Synthesis and characterization of new modified silica coated magnetite nanoparticles with bisaldehyde as selective adsorbents of Ag(i) from aqueous samples†
Abstract
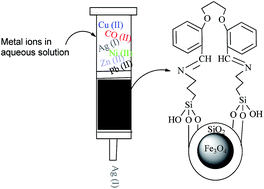
In this study, new silica-coated magnetic nanoparticles modified with bisaldehyde (BISA–APTSCMNPs) were synthesized using a normal method and a vice versa method. The crystal structure of the newly obtained nanoparticles was characterized by Fourier transform infrared spectroscopy (FT-IR), X-ray diffraction (XRD), Scanning Electron Microscopy (SEM), Transmission Electron Microscopy (TEM), Vibrating Sample Magnetometry (VSM), thermogravimetric analysis (TGA) and ultra-violet visible spectroscopy (UV-vis). The surface of the nanoparticles was modified with bisaldehyde (BISA–APTSCMNPs), and these were used for the highly selective removal of Ag(I) ion from an aqueous mixed metal ion solution containing Cu(II), Co(II), Ni(II), Zn(II) and Pb(II) using the syringe and batch techniques. Moreover, the silver ion desorption was most efficient in HCl.

 Please wait while we load your content...
Please wait while we load your content...