Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Nickel sulfide and phosphide electrocatalysts for hydrogen evolution reaction: challenges and future perspectives
Ali Shahroudi
 ,
Mahsa Esfandiari
and
Sajjad Habibzadeh
,
Mahsa Esfandiari
and
Sajjad Habibzadeh
 *
*
Surface Reaction and Advanced Energy Materials Laboratory, Chemical Engineering Department, Amirkabir University of Technology (Tehran Polytechnic), Tehran, Iran. E-mail: sajjad.habibzadeh@mail.mcgill.ca
First published on 17th October 2022
Abstract
The search for environmentally friendly and sustainable energy sources has become necessary to alleviate the issues associated with the consumption of fossil fuel such as air pollution and global warming. Furthermore, this is significant considering the exhaustible resources and burgeoning energy demand globally. In this regard, hydrogen, a clean fuel with high energy density, is considered a reliable alternative energy source. The hydrogen evolution reaction (HER) is one of the most promising methods to produce green hydrogen from water on a large scale. However, the HER needs effective electrocatalysts to address the concerns of energy consumption; thus, finding active materials has recently been the main focus of researchers. Among the various electrocatalysts, nickel sulfides and phosphides and their derivatives with low cost, high abundance, and relatively straightforward preparation have shown high HER activity. In this review, we compare the diverse methods in the synthesis of nickel sulfides and phosphides together with effective synthesis parameters. Also, the optimum conditions for the preparation of the desired active materials and their properties are provided. Then, the performance of nickel sulfide and phosphide electrocatalysts in the HER is addressed. The HER activity of the various crystalline phases is compared, and their most active crystalline phases are introduced. Finally, the present challenges and perspectives for future HER electrocatalysts are presented.
1. Introduction
Nowadays, due to the burgeoning world energy demand and exhaustible fossil fuel resources, renewable energies have received much attention.1,2 In the last two centuries, although fossil fuels as key energy carriers have played a significant role in industrial development, they are non-renewable sources and their formation through natural processes takes millions of years. Also, there are many environmental concerns including air pollution and global warming caused by combustion.3,4 Thus, finding sustainable energy sources to replace fossil fuels is vital.Renewable energies such as solar and wind energy are potential substitutes for fossil fuels. However, their intrinsic fluctuation has led to their low energy efficiency and has limited their usage. Accordingly, storing the energy from these types of sources in the chemical bonds of molecules is a viable solution.5 In this regard, one of the best energy storage molecules is hydrogen. The high energy density (about three times greater than standard fuels such as natural gas and gasoline) and clean hydrogen combustion demonstrate that it is as a considerable alternative to fossil fuels.6,7 In addition, currently, hydrogen plays a crucial role in many applications, making it and its production methods critical. Fig. 1 shows a graphical representation of the applications of hydrogen.
Hydrogen is mainly produced by natural gas and oil reforming or coal gasification. However, these processes consume a great amount of fossil fuels, emit CO2, and have low energy efficiency due to severe operational conditions. Also, the presence of carbon residue and sulfur contaminants leads to the production of hydrogen with low purity, which can easily poison sensitive catalysts.8 Thus, it is necessary to develop suitable strategies for the efficient and clean production of pure hydrogen.
The hydrogen evolution reaction (HER), the cathodic half-reaction of water electrolysis, is a clean and efficient alternative energy source, which produces highly pure hydrogen from water or aqueous solutions at ambient pressure and temperature without undesirable emissions. Similar to many other electrochemical processes, the HER requires electrocatalysts with high electrocatalytic activity, long-term stability, and low price for sustainable hydrogen production to overcome the energy barriers. Accordingly, Pt-group metals are the best electrocatalysts for the HER in terms of activity. However, their high cost and scarcity limit their use.9,10 Thus, significant research efforts have been devoted to finding effective non-noble metal electrocatalysts. In this regard, nickel sulfides, phosphides and their derivatives with low cost, high abundance, and relatively easy preparation have shown high activity toward the HER and are known as promising materials for the efficient electrocatalysis of the HER. However, these materials have not been studied in detail. Hence, in this review article, we provide a complete overview of these materials.
This review introduces the fundamentals of the HER and approaches for evaluating electrocatalysts in detail. Then, the different methods for the synthesis of nickel sulfides and phosphides, including the electrodeposition technique, solvothermal route, thermal decomposition, and vapor–solid reaction, are investigated and compared, their effective parameters and how they affect the properties of the synthesized materials are comprehensively discussed, and the best parameters for obtaining the desired materials and properties are provided. Then, the performance of nickel sulfide and phosphide electrocatalysts in the HER is studied, the HER activity of their different crystalline phases is compared, and their most active crystalline phase is introduced. Finally, the present challenges and perspectives for future works are presented.
2. Fundamentals of the HER
The overall water splitting reaction is as follows:
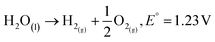 | (1) |
The HER is the cathodic half-reaction of the overall water splitting reaction, and its mechanism consists of two main steps. The first step is the electrochemical hydrogen ion adsorption reaction, which is known as the Volmer step (discharge reaction). In this step, a proton or water molecule reacts with an electron in acidic or alkaline media to produce an adsorbed hydrogen atom (H*) on the electrode surface (see reactions (2) and (3)).
Acidic medium:
| H+ + e− → H* | (2) |
Alkaline medium:
| H2O + e− → H* + OH− | (3) |
In the second step, molecular hydrogen is formed, which can occur through a chemical desorption reaction (Tafel reaction) or electrochemical desorption reaction (Heyrovsky reaction). In the Heyrovsky reaction, a hydrogen ion (H+) or water molecule in the electrolyte combines with an adsorbed hydrogen (H*) on the electrode and an electron to produce one hydrogen molecule.
Acidic medium:
| H* + H+ + e− → H2 | (4) |
Alkaline medium
| H* + H2O + e− → H2 + OH− | (5) |
Two adsorbed hydrogens (H*) combine to form molecular hydrogen in the Tafel reaction.
| H* + H* → H2 | (6) |
Thus, in general, the HER has two mechanisms, i.e., Volmer–Heyrovsky and Volmer–Tafel. The classification of the reactions based on acidic and alkaline media shows which reaction is dominant but does not mean that only the mentioned reaction proceeds in a particular medium. Also, hydrogen is produced in an electrolyte through both Volmer–Heyrovsky and Volmer–Tafel mechanisms, not just one of them. The Tafel plot can be employed to determine the dominant mechanism and the rate-determining step, which will be discussed in Section 3.2.
3. Evaluation criteria of HER electrocatalysts
3.1. Overpotential
In the equilibrium state, the net current density is zero, and to perform an electrolytic reaction at current densities greater than zero, energy more than the equilibrium electromotive force have to be applied to the system. This extra energy is used to overcome several energy barriers and resistances such as electron transfer resistance and mass transfer resistance. The difference between the applied and equilibrium electromotive forces is the overpotential (η).| η = Eapplied − Eequilibrium | (7) |
The overpotential is one of the main evaluation parameters of electrocatalysts. The lower the overpotential an electrocatalyst needs to deliver a specific current density, the lower the amount of energy is consumed, and thus the electrocatalyst shows a better performance. Commonly, electrocatalysts are evaluated and compared based on the required overpotentials at current densities of 1, 10 and 100 mA cm−2. The overpotential at 1 mA cm−2, which is mainly known as the onset potential, shows the intrinsic activity of electrocatalysts for triggering an electrochemical reaction and significantly affects the overall performance. The current density of 10 mA cm−2 is the current density on which photovoltaic cells usually operate, and the current density of 100 mA cm−2 has been selected as a criterion to show the performance of electrocatalysts on an industrial scale. These values are measured by linear sweep voltammetry (LSV). It should be noted that usually, the ohmic loss due to the resistance of the electrolyte (Rs) is compensated to investigate only the performance of electrocatalysts (ELSV = Emeasured − iRs).
In the HER field, the electric potential is often reported relative to the reversible hydrogen electrode (RHE). However, reference electrodes such as Ag/AgCl and saturated calomel electrode (SCE) are commonly used in practice. The following equations can be employed to convert the potential measured by these two reference electrodes with saturated electrolyte at 25 °C:11
| ERHE (V) = EAg/Agcl (V) + 0.199 + 0.059 × pH | (8) |
| ERHE (V) = ESCE (V) + 0.244 + 0.059 × pH | (9) |
In the case of the use of these two reference electrodes at different temperatures and concentrations, the above-mentioned equations must be corrected accordingly.
3.2. Tafel plot
According to the previous section, the overpotential is dependent on the current density. The relationship between the overpotential and current density, where electron transfer controls the electrochemical reaction (most cases), can be explained by the Butler–Volmer equation, as follows:
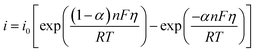 | (10) |
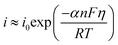 | (11) |
Extraction of η and converting the logarithm base from e to 10 give the following equation:
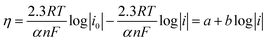 | (12) |
As is evident, the overpotential against the logarithm of current density is a straight line with the slope of 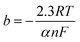 and intercept of
and intercept of 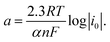 This linear equation and slope (b) are called the Tafel equation and Tafel slope, respectively, which were proposed for the first time in 1905 by Julius Tafel.
This linear equation and slope (b) are called the Tafel equation and Tafel slope, respectively, which were proposed for the first time in 1905 by Julius Tafel.
The values of a and b are usually obtained from LSV. Plotting the overpotential against the logarithm of current density gives a curve with a linear part, which can be interpolated with a line equation, and a and b are obtained from the intercept and slope of this line, respectively. The exchange current density can be determined from a according to its definition. It is worth mentioning that the Tafel slope value can also be obtained using EIS, which will be explained in Section 3.3.
The unit of Tafel slope is mV dec−1, which indicates how much overpotential has to be applied to increase the current density by 10-fold. Thus, the lower the Tafel slope of an electrocatalyst, the better its performance. The exchange current density is the current density at the equilibrium state (zero overpotential), which indicates the intrinsic activity of electrocatalysts. Thus, the higher the exchange current density of an electrocatalyst, the better its electrocatalytic performance.
Besides the above-mentioned application of the Tafel slope for the evaluation of electrocatalysts, it also can be used to determine the dominant HER mechanism and the rate-determining step (RDS) of the HER. The Tafel slopes of the Volmer, Heyrovsky, and Tafel reactions at 25 °C and assuming α = 0.5 are 118, 39 and 30 mV dec−1, respectively. Based on these values, the RDS of the HER can be specified. For example, if the Tafel slope of an electrocatalyst is more than 118 mV dec−1, the Volmer reaction (electrochemical adsorption) controls the HER or if the Tafel slope is between 118 and 39 mV dec−1, the HER mechanism is the Volmer–Heyrovsky, and the HER proceeds through a relatively fast Volmer reaction and a controlling Heyrovsky reaction.
3.3. Conductivity/charge transfer resistance
Conductivity or charge transfer resistance is one of the essential characteristics of electrocatalysts. Specifically, the higher the conductivity of an electrocatalyst, the less energy will be wasted during charge transfer. One of the most accurate and advanced techniques for measuring and comparing the charge transfer resistance of electrocatalysts is electrochemical impedance spectroscopy (EIS) (impedance in alternating current (AC) systems is equivalent to the resistance in direct current (DC) systems). In this technique, the impedance of electrocatalysts is measured by applying an AC voltage with a constant amplitude and frequency usually in the range of 100 kHz to 100 mHz. The use of an alternating current enables the effects of resistances caused by redox reactions to be considered or eliminated by adjusting the frequency. One of the outputs of EIS is the Nyquist plot, which depicts the imaginary parts of impedances against the real parts. In the case of electrocatalysts, which usually have both capacitive and resistive behavior, the Nyquist plot is approximately a semi-circle. The beginning of the plot (semi-circle) at high frequencies shows the resistance of the electrolyte. This is because redox reactions cannot proceed at high frequencies due to the quick change of the anode and cathode, and only the movement and the conductivity of ions are monitored. The end of the plot (semi-circle) at low frequencies (close to zero) in which the current is somehow direct shows all the resistances of the system, including the charge transfer resistance of the electrolyte and electrocatalyst. Thus, the diameter of the semi-circle indicates the charge transfer resistance of the electrocatalyst (note that in general, fitting techniques should be applied to extract the resistance values). To compare different electrocatalysts, the smaller the diameter of the EIS semi-circle of an electrocatalyst, the smaller its charge transfer resistance, and in terms of conductivity, it is the best electrocatalyst. Note that Nyquist plots and EIS are only addressed practically here, while their fundamentals are more than explained. Thus, it is suggested that EIS be studied in detail in the relevant sources.In addition to evaluating conductivity, a newly developed application of EIS is the calculation of the Tafel slope.12 To do this, EIS is performed at different amplitudes, and in each case, the charge transfer resistance (Rct) is extracted. Then, the amplitude is plotted versus 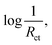 which gives a straight line, with its slope equal to the Tafel slope of the electrocatalyst. The Tafel slope calculated by this method is more accurate than that calculated using LSV data because only the charge transfer resistance of the electrocatalyst is included, and the other resistances of the system, such as the resistance of the electrolyte, are not considered.
which gives a straight line, with its slope equal to the Tafel slope of the electrocatalyst. The Tafel slope calculated by this method is more accurate than that calculated using LSV data because only the charge transfer resistance of the electrocatalyst is included, and the other resistances of the system, such as the resistance of the electrolyte, are not considered.
3.4. Stability and durability
Stability/durability is one of the most important properties of electrocatalysts, especially in industrial applications. This is because with the use of more stable and durable electrocatalysts, maintenance costs are lowered, and thus processes become more cost-effective. The stability of electrocatalysts is usually evaluated using two electrolysis approaches, i.e., galvanostatic polarization (chronopotentiometry) and potentiostatic polarization (chronoamperometry). In chronoamperometry/chronopotentiometry, the current density/overpotential is tracked against time at a constant overpotential/current density. After a period of time, if the change in potential or current density is negligible, the electrocatalyst is considered stable. It is worth mentioning that the currently used electrocatalysts in industrial water electrolyzers have shown a stable operation for as long as 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h.13 The durability of electrocatalysts can be assessed by continuous cyclic voltammetry (CV) tests. In this method, the performance of the electrocatalyst is evaluated by LSV plots before and after applying several continuous CV or LSV tests. The smaller the difference between the LSV curves of an electrocatalyst, the more durable it is.
000 h.13 The durability of electrocatalysts can be assessed by continuous cyclic voltammetry (CV) tests. In this method, the performance of the electrocatalyst is evaluated by LSV plots before and after applying several continuous CV or LSV tests. The smaller the difference between the LSV curves of an electrocatalyst, the more durable it is.
3.5. Electrochemical active surface area
Three types of surfaces can be considered for an electrocatalyst, as follows: (1) geometric surface, (2) physical specific surface area (SSA) and (3) electrochemical active surface area (ECSA). The geometric surface is the area that can be seen visually and measured using simple tools such as a ruler. This surface usually is used to report the current density relative to the surface area. The SSA is the total surface area of a material exposed per mass unit, which is measured by gas adsorption methods. However, the whole SSA is not active for reactions, and an electrocatalyst with a high SSA does not necessarily have high electrocatalytic activity. Thus, the surface area that is active for a reaction becomes important in the field of electrochemical reactions, which is called the electrochemical active surface area (ECSA). The higher the ECSA per unit of mass of an electrocatalyst, the more active sites it provides for the desired reaction, resulting in a better electrocatalytic performance. In the HER field, usually a CV test is used to calculate the ECSA. In this case, initially, the open circuit potential (OCP), which is the working potential of the electrode without applying any load to the system, is measured. The CV test is conducted at various scan rates in a potential range around the OCP. This potential range is selected because the current density is almost non-faradaic, and the electrical energy is consumed to store charges on the surface of the electrode. Then, the current density is plotted versus the scan rate, which gives a line with a slope equal to the electrochemical double-layer capacitance (Cdl) of the electrocatalyst. The selected current densities must be non-faradaic (charging) and almost in the plateau part of the CV curves selected at the OCP. To consider both the anodic and cathodic paths in CV, the difference between the cathodic and anodic current density is commonly plotted against scan rate. Accordingly, it must be noted that the Cdl is equal to half of the slope. The Cdl is directly proportional to the ECSA. Thus, the higher the Cdl, the higher the ECSA. Based on the type of materials and the capacitance per unit of area (Cs), the ECSA can be calculated according to the following equation:| ECSA = Cdl/Cs | (13) |
3.6. Turnover frequency
The electrocatalytic performance of electrocatalysts and their intrinsic activity is a function of their mass loading and SSA. Different electrocatalysts have different loadings and SSAs, making it difficult to compare their intrinsic activity. For example, an electrocatalyst with lower intrinsic activity but higher mass loading or SSA may perform better than an electrocatalyst with higher intrinsic activity but lower mass loading or SSA. Thus, comparing the intrinsic activity of electrocatalysts is challenging, and defining a parameter that can indicate the intrinsic activity of materials regardless of the mass loading and SSA is of great importance. The turnover frequency (TOF) is obtained by normalizing the amount of evolved hydrogen in relation to the number of active sites, which can indicate the intrinsic activity of electrocatalysts and used to compare different electrocatalysts in a meaningful manner. In the HER field, the TOF is defined as the number of hydrogen molecules produced per number of active sites in one second. According to this definition, the higher the TOF of an electrocatalyst, the higher its intrinsic activity. Because the number of produced H2 per unit time depends on the current density and overpotential, the TOF of different electrocatalysts is compared at the same overpotentials. At the same overpotentials, an electrocatalyst with a higher TOF has higher intrinsic activity toward the HER. It should be noted that the number of active sites is calculated using the ECSA based on some assumptions that make calculating the exact number of active sites inaccessible. Nevertheless, the TOF can be considered an acceptable parameter for comparing the intrinsic activity of different electrocatalysts.3.7. Hydrogen adsorption
Hydrogen adsorption is one of the most important aspects of HER electrocatalysis. Materials with moderate (neither too low nor too high) hydrogen adsorption ability show better performances in the HER than materials with too low or too high hydrogen adsorption ability. In detail, according to the Sabatier principle,14 the closer to zero the Gibbs free energy of hydrogen adsorption (ΔGH), the better the HER performance. This is because although materials with high hydrogen adsorption ability (ΔGH < 0) can adsorb hydrogen species such as H+ or H2O very well, they cannot properly release hydrogen in the desorption steps (Heyrovsky or Tafel reaction), thus limiting the reaction rate. Alternatively, materials with low hydrogen adsorption ability (ΔGH > 0) will face the problem from the first step (Volmer reaction) because they cannot adsorb reactants of the HER well. Therefore, the best electrocatalysts in terms of hydrogen adsorption are those that have a closer to zero ΔGH. In addition to being used as a criterion for hydrogen adsorption ability, ΔGH is also used in combination with the exchange current density to provide a good view for comparing the HER activity of different materials. For this purpose, the exchange current densities of materials are depicted versus their ΔGH, which gives a semi theoretical-semi experimental volcano-type plot. In the volcano plot, the best materials are at the top of the plot, which are Pt-group metals. Thus, the closer to the top of the volcano plot the location of an electrocatalyst, the better it is.4. Synthesis methods
4.1. Electrodeposition technique
Electrodeposition is an easy synthesis method with mild conditions in which both synthesis and deposition occur simultaneously. Briefly, in the electrodeposition technique, a precursor solution is prepared and used as the electrolyte. Then, by applying an electric field in the electrolyte in an electrochemical cell, cations move toward the cathode and deposit through a reduction reaction. The electrolyte is usually a modified Watts bath in the electrodeposition synthesis of nickel sulfides and phosphides. The conventional Watts bath, which is commonly used for nickel electroplating, consists of NiSO4·7H2O, NiCl2·6H2O, and H3BO3. For modification, sulfur and phosphorus precursors are added to the bath. Various precursors are used as the sulfur source such as thiourea (SC(NH2)2),15–21 sodium thiocyanate (NaSCN)22 and sodium thiosulfate (Na2S2O3·5H2O).23 However, in the case of the phosphorus precursor, NaH2PO2·H2O is generally always used.24–31 In addition to sulfur and phosphorus precursors, it is common to use salts such as NaCl,16–20,24,25 KCl21 and NaOAc (sodium acetate)26,27 to increase the conductivity of the electrolyte. However, the Watts bath and modifiers only ease the synthesis procedure, and in general, it is possible to synthesize nickel sulfides and phosphides via the electrodeposition method using a solution of only nickel and sulfur or phosphorus precursors. For example, Cao et al.31 synthesized Ni–P on nickel foam using an aqueous solution containing only NiCl2·6H2O and NaH2PO2·H2O as the nickel and phosphorus precursor, respectively.The parameters affecting the electrodeposition method include the type of electrolysis (galvanostatic, potentiostatic, cyclic voltammetry, etc.), current density (in galvanostatic mode), potential (in potentiostatic mode), number of cycles (in cyclic voltammetry mode), concentration of the precursors, electrodeposition time, temperature, pH and additives (conductivity adjusters, capping agents, etc.). The effects of these parameters are discussed below.
Han et al.18 investigated the effect of several parameters, including the concentration of the sulfur precursor, current density, pH of the electrolyte, and temperature on the sulfur content of the coating. Fig. 2a shows the effect of thiourea concentration at two different current densities. At concentrations below 100 g L−1, the sulfur content of the coating increased sharply as the thiourea concentration increased. However, at concentrations above 100 g L−1, the thiourea concentration had little effect on the sulfur content of the coating. A similar result also was obtained by Paseka.16
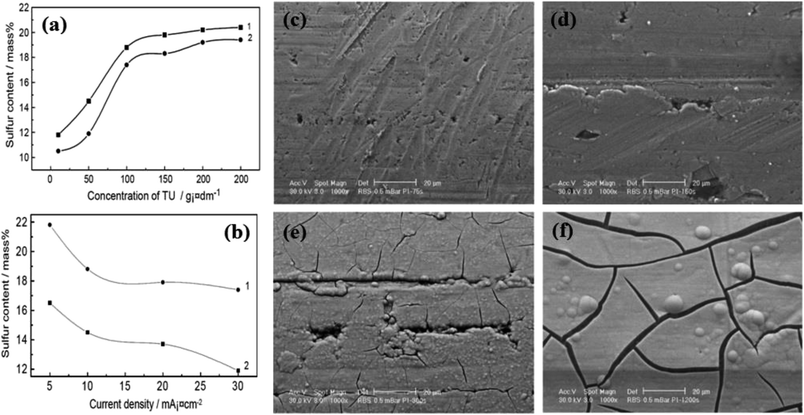 | ||
| Fig. 2 Effect of electrodeposition parameters on the sulfur content of the coating: (a) effect of the thiourea concentration at current densities of (1) 10 mA cm−2 and (2) 30 mA cm−2 and (b) effect of the electrodeposition current density at thiourea concentrations of (1) 100 g L−1 and (2) 50 g L−1 (reproduced from ref. 18 with permission from Elsevier, Copyright 2003). SEM images of the Ni–P electrode at different electrodeposition times of: (c) 75 s, (d) 150 s, (e) 300 s and (f) 1200 s (reproduced from ref. 17 with permission from Elsevier, Copyright 2001). | ||
Fig. 2b shows the effect of electrodeposition current density in the range of 5 to 30 mA cm−2 on the sulfur content of the deposited nickel sulfide. The sulfur content decreased with an increase in current density. This is because of the different polarizability of the Ni2+ ion and its complex with thiourea (Ni[CS(NH2)2]2+) at high current densities, leading to different deposition rates, and finally, a decrease in sulfur content. This result was also reported by Zhang et al.32 In the following, Han and coworkers investigated the effect of pH and temperature. They found that an increase in pH decreased the sulfur content due to the co-deposition of hydride ions at pH above 4. Also, an increase in temperature favored the dissolution of the deposited nickel and slightly increased the sulfur content deposited. Among these four factors, thiourea concentration and current density had the greatest effect on the sulfur content of the nickel sulfide coating, while pH and temperature had little effect on it. Anyway, the electrodeposition of nickel sulfides and phosphides is usually conducted in acidic media because it is assumed that sulfur and phosphorus tend to be electrodeposited more at low pH.
Although the temperature has little effect on the sulfur content of Ni–S electrocatalysts, the phosphorus content of Ni–P electrocatalysts dramatically depends on temperature. However, there are contradictory reports about how temperature affects the phosphorus content. In this regard, Burchardt25 examined the effect of temperature on the phosphorus content of Ni–P electrocatalysts. He reported that by increasing the temperature, the phosphorus content increased, whereas Wasalathanthri et al.30 reported that a decrease in temperature increased the phosphorus content. Thus, to date, it is only known that temperature affects the phosphorus content of Ni–P electrocatalysts, but it is not clear how it is influenced by temperature, which needs to be further investigated in future works.
Electrodeposition time is another parameter affecting the electrodeposition method. The electrodeposition time can affect the property of electrocatalysts by increasing their thickness and changing the morphology of the coating. In this regard, Paseka17 studied the effect of electrodeposition time on the morphology of Ni–P and Ni–S electrocatalysts. Fig. 2c–f shows the SEM images of Ni–P electrodes synthesized at different electrodeposition times. As can be seen, the amount of surface cracks increased with electrodeposition time. The calculation of the roughness factor for Ni–P and Ni–S electrodes at different electrodeposition times also confirmed this effect, where the increase in electrodeposition time increased the roughness factor of the electrodes.
Although electrodeposition mainly gives rise to amorphous structures, crystalline nickel sulfides have also been synthesized using this method. Current density is one of the critical factors determining the crystalline phase of nickel sulfide synthesized by the electrodeposition method. Zhang et al.32 synthesized amorphous nickel sulfide, Ni3S2 and NiS at 45 °C by changing the electrodeposition current density in the range of 6 to 12 mA cm−2. At current densities of 12, 10, 8 and 6 mA cm−2, amorphous, a mixture of amorphous and Ni3S2, almost pure Ni3S2 and a mixture of Ni3S2 and NiS phases were obtained, respectively. In fact, sulfur-rich phases were obtained at lower current densities. This is because as mentioned, more sulfur is deposited as the electrodeposition current density decreases. In another study, Murthy et al.33 synthesized a pure NiS crystalline phase via an electrodeposition method. They used cyclic voltammetry in the range of −1 to 0.4 V for deposition and reported that the activity of the resulting electrocatalyst in the HER depended on the number of cycles. Accordingly, in their work, the best sample was obtained at 25 cycles. It is worth mentioning that no report was found about the direct synthesis of crystalline phases of nickel phosphide by an electrodeposition method, which should be considered a subject to be studied in future works.
One of the advantages of electrodeposition is the facile synthesis of various alloys and composites. Typically, only a suitable precursor of the desired material is required together with the main precursors. In this regard, the synthesis of Co–Ni–S with CoCl2·6H2O18,34,35 or CoSO4·7H2O,36 Mn–Ni–S with MnSO4·2H2O,23 B–Ni–S with Na2B4O7·10H2O37 and Ni–P–Ag with AgNO3 (ref. 38) are some examples.
4.2. Solvothermal route
The solvothermal route is a popular and facile method for the synthesis of various materials, including nickel sulfides and phosphides. Concisely, in the standard solvothermal method, a solution of precursors is heated in an autoclave at a certain temperature for a specific time. If the solvent is water, this method also is called hydrothermal synthesis. The synthesis of nickel sulfides and phosphides via the solvothermal method has been conducted with water (hydrothermal method)39–47 and other solvents such as ethanol,48 oleylamine,49 ethanolamine,50 ethylene glycol,51 triethylene glycol,52 ethylenediamine53 and mixed solvents.54–59 The most used nickel precursor is nickel chloride,39,41,42,47,54,56,57 while other precursors such as nickel nitrate,39,59,60 nickel acetate44,45,49,55,61–63 and nickel foam39,48,50–53 have also been used. Similar to the electrodeposition method, for the synthesis of nickel sulfides, different sulfur precursors such as sulfur powder,40,49,51,61 thiourea39,40,62 and thioacetamide35 have been used, but only NaH2PO2·H2O41,42,56,57,59 and red phosphorus43–48,50,52–55,58,60 have been commonly used as the phosphorus precursor in the synthesis of nickel phosphides.There are several parameters affecting the solvothermal method, which are mainly synthesis time, temperature, pH, type of precursors, type of solvent, concentration of precursors, and additives (capping agents, reducing agents, shape controller, etc.). The effects of these factors, specifically on the synthesis of nickel sulfides and phosphides, are discussed below.
The synthesis time is one of the important parameters in the solvothermal method. The general effect of the synthesis time is through Ostwald ripening. Ostwald ripening is one of the particle growth mechanisms in the wet synthesis methods, including solvothermal route. Based on this mechanism, small particles have high solubility and the potential to dissolve in a solvent as they are synthesized due to their high surface energy. After dissolution, these particles heterogeneously grow on the larger particles with higher stability. The longer the synthesis time, the more these smaller particles dissolve and redeposit, leading to the formation of larger particles. Thus, one of the effects of prolonging the synthesis time in the solvothermal method is the formation of larger particles. It is worth mentioning that the Ostwald ripening mechanism, in addition to increasing the average particle size, also narrows the particle size distribution. Beside these general effects, the reaction time can also change the crystalline phase of the product. In the solvothermal synthesis of nickel phosphides, it seems that a longer reaction time favors the synthesis of Ni12P5 compared to Ni2P. Liu et al.60 reported that using a surfactant-aided solvothermal method at a synthesis time of 8, 16–24, and 36 h, spherical Ni2P, core–shell Ni2P/Ni12P5, and spherical Ni12P5 were achieved, respectively (Fig. 3a–e). This hypothesis was also seen in the work by Liu et al.,64 where under identical conditions and different reaction times of 24 and 48 h, almost pure Ni2P and Ni12P5 were achieved, respectively. The synthesis time can also influence the morphology of the product. In this regard, Ni et al.41 investigated the effect of the synthesis time on the morphology of the Ni12P5 product. According to Fig. 3f–h, by prolonging the synthesis time from 3 to 15 h, almost smooth spheres were converted to an immature porous structure, and then a compact porous superstructure.
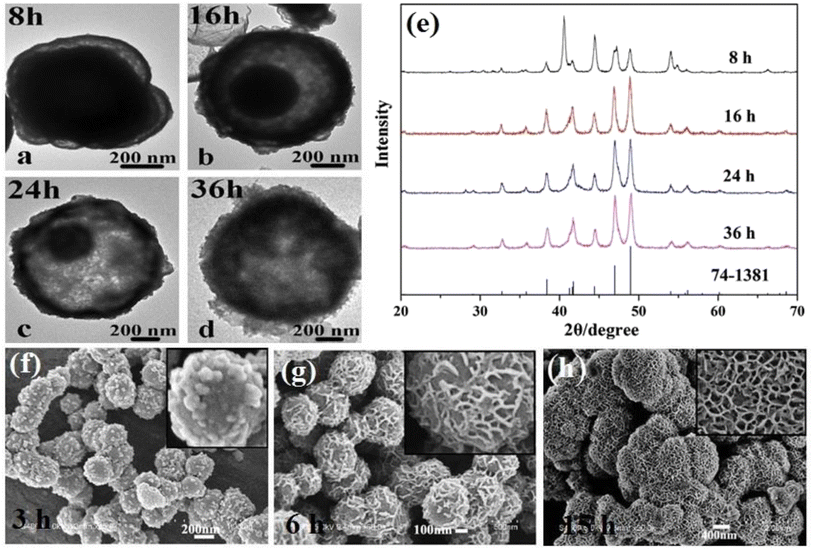 | ||
| Fig. 3 Effect of synthesis time on: (a)–(d) structure, (e) crystalline phase (reproduced from ref. 60 with permission from Elsevier, Copyright 2015), and (f)–(h) morphology of nickel phosphides.41 | ||
Temperature is another effective parameter in the solvothermal method. An increase in temperature directs the synthesis to the nucleation and formation of small particles. For example, Liu et al.47 showed that increasing the reaction temperature from 120 °C to 200 °C decreased the average particle size of Ni2P from 200 to 20 nm. In addition to the decrease in particle size, synthesis at higher temperatures has more potential to provide the needed crystallization energy, causing the growth of more grains. This can be seen in the sharper peaks of the XRD patterns, as was reported in work by Wang et al.,65 in which the crystallite size of bimetallic sulfide CoNi2S4 was calculated using the Scherrer equation, which increased from 9.3 to 14.3 nm with an increase of temperature from 160 °C to 240 °C. Generally, the solvothermal synthesis of nickel sulfides and phosphides is mainly conducted between 120 °C to 180 °C. Temperature can also change the crystalline phase of the product. In the solvothermal synthesis of nickel phosphides, it seems that higher temperatures are conducive to the synthesis of Ni12P5 compared to Ni2P. In a comprehensive study, Deng et al.43 investigated the synthesis of nickel phosphides via the solvothermal method under different conditions. They showed that with the use of different nickel precursors and different mixed solvents, an increase in temperature favored the synthesis of Ni12P5 relative to Ni2P (Fig. 4a–d). This was also observed in the work by Menezes et al.,44 where at constant conditions and different synthesis temperatures of 200 °C and 140 °C, Ni12P5 and Ni2P were formed, respectively.
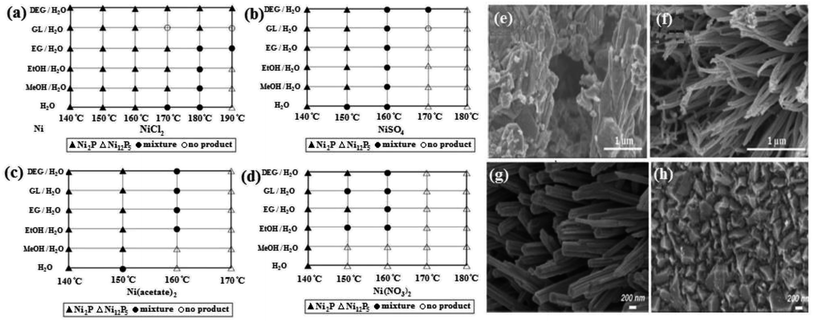 | ||
| Fig. 4 Synthesis of nickel phosphides via the solvothermal method with different nickel precursors: (a) NiCl2·6H2O, (b) NiSO4·6H2O, (c) Ni(acetate)2·4H2O and (d) Ni(NO3)2·6H2O (mixed solvents are a mixture with equal volume ratio and MeOH, EtOH, EG, GL, and DEG stand for methanol, ethanol, ethylene glycol, glycerol and diethylene glycol, respectively).43 SEM images of NiCo2S4: (e) without using nickel foam and (f) with use of nickel foam (reproduced from ref. 66 with permission from Wiley, Copyright 2016). SEM images of (g) V-doped Ni3S2 using Na3VO4·12H2O and (h) Ni3S2 (reproduced from ref. 68 with permission from the American Chemical Society, Copyright 2017). | ||
The pH of the solution is also one of the parameters affecting the solvothermal method. This parameter is crucial, especially in synthesizing nickel phosphides with NaH2PO2·H2O. This is because when NaH2PO2·H2O is dissolved in the solution, it dissociates into H2PO21− and Na+. H2PO21− needs OH− to produce PH3, which will react with Ni2+ to form nickel phosphides. In this regard, Ni et al.41 found that at pH below 4, no product was obtained, and thus they used NaHCO3 as the pH adjuster. The reactions for the synthesis of Ni2P and Ni12P5 with NaH2PO2·H2O are as follows:
PH3 generation reaction:
| 3H2PO−2 + OH− → PH3 + 2HPO2−3 + H2O | (14) |
Nickel phosphide formation reactions:
| 12Ni2+ + 5PH3 + 9e− → Ni12P5↓ + 15H + | (15) |
| 2Ni2+ + PH3 + e− → Ni2P↓ + 3H+ | (16) |
The direct deposition of catalysts on substrates without the use of a binder is one of the strategies to improve the ion/electron transfer rate because polymer binders such as Nafion and PVDF block the active sites and reduce the activity of the electrocatalysts. In the solvothermal method, if a suitable substrate such as nickel foam is placed directly in the autoclave, the synthesis and deposition coincide, and there is no need to use polymer binder. Nickel foam is one of the substrates with the potential to be used as both a substrate and nickel precursor.39,48,50–53 Moreover, nickel foam provides the opportunity to synthesize one-dimensional structures. Sivanantham et al.66 synthesized NiCo2S4 nanowire arrays using nickel foam as the substrate via a two-step solvothermal method. They reported that nickel foam was necessary for the growth of the one-dimensional structure because when NiCo2S4 was synthesized without nickel foam, an aggregated and irregular structure was obtained (Fig. 4e and f).
Similar to electrodeposition, the solvothermal method also has high ability to synthesize various complex compounds. Dhandapani et al.67 synthesized N-doped carbon-embedded Ni3S2 (Ni3S2/NC) nanocubes via the solvothermal method. They synthesized Ni3S2/NC by adding N-doped carbon dots together with Ni3S2 particles to an autoclave. Yu et al.52 simply coated Ni8P3 nanosheets with carbon using glucose as the carbon source. In another work, Qu et al.68 synthesized vanadium-doped Ni3S2 nanowire arrays via the solvothermal method. They found that using Na3VO4·12H2O salt, in addition to vanadium doped in the structure of Ni3S2, caused the growth of one-dimensional nanowires. This is because, under the same conditions and without using vanadium precursor, they observed the formation of Ni3S2 triangle-shaped nanoparticles (Fig. 4g and h). This result was also reported by Shang et al.69
4.3. Thermal decomposition
Thermal decomposition is a method for synthesis based on the decomposition of materials in which the precursors are heated to a specific temperature to break the existing chemical bonds and create new ones. In the thermal decomposition synthesis of nickel sulfides and phosphides, usually, nickel(II) acetylacetonate,70–79 Ni(NO3)2·6H2O,80 nickel(II) acetate (Ni(OAc)2·4H2O)81,82 and nickel foam83 as the nickel precursor, 1-dodecanethiol,84 cysteamine85 and thioacetamide80,83 as the sulfur precursor, trioctylphosphine (TOP),71–73,77–79,82,86 trioctylphosphine oxide (TOPO)70 and both of them74–76,81 as the phosphorus precursor, and oleylamine (OAm),84 1-octadecane (ODE), pentanediol,85 n-octyl ether,70,75 and triethylene glycol80 as the solvent have been used.The thermal decomposition synthesis of nickel sulfides and phosphides is usually carried out at temperatures of around 280 °C and 320 °C, respectively. This is because at these temperatures, the sulfur–carbon (S–C) and phosphorus–carbon (P–C) bonds in the organic precursors are broken.73,80,84,85 Thus, the temperature is usually a known and constant value in the thermal decomposition synthesis of nickel sulfides and phosphides. Accordingly, other factors such as the precursor ratio, type of solvent, synthesis time, injection rate, and additives are adjusted to synthesize materials with desired properties.
As mentioned, the precursor ratio and type of solvent are two parameters affecting the thermal decomposition method. Pan et al.84 investigated the effect of these factors on the structure of the synthesized nickel sulfides. According to their result, at the constant condition of 280 °C and 5 h synthesis time, the crystalline phase of the synthesized nickel sulfides depended on the nickel to sulfur precursor ratio (Ni![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S) and the type of solvent. Consequently, by using OAm and decreasing the Ni
S) and the type of solvent. Consequently, by using OAm and decreasing the Ni![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S ratio from 0.36 to 0.08, the crystalline phase changed from Ni7S6 to β-NiS. By lowering this ratio, more sulfur atoms diffused from the sulfur precursor to the nickel precursors, leading to the more sulfur-rich phase of NiS. Regarding the solvent effect, at a constant Ni
S ratio from 0.36 to 0.08, the crystalline phase changed from Ni7S6 to β-NiS. By lowering this ratio, more sulfur atoms diffused from the sulfur precursor to the nickel precursors, leading to the more sulfur-rich phase of NiS. Regarding the solvent effect, at a constant Ni![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S ratio of 0.18 using OAm and octadecane (ODE), Ni7S6 and a mixture of α-NiS and β-NiS were obtained, respectively. This result can be ascribed to the different capping abilities of these solvents. ODE has weaker capping ability than OAm, leading to the more effortless transfer of sulfur atoms and the formation of NiS (Fig. 5a). The effect of the precursor ratio also has been investigated in the synthesis of nickel phosphides. Pan et al.73 synthesized different crystalline phases of nickel phosphide at constant conditions and just by changing the molar ratio of TOP, as the phosphorus precursor, to Ni(acac)2, as the nickel precursor. Although they could synthesize pure Ni12P5 and Ni2P by changing the precursor ratio, pure Ni5P4 was not obtained in their experiments even by increasing the precursor molar ratio from 2.18 to 8.75. In another work, Li et al.71 completed the previous work and investigated the effect of both precursor ratio and synthesis time on the crystalline phase of the obtained nickel phosphide. They reported that when the molar ratio of TOP to Ni(acac)2 was 0.56, the product was pure Ni12P5. By increasing this ratio from 0.56 to 1.12, the pure Ni12P5 was converted to a mixture of Ni12P5 and Ni2P. A further increase in the ratio to 2.24 resulted in pure Ni2P. However, although at the ratio of 4.48, some weak peaks of Ni5P4 appeared, pure Ni5P4 was not achieved even by increasing the ratio to 5.6 (Fig. 5b). This showed that the desired crystalline phase was not achieved only by increasing the precursor ratio. Thus, they also investigated the effect of synthesis time, where they found that at the constant precursor ratio of 4.48 and increasing the synthesis time from 1 to 2 h, a mixture of Ni2P and Ni5P4 turned to pure Ni5P4 (Fig. 5c). Thus, it can be proposed that a higher P/Ni precursor ratio and longer synthesis time are favorable for synthesizing phosphorus-rich nickel phosphides.
S ratio of 0.18 using OAm and octadecane (ODE), Ni7S6 and a mixture of α-NiS and β-NiS were obtained, respectively. This result can be ascribed to the different capping abilities of these solvents. ODE has weaker capping ability than OAm, leading to the more effortless transfer of sulfur atoms and the formation of NiS (Fig. 5a). The effect of the precursor ratio also has been investigated in the synthesis of nickel phosphides. Pan et al.73 synthesized different crystalline phases of nickel phosphide at constant conditions and just by changing the molar ratio of TOP, as the phosphorus precursor, to Ni(acac)2, as the nickel precursor. Although they could synthesize pure Ni12P5 and Ni2P by changing the precursor ratio, pure Ni5P4 was not obtained in their experiments even by increasing the precursor molar ratio from 2.18 to 8.75. In another work, Li et al.71 completed the previous work and investigated the effect of both precursor ratio and synthesis time on the crystalline phase of the obtained nickel phosphide. They reported that when the molar ratio of TOP to Ni(acac)2 was 0.56, the product was pure Ni12P5. By increasing this ratio from 0.56 to 1.12, the pure Ni12P5 was converted to a mixture of Ni12P5 and Ni2P. A further increase in the ratio to 2.24 resulted in pure Ni2P. However, although at the ratio of 4.48, some weak peaks of Ni5P4 appeared, pure Ni5P4 was not achieved even by increasing the ratio to 5.6 (Fig. 5b). This showed that the desired crystalline phase was not achieved only by increasing the precursor ratio. Thus, they also investigated the effect of synthesis time, where they found that at the constant precursor ratio of 4.48 and increasing the synthesis time from 1 to 2 h, a mixture of Ni2P and Ni5P4 turned to pure Ni5P4 (Fig. 5c). Thus, it can be proposed that a higher P/Ni precursor ratio and longer synthesis time are favorable for synthesizing phosphorus-rich nickel phosphides.
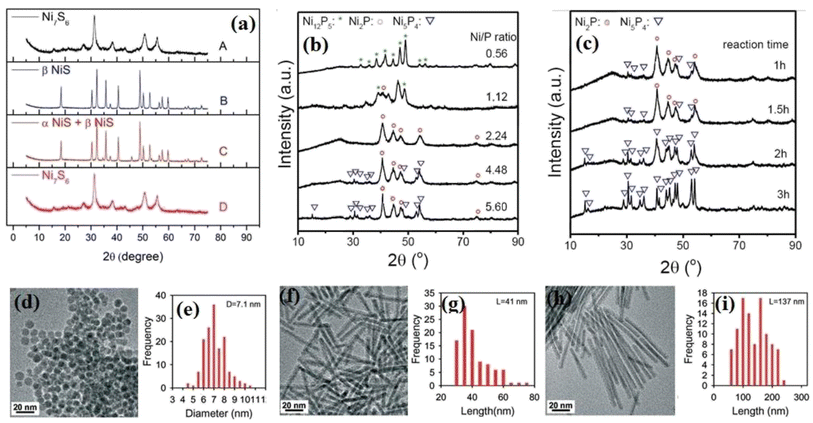 | ||
Fig. 5 (a) XRD pattern of nickel sulfides at different conditions: (A) Ni![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S = 0.18 in OAm, (B) Ni S = 0.18 in OAm, (B) Ni![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S = 0.08 in OAm, (C) Ni; S = 0.18 in ODE, and (D) Ni S = 0.08 in OAm, (C) Ni; S = 0.18 in ODE, and (D) Ni![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S = 0.18 in OAm.84 (b) XRD pattern of nickel phosphides at different precursor ratios and synthesis time of 2 h. (c) XRD pattern of nickel phosphides at different synthesis times and precursor ratio of (P/Ni) = 4.48 (reproduced from ref. 71 with permission from Wiley, Copyright 2018). SEM images and corresponding particle diameter (for spheres) or length (for nanorods) distributions of nickel phosphides at different injection rates: (d) and (e) all in one pot, (f) and (g) dropwise, and (h) and (i) with lower rate injection than that of (f) and (g).75 S = 0.18 in OAm.84 (b) XRD pattern of nickel phosphides at different precursor ratios and synthesis time of 2 h. (c) XRD pattern of nickel phosphides at different synthesis times and precursor ratio of (P/Ni) = 4.48 (reproduced from ref. 71 with permission from Wiley, Copyright 2018). SEM images and corresponding particle diameter (for spheres) or length (for nanorods) distributions of nickel phosphides at different injection rates: (d) and (e) all in one pot, (f) and (g) dropwise, and (h) and (i) with lower rate injection than that of (f) and (g).75 | ||
The injection rate of the precursors is another parameter affecting the thermal decomposition method. Li et al.74 reported that when a mixture of Ni(acac)2 and TOP was injected into a solution of TOPO in one pot at 330 °C, small particles with a diameter of around 10 nm were obtained, whereas when the mixture was injected dropwise in 1 h into the TOPO solution, nanorods with a length of up to 30 nm and an average diameter of 3 nm were achieved. In another work, Seo et al.75 synthesized nanospheres and nanorods of Ni2P by changing the injection rate. Ni2P nanospheres were obtained by adding all the TOP into a solution containing Ni(acac)2, while Ni2P nanorods were synthesized by continuous injection of a mixture of Ni(acac)2 and TOP into the TOPO solution. They also changed the nanorod length by adjusting the injection rate, and thus a lower injection rate favors the synthesis of longer nanorods (Fig. 5d–i). In a similar work, Chung et al.76 obtained short and long Ni2P nanowires by using this effect. Thus, it can be concluded that lower injection rates facilitate the formation of one-dimensional structures.
Reducing agents can ease the synthesis of nickel sulfides and the formation of more sulfur-rich phases. Zheng et al.80 synthesized NiS, NiS2, and Ni3S2 phases via a reducing agent-assisted thermal decomposition method. They synthesized NiS and NiS2 with a constant amount of precursors and exploited hydrazine hydrate (N2H4·H2O) as the reducing agent in the synthesis of NiS2 to increase the reducing ability of the solvent (triethylene glycol). Triethylene glycol has low reducing ability because the electron in the reduction of thioacetamide into S22− is produced through the slow oxidation of its hydroxyl groups into aldehydes. By using N2H4·H2O as the reducing agent, an [S–NHHN–S]2− complex is formed, which at high temperatures easily turns to S22− and causes the synthesis of a more sulfur-rich NiS2 phase.
4.4. Vapor–solid reaction
The vapor–solid reaction (VSR) is a standard method for synthesizing metal sulfides and phosphides, especially sulfur-rich and phosphorus-rich samples. In this method, sulfurization or phosphorization is typically conducted by heating a suitable sulfur or phosphorus precursor in a tube furnace to increase the vapor pressure of the precursor or to decompose it and create a sulfurizing or phosphorizing vapor. Then, the vapor is transferred to the nickel precursor location by flowing an inert/carrier gas such as nitrogen or argon inside the tube furnace. The vapor reacts with the solid-state nickel precursor (in general, metal precursor) and forms nickel sulfides or phosphides. Thus, this method is called vapor–solid reaction (wrongly, sometimes this method is called chemical vapor deposition (CVD)).This method always uses sulfur powder as the sulfur precursor. However, according to the synthesis procedure explained above, other sulfur precursors that have sufficient vapor pressure at moderate temperatures or sulfur precursors will be decomposed to a gas mixture containing sulfurizing components have the potential to be used as sulfur precursors. For example, Li et al.87 used a sulfur-containing resin and synthesized Ni3S2 nanowires via the VSR method. Regarding the phosphorus precursors, similar to the solvothermal method, only NaH2PO2·H2O and elemental red phosphorus were used as the phosphorus precursors. However, again, based on the VSR synthesis procedure, it seems that other phosphorus precursors with an appropriate vapor pressure at not too high temperature or decomposable phosphorus precursors that decompose to a gas mixture contain a phosphorizing component by heating to a specific temperature can also be used as potential phosphorus sources.
The VSR synthesis of nickel sulfides and phosphides can be carried out through one-step or two-step synthesis. In the one-step synthesis, nickel foam is used as the nickel precursor. In the two-step synthesis, initially, a nickel precursor (mostly Ni(OH)2) is synthesized mainly via the solvothermal method, but various methods such as electrodeposition,88 precipitation89–91 and electrospinning92 can generally be used. Then, the nickel precursor is placed in a tube furnace for sulfurization or phosphorization in the second step. These two routes each have advantages and disadvantages. For example, one-step VSR synthesis is more straightforward and shorter due to having one less step, but it is limited to the use of nickel foam as the nickel precursor and has limited flexibility to alter the morphology of the product. Alternatively, two-step synthesis can enable the synthesis of nickel sulfides and phosphides from different nickel precursors, synthesis of various composites, and tuning the morphology of the nickel precursor and the final product; however, it needs one more step, which makes the whole procedure more complicated and longer.
The main parameters affecting the VSR method are temperature, synthesis time, position of the precursors, amount of precursors, and type of precursors. The effects of these factors, in particular, on the VSR synthesis of nickel sulfides and phosphides are discussed in the following. Temperature plays a crucial role in the VSR method. As the temperature increases, the vapor pressure of the sulfur or phosphorus precursor increases, which results in a higher degree of sulfurization and phosphorization and the formation of more sulfur- or phosphorus-rich crystalline phases. Also, some materials with multi-step decomposition reactions experience the complete decomposition and generation of sulfurizing and phosphorizing gases at higher temperatures. For example, the decomposition of NaH2PO2 occurs in three steps, where the first step is triggered at about 310 °C and 0.3 mol PH3 is generated per 1 mol of NaH2PO2. Upon increasing the temperature to 450 °C, complete decomposition occurs, in which 0.4 mol PH3 is generated per 1 mol of NaH2PO2.93 Although the increase in temperature facilitates the formation of sulfur- and phosphorus-rich phases, very high temperatures can cause them to decompose again into nickel-rich phases. Thus, there is an optimum value for VSR temperature in the synthesis of sulfur- or phosphorus-rich phases. In this regard, Liu et al.94 reported that when the VSR synthesis using red phosphorus was conducted at 400 °C, almost no phosphorization occurred, which shows that this temperature was not as sufficient to appropriately sublime red phosphorus. However, nickel phosphides were formed when they increased the synthesis temperature to 500 °C and above it. In another work, Li et al.90 synthesized various nickel phosphides via the VSR method at different temperatures. They reported that pure Ni2P was achieved at 275 °C, and by increasing the temperature to 300 °C, a composite with the major content of Ni2P and minor contents of Ni5P4 and NiP2 was synthesized. An increase in temperature to 325 °C resulted in a mixture with a higher amount of Ni5P4 and NiP2 and lower content of Ni2P. However, a further increase in temperature to 375 °C gave rise to a mixed-phase of Ni2P, Ni5P4 and NiP2 with a higher content of Ni2P, and finally a higher synthesis temperature of 475 °C further increased the content of Ni2P (Fig. 6a).
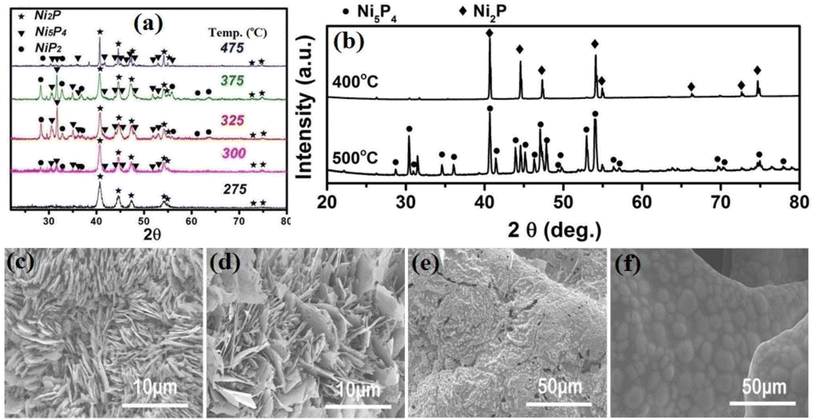 | ||
| Fig. 6 (a) XRD patterns of nickel phosphides at different temperatures (reproduced from ref. 90 with permission from the American Chemical Society, Copyright 2016) and (b) XRD patterns of nickel phosphides at different temperatures (reproduced from ref. 95 with permission from Wiley, Copyright 2015). SEM images of nickel phosphides at different temperatures of: (c) 400 °C, (d) 500 °C, (e) 600 °C and (f) 800 °C (reproduced from ref. 95 with permission from Wiley, Copyright 2015). | ||
In a similar work, Wang et al.95 synthesized nickel phosphides via the VSR method and examined the effect of temperature. They observed that by increasing the VSR temperature from 400 °C to 500 °C, the product changed from a mixture of Ni2P and Ni5P4 with higher content of Ni2P to a mixture of Ni2P and Ni5P4 with ca. 80 wt% of Ni5P4 (Fig. 6b). However, a further increase in temperature to 600 °C and 800 °C decreased the content of Ni5P4 to values even lower than that at 400 °C. They also observed that temperature could change the morphology in such a way that at temperatures of 400 °C and 500 °C, nanosheet arrays were formed, while an increase in temperature from 400 °C to 500 °C promoted the growth of nanosheets. However, a further increase in temperature to 600 °C and 800 °C caused the nanosheets to disappear (Fig. 6c–f). The effect of VSR temperature on morphology was also reported by Mishra et al.96 and Li et al.,87 where again it was confirmed that an increase in temperature promotes the growth of nanostructures. It should be noted that in the VSR synthesis of nickel phosphides using red phosphorus, after their synthesis, firstly, the tube furnace should be cooled to as low as about 250 °C and maintained at this temperature for a few hours to ensure that the highly reactive and flammable yellow phosphorus is converted to safe red phosphorus.88
The synthesis time in the VSR method can affect the crystalline phase and morphology of the products. A longer synthesis time provides the opportunity for complete sulfurization or phosphorization of the nickel precursor and the growth of the formed structures. Specifically, increasing the synthesis time increases the probability of the formation of phases with a higher content of sulfur and phosphorus, and also in the case of one-dimensional structures, it can lengthen them. Li et al.87 investigated the effect of the synthesis time on the morphology of the product. They reported that when the synthesis was conducted for 1 h, nano-seeds of Ni3S2 were formed on nickel foam. An increase in the synthesis time to 3 h led to the appearance of Ni3S2 nanowires, and finally performing synthesis for 6 h caused them to grow further (Fig. 7a–c). This result was also reported by Xiao et al.,97 who saw that by extending the synthesis time, the nickel phosphide nanowires gradually grew along the vertical direction of the Ni foam (see Fig. 7d–f). In another work, Mishra et al.96 reported that the synthesis time affects the progress of the phosphorization process in such a way that in their work, according to the amount of red phosphorus, 1 h was not sufficient for the complete phosphorization of nickel foam, and peaks related to nickel were observed in the XRD pattern. By increasing both the content of red phosphorus and synthesis time to 6 h, the nickel foam was turned entirely to nickel phosphide.
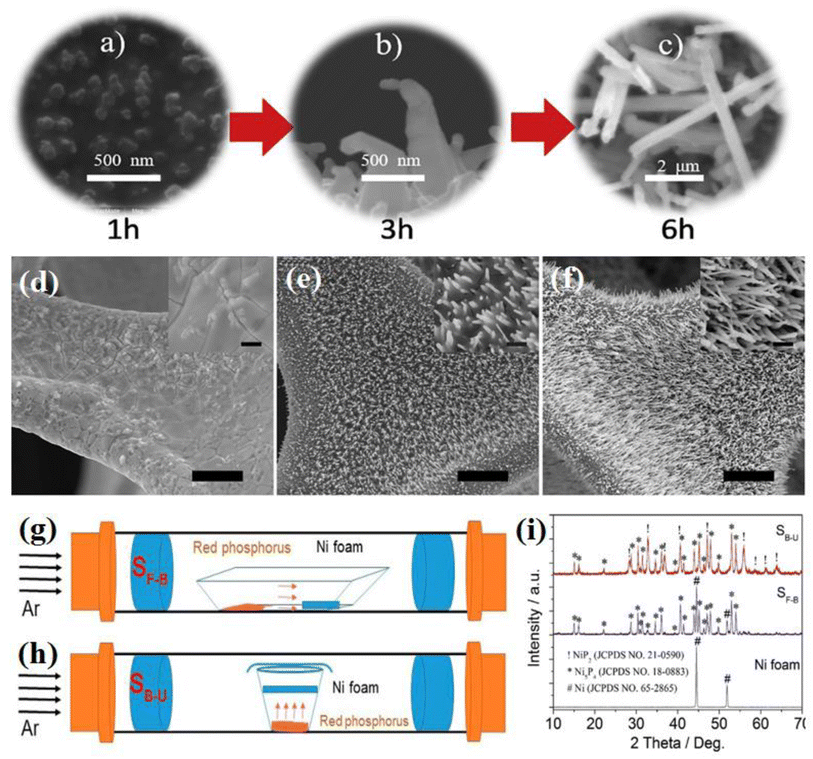 | ||
| Fig. 7 (a)–(c) SEM images of nickel sulfide at different synthesis times (reproduced from ref. 87 with permission from Elsevier, Copyright 2019). (d)–(f) SEM images of nickel foam at different synthesis times: all bars are 20 μm (inset: the corresponding enlargement, all bars are 2 μm).97 Schematic of precursor positions in the VSR method: (g) back-front, (h) bottom-up and (i) XRD pattern of nickel phosphides at different positions of the precursors (reproduced from ref. 94 with permission from Elsevier, Copyright 2019). | ||
In the VSR method, the position of the precursors in the tube furnace is also a factor affecting the synthesis. In most cases, the sulfur or phosphorus precursors are put upstream, while the nickel precursors are placed at the end or middle of the tube furnace (back-front position) (Fig. 7g). However, Li et al.87 synthesized Ni3S2 nanowires via VSR by placing nickel foam above a sulfur-bearing resin (bottom-up position) (Fig. 7h) or some works89,98–100 synthesized different nickel phosphides by preparing a mixture of nickel precursor and NaH2PO2 and putting the whole mixture in one location. In this regard, Liu et al.94 investigated the effect of the precursor position in the VSR method. They synthesized mixed-phase nickel phosphides in both back-front and bottom-up positions. The results showed that under the same conditions, the bottom-up position led to the complete phosphorization of nickel foam, whereas in the back-front position, phosphorization was not entirely performed, and there were peaks related to nickel in the XRD pattern of the sample (Fig. 7i). It is worth mentioning that the sample prepared in the bottom-up position had higher HER activity compared to the sample synthesized in the back-front position. According to the above discussion, the advantages and disadvantages of these synthesis methods are summarized in Table 1.
| Synthesis method | Advantages | Disadvantages |
|---|---|---|
| Electrodeposition | Low temperature | Low crystallinity and amorphous products |
| Flexibility in precursor selection | Limitations in the synthesis of various morphologies | |
| Ability to synthesize various composites | Difficulties in conducting powder characterization tests | |
| Short synthesis time | Limited to conductive substrates | |
| Binder-free | ||
| Facile control of loading | ||
| Solvothermal | Flexibility in precursor selection | Relatively long synthesis time |
| Facile synthesis procedure | Requires binder for the preparation of electrocatalyst (except cases that use particular substrates inside autoclave) | |
| Ability to synthesize various morphologies | ||
| Ability to synthesize various composites | ||
| Ability to synthesize various crystalline phases | ||
| Thermal decomposition | High crystallinity of products | High temperature |
| Ability to synthesize various crystalline phases | Limited to organic sulfur and phosphorus precursors | |
| Ability to synthesize various composites | Requiring inert atmosphere | |
| Ability to synthesize various morphologies | Requiring binder for the preparation of electrocatalysts | |
| Requiring a relatively complicated setup | ||
| Poor HSE | ||
| VSR | High crystallinity of products | High temperature |
| Ability to synthesize various composites (in two-step synthesis) | Requiring inert/carrier gas | |
| Binder-free (except for some two-step methods) | Limitations in the synthesis of various morphologies (in one-step synthesis) | |
| Relatively short synthesis time (in one-step synthesis) | Requiring a relatively complicated setup | |
| Poor HSE |
5. Performance in the HER
In this section, we investigate the performance of nickel sulfide and phosphide electrocatalysts in the HER. The main aim is to introduce the most active materials. In addition, the activation step in nickel sulfide electrocatalysts and the performance of nickel phosphide-based composites are also considered in detail. It should be noted that what is explained for the activation step of nickel sulfides and the effects of various materials on the properties of pristine nickel phosphides are also valid for nickel sulfide, and phosphide electrocatalysts and are not presented separately to refrain from repetition. Here, we provide a good view for finding materials with the best performance and illustrate how different materials can affect the performance of pure nickel sulfide and phosphide electrocatalysts.5.1. Nickel sulfide electrocatalysts
The performance of nickel sulfide electrocatalysts in the HER dramatically depends on their crystalline phase. In fact, their different properties compared to pure nickel can be attributed to their crystalline structure, not just the presence of sulfur.16 According to the literature, Ni3S2 appears to be the most active crystalline phase of nickel sulfides in alkaline media. In this regard, Jiang et al.49 investigated the HER performance of Ni3S2, NiS2, and NiS on a carbon substrate in 1.0 M KOH. According to Fig. 8a, although all three electrocatalysts were active in the HER, their performance was considerably different. The Ni3S2 electrocatalyst with the lowest onset potential was the most active sample, which needed an overpotential of −335 mV to deliver a current density of 10 mA cm−2. NiS2 and NiS required overpotentials of −454 and −474 mV at the same current density, respectively. The better performance of Ni3S2 compared to NiS and NiS2 can be related to the more optimum electrochemical hydrogen adsorption, as was confirmed by the corresponding Tafel plot of the electrocatalysts, where Ni3S2, NiS2 and NiS had Tafel slopes of 97, 128 and 124 mV dec−1, respectively (Fig. 8b). The higher conductivity of Ni3S2 was the second reason for its better performance, where according to the EIS plots, Ni3S2 with lowest semi-circle diameter had the highest conductivity (Fig. 8d). In addition, Ni3S2 possessed the largest ECSA among the electrocatalysts. Fig. 8c shows a comparison of the physical and electrochemical surface area of these three electrocatalysts. Although the NiS electrocatalyst had the largest physical surface area, its ECSA was the lowest. Thus, it is not merely important how large the physical surface area of an electrocatalyst is, it is also important how much of the surface is active for the reaction. The percentage of a surface that is active for the reaction can be calculated by dividing the ECSA by the physical surface area, which for the above-mentioned case showed that Ni3S2 was intrinsically more active than NiS2 and NiS in the HER in alkaline media. In a similar study, Zheng et al.80 considered the HER performance of Ni3S2, NiS2, and NiS on carbon paper in 1.0 M KOH. Based on Fig. 8e, Ni3S2 was the most active electrocatalyst, which needed ca. 50 and 110 mV lower overpotentials than NiS and NiS2 for delivering a current density of 10 mA cm−2, respectively. This superiority was further confirmed by the Tafel slope of these electrocatalysts (Fig. 8f). To investigate the reasons behind this performance, the ECSA and charge transfer resistance of these electrocatalysts were analyzed. The results showed that Ni3S2 had the largest ECSA and the lowest charge transfer resistance (Fig. 8g and h), respectively. The better performance of Ni3S2 in alkaline media also was confirmed by Zhang et al.,32 where again the better performance of Ni3S2 was attributed to its optimum electrochemical hydrogen adsorption (lower Tafel slope), higher conductivity and larger ECSA.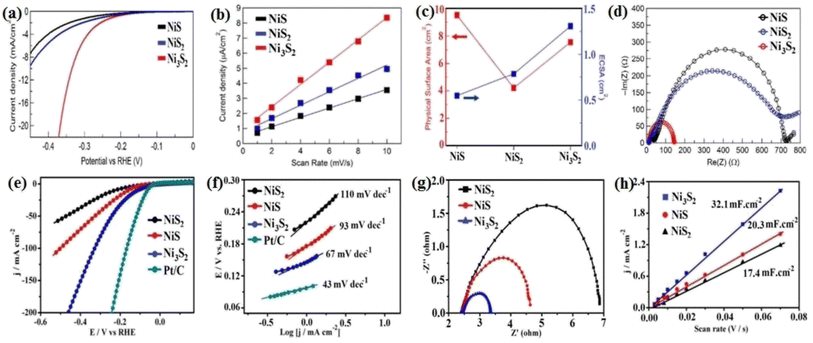 | ||
| Fig. 8 Ni3S2, NiS and NiS2 electrocatalysts: (a) LSV curves in 1.0 M KOH, (b) electrochemical double-layer capacitance, (c) comparison of SSA (red) and ECSA (blue),49 (d) EIS, (e) LSV curves in 1.0 M KOH, (f) corresponding Tafel plots, (g) EIS plots and (h) electrochemical double-layer capacitance.80 | ||
Although it seems that Ni3S2 is the most active crystalline phase of nickel sulfides in alkaline media, comparing the different phases of nickel sulfides in acidic media has been rarely investigated like in the work by Chung et al.85 Therefore, it is hard to conclude the activity trend of nickel sulfides in acidic media, and further studies have to be conducted in this field in the future.
Besides the crystalline phases, amorphous nickel sulfide electrocatalysts have been applied in the HER even earlier than the crystalline phases since the 1980s. In one of the primary works, in 1983, Vandenborre et al.15 investigated the performance of amorphous nickel sulfide electrocatalysts in the HER. They found that the thickness of the nickel sulfide layer affected the electrocatalytic performance, where as its thickness increased, the overpotentials decreased. In 2001, Paseka17 confirmed this finding. He reported that as the thickness increased, the electrocatalyst activity increased to a limiting thickness, at which the layer decomposed and became mechanically unstable. In another work, Paseka16 reported that his synthesized amorphous nickel sulfide was more active than Ni3S2. He prepared some nickel sulfide electrocatalysts with different sulfur contents on nickel foam via an electrodeposition method. Then, the electrocatalysts were heated at 150 °C and their performance in 1.0 M KOH was evaluated before and after heat treatment. The results showed that after heat treatment, the hydrogen adsorption ability of the samples decreased and the needed overpotentials at different current densities increased. The characterization of the heated electrocatalysts showed that the structure of most of them was converted to Ni3S2 without reducing the sulfur content. Thus, the amorphous nickel sulfides were more active than crystalline Ni3S2. Although this result does not mean that every amorphous nickel sulfide electrocatalyst necessarily has a better performance than crystalline nickel sulfides such as active Ni3S2 because amorphous electrocatalysts have specific and different performances due to their random nature, it shows the great potential of amorphous nickel sulfide electrocatalysts in the HER. The sulfur content of amorphous nickel sulfide electrocatalysts is one of the main factors affecting their activity. By increasing the sulfur content to the optimum amount of ca. 19%, the HER activity increased and the overpotential decreased, and a further increase in sulfur content lowered the activity of the electrocatalyst. One reason for this phenomenon is the morphological changes. By increasing the sulfur content to more than the optimum amount, the surface roughness of the electrode decreased, consequently lowering the number of exposed active sites and activity.17–20
Nickel sulfide and phosphide electrocatalysts (in general, all electrocatalysts) usually have an activation step in which their activity is significantly changed. Acid/base treatment or continuous polarization has been performed to study this activation step. In this regard, Lin et al.101 examined Ni3S2 loaded on multi-walled carbon nanotube (Ni3S2(X%)/MWCNT) electrocatalysts in which X indicates the weight percent of Ni3S2 in the composites. They immersed the Ni3S2(55%)/MWCNT electrocatalyst in a 30 wt% KOH solution for 12 h and conducted electrochemical measurements and characterization tests before and after the base treatment. In addition, they investigated the effect of continuous polarization on the activity of the electrocatalysts. Fig. 9a shows the LSV curves of Ni3S2(55%)/MWCNT at different polarization values. As can be seen, as the number of polarizations increased, the electrocatalyst activity gradually improved to the optimum number, at which the performance of the electrocatalyst became steady. Fig. 9b shows the Tafel plots of Ni3S2(55%)/MWCNT before and after 12 h of base treatment. It is evident that the KOH-treated electrocatalyst with a lower Tafel slope (102 mV dec−1) was more active than the as-synthesized sample (167 mV dec−1). They also reported that the base treatment of Ni3S2(83%)/MWCNT approximately doubled the delivered current density at the overpotential of −580 mV.
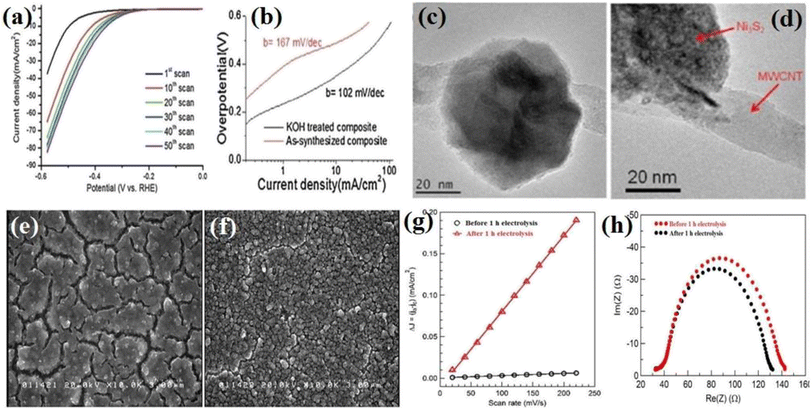 | ||
| Fig. 9 Ni3S2(55%)/MWCNT-NC: (a) LSV curves at different polarization values in 30 wt% KOH and (b) corresponding Tafel plots before and after 12 h base treatment in 30 wt% KOH. (c) TEM image before base treatment and (d) TEM image after base treatment (reproduced from ref. 101 with permission from Elsevier, Copyright 2014). Ni–S/FTO: (e) SEM image before 1 h electrolysis, (f) SEM image after 1 h electrolysis, (g) electrochemical double-layer capacitance and (h) EIS plots.21 | ||
To find the cause of this behavior, transmission electron microscopy (TEM) analysis was conducted. According to Fig. 9c and d, the surface of the KOH-treated Ni3S2(55%)/MWCNT-NC nanoparticle was rougher than the untreated particle. This showed that the base treatment changed the morphology of the electrocatalyst by increasing the surface area and exposed active sites. This finding was further confirmed by calculating the ECSA, where the ECSA of the KOH-treated electrocatalyst was 2-times greater than that of the untreated sample. Also, the EIS result showed that the charge transfer resistance of the KOH-treated electrocatalyst decreased by 7 times. Thus, base treatment and continuous polarization can increase the activity of electrocatalysts by increasing their surface area and exposed active sites and reducing the charge transfer resistance. This observation was also reported in other works.17,21,39,102 Jiang et al.21 showed that the surface cracks of Ni–S/FTO after 1 h of electrolysis in a neutral solution decreased, and the roughness and porosity increased (Fig. 9e and f) in such a way that the ECSA of the electrocatalyst after 1 h of electrolysis got 34 times higher than that of the as-synthesized sample (Fig. 9g). Also, the EIS plots showed that after electrolysis, the conductivity of Ni–S/FTO improved (Fig. 9h). In another work, Ouyang et al.39 investigated the effect of acid treatment of the nickel foam as the substrate. The results showed that Ni3S2 on the acid-treated nickel foam was more active than Ni3S2 on the pristine nickel foam, and again, this improved behavior was attributed to the enhanced surface. A summary of the performance of pure nickel sulfide electrocatalysts is presented in Table 2.
| Catalysta | Substrate | Synthesis method | Electrolyte | Loadingb (mg cm−2) | η10 (mV vs. RHE) | b (mV dec−1) | Ref. |
|---|---|---|---|---|---|---|---|
| a Ni–S stands for amorphous nickel sulfide.b For amorphous nickel sulfide, loading indicates the percentage of sulfur in the electrocatalyst. | |||||||
| Ni–S | Nickel | Electrodeposition | 1.0 M KOH | ∼4 | 281 | 150 | 35 |
| Ni–S | Nickel foam | Electrodeposition | 30% KOH | ∼18 | 190 | 166 | 20 |
| NiS | Glassy carbon | Thermal decomposition | 0.5 M H2SO4 | 0.199 | 250 | 51.2 | 84 |
| NiS | Carbon paper | Thermal decomposition | 1.0 M KOH | 0.6 | 160 | 93 | 80 |
| NiS | Carbon paper | Solvothermal | 0.5 M H2SO4 | 0.3 | 96 | 52 | 60 |
| NiS | Glassy carbon | Solvothermal | 1.0 M KOH | 0.283 | 474 | 124 | 49 |
| NiS | Nickel foam | VSR | 1.0 M KOH | 43 | 120 | 83 | 103 |
| Ni3S2 | Glassy carbon | Solvothermal | 1.0 M KOH | 0.283 | 335 | 97 | 49 |
| Ni3S2 | Ni foam | Solvothermal | 1.0 M KOH | 1.5 | 123 | 110 | 40 |
| Ni3S2 | Ni foam | Solvothermal | 1.0 M KOH | 19.3 | 123 | 92 | 51 |
| Ni3S2 | Carbon paper | Thermal decomposition | 1.0 M KOH | 0.6 | 112 | 67 | 80 |
| Ni3S2 | Ni foam | Thermal decomposition | 1.0 M KOH | 10 | 199.2 | 106.1 | 87 |
| NiS2 | Glassy carbon | Solvothermal | 1.0 M KOH | 0.283 | 335 | 128 | 49 |
| NiS2 | Glassy carbon | Solvothermal | 1.0 M KOH | 0.7 | 148 | 83 | 104 |
| NiS2 | Carbon cloth | Solvothermal-VSR | 1.0 M KOH | 4.1 | 149 | 104 | 105 |
| NiS2 | Nickel foam | Solvothermal-VSR | 1.0 M KOH | 1.6 | 67 | 72 | 102 |
| NiS2 | Carbon cloth | Precipitation-VSR | 1.0 M NaOH | 1.2 | 190 | 85 | 106 |
| NiS2 | Carbon paper | Thermal decomposition | 1.0 M KOH | 0.6 | 227 | 110 | 80 |
5.2. Nickel phosphide electrocatalysts
Nickel phosphides have nine known crystalline phases, including Ni3P, Ni8P3, Ni5P2, Ni12P5, Ni2P, Ni5P4, NiP, NiP2, and NiP3.107,108 Similar to nickel sulfides, the performance of crystalline nickel phosphide electrocatalysts in the HER is considerably phase-dependentin such a way that by increasing the phosphorus content of the compound, the HER activity and stability increase. In other words, crystalline phases with a higher phosphorus content show better performances in the HER. In this regard, Menezes et al.44 investigated the HER activity of Ni12P5 and Ni2P on fluorinated tin oxide (FTO) and nickel foam substrates with different loadings in 1.0 M KOH solution. They found that on both substrates and with different loadings, Ni2P was more active than Ni12P5 (Fig. 10a). This activity trend was also reported by Tian et al.,98 who investigated MOF-derived Ni12P5 and Ni2P (Fig. 10b). The result of the electrochemical double-layer capacitance and conductivity of the electrocatalysts showed that Ni2P had a lower charge transfer resistance and higher electrochemical double-layer capacitance than Ni12P5, indicating that the better performance of more phosphorus-rich nickel phosphides can be attributed to their higher conductivity and larger electrochemical active surface area (Fig. 10c and d). To investigate more phosphorus-rich phases, Wang et al.109 studied the HER activity of Ni2P and Ni5P4 on Ti foil in both acidic and alkaline media. They observed that Ni5P4 with smaller overpotentials and Tafel slope in both media showed better performances than Ni2P (Fig. 10e and f). They conducted an EIS test to investigate the reason behind the better performance of Ni5P4 (Fig. 10g). The results showed that Ni5P4 had a smaller charge transfer resistance than Ni2P. They also theoretically studied the hydrogen adsorption of Ni2P and Ni5P4 through density functional theory (DFT) tools (Fig. 10h). The results showed that the nickel and phosphorus sites in Ni5P4 had lower and closer to zero Gibbs free energy of hydrogen adsorption than Ni2P, which further confirmed the more optimum hydrogen adsorption of more phosphorus-rich nickel phosphides.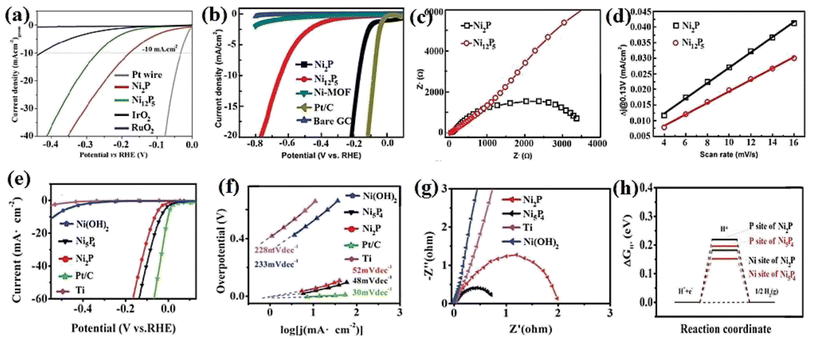 | ||
| Fig. 10 (a) LSV curves of Ni12P5 and Ni2P electrocatalysts on FTO in 1.0 M KOH with loadings of ∼1 mg cm−2 (reproduced from ref. 44 with permission from the American Chemical Society, Copyright 2017). MOF-derived Ni12P5 and Ni2P: (b) LSV curves in 0.5 M H2SO4, (c) EIS plots and (d) electrochemical double-layer capacitance.98 Ni2P and Ni5P4: (e) LSV curves in 0.5 M H2SO4 without iR compensation, (f) corresponding Tafel plots, (g) EIS plots and (h) DFT-calculated Gibbs free energy of hydrogen adsorption (reproduced from ref. 109 with permission from Wiley, Copyright 2017). | ||
The phase-dependent performance of nickel phosphide electrocatalysts also has been widely investigated by employing more phases of nickel phosphides. In this regard, Li et al.71 investigated the performance of Ni12P5, Ni2P, and Ni5P4 electrocatalysts in 0.5 M H2SO4 (Fig. 11a). They found that the higher the phosphorus content in an electrocatalyst, the better its activity. According to the Tafel plots of the electrocatalysts (Fig. 11b), they attributed the better performance of Ni5P4 to its better hydrogen binding energy. It is worth mentioning that they also normalized the LSV data relative to the surface area to determine whether the better performance of Ni5P4 is related to its higher surface area, where again, Ni5P4 had the best performance, showing that it is intrinsically more active than Ni2P and Ni12P5 (Fig. 11c). Kim et al.110 employed more phosphorus-rich phases of nickel phosphides and investigated Ni2P, Ni5P4, and NiP2 in 1.0 M NaOH. The LSV curves showed that NiP2 with lower needed overpotentials than other electrocatalysts was the most active phase (Fig. 11d). As previously proposed, it was seen here again that more phosphorus-rich phases of nickel phosphides had better hydrogen adsorption and higher conductivity according to the Tafel and EIS plots (Fig. 11e and f), respectively.
 | ||
| Fig. 11 Ni12P5, Ni2P, and Ni5P4: (a) LSV curves in 0.5 M H2SO4, (b) corresponding Tafel plots and (c) normalized LSV curves concerning the surface area in 0.5 M H2SO4 (reproduced from ref. 71 with permission from Wiley, Copyright 2018). Ni12P5, Ni2P and Ni5P4: (d) LSV curves of in 1.0 M NaOH, (e) corresponding Tafel plots and (f) EIS plots (reproduced from ref. 110 with permission from Elsevier, Copyright 2020). (g) Chronoamperometry test of the Ni2P (blue) and Ni5P4 (red) electrocatalysts: dash lines: 1.0 M NaOH solid lines: 0.5 M H2SO4, (h) nickel dissolution during chronopotentiometry test and (i)70 chronoamperometry test of Ni2P and NiP2 electrocatalysts (reproduced from ref. 110 with permission from Elsevier, Copyright 2020). | ||
In addition to activity, the stability and durability of nickel phosphides increase with an increase in phosphorus content in their structure. Laursen70 investigated the performance of Ni2P and Ni5P4 pellets in both acidic and alkaline media. In addition to reporting a similar trend for the activity of the above-mentioned crystalline phases, they studied the stability of the electrocatalysts. The results indicated that Ni5P4 had a stable performance in both media, whereas Ni2P showed a relatively unstable performance, especially at a longer electrolysis time (Fig. 11g). To determine the reason for this stability trend, they considered the compositional change of the electrocatalysts after electrolysis, where the results showed that a significant amount of nickel content of Ni2P (about 50%) was dissolved in 1.0 M H2SO4 solution after 20 h of chronopotentiometry test (compared with that of about 16% for Ni5P4) (Fig. 11h). Thus, the stability of nickel phosphides improves with an increase in phosphorus content in their structure due to their lower compositional changes. In another work, Kim et al.110 compared the stability of NiP2 and Ni2P in 1.0 M NaOH. The chronoamperometry test showed that NiP2 had better stability than Ni2P (Fig. 11i). They also showed that a considerable amount of the Ni2P electrocatalyst was dissolved in the electrolyte after 4 h of the chronopotentiometry test.
Considering all the reported data for comparing different crystalline phases of nickel phosphides, it can be concluded that by increasing the phosphorus content of the crystalline phases of nickel phosphides, their activity and stability in the HER increase. The better HER performance of more phosphorus-rich nickel phosphides is mainly attributed to their better electrochemical hydrogen adsorption (lower Tafel slope), higher conductivity (smaller EIS semi-circle), and higher ECSA (higher Cdl). Also, their better stability is related to their more stable compositional and morphological structure.
Although the activity of crystalline nickel phosphide electrocatalysts increases with an increase in the phosphorus content of the crystalline phase with no limitation, this is not the case in amorphous nickel phosphide electrocatalysts. The activity of the amorphous nickel phosphide electrocatalysts increases up to an optimum amount by increasing the phosphorus content, and then decreases. In this regard, Burchardt24 investigated the variation in the HER activity of amorphous nickel phosphide as a function of the phosphorus content of the coating. He found that the HER activity of the Ni–P electrocatalysts increased by increasing the phosphorus content up to about 17 at%, and then decreased in such a way that at the overpotential of −1.3 V vs. SCE, the delivered current density of the Ni–P electrode with 17.1 at% P was about 20-times greater than the Ni–P electrode with 27 at%. A similar result was also reported in his next study.25 However, the phosphorus content-dependence behavior of amorphous nickel phosphides has to be further studied in future works.
Nickel phosphides have high activity toward the HER. Nevertheless, their activity can be enhanced even more by combining specific materials. Various ideas have been implemented in this regard, such as the use of transition metals, heteroatoms, and carbonaceous materials. Next, we present several works on the use of nickel phosphide composites in the HER and reveal how they affect the properties of pristine nickel phosphides.
Because of their low price and abundant resources, transition metals have received significant attention as a potential substitute for platinum group metals. However, transition metals have no activity comparable to platinum group metals and are usually used in the form of sulfides, phosphides, etc. Nevertheless, due to their excellent hydrogen adsorption ability, high conductivity, and active sites toward the HER, they can be employed with active materials such as nickel sulfides and phosphides to improve the performance of the whole system. In this regard, Zhang et al.111 showed the great potential of transition metals for boosting the HER activity of nickel phosphides, where the incorporation of Co in Ni2P, named as self-supported NiCo2Px nanowire arrays with overpotentials of 11 and 58 mV at current densities of 1 and 10 mA cm−2, exhibited an even better performance than Pt sheets in 1.0 M KOH with values of 23 and 70 mV (Fig. 12a and b), respectively. The Tafel plots indicated that NiCo2Px had the lowest Tafel slopes among Pt sheets, Ni2P, and CoP, which means that the better performance of NiCo2Px can be ascribed to its modified hydrogen adsorption and close to zero Gibbs free energy of hydrogen adsorption (Fig. 12c). Ma et al.112 further proved the better performance of cobalt-nickel bimetallic phosphide than single metal phosphides of CoP and Ni2P in acidic and alkaline media. The EIS plots and electrochemical double-layer capacitances of the electrocatalysts showed that the better performance of cobalt–nickel phosphide could be related to the improved conductivity and enhancement of active sites. A similar result was also reported by Liu et al.113
 | ||
| Fig. 12 NiCo2Px nanowire arrays: (a) SEM image, (b) LSV curves in 1.0 M KOH and (c) corresponding Tafel plots (reproduced from ref. 111 with permission from Wiley, Copyright 2017). | ||
Wang et al.114 studied the incorporation of iron in nickel phosphide by doping iron in Ni2P nanosheet arrays. The results showed that the addition of iron improved both the HER and OER activity of pristine Ni2P (Fig. 13a). The Tafel plots indicated that iron doping decreased the Tafel slope and accelerated the HER kinetics by modifying the hydrogen adsorption (Fig. 13b). Huang et al. also investigated iron-tuned Ni2P and observed similar results. In another work, Wang et al.115 considered Mn doping of nickel phosphide by synthesizing Mn-doped NiP2 nanosheets. The LSV curves showed that Mn doping improved the HER activity of pristine NiP2 in a wide pH range from acidic to alkaline media (Fig. 13c). Similar to previous reports in the transition metal-doping of nickel phosphides, Mn doping also accelerated the HER kinetics, lowered the charge transfer resistance, and increased the ECSA (Fig. 13d–f). In similar studies, Zhang et al.116 and Jiang et al.117 investigated Mn-doped Ni2P and again reported an enhancement in the HER activity of pristine Ni2P through just the above-mentioned improvements. The modification of hydrogen adsorption by Mn doping was also theoretically confirmed by DFT calculations. According to Fig. 13g and h, by doping Mn in the structure of Ni2P, the Gibbs free energy of hydrogen adsorption of the nickel and phosphorus sites in Ni2P approached zero, thus accelerating the kinetics of the HER, and the activity of the electrocatalyst toward the HER increased.118 Man et al.81 investigated the transition metal-doping of nickel phosphides using various transition metals, including Mn, Co, Fe, and Mo, in a comprehensive study. The polarization curves of these electrocatalysts showed that generally, transition metal-doping improved the activity of pristine nickel phosphide (Ni2P), where molybdenum had the best and most significant effect on the HER activity of Ni2P (Fig. 13i).
 | ||
| Fig. 13 Iron-doped Ni2P: (a) LSV curves in 1.0 M KOH and (b) corresponding Tafel plots (reproduced from ref. 114 with permission from the American Chemistry Society, Copyright 2017). Mn-Doped NiP2: (c) LSV curves in 0.5 M H2SO4, (d) corresponding Tafel plots, (e) EIS plots and (f) electrochemical double-layer capacitance (reproduced from ref. 115 with permission from Elsevier, Copyright 2018). Gibbs free energy of hydrogen adsorption of (g) Ni2P and (h) Mn-incorporated Ni2P (red, blue, and green lines correspond to adsorption at P, Ni, and Mn sites, respectively) (reproduced from ref. 118 with permission from the American Chemistry Society, Copyright 2020). (i) LSV curves of transition metal-doped Ni2P (reproduced from ref. 81 with permission from Elsevier, Copyright 2019). | ||
Due to their high electron transfer ability, high specific surface area, and porous structure, carbonaceous materials are widely used in electrochemical applications, ranging from batteries and supercapacitors to electrocatalysts. However, although carbonaceous materials have high conductivity and surface area, they have no considerable active sites or HER activity. Consequently, they cannot be solely used as electrocatalysts and should be used in combination with active materials. In this regard, Pan et al.78 loaded Ni2P nanoparticles on carbon nanospheres with different loadings (Fig. 14a). The LSV curves and Tafel plots showed that increasing the amount of carbon nanospheres enhanced the activity of Ni2P up to an optimum amount (Fig. 14b and c). Although the specific surface area of the electrocatalysts increased by increasing the content of carbon nanospheres according to the calculated BET surface area of the electrocatalysts, the carbon nanospheres had almost no active sites. Thus, there was a trade-off between an increase in activity through the increase of surface area and improvement of conductivity, as was demonstrated by the EIS test, and a decrease in the number of active sites by lowering the content of Ni2P as the active material. A similar result was reported by Li et al.,45 where they used a nanohybrid of Ni2P film and carbon nanosheets. Yu et al.108 coated nanosheets of Ni8P3 with carbon in two different amounts via simple hydrothermal carbonization. The polarization curves showed that carbon coating improved the activity of Ni8P3, whereas extra carbon coating decreased the activity, which was due to the above-mentioned reason. However, the performance of both carbon-coated electrocatalysts was still better than that of the pristine Ni8P3 (Fig. 14d). The calculated electrochemical double-layer capacitance of the electrocatalysts showed that the Cdl increased slightly after the carbon coating, while based on the EIS plots, the main effect of the carbon coating was lowering the charge transfer resistance, as expected (Fig. 14e and f). In another work, Wang et al.119 electrodeposited amorphous nickel phosphide on a 3D porous carbon nanotube support. The results showed that while the carbon nanotube support had close to zero HER activity and low ECSA, the Ni–P on carbon nanotube support had high activity and ECSA in a wide pH range from acidic to alkaline media, again confirming that carbonaceous materials affect the electrochemical performance of materials through the improvement of electron transfer and surface area.
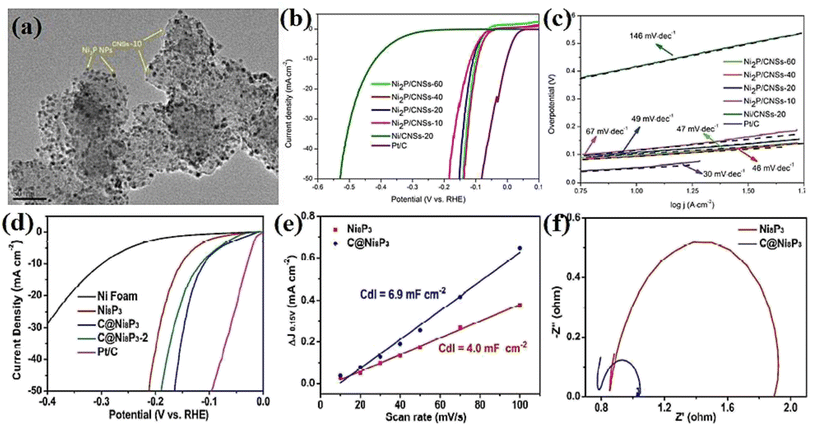 | ||
| Fig. 14 Ni2P supported on carbon nanospheres: (a) TEM image, (b) LSV curves in 0.5 M H2SO4 and (c) corresponding Tafel plots (reproduced from ref. 78 with permission from Elsevier, Copyright 2015). Carbon-coated Ni8P3: (d) LSV curves in 0.5 M H2SO4, (e) electrochemical double-layer capacitance and (f) EIS plots (reproduced from ref. 108 with permission from the America Chemistry Society, Copyright 2016). | ||
Heteroatoms are another group of materials that can be combined with nickel sulfide and phosphides to improve the HER activity of pristine materials. In organic chemistry, heteroatoms refer to elements other than carbon and hydrogen that can replace carbon in organic molecules. Here, heteroatoms can be considered as nitrogen (N), sulfur (S), phosphorus (P), and selenium (Se). Heteroatoms have shown high ability to boost the activity of transition metals toward the HER, where transition metal phosphides, sulfides, selenides, and nitrides have been shown to be the most promising materials and the main focus of researchers in the field of finding non-noble metal electrocatalysts for the HER. Heteroatoms can be further applied to improve the electrocatalytic properties of materials. In this regard, Chang et al.120 introduced sulfur-doped Ni5P4 nanoplate arrays with different sulfur contents as efficient electrocatalysts in acidic HER. The LSV curves showed that the activity increased by increasing the sulfur content from 2% to 6%, and then decreased from 6% to 10%, and thus 6% was the optimum sulfur content (Fig. 15a). The experimental results showed that the best performance of 6% S-doped Ni5P4 was related to its maximum ECSA, optimum electrochemical hydrogen adsorption (the lowest Tafel slope), and highest conductivity (Fig. 15b and c). DFT calculations further confirmed the superiority of S-doped Ni5P4 from a theoretical view. It is also worth mentioning that they synthesized pure Ni5P4 and NiS2 for comparison with 6% S-doped Ni5P4, where it showed a performance even better than pristine Ni5P4 and NiS2. In another work, Zhou et al.121 investigated selenium-doped NiP2 and phosphorus-doped NiSe2 and compared their activity with that of pure NiP2 and NiSe2. The LSV curves showed that the HER activity followed the order of Se-doped NiP2 > NiP2 > P-doped NiSe2 > NiSe2 (Fig. 15d), which indicates the positive effect of heteroatom doping, and also the superior activity of the nickel phosphide in comparison with nickel selenide.
 | ||
| Fig. 15 Sulfur-doped Ni5P4: (a) LSV curves in 0.5 M H2SO4, (b) EIS plots and (c) ECSA as a function of sulfur content (reproduced from ref. 120 with permission from the America Chemistry Society, Copyright 2018). (d) LSV curves of selenium-doped NiP2 (reproduced from ref. 121 with permission from the America Chemistry Society, Copyright 2015). Nickel phosphide nanoparticle–nitrogen-doped graphene hybrid: (e) LSV curves and (f) EIS plots (reproduced from ref. 77 with permission from Elsevier, copyright 2015). Nickel phosphide nanoparticles with nitrogen and phosphorus co-doped porous carbon: (g) LSV curves, (h) EIS plots and (i) electrochemical double-layer capacitance (reproduced from ref. 123 with permission from Elsevier, copyright 2020). | ||
Heteroatoms can also be combined with carbonaceous materials, significantly improving the performance of the base materials in various aspects. In this regard, Pan et al.77 combined Ni2P nanoparticles with reduced graphene oxide and nitrogen-doped reduced graphene oxide. The LSV curves indicated that nitrogen doping considerably decreased the onset potential, which could be attributed to the increase in conductivity, as confirmed by the EIS test (Fig. 15e and f). Also, the calculation of the active sites showed that the number of active sites for Ni2P on the nitrogen-doped reduced graphene oxide was about 10-times higher than that of Ni2P on reduced graphene oxide, which revealed that nitrogen doping has a significant effect on the increase of both conductivity and active sites. This result also was reported by Wang et al.122 for Ni2P embedded in nitrogen-doped carbon nanofibres. In another work, Ma et al.123 synthesized Ni2P nanoparticles on nitrogen and phosphorus co-doped carbon (Ni2P@NPC) and evaluated their HER activity in 1.0 M KOH. The polarization curves showed that the use of nitrogen and phosphorus co-doped carbon improved the activity of pristine Ni2P and activated the inactive bare carbon cloth (Fig. 15g). The combination of nitrogen and phosphorus co-doped carbon with Ni2P improved the conductivity and ECSA, and thus Ni2P@NPC had a Cdl of about 400% higher than that of Ni2P nanoparticles (Fig. 15h and i). A summary of the performance of pure nickel phosphide electrocatalysts is presented in Table 3.
| Catalysta | Substrate | Synthesis method | Electrolyte | Loadingb (mg cm−2) | η10 (mV vs. RHE) | b (mV dec−1) | Ref. |
|---|---|---|---|---|---|---|---|
| a Ni–P stands for amorphous nickel phosphide.b For amorphous nickel phosphide, loading indicates the percentage of the sulfur content of the electrocatalyst. | |||||||
| Ni–P | Nickel foam | Electrodeposition | 1.0 M KOH | 4.4 | 63 | 55 | 31 |
| Ni–P | Copper foam | Electrodeposition | 1.0 M KOH | 5 | 98 | 55 | 27 |
| Ni8P3 | Nickel foam | Solvothermal | 0.5 M H2SO4 | 1.7 | 152 | 86 | 52 |
| Ni12P5 | Glassy carbon | Thermal decomposition | 0.5 M H2SO4 | 1.99 | 280 | 75 | 73 |
| Ni12P5 | Carbon black | Thermal decomposition | 0.5 M H2SO4 | 0.12 | 182 | 63 | 71 |
| Ni2P | Glassy carbon | Solvothermal | 0.5 M H2SO4 | 0.429 | 295 | 115 | 45 |
| Ni2P | Ti foil | Thermal decomposition | 0.5 M H2SO4 | 1 | 78 | 41.4 | 82 |
| Ni2P | Carbon black | Thermal decomposition | 0.5 M H2SO4 | 0.12 | 135 | 62 | 71 |
| Ni2P | Glassy carbon | Thermal decomposition | 0.5 M H2SO4 | 0.35 | 137 | 49 | 73 |
| Ni2P | Glassy carbon | VSR | 0.5 M H2SO4 | 0.35 | 172 | 62 | 98 |
| Ni2P | Graphite | VSR | 1.0 M NaOH | 1.7 | 217 | 103 | 110 |
| Ni2P | Ti foil | VSR | 0.5 M H2SO4 | 4.2 | 56.6 | 52 | 109 |
| 1.0 M KOH | 4.2 | 62.2 | 61 | ||||
| Ni5P4 | Glassy carbon | Thermal decomposition | 0.5 M H2SO4 | 1.99 | 118 | 42 | 73 |
| Ni5P4 | Carbon black | Thermal decomposition | 0.5 M H2SO4 | 0.12 | 103 | 51 | 71 |
| Ni5P4 | Carbon paper | VSR | 0.5 M H2SO4 | 0.2 | 95 | 97.4 | 120 |
| Ni5P4 | Graphite | VSR | 1.0 M NaOH | 1.7 | 189 | 93.3 | 110 |
| Ni5P4 | Ti foil | VSR | 0.5 M H2SO4 | 4.6 | 35.4 | 48 | 109 |
| 1.0 M KOH | 4.6 | 47.1 | 56 | ||||
| NiP2 | Graphite | VSR | 1.0 M NaOH | 1.7 | 178 | 85.2 | 110 |
| NiP2 | Carbon cloth | VSR | 0.5 M H2SO4 | 4.4 | 84 | 52 | 115 |
| 1.0 M KOH | 4.4 | 122 | 77 | ||||
6. Challenges and future perspectives
6.1. General points for both nickel sulfides and phosphides
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300- and 34
300- and 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800-dimensional problems, respectively. However, density functional theory (DFT) as a powerful tool can make these calculations simpler. Investigating electrocatalysts in terms of hydrogen adsorption energy, activity, thermodynamic stability of their different crystalline planes, the effect of different dopants, and many other aspects by DFT gives a much better and clearer vision for future works, making use of all their advantages.
800-dimensional problems, respectively. However, density functional theory (DFT) as a powerful tool can make these calculations simpler. Investigating electrocatalysts in terms of hydrogen adsorption energy, activity, thermodynamic stability of their different crystalline planes, the effect of different dopants, and many other aspects by DFT gives a much better and clearer vision for future works, making use of all their advantages.6.2. Nickel sulfides
6.3. Nickel phosphides
Author contributions
Ali Shahroudi: conceptualization, data curation, investigation, project administration, supervision, visualization, writing original draft, writing review & editing. Mahsa Esfandiari: data curation, investigation, writing original draft. Sajjad Habibzadeh: conceptualization, project administration, supervision, writing review & editing.Conflicts of interest
There are no conflicts to declare.References
- M. Wang, W. Fu, L. Du, Y. Wei, P. Rao, L. Wei, X. Zhao, Y. Wang and S. Sun, Surface engineering by doping manganese into cobalt phosphide towards highly efficient bifunctional HER and OER electrocatalysis, Appl. Surf. Sci., 202015, 515, 146059 CrossRef CAS.
- Y. Zhang, B. Ouyang, J. Xu, S. Chen, R. S. Rawat and H. J. Fan, 3D porous hierarchical nickel–molybdenum nitrides synthesized by RF plasma as highly active and stable hydrogen-evolution-reaction electrocatalysts, Adv. Energy Mater., 2016, 6(11), 1600221 CrossRef.
- G. Tang, Y. Zeng, B. Wei, H. Liang, J. Wu, P. Yao and Z. Wang, Rational Design of Manganese Cobalt Phosphide with Yolk–Shell Structure for Overall Water Splitting, Energy Technol., 2019, 7(6), 1900066 CrossRef.
- Y. Ge, J. Chen, H. Chu, P. Dong, S. R. Craig, P. M. Ajayan, M. Ye and J. Shen, Urchin-like CoP with controlled manganese doping toward efficient hydrogen evolution reaction in both acid and alkaline solution, ACS Sustainable Chem. Eng., 2018, 6(11), 15162–15169 CrossRef CAS.
- J. Cai, Y. Song, Y. Zang, S. Niu, Y. Wu, Y. Xie, X. Zheng, Y. Liu, Y. Lin, X. Liu and G. Wang, N-induced lattice contraction generally boosts the hydrogen evolution catalysis of P-rich metal phosphides, Sci. Adv., 2020, 6(1), eaaw8113 CrossRef CAS PubMed.
- M. El-Shafie, S. Kambara and Y. Hayakawa, Hydrogen production technologies overview, Journal of Power and Energy Engineering, 2019, 7(01), 107 CrossRef.
- M. Wang, Y. Tuo, X. Li, Q. Hua, F. Du and L. Jiang, Mesoporous Mn-doped FeP: facile synthesis and enhanced electrocatalytic activity for hydrogen evolution in a wide pH range, ACS Sustainable Chem. Eng., 2019, 7(14), 12419–12427 CAS.
- H. Li, Y. Wang, W. Zhu and Z. Zhuang, Hollow bimetallic M-Fe-P (M= Mn, Co, Cu) nanoparticles as efficient electrocatalysts for hydrogen evolution reaction, Int. J. Hydrogen Energy, 2019, 44(41), 22806–22815 CrossRef CAS.
- S. Anantharaj, S. Kundu and S. Noda, Progress in nickel chalcogenide electrocatalyzed hydrogen evolution reaction, J. Mater. Chem. A, 2020, 8(8), 4174–4192 RSC.
- A. Wang, X. Chen, L. Cheng, X. Shen, W. Zhu, L. Li and J. Pang, Insights into the synergistic effect of multi-walled carbon nanotube decorated Mo-doped CoP 2 hybrid electrocatalysts toward efficient and durable overall water splitting, J. Mater. Chem. A, 2020, 8(34), 17621–17633 RSC.
- Handbook of reference electrodes, ed. G. Inzelt, A. Lewenstam and F. Scholz, Springer, Heidelberg, 2013 Search PubMed.
- H. Vrubel, T. Moehl, M. Grätzel and X. Hu, Revealing and accelerating slow electron transport in amorphous molybdenum sulphide particles for hydrogen evolution reaction, Chem. Commun., 2013, 49(79), 8985–8987 RSC.
- M. G. Kibria, J. P. Edwards, C. M. Gabardo, C. T. Dinh, A. Seifitokaldani, D. Sinton and E. H. Sargent, Electrochemical CO2 reduction into chemical feedstocks: from mechanistic electrocatalysis models to system design, Adv. Mater., 2019, 31(31), 1807166 CrossRef PubMed.
- P. Sabatier, La catalyse en chimie organique, 1920 Search PubMed.
- H. Vandenborre, P. Vermeiren and R. Leysen, Hydrogen evolution at nickel sulphide cathodes in alkaline medium, Electrochim. Acta, 1984, 29(3), 297–301 CrossRef CAS.
- I. Paseka, Sorption of hydrogen and kinetics of hydrogen evolution on amorphous Ni-Sx electrodes, Electrochim. Acta, 1993, 38(16), 2449–2454 CrossRef CAS.
- I. Paseka, Hydrogen evolution reaction on amorphous Ni–P and Ni–S electrodes and the internal stress in a layer of these electrodes, Electrochim. Acta, 2001, 47(6), 921–931 CrossRef CAS.
- Q. Han, K. Liu, J. Chen and X. Wei, A study on the electrodeposited Ni–S alloys as hydrogen evolution reaction cathodes, Int. J. Hydrogen Energy, 2003, 28(11), 1207–1212 CrossRef CAS.
- H. He, H. Liu, F. Liu and K. Zhou, Distribution of sulphur and electrochemical properties of nickel sulphur coatings electrodeposited on the nickel foam as hydrogen evolution reaction cathodes, Mater. Lett., 2005, 59(29–30), 3968–3972 CrossRef CAS.
- H. He, H. Liu, F. Liu and K. Zhou, Structures and electrochemical properties of amorphous nickel sulphur coatings electrodeposited on the nickel foam substrate as hydrogen evolution reaction cathodes, Surf. Coat. Technol., 2006, 201(3–4), 958–964 CrossRef CAS.
- N. Jiang, L. Bogoev, M. Popova, S. Gul, J. Yano and Y. Sun, Electrodeposited nickel-sulfide films as competent hydrogen evolution catalysts in neutral water, J. Mater. Chem. A, 2014, 2(45), 19407–19414 RSC.
- E. R. Gonzalez, L. A. Avaca, G. Tremiliosi-Filho, S. A. Machado and M. Ferreira, Hydrogen evolution reaction on Ni–S electrodes in alkaline solutions, Int. J. Hydrogen Energy, 1994, 19(1), 17–21 CrossRef CAS.
- Z. Shan, Y. Liu, Z. Chen, G. Warrender and J. Tian, Amorphous Ni–S–Mn alloy as hydrogen evolution reaction cathode in alkaline medium, Int. J. Hydrogen Energy, 2008, 33(1), 28–33 CrossRef CAS.
- T. Burchardt, The hydrogen evolution reaction on NiPx alloys, Int. J. Hydrogen Energy, 2000, 25(7), 627–634 CrossRef CAS.
- T. Burchardt, The effect of deposition temperature on the catalytic activity of Ni–P alloys toward the hydrogen reaction, Int. J. Hydrogen Energy, 2002, 27(3), 323–328 CrossRef CAS.
- N. Jiang, B. You, M. Sheng and Y. Sun, Bifunctionality and mechanism of electrodeposited nickel–phosphorous films for efficient overall water splitting, ChemCatChem, 2016, 8(1), 106–112 CrossRef CAS.
- Q. Liu, S. Gu and C. M. Li, Electrodeposition of nickel–phosphorus nanoparticles film as a Janus electrocatalyst for electro-splitting of water, J. Power Sources, 2015, 299, 342–346 CrossRef CAS.
- M. Popczyk, A. Budniok and A. Lasia, Electrochemical properties of Ni–P electrode materials modified with nickel oxide and metallic cobalt powders, Int. J. Hydrogen Energy, 2005, 30(3), 265–271 CrossRef CAS.
- Q. Ren, H. Jin, X. Xu, A. Liu, J. Li, J. Wang and S. Wang, Hydrogen evolution reaction catalyzed by nickel/nickel phosphide nanospheres synthesized through electrochemical methods, Electrochim. Acta, 2019, 298, 229–236 CrossRef CAS.
- R. N. Wasalathanthri, S. Jeffrey, N. Su, K. Sun and D. M. Giolando, Stoichiometric control of electrocatalytic amorphous nickel phosphide to increase hydrogen evolution reaction activity and stability in acidic medium, ChemistrySelect, 2017, 2(26), 8020–8027 CrossRef CAS.
- X. Cao, D. Jia, D. Li, L. Cui and J. Liu, One-step co-electrodeposition of hierarchical radial NixP nanospheres on Ni foam as highly active flexible electrodes for hydrogen evolution reaction and supercapacitor, Chem. Eng. J., 2018, 348, 310–318 CrossRef CAS.
- K. Zhang, W. Xiao, J. Li, J. Liu and C. Yan, Two-step preparation of porous nickel-sulfur electrode for hydrogen evolution in alkaline water electrolysis, Electrochim. Acta, 2017, 228, 422–427 CrossRef CAS.
- A. P. Murthy, J. Theerthagiri, J. Madhavan and K. Murugan, Electrodeposited carbon-supported nickel sulfide thin films with enhanced stability in acid medium as hydrogen evolution reaction electrocatalyst, J. Solid State Electrochem., 2018, 22(2), 365–374 CrossRef CAS.
- A. Irshad and N. Munichandraiah, Electrodeposited nickel–cobalt–sulfide catalyst for the hydrogen evolution reaction, ACS Appl. Mater. Interfaces, 2017, 9(23), 19746–19755 CrossRef CAS PubMed.
- Q. Han, K. Liu, J. Chen and X. Wei, Hydrogen evolution reaction on amorphous Ni–S–Co alloy in alkaline medium, Int. J. Hydrogen Energy, 2003, 28(12), 1345–1352 CrossRef CAS.
- T. C. Yuan, R. D. Li and K. C. Zhou, Electrocatalytic properties of Ni-S-Co coating electrode for hydrogen evolution in alkaline medium, Trans. Nonferrous Met. Soc. China, 2007, 17(4), 762–765 CrossRef CAS.
- Y. Wu, Y. Gao, H. He and P. Zhang, Novel electrocatalyst of nickel sulfide boron coating for hydrogen evolution reaction in alkaline solution, Appl. Surf. Sci., 2019, 480, 689–696 CrossRef CAS.
- K. Xiong, L. Huang, Y. Gao, H. Zhang, Y. Zhuo, H. Shen, Y. Wang, L. Peng and Z. Wei, Formation of a thin-layer of nickel hydroxide on nickel phosphide nanopillars for hydrogen evolution, Electrochem. Commun., 2018, 92, 9–13 CrossRef CAS.
- C. Ouyang, X. Wang, C. Wang, X. Zhang, J. Wu, Z. Ma, S. Dou and S. Wang, Hierarchically porous Ni3S2 nanorod array foam as highly efficient electrocatalyst for hydrogen evolution reaction and oxygen evolution reaction, Electrochim. Acta, 2015, 174, 297–301 CrossRef CAS.
- C. Tang, Z. Pu, Q. Liu, A. M. Asiri, Y. Luo and X. Sun, Ni3S2 nanosheets array supported on Ni foam: A novel efficient three-dimensional hydrogen-evolving electrocatalyst in both neutral and basic solutions, Int. J. Hydrogen Energy, 2015, 40(14), 4727–4732 CrossRef CAS.
- Y. Ni, K. Liao and J. Li, In situ template route for synthesis of porous Ni 12 P 5 superstructures and their applications in environmental treatments, CrystEngComm, 2010, 12(5), 1568–1575 RSC.
- A. R. Kucernak and V. N. Sundaram, Nickel phosphide: the effect of phosphorus content on hydrogen evolution activity and corrosion resistance in acidic medium, J. Mater. Chem. A, 2014, 2(41), 17435–17445 RSC.
- Y. Deng, Y. Zhou, Y. Yao and J. Wang, Facile synthesis of nanosized nickel phosphides with controllable phase and morphology, New J. Chem., 2013, 37(12), 4083–4088 RSC.
- P. W. Menezes, A. Indra, C. Das, C. Walter, C. Göbel, V. Gutkin, D. Schmeiβer and M. Driess, Uncovering the nature of active species of nickel phosphide catalysts in high-performance electrochemical overall water splitting, ACS Catal., 2017, 7(1), 103–109 CrossRef CAS.
- Y. Li, P. Cai, S. Ci and Z. Wen, Strongly Coupled 3D Nanohybrids with Ni2P/Carbon Nanosheets as pH-Universal Hydrogen Evolution Reaction Electrocatalysts, ChemElectroChem, 2017, 4(2), 340–344 CrossRef CAS.
- F. Yang, N. Kang, J. Yan, X. Wang, J. He, S. Huo and L. Song, Hydrogen evolution reaction property of molybdenum disulfide/nickel phosphide hybrids in alkaline solution, Metals, 2018, 8(5), 359 CrossRef.
- J. Liu, X. Chen, M. Shao, C. An, W. Yu and Y. Qian, Surfactant-aided solvothermal synthesis of dinickel phosphide nanocrystallites using red phosphorus as starting materials, J. Cryst. Growth, 2003, 252(1–3), 297–301 CrossRef CAS.
- W. Sun, Z. Fu, H. Shi, C. Jin, E. Liu, X. Zhang and J. Fan, Cu3P and Ni2P co-modified g-C3N4 nanosheet with excellent photocatalytic H2 evolution activities, J. Chem. Technol. Biotechnol., 2020, 95(12), 3117–3125 CrossRef CAS.
- N. Jiang, Q. Tang, M. Sheng, B. You, D. E. Jiang and Y. Sun, Nickel sulfides for electrocatalytic hydrogen evolution under alkaline conditions: a case study of crystalline NiS, NiS 2, and Ni 3 S 2 nanoparticles, Catal. Sci. Technol., 2016, 6(4), 1077–1084 RSC.
- J. Xia, K. Dhaka, M. Volokh, G. Peng, Z. Wu, Y. Fu, M. C. Toroker, X. Wang and M. Shalom, Nickel phosphide decorated with trace amount of platinum as an efficient electrocatalyst for the alkaline hydrogen evolution reaction, Sustainable Energy Fuels, 2019, 3(8), 2006–2014 RSC.
- H. Ren, Z. H. Huang, Z. Yang, S. Tang, F. Kang and R. Lv, Facile synthesis of free-standing nickel chalcogenide electrodes for overall water splitting, J. Energy Chem., 2017, 26(6), 1217–1222 CrossRef.
- J. Yu, Q. Li, N. Chen, C. Y. Xu, L. Zhen, J. Wu and V. P. Dravid, Carbon-coated nickel phosphide nanosheets as efficient dual-electrocatalyst for overall water splitting, ACS Appl. Mater. Interfaces, 2016, 8(41), 27850–27858 CrossRef CAS PubMed.
- X. Wang, Y. V. Kolen'ko and L. Liu, Direct solvothermal phosphorization of nickel foam to fabricate integrated Ni 2 P-nanorods/Ni electrodes for efficient electrocatalytic hydrogen evolution, Chem. Commun., 2015, 51(31), 6738–6741 RSC.
- K. Mi, Y. Ni and J. Hong, Solvent-controlled syntheses of Ni12P5 and Ni2P nanocrystals and photocatalytic property comparison, J. Phys. Chem. Solids, 2011, 72(12), 1452–1456 CrossRef CAS.
- C. Jin, C. Xu, W. Chang, X. Ma, X. Hu, E. Liu and J. Fan, Bimetallic phosphide NiCoP anchored g-C3N4 nanosheets for efficient photocatalytic H2 evolution, J. Alloys Compd., 2019, 803, 205–215 CrossRef CAS.
- R. Ding, X. Li, W. Shi, Q. Xu, L. Wang, H. Jiang, Z. Yang and E. Liu, Mesoporous Ni-P nanocatalysts for alkaline urea electrooxidation, Electrochim. Acta, 2016, 222, 455–462 CrossRef CAS.
- R. Ding, X. Li, W. Shi, Q. Xu and E. Liu, One-pot solvothermal synthesis of ternary Ni-Co-P micro/nano-structured materials for high performance aqueous asymmetric supercapacitors, Chem. Eng. J., 2017, 320, 376–388 CrossRef CAS.
- S. Ranganatha and N. Munichandraiah, Solvothermal synthesis of mesoporous NiCoP for high performance electrochemical supercapacitors, Mater. Chem. Phys., 2019, 224, 124–128 CrossRef CAS.
- H. M. El Sharkawy, D. M. Sayed, A. S. Dhmees, R. M. Aboushahba and N. K. Allam, Facile synthesis of nanostructured binary Ni–Cu phosphides as advanced battery materials for asymmetric electrochemical supercapacitors, ACS Appl. Energy Mater., 2020, 3(9), 9305–9314 CrossRef CAS.
- S. Liu, Y. Lin and J. Tong, Mixed surfactant controlled syntheses of solid Ni2P and yolk–shell Ni12P5 via a hydrothermal route and their photocatalytic properties, J. Alloys Compd., 2015, 644, 140–146 CrossRef CAS.
- Z. Fang, Y. Wang, Y. Zou, Z. Hao and Q. Dong, One-pot synthesis of nickel sulfide with sulfur powder as sulfur source in solution and their electrochemical properties for hydrogen evolution reaction, Inorg. Chem. Commun., 2017, 79, 1–4 CrossRef CAS.
- A. Wang, H. Li, J. Xiao, Y. Lu, M. Zhang, K. Hu and K. Yan, Integration of theory and experiment on mesoporous nickel sulfide microsphere for hydrogen evolution reaction, ACS Sustainable Chem. Eng., 2018, 6(12), 15995–16000 CrossRef CAS.
- Z. Qin, Y. Chen, Z. Huang, J. Su, Z. Diao and L. Guo, Composition-dependent catalytic activities of noble-metal-free NiS/Ni3S4 for hydrogen evolution reaction, J. Phys. Chem. C, 2016, 120(27), 14581–14589 CrossRef CAS.
- Z. Liu, X. Huang, Z. Zhu and J. Dai, A simple mild hydrothermal route for the synthesis of nickel phosphide powders, Ceram. Int., 2010, 36(3), 1155–1158 CrossRef CAS.
- D. Wang, X. Zhang, Z. Du, Z. Mo, Y. Wu, Q. Yang, Y. Zhang and Z. Wu, CoNi2S4 nanoparticles as highly efficient electrocatalysts for the hydrogen evolution reaction in alkaline media, Int. J. Hydrogen Energy, 2017, 42(5), 3043–3050 CrossRef CAS.
- A. Sivanantham, P. Ganesan and S. Shanmugam, Hierarchical NiCo2S4 nanowire arrays supported on Ni foam: an efficient and durable bifunctional electrocatalyst for oxygen and hydrogen evolution reactions, Adv. Funct. Mater., 2016, 26(26), 4661–4672 CrossRef CAS.
- B. Dhandapani, M. Jagannathan, M. S. AlSalhi, M. J. Aljaafreh and S. Prasad, N-doped carbon embedded Ni3S2 electrocatalyst material towards efficient hydrogen evolution reaction in broad pH range, Colloids Surf., A, 2020, 603, 125194 CrossRef CAS.
- Y. Qu, M. Yang, J. Chai, Z. Tang, M. Shao, C. T. Kwok, M. Yang, Z. Wang, D. Chua, S. Wang and Z. Lu, Facile synthesis of vanadium-doped Ni3S2 nanowire arrays as active electrocatalyst for hydrogen evolution reaction, ACS Appl. Mater. Interfaces, 2017, 9(7), 5959–5967 CrossRef CAS PubMed.
- X. Shang, K. L. Yan, Y. Rao, B. Dong, J. Q. Chi, Y. R. Liu, X. Li, Y. M. Chai and C. G. Liu, In situ cathodic activation of V-incorporated Ni x S y nanowires for enhanced hydrogen evolution, Nanoscale, 2017, 9(34), 12353–12363 RSC.
- A. B. Laursen, K. R. Patraju, M. J. Whitaker, M. Retuerto, T. Sarkar, N. Yao, K. V. Ramanujachary, M. Greenblatt and G. C. Dismukes, Nanocrystalline Ni 5 P 4: a hydrogen evolution electrocatalyst of exceptional efficiency in both alkaline and acidic media, Energy Environ. Sci., 2015, 8(3), 1027–1034 RSC.
- H. Li, S. Lu, J. Sun, J. Pei, D. Liu, Y. Xue, J. Mao, W. Zhu and Z. Zhuang, Phase-Controlled Synthesis of Nickel Phosphide Nanocrystals and Their Electrocatalytic Performance for the Hydrogen Evolution Reaction, Chem.–Eur. J., 2018, 24(45), 11748–11754 CrossRef CAS PubMed.
- E. J. Popczun, J. R. McKone, C. G. Read, A. J. Biacchi, A. M. Wiltrout, N. S. Lewis and R. E. Schaak, Nanostructured nickel phosphide as an electrocatalyst for the hydrogen evolution reaction, J. Am. Chem. Soc., 2013, 135(25), 9267–9270 CrossRef CAS PubMed.
- Y. Pan, Y. Liu, J. Zhao, K. Yang, J. Liang, D. Liu, W. Hu, D. Liu, Y. Liu and C. Liu, Monodispersed nickel phosphide nanocrystals with different phases: synthesis, characterization and electrocatalytic properties for hydrogen evolution, J. Mater. Chem. A, 2015, 3(4), 1656–1665 RSC.
- H. Li, W. Wang, Z. Gong, Y. Yu, H. Chen and J. Xia, Shape-controlled synthesis of nickel phosphide nanocrystals and their application as hydrogen evolution reaction catalyst, J. Phys. Chem. Solids, 2015, 80, 22–25 CrossRef CAS.
- B. Seo, D. San Baek, Y. J. Sa and S. H. Joo, Shape effects of nickel phosphide nanocrystals on hydrogen evolution reaction, CrystEngComm, 2016, 18(32), 6083–6089 RSC.
- Y. H. Chung, K. Gupta, J. H. Jang, H. S. Park, I. Jang, J. H. Jang, Y. K. Lee, S. C. Lee and S. J. Yoo, Rationalization of electrocatalysis of nickel phosphide nanowires for efficient hydrogen production, Nano Energy, 2016, 26, 496–503 CrossRef CAS.
- Y. Pan, N. Yang, Y. Chen, Y. Lin, Y. Li, Y. Liu and C. Liu, Nickel phosphide nanoparticles-nitrogen-doped graphene hybrid as an efficient catalyst for enhanced hydrogen evolution activity, J. Power Sources, 2015, 297, 45–52 CrossRef CAS.
- Y. Pan, Y. Liu and C. Liu, Nanostructured nickel phosphide supported on carbon nanospheres: synthesis and application as an efficient electrocatalyst for hydrogen evolution, J. Power Sources, 2015, 285, 169–177 CrossRef CAS.
- Y. Pan, W. Hu, D. Liu, Y. Liu and C. Liu, Carbon nanotubes decorated with nickel phosphide nanoparticles as efficient nanohybrid electrocatalysts for the hydrogen evolution reaction, J. Mater. Chem. A, 2015, 3(24), 13087–13094 RSC.
- X. Zheng, X. Han, Y. Zhang, J. Wang, C. Zhong, Y. Deng and W. Hu, Controllable synthesis of nickel sulfide nanocatalysts and their phase-dependent performance for overall water splitting, Nanoscale, 2019, 11(12), 5646–5654 RSC.
- H. W. Man, C. S. Tsang, M. M. Li, J. Mo, B. Huang, L. Y. Lee, Y. C. Leung, K. Y. Wong and S. C. Tsang, Transition metal-doped nickel phosphide nanoparticles as electro-and photocatalysts for hydrogen generation reactions, Appl. Catal., B, 2019, 242, 186–193 CrossRef CAS.
- M. Zhou, Y. Kang, K. Huang, Z. Shi, R. Xie and W. Yang, Ultra-small nickel phosphide nanoparticles as a high-performance electrocatalyst for the hydrogen evolution reaction, RSC Adv., 2016, 6(78), 74895–74902 RSC.
- M. Ledendecker, H. Schlott, M. Antonietti, B. Meyer and M. Shalom, Experimental and Theoretical Assessment of Ni-Based Binary Compounds for the Hydrogen Evolution Reaction, Adv. Energy Mater., 2017, 7(5), 1601735 CrossRef.
- Y. Pan, Y. Chen, X. Li, Y. Liu and C. Liu, Nanostructured nickel sulfides: phase evolution, characterization and electrocatalytic properties for the hydrogen evolution reaction, RSC Adv., 2015, 5(127), 104740–104749 RSC.
- D. Y. Chung, J. W. Han, D. H. Lim, J. H. Jo, S. J. Yoo, H. Lee and Y. E. Sung, Structure dependent active sites of Ni x S y as electrocatalysts for hydrogen evolution reaction, Nanoscale, 2015, 7(12), 5157–5163 RSC.
- Y. Lin, J. Zhang, Y. Pan and Y. Liu, Nickel phosphide nanoparticles decorated nitrogen and phosphorus co-doped porous carbon as efficient hybrid catalyst for hydrogen evolution, Appl. Surf. Sci., 2017, 422, 828–837 CrossRef CAS.
- J. Li, P. K. Shen and Z. Tian, One-step synthesis of Ni3S2 nanowires at low temperature as efficient electrocatalyst for hydrogen evolution reaction, Int. J. Hydrogen Energy, 2017, 42(10), 7136–7142 CrossRef CAS.
- X. Wang, W. Li, D. Xiong, D. Y. Petrovykh and L. Liu, Bifunctional nickel phosphide nanocatalysts supported on carbon fiber paper for highly efficient and stable overall water splitting, Adv. Funct. Mater., 2016, 26(23), 4067–4077 CrossRef CAS.
- R. Zhou, J. Zhang, Z. Chen, X. Han, C. Zhong, W. Hu and Y. Deng, Phase and composition controllable synthesis of nickel phosphide-based nanoparticles via a low-temperature process for efficient electrocatalytic hydrogen evolution, Electrochim. Acta, 2017, 258, 866–875 CrossRef CAS.
- J. Li, J. Li, X. Zhou, Z. Xia, W. Gao, Y. Ma and Y. Qu, Highly efficient and robust nickel phosphides as bifunctional electrocatalysts for overall water-splitting, ACS Appl. Mater. Interfaces, 2016, 8(17), 10826–10834 CrossRef CAS PubMed.
- J. Huo, Y. Chen, Y. Liu, J. Guo, L. Lu, W. Li, Y. Wang and H. Liu, Bifunctional iron nickel phosphide nanocatalysts supported on porous carbon for highly efficient overall water splitting, Sustainable Mater. Technol., 2019, 22, e00117 CrossRef CAS.
- P. Liu, J. Li, Y. Lu and B. Xiang, Facile synthesis of NiS2 nanowires and its efficient electrocatalytic performance for hydrogen evolution reaction, Int. J. Hydrogen Energy, 2018, 43(1), 72–77 CrossRef CAS.
- D. Liu, X. Li, L. Wei, T. Zhang, A. Wang, C. Liu and R. Prins, Disproportionation of hypophosphite and phosphite, Dalton Trans., 2017, 46(19), 6366–6378 RSC.
- S. Liu, C. Hu, C. Lv, J. Cai, M. Duan, J. Luo, J. Song, Y. Shi, C. Chen, D. Luo and A. Watanabe, Facile preparation of large-area self-supported porous nickel phosphide nanosheets for efficient electrocatalytic hydrogen evolution, Int. J. Hydrogen Energy, 2019, 44(33), 17974–17984 CrossRef CAS.
- X. Wang, Y. V. Kolen'ko, X. Q. Bao, K. Kovnir and L. Liu, One-step synthesis of self-supported nickel phosphide nanosheet array cathodes for efficient electrocatalytic hydrogen generation, Angew. Chem., 2015, 127(28), 8306–8310 CrossRef.
- I. K. Mishra, H. Zhou, J. Sun, K. Dahal, Z. Ren, R. He, S. Chen and Z. Ren, Highly efficient hydrogen evolution by self-standing nickel phosphide-based hybrid nanosheet arrays electrocatalyst, Mater. Today Phys., 2018, 4, 1–6 CrossRef.
- J. Xiao, Q. Lv, Y. Zhang, Z. Zhang and S. Wang, One-step synthesis of nickel phosphide nanowire array supported on nickel foam with enhanced electrocatalytic water splitting performance, RSC Adv., 2016, 6(109), 107859–107864 RSC.
- T. Tian, L. Ai and J. Jiang, Metal–organic framework-derived nickel phosphides as efficient electrocatalysts toward sustainable hydrogen generation from water splitting, RSC Adv., 2015, 5(14), 10290–10295 RSC.
- T. Wu, P. Wang, J. Qian, Y. Ao, C. Wang and J. Hou, Noble-metal-free nickel phosphide modified CdS/C 3 N 4 nanorods for dramatically enhanced photocatalytic hydrogen evolution under visible light irradiation, Dalton Trans., 2017, 46(40), 13793–13801 RSC.
- P. Kumar, A. P. Murthy, L. S. Bezerra, B. K. Martini, G. Maia and J. Madhavan, Carbon supported nickel phosphide as efficient electrocatalyst for hydrogen and oxygen evolution reactions, Int. J. Hydrogen Energy, 2021, 46(1), 622–632 CrossRef CAS.
- T. W. Lin, C. J. Liu and C. S. Dai, Ni3S2/carbon nanotube nanocomposite as electrode material for hydrogen evolution reaction in alkaline electrolyte and enzyme-free glucose detection, Appl. Catal., B, 2014, 154, 213–220 CrossRef.
- Q. Ma, C. Hu, K. Liu, S. F. Hung, D. Ou, H. M. Chen, G. Fu and N. Zheng, Identifying the electrocatalytic sites of nickel disulfide in alkaline hydrogen evolution reaction, Nano Energy, 2017, 41, 148–153 CrossRef CAS.
- W. Zhu, X. Yue, W. Zhang, S. Yu, Y. Zhang, J. Wang and J. Wang, Nickel sulfide microsphere film on Ni foam as an efficient bifunctional electrocatalyst for overall water splitting, Chem. Commun., 2016, 52(7), 1486–1489 RSC.
- P. Luo, H. Zhang, L. Liu, Y. Zhang, J. Deng, C. Xu, N. Hu and Y. Wang, Targeted synthesis of unique nickel sulfide (NiS, NiS2) microarchitectures and the applications for the enhanced water splitting system, ACS Appl. Mater. Interfaces, 2017, 9(3), 2500–2508 CrossRef CAS PubMed.
- C. Tang, Z. Pu, Q. Liu, A. M. Asiri and X. Sun, NiS2 nanosheets array grown on carbon cloth as an efficient 3D hydrogen evolution cathode, Electrochim. Acta, 2015, 153, 508–514 CrossRef CAS.
- X. Wu, B. Yang, Z. Li, L. Lei and X. Zhang, Synthesis of supported vertical NiS2 nanosheets for hydrogen evolution reaction in acidic and alkaline solution, RSC Adv., 2015, 5(42), 32976–32982 RSC.
- H. Okamoto, Ni-P (nickel-phosphorus), J. Phase Equilib. Diffus., 2010, 31(2), 200 CrossRef CAS.
- J. Yu, Q. Li, N. Chen, C. Y. Xu, L. Zhen, J. Wu and V. P. Dravid, Carbon-coated nickel phosphide nanosheets as efficient dual-electrocatalyst for overall water splitting, ACS Appl. Mater. Interfaces, 2016, 8(41), 27850–27858 CrossRef CAS PubMed.
- H. Wang, Y. Xie, H. Cao, Y. Li, L. Li, Z. Xu, X. Wang, N. Xiong and K. Pan, Flower-Like Nickel Phosphide Microballs Assembled by Nanoplates with Exposed High-Energy (0 0 1) Facets: Efficient Electrocatalyst for the Hydrogen Evolution Reaction, ChemSusChem, 2017, 10(24), 4899–4908 CrossRef CAS PubMed.
- W. S. Kim, D. K. Cho, H. W. Lim, K. H. Kim, S. H. Hong and J. Y. Kim, Phase-dependent hydrogen evolution activity of nickel phosphide nanosheet arrays in alkaline electrolytes, Electrochim. Acta, 2020, 344, 136116 CrossRef CAS.
- R. Zhang, X. Wang, S. Yu, T. Wen, X. Zhu, F. Yang, X. Sun, X. Wang and W. Hu, Ternary NiCo2Px nanowires as pH-universal electrocatalysts for highly efficient hydrogen evolution reaction, Adv. Mater., 2017, 29(9), 1605502 CrossRef PubMed.
- B. Ma, Z. Yang, Y. Chen and Z. Yuan, Nickel cobalt phosphide with three-dimensional nanostructure as a highly efficient electrocatalyst for hydrogen evolution reaction in both acidic and alkaline electrolytes, Nano Res., 2019, 12(2), 375–380 CrossRef CAS.
- T. Liu, X. Yan, P. Xi, J. Chen, D. Qin, D. Shan, S. Devaramani and X. Lu, Nickel–Cobalt phosphide nanowires supported on Ni foam as a highly efficient catalyst for electrochemical hydrogen evolution reaction, Int. J. Hydrogen Energy, 2017, 42(20), 14124–14132 CrossRef CAS.
- P. Wang, Z. Pu, Y. Li, L. Wu, Z. Tu, M. Jiang, Z. Kou, I. S. Amiinu and S. Mu, Iron-doped nickel phosphide nanosheet arrays: an efficient bifunctional electrocatalyst for water splitting, ACS Appl. Mater. Interfaces, 2017, 9(31), 26001–26007 CrossRef CAS PubMed.
- X. Wang, H. Zhou, D. Zhang, M. Pi, J. Feng and S. Chen, Mn-doped NiP2 nanosheets as an efficient electrocatalyst for enhanced hydrogen evolution reaction at all pH values, J. Power Sources, 2018, 387, 1–8 CrossRef CAS.
- Y. Zhang, Y. Liu, M. Ma, X. Ren, Z. Liu, G. Du, A. M. Asiri and X. Sun, A Mn-doped Ni 2 P nanosheet array: an efficient and durable hydrogen evolution reaction electrocatalyst in alkaline media, Chem. Commun., 2017, 53(80), 11048–11051 RSC.
- D. Jiang, W. Ma, R. Yang, B. Quan, D. Li, S. Meng and M. Chen, Nickel–manganese bimetallic phosphides porous nanosheet arrays as highly active bifunctional hydrogen and oxygen evolution electrocatalysts for overall water splitting, Electrochim. Acta, 2020, 329, 135121 CrossRef CAS.
- S. Sarkar, L. Dheer, C. P. Vinod, R. Thapa, U. V. Waghmare and S. C. Peter, Stress-Induced Electronic Structure Modulation of Manganese-Incorporated Ni2P Leading to Enhanced Activity for Water Splitting, ACS Appl. Energy Mater., 2020, 3(2), 1271–1278 CrossRef CAS.
- S. Wang, L. Zhang, X. Li, C. Li, R. Zhang, Y. Zhang and H. Zhu, Sponge-like nickel phosphide–carbon nanotube hybrid electrodes for efficient hydrogen evolution over a wide pH range, Nano Res., 2017, 10(2), 415–425 CrossRef CAS.
- J. Chang, K. Li, Z. Wu, J. Ge, C. Liu and W. Xing, Sulfur-doped nickel phosphide nanoplates arrays: a monolithic electrocatalyst for efficient hydrogen evolution reactions, ACS Appl. Mater. Interfaces, 2018, 10(31), 26303–26311 CrossRef CAS PubMed.
- J. Zhuo, M. Cabán-Acevedo, H. Liang, L. Samad, Q. Ding, Y. Fu, M. Li and S. Jin, High-performance electrocatalysis for hydrogen evolution reaction using Se-doped pyrite-phase nickel diphosphide nanostructures, ACS Catal., 2015, 5(11), 6355–6361 CrossRef CAS.
- M. Q. Wang, C. Ye, H. Liu, M. Xu and S. J. Bao, Nanosized metal phosphides embedded in nitrogen-doped porous carbon nanofibers for enhanced hydrogen evolution at all pH values, Angew. Chem., 2018, 130(7), 1981–1985 CrossRef.
- B. Ma, X. Duan, W. Han, X. Fan, Y. Li, F. Zhang, G. Zhang and W. Peng, Decorated nickel phosphide nanoparticles with nitrogen and phosphorus co-doped porous carbon for enhanced electrochemical water splitting, J. Colloid Interface Sci., 2020, 567, 393–401 CrossRef CAS PubMed.
- J. H. Gary, J. H. Handwerk, M. J. Kaiser and D. Geddes, Petroleum refining: technology and economics, CRC press, 2007 Search PubMed.
- Y. Pan, Y. Chen, Y. Lin, P. Cui, K. Sun, Y. Liu and C. Liu, Cobalt nickel phosphide nanoparticles decorated carbon nanotubes as advanced hybrid catalysts for hydrogen evolution, J. Mater. Chem. A, 2016, 4(38), 14675–14686 RSC.
- W. Chen, Z. Qin, B. McElhenny, F. Zhang, S. Chen, J. Bao, Z. M. Wang, H. Z. Song and Z. Ren, The effect of carbon quantum dots on the electrocatalytic hydrogen evolution reaction of manganese–nickel phosphide nanosheets, J. Mater. Chem. A, 2019, 7(37), 21488–21495 RSC.
- H. Okamoto, Ni-S (Nickel-Sulfur), J. Phase Equilib. Diffus., 2009, 30(1), 123 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2022 |