Hierarchical Ni–Fe layered double hydroxide/MnO2 sphere architecture as an efficient noble metal-free electrocatalyst for ethanol electro-oxidation in alkaline solution†
Zhijun Jia,
Yi Wang* and
Tao Qi
National Engineering Laboratory for Hydrometallurgical Cleaner Production Technology, Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100190, China. E-mail: wangyi@ipe.ac.cn; Fax: +86-10-82544848-802; Tel: +86-10-82544967
First published on 17th September 2015
Abstract
Ni–Fe layered double hydroxide (LDH) nanosheets and hierarchical Ni–Fe LDH@MnO2 spheres are synthesized by a facile and cost-effective approach for highly efficient ethanol electro-oxidation. The LDH@MnO2 microspheres display excellent catalytic activity and robust durability for ethanol electro-oxidation, compared with the Ni–Fe LDH nanosheets. According to the analyses for nitrogen adsorption isotherms and electrochemical impedance spectra (EIS), it is inferred that the performance enhancement could be attributed to the MnO2 increasing the concentration of OHads species on the Ni–Fe LDH surface. These OHads can react with C1ad intermediate species to produce CO2 or water soluble products, releasing the active sites on LDH for further electrochemical reactions. Therefore, it is expected that an effective noble-metal free catalyst for ethanol electro-oxidation could be obtained by tailoring structure and properties of LDHs and their composites.
Introduction
Considerable efforts have been devoted to exploiting green, sustainable and efficient power sources owing to the increasing global demand for energy, coupled with the depletion of fossil fuels and the associated detrimental environmental impact.1 Ethanol is a promising fuel for low temperature direct fuel cell reactions due to its high energy density, low toxicity, ease of storage and availability from different sources.2–6 In direct ethanol fuel cells (DEFCs), noble metal based materials (Pt, Pd and their alloys) are the most effective catalysts due to their superior properties in the adsorption and dissociation of small organic molecules, but the high cost, limited availability as well as unsatisfactory cycle life restrict their practical applications.2–13 In recent years, lots of explorations have been made to obtain advanced noble metal-based hetero-structures for improving the performance of DEFCs, such as graphene-supported Pt nanoparticles, free-standing Pd–Au bimetallic nanotubes and noble metal membranes.14–22 Despite all this progress, these catalysts are also noble metal-based. How to develop effective noble metal-free anode catalysts for ethanol electro-oxidation remains a challenging goal.Layered double hydroxides (LDHs) are a family of layered materials consisting of positively charged brucite-type octahedral layers where the charge-balancing anions and water molecules occupy the interlayer space.14,15 They have been widely used in the fields of electrochemical sensors,16 super-capacitors17 and alkaline secondary batteries,18 owing to their novel structure and desirable properties. Recently, LDHs have been reported as promising noble metal-free electrode materials for the electrocatalytic oxidation of alcohol in alkaline medium.14,15,19 However, a key challenge for LDHs in the application of anode catalysts is to enhance their electrochemical activity by tailoring their structures and properties, such as having a well-defined hierarchical architecture with high surface area and suitable pore-size distribution, in which all the electroactive species participate in the faradaic redox reaction and a fast mass transport and electron transfer are guaranteed, and finding a co-catalyst to influence intermediate adsorption–desorption.
Herein, we synthesized Ni–Fe LDH nanosheets and hierarchical Ni–Fe LDH@MnO2 microspheres by a facile and cost-effective approach for highly efficient ethanol electro-oxidation. Furthermore, we hope to gain essential insight into the synergistic mechanism for electro-catalytic activity enhancement.
Experimental
Ni–Fe LDH synthesis
In a typical synthesis, co-precipitated Ni–Fe LDH was synthesized by simultaneous dropwise addition of 30 mL of 1.0 M Ni(NO3)2 and 30 mL of 0.5 M Fe(NO3)3 solutions to 100 mL of 1.5 M Na2CO3 solution while constantly stirring. The pH was adjusted to ∼10 by addition of NaOH solution. Then the vessel was transferred to a water bath at 65 °C for 6 hours. Finally the precipitate was filtered, washed with deionized water, and dried in air at 80 °C.Ni–Fe LDH@MnO2 sphere synthesis
To synthesize the composite electrocatalyst, 200 mg of MnO2 spheres, prepared according to previous works,5 were dispersed into 100 mL deionized water and ultra-sonicated for 8 min. Then Ni–Fe LDH was synthesized and coated on the surface of MnO2 sphere. The composite was harvested and denoted as LDH@MnO2.Material characterization
Powder X-ray diffraction (XRD) patterns of the as-prepared samples were collected on SmartLab XRD diffractometer using a Cu Kα source, with a scan step of 0.02° and a scan range between 5 and 90°. The morphology of the samples was investigated using a transmission electron microscopy (TEM JEOL JEM-2010 HR-TEM). The accelerating voltage was 200 kV. The specific surface area was measured using the Brunauer–Emmett–Teller (BET) method based on the nitrogen adsorption–desorption isotherm at 77 K on a Micrometritics ASAP2020 sorption analyzer.Electrochemical characterization
Electrochemical measurements were performed on an electrochemical workstation (CHI 660D, CH Instruments Inc., Shanghai) using a traditional three-electrode mode. Pt plate was used as a counter electrode, Hg/HgO as a reference electrode, and glassy carbon (3 mm in diameter) coated with the as-prepared samples, as a working electrode. The working electrode was fabricated as follows: 4 mg of catalyst was dispersed in 2 mL of ethanol solution and sonicated for 5 min; 10 μL of the suspension was dripped onto the surface of a glassy carbon electrode and dried for 15 min; subsequently 2 μL of 0.5% Nafion solution (Sigma-Aldrich) was coated on the electrode surface and dried for another 5 min. Electrocatalytic oxidation of ethanol on the working electrode was measured in 1.0 M KOH + 1.0 M ethanol solution by cyclic voltammetry in the potential range from 0.2 to 0.7 V. Electrochemical impedance spectra (EIS) were measured at 0.5 V from 100 kHz to 0.01 Hz and the perturbing AC amplitude was 5 mV. Electrical current density was calculated by normalizing electrical current on the area of the 3 mm diameter glassy carbon electrode. The chronoamperometry (CA) was conducted at 0.5 V for 3600 s.Results and discussion
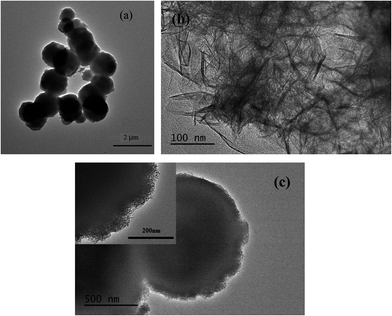
The typical XRD pattern of the as-prepared Ni–Fe LDH is presented in Fig. 1(a), showing the characteristic diffraction peaks at 2θ of 11.5, 23.3 and 34.6° corresponding to the (003), (006) and (012) plane reflections of 2D hydrotalcite-like materials, evidencing the formation of LDH particles (JCPSD card no.: 40-0215). As shown in Fig. 1(b), all diffraction peaks can be unambiguously assigned to the single-phase γ-MnO2 (JCPSD card no.: 14-0644). According to the XRD pattern shown in Fig. 1(c), it is found that the LDH@MnO2 sphere is the phase mixture of γ-MnO2 and Ni–Fe LDH. There is no new phase generated in the synthesis process.The TEM image, as shown in Fig. 2(a), displays MnO2 particles that are mainly spherical and the diameter of these microspheres is about 1–2 μm. Fig. 2(b) presents the morphology of Ni–Fe LDH and the nanosheet structure of this as-prepared sample is apparent. For LDH@MnO2, as shown in Fig. 2(c), it holds the spherical morphology of the MnO2 and is coated with Ni–Fe LDH nanosheets on its surface. Due to the adsorption of Ni2+ and Fe3+ on the MnO2 spheres surface, Ni–Fe LDH nanosheets could cover the MnO2 sphere uniformly when the precipitant was added. Furthermore, the heterogeneous interface between MnO2 and the solution could facilitate the nucleation of Fe–Ni LDH. This may be another reason for MnO2 to be covered uniformly with Fe–Ni LDH.
The N2 adsorption and desorption isotherms shown in Fig. 3(a) and (c) clearly indicate the presence of mesopores in the prepared samples, classified as type IV as defined by the International Union of Pure and Applied Chemistry (IUPAC). A hysteretic loop between the adsorption and desorption branches can be considered type H4, indicative of slit-like pores in the samples. The sample of Ni–Fe LDH shows a BET surface area of 208.33 m2 g−1, which is much larger than that of the LDH@MnO2 composite (103.47 m2 g−1).The pore volumes of the as-prepared Ni–Fe LDH and LDH@MnO2 microsphere are 0.883 and 0.208 cm3 g−1, respectively. As shown in Fig. 3(b) and (d), the pore size distributions from the adsorption branch of the isotherms calculated using the Barrett–Joyner–Halenda (BJH) method reveal that the pore sizes of Ni–Fe LDH concentrate around 5–30 nm and for LDH@MnO2, most of the pores have diameters under 20 nm.
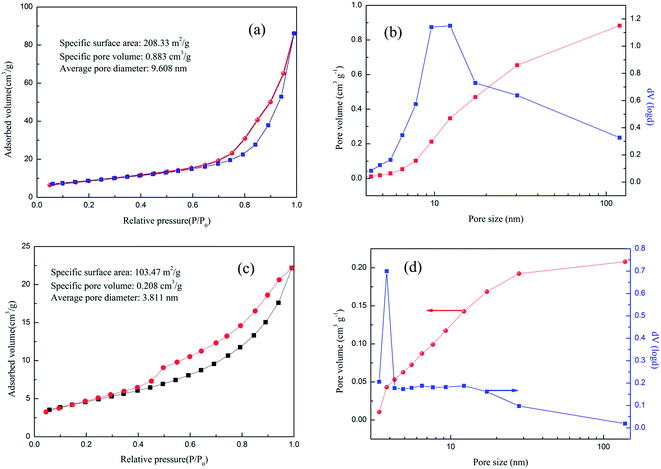 | ||
| Fig. 3 N2 adsorption–desorption isotherms of (a) Ni–Fe LDH; (c) LDH@MnO2 and pore size distribution of (b) Ni–Fe LDH; (d) LDH@MnO2. | ||
The catalytic activities of the as-prepared catalysts towards ethanol electro-oxidation were subsequently evaluated in an alkaline medium. The oxidation current was normalized to the electrode surface area; this allowed the current density to be directly used to compare the catalytic activity of different samples. The cyclic voltammograms of ethanol oxidation on the prepared catalysts are shown in Fig. 4. As shown in Fig. 4(a), the cyclic voltammograms of MnO2 in alkaline solutions indicate that MnO2 does not have catalytic activity for ethanol electro-oxidation. From Fig. 4(b) and (c), it is seen that the CVs of Ni–Fe LDH and LDH@MnO2 recorded in 1.0 M KOH solution without ethanol consist of a pair of redox peaks, corresponding to the reversible redox of Fe2+/Fe3+ associated with OH−. In the presence of 1.0 M ethanol, both Ni–Fe LDH and LDH@MnO2 display electrocatalytic behavior to ethanol oxidation. The onset potentials of the forward anodic peak for both Ni–Fe LDH and LDH@MnO2 were 0.557 V vs. Hg/HgO. Furthermore, as shown in Fig. 4(d), the electrocatalytic behavior for ethanol oxidation is significantly enhanced for LDH@MnO2 spheres in comparison with the Ni–Fe LDH nanosheets. Generally speaking, a larger specific surface area and pore volume can provide more electroactive sites as well as effective diffusion channels for electrolyte ions. In particular, abundant mesopores benefit the mass diffusion and electron transfer, which guarantees highly efficient electro-oxidation reactions. However, according to the analysis of the nitrogen adsorption isotherms (Fig. 3), though the LDH@MnO2 spheres possess a smaller specific surface area and mesopore volume than the Ni–Fe LDH nanosheets, the electrocatalytic activity of the former is remarkably superior to that of the latter. Therefore, the performance improvement of LDH@MnO2 cannot be attributed to the difference of surface and pore properties.
EIS was further applied to analyze the electrocatalytic performance of Ni–Fe LDH and LDH@MnO2. Fig. 4(e) represents the EIS plots of Ni–Fe LDH and LDH@MnO2 at 0.5 V in 1.0 M KOH + 1.0 M ethanol solution and the data were analyzed by an equivalent circuit shown in Fig. 4(f). In the equivalent circuit, Rs is the sum of resistance of electrolyte, electrode material and the contact resistance at the interface of the active material/current collector; Q is a constant angle element, which represents the double layer capacitance; Rt is the charge transfer resistance; Rc is the resistance of intermediate ad-layer and L is the inductance induced by the intermediate. The values of Rs, Q, Rt, Rc and L were calculated from the CNLS fitting of the experimental impedance spectra and their resulting values are listed in Table 1.
| Rs (Ω) | Q (Y s−n) | n | Rt (Ω) | Rc (Ω) | L (H) | |
|---|---|---|---|---|---|---|
| Ni–Fe LDH | 6.149 | 2.88 × 10−5 | 0.7833 | 2157 | 1747 | 1.463 × 104 |
| LDH@MnO2 | 6.682 | 2.523 × 10−5 | 0.7883 | 2053 | 1154 | 9610 |
According to the fitting results in Table 1, it can be seen that the resistances for LDH@MnO2, no matter whether Rt or Rc, are smaller than those for Ni–Fe LDH. In other words, the former has a smaller charge transfer resistance and intermediate ad-layer resistance. Moreover, it is found from the EIS results that mass diffusion is not a key factor for the electro-oxidation process and thus both samples may provide effective diffusion channels despite the differences of surface area and pore volume. Additionally, it is well known that the adsorption of OHads species onto MnO2 is more favorable.6 Therefore, it is inferred that MnO2 could increase the concentration of OHads species on Ni–Fe LDH surface, and these OHads can react with C1ad intermediate species to produce CO2 or water soluble products, releasing the active sites on LDH for further electrochemical reactions.5,13–15,23 This results in a better electrocatalytic performance in the ethanol electro-oxidation process than Ni–Fe LDH. Further investigations of the mechanism are ongoing at our lab.
To evaluate the electrocatalytic activity and stability of Ni–Fe LDH and LDH@MnO2 composite under continuous operating conditions, CV cycling tests were carried out in a 1.0 M KOH + 1.0 M ethanol solution, as shown in Fig. 4(g). After 100 potential cycles, 90.8% of the initial catalytic activity was still maintained for the LDH@MnO2 spheres. This is highly superior to the Ni–Fe LDH nanosheets (60.2%), indicating the greatly improved stability of the LDH@MnO2 spheres. In addition, the cycling stability of these two samples was also studied by testing CA curves. Fig. 4(h) shows the current density curves versus time recorded at 0.5 V for 3600 s. It was found that the oxidation current density on the LDH@MnO2 spheres is much higher than that on Ni–Fe LDH nanosheets over the whole time range, further demonstrating a significantly enhanced electrocatalytic activity. Moreover, this also indicates that the LDH@MnO2 catalyst possesses good long-term durability for ethanol electro-oxidation in alkaline media.
Conclusions
In this work, Ni–Fe LDH nanosheets and hierarchical Ni–Fe LDH@MnO2 spheres were synthesized by a facile and cost-effective approach for highly efficient ethanol electro-oxidation. According to the CV curves, it is shown that MnO2 has no catalytic activity for ethanol electro-oxidation, and the LDH@MnO2 microspheres display excellent catalytic activity and robust durability for ethanol electro-oxidation, compared with the Ni–Fe LDH nanosheets. This can be ascribed to MnO2 increasing the concentration of OHads species on the Ni–Fe LDH surface, these OHads can react with C1ad intermediate species to produce CO2 or water soluble products, releasing the active sites on LDH for further electrochemical reactions. It is expected that an effective noble-metal free catalyst for ethanol electro-oxidation could be obtained by tailoring the structure and properties of LDHs and their composites.Acknowledgements
The authors are grateful for the financial support by One Hundred Talent Program of Chinese Academy of Sciences, Chinese National Programs for High Technology Research and Development (2014AA06A513), as well as by the NSFC (51302264) of China.References
- Y. L. Wang, D. D. Zhang, W. Peng, L. Liu and M. G. Li, Electrocatalytic oxidation of methanol at Ni–Al layered double hydroxide film modified electrode in alkaline medium, Electrochim. Acta, 2011, 56, 5754–5758 CrossRef CAS PubMed.
- Y. Wang, T. S. Nguyen, C. Wang and X. Wang, Ethanol electrooxidation on Pt/C catalysts promoted with praseodymium oxide nanorods, Dalton Trans., 2009, 7606–7609 RSC.
- A. L. Wang, H. Xu, J. X. Feng, L. X. Ding, Y. X. Tong and G. R. Li, Design of Pd/PANI/Pd sandwich-structured nanotube array catalysts with special shape effects and synergistic effects for ethanol electrooxidation, J. Am. Chem. Soc., 2013, 135, 10703–10709 CrossRef CAS PubMed.
- J. D. Cai, Y. Z. Zeng and Y. L. Guo, Copper@palladium–copper core–shell nanospheres as a highly effective electrocatalyst for ethanol electro-oxidation in alkaline media, J. Power Sources, 2014, 270, 257–261 CrossRef CAS PubMed.
- Y. L. Wang, D. D. Zhang, M. Tang, S. D. Xu and M. G. Li, Electrocatalysis of gold nanoparticles/layered double hydroxides nanocomposites toward methanol electro-oxidation in alkaline medium, Electrochim. Acta, 2010, 55, 4045–4049 CrossRef CAS PubMed.
- Y. C. Zhao, L. Zhan, J. N. Tian, S. L. Nie and Z. Ning, MnO2 modified multi-walled carbon nanotubes supported Pd nanoparticles for methanol electro-oxidation in alkaline media, Int. J. Hydrogen Energy, 2010, 35, 10522–10526 CrossRef CAS PubMed.
- C. H. Cui, J. W. Yu, H. H. Li, M. R. Gao, H. W. Liang and S. H. Yu, Remarkable enhancement of electrocatalytic activity by tuning the interface of Pd–Au bimetallic nanoparticle tubes, ACS Nano, 2011, 5, 4211–4218 CrossRef CAS PubMed.
- R. Z. Jiang, D. T. Tran, J. P. McChure and D. Chu, A class of (Pd–Ni–P) electrocatalysts for the ethanol oxidation reaction in alkaline media, ACS Catal., 2014, 4, 2577–2586 CrossRef CAS.
- L. Z. Li, M. X. Chen, G. B. Huang, N. Yang, L. Zhang, H. Wang, Y. Liu, W. Wang and J. P. Gao, A green method to prepare Pd–Ag nanoparticles supported on reduced graphene oxide and their electrochemical catalysis of methanol and ethanol oxidation, J. Power Sources, 2014, 263, 13–21 CrossRef CAS PubMed.
- F. F. Ren, H. W. Wang, C. Y. Zhai, M. S. Zhu, R. R. Yue, Y. K. Du, P. Yang, J. K. Xu and W. S. Lu, Clean method for the synthesis of reduced graphene oxide-supported PtPd alloys with high electrocatalytic activity for ethanol oxidation in alkaline medium, ACS Appl. Mater. Interfaces, 2014, 6, 3607–3614 CAS.
- N. Tian, Z. Y. Zhou, N. F. Yu, L. Y. Wang and S. G. Sun, Direct electrodeposition of Tetrahexahedral Pd nanocrystals with high-index facets and high catalytic activity for ethanol electrooxidation, J. Am. Chem. Soc., 2010, 132, 7580–7581 CrossRef CAS PubMed.
- X. M. Chen, Z. X. Cai, X. Chen and M. Oyama, Green synthesis of graphene–PtPd alloy nanoparticles with high electrocatalytic performance for ethanol oxidation, J. Mater. Chem. A, 2014, 2, 315–320 CAS.
- Y. L. Wang, H. Q. Ji, W. Peng, L. Liu, F. Gao and M. G. Li, Gold nanoparticle-coated Ni/Al layered double hydroxides on glassy carbon electrode for enhanced methanol electro-oxidation, Int. J. Hydrogen Energy, 2012, 37, 9324–9329 CrossRef CAS PubMed.
- K. Tdanaga, Y. Furukawa, A. Hayashi and M. Tatsumisago, Direct ethanol fuel cell using hydrotalcite clay as a hydroxide ion conductive electrolyte, Adv. Mater., 2010, 22, 4401–4404 CrossRef PubMed.
- M. F. Shao, F. Y. Ning, J. W. Zhao, M. Wei, D. G. Evans and X. Duan, Hierarchical layered double hydroxide microspheres with largely enhanced performance for ethanol elecrooxideation, Adv. Funct. Mater., 2013, 23, 3513–3518 CrossRef CAS PubMed.
- L. J. Chen, B. Sun, X. D. Wang, F. M. Qiao and S. Y. Ai, 2D ultrathin nanosheets of Co–Al layered double hydroxides prepared in L-asparagine solution: enhanced peroxidase-like activity and colorimetric detection of glucose, J. Mater. Chem. B, 2013, 1, 2268–2274 RSC.
- J. Memon, J. H. Sun, D. L. Meng, W. Z. Ouyang, M. A. Memon, Y. Huang, S. K. Yan and J. X. Geng, Synthesis of graphene/Ni–Al layered double hydroxide nanowires and their application as an electrode material for supercapacitors, J. Mater. Chem. A, 2014, 2, 5060–5067 CAS.
- B. Yang, Z. H. Yang, R. J. Wang and Z. B. Feng, Silver nanoparticle deposited layered double hydroxide nanosheets as a novel and high-performing anode material for enhanced Ni–Zn secondary batteries, J. Mater. Chem. A, 2014, 2, 785–791 CAS.
- G. Karim-eezhad, S. Pashazadeh and A. Pashazadeh, Electrocatalytic oxidation of methanol and ethanol by carbon ceramic electrode modified with Ni/Al LDH nanoparticles, Chin. J. Catal., 2012, 33, 1809–1816 CrossRef.
- L. M. Yang, Y. H. Tang, S. L. Luo, C. B. Liu, H. J. Song and D. F. Yan, Palladium nanoparticles supported on vertically oriented reduced graphene oxide for methanol electro-oxidation, ChemSusChem, 2014, 7, 2907–2913 CrossRef CAS PubMed.
- C. B. Liu, H. Zhang, Y. H. Tang and S. L. Luo, Controllable growth of graphene/Cu composite and its nanoarchitecture-dependent electrocatalytic activity to hydrazine oxidation, J. Mater. Chem. A, 2014, 2, 4580–4587 CAS.
- L. M. Yang, D. F. Yan, C. B. Liu, H. J. Song, Y. H. Tang, S. L. Luo and M. J. Liu, Vertically oriented reduced graphene oxide supported dealloyed palladium–copper nanoparticles for methanol electrooxidation, J. Power Sources, 2015, 278, 725–732 CrossRef CAS PubMed.
- Z. J. Jia, Y. Wang and T. Qi, Pd nanoparticles supported on Mg–Al–CO3 layered double hydroxide as an effective catalyst for methanol electro-oxidation, RSC Adv., 2015, 5, 62142–62148 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra15718h |
| This journal is © The Royal Society of Chemistry 2015 |