Composite electrospun membranes containing a monodispersed nano-sized TiO2@Li+ single ionic conductor for Li-ion batteries
Jiang Caoab,
Yuming Shanga,
Li Wang*a,
Xiangming He*ac,
Lingfeng Dengd and
Hong Chenbd
aInstitute of Nuclear and New Energy Technology, Tsinghua University, Beijing 100084, PR China. E-mail: hexm@tsinghua.edu.cn; wang-l@tsinghua.edu.cn
bState Key Laboratory of Powder Metallurgy, Centre South University, Changsha 410083, PR China
cState Key Laboratory of Automotive Safety and Energy, Tsinghua University, Beijing 100084, PR China
dCollege of Material Science and Engineering, Central South University of Forestry and Technology, Changsha, Hunan 410004, China
First published on 23rd December 2014
Abstract
In this work, we report novel composite electrospun membranes for Li-ion batteries. A monodispersed nano-sized TiO2@Li+ single ionic conductor containing a P(AALi-MMA) polymer layer grafted on a nano-sized TiO2 surface was prepared and used as functional fillers in composite membranes. The obtained results show that our material, based on the incorporation of a nano-sized TiO2@Li+ single ionic conductor into a composite electrospun membrane is a new generation battery separator for application in lithium-ion batteries.
1. Introduction
Nowadays, Li-ion secondary batteries have been used as power sources in electric vehicles (EVs) and hybrid electric vehicles (HEVs) due to their high energy density, high power density and long cycle life.1,2 However, the majority of Li-ion batteries employ thin (∼25 μm) microporous polyolefin separators (e.g. Celgard and Tonen amongst others), of which the poor wetting capability, small porosities and small pore sizes usually cause a significant decrease in ionic conduction compared to pure liquid electrolyte solutions (e.g. the ionic conductivity of the electrolyte solution is 1.2 × 10−2 S cm−1, but that of a PE separator filled with the electrolyte solution is only about 0.65 × 10−3 S cm−1). The net results are significant decreases in charge–discharge properties of cells.3,4 In addition to the poor ionic conductivity of polyolefin porous separator, the poor thermal stability is another serious problem, because the shrinking of separator by heating often causes short circuit, which may lead to ignition and explosion of power lithium-ion batteries and affect the safety performance of EVs.5,6 Therefore, separators used in power lithium-ion batteries still need improvement especially in terms of ionic conductivity at room temperature and thermal stability.Electrospinning is widely regarded as an alternate, efficient and simple technology for the manufacture of new generation battery separators. Compared to the conventional microporous polyolefin separators, separators based on electrospinning can offer higher level of porosity, higher ionic conductivity and superior thermal stability.3–6 Very recent applications of electrospinning separators or polymer electrolyte membranes for Li-ion batteries have been successfully prepared with submicron fibers from such polymers as PVDF,7–9 PAN,8,10 PVDF-HFP.2,6
In this work, we synthesized a monodispersed inorganic–organic nano-sized TiO2@Li+ single ionic conductor. The obtained single ionic conductor was dispersed in a solution of PVDF-HFP (10 wt%) dissolved in DMF and then coated on a supporting layer of pristine PVDF-HFP electrospinning membranes to form composite membranes. The structures and properties of the composite electrospun membranes (CEMs) were investigated. The results reported in this work confirm the superior performance of the TiO2@Li+ single ionic conductor doped PVDF-HFP based CEMs compared to conventional PE separators.
2. Experimental
2.1 Synthesis of nano-sized TiO2@Li+ single ionic conductor
Fig. 1 illustrates the synthetic scheme of nano-sized TiO2@Li+ single ionic conductor prepared by in situ radical polymerization and crystallization. Tetrabutyl titanate (TBOT) is added into an aqueous alcohol solution, of which the pH is adjusted to 3–4 by using NH4OH. The mixture is then hydrolyzed at 65 °C to form titania sol. After completion of this reaction, vinyl triethoxy silane, acrylic acid (AA), methyl methacrylate (MMA) monomers and benzoyl peroxide (as initiator) are successively added into the titania sol to polymerize for 4 hours at 80 °C to obtain a titania–PAA–PMMA dispersion. Then the dispersion is poured into a high pressure reactor to heat treatment at 145 °C. After heating treatment, the TiO2–P(AALi-MMA) single ion conductor is performed by an acid–base reaction for PAA and LiOH. | ||
| Fig. 1 Reaction scheme of nano-sized TiO2@Li+single ionic conductor prepared by in situ radical polymerization and crystallization. | ||
2.2 Preparation of electrospun PVDF-HFP composite membranes
Pristine electrospun PVDF-HFP membranes (PEMs) prepared by a typical electrospinning method at room temperature, as described in previous reports.11,12 The thickness of the pristine electrospun membranes are controlled to about 35 μm. With PEMs as supporting layer, PVDF-HFP with TiO2@Li+ fillers (mass rate = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) are dissolved in N,N-dimethyl formamide (DMF) at 20 wt% concentration to form a homogenous casting solution. Then the solution is casted on one side of the supporting layer to form CEMs at room temperature. After casting, the CEMs are submerged into distilled water bath immediately.13 The characterization of the CEMs are carried out after further submerged in absolute alcohol for 2 h and then dried in vacuum oven at 80 °C for 24 hours. The thickness of the dried CEMs are about 45 μm.
1) are dissolved in N,N-dimethyl formamide (DMF) at 20 wt% concentration to form a homogenous casting solution. Then the solution is casted on one side of the supporting layer to form CEMs at room temperature. After casting, the CEMs are submerged into distilled water bath immediately.13 The characterization of the CEMs are carried out after further submerged in absolute alcohol for 2 h and then dried in vacuum oven at 80 °C for 24 hours. The thickness of the dried CEMs are about 45 μm.
2.3 Cell assembly and electrochemical measurement
LiCoO2 electrode is prepared by coating a NMP-based slurry containing LiCoO2, PVDF, acetylene black and graphite (mass rate = 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) on an Al foil. 1.0 M LiPF6 dissolving in 1
1) on an Al foil. 1.0 M LiPF6 dissolving in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v/v) mixture of ethylene carbonate (EC), diethylene carbonate (DEC) and dimethyl carbonate (DMC) is used as electrolyte solution. LiCoO2/Li 2032-type coin cells with PVDF-HFP electrospun composite membrane or PE separator are assembled in a an argon filled glove box, of which the C-rate performance are galvanostatically measured in a voltage range of 2.75–4.2 V at a constant charge current density of 0.1 C for the first 5 cycles and 0.5 C for all of the following cycles, and discharge is at various current densities ranging from 0.1 to 8 C.
1 (v/v/v) mixture of ethylene carbonate (EC), diethylene carbonate (DEC) and dimethyl carbonate (DMC) is used as electrolyte solution. LiCoO2/Li 2032-type coin cells with PVDF-HFP electrospun composite membrane or PE separator are assembled in a an argon filled glove box, of which the C-rate performance are galvanostatically measured in a voltage range of 2.75–4.2 V at a constant charge current density of 0.1 C for the first 5 cycles and 0.5 C for all of the following cycles, and discharge is at various current densities ranging from 0.1 to 8 C.
3. Results and discussion
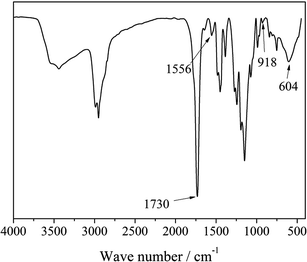
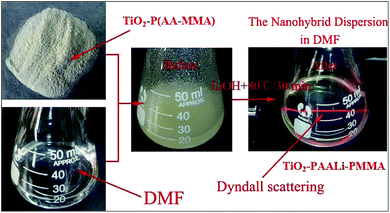
Fig. 2 shows the FTIR spectrum of TiO2@Li+ single ionic conductor. TiO2 is confirmed by –Ti–O–Ti– absorption band at 604 cm−1. The peaks at 1730 cm−1 and 1556 cm−1 are the characteristic absorption of –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and –COO–, respectively, which are attributed to P(AALi-MMA). The peak around 918 cm−1 is assigned to the stretching vibration band of –Si–O–Ti– which indicates that P(AALi-MMA) is grafted onto the TiO2 surface via vinyl triethoxy silane.13,14 Fig. 3 shows the dispersing process for TiO2@Li+ single ionic conductor. As 1 g TiO2–P(AA-MMA) nanohybrid disperses in 20 mL DMF solution, a light yellow emulsion can be formed. After adding a certain amount of LiOH into this emulsion, heating at 80 °C for 30 min, the emulsion transforms into a homogeneous and transparent solution, showing Tyndall scattering.13,14 Above results confirm that nano-sized TiO2@Li+ prepared by in situ radical polymerization and crystallization possesses well dispersity in DMF and it costs a little amount of energy to obtain a well dispersion without assistant of ultrasonication.
O and –COO–, respectively, which are attributed to P(AALi-MMA). The peak around 918 cm−1 is assigned to the stretching vibration band of –Si–O–Ti– which indicates that P(AALi-MMA) is grafted onto the TiO2 surface via vinyl triethoxy silane.13,14 Fig. 3 shows the dispersing process for TiO2@Li+ single ionic conductor. As 1 g TiO2–P(AA-MMA) nanohybrid disperses in 20 mL DMF solution, a light yellow emulsion can be formed. After adding a certain amount of LiOH into this emulsion, heating at 80 °C for 30 min, the emulsion transforms into a homogeneous and transparent solution, showing Tyndall scattering.13,14 Above results confirm that nano-sized TiO2@Li+ prepared by in situ radical polymerization and crystallization possesses well dispersity in DMF and it costs a little amount of energy to obtain a well dispersion without assistant of ultrasonication.
Fig. 4 shows the SEM images of PVDF-HFP based electrospun membranes. From Fig. 4(a), it can be found that PEM consists of nanofibers with smooth surfaces and well-controlled fiber diameter ranging from 200 to 400 nm. Meanwhile, the PEM exhibits big pore size and high porosities. After performing a CEM-separator by coating process (the casting solution soaks through the pristine PVDF-HFP electrospun membrane), as shown in Fig. 4(b)–(d), big pores are filled with TiO2@Li+/PVDF-HFP blends and an about 10 μm thickness of inorganic–organic nanocomposite layer is formed on the upper surface of the CEM, of which the total thickness is controlled to about 45 μm.
Fig. 5 shows the photographs of PE separator and CEM-separator after being exposed to 150 °C for 2 h. PE separator is transformed into a transparent film with thermal shrinkage of 25%, but the (area-based) dimensional change of CEM-separator is negligible after heating treatment at 150 °C heating treatment for 2 h. This confirms the superior thermal stability of CEM-separator as compared to PE separator. Table 1 lists the values of breaking strength, liquid uptake, ionic conductivity and lithium transport number of PVDF-HFP based electrospun membranes at room temperature. The stress at failure for the PVDF-HFP based electrospun membrane increase from approximately 8 MPa to approximately 39 MPa after compounding with TiO2@Li+/PVDF-HFP blends. This indicates that CEM-separator with acceptable mechanical strength can be used in commercial Li-ion batteries. However, an intriguing finding is that CEM-separator shows a decrease in the liquid uptake (280%), but increases in ionic conductivity (3.63 × 10−3 S cm−1) and lithium transport number (0.52) compared to PEM-separator. Generally speaking, improving liquid uptake can enhance the ionic conductivity of a polymer electrolyte membrane. Obviously, in here, this result is contradictory. Thus, it can be deduced that, when a composite membrane keeps enough liquid uptake, the improved ionic conductivity is related to the location of nano-sized fillers in membrane matrix but liquid uptake. In addition, the increased lithium transport number measured by combination of AC impedance and DC polarization for CEM-separator is attributed to the mobile lithium ions dissociating out of nano-sized TiO2@Li+ single ionic conductor, as well as the activated composite gel membrane.
| Sample | Breaking strength (MPa) | Liquid uptake (%) | Ionic conductivity (S cm−1) | Lithium transport number |
|---|---|---|---|---|
| PEM | 8 | 350 | 1.56 × 10−3 | 0.30 |
| CEM | 39 | 298 | 3.63 × 10−3 | 0.52 |
The higher ionic conductivity of Li-ion battery separator suggests a better rate performance and the C-rate performance of CEM-separator which is evaluated according to the assembled LiCoO2/Li cells with CEM-separator. As a contrast, the cells with PE separator are also assembled, and all the cells are charged under a voltage range of 2.75–4.2 V at a constant charge current density of 0.1 C for the first 5 cycles and 0.5 C for all of the following cycles and discharged at various current densities ranging from 0.1 to 8 C. Fig. 6(a) and (b) show the discharge curves for cells with PE separator and CEM-separator, respectively. It is obvious that the C-rate discharge performance of cells with CEM-separator is enhanced compared to that with PE separator. Fig. 6(c) summarizes the aforementioned discharge capacities of the two kind separators as a function of the discharge current density (i.e., the discharge C-rate). An intriguing finding is that, the cell with CEM separator exhibits a lower initial discharge capacity than the cell with PE separator at 0.1 C/0.1 C charge–discharge current density, but the capacity recovers after 3 cycles. Indeed, after the assembled composite membrane fully soaked in the liquid electrolyte in a cell, a composite gel polymer electrolyte membrane will be formed, and the interfacial compatibility between electrode and composite gel polymer electrolyte membrane play an important role in the capacity of a cell.15 Thus, a bad initial interface between the PVDF-HFP electrospun composite electrolyte membrane and the LiCoO2 electrode leads to a low initial capacity of the cell, but the gradual recovered capacity may be attributed to the gradual improved interfacial compatibility produced by monodispersed nano-TiO2 particle in membrane matrix during the charge–discharge process of cell.
4. Conclusions
A monodispersed nano-sized TiO2@Li+ single ionic conductor is successfully prepared by in situ radical polymerization and crystallization. The experimental results demonstrate that the PVDF-HFP based composite electrospun membranes containing TiO2@Li+ as fillers exhibit unique mechanical properties and battery performance, which confirms that the composite membranes are very promising separators for the use in rechargeable lithium-ion batteries, especially in power batteries.Acknowledgements
This work is supported by the MOST (Grant no. 2013CB934000, no. 2011CB935902, no. 2014DFG71590, no. 2010DFA72760, no. 2011CB711202, no. 2013AA050903, no. 2011AA11A257 and no. 2011AA11A254), the China Postdoctoral Science Foundation (Grant no. 2013M530599 and no. 2013M540929), the Tsinghua University Initiative Scientific Research Program (Grant no. 2010THZ08116, no. 2011THZ08139, no. 2011THZ01004 and no. 2012THZ08129), Beijing Municipal Program (Grant no. YETP0157, no. Z131100003413002 and no. Z131100003413001), State Key Laboratory of Automotive Safety and Energy (no. ZZ2012-011) and Suzhou (Wujiang) Automotive Research Institute (Project no. 2012WJ-A-01).References
- F. Croce, M. L. Focarete, J. Hassoun, I. Meschini and B. Scrosati, A safe, high-rate and high-energy polymer lithium-ion battery based on gelled membranes prepared by electrospinning, Energy Environ. Sci., 2011, 4, 921–927 CAS.
- B. S. Lalia, E. G. Burrieza, H. A. Arafat and R. Hashaikeh, Fabrication and characterization of polyvinylidenefluoride-co-hexafluoropropylene (PVDF-HFP) electrospun membranes for direct contact membrane distillation, J. Membr. Sci., 2013, 428, 104–115 CrossRef CAS PubMed.
- D. Bansal, B. Meyer and M. Salomon, Gelled membranes for Li and Li-ion batteries prepared by electrospinning, J. Power Sources, 2008, 178, 848–851 CrossRef CAS PubMed.
- Y. S. Lee, S. H. Ju, J. H. Kim, S. S. Hwang, J. M. Choi, Y. K. Sun, H. Kim, B. Scrosati and D. W. Kim, Composite gel polymer electrolytes containing core-shell structured SiO2(Li+) particles for lithium-ion polymer batteries, Electrochem. Commun., 2012, 17, 18–21 CrossRef CAS PubMed.
- S. J. Chun, E. S. Choi, E. H. Lee, J. H. Kim, S. Y. Lee and S. Y. Lee, Eco-friendly cellulose nanofiber paper-derived separator membranes featuring tunable nanoporous network channels for lithium-ion batteries, J. Mater. Chem., 2012, 22, 16618–16626 RSC.
- E. S. Choi and S. Y. Lee, Particle size-dependent, tunable porous structure of a SiO2/poly(vinylidene fluoride-hexafluoropropylene)-coated poly(ethylene terephthalate) nonwoven composite separator for a lithium-ion battery, J. Mater. Chem., 2011, 21, 14747–14754 RSC.
- J. R. Kim, S. W. Choi, S. M. Jo, W. S. Lee and B. C. Kim, Electrospun PVDF-based fibrous polymer electrolytes for lithium ion polymer batteries, Electrochim. Acta, 2004, 50, 69–75 CrossRef CAS PubMed.
- A. I. Gopalan, P. Santhosh, K. M. Manesh, J. H. Nho, S. H. Kim, C. G. Hwang, K. P. Lee, M. Manesh, J. H. Nho, S. H. Kim, C. G. Hwang and K. P. Leeparato, Development of electrospun PVDF, J. Membr. Sci., 2008, 325, 683–690 CrossRef CAS PubMed.
- S. W. Lee, S. W. Choi, S. M. Jo, B. D. Chin, D. Y. Kim and K. Y. Lee, Electrochemical properties and cycle performance of electrospun poly(vinylidene fluoride)-based fibrous membrane electrolytes for Li-ion polymer battery, J. Power Sources, 2006, 163, 41–46 CrossRef CAS PubMed.
- P. Carol, P. Ramakrishnan, B. John and G. Cheruvally, Preparation and characterization of electrospun poly(acrylonitrile) fibrous membrane based gel polymer electrolytes for lithium-ion batteries, J. Power Sources, 2011, 196, 10156–10162 CrossRef CAS PubMed.
- X. Li, G. Cheruvally, J. K. Kim, J. W. Choi, J. H. Ahn, K. W. Kim and H. J. Ahn, Polymer electrolytes based on an electrospun poly(vinylidene fluoride-co-hexafluoropropylene) membrane for lithium batteries, J. Power Sources, 2007, 167, 491–498 CrossRef CAS PubMed.
- A. I. Gopalan, K. P. Lee, K. M. Manesh and P. Santhosh, Poly(vinylidene fluoride)–polydiphenylamine composite electrospun membrane as high-performance polymer electrolyte for lithium batteries, J. Membr. Sci., 2008, 318, 422–428 CrossRef CAS PubMed.
- J. Cao, L. Wang, X. He, M. Fang, J. Gao, J. Li, L. Deng, H. Chen, G. Tian, J. Wanga and S. Fane, In situ prepared nano-crystalline TiO2-poly(methyl methacrylate) hybrid enhanced composite polymer electrolyte for Li-ion batteries, J. Mater. Chem. A, 2013, 1, 5955–5961 CAS.
- J. Cao, L. Wang, M. Fang, X. He, J. Li, J. Gao, L. Deng d, J. Wang and H. Chen, Structure and electrochemical properties of composite polymer electrolyte based on poly vinylidene fluoride-hexafluoropropylene/titania–poly(methyl methacrylate) for lithium-ion batteries, J. Power Sources, 2014, 246, 499–504 CrossRef CAS PubMed.
- J. Cao, L. Wang, M. Fang, Y. Shang, L. Deng, J. Yang, J. Li, H. Chen and X. He, Interfacial compatibility of gel polymer electrolyte and electrode on performance of Li-ion battery, Electrochim. Acta, 2013, 114, 527–532 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |