Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Nanomaterial-based drug delivery systems as promising carriers for patients with COVID-19
M. Abd Elkodous *ab,
S. O. Olojede
*ab,
S. O. Olojede c,
Mahmoud Morsi
c,
Mahmoud Morsi d and
Gharieb S. El-Sayyad
d and
Gharieb S. El-Sayyad *ef
*ef
aDepartment of Electrical and Electronic Information Engineering, Toyohashi University of Technology, Toyohashi, Aichi 441-8580, Japan. E-mail: mohamed.hamada.abdlekodous.xi@tut.jp
bCenter for Nanotechnology (CNT), School of Engineering and Applied Sciences, Nile University, Sheikh Zayed, Giza 16453, Egypt
cNanotechnology Platforms, Discipline of Clinical Anatomy, Nelson Mandela School of Medicine, University of KwaZulu-Natal, Durban, South Africa
dFaculty of Medicine, Menoufia University, Menoufia, Shebin El Kom, Egypt
eDrug Radiation Research Department, National Center for Radiation Research and Technology (NCRRT), Egyptian Atomic Energy Authority (EAEA), Cairo, Egypt. E-mail: Gharieb.Elsayyad@eaea.org.eg
fChemical Engineering Department, Military Technical College (MTC), Egyptian Armed Forces, Cairo, Egypt
First published on 2nd August 2021
Abstract
Once the World Health Organization (WHO) declared the COVID-19 outbreak to be pandemic, massive efforts have been launched by researchers around the globe to combat this emerging infectious disease. Here we review the most recent data on the novel SARS-CoV-2 pathogen. We analyzed its etiology, pathogenesis, diagnosis, prevention, and current medications. After that, we summarized the promising drug delivery application of nanomaterial-based systems. Their preparation routes, unique advantages over the traditional drug delivery routes and their toxicity though risk analysis were also covered. We also discussed in detail the mechanism of action for one example of drug-loaded nanomaterial drug delivery systems (Avigan-contained nano-emulsions). This review provides insights about employing nanomaterial-based drug delivery systems for the treatment of COVID-19 to increase the bioavailability of current drugs, reducing their toxicity, and to increase their efficiency.
Introduction
Severe Acute Respiratory Syndrome-Coronavirus-2 (SARS-CoV-2) has developed into a major pandemic and has contributed to the present surge in morbidity and mortality rates worldwide.1 Following the World Health Organization (WHO) declaration of SARS-COV-2 as a public health challenge of global concern,2 the international committee on Taxonomy of Viruses has classified SARS-COV-2 as the seventh member of the corona virus family associated with human infection. This classification follows previous outbreaks associated with Severe Acute Respiratory Syndrome-Coronavirus (SARS-CoV) in 2002 and Middle East Respiratory Syndrome-Coronavirus (MERS-CoV) in 2012.3 The original name of this new pathogen was 2019-novel coronavirus (2019-nCoV) as the pathogen associated with the infection. While, Coronavirus Disease-2019 (COVID-19) was first recommended in February 2020 by the WHO as Severe Acute Respiratory Syndrome-Coronavirus-2 (SARS-CoV-2) by the international committee on Taxonomy of Viruses.4The SARS-CoV-2 outbreak has spread throughout the world;5 it is currently a threat of morbidity and mortality worldwide as shown in Fig. 1. One of the early cases of SARS-CoV-2 infection was traced to the seafood wholesale market in Wuhan, China, where different species of live animals are sold;6 this finding suggests that the virus was transmitted from animals to humans. Thereafter, reports of human-to-human transmission of the virus skyrocketed, as subsequent diagnosis of SARS-CoV-2 infection occurred among individuals who had no exposure to animals.7
 | ||
| Fig. 1 The global new cases and death cases due to SARS-CoV-2 as of 27th May 2021. Adapted from European Centre for Disease Prevention and Control. Assessed on 27th May 2021. | ||
Etiology
Coronaviruses are enveloped, positive-sense single-stranded ribonucleic acid (RNA) viruses with a unique appearance resembling a solar corona due to projection of its characteristic club-like spikes. These viruses causes respiratory tract infections in humans, and are associated with enteritis in birds as well as a variety of diseases of pigs, bats, cows, dogs, cats, and chickens.8,9 SARS-CoV is a member of the group 2b beta coronaviruses. The Middle East Respiratory Syndrome-Coronavirus (MERS-CoV) was classified as within group 2c of beta corona viruses and is highly homologous to bat corona viruses HKU4 and HKU5 as documented in the literature.10Infection with SARS-CoV-2 (also a beta coronavirus) follows a pattern that is similar to that reported for SARS-CoV and MERS-CoV.11 Previous studies revealed that SARS-CoV utilizes angiotensin converting enzyme 2 (ACE2) as a receptor for cell entry; this finding provided solid support of evidence suggesting that SARS-CoV originated in bats.12 By contrast, the cellular receptor used by the MERS-CoV is the enzyme dipeptidyl peptidase 4 (DPP4). Of note, MERS-CoV can only initiate infection via the use of species-restricted orthologs of DPP4, including those from humans, rabbits, bats, horses, and camels.13 Emerging evidence has confirmed that angiotensin converting enzyme 2 (ACE2) is also the host cellular receptor employed by SARS-CoV-2; this finding is not surprising given the nucleotide sequence homology reported in comparison between SARS-CoV-2 and SARS.14–17
Genomic sequencing has implicated either the Chinese (Rhinolophus sinicus) or the intermediate horseshoe bat (Rhinolophus affinis) as potential reservoirs of SARS-CoV-2; the source of the infection may be linked to an intermediate host. Identification of the intermediate wildlife host and reservoir may be critical as means to avoid future cross-species transmission of SARS-CoV-2; sequences isolated from the horseshoe bat as intermediate host were found to be similar to the sequence of SARS-CoV-2.18,19 Furthermore, viruses with ∼85–92% nucleotide homology to SARS-CoV-2 were identified in pangolins (family Manidae) during recent counter-smuggling activities in southern China; these results suggest that pangolins may be a potential intermediate host of SARS-CoV-2.20
The SARS-CoV-2 infection results in a wide spectrum of signs and symptoms, primarily those affecting the respiratory and gastrointestinal tracts. Moreover, circulating blood and specifically certain ABO blood groups have been implicated in differential susceptibility to SARS-CoV-2 and its complications. Zhao et al. (2020) reported that individuals of blood group O were somewhat less vulnerable to infection with SARS-CoV-2; those with blood group A demonstrated higher susceptibility than those with any of the other ABO blood groups; this has been attributed to the presence of natural serum anti-A antibodies.19 In one report, the complete blood count (CBC) of a female patient infected with SARS-CoV-2 after one week of hospitalization revealed an unusual leuko-erythroblastosis.21
Human-to-human transmission of SARS-CoV-2 is via droplets and through the respiratory tract, analogous to that reported for SARS-CoV and MERS-CoV.22 SARS-CoV-2 has also been detected on inanimate objects23 as well as in feces from infected patients24 which likely contributes in increasing in local transmission.
At this time, there are few specific therapeutic modalities established for the treatment of SARS-CoV-2;25 given the high economic loss and increasing number of infection, there is a dire need for more effective and safe therapeutic modalities. While several vaccine formulations are currently in various phases of testing, the daily rise in the number of confirmed cases worldwide suggests that more efforts are required. As such, this review shed light on unique nanomaterial-based drug delivery systems, which have already been successfully employed to deliver anticancer, antimicrobial, and antiviral drugs, might be employed to amplify efficiencies to anti-SARS-CoV-2 antiviral drugs.
COVID-19 pathogenesis
The genome of Coronaviridae family viruses includes a 5′ cap structure and a 3′ poly-A tail with a genome length of 30–32 kb. Coronaviruses as a group have high genetic variability and recombination rates that facilitate generation of pathogenic and highly contagious strains.26,27With emerging evidence, diverse variants of SARS-CoV-2 are now rampant. Among these new variants 501Y.V1 (B.1.1.7) of the United Kingdom lineage, 501Y.V2 (B.1.351) described in South Africa, 501Y.V3 (B.1.1.248) reported in Brazil, and B.1.4427 that was identified in California, USA. More recently, SARS-CoV-2 variants such as B.1.617 and B.1.618 were discovered in India by Indian scientists.28,29
Although the pathogenesis of SARS-CoV-2 infection remains poorly understood, the virus is genetically and structurally related to SARS-COV. As such, a focus on the mechanisms underlying MERS-CoV and SARS-CoV pathogenesis may elucidate critical factors associated with COVID-19.30 Among the pathological findings that have been elucidated, SARS-CoV-2 reaches the alveolar epithelial cells of the lower lung via transit through the respiratory tract, beginning with mucous membranes in the nasopharynx.31,32
The SARS-CoV-2 envelope spike glycoprotein can recognize and bind to the ACE2 receptor of lung alveoli with higher affinity than that associated with SARS-CoV; the virus then invades the host cell via clathrin-mediated endocytosis.33 As SARS-CoV enters the target cells, the viral genome discharged and is ultimately translated into one basic protein and two polyproteins in the cytoplasm. The viral genome then undergoes replication; and the newly formed envelope glycoproteins are combined with the nucleocapsid within the endoplasmic reticulum and Golgi, which results in vesicles containing virus fragments. These vesicles fuse with the plasma membrane in order to export virus progeny which can then infect neighboring cells and/or enter the circulation to invade other cells that express the ACE2 receptor.34,35
Viral infection also results in extensive immune and inflammatory reactions in the host which can lead to severe tissue damage. Acute respiratory distress syndrome (ARDS) is the most common immunopathological complication of COVID-19.36 The pathogenic mechanisms underlying ARDS are complex and difficult to control; a number of published reports have suggested that the ensuing cytokine storm plays a pivotal role in the pathogenesis of ARDS due to the large and seemingly uncontrolled release of pro-inflammatory cytokines including interferon (IFN)-α, IFN-γ, interleukin (IL)-1β, IL-6, IL-12, IL-18, IL-3, tumor necrosis factor (TNF)-α, transforming growth factor (TGF)-β, IL-2, IL-10, and IL-1RA, and by increasing the synthesis and release of chemokines, including CCL2, CCL3, CXCL8, CXCL9, and CXCL10.30,37,38
Clinical presentation and diagnosis of COVID-19
The clinical spectrum of SARS-CoV-2 infection covers a wide range and varies from asymptomatic to the most severe manifestations involving hospitalization and respiratory support. The incubation period for COVID-19 is currently believed to be ≤14 days following exposure.39 Recent publications include reports of symptoms that vary from mild to severe. The most common clinical features associated with COVID-19 are fever, cough, severe dyspnea, sore throat, expectoration, muscle soreness, and fatigue; other symptoms noted less frequently are headache, hemoptysis, rhinorrhea, and gastrointestinal symptoms, including nausea, diarrhea, and vomiting.40–42Among confirmed cases, patients with generally mild symptoms are likely to recover after an illness of approximately one week in duration while those with severe and critical findings may progress to respiratory failure, acute cardiac injury, acute renal injury, shock, and multiple organ dysfunction syndrome (MODS).43 The most comorbidities reported most frequently among those with severe disease include cardiovascular disease, diabetes mellitus, hypertension, lung disease, cancer, chronic kidney disease, and obesity.43
Clinical diagnosis of SARS-CoV-2 infection is based on epidemiological history, clinical presentation, the reverse-transcription polymerase chain reaction (RT-PCR) to detect virus-specific nucleic acid, computed tomography (CT) scan, blood cultures, and immunological testing for virus antigen or serum anti-SARS-CoV-2 antibodies.30
An RT-PCR test is used to confirm suspected cases of COVID-19; this method involving enzymatic detection of SARS-CoV-2 RNA in sputum, nasal and throat swabs, or in broncho alveolar lavage fluid.44 The gene targets of the RT-PCR tests include sequences encoding structural proteins as the nucleocapsid (N), envelope (E), and spike (S) glycoprotein; other targets include RNA-dependent RNA polymerase (RdRp) and regions in the first open reading frame.45 On occasion, SARS-CoV-2 can be detected in the peripheral blood and on rectal swabs despite negative throat swab tests; this result suggests that these patients can act as carriers of infection and confirms the importance of testing samples from different tissues and body sites in order to confirm infection.46 Due to the comparatively high false-negative rates reported for the virus RT-PCR test, many clinicians recommend performing a CT scan for those with negative RT-PCR screening in cases of high clinical suspicion as findings using this modality are typically more sensitive.47
The typical CT image associated with SARS-COV-2 infection includes ground-glass opacity with consolidative abnormalities in more severe cases with a bilateral peripheral distribution, primarily involving the lower lobes.48,49 There are other diagnostic techniques, including serologic tests that can detect antibodies to SARS-CoV-2; these tests will ultimately lead to the identification of patients with current infection and importantly, who have recovered from apriorSARS-CoV-2 infection.30
Prevention of COVID-19 and surface disinfection
As there are few effective drugs or vaccines, the main strategies used to prevent SARS-CoV-2 infection focus on avoiding exposure to individuals who might be infected, blocking the route of transmission and providing protection for the highly-susceptible individuals.50–52Fundamental standard measures include use of a face mask when in public, being careful to avoid touching one's face with unwashed hands, following routine respiratory hygiene practices, such as covering coughs and sneezes with tissues, frequent hand washing with soap and water for at least 20 seconds, utilizing hand sanitizer that contains at least 60% alcohol, and maintaining a distance from suspected or confirmed cases; furthermore, healthcare providers must use N95 respirator masks or a filtering face piece 2 (FFP2) while interacting with suspected or confirmed cases of COVID-19.53
The common prevailing challenges in biomedical science are the drug-resistance of some infectious viruses, output growth protocols, and their efficiency in courses of toxicity, remedial time, and side impacts on the individual cells. Additionally, discovering the infection-causing viral pathogen, monitoring the disease, and inhibition of some fatal diseases are important for public health.54
Human-to-human spread of SARS-CoV-2 takes place within incubation periods of between 2 and 14 days;55 transmission takes place via aerosol droplets and also via contaminated hands or surfaces. There may be other forms of transmission that have not been identified yet. Coronavirus pathogens, including SARS-CoV, MERS-CoV, and SARS-CoV-2, can persist on inanimate surfaces such as metal, glass, or plastic for more than 9 days.56 There is a varied range of disinfectant solutions that can be used to eliminate pathogens from surfaces.57 All surfaces that may have been in contact with virus pathogens should be fully-cleaned with water and disinfectants.58
A recent research study revealed that silver (Ag) NPs have excellent antiviral potential and may be an exceptional choice for future development.59 Nanofiber-based face respirators loaded with Ag NPs served as notably successful antimicrobial and antiviral disinfectants and may become the principal individual defensive agents that can be used to prevent the spread of the infection. Furthermore, there are a number of broad-based research projects in progress that focus on the use of nanomaterials as part of an immunization strategy for COVID-19. With respect to diagnostics, nanotechnology has been used extensively for the development of sensors that might accelerate the rate of current COVID-19 tests.60
Preliminary studies have also focused on nanomedicines. Ag NPs may have some advantages over the traditional biological solutions for cleaning and disinfection due to lower volatility; activities tested at even the lowest concentrations display low minimum inhibitory concentrations for virus pathogens.59 Moreover, Ag NPs may play a major role in bacterial, and viral disinfection due to their capacity to enrich for reactive oxygen species (ROS);61 for example, Ag NPs can effectively-generate extracellular ROS which could destroy influenza virus (IV) infection as an example.62 Intensive research is needed to verify these properties and to deploy these agents in the fight against COVID-19.
Current medications used to treat COVID-19
Many research is focused on new drug development.63 Currently, various compounds and drugs are under clinical investigation for their efficacy in treating SARS-CoV-2. A recent study from Rosa et al.,64 highlighted 24 clinical trials that featured over 20 medications under exploration for the treatment of SARS-CoV-2; these can be classified into 8 main categories including (1) antiviral drugs that inhibit virus replication, including favipiravir (Avigan), remdesivir, umifenovir (Arbidol) and oseltamivir. (2) Immunomodulatory proteins such as interferons, (3) human antibodies including immune globulin (IVIG) and monoclonal bevacizumab (Avastin), (4) antiretroviral medications such as HIV-1 protease inhibitors, lopinavir and the CYP3A inhibitor, ritonavir (Norvir), (5) corticosteroids including methyl-prednisolone (Depo-Medrol), (6) bacterial-derived antibiotics such as azithromycin, (7) anti-parasite modalities, including ivermectin, and (8) traditional Chinese medicines (TCM). The complete details regarding the current medications used to treat COVID-19 are summarized in Table 1.| Medication | Molecular Formula | Chemical Formula | Treatment | Reaction mechanism | Limitation | Ref. |
|---|---|---|---|---|---|---|
| Favipiravir (Avigan), remdesivir, umifenovir (Arbidol) and oseltamivir | C5H4FN3O2, C27H35N6O8P, C22H25BrN2O3S, and C16H28N2O4, respectively | 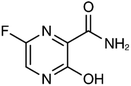 , , 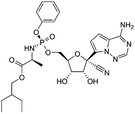 , , 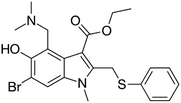 , and , and 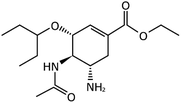 , respectively , respectively |
Antiviral drugs that inhibit virus replication | Favipiravir (as example) binds to and inhibits RNA dependent-RNA polymerase (RdRp), which ultimately-prevents viral transcription and replication | Clearly, it would have limitations in its use for pregnant and potentially-pregnant women. Blinded, controlled trials are needed to establish whether it will be useful overall | 65, 66 |
| Interferons | Interferon-alfa-2B; C16H17Cl3I2N3NaO5S | 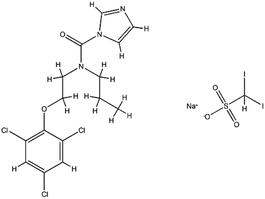 |
Immunomodulatory proteins made and released by host cells in response to the presence of several viruses | Interferon induces the expression of several hundred genes, names interferon-stimulated genes (ISGs) both in infected and neighboring cells. The products of the ISGs, in turn, allow the establishment of a so-called antiviral state, which is able to prevent, or at least limit, viral replication | The combination of interferon and ribavirin is now no longer used as safer, shorter highly-effective and more tolerable tablet only treatments are now available | 67 |
| Human antibodies including immune globulin (IVIG) | C6332H9826N1692O1980 S42 | — | Immunoglobulin therapy, also known as normal human immunoglobulin, is the use of a mixture of antibodies to treat a number of health conditions | Intravenous immune globulin (“IVIG”) is a product made up of antibodies that can be given intravenously (through a vein). Antibodies are proteins that a human body makes to help the fight infections viruses | One reason you might need IVIG is if your body does not make enough antibodies | 68 |
| HIV-1 protease inhibitors (Indinavir sulfate) | C36H49N5O8S | 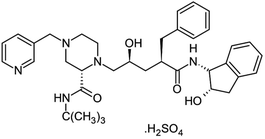 |
Antiretroviral medications used to treat HIV. The goal of these drugs is to reduce the amount of HIV virus in the body (called the viral load) to levels that are undetectable. This slows the progression of HIV and helps treat symptoms | Protease-mediated maturation of HIV-1 virus particles is essential for virus infectivity. Novel class of antiretroviral drug termed maturation inhibitors, which target cleavage sites. The exact mechanism is not clearly-understood, its substrate, and the chemical reaction of peptide bond cleavage had been detected | Unfortunately, most of the HIV-1 protease inhibitors are accompanied by side effects in long-term treatment | 69 |
| Corticosteroids-including methyl-prednisolone | C22H30O5 | 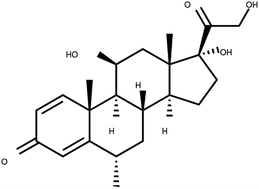 |
Clinical use of methylprednisolone is mainly due to its anti-inflammatory and immunosuppressive activity in the human body | Corticosteroid-resistant asthma is defined as a failure to improve FEV1 or PEF by over 15% after treatment with oral prednisolone 30–40 mg daily for 2 weeks | Corticosteroids including methylprednisolone can increase blood glucose, worsen pre-existing diabetes, and predispose those on long-term corticosteroid therapy to diabetes mellitus. Also, A course of broad-spectrum antibiotics must be achieved | 70 |
| Azithromycin | C38H72N2O12 | 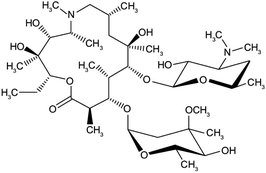 |
Bacterial-derived antibiotics used to treat Prevotella infections and decreases Prevotella-induced inflammation. Azithromycin has other attractive pharmacological and therapeutic properties in the search for COVID-19 drug therapy | Prevents bacteria from growing by interfering with their protein synthesis. It binds to the 50S subunit of the bacterial ribosome, thus inhibiting translation of mRNA. Azithromycin appears to decrease the COVID-19 virus entry into cells. In addition, it can enhance the immune response against viruses by several actions | There are mixed reports of effectiveness when azithromycin was used along with other medications to treat other viral respiratory infections | 71 |
| Ivermectin | C47H72O14 | 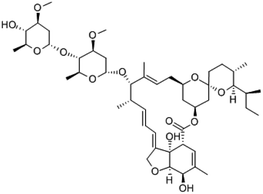 |
Anti-parasite modalities, including Ivermectin is an inhibitor of the SARS-CoV-2 in vitro. Ivermectin is FDA-approved for parasitic infections, and therefore has a potential for repurposing | Ivermectin binds to and destabilizes the Impα/β1 heterodimer thereby preventing Impα/β1 from binding to the viral protein (bottom) and preventing it from entering the nucleus. This likely results in reduced inhibition of the antiviral responses, leading to a normal, more efficient antiviral response. A single treatment able to effect ∼5000-fold reduction in virus at 48 h in cell culture | Since drugs that inhibit the enzyme CYP3A4 often also inhibit P-glycoprotein transport, the risk of increased absorption past the blood–brain barrier exists when ivermectin is administered along with other CYP3A4 inhibitors. These drugs include statins, HIV protease inhibitors, many calcium channel blockers, lidocaine, the benzodiazepines, and glucocorticoids such as dexamethasone | 72 |
Biomedical application of nanotechnology-based approaches in SARS-CoV-2 treatment
Since the last decade, the application of nanotechnology in the diagnosis, vaccine production, treatment and prevention of diseases have received excellent research attention.73 Food and Drug Administration (FDA) has approved nanocrystal formulations, polymeric-based nanoparticles such as polylactic-co-glycolic acid (PLGA) and polymeric micelles, as well as lipid-based nanoparticles like liposomes.74 More so, other nanoparticles such as inorganic, protein-based and metallic nanoparticles are in the pipeline of getting approval for their use in drug delivery.75,76In providing a solution to the menace of this ravaging SARS-CoV-2, clinical trials are ongoing to investigate and evaluate antiviral drugs, vaccines, immunomodulatory drugs, and neutralizing antibodies. The mechanism of these antiviral drugs is similar to the life cycle and structure of the SARS-CoV-2. For instance, Lopinavir/Ritonavir and Ivermectin inhibits viral protease. Arbidol Hydrochloride, rhACES, Leronlimab, block the membrane fusion. Also, Favipiravir/Tocilizumab and Remdesivir inhibit RNA polymerase, while EIDD-2801 provides broad-spectrum antiviral effects.74
Regarding these vaccines, irrespective of the mechanism of action, their effectiveness and stability depend on the delivery medium. The JNJ-78436735 and ChAdOx1 nCoV-19 employs non-replicating adenovirus vectors, INO-4800 needs a portable device known as CELLECTRA and lipid particles are utilized by mRNA-1273.74 Based on these delivery platforms, using the FDA approved nanoparticles may offer excellent results in vaccine delivery and the delivery of antiviral drugs.
In a most recent article, Chakravarty and Vora (2021) described carbon-based, inorganic metallic-based, lipid-based, and polymeric-based as nanoparticles that have been successfully employed to deliver antiviral drugs.77 Interestingly, in furtherance of its application as a drug delivery vehicle, some of these nanoparticles exhibit antiviral properties.74,78 Their size and surface characteristics make them suitable and preferred candidates to be encapsulated and loaded with different antiviral drugs.79 Although many antiviral drugs are currently in use to treat SARS-CoV-2, at the same time, experts have recommended the introduction of nanoparticles to deliver these drugs. The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is similar to the size of these nanoparticles, which are in nano-size of 60 to 140 nm. Hence, loading these antiviral drugs with nanoparticles may provide effective therapeutic outcomes.80 Most recently, dexamethasone nano-formulations, with inhalation and intravenous as the routes of administration to hyper-activated immune cells, have been suggested in the treatment of SARS-CoV-2.81
Emerging shreds of evidence have shown that comorbidity that comes with immunosuppression aggravate SARS-CoV-2 infection. Such an example is in patients undergoing immunotherapy.82 For this purpose, metallic and other two-dimensional nanomaterials will be a helpful platform to load these therapeutic agents to and deliver.
There are various classes of these two-dimensional nanomaterials available to deliver these antiviral drugs and vaccines for better therapeutic outcome in immunotherapy. Recently, these nanomaterials can be used as a medium to deliver different therapeutic agents such as photodynamic therapy (PDT), photothermal therapy and drug delivery. Chen and Colleagues (2020) synthesised a nontoxic, very stable nanocomposite made of tellurium–selenium (TeSe)-derived lateral heterojunction with a uniform size. The results of their study show accumulation of the formulated nanocomposite in the lung of the mice, tumour suppression, and induction of cancer cell apoptosis. More so, TeSe eradicated hepatocellular carcinoma and lung cancer in preclinical trials. Hence, the authors established the importance of TeSe nanocomposite as a PTT-based candidate to eliminate cancer.83 In another study in experimental mice, the synergistic effects of black phosphorus (BP)-based photothermal therapy loaded with anti-CD47 antibody-based immunotherapy was observed. Their findings demonstrated that a-CD47-BP-based photothermal therapy conjugate elicits local and systemic anticancer immune responses by activating adaptive and innate immunities.84
In recent times, silicon and black phosphorus represent some of the widely investigated inorganic nanomaterials for their biomedical applications, photothermal therapy, theranostics, drug delivery system and bio-imaging. Their ability in a wide range of applications has been attributed to the high drug loading capacity, biocompatibility, definite clearance pathways, less cytotoxicity, biodegradability, and excellent physicochemical properties.85 Phosphorus, as the core component of black phosphorus, is crucial to acid–base balance, important in energy metabolism and in the transfer of genetic materials. Hence, a coagent reason for the broad application of phosphorus and phosphorous-derived inorganic/two-dimensional nanoparticles in biomedical research.86
Furthermore, other inorganic nanomaterials such as gold nanorods, up conversion nanoparticle, black phosphorus nanosheets, MnO2, Mxene, single-walled carbon nanotubes, and graphene have been employed in photothermal therapy.87 Luo and Coworker have described black phosphorus as an alternative effective photothermal agent in cancer treatment owing to its high drug loading ability, excellent treatment effectiveness, and negligible cytotoxicity.88 A recent study utilized bath sonication and probe sonication to synthesized black phosphorus quantum dots and examined the photothermal effects of the nanocomposite fabricated on the C6 and MCF7 cancer. The result of this investigation revealed effective photothermal performance leading to stability in physiological medium, notable cell death and without cytotoxicity.89
To combat the ravaging SARS-CoV-2 pandemic, conjugating the currently available SARS-CoV-2 drugs with the FDA approved nanoparticles, inorganic nanoparticles, two-dimensional nanoparticles, biocompatible and biodegradable nanoparticles would be of great interest.
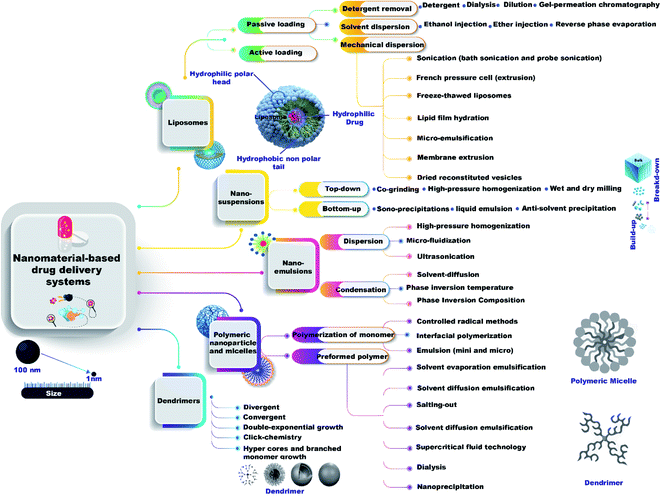
Nanomaterial-based drug delivery systems
Preparation of nanomaterial-based drug delivery systems
There are many methods to prepare nanomaterial-based drug delivery systems that can be divided into two main approaches, bottom-up and top-down (Fig. 2) as the following:Mechanical dispersion methods are divided into 7 classes, sonication (bath sonication and probe sonication), French pressure cell (extrusion), freeze-thawed liposomes, lipid film hydration (by hand shaking, non-hand shaking or freeze drying), micro-emulsification, membrane extrusion, and dried reconstituted vesicles.
Solvent dispersion method has three types (ether injection, ethanol injection, and reverse phase evaporation method). Detergent removal methods have four types (dialysis, detergent, gel-permeation chromatography, and dilution).
Unique advantages of nanomaterial-based drug delivery systems, their drug loading capacity, and the main differences for treating COVID-19
Due to the unique physicochemical properties of nanomaterials, their use in drug-delivery systems allows many superior advantages over the traditional routes for treating COVID-19 as the followingFor the drug loading capacity, Huil Gaoe et al.101 succeeded in synthesizing docetaxel-loaded nano-emulsion with a particle size of 72.3 nm. The average zeta potential was calculated to be −6.38 mV, the encapsulation performance was 93.1%, and the drug loading capacity had been assessed to be 2.87%.
Very few investigations have shown the mode of action of nano-emulsion of some essential oils on some deadly viruses.102 Most of the scientific papers prophesied that nano-emulsion of some essential oils could interfere with the virion envelope construction or mask the virus (COVID-19) building, which finally prevents COVID-19 adsorption and invasion into the host cells.102,103
Regarding drug loading capacity, Zhi Wang et al.,106 synthesized paclitaxel-ss-lysophospholipid (paclitaxel-liposome) with an average diameter of 234.9 nm and good stability (zeta potential of −29.1 mV), the highest paclitaxel loading capacity determined was about 7.97%.
It was reported that, liposomes (positively charged) possess elevated potential for mucosal vaccinations due to the retention in the nasal cavity (negatively charged) causing a powerful immune effect,107 pointing to the creation of greater levels of immunoglobulins to fight COVID-19.108
For the drug loading capacity, Ilaria Fratoddi et al.,112 prepared polymeric NPs bio-conjugated with dexamethasone (DXM) by a changed surfactant free emulsion system. The loading capacity was investigated as a function of various functionality standards of the copolymer and varying quantities of DXM drug, reaching up to 90% of DXM loading for the obtained polymeric NPs.
Polymer nanoparticles composed of chitosan (Cs) drew special attention for intranasal treatment because of their non-toxic characteristics, biocompatibility, ability to open up strong linkages with epithelial cells,113 and strength to be changed into fancied appearance and sizes.114 Simultaneous incorporation with therapeutic composites, Cs can improve assertiveness of polymeric NP in mucosal conditions and the entrance to the mucosal membrane to resist any COVID-19 infection in the mucosal environment.108
Regarding the drug loading capacity, Fei Wang et al.,118 prepared poly-propylenimine (PPI) dendrimers, and the acetylated PPI dendrimers with differing degrees of acetylation varying from 14.2% to 94.3% used to encapsulate drugs such as methotrexate sodium. The concentrations of methotrexate sodium in dendrimer/methotrexate complex raised linearly with the proportion of acetylation of PPI dendrimer up to 88.9%, and the drug loading capacity gave 1.2 mg ml−1.
With the significant interactions with some viruses, dendrimers exhibited improved antiviral potential, limiting the host's disease. So, they became a major candidate in managing viral conditions like HIV, COVID-19, and influenza virus disease.108,119 Chahal et al.,120 investigated dendrimer NP encapsulating an antigen-expressing replicon mRNA and allowed vital CD8+ T-cell and antibody responses to defend against dangerous pathogens, such as Ebola, and H1N1 influenza. The principal findings can be tested against COVID-19 and such impact can diminish the destructive attack of COVID-19.108
For the drug loading capacity, Jingyi Hong et al.,123 synthesized annonaceous acetogenins (ACGs) nano-suspensions possessing an average particle size of 144.4 nm, a zeta potential measured to be (−22.9 mV) and an extraordinary drug loading capacity of 46.17%.
Nano-suspensions can significantly inhibit the virus inside the host body and block the virus entry into the host cells.124 Due to their high surface area and the capacity to adhere multiple antigens and composites on their surface. In addition, nanomaterials such as gold NPs and carbon quantum dots (CQDs) were described as encouraging agents for interaction with COVID-19 and blocking their entry into host cells.108,124
Toxicity of nanomaterial-based drug delivery systems, with a risk analysis for therapeutic applications, and the animal experiments relating COVID-19
Due to the encouraging uses of nanomaterials-based drug delivery system, their toxicity to host cell and their risk analysis for therapeutic applications must be assessed as well as animal experiments relating COVID-19 the as the following:Regarding risk analysis for therapeutic applications, a fitting choice of ingredients is vital for an effective nano-emulsion formulation. Low-molar-volume oils are better than high-molar-volume oils, as they regularly confer more proper drug solubilization.127 New, innovative semisynthetic medium-chain derivatives, described as amphiphilic aggregates with surfactant characteristics, are favored. Consideration should be adjusted to the tolerability of the constituting excipients. Accordingly, recent attempts have concentrated on reducing the toxicity or inflammation of the nano-emulsion formulations. The influence of the surfactant/co-surfactant weight ratio in the nano-emulsion formulation must be considered. It is reasonable to obtain acceptable features by suitably modifying the level of surfactants, oil, and secondary surfactants to avoid their risk in therapy applications.127
For risk analysis, liposome particle size and drug encapsulation performance are two essential product features, and knowledge of the potential risks are critical. To accomplish this, two cause-and-effect diagrams (Ishikawa diagram) were created to recognize the possible causes for product variability. Following the risk analysis, the succeeding eight variables were classified as high-risk parts influencing liposome; drug encapsulation and particle size: lipid concentration, drug concentration, cholesterol concentration, buffer concentration, hydration time, sonication period, number of freeze–thaw cycles, and extrusion pressure.130
Pooladanda et al.131 synthesized iRGD conjugated nimbolide liposomes (iRGD-NIMLip) for treating COVID-19 related diseases and studied their effect in vivo (male C57BL/6 mice (5–6 weeks old)). The animal experiment assumed that iRGD-conjugated nimbolide liposomes treatment significantly repressed oxidative stress and cytokine storm associated with free-drug and showed higher activity than dexamethasone (DEX). The synthesized iRGD-NIMLip cancelled the LPS induced p65 NF-κB, Akt, MAPK, Integrin β3 and β5, STAT3, and DNMT1 expression. Finally, iRGD-NIMLip could be a hopeful novel drug delivery system to target critical pathological values recognized in COVID-19 associated cytokine storm.
Concerning the risk analysis, the sensitivity of the dendrimer structure to in vivo biodegradation may also affect the rate of dendrimer removal from the organization. Still, exterior functionalization with non-biologic collections (like non-natural amino acids) reduces dendrimer building disruption.139 The method suggested examining the surface adsorption of a collection of small-molecule inquiries and making a ‘surface adsorption index’ to foretell the binding of bio-molecules which represents a vital function in managing the bio-distribution behavior of dendrimers.140 After this, researchers have done physiologically based pharmacokinetic models to assume the mass-time bio-distribution outlines for various dendrimer.
Khaitov et al.,141 synthesized the qualified siRNA-peptide dendrimer (siR-7-EM/KK-46) formulation for treating COVID-19 related diseases and studied their effect in vivo (The Syrian hamsters (females, 4–5 weeks of age, 40–60 g weight)). The animal experiment assumed a meaningful decline of virus titer and lung inflammation in animals presented to the inhalation of siR-7-EM/KK-46 in vivo. Thus, they formed a therapeutic approach for COVID-19 based on the breath of a qualified siRNA-peptide dendrimer formulation.
Avigan as an example of COVID-19 current drugs
Biomedical experts in China announced promising results from a Japanese influenza drug that seemed to be effective for cases of SARS-CoV-2.145. Zhang Xinmin or China's science and technology ministry, reported overall promising results with favipiravir (the generic name of Avigan, produced by a subsidiary of Fujifilm) in 350 clinical cases tested in both Shenzhen and Wuhan.146Avigan is the trademarked name of the antiviral drug, favipiravir.147 This drug was first produced by Fujifilm Toyama Chemical Company and licensed in 2014 for medical use in Japan.148 It was particularly-recommended for the treatment of specific forms of influenza that were not adequately covered by other antiviral or vaccines.149 In addition to its role as an antiviral drug for the treatment of influenza, it is believed to have impact on several other viral diseases; its chemical formula is a pyrazinecarboxamide derivative and as such is similar in structure to other antiviral compounds, including T-1106 and T-1105.150
Favipiravir was registered for the treatment of new influenza (i.e., strains that are exceptionally-difficult to treat); this did not include routine seasonal influenza.151 In February 2020, the role of favipiravir as an agent for the treatment of SARS-CoV-2 infection was explored in China and in Japan for the treatment of new COVID-19 cases.152
With respect to the history of this drug compound, it was first recommended in China in 2019 for the treatment of clinically-significant coronavirus disease. Italy accepted the drug in March 2020 with the intent to perform clinical trials in three areas that were hard-hit by COVID-19.153 This drug is currently under consideration (April 2020) for three clinical projects to be carried out in the USA.
The mechanism of action underlying drug therapy with respect to COVID-19 is linked to its capacity to interfere with the activity of the viral enzyme RNA-dependent RNA polymerase;154 as such, favipiravir is believed to act by preventing virus replication inside the host cell.155 Other investigations reported that treatment with favipiravir results in fatal mutations during viral RNA transcription, which would lead to a reduction in the viable viral progeny.156 Favipiravir did not hinder host DNA and/or RNA organization in the mammalian cells and thus it is presumed to be safe for use and without any specific toxicity.157
Favipiravir is metabolized inside the host cell and converted to active favipiravir-ribofuranosyl-5′-triphosphate (favipiravir-RTP). The human enzyme hypoxanthine-guanine phosphoribosyl transferase (HGPRT) is responsible for activates favipiravir.158
Favipiravir is available for human consumption in the form of tablets (oral usage) or as a pro-drug that can be used for intravenous infusion.159 Scientists in Japan are currently using this drug in clinical investigations on coronavirus cases in an effort to reduce disease severity, as it may prevent ongoing SARS-CoV-2 replication in infected patients.160
Patients infected with COVID-19 in China who underwent treatment with Avigan became SARS-CoV-2 negative after a mean of 5 days.161 Radiographic studies revealed improvements with respect to lung findings in ∼90% of patients treated with favipiravir, compared to only 65% of those who did not receive drug treatment.162 Currently, use of favipiravir for COVID-19 patients requires administrative permission because it was designed and evaluated in patients infected with influenza.
Nanotechnology can foster the development of effective medications for COVID-19 either by acting as drug delivery carriers or by their high antimicrobial and antiviral abilities as shown in our previously-published article.163 The next section presents an example of a nano-drug composed of Avigan-encapsulated nanoemulsions.
Avigan-loaded nano-emulsions as example of nanodrugs
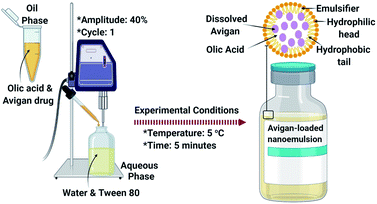
With increasing concerns regarding the number and severity of adverse events, recent studies have focused on new avenues and modalities for drug delivery. Toward this end, we propose a consideration of Avigan-loaded nano-emulsions as a promising nano-based delivery system for the treatment of COVID-19.Favipiravir is synthesized as a white to bright yellow powder.164,165 It is only marginally soluble in acetonitrile and methanol and slightly soluble in water and absolute ethanol. Also, favipiravir is slightly soluble in water buffered at pH 2 to 5.5 and sparingly soluble at pH 5.5 to 6.1.166 As such, favipiravir may be difficult to administer due to its limited solubility in water solubility. Drug solubility patterns have a direct impact on its pharmacokinetics, speed, and degree of absorption, bioavailability, and therapeutic potency; a more suitable therapeutic formulation will be required.167 As such, we present favipiravir-encapsulated nano-emulsions as promising drug delivery carriers for patients with COVID-19 as displayed in Fig. 3.
Nano-emulsions are kinetically-permanent and are dispersed in biphasic form as oil-in-water which is stabilized by an interfacial layer that includes an amphiphilic surfactant (Tween 80).168 Nano-emulsion formulations include submicron-sized particles that are typically 5 nm to 200 nm in diameter. Nano-emulsions present many possible benefits with respect to oral administration of active pharmaceutical constituents, including effective encapsulation of slightly water-soluble drugs such as favipiravir; they promote a strong balance vis-à-vis disconnection, coalescence, and flocculation. In addition, nano-emulsions can promote drug bioavailability and penetration.169
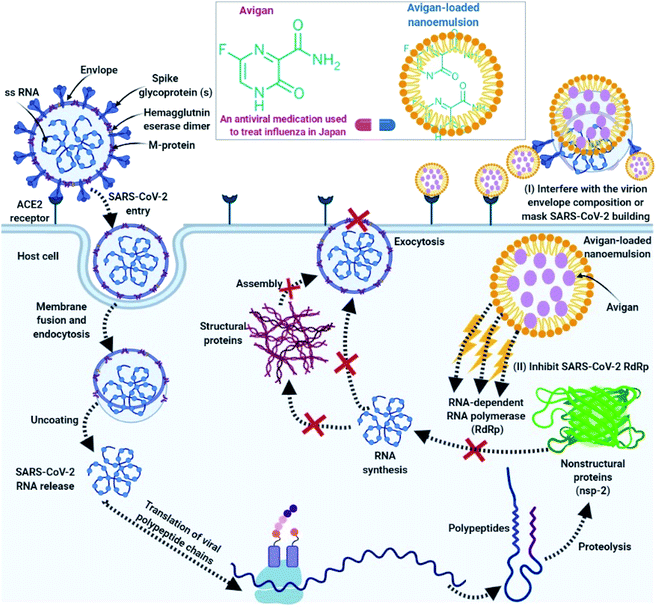
By encapsulating favipiravir inside the synthesized nano-emulsions, we can confirm that the slightly soluble drug will be centered in the core of the nano-emulsions and will be capable of binding by weak van der Waals forces170 as shown in Fig. 3. This will facilitate binding to the virus RNA-dependent RNA polymerase after its release from the nano-emulsions, and subsequently hinders virus replication through inhibiting the SARS-COV-2 RNA replication.
Proposed action mechanism
The mechanisms of action associated with the efficacy of nano-emulsions for the treatment of COVID-19 can be classified into two main categories:(I) Nano-emulsions have the capacity to interfere with the virion envelope composition or mask the virus (SARS-CoV-2) building, which finally prevents SARS-CoV-2 adsorption and invasion into the host cells.102
(II) Inside host cells, favipiravir carried by the nano-emulsion can interfere with the actions of the SARS-CoV-2 RNA-dependent RNA polymerase (RdRp).171
As such, we anticipate that favipiravir-loaded nano-emulsions could be capable of effectively-preventing SARS-CoV-2 adsorption and destroying its structure. This will prevent SARS-CoV-2 from surface-mediated attachment to the host cell as shown in Fig. 4.
Second, if the virus does in fact enter the host cell, favipiravir-loaded nano-emulsions will be able to interact with the viral polymerase (RNA-dependent RNA polymerase; Fig. 4).154 Favipiravir acts to prevent replication of the virus inside the host cell155 and generates fatal mutations during viral RNA transcription, thereby decreasing the population of viable viral progeny.156
Nanomaterial-based drug delivery systems as nanovaccines
Nanomedicine is a new and rapidly developing area, and the synthesized NPs can be applied in drug formulations due to their pharmacokinetics, security, efficiency, and targeting capability.76 Numerous drug-based NPs have been recorded in clinical cases. However, drug-based NPs meet a lot of difficulties because of their toxicity problems, demand for better validation, and lack of administrative guidelines.76 Nanotechnology has joined with vaccine progress in the fight toward infections produced by bacterial or viral diseases, as well as cancerous tumors, appearing in nanovaccines, which are important.172 Nanovaccines have many benefits over conventional vaccines as antigens can be encapsulated in nanocarriers to withdraw antigenic degeneration. Also, antigen-presenting cells (APCs) can immediately prepare and phagocytize nanovaccines.173Nanomaterial-based drug delivery systems have afforded one-of-a-kind possibilities to improve the therapeutic efficiency of cancer and SARS-CoV-2 vaccines (antigens), while molecular or nano-adjuvants and nano-carriers are usually applied in nanovaccines.174 In melanoma, colon cancer, and human papillomavirus E6/E7, nano-vaccines have caused significant immune responses that inhibited tumor growth.175 COVID-19 vaccines have been produced unprecedentedly, which would not have been possible without decades of fundamental research on delivery nanotechnology. Lipid-based nanoparticles have performed a crucial part in the progress of COVID-19 vaccines have therefore been regarded as the frontrunner in nanoscale drug delivery systems.176
Vaccination represents the most reliable line of defense against infectious disorders and is essential in reducing the pandemic extent of developing pathogens to which a population has insufficient immunity.177 In current years, mRNA vaccines have been introduced as the original frontier in vaccination, owing to their fast and straightforward growth while giving a safer alternative to conventional vaccine technologies such as live or attenuated viruses (SARS-CoV-2).178 Recent discoveries in mRNA vaccination have been through formulation with lipid nanoparticles (LNPs), which present both security and improving the delivery of mRNA vaccines in vivo.179 With the recent success of mRNA vaccines developed by Moderna and BioNTech/Pfizer against COVID-19, mRNA technology and lipid nanoparticles (LNP) have never received more attention.180 Emily H. Pilkington et al.,181 displays the most excellent mRNA-LNP vaccines that have just been accepted for emergency treatment and are in clinical cases, with a focus on the unusual construction of several COVID-19 vaccines, quicker than any other vaccine in history. Another study crated by Mai N. Vu et al.,182 stated that hemagglutinin functionalized liposomal vaccines improve the germinal center and follicular helper T cell immunity, which in turn obtains a solution of COVID- 19 invasion.
A key concept in nanomedicine is encapsulating therapeutic or diagnostic agents inside nanoparticles to prolong blood circulation time and to enhance interactions with targeted cells.183 During circulation and depending on the selected application (e.g., cancer drug delivery or immune modulators), NPs are required to possess low or high interactions with cells in human blood and blood vessels to minimize side effects or maximize delivery efficiency.184,185 Here, the first comprehensive method to analyze cellular interactions of both synthetic and commercially available NPs under human blood flow conditions in a microvascular network is developed. Importantly, this method allows to unravel the complex interplay of size, charge, and type of NPs on their cellular associations under the dynamic flow of human blood. This method offers a unique platform to study complex interactions of any type of NPs in human blood flow conditions and serves as a useful guideline for the rational design of liposomes and polymer NPs for diverse applications in nanomedicine.184
Poly ethylene glycol (PEG) is widely used as a gold standard in bio-conjugation and nanomedicine to prolong blood circulation time and improve drug efficacy. The conjugation of PEG to proteins, peptides, oligonucleotides (DNA, small interfering RNA (siRNA), microRNA (miRNA)) and NPs is a well-established technique known as PEGylation.186 We must note the importance of PEGylation in selective organ targeting of NPs, provides new insights into the structure–property relationship of LNPs, and offers a novel, simple, and practical PEGylation technology to prepare the next generation of safe and effective nanovaccines against COVID-19. Danijela Zukancic et al.,187 discovered that LNPs stabilized by 3% Tween 20, a surfactant with a branched PEG chain linking to a short lipid tail, achieved highly specific transfection at the lymph node.
Conclusion and future perspectives
In this article, we summarized recent data about COVID-19, etiology, pathogenesis, prevention routes, diagnosis, and the currently-available medications. After that, we opened the way of new nanomaterial-based approaches to increase the bioavailability and efficiency of COVID-19 drugs by their incorporation inside and/or onto the surface of 5 promising nanomaterial-based drug delivery systems. Their preparation routes and unique advantages were mentioned. Then, we discussed in detail, one potential nanoemulsion system containing Avigan drug. The proposed action mechanism of this unique nano-based drug was explained in the light of solid references. Many research studies and clinical trials are still under investigation for the development of effective and safe cure for COVID-19, some trials showed good promise. However, more investigation is required. Nanotechnology can foster the development of new nanodrugs and nanovaccines by acting as effective drug delivery systems.Author contributions
MAE, and GSE conceived the concept and idea of the present review, worked on the study design strategy and selected the topics to be discussed, draws the figures, did literature searches and screened titles and abstracts for relevance, abstracted the data from the eligible full text articles, analyzed and interpreted the data, and drafted the manuscript, revised the final draft of the manuscript. SOO, and MM worked on the study design strategy and selected the topics to be discussed, and did literature searches and screened titles and abstracts for relevance. All authors have read and approved the final draft.Compliance with ethical standards
Funding
Not applicable.Research involving human participation and/or animals
Not applicable.Informed consent
Not applicable.Ethical approval
Not applicable.Availability of data and materials
Not applicable.Conflicts of interest
The authors declare that they have no conflict of interest.References
- C. Chakraborty, A. Sharma, G. Sharma, M. Bhattacharya and S. Lee, Eur. Rev. Med. Pharmacol. Sci., 2020, 24, 4016–4026 CAS.
- WHO, World Health Organization, Geneva, 2020.
- M. Cascella, M. Rajnik, A. Cuomo, S. C. Dulebohn and R. Di Napoli, in Statpearls [internet], StatPearls Publishing, 2020 Search PubMed.
- K.-S. Yuen, Z.-W. Ye, S.-Y. Fung, C.-P. Chan and D.-Y. Jin, Cell Biosci., 2020, 10, 1–5 CrossRef PubMed.
- T. Zhang, Q. Wu and Z. Zhang, Curr. Biol., 2020, 30, 1346–1351 CrossRef CAS PubMed.
- Y. Dong, X. Mo, Y. Hu, X. Qi, F. Jiang, Z. Jiang and S. Tong, Pediatrics, 2020, 145, e20200702 CrossRef PubMed.
- J. Yang, Y. Zheng, X. Gou, K. Pu, Z. Chen, Q. Guo, R. Ji, H. Wang, Y. Wang and Y. Zhou, Int. J. Infect. Dis., 2020, 94, 91–95 CrossRef CAS PubMed.
- W. Zhou, W. Wang, H. Wang, R. Lu and W. Tan, BMC Infect. Dis., 2013, 13, 433 CrossRef PubMed.
- A. R. Fehr and S. Perlman, in Coronaviruses, Springer, 2015, pp. 1–23 Search PubMed.
- M. A. Müller, V. S. Raj, D. Muth, B. Meyer, S. Kallies, S. L. Smits, R. Wollny, T. M. Bestebroer, S. Specht and T. Suliman, mBio, 2012, 3(6), 1–5 CrossRef PubMed.
- N. Zhu, D. Zhang, W. Wang, X. Li, B. Yang, J. Song, X. Zhao, B. Huang, W. Shi and R. Lu, N. Engl. J. Med., 2020, 382, 727–733 CrossRef CAS PubMed.
- Y. Guan, B. Zheng, Y. He, X. Liu, Z. Zhuang, C. Cheung, S. Luo, P. Li, L. Zhang and Y. Guan, Science, 2003, 302, 276–278 CrossRef CAS PubMed.
- V. S. Raj, H. Mou, S. L. Smits, D. H. Dekkers, M. A. Müller, R. Dijkman, D. Muth, J. A. Demmers, A. Zaki and R. A. Fouchier, Nature, 2013, 495, 251–254 CrossRef CAS PubMed.
- R. Lu, X. Zhao, J. Li, P. Niu, B. Yang, H. Wu, W. Wang, H. Song, B. Huang and N. Zhu, Lancet, 2020, 395, 565–574 CrossRef CAS.
- L.-L. Ren, Y.-M. Wang, Z.-Q. Wu, Z.-C. Xiang, L. Guo, T. Xu, Y.-Z. Jiang, Y. Xiong, Y.-J. Li and X.-W. Li, Chin. Med. J., 2020, 133, 1015–1024 CrossRef PubMed.
- F. Wu, S. Zhao, B. Yu, Y.-M. Chen, W. Wang, Z.-G. Song, Y. Hu, Z.-W. Tao, J.-H. Tian and Y.-Y. Pei, Nature, 2020, 579, 265–269 CrossRef CAS PubMed.
- P. Zhou, X.-L. Yang, X.-G. Wang, B. Hu, L. Zhang, W. Zhang, H.-R. Si, Y. Zhu, B. Li, C.-L. Huang, H.-D. Chen, J. Chen, Y. Luo, H. Guo, R.-D. Jiang, M.-Q. Liu, Y. Chen, X.-R. Shen, X. Wang, X.-S. Zheng, K. Zhao, Q.-J. Chen, F. Deng, L.-L. Liu, B. Yan, F.-X. Zhan, Y.-Y. Wang, G.-F. Xiao and Z.-L. Shi, Nature, 2020, 579, 270–273 CrossRef CAS PubMed.
- J. S. Mackenzie and D. W. Smith, Microbiology Australia, 2020, 41, 45–50 CrossRef PubMed.
- P. Zhou, X.-L. Yang, X.-G. Wang, B. Hu, L. Zhang, W. Zhang, H.-R. Si, Y. Zhu, B. Li and C.-L. Huang, Nature, 2020, 579, 270–273 CrossRef CAS PubMed.
- T. T.-Y. Lam, M. H.-H. Shum, H.-C. Zhu, Y.-G. Tong, X.-B. Ni, Y.-S. Liao, W. Wei, W. Y.-M. Cheung, W.-J. Li and L.-F. Li, Nature, 2020, 1–6 Search PubMed.
- A. Mitra, D. M. Dwyre, M. Schivo, G. R. Thompson, S. H. Cohen, N. Ku and J. P. Graff, Am. J. Hematol., 2020, 1–2 Search PubMed.
- Q. Han, Q. Lin, Z. Ni and L. You, Influenza and Other Respiratory Viruses, 2020 Search PubMed.
- G. Kampf, D. Todt, S. Pfaender and E. Steinmann, J. Hosp. Infect., 2020, 104, 246–251 CrossRef CAS PubMed.
- Y. Chen, L. Chen, Q. Deng, G. Zhang, K. Wu, L. Ni, Y. Yang, B. Liu, W. Wang and C. Wei, J. Med. Virol., 2020 Search PubMed.
- C. Shen, Z. Wang, F. Zhao, Y. Yang, J. Li, J. Yuan, F. Wang, D. Li, M. Yang and L. Xing, Jama, 2020, 323, 1582–1589 CrossRef CAS PubMed.
- A. R. Fehr and S. Perlman, Methods Mol. Biol., 2015, 1282, 1–23 CrossRef CAS PubMed.
- F. Di Gennaro, D. Pizzol, C. Marotta, M. Antunes, V. Racalbuto, N. Veronese and L. Smith, Int. J. Environ. Res. Public Health, 2020, 17 Search PubMed.
- M. Cascella, M. Rajnik, A. Aleem, S. Dulebohn and R. Di Napoli, StatPearls, 2021 Search PubMed , available from: https://www.ncbi.nlm.nih.gov/books/NBK554776/.
- S. Cherian, V. Potdar, S. Jadhav, P. Yadav, N. Gupta, M. Das, P. Rakshit, S. Singh, P. Abraham and S. Panda, bioRxiv, 2021 DOI:10.1101/2021.04.22.440932.
- X. Li, M. Geng, Y. Peng, L. Meng and S. Lu, J. Pharm. Anal., 2020 DOI:10.1016/j.jpha.2020.03.001.
- C. Chen, X. R. Zhang, Z. Y. Ju and W. F. He, Zhonghua Shaoshang Zazhi, 2020, 36, E005 Search PubMed.
- F. Bennardo, C. Buffone and A. Giudice, Oral Oncol., 2020, 104659, DOI:10.1016/j.oraloncology.2020.104659.
- H. Wang, P. Yang, K. Liu, F. Guo, Y. Zhang, G. Zhang and C. Jiang, Cell Res., 2008, 18, 290–301 CrossRef CAS PubMed.
- S. Perlman and J. Netland, Nat. Rev. Microbiol., 2009, 7, 439–450 CrossRef CAS PubMed.
- E. de Wit, N. van Doremalen, D. Falzarano and V. J. Munster, Nat. Rev. Microbiol., 2016, 14, 523–534 CrossRef CAS PubMed.
- Z. Xu, L. Shi, Y. Wang, J. Zhang, L. Huang, C. Zhang, S. Liu, P. Zhao, H. Liu, L. Zhu, Y. Tai, C. Bai, T. Gao, J. Song, P. Xia, J. Dong, J. Zhao and F. S. Wang, Lancet Respir. Med., 2020, 8, 420–422 CrossRef CAS PubMed.
- A. E. Williams and R. C. Chambers, Am. J. Physiol.: Lung Cell. Mol. Physiol., 2014, 306, L217–L230 CrossRef CAS PubMed.
- R. Channappanavar and S. Perlman, Semin. Immunopathol., 2017, 39, 529–539 CrossRef CAS PubMed.
- P. Yang and X. Wang, Cell. Mol. Immunol., 2020 DOI:10.1038/s41423-020-0407-x.
- T. Lupia, S. Scabini, S. Mornese Pinna, G. Di Perri, F. G. De Rosa and S. Corcione, Journal of Global Antimicrobial Resistance, 2020, 21, 22–27 CrossRef PubMed.
- Y. Yang, F. Peng, R. Wang, K. Guan, T. Jiang, G. Xu, J. Sun and C. Chang, J. Autoimmun., 2020, 109, 102434 CrossRef CAS PubMed.
- C. Huang, Y. Wang, X. Li, L. Ren, J. Zhao, Y. Hu, L. Zhang, G. Fan, J. Xu, X. Gu, Z. Cheng, T. Yu, J. Xia, Y. Wei, W. Wu, X. Xie, W. Yin, H. Li, M. Liu, Y. Xiao, H. Gao, L. Guo, J. Xie, G. Wang, R. Jiang, Z. Gao, Q. Jin, J. Wang and B. Cao, Lancet, 2020, 395, 497–506 CrossRef CAS.
- Y. Cao, X. Liu, L. Xiong and K. Cai, J. Med. Virol., 2020 DOI:10.1002/jmv.25822.
- G. Lippi, A. M. Simundic and M. Plebani, Clin. Chem. Lab. Med., 2020 DOI:10.1515/cclm-2020-0285.
- R. A. Khailany, M. Safdar and M. Ozaslan, Gene Reports, 2020, 100682, DOI:10.1016/j.genrep.2020.100682.
- W. Wang, Y. Xu, R. Gao, R. Lu, K. Han, G. Wu and W. Tan, JAMA, 2020 DOI:10.1001/jama.2020.3786.
- Y. Pan, H. Guan, S. Zhou, Y. Wang, Q. Li, T. Zhu, Q. Hu and L. Xia, Eur. Radiol., 2020 DOI:10.1007/s00330-020-06731-x.
- T. Ai, Z. Yang, H. Hou, C. Zhan, C. Chen, W. Lv, Q. Tao, Z. Sun and L. Xia, Radiology, 2020, 200642, DOI:10.1148/radiol.2020200642.
- Y. Li and L. Xia, AJR, Am. J. Roentgenol., 2020, 1–7, DOI:10.2214/ajr.20.22954.
- G. Kampf, D. Todt, S. Pfaender and E. Steinmann, J. Hosp. Infect., 2020, 104, 246–251 CrossRef CAS PubMed.
- I. O. Ayenigbara, Central Asian Journal of Global Health, 2020, 9, e466 CrossRef PubMed.
- R. P. Saha, A. R. Sharma, M. K. Singh, S. Samanta, S. Bhakta, S. Mandal, M. Bhattacharya, S.-S. Lee and C. Chakraborty, Front. Pharmacol., 2020, 11 Search PubMed.
- S. P. Adhikari, S. Meng, Y. J. Wu, Y. P. Mao, R. X. Ye, Q. Z. Wang, C. Sun, S. Sylvia, S. Rozelle, H. Raat and H. Zhou, Infect. Dis. Poverty, 2020, 9, 29 CrossRef PubMed.
- B. Robson, Comput. Biol. Med., 2020, 103749 CrossRef CAS PubMed.
- Y. Bai, L. Yao, T. Wei, F. Tian, D.-Y. Jin, L. Chen and M. Wang, Jama, 2020, 323, 1406–1407 CrossRef CAS PubMed.
- G. Kampf, D. Todt, S. Pfaender and E. Steinmann, J. Hosp. Infect., 2020, 104, 246–251 CrossRef CAS PubMed.
- W. H. Organization, Water, sanitation, hygiene and waste management for COVID-19: technical brief, 03 March 2020, World Health Organization, 2020 Search PubMed.
- H. Fathizadeh, P. Maroufi, M. Momen-Heravi, S. Dao, S. Köse, K. Ganbarov, P. Pagliano, S. Espsoito and H. S. Kafil, Le Infezioni in Medicina, 2020, 28, 185–191 CAS.
- S. Deshmukh, S. Patil, S. Mullani and S. Delekar, Mater. Sci. Eng., C, 2019, 97, 954–965 CrossRef CAS PubMed.
- J. Yi, R. Mudumbai and W. Xu, 2020, arXiv preprint arXiv:2004.05759.
- A. I. El-Batal, F. M. Mosallam and G. S. El-Sayyad, J. Cluster Sci., 2018, 29, 1003–1015 CrossRef CAS.
- K.-K. Chen, M. Minakuchi, K. Wuputra, C.-C. Ku, J.-B. Pan, K.-K. Kuo, Y.-C. Lin, S. Saito and C.-S. Lin, 2019, 2019110328.
- A. Saha, A. R. Sharma, M. Bhattacharya, G. Sharma, S.-S. Lee and C. Chakraborty, Arch. Med. Res., 2020, 51, 595–597 CrossRef CAS PubMed.
- S. G. V. Rosa and W. C. Santos, Revista Panamericana de Salud Pública, 2020, 44 Search PubMed.
- S. S. Bag, S. Sinha and I. Saito, Medical Research Archives, 2020, 8, 1–21 CrossRef.
- A. Saha, A. R. Sharma, M. Bhattacharya, G. Sharma, S.-S. Lee and C. Chakraborty, Arch. Med. Res., 2020, 51, 585–586 CrossRef CAS PubMed.
- E. Sallard, F.-X. Lescure, Y. Yazdanpanah, F. Mentre, N. Peiffer-Smadja, A. Florence, Y. Yazdanpanah, F. Mentre, F.-X. Lescure and N. Peiffer-smadja, Antiviral Res., 2020, 104791 CrossRef CAS PubMed.
- A. Casadevall and L.-a. Pirofski, J. Clin. Invest., 2020, 130, 1545–1548 CrossRef CAS PubMed.
- S. Kumar, K. Zhi, A. Mukherji and K. Gerth, Viruses, 2020, 12, 486 CrossRef CAS PubMed.
- Y. Wang, W. Jiang, Q. He, C. Wang, B. Wang, P. Zhou, N. Dong and Q. Tong, Signal Transduction Targeted Ther., 2020, 5, 1–3 CrossRef CAS PubMed.
- A. B. Cavalcanti, F. G. Zampieri, R. G. Rosa, L. C. Azevedo, V. C. Veiga, A. Avezum, L. P. Damiani, A. Marcadenti, L. Kawano-Dourado and T. Lisboa, N. Engl. J. Med., 2020, 383, 2041–2052 CrossRef CAS PubMed.
- M. Bray, C. Rayner, F. Noël, D. Jans and K. Wagstaff, Antiviral Res., 2020, 178, 104805 CrossRef CAS PubMed.
- A. El-Sayed and M. Kamel, Environ. Sci. Pollut. Res., 2020, 27, 19200–19213 CrossRef CAS PubMed.
- Z. Tang, X. Zhang, Y. Shu, M. Guo, H. Zhang and W. Tao, Nano Today, 2021, 36, 101019 CrossRef CAS PubMed.
- D. Bobo, K. J. Robinson, J. Islam, K. J. Thurecht and S. R. Corrie, Nanomaterials and Neoplasms, 2021, 539–577 Search PubMed.
- D. Bobo, K. J. Robinson, J. Islam, K. J. Thurecht and S. R. Corrie, Pharm. Res., 2016, 33, 2373–2387 CrossRef CAS PubMed.
- M. Chakravarty and A. Vora, Drug delivery and translational research, 2020, pp. 1–40 Search PubMed.
- K. Maduray and R. Parboosing, Biological trace element research, 2020, pp. 1–18 Search PubMed.
- A. Maus, L. Strait and D. Zhu, Engineered Regeneration, 2021, 2, 31–46 CrossRef.
- N. Zhu, D. Zhang, W. Wang, X. Li, B. Yang, J. Song, X. Zhao, B. Huang, W. Shi and R. Lu, N. Engl. J. Med., 2020, 382, 727–733 CrossRef CAS PubMed.
- T. Lammers, A. M. Sofias, R. van der Meel, R. Schiffelers, G. Storm, F. Tacke, S. Koschmieder, T. H. Brümmendorf, F. Kiessling and J. M. Metselaar, Nat. Nanotechnol., 2020, 15, 622–624 CrossRef CAS PubMed.
- CDC, Morb. Mortal. Wkly. Rep., 2020, 69, 422 CrossRef PubMed.
- S. Chen, C. Xing, D. Huang, C. Zhou, B. Ding, Z. Guo, Z. Peng, D. Wang, X. Zhu and S. Liu, Sci. Adv., 2020, 6, eaay6825 CrossRef CAS PubMed.
- Z. Xie, M. Peng, R. Lu, X. Meng, W. Liang, Z. Li, M. Qiu, B. Zhang, G. Nie and N. Xie, Light: Sci. Appl., 2020, 9, 1–15 CrossRef PubMed.
- M. Qiu, A. Singh, D. Wang, J. Qu, M. Swihart, H. Zhang and P. N. Prasad, Nano Today, 2019, 25, 135–155 CrossRef CAS.
- Z. Tang, N. Kong, J. Ouyang, C. Feng, N. Y. Kim, X. Ji, C. Wang, O. C. Farokhzad, H. Zhang and W. Tao, Matter, 2020, 2, 297–322 CrossRef.
- Z. Xie, T. Fan, J. An, W. Choi, Y. Duo, Y. Ge, B. Zhang, G. Nie, N. Xie and T. Zheng, Chem. Soc. Rev., 2020, 49, 8065–8087 RSC.
- M. Luo, T. Fan, Y. Zhou, H. Zhang and L. Mei, Adv. Funct. Mater., 2019, 29, 1808306 CrossRef.
- Z. Sun, H. Xie, S. Tang, X. F. Yu, Z. Guo, J. Shao, H. Zhang, H. Huang, H. Wang and P. K. Chu, Angew. Chem., Int. Ed., 2015, 54, 11526–11530 CrossRef CAS PubMed.
- A. Maali and M. T. H. Mosavian, J. Dispersion Sci. Technol., 2013, 34, 92–105 CrossRef CAS.
- A. Akbarzadeh, R. Rezaei-Sadabady, S. Davaran, S. W. Joo, N. Zarghami, Y. Hanifehpour, M. Samiei, M. Kouhi and K. Nejati-Koshki, Nanoscale Res. Lett., 2013, 8, 102 CrossRef PubMed.
- C. I. C. Crucho and M. T. Barros, Mater. Sci. Eng., C, 2017, 80, 771–784 CrossRef CAS PubMed.
- M. Nikzamir, Y. Hanifehpour, A. Akbarzadeh and Y. Panahi, J. Inorg. Organomet. Polym. Mater., 2021, 31, 2246–2261 CrossRef CAS.
- S. Jacob, A. B. Nair and J. Shah, Biomater. Res., 2020, 24, 1–16 CrossRef PubMed.
- V. S. Mapari, R. P. Sable, S. P. Borkar, S. Bharat, S. B. S. Mulavakar and A. A. Sheikh, Int. J. Adv. Pharm. Biotech., 2020, 6, 8–11 CrossRef.
- P. Pandey, N. Gulati, M. Makhija, D. Purohit and H. Dureja, Recent Pat. Nanotechnol., 2020, 14, 276–293 CrossRef CAS PubMed.
- S. J. Choi and D. J. McClements, Food Sci. Biotechnol., 2020, 29, 149–168 CrossRef CAS PubMed.
- A. Naseema, L. Kovooru, A. K. Behera, K. P. Kumar and P. Srivastava, Adv. Colloid Interface Sci., 2020, 102318 Search PubMed.
- A. Bahuguna, S. Ramalingam and M. Kim, in Nanotechnology for Food, Agriculture, and Environment, Springer, 2020, pp. 39–61 Search PubMed.
- H. Soni and S. Sharma, Scholars International Journal of Anatomy and Physiology, 2021, 4, 6–13 Search PubMed.
- H. Gaoe, Z. Pang, S. Pan, S. Cao, Z. Yang, C. Chen and X. Jiang, Arch. Pharmacal Res., 2012, 35, 333–341 CrossRef CAS PubMed.
- J. S. Franklyne, P. M. Gopinath, A. Mukherjee and N. Chandrasekaran, Curr. Opin. Colloid Interface Sci., 2021, 101458 CrossRef CAS PubMed.
- A. Astani, J. Reichling and P. Schnitzler, Phytother. Res., 2010, 24, 673–679 CrossRef CAS PubMed.
- P. Yingchoncharoen, D. S. Kalinowski and D. R. Richardson, Pharmacol. Rev., 2016, 68, 701–787 CrossRef CAS PubMed.
- Y. Deng, X. Zhang, H. Shen, Q. He, Z. Wu, W. Liao and M. Yuan, Front. Bioeng. Biotechnol., 2020, 7, 489 CrossRef PubMed.
- Z. Wang, L. Ling, Y. Du, C. Yao and X. Li, Int. J. Pharm., 2019, 564, 244–255 CrossRef CAS PubMed.
- H. O. Alpar, S. Somavarapu, K. Atuah and V. Bramwell, Adv. Drug Delivery Rev., 2005, 57, 411–430 CrossRef CAS PubMed.
- R. Itani, M. Tobaiqy and A. Al Faraj, Theranostics, 2020, 10, 5932 CrossRef CAS PubMed.
- C. Shi, Y. He, M. Ding, Y. Wang and J. Zhong, Trends Food Sci. Technol., 2019, 87, 3–13 CrossRef CAS.
- M. Afsharzadeh, M. Hashemi, A. Mokhtarzadeh, K. Abnous and M. Ramezani, Artif. Cells, Nanomed., Biotechnol., 2018, 46, 1095–1110 CrossRef CAS PubMed.
- W. Wang, J. Ding, C. Xiao, Z. Tang, D. Li, J. Chen, X. Zhuang and X. Chen, Biomacromolecules, 2011, 12, 2466–2474 CrossRef CAS PubMed.
- I. Fratoddi, I. Venditti, C. Cametti, C. Palocci, L. Chronopoulou, M. Marino, F. Acconcia and M. V. Russo, Colloids Surf., B, 2012, 93, 59–66 CrossRef CAS PubMed.
- K. Sonaje, E.-Y. Chuang, K.-J. Lin, T.-C. Yen, F.-Y. Su, M. T. Tseng and H.-W. Sung, Mol. Pharmaceutics, 2012, 9, 1271–1279 CrossRef CAS PubMed.
- B. Y. Chu, M. Al Kobiasi, W. Zeng, D. Mainwaring and D. C. Jackson, Procedia Vaccinol., 2012, 6, 74–79 CrossRef.
- R. Gauro, M. Nandave, V. K. Jain and K. Jain, J. Nanopart. Res., 2021, 23, 1–20 CrossRef.
- Y. Lee, J. Lee, M. Kim, G. Kim, J. S. Choi and M. Lee, J. Controlled Release, 2021, 330, 907–919 CrossRef CAS PubMed.
- P. Kesharwani, V. Gajbhiye and N. K. Jain, Biomaterials, 2012, 33, 7138–7150 CrossRef CAS PubMed.
- F. Wang, X. Cai, Y. Su, J. Hu, Q. Wu, H. Zhang, J. Xiao and Y. Cheng, Acta Biomater., 2012, 8, 4304–4313 CrossRef CAS PubMed.
- Y. Kim, E. J. Park and D. H. Na, Arch. Pharmacal Res., 2018, 41, 571–582 CrossRef CAS PubMed.
- J. S. Chahal, O. F. Khan, C. L. Cooper, J. S. McPartlan, J. K. Tsosie, L. D. Tilley, S. M. Sidik, S. Lourido, R. Langer and S. Bavari, Proc. Natl. Acad. Sci., 2016, 113, E4133–E4142 CrossRef CAS PubMed.
- A. A. Noyes and W. R. Whitney, J. Am. Chem. Soc., 1897, 19, 930–934 CrossRef.
- M. R. Abdulbaqi, H. S. Taghi and Z. M. Jaafar, Benefits, 2021, 2, 11 Search PubMed.
- J. Hong, Y. Li, Y. Xiao, Y. Li, Y. Guo, H. Kuang and X. Wang, Colloids Surf., B, 2016, 145, 319–327 CrossRef CAS PubMed.
- S. Szunerits, A. Barras, M. Khanal, Q. Pagneux and R. Boukherroub, Molecules, 2015, 20, 14051–14081 CrossRef CAS PubMed.
- S. Sood, K. Jain and K. Gowthamarajan, Colloids Surf., B, 2014, 113, 330–337 CrossRef CAS PubMed.
- D. J. McClements and J. Rao, Crit. Rev. Food Sci. Nutr., 2011, 51, 285–330 CrossRef CAS PubMed.
- A. Azeem, M. Rizwan, F. J. Ahmad, Z. Iqbal, R. K. Khar, M. Aqil and S. Talegaonkar, AAPS PharmSciTech, 2009, 10, 69–76 CrossRef CAS PubMed.
- M. J. Parnham and H. Wetzig, Chem. Phys. Lipids, 1993, 64, 263–274 CrossRef CAS PubMed.
- F. Olson, E. Mayhew, D. Maslow, Y. Rustum and F. Szoka, Eur. J. Cancer Clin. Oncol., 1982, 18, 167–176 CrossRef CAS PubMed.
- X. Xu, M. A. Khan and D. J. Burgess, Int. J. Pharm., 2011, 419, 52–59 CrossRef CAS PubMed.
- V. Pooladanda, S. Thatikonda, O. Sunnapu, S. Tiwary, P. K. Vemula, M. V. N. K. Talluri and C. Godugu, Nanomedicine, 2021, 33, 102351 CrossRef CAS PubMed.
- N. Voigt, P. Henrich-Noack, S. Kockentiedt, W. Hintz, J. Tomas and B. A. Sabel, J. Nanopart. Res., 2014, 16, 2379 CrossRef PubMed.
- A. Kiss, V. Baksa, M. Bege, L. TÁlas, A. BorbÁs, I. Bereczki, G. BÁnfalvi and G. SzemÁn-Nagy, Anticancer Res., 2021, 41, 137–149 CrossRef CAS PubMed.
- A. Salvati, C. Åberg, T. dos Santos, J. Varela, P. Pinto, I. Lynch and K. A. Dawson, Nanomedicine, 2011, 7, 818–826 CrossRef CAS PubMed.
- M. F. Neerman, H.-T. Chen, A. R. Parrish and E. E. Simanek, Mol. Pharmaceutics, 2004, 1, 390–393 CrossRef CAS PubMed.
- L. Lennard, Eur. J. Clin. Pharmacol., 1992, 43, 329–339 CrossRef CAS PubMed.
- C.-M. J. Hu, S. Aryal and L. Zhang, Ther. Delivery, 2010, 1, 323–334 CrossRef CAS PubMed.
- G. Maio, P. D'Argenio, T. Stroffolini, A. Bozza, L. Sacco, M. E. Tosti, M. Intorcia, E. Fossi, G. D'Alessio and L. A. Kondili, J. Hepatol., 2000, 33, 116–120 CrossRef CAS PubMed.
- L. M. Kaminskas, D. E. V. Pires and D. B. Ascher, Sci. Rep., 2019, 9, 15465 CrossRef PubMed.
- J. Wolfram, Y. Yang, J. Shen, A. Moten, C. Chen, H. Shen, M. Ferrari and Y. Zhao, Colloids Surf., B, 2014, 124, 17–24 CrossRef CAS PubMed.
- M. Khaitov, A. Nikonova, I. Shilovskiy, K. Kozhikhova, I. Kofiadi, L. Vishnyakova, A. Nikolskii, P. Gattinger, V. Kovchina, E. Barvinskaia, K. Yumashev, V. Smirnov, A. Maerle, I. Kozlov, A. Shatilov, A. Timofeeva, S. Andreev, O. Koloskova, N. Kuznetsova, D. Vasina, M. Nikiforova, S. Rybalkin, I. Sergeev, D. Trofimov, A. Martynov, I. Berzin, V. Gushchin, A. Kovalchuk, S. Borisevich, R. Valenta, R. Khaitov and V. Skvortsova, Allergy, 2021, 1–15 Search PubMed.
- A. Murtaza, T. Rehman, Z. Zafar, S. Noreen, M. Iqbal, S. Hassan, I. Khawaish and M. H. A. Tarar, J. Pharm. Res. Int., 2020, 202–215 CrossRef.
- S. S. Yousaf, A. Isreb, I. Khan, E. Mewsiga, A. Elhissi, W. Ahmed and M. A. Alhnan, Pharm. Dev. Technol., 2021, 1–29 Search PubMed.
- S. Muthuirulappan and S. P. Francis, Asian Pac. J. Cancer Prev., 2013, 14, 2213–2216 CrossRef PubMed.
- H. Pearson, T. Clarke, A. Abbott, J. Knight and D. Cyranoski, Nature, 2003, 424, 121–126 CrossRef PubMed.
- X. Tang, C. Wu, X. Li, Y. Song, X. Yao, X. Wu, Y. Duan, H. Zhang, Y. Wang and Z. Qian, Natl. Sci. Rev., 2020, 7, 1012–1023 CrossRef.
- V. V. Zarubaev, E. A. Pushkina, S. S. Borisevich, A. V. Galochkina, A. V. Garshinina, A. A. Shtro, A. A. Egorova, A. S. Sokolova, S. L. Khursan and O. I. Yarovaya, Virology, 2018, 524, 69–77 CrossRef CAS PubMed.
- T. Nagata, A. K. Lefor, M. Hasegawa and M. Ishii, Disaster medicine and Public Health Preparedness, 2015, 9, 79–81 CrossRef PubMed.
- A. Sandoiu, Is the anti-flu drug Avigan effctive in treating COVID-19?, Medical News Today, 2020, https://www.medicalnewstoday.com/articles/anti-flu-drug-effective-in-treating-covid-19 Search PubMed.
- Y. Yang, F. Peng, R. Wang, K. Guan, T. Jiang, G. Xu, J. Sun and C. Chang, J. Autoimmun., 2020, 102434 CrossRef CAS PubMed.
- J. Dunning and P. Openshaw, Curr. Respir. Med. Rev., 2012, 8, 208–227 CrossRef.
- C. Chen, J. Huang, Z. Cheng, J. Wu, S. Chen, Y. Zhang, B. Chen, M. Lu, Y. Luo and J. Zhang, medRxiv, 2020, 1–27 Search PubMed.
- A. Saglietto, F. D'Ascenzo, G. B. Zoccai and G. M. De Ferrari, Lancet, 2020, 395, 1110–1111 CrossRef CAS.
- S. K. Dey, M. Saini, C. Dhembla, S. Bhatt, A. S. Rajesh, V. Anand, H. K. Das, S. Kundu, Suramin, Penciclovir and Anidulafungin bind nsp12, which governs the RNA-dependent-RNA polymerase activity of SARS-CoV-2, with higher interaction energy than Remdesivir, indicating potential in the treatment of Covid-19 infection, 2020, DOI:10.31219/osf.io/urxwh.
- C. K. Lumby, L. Zhao, M. Oporto, T. Best, H. Tutill, D. Shah, P. Veys, R. Williams, A. Worth and C. J. Illingworth, Clin. Infect. Dis., 2020, 71, 191–194 CrossRef PubMed.
- T. Baranovich, S.-S. Wong, J. Armstrong, H. Marjuki, R. J. Webby, R. G. Webster and E. A. Govorkova, J. Virol., 2013, 87, 3741–3751 CrossRef CAS PubMed.
- I. Giacchello, F. Musumeci, I. D’Agostino, C. Greco, G. Grossi and S. Schenone, Curr. Med. Chem., 2020, 28, 1068–1090 CrossRef PubMed.
- A. Stevaert and L. Naesens, Med. Res. Rev., 2016, 36, 1127–1173 CrossRef PubMed.
- K. Marte, The effect of type-I interferons, and the antiviral drugs ribavirin and favipiravir on rabies virus replication in mouse neuroblastoma (N2A) cells, 2015, http://hdl.handle.net/10832/1478 Search PubMed.
- L. Ferretti, C. Wymant, M. Kendall, L. Zhao, A. Nurtay, L. Abeler-Dörner, M. Parker, D. Bonsall and C. Fraser, Science, 2020, 368, eabb6936 CrossRef CAS PubMed.
- A. Chanda, Coronavirus: A study on possible treatment, 2020, DOI:10.31219/osf.io/7xrqw.
- S. Latif, M. Usman, S. Manzoor, W. Iqbal, J. Qadir, G. Tyson, I. Castro, A. Razi, M. N. K. Boulos and A. Weller, IEEE Transactions on Artificial Intelligence, 2020, 1, 85–103 Search PubMed.
- M. Abd Elkodous, G. S. El-Sayyad and M. M. Abdel-Daim, Environ. Sci. Pollut. Res., 2020 DOI:10.1007/s11356-020-11032-3.
- K. Sleeman, V. P. Mishin, V. M. Deyde, Y. Furuta, A. I. Klimov and L. V. Gubareva, Antimicrob. Agents Chemother., 2010, 54, 2517–2524 CrossRef CAS PubMed.
- Y. Furuta, B. B. Gowen, K. Takahashi, K. Shiraki, D. F. Smee and D. L. Barnard, Antiviral Res., 2013, 100, 446–454 CrossRef CAS PubMed.
- A. B. Janowski, H. Dudley and D. Wang, J. Clin. Virol., 2020, 123, 104247 CrossRef CAS PubMed.
- R. H. Müller, C. Jacobs and O. Kayser, Adv. Drug Delivery Rev., 2001, 47, 3–19 CrossRef.
- A. Gupta, in Nanoparticles for Biomedical Applications, Elsevier, 2020, pp. 371–384 Search PubMed.
- R. R. Bhosale, R. A. Osmani, P. P. Ghodake, S. M. Shaikh and S. R. Chavan, Indian J. Pharm. Biol. Res., 2014, 2, 122–127 CrossRef.
- A. Sharifi-Rad, J. Mehrzad, M. Darroudi, M. R. Saberi and J. Chamani, J. Biomol. Struct. Dyn., 2020, 1–15 CrossRef PubMed.
- L. N. de M. Ribeiro and B. B. Fonseca, Future Microbiol., 2020, 15, 1571–1582 CrossRef PubMed.
- C. G. Kim, Y.-C. Kye and C.-H. Yun, Pharmaceutics, 2019, 11, 612 CrossRef CAS PubMed.
- J. Xu, J. Lv, Q. Zhuang, Z. Yang, Z. Cao, L. Xu, P. Pei, C. Wang, H. Wu and Z. Dong, Nat. Nanotechnol., 2020, 15, 1043–1052 CrossRef PubMed.
- Y. Qiao, J. Wan, L. Zhou, W. Ma, Y. Yang, W. Luo, Z. Yu and H. Wang, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2019, 11, e1527 Search PubMed.
- W.-m. Yin, Y.-w. Li, Y.-q. Gu and M. Luo, Acta Pharmacol. Sin., 2020, 41, 902–910 CrossRef CAS PubMed.
- T. T. H. Thi, E. J. A. Suys, J. S. Lee, D. H. Nguyen, K. D. Park and N. P. Truong, Vaccines, 2021, 9, 359 CrossRef PubMed.
- N. C. Kyriakidis, A. López-Cortés, E. V. González, A. B. Grimaldos and E. O. Prado, npj Vaccines, 2021, 6, 1–17 CrossRef PubMed.
- L. Han, K. Peng, L.-Y. Qiu, M. Li, J.-H. Ruan, L.-L. He and Z.-X. Yuan, Front. Pharmacol., 2021, 12, 1149 Search PubMed.
- J. T. Granados-Riveron and G. Aquino-Jarquin, Biomed. Pharmacother., 2021, 111953 CrossRef.
- R. Noor, Current Clinical Microbiology Reports, 2021, 1–8 Search PubMed.
- E. H. Pilkington, E. J. A. Suys, N. L. Trevaskis, A. K. Wheatley, D. Zukancic, A. Algarni, H. Al-Wassiti, T. P. Davis, C. W. Pouton, S. J. Kent and N. P. Truong, Acta Biomater., 2021 DOI:10.1016/j.actbio.2021.06.023.
- M. N. Vu, H. G. Kelly, H.-X. Tan, J. A. Juno, R. Esterbauer, T. P. Davis, N. P. Truong, A. K. Wheatley and S. J. Kent, Adv. Healthcare Mater., 2021, 10, 2002142 CrossRef CAS PubMed.
- A. Tewabe, A. Abate, M. Tamrie, A. Seyfu and E. A. Siraj, Journal of Multidisciplinary Healthcare, 2021, 14, 1711 CrossRef PubMed.
- M. N. Vu, H. G. Kelly, A. K. Wheatley, S. Peng, E. H. Pilkington, N. A. Veldhuis, T. P. Davis, S. J. Kent and N. P. Truong, Small, 2020, 16, 2002861 CrossRef CAS PubMed.
- T. C. Jackson, N. M. Obiakor, I. N. Iheanyichukwu, O. O. Ita and A. S. Ucheokoro, J. Pharm. Pharmacol., 2021, 9, 127–132 Search PubMed.
- T. T. Hoang Thi, E. H. Pilkington, D. H. Nguyen, J. S. Lee, K. D. Park and N. P. Truong, Polymers, 2020, 12, 298 CrossRef PubMed.
- D. Zukancic, E. J. A. Suys, E. H. Pilkington, A. Algarni, H. Al-Wassiti and N. P. Truong, Pharmaceutics, 2020, 12, 1068 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2021 |