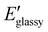Efficient catechol functionalization of high oleic sunflower oil for the preparation of fully biobased and extrudable acetal CANs
Timo
Sehn
a,
Mickaël
Du Fraysseix
b,
Cédric
Le Coz
 b,
Emmanuel
Ibarboure
b,
Michael A. R.
Meier
b,
Emmanuel
Ibarboure
b,
Michael A. R.
Meier
 *ac and
Audrey
Llevot
*ac and
Audrey
Llevot
 *b
*b
aInstitute of Biological and Chemical Systems – Functional Molecular Systems (IBCS-FMS), Karlsruhe Institute of Technology (KIT), 76131 Karlsruhe, Germany. E-mail: michael.meier2@kit.edu
bUniv. Bordeaux, CNRS, Bordeaux INP, LCPO UMR 5629, Pessac F-33600, France. E-mail: audrey.llevot@enscbp.fr
cInstitute of Organic Chemistry (IOC), Materialwissenschaftliches Zentrum (MZE), Karlsruhe Institute of Technology (KIT), Kaiserstraße 12, 76131 Karlsruhe, Germany
First published on 25th November 2025
Abstract
Herein, we introduce the synthesis of fully renewable and extrudable high oleic sunflower oil-based acetal containing covalent adaptable networks (CANs) via a catalyst and solvent-free click-like reaction between a bio-based polyol and divinyl ether, i.e. 1,4-cyclohexanedimethanol divinyl ether. High oleic sunflower oil was therefore first converted into the respective polyol via a simple H2SO4 catalyzed Friedel–Crafts alkylation using catechol within 30 minutes at 120 °C. After subsequent structural characterization of the polyol, acetal containing CANs showing high cross-linking densities, fast stress relaxation, and excellent malleability were synthesized without releasing any small-molecule byproducts. The presence of the catechol moiety is particularly interesting, as the presence of an adjacent phenolic group induces neighboring group participation effects and accelerates exchange reaction rates. The dynamic behavior of the new cross-linked materials was confirmed by stress relaxation measurements at different temperatures as well as by their reprocessability via compression molding and extrusion. Additionally, the materials were degraded under weak acidic conditions, and the starting biobased polyol was recovered in a yield of 72%, thus enabling a closed-loop chemical recycling of this monomer.
Introduction
Covalent adaptable networks (CANs), pioneered by Bowman and coworkers, are classified as dynamically covalently cross-linked materials, which exhibit the structure and improved thermomechanical properties of thermosets but upon a certain external stimulus, such as temperature or pressure, can feature the processability and recyclability of thermoplastics.1,2 This behavior can be explained by the occurrence of exchange reactions that can either proceed in an associative or in a dissociative fashion under specific conditions. Dissociative CANs show a temporary decrease in network connectivity upon a temperature trigger, as a result of bond cleavage preceding the formation of a new bond, reflected by the so-called sol–gel transition.3 CANs based on associative exchange reactions undergo this reversible rearrangement without reduced time-averaged cross-linking density and show similar viscosity dependence as vitreous silica. These materials were termed vitrimers by Leibler and coworkers.4 Noteworthy, some CANs based on dissociative exchange reactions also exhibit the same viscosity dependence as vitrimers.3 Common functional groups that can undergo reversible exchange reactions and therefore often find application in CANs are for instance disulfides,5–7 esters,8,9 ureas,10,11 imines,12,13 urethanes14,15 and vinylogous urethanes,16 among others. CANs reveal a high potential to extend the service life of cross-linked materials and may offer enhanced end-of-life characteristics, thus contributing to improved sustainability. In recent years, particularly the development of synthetic strategies following the 12 principles of green chemistry gained a significant importance to access CANs with a lower environmental impact.17–19 Especially, the use of renewable feedstocks, the development of less hazardous synthetic pathways, the investigation of recycling strategies, i.e. in a chemical or mechanical manner, and the potential biodegradability of the materials play a key role in this context.20–22Acetal moieties are well-known as acid labile protection groups in organic chemistry.23 Moreover, these structural motifs are also established in polymer science, for example in the synthesis of thermoplastic materials such as polyacetals.24–26 In this regard, particularly acid catalyzed reactions between divinyl ethers and diols appear as an efficient synthetic tool.24,25 In 2019, Zhu et al. introduced this chemistry into CANs by cross-linking a polyol, i.e. poly(styrene-co-styrene-OH), with commercial divinyl ethers yielding robust, highly malleable and recyclable CANs.27 At this point, it is worth to mention that the cross-linking reaction was herein thermally accelerated by applying elevated temperatures, i.e. up to 150 °C, and thus did not require the use of a catalyst.27 In 2020, the same group used a similar synthetic approach targeting materials with an increased stability and creep resistance by cross-linking a phenolic resin with 1,4-cyclohexanedimethanol divinyl ether.28 Interestingly, further investigations on acetal-based CANs revealed that neighboring group participation of phenolic hydroxyl moieties can significantly accelerate the acetal exchange reactions.29 Accordingly, Zhu et al. synthesized CANs combining high performance with fast reprocessability by cross-linking epigallocatechin gallate with tri(ethylene glycol) divinyl ether, thus enabling processing by extrusion.29 From a sustainability perspective, however, all synthetic approaches that deliver acetal-based CANs via a catalyst-free click reaction between a polyol and a divinyl ether still suffer from at least one component being fossil raw material based. Another strategy to prepare acetal CANs consists in synthesizing a monomer incorporating cyclic acetals and spyro diacetals units.30 Amongst the studies, in 2023, Lin and coworkers reported the synthesis of a fully biobased spyro diacetal monomer from vanillin and erythritol that they cross-linked with epoxydized soybean oil yielding fully biobased CANs.19 However, in this approach, dimethyl imidazole was required as external catalyst.
Thus, we herein introduce the synthesis of fully renewable and extrudable high oleic sunflower oil-based acetal CANs via a catalyst and solvent-free click reaction between a bio-based polyol and divinyl ether, i.e. 1,4-cyclohexanedimethanol divinyl ether. High oleic sunflower oil was first converted into a polyol by simple H2SO4 catalyzed Friedel–Crafts alkylation using catechol. After structural characterization by 1H, 13C, 2D NMR, ATR-IR, and ESI-MS, the polyol was cross-linked with different amounts of 1,4-cyclohexanedimethanol divinyl ether under catalyst and solvent-free conditions delivering acetal-based CANs with tunable cross-linking densities and fast stress relaxation. The reprocessing of the CANs was investigated by compression molding and extrusion. Finally, we also explored the chemical recycling of these acetal-containing CANs by taking advantage of the hydrolysable acetal groups with the objective to recover the newly synthesized sunflower oil-based polyol.
Experimental section
Materials and methods
High oleic sunflower oil (HOSO, Lesieur), 1,4-cyclohexanedimethanol divinyl ether (CVDE, mixture of isomers, Sigma Aldrich, 98%), catechol (Thermo Scientific, 99%), CDCl3 (Eurisotop, 99.8%), hydrochloric acid (Sigma Aldrich), sulfuric acid (Fisher Scientific, ≥95%) were used without further purification. All other solvents were employed in technical grade.Procedure for the synthesis of HOSO–catechol
A 250 mL two-neck round bottom flask equipped with a reflux condenser was charged with HOSO (5.00 g, 17.0 mmol of double bonds (based on the molecular weight of triolein (885.45 g mol−1), see details under Fig. S1), 1.00 equiv.), catechol (14.9 g, 136 mmol, 8.00 equiv.) and a stirring bar. The reaction setup was heated to 120 °C and the suspension was stirred until catechol was completely molten (Tm = 105 °C) and a homogenous reaction mixture was obtained. Then, H2SO4 (≥95%, 365 µL, 665 mg, 6.80 mmol, 0.40 equiv.) was slowly added and the reaction solution was rapidly stirred for 30 minutes at 120 °C. Subsequently, the mixture was cooled to room temperature, the residue was dissolved in THF and precipitated in 400 mL H2O (2×). After precipitation, the highly viscous crude polyol was resolubilized in Et2O and washed with saturated sodium bicarbonate solution (3 times) in order to remove residual sulfuric acid. Afterwards, the organic phase was dried over anhydrous sodium sulfate, the solvent was removed under reduced pressure and remaining traces of catechol were removed under high vacuum (1.8 × 10−3 mbar) at 150 °C. The product was obtained as dark red to black, highly viscous and sticky liquid (2.75 g, 39.9%).1 H NMR: (400 MHz, CDCl3) δ (ppm) = 7.10–6.35 (m, Hh, 9H), 5.77–5.30 (m, Hg, 6H), 5.31–5.00 (m, Hf, 1H), 4.49–3.91 (m, He, 4H), 2.96–2.82 (m, Hd, 1.5H), 2.64–2.47 (m, Hd, 1.5H), 2.40–2.19 (m, Hc, 6H), 1.83–0.95 (m, Hb, 84H), 0.94–0.77 (m, Ha, 9H).
13 C NMR: (126 MHz, CDCl3) δ (ppm) = 174 (Cg, 3C), 144–132 (Cf, 9C), 122–112 (Ce, 9C), 70.9–62.2 (Ce, 3C), 45.4 (Cd, 3C), 38.2 (Cc, 1C), 38.1–22.7 (Cb, 30C), 14.1 (Ca, 3C).
ATR-IR: ν (cm−1) = 2922 (vs), 2851 (s), 1720 (m), 1595 (w), 1508 (w), 1497 (w), 1464 (m), 1441 (w), 1364 (w), 1323 (w), 1277 (m), 1173 (s), 1159 (s), 1109 (m), 1043 (w), 868 (vw), 812 (w), 781 (w), 733 (m), 642 (vw), 623 (vw), 590 (vw), 453 (vw), 446 (vw), 432 (vw).
Calculation for the attachment of catechol units onto high oleic sunflower oil (HOSO)
The amount of attached catechol moieties onto the triglyceride structure of HOSO was calculated from 1H NMR by applying eqn (1):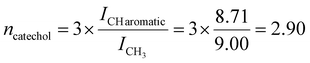 | (1) |
General procedure for the synthesis of acetal CANs
HOSO–catechol (500 mg, 2.47 mmol OH groups (calculated based on the molecular weight of a quantitatively modified triolein structure MHOSO–catechol = 1215.78 g mol−1), 1.00 equiv.) and different amounts of 1,4-cyclohexanedimethanol divinyl ether (CVDE) (Table 1) were poured into a rectangular Teflon mold:The mixture was subsequently carefully heated until both components could be manually mixed to reach a homogenous reaction mixture for the curing process. The mold was then placed into an oven for 1 h at 120 °C and 3 h at 150 °C to obtain the final dark red to black materials.
Swelling ratio and gel content determination
Each cross-linked material, i.e.P1-0.5, P2-0.7, and P3-0.9, (∼60 mg), was immersed in THF (1.5 mL) for 24 h at room temperature, enabling the solubilization of non-cross-linked oligomers. For each polymer, experiments were performed in triplicates. Subsequently, the materials were removed from the solvent and weighed. The swelling ratio is linked to the amount of solvent that is soaked into the material and can therefore be calculated according to eqn (2):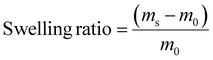 | (2) |
After, the solid materials were removed from the THF solution, dried under reduced pressure at 70 °C, and weighed. The gel content describes the percentage of mass that is insoluble in tetrahydrofuran and can therefore be calculated according to eqn (3):
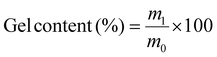 | (3) |
Reprocessing by compression molding
Reprocessing of P3-0.9 was conducted using a hot press Darragon. Therefore, the original P3-0.9 samples were cut into pieces, placed between two Teflon molds and hot pressed at 6 bar and 160 °C for 40 minutes.Processing by extrusion
Extrusion of P2-0.7 and P3-0.9 was performed using a Thermo Scientific HAAKE MiniCTW. P2-0.7 and P3-0.9 were cut into millimeter pieces and added in the extruder. After keeping the sample for 5 min at 190 °C, the extrusion speed was set to 20 rpm.Chemical recycling
25 mg of P3-0.9 was immersed in 0.05 M HCl water/acetone (1/9 v/v) for 2 h at 50 °C until full solubilization of the sample. Subsequently, the solvent mixture was removed under reduced pressure, the residue was resolubilized in dichloromethane (DCM) and the organic phase was washed with water (3 times). After drying the organic phase over sodium sulfate, DCM was removed under reduced pressure to obtain the degradation product in a recovery yield of 72%.Instrumentation
Tensile tests
The comparison of the polymer network mechanical properties before and after reprocessing was done using tensile tests on a TA Instruments RSA-III dynamic mechanical analyzer equipped with a 35 N load cell. Tensile stress and strain were obtained in static mode at a speed of 10 mm min−1. The rectangular shape sample dimensions were 1 cm wide and 2 cm long, with a thickness of 1 mm. These measurements were carried out on a minimum of 3 replicates per sample.Dynamic mechanical analysis (DMA)
A TA Instrument DMA 850 (dynamic mechanical analysis) was used to study the thermo-mechanical properties of P3-0.9 from −20 °C to 110 °C at a heating rate of 3 °C min−1. The measurements were performed with a traction set up at a frequency of 1 Hz, a strain sweep of 0.1% (linear domain), an initial static force of 0.1 N.Optical microscopy
Images were acquired using a digital microscope (VHX-X1F) from Keyence equipped with a high-performance camera (VHX-7020) with 20× zoom.Results and discussion
In this study, a fully renewable polyol based on high oleic sunflower oil (HOSO) was synthesized using a methodology previously established by our group, which entails in the attachment of catechol functionalities onto double bonds via acid catalyzed Friedel–Crafts alkylation (FCA).31 Catechol is accessible from the renewable resource lignin via demethylation and subsequent hydrogenation.32 In the current work, HOSO was reacted with an excess of catechol in the presence of H2SO4 (>95%) at 120 °C (Scheme 1). Quantitative conversion of the alkene moieties was achieved within 30 minutes, similar to the results reported for polybutadiene in the previously published study. | ||
| Scheme 1 Synthesis of a fully renewable polyol from high oleic sunflower oil (HOSO) via acid catalyzed Friedel–Crafts alkylation (FCA). | ||
Structural characterization of the synthesized polyol via nuclear magnetic resonance (NMR) and attenuated total reflection infrared (ATR-IR) spectroscopy was performed. As shown in Fig. 1A, 1H NMR confirmed the attachment of catechol moieties onto the triglyceride structure of HOSO by revealing characteristic magnetic resonances from 5.30 ppm to 5.77 ppm and from 6.35 ppm and 7.10 ppm, which can be assigned to the introduced hydroxyl and aromatic protons, respectively. Two additional characteristic magnetic resonances (arising at 2.57 ppm and 2.92 ppm), corresponding to the methine protons formed during the modification process, appeared in the spectrum and provided additional proof for the successful implementation of catechol units. The presence of more than one signal associated with the proton on the methine carbon can be explained by carbocation shifts and rearrangements occurring after protonation of the double bonds.33
 | ||
| Fig. 1 (A) 1H NMR (400 MHz) spectrum of HOSO–catechol in CDCl3 (*) at ambient temperature. (B) ATR-IR spectra of catechol (blue), HOSO (red), and HOSO–catechol (black). | ||
Furthermore, the 13C NMR spectrum of HOSO–catechol showed the expected magnetic resonances for catechol moieties in the aromatic region (112 ppm to 122 ppm and 132 ppm to 144 ppm), which was complemented by 2D NMR experiments, i.e. correlation spectroscopy (COSY), and heteronuclear single quantum coherence (HSQC) spectroscopy, confirming the correlations (Fig. S2–S4). Additional proof for the successful attachment of catechol units onto the triglyceride structure was provided by the magnetic resonance appearing at 38 ppm in the 13C NMR spectrum which could clearly be assigned as the tertiary aliphatic carbon on the triglyceride backbone after catechol attachment (Fig. S2). Moreover, the presence of the signal at 175 ppm confirms the presence of the carbonyl group of the triglyceride backbone. In our previous work, model reactions conducted on methyl-10-undecenoate showed that the ester moieties remained intact under the acidic conditions of the Friedel–Craft alkylation.31 As a result, we do not expect the occurrence of transesterification reactions during the catechol functionalization of HOSO performed in this study. ATR-IR spectroscopy further confirmed the attachment of catechol groups onto HOSO, especially with the appearance of two stretching vibration bands at 3418 cm−1 (O–H) and 1597 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Caromatic) supplemented by the disappearance of the C
Caromatic) supplemented by the disappearance of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibration band at 3007 cm−1 (Fig. 1B). Additional ESI-MS measurement revealed the mass of the deprotonated polyol, thus proofing the successful synthesis (Fig. S5). Subsequently, thermal properties of HOSO and the corresponding polyol were investigated via differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA). DSC measurement of HOSO revealed a melting point at −7 °C (Fig. S6). After the introduction of bulky catechol moieties into the HOSO structure, crystallization was suppressed and thus no melting point could be observed. Instead, the newly formed polyol (HOSO–catechol) displayed a glass transition, at −9 °C (Fig. S6). HOSO–catechol exhibits a relatively good thermal stability with a 5% weight loss observed at 296 °C, enabling bulk covalent adaptable network preparation without precursor degradation (Fig. S7).
C stretching vibration band at 3007 cm−1 (Fig. 1B). Additional ESI-MS measurement revealed the mass of the deprotonated polyol, thus proofing the successful synthesis (Fig. S5). Subsequently, thermal properties of HOSO and the corresponding polyol were investigated via differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA). DSC measurement of HOSO revealed a melting point at −7 °C (Fig. S6). After the introduction of bulky catechol moieties into the HOSO structure, crystallization was suppressed and thus no melting point could be observed. Instead, the newly formed polyol (HOSO–catechol) displayed a glass transition, at −9 °C (Fig. S6). HOSO–catechol exhibits a relatively good thermal stability with a 5% weight loss observed at 296 °C, enabling bulk covalent adaptable network preparation without precursor degradation (Fig. S7).
After structural characterization of the synthesized polyol, the preparation of fully renewable HOSO-based acetal CANs was targeted. Based on the 1H NMR spectrum, 2.9 catechol units in average, are attached onto the high oleic sunflower oil (HOSO) backbone. During the functionalization, the occurrence of oligomerization cannot be excluded but could not be evidenced. However, it does not affect the network formation provided that the functionality of the HOSO–catechol monomer is higher than 2. A thermally initiated, catalyst and solvent-free click reaction between a bifunctional vinyl ether (VE) and hydroxyl groups of the polyol was selected for cross-linking. The polyol was therefore reacted with 1,4-cyclohexanedimethanol divinyl ether (CVDE), which can be obtained from terpenes such as limonene and is therefore recognized as bio-based (Scheme 2).34,35
Based on already described approaches to prepare acetal-based CANs using a click reaction between vinyl ethers and hydroxyl groups, a OH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VE ratio of 1.0
VE ratio of 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 was selected first.20,28,29 The polyol and CVDE were mixed (OH 1
0.5 was selected first.20,28,29 The polyol and CVDE were mixed (OH 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 VE) without any additional solvent and a non-isothermal DSC measurement was conducted. Herein, a broad exothermic peak between 100 °C and 200 °C with a maximum at 147 °C was observed, indicating that the cross-linking reaction between vinyl ethers and hydroxyl groups occurred in this temperature range (Fig. S8). In order to reach a high cross-linking density, the curing procedure of the polymer network will include a first step at 120 °C for 1 h to provide enough time and flexibility for the starting materials to react quantitatively, followed by a second step for 3 h at 150 °C to complete the cross-linking. By applying these curing conditions, three materials, i.e.P1-0.5, P2-0.7, and P3-0.9, with different compositions, i.e. OH 1
0.5 VE) without any additional solvent and a non-isothermal DSC measurement was conducted. Herein, a broad exothermic peak between 100 °C and 200 °C with a maximum at 147 °C was observed, indicating that the cross-linking reaction between vinyl ethers and hydroxyl groups occurred in this temperature range (Fig. S8). In order to reach a high cross-linking density, the curing procedure of the polymer network will include a first step at 120 °C for 1 h to provide enough time and flexibility for the starting materials to react quantitatively, followed by a second step for 3 h at 150 °C to complete the cross-linking. By applying these curing conditions, three materials, i.e.P1-0.5, P2-0.7, and P3-0.9, with different compositions, i.e. OH 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 VE, OH 1
0.5 VE, OH 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.7 VE, and OH 1
0.7 VE, and OH 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.9 VE, were synthesized, respectively. As depicted in Fig. 2, the materials were obtained as dark red to black materials.
0.9 VE, were synthesized, respectively. As depicted in Fig. 2, the materials were obtained as dark red to black materials.
 | ||
| Fig. 2 Optical appearance of the fully renewable acetal CANs P1-0.5 (left), P2-0.7 (middle), and P3-0.9 (right) after removal from the mold directly after cross-linking. | ||
The structural characterization after the curing was performed by IR spectroscopy. The absorption ATR-IR spectra of the polyol and the CANs, i.e.P1-0.5, P2-0.7, and P3-0.9, were first normalized to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration band (1740 cm−1), which was expected not to undergo any change as the carbonyl moieties are not affected during the cross-linking process. The series of ATR-IR spectra of P1-0.5, P2-0.7, and P3-0.9 revealed a decrease of the O–H stretching vibration band (3418 cm−1) and an increase of the C–O–C stretching vibration band (1101 cm−1) characteristic of the acetal formation. These changes in band intensity depended on the amount of introduced cross-linker (Fig. 3). More precisely, P3-0.9, with the highest amount of cross-linker, revealed the most significant change in signal intensity of the O–H and C–O–C stretching vibration bands. These observations indicate a successful acetal formation reaction. Additionally, in all cases, no characteristic stretching vibration band corresponding to residual vinyl ether groups (1608 cm−1) could be observed in the ATR-IR spectra of the materials, suggesting an efficient and quantitative cross-linking.
O stretching vibration band (1740 cm−1), which was expected not to undergo any change as the carbonyl moieties are not affected during the cross-linking process. The series of ATR-IR spectra of P1-0.5, P2-0.7, and P3-0.9 revealed a decrease of the O–H stretching vibration band (3418 cm−1) and an increase of the C–O–C stretching vibration band (1101 cm−1) characteristic of the acetal formation. These changes in band intensity depended on the amount of introduced cross-linker (Fig. 3). More precisely, P3-0.9, with the highest amount of cross-linker, revealed the most significant change in signal intensity of the O–H and C–O–C stretching vibration bands. These observations indicate a successful acetal formation reaction. Additionally, in all cases, no characteristic stretching vibration band corresponding to residual vinyl ether groups (1608 cm−1) could be observed in the ATR-IR spectra of the materials, suggesting an efficient and quantitative cross-linking.
The gel contents of P1-0.5, P2-0.7, and P3-0.9 were evaluated according to eqn (3) in tetrahydrofuran (THF), in which both the polyol and the cross-linker are soluble. As shown in Fig. 4A, P2-0.7 and P3-0.9 revealed a high gel content of 99%, which confirmed the efficient cross-linking. However, P1-0.5 possessed a gel content of 72%, indicating that an appropriate amount of cross-linker, i.e. 0.50 or more equivalents of VE groups related to OH groups, is crucial to achieve solvent resistance of the material. Moreover, swelling tests in THF revealed a higher swelling ratio of 505 ± 29% for P1-0.5 compared to lower and similar values of 274 ± 42% and 238 ± 47%, respectively for P2-0.7 and P3-0.9. DSC measurements of the polymer networks revealed that the glass transition temperatures after cross-linking (−5 °C ≤ Tg ≤ −3 °C) were higher than the one of the starting polyol (Tg = −9 °C), which can be explained by the reduced chain mobility after cross-linking (Fig. 4B and S6). The obtained Tg values were in accordance with the current literature for cross-linked triglycerides. For instance, Meier et al. reported HOSO-based thermosets using the Passerini three-component reaction revealing Tgs between −18 and 9 °C.36
P1-0.5, which included the least amount of cross-linker and possessed therefore also a lower cross-linking density, revealed the lowest glass transition temperature of −5 °C (Fig. 4B). The increased cross-linking density of P2-0.7 and P3-0.9 led to a slight increase of Tg to −4 °C and −3 °C (Fig. 4B), respectively. The thermal stability of P1-0.5, P2-0.7, and P3-0.9 was subsequently investigated via TGA. Thermal degradation of the materials, which is defined as the temperature at which 5% weight loss occurs, was observed at 258 °C, 274 °C, and 248 °C for P1-0.5, P2-0.7, and P3-0.9, respectively, which is in line with the literature on acetal based covalent adaptable networks (Fig. S9).29
Acetal-based polymer networks such as P1-0.5, P2-0.7, and P3-0.9 belong to the category of covalent adaptable networks (CANs), as acetal groups can undergo exchange reactions like acetal metathesis and transacetalization, enabling reprocessing and reshaping under external stimuli such as temperature and pressure. The dynamic nature of the three polymer networks was assessed by stress relaxation experiments. In these measurements, a specific strain of 1%, which is comprised in the linear viscoelastic region at the measured temperatures, was applied to the materials and the evolution of the sample relaxation modulus was measured over time at different temperatures. As expected, the three polymer networks exhibited relatively fast stress relaxation at high temperature, which provides evidence of a liquid viscous characteristic induced by the implementation of exchangeable acetal units. The relaxation curves were fitted to a Kohlrausch–Williams–Watts (KWW) stretched exponential decay (eqn (4), Fig. 5, Fig. S10) and the corresponding relaxation times (τ*), stretching exponent (β), and fraction of the residual relaxation modulus Gres/G0 were extracted for each temperature (Table S1).37,38
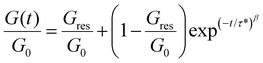 | (4) |
 | ||
| Fig. 5 (A–C) Stress relaxation experiments of P1-0.5, P2-0.7, and P3-0.9, respectively including the KWW fits. (D) Arrhenius plots of P1-0.5, P2-0.7, and P3-0.9. | ||
As shown in Fig. 5A–C, the relaxation of the materials occurred faster at higher temperatures. For instance, as the temperature increased from 140 °C to 170 °C, the relaxation times of P3-0.9 decreased from 674 to 217 s.
With the lower cross-linker content, P1-0.5 exhibited the lowest relaxation time of 23 s at 130 °C. This value increased to 408 s at 140 °C for P2-0.7 and to 674 s at 140 °C P3-0.9. This trend could be attributed to the concomitant increase of the cross-linking density and decrease of free hydroxyl groups, which enable transacetalization reactions, when increasing the cross-linker content. Having determined τ* values of P1-0.5, P2-0.7, and P3-0.9 at four different temperatures, the relationship between ln(τ*) and 1000/T was fitted to the Arrhenius equation and the corresponding viscous flow Ea was calculated from the slope (eqn (5)):
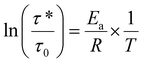 | (5) |
Here, τ* represents the characteristic stress relaxation time, τ0 is the Arrhenius factor, R the universal gas constant, and T the temperature. All synthesized materials exhibited an Arrhenius behavior typical of vitrimers with activation energies between 37.6 and 57.8 kJ mol−1 (Fig. 5D). Activation energies, affected both by the chemical kinetics of bond exchanges and the physical mobility of the polymer networks, describe the sensitivity of the viscosity of materials to temperature changes.28 Accordingly, a high Ea value indicates a strong decrease in viscosity with increasing temperature.28 In the series of the synthesized polymer networks, no trend was observed regarding the amount of introduced cross-linker. Indeed, P1-0.5 showed the highest Ea of 57.8 kJ mol−1 followed by P3-0.9 (55.9 kJ mol−1) and P2-0.7 (37.6 kJ mol−1). These values are the result of a balance between several parameters such as cross-linking density, amount of free OH groups, density of exchangeable units, and chain mobility that differ in the synthesized polymer networks. These values are lower than the activation energies in other systems containing acetals as dynamic linkages.28,29
The stress relaxation ability of covalent adaptable networks endows them with intrinsic reprocessability. This capacity was first evaluated through compression molding on the sample featuring the highest content of cross-linker P3-0.9. Therefore, P3-0.9 was cut into pieces and hot pressed for 40 minutes at 160 °C to yield a homogeneous reprocessed sample (Fig. 6A), which was subsequently characterized via ATR-IR (Fig. 6B), dynamic mechanical analysis (DMA), and tensile strength measurements (Fig. 6C and D) and compared to the native sample.
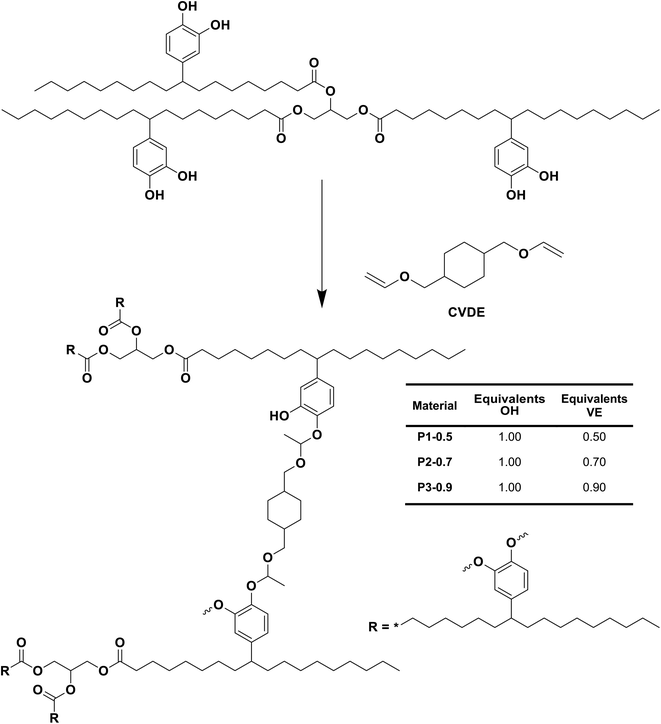
As shown in Fig. 6B, despite a slight increase of the OH band at 3400 cm−1, no significant change could be observed in the recorded ATR-IR spectrum of the P3-0.9_reprocessed sample compared to the native one, indicating that chemical structure was well preserved during reprocessing. Moreover, DMA revealed similar materials properties, i.e. storage modulus, loss modulus both in the glassy and rubbery state, and maximum of tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ (ΔTα = 2 °C) (Fig. 6C and Table 2) indicating no internal degradation of the polymer network structure. In line with DMA results, tensile tests of P3-0.9_reprocessed confirmed conservation of the mechanical properties after reprocessing, with only a slight increase of the Young′s moduli from 0.98 ± 0.06 MPa to 1.14 ± 0.06 MPa compared to pristine P3-0.9 (Fig. 6D and Table 2).
δ (ΔTα = 2 °C) (Fig. 6C and Table 2) indicating no internal degradation of the polymer network structure. In line with DMA results, tensile tests of P3-0.9_reprocessed confirmed conservation of the mechanical properties after reprocessing, with only a slight increase of the Young′s moduli from 0.98 ± 0.06 MPa to 1.14 ± 0.06 MPa compared to pristine P3-0.9 (Fig. 6D and Table 2).
Furthermore, we investigated the suitability of P2-0.7 and P3-0.9 for extrusion. A mini-extruder was filled with millimeter pieces of the CANs. After a residence time of 5 minutes at 190 °C, the covalent adaptable networks were extruded with a rotation speed of 20 rpm. In contrast to other acetal-based systems, which are not or only badly extrudable, P2-0.7 and P3-0.9 could successfully be extruded showing only minor cracks and defects on the surface (Fig. 7A, Fig. S11).28,29
Gel content and swelling ratio determinations, ATR-IR, DSC and TGA measurements after extrusion were performed on P2-0.7 (Table 3). The obtained gel content of 79% is slightly lower than the one of the native sample. The reduced gel content suggests that a certain degree of decross-linking occurred during extrusion. This observation is further supported by the slight increase of the OH band at 3400 cm−1 in the FTIR spectrum (Fig. 7B) and the small decrease of the Td,5% from 274 °C to 251 °C (Fig. S11), while the Tg stays unchanged (−4 °C) (Fig. S13). Extruded P2-0.7 featured a swelling ratio of 205 ± 10%, which lays in the same range as the one of the native sample, i.e. 274 ± 42%. Importantly, these variations are minor, and the gel content remains high overall, which indicates that the cross-linked structure of the polymer is mostly preserved and that the extrusion process does not significantly compromise the material integrity. The same characterizations performed on the extruded P3-0.9 sample (except the gel content determination due to technical issues) demonstrated analogous trends, thereby confirming the conclusions (Fig. S11–S13, Table 3). As shown by Zhu and coworkers, this extrusion capability is attributed to the neighboring group participation effect of the adjacent phenolic groups on the catechol unit. To the best of our knowledge, this study describes the first example of fully biobased extrudable CANs. This extrusion study provides a proof of concept demonstration of the biobased CAN processability. A dedicated process-oriented investigation will be required to further optimize and validate extrusion parameters and conditions at a larger scale.
| Gel contenta (%) | Swelling ratioa (%) |
T
g![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b (°C) b (°C) |
T
d,5%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c (°C) c (°C) |
|
|---|---|---|---|---|
| a Determined after 24 h immersion at room temperature in THF. b Determined by DSC. c Determined by TGA. N.D = not determined. | ||||
| P2-0.7 | 99 ± 2 | 274 ± 43 | −4 | 274 |
| P2-0.7-extrusion | 79 ± 2 | 205 ± 10 | −4 | 251 |
| P3-0.9 | N.D | N.D | −3 | 248 |
| P3-0.9-extrusion | N.D | N.D | −3 | 259 |
A key point of the twelve principles for circular chemistry introduced by Keijer et al. is to maximize atom circulation. Hence, the chemical recycling of the polymer network P3-0.9 to recover the starting HOSO-based polyol was subsequently targeted. In the literature, the hydrolysis of acetal moieties into hydroxyl and aldehyde groups in acidic environments has frequently been described and has also been implemented for the degradation of acetal-based polymer networks.17,27,39 Accordingly, P3-0.9 was placed in a 0.05 M HCl water/acetone (1/9, v/v) solution at 50 °C to degrade it into the starting HOSO-based polyol, 1,4-cyclohexanedimethanol and acetaldehyde (Fig. 8A). Full solubilization of P3-0.9 was observed after 2 h (Fig. 8B). The degradation product was extracted with a yield of 72% and characterized via1H, 13C NMR and ATR-IR spectroscopy. These techniques revealed the expected magnetic resonances and vibration bands of the starting HOSO-based polyol (Fig. S14, Fig. 8C, and Fig. S15, respectively). It is well-established that ester bonds can also be hydrolyzed under acidic conditions. However, the hydrolysis of acetal bonds proceeds under milder acidic conditions and within a shorter time compared to ester bonds.40 In this work, the integrity of the triglyceride core structure of the polyol was confirmed by the presence of a magnetic resonance around 174 ppm in the 13C NMR corresponding to the carbonyl of the ester moieties and the absence of a new peak that could be characteristic of the fatty acid formation, as well as by the presence C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration band around 1720 cm−1 in the ATR-IR spectrum. As a consequence, the ester bonds were not degraded under the previously mentioned acidic recycling conditions (Fig. S15). This was a key requirement that the recovered polyol can be further used for material synthesis.
O stretching vibration band around 1720 cm−1 in the ATR-IR spectrum. As a consequence, the ester bonds were not degraded under the previously mentioned acidic recycling conditions (Fig. S15). This was a key requirement that the recovered polyol can be further used for material synthesis.
Conclusions
In the current work, a fully renewable catechol containing high oleic sunflower oil-based polyol was synthesized within 30 minutes at 120 °C via a H2SO4 (≥95%) catalyzed Friedel–Crafts alkylation. With a Tg of −9 °C and a Td5% of 296 °C, the polyol could be readily employed for the bulk preparation of covalent adaptable networks. Subsequently, a thermally induced catalyst and solvent-free reaction between hydroxyl groups of the polyol and a bifunctional vinyl ether, i.e. 1,4-cyclohexanedimethanol divinyl ether, was implemented to synthesize fully renewable covalent adaptable networks (CANs) with different cross-linking densities. The successful cross-linking and the chemical composition of the CANs was herein confirmed by ATR-IR, swelling and gel content tests. The cross-linker content did not significantly influence the thermal properties (Tg and Td5%), but played an important role on gel content and the dynamic behavior assessed via stress relaxation measurements. Due to the neighboring group participation effect of the adjacent phenolic groups on the catechol unit, the CANs exhibited relatively short relaxation times. This characteristic provided the materials with good reprocessability via hot pressing and suitability for continuous processing by extrusion. In both cases, the chemical structure as well as the materials properties of the CAN could mostly be maintained. Moreover, the hydrolyzable acetal linkages could be selectively cleaved into hydroxyl and aldehyde groups in acidic conditions, to recover the starting biobased polyol including the conservation of the triglyceride structure. This feature can result in a closed-loop chemical recycling of the novel biobased polyol. As double bonds are common in naturally occurring compounds such as vegetable oils or terpenes, their functionalization to introduce catechol in an efficient manner is particularly relevant to prepare a broad range of fully biobased polyols. Interestingly, for covalent adaptable network synthesis, catechol can induce neighboring group participation effect and accelerate exchange reaction rates, thus opening the door to extrudable biobased materials, attribute that is crucial for industrial applications.Author contributions
T. S.: investigation, data collection, and writing the original draft; M. D. F.: rheology and sample reprocessing; C. L. C.: DMA analyses; E. I.: extrusion; M. A. R. M. and A. L.: conceptualization, supervision, validation, review and editing. All authors have read and approved the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information is available. See DOI: https://doi.org/10.1039/d5py00927h.Acknowledgements
The authors would like to thank the Karlsruhe House of Young Scientists (KHYS) for financial support and Mr Joshua Bedarride for his help with the optical microscopy.References
- C. J. Kloxin, T. F. Scott, B. J. Adzima and C. N. Bowman, Macromolecules, 2010, 43, 2643–2653 CrossRef CAS PubMed
.
- S. Kamarulzaman, Z. M. Png, E. Q. Lim, I. Z. S. Lim, Z. Li and S. S. Goh, Chem, 2023, 9, 2771–2816 CAS
.
- W. Denissen, J. M. Winne and F. E. Du Prez, Chem. Sci., 2016, 7, 30–38 RSC
.
- D. Montarnal, M. Capelot, F. Tournilhac and L. Leibler, Science, 2011, 334, 965–968 CrossRef CAS PubMed
.
- D. Yang, K. Zhao, R. Yang, S.-W. Zhou, M. Chen, H. Tian and D.-H. Qu, Adv. Mater., 2024, 36, 2403880 CrossRef CAS PubMed
.
- T. Wang, Y. Chen, B. Chen, M. J. Suazo, N. S. Purwanto and J. M. Torkelson, ACS Macro Lett., 2024, 13, 1147–1155 CrossRef CAS
.
- S. Guggari, F. Magliozzi, S. Malburet, A. Graillot, M. Destarac and M. Guerre, ACS Sustainable Chem. Eng., 2023, 11, 6021–6031 CrossRef CAS PubMed
.
- F. I. Altuna, V. Pettarin and R. J. Williams, Green Chem., 2013, 15, 3360–3366 RSC
.
- S. Debnath, S. Kaushal and U. Ojha, ACS Appl. Polym. Mater., 2020, 2, 1006–1013 CrossRef CAS
.
- J. Lyu, S. Lee, H. E. Bae, H. Jung, Y. I. Park, Y.-J. Jin, J.-E. Jeong and J. C. Kim, Angew. Chem., Int. Ed., 2024, 63, e202411397 CAS
.
- D. Yilmaz, M. Du Fraysseix, S. Lewandowski, S. Perraud, E. Ibarboure, A. Llevot and S. Carlotti, ACS Appl. Mater. Interfaces, 2024, 16, 21161–21174 CrossRef CAS
.
- Y. Ding, R. Miao, J. Liu, Z. Xin and C. Bao, ACS Appl. Polym. Mater., 2024, 6, 9008–9016 CrossRef CAS
.
- M. D. Fraysseix, S. Lewandowski, S. Perraud, S. Carlotti and A. Llevot, Macromol. Rapid Commun., 2025, 2500173 CrossRef CAS PubMed
.
- S. Geng, Z. Huang, Y. Chen, Y. Li, L. Zhang, M. Fei, R. Qiu and W. Liu, ACS Appl. Polym. Mater., 2024, 6, 14760–14771 CrossRef CAS
.
- Z. Fang, Y. Shi, H. Mu, R. Lu, J. Wu and T. Xie, Nat. Commun., 2023, 14, 1313 CrossRef CAS
.
- W. Denissen, M. Droesbeke, R. Nicolaÿ, L. Leibler, J. M. Winne and F. E. Du Prez, Nat. Commun., 2017, 8, 14857 CrossRef CAS
.
- Z. M. Png, J. Zheng, S. Kamarulzaman, S. Wang, Z. Li and S. S. Goh, Green Chem., 2022, 24, 5978–5986 RSC
.
- X.-L. Zhao, P.-X. Tian, Y.-D. Li and J.-B. Zeng, Green Chem., 2022, 24, 4363–4387 RSC
.
- W. Zhang, F. Gao, X. Chen, L. Shen, Y. Chen and Y. Lin, ACS Sustainable Chem. Eng., 2023, 11, 3065–3073 CrossRef CAS
.
- A. Moreno, M. Morsali and M. H. Sipponen, ACS Appl. Mater. Interfaces, 2021, 13, 57952–57961 CrossRef CAS
.
- T. Han, B. Ju and S. Zhang, Int. J. Biol. Macromol., 2024, 261, 129563 CrossRef CAS PubMed
.
- S. S. Kashyap, K. Borah, J. Shivam Shailesh Kumar, S. Sarmah, M. K. Bera, P. Basak and R. Narayan, ACS Appl. Polym. Mater., 2024, 6, 3894–3908 CrossRef CAS
.
- M. Schelhaas and H. Waldmann, Angew. Chem., Int. Ed. Engl., 1996, 35, 2056–2083 CrossRef CAS
.
- R. T. Ananthu, Y. An, Y. Tajima, H. Taguchi, T. Kikuchi, T. Kubo, H. Masunaga, S. Sasaki, A. Takahara and K. Satoh, Macromolecules, 2025, 58, 2599–2608 CrossRef CAS
.
- S. Samanta, D. R. Bogdanowicz, H. H. Lu and J. T. Koberstein, Macromolecules, 2016, 49, 1858–1864 CrossRef CAS
.
- A. C. Renner, S. S. Thorat and M. P. Sibi, RSC Sustainability, 2024, 2, 3669–3703 RSC
.
- Q. Li, S. Ma, S. Wang, W. Yuan, X. Xu, B. Wang, K. Huang and J. Zhu, J. Mater. Chem. A, 2019, 7, 18039–18049 RSC
.
- Q. Li, S. Ma, S. Wang, Y. Liu, M. A. Taher, B. Wang, K. Huang, X. Xu, Y. Han and J. Zhu, Macromolecules, 2020, 53, 1474–1485 CrossRef CAS
.
- Q. Li, S. Ma, P. Li, B. Wang, Z. Yu, H. Feng, Y. Liu and J. Zhu, Macromolecules, 2021, 54, 8423–8434 CrossRef CAS
.
- S. Yu, S. Wu, C. Zhang, Z. Tang, Y. Luo, B. Guo and L. Zhang, ACS Macro Lett., 2020, 9, 1143–1148 CrossRef CAS PubMed
.
- T. Sehn, N. Kolb, A. Azzawi and M. A. R. Meier, Macromolecules, 2024, 57, 10802–10811 CrossRef CAS
.
- K. H. Kim, K. Jeong, J. Zhuang, H. J. Jeong, C. S. Kim, B. Koo and C. G. Yoo, Ind. Crops Prod., 2021, 159, 113095 CrossRef CAS
.
- P.-C. Bian, W.-J. Xu, H.-Z. Gang, J.-F. Liu, B.-Z. Mu and S.-Z. Yang, Int. J. Mass Spectrom., 2017, 415, 85–91 CrossRef CAS
.
-
C. Berti, E. Binassi, M. Colonna, M. Fiorini, G. Kannan, S. Karanam, M. Mazzacurati, I. Odeh and M. Vannini, US2010168372A1, 2010
.
- N. Kasmi, M. Majdoub, G. Z. Papageorgiou and D. N. Bikiaris, Polym. Degrad. Stab., 2018, 152, 177–190 CrossRef CAS
.
- L. Santos Correa, S. Leidenheimer and M. A. R. Meier, Polym. Chem., 2025, 16, 821–832 RSC
.
- K. S. Fancey, J. Mater. Sci., 2005, 40, 4827–4831 CrossRef CAS
.
- L. Li, X. Chen, K. Jin and J. M. Torkelson, Macromolecules, 2018, 51, 5537–5546 CrossRef CAS
.
- L. Yue, Y.-L. Su, M. Li, L. Yu, S. M. Montgomery, X. Sun, M. G. Finn, W. R. Gutekunst, R. Ramprasad and H. J. Qi, Adv. Mater., 2023, 35, 2300954 CrossRef CAS PubMed
.
- G. Zhang, C. Tian, H. Feng, T. Tan, R. Wang and L. Zhang, Macromol. Rapid Commun., 2022, 43, 2100887 CrossRef CAS PubMed
.
| This journal is © The Royal Society of Chemistry 2026 |