Polyhedral oligomeric silsesquioxane-based hybrid materials and their applications
Hui
Zhou
a,
Qun
Ye
a and
Jianwei
Xu
*ab
aInstitute of Materials Research and Engineering, Agency for Science, Technology and Research (A*STAR), 2 Fusionopolis Way, Innovis, #08-03, 138634, Singapore. E-mail: jw-xu@imre.a-star.edu.sg
bDepartment of Chemistry, National University of Singapore, 3 Science Drive 3, 117543, Singapore
First published on 4th August 2016
Abstract
POSS are nano-sized stable 3-dimensional architectures which consist of alternate Si–O bonds to form cage structures with Si atoms as vertices. The mono- or multi-functional POSS could be prepared through modification of their reactive organic functional groups attached in the vertex positions. Depending on the structure and reactivity of those vertex groups, POSS are allowed to be introduced into virtually any existing polymer systems. This review paper summarizes the recent development on the preparation and properties of POSS-containing polymers. In addition, this review paper also gives an overview of the recent development in the area of a wide range of POSS-based composite systems for a variety of applications including fluorescence sensors, liquid crystals, photoresist materials, low dielectric constant materials, organic semiconductors, energy-related materials, drug and gene delivery systems, coating materials and special rubber materials.
1. Introduction
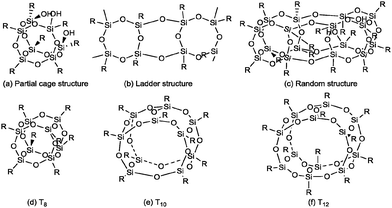
The silsesquioxane chemistry has been studied for more than half a century, but interest in this field has increased dramatically in recent years.1 The term silsesquioxane refers to the molecules, whose chemical structures follow the basic composition of (RSiO1.5)n. R is the vertex group for polyhedral molecules, including hydrogen, alkyl, alkene, aryl, arylene, etc.2 The molecular architecture of silsesquioxanes can be classified into two major categories: (I) non-caged structure and (II) caged structure. As shown in Scheme 1, the non-caged silsesquioxane molecules can be further classified into: (a) partial cage structure; (b) ladder structure, and (c) random structure.3 Cage-like silsesquioxanes are usually called polyhedral oligosilsesquioxanes or polyhedral oligomeric silsesquioxanes, abbreviated as POSS. This class of well-defined, highly symmetric molecules usually feature a nanoscopic size, approximately 1 to 3 nm in diameter when the vertex groups are included, which could be regarded as one of the smallest possible silica particles.4 POSS molecules with a T8 cubic inorganic core composed of silicon–oxygen (R8Si8O12) linkages are the most prevalent system studied. In this review paper, “T” and “Q” refer to the conventional nomenclature from the silicon NMR literature, with T and Q referring to silicon atoms bonded to three or four oxygen atoms, respectively.5Unlike traditional organic compounds, POSS derivatives are nonvolatile, odorless and environmentally friendly. The incorporation of POSS moieties into a polymeric material can dramatically improve its mechanical properties (e.g., strength, modulus, rigidity) and reduce its flammability, heat evolution, and viscosity during processing. These enhancements apply to a wide range of commercial thermoplastic polymers, high performance thermoplastic polymers, thermosetting polymers and other functional materials.4–6
Based on the dimensionality, nano-fillers can be classified as 1D, 2D, or 3D, depending on their geometrical symmetry. POSS are like other highly symmetric molecules, being roughly spherical and interacting with the polymer host in the 3D of the surrounding space. Due to their dispersion capability at the molecular level, POSS with non-reactive groups (Scheme 2), such as methyl, isobutyl, cyclopentyl, cyclohexyl, phenyl, are studied as nanoscopic fillers.7 Furthermore, one or more vertex groups can be substituted by a functional group through conventional chemical conversions. These versatile functional groups, such as methacrylate, acrylate, styrene, norbornene, amine, epoxy, alcohol, and phenol, provide possibilities to incorporate POSS into a polymer chain or network through polymerization or grafting (Schemes 3 and 4). Benefited by the development of polymer chemistry, the introduction of POSS into the polymer matrix can be more precisely controlled via chemical methodologies, for example, tethering POSS along the polymer chain by controlled polymerization, such as ATRP, RAFT, etc.8 With these developed polymerization strategies at hand, a large diversity of POSS–polymer architectures are possible to build up.
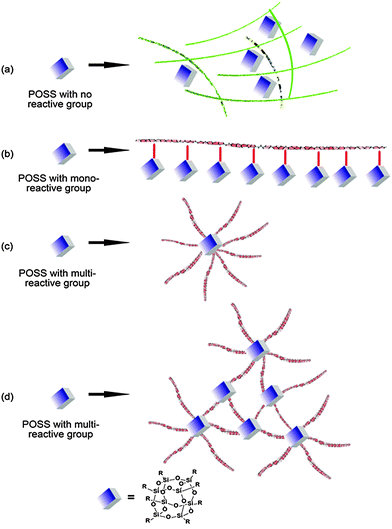
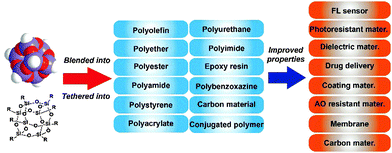
Typically, POSS could be integrated into composites by four main approaches (Fig. 1): (a) POSS with non-reactive groups as a blender in a polymer network; (b) mono-functional POSS as a tethering macromolecule to graft onto a polymer backbone, forming polymers with pedant POSS cages; (c) multi-functional POSS as the microinitiator to initiate polymerization from the surface of the POSS, giving a star-like macromolecule; (d) POSS with multi-reactive groups as a micro-initiator to produce a heavily cross-linked polymer network. POSS incorporated in the polymer matrix can change the matrix topology and subsequently tune its properties. From the microscopic view point, the nanoscopic size of the POSS molecule is comparable to the dimensions of polymeric segments in the condensed phase (molten or solid), yet nearly double the typical intermolecular spacing distance.9 The incorporation of POSS moieties into linear polymer chains or polymer networks will modify the local molecular interactions, local molecular topology, and the resulting polymer chain as well as segment mobility.10 The macroscopic physical properties manifest the microscopic modification, such as modulus, strength, glass transition temperature, thermal stability, and dimensional stability.
Recently, unique POSS structures have attracted the increasing attention of material scientists to investigate the structure–property relationship of such polymer composites. As nanosized building blocks, POSS and their derivatives have been widely studied for their self-assembly behaviour at the nanoscale. The directed self-assembly of POSS–polymer nanocomposites on the subnano/nanoscale is popular, and our latest review has focused on the recent developments on the self-assembly of POSS as well as their related applications.11a In addition, the chemistry of POSS,11b–f applications of POSS in nanocomposites,11g–i light-emitting diode materials,11j biomaterials11k–n and catalysis systems11o–r have been well documented in a lot of review papers and book chapters.11s However, it would be helpful to timely summarize the recent development of POSS-based hybrid materials on the host polymers, which may directly benefit material scientists to design and develop novel POSS-based hybrid materials with improved performance.
In this review paper, we have focused on the recent development of POSS in polymer composites, and their applications for high performance materials. Due to the limitation of space, most of the excellent research work done prior to the year 2010 is not included and only selected examples of recent work on POSS–polymer composites after 2010 are highlighted here (Fig. 2). This review paper summarizes the recent development in the preparation of POSS-embedded polymers, including polyolefin–POSS,12–15 polyether–POSS,16–21 polyester–POSS,22–29 polyamide–POSS,30–33 polystyrene–POSS,34–40 polyacrylate–POSS,11b,41–46 polyurethane–POSS,47–52 polyimide–POSS,53–58 epoxy resin–POSS,59–64 polybenzoxazine–POSS,65–71 carbon material–POSS,72–80 and conjugated polymer–POSS.81–82 In addition, this review paper also outlines the recent development in the applications of several high-performance POSS composite systems such as fluorescence sensors, liquid crystals, photoresist materials, low dielectric constant materials, organic semiconductors, energy-related materials, self-assembled block copolymers, drug or gene delivery systems, coating materials, special rubber materials, composite electrolytes, atomic oxygen resistant materials, piezoelectric polymers, thermoplastic elastomeric composites, catalytic systems, dielectric material for microelectronics, functional membranes and functional carbon materials.
2. POSS based polymers
2.1 Polyether–POSS
Polyethers are a type of hetero-chain polymer with oxygen linkages in the main chain, which exhibit semi-crystalline characteristics, including POM, PEO, PPO, PTMO, etc. Polyethers have two functional end groups, which enable reaction with functional parts in other molecules, such as POSS with reactive vertex groups, to form more complex structures (Table 1).Wang et al. reported a controlled cross-linking strategy to produce inorganic–organic hybrid hydrogels with tunable properties, which are formed by a disulfide-linked core/shell precursor with POSS and PEG shells.16 By applying thiol–disulfide exchange reaction with a pH-responsive ‘on/off’ function to control cross-linking reactions (Fig. 3a–d), hydrogels and aggregates with typical loose and compact structures are fabricated, wherein each POSS–(SS-PEG)8 molecule 1 (Fig. 3) is modeled as an isolated nano-cage with a 0.53 nm cubic core encircled by eight corner chains (up to 5 nm). Polu et al. studied the structural, thermal, mechanical and ionic conductivity properties of nanocomposite solid polymer electrolytes PEO/POSS–PEG 2 (Fig. 3) complexed with the LiN(–SO2CF3)2 (LiTFSI) salt.17 The incorporation of POSS–PEG restricted the segmental motion of polymer chains, and also improved the ionic conductivity of the solid polymer electrolyte to 5.05 × 10−5 S cm−1 at room temperature (23 °C) when 30 wt% of POSS–PEG was used. Kim et al. studied the effect of compositions and the PEG chain length of PEs 3 (Fig. 3) on the properties of the CE that consisted of a plasticizer (PEG-functionalized POSS derivatives), LiTFSI, and a hybrid star-shaped polymer SPP13 4 (Fig. 3) prepared by polymerization of poly(ethylene glycol) methyl ether methacrylate and methacryl–cyclohexyl–POSS.18 The CE in the solid state showed quite high ionic conductivity (4.5 × 10−5 S cm−1 at 30 °C), which was about three times larger than that of the matrix polymer (SPP13) electrolyte (1.5 × 10−5 S cm−1 at 30 °C). The CEs were electrochemically stable up to +4.2 V without decomposition. An all-solid-state lithium battery prepared from the CEs displayed a larger discharge capacity than that prepared from the SPP13 electrolyte at 60 °C. Wang et al. prepared the organic–inorganic nanocomposites by incorporation of a heptaphenyl POSS-capped PEO 5 telechelics into epoxy, which was synthesized via the Huisgen 1,3-dipolar cycloaddition between 3-azidopropylheptaphenyl POSS and α,ω-dialkynyl-terminated PEO.19 The incorporation of POSS-capped PEO telechelics significantly enhanced the surface hydrophobicity and reduced the surface free energy of nanocomposites, due to the enrichment of POSS cages at their surface. Le et al. reported the membranes made of Pebax/POSS 6, which were applied for ethanol recovery via pervaporation separation.20 The incorporation of octa(3-hydroxy-3-methylbutyldimethylsiloxy) (AL0136, POSS) nanoparticles improved the pervaporation performance of the hybrid membranes (Pebax/AL0136) significantly. At 2 wt% POSS loading, both the permeation flux and the separation factor of Pebax/AL0136 for ethanol/water reach the maximum values of 183.5 and 4.6 g m−2 h−1, respectively. Sharma et al. synthesized the ultra-low dielectric constant (k = 1.53) materials (POSS–PFCP) 7 with self-cleaning properties by incorporation of FD-POSS into PFCP.21 The increase in the weight percentages of FD-POSS resulted in a dramatic drop in the dielectric constant, as well as a significant increase in hydrophobicity and oleophobicity of POSS–PFCP composites. The ultra-low dielectric self-cleaning materials (θtilt = 38°) would be fabricated through an electrospun mixture of FD-POSS and PFCP polymers.
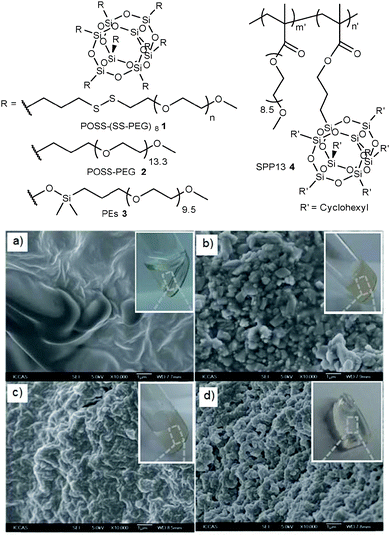 | ||
| Fig. 3 Schematic drawing of the chemical structure of POSS–(SS-PEG)81, POSS–PEG 2, PE 3 and SPP13 4. SEM images of the resulting hydrogels or aggregates after basification of POSS–(SS-PEG)81 solution for (a) 1, (b) 8, (c) 24 and (d) 240 h with addition of cysteamine. Adapted from ref. 16. Copyright 2013 Royal Society of Chemistry. | ||
2.2 Polyester–POSS
Aliphatic polyesters have received increasing attention owing to their biodegradability and biocompatibility. Among them, PCL and PLA are the most studied polyesters, which have potential as environmentally friendly packing materials and for biomedical applications.POSS-capped PCL has been synthesized via ROP using either monohydroxylated or multihydroxylated POSS and amino-functionalized POSS as the initiator. POSS molecules have also been incorporated into PLLA to obtain POSS containing PLLA hybrids using ROP initiated by hydroxy and amino-functionalized POSS (Table 2). Fan et al. reported the preparation of unimolecular micelles based on the hybrid copolymer POSS-(PAA-(PLLA-PEG)4)88 (Fig. 4), in which eight linear-dendritic-like arms poly(acrylic acid)–(poly(L-lactide)–poly(ethylene glycol))4 (PAA-(PLLA-PEG)4) are grafted onto an oligomeric silsesquioxane core. Polymer 8 was synthesized via the combination of single electron transfer living radical polymerization, ROP and thio-bromo “click” reactions.22 The TEM study revealed that the morphology of the self-assembled micelles takes a rod-like structure (Fig. 4a). Interestingly, the size of the micelles changes as the pH values vary, indicating its pH-responsive feature. This amphiphilic hybrid copolymer has been used as a good candidate for the controlled drug delivery of doxorubicin.
 | ||
| Fig. 4 Schematic drawing of the chemical structure of a multi-arm star-like block copolymer POSS-(PAA-(PLLA-b-PEG)4)88. (a) TEM images of the micelles formed from star-like amphiphilic copolymer 8. Adapted from ref. 22. Copyright 2015 Royal Society of Chemistry. (b) Preparation of sc-PLA/Pd fibers used as a catalyst in the Heck reaction. Adapted from ref. 29. Copyright 2014 American Chemical Society. | ||
| POSS | Composite | Incorporation type | Ref. | |
|---|---|---|---|---|
| 1 | Q-1 | POSS-(PAA-(PLLA-PEG)4)88 | Tethered | 22 |
| 2 | T-2 | O-POSS/PLA 9 | Blended | 23 |
| 3 | T-10 | Amino-POSS/PLLA 10 | Blended | 24 |
| 4 | T-10 | PLA-g-MA/POSS–NH211 | Tethered | 25 |
| 5 | T-11 | PLA-g-MA/POSS-OH 12 | Tethered | 25 |
| 6 | T-10 | PLLA-b-P(MA-POSS) 13 | Tethered | 27 |
| 7 | T-10 | PDLA-b-P(MA-POSS) 14 | Tethered | 27 |
| 8 | T-12 | MA-POSS/PLA 15 | Tethered | 28 |
| 9 | T-10 | POSS–PCL–POSS 16 | Tethered | 28 |
| 10 | T-10 | Amino-POSS/sc-PLA 17 | Blended | 29 |
Yilmaz et al. used the essential work of the fracture method to investigate the effect of the octavinylisobutyl based O-POSS addition to the PLA matrix on the fracture behaviour of O-POSS/PLA composites
9.23 The addition of O-POSS to PLA improved the toughness of O-POSS/PLA composites. Gardella et al. reported a catalytic system consisting of palladium nanoclusters homogenously dispersed on the surface of nanostructured polymer nanofibers (amino-POSS/PLLA)
10 based on PLLA and amino-functionalized POSS–NH2.24 The submicrometric dispersion of POSS–NH2 in the PLLA nanofibers fabricated by electrospinning could promote the formation of Pd nanoclusters, displaying relevant catalytic activity toward the hydrogenation of stilbene under heterogeneous conditions. Gardella et al. developed a method to prepare PLA and POSS-based bio-hybrid systems, which consist of a preliminary functionalization of the polymer matrix and a subsequent reaction with functionalized POSS to form (PLA-g-MA/POSS–NH2)
11 or (PLA-g-MA/POSS-OH)
12.25 The method could create maleic anhydride-grafted PLA (PLA-g-MA) and allow grafting of 0.7 wt% of MA onto the polymer backbone, avoiding a dramatic reduction of PLA molecular mass, while the reactivity of POSS-OH turned out to be much lower. By affecting the crystal nucleation density, the grafted POSS improved PLA crystallinity. The PLA-g-MA/POSS–NH2 films showed decreased surface water wettability. In order to improve the enhanced crystallization behaviour of aliphatic polyester-based nanocomposites, and thus enhance thermo-mechanical properties, Goffin et al. studied the effect of polyester-grafted POSS nanohybrids and neat POSS nanoparticles on the crystallization behaviour of their corresponding commercial polymeric matrices, including PCL and PLA.26 Polyester-grafted POSS nanohybrids were selectively synthesized through ROP of 3-caprolactone and L,L-lactide by amino-POSS, respectively. After melt-blending of POSS nanoparticles in the corresponding polymer, well-dispersed POSS nanoparticles acted as efficient nucleating sites, significantly increasing the crystallinity degree of both PCL and PLA matrices and thus improving thermo-mechanical properties of aliphatic polyester-based nanocomposites. To prepare stable hybrid nanoparticles in solution through self-assembly driven by the stereo-complexation between enantiomeric PLA chains in organic–inorganic hybrid diblock copolymers, Tan et al. synthesized hybrid diblock copolymers (PLLA-b-P(MA-POSS)
13 and PDLA-b-P(MA-POSS)
14) via ATRP of MA-POSS using either PLLA or PDLA as a macroinitiator.27 When compared to the mixture of PLLA and PDLA, the stereo-complexed nanoparticles prepared from 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 weight % of PLLA-b-P(MA-POSS) and PDLA-b-P(MA-POSS) exhibited significantly improved stability, which formed a stable hybrid nanoparticle dispersion for 30 days. The improved stability may be due to the bulky POSS nano-cages which sterically hinder the formation of larger nanoparticles formed by block copolymers. Ferńandez et al. studied the effects of covalent incorporation of POSS into polymers on their polymer thermal and surface properties, in which the telechelic POSS-containing PLA (POSS–PLLA)
15 and ditelechelic POSS-containing PCL (POSS–PCL–POSS)
16 were prepared by click coupling between alkyne moiety-functionalized POSS and azide end-functionalized PLLA and bis-azide end-functionalized PCL.28 After incorporation with POSS, both POSS–PLLA and POSS–PCL–POSS exhibited increased Tg and retarded crystallization as well. Surface hydrophobicity and thermal and thermal oxidative stability were also significantly enhanced after the introduction of POSS. Monticelli et al. reported an approach to functionalize electrospun stereocomplex PLA (sc-PLA)-based fibers (amino-POSS/sc-PLA)
17 by introducing functionalized POSS into the electrospinning solution containing PLLA and PDLA.29 The amino groups of the sc-PLA fibers functionalized by POSS–NH2 could promote specific interactions with a metal precursor, i.e., PdCl2, which forms metal nanoclusters, homogeneously dispersed on the fiber surface after subsequent reduction (Fig. 4b). In addition, the sc-PLA fibers exhibited higher thermal and chemical resistance when compared to those based solely on PLLA, which broadened the applications of the catalytic system. With those advantages, the sc-PLA/Pd fibers displayed high activity in the Heck reaction, with easy recovery and reuse for multiple catalytic cycles.
50 weight % of PLLA-b-P(MA-POSS) and PDLA-b-P(MA-POSS) exhibited significantly improved stability, which formed a stable hybrid nanoparticle dispersion for 30 days. The improved stability may be due to the bulky POSS nano-cages which sterically hinder the formation of larger nanoparticles formed by block copolymers. Ferńandez et al. studied the effects of covalent incorporation of POSS into polymers on their polymer thermal and surface properties, in which the telechelic POSS-containing PLA (POSS–PLLA)
15 and ditelechelic POSS-containing PCL (POSS–PCL–POSS)
16 were prepared by click coupling between alkyne moiety-functionalized POSS and azide end-functionalized PLLA and bis-azide end-functionalized PCL.28 After incorporation with POSS, both POSS–PLLA and POSS–PCL–POSS exhibited increased Tg and retarded crystallization as well. Surface hydrophobicity and thermal and thermal oxidative stability were also significantly enhanced after the introduction of POSS. Monticelli et al. reported an approach to functionalize electrospun stereocomplex PLA (sc-PLA)-based fibers (amino-POSS/sc-PLA)
17 by introducing functionalized POSS into the electrospinning solution containing PLLA and PDLA.29 The amino groups of the sc-PLA fibers functionalized by POSS–NH2 could promote specific interactions with a metal precursor, i.e., PdCl2, which forms metal nanoclusters, homogeneously dispersed on the fiber surface after subsequent reduction (Fig. 4b). In addition, the sc-PLA fibers exhibited higher thermal and chemical resistance when compared to those based solely on PLLA, which broadened the applications of the catalytic system. With those advantages, the sc-PLA/Pd fibers displayed high activity in the Heck reaction, with easy recovery and reuse for multiple catalytic cycles.
2.3 Polyamide–POSS
PA are a class of polymers that contain amide linkages along their macromolecular contour. Typically, the amide bond is formed through condensation reaction of a carboxylic group and an amine group. PA can be categorized as natural type and artificial type. The natural PA can be widely found in the natural world as proteins, including wool, silk, etc. Artificial PA can be generated through chemical polymerization, such as nylons, aramids and poly(N-isopropyl acrylamide). Owing to their high durability and strength, the artificial PA are widely used in textiles, the automotive industry, carpets and sportswear. The transportation industry is the major consumer of PA, which accounts for 35% of all PA consumption. POSS are commonly incorporated into PA to restrict the mobility of the respective PA (Table 3).Kuo et al. synthesized a series of POSS–helical polypeptide copolymers through the ROP of γ-benzyl-L-glutamate N-carboxyanhydride and aminopropyl isobutyl-POSS (Fig. 5).30 The self-assemblies of the copolymers were investigated and Fig. 5a presents TEM images of fibrous structures formed from the pure POSS–PBLG56 copolymer 18. The PBLG53 gel featured irregular fiber aggregation due to fiber intertwining caused by strong dipolar π–π interactions between the phenyl groups and the PBLG rods. While the POSS–PBLG56 gel featured ordered micro-fibers due to the POSS incorporation, the POSS–PBLG copolymer self-assembled in an anti-parallel manner to minimize steric hindrance between the POSS blocks. The polarized optical microscope images indicated smectic ordering of the POSS–PBLG56 copolymers in solutions. Thus, hierarchical self-organization could be useful for biomimetic applications and provide a new method for the construction of micrometer-scale materials.
 | ||
| Fig. 5 (a) Schematic drawing of the chemical structure of POSS–PBLG copolymers (left), TEM images of POSS–PBLG5618 (middle), and the nanoribbon formed in the network structure of the toluene gel of POSS–PBLG56. Adapted from ref. 30. Copyright 2009 American Chemical Society. (b) Schematic drawing of the chemical structure of nylon 6 and amino-POSS. Computer modulation to illustrate the structure of nylon 6–POSS blends. Adapted from ref. 33. Copyright 2012 American Chemical Society. | ||
Cost-effective and environmentally-friendly POSS/PA bionanocomposite 19 membranes with no cracks or voids were prepared by Gnanasekaran et al. for gas transport studies, which indicated that POSS concentration significantly affected the physical and gas transport properties of the membranes.31 The improved properties are due to the increased Tg, surface roughness and density of the membranes, and the decreased fractional free volume and surface free energy of the membranes upon increasing the POSS content. With those advantages, those POSS/PA materials displayed potential for application in the food packaging and the petrochemical industries. Matsumoto et al. prepared aromatic PA dendrimers 20 up to the sixth generation with a high yield through condensation of octa(n-propylamine)–POSS and 3,5-bis(trifluoroacetamido)benzoic acid. The dendrimers have squashed shapes on the substrate even for the sixth generation.32 The fluorescence intensity of dendrimers increased with the increase in the generation number, and dendrimers terminated with trifluoroacetamide showed specific fluorescence emission at 445 nm under UV irradiation. In order to better understand the role of processing in polymer property enhancement, Milliman et al. prepared a series of melt-blends (Nylon 6/POSS) 21 from nylon 6 and an aminopropylisobutyl POSS (Fig. 5b).33 Compared to injection moulded blends, the melt-spun composite fibers of the same composition exhibit an enhanced modulus and strength, and reach the maximum property enhancement when 2.5 wt% POSS is loaded. The property improvements may be due to the POSS–polymer interactions on the molecular scale, which subsequently causes physical cross-linking in melt–spun composites.
2.4 Polystyrene–POSS
PS is a large-scale commercially produced polymer, which is synthesized through free radical polymerization of styrene. Due to its advantageous properties, such as easy processing and functionalization, good solubility in solvents, outstanding thermal stability and a gentle substrate as a supporter, PS is widely used in a number of aspects.PS is also a good polymer matrix for POSS-containing nanomaterials, both blended and tethered on the chains. POSS can be incorporated into PS using various methods to form hybrid PS with different molecular architectures (Table 4), including ATRP, RAFT, anion polymerization etc. In addition, a triblock copolymer with pendent POSS can be synthesized by a hydrosilylation reaction using commercial SBS. Li et al. reported a convenient method to prepare hybrid PS from mono-vinyl-substituted POSS and commercial PS via a straightforward Friedel–Crafts reaction (Fig. 6).34 The hybrid PS (POSS/PSS) 22 exhibits different properties from pristine PS, such as solubility, crystalline and thermal properties. POSS would be well-dispersed in the PS matrix at a nearly molecular level and no macrophase separation was observed. The hybrid PS could self-assemble into a series of spheres in ethanol, and the diameters of these spheres decreased with increasing POSS loading.
 | ||
| Fig. 6 Schematic drawing of the chemical structure of PS/POSS composites 22, PSMA/Ti-POSS–NH224, PSMA/POSS 25, PSMIPOSS-b-PS 26, and the giant surfactant with ACPOSS 27. TEM images of PSMIPOSS-b-PS 26 with different POSS content: (a) PSMIPOSS6-b-PS351, (b) PSMIPOSS15-b-PS174, (c) PSMIPOSS18-b-PS109, and (d) PSMIPOSS25-b-PS80. Adapted from ref. 38. Copyright 2014 Royal Society of Chemistry. | ||
POSS have been incorporated into PS nanocomposites to improve their resistance to photo-degradation.35 Under photo irradiation, the POSS–PS 23 developed a lower level of carbonyl and hydroxy groups with the same exposure time when compared to pristine PS, suggesting that POSS molecules play a protective role in probably extending the in-use lifetime of the polymeric matrix. The protection efficiency is largely dependent on the POSS structure, and in general the open-cage POSS-triols show a more pronounced protection efficiency than the closed-cage POSS, indicating the active role of free OH groups of POSS-triol in the PS stabilisation process. Cozza et al. developed a catalytic system derived from electrospun polymer nanofibers with modification of a metal-containing POSS, in which the titanium-based POSS with an amino group as a reactive side group was contacted with PSMA nanofibers.36 The PSMA/Ti–POSS–NH2 system 24 exhibited catalytic activity in the degradation of sulforhodamine B under UV light. A method for the preparation of surface modified polymer films was reported by Monticell et al., including POSS vaporization and reaction on the polymer surface.37 POSS–NH2 was successfully grafted on PSMA, which showed an efficient method to produce a POSS-rich polymer layer (PSMA/POSS) 25 on the vapor-treated surface. The thickness of those layers would be controlled by POSS diffusion and tailored by treatment time as well. Zhang et al. reported an efficient a one-pot synthesis method of the PSMIPOSS alternating copolymers and poly(styrene-alt-maleimide isobutyl POSS)-block-polystyrene (PSMIPOSS-b-PS) block copolymers 26via RAFT polymerization.38 By tuning the weight fraction of MIPOSS from 13% to 64%, the PSMIPOSS-b-PS block copolymers showed a series of short-range order phase transitions from the POSS sphere, POSS cylinder to lamella structures (Fig. 6a–d). Using thiol-Michael “click” chemistry, a giant surfactant with ACPOSS head was conveniently constructed from commercially available acrylo POSS and PS by Li et al.39 Various functional thiols were attached onto the head of the ACPOSS–PS 27 conjugate by thiol-Michael and thiol–ene reactions. The thiol molecules include 2-mercaptoethanol, 1H,1H,2H,2H-perfluoro-1-decanethiol, 1-thio-β-D-glucose tetraacetate, and 2-naphthalenethiol. The advantages of the thiol-Michael chemistry, such as mild reaction conditions, high efficiency, and broad functional group tolerance, would expand the scope of POSS-based giant surfactants for their application in nanofabrication. Tan et al. studied the rheological behavior of three-arm and six-arm star PS (SPS) with a small amount of POSS.40 The SPS/POSS composites 28 showed reduced melt viscosity in the unentangled SPS matrix, and increased viscosity in the entangled SPS matrix. The Ma of SPS played a key role in the melt viscosity of the composites. When the Ma of SPS was smaller than the critical molecular weight for entanglement of PS, the melt viscosity of the SPS/POSS composites with a low POSS content was lower than that of pure SPS. The SPS/POSS composites were consistent with the Cox–Merz empirical relationship when the POSS content was low (1 wt%).
2.5 Polyacrylate–POSS
Polyacrylates are a type of polymer synthesized from various acrylate monomers. The most commonly used and studied is PMMA, which is a transparent polymeric material possessing many desirable properties including light weight, high light transmittance, chemical resistance, colorlessness, resistance to weathering corrosion, and good insulating properties. Due to their high technological importance, similar to PS, they have been employed as a matrix for a variety of functional materials. In general, an acrylate substituted POSS monomer can be synthesized to contain one or more polymerizable functional groups. By polymerization, POSS has been incorporated into polyacrylate to form new materials with desired properties (Table 5). For example, linear polymers with incorporated POSS tended to prevent or reduce segmental segregation or mobility; therefore the resultant materials are generally transparent and brittle. In addition, glass transition and decomposition temperatures of materials will increase upon increasing the POSS content.Recently, the polyacrylate–POSS based porous materials have been well explored for diverse applications, such as gas storage, gas separation, sensing and superhydrophobic coating. We have developed a new type of AIE active copolymer (polyacrylate–POSS) 29 for explosive vapour detection (Fig. 7). As shown in Fig. 7a, the porous films were readily fabricated by electrospinning with a concentration of 2% in acetone–chloroform. Unlike fluorescent conjugated polymers, the porous AIE polymer films showed less dependence of fluorescence response to nitro-compounds on film thickness even though they have a thickness as high as 560 ± 60 nm. This is due to the presence of POSS moieties, facilitating the formation of porous structures and subsequently leading to a large response to explosive vapour (Fig. 7b). Moreover, less dependence of response to nitro-compounds on film thickness avoids a tedious film fabrication method to control film thickness. Finally, electrospun AIE active films displayed remarkable fluorescence quenching sensitivity to TNT and DNT vapour compared to the corresponding dense films, making them promising for potential applications in the detection of explosives.
 | ||
| Fig. 7 Schematic drawing of the chemical structure of AIE polyacrylate–POSS polymers 29, hybrid branched (BCPs)-graft copolymers 32, linear (LCPs)-graft copolymers 33, poly(PEGMA–PPGMA–POSSMA) (PEPS) 34, and star-shaped POSS-containing diblock copolymers with 16-arms 35. (a) SEM images of films F4 fabricated by 29. The inserted photo in (b) is the fluorescence image of the film F4 taken under a fluorescence microscope with a 337 nm excitation. (b) Time-dependent fluorescence quenching of porous film F4 upon exposure to saturated vapour of nitro compounds. The insets display the photos of F4 upon exposure to analyte saturated vapour under UV light (365 nm) illumination. Adapted from ref. 41. Copyright 2014 Royal Society of Chemistry. | ||
Yuan et al. prepared a series of star-shaped hybrid P(2-(2-methoxyethoxy)ethylmethacrylate)-co-oligo(ethylene glycol) methacrylate (P(MEO2MA-co-OEGMA)) polymers with the POSS core (POSS-(P(MEO2MA-co-OEGMA))8) 30 by ATRP.42 Owing to the amphiphilic properties, the hybrid polymers would self-assemble into micelles in an aqueous solution. Altering the ratio of MEO2MA and OEGMA in hybrid polymers led to the increase in the responsive temperature of hybrid polymeric micelles from 29.7 to 39.1 °C. Thus, these amphiphilic hybrid polymers have been regarded as potential materials for applications in nano-carriers, nano-reactors, smart materials and biomedical fields. Ramirez et al. prepared the first examples of covalently bound F-POSS nanocomposites though RAFT polymerization of the F-POSS macromer with MMA, which possessed excellent wetting-resistant behaviour and improved surface robustness.43 The cotton fabrics coated with F-POSS/MMA copolymers 31 displayed superhydrophobic and oleophobic behaviour, indicating their potential value for application in low surface energy materials. Shim et al. synthesized a series of hybrid branched (BCPs) 32 and linear (LCPs)-graft copolymers 33 though RAFT polymerization of PEGMA, MA-POSS and EGDMA, which were applied as SPE materials in high-temperature lithium-ion batteries.44 Both BCPs and LCPs showed dimensional stability when MA-POSS contents were higher than 21 mol%, which maintained their original shape and storage modulus even if the temperature increased up to 90 °C. However, the BCP electrolyte exhibited a much higher ionic conductivity value (1.6 × 10−4 S cm−1 at 60 °C) than that of the LCP electrolyte (5.6 × 10−5 S cm−1 at 60 °C) under the same conditions, due to the higher chain mobility of the branched-graft copolymer electrolyte. All-solid-state batteries with the BCP electrolyte displayed reasonable cell performance without safety problems at 60 °C, which showed the high potential of BCPs as SPE materials for high-temperature batteries. Li et al. prepared a series of thermo-responsive amphiphilic hybrid copolymers through ATRP of PEGMA and MA-POSS together with PPGMA.45 The poly(PEGMA–PPGMA–POSSMA) (PEPS) 34 hybrid copolymers in aqueous media could form core–shell structures and possess a thick hydration layer. The micelles of PEPS showed a more enhanced stability than their counterparts without POSS. The thermo-responsiveness of the PPG brush in PEPS hybrid micelles could be used to spontaneously capture and protect the unfolded protein for preventing undesirable proteins from aggregation during the heat-induced denaturation process, in which the protection efficiencies for GFP, lipase and lysozyme were 81.4%, 89.3% and 88.7%, respectively. Considering those advantages together with the good cell biocompatibility, the PEPS hybrid micelles displayed high potential as artificial chaperones for protecting unfolded proteins from toxic aggregation at high temperatures. The star-shaped POSS-containing diblock copolymers with 16-arms (s-POSS-PMMA-b-P(MA-POSS)) 35 were synthesized by Pan et al. from MMA and MA-POSS using octakis(dibromoethyl) POSS (POSS-(Br)16) as a cetylfunctional initiator.46 The incorporation of MA-POSS obviously enhanced hydrophobic/oleophobic performance and thermal stability of copolymers, and also significantly improved the surface roughness of films. Due to the effect of solvents on the self-assembled films, those diblock copolymers could be used as a solvent dependent coating material.
2.6 Polyurethane–POSS
PU is a copolymer containing urethane bonds along its macromolecular structure. The urethane bonds are formed through condensation reaction of diols and diisocyanates. In most of the common structures, the PU chain is composed of two segments, namely the soft segments made by a single flexible macrodiol, and the hard segments made by condensation of a diisocyanate and a short rigid diol. The immiscibility of those two segments results in the microphase separated morphology of PU. Typically, the DMS depends on the chemical nature of the components, their molecular weight and the thermal properties. The DMS would be tuned by choosing the building blocks for segments, including diisocyanates, chain extenders and macrodiols. The segmental dynamics of PU is significantly affected by the DMS. Generally, low DMS materials show slower dynamics (higher Tg) than their highly phase separated counterparts. Thus, the effect of POSS on microphase separation may be crucial for the molecular mobility of PU hybrids. By substitution of either a chain extender or a macro-diol with a POSS moiety with two OH groups, POSS would be incorporated into the PU hybrid. In addition, POSS with eight OH or OCN reactive vertex groups are widely used for the formation of PU hybrids with a cross-linked structure (Table 6).Mahapatra et al. reported the synthesis of thermoplastic HBP elastomer hybrids containing POSS (HBP–POSS) 36 by the A2 + B3 approach.47 These hybrid nanomaterials with different compositions were obtained by tuning the ratio of POSS-diol, triethanolamine, PCL-diol, and 4,4′-methylenebis(phenyl isocyanate) together with a chain extender. The SEM images indicated that the HBP matrix with non-agglomerated homogeneous dispersion could be obtained through covalent attachment of POSS (Fig. 8a–c). Nevertheless, after the introduction of POSS into the hybrids, the mechanical properties and thermal stability of HBP are significantly improved relative to hybrids without POSS (Fig. 8d). Sasi Kumar et al. prepared an interlayer low-k dielectric material for microelectronics applications, which was based on POSS reinforced PU–PBZ nanocomposites (POSS–PU–PBZ) 37. The incorporation of POSS into the polymer matrix significantly reduced the value of the dielectric constant.48 However, the reduction of the dielectric constant by POSS is limited up to its concentration in POSS–PU–PBZ, and beyond 30% (k 1/4 1.94) the reverse trend was observed due to agglomeration of POSS nanoparticles. The thermal stability of the resulting POSS reinforced PU–PBZ nanocomposites also increased to an appreciable extent. Lungu et al. used DSC to follow the degree of conversion for the methacrylic groups in the case of some hybrid systems (UDMA–POSS) 38 based on the urethane UDMA monomer and various POSS structures (HISO–POSS, CPENTYLPOSS, and MA-POSS).49 The chemical structure of the POSS compounds would directly influence the DC value. The introduction of the POSS compound within the UDMA matrix leads to a decrease of the hybrid material transparency due to the formation of agglomerates. Przdka et al. reported hybrid elastomers (PU-NCOPOSS) 39, in which NCOPOSS was used as the crosslinking agent for the PU systems.50 The incorporation of a small amount (1.2–2.5 wt%) of NCOPOSS into the formulation resulted in an improvement or deterioration in the mechanical properties of the polymer, depending on the diisocyanate used. A further increase in the NCOPOSS concentration resulted only in the loss of mechanical properties because of an over-crosslinking or wastage of POSS-deriving NCO groups in the reaction. Bakhshi et al. developed a new nanocomposite polymer with POSS and PCU, which is an anti-thrombogenic and a non-biodegradable polymer with in situ endothelialization properties.51 The nitinol (NiTi) surface coated with POSS–PCU 40 exhibited enhanced surface resistance and improved biocompatibility. In addition, the modified NiTi showed far greater resistance to decay in peel strength compared to the non-modified NiTi. Zhang et al. investigated the gelation process of N-phenylaminomethyl-POSS/PU nanocomposites 41 during curing and in the stable state after curing by rheology.52 Rising curing temperature or increasing POSS concentration leads to a decrease in the gelation time of composites and the gel stiffness. After the completion of the curing reaction, the critical concentration of POSS beyond which the gelation of POSS/PU composites would occur was found to be around 2.5 wt%. The incorporation of multifunctional POSS into PU increased the homogeneity of crosslinking POSS/PU composites.
 | ||
| Fig. 8 Schematic drawing of the chemical structure of thermoplastic hyperbranched polyurethane HBP 36. FE-SEM cross-sectional images of 36: (a) HBP–POSS2.5, (b) HBP–POSS5.0, and (c) HBP–POSS7.5 hybrids. (d) Moduli of (I) pure HBP, (II) HBP–POSS2.5, (III) HBP–POSS5.0 and (IV) HBP–POSS7. Adapted from ref. 47. Copyright 2012 Elsevier Ltd. | ||
2.7 Polyimide–POSS
Due to their excellent mechanical properties and high temperature durability, PI have been widely used in spacecraft external surfaces as thermal blankets in solar arrays, and space inflatable structures. However, protective layers are still widely used to protect PI from AO. POSS with various functionalities have been used as simultaneous enhancement materials in PI-based nanocomposites to improve their thermo-mechanical and AO resistant properties (Table 7).11f,53 Upon AO exposure, POSS-PI would form a SiO2 passivating layer to prevent AO from eroding the bulk matrix, and thus increase AO resistance. Diamine POSS and octaamine POSS are common candidates for the preparation of POSS-PI.Lei et al. reported the synthesis of a diamine POSS and its incorporation into PI molecular backbones to fabricate POSS-PI 42 through co-polymerization (Fig. 9).54 As shown in SEM images (Fig. 9a–c), upon AO exposure, the surface of pristine PI becomes significantly roughened and displays a ‘carpet-like’ morphology, while PI with 21.9 wt% POSS exhibit less root-mean-square surface roughness. PI with a higher POSS amount are likely to form a much denser and more connected silica passivating layer on the membrane surface after AO exposure, which significantly improves the AO durability of POSS-PI. Finally, a hybrid membrane with 29.7 wt% POSS loading shows the best atomic AO resistance with the lowest erosion yield of 0.9 × 10−25 cm3 per atom. Thus, POSS increases AO survivability of the resulting hybrid materials, and at the same time, the PI main chain provides desirable thermal stability and mechanical strength. With both of the above advantages, POSS-PI may find wide uses as surface protective materials onboard spacecraft to resist AO attack in the low earth orbit environment.
 | ||
| Fig. 9 Schematic drawing of the chemical structure of POSS-PI 42, linear sulfonated PI 45, and glycidyl–POSS hybrid membranes 46. Select SEM images of polyimide membranes 42 after exposure to an atomic oxygen fluence of 3.87 × 1020 O atoms per cm2: (a) pristine polyimide, (b) 8.8 wt% POSS polyimide, (c) 21.9 wt% POSS polyimide. Note: the scale bar at the bottom of each image indicates 1 µm. Adapted from ref. 54. Copyright 2014 Elsevier Ltd. | ||
Duo et al. prepared a PI-POSS hybrid nanocomposite by copolymerization of trisilanolphenyl-POSS, 4,4′-oxydianiline, and pyromellitic dianhydride.55 The introduction of POSS into the PI could dramatically improve the AO resistance of PI films in the low earth orbit environment. For example, the erosion yield of the hybrid film with 20 wt% PI/POSS 43 was 1.2 × 10−25 cm3 per atom, which is equivalent to 4.3% of the pure PI film. Le et al. prepared a novel miscible polymer blend of copoly(1,5-naphthalene/3,5-benzoic acid-2,2′-bis(3,4-dicarboxy phenyl))hexafluoropropanedimide (6FDA-NDA/DABA) and its sulfonated PI (POSS-PIs) 44, which has been applied as a water-selective layer to develop dual-layer hollow fiber membranes for ethanol dehydration via pervaporation.56 POSS modification is the most effective way to enhance the membrane selectivity due to the anti-swelling properties of POSS inorganic nanoparticles, mobility restriction of polymer chains and narrowed d-space in a polymer structure. Compared to various membranes in the literature, the hollow fibers have superior fluxes of 42.0 kg m−2 h−1 and a good separation factor of 4200 for ethanol dehydration. Wu et al. reported exchange membranes through introducing phenyl8–bisaniline–POSS into the main backbone of linear sulfonated PI (SPI's) 45 at concentration levels of 3, 5 and 10 mol%.57 Compared to other POSS hybrid membranes fabricated using direct blending or cross-linking methods, the ultrafine POSS aggregates show a more uniform distribution and the obtained POSS–SPI copolymers exhibit improved properties as proton exchange membranes. The POSS–SPI membranes with 5 and 10 mol% POSS show higher conductivity in comparison to the POSS-free SPI (0.114 and 0.132 S cm−1vs. 0.0998 S cm−1) at 80 °C. Moreover, the obtained POSS–SPI membranes also possess enhanced oxidative and hydrolytic stabilities, a low swelling ratio combined with high water uptake, adequate thermal stability and mechanical properties, as well as low methanol permeability. Konietzny et al. reported the glycidyl–POSS hybrid membranes (glycidyl-POSS-PIs) 46, which are synthesized through cross-linking of glycidyl–POSS and polyimides.58 The membranes show lower fluxes due to cross-linking and higher selectivity at higher temperature.
2.8 Epoxy resin–POSS
Epoxy resins are widely used as thermosets, such as metal coatings, electronics/electrical components, high tension electrical insulators, fiber-reinforced plastic materials and structural adhesives. Typically, epoxy resins are formed through epoxy ring opening of diepoxide and diamine. POSS have been incorporated into epoxy resins as POSS-epoxies for improved properties, which would be classified into two major types in terms of functionality of POSS (Table 8). Mono-functional POSS-epoxies are incorporated into epoxy resin backbones to improve their thermal properties.59 Multifunctional POSS-epoxies are incorporated into the precursor polymer composed of di- and/or tetra-functionalized epoxies to form an epoxy resin network. The glass transition temperature of epoxy resins would be increased through POSS incorporation, because POSS cages enhance their ability to hinder the segmental motion of molecular chains and network junctions.60Wang et al. reported the synthesis of copolymer-poly(hydroxylether of bisphenol A) (PH-POSS) 47 through polymerization of diepoxy and bisphenol A, which contains POSS in the main chains (Fig. 10).61 By adjusting the ratio of 3,13-diglycidyloxypropyloctaphenyl double-decker silsesquioxane (denoted as diglycidyloxypropyl DDSQ) to DGEBA, the PH copolymers with variable contents of POSS in the main chains were obtained. DSC and TGA indicate the significant improvement of Tg and thermal stability for PH-POSS (Fig. 10a). In addition, the surface hydrophobicity of PH-POSS was significantly increased when compared to the reference PH, due to the incorporation of POSS into polymer main chains (Fig. 10b). Sethuraman et al. used NH2–POSS in different weight percentages (1%, 3% and 5% wt) to reinforce the DGEBA epoxy resin (nBGE–BDGE–POSS) 48, which was cured by diamines, including bisphenol-A based ether diamine, octane diol based ether diamine, and capron based diamine.62 The resulting epoxy matrix can be used in the form of coating, encapsulation, or sealant for different industrial and engineering applications for better performance and improved longevity. Ni et al. prepared hybrid copolymers (nBGE–BDGE–POSS) 49 by reaction of POSS–NH2 with n-butyl glycidyl ether (nBGE) and 1,4-butanediol diglycidyl ether (BDGE) with varied feed ratios.63 The resulting copolymers were further used to enhance the toughening and thermal properties of the epoxy resin. The enhancement was ascribed to the nano-scale effect of the POSS structure and the formation of the anchor structure in the cured network. Matĕjka et al. prepared the epoxy–POSS hybrid networks with POSS bound as pendant cages or with untethered POSS dispersed in the matrix.64 For the hybrids with tethered POSS (epoxy-POSS) 50, physical crosslinks in the pregel stage of the network build-up were developed in situ.
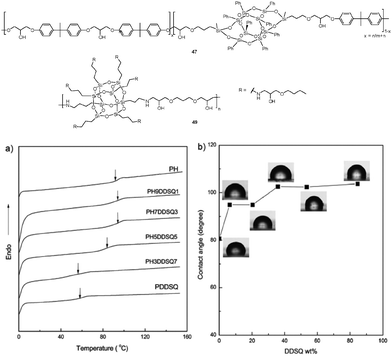 | ||
| Fig. 10 Schematic drawing of the chemical structure of poly(hydroxylether of bisphenol A) 47, and hybrid copolymers 49. (a) DSC curves of PH and POSS-containing PH copolymers 47. (b) Plot of surface water contact angles as a function of the content of POSS in copolymers 47. Adapted from ref. 61. Copyright 2011 Royal Society of Chemistry. | ||
2.9 Polybenzoxazine–POSS
PBZ are a class of thermosetting phenolic resins that have been recently developed for use in electronics, aerospace, matrices for composite materials, high performance adhesives, nano-imprinting technologies and other industries as an attractive alternative to other novolac-type phenolic resins. In order to tune the rigidity of PBZ in certain applications, copolymerization and chemical blending are used to toughen PBZ.65 Epoxy is a potential modifier for the PBZ matrix for toughening the rigid properties of PBZ. Polymers with silica coated surfaces exhibit good radiation resistance. However, inherent or debris-induced defects on the coating surface will permit UV penetration and undercut the underlying polymer substrate. Thus, it is necessary to develop new polymeric materials that can sustain such erosion and still function under the harsh conditions. Incorporation of POSS into the polymers through covalent bonding would be a promising approach to generate such functional polymers (Table 9).66Selvi et al. reported a method to develop semi-organic precursors, POSS reinforced PBZ/EP nanocomposites
51, which were obtained by thermal curing of POSS and the BZ/DGEBA EP matrix (Fig. 11). On varying the weight percentage of POSS (0, 1.0, 3.0, and 5.0%) to BZ and DGEBA EP (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, w/w), only the 5.0% POSS reinforced PBZ/EP displayed an obviously improved tensile strength.67 The SEM analysis shows that a passive inert silica protective layer has been formed after radiation, protecting the composite materials from further deterioration due to radiation (Fig. 11a–f). POSS reinforced PBZ/EP nanocomposites also possess better thermal and low dielectric properties than those of the neat PBZ/EP matrix. Huang et al. prepared a PBZ matrix (PBZ–POSS)
52 with improved thermal and mechanical properties through ring opening polymerization of allyl-functionalized benzoxazine and multifunctional POSS bearing eight vinyl-terminated benzoxazine groups (VBa–POSS).68 Other PBZ–POSS network nanocomposites were also synthesized by Wu et al. through polymerization of multifunctional benzoxazine POSS (OBZ–POSS), which was prepared from octa-azido functionalized POSS (OVBN3–POSS) with 3,4-dihydro-3-(prop-2-ynyl)-2H-benzoxazine (P-pa) via a click reaction.69 The improved thermal and mechanical properties of PBZ/POSS hybrid materials
53 are due to the incorporation of a large number of POSS units into their PBZs. Tseng et al. prepared PBZ–POSS nanocomposites
54 through copolymerization of furan-containing benzoxazine compounds and methylmethacrylate–POSS.70 POSS orientation reduces the dielectric constants of the nanocomposites to about 1.9, and thus the nanocomposites exhibit ultra-low dielectric constants. Chandramohan et al. reported the PBZ nanocomposites reinforced by octakis(dimethylsiloxypropylglycidylether)silsesquioxane (OG-POSS) (PBZ–OGPOSS)
55, which are prepared by ROP via thermal curing of benzoxazine precursors and OGPOSS (up to 10 wt%) and showed improved thermal stability.71
1, w/w), only the 5.0% POSS reinforced PBZ/EP displayed an obviously improved tensile strength.67 The SEM analysis shows that a passive inert silica protective layer has been formed after radiation, protecting the composite materials from further deterioration due to radiation (Fig. 11a–f). POSS reinforced PBZ/EP nanocomposites also possess better thermal and low dielectric properties than those of the neat PBZ/EP matrix. Huang et al. prepared a PBZ matrix (PBZ–POSS)
52 with improved thermal and mechanical properties through ring opening polymerization of allyl-functionalized benzoxazine and multifunctional POSS bearing eight vinyl-terminated benzoxazine groups (VBa–POSS).68 Other PBZ–POSS network nanocomposites were also synthesized by Wu et al. through polymerization of multifunctional benzoxazine POSS (OBZ–POSS), which was prepared from octa-azido functionalized POSS (OVBN3–POSS) with 3,4-dihydro-3-(prop-2-ynyl)-2H-benzoxazine (P-pa) via a click reaction.69 The improved thermal and mechanical properties of PBZ/POSS hybrid materials
53 are due to the incorporation of a large number of POSS units into their PBZs. Tseng et al. prepared PBZ–POSS nanocomposites
54 through copolymerization of furan-containing benzoxazine compounds and methylmethacrylate–POSS.70 POSS orientation reduces the dielectric constants of the nanocomposites to about 1.9, and thus the nanocomposites exhibit ultra-low dielectric constants. Chandramohan et al. reported the PBZ nanocomposites reinforced by octakis(dimethylsiloxypropylglycidylether)silsesquioxane (OG-POSS) (PBZ–OGPOSS)
55, which are prepared by ROP via thermal curing of benzoxazine precursors and OGPOSS (up to 10 wt%) and showed improved thermal stability.71
 | ||
| Fig. 11 Schematic drawing of the chemical structure of PBZ/EP/POSS nanocomposites 51, and OBZ-POSS 53. SEM images for before UV radiation of (a) neat PBZ/EP, (b) 1.0 wt% and (c) 5.0 wt% of PBZ/EP/POSS and after UV radiation of (d) neat PBZ/EP, (e) 1.0 wt% and (f) 5.0 wt% of PBZ/EP/POSS 51. Adapted from ref. 67. Copyright 2014 Royal Society of Chemistry. | ||
2.10 Conjugated polymer–POSS
Fluorescence imaging techniques have been widely used in biomedical imaging because of their multiple advantages, such high sensitivity, fast response, high emissive intensity and low cost. Their bio-imaging applications always suffer from photolimiting interference in biological media, such as scattering, absorption and auto-fluorescence. These limitations are expected to be overcome by employing FR/NIR fluorescent probes, because the emission region of FR/NIR (650–900 nm) can provide a unique solution to overcome the current limitation, including minimal interferential absorption, low biological autofluorescence and high tissue penetration. Conjugated polymers are macromolecules with π-conjugated backbones and are readily tailored with desired electrical and optical properties, such as high quantum yields and emission bands. However, conjugated polymers with FR/NIR fluorescence have rarely been used in real bio-imaging application because of their significantly reduced fluorescence quantum yields in aqueous media with respect to those in organic solvents due to severe aggregation and charge transfer induced fluorescence quenching. The introduction of rigid and bulky POSS moieties in organic luminescent dyes, luminescent oligomers or polymers promotes their quantum efficiencies in photoluminescence and electroluminescence, due to suppression of self-quenching in their aggregate state.Ruan et al. reported the synthesis of POSS containing organometallic polymers (P2-Co) 56 through a solid-state pyrolysis process, where POSS would aid in the formation of Co nanoparticles with uniform size (∼8 nm).81 Liu et al. reported the POSS-pendanted far-red/NIR fluorescent conjugated polymer poly{[9,9-di(hexyl)fluorene]-alt-co-[4,7-bis(thiophene-2-yl)-2,1,3-benzothiazole]} (PFDBT–POSS, Fig. 12) 57, which could form uniform nanoparticles under aqueous conditions using the matrix of DSPE–PEG2000 and DSPE–PEG2000–Mal.82 The obtained PFDBT–POSS–Mal NPs show maximum absorption at 536 nm and FR/NIR emission centered at 687 nm. By attachment of the POSS moieties onto the side chains, the fluorescence quantum yield of the conjugated polymer increased significantly from 2% to 13.6% in water, indicating that the presence of POSS could help mitigate fluorescence quenching. The obtained nanoparticle probe PFDBT–POSS-Affibody was successfully applied in the detection and cellular imaging of HER2-over expressed cancer cells using SKBR-3 as an example with low cytotoxicity and high photostability (Fig. 12a and b).
 | ||
| Fig. 12 Schematic drawing of PFDBT–POSS polymer 56, and PFDBTPOSS 57. CLSM fluorescence (a) and fluorescence/transmission overlay (b) images of fixed SKBR-3 cells incubated overnight with 5 nM PFDBT–POSS-Affibody NPs at 37 °C. All the images share the same scale bar of 30 mm. Adapted from ref. 82. Copyright 2013 Royal Society of Chemistry. | ||
2.11 Carbon materials-POSS
CF have been widely blended as reinforcements in composites, such as CF reinforced polymers or carbon composites in aerospace, marine and automobile industries, due to their high modulus, strong tensile strength, low density, excellent chemical resistance, high temperature tolerance and low thermal expansion. Typically, the CF reinforced polymer matrix composites contain three parts: reinforcing CF, the hosting matrix, and the interphase between the CF and the matrix. Among them, the physicochemical properties of the interphase contribute critically to the performance of composites. In addition, the poor wettability between CF and the polymer would result in weak interfacial adhesion between fibers and the matrix. Therefore, a lot of attempts, such as physical coating treatment and chemical grafting, have been reported to increase the surface wettability, surface roughness or chemical bonding aiming to improve the interfacial strength between the CF and the matrix. The roughness and wettability of the CF could be improved by grafting POSS onto the CF surface. Thus, the introduction of POSS cages can improve the mechanical properties of the composites including strength, modulus and rigidity through improving the interface adhesion of the composites. The introduced POSS can also reduce the flammability of the final finishing, heat evolution, and viscosity during processing.Jiang et al. reported the effective enhancement of the interfacial strength of CF reinforced unsaturated polyester resin (UPR) composites 58 through uniformly grafting methacryl and methacryl-isobutyl POSS on the CF surface by a silanization reaction (Fig. 13).72 The wettability and roughness of the CF grafted with both kinds of POSS were almost the same, while higher than that of the as-received CF. For the UPR composites reinforced with methacryl POSS (multifunctional) grafted carbon fibers, the ILSS, IFSS and the impact energy were dramatically increased compared with that of the UPR composites reinforced with methacryl-isobutyl POSS (monofunctional) grafted carbon fibers. The ILSS, IFSS and impact energy of the UPR composites reinforced with methacryl POSS grafted CFs were 67, 93 MPa and 1.72 J, respectively, much higher than those of the UPR composites reinforced with methacryl-isobutyl POSS grafted carbon fibers (62, 87 MPa and 1.43 J).
 | ||
| Fig. 13 Schematic of the grafting procedures of POSS grafted CF 58 and the image of the un-sizing CF. AFM three-dimensional images of the (a) as-received CF, (b) methacryl POSS T-7 grafted CF. Morphologies of the fractured surface of the UPR composites reinforced with (c) untreated CF and (d) T-7 grafted CF. Adapted from ref. 72. Copyright 2014 Royal Society of Chemistry. | ||
Zhao et al. reported the application of POSS as coupling agents to functionalize conventional carbon fibers, where POSS could be grafted uniformly onto the fiber surface, resulting in increased surface roughness.73 Wang et al. reported a convenient method to reduce and functionalize GO with refluxing GO and OapPOSS without the use of any reducing agents (Fig. 14).74 The adsorption and barrier effect of reduced graphene oxide inhibit the heat and gas release and promoted graphitized carbon formation. At the same time OapPOSS improves the thermal oxidative resistance of the char layer. Thus the synergistic effect of OapPOSS–rGO 59 dramatically reduces fire hazards. Li et al. prepared a composite (rGO–OapPOSS) 60 through polymerization of octa-methylmethacrylate-POSS and 1-allyl-3-methylimidazolium hexafluorophosphate ionic liquid on the filler of CNTs.75 The resulting composite showed a high dielectric constant and a low dielectric loss due to the in situ formed POSS coating layer on the CNT surface. Yadav et al. reported a simple and convenient approach to efficiently functionalize a wide variety of nanostructured materials on the surface of CNTs, in which a hybrid material consisting of POSS–CNT 61 was synthesized by entailing click coupling between azide moiety-functionalized POSS and alkyne-functionalized multi-walled CNTs.76 Yu et al. reported a graphene hybrid material functionalized with Oap-POSS (Oap-POSS-g-GO) 62.77 Besides the significantly improved thermal stability compared to GO (Td from 75 to 395 °C), the dielectric constant and dielectric loss of OapPOSS-g-GO/epoxy composites dropped by 9% and 49%, respectively, compared to the neat epoxy composites with only 0.2 wt% incorporation of OapPOSS-g-GO. Tang et al. prepared a hybrid nanopaper through a paper making process from CNF and/or clay, POSS and APP.78 The addition of clay in the hybrid paper increases the char residues of the nanocomposites, and the peak heat release rate (PHRR) decreases dramatically in the case of laminate composites incorporating CNF/clay/APP 63 hybrid paper. Nezakati et al. reported the preparation of an electrically conductive polymer (POSS–PCL/graphene) 64 through incorporation of POSS into a modified poly[caprolactone based urea-urethane] (PCL)/graphene hybrid nanocomposite.79 Multilayer graphene flakes (8 nm) were homogeneously dispersed into POSS–PCL with a concentration of 0.1, 2, 5, and 10 wt%. The impedance spectroscopy of 5.0 wt% and higher concentration of graphene in POSS–PCL represents major improvement in conductivity over pristine POSS–PCL. Zhao et al. prepared a carbon fiber/POSS/carbon nanotube (CF–POSS–CNT) 65 hybrid reinforcement by grafting CNTs onto the carbon fiber surface using octaglycidyldimethylsilyl POSS as the linkage.80 POSS and CNTs could be grafted uniformly on the fiber surface and significantly increase the fiber surface roughness. POSS and CNTs effectively enhance the interfacial adhesion of the composites by improving resin wettability, increasing chemical bonding and mechanical interlocking (Table 10).
 | ||
| Fig. 14 Schematic illustration of functionalized and reduced graphene oxide (OapPOSS–rGO) 59 by OapPOSS. Photograph of OapPOSS–rGO dispersions in tetrahydrofuran. TEM images of (a) GO, and (b) OapPOSS–rGO composites 59. Adapted from ref. 74. Copyright 2012 Royal Society of Chemistry. | ||
| POSS | Composite | Incorporation type | Ref. | |
|---|---|---|---|---|
| 1 | T-16 | P2-Co 56 | Tethered | 81 |
| 2 | T-16 | PFDBT–POSS 57 | Tethered | 82 |
| 3 | T-28, T-12 | CF-UPR 58 | Tethered | 72 |
| 4 | T-33 | rGO–OapPOSS 59 | Tethered | 74 |
| 5 | T-28 | POSS/iMWNT 60 | Tethered | 75 |
| 6 | T-15 | POSS–CNT 61 | Tethered | 76 |
| 7 | T-33 | Oap-POSS-g-GO 62 | Tethered | 77 |
| 8 | T-29 | CNF/clay/APP 63 | Tethered | 78 |
| 9 | T-PCL | POSS–PCL/graphene 64 | Blended | 79 |
| 10 | Q-5 | CF–POSS–CNT 65 | Tethered | 80 |
2.12 Other types of polymer–POSS
Polyolefins are produced commercially on a large scale by industry and widely used as basic matrices. Polyolefins are essentially macro-hydrocarbons, which are prepared by polymerization of alkenes, such as ethylene and propylene. POSS have been introduced into polyolefins to form polyolefin POSS composites or polyolefin POSS hybrid materials by many strategies, such as blending, ring-opening metathesis copolymerization and copolymerization (Table 11).Pan et al. reported a mesogen-jacketed liquid crystalline polymer (MJLCP, PNb10POSS) 66 prepared through ring-opening metathesis polymerization of norbornene, in which POSS are incorporated into the side chain of the polymer (Fig. 15).12 The phase behavior of PNb10POSS was investigated by several methodologies, such as DSC, polarized light microscopy, one-dimensional wide-angle X-ray scattering, synchrotron radiation small-angle X-ray scattering, two-dimensional wide-angle X-ray diffraction, and high resolution TEM. PNb10POSS shows various phase structures including an angstrom POSS (Cr), a hexagonal columnar (Colh) phase and the Cr coexisting, and the Colh phase at different temperatures, because of competitive self-assemblies between MJLCP and POSS moieties. With the tremendous effect of POSS crystals on the liquid crystalline behavior of the MJLCP, a MJLCP-based nanohybrid liquid crystalline polymer is produced with the complex phase behavior (Fig. 15a). The polymer self-assembles into an organic–inorganic hybrid inclusion complex on the sub-10 nm scale, which therefore provides a new approach for the design and synthesis of ordered structures constructed by self-assembly on the sub-10 nm scale (Fig. 15a). Xue et al. prepared POSS-containing EPDM composite fibers (EPDM/POSS) by electrospinning the melt-blended mixture of ph-POSS molecules and EPDM.13 The resulting EPDM/POSS composite fibers 67 exhibited a micron grade diameter and fibrous structural morphology. The incorporation of POSS significantly increases the thermal decomposition temperature of composite fibers and greatly reduces the total heat release when compared to pure EPDM. Those advantages would be useful to produce special rubber materials for high temperature application. PVDF is a kind of important piezoelectric polymer, which is widely used in the spacecraft industry. AO collision degradation is regarded as an important issue for PVDF used in spacecrafts. To better understand the PVDF erosion behaviors on AO impacts and to improve the PVDF stability against AO impacts, Zeng et al. introduced a POSS compound, FP-POSS, into the PVDF matrix. The PVDF/FP-POSS matrix 68 displayed greatly enhanced stability against AO impact and reduced the temperature rise, mass loss, and the erosion yield of PVDF greatly, due to the stable cage-like Si–O frame in FP-POSS molecules.14 This work provided valuable information to optimize the PVDF structure and composition for effective protection against AO impacts in the LEO environment. Bai et al. fabricated the nanostructured thermoplastic elastomeric composites based on SBS cross-linked by POSS (SBS/POSS, Fig. 15b) 69, in which the thiol groups in POSS molecules and the double bonds in the soft block segment of SBS were cross-linked via UV-induced thiol–ene reaction.15 POSS formed the cross-linking centers for SBS, and the covalent linkages between POSS and SBS promoted the compatibility of the composite material, resulting in well-ordered micro-phase separation at the nanoscale for the composite films. The incorporation of POSS significantly improved the mechanical properties of SBS, due to the morphological changes from the circle inland structure (pure SBS) to the lamella structures (SBS/POSS).
 | ||
| Fig. 15 Schematic drawing of the chemical structure of PNb10POSS 66 and SBS/POSS 69. (a) PLM micrographs of 66 at 130 °C (left), HR-TEM micrograph of 66 (right). Adapted from ref. 12. Copyright 2015 Royal Society of Chemistry. (b) AFM phase images of samples 69 with different POSS weight percentages. Adapted from ref. 15. Copyright 2014 Royal Society of Chemistry. | ||
3. Patented POSS–polymer nanocomposites and technology
Polymer nanocomposites have received increasing attention in the past two decades due to their commercial and military value. In this section, we will highlight a number of recent patented materials and technologies on POSS–polymer nanocomposites. POSS grafted-polyolefin was used in cosmetic compositions by L'Oréal to make keratinous tissue to preserve long-wear with greater comfort, reduced tackiness and enriched colour.83 Graftable POSS was also used as a cosmetic composition by Avon, where POSS was cross-linked in situ to form a film on mammalian keratinous materials, and those optimized cosmetic compositions showed improved properties, such as non-transferable and long-lasting with a comfortable feeling of use to consumers over long periods of wear time.84 A support element was prepared by Pall Corporation based on a polyphenylene sulfide resin with incorporation of POSS additives. The introduced POSS additives significantly improved the mechanical strength and drainage of the support element and they were compatible with resin extrusion processes. In addition, no extra separate dye lubricant was needed during the extrusion because the added POSS can act as a lubricant.85 An intermediate transfer member in an electrostatographic apparatus was prepared by Xerox Corporation, in which the surface layer fabricated by polyimide was composed of POSS. The obtained layer possessed acceptable functional resistivity, good scratch resistivity, excellent layer/layer adhesion for multi-layered members, excellent surface tension characteristics, and a hydrophobic nature.86 A composite was formulated from POSS and a soluble liquid crystal thermosetting oligomer, which was used as an insulation material for printed circuit boards.87 The composite displayed improved properties, such as better insulation, rigidity, high heat resistance, and low coefficient of thermal expansion, making it suitable as an insulation layer of a printed circuit board.4. Conclusions and perspectives
In this review, we have summarized the diverse and significant research on POSS–polymer nanocomposites occurring in the past several years. The POSS–polymer nanocomposites highlighted in this review have many potential applications, including but not limited to the enhancement of thermal stability and mechanical strength of the materials, efficient drug and gene delivery with low toxicity, suppressing the π–π stacking in luminescent oligomers or polymers to improve their optical purity and the increase of quantum efficiencies in photoluminescence and electroluminescence. The aggregation or crystallization behaviour of POSS in nanocomposites plays an important role in manipulating the physical properties of materials, such as viscosity and melt elasticity. Most importantly, the nature of vertex groups in POSS has been found to regulate the interactions between the POSS moiety and the polymer segments, and subsequently impact the microstructure and rheology of nanocomposites. Although numerous POSS derivatives with functional vertex groups have been developed to improve material performances, there are several desired features yet to be achieved and require further exploration of POSS materials. The precise functionalization of POSS is one issue for the future POSS chemistry, and POSS monomers with such precise functional groups are still scarce, but they are very useful for material scientists to design and synthesise polymer nanocomposites. In addition, the directed self-assembly of POSS–polymers nanocomposites on the subnano/nanoscale has attracted interest from electronics industries, especially the microelectronics sector, which is regarded as a promising nano-patterning technology to generate next-generation microelectronics with higher resolution and fewer defects. The polymers with POSS have also shown significantly improved photo-oxidative stability and AO stability, which will be further utilized in various industrial applications, such as high-resolution lithography and spacecraft materials. We expect that fundamental science as well as industrial applications of POSS–polymer nanocomposites will continue to grow, and offer new functional materials with competitive performance and reasonable cost for the application of energy devices, aerospace materials, coatings, catalysts, cosmetics and medicine (Fig. 16).Acronyms
| AIE | Aggregation induced emission |
| ACPOSS | Acryloxyl-functionalized POSS |
| AL0136 | Octa(3-hydroxy-3-methylbutyldimethylsiloxy) |
| AFM | Atomic force microscopy |
| AO | Atomic oxygen |
| APP | Ammonium polyphosphate |
| ATRP | Atom transfer radical polymerization |
| CE | Composite electrolytes |
| CF | Carbon fibers |
| CNT | Carbon nanotube |
| DNT | 2,4-Dinitrotoluene |
| DMS | Degree of micro phase separation |
| DSC | Differential scanning calorimetry |
| DGEBA | Diglycidyl ether bisphenol A |
| EPDM | Ethylene–propylene–diene monomer |
| EGDMA | Ethylene glycol dimethacrylate |
| FR/NIR | Far-red/near-infrared |
| FP-POSS | (3,3,3-Trifluoropropyl)8Si8O12 |
| FD-POSS | Fluorodecyl-POSS |
| GO | Graphene oxide |
| HBP | Hyperbranched polyurethane |
| IFSS | Interfacial shear strength |
| Ma | Molecular weight of the arm |
| PEO | Poly(ethylene oxide) |
| PFCP | Perfluorocyclopentenyl aryl ether polymers |
| PI | Polyimides |
| PMMA | Poly(methyl methacrylate) |
| POM | Poly(oxymethylene) |
| POSS | Polyhedral oligomeric silsesquioxane |
| PPO | Poly(propylene oxide) |
| PS | Poly(styrene) |
| PTMO | Poly(tetramethyleneoxide) |
| PSMA | Poly(styrene-co-maleic anhydride) |
| PSMIPOSS | Poly(styrene-alt-maleimide isobutyl POSS) |
| PU | Polyurethane |
| PVDF | Poly(vinylidene fluoride) |
| RAFT | Reversible addition fragmentation chain transfer polymerization |
| OapPOSS | Octa-aminophenyl-POSS |
| PA | Polyamide |
| PBZ | Polybenzoxazines |
| PBLG | Poly(γ-benzyl-L-glutamate) |
| PC | Poly(carbonate) |
| PCL | Poly(ε-caprolactone) |
| PLA | Polylactide |
| PCU | Poly(carbonate-urea)urethane |
| PLLA | Poly(L-lactic acid) |
| PEG | Poly(ethylene glycol) |
| SBS | Polystyrene–butadiene–polystyrene |
| SEM | Scanning electron microscopy |
| SPE | Solid polymer electrolyte |
| TEM | Transmission electron microscopy |
| T g | Glass transition temperatures |
| TNT | 2,4,6-Trinitrotoluene |
| TGA | Thermal gravity analysis |
| UDMA | Dimethacrylate |
| 1D | One-dimensional |
| 2D | Two-dimensional |
| 3D | Three-dimensional |
Acknowledgements
The authors would like to acknowledge the financial support from the Institute of Materials Research and Engineering (IMRE), Agency for Science, Technology and Research (A*STAR).Notes and references
- D. W. Scott, J. Am. Chem. Soc., 1946, 68, 356–358 CrossRef CAS.
- R. H. Baney, M. Itoh, A. Sakakibara and T. Suzuki, Chem. Rev., 1995, 95, 1409–1430 CrossRef CAS.
- G. Li, L. Wang, H. Ni and C. U. Pittman Jr, J. Inorg. Organomet. Polym., 2001, 11, 123–154 CrossRef CAS.
- J. Wu and P. T. Mather, Polym. Rev., 2009, 49, 25–63 CrossRef CAS.
- C. B. Hurd, J. Am. Chem. Soc., 1946, 68, 364–370 CrossRef CAS.
- Y. Kawakami, Y. Kakihana, A. Miyazato, S. Tateyama and M. A. Hoque, Adv. Polym. Sci., 2010, 55, 1–44 Search PubMed.
- (a) P. H. T. Vollenberg and D. Heikens, Polymer, 1989, 30, 1656–1662 CrossRef CAS; (b) Z. S. Petrović, I. Javni, A. Waddon and G. Bánhegyi, J. Appl. Polym. Sci., 2000, 76, 133–151 CrossRef; (c) A. L. Andrady, T. C. Merkel and L. G. Toy, Macromolecules, 2004, 37, 4329–4331 CrossRef CAS; (d) K. S. Cho, J. I. Hong and C. I. Chung, Polym. Eng. Sci., 2004, 44, 1702–1706 CrossRef CAS.
- (a) J. D. Lichtenhan, Comments Inorg. Chem., 1995, 17, 115–130 CrossRef CAS; (b) A. Voigt, R. Murugavel and H. W. Roesky, Organometallics, 1996, 15, 5097–5101 CrossRef CAS; (c) M. W. Ellsworth and D. L. Gin, Polym. News, 1999, 24, 331–341 CAS; (d) J. Q. Zhao, F. Fu and S. M. Liu, Polym. Polym. Compos., 2008, 16, 483–500 CAS; (e) C. U. Pittman Jr, G. Z. Li and H. Ni, Macromol. Symp., 2003, 196, 301–325 CrossRef.
- M. Rubinstein and R. H. Colby, Polymer Physics, Oxford University Press, New York, 2003 Search PubMed.
- (a) S. Bizet, J. Galy and J. F. Gerard, Polymer, 2006, 47, 8219–8227 CrossRef CAS; (b) X. Zhang, E. R. Chan and S. C. Glotzer, J. Chem. Phys., 2005, 123, 184718 CrossRef PubMed; (c) A. Striolo, C. McCabe and P. T. Cummings, J. Chem. Phys., 2006, 125, 104904 CrossRef PubMed; (d) R. R. Patel, R. Mohanraj and C. U. Pittman, J. Polym. Sci., Part B: Polym. Phys., 2006, 44, 234–248 CrossRef CAS; (e) E. R. Chan, X. Zhang, C. Y. Lee, M. Neurock and S. C. Glotzer, Macromolecules, 2005, 38, 6168–6180 CrossRef CAS.
- (a) Q. Ye, H. Zhou and J. Xu, Chem. – Asian J., 2016, 11, 1322–1337 CrossRef CAS PubMed; (b) R. H. Baney, M. Itoh, A. Sakakibara and T. Suzuki, Chem. Rev., 1995, 95, 1409–1430 CrossRef CAS; (c) R. M. Laine, J. Mater. Chem., 2005, 15, 3725–3744 RSC; (d) D. B. Cordes, P. D. Lickiss and F. Rataboul, Chem. Rev., 2010, 110, 2081–2173 CrossRef CAS PubMed; (e) F. Wang, X. Lu and C. He, J. Mater. Chem., 2011, 21, 2775–2782 RSC; (f) A. Fina, O. Monticelli and G. Camino, J. Mater. Chem., 2010, 20, 9297–9305 RSC; (g) A. Fina, O. Monticelli and G. Camino, J. Mater. Chem., 2010, 20, 9297–9305 RSC; (h) S. W. Kuo and F. C. Chang, Prog. Polym. Sci., 2011, 36, 649–1696 CrossRef; (i) K. N. Raftopoulos and K. Pielichowski, Prog. Polym. Sci., 2016, 52, 136–187 CrossRef CAS; (j) K. L. Chan, P. Sonar and A. Sellinger, J. Mater. Chem., 2009, 19, 9103–9120 RSC; (k) R. Y. Kannan, H. J. Salacinski, P. E. Butler and A. M. Seifalian, Acc. Chem. Res., 2005, 38, 879–884 CrossRef CAS PubMed; (l) J. Wu and P. T. Mather, J. Macromol. Sci., Polym. Rev., 2009, 49, 25–63 CAS; (m) K. Tanaka and Y. Chujo, J. Mater. Chem., 2012, 22, 1733–1746 RSC; (n) K. Tanaka and Y. Chujo, Bull. Chem. Soc. Jpn., 2013, 86, 1231–1239 CrossRef CAS; (o) H. C. L. Abbenhuis, Chem. – Eur. J., 2000, 6, 25–32 CrossRef CAS PubMed; (p) R. Duchateau, Chem. Rev., 2002, 102, 3525–3542 CrossRef CAS PubMed; (q) E. A. Quadrelli and J. M. Basset, Coord. Chem. Rev., 2010, 254, 707–728 CrossRef CAS; (r) M. C. Kung, M. V. Riofski, M. N. Misasghi and H. H. Kung, Chem. Commun., 2014, 50, 3262–3276 RSC; (s) Applications of Polyhedral Oligomeric Silsesquioxane, ed. C. Hartmann-Thompson, Springer, Netherlands, 2011 Search PubMed.
- P. P. Hou, K. H. Gu, Y. F. Zhu, Z. Y. Zhang, Q. Wang, H. B. Pan, S. Yang, Z. Shen and X. H. Fan, RSC Adv., 2015, 5, 70163–70171 RSC.
- M. Xue, X. Zhang, L. Ma, Z. Gu, Y. Lin, C. Bao and X. Tian, J. Appl. Polym. Sci., 2013, 128, 2395–2401 CrossRef CAS.
- F. Zeng, C. Peng, Y. Liu and J. Qu, J. Phys. Chem. A, 2015, 119, 8359–8368 CrossRef CAS PubMed.
- J. Bai, Z. Shi, J. Yin and M. Tian, Polym. Chem., 2014, 5, 6761–6769 RSC.
- X. Wang, D. Li, F. Yang, H. Shen, Z. Li and D. Wu, Polym. Chem., 2013, 4, 4596–4600 RSC.
- A. R. Polu and H. W. Rhee, J. Ind. Eng. Chem., 2015, 31, 323–329 CrossRef CAS.
- D.-G. Kim, J. Shim, J. H. Lee, S.-J. Kwon, J.-H. Baik and J.-C. Lee, Polymer, 2013, 54, 5812–5820 CrossRef CAS.
- L. Wang and S. Zheng, Mater. Chem. Phys., 2012, 136, 744–754 CrossRef CAS.
- N. L. Le, Y. Wang and T.-S. Chung, J. Membr. Sci., 2011, 379, 174–183 CrossRef CAS.
- B. Sharma, R. Verma, C. Baur, J. Bykova, J. M. Mabry and D. W. Smith Jr., J. Mater. Chem. C, 2013, 1, 7222–7227 RSC.
- X. Fan, Z. Wang and C. He, J. Mater. Chem. B, 2015, 3, 4715–4722 RSC.
- S. Yilmaz, M. Kodal, T. Yilmaz and G. Ozkoc, Composites, Part B, 2014, 56, 527–535 CrossRef CAS.
- L. Gardella, A. Basso, M. Prato and O. Monticelli, ACS Appl. Mater. Interfaces, 2013, 5, 7688–7692 CAS.
- L. Gardella, S. Colonna, A. Fina and O. Monticelli, Colloid Polym. Sci., 2014, 292, 3271–3278 CAS.
- A.-L. Goffin, E. Duquesne, J.-M. Raquez, H. E. Miltner, X. Ke, M. Alexandre, G. Van Tendeloo, B. Van Mele and P. Dubois, J. Mater. Chem., 2010, 20, 9415–9422 RSC.
- B. H. Tan, H. Hussain, Y. W. Leong, T. T. Lin, W. W. Tjiu and C. B. He, Polym. Chem., 2013, 4, 1250–1259 RSC.
- M. J. Ferńandez, M. D. Ferńandez and M. Cobos, RSC Adv., 2014, 4, 21435–21449 RSC.
- O. Monticelli, M. Putti, L. Gardella, D. Cavallo, A. Basso, M. Prato and S. Nitti, Macromolecules, 2014, 47, 4718–4727 CrossRef CAS.
- S.-W. Kuo, H.-F. Lee, W.-J. Huang, K.-U. Jeong and F.-C. Chang, Macromolecules, 2009, 42, 1619–1626 CrossRef CAS.
- D. Gnanasekaran, A. Shanavas, W. W. Focke and R. Sadiku, RSC Adv., 2015, 5, 11272–11283 RSC.
- K. Matsumoto, K. Nishi, K. Ando and M. Jikei, Polym. Chem., 2015, 6, 4758–4765 RSC.
- H. W. Milliman, H. Ishida and D. A. Schiraldi, Macromolecules, 2012, 45, 4650–4657 CrossRef CAS.
- L. Li and H. Liu, RSC Adv., 2014, 4, 46710–46717 RSC.
- N. Tz. Dintcheva, E. Morici, R. Arrigo, F. P. La Mantia, V. Malatesta and J. J. Schwab, Polym. Degrad. Stab., 2012, 97, 2313–2322 CrossRef.
- E. S. Cozza, V. Bruzzo, F. Carniato, E. Marsano and O. Monticelli, ACS Appl. Mater. Interfaces, 2012, 4, 604–607 CAS.
- O. Monticelli, A. Fina, E. S. Cozza, M. Prato and V. Bruzzo, J. Mater. Chem., 2011, 21, 18049–18054 RSC.
- Z. Zhang, L. Hong, Y. Gao and W. Zhang, Polym. Chem., 2014, 5, 4534–4541 RSC.
- Y. Li, H. Su, X. Feng, Z. Wang, K. Guo, C. Wesdemiotis, Q. Fu, S. Z. D. Cheng and W.-B. Zhang, Polym. Chem., 2014, 5, 6151–6162 RSC.
- H. Tan, J. Zheng, D. Xu, D. Wan, J. Qiu and T. Tang, J. Phys. Chem. B, 2014, 118, 5229–5239 CrossRef CAS PubMed.
- H. Zhou, Q. Ye, W. T. Neo, J. Song, H. Yan, Y. Zong, B. Z. Tang, T. S. A. Hor and J. Xu, Chem. Commun., 2014, 50, 13785–13788 RSC.
- W. Yuan, T. Shen, X. Liu and J. Ren, Mater. Lett., 2013, 111, 9–12 CrossRef CAS.
- S. M. Ramirez, Y. J. Diaz, C. M. Sahagun, M. W. Duff, O. B. Lawal, S. T. Iacono and J. M. Mabry, Polym. Chem., 2013, 4, 2230–2234 RSC.
- J. Shim, D. G. Kim, J. H. Lee, J. H. Baik and J. C. Lee, Polym. Chem., 2014, 5, 3432–3442 RSC.
- Z. Li, B. H. Tan, G. Jin, K. Li and C. He, Polym. Chem., 2014, 5, 6740–6753 RSC.
- A. Pan, S. Yang, L. He and X. Zhao, RSC Adv., 2014, 4, 27857–27866 RSC.
- S. S. Mahapatra, S. K. Yadav and J. W. Cho, React. Funct. Polym., 2012, 72, 227–232 CrossRef CAS.
- R. Sasi kumar and M. Alagar, RSC Adv., 2015, 5, 33008 RSC.
- A. Lungu, N. M. Şulcă, E. Vasile, N. Badea, C. Pârvu and H. Iovu, J. Appl. Polym. Sci., 2011, 121, 2919–2926 CrossRef CAS.
- D. Przdka, J. Jczalik, E. Andrzejewska, B. Marciniec, M. Dutkiewicz and M. Szłapka, React. Funct. Polym., 2013, 73, 114–121 CrossRef.
- R. Bakhshi, A. Darbyshire, J. E. Evans, Z. You, J. Lu and A. M. Seifalian, Colloids Surf., B, 2011, 86, 93–105 CrossRef CAS PubMed.
- Q. H. Zhang, X. Huang, X. L. Wang, X. D. Jia and K. Xi, Polymer, 2014, 55, 1282–1291 CrossRef CAS.
- (a) T. K. Minton, M. E. Wright, S. J. Tomczak, S. A. Marquez, L. H. Shen, A. L. Brunsvold, R. Cooper, J. M. Zhang, V. Vij, A. J. Guenthner and B. J. Petteys, ACS Appl. Mater. Interfaces, 2012, 4, 492–502 CrossRef CAS PubMed; (b) K. Tanaka and Y. Chujo, J. Mater. Chem., 2012, 22, 1733–1746 RSC; (c) A. F. Luo, X. S. Jiang, H. Lin and J. Yin, J. Mater. Chem., 2011, 21, 12753–12760 RSC; (d) H. Lin, X. Wan, X. Jiang, Q. Wnag and J. Yin, Adv. Funct. Mater., 2011, 21, 2960–2967 CrossRef CAS.
- X.-F. Lei, M.-T. Qiao, L.-D. Tian, P. Yao, Y. Ma, H.-P. Zhang and Q.-Y. Zhang, Corros. Sci., 2015, 90, 223–238 CrossRef CAS.
- S. Duo, H. Ke, T. Liu, M. Song and M. Li, Nucl. Instrum. Methods Phys. Res., Sect. B, 2013, 307, 324–327 CrossRef CAS.
- N. L. Le and T.-S. Chung, J. Membr. Sci., 2014, 454, 62–73 CrossRef CAS.
- Z. Wu, S. Zhang, H. Li, Y. Liang, Z. Qi, Y. Xu, Y. Tang and C. Gong, J. Power Sources, 2015, 290, 42–52 CrossRef CAS.
- R. Konietzny, T. Koschine, K. Rätzke and C. Staudt, Sep. Purif. Technol., 2014, 123, 175–182 CrossRef CAS.
- (a) L. Matĕjka, I. Amici Kroutilová, J. D. Lichtenhan and T. S. Haddad, Eur. Polym. J., 2014, 52, 117–126 CrossRef; (b) Y. Xu, Y. Ma, Y. Deng, C. Yang, J. Chen and L. Dai, Mater. Chem. Phys., 2011, 125, 174–183 CrossRef CAS; (c) Y. C. Chiu, L. Riang, I. C. Chou, C. C. M. Ma, C. L. Chiang and C. C. Yang, J. Polym. Sci., Part B: Polym. Phys., 2010, 48, 643–652 CrossRef CAS.
- (a) J. Boček, L. Matčjka, V. Mentlík, P. Trnka and M. Šlouf, Eur. Polym. J., 2011, 47, 861–872 CrossRef; (b) P. Musto, M. Abbate, M. Pannico, G. Scarinzi and G. Ragosta, Polymer, 2012, 53, 5016–5036 CrossRef CAS; (c) G.-X. Chen, L. Si, P. Lu and Q. Li, J. Appl. Polym. Sci., 2012, 125, 3929–3935 CrossRef CAS; (d) V. Pistor, B. G. Soares and R. S. Mauler, Polymer, 2013, 54, 2292–2298 CrossRef CAS.
- L. Wang, C. Zhang and S. Zheng, J. Mater. Chem., 2011, 21, 19344–19352 RSC.
- K. Sethuraman, P. Prabunathan and M. Alagar, RSC Adv., 2014, 4, 45433–45441 RSC.
- C. Ni, G. Ni, L. Zhang, J. Mi, B. Yao and C. Zhu, J. Colloid Interface Sci., 2011, 362, 94–99 CrossRef CAS PubMed.
- L. Matĕjka, P. Murias and J. Pleštil, Eur. Polym. J., 2012, 48, 260–274 CrossRef.
- (a) M. R. Vengatesan, S. Devaraju, K. Dinakaran and M. Alagar, J. Mater. Chem., 2012, 22, 7559–7566 RSC; (b) M. R. Vengatesan, S. Devaraju, M. Selvi, A. Chandramohan, A. Ashok Kumar and M. Alagar, J. Mol. Struct., 2012, 1027, 162–166 CrossRef CAS; (c) M. R. Vengatesan, S. Devaraju, K. Dinakaran, J. K. Song and M. Alagar, Polym. Int., 2012, 62, 127–133 CrossRef.
- (a) N. Xu, E. J. Stark, P. I. Carver, P. Sharps, J. Hu and C. Hartmann-Thompson, J. Appl. Polym. Sci., 2013, 130, 3849–3861 CAS; (b) D. Shuwang, S. Mimi, L. Tingzhi, L. Ying and L. Meishuan, Adv. Mater. Res., 2011, 336, 189–193 Search PubMed.
- M. Selvi, S. Devaraju, M. R. Vengatesan, J. S. Go, M. Kumard and M. Alagar, RSC Adv., 2014, 4, 8238–8244 RSC.
- K. W. Huang and S. W. Kuo, Macromol. Chem. Phys., 2010, 211, 2301–2311 CrossRef CAS.
- Y. C. Wu and S. W. Kuo, Polymer, 2010, 51, 3948–3955 CrossRef CAS.
- M. C. Tseng and Y. L. Liu, Polymer, 2010, 51, 5567–5575 CrossRef CAS.
- A. Chandramohan, S. Devaraju, M. R. Vengatesan and M. Alagar, J. Polym. Res., 2012, 19, 9903–9912 CrossRef.
- D. Jiang, L. Xing, L. Liu, X. Yan, J. Guo, X. Zhang, Q. Zhang, Z. Wu, F. Zhao, Y. Huang, S. Wei and Z. Guo, J. Mater. Chem. A, 2014, 2, 18293–18303 CAS.
- F. Zhao and Y. Huang, J. Mater. Chem., 2011, 21, 3695–3703 RSC.
- X. Wang, L. Song, H. Yang, W. Xing, B. Kandola and Y. Hu, J. Mater. Chem., 2012, 22, 22037–22043 RSC.
- Q. Li, P. Peng, G.-X. Chen and S. W. Yoon, J. Mater. Chem. C, 2014, 2, 8216–8221 RSC.
- S. K. Yadav, S. S. Mahapatra, H. J. Yoo and J. W. Cho, Nanoscale Res. Lett., 2011, 6, 122–127 CrossRef PubMed.
- W. Yu, J. Fu, X. Dong, L. Chen and L. Shi, Compos. Sci. Technol., 2014, 92, 112–119 CrossRef CAS.
- Y. Tang, J. Zhuge, J. Lawrence, J. Mckee, J. H. Gou, C. Ibeh and Y. Hu, Polym. Degrad. Stab., 2011, 96, 760–770 CrossRef CAS.
- T. Nezakati, A. Tan and A. M. Seifalian, J. Colloid Interface Sci., 2014, 435, 145–155 CrossRef CAS PubMed.
- F. Zhao, Y. Huang, L. Liu, Y. Bai and L. Xu, Carbon, 2011, 49, 2624–2632 CrossRef CAS.
- Z. Ruan, W. Rong, X. Zhan, Q. Li and Z. Li, Polym. Chem., 2014, 5, 5994–6002 RSC.
- J. Liu, G. Feng, D. Ding and B. Liu, Polym. Chem., 2013, 4, 4326–4334 RSC.
- E. Dimotakis, H. S. Bui and M. Kanji, EP 2648694A2, L'Oréal, SA, 2013.
- (a) P. Maitra and T. Zheng, US8133478 B2, Avon Products Inc., 2012; (b) P. Maitra and T. Zheng, US8545823 B2, Avon Products Inc., 2013.
- K. R. Kathan, J. A. Liddy and M. J. Adams, US20140332461A1, Pall Corporation, 2014.
- (a) D. M. Pietrantoni, J. Wu, J. H. Herko, L. Zhang, L. Ma, F. J. Lopez and K. B. Tallman, US8025967 B1, Xerox Corporation, 2011; (b) D. M. Pietrantoni, Y. Tong, M. S. Roetker, F. J. Lopez, K. B. Tallman, J. H. Herko and J. Wu, US8609233 B2, Xerox Corporation, 2013; (c) J. Wu, L. Zhang, L. Ma and R. Ehmann, US8435632 B2, Xerox Corporation, 2013.
- S. Y. Ji, S. H. Kim and S. J. Ham, US20140187687, Samsung Electro-Mechanics Co., Ltd., 2014.
| This journal is © the Partner Organisations 2017 |