. L. Long, C. Ma, Z. H. Zhang, J. J. Li and H. W. Zhang, J. Membr. Sci., 2021, 625, 119154 CrossRef CAS.
- H. Z. Zhang, Z. L. Xu and Q. Shen, Desalination, 2021, 498, 114802 CrossRef CAS.
- M. D. Wang, W. X. Guo, Z. Y. Jiang and F. S. Pan, Chin. J. Chem. Eng., 2020, 28, 1039–1045 CrossRef CAS.
- X. Zhang, F. Y. Cheng, H. Z. Zhang, Z. L. Xu, S. M. Xue, X. H. Ma and X. R. Xu, J. Membr. Sci., 2020, 601, 117916 CrossRef.
- G. H. Gong, P. Wang, Z. Y. Zhou and Y. X. Hu, ACS Appl. Mater. Interfaces, 2019, 11, 7349–7356 CrossRef CAS PubMed.
- N. Kato, R. R. Gonzales, A. Nishitani, Y. Negi, T. Ono and H. Matsuyama, Colloids Surf., A, 2021, 620, 126538 CrossRef CAS.
- S. S. Yang, H. H. Li, X. Zhang, S. J. Du, J. M. Zhang, B. W. Su, X. L. Gao and B. Mandal, J. Membr. Sci., 2020, 614, 118433 CrossRef CAS.
- Y. Y. Li, C. Li, S. X. Li, B. W. Su, L. H. Han and B. Mandal, J. Mater. Chem. A, 2019, 7, 13315–13330 RSC.
- L. Y. Wang, S. X. Duan, M. Q. Fang, J. Liu, J. He, J. D. Li and J. D. Lei, RSC Adv., 2016, 6, 71250–71261 RSC.
- J. L. Guo, M. H. Huang, L. J. Meng, N. Jiang, S. Y. Zheng, M. Y. Shao and X. B. Luo, Environ. Res., 2021, 195, 110791 CrossRef CAS PubMed.
- A. A. Shah, Y. H. Cho, H. G. Choi, S. E. Nam, J. F. Kim, Y. Kim, Y. I. Park and H. Park, J. Ind. Eng. Chem., 2019, 73, 276–285 CrossRef CAS.
- X. W. Liu, Y. Cao, Y. X. Li, Z. L. Xu, Z. Li, M. Wang and X. H. Ma, J. Membr. Sci., 2019, 576, 26–35 CrossRef CAS.
- Z. Zhang, X. Shi, R. Wang, A. Xiao and Y. Wang, Chem. Sci., 2019, 10, 9077–9083 RSC.
- Q. Q. Song, Y. Q. Lin, T. Ueda, T. Istirokhatun, Q. Shen, K. C. Guan, T. Yoshioka and H. Matsuyama, J. Mater. Chem. A, 2021, 9, 26159–26171 RSC.
- Y.-S. Li, H. Bux, A. Feldhoff, G.-L. Li, W.-S. Yang and J. Caro, Adv. Mater., 2010, 22, 3322–3326 CrossRef CAS PubMed.
- Y.-S. Li, F.-Y. Liang, H. Bux, A. Feldhoff, W.-S. Yang and J. Caro, Angew. Chem., Int. Ed., 2010, 49, 548–551 CrossRef CAS PubMed.
- J. Li, M. Zhang, W. Feng, L. Zhu and L. Zhang, J. Membr. Sci., 2020, 601, 117951 CrossRef.
- H. Yang, N. Wang, L. Wang, H.-X. Liu, Q.-F. An and S. Ji, J. Membr. Sci., 2018, 545, 158–166 CrossRef CAS.
- Y. Lu, Z. Qin, N. Wang, Q.-F. An and H. Guo, J. Membr. Sci., 2021, 620, 118827 CrossRef CAS.
- K. Vanherck, G. Koeckelberghs and I. F. J. Vankelecom, Prog. Polym. Sci., 2013, 38, 874–896 CrossRef CAS.
- P. Hu, J. T. He, B. Z. Tian, Z. W. Xu, T. Yuan, H. X. Sun, P. Li and Q. J. Niu, Desalination, 2020, 496, 114340 CrossRef CAS.
- R. Wang, X. Shi, A. Xiao, W. Zhou and Y. Wang, J. Membr. Sci., 2018, 566, 197–204 CrossRef CAS.
- X.-H. Ma, Z. Yang, Z.-K. Yao, H. Guo, Z.-L. Xu and C. Y. Tang, Environ. Sci. Technol. Lett., 2018, 5, 117–122 CrossRef CAS.
- R. Chowdhury Maqsud, J. Steffes, D. Huey Bryan and R. McCutcheon Jeffrey, Science, 2018, 361, 682–686 CrossRef CAS PubMed.
- S. M. Yang, J. Q. Wang, L. F. Fang, H. B. Lin, F. Liu and C. Y. Y. Tang, J. Membr. Sci., 2020, 602, 117971 CrossRef.
- S. T. Xu, H. B. Lin, G. L. Li, J. Q. Wang, Q. Han and F. Liu, Chem. Eng. J., 2022, 427, 132009 CrossRef CAS.
- Y. F. Hao, Q. Li, B. Q. He, B. Liao, X. H. Li, M. Y. Hu, Y. H. Ji, Z. Y. Cui, M. Younas and J. X. Li, J. Mater. Chem. A, 2020, 8, 5275–5283 RSC.
- X. Yang, Membranes, 2020, 10(1), 12 CrossRef CAS PubMed.
- F. Soyekwo, Q. G. Zhang, R. S. Gao, Y. Qu, R. X. Lv, M. M. Chen, A. M. Zhu and Q. L. Liu, J. Mater. Chem. A, 2017, 5, 583–592 RSC.
- X. Zhang, Y. Zeng, C. Shen, Z. X. Fan, Q. Meng, W. Z. Zhang, G. L. Zhang and C. J. Gao, ACS Appl. Mater. Interfaces, 2021, 13, 48679–48690 CrossRef CAS PubMed.
- A. R. Hind, S. K. Bhargava and A. McKinnon, Adv. Colloid Interface Sci., 2001, 93, 91–114 CrossRef CAS PubMed.
- K. L. A. Chan and S. G. Kazarian, Anal. Chem., 2012, 84, 4052–4056 CrossRef CAS PubMed.
- J. Han, Y. He, M. Xiao, G. Ma and J. Nie, Polym. Adv. Technol., 2009, 20, 607–612 CrossRef CAS.
- D. Ren, J. I. N. Yeo, T.-Y. Liu and X. Wang, Polym. Chem., 2019, 10, 2769–2773 RSC.
- P. S. Singh, A. P. Rao, P. Ray, A. Bhattacharya, K. Singh, N. K. Saha and A. V. R. Reddy, Desalination, 2011, 282, 78–86 CrossRef CAS.
- Y. Li, X. You, Y. Li, J. Yuan, J. Shen, R. Zhang, H. Wu, Y. Su and Z. Jiang, J. Mater. Chem. A, 2020, 8, 23930–23938 RSC.
- C. Liu, J. Yang, B.-B. Guo, S. Agarwal, A. Greiner and Z.-K. Xu, Angew. Chem., Int. Ed., 2021, 60, 14636–14643 CrossRef CAS PubMed.
- Y. Lin, X. Yao, Q. Shen, T. Ueda, Y. Kawabata, J. Segawa, K. Guan, T. Istirokhatun, Q. Song, T. Yoshioka and H. Matsuyama, Nano Lett., 2021, 21, 6525–6532 CrossRef CAS PubMed.
- X. You, K. Xiao, H. Wu, Y. Li, R. Li, J. Yuan, R. Zhang, Z. Zhang, X. Liang, J. Shen and Z. Jiang, iScience, 2021, 24, 102369 CrossRef CAS PubMed.
- V. Freger, Langmuir, 2003, 19, 4791–4797 CrossRef CAS.
- S. Karan, Z. Jiang and A. Livingston, Science, 2015, 348, 1347–1351 CrossRef CAS PubMed.
- C. Shen, L. Bian, P. Zhang, B. An, Z. Cui, H. Wang and J. Li, J. Membr. Sci., 2020, 601, 117949 CrossRef.
- X.-H. Ma, Z. Yang, Z.-K. Yao, Z.-L. Xu and C. Y. Tang, J. Membr. Sci., 2017, 525, 269–276 CrossRef CAS.
- K. Ariga, Y. Lvov and T. Kunitake, J. Am. Chem. Soc., 1997, 119, 2224–2231 CrossRef CAS.
- X. Song, S. Qi, C. Y. Tang and C. Gao, J. Membr. Sci., 2017, 540, 10–18 CrossRef CAS.
- X. Li, R. Wang, F. Wicaksana, Y. Zhao, C. Tang, J. Torres and A. G. Fane, Colloids Surf., B, 2013, 111, 446–452 CrossRef CAS PubMed.
- L. Chem, Seawater Reverse Osmosis (SWRO) Membranes, https://www.lgwatersolutions.com/en/product/seawater-ro Search PubMed.
- L. Chem, Brackish Water Reverse Osmosis (BWRO) Membranes, https://www.lgwatersolutions.com/en/product/brackish-water-ro Search PubMed.
| This journal is © The Royal Society of Chemistry 2022 |